Abstract
Exposure to prolonged, uncontrollable stress reduces reward-seeking behavior, resulting in anhedonia in neuropsychiatric disorders, such as posttraumatic stress disorder. However, it is unclear to what degree stressed subjects lose interest in rewards themselves or in reward-related cues that instigate reward-seeking behavior. In the present study, we investigated the effects of single prolonged stress (SPS) on cue-directed behavior in two different procedures: Pavlovian conditioned approach (PCA) and cue-induced reinstatement of cocaine-seeking. In Experiment 1, rats were exposed to SPS and tested for the acquisition of sign-tracking (cue-directed) and goal-tracking (reward-directed) behaviors during a PCA procedure. In Experiment 2, rats were exposed to SPS and tested for the expression of sign- and goal-tracking as well as cue-induced reinstatement of cocaine-seeking. Because dopaminergic activity in the nucleus accumbens is known to play a central role in many cue-directed behaviors, including both sign-tracking and cue-induced reinstatement, Experiment 3 used in vivo microdialysis to measure the effect of SPS on baseline and evoked dopamine levels in the nucleus accumbens. SPS decreased sign-tracking and increased goal-tracking during the acquisition of PCA behavior without affecting reward consumption. In addition, SPS decreased cue-induced reinstatement without affecting cocaine self-administration. Finally, SPS decreased evoked but not baseline levels of dopamine in the nucleus accumbens. These results suggest that SPS decreases the motivational, but not consummatory, aspects of reward-seeking behavior, which may result from long-term, SPS-induced reductions in dopamine release in the nucleus accumbens.
Keywords: Pavlovian conditioned approach, anhedonia, dopamine, nucleus accumbens, incentive salience, motivation
1. Introduction
Exposure to prolonged, uncontrollable stress can lead to the development of neuropsychiatric disorders, such as posttraumatic stress disorder (PTSD). One feature of PTSD is anhedonia, or the pathological lack of interest or pleasure in once desirable activities. Stress-induced anhedonia is typically modeled in animals by exposing subjects to prolonged or repeated uncontrollable stressors and then measuring the resultant decreases in reward-related behavior. For example, rats exposed to prolonged stress show decreases in exploratory behavior, sexual behavior, consumption of sweetened liquids, conditioned place preference for palatable foods and drug rewards, and operant responses for rewarding brain stimulation (Gronli et al., 2005; Moreau et al., 1992; Papp et al., 1991; Zacharko et al., 1983). The standard interpretation has been that in these situations reward-related activities are diminished, because, as thought for depressed patients, they no longer find these activities pleasurable.
However, the concept of anhedonia as a diminished capacity for pleasure has been recently challenged (Treadway and Zald, 2011). The most common way to assess anhedonia is simply to ask patients whether they are experiencing decreased enjoyment of activities that they would normally find pleasurable, and affected individuals reliably report a lack of pleasure (Watson and Naragon-Gainey, 2010). Yet, people’s estimates of their own subjective enjoyment of future, past, or hypothetical activities are often inaccurate and can be heavily influenced by their own past decisions on whether to engage in those activities (Ariely and Norton, 2008; Brehm, 1956; Wenze et al., 2012; Wilson and Gilbert, 2005; Wilson et al., 2003). Several clinical studies have found that patients endorsing anhedonia often show normal hedonic responses to rewarding stimuli when affect is measured in real time (Klein, 1987; Kring and Moran, 2008; Strauss and Gold, 2012; Taylor et al., 2012; Treadway and Zald, 2011). It has been suggested, therefore, that the symptomatic deficit in patients may primarily be their motivation to pursue rewards, rather than their hedonic capacity to enjoy rewards (Myin-Germeys et al., 2000; Treadway and Zald, 2013).
A crucial process in generating motivated behavior is the attribution of incentive-motivational value to cues in the environment associated with reward (Berridge, 2004; Bindra, 1974). Although it is often difficult to dissociate the predictive properties of cues from their incentive-motivational properties, Pavlovian conditioned approach (PCA) procedures allow one to do so by separating in space the cue that predicts an impending reward from the location of reward delivery. For example, in the procedure used here, extension of a retractable lever, situated a few centimeters away from a pellet magazine, response-independently predicts the delivery of food pellets. Thus, on any given trial the rat may choose to interact with the lever (sign-tracking) or enter the magazine (goal-tracking) when the lever is extended, even though neither action influences reward delivery (Flagel et al., 2009). For sign-trackers, the reward-related cue acquires incentive-motivational value, evidenced by their propensity to approach and interact with it as well as their willingness to work for it during a conditioned reinforcement test (Robinson and Flagel, 2009). In contrast, for goal-trackers, the reward-related cue acquires predictive value, but it does not appear to become particularly attractive or rewarding for them (i.e., the cue itself does not acquire incentive-motivational value).
Single prolonged stress (SPS) is the serial application of three stressors (restraint, forced swim, and ether exposure), which has been reliably used to model PTSD-like behaviors (Knox et al., 2012a) and depression-like behaviors, such as “despair” in the forced swim test (Serova et al., 2013a, 2013b). In the present study, we assessed the effects of SPS on the incentive-motivational properties of food- and cocaine-related reward cues. First, we measured sign-tracking, goal-tracking, and consummatory behaviors to compare and contrast the incentive properties of food-related cues versus food as a primary reward in rats exposed to SPS. To test whether these findings would extend to cue-induced behaviors toward other primary rewards, we assessed cue-induced reinstatement of cocaine-seeking behavior in SPS-exposed rats. Finally, because decreased dopamine (DA) transmission has been proposed to mediate stress-induced reductions in reward-seeking behavior (Bekris et al., 2005; Cabib and Puglisi-Allegra, 2012; Pascucci et al., 2007; Puglisi-Allegra et al., 1991) and DA release within the nucleus accumbens (NAc) can selectively affect cue-directed behaviors while sparing primary reward responses (Berridge and Robinson, 1998; Flagel et al., 2011; Saunders and Robinson, 2012), we used in vivo microdialysis to measure baseline and evoked levels of DA within the NAc of SPS-exposed rats.
2. Experimental Procedures
2.1. Animals
Adult male Sprague Dawley rats (275–300 g) were purchased from Charles River and Harlan Laboratories. Rats were selected from these two vendors to maximize variability in sign-and goal-tracking behaviors (Fitzpatrick et al., 2013). Animals were maintained on a 12:12-hr light/dark cycle, and housed individually with food and water available ad libitum for the duration of experimentation. All procedures were approved by the University Committee on the Use and Care of Animals (University of Michigan; Ann Arbor, MI).
2.2. Experimental timeline
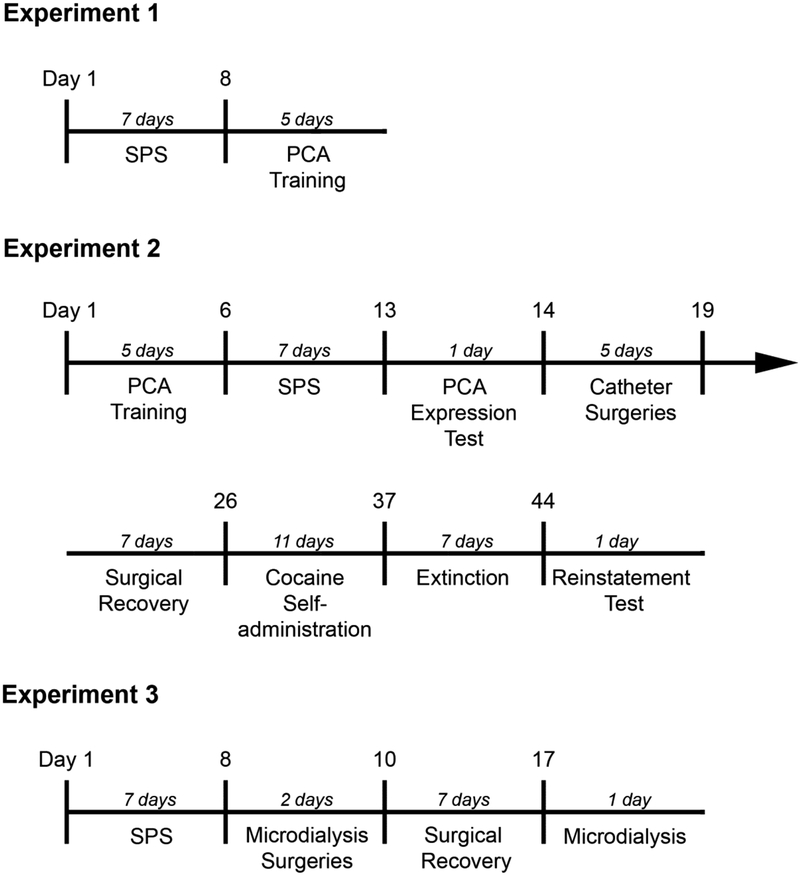
Figure 1 shows the timeline for the three experiments in the present study. In Experiment 1, rats were exposed to SPS followed by five daily PCA training sessions. In Experiment 2, rats underwent five daily PCA training sessions followed by SPS and a single PCA training session to measure the expression of PCA behavior. Next, rats were implanted with indwelling intravenous catheters, allowed to recover for one week, and then tested for cocaine self-administration, extinction, and cue-induced reinstatement. Rats were counterbalanced into SPS and control groups as equally as possible from both vendors in Experiment 1 (Harlan: SPS = 12, Control = 12; Charles River: SPS = 12, Control = 12) and Experiment 2 (Harlan: SPS = 10, Control = 11; Charles River: SPS = 6, Control = 8). In Experiment 3, rats were exposed to SPS, implanted with in vivo microdialysis probes, then tested one week later (approximately two weeks after the administration of SPS) for baseline and evoked DA levels in the NAc. In Experiment 3, rats were only purchased from Charles River (SPS = 5, Control = 6).
Figure 1.
The schematic illustration describes each experimental timeline. Experiment 1 investigated the effect of single prolonged (SPS) stress on the acquisition of Pavlovian conditioned approach (PCA) behavior. Experiment 2 investigated the effect of SPS on the expression of PCA as well as the acquisition, extinction, and cue-induced reinstatement of cocaine self-administration. Experiment 3 investigated the effect of SPS on K+-induced dopamine release in the nucleus accumbens.
2.3. Pavlovian conditioned approach (PCA): Apparatus and procedure
Sixteen modular conditioning chambers (24.1 cm width × 20.5 cm depth ×29.2 cm height; MED Associates, Inc.; St. Albans, VT) were used for Pavlovian conditioning. Each chamber was situated in a sound-attenuating cubicle equipped with a ventilation fan to provide ambient background noise. Each chamber was equipped with a pellet magazine, a retractable lever (counterbalanced on the left or right side of the magazine), and a red house light on the wall opposite the magazine. The magazine contained an infrared sensor to detect magazine entries, and the lever was calibrated to detect lever deflections in response to 10 g of applied weight. Whenever the lever was extended into the chamber, an LED mounted inside the lever mechanism illuminated the slot through which the lever protruded.
For two days prior to the start of training, rats were familiarized with banana-flavored pellets (45 mg; Bioserv; Frenchtown, NJ) in their home cages. Rats were then placed into the test chambers for one pretraining session during which the red house light remained on but the lever was retracted. Twenty-five food pellets were delivered on a variable time (VT) 30-s schedule (i.e., one pellet was delivered on average every 30 s, but varied 0–60 s). Each trial during a test session consisted of presentation of the illuminated lever (conditioned stimulus; CS) into the chamber for 8 s on a VT 90-s schedule (i.e., the lever was presented on average every 90 s, but varied 30–150 s between CS presentations). Retraction of the lever was immediately followed by the response-independent delivery of one food pellet (unconditioned stimulus; US) into the magazine. The beginning of the next inter-trial interval commenced immediately after pellet delivery. Each test session consisted of 25 trials of a CS-US pairing. All rats consumed all pellets that were delivered. Rats were not food deprived at any point during experimentation.
2.3. Single prolonged stress (SPS)
Rats were exposed to the SPS procedure as previously described (Liberzon et al., 1997), and an equal number of control rats were placed in a novel room and left undisturbed for an equivalent time (~3 h). SPS consisted of the serial application of restraint, forced swim, and ether exposure until general anesthesia. Rats were restrained for two hours, followed immediately by a 20-min forced swim in room temperature (20–25°C) water. Forced swim occurred with eight rats at a time in an 18-gal plastic tub, filled two-thirds from the bottom with water. After the forced swim, rats were dried with towels and allowed to recuperate for 15 min on heating pads. Next, rats were exposed to ether (75 mL) in a container within a fume hood until the loss of consciousness. Serial exposure to all three stressors is critical to the effects of SPS, because partial exposure to some stressors (e.g., restraint and forced swim) or substitution of stressors (e.g., isoflurane for ether) abolishes stress-induced behavioral alterations (Knox et al., 2012b). Following SPS, rats were returned to the housing colony and left undisturbed in their home cages for seven days prior to PCA training.
2.4. Catheterization surgery
Rats were prepared with indwelling i.v. catheters (see Supplemental Experimental Procedures).
2.5. Cocaine self-administration
In Experiment 2, cocaine self-administration sessions began one week after surgery. Chambers were equipped with a red house light, two nose-poke ports, and a single-speed syringe pump attached to the door of the sound-attenuating cubicle. Drug-delivery tubing was attached to the syringe pump and connected to rats using a drug-delivery arm mounted above the conditioning chamber. A nose poke into the active port resulted in an i.v. injection of cocaine hydrochloride (0.5 mg/kg/infusion in 25 μL saline delivered over 1.6 s) on a fixed-ratio (FR) 1 schedule. The light inside the active port was illuminated during a 20-s timeout period following infusions, serving as the CS signaling cocaine infusion. Responses into the inactive port had no consequence. To ensure that all rats received an equal number of drug infusions, and therefore the same number of CS-US pairings, rats were initially allowed to take 10 infusions (i.e., session length was determined by the time to self-administer 10 infusions). This infusion criterion (IC) was repeated for three daily sessions, after which the IC was increased to 20 for 3 daily sessions, and then 40 for 5 daily sessions. Rats that failed to reach criterion (n = 4) or maintain catheter patency (n = 3) were removed from further analysis.
After rats achieved an IC of 40 and showed stable self-administration behavior (as determined by similar daily inter-infusion intervals), they underwent extinction training sessions (60 min) for seven days. During these sessions, the rats remained attached to the infusion pump, however, nose pokes in the active port did not result in CS presentation or cocaine infusion. Rats that performed more nose pokes in the active port during the last extinction session than during the last session of cocaine self-administration (n = 3: SPS = 1, Control = 2) were removed from further analysis. Twenty-four hours after the last extinction training session, rats underwent a cue-induced reinstatement test (35 min) during which the rats remained attached to the infusion pump, the active port CS was illuminated for 5 s noncontingently at the start of the session, and subsequent nose pokes into the active port resulted in CS presentation for 5 s without cocaine infusion.
2.6. In vivo microdialysis
Rats were surgically prepared for in vivo microdialysis as previously described (Becker and Rudick, 1999) to measure baseline and evoked DA levels in the NAc. Commercially available microdialysis probes were used for the experiment (MAB 6.14.2; 2 mm, 15 kDa cut-off PES membrane; SciPro, Inc.; Sanborn, NY). All probes were tested for in vitro recovery less than one week before the day of the experiment. During recovery testing, a Ringer’s solution (145 mM NaCl; 2.7 mM KCl; 1 mM MgSO4; 1.2 mM CaCl2; 1.55 mM Na2HPO4 and 0.445 mM NaH2PO4; pH = 7.3) was pumped through the probes at a flow rate of 1.5 μL/min. The probes were immersed in a DA standard solution warmed to 37 ± 1°C. Samples were collected every 5 min, and the DA recovery percentage was determined relative to the standard concentration. Only probes that had greater than 10% recovery were used.
Rats were surgically implanted with a unilateral microdialysis guide cannula (MAB 6.14 G; SciPro, Inc.) aimed at the NAc core-medial shell boundary (AP: +1.7 mm, measured from bregma; ML: ± 1.4 mm; DV: −6.8 mm, measured from the skull surface), and a dummy probe extending 2 mm below the guide was inserted. Rats recovered for one week before the microdialysis experiment. The dummy probe was removed one day before microdialysis, and the microdialysis probe was inserted and secured in place. Rats were placed in the microdialysis chamber (Med Associates, Inc.) with food and water provided ad libitum. Ringer’s solution was perfused through the probe at a constant rate of 0.4 μL/min overnight and 1.5 μL/min on the day of testing using a Harvard Apparatus pump (Instech Laboratories, Inc.; Plymouth Meeting, MA). On the day of testing, samples were collected every 10 min. After the collection of three consistent baseline samples with stable DA concentration, the perfusion fluid was switched to a high-K+ Ringer’s solution with a KCl concentration of 75 mM for 10 min. High-K+ perfusion is an extraphysiological stimulus that reliably releases DA, which is believed to occur primarily through depolarization-induced exocytosis of synaptic vesicles (Arbuthnott et al., 1990a, 1990b). Next, the perfusate was switched back to the original Ringer’s solution, and samples were collected for an additional 30 min.
DA content of the dialysate was determined using high-performance liquid chromatography with electrochemical detection. A C-18 ESA (ESA Biosciences, Inc.; Chelmsford, MA) column (HR-80X3.2; 3 μm particle size, 80 mm length) was used to separate DA in the samples at 27°C by pumping a mobile phase consisting of 75 mM NaH2PO4, 0.2 mM EDTA, 1.4 mM OSA (1-ocatanesulfonic acid sodium salt monohydrate), and 19% methanol (pH = 4.7) at a flow rate of 0.7 mL/min. Potentials of −75 mV and 100 mV were applied to a dual coulometric analytical cell (ESA Model #5014B; ESA Biosciences, Inc.), and the latter potential was used to determine DA content. Current in the analytical cell was detected by a Coulochem II/III detector (ESA Biosciences, Inc.).
2.7. Statistical analysis
PCA behavior was scored using an index that combines the number, latency, and probability of lever presses and magazine entries during CS presentations (Meyer et al., 2012). Briefly, we averaged the response bias (i.e., number of lever presses and magazine entries for a session; [lever presses – magazine entries] / [lever presses + magazine entries]), latency score (i.e., average latency to perform a lever press or magazine entry during a session; [magazine entry latency – lever press latency]/8), and probability difference (i.e., proportion of lever presses or magazine entries; lever press probability – magazine entry probability) for each session. The index scores behavior from +1.0 (absolute sign-tracking) to −1.0 (absolute goal-tracking) with 0 representing no bias. In addition, latency of pellet retrieval was measured as the time elapsed between the retraction of the lever-CS and the first magazine entry during the non-CS period.
SPSS (Version 24; IBM, Inc.) was used for all statistical analysis. Repeated measures were analyzed using a linear mixed model with a covariance structure selected using Akaike’s information criterion (i.e., the lowest criterion value represents the highest quality statistical model using a given covariance structure). Group differences were analyzed using independent samples t-test or two-way analysis of variance (ANOVA) when appropriate. With significant effects or interactions, multiple comparisons were performed using the Sidak correction.
3. Results
3.1. SPS decreases sign-tracking and increases goal-tracking during the acquisition of PCA behavior
In Experiment 1, SPS was administered one week prior to the start of PCA training. During training, rats were presented with a lever-CS for 8 s followed by the response-independent delivery of a food pellet for 25 trials over five daily sessions. SPS decreased PCA index scores over the five training sessions (Supplemental Figure 1; effect of Stress: F(1,57.82) = 9.84, p = 0.003; effect of Session: F(1,177.3) = 9.19, p = 9.02 × 10−7; interaction of Stress × Session: F(1,177.3) = 0.94, p = 0.44), indicating a bias towards goal-tracking. Post-hoc comparisons revealed that differences in PCA index scores between SPS-exposed and control rats presented during Sessions 3–5 (p < 0.05). Averaged PCA index scores from Session 4–5 are routinely used to phenotype rats as sign-trackers (score ≥ 0.5), intermediate-responders (0.5 > score > −0.5), and goal-trackers (score ≤ −0.5). Compared to the control group, the SPS-exposed group had less rats that would normally be classified as sign-trackers and more rats that would be classified as intermediate-responders and goal-trackers (Supplemental Figure 2).
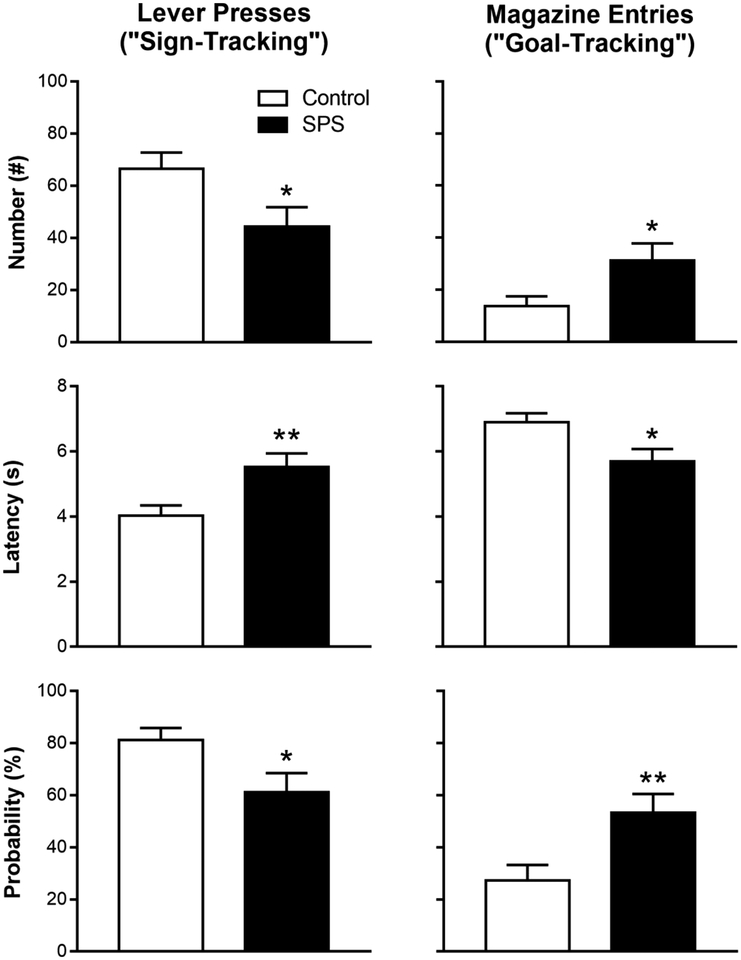
Figure 2 shows that the decrease in PCA index scores in SPS-exposed rats resulted from decreased sign-tracking behavior and increased goal-tracking behavior. SPS decreased the number (effect of Stress: t(1,46) = 2.28, p = 0.027), latency (effect of Stress: t(1,46) = −2.92, p = 0.005), and probability (effect of Stress: t(1,46) = 2.28, p = 0.026) of lever presses and increased the number (effect of Stress: t(1,46) = −2.35, p = 0.023), latency (effect of Stress: t(1,46) = 2.59, p = 0.013), and probability (effect of Stress: t(1,46) = −2.77, p = 0.008) of magazine entries. In contrast to the effects of SPS on behavior while the lever was extended just before food delivery, SPS had no effect on the latency of rats to enter the magazine after the lever retracted, and all rats ate all pellets during every session (data not shown; effect of Stress: F(1,63.58) = 6.73 × 10−5, p = 0.99; effect of Session: F(4,150.3) = 8.21, p = 5.16 × 10−6; interaction of Stress × Session: F(4,150.27) = 1.06, p = 0.38). Moreover, SPS had no effect on magazine entries outside the CS-period (data not shown; effect of Stress: t(1,46) = 0.12, p = 0.91). These latter findings further suggest that SPS did not produce non-specific motor deficits or decrease discrimination between conditioned responding during CS and non-CS periods. Moreover, the food itself maintained its motivational value. Thus, SPS decreased cue-directed (sign-tracking) behavior and shifted behavior toward reward-directed (goal-tracking) behavior.
Figure 2.
The lever press and magazine entry number, latency, and probability was averaged during Session 4 and 5 in single prolonged stress (SPS)-exposed and control rats. Data are presented as mean and S.E.M. * - p < 0.05, ** - p < 0.01.
3.2. SPS does not affect the expression of PCA behavior
In Experiment 2, rats underwent five daily PCA training sessions, SPS (or control treatment), then a single PCA session to test for the expression of PCA behavior. SPS did not affect the expression of PCA behavior (Supplemental Figure 3; interaction of Session × Stress: F(1,40) = 0.44, p = 0.83). In other words, after PCA behavior had been acquired, rats were unaffected by subsequent exposure to SPS.
3.3. SPS does not affect the acquisition or extinction of cocaine self-administration, but it decreases cue-induced reinstatement
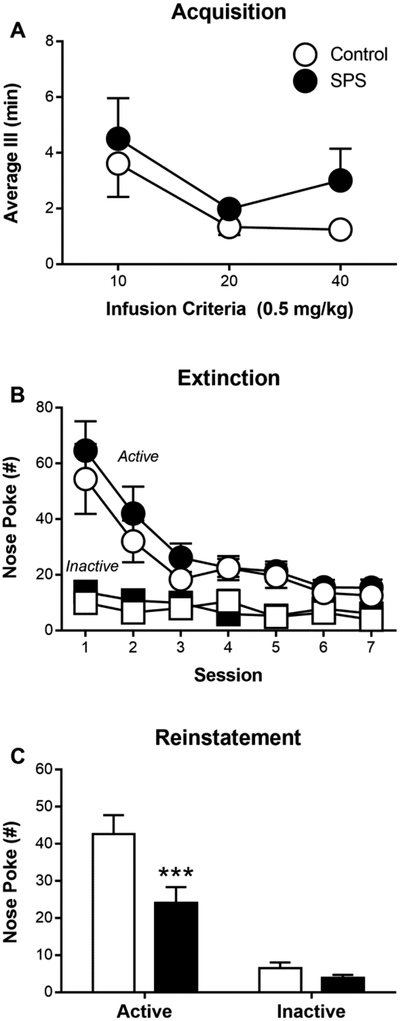
Following the PCA expression test in Experiment 2, rats were implanted with indwelling i.v. catheters, allowed to recover for seven days, and then trained to nose poke for i.v. infusions of cocaine (US). The infusion of cocaine was paired with the illumination of the active port (CS), and each rat received a fixed number of i.v. cocaine infusions. This protocol was adopted to ensure that all rats received the same number of CS-US pairings. Consequently, group differences in the rate of cocaine self-administration could be determined by the average inter-infusion interval. SPS did not affect the average inter-infusion interval across each IC (Figure 3A; effect of Stress: F(1,25.57) = 1.53; p = 0.23; interaction of Criterion × Stress: F(1,53.06) = 0.27; p = 0.76). Moreover, SPS did not affect the number of active or inactive nose pokes (effect of Stress: F(1,43.16) = 0.09; p = 0.77), and both groups successfully discriminated between inactive and active ports (Supplemental Figure 4; effect of Port: F(1,75.95) = 73.76; p = 8.24 × 10−13).
Figure 3.
Single prolonged stress (SPS)-exposed (n = 16) and control (n = 19) rats underwent acquisition, extinction, and reinstatement of cocaine self-administration. (A) Cocaine self-administration involved presentation of a cocaine-CS following an active (but not inactive) nose-poke, and rats received a set number of cocaine infusions: 10 (3 days), 20 (3 days), and 40 (5 days) infusions/session. Because rats only had to nose poke one time in the active nose poke port to receive a cocaine infusion, the average inter-infusion interval (III) provides a more informative description on the acquisition of cocaine self-administration. (B) Extinction training lasted seven sessions (60 min), during which active nose-pokes no longer resulted in cocaine infusions or cocaine-CS presentations. (C) During the cue-induced reinstatement test (35 min), active nose-pokes now resulted in presentation of the cocaine cue. Inactive nose-pokes never resulted in cocaine or cocaine cue during acquisition, extinction, or reinstatement. Data presented as mean and S.E.M. *** - p < 0.001.
After acquisition of cocaine self-administration, rats underwent seven days of extinction training during which active nose pokes did not result in either cocaine-CS presentation (i.e., active port illumination) or cocaine infusion. Figure 3B shows that SPS did not affect responding to the active port (effect of Stress: F(1,35.03) = 0.90; p = 0.35), and both groups extinguished responding to the active port over the course of extinction training (effect of Session: F(1,158.3) = 12.58; p = 1.41 × 10−11). Twenty-four hours after the final extinction training session, rats were tested for cue-induced reinstatement. During this test, active nose pokes resulted in presentation of the cocaine-CS without cocaine infusion. SPS-exposed and control rats differed in their nose-poke responses during the test for cue-induced reinstatement (Figure 3C; interaction of Stress × Port: F(1,66) = 5.27; p = 0.025). Post-hoc comparisons revealed that both SPS-exposed (p < 0.001) and control (p < 0.001) rats performed more active than inactive nose pokes; however, SPS decreased the number of active nose pokes (p < 0.001) while not affecting inactive nose pokes (p > 0.05).
3.4. SPS decreases evoked but not baseline DA levels in the NAc
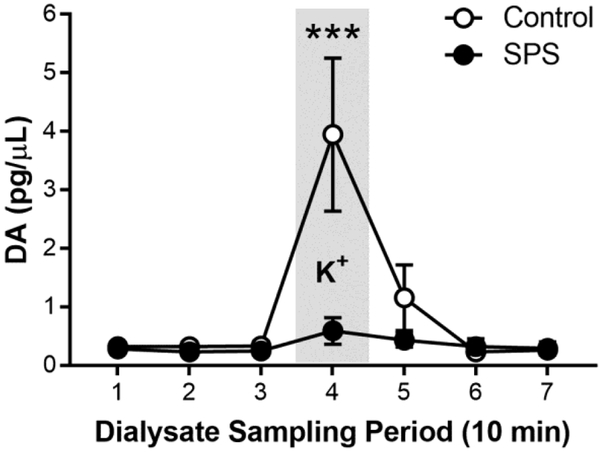
In Experiment 3, a separate cohort of rats were exposed to SPS followed by intracranial implantation of microdialysis probes targeted unilaterally at the NAc (core/medial shell boundary). Following a postsurgical recovery period of seven days (for a total of 14 days after the SPS procedure), DA levels in the NAc of both SPS-exposed and control rats were measured during seven 10-min sampling periods: baseline (Periods 1–3), K+ stimulation (Period 4), and post-stimulation recovery (Periods 5–7). SPS decreased DA release in response to K+ stimulation (Figure 4; Stress × Session; F(5,49.65) = 4.22; p = 0.003). Post-hoc comparisons revealed that SPS did not affect DA levels during baseline (p > 0.05) or post-stimulation recovery (p > 0.05); however, SPS decreased DA during the K+-stimulation sampling period (p < 0.001). In addition, control rats had significantly higher DA release during K+ stimulation compared to baseline (p < 0.001), and DA levels decreased during post-stimulation recovery (p < 0.001). On the other hand, DA release during K+ stimulation in SPS-exposed rats was not significantly different from DA levels during baseline (p > 0.05) or post-stimulation recovery (p > 0.05).
Figure 4.
Two weeks following single prolonged stress (SPS), dopamine (DA) levels in the nucleus accumbens were measured in SPS-exposed (n = 5) and control (n = 6) rats by in vivo microdialysis. Samples were collected over seven 10-minute periods: baseline (Period 1–3), K+-evoked stimulation (75 mM; Period 4), and post-stimulation recovery (Periods 5–7. Data presented as mean and S.E.M. *** - p < 0.001.
4. Discussion
In the present study, a single exposure to prolonged, uncontrollable stress in rats produced a long-lasting reduction specifically in cue-directed behavior. In Experiment 1, SPS decreased cue-directed behavior (sign-tracking) and increased behavior towards the location of reward delivery (goal-tracking) during the acquisition of PCA behavior; however, SPS did not affect the expression of either sign- or goal-tracking behaviors. In Experiment 2, cue-induced reinstatement of cocaine-seeking behavior was decreased without affecting self-administration or extinction. In Experiment 3, SPS decreased DA release within the NAc at a time point comparable to when sign-tracking behavior decreased during the acquisition of PCA behavior. Because the decreased cue-induced reinstatement was observed at an even later time point, our results suggest that SPS-induced reductions in accumbal DA release may endure longer than our measurements in Experiment 3. Given that blocking dopaminergic signaling in the NAc decreases both sign-tracking and cue-induced reinstatement of cocaine-seeking (Saunders et al., 2012, 2013), these results suggest that reduced dopaminergic activity in the NAc could contribute to SPS-induced reductions in cue-directed behavior at both timepoints and possibly beyond.
Interestingly, SPS (1) increased the acquisition of goal-tracking behavior (and decreased the acquisition of sign-tracking behavior) and (2) did not affect the expression of either sign- or goal-tracking behaviors. Regarding the acquisition of PCA behavior, one possibility is that altered dopaminergic tone within the NAc shifted conditioned responding away from reward-distal cues (e.g., the lever) to reward-proximal cues (e.g., the pellet magazine; Simon et al., 2009; Holden and Peoples, 2010). In addition, SPS may not have affected the expression of PCA behavior, because conditioned approach becomes DA-independent after extended training (Clark et al., 2013). Although it is possible that SPS-induced changes in the expression of PCA behavior require a longer incubation period than other behavioral and physiological changes, e.g. 14 days instead of 7 days, the current results are consistent with other previous studies showing that PCA behavior is often much more susceptible to manipulation during acquisition than it is after several days of training (Fitzpatrick et al., 2016).
The fact that SPS decreased sign-tracking behavior and increased goal-tracking behavior during acquisition could have important implications for anhedonia in patients. Many patients with anhedonia enjoy primary rewards, but complain bitterly about not wanting to obtain them (Klein, 1987). The long-standing definition of anhedonia as the inability to experience pleasure has recently been challenged as accumulating evidence suggests that pursuing rewards involve aspects of “wanting”, “liking”, and learning (Berridge and Robinson, 2003; Thomsen, 2013). Interest has arisen in the heterogeneity of anhedonia symptoms, leading to sub-classifications such as consummatory anhedonia (i.e., deficits in hedonic responses to reward) and motivational anhedonia (i.e., diminished motivation to pursue reward; Treadway and Zald, 2011). In the present study, it appears that SPS impairs motivational but not consummatory aspects of reward-seeking behaviors. One limitation of the current study, however, is that pleasure and “liking” cannot be directly measured using PCA and cocaine-self administration procedures. Future studies can explore “liking” by investigating, for instance, how SPS affects behavioral responses to oral sucrose administration (Peciña and Berridge, 2000).
One striking feature of the SPS-induced decreases in cue-directed behavior is the persistence of the effects over time. The reductions in sign-tracking and evoked dopaminergic activity within the NAc were observed approximately two weeks after SPS, and the decrease in reinstatement of cocaine-seeking behavior was observed five weeks after SPS. Although studies of neurochemical changes after a single stressor tend to focus on acute responses, our findings agree with previous studies reporting long-term effects of “chronic” exposure to repeated stressors over multiple days (Gambarana et al., 1999; Mangiavacchi et al., 2001; Shimamoto et al., 2011). In support of this, neither acute restraint stress (Puglissi-Allegra et al., 1991) or ether-exposure (Schwarting and Huston, 1987) alter DA concentrations in the ventral striatum. On the other hand, although acute swim stress increases DA concentrations in the ventral striatum 150 min after exposure, DA concentrations return to baseline levels at 210 min (Yadid et al., 2001). Therefore, it seems that the acute, serial application of all three stressors is critical to producing the observed chronic-like state of reduced dopaminergic activity in the ventral striatum.
The effects of SPS on cue-directed behaviors and DA release within the NAc complement a long line of research demonstrating that reduced DA transmission in the NAc is related to motivational deficits (Berridge and Robinson, 1998; Ikemoto and Panksepp, 1999; Roberts et al., 1977; Salamone and Correa, 2002). The effects of stress on DA within the NAc are dependent on the nature and timing of the stress: brief or controllable stressors increase NAc DA release, while prolonged, uncontrollable stressors typically decrease NAc DA release (Cabib and Puglisi-Allegra, 2012). Our finding that SPS falls into the latter category agrees with other reports that SPS reduces behavioral sensitization to methamphetamine, cocaine-conditioned place preference and sucrose preference, and striatal dopamine content (Eagle and Perrine, 2013; Enman et al., 2015), though there may be heterogeneity in individual responses to SPS (Toledano et al., 2013). However, it should be noted that the temporal and spatial resolution of in vivo microdialysis does not permit isolation of DA release on sub-second timescales. In future studies, fast-scan cyclic voltammetry can be used to more precisely measure DA release in the NAc, for instance, surrounding CS and US presentations during PCA training.
Similar to our results, patients experiencing anhedonia have reduced activity in the ventral striatum during reward conditioning (Kumar et al., 2008). Even after recovery from neuropsychiatric disorders characterized by anhedonia, many patients still have reduced activity in the ventral striatum in response to reward-related sensory cues, despite subjectively rating the pleasantness, intensity, and desirability of the rewarding stimulus the same as control subjects (McCabe et al., 2009). Clinically, increasing neural activity in the NAc through deep brain stimulation (Bewernick et al., 2010) or administering DA agonists (Lemke et al., 2006; Reichmann et al., 2006) have been shown to have pro-motivational effects and reduce anhedonia in patients. Our results provide insight into behavioral and neurobiological mechanisms explaining the utility of these treatments, and suggest that future investigations of anhedonia should focus on restoring cue-directed incentive motivation in patients suffering from PTSD and other neuropsychiatric disorders.
5. Conclusion
In the present study, we demonstrated that SPS decreases two forms of cue-directed behavior: the acquisition of sign-tracking during a PCA procedure and cue-induced reinstatement of cocaine-seeking. Importantly, reward-directed behaviors, such as the consumption of food pellets or self-administration of cocaine were relatively unaffected by exposure to SPS. In addition, we demonstrated that SPS decreases DA release in the NAc, which may underlie the observed reductions in sign-tracking and cue-induced reinstatement, especially given that both behaviors are dependent on dopaminergic activity in the NAc. These findings have important implications for classifying and treating anhedonia observed in PTSD and other neuropsychiatric disorders by suggesting uncontrollable stress impairs cue-directed motivational, but not consummatory, aspects of reward-seeking behavior.
Supplementary Material
Research Highlights.
Single prolonged stress (SPS) decreases the acquisition of sign-tracking behavior
SPS increases the acquisition of goal-tracking behavior
SPS attenuates cue-induced reinstatement of cocaine-seeking behavior
SPS blocks K+-evoked dopamine release in the nucleus accumbens
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arbuthnott GW, Fairbrother IS, Butcher SP, 1990a. Brain microdialysis studies on the control of dopamine release and metabolism in vivo. J. Neurosci. Methods 34(1–3), 73–81. [DOI] [PubMed] [Google Scholar]
- Arbuthnott GW, Fairbrother IS, Butcher SP, 1990b. Dopamine release and metabolism in the rat striatum: an analysis by ‘in vivo’ brain microdialysis. Pharmacol. Ther 48(3), 281–93. [DOI] [PubMed] [Google Scholar]
- Ariely D, Norton MI, 2008. How actions create--not just reveal--preferences. Trends Cogn. Sci 12(1), 13–6. [DOI] [PubMed] [Google Scholar]
- Becker JB, Rudick CN, 1999. Rapid effects of estrogen or progesterone on the amphetamine-induced increase in striatal dopamine are enhanced by estrogen priming: a microdialysis study. Pharmacol. Biochem. Behav 64(1), 53–7. [DOI] [PubMed] [Google Scholar]
- Bekris S, Antoniou K, Daskas S, Papadopoulou-Daifoti Z, 2005. Behavioural and neurochemical effects induced by chronic mild stress applied to two different rat strains. Behav. Brain Res 161(1), 45–59. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, 2003. Parsing reward. Trends Neurosci 26(9): 507–13. [DOI] [PubMed] [Google Scholar]
- Berridge KC, 2004. Motivation concepts in behavioral neuroscience. Physiol. Behav 81(2), 179–209. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, 1998. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res. Brain Res. Rev 28(3), 309–69. [DOI] [PubMed] [Google Scholar]
- Bewernick BH, Hurlemann R, Matusch A, Kayser S, Grubert C, Hadrysiewicz B, Axmacher N, Lemke M, Cooper-Mahkorn D, Cohen MX, Brockmann H, Lenartz D, Sturm V, Schlaepfer TE, 2010. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol. Psychiatry 67(2), 110–6. [DOI] [PubMed] [Google Scholar]
- Bindra D, 1974. A motivational view of learning, performance, and behavior modification. Psychol. Rev 81(3), 199–213. [DOI] [PubMed] [Google Scholar]
- Brehm JW, 1956. Postdecision changes in the desirability of alternatives. J. Abnorm. Psychol 52(3), 384–9. [DOI] [PubMed] [Google Scholar]
- Cabib S, Puglisi-Allegra S, 2012. The mesoaccumbens dopamine in coping with stress. Neurosci. Biobehav. Rev 36(1), 79–89. [DOI] [PubMed] [Google Scholar]
- Clark JJ, Collins AL, Sanford CA, Phillips PE, 2013. Dopamine encoding of Pavlovian incentive stimuli diminishes with extended training. J. Neurosci 33(8), 3526–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle AL, Perrine SA, 2013. Methamphetamine-induced behavioral sensitization in a rodent model of posttraumatic stress disorder. Drug Alcohol Depend 131(1–2), 36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enman NM, Arthur K, Ward SJ, Perrine SA, Unterwald EM, 2015. Anhedonia, reduced cocaine reward, and dopamine dysfunction in a rat model of posttraumatic stress disorder. Biol. Psychiatry 78(12), 871–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick CJ, Gopalakrishnan S, Cogan ES, Yager LM, Meyer PJ, Lovic V, Saunders BT, Parker CC, Gonzales NM, Aryee E, Flagel SB, Palmer AA, Robinson TE, Morrow JD, 2013. Variation in the form of Pavlovian conditioned approach behavior among outbred male Sprague-Dawley rats from different vendors and colonies: sign-tracking vs. goal-tracking. PloS One 8(10), e75042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick CJ, Creeden JF, Perrine SA, Morrow JD, 2016. Lesions of the ventral hippocampus attenuate the acquisition but not expression of sign-tracking behavior in rats. Hippocampus 26(11), 1424–34. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Akil H, Robinson TE, 2009. Individual differences in the attribution of incentive salience to reward-related cues: implications for addiction. Neuropharmacology 56(Suppl. 1), 139–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, Akers CA, Clinton SM, Phillips PE, Akil H, 2011. A selective role for dopamine in stimulus-reward learning. Nature 469(7328), 53–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambarana C, Masi F, Tagliamonte A, Scheggi S, Ghiglieri O, De Montis MG, 1999. A chronic stress that impairs reactivity in rats also decreases dopaminergic transmission in the nucleus accumbens: a microdialysis study. J. Neurochem 72(5), 2039–46. [DOI] [PubMed] [Google Scholar]
- Gronli J, Murison R, Fiske E, Bjorvatn B, Sorensen E, Portas CM, Ursin R, 2005. Effects of chronic mild stress on sexual behavior, locomotor activity and consumption of sucrose and saccharine solutions. Physiol. Behav 84(4), 571–7. [DOI] [PubMed] [Google Scholar]
- Holden JM, Peoples LL, 2010. Effects of acute amphetamine exposure on two kinds of Pavlovian conditioned approach behavior. Behav. Brain Res 208(1), 270–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J, 1999. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res. Brain Res. Rev 31(1), 6–41. [DOI] [PubMed] [Google Scholar]
- Klein DN, 1987. Depression and anhedonia, in: Clark DC, Fawcett J (Eds.), Anhedonia and Affect Deficit States. PMA Publishing Corporation, New York. [Google Scholar]
- Knox D, George SA, Fitzpatrick CJ, Rabinak CA, Maren S, Liberzon I, 2012a. Single prolonged stress disrupts retention of extinguished fear in rats. Learn. Mem 19(2), 43–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox D, Nault T, Henderson C, Liberzon I, 2012b. Glucocorticoid receptors and extinction retention deficits in the single prolonged stress model. Neuroscience 223, 163–73. [DOI] [PubMed] [Google Scholar]
- Kring AM, Moran EK, 2008. Emotional response deficits in schizophrenia: insights from affective science. Schizophr. Bull 34(5), 819–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Waiter G, Ahearn T, Milders M, Reid I, Steele JD, 2008. Abnormal temporal difference reward-learning signals in major depression. Brain 131(Pt. 8), 2084–93. [DOI] [PubMed] [Google Scholar]
- Lemke MR, Brecht HM, Koester J, Reichmann H, 2006. Effects of the dopamine agonist pramipexole on depression, anhedonia and motor functioning in Parkinson’s disease. J. Neurol. Sci 248(1–2), 266–70. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Krstov M, Young EA, 1997. Stress-restress: effects on ACTH and fast feedback. Psychoneuroendocrinology 22(6), 443–53. [DOI] [PubMed] [Google Scholar]
- Mangiavacchi S, Masi F, Scheggi S, Leggio B, De Montis MG, Gambarana C, 2001. Long-term behavioral and neurochemical effects of chronic stress exposure in rats. J. Neurochem 79(6), 1113–21. [DOI] [PubMed] [Google Scholar]
- McCabe C, Cowen PJ, Harmer CJ, 2009. Neural representation of reward in recovered depressed patients. Psychopharmacology (Berl.) 205(4), 667–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer PJ, Lovic V, Saunders BT, Yager LM, Flagel SB, Morrow JD, Robinson TE, 2012. Quantifying individual variation in the propensity to attribute incentive salience to reward cues. PloS One 7(6), e38987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau JL, Jenck F, Martin JR, Mortas P, Haefely WE, 1992. Antidepressant treatment prevents chronic unpredictable mild stress-induced anhedonia as assessed by ventral tegmentum self-stimulation behavior in rats. Eur. Neuropsychopharmacol 2(1), 43–9. [DOI] [PubMed] [Google Scholar]
- Myin-Germeys I, Delespaul PA, deVries MW, 2000. Schizophrenia patients are more emotionally active than is assumed based on their behavior. Schizophr. Bull 26(4), 847–54. [DOI] [PubMed] [Google Scholar]
- Papp M, Willner P, Muscat R, 1991. An animal model of anhedonia: attenuation of sucrose consumption and place preference conditioning by chronic unpredictable mild stress. Psychopharmacology (Berl.) 104(2), 255–9. [DOI] [PubMed] [Google Scholar]
- Pascucci T, Ventura R, Latagliata EC, Cabib S, Puglisi-Allegra S, 2007. The medial prefrontal cortex determines the accumbens dopamine response to stress through the opposing influences of norepinephrine and dopamine. Cereb. Cortex 17(12), 2796–804. [DOI] [PubMed] [Google Scholar]
- Peciña S, Berridge KC, 2000. Opioid sites in nucleus accumbens shell mediates eating and hedonic “liking” for food: map based on microinjection Fos plumes. Brain Res 863(1–2): 71–86. [DOI] [PubMed] [Google Scholar]
- Puglisi-Allegra S, Imperato A, Angelucci L, Cabib S, 1991. Acute stress induces time-dependent responses in dopamine mesolimbic system. Brain Res 554(1–2), 217–22. [DOI] [PubMed] [Google Scholar]
- Reichmann H, Odin P, Brecht HM, Koster J, Kraus PH, 2006. Changing dopamine agonist treatment in Parkinson’s disease: experiences with switching to pramipexole. J. Neural Transm Suppl. 71, 17–25. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Corcoran ME, Fibiger HC, 1977. On the role of ascending catecholaminergic systems in intravenous self-administration of cocaine. Pharmacol. Biochem. Behav 6(6), 615–20. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Flagel SB, 2009. Dissociating the predictive and incentive motivational properties of reward-related cues through the study of individual differences. Biol. Psychiatry 65(10), 869–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M, 2002. Motivational views of reinforcement: implications for understanding the behavioral functions of nucleus accumbens dopamine. Behav. Brain Res 137(1–2), 3–25. [DOI] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE, 2010. A cocaine cue acts as an incentive stimulus in some but not others: implications for addiction. Biol. Psychiatry 67(8), 730–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE, 2012. The role of dopamine in the accumbens core in the expression of Pavlovian-conditioned responses. Eur. J. Neurosci 36(4), 2521–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Yager LM, Robinson TE, 2013. Cue-evoked cocaine “craving”: role of dopamine in the accumbens core. J. Neurosci 33(35), 13989–4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarting R, Huston JP, 1987. Short-term effects of ether, equithesin, and droperidol/fentanyl on cathecholamine and indolamine metabolism in the brain of the rat. Neuropharmacology. 26(5), 457–61. [DOI] [PubMed] [Google Scholar]
- Serova LI, Laukova M, Alaluf LG, Sabban EL, 2013a. Intranasal infusion of melanocortin receptor four (MC4R) antagonist to rats ameliorates development of depression and anxiety related symptoms induced by single prolonged stress. Behav. Brain Res 250, 139–47. [DOI] [PubMed] [Google Scholar]
- Serova LI, Tillinger A, Alaluf LG, Laukova M, Keegan K, Sabban EL, 2013b. Single intranasal neuropeptide Y infusion attenuates development of PTSD-like symptoms to traumatic stress in rats. Neuroscience 236, 298–312. [DOI] [PubMed] [Google Scholar]
- Shimamoto A, Debold JF, Holly EN, Miczek KA, 2011. Blunted accumbal dopamine response to cocaine following chronic social stress in female rats: exploring a link between depression and drug abuse. Psychopharmacology (Berl.) 218(1), 271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, Mendez IA, and Setlow B, 2009. Effects of prior amphetamine exposure on approach strategy in appetitive Pavlovian conditioning in rats. Psychopharmacology (Berl.) 202(4): 699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Gold JM, 2012. A new perspective on anhedonia in schizophrenia. Am. J. Psychiatry 169(4), 364–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SF, Kang J, Brege IS, Tso IF, Hosanagar A, Johnson TD, 2012. Meta-analysis of functional neuroimaging studies of emotion perception and experience in schizophrenia. Biol. Psychiatry 71(2), 136–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen KR, 2015. Measuring anhedonia: impaired ability to pursue, experience, and learn about reward. Front. Psychol 6, 1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledano D, Tassin JP, Gisquet-Verrier P, 2013. Traumatic stress in rats induces noradrenergic-dependent long-term behavioral sensitization: role of individual differences and similarities with dependence on drugs of abuse. Psychopharmacology (Berl.) 230(3), 465–76. [DOI] [PubMed] [Google Scholar]
- Treadway MT, Zald DH, 2011. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci. Biobehav. Rev 35(3), 537–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Zald DH, 2013. Parsing anhedonia: translational models of reward-processing deficits in psychopathology. Curr. Dir. Psychol. Sci 22(3), 244–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Naragon-Gainey K, 2010. On the specificity of positive emotional dysfunction in psychopathology: evidence from the mood and anxiety disorders and schizophrenia/schizotypy. Clin. Psychol. Rev 30(7), 839–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenze SJ, Gunthert KC, German RE, 2012. Biases in affective forecasting and recall in individuals with depression and anxiety symptoms. Pers. Soc. Psychol. Bull 38(7), 895–906. [DOI] [PubMed] [Google Scholar]
- Wilson TD, Gilbert DT, 2005. Affective forecasting: knowing what to want. Curr. Dir. Psychol. Sci 14(3), 131–4. [Google Scholar]
- Wilson TD, Meyers J, Gilbert DT, 2003. “How happy was I, anyway?” A retrospective impact bias. Soc. Cognition 21(6), 421–46. [Google Scholar]
- Yadid G, Overstreet DH, Zangen A, 2001. Limbic dopaminergic adaptation to a stressful stimulus in a rat model of depression. Brain Res 896(1–2), 43–7. [DOI] [PubMed] [Google Scholar]
- Zacharko RM, Bowers WJ, Kokkinidis L, Anisman H, 1983. Region-specific reductions of intracranial self-stimulation after uncontrollable stress: possible effects on reward processes. Behav. Brain Res 9(2), 129–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.