Abstract
Dynorphin (DYN), and its receptor, the kappa opioid receptor (KOR) are involved in drug seeking and relapse but the mechanisms are poorly understood. One hypothesis is that DYN/KOR activation promotes drug seeking through increased impulsivity, because many stimuli that induce DYN release increase impulsivity. Here, we systematically compare the effects of drugs that activate DYN/KOR on performance on the 5-choice serial reaction time task (5-CSRTT), a test of sustained attention and impulsivity. In Experiment 1, we determined the effects of U50,488 (0, 2.5, 5 mg/kg), yohimbine (0, 1.25, 5 mg/kg), and nicotine (0, 0.15, 0.3 mg/kg) on 5-CSRTT performance. In Experiment 2, we determined the effects of alcohol (0, 0.5, 1.0, 1.5 g/kg) on 5-CSRTT performance before and after voluntary, intermittent alcohol exposure. In Experiment 3, we determined the potential role of KOR in the pro-impulsive effects of yohimbine (1.25 mg/kg) and nicotine (0.3 mg/kg) by the prior administration of the KOR antagonist nor-BNI (10 mg/kg). Premature responding, the primary measure of impulsivity, was reduced by U50,488 and alcohol, but these drugs had a general suppressive effect. Yohimbine and nicotine increased premature responding. Yohimbine-, but not nicotine-induced increases in premature responding were blocked by nor-BNI, suggesting that impulsivity induced by yohimbine is KOR dependent. This may suggests a potential role for KOR-mediated increases in impulsivity in yohimbine-induced reinstatement.
Keywords: Impulsivity, Alcohol, Yohimbine, Nicotine, Stress, Kappa opioid
1. Introduction
The endogenous opioid dynorphin (DYN), and its receptor, the kappa opioid receptor (KOR) play important roles in stress-related behaviors and are also implicated in drug seeking and relapse [1, 2]. Stimulation of KOR, such as with the selective agonist U50,488, produce aversive and dysphoric effects [3–5] and also induces relapse to drug seeking [6–9]. In contrast, KOR antagonists have anti-stress effects, including blockade of stress-induced reinstatement of drug seeking [7, 8, 10–12] and withdrawal-induced alcohol intake and anxiety [13–15].
Treatments that induce reinstatement, including priming doses of drugs or exposure to stress increase DYN [1] and are also associated with increased impulsivity [16, 17]. Impulsivity may be a predisposing factor in acquisition of drug self-administration has also been linked to reinstatement [18–21]. The pharmacological stressor yohimbine reinstates drug-seeking and also increases impulsivity in the 5-choice serial reaction time task (5-CSRTT) [22, 23]. We showed that the KOR antagonist nor-binaltorphimine (nor-BNI) blocks yohimbine-induced reinstatement of alcohol and nicotine seeking [7, 8]. This leads to the hypothesis that KOR antagonism may block yohimbine-induced reinstatement by reducing its pro-impulsive effects.
Although the effects of KOR agonists on impulsivity have been examined, the effects reported are mixed, and may depend on the task employed. In the 5-CSRTT, the KOR agonist U69,593 did not significantly affect premature response rates but increased omissions and response latencies, perhaps reflecting depressive effects of KOR stimulation [24, 25]. Consistent with this, the KOR agonist U50,488 also increased omissions and response latencies, but premature responding data were not reported [25]. In other paradigms, U50,488 increased impulsive-like responding in the stop signal reaction time task [26] and differential reinforcement of low rate responding (DRL) procedures [27] but was without effect in the delay discounting procedure [26].
Here, to help further understand the role of KOR in impulsivity, we will compare the effects of 4 drugs that affect the DYN/KOR systems, including the KOR agonist U50,488, the alpha-2 antagonist yohimbine, nicotine and alcohol on 5-CSRTT responding. U50,488 is a selective KOR agonist [28], while yohimbine, nicotine and alcohol activate DYN-containing brain pathways [29–31]. All of these drugs induce reinstatement, and we showed that for yohimbine, its effects on reinstatement are KOR-dependent [7, 8]. Our determination of the effects of these drugs on impulsivity may also help to understand the role of impulsivity as a potential mediator of their effects on reinstatement, as well as, more generally, the role of DYN/KOR in impulsivity.
Consistent with previous data, we found that premature responding was increased by yohimbine and nicotine [23, 32]. Premature responding was reduced by U50,488 and alcohol, but this was likely due to their general suppressive effects [24, 25, 33]. We then determined the KOR dependency of yohimbine-and nicotine-induced increases in impulsivity by assessing whether their pro-impulsive effects are blocked by the selective KOR antagonist nor-BNI.
2. Materials and methods
2.1. Subjects
Twenty-eight male Long-Evans rats weighing 225–250 g at the start of the experiments were obtained from Charles River (Kingston, NY). They were housed individually under a 12:12 reversed light-dark cycle (lights on from 7:00 p.m. to 7:00 a.m.; 21±1°C). They were fed 15 g of rat chow (4 pellets), 2 h after the daily experimental sessions in the home cage; water was freely available. Experimental procedures followed the NIH “Principles of laboratory animal care” (Eighth edition, 2011) and were approved by the animal care committee of the CAMH. One rat was excluded because it could not reach the performance criteria on the 5-CSRTT.
2.2. Drugs
U50,488 (Tocris), yohimbine (Sigma Aldrich) and nor-BNI HCl, (NIDA Drug Supply Program) were dissolved in sterile distilled water. Nicotine hydrogen tartrate (MP Biomedicals) was dissolved in saline and adjusted to pH 7, and solutions of alcohol were prepared by diluting 95% alcohol (Commercial Alcohols) in saline (for injection) or tap water (for drinking). Drug doses (free-base) and pre-treatment times were based on published studies [7, 8, 23, 24, 32–37].
2.3. Five-choice serial reaction time task
2.3.1. Apparatus:
Training and testing was conducted in 8 operant conditioning boxes (Med Associates), measuring 33 cm x 31 cm x 29 cm, with stainless steel grid floors. The rear wall of each chamber contained an array of five 2.5 cm square apertures located above the floor. Infrared photo detectors were located at the entrance to each aperture and a yellow LED stimulus light was centered behind each. A reinforcer magazine was centered on the front wall, containing a photo detector at the entrance, and a magazine light mounted on its roof. A pellet dispenser delivered reinforcers (45 mg sucrose, Bioserv) to the magazine. The boxes were controlled by an IBM-compatible computer running Med-PC for Windows.
2.3.2. Training:
Initially, three 30 min training sessions were given in which rats were placed in the test boxes with the magazine light illuminated and pellets dispensed on a random time 30 s schedule. Subsequently, animals were placed in the chamber with one of the 5 response apertures illuminated. A response in that aperture extinguished that light, illuminated the magazine light and resulted in a pellet reward. Sessions lasted until 60 trials had been completed, or 30 min elapsed. When each animal had successfully acquired this task (approximately 5 d), training on the five-choice serial reaction time task began, requiring the rat to discriminate brief visual stimuli presented randomly in one of five spatial locations [38].
The start of the session began with illumination of the houselight and the magazine light for 2.5 s and pellet delivery. A nose poke in the magazine began the first trial. After a fixed inter-trial interval (ITI), one of the 5 light stimuli was illuminated for a brief period; a response in that hole while the light was on, or during a 5 s limited hold period, resulted in delivery of a pellet, and illumination of the magazine light for 2.5 s. A nose-poke into the magazine to collect the reinforcer initiated the ITI to the next trial. Incorrect responses in any of the other 4 holes were not reinforced but were followed by a 5 s time out period of darkness; failures to respond within the limited hold period (omissions) were also followed by a 5 s time out. At the end of the time out periods the magazine light was turned on and a nose poke in the magazine began the next trial. Responses made during the ITI were recorded as premature responses, and were followed by a time out. Magazine responses at the end of these time out periods restarted the same trial. Training began with a stimulus duration of 60 s and a limited hold of 5 s. These parameters were altered during training, dependent upon performance, until the final parameters were reached, which were a 1s stimulus duration and a 5s limited hold [39, 40]. The length of the timeout was always 5 s; the ITI was also held constant at 5 s. Generally, sessions lasted for 30 min, or until rats had completed 100 trials, with each stimulus presented 20 times in a random order. Training took approximately 60 days until rats responded consistently with an accuracy of >80% and <20% omissions.
2.4. Experiment 1: Effects of U50,488, yohimbine and nicotine on the 5-choice serial reaction time task
2.4.1. Experiment 1A, U50,488:
Nine rats were trained on the 5-CSRTT as described above. They received 5 daily drug-free 5-CSRTT sessions preceded by vehicle injections (water, S.C.). They then received, in counterbalanced order, injections of water vehicle or U50,488 (2.5 and 5 mg/kg S.C.) 45 min prior to the test sessions. In this and the rest of the experiments, rats were given 2–3 drug-free training days between each dose.
2.4.2. Experiment 1B, Yohimbine:
Rats then received 5 daily drug-free 5-CSRTT sessions preceded by vehicle injections (water, I.P.). They then received injections of water or yohimbine (1.25 and 2.5 mg/kg I.P.) in counterbalanced order 30 min prior to the test sessions.
2.4.3. Experiment 1C, Nicotine:
Rats then received 5 daily 5-CSRTT sessions preceded by vehicle injections (saline, S.C). Two h after each of the last 4 sessions, they received injections of nicotine (0.4 mg/kg S.C) in their home cages in order to make them tolerant to its locomotor depressant effects. They received injections of saline or nicotine (0.15 and 0.3 mg/kg S.C.) in counterbalanced order 15 min prior to the test sessions.
2.5. Experiment 2: Effects of alcohol on the 5-choice serial reaction time task
We first determined the dose-response of acute injections of alcohol on performance in the 5-CSRTT in alcohol naïve rats. Then, since alcohol’s effects change with repeated exposure, a second alcohol dose response was conducted in the same rats after 2 weeks of intermittent alcohol exposure. Ten rats were trained on the 5-CSRTT as described above, and after stable performance received 5 daily 5-CSRTT sessions preceded by vehicle injections (saline, I.P.). Rats then received, in counterbalanced order, injections of saline or alcohol (0.5, 1.0 and 1.5 g/kg I.P.) 15 min prior to the test sessions. Rats were then subject to intermittent access to 20% (v/v) alcohol in bottles in the home cage for 24 h every second day for 2 weeks. The rats continued to receive 5-CSRTT training every second day prior to alcohol exposure during the intermittent access period. Rats then received the second alcohol dose response (saline, 0.5 and 1.0 g/kg alcohol I.P.) on 5-CSRTT performance. The high dose of 1.5 g/kg was excluded from the post-intermittent access testing due to its strong suppressive effects on 5-CSRTT performance.
2.6. Experiment 3: Effects of nor-BNI on yohimbine- and nicotine-induced increases in impulsivity in the 5-choice serial reaction time task
The rats from Exp. 1 and 2 were assigned to 3 groups. Seven rats from Exp. 2 were assigned to the group that would be tested with yohimbine and nor-BNI and 5 from Exp.1 were assigned to the group to be tested with nicotine and nor-BNI. The remaining 5 rats (2 from Exp. 1 and 3 from Exp. 2) were assigned to the group to be tested with vehicle and nor-BNI. Rats received 5 days of 5-CSRTT sessions preceded by vehicle injections. On the first test day, rats received an injection of the nor-BNI vehicle prior to injections of vehicle, yohimbine or nicotine given at the same pretreatment times described above, and a 5-CSRTT test. Rats then received two daily drug-free 5-CSRTT sessions. The day after, all rats were injected with nor-BNI (10 mg/kg I.P.) and then vehicle, yohimbine or nicotine followed by a 5-CSRTT test. Nor-BNI was injected 1 h prior to the test sessions.
2.7. Presentation and Statistical Analysis
Measures of performance on the 5-CSRTT collected were trials completed, % accuracy, number of omissions (failure to respond during limited hold period), premature responses (responding before presentation of target stimulus), perseverative responses (responses made after a correct response, prior to reward collection), latency to make a correct response and latency to collect the reward. % accuracy was calculated as correct responses/(correct + incorrect responses) x 100. Data from Exp. 1 were analyzed with repeated measures one-way ANOVA with the repeated factor of Drug dose (U50,488: 0, 2.5, 5; Yohimbine: 0, 1.25, 2.5; Nicotine: 0, 0.15, 0.3 mg/kg). The data prior to and after intermittent access to alcohol in Exp. 2 were analyzed with repeated measures one-way ANOVA with the factor of Alcohol dose (Prior to intermittent access: 0, 0.5, 1.0, 1.5 g/kg; After intermittent access: 0, 0.5, 1.0 g/kg). Data from Exp. 3 were analyzed with mixed two-way ANOVA with the between factor of drug dose (vehicle, 1.25 mg/kg yohimbine, 0.3 mg/kg nicotine) and the repeated factor of nor-BNI dose (vehicle, 10 mg/kg nor-BNI). Significant effects (p<0.05) from the ANOVAs were followed with Fisher’s PLSD post-hoc tests.
3. Results
3.1. Experiment 1: Effects of U50,488, yohimbine and nicotine on the 5-choice serial reaction time task
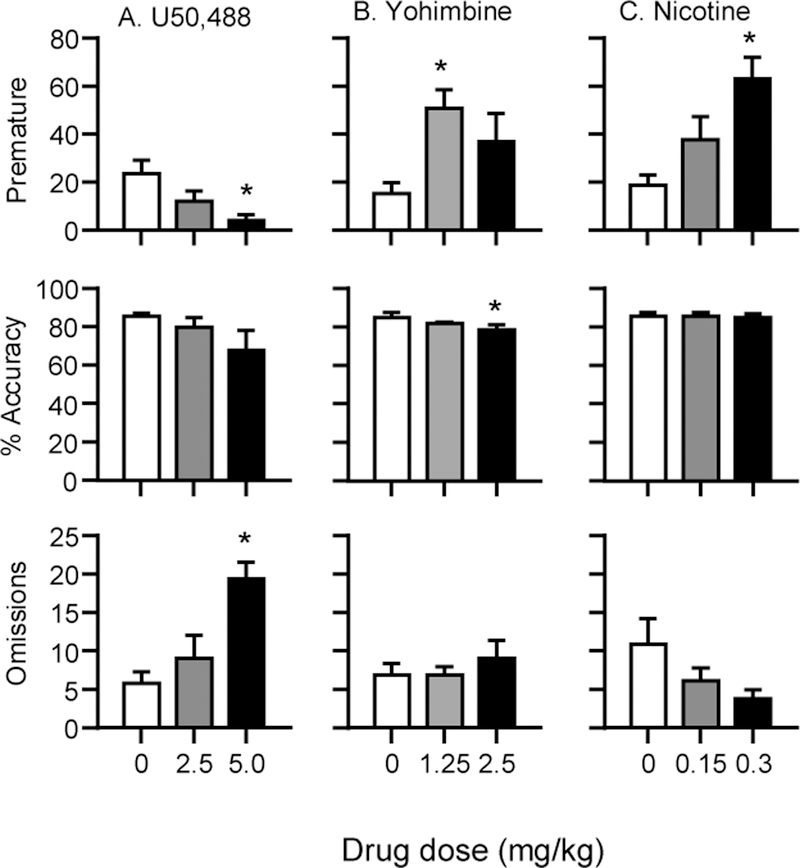
Figure 1 shows the effects of U50,488 (A), yohimbine (B) and nicotine (C) on the mean±SEM number of premature responses (top panel), % accuracy (middle panel) and omissions (lower panel).
Figure 1. Dose-response of U50,488, yohimbine and nicotine on premature responses, % accuracy and omissions in the 5-CSRTT in Exp. 1.
(A) U50,488 dose-response. (B) Yohimbine dose-response (C) Nicotine dose-response. Values are mean±SEM premature responses, % accuracy and omissions. * Different from vehicle (0 dose), p<0.05, U50,488: n=9, yohimbine: n=8, nicotine: n=9.
3.1.1. U50,488:
There was a significant main effect of U50,488 dose (F(2,16)=8.2, p=0.004) on premature responses. Post hoc tests showed that, compared to vehicle, 5 mg/kg U50,488 significantly reduced premature responses (p<0.05)(1A). U50,488 significantly increased omissions (F(2,16)=11.16, p<0.001) which were significantly higher at the 5 mg/kg dose compared to vehicle(p<0.05). U50,488 significantly decreased the numbers of trials completed (F(2,16)=10.0, p=0.002); post hoc tests showed fewer trials were completed at the 5 mg/kg compared to vehicle (p<0.05) (Table 1A). U50,488 did not significantly affect % accuracy (F(2,16)=2.19, p=0.14), perseverative responding (F(2,16)=0.84, p=0.45), latency to make a correct response (F(2,16)=1.89, p=0.18), or latency to collect the rewards (F(2,16)=0.11, p=0.89).
Table 1. Dose-response of U50,488, yohimbine and nicotine on additional parameters in the 5-CSRTT in Exp. 1.
(A) U50,488 dose-response. (B) Yohimbine dose-response (C) Nicotine dose-response. Values are mean±SEM trials completed, perseverative responses and the latencies for correct responses and to obtain a reward.
| A. U50,488 dose (mg/kg) |
B. Yohimbine dose (mg/kg) |
C. Nicotine dose (mgAg) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 2.5 | 5.0 | 0 | 1.25 | 2.5 | 0 | 0.15 | 0.3 | |
| Trials completed | 91.3 ± 6.9 | 64.2 ± 11.7 | 35.2 ± 6.1* | 96.1 ± 2.6 | 94.1 ±2.4 | 85.9 ± 9.4 | 92.8 ± 4.7 | 95.1 ± 3.6 | 91.6 ± 3.7 |
| Perseverative | 22.8 ± 5.1 | 20.4 ± 4.7 | 17.8 ±5.3 | 25.0 ± 6.6 | 20.3 ± 6.4 | 19.3 ± 6.8 | 24.3 ± 8.6 | 25.4 ± 9.3 | 25.9 ± 9.9 |
| Correct latency | 0.61 ± 0.04 | 0.67 ± 0.07 | 0.96 ±0.21 | 0.64 ± 0.03 | 0.57 ± 0.03 | 0.65 ± 0.03 | 0.60 ± 0.04 | 0.54 ± 0.04 | 0.54 ± 0.04 |
| Reward latency | 2.27 ± 0.49 | 2.23 ± 0.44 | 1.99 ± 0.28 | 2.02 ± 0.36 | 2.04 ± 0.63 | 1.67 ± 0.17 | 1.57 ± 0.31 | 1.84 ± 0.26 | 1.82 ± 0.24 |
Different from vehicle (0 dose), p<0.05, n=9 for U50,488 and nicotine and 8 for yohimbine.
3.1.2. Yohimbine:
Yohimbine significantly increased premature responding (F(2,14)=11.8, p=0.001), which was significantly greater than vehicle at the 1.25 mg/kg dose (p<0.05) (1B). Yohimbine produced a small but significant decrease in % accuracy (F(2,14)=4.6, p=0.028), which was significant at the 2.5 mg/kg dose (p<0.05). Yohimbine did not significantly affect numbers of trials completed (F(2,14)=0.14, p=0.87), numbers of omissions (F(2,14)=0.52, p=0.60), perseverative responding (F(2,14)=1.30, p=0.30), latency to make a correct response (F(2,14)=3.11, p=0.076), or latency to collect the rewards (F(2,14)=3.11, p=0.08) (Figure 1B and Table 1B).
3.1.3. Nicotine:
Nicotine increased premature responding as reflected in a significant main effect of Nicotine dose (F(2,16)=16.9, p=0.000). Post hoc tests showed that premature responding was significantly higher at the 0.3 mg/kg dose than vehicle (p<0.05)(1C). Nicotine did not significantly affect numbers of trials completed (F(2,16)=0.19, p=0.83), % accuracy (F(2,16)=0.13, p=0.88), numbers of omissions (F(2,16)=3.32, p=0.062), perseverative responding (F(2,16)=0.16, p=0.85), latency to make a correct response (F(2,16)=3.29, p=0.064), or latency to collect the rewards (F(2,16)=0.56, p=0.58) (Figure 1C and Table 1C).
3.2. Experiment 2: Effects of alcohol on the 5-choice serial reaction time task
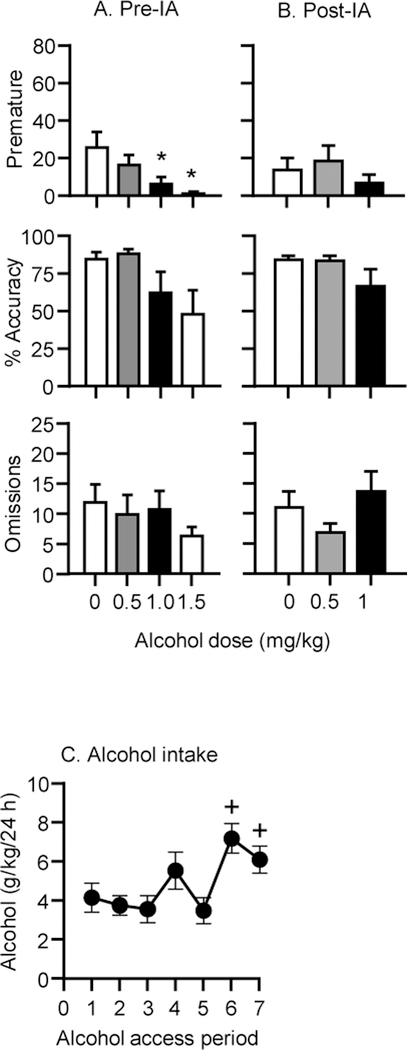
Figure 2 shows the effects of alcohol injections in alcohol naïve rats (A) and in the same rats following 2 weeks of intermittent access to alcohol (B) on mean±SEM number of premature responses, % accuracy and omissions in the 5-CSRTT. Figure 2C shows alcohol intake over the 2 weeks of intermittent access to alcohol.
Figure 2. Dose-response of alcohol in naïve rats and after voluntary intermittent access to alcohol on premature responding, % accuracy and omissions in the 5-CSRTT in Exp. 2.
(A) Naïve rats (B) after intermittent access to alcohol. (C) Alcohol intake over the 2 weeks of intermittent alcohol access (IA). Values are mean±SEM. * Different from vehicle (0 dose), + different from alcohol access period 1, ps<0.05. n=10.
3.2.1. Naïve rats:
Compared to vehicle, alcohol significantly reduced premature responses in naïve rats (Alcohol dose: F(3,27)=5.9, p=0.003) (2A), which was significant at the 1.0 and 1.5 g/kg doses (p<0.05). There was also a significant main effect of Alcohol dose on % accuracy (F(3,27)=3.05, p=0.046), although the post hoc tests did not reveal any significant differences among doses (p>0.05). Administration of alcohol to naïve rats also reduced numbers of trials completed (F(3,27)=34.5, p=0.000) and perseverative responding (F(3,27)=11.5, p=0.000), which, compared to vehicle, were significantly lower at the 1 and 1.5 g/kg doses (p<0.05). Alcohol administration to alcohol naïve rats did not significantly affect numbers of omissions (F(3,27)=0.76, p=0.53), latency to make a correct response (F(3,27)=0.49, p=0.69), or latency to collect the rewards (F(3,27)=0.79, p=0.51) Table 2A.
Table 2. Dose-response of alcohol in naïve rats and after intermittent access to alcohol on additional parameters in the 5-CSRTT in Exp. 2.
(A) Naïve rats (B) after intermittent access. Values are mean±SEM trials completed, perseverative responses and the latencies for correct responses and to obtain a reward.
| A. Alcohol naive |
B. Post-intermittent access |
||||||
|---|---|---|---|---|---|---|---|
| Alcohol dose (g/kg) | 0 | 0.5 | 1.0 | 1.5 | 0 | 0.5 | 1.0 |
| Trials completed | 98.1 ± 1.7 | 98.7 ± 1.3 | 59.1 ± 13.9* | 11.1 ± 4.2* | 87.9 ± 8.6 | 83.3 ± 9.4 | 60.0 ± 11.3 |
| Perseverative | 14.1 ± 3.6 | 14.5 ± 4.5 | 8.1 ± 3.1* | 1.4 ± 1.4* | 12.6 ± 3.9 | 11.5 ± 4.2 | 7.3 ± 3.6* |
| Correct latency | 0.58 ± 0.02 | 0.63 ± 0.03 | 0.54 ± 0.13 | 0.47 ± 0.16 | 0.67 ± 0.05 | 0.63 ± 0.04 | 0.58 ±0.11 |
| Reward latency | 1.86 ± 0.17 | 1.74 ± 0.18 | 1.43 ± 0.39 | 1.34 ± 0.47 | 1.67 ± 0.18 | 1.63 ± 0.14 | 1.45 ± 0.30 |
Different from vehicle (0 dose), p<0.05. n=10.
3.2.2. Post intermittent alcohol access:
Figure 2B shows the effects of alcohol injections on premature responding, % accuracy and omissions in the rats after intermittent access to alcohol. The alcohol injections did not affect these measures (premature responding: F(2,18)=1.23, p=0.29, % accuracy: F(2,18)=2.12, p=0.15, % omissions: F(2,18)=2.99, p=0.076). Alcohol injections after intermittent access significantly reduced perseverative responses (F(2,18)=4.4. p=0.027) at the 1.0 g/kg dose (p<0.05) but did not significantly affect the numbers of trials completed (F(2,18)=3.26, p=0.062), latency to make a correct response (F(2,18)=0.80, p=0.46) or to collect a reward (F(2,18)=0.87, p=0.44).
Figure 2C shows the alcohol intake of the rats across the 7–24 h intermittent access periods. There was a significant effect of Access period on alcohol intake (F(6,54)=10.3, p=0.000). Alcohol intake during access periods 6 and 7 were significantly higher than during period 1 (ps<0.05).
3.3. Experiment 3: Effects of nor-BNI on yohimbine- and nicotine-induced increases in impulsivity in the 5-choice serial reaction time task
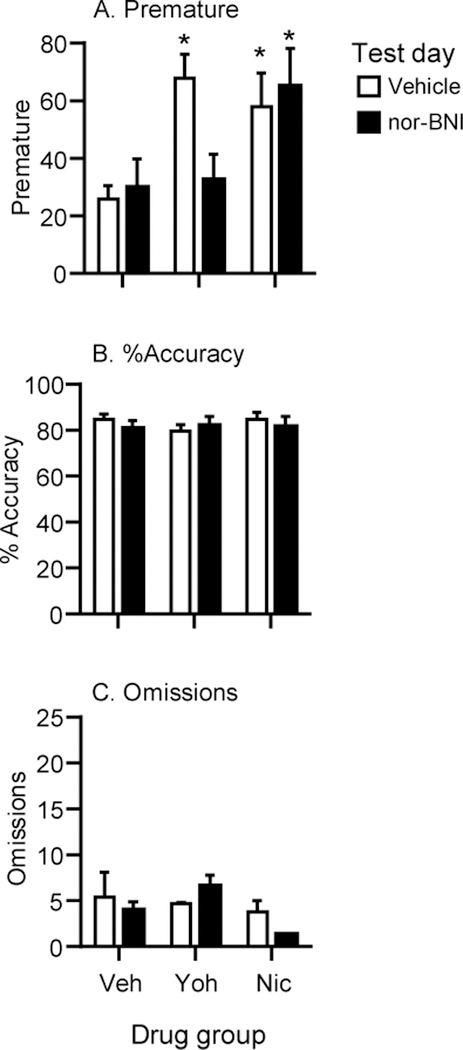
Figure 3 shows mean±SEM number of premature responses (A), % accuracy (B) and omissions (C) in rats treated with vehicle or nor-BNI prior to injections of vehicle, yohimbine or nicotine. For premature responding, ANOVA revealed a significant Drug x nor-BNI dose interaction (F(2,14)=7.5, p=0.006). Post hoc tests showed that in the test with nor-BNI vehicle, yohimbine and nicotine significantly increased premature responding compared to the vehicle group (ps<0.05) (3A). The injection of nor-BNI prior to the second test blocked the yohimbine-induced increases in premature responding, but did not affect the nicotine-induced increases (p>0.05) (3A). There was no significant Drug x nor-BNI dose interactions in the analyses of % accuracy (F(2,14)=1.39, p=0.283), omissions (F(2,14)=1.48, p=0.26), trials completed (F(2,14)=1.78, p=0.21), perseverative responses (F(2,14)=1.68, p=0.22), correct response latencies (F(2,14)=0.99, p=0.39) or latencies to collect the reward (F(2,14)=3.33, p=0.065) (Figure 3B, C and Table 3).
Figure 3. Effects of nor-BNI on yohimbine and nicotine-induced effects in the 5-CSRTT in Exp.3.
(A) Premature responding. (B) % accuracy. (C) omissions. Rats were injected with vehicle (Veh), yohimbine (Yoh) (1.25 mg/kg) or nicotine (Nic) (0.3 mg/kg) and tested in the 5-CSRTT on two separate tests. Prior to the first test, they were injected with the vehicle for nor-BNI (open bars); prior to the second test, they were injected with nor-BNI (10 mg/kg) (closed bars). Values are mean±SEM. * Different from vehicle (0 dose nor-BNI), p<0.05, vehicle: n=5, yohimbine: n=7, nicotine: n=5.
Table 3. Effects of nor-BNI on yohimbine and nicotine-induced effects on additional parameters in the 5-CSRTT in Exp.3.
(A) Vehicle. (B) Yohimbine. (C) Nicotine. Values are mean±SEM trials completed, perseverative responses and the latencies for correct responses and to obtain a reward.
| A. Vehicle |
B. Yohimbine |
C. Nicotine |
||||
|---|---|---|---|---|---|---|
| Vehicle | Nor-BNI | Vehicle | Nor-BNl | Vehicle | Nor-BNI | |
| Trials completed | 85.0 ± 9.8 | 78.0 ± 15.2 | 90.0 ± 4.5 | 97.6 ± 1.7 | 95.2 ± 3.9 | 90.2 ± 7.0 |
| Perseverative | 10.8 ± 5.3 | 10.0 ± 2.4 | 12.1 ± 4.7 | 15.7 ± 7.6 | 25.8 ± 12.8 | 18.4 ± 9.4 |
| Correct latency | 0.57 ± 0.05 | 0.61 ± 0.04 | 0.54 ± 0.03 | 0.59 ± 0.03 | 0.45 ± 0.03 | 0.46 ± 0.03 |
| Reward latency | 2.13 ± 0.37 | 1.40 ± 0.15 | 1.59 ± 0.23 | 1.91 ± 0.34 | 2.11 ± 0.48 | 1.86 ± 0.29 |
Different from vehicle (0 dose), p<0.05. Vehicle: n=5, yohimbine: n=7, nicotine: n=5.
4. Discussion
We compared the effects of four drugs that induce reinstatement of drug and alcohol seeking and are known to have effects on the DYN/KOR systems, on performance on the 5-CSRTT, a test of sustained attention that is also used to measure impulsivity. Two of these drugs, the KOR agonist U50,488 and alcohol, reduced impulsive responding [24, 25, 33, 41], an effect likely due their general suppressive effects, as supported by reductions in the numbers of completed trials. The other two drugs we tested, yohimbine and nicotine, increased impulsivity in the 5-CSRTT as shown previously [23, 32, 40, 42, 43]. We followed up on these results and established that the pro-impulsive effects of yohimbine, but not nicotine in the 5-CSRTT are mediated by KOR.
We found that U50,488 decreased premature responding in the 5-CSRTT. The only published study on the effects of U50,488 in the 5-CSRTT did not present premature responding data [25], but another KOR agonist, U69,593 was reported to cause modest, non-significant decreases in premature responding [25]. We found that U50,488 did not significantly affect accuracy, but increased the number of omitted trials and reduced the numbers of trials completed, suggesting generalized suppressive effects which is consistent with previous work with U50,488 [25] and U69,593 [24]. Finally, we did not find U50,488 to affect response latencies, but another study reported that U69,593 significantly increased the latency to make a correct response [24]. Our data is in general, consistent with the idea that KOR agonists have a generalized suppressive effect on the 5-CSRTT.
Only two studies have reported on the effects of alcohol on the 5-CSRTT in rats [33, 41]. In alcohol-naïve rats, our findings of reduced premature responding at 1.0 and 1.5 g/kg doses of alcohol, and increases in omissions at higher doses are consistent with this previous work. We also showed decreases in perseverant responses which has not been previously reported. We found a significant reduction in % accuracy, expressed as a significant main effect of Alcohol dose, whereas previous studies only displayed trends towards decreases at the higher doses tested [33, 41].
We also determined the effects of alcohol on 5-CSRTT performance in the rats after 2 weeks of intermittent alcohol exposure. In contrast to the decreased premature and perseverant responding and decreased % accuracy noted in alcohol naïve rats, we found significant alcohol-induced reductions only in perseverant responses after intermittent access to alcohol, suggesting that the effects of alcohol may have been attenuated by intermittent exposure. Some limitations make interpreting these findings difficult. In alcohol naïve rats, we tested vehicle and three doses of alcohol, and after intermittent access, vehicle and the two lower doses. Another is that after intermittent access, there was a downward shift in baseline premature responding (vehicle condition), which may have masked any inhibitory effects of alcohol on premature responding. Another is that a control group that did not receive intermittent alcohol access was not included. Finally, the duration of intermittent exposure was relatively short (7 24 h exposures over 2 weeks) and it is possible that with longer voluntary exposure, or with exposure to high doses of alcohol, such as with alcohol vapor, we would have obtained clearer results.
The present results and previous work with U50,488, other KOR agonists and alcohol on the 5-CSRTT revealed primarily inhibitory effects of these compounds on premature responding. However, the work on these drugs employing other tasks that measure impulsivity have produced different results. Intracerebroventricular injection of U50,488 increased impulsivity in a response inhibition procedure, the stop-signal reaction time task, in which rats must inhibit an operant response that is already initiated when cued to do so. However higher doses were without effect [26]. In mice, systemic U50,488 increased impulsive responding in a DRL procedure [27]. U50,488 did not affect impulsivity in a delay of reward task [26], in that it did not affect the probability of rats selecting a small, immediate reward versus a larger, delayed reward. Alcohol has also been noted to produce different effects on impulsivity in other paradigms. It has been shown to increase impulsivity in delay of reward and stop-signal tasks [17, 44–46] but see [47].
The precise nature of these task-related differences are not known. Impulsivity is a multi-faceted construct, and behavioral and neurobiological studies suggest there are 2 main forms, choice impulsivity and motor impulsivity. Delay tasks are thought to measure impulsive choice, whereas stop-signal tasks index motor impulsivity, the ability to inhibit motor responses [48]. The type of impulsivity indexed by the 5-CSRTT has been suggested to overlap with that of delay and stop-signal tasks [22, 48]. Examination of multiple paradigms may be necessary to determine the effects of a given drug on impulsivity.
Consistent with previous reports on its effects in the 5-CSRTT, we found that yohimbine increased premature responding, with the dose response curve tending to follow an inverted U shape [23, 42, 43]. We also found yohimbine to slightly but significantly reduce % accuracy at the 2.5 mg/kg dose, an effect not previously reported.
We found that nicotine significantly increased premature responding, which agrees with previous work [32, 40, 49, 50]. Other studies found either no effect [51], or nicotine-induced decreases [33] in premature responding. The reasons for these differences are not known. It does not appear to be related to the acute motor suppressant effects of nicotine, as the rats these 2 studies received nicotine injections in the home cage prior to the 5-CSRTT tests with nicotine [33, 51], as we did in our study, nor to strain effects.
Since KOR are implicated in the effects of yohimbine and nicotine on reinstatement [7, 8, 52], and stress or nicotine exposure can increase levels of central DYN [29, 53], in the final experiment we examined the involvement of KOR in the pro-impulsive effects of yohimbine and nicotine. To this end, we determined the effects of the selective KOR antagonist nor-BNI on the increased premature responding in the 5-CSRTT produced by yohimbine and nicotine. We found that nor-BNI blocked the pro-impulsive effects of yohimbine, but was without effect on nicotine-induced increases in impulsivity.
These effects of nor-BNI on yohimbine-induced impulsivity may be relevant to understanding the potential mechanisms underlying yohimbine-induced reinstatement. Our data suggest that yohimbine-induced release of DYN and subsequent activation of KOR may be responsible for the enhanced impulsivity produced by yohimbine. This increased impulsivity may contribute to yohimbine-induced reinstatement, which is consistent with our work showing that nor-BNI blocks yohimbine-induced reinstatement of alcohol and nicotine seeking [7, 8]. The function of KOR in the effects of stressors on the 5-CSRTT is poorly understood. The one study that examined this showed that the KOR antagonist JDTic did not affect I.C.V. corticotropin-releasing factor (CRF)-induced reduction of premature responding, although it did block the impairments produced by CRF on other measures of task performance [54].
A potential issue in interpreting these effects of nor-BNI on yohimbine-induced increases in impulsivity is the selectivity of nor-BNI for KOR when injected 1 h before testing. Nor-BNI is a widely used selective KOR antagonist [55], but has been reported to also bind to mu opioid receptors for several hours after systemic injections [56, 57], but see Narita [58] for data that do not support this. Support for the selectivity of nor-BNI for KOR early after injection comes from studies showing that yohimbine-induced reinstatement of drug seeking is blocked by nor-BNI [7, 8] given 1–2 h prior to testing, but not by the mu opioid receptor antagonist naltrexone [59]. Taking these results together, the most straightforward interpretation of our data is that the effects of nor-BNI on yohimbine-induced increases in impulsivity are mediated by selective KOR blockade.
KOR antagonism with nor-BNI blocked yohimbine-induced increases in impulsivity, suggesting that the dynorphin released by yohimbine stimulates KOR and increases impulsivity. However, the KOR agonist U50,488 decreased impulsivity, an effect likely mediated by a general suppressive effect. The reasons for this pattern of results are not known, but it could be related to differences in how KOR are stimulated by the two drugs. Yohimbine evokes the release of endogenous DYN, that in turn simulates KOR, while U50,488 stimulates KOR directly. These distinct mechanisms could lead to stimulation of different populations of KOR in the brain, and the differential effects on impulsivity vs general suppressive effects. Although the brain regions responsible for either of these effects are not clear, a potential candidate for the pro-impulsive effects of yohimbine is the orbitofrontal cortex (OFC). The OFC contains DYN and KOR [60–62] and it plays a key role in yohimbine-induced increases in impulsivity in the 5-CSRTT [23].
A potential alternative explanation of increased premature responding produced by yohimbine and nicotine is that rats use a timing strategy to guide when they make their responses [63] and that drug-induced increases in premature responses reflect disruption of this strategy. Our present design cannot rule this out because we tested only a single ITI. In future studies, we will systematically vary the ITI duration and observe its effects on the yohimbine and nicotine-induced premature responding, and the potential role of KOR in this, to help resolve this issue.
Yohimbine increases impulsivity in the 5-CSRTT and reinstates drug seeking, and both of these effects of yohimbine are blocked by nor-BNI. Although we did not compare reinstatement with impulsivity in the present study, we speculate that KOR-mediated impulsivity could contribute to yohimbine’s effects on reinstatement. To our knowledge, only one study has examined the relationship between yohimbine-induced increases in impulsivity and yohimbine-induced reinstatement. In this study, yohimbine-induced reinstatement of cocaine seeking was not significantly correlated with the increased premature responding in the 5-CSRTT [22]. However, these correlational results do not completely rule out a role for impulsivity in the effects of yohimbine on reinstatement, because yohimbine increases both motor [23, 42] and choice impulsivity [64]. Previous work showed that both forms are positively associated with reinstatement [19, 21, 65], so it is possible that increased impulsive choice may also contribute to yohimbine-induced reinstatement. Further studies aimed at determining the relative contribution motor and choice impulsivity may be needed to fully understand the role of KOR in yohimbine-induced reinstatement.
In conclusion, we found the selective KOR agonist U50,488 and alcohol reduced impulsivity in the 5-CSRTT, likely due to a generalized suppressive effect as evidenced by the reduction in completed trials. Yohimbine and nicotine increased impulsivity. The pro-impulsive effects of yohimbine, but not nicotine are mediated by KOR. Taken together with our previous work showing the KOR-dependency of yohimbine-induced reinstatement of alcohol seeking, our data suggests that KOR-dependent increases in impulsivity may contribute to yohimbine-induced reinstatement.
Acknowledgements:
The study was supported by NIAAA-NIH funds to ADL (R01-AA024341).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Compliance with ethical standards: All experimental procedures described were conducted in full compliance with the National Institutes of Health Principles of Laboratory Animal Care (8th Edition, 2011) and approved by the local Animal Care Committee of the Centre for Addiction and Mental Health.
Conflict of interest: The authors declare that they have no competing interests.
References
- 1.Chavkin C and Koob GF, Dynorphin, Dysphoria, and Dependence: the Stress of Addiction. Neuropsychopharmacology, 2016. 41(1): p. 373–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shippenberg TS, Zapata A, and Chefer VI, Dynorphin and the pathophysiology of drug addiction. Pharmacol Ther, 2007. 116(2): p. 306–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bechara A and van der Kooy D, Kappa receptors mediate the peripheral aversive effects of opiates. Pharmacol Biochem Behav, 1987. 28(2): p. 227–33. [DOI] [PubMed] [Google Scholar]
- 4.McLaughlin JP, et al. , Prior activation of kappa opioid receptors by U50,488 mimics repeated forced swim stress to potentiate cocaine place preference conditioning. Neuropsychopharmacology, 2006. 31(4): p. 787–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bals-Kubik R, Herz A, and Shippenberg TS, Evidence that the aversive effects of opioid antagonists and kappa-agonists are centrally mediated. Psychopharmacology (Berl), 1989. 98(2): p. 203–6. [DOI] [PubMed] [Google Scholar]
- 6.Al-Hasani R, et al. , Locus coeruleus kappa-opioid receptors modulate reinstatement of cocaine place preference through a noradrenergic mechanism. Neuropsychopharmacology, 2013. 38(12): p. 2484–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Funk D, Coen K, and Le AD, The role of kappa opioid receptors in stress-induced reinstatement of alcohol seeking in rats. Brain Behav, 2014. 4(3): p. 356–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grella SL, et al. , Role of the kappa-opioid receptor system in stress-induced reinstatement of nicotine seeking in rats. Behav Brain Res, 2014. 265: p. 188–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Redila VA and Chavkin C, Stress-induced reinstatement of cocaine seeking is mediated by the kappa opioid system. Psychopharmacology (Berl), 2008. 200(1): p. 59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beardsley PM, et al. , Differential effects of the novel kappa opioid receptor antagonist, JDTic, on reinstatement of cocaine-seeking induced by footshock stressors vs cocaine primes and its antidepressant-like effects in rats. Psychopharmacology (Berl), 2005. 183(1): p. 118–26. [DOI] [PubMed] [Google Scholar]
- 11.Jackson KJ, et al. , Effects of the kappa opioid receptor antagonist, norbinaltorphimine, on stress and drug-induced reinstatement of nicotine-conditioned place preference in mice. Psychopharmacology (Berl), 2012. [DOI] [PMC free article] [PubMed]
- 12.Sperling RE, et al. , Endogenous kappa-opioid mediation of stress-induced potentiation of ethanol-conditioned place preference and self-administration. Psychopharmacology (Berl), 2010. 210(2): p. 199–209. [DOI] [PubMed] [Google Scholar]
- 13.Walker BM and Koob GF, Pharmacological evidence for a motivational role of kappa-opioid systems in ethanol dependence. Neuropsychopharmacology, 2008. 33(3): p. 643–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walker BM, Zorrilla EP, and Koob GF, Systemic kappa-opioid receptor antagonism by nor-binaltorphimine reduces dependence-induced excessive alcohol self-administration in rats. Addict Biol, 2011. 16(1): p. 116–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valdez GR and Harshberger E, kappa opioid regulation of anxiety-like behavior during acute ethanol withdrawal. Pharmacol Biochem Behav, 2012. 102(1): p. 44–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bari A and Robbins TW, Inhibition and impulsivity: behavioral and neural basis of response control. Prog Neurobiol, 2013. 108: p. 44–79. [DOI] [PubMed] [Google Scholar]
- 17.Poulos CX, Parker JL, and Le DA, Increased impulsivity after injected alcohol predicts later alcohol consumption in rats: evidence for “loss-of-control drinking” and marked individual differences. Behav Neurosci, 1998. 112(5): p. 1247–57. [DOI] [PubMed] [Google Scholar]
- 18.Anker JJ, et al. , Impulsivity predicts the escalation of cocaine self-administration in rats. Pharmacol Biochem Behav, 2009. 93(3): p. 343–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Economidou D, et al. , High impulsivity predicts relapse to cocaine-seeking after punishment-induced abstinence. Biol Psychiatry, 2009. 65(10): p. 851–6. [DOI] [PubMed] [Google Scholar]
- 20.Perry JL, Nelson SE, and Carroll ME, Impulsive choice as a predictor of acquisition of IV cocaine self-administration and reinstatement of cocaine-seeking behavior in male and female rats. Exp Clin Psychopharmacol, 2008. 16(2): p. 165–77. [DOI] [PubMed] [Google Scholar]
- 21.Broos N, et al. , Trait impulsive choice predicts resistance to extinction and propensity to relapse to cocaine seeking: a bidirectional investigation. Neuropsychopharmacology, 2012. 37(6): p. 1377–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Broos N, et al. , Dissociable effects of cocaine and yohimbine on impulsive action and relapse to cocaine seeking. Psychopharmacology (Berl), 2017. 234(22): p. 3343–3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun H, et al. , Yohimbine increases impulsivity through activation of cAMP response element binding in the orbitofrontal cortex. Biol Psychiatry, 2010. 67(7): p. 649–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paine TA, et al. , Sensitivity of the five-choice serial reaction time task to the effects of various psychotropic drugs in Sprague-Dawley rats. Biol Psychiatry, 2007. 62(6): p. 687–93. [DOI] [PubMed] [Google Scholar]
- 25.Shannon HE, et al. , Effects of kappa opioid receptor agonists on attention as assessed by a 5-choice serial reaction time task in rats. Neuropharmacology, 2007. 53(8): p. 930–41. [DOI] [PubMed] [Google Scholar]
- 26.Walker BM and Kissler JL, Dissociable effects of kappa-opioid receptor activation on impulsive phenotypes in wistar rats. Neuropsychopharmacology, 2013. 38(11): p. 2278–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abraham AD, et al. , Kappa Opioid Receptor Activation in Dopamine Neurons Disrupts Behavioral Inhibition. Neuropsychopharmacology, 2017. [DOI] [PMC free article] [PubMed]
- 28.Clark JA and Pasternak GW, U50,488: a kappa-selective agent with poor affinity for mu1 opiate binding sites. Neuropharmacology, 1988. 27(3): p. 331–2. [DOI] [PubMed] [Google Scholar]
- 29.Carboni L, et al. , Repeated nicotine exposure modulates prodynorphin and pronociceptin levels in the reward pathway. Drug Alcohol Depend, 2016. 166: p. 150–8. [DOI] [PubMed] [Google Scholar]
- 30.Zhou Y, et al. , Involvement of dynorphin and kappa opioid receptor in yohimbine-induced reinstatement of heroin seeking in rats. Synapse, 2013. 67(6): p. 358–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.D’Addario C, et al. , Different alcohol exposures induce selective alterations on the expression of dynorphin and nociceptin systems related genes in rat brain. Addict Biol, 2013. 18(3): p. 425–33. [DOI] [PubMed] [Google Scholar]
- 32.Higgins GA, et al. , The 5-HT2C receptor agonist lorcaserin reduces nicotine self-administration, discrimination, and reinstatement: relationship to feeding behavior and impulse control. Neuropsychopharmacology, 2012. 37(5): p. 1177–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bizarro L, Patel S, and Stolerman IP, Comprehensive deficits in performance of an attentional task produced by co-administering alcohol and nicotine to rats. Drug Alcohol Depend, 2003. 72(3): p. 287–95. [DOI] [PubMed] [Google Scholar]
- 34.Kissler JL and Walker BM, Dissociating Motivational From Physiological Withdrawal in Alcohol Dependence: Role of Central Amygdala kappa-Opioid Receptors. Neuropsychopharmacology, 2016. 41(2): p. 560–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muschamp JW, et al. , Activation of CREB in the nucleus accumbens shell produces anhedonia and resistance to extinction of fear in rats. J Neurosci, 2011. 31(8): p. 3095–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matta SG, et al. , Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl), 2007. 190(3): p. 269–319. [DOI] [PubMed] [Google Scholar]
- 37.Hahn B, Shoaib M, and Stolerman IP, Nicotine-induced enhancement of attention in the five-choice serial reaction time task: the influence of task demands. Psychopharmacology (Berl), 2002. 162(2): p. 129–37. [DOI] [PubMed] [Google Scholar]
- 38.Robbins TW, The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology, 2002. 163 p. 362. [DOI] [PubMed] [Google Scholar]
- 39.Fletcher PJ, et al. , Impulsive action induced by amphetamine, cocaine and MK801 is reduced by 5-HT(2C) receptor stimulation and 5-HT(2A) receptor blockade. Neuropharmacology, 2011. 61(3): p. 468–77. [DOI] [PubMed] [Google Scholar]
- 40.Hahn B, Riegger KE, and Elmer GI, Strain dependency of the effects of nicotine and mecamylamine in a rat model of attention. Psychopharmacology (Berl), 2016. 233(8): p. 1427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Semenova S, Attention, impulsivity, and cognitive flexibility in adult male rats exposed to ethanol binge during adolescence as measured in the five-choice serial reaction time task: the effects of task and ethanol challenges. Psychopharmacology (Berl), 2012. 219(2): p. 433–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adams WK, et al. , Dissociable effects of systemic and orbitofrontal administration of adrenoceptor antagonists on yohimbine-induced motor impulsivity. Behav Brain Res, 2017. 328: p. 19–27. [DOI] [PubMed] [Google Scholar]
- 43.Torregrossa MM, Xie M, and Taylor JR, Chronic corticosterone exposure during adolescence reduces impulsive action but increases impulsive choice and sensitivity to yohimbine in male Sprague-Dawley rats. Neuropsychopharmacology, 2012. 37(7): p. 1656–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tomie A, et al. , Ethanol induces impulsive-like responding in a delay-of-reward operant choice procedure: impulsivity predicts autoshaping. Psychopharmacology (Berl), 1998. 139(4): p. 376–82. [DOI] [PubMed] [Google Scholar]
- 45.Hellemans KG, Nobrega JN, and Olmstead MC, Early environmental experience alters baseline and ethanol-induced cognitive impulsivity: relationship to forebrain 5-HT1A receptor binding. Behav Brain Res, 2005. 159(2): p. 207–20. [DOI] [PubMed] [Google Scholar]
- 46.Evenden JL and Ryan CN, The pharmacology of impulsive behaviour in rats VI: the effects of ethanol and selective serotonergic drugs on response choice with varying delays of reinforcement. Psychopharmacology (Berl), 1999. 146(4): p. 413–21. [DOI] [PubMed] [Google Scholar]
- 47.Moschak TM and Mitchell SH, Sensitivity to reinforcer delay predicts ethanol’s suppressant effects, but itself is unaffected by ethanol. Drug Alcohol Depend, 2013. 132(1–2): p. 22–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dalley JW, Everitt BJ, and Robbins TW, Impulsivity, compulsivity, and top-down cognitive control. Neuron, 2011. 69(4): p. 680–94. [DOI] [PubMed] [Google Scholar]
- 49.Wiskerke J, et al. , On the Role of Cannabinoid CB1- and mu-Opioid Receptors in Motor Impulsivity. Front Pharmacol, 2012. 3: p. 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grottick AJ and Higgins GA, Effect of subtype selective nicotinic compounds on attention as assessed by the five-choice serial reaction time task. Behav Brain Res, 2000. 117(1–2): p. 197–208. [DOI] [PubMed] [Google Scholar]
- 51.Semenova S, Stolerman IP, and Markou A, Chronic nicotine administration improves attention while nicotine withdrawal induces performance deficits in the 5-choice serial reaction time task in rats. Pharmacol Biochem Behav, 2007. 87(3): p. 360–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nygard SK, et al. , Stress-Induced Reinstatement of Nicotine Preference Requires Dynorphin/Kappa Opioid Activity in the Basolateral Amygdala. J Neurosci, 2016. 36(38): p. 9937–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Berrendero F, et al. , Neurobiological mechanisms involved in nicotine dependence and reward: participation of the endogenous opioid system. Neurosci Biobehav Rev, 2010. 35(2): p. 220–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Van’t Veer A, et al. , Corticotropin-releasing factor (CRF)-induced disruption of attention in rats is blocked by the kappa-opioid receptor antagonist JDTic. Neuropsychopharmacology, 2012. 37(13): p. 2809–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Portoghese PS, Lipkowski AW, and Takemori AE, Binaltorphimine and nor-binaltorphimine, potent and selective kappa-opioid receptor antagonists. Life Sci, 1987. 40(13): p. 1287–92. [DOI] [PubMed] [Google Scholar]
- 56.Broadbear JH, et al. , Differential effects of systemically administered nor-binaltorphimine (nor-BNI) on kappa-opioid agonists in the mouse writhing assay. Psychopharmacology (Berl), 1994. 115(3): p. 311–9. [DOI] [PubMed] [Google Scholar]
- 57.Endoh T, et al. , Nor-binaltorphimine: a potent and selective kappa-opioid receptor antagonist with long-lasting activity in vivo. Arch Int Pharmacodyn Ther, 1992. 316: p. 30–42. [PubMed] [Google Scholar]
- 58.Narita M, et al. , Effects of kappa-agonist on the antinociception and locomotor enhancing action induced by morphine in mice. Jpn J Pharmacol, 1993. 62(1): p. 15–24. [DOI] [PubMed] [Google Scholar]
- 59.Stopponi S, et al. , Activation of PPARgamma by pioglitazone potentiates the effects of naltrexone on alcohol drinking and relapse in msP rats. Alcohol Clin Exp Res, 2013. 37(8): p. 1351–60. [DOI] [PubMed] [Google Scholar]
- 60.Mansour A, et al. , Immunohistochemical localization of the cloned kappa 1 receptor in the rat CNS and pituitary. Neuroscience, 1996. 71(3): p. 671–90. [DOI] [PubMed] [Google Scholar]
- 61.Mansour A, et al. , Kappa 1 receptor mRNA distribution in the rat CNS: comparison to kappa receptor binding and prodynorphin mRNA. Mol Cell Neurosci, 1994. 5(2): p. 124–44. [DOI] [PubMed] [Google Scholar]
- 62.Ploj K, Roman E, and Nylander I, Long-term effects of maternal separation on ethanol intake and brain opioid and dopamine receptors in male Wistar rats. Neuroscience, 2003. 121(3): p. 787–99. [DOI] [PubMed] [Google Scholar]
- 63.Cope ZA, et al. , Premature responses in the five-choice serial reaction time task reflect rodents’ temporal strategies: evidence from no-light and pharmacological challenges. Psychopharmacology (Berl), 2016. 233(19–20): p. 3513–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schwager AL, Haack AK, and Taha SA, Impaired flexibility in decision making in rats after administration of the pharmacological stressor yohimbine. Psychopharmacology (Berl), 2014. 231(20): p. 3941–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Diergaarde L, et al. , Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeking in rats. Biol Psychiatry, 2008. 63(3): p. 301–8. [DOI] [PubMed] [Google Scholar]