Abstract
Smokers that begin during adolescence are more likely to develop nicotine dependence than those who begin as adults. However, the factors that contribute to this remain largely unknown. Here we utilized a novel operant oral nicotine self-administration procedure in mice to assess the consequences of adolescent nicotine exposure on nicotine and saccharin (non-drug) reinforcement in adults. Animals were given non-contingent exposure to either saline or nicotine using the osmotic minipumps during both adolescence and adulthood for 2 weeks. Reinforcing efficacy for oral nicotine and saccharin was assessed using the progressive ratio schedule 2-weeks following the washout period in adults. Non-contingent nicotine exposure in adolescence drastically increased operant responding for oral nicotine but reduced responding for oral saccharin in the group re-exposed to nicotine in adulthood. Interestingly, adolescent nicotine-exposed mice that received saline exposure as adults exhibited higher preference for oral saccharin. However, breakpoints for oral nicotine in these mice remained comparable to control animals. Surprisingly, both adolescent and adult nicotine exposure increased inactive lever responding during self-administration presumably reflecting impulsive responding. Our data suggest that adolescent nicotine exposure produces an increase in reinforcement sensitivity in adulthood as reflected by increased saccharin self-administration but this sensitivity becomes biased towards nicotine self-administration when re-exposed to nicotine in adulthood. Moreover, nicotine/saccharin reinforcement could be impacted by changes in cognitive control, such as increased impulsivity. These distinct behavioral mechanisms may act in concert to facilitate maladaptive nicotine taking in smokers that initiate nicotine use during adolescence.
Keywords: adolescence, nicotine, motivated behavior, oral self-administration, reinforcement, mice
1. Introduction
Adolescence is a developmental period that is marked by increased sensation seeking and immature ability to self-regulate behavior [1–3]. Due to the lack of development of areas crucial to behavioral regulation, adolescence also is a susceptible period for tobacco smoking initiation. There is considerable evidence indicating that smoking adolescents are more likely to develop nicotine addiction than those who begin smoking as adults [4]; however, the underlying behavioral mechanism for this phenomenon remains unknown.
Age-related differences in the reinforcing effects of nicotine and physiological symptoms of nicotine withdrawal have been reported. Numerous rodent studies indicated that adolescents experience enhanced conditioned rewarding effects of nicotine and self-administer more nicotine than adults [5–10]. Moreover, studies involving animal models of nicotine withdrawal reported that adolescent rats have more benign somatic and affective measures of withdrawal relative to adult nicotine-exposed rats [11, 12]. Together these investigations supported the notion that an imbalance between an oversensitive reward system and reduced ability to experience negative aversive effects of withdrawal contributes to the smoking behavior in adolescents. Contrary to this view, the withdrawal-based theories of addiction emphasized that motivation to reinstate tobacco use during acute abstinence is mediated by withdrawal symptoms [13]. Moreover, escalation of smoking behavior observed in nicotine addicts is suggested to be facilitated by withdrawal symptoms during the intermittent tobacco use [14]. Thus, it remains unclear what psychological mechanisms accounts for greater propensity for nicotine intake during adulthood and beyond in smokers that had been exposed to nicotine during adolescence, a period associated with less aversive withdrawal symptoms. Moreover, maladaptive patterns of smoking behavior persist in nicotine addicts even after the acute abstinence symptoms are dissipated. Likewise, if the magnitude of drug reinforcement is lower in adults than adolescents, it is unknown what mechanisms drive life-long maladaptive nicotine use in addicts that begin smoking in early life.
Previous studies employing the self-administration, conditioned place preference and operant visual reinforcement paradigms reported escalated nicotine intake, heightened reward responsiveness and augmented reinforcement enhancing effects of nicotine in adult rodents that were exposed to nicotine during adolescence [15–17]. Adolescent nicotine exposure has also been shown to enhance the reinforcing effects of other drugs of abuse such as cocaine in adult rodents [18, 19]. On the other hand, adult but not adolescent nicotine exposure increased alcohol self-administration in adulthood [20, 21]. Moreover, adolescent nicotine did not alter instrumental responding for natural rewards [22]. Thus it is possible that both adolescent and adult nicotine exposure may exert dissociated effects on reward-related enhancement of motivational processes, and that interaction between age-specific effects on these processes determines subsequent nicotine abuse. However, there are no studies that systematically determined how age-specific exposure to chronic nicotine would impact motivated behaviors in adulthood.
Although traditional self-administration animal models of intravenous delivery of psychostimulant drugs provided a wealth of information on maladaptive addiction-related behaviors, low reinforcing properties nicotine compared to these drugs [23] make intravenous self-administration very challenging specifically in the mouse models. Additionally, intravenous nicotine delivery does not completely translate to nicotine self-administration in humans who ingests nicotine either through the inhalational (smoking) or oral (chewing gum) route. Here we developed a novel operant oral nicotine self-administration procedure in mice to assess the consequences of adolescent nicotine exposure on nicotine- and saccharin-taking behavior in adults. Animals were given non-contingent saline/nicotine exposure during both adolescence and adulthood prior to operant behavioral testing as repeated nicotine exposure may alter addiction-like behaviors [24]. The behavioral testing was conducted in adult animals after prolonged washout period to eliminate any potential influence of acute nicotine withdrawal syndrome on motivated behavior. Our results indicate for the first time that adolescent nicotine exposure produces a generalized increase in reward sensitivity in adulthood as reflected by increased saccharin reinforcement but this sensitivity becomes biased towards nicotine following adult re-exposure. Moreover, chronic nicotine-induced alterations in cognitive processes and positive affect in an age-specific manner could contribute to these behavioral effects.
2. Materials and methods
2.1. Animals
Male and female C57BL/6J mice were procured from the Jackson Laboratory (Bar Harbor, ME) and were preadolescent (PND23) at the time of arrival. Animals were housed in a humidity/temperature controlled colony room with a 12h light/dark cycle (lights on at 07:00) and had access to food and water ad libitum. The experiments were started at PND33-35 with the implantation of osmotic minipumps for chronic nicotine delivery (see details below). This age corresponds to the mid-adolescence phase in mice and is characterized by pubertal maturation [25]. Mice remained single-housed throughout the duration of study. They had free access to food and water during the nicotine exposure phase but were water restricted (5 min/day) for the operant behavioral training and testing. All experimental procedures were approved by the Institutional Animal Care and Use Committee of Temple University and were in accordance with the National Institute of Health guidelines. A total of 39 mice were used for the study. Three animals were excluded due to deteriorating health following either the adolescent or adult osmotic minipump surgeries.
2.2. Chronic nicotine administration and experimental design
As noted above, chronic nicotine administration in adolescent mice was started at PND33-35. Osmotic minipumps were implanted for non-contingent nicotine/saline exposure in isoflurane-anesthetized mice as described in our previous studies [52, 55]. Briefly, sterile pumps (model 1002, DURECT Corporation., Cupertino, CA) were filled either with nicotine hydrogen tartrate (Sigma Co., St Louis, MO) dissolved in sterile saline to deliver 3 mg/kg/d of nicotine (as free base) solution at a rate of 0.25μL/h for 2 weeks. The adolescent nicotine dose (3.0 mg/kg/d) selected for this study was used to model mild nicotine exposure. Moreover, chronic nicotine administration at this dose has previously been shown to produce withdrawal-associated learning deficits in older adolescent mice [26]. Control animals were implanted with minipumps filled with sterile saline. All pumps were inserted subcutaneously into the back by incision just below the neck region. After insertion, the wounds were closed with sutures. The pumps were removed after 2 weeks of chronic nicotine/saline administration. The adolescent nicotine and adolescent saline mice remained nicotine-free for a period of 2 weeks following which they were randomly assigned into adult nicotine and saline groups respectively. Adolescent saline animals were re-implanted with sterile osmotic minipumps for non-contingent delivery of either saline (sal/sal: N=8; 5 males and 3 females) or nicotine (sal/nic: N=10; 5 males and 5 females) for 2 weeks during adulthood (PND61-63). Likewise, adolescent nicotine animals received adult nicotine exposure of either saline (nic/sal: N=8; 4 males and 4 females) or nicotine (nic/nic: N=10; 5 males and 5 females). Chronic nicotine exposure was given at a higher dose (6.3 mg/kg/d) to both sal/nic and nic/nic mice during adulthood. The adult nicotine dose was selected based on the previous studies that reported the desensitization of nicotinic acetylcholine receptors (nAChRs), reward sensitization and effects on cognition in adult mice [26–28]. Chronic nicotine administration in the dose range used in our study has previously been shown to produce plasma nicotine levels similar to human smokers [26, 29, 30]. Moreover, the low-high nicotine exposure sequence was adopted to model the escalation of nicotine use commonly seen in adolescents that initially smoke for recreational use and transition to regular smokers in adulthood [31]. The pumps were removed 2 weeks after the second nicotine/saline exposure and the animals underwent another washout period of 2 weeks, following which the operant behavioral experiments were conducted (see Experimental design in Fig 1). The 2-week washout period was based on a previous study that used a multiple nicotine exposure paradigm using osmotic minipumps with intermittent nicotine-free periods in mice to model cycles of smoking and relapse [24]. The delivery of saline/nicotine solutions through the minipumps was verified by measuring the residual volume following surgical removal at the end of the exposure period for each time point.
Figure 1.
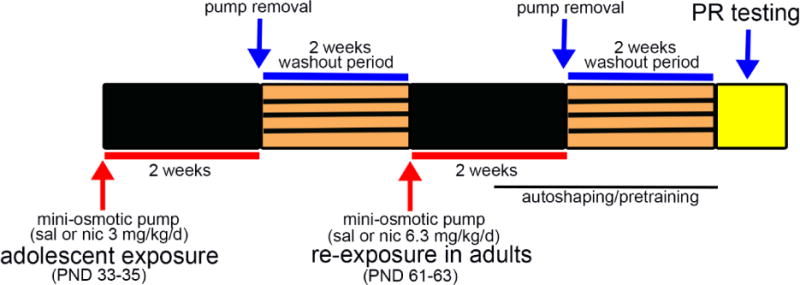
Schematic of experimental design. Adolescent mice (PND 33-35) were implanted with mini-osmotic pumps to deliver 3.0mg/kg/d nicotine (nic) or saline (sal) for 2 weeks. After pump removal, a 2-week washout period was given. Adult (PND 61-63) mice that previously received sal were re-exposed either to chronic sal or nic (6.3mg/kg/d) for another 2 weeks. Likewise, adolescent nic-exposed mice received either sal or nic (6.3 mg/kg/d) during adulthood. PR testing to examine saccharin and nicotine reinforcement (see Methods) was conducted following the second 2-week washout period. The autoshaping and pretraining for PR testing was initiated in animals moving into the second week of re-exposure.
Mice (N=5 per condition) were chosen at random to observe for somatic signs of withdrawal 24 h and 14 d after the adolescent nicotine or saline exposure as described previously [32, 33]. As noted earlier, the 2-week w period was chosen to eliminate any potential influence of acute abstinence syndrome on adult behavior as previous work showed that withdrawal deficits in learning in mice lasted 4 days [56]. The observations for somatic signs were made in animals for 20-min in the home cage by monitoring the following withdrawal symptoms: head shakes, paw tremors, retropulsion, writhing, scratching, backing, piloerection, and Straub tail. Somatic signs were calculated as total scores based on the number of signs displayed by mice during the 20-min observation period by an experimenter blind to the treatment conditions.
2.3. Operant oral self-administration of saccharin and nicotine
Behavioral training and testing for progressive ratio (PR) of reinforcement for oral saccharin (sweetened water with saccharin; natural reinforcer) and oral nicotine (sweetened with saccharin) was conducted in mouse operant conditioning chambers (MED Associates; St. Albans, VT, USA). The chambers were equipped with a standard grid floor and house light (28V, 100mA), and a panel consisting of two large cue lights (2.5cm; 28V, 100mV), a central reward port attached to a fluid dipper, and two ultra-sensitive retractable levers were used. Control of all events, including light presentation, lever operations, and reward delivery, utilized a SmrtCtrl interface running MED-PC IV software on Dell PC (Optiplex 960). Mice were progressively water-restricted to 5 min of water/day. Operant training was conducted 7 days/week between 9:00 and 16:00 h. Food pellets (PMI LabDiet) were available ad libitum during the behavioral experiments.
The oral self-administration procedure for sweetened nicotine solution was adapted from an operant procedure previously established in rats [34]. Briefly, partially water-deprived animals were autoshaped on a fixed ratio-1 (FR-1) schedule of reinforcement to acquire lever pressing and subsequent reward delivery (10μl of 0.066% saccharin solution). Autoshaping was followed by a pretraining phase where the animals were trained to discriminate an active (rewarded) from an inactive lever (non-rewarded) lever. Training sessions consisting of 30 trials were divided into 3 blocks of 10 trials with each block following an FR-1, FR-3 and FR-5 schedule of reinforcement in succession. After each successful response, the light associated with the active lever was illuminated to signal the completion of the ratio and the dipper was activated for reward delivery. Responses to the inactive lever were not rewarded. The animals were considered to attain criterion when 30 rewards were obtained within 45 min for 3 consecutive days. The PR testing was conducted as described earlier in mice [35]. The first trial was rewarded with a single active lever press response and subsequent trials followed an accumulating FR-2 schedule of reinforcement (e.g., 1, 3, 5, 7…, X+2). The session continued until a mouse failed to obtain a reward within 5 minutes after earning its last reward. The breakpoint was considered to be the last successfully completed ratio. To assess the PR of natural reinforcement, a 2% saccharin solution was presented as a reward. For oral nicotine reinforcement, a 2% saccharin solution containing 100μM nicotine was used. PR testing was conducted for 3 consecutive days for each reinforcement condition and a counterbalanced design was followed to ensure that there are no sequence effects. Authoshaping and pretraining was initiated during the second week of adult subcutaneous chronic nicotine administration and the PR testing was conducted after a 2-week washout period (see experimental design in Fig 1).
2.4. Statistical analysis
Statistical analyses were performed using SPSS/PC+ version 24.0 (IBM-SPSS, Armonk, NY, USA). A general linear model repeated measure analysis of variance (ANOVA) was used to analyze three day averages for active lever presses, inactive lever presses, number of earned reinforcers, and break-point ratios for saccharin and nicotine PR testing sessions. Adolescent nicotine (saline vs nicotine; 2 levels), adult nicotine (saline vs nicotine; 2 levels) and sex (male vs female; 2 levels) were used as between subject factors while reinforcement (oral saccharin vs oral nicotine; 2 levels) was used as a within subject factor. Tukey’s HSD post hoc tests were applied for group comparisons when significant interactions were observed. A two-way ANOVA was used to compare somatic signs of withdrawal following adolescent nicotine exposure at 24 h and 14 d time points. For all statistical tests, p values <0.05 were considered statistically significant.
3. Results
3.1. Physical symptoms following adolescent nicotine exposure
Somatic signs of withdrawal were examined 24 hr. and 14 d following nicotine and saline exposure during adolescence. Total scores for physical symptoms differed significantly between the two time points (main effect: F(1,8)=50.0, p<0.001) and this effect interacted with adolescent nicotine exposure (F(1,8)=42.78, p<0.001). Post hoc analysis indicated that somatic symptom scores were significantly higher at 24 hr. time point in mice exposed to nicotine during adolescence as compared to the saline controls (F(1,8)=67.1, p<0.001). However, total scores remained comparable between the two groups 14 days later (F(1,8)=1.67, p>0.22) indicating that the acute withdrawal symptoms returned to baseline levels before the adult manipulations were given and before the behavioral assessments were conducted.
3.2. Counterbalancing of saccharin and nicotine PR sessions
Adolescent saline-exposed (sal/sal and sal/nic) and adolescent nicotine-exposed (nic/sal and nic/nic) mice were tested for their level of motivation to attain either a saccharin or nicotine reinforcement in a counterbalanced fashion. We neither observed a main effect of sequence of reinforcement (all F(1,28)<0.85, all p>0.37) nor any interaction with the behavioral variables (active lever presses, inactive lever presses, number of reinforcers and breakpoint; all F(1,28)<1.24, all p>0.28). Moreover, the number of training sessions to attain criterion for PR testing (see Methods) did not differ between adolescent saline- and adolescent nicotine-exposed mice (F(1,28)=0.41, p>0.73) indicating that instrumental learning prior to PR testing remained similar across the groups. Thus, we can conclude that our reinforcement sequence counterbalancing for oral saccharin/nicotine PR testing was successful.
3.3. Effect of adolescent and adult nicotine exposure on active and inactive lever responses
We examined the number of active lever presses made during the PR testing sessions and uncovered that mice exposed to nicotine during adolescence (nic/sal + nic/nic) responded more on active levers as compared to mice exposed to saline (sal/sal + sal/nic) during adolescence (main effect of adolescent nicotine: F(1,28)=4.99, p<0.05; Fig 2). Although the total number of active lever presses did not differ by reinforcement (oral saccharin: 633.82 ± 120.53; oral nicotine: 674.39 ± 69.61; main effect: F(1,28)=0.07, p>0.78), a significant adult nicotine × reinforcement interaction was observed (F(1,28)=30.26, p<0.001). Furthermore, the effects of adolescent nicotine interacted with adult nicotine exposure and reinforcement (adolescent nicotine × adult nicotine × reinforcement interaction: F(1,28)=8.62, p<0.01). Post hoc analysis of these interactions revealed that active lever responding for nicotine reinforcement remained significantly higher in nic/nic mice as compared to the sal/sal and sal/nic groups, respectively (Tukey’s HSD: p<0.05 for both comparisons; Fig 2). Interestingly, active lever responses for nicotine-taking did not significantly differ between the nic/nic and sal/nic groups (p>0.05). Comparisons of active lever responses by reinforcement for each treatment group indicated that nic/nic mice made more active lever responses when presented with oral nicotine as compared to oral saccharin (p<0.01). A similar trend for higher preference for active lever responding for nicotine vs saccharin was noted in the sal/nic group (Fig 2); although this effect did not reach significance (p>0.05). Surprisingly, nic/sal animals made significantly more active lever responses for the oral saccharin as compared to oral nicotine (p<0.01). Moreover, active lever responding for saccharin remained significantly higher in these animals as compared to sal/sal, sal/nic and nic/nic mice (all p<0.05; Fig 2).
Figure 2.
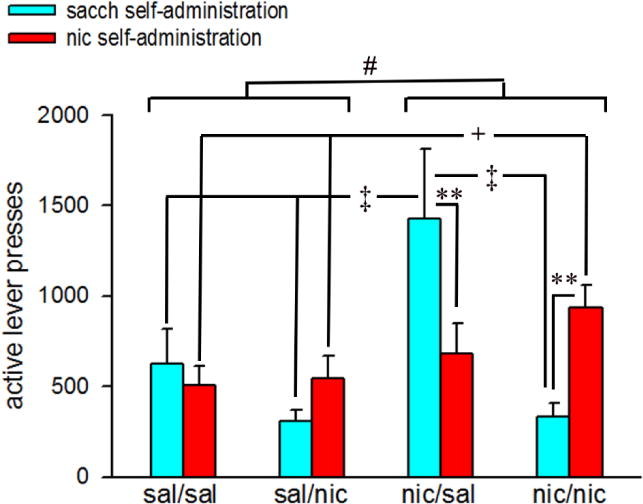
Active lever responding for saccharin and nicotine reinforcement in the PR testing procedure. Bars depict mean ± SEMs for 3-day averages of active lever presses for each saline/nicotine exposure condition. #, p<0.05 main effect of adolescent nicotine. Post hoc tests for adolescent nicotine × adult nicotine × reinforcement interactions: **, p<0.01 sacch reinforcement vs nic reinforcement (within subjects) comparisons for nic/sal and nic/nic; ‡, p<0.05 nic/sal vs sal/sal, sal/nic and nic/nic (sacch reinforcement; between subjects); +, p<0.05 sal/sal vs nic/nic (nic reinforcement; between subjects). sal/sal: adolescent saline-exposed mice re-exposed to saline in adulthood; sal/nic: adolescent saline-exposed mice re-exposed to nicotine in adulthood; nic/sal: adolescent nicotine-exposed mice re-exposed to saline in adulthood; nic/nic: adolescent nicotine-exposed mice re-exposed to nicotine in adulthood; sacch: saccharin; nic: nicotine.
Although the active lever responding remained indifferent between male and female mice (main effect of sex: F(1,28)=0.002, p>0.95), a significant sex × adolescent nicotine × reinforcement interaction was noted (F(1,28)=8.97, p<0.01). The data for these interactions are summarized in Table 1. Female mice exposed to nicotine during adolescence (nic/sal + nic/nic) exhibited significantly higher responding for oral nicotine (p<0.01) but not saccharin (p>0.05) as compared to the adolescent saline-exposed females (sal/sal + sal/nic) responding for nicotine reinforcement. Conversely, male adolescent nicotine-exposed mice (nic/sal + nic/nic) pressed the active lever significantly more for the saccharin as compared to adolescent saline-exposed male mice (sal/sal + sal/nic) responding for oral saccharin (p<0.01). However, responding for oral nicotine remained generally higher in adolescent saline males as compared to responding for saccharin, and this measure remained comparable to adolescent females exposed to non-contingent nicotine (p>0.05). This may indicate that under normal conditions, nicotine might be more reinforcing in males as compared to females [36, 37]. Active lever presses for nicotine PR sessions remained significantly lower in both male and female adolescent saline-exposed (sal/sal + sal/nic) mice as compared to saccharin PR sessions for male adolescent nicotine-exposed (nic/sal + nic/nic) mice (p<0.05 for both male adolescent-saline vs male adolescent-nicotine and female adolescent-saline vs male adolescent-nicotine, respectively; Table 1). Additionally, active lever responding for nicotine in adolescent nicotine-exposed female mice (nic/sal + nic/nic) remained substantially higher than adolescent saline-exposed males (sal/sal + sal/nic) responding for saccharin (p<0.01). Active lever responding in males and females was not affected by adult nicotine exposure (sex × adult nicotine interaction: F(1,28)=0.20, p>0.65; sex × adult nicotine × reinforcement interaction: F(1,28)=2.75, p>0.10)
Table 1.
Effect of adolescent nicotine treatment on active lever press responses in male and female mice.
| adolescent exposure | sex | active lever responses | |
|---|---|---|---|
| sacch reinforcement | nic reinforcement | ||
| adolescent sal (sal/sal + sal/nic) | male | 361.38±183.08 | 598.82 ±127.16‡‡ |
| female | 631.32±211.40 | 453.57 ±146.82‡‡ | |
| adolescent nic (nic/sal + nic/nic) | male | 1039.34±194.18‡ | 695.09 ±134.86 |
| female | 720.34±194.18 | 917.94 ±134.86**,‡ | |
Data are Mean ± SEMs for sex × adolescent nicotine × reinforcement interactions.
p<0.01 vs female adolescent sal (nic reinforcement);
p<0.05 vs male adolescent sal (sacch reinforcement);
p<0.01 vs male adolescent nic (sacch reinforcement). sal: saline, nic: nicotine; sacch: saccharin.
In general, the total number of inactive lever responses remained significantly lower than the active lever responses (F(1,71)=59.37, p<0.001; Fig 3A). Moreover, responding on the inactive lever was neither affected by reinforcement (main effect: F(1,20)=0.09, p>0.76) nor adult nicotine exposure (main effect: F(1,20)=0.15, p>0.69). However, adolescent nicotine exposed mice responded significantly more on the inactive levers in adulthood as compared to adolescent saline-exposed mice (main effect of adolescent nicotine: nic/sal + nic/nic: 56.86±6.27; sal/sal + sal/nic: 34.87±6.16; F(1,20)=6.28, p<0.03; Fig 3B). Furthermore, we observed a significant 3-way adolescent nicotine × adult nicotine × reward interaction (F(1,20)=4.29, p<0.05) on inactive lever responses. Exploration of the interaction effects revealed that inactive lever responding during nicotine PR sessions remained substantially higher in both nic/sal and nic/nic mice as compared to the sal/sal group (p<0.01 for both comparisons). Surprisingly, nic/sal mice also made more inactive lever presses in the saccharin PR sessions as compared to the other three groups (p<0.01 vs sal/sal and nic/nic; p<0.05 vs sal/nic; Fig 3B). Post hoc comparisons between the sal/sal and sal/nic group show that adult nicotine exposure increased inactive lever responses in both oral saccharin and oral nicotine PR sessions (both p<0.01; Fig 3B). However, inactive lever responses for both reinforcements remained comparable between sal/nic and nic/nic groups (both p>0.05). Inactive lever responding was neither affected by sex (F(1,28)=0.27, p>0.60) nor this effect interacted with adolescent nicotine treatment, adult nicotine treatment and reinforcement (sex × adolescent nicotine × adult nicotine × reinforcement interaction: F(1,28)=1.87, p>0.18).
Figure 3.
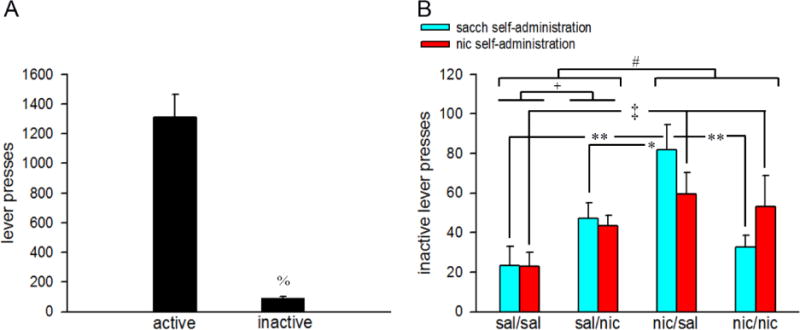
Inactive lever responding in the saccharin and nicotine PR testing sessions. A) The total number of inactive lever presses remained substantially lower as compared to active lever presses. B) Bars depict 3-day averages of inactive lever presses for each saline/nicotine exposure condition. All data are mean ± SEMs. %, p<0.001 vs active levers. #, p<0.05 main effect of adolescent nicotine. Post hoc tests for adolescent nicotine × adult nicotine × reinforcement interactions: +, p<0.05 sal/sal vs sal/nic (both reinforcements; between subjects); *, **, p<0.05, 0.01 nic/sal vs sal/sal, sal/nic and nic/nic (sacch reinforcement; between subjects); ‡, p<0.05 sal/sal vs nic/nic (nic reinforcement; between subjects). sal/sal: adolescent saline-exposed mice re-exposed to saline in adulthood; sal/nic: adolescent saline-exposed mice re-exposed to nicotine in adulthood; nic/sal: adolescent nicotine-exposed mice re-exposed to saline in adulthood; nic/nic: adolescent nicotine-exposed mice re-exposed to nicotine in adulthood; sacch: saccharin; nic: nicotine.
3.4. Nicotine and saccharin reinforcers
The total number of reinforcers earned did not differ between oral nicotine and oral saccharin PR sessions (F(1,20)=2.95, p>0.09); however, this effect interacted with the adult nicotine exposure (F(1,20)= 40.48, p<0.001). Additionally, we observed a significant 3-way interaction between adolescent nicotine × adult nicotine × reinforcement for total reinforcers (F(1,20)=13.09, p=0.001). Data for these interactions are shown in Fig 4. Multiple comparisons show that nic/nic mice earned significantly more nicotine reinforcers as compared to sal/sal mice (Tukey’s HSD: p<0.01). However an opposite effect was observed for saccharin reinforcers; nic/nic mice earned significantly reduced number of saccharin reinforcers as compared to the sal/sal animals (p<0.05). There was a trend for higher nicotine reinforcers vs saccharin reinforcers in the sal/nic group (p<0.07). In line with active lever responding, nic/sal animals exhibited a higher earning of saccharin reinforcers (p<0.05 vs sal/sal, sal/nic and nic/nic mice). However, the total number of nicotine reinforcers remained comparable between nic/sal and other groups (all p>0.05).
Figure 4.
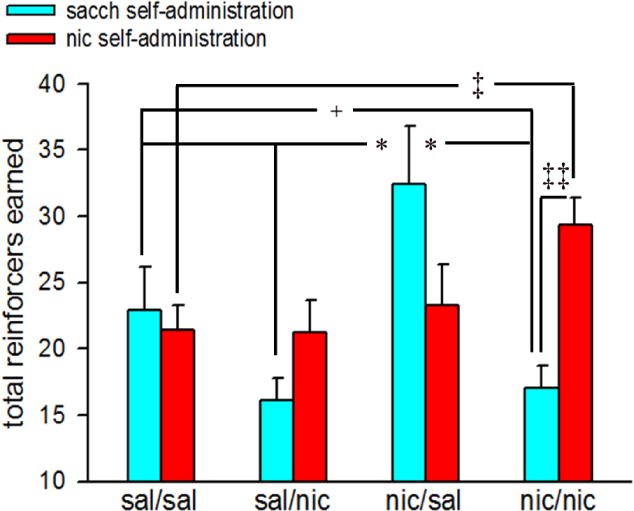
Effects of adolescent and adult nicotine exposure on saccharin and nicotine reinforcers. Bars depict mean ± SEMs for 3-day PR testing averages of total reinforcers earned for each saline/nicotine exposure condition. Post hoc tests for adolescent nicotine × adult nicotine × reinforcement interactions: ‡‡, p<0.01 sacch reinforcement vs nic reinforcement (within subjects) comparison for nic/nic; *, p<0.05 nic/sal vs sal/sal, sal/nic and nic/nic (sacch reinforcement; between subjects); +, p<0.05 sal/sal vs nic/nic (sacch reinforcement; between subjects); ‡, p<0.05 sal/sal vs nic/nic (nic reinforcement; between subjects). sal/sal: adolescent saline-exposed mice re-exposed to saline in adulthood; sal/nic: adolescent saline-exposed mice re-exposed to nicotine in adulthood; nic/sal: adolescent nicotine-exposed mice re-exposed to saline in adulthood; nic/nic: adolescent nicotine-exposed mice re-exposed to nicotine in adulthood; sacch: saccharin; nic: nicotine.
Investigation of total reinforcers in male and female animals indicated a significant sex × adolescent nicotine × reinforcement interaction (F(1,28)=5.43, p<0.04). Post hoc comparisons for these interactions are presented in Table 2. Adolescent nicotine-exposed female mice (nic/sal + nic/nic) earned significantly higher nicotine reinforcers as compared to nicotine reinforcers in both male and female adolescent saline-exposed mice (sal/sal + sal/nic; both sexes p<0.05). Moreover, total nicotine reinforcers earned in female mice that were exposed to nicotine during adolescence remained substantially higher than saccharin reinforcers in males exposed to saline during adolescence (p<0.05). In comparison to saccharin reinforcers in adolescent saline-exposed males, both male and female mice exposed to nicotine during adolescence earned significantly higher saccharin reinforcers (both p<0.05). Adult nicotine exposure did not affect total saccharin and nicotine reinforcers in males and females (sex × adult nicotine × reinforcement interaction: F(1,28)=1.20, p>0.28).
Table 2.
Effect of adolescent nicotine treatment on total reinforcers in male and female mice.
| adolescent exposure | sex | number of reinforcers | |
|---|---|---|---|
| sacch reinforcement | nic reinforcement | ||
| adolescent sal (sal/sal + sal/nic) | male | 17.43±2.54 | 22.0 ±2.28 |
| female | 22.78±2.93 | 22.49 ±2.64 | |
| adolescent nic (nic/sal + nic/nic) | male | 25.35±2.69‡ | 24.33 ±2.42 |
| female | 24.11±2.69‡ | 28.29 ±2.42*,‡,# | |
Data are Mean ± SEMs for sex × adolescent nicotine × reward interactions.
p<0.05 vs female adolescent sal (nic reinforcement);
p<0.05 vs male adolescent sal (sacch reinforcement);
p<0.05 vs male adolescent sal (nic reinforcement). sal: saline, nic: nicotine; sacch: saccharin.
3.5. Breakpoints for oral nicotine and oral saccharin
The breakpoints for PR sessions differ significantly by reinforcement (saccharin vs nicotine: F(1,28)=4.72, p<0.05) and by adult nicotine exposure (F(1,28)=4.98, p<0.04), and there was a significant interaction between the two factors (F(1,28)=30.01, p<0.001). Moreover, the effects of adolescent nicotine exposure, adult nicotine exposure and reinforcement significantly interacted with each other (F(1,28)=9.83, p<0.01). Multiple post hoc comparisons indicated that while breakpoints for oral nicotine significantly increased as compared to saccharin in sal/nic mice (p<0.05), the magnitude of increase in reward strength for oral nicotine was even higher in the nic/nic group (p<0.01; Fig 5). On the other hand, mice exposed to nicotine during adolescence and saline during adulthood (nic/sal) displayed a significant increase in breakpoints for oral saccharin (p<0.01 vs oral nicotine; Fig 5). Additionally, saccharin breakpoints remained significantly higher in these animals as compared to mice that received nicotine as adults (p<0.001 vs sal/nic and nic/nic, respectively). The breakpoints attained for saccharin and nicotine remained comparable in the sal/sal group (p>0.05). However, a significant decrease in saccharin breakpoints was observed in both sal/nic and nic/nic groups (both p<0.01 vs sal/sal; Fig 5). Male and female mice displayed similar break point ratios (main effect of sex: F(1,28)=1.12, p>0.28). Moreover, sex × adolescent nicotine × reinforcement and sex × adult nicotine × reinforcement interactions did not reach significance (p>0.09 for both interactions).
Figure 5.
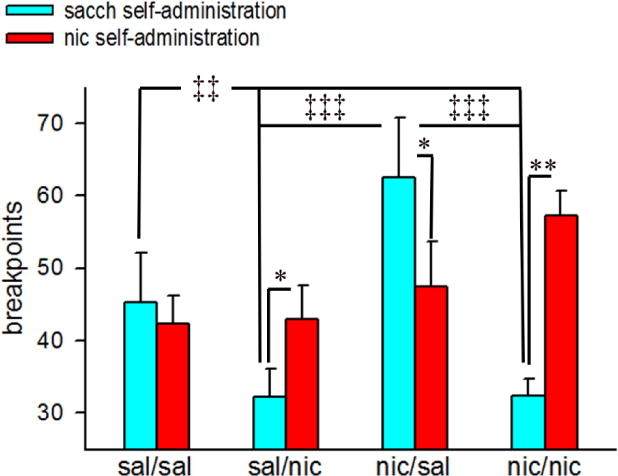
Effects of adolescent and adult nicotine exposure on breakpoints. Bars depict mean ± SEMs for 3-day PR testing averages of breakpoints for each saline/nicotine exposure condition. Post hoc tests for adolescent nicotine × adult nicotine × reinforcement interactions: *, **, p<0.05, 0.01 sacch reinforcement vs nic reinforcement (within subjects) comparisons for sal/nic, nic/sal and nic/nic; ‡‡, p<0.01 sal/sal vs nic/nic (sacch reinforcement; between subjects); ‡‡‡, p<0.001 nic/sal vs sal/nic and nic/nic (sacch reinforcement; between subjects). sal/sal: adolescent saline-exposed mice re-exposed to saline in adulthood; sal/nic: adolescent saline-exposed mice re-exposed to nicotine in adulthood; nic/sal: adolescent nicotine-exposed mice re-exposed to saline in adulthood; nic/nic: adolescent nicotine-exposed mice re-exposed to nicotine in adulthood; sacch: saccharin; nic: nicotine.
4. Discussion
In the present study, we utilized a novel oral self-administration paradigm in mice to better understand how previous exposure to nicotine during adolescence affects nicotine and saccharin (non-drug) reinforcement during adulthood. As mentioned earlier, modelling i.v. nicotine self-administration has proven to be a challenge in mouse models due to nicotine’s low reinforcing properties [23]. Our paradigm allowed for reliable nicotine self-regulation which, although not analogous to inhalational nicotine consumption (smoking in humans), mimics motivated behaviors associated with oral nicotine consumption in humans. Moreover, our task allows direct comparison of nicotine and saccharin to examine drug vs non-drug reinforcement within subjects that is lacking in traditional self-administration tasks.
Our results show that non-contingent nicotine exposure given to adolescent mice drastically increased operant responding for rewards during adulthood. This, however, was mediated by whether mice received a second exposure to nicotine in adulthood. For mice exposed to nicotine in both adolescence and adulthood, non-contingent nicotine exposure preferentially increased nicotine reinforcement but not saccharin (non-drug) reinforcement. Although the nicotine solution was sweetened with saccharin to mask its bitter taste and make it palatable for animals, the concentration of saccharin for the two reinforcers (sweetened water and nicotine solution) was kept similar (2%) for the PR testing. Thus, higher active lever responding in nicotine PR sessions observed in nic/nic mice clearly indicates a stronger motivation to consume nicotine than saccharin in these animals as compared to their counterparts that received saline exposure during adolescence and adulthood. Consistent with the previous studies [15–17], our data indicate that adolescent nicotine exposure preferentially enhances the reinforcing properties of nicotine in adulthood. Interestingly, adolescent nicotine-exposed mice that received saline exposure as adults exhibited higher preference for oral saccharin than oral nicotine. These results align with a recent study that reported higher saccharin reinforcement during adulthood in adolescent mice that were pretreated with nicotine but not in adult nicotine pretreated mice [20]. It should be noted that the motivation to attain nicotine rewards in nic/sal mice remained comparable in sal/sal animals while it increased in nic/nic mice. Taken together, our findings suggest for the first time that adolescent and adult nicotine exposure differentially impacts the sensitivity to natural reinforcement and nicotine reinforcement in that adolescent nicotine exposure may potentiate the reinforcement sensitivity in general, but a second nicotine exposure may specifically shift the preference towards nicotine self-administration.
Non-contingent nicotine exposure in adulthood per se was also associated with higher breakpoints for oral nicotine as compared to oral saccharin. This may suggest that nicotine by itself possess a high reinforcing efficacy as suggested earlier [38]. However, the motivation to self-administer saccharin and nicotine remained similar in the sal/sal group. Repeated non-contingent exposure of nicotine was previously shown to potentiate the hedonic value of nicotine reward in adult mice [24]. Thus, a prior nicotine exposure may be critical for sensitization of the nicotine reward system. This contention is further supported by the observation that adolescent nicotine exposure potentiated the effect of adult nicotine exposure on break-points in nicotine PR sessions. Therefore, it is conceivable that adolescent nicotine exposure might serve as an intervening variable that influences the impact of adult nicotine on instrumental motivational processes subserving persistent nicotine intake.
Reinforcing effects of nicotine occur by stimulation of nAChRs, and the density and functionality of these receptors in different brain regions, including those that are engaged in regulating reward and motivation, was higher in adolescent mice as compared to the adult mice [5]. Thus, age-specific nicotine-induced alterations in reward sensitivity may involve changes in nAChRs and downstream signaling mechanisms. It is possible that observed differences in motivated behaviors might have occurred due to different doses (3mg/kg/d vs 6.3 mg/kg/d) of nicotine that were used to expose mice during the adolescence and adult period rather than the age of the animals. However, this seems less likely to be the case because plasma nicotine levels in adolescent mice were about 2 times higher than adult mice at a comparable dose of nicotine [39]. Thus pharmacokinetic differences might have compensated for any variation in motivated behavior that might have occurred due to differences in the dose.
A plethora of evidence from human and rodent studies indicates that cessation of chronic nicotine produces a state of anhedonia (reduced experience of pleasure) that is mostly associated with reduction in reward value for non-drug reinforcers [40–43]. Moreover, an imbalance in the relative reward value of nicotine versus monetary incentives (non-drug reinforcers) was observed in smokers with anhedonia [44]. In the current study, compared to the sal/sal mice, both sal/nic and nic/nic mice exhibited lower motivation to obtain saccharin reward while nicotine reinforcement was substantially enhanced in these animals. As noted previously, all behavioral testing was conducted following 2 weeks of nicotine-free period during adulthood when the acute nicotine abstinence phase was subsided. Anhedonia occurs as a consequence of dysregulation of the reward circuits and these affective symptoms have been observed during the protracted withdrawal associated with long-term substance use [45]. Although firm conclusions could not be drawn from the current data, it is plausible that reduction in positive affect associated with prolonged nicotine deprivation in adulthood but not adolescence might have contributed to the relative bias in the motivation to attain nicotine reinforcers over saccharin reinforcers.
Although, the interactions between sex, adolescent nicotine exposure and reward for breakpoints did not reach significance in our study presumably due to high behavioral variation and lower sample size, significant interactions effects between these variables on active lever responding and total reinforcers earned were noted. Specifically, female mice that receive non-contingent nicotine exposure during adolescence exhibited higher active lever responding for oral nicotine but not saccharin and consumed more nicotine as adults. This observation supports the results of a previous study that reported higher nicotine intake in adult female rats that initiated nicotine self-administration during adolescence as compared to those female rats that initiated self-administration in adulthood [16]. In another study, sex differences on the effects of adolescent nicotine exposure on conditioned nicotine reward were noted; the magnitude of nicotine reward in adolescent female rats was higher than adult female rats while this age-specific effect was not observed in male rats [46]. This further supports our observation that adolescent nicotine impacted the reward system differentially between male and female mice. Furthermore, our data also align well with human studies on smoking behavior that illustrate that females are less like to quit smoking and experience higher rates of relapse [47]. As discussed earlier, we observed a higher reinforcement for saccharin in the nic/sal group and this effect was more prominent in male mice. Moreover, nicotine reinforcement remained unaffected between saline- and nicotine-exposed adolescent male mice. Previous research in human subjects indicated that nicotine’s reinforcement-enhancing effects for non-drug rewards [48]. Likewise, adolescent nicotine exposure in male rats facilitated saccharin self-administration but not drug (alcohol) self-administration during adulthood in these animals [20]. The effects of adolescent nicotine exposure on the sensitization of the reward system are more generalized in males while they are more specific for drug reward in the females. However, this hypothesis warrants further investigation. Likewise, it remains to be seen whether sex differences in the effects of adolescent nicotine in adult motivated behaviors are driven by pharmacokinetics of nicotine or pharmacodynamics changes that involve differences in nAChR densities.
Interactions between the effects of adolescent and adult nicotine exposure on inactive lever responses during the PR sessions were surprising observations because these levers had no programmed consequence. Adult nicotine-exposure dramatically increased inactive lever presses during both the saccharin and nicotine self-administration sessions. Likewise, animals exposed to nicotine during adolescence regardless of adult nicotine exposure displayed a more pronounced increase in responding to the inactive levers; albeit reward-specific differences were also prominent. Specifically, the effects on inactive lever responding in saccharin self-administration sessions were moderated by adolescent nicotine exposure. Higher responding on inactive levers in self-administration paradigms could be indicative of non-specific behavioral activation and not the generalization effect that is typically seen during the earlier stages of instrumental conditioning [49]. Behavioral activation system is known to drive inhibitory control and impulsivity [50]. Thus increased inactive lever responding might reflect a higher state of impulsivity resulting in reward-based decision-making deficits (i.e. difficulty in selecting an appropriate reward strategy as a function of increasing schedule of reinforcement). This parsimonious explanation for the observed behavior is in accordance with previous studies that reported deficits in behavioral inhibition and cognitive control with chronic nicotine in adult rodents [33, 51, 52]. Adolescent nicotine exposure is known to exert detrimental effects on the prefrontal cortex and cognitive processes mediated by this brain region [53]. Moreover, adolescent rats exhibited more perseverative behavior than adults during extinction in an instrumental learning task and these age-specific differences were modulated by certain motivational factors [54]. It is plausible that nicotine experience during adolescence and adulthood might differentially impact motivated nicotine-taking behaviors by altering distinct components of impulse control.
There are several caveats associated with our study that requires a discussion. First, osmotic mini-pumps were employed to provide chronic nicotine exposure in mice during adolescence and adulthood. Because the half-life of nicotine in mice is relatively short (~10 min), non-contingent delivery of nicotine through subcutaneous mini-osmotic pumps is an efficient way to maintain plasma nicotine/cotinine concentration typically observed in human smokers and to induce nicotine dependence-like behaviors [26,29,30,57–59]. However, nicotine delivery via this approach does not truly reflect the pulsatile pattern of nicotine delivery in humans and this remains a shortcoming of this study. Second, the duration of nicotine exposure was higher for the nic/nic group (4 weeks; 2-weeks as adolescents and 2-weeks as adults) as compared to the nic/sal and sal/nic groups (2 weeks either as adolescents or adults). Therefore, we cannot entirely dismiss the possibility that longer duration of nicotine exposure in nic/nic mice rather than the timing of exposure (adolescence vs adult) might have contributed to observed differences in nicotine vs saccharin reinforcement in these animals. Third, nicotine is known to exert conditioned reinforcement effects [60–62]. Because nicotine solution was paired with saccharin for the nicotine PR testing, it is possible that animals responded for nicotine rewards due to conditioned enhancing effects on saccharin solution. As noted in Fig 4, the total number of saccharin and saccharin + nicotine reinforcers did not differ in the sal/sal (control) mice. Therefore, reinforcement enhancement as a consequence of the pairing of saccharin and nicotine solution seems unlikely. However, we cannot dismiss the possibility that previous non-contingent nicotine exposure (either as adolescent or adult or both) might have facilitated conditioned reinforcing effects of nicotine resulting in higher responding for nicotine + saccharin solution in the sal/nic and nic/nic mice.
Finally, it is noteworthy that the physical and negative affective symptoms such as anxiety-like behavior observed following acute nicotine withdrawal in adult mice did not last for more than a week [32, Cole, Gould and Parikh, unpublished observation]. Likewise, somatic symptoms were observed 24 hrs. following the removal of nicotine-containing osmotic minipumps in adolescent mice but these symptoms were not discernible after 2 weeks of washout period (see Results). This is in line with previous work from our group showing withdrawal symptoms in mice last approximately 4 days [56]. Thus, the effects of adolescent and adult nicotine exposure observed on instrumental motivational processes seem unlikely to have occurred as a consequence of acute nicotine abstinence syndrome. However, we could not rule out if cognitive and other affective components (e.g. anhedonia; see above) associated with protracted nicotine withdrawal syndrome that could be present 2-weeks post-nicotine cessation contributed to the observed effects on motivational processes.
In summary, the presented findings imply that adolescent nicotine exposure enhances nicotine reward responsiveness after further nicotine experience in adulthood. Long-term nicotine exposure in adulthood alone could also impact motivated behaviors presumably by blunting the affective response through the disruption of reward circuits. The adult behavioral effects could presumably be driven by cessation of nicotine and prolonged abstinence. Furthermore, nicotine- and saccharin-taking behavior could be impacted by alterations in cognitive processes that may occur as a consequence of prior nicotine exposure either during adolescence or adulthood. Because of the overlapping connectivity of brain circuits implicated in motivation, cognition and affect, it is possible that chronic nicotine-induced neuroadaptations in these circuits in an age-specific manner may eventually act in concert to escalate nicotine taking and seeking in adults that have been previously exposed to nicotine during adolescence. Lastly, our data for the first time shows the utility of using an oral nicotine self-administration paradigm in mice to study maladaptive nicotine-taking behaviors and the underlying neural substrates.
Highlights.
Adolescent nicotine exposure increased saccharin reinforcement in adulthood.
Adult nicotine re-exposure biased the sensitivity towards nicotine reward.
Both adolescent and adult nicotine exposure increased impulsive responding.
Adolescent and adult nicotine experience activated distinct behavioral mechanisms.
These mechanisms acted in concert to facilitate maladaptive nicotine taking.
Acknowledgments
This work was supported by grants from the National Institute of Health (DA037421 and DA017949).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest
The authors declare no conflict of interest.
References
- 1.Steinberg L, Morris AS. Adolescent development. Annual review of psychology. 2001;52:83–110. doi: 10.1146/annurev.psych.52.1.83. [DOI] [PubMed] [Google Scholar]
- 2.Steinberg L. Risk taking in adolescence: what changes, and why? Annals of the New York Academy of Sciences. 2004;1021:51–58. doi: 10.1196/annals.1308.005. [DOI] [PubMed] [Google Scholar]
- 3.Steinberg L. Cognitive and affective development in adolescence. Trends Cogn Sci. 2005;9:69–74. doi: 10.1016/j.tics.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Lydon DM, Wilson SJ, Child A, Geier CF. Adolescent brain maturation and smoking: what we know and where we’re headed. Neurosci Biobehav Rev. 2014;45:323–342. doi: 10.1016/j.neubiorev.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kota D, Martin BR, Robinson SE, Damaj MI. Nicotine dependence and reward differ between adolescent and adult male mice. J Pharmacol Exp Ther. 2007;322:399–407. doi: 10.1124/jpet.107.121616. [DOI] [PubMed] [Google Scholar]
- 6.Kota D, Sanjakdar S, Marks MJ, Khabour O, Alzoubi K, Damaj MI. Exploring behavioral and molecular mechanisms of nicotine reward in adolescent mice. Biochem Pharmacol. 2011;82:1008–1014. doi: 10.1016/j.bcp.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levin ED, Lawrence SS, Petro A, Horton K, Rezvani AH, Seidler FJ, Slotkin TA. Adolescent vs. adult-onset nicotine self-administration in male rats: duration of effect and differential nicotinic receptor correlates. Neurotoxicol Teratol. 2007;29:458–465. doi: 10.1016/j.ntt.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torres OV, Tejeda HA, Natividad LA, O’Dell LE. Enhanced vulnerability to the rewarding effects of nicotine during the adolescent period of development. Pharmacology, biochemistry, and behavior. 2008;90:658–663. doi: 10.1016/j.pbb.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vastola BJ, Douglas LA, Varlinskaya EI, Spear LP. Nicotine-induced conditioned place preference in adolescent and adult rats. Physiol Behav. 2002;77:107–114. doi: 10.1016/s0031-9384(02)00818-1. [DOI] [PubMed] [Google Scholar]
- 10.Belluzzi JD, Lee AG, Oliff HS, Leslie FM. Age-dependent effects of nicotine on locomotor activity and conditioned place preference in rats. Psychopharmacology. 2004;174:389–395. doi: 10.1007/s00213-003-1758-6. [DOI] [PubMed] [Google Scholar]
- 11.O’Dell LE, Bruijnzeel AW, Ghozland S, Markou A, Koob GF. Nicotine withdrawal in adolescent and adult rats. Ann N Y Acad Sci. 2004;1021:167–174. doi: 10.1196/annals.1308.022. [DOI] [PubMed] [Google Scholar]
- 12.O’Dell LE, Torres OV, Natividad LA, Tejeda HA. Adolescent nicotine exposure produces less affective measures of withdrawal relative to adult nicotine exposure in male rats. Neurotoxicol Teratol. 2007;29:17–22. doi: 10.1016/j.ntt.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aguirre CG, Madrid J, Leventhal AM. Tobacco withdrawal symptoms mediate motivation to reinstate smoking during abstinence. J Abnorm Psychol. 2015;124:623–634. doi: 10.1037/abn0000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doubeni CA, Reed G, Difranza JR. Early course of nicotine dependence in adolescent smokers. Pediatrics. 2010;125:1127–1133. doi: 10.1542/peds.2009-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adriani W, Deroche-Gamonet V, Le Moal M, Laviola G, Piazza PV. Preexposure during or following adolescence differently affects nicotine-rewarding properties in adult rats. Psychopharmacology (Berl) 2006;184:382–390. doi: 10.1007/s00213-005-0125-1. [DOI] [PubMed] [Google Scholar]
- 16.Levin ED, Rezvani AH, Montoya D, Rose JE, Swartzwelder HS. Adolescent-onset nicotine self-administration modeled in female rats. Psychopharmacology (Berl) 2003;169:141–149. doi: 10.1007/s00213-003-1486-y. [DOI] [PubMed] [Google Scholar]
- 17.Weaver MT, Geier CF, Levin ME, Caggiula AR, Sved AF, Donny EC. Adolescent exposure to nicotine results in reinforcement enhancement but does not affect adult responding in rats. Drug Alcohol Depend. 2012;125:307–312. doi: 10.1016/j.drugalcdep.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alajaji M, Lazenka MF, Kota D, Wise LE, Younis RM, Carroll FI, Levine A, Selley DE, Sim-Selley LJ, Damaj MI. Early adolescent nicotine exposure affects later-life cocaine reward in mice. Neuropharmacology. 2016;105:308–317. doi: 10.1016/j.neuropharm.2016.01.032. [DOI] [PubMed] [Google Scholar]
- 19.Dickson PE, Miller MM, Rogers TD, Blaha CD, Mittleman G. Effects of adolescent nicotine exposure and withdrawal on intravenous cocaine self-administration during adulthood in male C57BL/6J mice. Addict Biol. 2014;19:37–48. doi: 10.1111/j.1369-1600.2012.00496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bianchi PC, Carneiro de Oliveira PE, Palombo P, Leao RM, Cogo-Moreira H, Planeta CDS, Cruz FC. Functional inactivation of the orbitofrontal cortex disrupts context-induced reinstatement of alcohol seeking in rats. Drug Alcohol Depend. 2018;186:102–112. doi: 10.1016/j.drugalcdep.2017.12.045. [DOI] [PubMed] [Google Scholar]
- 21.Kemppainen H, Hyytia P, Kiianmaa K. Behavioral consequences of repeated nicotine during adolescence in alcohol-preferring AA and alcohol-avoiding ANA rats. Alcohol Clin Exp Res. 2009;33:340–349. doi: 10.1111/j.1530-0277.2008.00838.x. [DOI] [PubMed] [Google Scholar]
- 22.McQuown SC, Belluzzi JD, Leslie FM. Low dose nicotine treatment during early adolescence increases subsequent cocaine reward. Neurotoxicol Teratol. 2007;29:66–73. doi: 10.1016/j.ntt.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manzardo AM, Stein L, Belluzzi JD. Rats prefer cocaine over nicotine in a two-lever self-administration choice test. Brain Res. 2002;924:10–19. doi: 10.1016/s0006-8993(01)03215-2. [DOI] [PubMed] [Google Scholar]
- 24.Hilario MR, Turner JR, Blendy JA. Reward sensitization: effects of repeated nicotine exposure and withdrawal in mice. Neuropsychopharmacology. 2012;37:2661–2670. doi: 10.1038/npp.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brust V, Schindler PM, Lewejohann L. Lifetime development of behavioural phenotype in the house mouse (Mus musculus) Front Zool. 2015;12(Suppl 1):S17. doi: 10.1186/1742-9994-12-S1-S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Portugal GS, Wilkinson DS, Turner JR, Blendy JA, Gould TJ. Developmental effects of acute, chronic, and withdrawal from chronic nicotine on fear conditioning. Neurobiol Learn Mem. 2012;97:482–494. doi: 10.1016/j.nlm.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gould TJ, Wilkinson DS, Yildirim E, Blendy JA, Adoff MD. Dissociation of tolerance and nicotine withdrawal-associated deficits in contextual fear. Brain Res. 2014;1559:1–10. doi: 10.1016/j.brainres.2014.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turner JR, Castellano LM, Blendy JA. Parallel anxiolytic-like effects and upregulation of neuronal nicotinic acetylcholine receptors following chronic nicotine and varenicline. Nicotine Tob Res. 2011;13:41–46. doi: 10.1093/ntr/ntq206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.AlSharari SD, Akbarali HI, Abdullah RA, Shahab O, Auttachoat W, Ferreira GA, White KL, Lichtman AH, Cabral GA, Damaj MI. Novel insights on the effect of nicotine in a murine colitis model. J Pharmacol Exp Ther. 2013;344:207–217. doi: 10.1124/jpet.112.198796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davis JA, James JR, Siegel SJ, Gould TJ. Withdrawal from chronic nicotine administration impairs contextual fear conditioning in C57BL/6 mice. J Neurosci. 2005;25:8708–8713. doi: 10.1523/JNEUROSCI.2853-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tucker JS, Ellickson PL, Klein DJ. Predictors of the transition to regular smoking during adolescence and young adulthood. J Adolesc Health. 2003;32:314–324. doi: 10.1016/s1054-139x(02)00709-7. [DOI] [PubMed] [Google Scholar]
- 32.Damaj MI, Kao W, Martin BR. Characterization of spontaneous and precipitated nicotine withdrawal in the mouse. J Pharmacol Exp Ther. 2003;307:526–534. doi: 10.1124/jpet.103.054908. [DOI] [PubMed] [Google Scholar]
- 33.Parikh V, Cole RD, Patel PJ, Poole RL, Gould TJ. Cognitive control deficits during mecamylamine-precipitated withdrawal in mice: Possible links to frontostriatal BDNF imbalance. Neurobiol Learn Mem. 2016;128:110–116. doi: 10.1016/j.nlm.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith A, Roberts DC. Oral self-administration of sweetened nicotine solutions by rats. Psychopharmacology (Berl) 1995;120:341–346. doi: 10.1007/BF02311182. [DOI] [PubMed] [Google Scholar]
- 35.Brunzell DH, Chang JR, Schneider B, Olausson P, Taylor JR, Picciotto MR. beta2-Subunit-containing nicotinic acetylcholine receptors are involved in nicotine-induced increases in conditioned reinforcement but not PR responding for food in C57BL/6 mice. Psychopharmacology (Berl) 2006;184:328–338. doi: 10.1007/s00213-005-0099-z. [DOI] [PubMed] [Google Scholar]
- 36.Perkins KA, Doyle T, Ciccocioppo M, Conklin C, Sayette M, Caggiula A. Sex differences in the influence of nicotine dose instructions on the reinforcing and self-reported rewarding effects of smoking. Psychopharmacology (Berl) 2006;184:600–607. doi: 10.1007/s00213-005-0103-7. [DOI] [PubMed] [Google Scholar]
- 37.al’Absi M, Nakajima M, Allen S, Lemieux A, Hatsukami D. Sex differences in hormonal responses to stress and smoking relapse: a prospective examination. Nicotine Tob Res. 2015;17:382–389. doi: 10.1093/ntr/ntu340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Le Foll B, Wertheim C, Goldberg SR. High reinforcing efficacy of nicotine in non-human primates. PLoS One. 2007;2:e230. doi: 10.1371/journal.pone.0000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pietila K, Laakso I, Ahtee L. Chronic oral nicotine administration affects the circadian rhythm of dopamine and 5-hydroxytryptamine metabolism in the striata of mice. Naunyn Schmiedebergs Arch Pharmacol. 1995;353:110–115. doi: 10.1007/BF00168923. [DOI] [PubMed] [Google Scholar]
- 40.Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–79. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- 41.Bruijnzeel AW. Reward Processing and Smoking. Nicotine Tob Res. 2017;19:661–662. doi: 10.1093/ntr/ntw303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Powell J, Dawkins L, Davis RE. Smoking, reward responsiveness, and response inhibition: tests of an incentive motivational model. Biol Psychiatry. 2002;51:151–163. doi: 10.1016/s0006-3223(01)01208-2. [DOI] [PubMed] [Google Scholar]
- 43.Snuggs S, Hajek P. Responsiveness to reward following cessation of smoking. Psychopharmacology (Berl) 2013;225:869–873. doi: 10.1007/s00213-012-2874-y. [DOI] [PubMed] [Google Scholar]
- 44.Leventhal AM, Trujillo M, Ameringer KJ, Tidey JW, Sussman S, Kahler CW. Anhedonia and the relative reward value of drug and nondrug reinforcers in cigarette smokers. J Abnorm Psychol. 2014;123:375–386. doi: 10.1037/a0036384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hatzigiakoumis DS, Martinotti G, Giannantonio MD, Janiri L. Anhedonia and substance dependence: clinical correlates and treatment options. Front Psychiatry. 2011;2:10. doi: 10.3389/fpsyt.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lenoir M, Starosciak AK, Ledon J, Booth C, Zakharova E, Wade D, Vignoli B, Izenwasser S. Sex differences in conditioned nicotine reward are age-specific. Pharmacol Biochem Behav. 2015;132:56–62. doi: 10.1016/j.pbb.2015.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harris KK, Zopey M, Friedman TC. Metabolic effects of smoking cessation. Nat Rev Endocrinol. 2016;12:299–308. doi: 10.1038/nrendo.2016.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perkins KA, Karelitz JL, Boldry MC. Nicotine Acutely Enhances Reinforcement from Non-Drug Rewards in Humans. Front Psychiatry. 2017;8:65. doi: 10.3389/fpsyt.2017.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ikemoto S, Wise RA. Rewarding effects of the cholinergic agents carbachol and neostigmine in the posterior ventral tegmental area. J Neurosci. 2002;22:9895–9904. doi: 10.1523/JNEUROSCI.22-22-09895.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Quilty LC, DeYoung CG, Oakman JM, Bagby RM. Extraversion and behavioral activation: integrating the components of approach. J Pers Assess. 2014;96:87–94. doi: 10.1080/00223891.2013.834440. [DOI] [PubMed] [Google Scholar]
- 51.Kolokotroni KZ, Rodgers RJ, Harrison AA. Effects of chronic nicotine, nicotine withdrawal and subsequent nicotine challenges on behavioural inhibition in rats. Psychopharmacology (Berl) 2012;219:453–468. doi: 10.1007/s00213-011-2558-z. [DOI] [PubMed] [Google Scholar]
- 52.Ortega LA, Tracy BA, Gould TJ, Parikh V. Effects of chronic low- and high-dose nicotine on cognitive flexibility in C57BL/6J mice. Behav Brain Res. 2013;238:134–145. doi: 10.1016/j.bbr.2012.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goriounova NA, Mansvelder HD. Nicotine exposure during adolescence alters the rules for prefrontal cortical synaptic plasticity during adulthood. Front Synaptic Neurosci. 2012;4:3. doi: 10.3389/fnsyn.2012.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sturman DA, Mandell DR, Moghaddam B. Adolescents exhibit behavioral differences from adults during instrumental learning and extinction. Behav Neurosci. 2010;124:16–25. doi: 10.1037/a0018463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cole RD, Poole RL, Guzman DM, Gould TJ, Parikh V. Contributions of beta2 subunit-containing nAChRs to chronic nicotine-induced alterations in cognitive flexibility in mice. Psychopharmacology (Berl) 2015;232:1207–1217. doi: 10.1007/s00213-014-3754-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gould TJ, Portugal GS, Andre JM, Tadman MP, Marks MJ, Kenney JW, Yildirim E, Adoff M. The duration of nicotine withdrawal-associated deficits in contextual fear conditioning parallels changes in hippocampal high affinity nicotinic acetylcholine receptor upregulation. Neuropharmacology. 2012;62:2118–2125. doi: 10.1016/j.neuropharm.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC, Gardiner PS, Grady SR, Heberlein U, Leonard SS, Levin ED, Lukas RJ, Markou A, Marks MJ, McCallum SE, Parameswaran N, Perkins KA, Picciotto MR, Quik M, Rose JE, Rothenfluh A, Schafer WR, Stolerman IP, Tyndale RF, Wehner JM, Zirger JM. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology. 2007;190:269–319. doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- 58.Leach PT, Holliday E, Kutlu MG, Gould TJ. Withdrawal From Chronic Nicotine Reduces Thyroid Hormone Levels and Levothyroxine Treatment Ameliorates Nicotine Withdrawal-Induced Deficits in Hippocampus-Dependent Learning in C57BL/6J Mice. Nicotine Tob Res. 2015;17:690–696. doi: 10.1093/ntr/ntu229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cohen A, George O. Animal models of nicotine exposure: relevance to second-hand smoking, electronic cigarette use, and compulsive smoking. Frontiers in psychiatry. 2013;4:41. doi: 10.3389/fpsyt.2013.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Olausson P, Jentsch JD, Taylor JR. Nicotine enhances responding with conditioned reinforcement. Psychopharmacology (Berl) 2004;171:173–178. doi: 10.1007/s00213-003-1575-y. [DOI] [PubMed] [Google Scholar]
- 61.Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib M, Craven L, Palmatier MI, Liu X, Sved AF. Operant responding for conditioned and unconditioned reinforcers in rats is differentially enhanced by the primary reinforcing and reinforcement-enhancing effects of nicotine. Psychopharmacology (Berl) 2006;189:27–36. doi: 10.1007/s00213-006-0522-0. [DOI] [PubMed] [Google Scholar]
- 62.Barrett ST, Bevins RA. Nicotine Enhances Operant Responding for Qualitatively Distinct Reinforcers Under Maintenance and Extinction Conditions. Pharmacol Biochem Behav. 2013;114–115:9–15. [PMC free article] [PubMed] [Google Scholar]


