Abstract
Studies on cannabinoids have reported contradictory findings, showing both aversion and rewarding outcomes in conditioned place preference (CPP). Various possibilities have been suggested to explain the aversive properties of cannabinoids, including the pharmacokinetics profile and dose selection. In this study, we have established a CPP method to investigate the effects of modulating astroglial glutamate transporters in cannabinoid dependence using a cannabinoid receptor 1 (CB1R) agonist, CP 55,940 (CP). Previous reports using CPP paradigm demonstrated the involvement of glutamatergic system in seeking behavior of several drugs of abuse such as cocaine, heroin and nicotine. Glutamate homeostasis is maintained by several astroglial glutamate transporters, such as glutamate transporter 1 (GLT-1), cystine/glutamate transporter (xCT) and glutamate aspartate transporter (GLAST). In this study, we investigated the effects of Ampicillin/Sulbactam, β-lactam compounds known to upregulate GLT-1 and xCT, on cannabinoid seeking behavior using CP. We found first that one prime dose of CP induced CP reinstatement; this effect was associated, in part, with significant downregulation of xCT expression in the nucleus accumbens, dorsomedial prefrontal cortex and amygdala. Moreover, GLT-1 expression was downregulated in the amygdala. Importantly, Ampicillin/Sulbactam treatment during the extinction phase attenuated CP-induced reinstatement and restored the expression of GLT-1 and xCT in mesocorticolimbic brain regions. These findings suggest that β-lactams may play a potential therapeutic role in attenuating dependence to cannabinoids, in part, through upregulation of GLT-1 and xCT.
Keywords: Cannabinoids, CP 55, 940, CPP, GLT-1, xCT, Glutamate, Ampicillin/Sulbactam
1. Introduction
Evidence from several studies revealed clear reinforcing properties with cannabinoid receptor 1 (CB1R) agonists using intracerebral and intravenous self-administration in animal models (1-4). Cannabinoid agonists exposure exhibited an increase in the dopamine firing in several brain reward regions, including the nucleus accumbens (NAc) (5) and prefrontal cortex (PFC) (6). In addition, a number of studies demonstrated the involvement of CB1Rs on modulating drugs of abuse, including nicotine, methamphetamine, cocaine, morphine, and ethanol (7-14). Previous reports have considered the dopaminergic system as the primary mediator for drug-induced reinstatement (15, 16); however, the importance of the glutamatergic system in the reinstatement of multiple drugs of abuse has been clearly proven (17, 18).
Glutamate is the most prevalent excitatory neurotransmitter in the brain that displayed a critical role in mediating several drugs of abuse, including ethanol, methamphetamine, nicotine, and cocaine (19-22). Repeated drug exposure prominently disrupts glutamate homeostasis in the NAc and PFC (19, 23, 24). NAc receives dense glutamatergic innervation from the PFC, hippocampus (Hipp) and amygdala (Amg) (25). A disruption of glutamatergic clearance in the NAc has been associated with relapse behavior to different drugs of abuse (19, 20, 24, 26). Furthermore, the medial PFC has been suggested to be involved in the drug-seeking behavior (27). Additionally, the functional integrity of the dorsomedial PFC (dmPFC) is necessary for cocaine-seeking behavior (28). Enhancement of glutamatergic function in the dmPFC and the Amg is crucial for mediating cocaine-seeking behavior in rats (29-31). Glutamate homeostasis is controlled by a group of glutamate transporters; among these transporters, glutamate transporter 1 (GLT-1), which regulates the majority of extracellular glutamate in the brain (32-34). Whereas, cystine /glutamate transporter (xCT) has a crucial role in maintaining basal glutamate concentration in the brain (35, 36). Glutamate/aspartate transporter (GLAST) is another glutamate transporter that controls synaptic glutamate concentration and is expressed mainly in the cerebellum (37). To the best of our knowledge, less is known about the involvement of the glutamatergic system in cannabinoid dependence and relapse.
Several studies have shown controversial outcomes regarding the effect of acute exposure to CB1R agonists on the glutamatergic system. Of note, acute activation of CB1Rs exhibited a reduction in glutamate release and synaptic transmission (38-41). However, other reports revealed elevation in the extracellular glutamate concentration following acute activation of CB1Rs (42-44). Importantly, with most drugs of abuse, cue-induced reinstatement is associated with disruption of glutamate homeostasis, in part through downregulation of its transporters, such as GLT-1 and xCT (20, 26, 45). Studies from ours and others reported the crucial role of β-lactams on restoring glutamate homeostasis and attenuating drug-seeking behavior (19, 26, 46-48). Moreover, β-lactams were successfully used to attenuate methamphetamine-, nicotine-, and cocaine-seeking behavior, in part, through restoring the expression of astroglial glutamate transporters, including GLT-1 and xCT (20-22, 49). Thus, we investigated the effects of β-lactam compounds, Ampicillin/Sulbactam (A/S), on reinstatement to CB1R agonist, CP 55,940 (CP), using conditioned place preference paradigm (CPP).
Conditioned place preference (CPP) procedure is a standard preclinical behavioral paradigm to investigate the rewarding, relapse and aversive effects of drugs of abuse (50). Using CPP, cannabinoids appear atypical as drugs of abuse since controversial data exist concerning their ability to produce CPP in rat models (51-54). Various possibilities have been proposed to clarify the rewarding properties of cannabinoids using the CPP paradigm. One possible explanation is due to the methodological variation such as selection of dose, use of prime dose (55) and pharmacokinetic profile of cannabinoids (53). The purpose of this study is to investigate the reinforcing properties of CB1R agonist CP at a dose of 20 μg/kg (i.p.) using the CPP paradigm. Moreover, A/S β-lactams were used to attenuate the CP-induced reinstatement behavior. We hypothesized that one prime dose of CP can induce CP-seeking behavior, and this effect can be attenuated with A/S pretreatment during the extinction phase. We also investigated the possible role of CP-induced reinstatement and A/S administration on astroglial glutamate transporters (GLT-1, xCT, and GLAST). To exclude conditioned place aversion as a possible effect for A/S, we performed a second experiment to investigate A/S effect alone using the CPP procedure.
2. Materials and methods
2.1. Animals
Male alcohol-preferring (P) rats were used in this study as an animal model of drug dependence. P rats were used in this study to investigate CB1R agonist dependence and pharmacological modulation of astroglial glutamate transporters as it was performed in a study from our laboratory that investigated the reinforcing effect of cocaine using this rat model (49). Moreover, a study using P rats demonstrated the involvement of CB1R activation in regulating ethanol-seeking behavior (56). P rats were obtained from Indiana University, School of Medicine (Indianapolis, IN, USA). All animals were housed in a plastic corn-cob bedding tubs and had access to food and water ad lib throughout the experiment. The room temperature was maintained at 21°C and 50% humidity with a 12-hour light-dark cycle. Rats were then divided into three subgroups: 1) The vehicle control group received vehicle during the conditioning and extinction phases; 2) the CP-Vehicle group received CP during the conditioning phase and vehicle during the extinction phase; and 3) the CP-A/S group received CP during the conditioning phase and A/S during the extinction phase. The CP-Vehicle and CP-A/S groups received one prime dose of CP during the reinstatement phase. All animal procedures were in compliance and approved by the Institutional Animal Care and Use Committee of The University of Toledo in accordance with the guidelines of the Institutional Animal Care and Use Committee of the National Institutes of Health and the Guide for the Care and Use of Laboratory Animals.
2.2. Drugs
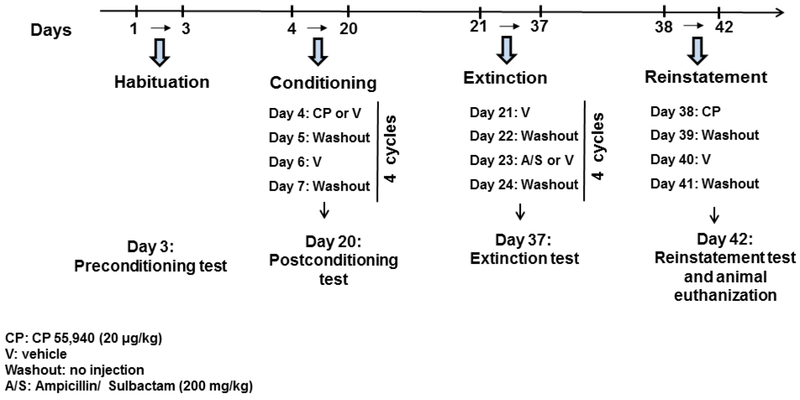
CP (CP 55,940) was purchased from Sigma-Aldrich (St. Louis, MO) and dissolved in dimethyl sulfoxide (DMSO)/ saline. The rationale for using CB1R agonist, CP, is based on the fact that previous reports on CP revealed a reliable drug-seeking behavior in rodents (1, 54). A/S was obtained from Fresenius Kabi (Lake Zurich, IL) in a 2 (A): 1 (S) ratio. CP 55,940 at dose of 20 μg/kg and A/S (200 mg/kg) were administered intraperitoneally (i.p.) during CPP phases (Figure 1).
Figure 1.
Experimental schedule for the habituation, conditioning, extinction, and reinstatement phases of CPP. Rats had free access to water and food throughout the experiment (washout: no injection or any experiment procedure, V: Vehicle, CP: CP 55,940, A/S: Ampicillin/ Sulbactam).
2.3. Experimental design
2.3.1. Conditioned place preference paradigm:
The CPP apparatus contains three chambers: two conditioning chambers and one neutral middle chamber. The conditioning chambers were designed with different patterned walls and floor textures for animals to distinguish between them. The middle chamber is featureless and separated from the two conditioning chambers by two guillotine Plexiglas doors.
2.3.2. Phases of CPP paradigm:
1-. Habituation:
The experimental schedule is illustrated in Fig. 1. On Days 1-3, rats were placed in the middle chamber for three minutes. Then, the guillotine doors were opened and rats had free access to explore the chambers for 30 minutes. On Day 3, the time spent in each chamber was recorded using a digital camera and then calculated. Rats were excluded from the study if they spent more than 67% of the total time in one chamber, as adopted in previous studies (49, 57). Rats were then randomly divided to receive CP in chamber 1 or chamber 2.
2-. Conditioning:
During the conditioning phase (Days 4-19), rats in the CP-Vehicle and CP-A/S groups received CP doses (i.p.) and vehicle (i.p.) in the following cycle (Day 4: CP, Day 5: washout, Day 6: Vehicle, and Day 7: Washout). This cycle was repeated four times, as reported previously (53). Animals in the vehicle group received the vehicle starting on Day 4 and throughout the last day of the experiment. Rats received the CP i.p. injection and were placed in the home cage for three hours. Then, rats were placed in the corresponding chamber with the door closed for 30 minutes. This paradigm was proposed to avoid a possible dysphoric effect and to follow the pharmacokinetic profile of CP, which has a relatively long half-life (51, 55). On Day 20 (postconditioning test day), rats did not receive any i.p. injection and were placed in the middle chamber with the guillotine doors closed for three minutes. Both doors were then opened and rats were allowed to freely explore the CPP apparatus for a 20-minute session. This session was recorded by digital camera, and the time spent in each chamber was calculated by blinded observers to measure the drug conditioning effect.
3-. Extinction:
On Day 21, rats in the CP-Vehicle and CP-A/S groups received A/S or vehicle, according to their corresponding groups, and were returned to CP-paired chamber with door closed for 30 minutes session. Rats in either group received the dosing cycle as follows: Day 21: vehicle, Day 22: washout, Day 23: A/S or vehicle, Day 24: washout for four cycles. On Day 37, the extinction test was conducted, and the time spent in each chamber was calculated. If the time spent in the CP-paired chamber did not decrease by 25% following the extinction phase, rats were not considered extinguished and were withdrawn from the study, as was performed in previous work (21, 49).
4-. Reinstatement:
On Day 38, rats in the CP-Vehicle or CP-A/S groups received a single dose of CP or vehicle and were returned to the home cage for three hours and then placed in the assigned CP-paired chamber with the door closed for a 30-minute session. Rats in either group received a dosing cycle as follows: Day 38: CP, Day 39: washout, Day 40: vehicle, Day 41: washout. On Day 42, the reinstatement test was performed, and the time spent in each chamber was calculated.
2.4. Effects of Ampicillin/Sulbactam treatment on CPP
We investigated the effect of A/S i.p. injections alone on conditioning training in P rats. The habituation phase and preconditioning test were conducted to identify initial preference for each chamber. Rats then received A/S i.p. injections and were placed in the A/S-paired chamber. The next day, the same group of rats received the vehicle (saline) i.p. injections and was placed in the other chamber. This cycle was repeated four times. After completion of the conditioning training, the postconditioning test was performed, and time spent in each chamber was calculated by a blinded observer to the assigned chambers.
2.5. Brain tissue harvesting
Animals were euthanized using carbon dioxide inhalation after the reinstatement test (Day 42) and decapitated using a guillotine. Brains were then removed and immediately stored at −80°C. Stereotaxic microdissections were carried out to isolate the NAc, dmPFC, Hipp and Amg according to the Rat Brain Atlas (58) and were stored at −80°C for Western blot analysis.
2.6. Western blot
Isolated brain regions were examined for changes in GLT-1, xCT and GLAST expression relative to total β-tubulin using the Western blotting procedure. Briefly, extracted brain regions were lysed using regular lysis buffer, as described in a previous study from our laboratory (59). Isolated protein samples were then mixed with 5X laemmli loading dye and loaded in polyacrylamide gels for the electrophoresis separation. Proteins were then transferred on PVDF membranes electrophoretically (Bio-Rad, Hercules, CA). Membranes were blocked with 3% fat-free milk in TBST (50 mM Tris HC1; 150 mM NaCl, pH 7.4; 0.1% Tween 20) for 30 minutes at room temperature. Membranes were then incubated overnight at 4°C with one of the following primary antibodies: guinea pig anti-GLT1 (1:5000), rabbit anti-xCT antibody (1:1,000), and rabbit anti-EAAT1 (GLAST) antibody (1:5,000). Membranes were then washed with TBST and blocked with 3% milk in TBST for 30 minutes. Membranes were further incubated with secondary antibodies for 90 minutes at room temperature. The corresponding secondary antibodies were: guinea pig anti-GLT-1 secondary antibody (1:5,000) and rabbit anti-xCT secondary antibody (1:5000). β-tubulin was used as a loading control (1:5,000). Membranes were then exposed to the chemiluminescent substrate and further developed on the SRX-101A machine. The detected bands were quantified using the MCID system, and the results were expressed as a percentage of the ratio of tested protein/β-tubulin, relative to the vehicle control group (100% control-value), as performed in previous work from our laboratory (46, 47).
2.7. Statistical analysis
Time spent in different CPP phases was analyzed using two-way repeated measures ANOVA (Time x Chamber). Additionally, one-way ANOVA was used to analyze Western blot data (GLT-1/β-tubulin, xCT/β-tubulin and GLAST/β-tubulin). A Newman-Keuls multiple comparisons post hoc test was applied whenever a significant difference was revealed. Western blot densities from the vehicle group were converted to 100% as performed in previous studies from our laboratory (47, 60). All statistical analyses were calculated using GraphPad prism with p < 0.05 as level of significance.
3. Results
3.1. Effect of Ampicillin/Sulbactam treatment on CP 55,940-induced reinstatement
A two-way repeated measure ANOVA analysis was conducted to investigate the reinstatement effect of CP and the ability of A/S treatment to attenuate the cue-induced reinstatement of CP-seeking.
a. CP 55,940-vehicle treated group
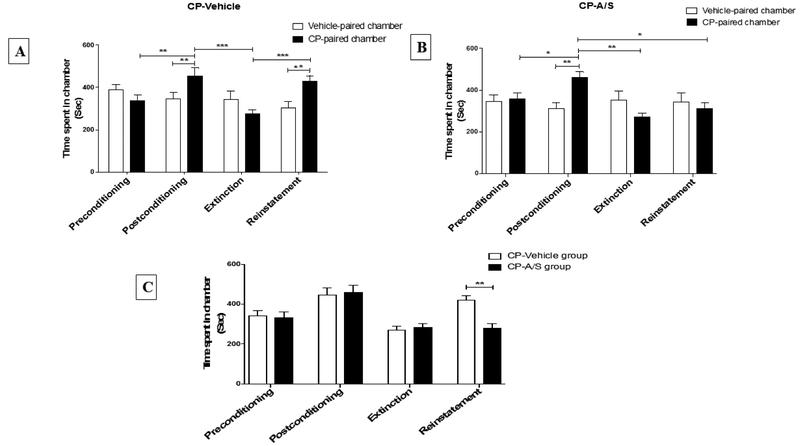
A two-way repeated measure ANOVA revealed a significant effect of time [F (3, 15) = 4.046, p = 0.0271], a non-significant effect of chamber [F (1, 5) = 0.4873, p = 0.5163], and a significant interaction between time x chamber [F (3, 15) = 16.28, p < 0.0001]. Newman-Keuls multiple comparisons post hoc test revealed a significant increase in time spent in the CP-paired chamber following conditioning training as compared to preconditioning (p < 0.01). Moreover, there was a significant increase in time spent in the CP-paired chamber as compared to the vehicle-paired chamber in the postconditioning test (p < 0.01). However, this drug acquisition was significantly reduced during the extinction period (administration of vehicle for four dose cycles) as compared to postconditioning tests (p < 0.001). After extinction, the prime i.p. injection of CP produced a reinstatement to CP-seeking behavior and significantly increased the time spent in the CP-paired chamber as compared to the extinction test (p < 0.001). In addition, there was a significant increase in time spent in the CP-paired chamber as compared to the vehicle-paired chamber following the reinstatement test (p < 0.01) (Fig. 2A).
Figure 2.
Effect of A/S on CP-induced reinstatement in (A) CP-vehicle and (B) CP-A/S. statistical analysis revealed a significant increase in time spent in CP-paired chamber during the conditioning phase as compared to preconditioning phase. Moreover, time spent in CP-paired chamber was significantly increased as compared to vehicle-paired chamber after conditioning training. In addition, one prime dose of CP induced a seeking behavior and increased time spent in CP-paired chamber as compared to extinction test. Time spent in CP-paired chamber was significantly increased as compared to vehicle-paired chamber following the reinstatement test. (B) CP-A/S treated group: statistical analysis revealed significant conditioning effect of CP as compared to preconditioning test. In addition, time spent in CP-paired chamber was significantly increased as compared to vehicle-paired chamber after conditioning training. Following extinction phase, A/S administration successfully attenuated the CP-induced reinstatement. In addition, there was a significant reduction in time spent in the CP-paired chamber in the reinstatement test as compared to postconditioning test. (C) CP-Vehicle and CPA/S groups: statistical analysis revealed a significant decrease in time spent in CP-paired chamber in CP-A/S group as compared to CP-Vehicle group during reinstatement test. Values shown as means ± S.E.M. (* comparison between time spent in CP-paired chamber during different CPP phases, *p < 0.05, **p < 0.01, ***p < 0.001), (n = 6-9 for each group).
b. CP 55,940-Ampicillin/Sulbactam treated group:
A two-way repeated measure ANOVA revealed a significant effect of time [F (3, 24) = 4.159, p = 0.0166], a non-significant effect of chamber [F (1, 8) = 0.1026, p = 0.7570], and a significant interaction between time x chamber [F (3, 24) = 6.457, p = 0.0023]. Newman-Keuls multiple comparisons post hoc test revealed a significant increase in time spent in the CP-paired chamber following conditioning training, as compared to preconditioning (p < 0.05). Moreover, there was a significant increase in time spent in CP-paired chamber as compared to the vehicle-paired chamber in the postconditioning test (p < 0.01). Time spent in the CP-paired chamber was significantly reduced during the extinction period (following administration of A/S) as compared to the postconditioning test (p < 0.01). A prime dose of CP did not stimulate the reinstatement to CP-seeking behavior, and time spent in the CP-paired chamber was not significantly changed as compared to the extinction test (p > 0.05) (Fig. 2B).
c. Ampicillin/Sulbactam effect on time spent in CP 55,940-paired chamber
A two-way repeated measure ANOVA revealed a significant effect of time [F (3, 18) = 32.53, p < 0.0001], a non-significant effect of chamber [F (1, 6) = 2.777, p = 0.1467], and a significant interaction between time x chamber [F (3, 18) = 5.044, p = 0.0104]. Newman-Keuls multiple comparisons post hoc test revealed a significant decrease in time spent in the CP-paired chamber in the CP-A/S group during the reinstatement test as compared to the CP-vehicle group (p < 0.01) (Fig. 2C).
3.2. Effect of Ampicillin/Sulbactam treatment alone on time spent using CPP.
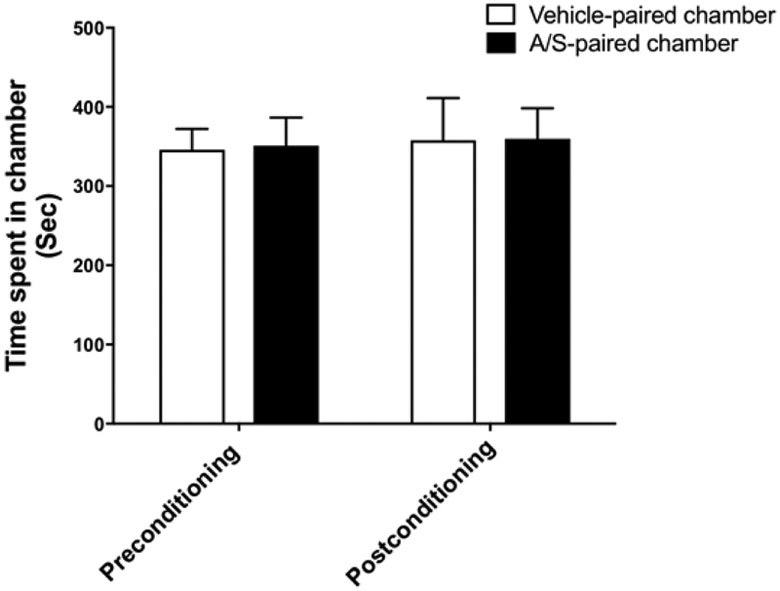
A two-way repeated measure ANOVA analysis revealed no significant difference in time spent in the A/S-paired chamber and vehicle-paired chamber in preconditioning and conditioning preference tests, for time [F (1, 4) = 0.3686, P = 0.5766], for chamber [F (1, 4) = 0.003476, P = 0.9558], or for time x chamber interaction [F (1, 4 = 0.002291, P = 0.9641], (Fig. 3).
Figure 3.
Effect of A/S i.p. injections on conditioning phase. Statistical analysis revealed no significant change in time spent between A/S-paired chamber and the vehicle-paired chamber in preconditioning and postconditioning phases. Values shown as means ± S.E.M (p > 0.05), (n = 5), (A/S: Ampicillin/Sulbactam).
3.3. Effect of CP 55,940 and Ampicillin/Sulbactam on GLT-1, xCT and GLAST expression in the NAc.
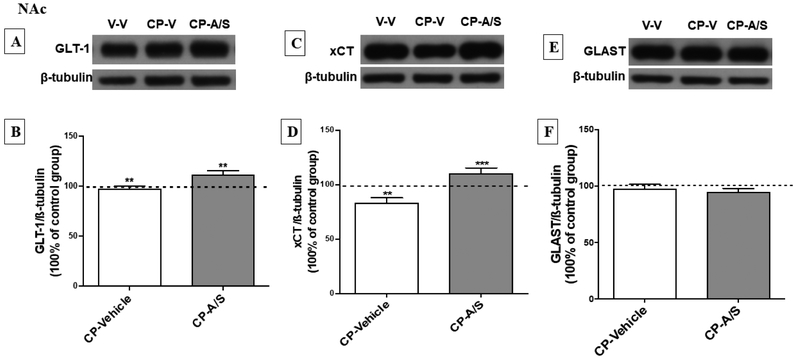
One-way ANOVA analysis followed by Newman-Keuls multiple comparisons post hoc test revealed a significant upregulation in the expression of GLT-1 in the CP-A/S group as compared to the vehicle (p < 0.01) and CP-Vehicle (p < 0.01) groups [F (2, 14) = 8.833, p = 0.0021, Fig. 4A, B]. Importantly, statistical analysis revealed a significant downregulation in xCT expression in the CP-Vehicle group as compared to vehicle (p < 0.01). However, the statistical analysis revealed a significant upregulation of xCT expression in the CP-A/S as compared to the CP-Vehicle group (p < 0.001), [F (2, 14) = 13.52, p = 0.0003, Fig. 4C, D]. However, there was no difference in GLAST expression among vehicle, CP-Vehicle or CP-A/S groups [F (2, 14) = 0.7983, p = 0.4654, Fig. 4E, F].
Figure 4.
Effect of CP and A/S on GLT-1, xCT and GLAST expression in the nucleus accumbens (NAc). (A, C, E) Immunoblots for GLT-1/ β-tubulin, xCT/ β-tubulin and GLAST/β-tubulin, respectively. (B) Statistical analysis exhibited significant upregulation in the expression of GLT-1 in CP-A/S group as compared to CP-Vehicle and Vehicle-Vehicle groups. (D) Statistical analysis revealed significant downregulation in the expression of xCT in CP-Vehicle treated group as compared to Vehicle-Vehicle and CP-A/S groups. (F) GLAST expression was not changed among the treated groups in the NAc. (**p < 0.01 and ***p < 0.001), (n = 6-7 for each group), values shown as means ± S.E.M, (V: Vehicle; A/S: Ampicillin/Sulbactam; CP: CP 55,940).
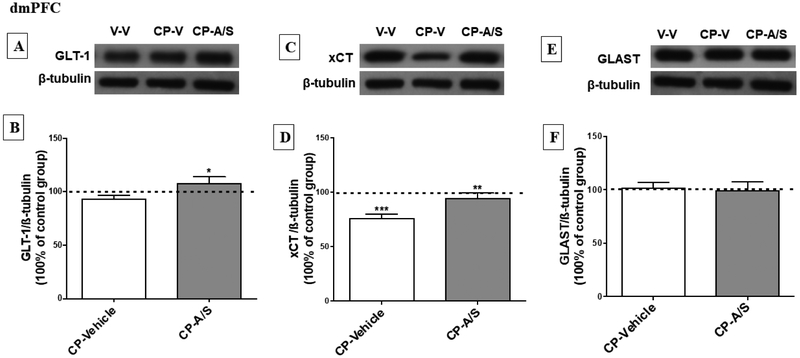
3.4. Effect of CP 55,940 and Ampicillin/Sulbactam on GLT-1, xCT and GLAST expression in the dmPFC.
One-way ANOVA analysis followed by Newman-Keuls multiple comparisons post hoc test revealed a significant upregulation in the expression of GLT-1 in the CP-A/S group as compared to the CP-Vehicle (p < 0.05) treated group [F (2, 14) = 3.698, p = 0.0451, Fig. 5A, B]. Moreover, a significant downregulation of xCT expression was found in the CP-Vehicle group as compared to vehicle (p < 0.001). However, statistical analysis revealed a significant upregulation of xCT expression in the CP-A/S as compared to the CP-Vehicle group (p < 0.01), [F (2, 14) = 13.65, p = 0.0002, Fig. 5C, D]. However, there was no difference in GLAST expression among the vehicle, CP-Vehicle or CP-A/S groups [F (2, 14) = 0.04296, p = 0.9580, Fig. 5E, F].
Figure 5.
Effect of CP and A/S on GLT-1, xCT and GLAST expression in the dorsomedial prefrontal cortex (dmPFC). (A, C, E) Immunoblots for GLT-1/ β-tubulin, xCT/ β-tubulin and GLAST/ β-tubulin, respectively. (B) Statistical analysis revealed a significant upregulation of GLT-1 expression in CP-A/S treated group as compared to CP-Vehicle group. (D) xCT expression was downregulated in CP-Vehicle group as compared to Vehicle-Vehicle and CP-A/S groups. (F) Statistical analysis of GLAST expression revealed no change among all groups in the dmPFC. (*p < 0.05, **p < 0.01 and ***p < 0.001), (n = 6-7 for each group), values shown as means ± S.E.M, (V: Vehicle; A/S: Ampicillin/Sulbactam; CP: CP 55,940).
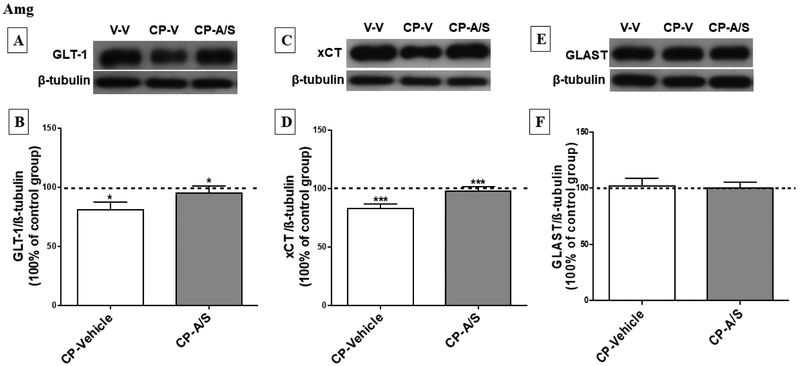
3.5. Effect of CP 55,940 and Ampicillin/Sulbactam on GLT-1, xCT and GLAST expression in the Amg.
One-way ANOVA analysis followed by Newman-Keuls multiple comparisons post hoc test revealed significant downregulation in the expression of GLT-1 in the CP-Vehicle treated group as compared to vehicle (p < 0.05). However, statistical analysis revealed a significant upregulation of GLT-1 expression in the CP-A/S as compared to the CP-Vehicle group (p < 0.05), [F (2, 14) = 4.432, p = 0.0307, Fig. 6A, B]. In addition, there was a significant downregulation in xCT expression in the CP-vehicle group as compared to the vehicle (p < 0.001) and CP-A/S (p < 0.001) treated groups [F (2, 14) = 13.33, p = 0.0003, Fig. 6C, D]. However, there was no difference in GLAST expression among the vehicle, CP-Vehicle and CPA/S groups [F (2, 14) = 0.08035, p = 0.9231, Fig. 6E, F].
Figure 6.
Effect of CP and A/S on GLT-1, xCT and GLAST expression in the amygdala (Amg). (A, C, E) Immunoblots for GLT-1/ β-tubulin, xCT/ β-tubulin and GLAST/ β-tubulin, respectively. (B) Statistical analysis revealed a significant downregulation in the expression of GLT-1 in CP-Vehicle group as compared to vehicle and CP-A/S groups. (D) Moreover, statistical analysis exhibited significant downregulation in xCT expression in CP-Vehicle group as compared to Vehicle-Vehicle and CP-A/S groups. (F) GLAST expression in the Amg was not changed following the CP or A/S treatment. (*p < 0.05 and ***p < 0.001), (n = 6-7 for each group), values shown as means ± S.E.M, (A/S: Ampicillin/Sulbactam, CP: CP 55,940).
3.6. Effect of CP 55,940 and Ampicillin/Sulbactam on GLT-1, xCT and GLAST expression in the Hipp.
One-way ANOVA analysis revealed no change in the expression of GLT-1 [F (2, 14) = 1.680, p = 0.2143, Fig. 7A, B], or xCT expression in the Hipp [F (2, 14) = 1.020, p = 0.3806, Fig. 7C, D]. Statistical analysis revealed no change in GLAST expression among the vehicle, CP-Vehicle or CP-A/S groups [F (2, 14) = 0.7808, p = 0.4703, Fig.7E, F].
Figure 7.
Effect of CP and A/S on GLT-1, xCT and GLAST expression in the hippocampus (Hipp). (A, C, E) Immunoblots for GLT-1/ β-tubulin, xCT/ β-tubulin and GLAST/ β-tubulin, respectively. (B), (D) and (F) Statistical analysis revealed no change in the GLT-1, xCT or GLAST expression among Vehicle-Vehicle, CP-Vehicle and CP-A/S groups, (p > 0.05), (n = 6-7 for each group), values shown as means ± S.E.M, (V: Vehicle; A/S: Ampicillin/Sulbactam; CP: CP 55,940).
4. Discussion
Several studies have reported contradictory findings on cannabinoid dependence, showing both aversion and rewarding outcomes using CPP (51, 53-55). These outcomes referred to cannabinoid’s unique pharmacokinetic properties, particularly with respect to their anxiolytic and anxiogenic outcomes (61, 62). Previous reports on cannabinoids demonstrated that any slight modification in the CPP procedure might produce a dramatic shift in CPP outcomes [for review, see ref. (63)]. For example, aversive effects were reported when CP was administered daily for four days in conditioning training (51), which could account for the failure to observe a positive CPP effect. Moreover, the narrow interval between cannabinoids injections can produce a similar dysphoric effect on the brain’s reward threshold, which may mask the rewarding effect, as proposed in previous report (53). Interestingly, a study that incorporated a 24-hour washout period revealed a positive CPP of Δ9-tetrahydrocannabinol (THC) in a rat model (53). Therefore, in this study we introduced a washout period after the CP injection to increase the interval between the i.p. injections of CP and reduce the potential rebound dysphoria with a short interval dosing procedure. Another novel adjustment in this CPP procedure was to introduce the three-hour i.p. injection of CP to the placement interval. This adjustment was included to reduce the potential early aversive effects of cannabinoids prior to the conditioning session (51, 54, 64). Another report incorporated the long pairing session (45 min) during the conditioning phase to minimize the possible aversive effects of cannabinoids (55).
Several studies have revealed that lower doses of cannabinoids show a greater tendency to produce rewarding effects (54, 55). A study that investigated multiple doses of CP revealed reinforcing properties with a 20 μg/kg dose in a rat model (54). In the present study, we exhibited that CP, at the dose of 20 μg/kg, can produce rewarding effects, where others showed aversive outcomes with CP at doses of 10 and 100 μg/kg (i.p.), and THC at doses of 0.75 and 1.5 mg/kg (i.p.) (51, 52). Thus, reinforcing properties of cannabinoids may be referred to the dose and CPP paradigm selection used in this study. In line with our findings, clear rewarding properties were reported with CP using the same dose in a rat animal model (54). This study is the first to report the cannabinoid’s ability to trigger reinstatement in a P rat model. These results supplement the evidence of cannabinoids’ reinforcing properties such as self-administration (1, 65-67) or conditioning properties (55, 64). It is important to note that CP is considered 30-times more potent than THC [20], thus, we tested a dose of 20 μg/kg, which may produce a similar effect to 0.5-2 mg/kg dose of THC as shown in previous study [21], Furthermore, there is possibility that CP may also activate CB2Rs, however, it is suggested that CB1Rs activation by THC is the major pharmacological mechanism for the rewarding properties of cannabinoid related drugs (55). In fact, CB2Rs are most abundantly found in the immune cells (68). In this study, we were interested in targeting astroglial glutamate transporters to modulate reinstatement to CP using the CPP paradigm. The involvement of the glutamatergic system in context-specific aspects of drug dependence, relapse and reinstatement is clearly proven [for review, see ref. (17, 18, 69-71)]. Studies of ours and others reported attenuation of cocaine-, nicotine-, and methamphetamine-induced reinstatement of CPP, in part, through restoration of astroglial glutamate transporters, GLT-1 and xCT (21, 22, 49). In addition, the use of N-methyl-D-aspartic acid (NMDA) receptors antagonists has been associated with attenuation of cocaine- and morphine-induced reinstatement (72, 73). Regarding glutamate involvement in cannabinoids, a study demonstrated the evidence for elevation in the extracellular glutamate concentration in the PFC by a CB1R agonist, WIN 55,212-2 (43). This effect on extracellular glutamate was counteracted by pretreatment with SR 141716. Importantly, another study exhibited elevation in glutamate and dopamine extracellular concentrations in the PFC following administration of THC (42). Also, recent studies have suggested that the glutamatergic system can be involved in cannabinoid dependence and tolerance (74, 75). However, to the best of our knowledge, less is known about the effects of ceftriaxone treatment on the attenuation of cannabinoids-seeking behavior. Our study was the first to investigate the effects of β-lactam compounds, known to upregulate astroglial glutamate transporters, on the attenuation of cannabinoids-seeking behavior. Together, these studies demonstrate the involvement of the astroglial glutamate transporters in dependence and relapse mechanisms of several drugs of abuse, including cannabinoids.
In the NAc, the xCT system is responsible for mediating the majority of extracellular basal glutamate, and its function was reported to be reduced following cocaine exposure (35, 76). Importantly, reports on cocaine-induced drug seeking behavior concluded that xCT is crucial in regulating synaptic glutamate release and thereby regulates reinstatement to cocaine-seeking behavior (36, 77). Another report demonstrated that xCT knockdown inhibits the prevention of cocaine-triggered reinstatement by ceftriaxone (78). Importantly, several findings showed that restoring xCT function with N-acetylcysteine attenuated cocaine- (76, 79) and heroine- (80) induced reinstatement in animals. In the present study, we revealed for the first time that CP exposure downregulated xCT expression in the NAc, dmPFC and Amg. We assumed here that downregulation of xCT during the reinstatement phase may facilitate the CP-induced reinstatement, and restoring its expression may, in part, attenuate cannabinoid reinstatement. Together, findings from this study support the potential role of xCT in reinstatement to drugseeking behavior and introduce a novel role of the glutamatergic system on reinstatement to cannabinoids.
The Amg appears to be a crucial part of the rewarding circuit, which has a role in maintaining drug-seeking behavior (81, 82). Inactivation of the basolateral Amg abolished the heroin-induced reinstatement behavior in a rat animal model (83). In addition, selective inactivation of the basolateral Amg impaired the reinstatement of extinguished cocaine-seeking behavior in rats (29, 84). Moreover, several studies have reported a significant role of the Amg dopaminergic transmission in mediating cocaine self-administration behavior (85, 86). Thus, latter studies revealed that injection of dopamine receptor 1 (D1) antagonist, SCH 23390, into the Amg increased the rate of cocaine self-administration behavior. Additionally, studies suggested the involvement of the Amg in cannabinoid-related stress and anxiety behaviors (87, 88). Thus, we investigate for any changes in the expression of target proteins in the Amg on reinstatement to CP. We revealed a significant downregulation in the expression of GLT-1 in the CP-Vehicle group as compared to the vehicle control group in the Amg. It is important to note studies revealed that CB1Rs are expressed more predominantly in the GABAergic synapses than in the glutamatergic synapse in the Amg (89, 90). However, another study revealed a comparable effect of CB1R agonists on both GABAergic and glutamatergic synapses (91). This differential role of CB1R in the Amg may account for this significant effect on the expression of GLT-1; therefore, further investigation is required to determine the pharmacological mechanism of CB1R in different brain rewarding regions. Moreover, there was a trend for GLT-1 downregulation in other brain regions, such as the NAc, dmPFC, and Hipp; however, statistical analyses revealed no significant difference between the CP-Vehicle and control groups. This effect of CP-induced reinstatement on GLT-1 in different brain regions might be due to either a decrease in the activity of GLT-1 or other unknown pharmacological effects that warrant further investigation. In line with our findings, a recent report hypothesized a significant role of GLT-1 upregulation by ceftriaxone in the prevention of cannabinoid dependence (74). Moreover, a study using a slice preparation from Sprague Dawley rats proposed that CB1R activation may decrease glutamate uptake, which may lead to an increase in the synaptic glutamate concentration (92). A previous report from our laboratory reported downregulation of GLT-1 following the reinstatement to cocaine and A/S attenuated cocaine-seeking behavior, in part, by restoring GLT-1 expression in P rats (49). Moreover, increasing GLT-1 expression by MS-153, a GLT-1 activator, attenuated the acquisition of morphine, methamphetamine and cocaine in mice (93). Our present study is the first to reveal the effect of CP-induced reinstatement on GLT-1 expression in rats.
Several reports have investigated the role of the cannabinoid system in the Hipp. Of note, CB1Rs have been introduced as the major cannabinoid receptors in the glutamatergic synapse in the Hipp and cerebellum (94). Another study revealed the inhibitory effect on glutamatergic transmission following exposure of CB1R agonist in cultured rat hippocampal neurons (40). Furthermore, a previous study suggested the involvement of novel-cannabinoid sensitive receptors in the mediation of glutamatergic transmission in the Hipp (95). CB1Rs are highly expressed in the Hipp (96); however, the role of this neuronal population in the intracellular signaling effect of cannabinoids is still unknown. In the present study, statistical analysis revealed no change in the expression of GLT-1 or xCT in the CP-Vehicle treated group as compared to the vehicle and CP-A/S groups in the Hipp. Another finding demonstrated that inactivation of Hipp abolished contextual reinstatement, but failed to attenuate conditioned stimuli or cocaine-induced reinstatement of cocaine-seeking behavior (84). Further studies are warranted to investigate the functional connectivity between the Hipp or other brain regions and reinstatement to cannabinoids.
To the best of our knowledge, most previous studies have introduced the modulation of CB1Rs or opioid receptors as the suggested approach to attenuate the reinforcing properties of cannabinoids (54, 97). In the present study, we introduced, for the first time, the ability of β-lactams to attenuate CP-induced seeking behavior. We tested β-lactams that contain Ampicillin and Sulbactam in a 2:1 (Ampicillin: Sulbactam) ratio. Both compounds have a central β-lactam core, which is proposed to be the factor for the upregulatory effect of GLT-1 expression (87). A previous report from our laboratory revealed the upregulatory effect of Ampicillin on GLT-1 and xCT expression in the NAc and PFC and this effect was associated, at least in part, with a reduction of ethanol consumption in P rats (98). Interestingly, another report determined the effective role of A/S in the prevention of reinstatement to cocaine-seeking behavior (49). This effect was associated, in part, with the restoration of astroglial glutamate transporters such as GLT-1 and xCT in the mesocorticolimbic brain regions, including the NAc and dmPFC. Similarly, we revealed that A/S i.p. injections during the extinction phase successfully attenuated CP-seeking behavior during the reinstatement phase. Moreover, this effect was associated with the restoration of GLT-1 expression in the Amg, and xCT in the NAc, dmPFC and Amg. Importantly, A/S administration revealed a significant upregulation of GLT-1 expression in those brain regions. We assumed this upregulation of GLT-1 in the NAc and dmPFC might be associated, in part, with attenuation of the CP-induced reinstatement. Further studies are warranted to investigate the effects of different doses of CP on the expression of GLT-1 in different brain regions. In addition, we showed that A/S administration alone did not reveal any significant change in time spent using CPP. Thus, we concluded that A/S effect on CP-induced reinstatement is not due to any confounding factors, such as possible conditioned place aversion. This is in agreement with recent reports that suggested that ceftriaxone, a β-lactam antibiotic, alone did not elicit any change in conditioning training (21). Further studies are warranted to investigate the effect of A/S at different doses using our established CPP paradigm as well as on the expression of astroglial glutamate transporters.
Statistical analysis revealed no change in the expression of GLAST among the treated groups in the NAc, dmPFC, Amg and Hipp. These findings might be explained by the fact that GLAST is the predominant astroglial glutamate transporter in the cerebellum (37) and peripheral organs such as the retina (99) and the inner ear (100). Furthermore, previous reports from our laboratory demonstrated that other drugs of abuse, such as ethanol, cocaine and methamphetamine did not reveal any differences in GLAST expression as compared to the control group (47, 49, 101).
In conclusion, our study supports the previous reports on the rewarding and reinforcing properties of cannabinoids in an animal model. We revealed, for the first time, the possibility of a cannabinoid-triggered reinstatement property using the CPP procedure in a rat animal model. Importantly, we showed that the glutamatergic system is, in part, associated with reinstatement to cannabinoids. This study also presented the ability of β-lactam compounds to attenuate cannabinoid-triggered reinstatement, at least in part through restoration of astroglial glutamate transporters and glutamate homeostasis. These findings verify the crucial role of the glutamatergic system in reinstatement and relapse to most drugs of abuse, including cannabinoids, and that restoring their activity may, in part, attenuate the reinstatement properties of drugs of abuse.
Acknowledgements
The work was supported in part by Award Number R01AA019458 (Y.S.) from the National Institutes on Alcohol Abuse and Alcoholism and fund provided by The University of Toledo. AYH was supported by a scholarship from King Saud bin Abdulaziz University for health sciences, College of Medicine, Jeddah, Saudi Arabia. FSA was supported by a scholarship from Umm Al-Qura University, College of Pharmacy & Pharmaceutical Sciences, Makkah, Saudi Arabia. The authors would like to thank Charisse Montgomery for editing this manuscript.
Footnotes
Funding and disclosure
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Braida D, Pozzi M, Parolaro D, Sala M. Intracerebral self-administration of the cannabinoid receptor agonist CP 55,940 in the rat: interaction with the opioid system. European journal of pharmacology. 2001;413(2):227–34. [DOI] [PubMed] [Google Scholar]
- 2.Martellotta M, Cossu G, Fattore L, Gessa G, Fratta W. Self-administration of the cannabinoid receptor agonist WIN 55,212-2 in drug-naive mice. Neuroscience. 1998;85(2):327–30. [DOI] [PubMed] [Google Scholar]
- 3.Fattore L, Cossu G, Martellotta CM, Fratta W. Intravenous self-administration of the cannabinoid CB1 receptor agonist WIN 55,212-2 in rats. Psychopharmacology (Berl). 2001;156(4):410–6. [DOI] [PubMed] [Google Scholar]
- 4.Spano MS, Fattore L, Cossu G, Deiana S, Fadda P, Fratta W CB1 receptor agonist and heroin, but not cocaine, reinstate cannabinoid- seeking behaviour in the rat. British journal of pharmacology. 2004;143(3):343–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gessa G, Melis M, Muntoni A, Diana M. Cannabinoids activate mesolimbic dopamine neurons by an action on cannabinoid CB 1 receptors. European journal of pharmacology. 1998;341(l):39–44. [DOI] [PubMed] [Google Scholar]
- 6.Gardner EL, Lowinson JH. Marijuana's interaction with brain reward systems: update 1991. Pharmacology Biochemistry and Behavior. 1991. ;40(3):571−80. [DOI] [PubMed] [Google Scholar]
- 7.Chaperon F, Soubrié P, Puech AJ, Thiébot M-H. Involvement of central cannabinoid (CB1) receptors in the establishment of place conditioning in rats. Psychopharmacology. 1998; 13 5(4):324–32. [DOI] [PubMed] [Google Scholar]
- 8.De Vries TJ, Shaham Y, Homberg JR, Crombag H, Schuurman K, Dieben J, et al. A cannabinoid mechanism in relapse to cocaine seeking. Nature medicine. 2001;7(10):1151–4. [DOI] [PubMed] [Google Scholar]
- 9.Arnone M, Maruani J, Chaperon F, Thiébot M-H, Poncelet M, Soubrié P, et al. Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology. 1997; 132(1): 104–6. [DOI] [PubMed] [Google Scholar]
- 10.Vinklerová J, Nováková J, Šulcová A. Inhibition of methamphetamine self-administration in rats by cannabinoid receptor antagonist AM 251. Journal of Psychopharmacology. 2002;16(2):139–43. [DOI] [PubMed] [Google Scholar]
- 11.Hungund BL, Szakall I, Adam A, Basavarajappa BS, Vadasz C. Cannabinoid CB1 receptor knockout mice exhibit markedly reduced voluntary alcohol consumption and lack alcohol-induced dopamine release in the nucleus accumbens. Journal of neurochemistry. 2003;84(4):698–704. [DOI] [PubMed] [Google Scholar]
- 12.Forget B, Hamon M, Thiébot M-H Cannabinoid CB1 receptors are involved in motivational effects of nicotine in rats. Psychopharmacology. 2005;181(4):722–34. [DOI] [PubMed] [Google Scholar]
- 13.Singh M, Verty A, McGregor I, Mallet P A cannabinoid receptor antagonist attenuates conditioned place preference but not behavioural sensitization to morphine. Brain research. 2004; 1026(2):244–53. [DOI] [PubMed] [Google Scholar]
- 14.Cossu G, Ledent C, Fattore L, Imperato A, Böhme GA, Parmentier M, et al. Cannabinoid CB 1 receptor knockout mice fail to self-administer morphine but not other drugs of abuse. Behavioural brain research. 2001;118(1):61–5. [DOI] [PubMed] [Google Scholar]
- 15.Self DW. Neural substrates of drug craving and relapse in drug addiction. Annals of medicine. 1998;30(4):379–89. [DOI] [PubMed] [Google Scholar]
- 16.Willuhn I, Wanat MJ, Clark JJ, Phillips PE. Dopamine signaling in the nucleus accumbens of animals self-administering drugs of abuse. Behavioral neuroscience of drug addiction: Springer; 2010. p. 29–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tzschentke T, Schmidt W. Glutamatergic mechanisms in addiction. Molecular psychiatry. 2003;8(4):373–82. [DOI] [PubMed] [Google Scholar]
- 18.Knackstedt LA, Kalivas PW. Glutamate and reinstatement. Current opinion in pharmacology. 2009;9(1):59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Das SC, Yamamoto BK, Hristov AM, Sari Y. Ceftriaxone attenuates ethanol drinking and restores extracellular glutamate concentration through normalization of GLT-1 in nucleus accumbens of male alcohol-preferring rats. Neuropharmacology. 2015;97:67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sari Y, Smith KD, Ali PK, Rebec GV. Upregulation of GLT1 attenuates cue-induced reinstatement of cocaine-seeking behavior in rats. The Journal of Neuroscience. 2009;29(29):9239–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abulseoud OA, Miller JD, Wu J, Choi D-S, Holschneider DP. Ceftriaxone upregulates the glutamate transporter in medial prefrontal cortex and blocks reinstatement of methamphetamine seeking in a condition place preference paradigm. Brain research. 2012;1456:14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alajaji M, Bowers M, Knackstedt L, Damaj M. Effects of the beta-lactam antibiotic ceftriaxone on nicotine withdrawal and nicotine-induced reinstatement of preference in mice. P sychopharmacology. 2013;228(3):419–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. The Journal of neuroscience. 2003;23(8):3531–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melendez RI, Hicks MP, Cagle SS, Kalivas PW. Ethanol exposure decreases glutamate uptake in the nucleus accumbens. Alcoholism: Clinical and Experimental Research. 2005;29(3):326–33. [DOI] [PubMed] [Google Scholar]
- 25.Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nature Reviews Neuroscience. 2013;14(9):609–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knackstedt LA, Melendez RI, Kalivas PW. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biological psychiatry. 2010;67(1):81–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Capriles N, Rodaros D, Sorge RE, Stewart J. A role for the prefrontal cortex in stress-and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2003;168(1-2):66–74. [DOI] [PubMed] [Google Scholar]
- 28.McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drugseeking behavior. Journal of Neuroscience. 2001;21(21):8655–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology. 2003;168(l-2):57–65. [DOI] [PubMed] [Google Scholar]
- 30.McFarland K, Davidge SB, Lapish CC, Kalivas PW. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. Journal of Neuroscience. 2004;24(7): 1551–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berglind WJ, Whitfield TW, LaLumiere RT, Kalivas PW, McGinty JF. A single intra-PFC infusion of BDNF prevents cocaine-induced alterations in extracellular glutamate within the nucleus accumbens. Journal of Neuroscience. 2009;29(12):3715–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, et al. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996; 16(3):675–86. [DOI] [PubMed] [Google Scholar]
- 33.Rothstein JD, Van Kammen M, Levey AI, Martin LJ, Kuncl RW. Selective loss of glial glutamate transporter GLT- 1 in amyotrophic lateral sclerosis. Annals of neurology. 1995;38(l):73–84. [DOI] [PubMed] [Google Scholar]
- 34.Danbolt NC. Glutamate uptake. Progress in neurobiology. 2001. ;65(1): 1–105. [DOI] [PubMed] [Google Scholar]
- 35.Baker D, Shen H, Kalivas P. Cystine/glutamate exchange serves as the source for extracellular glutamate: modifications by repeated cocaine administration. Amino acids. 2002;23(l-3):161–2. [DOI] [PubMed] [Google Scholar]
- 36.Moran MM, McFarland K, Melendez RI, Kalivas PW, Seamans JK. Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. The Journal of neuroscience. 2005;25(27):6389–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lehre KP, Danbolt NC. The number of glutamate transporter subtype molecules at glutamatergic synapses: chemical and stereological quantification in young adult rat brain. Journal of Neuroscience. 1998; 18(21):8751–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gerdeman G, Lovinger DM. CB1 cannabinoid receptor inhibits synaptic release of glutamate in rat dorsolateral striatum. Journal of neurophysiology. 2001. ;85(1):468–71. [DOI] [PubMed] [Google Scholar]
- 39.Huang CC, Lo SW, Hsu KS. Presynaptic mechanisms underlying cannabinoid inhibition of excitatory synaptic transmission in rat striatal neurons. The Journal of physiology. 2001;532(3):731–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen M, Piser TM, Seybold VS, Thayer SA. Cannabinoid receptor agonists inhibit glutamatergic synaptic transmission in rat hippocampal cultures. Journal of Neuroscience. 1996;16(14):4322–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Al- Hayani A, Davies SN. Cannabinoid receptor mediated inhibition of excitatory synaptic transmission in the rat hippocampal slice is developmentally regulated. British journal of pharmacology. 2000;131(4):663–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pistis M, Ferraro L, Pira L, Flore G, Tanganelli S, Gessa GL, et al. Δ 9-Tetrahydrocannabinol decreases extracellular GABA and increases extracellular glutamate and dopamine levels in the rat prefrontal cortex: an in vivo microdialysis study. Brain research. 2002;948(l):155–8. [DOI] [PubMed] [Google Scholar]
- 43.Ferraro L, Tomasini MC, Gessa GL, Bebe BW, Tanganelli S, Antonelli T. The cannabinoid receptor agonist WIN 55,212-2 regulates glutamate transmission in rat cerebral cortex: an in vivo and in vitro study. Cerebral Cortex. 2001;11(8):728–33. [DOI] [PubMed] [Google Scholar]
- 44.Tomasini MC, Ferraro L, Bebe BW, Tanganelli S, Cassano T, Cuomo V, et al. Δ9-tetrahydrocannabinol increases endogenous extracellular glutamate levels in primary cultures of rat cerebral cortex neurons: Involvement of CB1 receptors. Journal of neuroscience research. 2002;68(4):449–53. [DOI] [PubMed] [Google Scholar]
- 45.Qrunfleh AM, Alazizi A, Sari Y. Ceftriaxone, a beta-lactam antibiotic, attenuates relapse-like ethanol-drinking behavior in alcohol-preferring rats. Journal of psychopharmacology. 2013:0269881113482529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Althobaiti YS, Almalki AH, Das SC, Alshehri FS, Sari Y. Effects of repeated high-dose methamphetamine and ceftriaxone post-treatments on tissue content of dopamine and serotonin as well as glutamate and glutamine. Neuroscience Letters. 2016;634:25–31. [DOI] [PubMed] [Google Scholar]
- 47.Hakami AY, Hammad AM, Sari Y. Effects of amoxicillin and Augmentin on cystine-glutamate exchanger and glutamate transporter 1 isoforms as well as ethanol intake in alcohol-preferring rats. Frontiers in neuroscience. 2016; 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim J, John J, Langford D, Walker E, Ward S, Rawls SM. Clavulanic acid enhances glutamate transporter subtype I (GLT-1) expression and decreases reinforcing efficacy of cocaine in mice. Amino acids. 2016;48(3):689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hammad AM, Alasmari F, Althobaiti YS, Sari Y. Modulatory effects of Ampicillin/Sulbactam on glial glutamate transporters and metabotropic glutamate receptor 1 as well as reinstatement to cocaine-seeking behavior. Behavioural Brain Research. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prus AJ, James JR, Rosecrans JA. Conditioned place preference. 2009. [Google Scholar]
- 51.McGregor IS, Issakidis CN, Prior G. Aversive effects of the synthetic cannabinoid CP 55,940 in rats. Pharmacology Biochemistry and Behavior. 1996;53(3):657–64. [DOI] [PubMed] [Google Scholar]
- 52.Parker LA, Gillies T. THC-induced place and taste aversions in Lewis and Sprague-Dawley rats. Behavioral neuroscience. 1995; 109(1):71. [DOI] [PubMed] [Google Scholar]
- 53.Lepore M, Vorel SR, Lowinson J, Gardner EL. Conditioned place preference induced by Δ 9-tetrahydrocannabinol: comparison with cocaine, morphine, and food reward. Life sciences. 1995;56(23):2073–80. [DOI] [PubMed] [Google Scholar]
- 54.Braida D, Pozzi M, Cavallini R, Sala M. Conditioned place preference induced by the cannabinoid agonist CP 55,940: interaction with the opioid system. Neuroscience. 2001;104(4):923–6. [DOI] [PubMed] [Google Scholar]
- 55.Valjent E, Maldonado R. A behavioural model to reveal place preference to Δ9-tetrahydrocannabinol in mice. Psychopharmacology. 2000;147(4):436–8. [DOI] [PubMed] [Google Scholar]
- 56.Getachew B, Hauser SR, Dhaher R, Katner SN, Bell RL, Oster SM, et al. CB1 receptors regulate alcohol-seeking behavior and alcohol self-administration of alcohol-preferring (P) rats. Pharmacology Biochemistry and Behavior. 2011;97(4):669–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fujio M, Nakagawa T, Sekiya Y, Ozawa T, Suzuki Y, Minami M, et al. Gene transfer of GLT- 1, a glutamate transporter, into the nucleus accumbens shell attenuates methamphetamine- and morphine- induced conditioned place preference in rats. European Journal of Neuroscience. 2005;22(ll):2744–54. [DOI] [PubMed] [Google Scholar]
- 58.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6th ed. Amsterdam; Boston;: Academic Press/Elsevier; 2007. [Google Scholar]
- 59.Sari Y, Sakai M, Weedman JM, Rebec GV, Bell RL. Ceftriaxone, a beta-lactam antibiotic, reduces ethanol consumption in alcohol-preferring rats. Alcohol and alcoholism. 2011;46(3):239–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alhaddad H, Das SC, Sari Y. Effects of ceftriaxone on ethanol intake: a possible role for xCT and GLT-1 isoforms modulation of glutamate levels in P rats. Psychopharmacology. 2014;231(20):4049–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Onaivi ES, Green MR, Martin BR. Pharmacological characterization of cannabinoids in the elevated plus maze. Journal of Pharmacology and Experimental Therapeutics. 1990;253(3):1002–9. [PubMed] [Google Scholar]
- 62.de Fonseca FR, Carrera MRA, Navarro M, Koob GF, Weiss F Activation of corticotropin-releasing factor in the limbic system during cannabinoid withdrawal. Science. 1997;276(5321):2050–4. [DOI] [PubMed] [Google Scholar]
- 63.Tanda G, Goldberg SR. Cannabinoids: reward, dependence, and underlying neurochemical mechanisms—a review of recent preclinical data. Psychopharmacology. 2003;169(2):115–34. [DOI] [PubMed] [Google Scholar]
- 64.Braida D, Iosue S, Pegorini S, Sala M. Δ 9-Tetrahydrocannabinol-induced conditioned place preference and intracerebroventricular self-administration in rats. European journal of pharmacology. 2004;506(l):63–9. [DOI] [PubMed] [Google Scholar]
- 65.Tanda G, Munzar P, Goldberg SR. Self-administration behavior is maintained by the psychoactive ingredient of marijuana in squirrel monkeys. Nature neuroscience. 2000;3(11): 1073–4. [DOI] [PubMed] [Google Scholar]
- 66.Martellotta MC, Cossu G, Fattore L, Gessa GL, Fratta W. Self-administration of the cannabinoid receptor agonist WIN 55,212–2 in drug-naive mice. Neuroscience. 1998;85(2):327–30. [DOI] [PubMed] [Google Scholar]
- 67.Justinova Z, Tanda G, Redhi GH, Goldberg SR. Self-administration of Δ9-tetrahydrocannabinol (THC) by drug naive squirrel monkeys. Psychopharmacology. 2003; 169(2): 135–40. [DOI] [PubMed] [Google Scholar]
- 68.Klein TW, Newton C, Larsen K, Lu L, Perkins I, Nong L, et al. The cannabinoid system and immune modulation. J Leukoc Biol. 2003;74(4):486–96. [DOI] [PubMed] [Google Scholar]
- 69.Kalivas PW, LaLumiere RT, Knackstedt L, Shen H. Glutamate transmission in addiction. Neuropharmacology. 2009;56:169–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rao P, Satemos H, Goodwani S, Sari Y. Effects of ceftriaxone on GLT1 isoforms, xCT and associated signaling pathways in P rats exposed to ethanol. Psychopharmacology. 2015:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goodwani S, Satemos H, Alasmari F, Sari Y. Metabotropic and ionotropic glutamate receptors as potential targets for the treatment of alcohol use disorder. Neuroscience & Biobehavioral Reviews. 2017;77:14–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Do Couto BR, Aguilar M, Manzanedo C, Rodriguez-Arias M, Minarro J. NMDA glutamate but not dopamine antagonists blocks drug-induced reinstatement of morphine place preference. Brain research bulletin. 2005;64(6):493–503. [DOI] [PubMed] [Google Scholar]
- 73.Maldonado C, Rodriguez-Arias M, Castillo A, Aguilar M, Minarro J. Effect of memantine and CNQX in the acquisition, expression and reinstatement of cocaine-induced conditioned place preference. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2007;31(4):932–9. [DOI] [PubMed] [Google Scholar]
- 74.Ulugol A Reduction of dependence to cannabinoids by GLT-1 activating property of the beta-lactam antibiotic. Medical hypotheses. 2013;80(3):247–8. [DOI] [PubMed] [Google Scholar]
- 75.Gunduz O, Oltulu C, Ulugol A. Role of GLT-1 transporter activation in prevention of cannabinoid tolerance by the beta-lactam antibiotic, ceftriaxone, in mice. Pharmacology Biochemistry and Behavior. 2011. ;99(1): 100–3. [DOI] [PubMed] [Google Scholar]
- 76.Baker DA, McFarland K, Lake RW, Shen H, Xing-Chun T, Toda S, et al. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nature neuroscience. 2003;6(7):743. [DOI] [PubMed] [Google Scholar]
- 77.Moussawi K, Zhou W, Shen H, Reichel CM, See RE, Carr DB, et al. Reversing cocaine- induced synaptic potentiation provides enduring protection from relapse. Proceedings of the National Academy of Sciences. 2011; 108(1):385–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.LaCrosse AL, O'Donovan SM, Sepulveda-Orengo MT, McCullumsmith RE, Reissner KJ, Schwendt M, et al. Contrasting the Role of xCT and GLT-1 Upregulation in the Ability of Ceftriaxone to Attenuate the Cue-Induced Reinstatement of Cocaine Seeking and Normalize AMPA Receptor Subunit Expression. Journal of Neuroscience. 2017;37(24):5809–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kau KS, Madayag A, Mantsch JR, Grier MD, Abdulhameed O, Baker DA. Blunted cystine-glutamate antiporter function in the nucleus accumbens promotes cocaine-induced drug seeking. Neuroscience. 2008;155(2):530–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou W, Kalivas PW. N-acetylcysteine reduces extinction responding and induces enduring reductions in cue-and heroin-induced drug-seeking. Biological psychiatry. 2008;63(3):338–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Meil WM, See RE. Lesions of the basolateral amygdala abolish the ability of drug associated cues to reinstate responding during withdrawal from self-administered cocaine. Behavioural brain research. 1997;87(2): 139–48. [DOI] [PubMed] [Google Scholar]
- 82.Grimm JW, See RE. Dissociation of primary and secondary reward-relevant limbic nuclei in an animal model of relapse. Neuropsychopharmacology. 2000;22(5):473. [DOI] [PubMed] [Google Scholar]
- 83.Fuchs RA, See RE. Basolateral amygdala inactivation abolishes conditioned stimulus-and heroin-induced reinstatement of extinguished heroin-seeking behavior in rats. Psychopharmacology. 2002;160(4):425–33. [DOI] [PubMed] [Google Scholar]
- 84.Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, et al. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30(2):296–309. [DOI] [PubMed] [Google Scholar]
- 85.Hurd Y, McGrego A, Ponten M. In vivo Amygdala Dopamine Levels Modulate Cocaine Self- administration Behaviour in the Rat: D1 Dopamine Receptor Involvement. European Journal of Neuroscience. 1997;9(12):2541–8. [DOI] [PubMed] [Google Scholar]
- 86.McGregor A, Roberts DC. Dopaminergic antagonism within the nucleus accumbens or the amygdala produces differential effects on intravenous cocaine self-administration under fixed and progressive ratio schedules of reinforcement. Brain research. 1993;624(l-2):245–52. [DOI] [PubMed] [Google Scholar]
- 87.Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, et al. β-Lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433(7021):73–7. [DOI] [PubMed] [Google Scholar]
- 88.Ganon-Elazar E, Akirav I. Cannabinoid receptor activation in the basolateral amygdala blocks the effects of stress on the conditioning and extinction of inhibitory avoidance. Journal of Neuroscience. 2009;29(36): 11078–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Katona I, Rancz EA, Acsády L, Ledent C, Mackie K, Hájos N, et al. Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. Journal of Neuroscience. 2001;21(23):9506–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Matsuda LA, Bonner TI, Lolait SJ. Localization of cannabinoid receptor mRNA in rat brain. Journal of Comparative Neurology. 1993;327(4):535–50. [DOI] [PubMed] [Google Scholar]
- 91.Azad SC, Eder M, Marsicano G, Lutz B, Zieglgänsberger W, Rammes G. Activation of the cannabinoid receptor type 1 decreases glutamatergic and GABAergic synaptic transmission in the lateral amygdala of the mouse. Learning & memory. 2003; 10(2): 116–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brown TM, Brotchie JM, Fitzjohn SM. Cannabinoids decrease corticostriatal synaptic transmission via an effect on glutamate uptake. Journal of Neuroscience. 2003;23(35): 11073–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nakagawa T, Fujio M, Ozawa T, Minami M, Satoh M. Effect of MS-153, a glutamate transporter activator, on the conditioned rewarding effects of morphine, methamphetamine and cocaine in mice. Behavioural brain research. 2005;156(2):233–9. [DOI] [PubMed] [Google Scholar]
- 94.Kawamura Y, Fukaya M, Maejima T, Yoshida T, Miura E, Watanabe M, et al. The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic sites in the hippocampus and cerebellum. Journal of Neuroscience. 2006;26(11):2991–3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hájos N, Ledent C, Freund TF. Novel cannabinoid-sensitive receptor mediates inhibition of glutamatergic synaptic transmission in the hippocampus. Neuroscience. 2001; 106(1): 1–4. [DOI] [PubMed] [Google Scholar]
- 96.Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, De Costa BR, et al. Cannabinoid receptor localization in brain. Proceedings of the national Academy of sciences. 1990;87(5): 1932–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ghozland S, Matthes HW, Simonin F, Filliol D, Kieffer BL, Maldonado R. Motivational effects of cannabinoids are mediated by μ-opioid and κ-opioid receptors. Journal of Neuroscience. 2002;22(3): 1146–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Alasmari F, Abuhamdah S, Sari Y. Effects of ampicillin on cystine/glutamate antiporter and glutamate transporter 1 isoforms as well as ethanol drinking in male P rats. Neuroscience letters. 2015;600:148–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lehre KP, Davanger S, Danbolt NC. Localization of the glutamate transporter protein GLAST in rat retina. Brain research. 1997;744(1): 129–37. [DOI] [PubMed] [Google Scholar]
- 100.Takumi Y, Matsubara A, Danbolt N, Laake J, Storm-Mathisen J, Usami S, et al. Discrete cellular and subcellular localization of glutamine synthetase and the glutamate transporter GLAST in the rat vestibular end organ. Neuroscience. 1997;79(4):1137–44. [DOI] [PubMed] [Google Scholar]
- 101.Alshehri FS, Althobaiti YS, Sari Y. Effects of administered ethanol and methamphetamine on glial glutamate transporters in rat striatum and hippocampus. Journal of Molecular Neuroscience. 2017;61(3):343–50. [DOI] [PMC free article] [PubMed] [Google Scholar]