Abstract
Prostaglandins are synthesized through the metabolism of arachidonic acid via the cyclooxygenase pathway. There are five primary prostaglandins, PGD2, PGE2, PGF2, PGI2, and thromboxane B2, that all signal through distinct seven transmembrane, Gprotein coupled receptors. The receptor through which the prostaglandins signal determines their immunologic or physiologic effects. For instance, the same prostaglandin may have opposing properties, dependent upon the signaling pathways activated. In this article, we will detail how inhibition of cyclooxygenase metabolism and regulation of prostaglandin signaling regulates allergic airway inflammation and asthma physiology. Possible prostaglandin therapeutic targets for allergic lung inflammation and asthma will also be reviewed, as informed by human studies, basic science, and animal models.
Keywords: prostaglandin, cyclooxygenase, lung, allergy, asthma
Prostaglandins are lipid products synthesized from nuclear and plasma membranes via by the metabolism of cyclooxygenase (COX) enzymes through the arachidonic acid metabolic pathway.(Ricciotti & Fitzgerald, 2011) These lipid mediators were identified in the 1930s and introductory studies focused on blood pressure regulation and constriction of smooth muscle.(Goldblatt, 1933; von Euler, 2014) Piper and Vane first suggested that prostaglandins regulated allergic disease in 1969.(Piper & Vane, 1969) They reported that anaphylaxis induced the production of prostaglandin (PG)E2 and PGF2α from guinea pig lungs and their synthesis was blunted by low doses of the COX inhibitors aspirin and indomethacin. Since that discovery, a multitude of pro- and anti-allergic effects was credited to prostaglandins. Initial investigations were handicapped by the short biologic half-lives of the prostaglandins, which can range from seconds to a few minutes. Understanding of how prostaglandins modulate allergeninduced inflammatory disease accelerated over the last 15 years, resulting from the generation of many transgenic mouse models whereby either a prostaglandin receptor gene or synthase are either overexpressed or eliminated. Additionally, improvement in methods of production of prostaglandin agonists that have more sustained biologic actions than a native prostaglandin, as well as specific receptor antagonists, greatly advanced knowledge of how this class of pharmacologic agents modulate allergic diseases. In this article, we will detail the pathways of prostaglandin generation, review studies that affirm the existence of these lipids in allergic inflammatory states, and discuss in vivo intervention studies in humans and recent murine studies that illuminate the activity of these mediators in the pathogenesis of allergic disease. These studies illustrate the potential of individual prostaglandins as possible future therapeutic targets for treatment of allergic diseases and asthma.
Generation of prostaglandins by phospholipase A2
Arachidonic acid is the antecedent in the generation of the prostaglandins and leukotrienes and termed eicosanoids as the Greek word for twenty is “eikosi”, the quantity of carbon atoms in arachidonic acid. There are multiple phospholipase A2 (PLA2) enzymes that hydrolyze fatty acids at the sn-2 position of membrane phospholipids, producing free fatty acids, including arachidonic acid.(Dennis, Cao, Hsu, Magrioti, & Kokotos, 2011) Six classes of PLA2s, secretory PLA2s (sPLA2), cytosolic PLA2s (cPLA2), Ca2+ independent PLA2 (iPLA2), platelet-activating factor acetylhydrolases (PAF-AH), lysosomal PLA2s, and adipose-specific PLA2 have been identified.(Dennis et al., 2011) Classification of the PLA2s is defined by the catalytic mechanism of the particular PLA2, as well as the functional and structural characteristics. Sixteen groups of PLA2 have been described; those resulting in lipid mediator generation include group IIA, group IVA, group V, group VI and group X.(Balestrieri et al., 2006; Dennis et al., 2011) The sPLA2s engage in paracrine or autocrine formation of arachidonic acid from the outer leaflet of plasma membranes. Therefore, the PLA2 enzymes are essential in generating arachidonic acid from membrane phospholipids.
CYCLOOXYGENASE PATHWAY
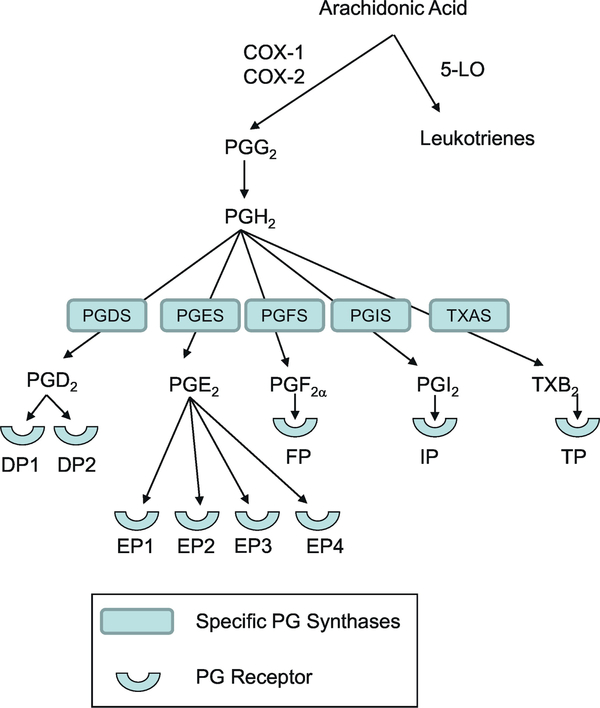
Both the COX and lipoxygenase (LO) pathways oxidatively metabolize arachidonic acid; however, the COX pathway is the focal point of this review.(W. L. Smith, Urade, & Jakobsson, 2011) COX catalyzes an initial cyclooxygenase reaction leading to the insertion of two oxygen molecules into arachidonic acid to generate prostaglandin PGG2, followed by an endoperoxidase reaction reducing PGG2 to PGH2 (Figure 1). PGH2 is the precursor for PGD2, PGE2, PGF2α, PGI2, and thromboxane A2 (TXA2) that are generated by tissue specific enzymes and isomerases. COX-1 and COX-2 are the two functional COX enzymes in humans. A third cyclooxygenase enzyme, COX-3, is encoded by the COX-1 gene, however, COX-3 is not believed to be functional in humans. COX-1 and COX-2 are derived from distinct genes and have distinctive functions based on their divergent temporal and tissue expression.(W. L. Smith et al., 2011) The COX-1 gene exists chromosome 9 in humans and is constitutively expressed in most tissues. COX-1 participates in homeostatic prostanoid synthesis, but may be induced in specific situations.(Kang, Mbonye, Delong, Wada, & Smith, 2007) Conversely, COX-2 expression is typically induced and the induction is transient. The COX-2 gene is located on human chromosome 1. Interleukin (IL)-1, IL-2, and TNF-α, as well as by lipopolysaccharide (LPS) induce the expression of COX-2.(Kang et al., 2007) COX-2 is predominantly an inducible enzyme, yet constitutive expression is noted in cultured human lung epithelial cells, cortical collecting duct cells in the thick ascending limb of the kidney, pancreatic islet cells, and in human gastric carcinoma.(Ferguson, Hebert, & Laneuville, 1999; Sorli et al., 1998; Soslow et al., 2000) Their major therapeutic effect of nonsteroidal anti-inflammatory drugs (NSAIDs) results from blunting COX-2 activity, whereas inhibition of COX-1 produces some of their undesired side effects.(Kang et al., 2007) It is important to note that COX-2 inhibition may be deleterious. For instance, cardiovascular disease was increased in patients ingesting COX-2-specific inhibitors, most likely from inhibiting the synthesis of the vasodilator PGI2, whereas the vasoconstrictive activities of the COX-1 product TXA2 were not inhibited.(Fitzgerald, 2004)
Figure 1.
Synthesis of prostaglandins. Arachidonic acid is metabolized by the cyclooxygenase enzymes sequentially to PGG2 and then PGH2. The individual prostaglandin synthases convert PGH2 into the five primary prostanoids, PGD2, PGE2, PGF2α, PGI2, and TXA2. Each of these prostanoids signal through distinct G protein coupled receptors (GPCR).
The COX pathway in human allergic inflammation
COX-2 expression in human airways has been examined to help define its role in the pathogenesis of allergic disease; yet, the results have been contradictory. One study reported a fourfold increase in COX-2 immunostaining in the bronchial epithelium of asthmatic subjects compared to healthy controls;(Sousa et al., 1997) however, another study found no difference.(Demoly et al., 1997) COX-2 mRNA expression and immunoreactive protein were increased in the airway epithelium of asthmatics that had not been treated with corticosteroids compared with non-asthmatic controls, suggesting that this medication class may inhibit COX-2 activity. In support of this concept, subjects with asthma treated with corticosteroids had decreased COX-2 expression compared to non-treated asthmatics.(Redington et al., 2001) The relationship between the expression of COX-2 and the cytokines involved in allergic disease is complicated. For instance, IL-4 and IL-13 blunted bronchial epithelial cells production of PGE2 by inhibiting both COX-2 and microsomal PGE synthase (mPGES) through JAK1 and STAT6 signaling.(W. Cho, Kim, Jeoung, Kim, & Choe, 2011) As a consequence, in patients with asthma, augmented TNF-α expression could induce COX-2, whereas IL-4 and IL-13 might inhibit COX-2 expression. It is possible that the inhibition of COX-2 expression by corticosteroids might be an indirect action of IL-4 and IL-13, yet in contrast, TNF-α might induce COX-2. This is supported by in vitro data in which COX-2 immunoreactivity in cultured airway epithelial cells was blunted by corticosteroid treatment.(Aksoy, Li, Borenstein, Yi, & Kelsen, 1999) Corticosteroids inhibited basal and bradykinin-induced levels of PGE2 in airway epithelial cells, implying that COX-2 is a primary source of PGE2 in the airway epithelium.(Aksoy et al., 1999) As will be detailed later, PGE2 has robust anti-inflammatory properties via signaling through its EP2 receptor. Decreased expression of COX-2 by corticosteroids may downregulate PGE2 production, likely removing PGE2-mediated restraining effect on inflammation. This is one plausible mechanism through which corticosteroids do not inhibit inflammation and could result in corticosteroid-resistant asthma. There is debate in the in vivo effect of corticosteroids on the expression of COX-1 and COX-2 in nasal polyps. While prednisone increased COX-2 mRNA expression in polyp tissue after two weeks of therapy, COX-1 mRNA expression was not altered.(Pujols et al., 2009) In contrast, topical corticosteroids significantly inhibited COX-1 expressing nasal polyp cells; however, they had no effect on COX-2 expressing cells in nasal polyps.(Ebbens et al., 2009)
COX-1 and COX-2 mRNA is not expressed by structural cells in the airway, but also by resting human T lymphocytes.(Iniguez, Punzon, & Fresno, 1999) While T cell activation did not alter COX-1 expression, T cell stimulation increased COX-2 mRNA levels with induced COX-2 protein and cyclooxygenase activity.(Iniguez et al., 1999) A number of airway cells, including macrophages, endothelial cells, airway fibroblasts, airway epithelial cells, airway smooth muscle cells, mast cells, and eosinophils have the potential for inducible COX-2 expression.(Kang et al., 2007; Sousa et al., 1997) Therefore, both resident airway cells and adaptive immune cells are capable of expressing COX.
Allergic inflammation increases the expression of COX products. There was a significant increase in prostanoids in the bronchoalveolar (BAL) fluid of subjects with allergic asthma compared to healthy control subjects without asthma. Further, prostanoid production is induced by airway allergen challenge. A 12- to 22-fold increase in BAL fluid PGD2 and PGF2α levels occurred in subjects with allergic asthma compared to nonallergic subjects, with a log increase in these same metabolites in subjects with allergic asthma compared to subjects without asthma who had allergic rhinitis.(M. C. Liu et al., 1990) Segmental allergen challenge, a process where an allergen to which the subject is sensitized is instilled via bronchoscopy to a segment of the lung, significantly increased the levels of PGD2, thromboxane (Tx) B2, and 6-keto-PGF1α, a PGI2 metabolite.(M. C. Liu et al., 1991) Prednisone treatment for three days prior to segmental allergen challenge did not change the prostanoid concentrations in the BAL fluid, implying that corticosteroids were unable to inhibit COX pathway activation resulting from an allergic inflammatory stimulus,(M. C. Liu et al., 2001) supporting the findings in patients with nasal polyps treated with prednisone as discussed in the last paragraph.
Inhibiting the COX pathway with medications such as indomethacin that inhibit both COX-1 and COX-2 has been investigated to determine the role of COX products on airway inflammation and physiologic changes resulting from allergen challenge. Indomethacin did not alter lung function before allergen challenge in subjects with allergic asthma or in allergic rhinitis who did not have asthma.(Fish, Ankin, Adkinson, & Peterman, 1981) In contrast, indomethacin treatment reduced the forced expiratory volume in one second (FEV1) and specific airway conductance in nonasthmatic subjects with allergic rhinitis following inhaled allergen challenge.(Fish et al., 1981) Indomethacin administration before allergen challenge caused a significant, but small, decrement in specific airway conductance in subjects with allergic asthmatic subjects compared to placebo; however, this non-specific COX inhibitor did not alter allergeninduced alterations in FEV1.(Fish et al., 1981) Indomethacin treatment did not change airway responsiveness to histamine, nor indomethacin modulate the immediate or late phase pulmonary response to allergen challenge in allergic asthmatics.(Kirby, Hargreave, Cockcroft, & O'Byrne, 1989; Sladek et al., 1990) In subjects with exercise-induced bronchoconstriction (EIB), bronchoconstriction after exercise was not altered by indomethacin treatment; however, indomethacin prevented refractoriness after exercise.(O'Byrne & Jones, 1986) In contrast, inhaled indomethacin significantly attenuated EIB in children with asthma.(Shimizu, Mochizuki, Shigeta, & Morikawa, 1997) Further, indomethacin significantly inhibited the mean maximal decrease in arterial oxygen saturation following exercise. These data imply that a reduction in local prostaglandin synthesis may be a mechanism by which inhaled indomethacin protected against exercise-induced airway dysfunction. Etoricoxib, a COX2 inhibitor, did not alter either baseline lung function or airway responsiveness to allergen or methacholine in 16 subjects with mild allergic asthma who underwent increasing dose inhalational challenges with allergen or methacholine.(Daham et al., 2014) These investigators reported that a selective COX-2 inhibitor had no effects on sputum eosinophils, allergen-induced airflow obstruction, basal lung function, or methacholine responsiveness. The complex effect of COX inhibition on lung function reflects the tissue-specific diversity of the individual prostanoids and the receptors through which they signal (see below). It is evident that some prostanoids may counteract the actions of others, or even the same prostanoid may have opposing physiologic or immunologic effects depending on the specific receptor through which it signals.
Animal studies of the COX pathway in allergic inflammation
Transgenic mice generated with targeted deletions of the COX-1 and COX-2 genes and then subjected to models of OVA sensitization and challenge have provided important information on how COX products regulate allergic inflammation. OVAsensitized and challenged COX-1 knock out (KO) mice had increased lung eosinophilia, augmented serum IgE levels, greater airway responsiveness, heightened numbers of CD4+ and CD8+ T cells, exaggerated levels of Th2 cytokines, and amplified concentrations of eotaxin and thymus- and activation-regulated chemokine (TARC, CCL17) compared to both COX-2 KO and WT mice.(Carey et al., 2003; Zeldin et al., 2001) These data imply that COX-1-derived PGs are essential in preserving homeostasis during allergic airway inflammation. COX-1 inhibition augmented allergic airway inflammation and airway responsiveness, suggesting that overexpression of COX-1 decreases allergic airway inflammation and inhibits airway responsiveness. Airway epithelial cell targeted COX-1 overexpression inhibited basal airway responsiveness; however, allergic inflammation was unchanged.(Card et al., 2006) The importance of COX-2 in regulating allergen-induced airway inflammation and bronchomotor tone was investigated in animal models. Allergen challenged COX-2 KO mice on a C57BL/6 background had increased serum IgE levels, vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1) expression compared to WT mice; however, airway eosinophils or airway responsiveness were not different between the two groups of mice.(Carey et al., 2003; Zeldin et al., 2001) Reinforcing this result, another group communicated that COX-2 KO mice, also on a C57BL/6 background, had augmented allergen-induced lung eosinophilia compared to WT mice.(Nakata et al., 2005) COX-2 KO mice had a significantly greater percentage of IL-9 expressing CD4+ cells in the lung, BAL fluid, lymph nodes and blood compared to WT mice resulting from ovalbumin sensitization and challenge.(Li et al., 2013) Additionally, COX-2 KO mice, or WT mice treated with COX-2 inhibitors (NS-398, CAY 10404 and SC-5812), had augmented BAL IL-9, serum IL-9, and lung IL-17RB expression compared to either WT controls or WT mice treated with placebo, respectively. These increases in COX-2 inhibitor enhanced IL-9 and lung IL-17RB expression were reduced by PGD2 and PGE2, which also inhibited human and mouse Th9 cell differentiation in vitro.(Li et al., 2013)
Experiments utilizing pharmacologic inhibition complement and, in general, reinforce the transgenic mouse models. WT BALB/c mice treated with the COX inhibitor indomethacin during both OVA sensitization and challenge had increased lung Th2 cytokines, augmented lung eosinophilia, and greater airway responsiveness to methacholine compared to vehicle-treated mice.(Peebles Jr. et al., 2000) BAL cysteinyl leukotriene (cysLT) levels were increased as a result of indomethacin treatment, yet 5-LO KO mice on a 129 genetic background that could not generate leukotrienes also had increased allergen-induced inflammation with indomethacin treatment. These results essentially eliminate indomethacin-enhanced leukotriene production as a cause for the exaggerated inflammatory response.(R.S. Peebles, Jr. et al., 2005) The increased allergic inflammation with indomethacin treatment was CD4+ cell-dependent, but was independent of IL-4, IL-4 receptor alpha, and STAT6, key elements in the Th2 signaling pathway.(Hashimoto et al., 2005) This heightened allergic phenotype was not indomethacin-specific, in that COX-1 and COX-2 inhibitors independently increased allergen-induced lung IL-13 and methacholine responsiveness compared to vehicletreated mice.(R.S. Peebles, Jr. et al., 2002) COX-2 inhibition in a murine model of atopic dermatitis induced by epicutaneous OVA sensitization produced heightened eosinophil skin infiltration, augmented total and antigen specific IgE, and a systemic Th2 response to antigen.(Laouini et al., 2005) The role of COX-2 in modulating airway tone has been examined in guinea pig models. COX-2 was induced in guinea pigs as a result of allergic inflammation and celecoxib, a COX-2 inhibitor, significantly reduced allergen-induced bronchoconstriction and generation of COX products.(Oguma et al., 2002; Selg, Lastbom, Ryrfeldt, Kumlin, & Dahlen, 2008) Additionally, COX-2 inhibition abolished PGE2-induced contraction.(Safholm, Dahlen, Delin, et al., 2013) In summary, several studies show that COX inhibition during the development of allergic disease augmented allergen-induced inflammation and airway responsiveness, suggesting that a COX product inhibits allergic inflammation and may be a therapeutic target for atopic diseases such as asthma and atopic dermatitis.
It is important to note that in the majority of these animal models of allergendriven inflammation, COX was inhibited prior to the initial antigen exposure throughout allergen challenge. In human studies utilizing indomethacin, COX inhibition occurred only during allergen challenge, long after initial antigen exposure and after the regulatory elements of allergic inflammation in the lung had been set in place. It is important to recognize that there are important differences between mouse and human airway physiology. For example, PGD2 causes bronchoconstriction in humans, yet it fails to constrict mouse airways.(Martin, Gerard, Galli, & Drazen, 1988) Therefore, animal models of allergic lung disease, in which COX activity is pharmacologically inhibited or knocked out by gene deletion, might be better suited to examine the immunologic function of PGs, instead of the direct effects on end-organ physiology that are more often studied in human investigations.
Individual PGs
Prostaglandin D2
PGD2 is the major mast cell-derived PG and is produced in nanogram quantities in response to IgE-mediated activation. (W. L. Smith et al., 2011) Eosinophils also produce PGD2.(Luna-Gomes et al., 2011) Two different enzymes that synthesize PGD2 are hematopoietic- and lipocalin- PGD2 synthases (H-PGDS and L-PGDS, respectively). H-PGDS produces PGD2 in mast cells and other hematopoietic cells. In contrast, LPGDS is expressed in oligodendrocytes, the choroid plexus, organs of the male genital tract, leptomeninges, and in the hearts of humans and monkeys. L-PGDS gene expression in the central nervous system is modulated by glucocorticoid, thyroid, and estrogen hormones, whereas estrogen regulates L-PGDS expression in the heart. Human placenta, lung, adipose tissue, and fetal liver express H-PGDS at high levels, while lower levels are expressed in the bone marrow, heart, lymph nodes, and appendix. Not only do human mast cells express H-PGDS, but it is also expressed by CD4+ Th2 lymphocytes, CD8+ Tc2 cells, megakaryocytes, dendritic cells (DCs), histiocytes, and Kupffer cells. PGD2 can be metabolized to PGF2α, 9α,11β-PGF2 (the stereoisomer of PGF2α), and the J series of PGs, including PGJ2, Δ12-PGJ2, and 15d-PGJ2.(W. L. Smith et al., 2011)
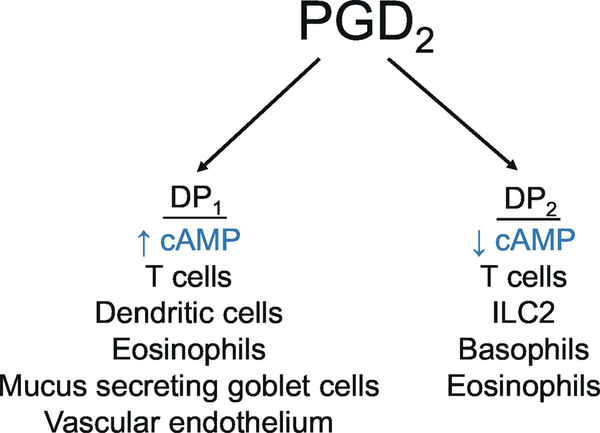
All of the PGs signal through distinct seven transmembrane, G-protein coupled receptors (GPCRs). PGD2 signals through receptors termed DP1 and DP2 (Figure 1). (W. L. Smith et al., 2011) DP1 is expressed on mucus-secreting goblet cells in the nasal and colonic mucosa, nasal serous glands, vascular endothelium, Th2 cells, DCs, basophils, and eosinophils (Figure 2). DP1 stimulation activates adenylate cyclase, resulting in an increase in intracellular cAMP levels and protein kinase A activity. Chemoattractant receptor-like molecule expressed on Th2 cells (CRTH2) is another name for DP2. In addition to PGD2, other DP2 agonists include Δ12-PGJ2; 15-deoxy-Δ12,14PGJ2 (15d-PGJ2); 13,15-dihydro-15-keto-PGD2; 11-dehydro-TXB2; and the COX inhibitor indomethacin.(Hirai et al., 2001; Sugimoto, Shichijo, Okano, & Bacon, 2005) Immune cells such as eosinophils, basophils, group 2 innate lymphoid cells (ILC2), and the T cell subsets CD4+ Th2 and CD8+ Tc2 cells also express DP2. PGD2 stimulates chemotaxis in immune cells in a DP2-dependent manner. DP2 is preferentially expressed by IL-4+/IL-13+ T cells in comparison to IFN-γ+ T cells in BAL fluid of subjects with asthmatic.(Mutalithas et al., 2010) Signaling through DP2 in eosinophils upregulates their release from bone marrow, activates their respiratory burst, increases the chemotactic response to other chemokines such as eotaxin, and primes them for degranulation. In addition, DP2 signaling augmented microvascular permeability, depletion of goblet cells, and constricted coronary arteries. In contrast to DP1 signaling, stimulation through DP2 decreased intracellular cAMP.(W. L. Smith et al., 2011) Hence, PGD2 signaling through DP2, via suppression of cAMP, might facilitate allergic inflammation by increasing chemotaxis and mediator release by effector cells. PGD2 and its immediate metabolite, 9α, 11β-PGF2 contracted smooth muscle, presumably by signaling through the thromboxane TP receptor.(Johnston, Freezer, Ritter, O'Toole, & Howarth, 1995; Larsson, Hagfjard, Dahlen, & Adner, 2011)
Figure 2.
PGD2 signals through two GCPR, termed DP1 and DP2. PGD2 signaling through DP1 increases cAMP, while signaling through DP2 decreases cAMP.
Human studies of PGD2 in allergic inflammation
Allergen inhalation challenge of human allergic asthmatic subjects increased PGD2 in BAL fluid.(Murray, Webb, O'Callaghan, Swarbrick, & Milner, 1992) PGD2 levels were increased in the BAL fluid from patients with severe asthma, even at baseline in the absence of allergen challenge.(Fajt et al., 2013) Whereas PGD2 is the most abundant PG produced by mast cells, epithelial hematopoietic prostaglandin D synthase (HPGDS) mRNA and immunohistochemistry (IHC) was significantly greater in subjects with severe asthma compared to healthy persons. DP2 mRNA and IHC were also greater in patients with severe asthma in contrast to healthy controls. Asthma exacerbations, poor asthma control, and markers of Th2 inflammation were associated with higher PGD2 levels, HPGDS, and DP2.(Fajt et al., 2013) PGD2 was higher in the nasal lavage from subjects with allergic rhinitis,(Naclerio et al., 1983) in tears from patients experiencing allergic conjunctivitis,(Proud et al., 1990) and in blister fluid from patients with skin late phase reactions.(Charlesworth, Kagey-Sobotka, Schleimer, Norman, & Lichtenstein, 1991) In asthmatic patients, the stable urinary PGD2 metabolite, 9α,11β-PGF2, was not changed by treatment with the COX-2 specific inhibitor celecoxib for 3 days, implying that PGD2 is largely produced by COX-1.(Daham et al., 2011) However, aspirin challenge of individuals with aspirin-exacerbated respiratory disease (AERD) did not reduce PGD2 concentration in BAL fluid. PGD2 is a potent vasodilator and bronchoconstrictor, and potentiated airway responsiveness.60 Intranasal administration of PGD2 increased nasal resistance 10-fold more potently than histamine and 100-fold greater compared to bradykinin.(Doyle, Boehm, & Skoner, 1990) PGD2 administration upregulated vascular leakage in the skin and conjunctiva,(Flower, Harvey, & Kingston, 1976) and while resulting in eosinophil influx in the conjunctiva(Woodward et al., 1990) and trachea, (Emery, Djokic, Graf, & Nadel, 1989) suggesting a pathogenic role in allergic disease. PGD2’s vascular effects mostly reflect dilation regulated by DP1, while recruitment of effector cells is more likely to a function of chemotaxis via DP2.(Hirai et al., 2001; Monneret, Gravel, Diamond, Rokach, & Powell, 2001) DP2 also modulates airway epithelial cell function. 13, 14-dihydro-15-keto PGD2 increased epithelial cell migration in vitro and augmented the number of goblet-like cells and terminallydifferentiated cells at air liquid interface in culture, whereas the effect of 13, 14-dihydro-15-keto PGD2 was blocked by the DP2-selective antagonist AZD6430.(Stinson, Amrani, & Brightling, 2015) In regard to smooth muscle contraction by PGD2 released upon allergen exposure, TP receptor antagonists such as GR32191 partially antagonized the early bronchoconstrictor response, with other constrictor mediators, such as histamine and LTC4/LTD4, contributing to make up the difference.(Beasley et al., 1989)
HPGDS is expressed by CD4 Th2 cells.(Mitson-Salazar et al., 2016) CD4 T cells expressing HPGDS, DP2, and CD161 have been named pathogenic effector Th2 cells because they secrete significantly increased IL-5 and IL-13 compared to cells that do not express HPGDS or CD161. Pathogenic effector CD4 T cells were highly correlated with blood eosinophilia and present in 30- to 40-fold greater numbers in subjects with eosinophilic gastrointestinal disease and subjects with atopic dermatitis in comparison to nonallergic subjects. Pathogenic effector CD4 T cells have significantly increased expression of receptors for TSLP, IL-25, and IL-33 and augmented responsiveness to these cytokines compared to CD4 cells that do not express HPGDS. Additionally, pathogenic effector CD4 T cells express gut and skin-homing receptors. These data suggest that pathogenic effector CD4 cells may be a pro-inflammatory CD4 cell type that may have an important role in promoting allergic eosinophilic inflammation.
One of the most intriguing new developments in allergic disease was the discovery of innate lymphoid cells (ILC), which secrete high levels of cytokines critical in the pathogenesis of the allergen-driven inflammatory response.(R. S. Peebles, Jr., 2013) ILC2 secrete large quantities of IL-5 and IL-13 in response to the epithelialderived cytokines IL-25, IL-33, and thymic stromal lymphopoietin (TSLP). IL-5 and IL-13 are central to inducing and maintaining the allergic phenotype and have been targets of biologic agents used in asthma treatment trials. IL-5 is a powerful eosinophil growth, differentiation, and survival factor and is important in eosinophil chemotaxis. IL-13 is a central mediator in asthma pathogenesis, causing goblet cell metaplasia, mucus production, smooth muscle constriction, and airway responsiveness.(Wills-Karp et al., 1998) PGD2 stimulated human peripheral blood ILC2 to produce large amounts of IL-13 in response to IL-25 and IL-33, whereas the addition of IL-25 and IL-33 to PGD2 caused a synergistic increase in IL-13 expression by ILC2.(Barnig et al., 2013) In these experiments, PGD2 induced IL-13 secretion by ILC2 predominantly via activation of DP2.(Barnig et al., 2013) Another group similarly reported that PGD2 enhanced human ILC2 function.(Xue et al., 2014) PGD2 binding to DP2 upregulated ILC2 migration and production of Th2-like cytokines. PGD2 activation through DP2 heightened ILC2 surface expression of the receptor subunits for IL-33 and IL-25, ST2 and IL-17RA, respectively.(Xue et al., 2014) CysLTs, particularly LTE4, enhances the activation of ILC2 by PGD2.(Salimi et al., 2017) LTE4 augmented Type 2 cytokine production stimulated by several mediators, including PGD2, IL-25, IL-33, and TSLP. The increase in ILC2 production of Type 2 cytokines induced by IL-25 and IL-33 was augmented by the addition of IL-2 to the culture and was likely a result of heightened IL-25 and IL-33 signaling as IL-2 induced the expression of the receptors of those cytokines on ILC2. LTE4 induced augmentation of ILC2 function was inhibited by montelukast, a cysLT receptor 1 (cysLT1) antagonist.(Salimi et al., 2017) LTE4 binds to cysLT1 with low affinity and a new LTE4 receptor, cysLT3, also known as GPR99, was recently discovered to have much higher affinity.(Bankova et al., 2016; Kanaoka, Maekawa, & Austen, 2013)
There is increasing evidence that PGD2 is important in AERD pathogenesis. Levels of the stable urinary PGD2 metabolite (PGD-M) at baseline were higher in subjects with AERD who could not tolerate aspirin desensitization compared to those that were successfully desensitized to aspirin.(Cahill, Bensko, Boyce, & Laidlaw, 2015) During reactions to aspirin administration, PGD-M levels significantly increased in subjects who did not tolerate aspirin desensitization compared to those that did. A clinical endpoint of aspirin challenge is changes in pulmonary function and FEV1 inversely correlated with levels of both PGD-M and leukotriene E4.(Cahill et al., 2015) These data reveal that the inability to tolerate aspirin desensitization was associated with higher PGD-M levels. Nasal polyp TSLP mRNA expression strongly correlated with mRNA encoding HPGDS and urinary PGD-M. The active form of TSLP was greater in nasal polyps from subjects with AERD in comparison to aspirin tolerant control subjects. Recombinant TSLP stimulated PGD2 generation by cultured mast cells. These data imply that PGD2 produced by mast cells is a major effector of Type 2 immune responses driven by TSLP in the setting of AERD, and that targeting either PGD2, TSLP, or both, could have beneficial effects in AERD patients, especially for those not successfully desensitized to aspirin.
The therapeutic effects of DP2 antagonists have been investigated in humans with asthma and other allergic diseases. In a randomized, double-blind, placebo-controlled trial in subjects with moderate-persistent asthma, the DP2 antagonist OC000459 significantly improved both quality of life and night-time symptom score.(Barnes et al., 2012) There was also a significant reduction in geometric mean sputum eosinophil count in the DP2 antagonist group compared to pre-treatment baseline, although this decrease was not significant compared to the placebo-treated group. The DP2 antagonist OC000459 has also been examined in a randomized, double-blind placebo-controlled trial of adult patients with active, corticosteroid-dependent, or corticosteroid-refractory eosinophilic esophagitis (EoE).(Straumann et al., 2013) After 8-weeks of treatment with OC000459, there was a significant decrease in the number of eosinophils per high power field (115 to 73), while placebo had no effect. Further, OC000459 treatment improved physicians’ assessment of disease activity.(Straumann et al., 2013) There were no serious adverse events in the subjects treated with OC000459. The DP2 antagonist BI 671800 has also been examined in patients with seasonal allergic rhinitis.(Krug et al., 2014) In a randomized, double-blind, placebo-controlled partial cross-over study, patients with a positive skin test to Dactylis glomerata pollen were exposed to out of season allergen in an environmental challenge chamber for 6 hours. BI 671800 at a dose of 400 mg twice daily, but not at lower doses, significantly improved nasal symptom scores, reduced nasal eosinophils, inhibited nasal IL-4 and eotaxin levels, and reduced ex vivo PGD2-mediated eosinophil shape change in a dose-related manner.(Krug et al., 2014) BI 671800 was also examined in 2 separate trials in patients with asthma.(Hall et al., 2015) In the first trial, BI 671800 increased FEV1 by 3.08% (50 mg twice daily dose), 3.59% (200 mg twice daily dose), and 3.98% (400 mg twice daily dose), and these increases were all significantly greater than the change in FEV1 seen with placebo. In this same trial, inhaled fluticasone propionate 220 μg twice daily increased FEV1 by 8.62%. There were no significant change in asthma control questionnaire (ACQ) with any dose of BI 6718000, while inhaled fluticasone propionate significantly improved asthma symptom scores. In the second trial, BI 671800 at a dose of 400 mg twice daily significantly increased FEV1 by 3.87% compared to placebo, whereas montelukast did not. BI 671800 at a dose of 400 mg twice daily significantly increased the mean ACQ score (-0.28), although this increase is not deemed to be clinically significant, whereas the montelukast treated arm did not have a change in ACQ score compared to placebo.(Hall et al., 2015) In a more recent phase IIa, 12-week, randomized, double-blind, three period, four-treatment, incomplete block crossover trial, BI 6718000 was administered either as a single 400 mg dose in the morning or evening, or 200 mg twice daily versus placebo, with fluticasone propionate at 44μg twice daily.(Miller et al., 2017) There were no statistically significant or clinically meaningful differences in the ACQ scores compared to placebo.(Miller et al., 2017) In an exploratory phase II, double-blind, randomized, placebo-controlled multicenter trial, the oral DP2 antagonist QAW039 (fevipiprant) was examined in patients with mild-to-moderate uncontrolled allergic asthma.(Erpenbeck et al., 2016) While there was no benefit with QAW039 in the entire study population, a subgroup analysis revealed that patients with an FEV1<70% predicted at baseline had a significant improvement in trough FEV1 and ACQ7 score compared to placebo. QAW039 was also studied in a single-center, randomized, double-blind parallel-group, placebo-controlled trial in patients with persistent, moderate-to-severe asthma and an elevated eosinophil count (≥2%).(Gonem et al., 2016) QAW039 treated patients had a decrease in the mean sputum eosinophil percentage by 4.5-fold, and this was significantly greater than the change in sputum eosinophils in the placebo-treated patients.(Gonem et al., 2016) The DP2 antagonist AZD1981 was examined in adults with asthma in two randomized, placebo-controlled, parallel-group trial.(Kuna, Bjermer, & Tornling, 2016) In study 1, patients with stable asthma were withdrawn from inhaled corticosteroids and randomized to AZD1981 1000mg twice daily or placebo. This treatment had no significant effect on morning peak expiratory flow. In study 2, patients with uncontrolled asthma despite inhaled corticosteroid therapy were randomized to 50 mg, 400 mg, or 1000 mg AZD1981 or placebo. In this study, all doses of AZD1981 significantly increased ACQ-5 scores, but there was no dose-response relationship.(Kuna et al., 2016) Additional studies will be important to confirm the clinical usefulness DP2 antagonism in asthma. The combination of DP2 and TP antagonists have been used for the treatment of rhinitis with resulting decrease in eosinophilia, nasal mucosa edema, and symptoms; future studies will identify if they have a therapeutic role in asthma treatment.(Kupczyk & Kuna, 2017)
Animal studies of PGD2 in allergic inflammation
Data from mouse investigations reveal a complex role for PGD2 in experimental allergic disease.(Matsuoka et al., 2000) Overexpression of L-PGDS increased BAL fluid levels of Th2 cytokines, eotaxin, eosinophils, and lymphocytes after allergen sensitization and challenge in comparison to nontransgenic littermates.(Fujitani et al., 2002) Aerosolized PGD2 treatment a day prior to inhalational challenge with low-dose antigen increased eosinophils, lymphocytes, and macrophages, as well as IL-4 and IL-5, in the BAL fluid of sensitized mice.(Honda et al., 2003) These results suggest that PGD2 increases pulmonary Th2 responses. However, genetic deficiency in HPGDS exacerbated all of the manifestations of oral ovalbumin administration in ovalbumin-sensitized animals compared to WT mice in a mouse model of food allergy.(Nakamura et al., 2015) Adoptive transfer of mast cells expressing HPGDS into mast cell KO mice increased mast cell hyperplasia and allergic inflammation. HPGDS deficient mice had more profound anaphylaxis than WT mice, with mast cell-derived PGD2 inhibiting vascular hyperpermeability.(Nakamura et al., 2017) These data imply that HPGDS deficiency increases food antigen-induced mast cell hyperplasia and that PGD2 restrains food allergy in mice.
Mouse studies examining the role of signaling through DP1 in allergic inflammation have been contradictory. While DP1 agonist increased allergen-induced sneezing compared to placebo in a model of Japanese cedar pollen-induced allergic rhinitis, this endpoint was reduced in DP1 knockout mice compared to WT mice.(Nakano et al., 2016) These investigators also reported a DP1 antagonist completely inhibited PGD2-induced augmentation of electrical and histamine-induced excitability of trigeminal ganglion excitability in guinea pigs.(Nagira et al., 2016) Allergen sensitized and challenged DP1 KO mice had significantly inhibited airway responsiveness and BAL concentrations of IL-4, IL-5, and IL-13 compared to WT mice, while there was no difference in the BAL levels of IFN-γ.(Matsuoka et al., 2000) Further, DP1 KO mice had reduced BAL eosinophils and lymphocytes compared to WT mice, suggesting that DP1 signaling was critical for the full expression of allergic inflammation.(Matsuoka et al., 2000) In contrast, the DP1 agonist BW245C reduced lung DC function, as well as the ability of DCs to activate T cell proliferation and DC recruitment to the lungs.(Hammad et al., 2003; Hammad et al., 2007) Mice treated with BW245C, or mice adoptively transferred DP1-treated DCs, had increased Foxp3+ CD4+ T regulatory cells that suppressed inflammation in an IL-10–dependent manner.(Hammad et al., 2007) The reduced allergic inflammation caused by the DP1 agonist through diminished DC function was modulated by cyclic AMP-dependent protein kinase A.(Hammad et al., 2007) Furthermore, chimeric mice lacking DP1 expression on hematopoietic cells had augmented airway inflammation following allergen challenge, implying a critical homeostatic role of DP1 and endogenous PGD2.(Hammad et al., 2007) DP1, but not DP2, signaling stimulated single airway C-fibers in mice, guinea pigs, and human vagal afferents. (S. A. Maher et al., 2015) These data imply that inhibiting DP1 signaling could be a therapeutic target for asthma-related cough symptoms. Taken together, these results imply that DP1 signaling promotes effector responses through structural cells, but inhibits DC function during the sensitization phase to inhibit allergic inflammatory process.
Experiments in different species support the notion that DP2 signaling augments allergic inflammation. The DP2 receptor antagonist AM211 inhibited OVA-induced airway eosinophilia in guinea pigs, while reducing the number of sneezes in mice resulting from intranasal allergen challenge.(Bain et al., 2011) The DP2 antagonist ARRY-063 significantly inhibited increases in the respiratory frequency resulting from challenges with the combination of ovalbumin and PGD2 in both the early and late phases in ovalbumin-sensitized mice.(Shiraishi, Takeda, Domenico, & Gelfand, 2014) Further, a different DP2 antagonist, MK-7246, inhibited antigen-induced late phase bronchoconstriction and airway responsiveness in sheep, in addition to reducing antigen-induced eosinophilia in both sheep and monkeys.(Gervais et al., 2011) The DP2 antagonist OC000459 almost fully ablated Aspergillus fumigatus-induced airway eosinophilia and airway responsiveness in Wistar rats.(H. Liu et al., 2014) Finally, a potently selective alkynylphenoxyacetic acid DP2 antagonist administered orally inhibited OVA-induced airway eosinophilia in mice.(Crosignani et al., 2011) These studies strongly suggest that PGD2 signaling through DP2 enhances allergic inflammation, and blocking receptor signaling blunts inflammatory responses in animals.
Prostaglandin E2
PGH2 may be metabolized to PGE2 by three distinct enzymes, microsomal PGE synthase-1 (mPGES-1), mPGES-2, and cytosolic PGE synthase (cPGES). (W. L. Smith et al., 2011) mPGES-1 is membrane-associated, localized to the perinuclear area, has a trimeric structure, and is glutathione-dependent. PGE2 production was significantly increased in cells co-transfected with both mPGES-1 and COX-2, implying that mPGE-2 preferentially couples with COX-2 to synthesize PGE2 when COX-2 is active. mPGES-1 metabolizes PGH2 produced from COX-1; however, exogenous administration of arachidonic acid is required for this effect. Arachidonic acid synthesized by mast cell group IVA cPLA2 caused PGE2 production by mouse fibroblast mPGES-1.(Ueno et al., 2011) cPGES expression was largely constitutive and not induced by inflammatory stimuli.(Sugimoto et al., 2005; Tanioka, Nakatani, Semmyo, Murakami, & Kudo, 2000) In comparison to mPGES-1, cPGES coupled more efficiently with COX-1 than with COX-2 in generating PGE2. These data imply that cPGE2 may provide PGE2 that is necessary for cellular homeostasis, as mPGES-1 KO mice had significantly decreased basal PGE2 production in most organs. Interestingly, mPGES-1 activity is reduced in transformed cell lines by cysLT1 antagonists;(Kahnt et al., 2013) however, this has not been confirmed either in primary cells or in vivo. Studies in KO mice do not confirm that either cPGES or mPGES-2 are critical PGESs enzymes in vivo. cPGES is localized to the cytosol. There was evidence that cPGES translocated from the cytosol to the nuclear membrane to assemble with COX-1 in PGE2 production; however, cPGES had a slight preference to interact with COX-2.(Park, Pillinger, & Abramson, 2006) Dexamethasone reduced cPGES activation.(Park et al., 2006) mPGES-2 is expressed constitutively in many cells and tissues.(Park et al., 2006) In transfected cells, mPGES-2 utilizes PGH2 produced from COX-1 and COX-2 with equal efficiency. Local PGE2 concentrations are modulated by COX-2 driven production and degradation of PGE2 by 15-hydroxyprostaglandin dehydrogenase (15-PGDH).(Kalinski, 2012)
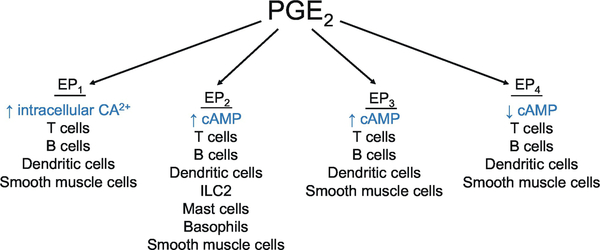
PGE2 signals through four distinct GPCRs, named EP receptors 1 through 4 (Figure 3).(W. L. Smith et al., 2011) Each EP receptor has a distinct G protein coupling preference and downstream signal activation, and some of these signals counteract one another. The four receptor subtypes are present in the lung and other organs associated with allergic inflammation.(W. L. Smith et al., 2011) EP1 receptor signaling increased cell Ca2+ and caused smooth muscle contraction. EP2 and EP4 receptor activation upregulated the concentration of intracellular cAMP, resulting in smooth muscle relaxation.(Coleman, Smith, & Narumiya, 1994) EP2 is highly expressed in the uterus, lung and spleen.(R. M. Breyer, Bagdassarian, Myers, & Breyer, 2001) Activation of the EP2 receptor reduced mast cell mediator release. Expression of EP4 is greatest in the kidney and peripheral blood leukocytes; however, EP4 expression at high levels also occurs in the thymus, lung and several other tissues.(An, Yang, Xia, & Goetzl, 1993) EP3 receptor signaling led to smooth muscle contraction by reducing the rate of cAMP synthesis.(Adam et al., 1994) EP3 receptors are unique as multiple splice variants produce alternate sequences in the C-terminal tail of this receptor subtype.(R. M. Breyer et al., 2001) However, the functional importance of these alternative splice variants is not clearly defined. Usually, signaling through these splice variants of EP3 reduced cAMP generation, while signaling through EP2 and EP4 increase cAMP.(R. M. Breyer et al., 2001) Therefore, PGE2 signaling may have opposing effects in different tissues depending upon the relative contributions of the receptors activated in a specific context.
Figure 3.
PGE2 signals through four GCPR, termed EP1, EP2, EP3, and EP4. Signaling through EP1 increases intracellular Ca2+, signaling through EP2 increases cAMP, signaling through EP3 decreases cAMP, and signaling through EP4 increases cAMP.
Human studies of PGE2 in allergic inflammation
PGE2 is one of the most abundant COX products synthesized by airway epithelium and smooth muscle.(Churchill et al., 1989; Delamere et al., 1994) Several reports imply that endogenous PGE2 is bronchoprotective in human asthma.(Pavord & Tattersfield, 1995) PGE2 synthesized by epithelial cells inhibited vagal cholinergic contraction of airway smooth muscle.(Barnett, Jacoby, Nadel, & Lazarus, 1988) Bronchial epithelial cell-synthesized PGE2 also inhibited DC migration and pro-inflammatory cytokine protein production.(Schmidt et al., 2011) PGE2 inhibited dendritic cell migration by signaling through the EP4 receptor, as DCs treated with an EP4 antagonist as well as DCs from EP4 KO mice had reduced inhibition by airway epithelial cells with respect to secretion of proinflammatory cytokines. Sputum levels of PGE2 from asthmatics were inversely correlated to sputum eosinophil counts. These data imply that PGE2 may restrain airway eosinophilia.(Aggarwal, Moodley, Thompson, & Misso, 2010; Pavord et al., 1999) Further, PGE2 inhalation reduced the pulmonary early and late phase responses to inhaled allergen challenge.(Gauvreau, Watson, & O'Byrne, 1999; Pavord, Wong, Williams, & Tattersfield, 1993) Inhaled PGE2 inhibited methacholine airway reactivity and reduced airway eosinophilia following inhaled allergen challenge.(Gauvreau et al., 1999) PGE2 also blunted exercise-induced and aspirininduced bronchoconstriction in patients sensitive to these challenges.(Melillo, Woolley, Manning, Watson, & O'Byrne, 1994; Sestini et al., 1996) While PGE2 significantly protected against reduction in pulmonary function in challenge models, baseline FEV1 or methacholine reactivity were not affected.(Pavord et al., 1993) Therefore, PGE2 seems to have impressive immunomodulatory properties, yet does not directly regulate airway caliber. This concept is supported by the finding that PGE2 inhalation before segmental allergen challenge reduced the mast cell products PGD2 and cysLT in BAL fluid.(Hartert et al., 2000) EP4 receptor signaling in human, guinea pig, and rat airways promoted smooth muscle relaxation,(Buckley et al., 2011) while EP3 receptor signaling induced PGE2-mediated cough. (S.A. Maher, Birrell, & Belvisi, 2009) PGE2 combined with albuterol, a β2-adrenergic receptor agonist, inhibited human airway smooth muscle migration and mitogenesis,(Goncharova et al., 2012; Yan et al., 2011) confirming the multitude of effects that PGE2 has on airway function. It is important to note one report in which PGE2 directly regulated human bronchoconstrictor responses. Low concentrations of PGE2 relaxed human small airways that had been precontracted by histamine, and this was inhibited by the EP4 antagonist ONO-AE3–208.(Safholm et al., 2015) Higher concentrations of PGE2 (10–100 μmol/L) contracted small airways, but not to the same degree as caused by either a TP receptor agonist, PGF2α, or PGD2. EP2 signaling reduced mast cell-mediated bronchoconstriction caused by anti-IgE challenge in the presence of TP and EP4 antagonists. Therefore, PGE2 has variable effects on airway tone depending upon the concentration of PGE2 and the receptor through which it signals.
The rapid metabolism of PGE2 has led investigators to utilize a stable orally active PGE1 analogue, misoprostol, when investigating allergic airway inflammation and lung function in humans. Unfortunately, in these studies misoprostol has had little effect. Misoprostol did not alter β2 agonist use, pulmonary function, or asthma severity score in subjects with AERD.(Wasiak & Szmidt, 1999) In subjects with mild asthma, misoprostol did not change either baseline lung function or histamine reactivity; yet, there were important gastrointestinal side effects in one-third of subjects.(Harmanci, Ozakyol, Ozdemir, Elbek, & Isik, 1998) It is important to consider that misoprostol is significantly less potent than PGE2 in activating adenylate cyclase.(Pawlotsky, Ruszniewski, Reyl-Desmars, Bourgeois, & Lewin, 1993)
While PGE2 inhibited eosinophilia and allergen challenge early- and late-phase responses, in vitro studies reveal that PGE2 may either stimulate or suppress immune cell function. PGE2 inhibited T cell production of the Th1 cytokines IL-2 and IFN-γ in vitro, promoting T cell polarization toward a Th2 cytokine profile.(Betz & Fox, 1991; Hilkens et al., 1995; Katamura et al., 1995; Snijdewint, Kalinski, Wierenga, Bos, & Kapsenberg, 1993) These in vitro data imply that PGE2 driven Type 2 cytokine production might be modulated during antigen presentation. Myeloid DCs matured in the presence of IFN-γ resulted in Th1 CD4+ T lymphocyte responses, while DCs matured in PGE2 promoted Th2 responses.(Vieira, de Jong, Wierenga, Kapsenberg, & Kalinski, 2000) While PGE2 induced Th2 cytokine secretion, primarily through its activities during antigen presentation, does not necessarily contradict in vivo human studies that suggested PGE2 has anti-inflammatory properties. For instance, PGE2 in combination with IL-23, induced polarization and expansion of CD4+ Th17 cells, in addition to secreting Th17 cytokines.(Chizzolini et al., 2008)
Not only does PGE2 regulate CD4+ Th1 and Th2 differentiation, it also modulates the function of other cells involved in asthma pathogenesis. Both PGE2 and cAMP reduced spontaneous eosinophil apoptosis, as did an EP2 agonist, in vitro.(Peacock, Misso, Watkins, & Thompson, 1999) This suggests that by prolonging eosinophil survival PGE2 could promote the inflammatory potential of these cells in asthma. In contrast, PGE2 inhibited IL-5-mediated survival, eosinophil chemotaxis, aggregation, and degranulation.(Kita, Abu-Ghazaleh, Gleich, & Abraham, 1991; Teixeira, al Rashed, Rossi, & Hellewell, 1997) PGE2 blunted of eosinophil trafficking via EP2 signaling.(Sturm et al., 2008) Therefore, further studies are necessary to determine the importance of these in vitro results to in vivo disease states.
PGE2 also modulated granulocyte macrophage-colony stimulating factor (GM-CSF) production by human airway smooth muscle cells(Lazzeri et al., 2001). Indomethacin increased GM-CSF production by cultured human airway smooth muscle cells, while exogenous PGE2 decreased this indomethacin-induced GM-CSF production. These results suggest that PGE2 inhibited GM-CSF secretion and the inflammation associated with this cytokine.(Lazzeri et al., 2001) However, PGE2 augmented IL-6 and GM-CSF production by IgE-mediated degranulation mast cells through the EP1 and EP3 receptors.(Gomi, Zhu, & Marshall, 2000) The effect of PGE2 on mast cell production of differing mediators is not clearly defined. PGE2 reduced(Hogaboam, Bissonnette, Chin, Befus, & Wallace, 1993; Kaliner & Austen, 1974; Peachell, MacGlashan, Lichtenstein, & Schleimer, 1988) or enhanced(Leal-Berumen, O'Byrne, Gupta, Richards, & Marshall, 1995; Nishigaki et al., 1995) the release of histamine and other inflammatory mediators from mast cells. Quite possibly, these results are a function of the relative dominance of EP3 (activating) versus EP2 (inhibitory) signaling in a specific mast cell population. While PGE2 activated human mast cells via EP3 signaling, it inhibited activation through the EP2-PKA signaling pathway.(Feng, Beller, Bagga, & Boyce, 2006)
COX-1, but not COX-2, inhibition of PGE2 has an important role in AERD-mediated bronchoconstriction.(Mastalerz et al., 2008) COX-1 inhibition inhibits synthesis of PGE2 that blunts 5-LO-mediated cysLT production.(Harizi, Juzan, Moreau, & Gualde, 2003) Reduction of PGE2 production by COX inhibition, with the resultant increase in cysLT, promotes the bronchoconstriction that occurs with NSAID ingestion.(Drazen, 1998) Inhaled PGE2 reduced the increased urinary LTE4 and bronchoconstriction caused by aspirin challenge in subjects with AERD.(Sestini et al., 1996; Szczeklik, Mastalerz, Nizankowska, & Cmiel, 1996) COX-2 inhibitors did not cause symptoms in AERD subjects, implying that COX-1 mediated PGE2 production is protective.(Gyllfors et al., 2003)
A leading proposed mechanism of AERD pathophysiology is that subjects have differential metabolism of arachidonic acid, resulting in decreased PGE2 production. For example, epithelial cells from polyp tissues from AERD subjects produced significantly reduced PGE2 in comparison to nasal epithelial cells from aspirin tolerant subjects.(Kowalski et al., 2000) Related to this reduction in PGE2, incubation of these epithelial cells from AERD subjects produced significantly increased 15-hydroyeicostetraenoic acid, a product of 15-LO.(Kowalski et al., 2000) Similarly, nasal tissue from AERD subjects with nasal polyposis had decreased COX-2 mRNA expression and PGE2 synthesis, but had increased LTC4 synthase (the enzyme that converts LTA4 to LTC4), 5-LO mRNA, and cysLT levels, in comparison to healthy controls or those with only chronic rhinosinusitis.(Perez-Novo, Watelet, Claeys, Van, & Bachert, 2005) This decreased PGE2 production in AERD subjects is not limited to nasal tissue, as airway fibroblasts from AERD subjects had reduced PGE2 production compared to healthy controls. In this study, there was reduced COX-1, but not COX-2, protein in the airway fibroblasts from AERD subjects compared to those from healthy controls.(Pierzchalska, Szabo, Sanak, Soja, & Szczeklik, 2003) Nasal tissue fibroblasts from AERD subjects produced significantly reduced PGE2 after IL-1β stimulation compared to healthy subjects or those with nasal polyps that were aspirin tolerant.(Roca-Ferrer et al., 2011)
Not only was there reduced PGE2 production in tissue from AERD subjects compared to healthy controls, but also aberrant expression of PGE2 receptors in tissues from AERD subjects. There was a reduction in the density of EP2, and an increase in cysLT receptors, in nasal polyp tissue from AERD subjects compared to aspirin tolerant subjects.(Adamusiak et al., 2012) There was reduced EP2 expression on T cells, mast cells, neutrophils, and macrophages from subjects with AERD compared to subjects with aspirin tolerant asthma.(Corrigan et al., 2012) Likewise, there was reduced EP2 expression and resistance to PGE2 in nasal polyp fibroblasts from AERD subjects.(Cahill et al., 2016) There was also a significant decrease in the percentage of mast cells, eosinophils, neutrophils, and T cells expressing EP2, but not EP1, EP3, or EP4 in nasal biopsies from AERD subjects compared to aspirin tolerant controls.(Ying et al., 2006) While there was no difference in EP4 expression on eosinophils between AERD subjects and healthy control, inhibition of eosinophil chemotaxis by PGE2 or an EP4 receptor agonist (CAY 10598) was reduced in eosinophils from AERD subjects compared to healthy controls.(Luschnig et al., 2014) The oral PGE1 analogue, misoprostol, did not protect against NSAID-induced AERD symptoms;(Walters, Simon, Woessner, Wineinger, & White, 2017) however, newer PGE2 agonists should be examined to evaluate this pathway for treatment of AERD.
Candidate gene approaches investigating AERD revealed that single nucleotide polymorphisms (SNPs) in the EP2 gene confer susceptibility to AERD. Evaluation of allelic association of 370 SNPs of genes that modulate the arachidonic acid metabolic cascade revealed multiple SNPs in the EP2 gene that significantly associated with AERD.(Jinnai et al., 2004) SNPs in the EP2 promoter gene, uS5, uS5b, and uS7, significantly associated with AERD and analysis of haplotypes revealed a significant association with AERD. The most significantly associated SNP, uS5, located in the regulatory region of the EP2 gene, was in a STATs-binding consensus sequence (AERD 31.1% versus control 22.1% [permutation P=0.0016] or versus aspirin-tolerant asthma 22.2% [permutation P=0.0017]). In an in vitro reporter assay, the site containing the uS5 allele had a reduction in transcription activity. These data imply that the uS5 allele is a target of a transcription repressor protein.(Jinnai et al., 2004) A functional SNP of the EP2 gene associated with risk of AERD should inhibit transcription, leading to a reduction of the ability of PGE2 to restrain the inflammation that underlies AERD. In another report, genetic polymorphisms in EP2, EP3, EP4, the PGI2 receptor (IP), and the thromboxane A receptor (TP) associated with AERD.(Kim et al., 2007) In summary, there is ample data implying that a reduction in PGE2 production and blunted expression of EP2 on a variety of cell types is pathogenic in AERD. Genetic variability of EP4 may also be a risk factor for aspirin-intolerant chronic urticaria (AICU). There was a significantly greater frequency of AICU patients who had the GG phenotype at -1254 G>A compared with healthy controls.(Palikhe et al., 2012) Similarly, the minor allele frequency, G allele was significantly greater in AICU patients compared to healthy controls.
PGE2 may have a protective role in exercise-induced bronchoconstriction (EIB).(Torres-Atencio, Ainsua-Enrich, de Mora, Picado, & Martin, 2014) One possible mechanism of EIB pathogenesis is increased airway fluid osmolarity as a result of water evaporation during exercise, which also results in airway cooling. The augmented airway fluid osmolarity stimulates mast cells to release inflammatory mediators that causes airway smooth muscle bronchoconstriction. PGE2 produced by mast cells lengthen the refractory period seen in patients with EIB. In human mast cell lines, a hyperosmolar state caused by culturing the mast cells in mannitol, induced mast cell degranulation and this was reduced by PGE2 signaling through EP2 and EP4.(Torres-Atencio et al., 2014)
While PGD2 signaling promotes ILC2 function, PGE2 signaling inhibits human ILC2 activation. PGE2 reduced the secretion of IL-5 and IL-13 from ILC2 isolated from human tonsils and peripheral blood resulting from stimulation with a combination of IL-25, IL-33, and TSLP, while suppressing the expression of GATA-3, the master transcription factor for the production of IL-5 and IL-13.(Maric et al., 2017) Additionally, PGE2 reduced the expression of CD25, the IL-2 receptor α chain, which was associated with decreased ILC2 proliferation. The effect of PGE2 on ILC2 functional suppression was confirmed through the use selective EP2 and EP4 agonists, the receptors for which were both expressed on ILC2.
Animal studies of PGE2 in allergic inflammation
Animal models of allergen-induced airway inflammation have been inconclusive as to whether PGE2 signaling promotes or inhibits allergic inflammation. The animal models of EP receptor deficient mice have resulted in different conclusions even in mice with the same EP receptor genetic deletion. In an OVA-sensitization and challenge model, EP3 KO mice had augmented allergic inflammation compared to WT mice, while there was no effect in the lung allergic inflammation between WT, EP1 KO, EP2 KO, and EP4 KO mice.(Kunikata et al., 2005) EP3 KO mice had increased airway eosinophils, neutrophils, and lymphocytes, as well as increased IL-4, IL-5, and IL-13 in BAL fluid compared to WT mice.(Kunikata et al., 2005) This result was supported by the EP3 agonist AE-248 significantly inhibiting allergic airway cellularity.(Kunikata et al., 2005) In ex vivo experiments, lungs from OVA-sensitized and challenged EP3-deficient or WT mice were challenged with OVA, resulting in significantly decreased histamine and cysLT in lungs from WT mice treated with an EP3 agonist. These results imply that PGE2 signals through EP3 on mast cells in vivo to inhibit mediator release.(Kunikata et al., 2005) However, these data would not have been predicted from in vivo analyses, since EP3 receptor signaling causes mast cell activation in vitro.(Feng et al., 2006) Another group published that PGE2 augmented allergic airway inflammation in that EP2-deficient mice had decreased allergic airway inflammation and a reduction in IgE production.(Gao et al., 2016) Further, PGE2 enhanced activation of STAT6 induced by IL-4 in an EP2-dependent manner and increased IgE class switching, generation of IgE bearing B lymphocytes, and IgE secretion by B cells that had been stimulated with LPS and IL-4. This is in opposition to a report in which an EP2 antagonist exacerbated, while an EP2 agonist prevented, dust mite-induced inflammation and airway responsiveness, implying that EP2 signaling restrains the allergic inflammatory response.(Serra-Pages et al., 2015) Further, other investigators found that PGE2 inhibited allergic sensitization and lung inflammation through EP2 signaling on T cells.(Zaslona et al., 2014) In this report, splenocytes and lung lymph node cells from sensitized EP2-deficient mice secreted greater IL-13 than cells from WT mice. These investigators also reported that misoprostol treatment of WT mice, but not EP2-deficient mice, during the sensitization phase blunted allergic inflammation in the ovalbumin model.
Additional reports suggest that PGE2 inhibits allergen-challenge airway inflammation in mice. PGE2 administered subcutaneously blunted lung eosinophilia and Th2 cytokine production in a house dust mite model of allergic inflammation.(Herrerias et al., 2009) Further, PGE2-treated mice had reduced house dust mite-induced lung eosinophils and decreased YM1 serum levels than vehicle-treated animals.(Draijer et al., 2016) Intranasal PGE2 reduced allergic airway inflammation in mice when administered prior to allergen challenge during the last 5 days of 10 consecutive days of house dust mite-challenge.(Torres et al., 2013) Adoptive transfer of PGE2-treated macrophages in this model reduced lung-infiltrating eosinophils, likely by promoting macrophage IL-10 production. Interestingly, PGE2 seemingly has differing effects on mouse mast cell function in vitro compared to other cells involved in the allergic inflammatory response. For instance, PGE2 stimulated mast cell chemotaxis and cytokine production via mTORC2 activation.(Kuehn, Jung, Beaven, Metcalfe, & Gilfillan, 2011) PGE2 signaling through EP3 induced mast cell chemotaxis.(Weller et al., 2007) Adoptive transfer of adipose-derived stem cells that produce PGE2 reduced allergic airway inflammation and this inhibitory effect of the adipose-derived stem cell transfer was abrogated by a PGE2 inhibitor. (K. S. Cho et al., 2015) EP4 signaling also protected against airway inflammation. In three separate systems, LPS, ovalbumin, and cigarette smoke, mice deficient in EP4 had augmented airway inflammation, revealing that PGE2 signaling through EP4 inhibited the inflammatory responses.(Birrell et al., 2015)
PGE2 production is decrease in chronic allergen exposure, probably a consequence of allergic inflammation, and the aftermath of this reduced PGE2 is augmented airway remodeling. In a model of chronic allergen challenge, there was an inverse relationship between the number of aeroallergen challenges with lung fibroblast COX-2 and mPGES-1 expression, leading to inhibited production of cytokine-induced PGE2.(Stumm, Wettlaufer, Jancar, & Peters-Golden, 2011) mPGES-1 synthesized PGE2 did not modulate allergic sensitization or T cell effector responses with house dust mite challenge between mPGES-1 KO and WT mice.(Lundequist et al., 2010) However, mPGES-1 KO mice had a greater number of allergen challenge-induced vascular smooth muscle cells and thickness of intrapulmonary vessels.(Lundequist et al., 2010) These results imply that PGE2 synthesized by mPGES-1 reduced remodeling of the pulmonary vasculature during allergen-induced lung inflammation; however, these results may not be translatable to human disease.
PGE2 also controls airway tone in mice. Immunologically naïve mice that are deficient in 15-PGDH, the major enzyme in PGE2 catabolism, had increased levels of PGE2 and inhibited methacholine-induced bronchoconstrictor responses.(Hartney et al., 2006) Likewise, mice that had greater PGE2 production, resulting from over-expression of PGE2 synthase in the lung, had inhibited methacholine-induced airway constriction.(Hartney et al., 2006) Therefore, PGE2 defended against lower airway bronchoconstriction, with work from other investigators suggesting EP2 signaling mediates this effect. Pretreatment with aerosolized PGE2 reduced methacholine-induced bronchoconstriction in WT, but not EP2 KO mice.(Sheller, Mitchell, Meyrick, Oates, & Breyer, 2000) This notion was strengthened data revealing that PGE2-induced bronchodilation resulted from direct activation of EP2 receptors on airway smooth muscle, while PGE2 signaling through EP1 and EP3 caused bronchoconstriction.(Tilley et al., 2003) This data was supported by a guinea pig study in which an EP1 antagonist (ONO-8130) blocked initial PGE2-mediated contraction and an EP2 receptor antagonist (PF-04418948) inhibited the resulting PGE2-mediated relaxation. In this report, endogenous PGE2, predominantly synthesized by COX-2, sustained spontaneous guinea pig tracheal tone by balancing contractile EP1 receptors and relaxant EP2 receptors. In vitro, PGE2 activated EP1/EP2 mediated relaxation of intrapulmonary airways and was more potent than salbutamol in antagonizing submaximal pre-contractions to methacholine, serotonin, or endothelin-1.(FitzPatrick, Donovan, & Bourke, 2014) In sum, these studies imply that PGE2 modulates homeostasis of bronchomotor tone and pulmonary immune responses by activating different respective receptors. The animal data cited above suggests that agents that either stimulate EP2, or that antagonize EP1 and EP3, could be therapeutic strategies for asthma.
In vivo mouse experiments reinforce the notion that PGE2 is essential in protection against AERD. mPGES-1 KO mice with dust mite-induced airway inflammation had increased airways resistance, augmented mast cell activation, and enhanced cysLT production following lysine aspirin challenge.(T. Liu, Laidlaw, Katz, & Boyce, 2013) The stable PGE2 analog, 16, 16-dimethyl PGE2, significantly inhibited lysine aspirin-induced airways resistance, mast cell histamine release, and cysLT production. EP2 and EP4 receptor agonists had similar protective effects as 16, 16-dimethyl PGE2 on histamine and cysLT release, while an EP2 agonist inhibited airways resistance to a greater degree than an EP4 agonist. In this experiment, lysine aspirininduced airways resistance and histamine release was dependent on cysLT, supporting that PGE2 negatively regulates lysine aspirin-induced LT-mediated airway constriction and inflammation. Additional studies showed that lysine aspirin-induced cysLT and mast cell activation were dependent upon platelets adhering to granulocytes and signaling through the thromboxane receptor TP. (T. Liu et al., 2013) This group also reported that signaling through cysLT2 was essential for aspirin-induced inflammation in a mouse model of AERD. (T. Liu et al., 2018) Hence, COX-1 mediated inhibition of PGE2 synthesis augments mast cell activation and platelet-mediated TP-dependent cysLT generation. In another animal model of AERD generated by dust mite priming, PGE synthase (mPGES)-deficient mice had greater IL-33 protein expression in the airway epithelium and significantly increased eosinophilic bronchovascular inflammation compared to WT animals.(T. Liu, Kanaoka, et al., 2015) Deletion of LTC4 synthase, the terminal enzyme essential for cysLT generation, prevented the augmented IL-33 in the mPGES-deficient mice. PGE2 regulation of IL-33 production may be tissue specific. For example, endogenous PGE2 augmented macrophage production of IL-33 via an EP2/EP4-cAMP-EPAV-dependent pathway.(Samuchiwal, Balestrieri, Raff, & Boyce, 2017) The interaction between the cysLT and PGE2 is dependent upon the EP receptor through which PGE2 signals. For example, LTD4 and PGE2 synergized in potentiating vascular inflammation in a mast cell-dependent manner via cysLT1 and EP3 signaling.(Kondeti et al., 2016) This synergism was mediated through Gi, protein kinase G and Erk. The LTD4 and PGE2 potentiated effects were partially sensitive to cysLT1 or EP3 antagonists, yet were completely inhibited by simultaneous treatment both in vitro and in vivo.
PGE2 signaling on inflammatory responses has also been examined in other models of allergen-induced inflammation. In a model of passive cutaneous anaphylaxis, butaprost, an EP2 selective agonist, reduced mast cell-mediated FcεRI-induced immediate hypersensitivity.(Serra-Pages et al., 2012) EP2 signaling on mast cells increased cAMP production while inhibiting FcεRI-mediated calcium flux. PGE2’s effect on FcεRImediated mast cell degranulation varied between activating and restraining, dependent on the relative ratio of EP2 to EP3 expression, with restraint only in cells having an increased EP2 to EP3 ratio.
While PGE2 decreases allergic airway inflammation in some animal models, is evidence suggests PGE2 enhances allergic contact dermatitis. PGE2 induced IL-22 T cell production through EP2 and EP4 signaling via cAMP signaling.(Robb et al., 2017) EP4 deficient mice had reduced hapten-induced IL-22 production in vivo and had decreased atopic-like skin inflammation in an oxazolone–induced allergic contact dermatitis model.
Prostaglandin F2α
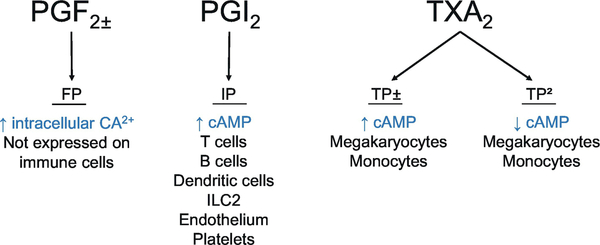
PGF2α is produced by PGF synthase (PGFS).(Komoto, Yamada, Watanabe, Woodward, & Takusagawa, 2006) PGFS has two main activities. First, PGFS catalyzes the formation of PGF2α from PGH2 by PGH2 9,11-endoperoxide reductase in the presence of NADPH. Second, PGFS catalyzes the conversion of PGF2α from PGD2 by PGD2 11-ketoreductase.(Komoto et al., 2006) PGFS is expressed in lung and peripheral blood lymphocytes, implying a potential role in allergic diseases such as asthma.(Suzuki-Yamamoto et al., 1999) PGFS is inhibited by non-steroidal anti-inflammatory drugs (NSAIDS) and this could partially explain the NSAID-mediated protective effect in some gastrointestinal tumors where PGFS activity is high.(Komoto et al., 2006) PGF2α has a single receptor, termed FP (Figure 4) that is the most promiscuous of the GPCRs in binding the principal prostaglandins. PGD2 and PGE2 bind to FP at nanomolar concentrations.(Hata & Breyer, 2004) Selective FP agonists such as fluprostenol and latanoprost are used in clinical settings because of these agents’ ocular hypotensive properties.(Hata & Breyer, 2004) PGF2α has important functions in renal physiology, reproduction, and modulation of intraocular pressure. FP receptor mRNA expression is greatest in the ovarian corpus luteum, followed by the kidney, and there is lower-level expression in the lung, stomach, and heart. (M. D. Breyer & Breyer, 2001) FP expression has not been detected in the spleen, thymus, or immune cells. Thus, in contrast to the other prostaglandins, PGF2α-FP signaling does not seem to strong regulatory role in inflammatory and immunological processes.(Hata & Breyer, 2004)
Figure 4.
PGF2α signals through FP to increase intracellular Ca2+. PGI2 signaling through IP increases cAMP. TXA2 signaling through TPα increases cAMP, while signaling through TPβ decreases cAMP.
Human studies of PGF2α
PGF2α has not been investigated to the same degree as PGD2 or PGE2 in allergic disease and asthma. PGF2α inhalation decreased specific airway conductance in both control and asthmatic subjects in a dose-dependent fashion.(Mathe, Hedqvist, Holmgren, & Svanborg, 1973; A. P. Smith & Cuthbert, 1972; A. P. Smith, Cuthbert, & Dunlop, 1975) There is relatively small inter-individual variation in healthy control subjects in response to inhaled PGF2α; however, wide variation in the pulmonary function response to PGF2α in asthmatics exists.(A. P. Smith et al., 1975) Asthmatics who inhaled PGF2α had wheezing, coughing and chest irritation within 3 to 4 minutes, with watery sputum occurring shortly thereafter.(A. P. Smith et al., 1975) Maximal decrease in specific airway conductance occurred 6 minutes after inhalation of after PGF2α and recovery occurred within 30 minutes.(A. P. Smith et al., 1975) Subjects with asthma experienced an approximate 150-fold greater sensitivity to PGF2α than did healthy subjects; however, asthmatics were only 8.5-fold more sensitive to histamine than nonasthmatic subjects.(A. P. Smith et al., 1975) There was reduced variation in individual responses to histamine compared to inhaled PGF2α challenge; however, a correlation existed in the sensitivity to these mediators with each other.(A. P. Smith et al., 1975) In general, women had less bronchoconstrictor responses to PGF2α compared to men.(A. P. Smith et al., 1975) Both PGE2 and isoprenaline shortened recovery from the decrease in pulmonary function elicited by inhalation of PGF2α, but neither atropine, disodium cromoglycate, nor flufenamic acid ablated PGF2α-induced bronchoconstriction.(A. P. Smith et al., 1975) PGF2α, and PGE2 as well, inhibited exhaled nitric oxide (NO) concentrations in both healthy subjects and those with asthma; however, the interpretation of this outcome is unknown.(Kharitonov, Sapienza, Barnes, & Chung, 1998) While FP is not expressed on immune cells, there is evidence that PGF2α may regulate airway inflammation. In asthma subjects, the degree of sputum eosinophilia correlated with the log sputum PGF2α concentrations and there was an inverse correlation between sputum eosinophilia and PGE2 levels. However, there was no correlation between sputum eosinophilia and sputum levels of cysLT, thromboxane, and PGD2.(Pavord et al., 1999)
Two studies investigated the ratio of plasma LTE4/PGF2α in asthma. In the first, elderly patients with asthma (age 60–85 years) were treated for 12 weeks with inhaled budesonide 400 μg plus montelukast or inhaled budesonide 800 μg.(Ban, Ye, et al., 2017) The plasma LTE4/PGF2α ratio and the blood eosinophil count increased in patients who had asthma exacerbations during a 12-week study period compared to the asthma subjects who did not have an exacerbation during the study period. In the second study of 45 patients with AERD and 44 patients with aspirin-tolerant asthma, the serum levels of LTE4 and LTE4/PGF2α were significantly greater in AERD subjects following lysine aspirin bronchoprovocation testing compared to aspirin-tolerant subjects.(Ban, Cho, et al., 2017) Serum baseline levels of LTE4 and LTE4/PGF2α discriminated AERD from aspirin-tolerant asthma.
Animal studies of PGF2α in allergic inflammation
To the best of my knowledge, no published studies exist that examine the effect of PGF2α administration or signaling through the FP receptor in the mouse allergen challenge model. An FP-deficient mouse exists and these mice had attenuated bleomycin-induced pulmonary fibrosis independent of TGF-β expression.(Oga et al., 2009) It would be interesting to determine if FP-deficient mice are protected from collagen deposition and airway wall remodeling resulting from chronic allergen challenge exposure.
Prostaglandin I2
PGI2 is synthesized from PGH2 by PGI synthase (PGIS) and the gene encoding PGIS is located on chromosome 20q13.11–13.(Nakayama, 2006) PGIS expression is high in the heart, lung, smooth muscle, kidney, and ovary, with moderate levels of expression in the brain, pancreas, and prostate.(Nakayama, 2006) There is low level PGIS expression in leukocytes, the placenta, and the spleen.(Nakayama, 2006) PGI2 signals through a GPCR receptor termed IP (Figure 4). (R. M. Breyer et al., 2001) PGI2 signaling through IP activates adenylate cyclase via Gs in a dose-dependent manner, resulting in increased cAMP production.(R. M. Breyer, Kennedy, Zhang, & Breyer, 2000) The increase in intracellular cAMP mediates PGI2 inhibition of platelet aggregation, dispersing existing platelet aggregates both in vitro and in human circulation. (R. M. Breyer et al., 2000) IP mRNA is expressed to the greatest degree in the thymus, while high levels of IP mRNA are found in spleen, heart, lung, and neurons in the dorsal root ganglia. Mouse bone marrow-derived dendritic cells (BMDCs) also express IP.(Zhou, Hashimoto, et al., 2007) The PGI2 analogs iloprost and cicaprost blocked BMDC production of proinflammatory chemokines (MIP-1alpha, MCP-1) and cytokines (IL-12, TNF-α, IL-1alpha, IL-6); however, these analogs augmented the secretion of the immunoinhibitory cytokine IL-10 by BMDCs.(Zhou, Hashimoto, et al., 2007) The regulatory effect of cytokine secretion by BMDCs was associated with IPdependent increase in intracellular cAMP and reduction of NF-κB activity.(Zhou, Hashimoto, et al., 2007) Iloprost and cicaprost also reduced LPS-induced BMDC expression of CD86, CD40, and MHC class II molecules and inhibited the ability of BMDCs to stimulate antigen-specific CD4+ T cell proliferation and production of Th2 cytokines.(Zhou, Hashimoto, et al., 2007) Iloprost increased human DC IL-10 production, and in co-culture experiments of iloprost-treated DCs and naïve T cells, T regulatory cells were induced.(Muller et al., 2010) IP is expressed in mouse T cells, as are the PGE2 receptor (EP) subtypes and the thromboxane receptor (TP).(Narumiya, Sugimoto, & Ushikubi, 1999) Further, IP is expressed by kidney smooth muscle and epithelial cells.(Komhoff, Lesener, Nakao, Seyberth, & Nusing, 1998) Messenger RNA for IP is expressed in both CD4+ Th1 and Th2 cells.(Zhou, Blackwell, et al., 2007) Therefore, IP is present on several different cell types, including those essential for the adaptive immune response.
Human studies of PGI2 in allergic inflammation
PGI2 and PGD2 were the major COX products produced in antigen-induced Type I hypersensitivity reactions in human lung parenchyma, at 3- to 7-fold increased concentrations compared to other PGs.(Schulman, Newball, Demers, Fitzpatrick, & Adkinson, 1981) The PGI2 metabolite 6-keto-PGF1α was measured in concentrations 2-to-3-fold greater than all the other PGs in both airway and subpleural lung fragments in an in vitro anaphylaxis assay of passively sensitized human lung.(Schulman, Adkinson, & Newball, 1982) Unexpectedly, plasma 6-keto-PGF1α was increased following antigen challenge in which asthmatic subjects were pretreated with indomethacin.(Shephard, Malan, Macfarlane, Mouton, & Joubert, 1985) Thus, PGI2 is synthesized at a high level in pulmonary allergic inflammatory responses, likely a result of activated endothelial cells that express almost exclusively the PGIS present in the human airway.
The majority of the intervention studies investigating the modulatory effect of PGI2 in human asthma were performed over 20 years ago. An important drawback of these older reports is that PGI2 (half-life 3–5 minutes) was used, rather than the more stable analogs that have been recently developed. These older reports may not accurately reflect the therapeutic capability of the currently available PGI2 agonists. In a study from 1979, PGI2 pretreatment had no effect on allergen-induced immediate phase bronchoconstriction.(Bianco, Robuschi, Grugni, Ceserani, & Gandolfi, 1979) In older another study, PGI2 protected against exercise and ultrasonic water-induced bronchoconstriction; however, it again had no effect on allergen-induced airway reactivity.(Bianco, Robuschi, Ceserani, & Gandolfi, 1980) Inhaled PGI2 did not have an effect on specific airway conductance; however, consistent bronchodilation occurred in two asthma subjects. In this study, PGI2 had a significant effect of on the cardiovascular system. Inhaled PGI2 decreased both diastolic (20±3 mmHg) and systolic (8±2 mmHg) blood pressure, and increased pulse rate (29±3 beats per minute).(C. Hardy, Robinson, Lewis, Tattersfield, & Holgate, 1985) Intravenous PGI2 administration had no effect on the decrease in airflow induced by aspirin in subjects with AERD.(Nizankowska, Czerniawska-Mysik, & Szczeklik, 1986) Contradictory results of the effect of inhaled PGI2 in subjects with mild asthma have been reported.(C. C. Hardy, Bradding, Robinson, & Holgate, 1988) In these studies PGI2 did not change specific airway conductance, yet resulted in a concentration-dependent reduction in FEV1. In contrast, these same investigators published that PGI2 protected against PGD2- or methacholine-induced bronchoconstriction. These investigators posited that these disparate findings could be related to PGI2’s marked vasodilator effect, with ensuing airway narrowing through mucosal blood engorgement, while this same phenomenon possibly reducing the spasmogenic properties of other inhaled mediators by augmenting their clearance from the airways. The oral PGI2 analog OP-41483 did not change FEV1 or airways responsiveness to methacholine in stable asthmatics.(Fujimura, Ozawa, & Matsuda, 1991) This last report was published in 1991 and, to our knowledge, there has been only one other published manuscript investigating PGI2 in human pulmonary allergic inflammation or asthma. In this report, the utility of administering inhaled iloprost to subjects with mild atopic asthma was investigated.(Majeski, Hoskins, Dworski, & Sheller, 2012) Subjects inhaled iloprost four times daily at either 2.5 or 5 μg for 2 weeks in a safety study. Chronic iloprost inhalation did not reduce spirometry or methacholine responsiveness.(Majeski et al., 2012) Importantly, both inhaled PGE2 and PGI2 induce cough.(Grace, Birrell, Dubuis, Maher, & Belvisi, 2012; Parikh, Rajagopal, Fortin, Tapson, & Poms, 2016) The therapeutic potential of newer, more stable PGI2 analogs in asthma, particularly oral agents, that have been approved for use in pulmonary hypertension, remains unexplored.
In vitro studies show that PGI2 inhibits the function of human cells that are critical to allergic inflammatory responses. Cicaprost decreased IL-5 and IL-13 production by human ILC2 isolated from peripheral blood.(Zhou, Toki, et al., 2016) PGI2 produced by the endothelium was essential for the maintenance of the endothelial barrier function and markedly blunted human eosinophil migration, yet had no effect on neutrophil migration. The IP antagonist Cay10441 abrogated the inhibitory effect of PGI2 on eosinophil migration.(Konya et al., 2010) These properties of PGI2 reflect its ability to inhibit the function of inflammatory cells that contribute to allergic inflammation.
Animal studies of PGI2 in allergic inflammation
Mouse models reveal that endogenous PGI2 signaling through IP inhibits allergic airway inflammation. IP KO mice had greater lung production of IL-4 and IL-5, serum antigen-specific and total IgE levels, and airway cellularity compared to WT mice in a model of short-term OVA challenge.(Takahashi et al., 2002) In a model of chronic allergen challenge, IP KO mice had heightened Th2 cytokine levels, airway eosinophils and lymphocytes, and hydroxyproline concentrations compared to WT mice.(Nagao et al., 2003) Endogenous PGI2 reduced STAT6-independent lung chemokine (CCL1, CCL17, CCL22, and CXCL12) and Th2 cytokine levels, while reducing CD4+ cell proliferation and IL-2 production in vitro.(Zhou, Zhang, et al., 2016) Pharmacologic COX-2 inhibition of PGI2 also increased allergic inflammation in mice, and adoptive transfer of ovalbumin-specific T cells that were treated with the PGI2 analog carbaprostacyclin increased T cells production of IL-10 production, which reversed the heightened Th2 inflammation.(Jaffar, Wan, & Roberts, 2002) Using this same mouse model of adoptive transfer of ovalbumin-specific CD4+ Th2 cells, PGI2 signaling restrained allergic inflammation by blocking allergen-challenge driven recruitment of CD4+ Th2 cells into the airways.(Jaffar, Ferrini, Buford, Fitzgerald, & Roberts, 2007) While PGI2 restrains allergic inflammation, three groups have shown that PGI2 promotes Th17 responses in mice.(Jaffar, Ferrini, Shaw, Fitzgerald, & Roberts, 2011; Li et al., 2011; Zhou et al., 2012) The concept that endogenous PGI2 signaling limits allergen-induced inflammation by promoting immune tolerance was supported by the finding that COX inhibition ablated immune tolerance through suppression of PGI2-IP signaling, and that the PGI2 analog cicaprost blocked the anti-tolerance effect of COX inhibition.(Zhou et al., 2014) Administration of the sustained-release PGI2 analog ONO-1301M, that also had thromboxane A2 synthase inhibitory activity blocked airways responsiveness, Th2 cytokine production, airway eosinophils, airway smooth muscle hypertrophy, goblet cell metaplasia, and submucosal fibrosis in chronic house dust mite and ovalbumin models of allergic inflammation.(Kimura et al., 2013; Yamabayashi et al., 2012) PGI2 not only restrains the adaptive allergic response, but PGI2 signaling through IP additionally reduced innate immunity-mediated allergic inflammation. In a mouse model of 4 consecutive days of airway challenge with Alternaria alternata extract, endogenous PGI2 signaling significantly reduced the number of lung IL-5 and IL-13-expressing ILC2 and mucous metaplasia, while inhaled cicaprost inhibited these same inflammatory endpoints.(Zhou, Toki, et al., 2016) IP KO mice had augmented inflammatory and physiologic changes compared to WT mice in the model of bleomycin-induced fibrosis.(Lovgren et al., 2006) In a different model of bleomycin-induced lung injury, mice that overexpressed PGIS in airway epithelial cells were protected against lung injury and had reduced production of F2-isoprostanes, a marker of oxidant injury. In these experiments, PGI2 stimulated the expression of NAD(P)H:quinone oxidoreductase type I (NQO1), an enzyme that prevents generation of reactive oxidant species.(Zhou et al., 2011)
In support of the notion that PGI2 limits airway inflammation, inhaled iloprost decreased maturation and migration of lung DCs to mediastinal lymph nodes after intranasal antigen administration, decreasing induction of an allergen-specific Th2 responses in these nodes.(Idzko et al., 2007) Iloprost-treated DCs also reduced Th2 differentiation from naive T cells and restrained effector cytokine production in primed Th2 cells.(Idzko et al., 2007) Not only did PGI2 downregulate mature DC function, but it also decreased the function of DCs. Cicaprost reduced uptake of FITC-labeled OVA by immature BMDCs.(Toki et al., 2013) Further, cicaprost augmented immature BMDC dissolution of podosomes, focal adhesion structures necessary for DC adherence to extracellular matrix in the lung and other tissues.(Toki et al., 2013) With podosomes dissolution, the DC is no longer tethered to the epithelium and can migrate to the regional lymph node. Podosome dissolution typically only takes place after the DC has taken up antigen, but PGI2-regulated podosome dissolution allows the DC to leave the environment-epithelial cell interface prior to antigen uptake. Cicaprost also augmented pro-MMP-9 production that has a critical role in DC egress from mucosal surfaces to draining lymph nodes.(Toki et al., 2013) Lastly, cicaprost promoted DC surface CCR7 expression and resulting chemotactic migration toward CCL19 and CCL21 produced in the lymph nodes T cell zone. These in vitro results imply that cicaprost promoted immature DCs migration from mucosal surface to draining lymph nodes. This notion was supported by migration of immature green fluorescent protein expressing BMDCs to draining lymph nodes that was enhanced by pretreatment with cicaprost. Cicaprost-mediated reduction in antigen uptake by immature DCs, enhanced podosome dissolution, heightened pro-MMP-9 production, and increased CCR7 expression were all IP-dependent.(Toki et al., 2013) These results reveal that PGI2 inhibits DC-mediated immune activation by enhancing immature DC migration and by decreasing antigen uptake, providing two additional potential mechanisms by which PGI2 may be therapeutically beneficial in allergic diseases, such as asthma. Comparable to the inhibitory effect of PGI2 on human eosinophil migration, PGI2 also reduced the mobilization of eosinophils from the bone marrow of guinea pigs, while blocking the shape change necessary for eosinophil locomotion.(Sturm, Schuligoi, Konya, Sturm, & Heinemann, 2011) Lastly, cicaprost reduced IL-33-induced mouse ILC2 production of IL-5 and IL-13 in vitro.(Zhou, Toki, et al., 2016)
These results in animal models of allergic inflammation are encouraging for the use of PGI2 in the treatment of allergic airway inflammation; however, cost and difficulty in drug delivery are currently obstacles.(Boswell, Zhou, Newcomb, & Peebles, 2011; Dorris & Peebles, 2012) The development of less expensive and longer acting agonists, particularly oral agents, may make stable analogs of PGI2 a viable therapeutic option.
Thromboxane A2
Thromboxane A2 (TXA2) is the predominant arachidonic acid metabolism product synthesized by platelets and is a potent platelet aggregating agent.(Whittle & Moncada, 1983) Thromboxane synthase (TXAS) is an endoplasmic reticulum membrane protein that catalyzes the conversion of prostaglandin H2 to thromboxane A2.(Miyata et al., 1994) TXAS is on q33-q34 of the long arm of chromosome 7 in humans.(Miyata et al., 1994) TXAS is expressed at high levels in lung, liver, kidney, and blood cells, including megakaryocytes and monocytes.(Miyata et al., 1994) Lower, but still significant, levels of TXAS mRNA are observed in placenta, kidney, and thymus.(Miyata et al., 1994) TXA2 is principally produced by platelets, macrophages, monocytes, neutrophils and lung parenchyma.(Ruan, 2004) Subsequent to its formation, TXA2 is nonezymatically hydrolyzed to thromboxane B2, which is then metabolized to the principle urinary metabolites 2,3-dinor-thromboxane B2 and 11-dehydro-thromboxane B2.(Roberts, Sweetman, & Oates, 1981) The TXA2 receptor is named TP (Figure 4) and isoforms have been identified, TPα and TPβ, which are produced by alternative splicing occurring in the carboxy-terminal region after the seventh transmembrane domain.(Raychowdhury et al., 1994) These isoforms couple to a Gq protein, leading to phospholipase C activation, calcium release, and activation of protein kinase C.(Huang, Ramamurthy, Lin, & Le Breton, 2004) Interestingly, these receptor isoforms couple oppositely to adenylate cyclase, as TPα stimulates adenylate cyclase while TPβ inhibits this enzyme.(Hirata, Ushikubi, Kakizuka, Okuma, & Narumiya, 1996) The TP receptors are localized to the plasma membrane and cytosolic compartments and are chiefly distributed to organs rich in vasculature such as lung, heart and kidney.(Hata & Breyer, 2004) These GPCRs are involved in a myriad of physiological and pathological processes, which include vasoconstriction that has been implicated in vascular diseases such as hypertension, atherosclerosis, stroke, and myocardial infarction.(Grosser, Fries, & Fitzgerald, 2006)
Human studies of TXA2 in allergic inflammation
TXA2 has a half-life of approximately 30 seconds,(Roberts, Sweetman, Lewis, Austen, & Oates, 1980) and because of the unstable nature of this lipid there is a dearth of in vivo studies investigating the effect of TXA2 in the human airway. TXB2 did not elicit bronchoconstriction of human airway in vivo;(Taylor et al., 1991) however, TXA2 was a potent stimulant of in vitro smooth muscle constriction.(Whittle & Moncada, 1983) TXA2 potentially regulates the physiology involved in acute asthma exacerbations. TXA2 metabolite concentrations were increased 4–6 fold in the urine of patients admitted to the hospital with asthma compared to non-smoking controls admitted for other diagnoses.(Taylor et al., 1991) Subjects with allergic asthma challenged with inhaled allergen had a significant increase in urinary excretion of TXA2 products;(Lupinetti, Sheller, Catella, & Fitzgerald, 1989; Sladek et al., 1990) however, another group did not report similar results.(Taylor et al., 1991) Inhibition of platelet COX by low dose aspirin reduced the increase in urinary 2,3-dimer thromboxane, supporting the notion that allergen inhalation causes platelet activation. Subjects with allergic asthma pre-treated with indomethacin prior to inhaled allergen challenge had in a significant reduction in urinary TXA2 metabolites; however, there was no change in pulmonary function.(Sladek et al., 1990) Subjects who have airway hyperresponsiveness following ozone exposure had an increase in TXA2 in BAL, as well as airway neutrophilia.(Seltzer et al., 1986) Likewise, LTB4 inhalation also increased levels of TXA2 and neutrophils in BAL fluid.(O'Byrne et al., 1985)
Short-term asthma studies and animal challenge models have used TXA2 antagonists to determine the effect of TXA2 on pulmonary function and airway reactivity. In an uncontrolled study, the TP antagonist seratrodast (AA-2414) significantly inhibited bronchial reactivity in subjects with asthma after 4 weeks of once daily therapy compared to a pre-treatment baseline.(Aizawa et al., 1998) Seratrodast did not change either exhaled nitric oxide or the percentage of eosinophils in sputum.(Aizawa et al., 1998) In a follow-up double blind, placebo-controlled study of asthma subjects treated for four weeks, seratrodast significantly improved symptom score, peak expiratory flow (PEF) rates, diurnal variation of PEF, and bronchial responsiveness compared to placebo.(Hoshino, Sim, Shimizu, Nakayama, & Koya, 1999) These improvements were associated with a reduction in the number of submucosal eosinophils on bronchial biopsy.(Hoshino et al., 1999) Seratrodast significantly reduced the number of cells in the epithelium expressing the chemokines RANTES (CCL5) and macrophage inflammatory protein (MIP)-1α (CCL3). Seratrodast also decreased the number of cells in the submucosa expressing monocyte chemotactic protein-3, RANTES, MIP-1α, and eotaxin (CCL11).(Hoshino et al., 1999) These data suggest that TXA2 antagonism blocks allergic inflammation in the lung; however, the mechanisms are not well defined.
Functional variants in the TXA2 pathway may impact the pathogenesis of hypersensitivity conditions. Comprehensive sequencing of the TBXA2R gene in 48 Japanese subjects identified a set of variants in intron 1 in linkage disequilibrium with c.795 T>C rs1131882 that was reported to be associated with asthma.(Takeuchi et al., 2013) Haplotypes containing the minor alleles of SNP2 (C>T rs2238632) and SNP3 (C>T rs2238632) had augmented transcriptional activity and were associated with lower lung function (baseline FEV1/FVC, FEF25–75, and postbronchodilator FEV1/FVC) in childhood-onset asthma compared to other haplotypes. TXA1 synthase (TBXAS1) has been associated with acute urticarial induced by NSAIDs. There was a significant association for rs6962291 under the log-additive genetic model that remained significant after correction for multiple comparisons.(Vidal et al., 2013) This SNP was associated with a protective role in relation to aspirin intolerance in asthma patients.(Oh et al., 2011) A meta-analysis found that the TBXA2R +924C/T polymorphism is associated with asthma risk, and that the TBXA2R +795C/T polymorphism may be a risk factor for AERD.(Pan, Li, Xie, & Li, 2016)
Animal studies of TXA2 in allergic inflammation
Both the TXA2 synthase inhibitor OKY-046 and the TP receptor antagonist S-1452 decreased total cells and eosinophils in BAL fluid in a dose response relationship in OVA-sensitized and challenged mice.193 Treatment with either a TXA2 synthase inhibitor or a TP receptor antagonist significantly decreased pro-inflammatory cytokine production in the setting of antigen-specific activation of splenic mononuclear cells from sensitized mice in ex vivo experiments.193 Genetic deletion of TP receptors from mPGES-1-deficient mice blocked dust mite-induced pulmonary eosinophilia, airway hyperresponsiveness, Th2 cytokine generation, and vascular remodeling.(T. Liu et al., 2012) Therefore, the pathogenic contributions from TXA2 may be amplified when local concentrations of PGE2 are low. This notion is supported by the result that antagonizing EP1 (ONO-8130) and EP2 (PF-04418948) receptor signaling showed that TP mediated a component of antigen-induced contraction of the guinea pig trachea.(Safholm, Dahlen, & Adner, 2013) The available animal data imply that blocking TP signaling, either through a receptor antagonist or neutralizing TXA2, may be a therapeutic target in the treatment of asthma.
TP receptor signaling is critical for cysLT-mediated airway effects. Intranasal administration of LTC4 to allergen-sensitized mice augmented the airway eosinophilia, yet decreased the number of peripheral blood eosinophils in a TP-specific fashion.(T. Liu, Garofalo, et al., 2015) LTC4 heightened ICAM-1 and VCAM-1 in an aspirin and TPdependent manner. Hematopoietic and nonhematopoietic TP expression was critical for LTC4 to elicit eosinophil recruitment. Therefore, both autocrine and paracrine functions of TXA2 act downstream of LTC4 signaling via cysLT2 on platelets to increase eosinophil recruitment through pulmonary vascular adhesion pathways. These results suggest that TP antagonists may be useful in asthma subjects who have high levels of cysLT production.
Conclusion
PGs are a varied array of lipid products synthesized rapidly by both hematopoietic and structural cells in response to endogenous and environmental stimuli. PGs regulate host homeostatic, immunologic, and inflammatory functions by signaling through specific GPCRs. PG-specific receptor-deficient mice and receptor-selective agonists have provided the opportunity to determine the biologic activity of these molecules. Development of specific enzyme inhibitors and receptor antagonists for therapeutic use continues and their use in animal studies strongly support targeting of these pathways in human allergic diseases such as asthma.
Acknowledgments
This work was supported by National Institutes of Health Grants U19 AI095227, R01 AI 124456, R01 AI 111820, and Veteran Affairs Grant I01BX000624.
Abbreviations
- AERD
aspirin-exacerbated respiratory disease
- ACQ
asthma control questionnaire
- BAL
bronchial alveolar lavage
- CRTH2
chemoattractant receptor-like molecule expressed on Th2 cells
- COX
cyclooxygenase
- cysLT
cysteinyl leukotriene
- cPGES
cytosolic PGE synthase
- EoE
eosinophilic esophagitis
- FEV1
forced expiratory volume in one second
- GM-CSF
granulocyte macrophage-colony stimulating factor
- ILC
innate lymphoid cells
- IL
interleukin
- KO
knockout
- LT
leukotrienes
- LPS
lipopolysaccharide
- LO
lipoxygenase
- mPGES
microsomal PGE synthase
- PLA2
phospholipase A2
- PAF-AH
platelet-activating factor acetylhydrolases
- PG
prostaglandin
- Th2
T helper cells type 2
- TSLP
thymic stromal lymphopoietin
Footnotes
Financial disclosures
The author declares that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Adam M, Boie Y, Rushmore TH, Muller G, Bastien L, McKee KT, . . . Abramovitz M (1994). Cloning and expression of three isoforms of the human EP3 prostanoid receptor. FEBS Letters, 338(2), 170–174. [DOI] [PubMed] [Google Scholar]
- Adamusiak AM, Stasikowska-Kanicka O, Lewandowska-Polak A, Danilewicz M, Wagrowska-Danilewicz M, Jankowski A, . . . Pawliczak R (2012). Expression of arachidonate metabolism enzymes and receptors in nasal polyps of aspirinhypersensitive asthmatics. Int. Arch. Allergy Immunol, 157(4), 354–362. doi:000329744[pii];10.1159/000329744[doi] [DOI] [PubMed] [Google Scholar]
- Aggarwal S, Moodley YP, Thompson PJ, & Misso NL (2010). Prostaglandin E2 and cysteinyl leukotriene concentrations in sputum: association with asthma severity and eosinophilic inflammation. Clin. Exp. Allergy, 40(1), 85–93. doi:CEA3386[pii];10.1111/j.1365-2222.2009.03386.x[doi] [DOI] [PubMed] [Google Scholar]
- Aizawa H, Inoue H, Nakano H, Matsumoto K, Yoshida M, Fukuyama S, . . . Hara N (1998). Effects of thromboxane A2 antagonist on airway hyperresponsiveness, exhaled nitric oxide, and induced sputum eosinophils in asthmatics. Prostaglandins Leukot. Essent. Fatty Acids, 59(3), 185–190. [DOI] [PubMed] [Google Scholar]
- Aksoy MO, Li X, Borenstein M, Yi Y, & Kelsen SG (1999). Effects of topical corticosteroids on inflammatory mediator-induced eicosanoid release by human airway epithelial cells. J. Allergy Clin. Immunol, 103(6), 1081–1091. [DOI] [PubMed] [Google Scholar]
- An S, Yang J, Xia M, & Goetzl EJ (1993). Cloning and expression of the EP2 subtype of human receptors for prostaglandin E2. Biochemical & Biophysical Research Communications, 197(1), 263–270. [DOI] [PubMed] [Google Scholar]
- Bain G, Lorrain DS, Stebbins KJ, Broadhead AR, Santini AM, Prodanovich P, . . . Evans JF (2011). Pharmacology of AM211, a potent and selective prostaglandin D2 receptor type 2 antagonist that is active in animal models of allergic inflammation. J Pharmacol. Exp. Ther, 338(1), 290–301. doi:jpet.111.180430[pii];10.1124/jpet.111.180430[doi] [DOI] [PubMed] [Google Scholar]
- Balestrieri B, Hsu VW, Gilbert H, Leslie CC, Han WK, Bonventre JV, & Arm JP (2006). Group V secretory phospholipase A2 translocates to the phagosome after zymosan stimulation of mouse peritoneal macrophages and regulates phagocytosis. J. Biol. Chem, 281(10), 6691–6698. doi:M508314200[pii];10.1074/jbc.M508314200[doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ban GY, Cho K, Kim SH, Yoon MK, Kim JH, Lee HY, . . . Park HS (2017). Metabolomic analysis identifies potential diagnostic biomarkers for aspirin-exacerbated respiratory disease. Clin Exp Allergy, 47(1), 37–47. doi: 10.1111/cea.12797 [DOI] [PubMed] [Google Scholar]
- Ban GY, Ye YM, Kim SH, Hur GY, Kim JH, Shim JJ, . . . group P (2017). Plasma LTE4/PGF2alpha Ratio and Blood Eosinophil Count Are Increased in Elderly Asthmatics With Previous Asthma Exacerbation. Allergy Asthma Immunol Res, 9(4), 378–382. doi: 10.4168/aair.2017.9.4.378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankova LG, Lai J, Yoshimoto E, Boyce JA, Austen KF, Kanaoka Y, & Barrett NA (2016). Leukotriene E4 elicits respiratory epithelial cell mucin release through the G-protein-coupled receptor, GPR99. Proc Natl Acad Sci U S A, 113(22), 6242–6247. doi: 10.1073/pnas.1605957113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes N, Pavord I, Chuchalin A, Bell J, Hunter M, Lewis T, . . . Perkins CM (2012). A randomized, double-blind, placebo-controlled study of the CRTH2 antagonist OC000459 in moderate persistent asthma. Clin. Exp. Allergy, 42(1), 38–48. doi: 10.1111/j.1365-2222.2011.03813.x[doi] [DOI] [PubMed] [Google Scholar]
- Barnett K, Jacoby DB, Nadel JA, & Lazarus SC (1988). The effects of epithelial cell supernatant on contractions of isolated canine tracheal smooth muscle. Am. Rev. Respir. Dis, 138(4), 780–783. [DOI] [PubMed] [Google Scholar]
- Barnig C, Cernadas M, Dutile S, Liu X, Perrella MA, Kazani S, . . . Levy BD (2013). Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma. Sci. Transl. Med, 5(174), 174ra126. doi:5/174/174ra26[pii];10.1126/scitranslmed.3004812[doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley RC, Featherstone RL, Church MK, Rafferty P, Varley JG, Harris A, . . . Holgate ST (1989). Effect of a thromboxane receptor antagonist on PGD2- and allergen-induced bronchoconstriction. J. Appl. Physiol, 66(4), 1685–1693. [DOI] [PubMed] [Google Scholar]
- Betz M, & Fox BS (1991). Prostaglandin E2 inhibits production of Th1 lymphokines but not of Th2 lymphokines. Journal of Immunology, 146(1), 108–113. [PubMed] [Google Scholar]
- Bianco S, Robuschi M, Ceserani R, & Gandolfi C (1980). Effects of prostacyclin on aspecifically and specifically induced bronchoconstriction in asthmatic patients. Eur. J. Respir. Dis. Suppl, 106, 81–87. [PubMed] [Google Scholar]
- Bianco S, Robuschi M, Grugni A, Ceserani R, & Gandolfi C (1979). Effect of prostacyclin on antigen induced immediate bronchoconstriction in asthmatic patients. Prostaglandins Med, 3(1), 39–45. [DOI] [PubMed] [Google Scholar]
- Birrell MA, Maher SA, Dekkak B, Jones V, Wong S, Brook P, & Belvisi MG (2015). Anti-inflammatory effects of PGE2 in the lung: role of the EP4 receptor subtype. Thorax, 70(8), 740–747. doi: 10.1136/thoraxjnl-2014-206592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boswell MG, Zhou W, Newcomb DC, & Peebles RS Jr. (2011). PGI2 as a regulator of CD4+ subset differentiation and function. Prostaglandins Other Lipid Mediat, 96(1–4), 21–26. doi:S1098-8823(11)00073-6[pii];10.1016/j.prostaglandins.2011.08.003[doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breyer MD, & Breyer RM (2001). G protein-coupled prostanoid receptors and the kidney. Annu. Rev. Physiol, 63, 579–605. [DOI] [PubMed] [Google Scholar]
- Breyer RM, Bagdassarian CK, Myers SA, & Breyer MD (2001). Prostanoid receptors: subtypes and signaling. Annu. Rev. Pharmacol. Toxicol, 41, 661–690. [DOI] [PubMed] [Google Scholar]
- Breyer RM, Kennedy CR, Zhang Y, & Breyer MD (2000). Structure-function analyses of eicosanoid receptors. Physiologic and therapeutic implications. Ann. N. Y. Acad. Sci, 905, 221–231. [DOI] [PubMed] [Google Scholar]
- Buckley J, Birrell MA, Maher SA, Nials AT, Clarke DL, & Belvisi MG (2011). EP4 receptor as a new target for bronchodilator therapy. Thorax, 66(12), 1029–1035. doi:thx.2010.158568[pii];10.1136/thx.2010.158568[doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill KN, Bensko JC, Boyce JA, & Laidlaw TM (2015). Prostaglandin D(2): a dominant mediator of aspirin-exacerbated respiratory disease. J Allergy Clin Immunol, 135(1), 245–252. doi: 10.1016/j.jaci.2014.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill KN, Raby BA, Zhou X, Guo F, Thibault D, Baccarelli A, . . . Laidlaw TM (2016). Impaired E Prostanoid2 Expression and Resistance to Prostaglandin E2 in Nasal Polyp Fibroblasts from Subjects with Aspirin-Exacerbated Respiratory Disease. Am J Respir Cell Mol Biol, 54(1), 34–40. doi: 10.1165/rcmb.2014-0486OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card JW, Carey MA, Bradbury JA, Graves JP, Lih FB, Moorman MP, . . . Zeldin DC (2006). Cyclooxygenase-1 overexpression decreases Basal airway responsiveness but not allergic inflammation. J Immunol, 177(7), 4785–4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey MA, Germolec DR, Bradbury JA, Gooch RA, Moorman MP, Flake GP, . . . Zeldin DC (2003). Accentuated T helper type 2 airway response after allergen challenge in cyclooxygenase-1-/- but not cyclooxygenase-2-/- mice. Am. J Respir. Crit Care Med, 167(11), 1509–1515. [DOI] [PubMed] [Google Scholar]
- Charlesworth EN, Kagey-Sobotka A, Schleimer RP, Norman PS, & Lichtenstein LM (1991). Prednisone inhibits the appearance of inflammatory mediators and the influx of eosinophils and basophils associated with the cutaneous late- phase response to allergen. J. Immunol, 146(2), 671–676. [PubMed] [Google Scholar]
- Chizzolini C, Chicheportiche R, Alvarez M, de RC, Roux-Lombard P, Ferrari-Lacraz S, & Dayer JM (2008). Prostaglandin E2 synergistically with interleukin-23 favors human Th17 expansion. Blood, 112(9), 3696–3703. doi:blood-2008-05-155408[pii];10.1182/blood-2008-05-155408[doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KS, Lee JH, Park MK, Park HK, Yu HS, & Roh HJ (2015). Prostaglandin E2 and Transforming Growth Factor-beta Play a Critical Role in Suppression of Allergic Airway Inflammation by Adipose-Derived Stem Cells. PLoS One, 10(7), e0131813. doi: 10.1371/journal.pone.0131813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho W, Kim Y, Jeoung DI, Kim YM, & Choe J (2011). IL-4 and IL-13 suppress prostaglandins production in human follicular dendritic cells by repressing COX-2 and mPGES-1 expression through JAK1 and STAT6. Mol. Immunol, 48(6–7), 966–972. doi:S0161-5890(11)00030-7[pii];10.1016/j.molimm.2011.01.007[doi] [DOI] [PubMed] [Google Scholar]
- Churchill L, Chilton FH, Resau JH, Bascom R, Hubbard WC, & Proud D (1989). Cyclooxygenase metabolism of endogenous arachidonic acid by cultured human tracheal epithelial cells. Am. Rev. Respir. Dis, 140(2), 449–459. [DOI] [PubMed] [Google Scholar]
- Coleman RA, Smith WL, & Narumiya S (1994). International Union of Pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. [Review] [242 refs]. Pharmacological Reviews, 46(2), 205–229. [PubMed] [Google Scholar]
- Corrigan CJ, Napoli RL, Meng Q, Fang C, Wu H, Tochiki K, . . . Ying S (2012). Reduced expression of the prostaglandin E2 receptor E-prostanoid 2 on bronchial mucosal leukocytes in patients with aspirin-sensitive asthma. J Allergy Clin. Immunol, 129(6), 1636–1646. doi:S0091-6749(12)00270-9[pii];10.1016/j.jaci.2012.02.007[doi] [DOI] [PubMed] [Google Scholar]
- Crosignani S, Pretre A, Jorand-Lebrun C, Fraboulet G, Seenisamy J, Augustine JK, . . . Johnson Z (2011). Discovery of potent, selective, and orally bioavailable alkynylphenoxyacetic acid CRTH2 (DP2) receptor antagonists for the treatment of allergic inflammatory diseases. J. Med. Chem, 54(20), 7299–7317. doi: 10.1021/jm200866y[doi] [DOI] [PubMed] [Google Scholar]
- Daham K, James A, Balgoma D, Kupczyk M, Billing B, Lindeberg A, . . . Dahlen B (2014). Effects of selective COX-2 inhibition on allergen-induced bronchoconstriction and airway inflammation in asthma. J Allergy Clin Immunol, 134(2), 306–313. doi: 10.1016/j.jaci.2013.12.002 [DOI] [PubMed] [Google Scholar]
- Daham K, Song WL, Lawson JA, Kupczyk M, Gulich A, Dahlen SE, . . . Dahlen B (2011). Effects of celecoxib on major prostaglandins in asthma. Clin. Exp. Allergy, 41(1), 36–45. doi: 10.1111/j.1365-2222.2010.03617.x[doi] [DOI] [PubMed] [Google Scholar]
- Delamere F, Holland E, Patel S, Bennett J, Pavord I, & Knox A (1994). Production of PGE2 by bovine cultured airway smooth muscle cells and its inhibition by cyclo-oxygenase inhibitors. Br. J. Pharmacol, 111(4), 983–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demoly P, Jaffuel D, Lequeux N, Weksler B, Creminon C, Michel FB, . . . Bousquet J (1997). Prostaglandin H synthase 1 and 2 immunoreactivities in the bronchial mucosa of asthmatics. Am. J. Respir. Crit Care Med, 155(2), 670–675. [DOI] [PubMed] [Google Scholar]
- Dennis EA, Cao J, Hsu YH, Magrioti V, & Kokotos G (2011). Phospholipase A2 enzymes: physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem. Rev, 111(10), 6130–6185. doi: 10.1021/cr200085w[doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorris SL, & Peebles RS Jr. (2012). PGI(2) as a Regulator of Inflammatory Diseases. Mediators. Inflamm, 2012, 926968. doi: 10.1155/2012/926968[doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle WJ, Boehm S, & Skoner DP (1990). Physiologic responses to intranasal dose-response challenges with histamine, methacholine, bradykinin, and prostaglandin in adult volunteers with and without nasal allergy. J. Allergy Clin. Immunol, 86(6 Pt 1), 924–935. [DOI] [PubMed] [Google Scholar]
- Draijer C, Boorsma CE, Reker-Smit C, Post E, Poelstra K, & Melgert BN (2016). PGE2-treated macrophages inhibit development of allergic lung inflammation in mice. J Leukoc Biol, 100(1), 95–102. doi: 10.1189/jlb.3MAB1115-505R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drazen JM (1998). Leukotrienes as mediators of airway obstruction. Am J Respir Crit Care Med, 158(5 Pt 3), S193–200. doi: 10.1164/ajrccm.158.supplement_2.13tac180 [DOI] [PubMed] [Google Scholar]
- Ebbens FA, Maldonado M, de Groot EJ, Alobid I, van Drunen CM, Picado C, . . . Mullol J (2009). Topical glucocorticoids downregulate COX-1 positive cells in nasal polyps. Allergy, 64(1), 96–103. doi:ALL1815[pii];10.1111/j.1398-9995.2008.01815.x[doi] [DOI] [PubMed] [Google Scholar]
- Emery DL, Djokic TD, Graf PD, & Nadel JA (1989). Prostaglandin D2 causes accumulation of eosinophils in the lumen of the dog trachea. J. Appl. Physiol, 67(3), 959–962. [DOI] [PubMed] [Google Scholar]
- Erpenbeck VJ, Popov TA, Miller D, Weinstein SF, Spector S, Magnusson B, . . . Beier J (2016). The oral CRTh2 antagonist QAW039 (fevipiprant): A phase II study in uncontrolled allergic asthma. Pulm Pharmacol Ther, 39, 54–63. doi: 10.1016/j.pupt.2016.06.005 [DOI] [PubMed] [Google Scholar]
- Fajt ML, Gelhaus SL, Freeman B, Uvalle CE, Trudeau JB, Holguin F, & Wenzel SE (2013). Prostaglandin D(2) pathway upregulation: relation to asthma severity, control, and TH2 inflammation. J Allergy Clin Immunol, 131(6), 1504–1512. doi: 10.1016/j.jaci.2013.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng C, Beller EM, Bagga S, & Boyce JA (2006). Human mast cells express multiple EP receptors for prostaglandin E2 that differentially modulate activation responses. Blood, 107(8), 3243–3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson S, Hebert RL, & Laneuville O (1999). NS-398 upregulates constitutive cyclooxygenase-2 expression in the M-1 cortical collecting duct cell line. J Am. Soc Nephrol, 10(11), 2261–2271. [DOI] [PubMed] [Google Scholar]
- Fish JE, Ankin MG, Adkinson NF Jr., & Peterman VI (1981). Indomethacin modification of immediate-type immunologic airway responses in allergic asthmatic and non-asthmatic subjects: evidence for altered arachidonic acid metabolism in asthma. Am. Rev. Respir. Dis, 123(6), 609–614. [DOI] [PubMed] [Google Scholar]
- Fitzgerald GA (2004). Coxibs and cardiovascular disease. N. Engl. J. Med, 351(17), 1709–1711. doi:NEJMp048288[pii];10.1056/NEJMp048288[doi] [DOI] [PubMed] [Google Scholar]
- FitzPatrick M, Donovan C, & Bourke JE (2014). Prostaglandin E2 elicits greater bronchodilation than salbutamol in mouse intrapulmonary airways in lung slices. Pulm Pharmacol Ther, 28(1), 68–76. doi: 10.1016/j.pupt.2013.11.005 [DOI] [PubMed] [Google Scholar]
- Flower RJ, Harvey EA, & Kingston WP (1976). Inflammatory effects of prostaglandin D2 in rat and human skin. Br. J. Pharmacol, 56(2), 229–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura M, Ozawa S, & Matsuda T (1991). Effect of oral administration of a prostacyclin analog (OP-41483) on pulmonary function and bronchial responsiveness in stable asthmatic subjects. J. Asthma, 28(6), 419–424. [DOI] [PubMed] [Google Scholar]
- Fujitani Y, Kanaoka Y, Aritake K, Uodome N, Okazaki-Hatake K, & Urade Y (2002). Pronounced eosinophilic lung inflammation and Th2 cytokine release in human lipocalin-type prostaglandin D synthase transgenic mice. J. Immunol, 168(1), 443–449. [DOI] [PubMed] [Google Scholar]
- Gao Y, Zhao C, Wang W, Jin R, Li Q, Ge Q, . . . Zhang Y (2016). Prostaglandins E2 signal mediated by receptor subtype EP2 promotes IgE production in vivo and contributes to asthma development. Sci Rep, 6, 20505. doi: 10.1038/srep20505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauvreau GM, Watson RM, & O'Byrne PM (1999). Protective effects of inhaled PGE2 on allergen-induced airway responses and airway inflammation. American Journal of Respiratory & Critical Care Medicine, 159(1), 31–36. [DOI] [PubMed] [Google Scholar]
- Gervais FG, Sawyer N, Stocco R, Hamel M, Krawczyk C, Sillaots S, . . . O'Neill GP (2011). Pharmacological characterization of MK-7246, a potent and selective CRTH2 (chemoattractant receptor-homologous molecule expressed on T-helper type 2 cells) antagonist. Mol. Pharmacol, 79(1), 69–76. doi:mol.110.068585[pii];10.1124/mol.110.068585[doi] [DOI] [PubMed] [Google Scholar]
- Goldblatt MW (1933). A depressor substance in seminal fluid. J Soc Chem Ind (Lond), 52, 1056–1057. [Google Scholar]
- Gomi K, Zhu FG, & Marshall JS (2000). Prostaglandin E2 selectively enhances the IgE-mediated production of IL- 6 and granulocyte-macrophage colonystimulating factor by mast cells through an EP1/EP3-dependent mechanism. J. Immunol, 165(11), 6545–6552. [DOI] [PubMed] [Google Scholar]
- Goncharova EA, Goncharov DA, Zhao H, Penn RB, Krymskaya VP, & Panettieri RA Jr. (2012). beta2-adrenergic receptor agonists modulate human airway smooth muscle cell migration via vasodilator-stimulated phosphoprotein. Am. J. Respir. Cell Mol. Biol, 46(1), 48–54. doi:46/1/48[pii];10.1165/rcmb.2011-0217OC[doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonem S, Berair R, Singapuri A, Hartley R, Laurencin MFM, Bacher G, . . . Brightling CE (2016). Fevipiprant, a prostaglandin D2 receptor 2 antagonist, in patients with persistent eosinophilic asthma: a single-centre, randomised, double-blind, parallel-group, placebo-controlled trial. Lancet Respir Med, 4(9), 699–707. doi: 10.1016/S2213-2600(16)30179-5 [DOI] [PubMed] [Google Scholar]
- Grace M, Birrell MA, Dubuis E, Maher SA, & Belvisi MG (2012). Transient receptor potential channels mediate the tussive response to prostaglandin E2 and bradykinin. Thorax, 67(10), 891–900. doi: 10.1136/thoraxjnl-2011-201443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosser T, Fries S, & Fitzgerald GA (2006). Biological basis for the cardiovascular consequences of COX-2 inhibition: therapeutic challenges and opportunities. J Clin Invest, 116(1), 4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyllfors P, Bochenek G, Overholt J, Drupka D, Kumlin M, Sheller J, . . . Dahlen B (2003). Biochemical and clinical evidence that aspirin-intolerant asthmatic subjects tolerate the cyclooxygenase 2-selective analgetic drug celecoxib. J Allergy Clin. Immunol, 111(5), 1116–1121. doi:S0091674903010728[pii] [DOI] [PubMed] [Google Scholar]
- Hall IP, Fowler AV, Gupta A, Tetzlaff K, Nivens MC, Sarno M, . . . Rand Sutherland E (2015). Efficacy of BI 671800, an oral CRTH2 antagonist, in poorly controlled asthma as sole controller and in the presence of inhaled corticosteroid treatment. Pulm Pharmacol Ther, 32, 37–44. doi: 10.1016/j.pupt.2015.03.003 [DOI] [PubMed] [Google Scholar]
- Hammad H, de Heer HJ, Soullie T, Hoogsteden HC, Trottein F, & Lambrecht BN (2003). Prostaglandin D2 inhibits airway dendritic cell migration and function in steady state conditions by selective activation of the D prostanoid receptor 1. J Immunol, 171(8), 3936–3940. [DOI] [PubMed] [Google Scholar]
- Hammad H, Kool M, Soullie T, Narumiya S, Trottein F, Hoogsteden HC, & Lambrecht BN (2007). Activation of the D prostanoid 1 receptor suppresses asthma by modulation of lung dendritic cell function and induction of regulatory T cells. J Exp. Med, 204(2), 357–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy C, Robinson C, Lewis RA, Tattersfield AE, & Holgate S (1985). Airway and cardiovascular responses to inhaled prostacyclin in normal and asthmatic subjects. American Review of Respiratory Disease, 131(1), 18–21. [DOI] [PubMed] [Google Scholar]
- Hardy CC, Bradding P, Robinson C, & Holgate ST (1988). Bronchoconstrictor and antibronchoconstrictor properties of inhaled prostacyclin in asthma. J. Appl. Physiol, 64(4), 1567–1574. [DOI] [PubMed] [Google Scholar]
- Harizi H, Juzan M, Moreau JF, & Gualde N (2003). Prostaglandins inhibit 5-lipoxygenase-activating protein expression and leukotriene B4 production from dendritic cells via an IL-10-dependent mechanism. J Immunol, 170(1), 139–146. [DOI] [PubMed] [Google Scholar]
- Harmanci E, Ozakyol A, Ozdemir N, Elbek O, & Isik R (1998). Misoprostol has no favorable effect on bronchial hyperresponsiveness in mild asthmatics. Allerg. Immunol. (Paris), 30(9), 298–300. [PubMed] [Google Scholar]
- Hartert TV, Dworski RT, Mellen BG, Oates JA, Murray JJ, & Sheller JR (2000). Prostaglandin E(2) decreases allergen-stimulated release of prostaglandin D(2) in airways of subjects with asthma. Am. J. Respir. Crit Care Med, 162(2 Pt 1), 637–640. [DOI] [PubMed] [Google Scholar]
- Hartney JM, Coggins KG, Tilley SL, Jania LA, Lovgren AK, Audoly LP, & Koller BH (2006). Prostaglandin E2 protects lower airways against bronchoconstriction. Am. J Physiol Lung Cell Mol. Physiol, 290(1), L105–L113. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Sheller JR, Morrow JD, Collins RD, Goleniewska K, O'Neal J, . . . Peebles RS Jr. (2005). Cyclooxygenase inhibition augments allergic inflammation through CD4-dependent, STAT6-independent mechanisms. J. Immunol, 174(1), 525–532. [DOI] [PubMed] [Google Scholar]
- Hata AN, & Breyer RM (2004). Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacol. Ther, 103(2), 147–166. [DOI] [PubMed] [Google Scholar]
- Herrerias A, Torres R, Serra M, Marco A, Roca-Ferrer J, Picado C, & de MF (2009). Subcutaneous prostaglandin E(2) restrains airway mast cell activity in vivo and reduces lung eosinophilia and Th(2) cytokine overproduction in house dust mite-sensitive mice. Int. Arch. Allergy Immunol, 149(4), 323–332. doi:000205578[pii];10.1159/000205578[doi] [DOI] [PubMed] [Google Scholar]
- Hilkens CM, Vermeulen H, van Neerven RJ, Snijdewint FG, Wierenga EA, & Kapsenberg ML (1995). Differential modulation of T helper type 1 (Th1) and T helper type 2 (Th2) cytokine secretion by prostaglandin E2 critically depends on interleukin-2. European Journal of Immunology, 25(1), 59–63. [DOI] [PubMed] [Google Scholar]
- Hirai H, Tanaka K, Yoshie O, Ogawa K, Kenmotsu K, Takamori Y, . . . Nagata K (2001). Prostaglandin D2 Selectively Induces Chemotaxis in T Helper Type 2 Cells, Eosinophils, and Basophils via Seven-Transmembrane Receptor CRTH2. J. Exp. Med, 193(2), 255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata T, Ushikubi F, Kakizuka A, Okuma M, & Narumiya S (1996). Two thromboxane A2 receptor isoforms in human platelets. Opposite coupling to adenylyl cyclase with different sensitivity to Arg60 to Leu mutation. J Clin Invest, 97(4), 949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogaboam CM, Bissonnette EY, Chin BC, Befus AD, & Wallace JL (1993). Prostaglandins inhibit inflammatory mediator release from rat mast cells. Gastroenterology, 104(1), 122–129. [DOI] [PubMed] [Google Scholar]
- Honda K, Arima M, Cheng G, Taki S, Hirata H, Eda F, . . . Fukuda T (2003). Prostaglandin D2 reinforces Th2 type inflammatory responses of airways to lowdose antigen through bronchial expression of macrophage-derived chemokine. J Exp. Med, 198(4), 533–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino M, Sim J, Shimizu K, Nakayama H, & Koya A (1999). Effect of AA-2414, a thromboxane A2 receptor antagonist, on airway inflammation in subjects with asthma. J Allergy Clin Immunol, 103(6), 1054–1061. [DOI] [PubMed] [Google Scholar]
- Huang JS, Ramamurthy SK, Lin X, & Le Breton GC (2004). Cell signalling through thromboxane A2 receptors. Cell Signal, 16(5), 521–533. [DOI] [PubMed] [Google Scholar]
- Idzko M, Hammad H, van Nimwegen M, Kool M, Vos N, Hoogsteden HC, & Lambrecht BN (2007). Inhaled iloprost suppresses the cardinal features of asthma via inhibition of airway dendritic cell function. J. Clin. Invest, 117(2), 464–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iniguez MA, Punzon C, & Fresno M (1999). Induction of cyclooxygenase-2 on activated T lymphocytes: regulation of T cell activation by cyclooxygenase-2 inhibitors. J. Immunol, 163(1), 111–119. [PubMed] [Google Scholar]
- Jaffar Z, Ferrini ME, Buford MC, Fitzgerald GA, & Roberts K (2007). Prostaglandin I2-IP signaling blocks allergic pulmonary inflammation by preventing recruitment of CD4+ Th2 cells into the airways in a mouse model of asthma. J Immunol, 179(9), 6193–6203. doi:179/9/6193[pii] [DOI] [PubMed] [Google Scholar]
- Jaffar Z, Ferrini ME, Shaw PK, Fitzgerald GA, & Roberts K (2011). Prostaglandin I(2)promotes the development of IL-17-producing gammadelta T cells that associate with the epithelium during allergic lung inflammation. J. Immunol, 187(10), 5380–5391. doi:jimmunol.1101261[pii];10.4049/jimmunol.1101261[doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffar Z, Wan KS, & Roberts K (2002). A key role for prostaglandin I2 in limiting lung mucosal Th2, but not Th1, responses to inhaled allergen. J. Immunol, 169(10), 5997–6004. [DOI] [PubMed] [Google Scholar]
- Jinnai N, Sakagami T, Sekigawa T, Kakihara M, Nakajima T, Yoshida K, . . . Inoue I (2004). Polymorphisms in the prostaglandin E2 receptor subtype 2 gene confer susceptibility to aspirin-intolerant asthma: a candidate gene approach. Hum. Mol. Genet, 13(24), 3203–3217. doi:ddh332[pii];10.1093/hmg/ddh332[doi] [DOI] [PubMed] [Google Scholar]
- Johnston SL, Freezer NJ, Ritter W, O'Toole S, & Howarth PH (1995). Prostaglandin D2-induced bronchoconstriction is mediated only in part by the thromboxane prostanoid receptor. Eur. Respir. J, 8(3), 411–415. [DOI] [PubMed] [Google Scholar]
- Kahnt AS, Rorsch F, Diehl O, Hofmann B, Lehmann C, Steinbrink SD, . . . Maier TJ (2013). Cysteinyl leukotriene-receptor-1 antagonists interfere with PGE2 synthesis by inhibiting mPGES-1 activity. Biochem. Pharmacol, 86(2), 286–296. doi:S0006-2952(13)00289-X[pii];10.1016/j.bcp.2013.05.005[doi] [DOI] [PubMed] [Google Scholar]
- Kaliner M, & Austen KF (1974). Cyclic AMP, ATP, and reversed anaphylactic histamine release from rat mast cells. J. Immunol, 112(2), 664–674. [PubMed] [Google Scholar]
- Kalinski P (2012). Regulation of immune responses by prostaglandin E2. J Immunol, 188(1), 21–28. doi:188/1/21[pii];10.4049/jimmunol.1101029[doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaoka Y, Maekawa A, & Austen KF (2013). Identification of GPR99 protein as a potential third cysteinyl leukotriene receptor with a preference for leukotriene E4 ligand. J Biol Chem, 288(16), 10967–10972. doi: 10.1074/jbc.C113.453704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang YJ, Mbonye UR, Delong CJ, Wada M, & Smith WL (2007). Regulation of intracellular cyclooxygenase levels by gene transcription and protein degradation. Prog. Lipid Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katamura K, Shintaku N, Yamauchi Y, Fukui T, Ohshima Y, Mayumi M, & Furusho K (1995). Prostaglandin E2 at priming of naive CD4+ T cells inhibits acquisition of ability to produce IFN-gamma and IL-2, but not IL- 4 and IL-5. Journal of Immunology, 155(10), 4604–4612. [PubMed] [Google Scholar]
- Kharitonov SA, Sapienza MA, Barnes PJ, & Chung KF (1998). Prostaglandins E2 and F2alpha reduce exhaled nitric oxide in normal and asthmatic subjects irrespective of airway caliber changes. Am. J. Respir. Crit Care Med, 158(5 Pt 1), 1374–1378. [DOI] [PubMed] [Google Scholar]
- Kim SH, Kim YK, Park HW, Jee YK, Kim SH, Bahn JW, . . . Min KU (2007). Association between polymorphisms in prostanoid receptor genes and aspirin-intolerant asthma. Pharmacogenet. Genomics, 17(4), 295–304. doi: 10.1097/01.fpc.0000239977.61841.fe[doi];01213011-200704000-00008[pii] [DOI] [PubMed] [Google Scholar]
- Kimura Y, Koya T, Kagamu H, Shima K, Sakamoto H, Kawakami H, . . . Narita I (2013). A single injection of a sustained-release prostacyclin analog (ONO-1301MS) suppresses airway inflammation and remodeling in a chronic house dust mite-induced asthma model. Eur J Pharmacol, 721(1–3), 80–85. doi: 10.1016/j.ejphar.2013.09.051 [DOI] [PubMed] [Google Scholar]
- Kirby JG, Hargreave FE, Cockcroft DW, & O'Byrne PM (1989). Effect of indomethacin on allergen-induced asthmatic responses. J. Appl. Physiol, 66(2), 578–583. [DOI] [PubMed] [Google Scholar]
- Kita H, Abu-Ghazaleh RI, Gleich GJ, & Abraham RT (1991). Regulation of Iginduced eosinophil degranulation by adenosine 3',5'- cyclic monophosphate. J. Immunol, 146(8), 2712–2718. [PubMed] [Google Scholar]
- Komhoff M, Lesener B, Nakao K, Seyberth HW, & Nusing RM (1998). Localization of the prostacyclin receptor in human kidney. Kidney Int, 54(6), 1899–1908. [DOI] [PubMed] [Google Scholar]
- Komoto J, Yamada T, Watanabe K, Woodward DF, & Takusagawa F (2006). Prostaglandin F2alpha formation from prostaglandin H2 by prostaglandin F synthase (PGFS): crystal structure of PGFS containing bimatoprost. Biochemistry, 45(7), 1987–1996. [DOI] [PubMed] [Google Scholar]
- Kondeti V, Al-Azzam N, Duah E, Thodeti CK, Boyce JA, & Paruchuri S (2016). Leukotriene D4 and prostaglandin E2 signals synergize and potentiate vascular inflammation in a mast cell-dependent manner through cysteinyl leukotriene receptor 1 and E-prostanoid receptor 3. J Allergy Clin Immunol, 137(1), 289–298. doi: 10.1016/j.jaci.2015.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konya V, Sturm EM, Schratl P, Beubler E, Marsche G, Schuligoi R, . . . Heinemann A (2010). Endothelium-derived prostaglandin I(2) controls the migration of eosinophils. J Allergy Clin. Immunol, 125(5), 1105–1113. doi:S0091-6749(09)01794-1[pii];10.1016/j.jaci.2009.12.002[doi] [DOI] [PubMed] [Google Scholar]
- Kowalski ML, Pawliczak R, Wozniak J, Siuda K, Poniatowska M, Iwaszkiewicz J, . . . Kaliner MA (2000). Differential metabolism of arachidonic acid in nasal polyp epithelial cells cultured from aspirin-sensitive and aspirin-tolerant patients. Am. J Respir. Crit Care Med, 161(2 Pt 1), 391–398. doi: 10.1164/ajrccm.161.2.9902034[doi] [DOI] [PubMed] [Google Scholar]
- Krug N, Gupta A, Badorrek P, Koenen R, Mueller M, Pivovarova A, . . . Wood C (2014). Efficacy of the oral chemoattractant receptor homologous molecule on TH2 cells antagonist BI 671800 in patients with seasonal allergic rhinitis. J Allergy Clin Immunol, 133(2), 414–419. doi: 10.1016/j.jaci.2013.10.013 [DOI] [PubMed] [Google Scholar]
- Kuehn HS, Jung MY, Beaven MA, Metcalfe DD, & Gilfillan AM (2011). Prostaglandin E2 activates and utilizes mTORC2 as a central signaling locus for the regulation of mast cell chemotaxis and mediator release. J. Biol. Chem, 286(1), 391–402. doi:M110.164772[pii];10.1074/jbc.M110.164772[doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuna P, Bjermer L, & Tornling G (2016). Two Phase II randomized trials on the CRTh2 antagonist AZD1981 in adults with asthma. Drug Des Devel Ther, 10, 2759–2770. doi: 10.2147/dddt.s105142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunikata T, Yamane H, Segi E, Matsuoka T, Sugimoto Y, Tanaka S, . . . Narumiya S (2005). Suppression of allergic inflammation by the prostaglandin E receptor subtype EP3. Nat. Immunol, 6(5), 524–531. [DOI] [PubMed] [Google Scholar]
- Kupczyk M, & Kuna P (2017). Targeting the PGD2/CRTH2/DP1 Signaling Pathway in Asthma and Allergic Disease: Current Status and Future Perspectives. Drugs, 77(12), 1281–1294. doi: 10.1007/s40265-017-0777-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laouini D, Elkhal A, Yalcindag A, Kawamoto S, Oettgen H, & Geha RS (2005). COX-2 inhibition enhances the TH2 immune response to epicutaneous sensitization. J Allergy Clin Immunol, 116(2), 390–396. [DOI] [PubMed] [Google Scholar]
- Larsson AK, Hagfjard A, Dahlen SE, & Adner M (2011). Prostaglandin D(2) induces contractions through activation of TP receptors in peripheral lung tissue from the guinea pig. Eur. J. Pharmacol, 669(1–3), 136–142. doi:S0014-2999(11)00885-5[pii];10.1016/j.ejphar.2011.07.046[doi] [DOI] [PubMed] [Google Scholar]
- Lazzeri N, Belvisi MG, Patel HJ, Yacoub MH, Fan CK, & Mitchell JA (2001). Effects of prostaglandin E2 and cAMP elevating drugs on GM-CSF release by cultured human airway smooth muscle cells. Relevance to asthma therapy. Am. J. Respir. Cell Mol. Biol, 24(1), 44–48. [DOI] [PubMed] [Google Scholar]
- Leal-Berumen I, O'Byrne P, Gupta A, Richards CD, & Marshall JS (1995). Prostanoid enhancement of interleukin-6 production by rat peritoneal mast cells. J. Immunol, 154(9), 4759–4767. [PubMed] [Google Scholar]
- Li H, Bradbury JA, Dackor RT, Edin ML, Graves JP, Degraff LM, . . . Zeldin DC (2011). Cyclooxygenase-2 (COX-2) Regulates Th17 Cell Differentiation During Allergic Lung Inflammation. Am. J Respir. Crit Care Med. doi:201010-1637OC[pii];10.1164/rccm.201010-1637OC[doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Edin ML, Bradbury JA, Graves JP, DeGraff LM, Gruzdev A, . . . Zeldin DC (2013). Cyclooxygenase-2 inhibits T helper cell type 9 differentiation during allergic lung inflammation via down-regulation of IL-17RB. Am J Respir Crit Care Med, 187(8), 812–822. doi: 10.1164/rccm.201211-2073OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Zheng M, Qiao J, Dang Y, Zhang P, & Jin X (2014). Role of prostaglandin D2 /CRTH2 pathway on asthma exacerbation induced by Aspergillus fumigatus. Immunology, 142(1), 78–88. doi: 10.1111/imm.12234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu MC, Bleecker ER, Lichtenstein LM, Kagey-Sobotka A, Niv Y, McLemore TL, . . . Hubbard WC (1990). Evidence for elevated levels of histamine, prostaglandin D2, and other bronchoconstricting prostaglandins in the airways of subjects with mild asthma. Am. Rev. Respir. Dis, 142(1), 126–132. [DOI] [PubMed] [Google Scholar]
- Liu MC, Hubbard WC, Proud D, Stealey BA, Galli SJ, Kagey-Sobotka A, . . . Lichtenstein LM (1991). Immediate and late inflammatory responses to ragweed antigen challenge of the peripheral airways in allergic asthmatics. Cellular, mediator, and permeability changes. Am. Rev. Respir. Dis, 144(1), 51–58. [DOI] [PubMed] [Google Scholar]
- Liu MC, Proud D, Lichtenstein LM, Hubbard WC, Bochner BS, Stealey BA, . . . Schleimer RP (2001). Effects of prednisone on the cellular responses and release of cytokines and mediators after segmental allergen challenge of asthmatic subjects. J. Allergy Clin. Immunol, 108(1), 29–38. [DOI] [PubMed] [Google Scholar]
- Liu T, Barrett NA, Kanaoka Y, Yoshimoto E, Garofalo D, Cirka H, . . . Boyce JA (2018). Type 2 Cysteinyl Leukotriene Receptors Drive IL-33-Dependent Type 2 Immunopathology and Aspirin Sensitivity. J Immunol, 200(3), 915–927. doi: 10.4049/jimmunol.1700603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Garofalo D, Feng C, Lai J, Katz H, Laidlaw TM, & Boyce JA (2015). Platelet-driven leukotriene C4-mediated airway inflammation in mice is aspirin-sensitive and depends on T prostanoid receptors. J Immunol, 194(11), 5061–5068. doi: 10.4049/jimmunol.1402959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Kanaoka Y, Barrett NA, Feng C, Garofalo D, Lai J, . . . Boyce JA (2015). Aspirin-Exacerbated Respiratory Disease Involves a Cysteinyl Leukotriene-Driven IL-33-Mediated Mast Cell Activation Pathway. J Immunol, 195(8), 3537–3545. doi: 10.4049/jimmunol.1500905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Laidlaw TM, Feng C, Xing W, Shen S, Milne GL, & Boyce JA (2012). Prostaglandin E2 deficiency uncovers a dominant role for thromboxane A2 in house dust mite-induced allergic pulmonary inflammation. Proc. Natl. Acad. Sci. U. S. A, 109(31), 12692–12697. doi:1207816109[pii];10.1073/pnas.1207816109[doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Laidlaw TM, Katz HR, & Boyce JA (2013). Prostaglandin E2 deficiency causes a phenotype of aspirin sensitivity that depends on platelets and cysteinyl leukotrienes. Proc. Natl. Acad. Sci. U. S. A, 110(42), 16987–16992. doi:1313185110[pii];10.1073/pnas.1313185110[doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovgren AK, Jania LA, Hartney JM, Parsons KK, Audoly LP, Fitzgerald GA, . . . Koller BH (2006). COX-2-derived prostacyclin protects against bleomycin-induced pulmonary fibrosis. Am. J Physiol Lung Cell Mol. Physiol, 291(2), L144–L156. [DOI] [PubMed] [Google Scholar]
- Luna-Gomes T, Magalhaes KG, Mesquita-Santos FP, Bakker-Abreu I, Samico RF, Molinaro R, . . . Bandeira-Melo C (2011). Eosinophils as a novel cell source of prostaglandin D2: autocrine role in allergic inflammation. J Immunol, 187(12), 6518–6526. doi:jimmunol.1101806[pii];10.4049/jimmunol.1101806[doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundequist A, Nallamshetty SN, Xing W, Feng C, Laidlaw TM, Uematsu S, . . . Boyce JA (2010). Prostaglandin E(2) exerts homeostatic regulation of pulmonary vascular remodeling in allergic airway inflammation. J. Immunol, 184(1), 433–441. doi:184/1/433[pii];10.4049/jimmunol.0902835[doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupinetti MD, Sheller JR, Catella F, & Fitzgerald GA (1989). Thromboxane biosynthesis in allergen-induced bronchospasm. Evidence for platelet activation. Am. Rev. Respir. Dis, 140(4), 932–935. [DOI] [PubMed] [Google Scholar]
- Luschnig P, Frei R, Lang-Loidolt D, Rozsasi A, Tomazic PV, Lippe IT, . . . Heinemann A (2014). Altered inhibitory function of the E-type prostanoid receptor 4 in eosinophils and monocytes from aspirin-intolerant patients. Pharmacology, 94(5–6), 280–286. doi: 10.1159/000369827 [DOI] [PubMed] [Google Scholar]
- Maher SA, Birrell MA, Adcock JJ, Wortley MA, Dubuis ED, Bonvini SJ, . . . Belvisi MG (2015). Prostaglandin D2 and the role of the DP1, DP2 and TP receptors in the control of airway reflex events. Eur Respir J, 45(4), 1108–1118. doi: 10.1183/09031936.00061614 [DOI] [PubMed] [Google Scholar]
- Maher SA, Birrell MA, & Belvisi MG (2009). Prostaglandin E2 mediates cough via the EP3 receptor: implications for future disease therapy. Am. J. Respir. Crit Care Med, 180(10), 923–928. doi:200903-0388OC[pii];10.1164/rccm.200903-0388OC[doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeski E, Hoskins A, Dworski R, & Sheller JR (2012). Iloprost inhalation in mild asthma. J. Asthma, 49(9), 961–965. doi: 10.3109/02770903.2012.724130[doi] [DOI] [PubMed] [Google Scholar]
- Maric J, Ravindran A, Mazzurana L, Bjorklund AK, Van Acker A, Rao A, . . . Mjosberg J (2017). PGE2 suppresses human group 2 innate lymphoid cell function. J Allergy Clin Immunol. doi: 10.1016/j.jaci.2017.09.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TR, Gerard NP, Galli SJ, & Drazen JM (1988). Pulmonary responses to bronchoconstrictor agonists in the mouse. Journal of Applied Physiology, 64(6), 2318–2323. [DOI] [PubMed] [Google Scholar]
- Mastalerz L, Sanak M, Gawlewicz-Mroczka A, Gielicz A, Cmiel A, & Szczeklik A (2008). Prostaglandin E2 systemic production in patients with asthma with and without aspirin hypersensitivity. Thorax, 63(1), 27–34. doi:thx.2007.080903[pii];10.1136/thx.2007.080903[doi] [DOI] [PubMed] [Google Scholar]
- Mathe AA, Hedqvist P, Holmgren A, & Svanborg N (1973). Bronchial hyperreactivity to prostaglandin F 2 and histamine in patients with asthma. Br. Med. J, 1(5847), 193–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka T, Hirata M, Tanaka H, Takahashi Y, Murata T, Kabashima K, . . . Narumiya S (2000). Prostaglandin D2 as a mediator of allergic asthma. Science, 287(5460), 2013–2017. [DOI] [PubMed] [Google Scholar]
- Melillo E, Woolley KL, Manning PJ, Watson RM, & O'Byrne PM (1994). Effect of inhaled PGE2 on exercise-induced bronchoconstriction in asthmatic subjects. Am. J. Respir. Crit Care Med, 149(5), 1138–1141. [DOI] [PubMed] [Google Scholar]
- Miller D, Wood C, Bateman E, LaForce C, Blatchford J, Hilbert J, . . . Fowler A (2017). A randomized study of BI 671800, a CRTH2 antagonist, as add-on therapy in poorly controlled asthma. Allergy Asthma Proc, 38(2), 157–164. doi: 10.2500/aap.2017.38.4034 [DOI] [PubMed] [Google Scholar]
- Mitson-Salazar A, Yin Y, Wansley DL, Young M, Bolan H, Arceo S, . . . Prussin C (2016). Hematopoietic prostaglandin D synthase defines a proeosinophilic pathogenic effector human T(H)2 cell subpopulation with enhanced function. J. Allergy Clin. Immunol, 137(3), 907–918. doi:S0091-6749(15)01186-0[pii];10.1016/j.jaci.2015.08.007[doi] [DOI] [PubMed] [Google Scholar]
- Miyata A, Yokoyama C, Ihara H, Bandoh S, Takeda O, Takahashi E, & Tanabe T (1994). Characterization of the human gene (TBXAS1) encoding thromboxane synthase. Eur. J Biochem, 224(2), 273–279. [DOI] [PubMed] [Google Scholar]
- Monneret G, Gravel S, Diamond M, Rokach J, & Powell WS (2001). Prostaglandin D2 is a potent chemoattractant for human eosinophils that acts via a novel DP receptor. Blood, 98(6), 1942–1948. [DOI] [PubMed] [Google Scholar]
- Muller T, Durk T, Blumenthal B, Herouy Y, Sorichter S, Grimm M, . . . Idzko M (2010). Iloprost has potent anti-inflammatory properties on human monocytederived dendritic cells. Clin. Exp. Allergy, 40(8), 1214–1221. doi: CEA3558[pii]; 10.1111/j.1365-2222.2010.03558.x[doi] [DOI] [PubMed] [Google Scholar]
- Murray M, Webb MS, O'Callaghan C, Swarbrick AS, & Milner AD (1992). Respiratory status and allergy after bronchiolitis. Archives of Disease in Childhood, 67(4), 482–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutalithas K, Guillen C, Day C, Brightling CE, Pavord ID, & Wardlaw AJ (2010). CRTH2 expression on T cells in asthma. Clin. Exp. Immunol, 161(1), 34–40. doi:CEI4161[pii]; 10.1111/j.1365-2249.2010.04161.x[doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naclerio RM, Meier HL, Kagey-Sobotka A, Adkinson NF Jr., Meyers DA, Norman PS, & Lichtenstein LM (1983). Mediator release after nasal airway challenge with allergen. Am. Rev. Respir. Dis, 128(4), 597–602. [DOI] [PubMed] [Google Scholar]
- Nagao K, Tanaka H, Komai M, Masuda T, Narumiya S, & Nagai H (2003). Role of prostaglandin I2 in airway remodeling induced by repeated allergen challenge in mice. Am. J. Respir. Cell Mol. Biol. [DOI] [PubMed] [Google Scholar]
- Nagira Y, Goto K, Tanaka H, Aoki M, Furue S, Inagaki N, . . . Shichijo M (2016). Prostaglandin D2 Modulates Neuronal Excitation of the Trigeminal Ganglion to Augment Allergic Rhinitis in Guinea Pigs. J Pharmacol Exp Ther, 357(2), 273–280. doi: 10.1124/jpet.115.231225 [DOI] [PubMed] [Google Scholar]
- Nakamura T, Fujiwara Y, Yamada R, Fujii W, Hamabata T, Lee MY, . . . Murata T (2017). Mast cell-derived prostaglandin D2 attenuates anaphylactic reactions in mice. J Allergy Clin Immunol, 140(2), 630–632.e639. doi: 10.1016/j.jaci.2017.02.030 [DOI] [PubMed] [Google Scholar]
- Nakamura T, Maeda S, Horiguchi K, Maehara T, Aritake K, Choi BI, . . . Murata T (2015). PGD2 deficiency exacerbates food antigen-induced mast cell hyperplasia. Nat Commun, 6, 7514. doi: 10.1038/ncomms8514 [DOI] [PubMed] [Google Scholar]
- Nakano Y, Kidani Y, Goto K, Furue S, Tomita Y, Inagaki N, . . . Shichijo M (2016). Role of Prostaglandin D2 and DP1 Receptor on Japanese Cedar Pollen-Induced Allergic Rhinitis in Mice. J Pharmacol Exp Ther, 357(2), 258–263. doi: 10.1124/jpet.115.229799 [DOI] [PubMed] [Google Scholar]
- Nakata J, Kondo M, Tamaoki J, Takemiya T, Nohara M, Yamagata K, & Nagai A (2005). Augmentation of allergic inflammation in the airways of cyclooxygenase-2-deficient mice. Respirology, 10(2), 149–156. [DOI] [PubMed] [Google Scholar]
- Nakayama T (2006). Prostacyclin analogues: prevention of cardiovascular diseases. Cardiovasc. Hematol. Agents Med. Chem, 4(4), 351–359. [DOI] [PubMed] [Google Scholar]
- Narumiya S, Sugimoto Y, & Ushikubi F (1999). Prostanoid receptors: structures, properties, and functions. Physiol Rev, 79(4), 1193–1226. [DOI] [PubMed] [Google Scholar]
- Nishigaki N, Negishi M, Honda A, Sugimoto Y, Namba T, Narumiya S, & Ichikawa A (1995). Identification of prostaglandin E receptor 'EP2' cloned from mastocytoma cells EP4 subtype. FEBS Lett, 364(3), 339–341. [DOI] [PubMed] [Google Scholar]
- Nizankowska E, Czerniawska-Mysik G, & Szczeklik A (1986). Lack of effect of i.v. prostacyclin on aspirin-induced asthma. Eur. J. Respir. Dis, 69(5), 363–368. [PubMed] [Google Scholar]
- O'Byrne PM, & Jones GL (1986). The effect of indomethacin on exercise-induced bronchoconstriction and refractoriness after exercise. Am. Rev. Respir. Dis, 134(1), 69–72. [DOI] [PubMed] [Google Scholar]
- O'Byrne PM, Leikauf GD, Aizawa H, Bethel RA, Ueki IF, Holtzman MJ, & Nadel JA (1985). Leukotriene B4 induces airway hyperresponsiveness in dogs. J. Appl. Physiol, 59(6), 1941–1946. [DOI] [PubMed] [Google Scholar]
- Oga T, Matsuoka T, Yao C, Nonomura K, Kitaoka S, Sakata D, . . . Narumiya S (2009). Prostaglandin F(2alpha) receptor signaling facilitates bleomycin-induced pulmonary fibrosis independently of transforming growth factor-beta. Nat. Med, 15(12), 1426–1430. doi:nm.2066[pii];10.1038/nm.2066[doi] [DOI] [PubMed] [Google Scholar]
- Oguma T, Asano K, Shiomi T, Fukunaga K, Suzuki Y, Nakamura M, . . . Yamaguchi K (2002). Cyclooxygenase-2 expression during allergic inflammation in guinea-pig lungs. Am. J. Respir. Crit Care Med, 165(3), 382–386. [DOI] [PubMed] [Google Scholar]
- Oh SH, Kim YH, Park SM, Cho SH, Park JS, Jang AS, . . . Park CS (2011). Association analysis of thromboxane A synthase 1 gene polymorphisms with aspirin intolerance in asthmatic patients. Pharmacogenomics, 12(3), 351–363. doi: 10.2217/pgs.10.181 [DOI] [PubMed] [Google Scholar]
- Palikhe NS, Sin HJ, Kim SH, Sin HJ, Hwang EK, Ye YM, & Park HS (2012). Genetic variability of prostaglandin E2 receptor subtype EP4 gene in aspirin-intolerant chronic urticaria. J Hum Genet, 57(8), 494–499. doi: 10.1038/jhg.2012.55 [DOI] [PubMed] [Google Scholar]
- Pan Y, Li S, Xie X, & Li M (2016). Association between thromboxane A2 receptor polymorphisms and asthma risk: A meta-analysis. J Asthma, 53(6), 576–582. doi: 10.3109/02770903.2015.1126849 [DOI] [PubMed] [Google Scholar]
- Parikh KS, Rajagopal S, Fortin T, Tapson VF, & Poms AD (2016). Safety and Tolerability of High-dose Inhaled Treprostinil in Pulmonary Hypertension. J Cardiovasc Pharmacol, 67(4), 322–325. doi: 10.1097/FJC.0000000000000357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JY, Pillinger MH, & Abramson SB (2006). Prostaglandin E2 synthesis and secretion: the role of PGE2 synthases. Clin Immunol, 119(3), 229–240. [DOI] [PubMed] [Google Scholar]
- Pavord ID, & Tattersfield AE (1995). Bronchoprotective role for endogenous prostaglandin E2. Lancet, 345(8947), 436–438. [DOI] [PubMed] [Google Scholar]
- Pavord ID, Ward R, Woltmann G, Wardlaw AJ, Sheller JR, & Dworski R (1999). Induced sputum eicosanoid concentrations in asthma. Am. J. Respir. Crit Care Med, 160(6), 1905–1909. [DOI] [PubMed] [Google Scholar]
- Pavord ID, Wong CS, Williams J, & Tattersfield AE (1993). Effect of inhaled prostaglandin E2 on allergen-induced asthma. Am. Rev. Respir. Dis, 148(1), 87–90. [DOI] [PubMed] [Google Scholar]
- Pawlotsky JM, Ruszniewski P, Reyl-Desmars F, Bourgeois M, & Lewin MJ (1993). Effects of PGE2, misoprostol, and enprostil on guinea pig enterocyte adenylate cyclase. Clinical implications. Dig. Dis. Sci, 38(2), 316–320. [DOI] [PubMed] [Google Scholar]
- Peachell PT, MacGlashan DW Jr., Lichtenstein LM, & Schleimer RP (1988). Regulation of human basophil and lung mast cell function by cyclic adenosine monophosphate. J. Immunol, 140(2), 571–579. [PubMed] [Google Scholar]
- Peacock CD, Misso NL, Watkins DN, & Thompson PJ (1999). PGE 2 and dibutyryl cyclic adenosine monophosphate prolong eosinophil survival in vitro. J. Allergy Clin. Immunol, 104(1), 153–162. [DOI] [PubMed] [Google Scholar]
- Peebles RS Jr., Dworski R, Collins RD, Jarzecka AK, Mitchell DB, Graham BS, & Sheller JR (2000). Cyclooxygenase inhibition increases interleukin 5 and interleukin 13 production and airway hyperresponsiveness in allergic mice. Am J Respir Crit Care Med, 162, 676–681. [DOI] [PubMed] [Google Scholar]
- Peebles RS Jr. (2013). A new horizon in asthma: inhibiting ILC function. Sci Transl Med, 5(174), 174fs177. doi: 10.1126/scitranslmed.3005881 [DOI] [PubMed] [Google Scholar]
- Peebles RS Jr., Hashimoto K, Morrow JD, Dworski R, Collins RD, Hashimoto Y, . . . Sheller JR (2002). Selective cyclooxygenase-1 and -2 inhibitors each increase allergic inflammation and airway hyperresponsiveness in mice. Am. J. Respir. Crit Care Med, 165(8), 1154–1160. [DOI] [PubMed] [Google Scholar]
- Peebles RS Jr., Hashimoto K, Sheller JR, Moore ML, Morrow JD, Ji S, . . . Zhou W (2005). Allergen-induced airway hyperresponsiveness mediated by cyclooxygenase inhibition is not dependent on 5-lipoxygenase or IL-5, but is IL-13 dependent. J Immunol, 175(12), 8253–8259. [DOI] [PubMed] [Google Scholar]
- Perez-Novo CA, Watelet JB, Claeys C, Van CP, & Bachert C (2005). Prostaglandin, leukotriene, and lipoxin balance in chronic rhinosinusitis with and without nasal polyposis. J Allergy Clin. Immunol, 115(6), 1189–1196. doi:S0091674905004239[pii];10.1016/j.jaci.2005.02.029[doi] [DOI] [PubMed] [Google Scholar]
- Pierzchalska M, Szabo Z, Sanak M, Soja J, & Szczeklik A (2003). Deficient prostaglandin E2 production by bronchial fibroblasts of asthmatic patients, with special reference to aspirin-induced asthma. J Allergy Clin. Immunol, 111(5), 1041–1048. doi:S0091674903011795[pii] [DOI] [PubMed] [Google Scholar]
- Piper PJ, & Vane JR (1969). Release of additional factors in anaphylaxis and its antagonism by anti-inflammatory drugs. Nature, 223(5201), 29–35. [DOI] [PubMed] [Google Scholar]
- Proud D, Sweet J, Stein P, Settipane RA, Kagey-Sobotka A, Friedlaender MH, & Lichtenstein LM (1990). Inflammatory mediator release on conjunctival provocation of allergic subjects with allergen. J. Allergy Clin. Immunol, 85(5), 896–905. [DOI] [PubMed] [Google Scholar]
- Pujols L, Benitez P, Alobid I, Martinez-Anton A, Roca-Ferrer J, Mullol J, & Picado C (2009). Glucocorticoid therapy increases COX-2 gene expression in nasal polyps in vivo. Eur. Respir. J, 33(3), 502–508. doi:09031936.00017408[pii];10.1183/09031936.00017408[doi] [DOI] [PubMed] [Google Scholar]
- Raychowdhury MK, Yukawa M, Collins LJ, McGrail SH, Kent KC, & Ware JA (1994). Alternative splicing produces a divergent cytoplasmic tail in the human endothelial thromboxane A2 receptor. J Biol. Chem, 269(30), 19256–19261. [PubMed] [Google Scholar]
- Redington AE, Meng QH, Springall DR, Evans TJ, Creminon C, Maclouf J, . . . Polak JM (2001). Increased expression of inducible nitric oxide synthase and cyclo- oxygenase-2 in the airway epithelium of asthmatic subjects and regulation by corticosteroid treatment. Thorax, 56(5), 351–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciotti E, & Fitzgerald GA (2011). Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol, 31(5), 986–1000. doi:31/5/986[pii];10.1161/ATVBAHA.110.207449[doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb CT, McSorley HJ, Lee J, Aoki T, Yu C, Crittenden S, . . . Yao C (2017). Prostaglandin E2 stimulates adaptive IL-22 production and promotes allergic contact dermatitis. J Allergy Clin Immunol. doi: 10.1016/j.jaci.2017.04.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts LJ, Sweetman BJ, Lewis RA, Austen KF, & Oates JA (1980). Increased production of prostaglandin D2 in patients with systemic mastocytosis. N. Engl. J. Med, 303(24), 1400–1404. [DOI] [PubMed] [Google Scholar]
- Roberts LJ, Sweetman BJ, & Oates JA (1981). Metabolism of thromboxane B2 in man. Identification of twenty urinary metabolites. J. Biol. Chem, 256(16), 8384–8393. [PubMed] [Google Scholar]
- Roca-Ferrer J, Garcia-Garcia FJ, Pereda J, Perez-Gonzalez M, Pujols L, Alobid I, . . . Picado C (2011). Reduced expression of COXs and production of prostaglandin E(2) in patients with nasal polyps with or without aspirin-intolerant asthma. J Allergy Clin. Immunol, 128(1), 66–72. doi:S0091-6749(11)00277-6[pii];10.1016/j.jaci.2011.01.065[doi] [DOI] [PubMed] [Google Scholar]
- Ruan KH (2004). Advance in understanding the biosynthesis of prostacyclin and thromboxane A2 in the endoplasmic reticulum membrane via the cyclooxygenase pathway. Mini. Rev. Med. Chem, 4(6), 639–647. [DOI] [PubMed] [Google Scholar]
- Safholm J, Dahlen SE, & Adner M (2013). Antagonising EP1 and EP2 receptors reveal that the TP receptor mediates a component of antigen-induced contraction of the guinea pig trachea. Eur J Pharmacol, 718(1–3), 277–282. doi: 10.1016/j.ejphar.2013.08.021 [DOI] [PubMed] [Google Scholar]
- Safholm J, Dahlen SE, Delin I, Maxey K, Stark K, Cardell LO, & Adner M (2013). PGE2 maintains the tone of the guinea pig trachea through a balance between activation of contractile EP1 receptors and relaxant EP2 receptors. Br. J Pharmacol, 168(4), 794–806. doi: 10.1111/j.1476-5381.2012.02189.x[doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safholm J, Manson ML, Bood J, Delin I, Orre AC, Bergman P, . . . Adner M (2015). Prostaglandin E2 inhibits mast cell-dependent bronchoconstriction in human small airways through the E prostanoid subtype 2 receptor. J Allergy Clin Immunol, 136(5), 1232–1239 e1231. doi: 10.1016/j.jaci.2015.04.002 [DOI] [PubMed] [Google Scholar]
- Salimi M, Stoger L, Liu W, Go S, Pavord I, Klenerman P, . . . Xue L (2017). Cysteinyl leukotriene E4 activates human group 2 innate lymphoid cells and enhances the effect of prostaglandin D2 and epithelial cytokines. J Allergy Clin Immunol, 140(4), 1090–1100.e1011. doi: 10.1016/j.jaci.2016.12.958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuchiwal SK, Balestrieri B, Raff H, & Boyce JA (2017). Endogenous prostaglandin E2 amplifies IL-33 production by macrophages through an E prostanoid (EP)2/EP4-cAMP-EPAC-dependent pathway. J Biol Chem, 292(20), 8195–8206. doi: 10.1074/jbc.M116.769422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt LM, Belvisi MG, Bode KA, Bauer J, Schmidt C, Suchy MT, . . . Dalpke AH (2011). Bronchial epithelial cell-derived prostaglandin E2 dampens the reactivity of dendritic cells. J. Immunol, 186(4), 2095–2105. doi:jimmunol.1002414[pii];10.4049/jimmunol.1002414[doi] [DOI] [PubMed] [Google Scholar]
- Schulman ES, Adkinson NF Jr., & Newball HH (1982). Cyclooxygenase metabolites in human lung anaphylaxis: airway vs. parenchyma. J Appl. Physiol, 53(3), 589–595. [DOI] [PubMed] [Google Scholar]
- Schulman ES, Newball HH, Demers LM, Fitzpatrick FA, & Adkinson NF Jr. (1981). Anaphylactic release of thromboxane A2, prostaglandin D2, and prostacyclin from human lung parenchyma. Am. Rev. Respir. Dis, 124(4), 402–406. [DOI] [PubMed] [Google Scholar]
- Selg E, Lastbom L, Ryrfeldt A, Kumlin M, & Dahlen SE (2008). Effects of selective and non-selective COX inhibitors on antigen-induced release of prostanoid mediators and bronchoconstriction in the isolated perfused and ventilated guinea pig lung. Prostaglandins Leukot. Essent. Fatty Acids, 78(2), 89–97. doi:S0952-3278(08)00002-1[pii];10.1016/j.plefa.2008.01.001[doi] [DOI] [PubMed] [Google Scholar]
- Seltzer J, Bigby BG, Stulbarg M, Holtzman MJ, Nadel JA, Ueki IF, . . . Boushey HA (1986). O3-induced change in bronchial reactivity to methacholine and airway inflammation in humans. J. Appl. Physiol, 60(4), 1321–1326. [DOI] [PubMed] [Google Scholar]
- Serra-Pages M, Olivera A, Torres R, Picado C, de Mora F, & Rivera J (2012). Eprostanoid 2 receptors dampen mast cell degranulation via cAMP/PKA-mediated suppression of IgE-dependent signaling. J Leukoc Biol, 92(6), 1155–1165. doi: 10.1189/jlb.0212109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra-Pages M, Torres R, Plaza J, Herrerias A, Costa-Farre C, Marco A, . . . de Mora F (2015). Activation of the Prostaglandin E2 receptor EP2 prevents house dust mite-induced airway hyperresponsiveness and inflammation by restraining mast cells' activity. Clin Exp Allergy, 45(10), 1590–1600. doi: 10.1111/cea.12542 [DOI] [PubMed] [Google Scholar]
- Sestini P, Armetti L, Gambaro G, Pieroni MG, Refini RM, Sala A, . . . Robuschi M (1996). Inhaled PGE2 prevents aspirin-induced bronchoconstriction and urinary LTE4 excretion in aspirin-sensitive asthma. Am. J. Respir. Crit Care Med, 153(2), 572–575. [DOI] [PubMed] [Google Scholar]
- Sheller JR, Mitchell D, Meyrick B, Oates J, & Breyer R (2000). EP(2) receptor mediates bronchodilation by PGE(2) in mice. J. Appl. Physiol, 88(6), 2214–2218. [DOI] [PubMed] [Google Scholar]
- Shephard EG, Malan L, Macfarlane CM, Mouton W, & Joubert JR (1985). Lung function and plasma levels of thromboxane B2, 6-ketoprostaglandin F1 alpha and beta-thromboglobulin in antigen-induced asthma before and after indomethacin pretreatment. Br. J. Clin. Pharmacol, 19(4), 459–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Mochizuki H, Shigeta M, & Morikawa A (1997). Effect of inhaled indomethacin on exercise-induced bronchoconstriction in children with asthma. Am J Respir Crit Care Med, 155(1), 170–173. doi: 10.1164/ajrccm.155.1.9001307 [DOI] [PubMed] [Google Scholar]
- Shiraishi Y, Takeda K, Domenico J, & Gelfand EW (2014). Role of prostaglandin D2 and CRTH2 blockade in early- and late-phase nasal responses. Clin Exp Allergy, 44(8), 1076–1082. doi: 10.1111/cea.12280 [DOI] [PubMed] [Google Scholar]
- Sladek K, Dworski R, Fitzgerald GA, Buitkus KL, Block FJ, Marney SR Jr., & Sheller JR (1990). Allergen-stimulated release of thromboxane A2 and leukotriene E4 in humans. Effect of indomethacin. Am. Rev. Respir. Dis, 141(6), 1441–1445. [DOI] [PubMed] [Google Scholar]
- Smith AP, & Cuthbert MF (1972). Prostaglandins and resistance to beta adrenoceptor stimulants. Br. Med. J, 2(806), 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AP, Cuthbert MF, & Dunlop LS (1975). Effects of inhaled prostaglandins E1, E2, and F2alpha on the airway resistance of healthy and asthmatic man. Clin. Sci. Mol. Med, 48(5), 421–430. [DOI] [PubMed] [Google Scholar]
- Smith WL, Urade Y, & Jakobsson PJ (2011). Enzymes of the cyclooxygenase pathways of prostanoid biosynthesis. Chem. Rev, 111(10), 5821–5865. doi: 10.1021/cr2002992[doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijdewint FG, Kalinski P, Wierenga EA, Bos JD, & Kapsenberg ML (1993). Prostaglandin E2 differentially modulates cytokine secretion profiles of human T helper lymphocytes. Journal of Immunology, 150(12), 5321–5329. [PubMed] [Google Scholar]
- Sorli CH, Zhang HJ, Armstrong MB, Rajotte RV, Maclouf J, & Robertson RP (1998). Basal expression of cyclooxygenase-2 and nuclear factor-interleukin 6 are dominant and coordinately regulated by interleukin 1 in the pancreatic islet. Proc. Natl. Acad. Sci. U. S. A, 95(4), 1788–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soslow RA, Dannenberg AJ, Rush D, Woerner BM, Khan KN, Masferrer J, & Koki AT (2000). COX-2 is expressed in human pulmonary, colonic, and mammary tumors. Cancer, 89(12), 2637–2645. [DOI] [PubMed] [Google Scholar]
- Sousa A, Pfister R, Christie PE, Lane SJ, Nasser SM, Schmitz-Schumann M, & Lee TH (1997). Enhanced expression of cyclo-oxygenase isoenzyme 2 (COX-2) in asthmatic airways and its cellular distribution in aspirin-sensitive asthma. Thorax, 52(11), 940–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinson SE, Amrani Y, & Brightling CE (2015). D prostanoid receptor 2 (chemoattractant receptor-homologous molecule expressed on TH2 cells) protein expression in asthmatic patients and its effects on bronchial epithelial cells. J Allergy Clin Immunol, 135(2), 395–406. doi: 10.1016/j.jaci.2014.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straumann A, Hoesli S, Bussmann C, Stuck M, Perkins M, Collins LP, . . . Simon HU (2013). Anti-eosinophil activity and clinical efficacy of the CRTH2 antagonist OC000459 in eosinophilic esophagitis. Allergy, 68(3), 375–385. doi: 10.1111/all.12096 [DOI] [PubMed] [Google Scholar]
- Stumm CL, Wettlaufer SH, Jancar S, & Peters-Golden M (2011). Airway remodeling in murine asthma correlates with a defect in PGE2 synthesis by lung fibroblasts. Am. J. Physiol Lung Cell Mol. Physiol, 301(5), L636–L644. doi:ajplung.00158.2011[pii];10.1152/ajplung.00158.2011[doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm EM, Schratl P, Schuligoi R, Konya V, Sturm GJ, Lippe IT, . . . Heinemann A (2008). Prostaglandin E2 inhibits eosinophil trafficking through E-prostanoid 2 receptors. J. Immunol, 181(10), 7273–7283. doi:181/10/7273[pii] [DOI] [PubMed] [Google Scholar]
- Sturm EM, Schuligoi R, Konya V, Sturm GJ, & Heinemann A (2011). Inhibitory effect of prostaglandin I2 on bone marrow kinetics of eosinophils in the guinea pig. J Leukoc. Biol, 90(2), 285–291. doi:jlb.0211087[pii];10.1189/jlb.0211087[doi] [DOI] [PubMed] [Google Scholar]
- Sugimoto H, Shichijo M, Okano M, & Bacon KB (2005). CRTH2-specific binding characteristics of [3H]ramatroban and its effects on PGD2-, 15-deoxy-Delta12, 14-. Eur. J Pharmacol, 524(1–3), 30–37. doi:S0014-2999(05)00911-8[pii];10.1016/j.ejphar.2005.09.005[doi] [DOI] [PubMed] [Google Scholar]
- Suzuki-Yamamoto T, Nishizawa M, Fukui M, Okuda-Ashitaka E, Nakajima T, Ito S, & Watanabe K (1999). cDNA cloning, expression and characterization of human prostaglandin F synthase. FEBS Lett, 462(3), 335–340. [DOI] [PubMed] [Google Scholar]
- Szczeklik A, Mastalerz L, Nizankowska E, & Cmiel A (1996). Protective and bronchodilator effects of prostaglandin E and salbutamol in aspirin-induced asthma. Am. J Respir. Crit Care Med, 153(2), 567–571. doi: 10.1164/ajrccm.153.2.8564099[doi] [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Tokuoka S, Masuda T, Hirano Y, Nagao M, Tanaka H, . . . Nagai H (2002). Augmentation of allergic inflammation in prostanoid IP receptor deficient mice. Br. J. Pharmacol, 137(3), 315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi K, Mashimo Y, Shimojo N, Arima T, Inoue Y, Morita Y, . . . Suzuki Y (2013). Functional variants in the thromboxane A2 receptor gene are associated with lung function in childhood-onset asthma. Clin Exp Allergy, 43(4), 413–424. doi: 10.1111/cea.12058 [DOI] [PubMed] [Google Scholar]
- Tanioka T, Nakatani Y, Semmyo N, Murakami M, & Kudo I (2000). Molecular identification of cytosolic prostaglandin E2 synthase that is functionally coupled with cyclooxygenase-1 in immediate prostaglandin E2 biosynthesis. J Biol. Chem, 275(42), 32775–32782. [DOI] [PubMed] [Google Scholar]
- Taylor IK, Ward PS, O'Shaughnessy KM, Dollery CT, Black P, Barrow SE, . . . Fuller RW (1991). Thromboxane A2 biosynthesis in acute asthma and after antigen challenge. Am. Rev. Respir. Dis, 143(1), 119–125. [DOI] [PubMed] [Google Scholar]
- Teixeira MM, al Rashed S, Rossi AG, & Hellewell PG (1997). Characterization of the prostanoid receptors mediating inhibition of PAF-induced aggregation of guinea-pig eosinophils. Br. J. Pharmacol, 121(1), 77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilley SL, Hartney JM, Erikson CJ, Jania C, Nguyen M, Stock J, . . . Koller BH (2003). Receptors and pathways mediating the effects of prostaglandin E2 on airway tone. Am. J Physiol Lung Cell Mol. Physiol, 284(4), L599–L606. [DOI] [PubMed] [Google Scholar]
- Toki S, Goleniewska K, Huckabee MM, Zhou W, Newcomb DC, Fitzgerald GA, . . . Peebles RS Jr. (2013). PGI2 signaling inhibits antigen uptake and increases migration of immature dendritic cells. J. Leukoc. Biol. doi:jlb.1112559[pii];10.1189/jlb.1112559[doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Atencio I, Ainsua-Enrich E, de Mora F, Picado C, & Martin M (2014). Prostaglandin E2 prevents hyperosmolar-induced human mast cell activation through prostanoid receptors EP2 and EP4. PLoS One, 9(10), e110870. doi: 10.1371/journal.pone.0110870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres R, Herrerias A, Serra-Pages M, Marco A, Plaza J, Costa-Farre C, . . . de Mora F (2013). Locally administered prostaglandin E2 prevents aeroallergeninduced airway sensitization in mice through immunomodulatory mechanisms. Pharmacol Res, 70(1), 50–59. doi: 10.1016/j.phrs.2012.12.008 [DOI] [PubMed] [Google Scholar]
- Ueno N, Taketomi Y, Yamamoto K, Hirabayashi T, Kamei D, Kita Y, . . . Murakami M (2011). Analysis of two major intracellular phospholipases A(2) (PLA(2)) in mast cells reveals crucial contribution of cytosolic PLA(2)alpha, not Ca(2+)-independent PLA(2)beta, to lipid mobilization in proximal mast cells and distal fibroblasts. J. Biol. Chem, 286(43), 37249–37263. doi:M111.290312[pii];10.1074/jbc.M111.290312[doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal C, Porras-Hurtado L, Cruz R, Quiralte J, Cardona V, Colas C, . . . Carracedo A (2013). Association of thromboxane A1 synthase (TBXAS1) gene polymorphism with acute urticaria induced by nonsteroidal anti-inflammatory drugs. J Allergy Clin Immunol, 132(4), 989–991. doi: 10.1016/j.jaci.2013.04.045 [DOI] [PubMed] [Google Scholar]
- Vieira PL, de Jong EC, Wierenga EA, Kapsenberg ML, & Kalinski P (2000). Development of Th1-inducing capacity in myeloid dendritic cells requires environmental instruction. Journal of Immunology, 164(9), 4507–4512. [DOI] [PubMed] [Google Scholar]
- von Euler US (2014). A depressor substance in the vesicular gland. J Physiol, 84, 21P. [Google Scholar]
- Walters KM, Simon RA, Woessner KM, Wineinger NE, & White AA (2017). Effect of misoprostol on patients with aspirin-exacerbated respiratory disease undergoing aspirin challenge and desensitization. Ann Allergy Asthma Immunol, 119(1), 71–76. doi: 10.1016/j.anai.2017.05.003 [DOI] [PubMed] [Google Scholar]
- Wasiak W, & Szmidt M (1999). A six week double blind, placebo controlled, crossover study of the effect of misoprostol in the treatment of aspirin sensitive asthma. Thorax, 54(10), 900–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller CL, Collington SJ, Hartnell A, Conroy DM, Kaise T, Barker JE, . . . Williams TJ (2007). Chemotactic action of prostaglandin E2 on mouse mast cells acting via the PGE2 receptor 3. Proc. Natl. Acad. Sci. U. S. A, 104(28), 11712–11717. doi:0701700104[pii];10.1073/pnas.0701700104[doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle BJ, & Moncada S (1983). Pharmacological interactions between prostacyclin and thromboxanes. Br. Med. Bull, 39(3), 232–238. [DOI] [PubMed] [Google Scholar]
- Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, & Donaldson DD (1998). Interleukin-13: central mediator of allergic asthma. Science, 282(5397), 2258–2261. [DOI] [PubMed] [Google Scholar]
- Woodward DF, Hawley SB, Williams LS, Ralston TR, Protzman CE, Spada CS, & Nieves AL (1990). Studies on the ocular pharmacology of prostaglandin D2. Invest Ophthalmol. Vis. Sci, 31(1), 138–146. [PubMed] [Google Scholar]
- Xue L, Salimi M, Panse I, Mjosberg JM, McKenzie AN, Spits H, . . . Ogg G (2014). Prostaglandin D2 activates group 2 innate lymphoid cells through chemoattractant receptor-homologous molecule expressed on TH2 cells. J Allergy Clin. Immunol, 133(4), 1184–1194. doi:S0091-6749(13)01771-5[pii];10.1016/j.jaci.2013.10.056[doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamabayashi C, Koya T, Kagamu H, Kawakami H, Kimura Y, Furukawa T, . . . Narita I (2012). A novel prostacyclin agonist protects against airway hyperresponsiveness and remodeling in mice. Am J Respir Cell Mol Biol, 47(2), 170–177. doi: 10.1165/rcmb.2011-0350OC [DOI] [PubMed] [Google Scholar]
- Yan H, Deshpande DA, Misior AM, Miles MC, Saxena H, Riemer EC, . . . Penn RB (2011). Anti-mitogenic effects of beta-agonists and PGE2 on airway smooth muscle are PKA dependent. FASEB J, 25(1), 389–397. doi:fj.10-164798[pii];10.1096/fj.10-164798[doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying S, Meng Q, Scadding G, Parikh A, Corrigan CJ, & Lee TH (2006). Aspirin-sensitive rhinosinusitis is associated with reduced E-prostanoid 2 receptor expression on nasal mucosal inflammatory cells. J Allergy Clin. Immunol, 117(2), 312–318. doi:S0091-6749(05)02331-6[pii];10.1016/j.jaci.2005.10.037[doi] [DOI] [PubMed] [Google Scholar]
- Zaslona Z, Okunishi K, Bourdonnay E, Domingo-Gonzalez R, Moore BB, Lukacs NW, . . . Peters-Golden M (2014). Prostaglandin E(2) suppresses allergic sensitization and lung inflammation by targeting the E prostanoid 2 receptor on T cells. J Allergy Clin Immunol, 133(2), 379–387. doi: 10.1016/j.jaci.2013.07.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeldin DC, Wohlford-Lenane C, Chulada P, Bradbury JA, Scarborough PE, Roggli V, . . . Schwartz DA (2001). Airway inflammation and responsiveness in prostaglandin H synthase- deficient mice exposed to bacterial lipopolysaccharide. Am. J. Respir. Cell Mol. Biol, 25(4), 457–465. [DOI] [PubMed] [Google Scholar]
- Zhou W, Blackwell TS, Goleniewska K, O'Neal JF, Fitzgerald GA, Lucitt M, . . . Peebles RS Jr. (2007). Prostaglandin I2 analogs inhibit Th1 and Th2 effector cytokine production by CD4 T cells. J Leukoc. Biol, 81(3), 809–817. [DOI] [PubMed] [Google Scholar]
- Zhou W, Dowell DR, Geraci MW, Blackwell TS, Collins RD, Polosukhin VV, . . . Peebles RS Jr. (2011). PGI synthase overexpression protects against bleomycin-induced mortality and is associated with increased Nqo 1 expression. Am. J. Physiol Lung Cell Mol. Physiol, 301(4), L615–L622. doi:ajplung.00224.2010[pii];10.1152/ajplung.00224.2010[doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Dowell DR, Huckabee MM, Newcomb DC, Boswell MG, Goleniewska K, . . . Peebles RS Jr. (2012). Prostaglandin I2 signaling drives Th17 differentiation and exacerbates experimental autoimmune encephalomyelitis. PLoS. One, 7(5), e33518. doi: 10.1371/journal.pone.0033518[doi];PONE-D-10-03779[pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Goleniewska K, Zhang J, Dulek DE, Toki S, Lotz MT, . . . Peebles RS Jr. (2014). Cyclooxygenase inhibition abrogates aeroallergen-induced immune tolerance by suppressing prostaglandin I receptor signaling. J. Allergy Clin. Immunol, 134(3), 698–705. doi:S0091-6749(14)00806-9[pii];10.1016/j.jaci.2014.06.004[doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Hashimoto K, Goleniewska K, O'Neal JF, Ji S, Blackwell TS, . . . Peebles RS Jr. (2007). Prostaglandin I2 analogs inhibit proinflammatory cytokine production and T cell stimulatory function of dendritic cells. J Immunol, 178(2), 702–710. [DOI] [PubMed] [Google Scholar]
- Zhou W, Toki S, Zhang J, Goleniewksa K, Newcomb DC, Cephus JY, . . . Peebles RS Jr. (2016). Prostaglandin I2 Signaling and Inhibition of Group 2 Innate Lymphoid Cell Responses. Am. J Respir. Crit Care Med, 193(1), 31–42. doi: 10.1164/rccm.201410-1793OC[doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Zhang J, Goleniewska K, Dulek DE, Toki S, Newcomb DC, . . . Peebles RS Jr. (2016). Prostaglandin I2 Suppresses Proinflammatory Chemokine Expression, CD4 T Cell Activation, and STAT6-Independent Allergic Lung Inflammation. J. Immunol, 197(5), 1577–1586. doi:jimmunol.1501063[pii];10.4049/jimmunol.1501063[doi] [DOI] [PMC free article] [PubMed] [Google Scholar]