Abstract
Whereas hypertension, diabetes, and dyslipidemia are age-related risk factors for cardiovascular disease (CVD) and chronic kidney disease (CKD), aging alone is an independent risk factor. With advancing age, the heart and kidney gradually but significantly undergo inflammation and subsequent fibrosis, which eventually results in an irreversible decline in organ physiology. Through cardiorenal network interactions, cardiac dysfunction leads to and responds to renal injury, and both facilitate aging effects. Thus, a comprehensive strategy is needed to evaluate the cardiorenal aging network. Common hallmarks shared across systems include extracellular matrix (ECM) accumulation, along with upregulation of matrix metalloproteinases (MMPs) including MMP-9. The wide range of MMP-9 substrates, including ECM components and inflammatory cytokines, implicates MMP-9 in a variety of pathological and age-related processes. In particular, there is strong evidence that inflammatory cell-derived MMP-9 exacerbates cardiorenal aging. This review explores the potential therapeutic targets against CVD and CKD in the elderly, focusing on ECM and MMP roles.
Keywords: Cardiorenal aging, Inflammaging, Fibrosis, Extracellular matrix, Matrix metalloproteinase-9
1. Introduction
Aging is an independent risk factor for cardiovascular disease (CVD) and chronic kidney disease (CKD), and elderly patients diagnosed with these diseases have poor clinical outcomes. CVD and CKD not only share many age-related risk factors including hypertension and diabetes but also interact in an interdependent and bidirectional manner (Abadir et al., 2011; Boudoulas, Triposkiadis, Parissis, Butler, & Boudoulas, 2017; Ebert et al., 2017). In elderly individuals, the immune response to new pathogens is often impaired, and this diminished immune response is termed immunosenescence (Oishi & Manabe, 2016). Immunosenescence is characterized by an elevated basal systemic in-flammatory state. Higher levels of circulating proinflammatory cytokines are observed in the elderly even in the absence of overt infection and chronic disease, a process termed inflammaging (Bruunsgaard & Pedersen, 2003).
Inflammation induces the production and release of fibrogenic cytokines and growth factors, leading to fibrosis (Wynn, 2008). The accumulation of fibrillar collagens leads to irreversible dysfunction and results in heart failure and end-stage renal failure (Biernacka & Frangogiannis, 2011; Wynn, 2008; Zhou et al., 2008). Although matrix metalloproteinases (MMPs) are traditionally believed to suppress fibrosis because of their proteolytic activity, MMPs, particularly MMP-9, play key roles in stimulating fibrillar extracellular matrix (ECM) collagen accumulation (Giannandrea & Parks, 2014; Horn et al., 2012; Horn & Trafford, 2016; Tan & Liu, 2012).
In this review, we summarize current findings regarding the mechanisms of cardiorenal aging and fibrosis, including recent reports showing common mechanisms that may provide comprehensive anti-aging strategies. This review focuses on the roles of ECM and MMPs as common underlying mediators. Assessment of age-related alterations in cardiac and renal ECM and MMPs may provide common and distinct therapeutic targets for CVD and CKD.
2. Cardiorenal interactions
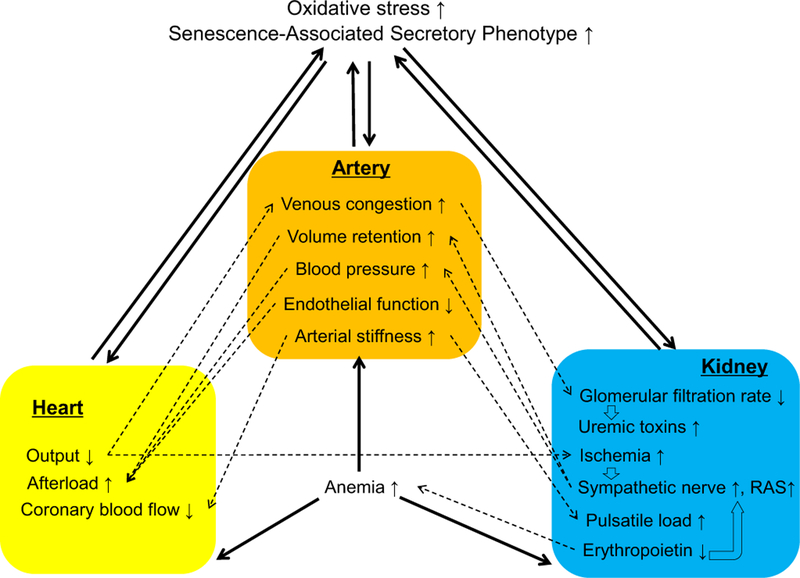
Approximately 25% patients with chronic heart failure have renal dysfunction, and renal dysfunction is one of the most important predictors of mortality, prolonged hospitalization, and rehospitalization in patients with heart failure (Forman et al., 2004; Gottlieb et al., 2002; Hillege et al., 2000; Hillege et al., 2006). Conversely, a major cause of death in patients with primary renal disease is CVD, and reversal of renal function by renal transplantation results in normalization of cardiac physiology (Tonelli et al., 2006; Wali et al., 2005). Cardiorenal interactions include not only secondary effects of common risk factors, including aging, hypertension, diabetes mellitus, and atherosclerosis, but also a vicious bidirectional effect. In this section, by reviewing the mechanisms of cardiorenal interaction which are reported previously in the settings of CVD and CKD, we would like to strengthen the importance to protect both the heart and kidney in the elderly stage (Fig. 1).
Fig. 1.
Mechanisms of cardiorenal interaction, showing the association among cardiac, renal and vascular dysfunction. While aging itself and common diseases such as hypertension, diabetes, and dyslipidemia are important risk factors for cardiovascular disease and chronic kidney disease, cardiac, renal, and vascular dysfunction exacerbate each other. Hatched lines show physiological interactions, and solid lines indicate the adverse interactions among organs.
2.1. Cardiac-renal interactions
Reduced renal perfusion, due to reduced cardiac output in heart failure, is likely to lead to the progressive decline in glomerular filtration rate (GFR). However, ESCAPE (Evaluation Study of Congestive Heart failure and Pulmonary Catherization Effectiveness) found no correlation between cardiac index (hemodynamic variables) and renal function (serum creatinine) (Bhatia et al., 2006; Nohria et al., 2008). Instead, venous congestion, rather than arterial blood flow, is likely to be an important mediator. Elevated central venous and intraabdominal pressure causes an insufficient pressure gradient across capillary networks, including those in the renal circulation, resulting in reduced renal blood flow and GFR (Bock & Gottlieb, 2010; Mullens et al., 2008; Winton, 1931).
Decline in cardiac output and consequent renal and pressure-sensing baroreceptor blood flow reduction cause renal ischemia and stimulate sympathetic nerves and the renin-angiotensin-aldosterone system, which causes sodium and volume retention and left ventricular remodeling. Renal sympathetic denervation shows significant improvements in resistant hypertension, left ventricular hypertrophy, and diastolic function without significant relation to blood pressure and heart rate, suggesting that renal sympathetic activation affects both vascular and cardiac function (Schirmer et al., 2014). As described below, angiotensin II and aldosterone induce oxidative stress and inflammation, resulting in excessive fibrosis in both organs.
Natriuretic peptides, such as atrial natriuretic peptide, brain natriuretic peptide, C-type natriuretic peptide, and N-terminal prohormone of brain natriuretic peptide, are neurohormones released from the heart, brain, and other organs to compensate cardiovascular derangement (Federico, 2010). Natriuretic peptides exert opposite effects to activate the sympathetic nerves and the renin-angiotensin-aldosterone system, because they decrease central venous pressure, sodium retention, and systemic and intrarenal vascular resistance and improve myocardial contractility and GFR. Maintaining and activating the natriuretic peptides could put a brake on adverse cardiorenal interactions.
2.2. Renal-cardiac interaction
Renal dysfunction causes sodium and water retention and hyper-tension, resulting in increased cardiac afterload and reduced catecholamine clearance to further activate sympathetic nerves (Laederach & Weidmann, 1987). Protein-bound uremic toxins, such as indoxyl sulfate and p-cresyl sulfate, cause cardiovascular and renal injury by inducing oxidative stress (Lekawanvijit, Kompa, Wang, Kelly, & Krum, 2012).
While erythropoietin increases in response to anemia, insensitivity to erythropoietin in heart failure causes inflammation and advanced kidney diseases result in anemia by the absolute deficiency of erythropoietin (Bock & Gottlieb, 2010). Since erythropoietin can protect cardiac, vascular, and renal cells by its anti-apoptotic, anti-oxidative, and anti-inflammatory effects, erythropoietin deficiency may exacerbate cardiorenal interactions by not only inducing ischemia but also taking away cell protective actions (Toba et al., 2009; Toba et al., 2010; Toba et al., 2011; Toba et al., 2012; Wang et al., 2013).
2.3. Vascular-cardiac interactions
Age-related arterial alterations include endothelial dysfunction, wall thickening, and narrowing diameter. The presence of endothelial dys-function is an independent risk factor for both progression to and adverse outcomes after heart failure development (Fischer et al., 2005; Heitzer, Baldus, von Kodolitsch, Rudolph, & Meinertz, 2005; Meyer et al., 2005). Cushioning effects of the elastic arteries are needed for their expansion during systole and delivery of blood to the coronary arteries during diastole. Arterial stiffness of the coronary artery impairs coronary blood flow, because the coronary arteries are perfused during diastole (O’Rourke & Hashimoto, 2007; Zieman, Melenovsky, & Kass, 2005). Increased stiffness of larger arteries increases afterload (Tomiyama & Yamashina, 2015).
Arterial-ventricular coupling (EA/ELV) is maintained within a narrow range to provide optimal energetic efficiency at the expense of mechanical efficacy. Whereas this coupling at rest is optically maintained during healthy aging, the coupling during acute maximal exercise is impaired with age. Arterial stiffness, in particular the larger arteries, contributes to increases in resting EA, leading to abnormal coupling (Tomiyama & Yamashina, 2015).
2.4. Vascular-renal interactions
Increased blood pressure is a critical risk factor for renal damage, and arterial stiffening results in a decrease of the pressure gradient in the arterial tree, where the pressure at more distal sites is higher than more central sites (O’Rourke & Hashimoto, 2007; Tomiyama & Yamashina, 2010).
Increased aortic stiffness attenuates its shock absorbing effects against cardiac constriction, resulting in increases in the transmission of the heart-generated pulsatile energy to the peripheral circulation, including renal circulation. This phenomena lead to pulsatile nephropathy, which results in decline of renal function (O’Rourke & Hashimoto, 2007; O’Rourke, Safar, & Dzau, 2010; Tomiyama & Yamashina, 2010).
2.5. Senescence-associated secretory phenotype (SASP)
Senescent cells produce a complex mixture of secreted factors that include proinflammatory cytokines, chemokines, growth factors, and proteases (Coppe, Desprez, Krtolica, & Campisi, 2010; Kuilman & Peeper, 2009). The SASP secretion pattern is stimulated by various mechanisms, including sustained DNA damage, oxidative stress, and NF-κB (McHugh & Gil, 2018). One important function of SASP is recruiting immune cells to eliminate antigens and senescent cells and to activate cell proliferation and differentiation of progenitor and stem cells to repair tissues. However, senescent cells that are not repaired successfully secrete more SASP, further promoting cell senescence (Wang, Cai, & Chen, 2017). The SASP can accelerate both autocrine and paracrine senescent responses, suggesting important roles as a mediator of cardiorenal interaction and inflammaging (Acosta et al., 2013; McHugh & Gil, 2018).
3. Characterization of aging effects on heart and kidney
The heart and kidney undergo progressive and inevitable senescence, characterized by a gradual decline in cell structure and physiology that are independent of changes due to concomitant hypertension or diabetes (Table 1) (Lakatta, 2015). Normal aging is gradual, with small individual effect size, such that values are often still within the physiological range. The superimposition of age-related diseases on aging amplifies and accelerates functional decline to result in pathology (Lakatta, 2015).
Table 1.
Characterization of Aging Effects on Physiology and Structure in Heart and Kidney.
| Age-related changes | ||
|---|---|---|
| Heart | Physiology | Diastolic function ↓, Systolic function ↔, Maximum heart rate ↓ |
| End diastolic and systolic volume in response to postural maneuvers and during exercise ↓ | ||
| Structure | Left ventricular concentric hypertrophy ↑, Cardiomyocyte number 4, Cardiomyocyte size ↓, Amyloid and lipofuscin accumulation ↑, Vessel density ↓, Inflammation ↑, Perivascular and interstitial fibrosis ↑ | |
| Kidney | Physiology | |
| Glomerular | Glomerular filtration rate ↓, Hyperfiltration ↑ | |
| Tubular | Urine concentration ↓, Electrode reabsorption and secretion regulation ↓ | |
| Acid secretion↔ | ||
| Endocrine | Renin release ↓, Erythropoietin production ↔, Conversion from 25-hydroxyvitamin D to 1,25-dihydroxyvitamin D ↓ | |
| Structure | ||
| Global | Kidney mass ↓, Parenchymal thickness ↓, Cortex volume ↓, Medullary volume ↑ | |
| Renal sinus fat ↑, Parenchymal, parapelvic, and hyperdense cysts ↑, Angiomyolipomas ↑ | ||
| Nephron number ↓, Inflammation ↑ | ||
| Glomerular | Global glomerulosclerosis ↑, Podocyte injury and loss ↑, Basement membrane thickness ↑ | |
| Tubular | Tubular atrophy ↑, Intratubular cast ↑, Peritubular capillary ↓, Tubulointerstitial fibrosis ↑, Basement membrane thickness ↑ | |
| Vascular | Arteriosclerosis ↑, Alomerular arterioles ↑ |
↑ increase, ↓decrease, ↔ unchanged.
3.1. Heart
3.1.1. Physiology
Early diastolic filling begins to slow beginning around 20 years of age in humans, with a reduction of about 50% by the age of 80 (Cannata, Camparini, Sinagra, Giacca, & Loffredo, 2016). This reduction in diastolic filling is attributed to ECM accumulation within the myocardium, decreased elasticity of the left ventricle (LV), and a delay in active relaxation due to residual myofilament Ca2+ activation (Horn & Trafford, 2016; Lakatta, 2003).
The decrease in early to late (E/A) relaxation ratio in the elderly is termed diastolic dysfunction and is a hallmark of cardiac aging (Dai, Chen, Johnson, Szeto, & Rabinovitch, 2012; Ferrari, Radaelli, & Centola, 2003; Steenman & Lande, 2017). Diastolic dysfunction predisposes an individual to develop heart failure with preserved ejection fraction. More than half of heart failure cases in the elderly (>75 years old) are diagnosed as diastolic heart failure (de Freitas, Batlouni, & Gamarsky, 2012). Decreased activity and protein levels of sarcoplasmic reticulum Ca2+ ATPase pump (SERCA), which causes relaxation of the cardiac muscle following the excitatory effects of high cytosolic Ca2+, in the aged heart may account for prolongation of relaxation and thus diastolic dysfunction (Lakatta, 2003).
To maintain LV filling in late diastole, atrial contraction gradually increases with age, which induces atrial hypertrophy and increases the risk for atrial fibrillation (Steenman & Lande, 2017). Despite a decline of diastolic function with age, LV ejection fraction, a most commonly used clinical parameter for LV systolic function, and stroke volume are relatively preserved at rest (Lakatta & Levy, 2003).
Cardiac reserve shows age-related reduction, which is involved in a decrease in maximum heart rate due to a dropout of pacemaker cells and a decreased responsiveness to β-adrenergic receptor and a deficit in the ability to reduce end-systolic volume achieved during exercise (Lakatta, 2015; Roh, Rhee, Chaudhari, & Rosenzweig, 2016; Steenman & Lande, 2017; Sun et al., 2017). The additional LV filling time causes the aged LV to expand to a larger size during diastole, which allows the aged LV to pump out about as much blood as the young LV despite the larger systolic chamber.
3.1.2. Structure
Age-related structural change is characterized by LV concentric hypertrophy (Chiao & Rabinovitch, 2015; Ferrari et al., 2003; Horn & Trafford, 2016; Lakatta & Levy, 2003). The increase in LV wall thickness is seen as a compensatory response after cardiomyocyte loss due to increased apoptosis and necrosis (Dai & Rabinovitch, 2009; Zamilpa, Navarro, Flores, & Griffey, 2014). This compensation contributes to the enlargement of residual cardiomyocytes (Horn & Trafford, 2016). The loss of cardiomyocytes with age stimulates the accumulation of fibrillar ECM components within the interstitial space (Horn & Trafford, 2016). Age-related increases in LV afterload, due to arterial stiffening and subsequent hypertension, also exacerbate LV hypertrophy and prolonged LV relaxation in diastole (Lakatta, 2003, 2015; North & Sinclair, 2012).
Amyloid and lipofuscin accumulate in aged cardiomyocytes (Shioi & Inuzuka, 2012). The presence of amyloid strongly correlates with the age at time of death in the >85 year old population (Tanskanen et al., 2008). Lipofuscin is an autofluorescent substance composed primarily of crosslinked protein and lipid residues that accumulates over time in lysosomal of cardiomyocytes due to an imbalance between protein damage and clearance of the damaged proteins (Terman & Brunk, 1998). Lipofuscin induces cell death and inhibits mitochondrial function, suggesting deleterious cardiomyocyte roles for lipofuscin in age-related cardiac dysfunction (Han, Li, & Liu, 2013).
3.1.3. Cardiac inflammaging and fibrosis
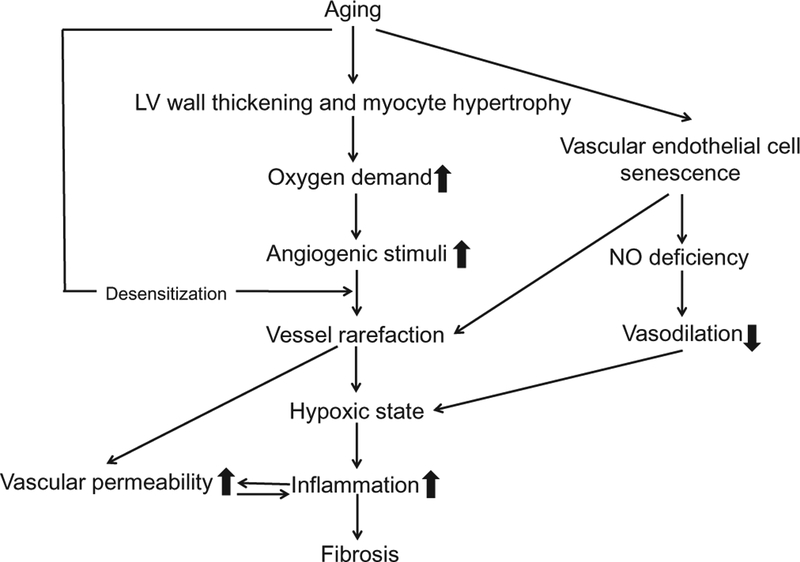
Age-related LV wall thickening and myocyte hypertrophy results in an additional oxygen demand (Fig. 2). Higher plasma concentrations of von Willebrand factor and LV expression of vascular endothelial cell growth factor (VEGF) are observed in aged mice and are indicative of an increased stimulation for angiogenesis (Yabluchanskiy et al., 2014). Despite an increase in proangiogenic stimuli, the number of vessels in the aged LV was not increased (Yabluchanskiy et al., 2014).
Fig. 2.
Mechanistic figure showing the development process of inflammation and fibrosis in the aging left ventricle (LV). Age-related LV wall thickness and myocyte hypertrophy generate an additional oxygen demand, which in turn increases the production of angiogenic stimuli. Despite the production of angiogenic stimuli, blood vessel numbers do not increase in aging LV. Together with nitric oxide deficiency due to endothelial cell senescence, vessel rarefaction leads to an hypoxic state, which triggers inflammation and subsequent fibrosis.
Insufficient vascularization leads to an imbalance of oxygen supply and consequently a hypoxic environment. In addition to vessel rarefaction, nitric oxide (NO) deficiency in coronary artery and arterioles, due to endothelial cell senescence, endothelial NO synthase (eNOS) inactivation, reactive oxygen species (ROS)-induced decline in NO bioavail-ability, and vasoconstriction, exacerbates ischemic statement in the aged heart (North & Sinclair, 2012). Hypoxic state triggers an inflammatory response in the myocardium. LV vascular permeability, the excessive leakage of fluid and proteins from vessels to the interstitial space, increases with age, which is associated with endothelial cell apoptosis, oxidative stress, and inflammation (Yabluchanskiy et al., 2014). High susceptibility to ischemia/reperfusion injury in aged hearts may be due to the potentially high vascular permeability (Oakley & Oakley & Tharakan, 2014).
Vascular cell adhesion molecule 1 and integrin αE mRNAs, both of which are involved in adhesion of inflammatory cells, increase with age in mouse LV (1.9-fold for both) (Toba et al., 2016). Indeed, macrophage numbers are approximately 4-fold age-related increased in the LVs of N18 month old mice compared to 3–5 month old mice. Age-related cardiac inflammation is a milder response than what is observed after pathological conditions, in which peak macrophage infiltration increases by approximately 100-fold with myocardial infarction and 10-fold with hypertensive heart failure (Halade, Jin, & Lindsey, 2013; Xia et al., 2009). Changes in vascular cell adhesion molecule 1 and integrin αE mRNAs in the myocardial setting (infarct area of day 5 after myocar-dial infarction in mice) are more drastic than normal aging and show 5.3- and 4.0-fold increases, respectively (Zamilpa et al., 2012). Since neutrophil numbers do not show such dramatic age-related changes, macrophages are the prominent player in cardiac inflammaging (Chiao et al., 2012; Toba et al., 2015).
In murine LV, the mRNA levels of pro-inflammatory cytokines and their receptors such as chemokine (C-C motif) ligand (Ccl)5, chemokine (C-X3-C motif) ligand 1, CC chemokine receptor (Ccr)2, Ccxr3, and macrophage migration inhibitory factor (Mif) are increased in an age-dependent manner with 2.3-fold, 1.6-fold, 2.0-fold, and 3.6-fold increases (young vs. old mice), respectively (Toba et al., 2015). In infarct area of day5 post-myocardial infarction, Cx3cl1 and Ccr2 mRNA levels show 1.8-fold and 4.4-fold increases (Zamilpa et al., 2012). Ccl5, chemokine (C-X3-C motif) ligand 1, Ccr2, and Cxcr3 play roles in inducing migration and recruitment of inflammatory cells (Altin & Schulze, 2011; Dawson, Miltz, Mir, & Wiessner, 2003; Ferrandi et al., 2007; Houard et al., 2009). Mif, which regulates the release of pro-inflammatory mediators, is released in response to a brief hypoxia in the heart (Koga et al., 2011). In old healthy subjects, plasma monocyte chemoattractant protein (MCP)-1, Ccl5, and Mif levels are higher than young controls (Mansfield et al., 2012; Rammos et al., 2014).
Macrophages are classified into pro-inflammatory (classically activated) M1 and anti-inflammatory (alternatively activated) M2 macrophages in vitro, a naming system that overly simplifies the in vivo scenario but is used here for simplicity. Cardiac tissue resident macrophages express a number of anti-inflammatory M2 macrophage markers in the young mice and are important for maintenance of cardiac homeostasis (Pinto et al., 2012). Markers of M1 macrophages include interleukin (IL)-6 and tumor necrosis factor (TNF) −α, and are increased with age, while the M2 marker Fizz-1 is decreased with age, indicating M1 > M2 polarization in the aged LV (Toba et al., 2015). The increase in pro-inflammatory cytokines from M1 macrophages likely stimulates the further accumulation of macrophages in the LV.
Cardiac inflammation causes ECM fibrillar collagen accumulation by stimulating the release of fibrogenic cytokines and growth factors (Wynn, 2008), particularly transforming growth factor (TGF)-β (Biernacka & Frangogiannis, 2011). In humans without CVD history, collagen content increases from 3.9 ± 0.8% at 20–25 years of age to 5.9 ±0.8% at 67–87 years of age; and similar rates of change are seen in mice (Chiao et al., 2012; Gazoti Debessa, Mesiano Maifrino, & Rodrigues de Souza, 2001; Toba et al., 2016; Toba et al., 2017). Of note, mice do not develop hypertension with age, making them a good model to study aging only effects. In the aged murine LV, TGF-β-induced protein (Tgfbi) and phosphorylated Smad2 increase and correlate with the modest but significant picrosirius red-positive collagen deposition (Chiao et al., 2012). Mechanisms of TGF-β-induced fibrosis include enhanced fibroblast proliferation and myofibroblast transdifferentiation, ECM protein synthesis, and matrix preservation by suppressing MMP activity by inducing protease inhibitors (Biernacka & Frangogiannis, 2011).
Fibroblasts are a predominant non-cardiomyocyte cell type in the heart (human, rat, mice) and play a pivotal role in regulating ECM maintenance (Camelliti, Borg, & Kohl, 2005). TGF-β is the primary fibrogenic factor that promotes cardiac fibrosis not only by inducing synthesis of ECM proteins and decreasing collagen degradation but also by cardiac fibroblast transdifferentiation to myofibroblast. TGF-β signaling play a crucial role in myofibroblast differentiation by inducing α-smooth muscle actin transcription (Pardali, Sanchez-Duffhues, Gomez-Puerto, & Dijke, 2017). Cardiac fibroblasts derive from activation and proliferation of resident fibroblasts and recruitment from other cell types (Camelliti et al., 2005). Mice deficient in plasminogen activator inhibitor-1 exhibit increased collagen deposition and TGF-β-induced epithelial-mesenchymal transition (EMT) in the aged heart, indicating that EMT contributes to age-related cardiac fibrosis (Ghosh et al., 2010).
The rate of newly-synthesized collagen in the rat myocardium remains steady until the age of 15 months and then begins to decreases around 24 months (Mays, McAnulty, Campa, & Laurent, 1991). ECM post-translational modifications play more critical roles in collagen accumulation than new synthesis. This is consistent with the phenotype of aging LV fibroblasts, which exhibit decreased proliferative and migratory capacities (Lindsey et al., 2005). Collagen solubility changes with age in the LV, indicating an alteration in collagen type and degree of crosslinking. In human hearts, the number of large diameter collagen fibrils increases with age, indicating more collagen crosslinking (Mendes et al., 2012). Lysyl oxidase, which stabilizes fibrillar collagen by producing covalent crosslinking of collagen fibrils, increases with age (Thomas, Cotter, Li, McCormick, & Gosselin, 2001).
Collagen type I and III are the most abundant collagens in the heart, with collagen I predominating (Meschiari, Ero, Pan, Finkel, & Lindsey, 2017). Old hearts exhibit the decreased ratio of collagen I to collagen III content. Since collagen I has high tensile strength and collagen III is more distensible, the alteration of collagen types as well as increased total collagen content may contribute to age-related myocardial stiffness (Meschiari et al., 2017). An increase in the collagen III proportion is also reported (Table 2) (Biernacka & Frangogiannis, 2011).
Table 2.
Summary of Age-related Changes in ECM in Heart and Kidney.
| Change with age | Sample type | Species | References | ||
|---|---|---|---|---|---|
| Heart | Collagen I | ↑ | Protein | C57BL/6, human | (Cieslik et al., 2011; Gazoti Debessa et al., 2001) |
| ↓ | mRNA | C57BL/6, SD rat, Fischer rat, FBNF1 rat | (Annoni et al, 1998; Kwak et al, 2011; Thomas, Zimmerman, Hansen, Martin, & McCormick 2000; Toba et al, 2016) | ||
| ↔ | mRNA | Sheep | (Horn et al, 2012) | ||
| ↓ | mRNA | C57BL/6J | (Chiao et al, 2012) | ||
| Collagen III | ↓ | protein | C57BL6/J | (Padmanabhan Iyer et al, 2016) | |
| ↓ | mRNA | C57BL/6, SD rat, Fischer rat, C57BL/6J | (Annoni et al, 1998; Chiao et al, 2012; Thomas et al, 2000; Toba et al, 2016) | ||
| Collagen IV | ↓ | mRNA | C57BL/6 | (Toba et al., 2016) | |
| Collagen V | ↓ | mRNA | C57BL/6 | (Toba et al., 2016) | |
| Collagen XV | ↓ | Protein | C57BL6/J | (Padmanabhan Iyer et al, 2016) | |
| Fibronectin | ↓ | mRNA | C57BL/6 | (Toba et al., 2016) | |
| Laminin | ↑ | mRNA | C57BL/6 | (Toba et al., 2016) | |
| ↓ | mRNA | C57BL/6 | (Toba et al., 2016) | ||
| Osteopontin | ↓ | mRNA | C57BL/6, SD rat | (Chiao et al, 2012; Graf et al, 1997) | |
| SPARC | ↑ | Protein | C57BL/6,C57B16/SV129, sheep | (Bradshaw et al, 2010; Horn et al, 2012; Toba et al, 2016) | |
| ↓ | mRNA | C57BL/6 | (Toba et al., 2016) | ||
| Thrombospondin-1 | ↑ | Protein | Kunming mouse | (Cai, Yuan, Fei, & Zhao, 2012) | |
| Kidney | Collagen I | ↑ | Protein | SD rat, C57BL/6 | (Gagliano et al., 2000; Hou et al., 2016) |
| ↔ | Protein | Human | (Eikmans, Baelde, de Heer, & Bruijn, 2001) | ||
| ↑ | mRNA | Human, Fischer rat | (Eikmans et al, 2001; Oelusarz et al, 2013) | ||
| → | mRNA | Fischer rat, SD rat | (Gagliano et al., 2000; Sangaralingham et al, 2016) | ||
| ↓ | mRNA | Wistar rat | (Hultstrom et al., 2012) | ||
| Collagen III | ↑ | Protein | NIH mouse, C57BL/6 | (Hou et al, 2016; Zhang et al, 2006) | |
| ↓ | Protein | SDrat | (Gagliano et al., 2000) | ||
| ↑ | mRNA | Fischer rat | (Oelusarz et al., 2013) | ||
| → | mRNA | SDrat | (Gagliano et al., 2000) | ||
| ↓,↔ | mRNA | Fischer rat | (Sangaralingham et al., 2016) | ||
| ↓ | mRNA | Wistar rat | (Hultstrom et al., 2012) | ||
| Collagen IV | ↑ | Protein | NIH mouse, human, mouse, DSrat | (Eikmans et al, 2001; Maric et al, 2004; Schneider et al, 2017; Zhang et al, 2006) | |
| ↔ | mRNA | Human | (Eikmans et al., 2001) | ||
| ↓ | mRNA | Wistar rat | (Hultstrom et al., 2012) | ||
| Collagen VI | ↓ | mRNA | Wistar rat | (Hultstrom et al., 2012) | |
| Fibronectin | ↑ | Protein | C57BL/6 | (Hou et al., 2016) | |
| ↓ | mRNA | Wistar rat | (Hultstrom et al., 2012) | ||
| Laminin | ↑ | Protein | DSrat | (Maric et al., 2004) | |
| Osteopontin | ↑ | mRNA | Wistar rat | (LiangS Barnes, 1995) | |
| SPARC | ↓ | mRNA | Wistar rat | (Hultstrom et al., 2012) | |
| Thrombospondin-1 | ↑ | Protein | SDrat | (Kang et al., 2001) |
↑ increased, ↓decreased, ↔ unchanged, ECM extracellular matrix, SPARC secreted protein acidic and rich in cysteine, SD Sprague-Dawley, DS Dahl salt-sensitive.
Age-related alterations in the immune system, characterized by a shift from Th1 to Th2 cytokines, occur in humans and animal models (Cieslik et al., 2011). Along with the increased shift to a Th2 phenotype, numbers of CD45+ myeloid-derived fibroblasts that contain procollagen I increase, along with the development of diastolic dysfunction in the aging mouse heart, indicating immunoinflammatory dysregulation in the aging heart stimulates interstitial fibrosis (Cieslik et al., 2011).
3.2. Kidney
3.2.1. Physiology
In human subjects, GFR shows an age-related decline (averaging 0.75–1.0 ml/min/1.73m2/year) from the age of 30–40 years (Bolignano, Mattace-Raso, Sijbrands, & Zoccali, 2014; Denic, Glassock, & Rule, 2016; Rule et al., 2010; Wetzels, Kiemeney, Swinkels, Willems, & Heijer, 2007; Zhou, Saxena, Liu, Vaziri, & Silva, 2008). Functional reserve is declines from 20% in the young to approximately 15% in the elderly (Fliser, Zeier, Nowack, & Ritz, 1993). Effective renal perfusion, an important determinant of GFR, decreases about 10% per decade in healthy elderly volunteers after the age of 30 (Fliser et al., 1993). The decrease is profound in the cortex, largely due to an increase in renal artery resistance that may result from blunted vasodilatory responses to acetylcholine with intact or enhanced responses to vasoconstrictors such as angiotensin (Fliser et al., 1997; Gekle, 2017; Goldberg & Finkelstein, 1987).
The filtration fraction (ratio of GFR to renal plasma flow) is slightly enhanced with age and starts to significantly increase from the age of 60. An enhanced filtration fraction indicates the extent of drop in renal blood flow is larger compared to the GFR drop and that the age-related increase in vascular resistance occurs mainly in efferent arterioles. Increased filtration fraction, depending on the resistance in efferent arterioles, results in glomerular pressure overload and hyperfiltration (Gekle, 2017). These changes damage glomerular capillaries, resulting in elevated albumin excretion, even though the increase is still preserved within the normal physiological range (Fliser et al., 1997).
Age-related sclerotic changes may reduce intrarenal pressure and shift the pressure-natriuresis relationship to rightward because additional systemic blood pressure elevation is needed to excrete sodium. In addition, atrophy of nephrons may cause the requirement of higher systemic blood pressure, while less sodium intake may require less blood pressure, which blunting the slope (Levy et al., 2009). These changes may induce hypertension, in particular salt-sensitive hypertension, in elderly population.
The decreased expression of the most important water channel, AQP-2, and medullary hypotonicity in the elderly (humans and animal models) suggest that the abilities of concentrating urine is impaired despite a normal circulating level of vasopressin (Bolignano et al., 2014; Preisser et al., 2000). The proximal sodium reabsorption is enhanced, and reabsorption at the thick ascending loop of Henle and the distal tubes is reduced, suggesting a reduced control range. The protein levels of the Na+ −K+ −2Cl− cotransporter NKCC2/BSC1 and the epithelial sodium channel ENaC and reduced in the aged rats compared to the young (Tian et al., 2006). The reduced levels of these sodium transporters may also, at least in part, contribute to the reduced maximal urine osmolality in aged rats. Together with reduced levels of renin and aldosterone and diminished responses to their stimuli, the elderly are at a high risk of volume depletion and sodium excretion (Gekle, 2017; Schaeffner et al., 2012). Potassium secretion is impaired, which corresponds to impaired sodium reabsorption across the Na+/K+ -ATPase transporters in the distal nephron and collecting duct, explaining the high risk for hyperkalemia in the elderly (Schaeffner et al., 2012).
Endocrine function, including renin release, is impaired with age, but serum erythropoietin levels are normal or slightly increased to compensate for age-related blood loss or increased erythropoietin resistance (Bolignano et al., 2014; Fliser et al., 1997; Gekle, 2017).
3.2.2. Structure
Arterial sclerosis (intimal fibrosis of the interlobular arteries) is found in the aged kidneys, which leads to the regional ischemia in nephrons, arteriolar hyaline, and fibrotic changes in the smaller distal branches (Takazakura et al., 1972). Deposition of hyaline and matrix in the mesangium leads to expansion of the mesangial compartment, collapse of glomerular and capillary tufts and intracapsular fibrosis (glomerulosclerosis), and globally sclerotic glomeruli may eventually atrophy. Corresponding tubules are atrophied; proximal convoluting tubes are shortened, and the size of the proximal tubular epithelial cells decreases (Abdel-Kader & Palevsky, 2009). Fibrillar collagens accumulate in tubulointerstitial space (Denic et al., 2016).
Glomerulosclerosis occurs primarily in the surface cortex, with losing the cortical glomeruli 30% - 50% by the age of 70 years (Abdel-Kader & Palevsky, 2009), which eventually contributes to the loss of parenchyma in the cortex (Emamian, Nielsen, Pedersen, & Ytte, 1993; Gourtsoyiannis, Prassopoulos, Cavouras, & Pantelidis, 1990; Kaplan, Pasternack, Shah, & Gallo, 1975). The remaining glomeruli are hypertrophied to compensate for the decline of functional glomeruli, and the medullary volume increases through the age of 40–50 years, in part due to the deposition of renal sinus fat and the hypertrophy of remaining and compensating functional juxtamedullary glomeruli and tubules (Bolignano et al., 2014; Zhou, Saxena, et al., 2008).
Podocytes support and maintain glomerular basement membrane filtration mechanisms, and with age become hypertrophied and detached (Bolton & Sturgill, 1980; Ortmann et al., 2004; Wiggins, 2009). Another type of arterial change seen in the juxtamedullary area is alomerular arterioles, where the afferent and efferent arteries communicate directly each other due to the loss of glomeruli (Takazakura et al., 1972).
3.2.3. Renal inflammaging and fibrosis
In the aged kidney, VEGF decreases and anti-angiogenic factors angiostatin and thrombospondin-1 increase, with decreased peritubular capillaries (Satoh et al., 2013). Mitochondrial dysfunction and NO deficiency are associated with the decline in VEGF and upregulation of angiostatin (Kang et al., 2001; Satoh et al., 2011). Endostatin, an anti-angiogenic factor, is elevated in the blood and kidney of aging mice, and elevated endostatin associates with the degree of renal injury in elderly patients (Lin et al., 2014; Ruge et al., 2014). Peritubular capillary loss contributes to interstitial hypoxia, oxidative stress, MCP-1 and IL-18 upregulation, and tubulointerstitial fibrosis, with proteinuria and increases in serum creatinine, suggesting the critical role of vessel rarefaction in renal aging (Satoh et al., 2011; Satoh et al., 2013).
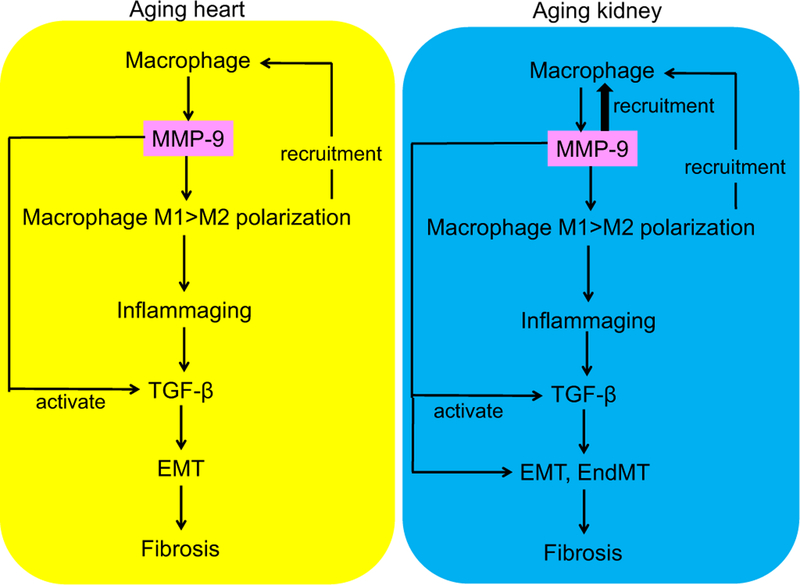
Accumulation of macrophages and lymphocytes is observed in the aged kidney, along with increased expression of intracellular adhesion molecule (ICAM)-1 and osteopontin. The number of F4/80-positive monocytes and macrophages doubles in mice from 3 months to 24 months old (Zhang et al., 2006). This increase in macrophage number is much lower than the diseased kidney. For example in the unilateral ureteral obstruction (UUO) mouse model, kidneys have 100–120 F4/80-positive cells/×200 field, compared to 5–10 F4/80-positive cells/×200 field for sham (Tan et al., 2013). The M1 macrophage marker inducible NOS increases and the M2 macrophage marker arginase 1 decreases with age in the kidney, indicating M1 > M2 polarization (Son et al., 2017). Chronic inflammation induces renal cell senescence, and senescent cells secrete cytokines and chemokines to propagate the cycle of inflammation (Mei & Zheng, 2009).
Inflammatory cells release cytokines and growth factors, including platelet-derived growth factor (PDGF)-β, IL-1β, TNF-α, IL-6, and TGF-β (Bolignano et al., 2014; Hewitson, 2012). TGF-β is a potent fibrogenic growth factor produced from all cell types in the kidney, and the mechanisms of TGF-β-induced fibrosis is inducing myofobroblast formation from fibroblasts, pericytes, endothelial cells in vitro, stimulating the ECM gene transcription in tubular, endothelial, and mesangial cells and stimulating cell proliferation and ECM accumulation via activation of connective tissue growth factor (Lawson, Elliott, Wheeler-Jones, Syme, & Jepson, 2015). TGF-β induces EMT via Smad2/3 and p38 mitogen-activated protein (MAP) kinase signaling. In addition to fibrogenic effects, TGF-β causes tubular epithelial cell apoptosis and inflammatory cell accumulation.
The number of macrophages correlates with the degree of interstitial fibrosis in the aging kidney, and glomerulosclerosis and tubulointerstitial fibrosis are major hallmarks of renal aging (Denic et al., 2016; Gagliano et al., 2000; Lim et al., 2012; Maric, Sandberg, & Hinojosa-Laborde, 2004). The picrosirius red stained fibrosis area is about 7.5% in 2 month old male Fischer rats and doubles to about 15% at 20 months (Sangaralingham et al., 2016). Collagen I, III, and IV, laminin, and fibronectin levels are higher in the aged kidney (Table 2) (Gagliano et al., 2000; Hou et al., 2016; Maric et al., 2004; Zhang et al., 2006).
Aging kidneys exhibit increased ECM crosslinking and a 2–4 fold significant increase in transglutaminase 2, an enzyme that catalyze Ca2+ dependent cross-links ECM proteins (Lin et al., 2016). Transglutaminase 2 is a molecular partner of endostatin, and overexpression of endostatin in young mice leads to interstitial fibrosis. Subcapsular injection of transglutaminase 2 or endostatin into a young kidney results in cellular senescence, and cotreatment exerts cumulative effects resulting in apoptosis, illustrating that endostatin and transglutaminase 2 synergistically promote fibrosis (Lin et al., 2016).
In normal adults, senescent resident renal cells are replaced by proliferating resident cells or differentiated stem cells. Stem cells have a potential to differentiate into tubular epithelial cells, endothelial cells, mesangial cells, and podocytes. Stem cell aging delays this replacement process, leading to renal fibrosis. Young bone marrow cell transplantation into radiated old mice reduces renal fibrosis and senescence markers (Yang et al., 2011). Thus, stem cell aging as well as parenchymal cell aging contributes to age-related renal fibrosis (Yang et al., 2011).
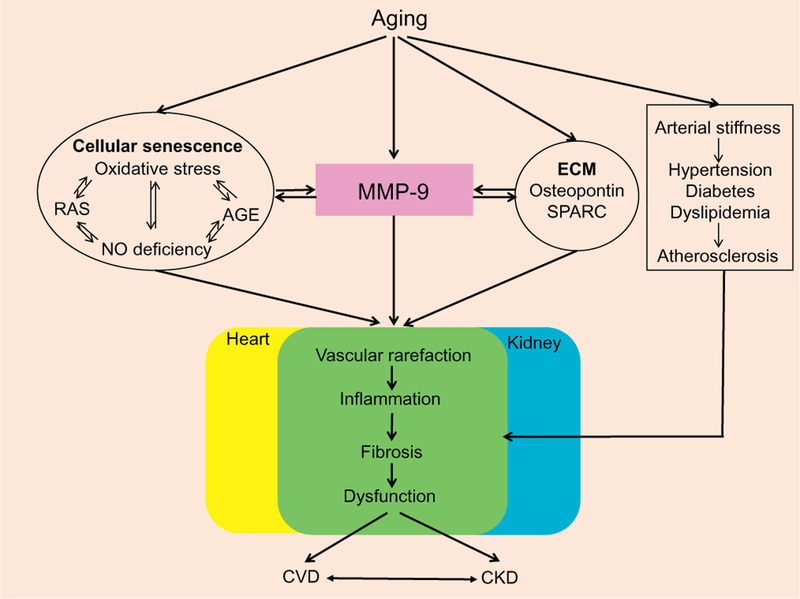
4. Common mechanisms of cardiac and renal aging
Among possible biological mechanisms of aging, the heart and kidney share a number of commonalities, which lead to cellular senescence and functional disorder.
4.1. Oxidative stress
In the 1950s, Harman et al. proposed the free radical theory of aging, where aging is a generative process driven by the oxidative-damaged macromolecules (proteins, lipids, and DNA) (Harman, 1956). An increased systemic oxidative stress marker and a reduced anti-oxidant enzyme activity are observed in the healthy elderly compared to young subjects (Bouzid, Hammouda, Matran, Robin, & Fabre, 2014). Once the imbalance between the production of ROS and its quenching system occurs, oxidative stress results in cellular oxidative damage. ROS triggers downstream inflammatory signaling and cascades related to cellular proliferation and growth signals, which further generate ROS (Benigni, Cassis, & Remuzzi, 2010; Lithgow & Kirkwood, 1996).
Impaired electron transport function in mitochondria contributes to elevated electron leakage and ROS generation (Dai, Rabinovitch, & Ungvari, 2012). Mitochondria DNA is susceptible to oxidation, because it lacks protective histones and in proximity to high ROS levels (Yakes & Van Houten, 1997). The heart requires a large amount of energy, and mitochondria are critical to provide ATP to meet the demands of the myocardium (Dai, Rabinovitch et al., 2012). Mitochondria of aged murine hearts are more damaged and produce more ROS than young hearts (Roh et al., 2016). Mice with mitochondrial DNA mutation show exacerbated cardiac aging phenotypes, which are partially suppressed by mitochondrial catalase overexpression (Dai et al., 2010; Kujoth et al., 2005).
Because reabsorption of fluid and minerals by the renal tubular cells demand energy and consume ATP, mitochondria are abundant in renal tubular cells (Eirin, Lerman, & Lerman, 2017). Mitochondrial ROS production is greater in old rat kidney with impaired mitochondrial function, which is suppressed by enalapril and losartan (de Cavanagh et al., 2003).
NADPH oxidase is composed with membrane-bound subunits (a gp91phox homologue, NOX and p22phox) and cytosolic subunits (p67, p47, Noxa1, Rac)(Sahoo, Meijles, & Pagano, 2016). Various factors including angiotensin II, mechanical forces, environmental factors, and agonistic stimulation by aldosterone, endothelin-1, PDGF, TGF-ß, and TNF-α, most of which relate to pathological states and increase with age, stimulate NADPH oxidase, and produce ROS (Wang et al., 2010).
Aged cardiomyocytes show enhanced p47phox and ROS generation with prolonged TR(90), reduced tolerance to high stimulus frequency, and slowed intracellular Ca2+ rise and clearing rates (Ren et al., 2010). NOX4 is a major source of ROS in mitochondria of failing hearts, and cardiac-specific overexpression of NOX4 results in mitochondrial dysfunction, oxidative stress, and exacerbated age-related cardiac phenotypes in mice (Ago et al., 2010).
In old rats, renal ROS, dysfunction, and structural injury as well as cell senescence markers are increased compared to the young and associated with overexpression of NOX4 and p22phox in the kidney (Simao et al., 2011). The senescence-accelerated mouse prone-8 (SAMP8) shows higher levels of renal NOX2 expression and NADPH oxidase activity at the age of 1 month than the senescence-accelerated-resistant mouse (Baltanas et al., 2013).
Catalase, Cu/Zn-super oxide dismutase, and Mn-super oxide dismutase levels all decrease, and the renal antioxidant potential declines, with age in the rat kidney and plasma (Hou et al., 2016; Lim et al., 2012). These changes may contribute to the marked increase in oxidative stress and more severe renal injury after ischemia/reperfusion compared to young controls (Wang, Bonventre, & Parrish, 2014). Renal glutathione levels in aged rats is not different from young controls at baseline, but levels decrease more rapidly after ischemia/reperfusion in old rats (Shimizu, Araujo, Borges, de Tolosa, & Seguro, 2004). Induction of heme oxygenase-1 is blunted in the aged kidney, leading to worse ischemic injury (Ferenbach et al., 2011). Disorders in antioxidant system may explain high susceptibility of aging kidney to nephrotoxicity.
4.2. Renin-angiotensin system (RAS)
Numerous evidence demonstrates the protective effects of RAS inhibition on progressive cardiac and renal dysfunction in animals and humans (Lakatta, 2015; Sargento, Simoes, Longo, Lousada, & Reis, 2016; Spannella et al., 2018). Plasma renin activity and aldosterone levels show age-related decreases, due to limited synthesis and release of renin. Despite a suppressed systemic RAS, pharmacological inhibition of RAS with angiotensin converting enzyme (ACE) inhibitors, AT1 receptor blockers, or AT1 receptor deletion attenuates age-related cardiac and renal alterations and increases survival, suggesting an exacerbating role of local RAS in cardiorenal aging (Benigni et al., 2009; de Cavanagh, Inserra, & Ferder, 2015; Ferder, Romano, Ercole, Stella, & Inserra, 1998). Indeed, the protein expression of angiotensin II, AT1 receptor, ACE, and aldosterone are markedly increased in hearts of older rats, while kidney localized RAS has been reported to both increase or decrease in aged kidneys, depending on the study (Li, Cao, Bai, Lin, & Shi, 2010; Lu, Li, Li, Li, & Wang, 1996; Schulman et al., 2010).
By binding to AT1 receptor, angiotensin II exerts various effects, including induction in inflammation, cell proliferation, ECM synthesis, and ROS production (Benigni et al., 2010; de Cavanagh et al., 2015; Feng, Wang, & Li, 2011; Ferrario, 2016; Ito et al., 2007; Mezzano, Ruiz-Ortega, & Egido, 2001; Wang & Shah, 2015). Angiotensin II-induced NADPH oxidase activation plays pivotal roles in both cardiac and renal aging (Benigni et al., 2010; Ito et al., 2007). For example, the increased levels of NADPH oxidase expression, NADPH oxidase-derived ROS, cardiomyocyte hypertrophy, and vascular remodeling in aged rat hearts are reproduced by the angiotensin II infusion in young rats (Wang, Zhang, et al., 2010). Regular exercise training improves cardiac function in elderly populations and recuses collagen deposition in rat hearts, which relates to the downregulation of cardiac angiotensin II and AT1 receptor and the concomitant suppression of NADPH oxidase-derived superoxide production (Lee, Kwak, Hord, Kim, & Lawler, 2015).
Aldosterone, the final product of the RAS pathway, induces the over-expression of cell senescence markers, including β-galactosidase, p21, and p53, as well as the reduction in an anti-senescence molecule Sirtuin1 through mineralocorticoid receptor stimulation in rat kidneys (Fan et al., 2011). Aldosterone infusion induces the overexpression of gp91phox, p47phox, and p67phox and increased oxidant levels in the kidney (Shibata, Nagase, Yoshida, Kawachi, & Fujita, 2007).
Chymase is a mast cell-derived serine protease that catalyzes conversion of angiotensin I to angiotensin II in humans, dogs, and hamsters (Miyazaki & Takai, 2006). Chymase inhibition suppresses cardiac fibrosis in a model of cardiomyopathy and improves hemodynamics and survival rates after myocardial infarction (Takai, Jin, & Miyazaki, 2012). Substrates of chymase include not only angiotensin I but also latent forms of TGF-β1 and MMP-9, producing their active forms (Takai, Jin, & Miyazaki, 2010). Chymase expression and activity increase with age and are reduced by exercise (Froogh et al., 2017). There are a wide range of effects chymase has in the heart that may accelerate cardiac aging.
The existence of intramitochondrial RAS is reported to regulate mitochondrial functions such as NO production and respiration through AT2 receptor stimulation (Abadir et al., 2011). Mitochondrial AT2 receptor is abundant in renal tubular cells from young mice, while mitochondrial AT1 receptor expression is very rare or not observed. Mitochondrial AT2 receptor decreases with age in the renal tubular cells, paralleled by increased levels of the mitochondrial AT1 receptor. Chronic treatment with losartan prevented the age-related decrease in mitochondrial AT2 receptor expression (Abadir et al., 2011).
Investigations into renal RAS will provide efficient and a wide range of potential therapeutic targets against cardiac and renal aging. Together with its hemodynamic and metabolic effects, RAS inhibitors may become comprehensive anti-aging agents against cardiorenal aging.
4.3. NO deficiency
One of the most important roles of endothelial cells of large arteries is producing NO, a critical vasodilator. Aged endothelial cells exhibit a decline in eNOS activity and NO production (Ma et al., 2014; Trinity et al., 2016). NO deficiency facilitates endothelial cell senescence (Ghebre et al., 2016). Because NO inhibits vascular inflammation, platelet aggregation, and aberrant cell proliferation, NO deficiency and inactivation results in significant vasomotor dysfunction and atherosclerotic vascular change (Kawashima & Yokoyama, 2004).
NO deficient mice show increased mortality and a shorter lifespan, supporting the favorable effects of NO to suppress aging. Nevertheless, the expression of NOS is reported to be both increased and decreased with age (Cau, Carneiro, & Tostes, 2012). Even if NOS levels increase with age, eNOS concentrations do not always correlate with the amount of NO; uncoupled eNOS produces superoxide (Lee, Zeeshan, Kim, & Chae, 2017). Oxidative stress in aging causes a functional inactivation of NO, and peroxynitrite, synthesized by binding superoxide to NO, and is also a very strong oxidant.
Asymmetric dimethylarginine (ADMA) is an endogenous NOS inhibitor, which is synthesized when arginine residues in proteins are methylated and then hydrolyzed (Vallance & Leiper, 2004). ADMA levels increase in aging rats and humans (Sverdlov, Ngo, Chan, Chirkov, & Horowitz, 2014). In the elderly, a high level of ADMA predicts cardiovascular events and death and correlates positively with reduced renal per-fusion, suggesting a key role of ADMA in cardiorenal aging (Zoccali, 2006).
Aged animals and humans exhibit reduced endothelium-dependent vasodilation in a number of arteries including the coronary artery. With age, coronary flow reserve is impaired, in part due to the multiple protective actions of NO that are blunted, leading to atherosclerotic changes in the coronary artery.
NO also plays an important role in cardiac contractility and Ca2+ channel functions in cardiomyocytes, either by cyclic guanosine monophosphate (cGMP) formation or by cGMP-independent protein modifications (S-nitrosation). The number and the activity of cardiac L-type Ca2+ channel increases, and L-type current inactivates more slowly, in myocytes with age. The prolonged Ca2+ transience and contraction may underlie the reduction in early diastolic filling and diastolic dysfunction. A dietary nitrate supplementation improves diastolic function associated with an accelerated cardiomyocyte calcium handling and augmented NO-cGMP-protein kinase G signaling in aged mice (Borlaug, 2016; Rammos et al., 2016).
In the aged rat kidney, NO content and acetylcholine-induced endothelium-dependent vasodilation is reduced, resulting in increased vasoconstriction and reduced renal perfusion (Long et al., 2005). Ischemia/reperfusion causes more severe functional and structural damage with a drastic increase in vasoconstriction in the kidney of old rats (Wang et al., 2014). Administration of the NO precursor L-arginine attenuates ischemia/reperfusion-induced renal injury in both young and old rats, to a greater extent in old rats, suggesting a significant role of NO in increased susceptibility to ischemia in the aged kidney (Sabbatini et al., 1994).
In addition to regulation of renal and glomerular hemodynamics, NO exerts other important physiological functions, including the maintenance of medullary perfusion, mediation of pressure-natriuresis, blunting of tubuloglomerular feedback, inhibition of tubular sodium re-absorption, and modulation of renal sympathetic nerve activity. Age-related NO deficiency causes subclinical renal pathology in all of these processes (Mount & Power, 2006).
4.4. Advanced glycation end product (AGE)
AGEs accumulate in various aged organs and tissues including the heart and kidney (Son et al., 2017). During normal aging, AGEs accumulate primarily in proteins with long turnover rates (e.g., collagens), because the glycation reaction proceeds slowly (Simm et al., 2015). Serum AGE levels increase with age in healthy adults and correlate with circulating oxidative and inflammatory markers (e.g., 8-isoprostanes, vascular cell adhesion molecule 1, TNF-α, and C-reactive protein) (Uribarri et al., 2007). Plasma AGE levels correlate with all-cause and CVD mortality in the elderly (≥65 year old men and women) and diastolic dysfunction in aging men (43–78 years old) undergoing coronary artery bypass graft surgery (Campbell et al., 2012). Reduction of AGEs by dietary control suppresses development of CKD and improves survival rates (Vlassara et al., 2009).
Glycated enzymes and growth factors are unable to exert their essential biological activities, resulting in functional decline. For example, AGEs inhibit eNOS activation and impair endothelium-dependent vasodilation (Xu et al., 2003). AGEs also cause diastolic dysfunction by prolonging Ca2+ transient decay time, which is associated with crosslinked AGE modification of sarcoplasmic reticulum proteins in cardiomyocytes (Neviere, Yu, Wang, Tessier, & Boulanger, 2016).
Glycation-induced changes in protein structure and function also induce crosslinking of collagens elastic proteins, leading to tissue stiffening. AGE actions to reduce NO availability and enhance myocardial stiffness may contribute to diastolic dysfunction and high susceptibility to ischemic injury in aged human and animals (Ramasamy & Schmidt, 2012).
Another important mechanism of AGEs-induced aging exerts through the receptor of AGE (RAGE). The binding of AGEs to RAGE induces several intracellular signaling pathways, including MAP kinase, Janus kinase/signal transducers and activators of transcription, and activation of nuclear factor-κB (Heidland, Sebekova, & Schinzel, 2001; Simm et al., 2015; Stinghen, Massy, Vlassara, Striker, & Boullier, 2016). AGE binding to RAGE activates these signaling pathways in cardiac and renal cells by upregulating ROS (Inagi, 2016; Lu et al., 2004; Neviere et al., 2016). NADPH oxidase activation is the principle mechanism of ROS generation by the AGE-RAGE axis (Neviere et al., 2016). These signaling pathways increase synthesis of various ECM proteins, including collagens, fibronectin, and laminin through upregulation of growth factors (e.g. TGF-β), and production of inflammatory cytokines and adhesion molecules (Inagi, 2016; Lu et al., 2004; Neviere et al., 2016).
RAGE null mice are resistant to ischemia/reperfusion-induced cardiac cell apoptosis, as shown by decreased caspase 3 activity and cytochrome c and decreased Bcl-xL, along with suppressed phosphorylation of JNK and STAT5 (Tsoporis et al., 2010). RAGE-induced inhibition of autophagy through ERK and AKT pathways also induces cardiomyocyte apoptosis (Neviere et al., 2016). Age-related AGE/RAGE accumulation, therefore, may explain the high sensitivity of aging hearts to ischemic damage.
AGEs and their precursors are excreted from the body by kidney (glomerular) filtration. Methylglyoxal and 3-deoxyglucosone, representative precursors of AGEs, are uremic toxins. The age-related decline in GFR induces deficient clearance and excessive accumulation of AGEs and uremic toxins, which leads to vicious cycle leading to further renal injury.
The glycoxalase system catalyzes toxic metabolites (e.g. AGE precursors) and subsequently protects organs from glycative stress (Inagi, 2016). In older rat kidney, glycoxalase 1 activity is lower, concomitant with higher oxidative stress markers, compared to younger rats (Ikeda et al., 2011). Glycoxalase transgenic overexpression blunts age-dependent increases in oxidant markers in the kidney and urine and reduces senescent marker expression in tubules, age-related interstitial thickening, and renal dysfunction (Ikeda et al., 2011). Glycoxalase 1 protects against vascular endothelial cell senescent phenotypes and age-related NO deficiency, which may also contribute to not only renal protection but also cardiac protection (Inagi, 2016). Preserving the defense system against AGEs may be one therapeutic method for cardiorenal aging.
4.5. Lifestyle approaches to anti-aging
Regular exercise, healthy diet, and caloric restriction are the most evidenced lifestyle approaches to anti-aging (Seals, Brunt, & Rossman, 2018). During aging, regular exercise reduces risk of all-cause mortality, chronic diseases, and premature death (Baltaci, Mogulkoc, & Baltaci, 2016; Mora & Valencia, 2018). Exercise leads to anti-oxidant protections of organism both in young and old subjects (Lawler & Powers, 1998; Leeuwenburgh & Heinecke, 2001). Physically active elderly individuals exert similar levels of antioxidant activity and lipid peroxidation to those of young sedentary subjects. Furthermore, muscle cells release cytokines, interleukins, and other proteins that play important roles in the protection against low-grade inflammation such as atherosclerosis (Golbidi, Badran, & Laher, 2012; Pedersen, 2011). Increased physical activity achieving a 30% energy deficit compared to controls increases average life span but does not expand maximal lifespan in rodents. While there are no data whether physical activity will expand lifespan in humans, it may be achieved at least in part by preventing chronic disease (Blair et al., 1989; Holloszy, 1997; Holloszy, Smith, Vining, & Adams, 1985). In addition to increasing endogenous antioxidants by exercise, nutritional antioxidants, including vitamin E, vitamin C, carotenoids, ubiquinone, polyphenols, and flavonoids, have been the focus of attention to reduce oxidative stress (Simioni et al., 2018). The mechanisms of exogenous antioxidant actions are neutralizing free radicals, repairing oxidized membranes, decreasing ROS production, and neutralizing ROS by short-chain free fatty acids and cholesteryl esters via lipid metabolism (Berger, 2005).
Caloric restriction is the only reproducible strategy that delays senescence and prolongs lifespan in yeast, worms, and rodents (Alfaras et al., 2016). In both the heart and the kidney, caloric restriction is reported to delay the aging process by increasing Sirtuins, which are demonstrated to moderate aging in yeast to mammals (Haigis & Sinclair, 2010).
In addition to reduced metabolic rate, alteration in insulin sensitivity, hormonal secretion, sympathetic nerve activation, and gene expression may contribute to anti-aging effects of caloric restriction (Heilbronn & Ravussin, 2003). Caloric restriction exerts anti-aging effects by reducing oxidative stress (Lee & Yu, 1990; Sohal & Weindruch, 1996). Short-term caloric restriction also reduces markers for inflammation (e.g. C-reactive protein) in obese and non-obese subjects, whereas the effects of long-term caloric restriction in human are unknown (Bastard et al., 2000; Heilbronn, Noakes, & Clifton, 2001; Mavri et al., 2001; Velthuis-te Wierik, Meijer, Kluft, & van den Berg, 1995). Recent clinical study reports that subjects who have sleeping difficulty have a shorter relative telomere length compared to those with no sleeping difficulties, suggesting that poor sleep may be an accelerator of or response to aging (Zgheib et al., 2018).
5. Common ECM targets for cardiac and renal aging
The ECM provides structural support for cells, and the major component of cardiac and renal ECM is collagen. The ECM also regulates inflammation and fibrosis, in part by serving as a reservoir for the sequestration of growth factors and inflammatory cytokines. Below, we discuss ECM proteins relevant to both cardiac and renal physiology and pathophysiology.
5.1. Osteopontin
Osteopontin, initially identified as a bone matrix protein, is increased in various pathological states in both the heart and kidney (Irita et al., 2011; Singh, Foster, Dalal, & Singh, 2010; Xie et al., 2001). Osteopontin promotes inflammation via cell adhesion, chemotaxis, and signal transduction including NF-κB activation, and angiotensin II is reported to increase osteopontin expression (Singh et al., 2010; Xie et al., 2001).
In the normal adult heart, osteopontin expression is low and does not show an age-related increase in the absence of injury (Table 2) (Singh et al., 2010). Following ischemia/reperfusion, osteopontin protein is 1.5-fold higher in old (6 years old) compared to young (2 years old) dogs (Jugdutt, Palaniyappan, Uwiera, & Idikio, 2009). The higher susceptibility to injury in the elderly may be due in part to the exacerbated induction of osteopontin. On the other hand, old mice (>2 years old) show worse systolic dysfunction and dilative and hypertrophic remodeling post-myocardial infarction and reperfusion compared to young mice (2–3 months old), which is associated with decreased and delayed neutrophil and macrophage infiltration and reduced osteopontin expression in the infarcted myocardium (Bujak et al., 2008).
Osteopontin expression in the adult human kidney is present at low levels and localized to distal tubular segments. The degree of tubular expression correlates with the number of macrophages and monocytes in the kidney (Bujak et al., 2008; Xie et al., 2001). Renal osteopontin expression shows age-related increases (Table 2), which is correlated with blood nitrogen levels, suggesting a role for osteopontin in age-related renal dysfunction (Liang & Barnes, 1995). Macrophage accumulation in the kidney may be caused by osteopontin and may cause further osteopontin generation. Though renoprotective effects of osteopontin in the recovery process after ischemia injury are reported, osteopontin may be one of the critical promoters of renal aging (Xie et al., 2001).
5.2. Secreted protein acidic and rich in cysteine (SPARC)
SPARC is expressed during morphogenesis and re-induced during tissue remodeling (Brekken & Sage, 2000). SPARC is a procollagen-binding matricellular protein, which chaperones procollagen processing (Bornstein & Sage, 2002; Bradshaw & Sage, 2001). SPARC is induced under fibrotic conditions in a variety of organs and tissues, including the heart and kidney (Pichler et al., 1996; Wu et al., 1997). SPARC increases the aged LV (Table 2), along with increases in myocyte hyper-trophy, LV stiffness, and insoluble fibrillar collagen content (Bradshaw et al., 2009; Bradshaw et al., 2010). Furthermore, SPARC promotes not only post-translational processing but also collagen I production and a disintegrin and metalloproteinase with thrombospondin-like motifs 1 (ADAMTS1) induction in cardiac fibroblasts (Toba et al., 2016). ADAMTS1 is an ECM protease that increases with age in the mouse LV, along with a decrease in its substrate, versican (Toba et al., 2016).
SPARC deletion reduces inflammation in a lipopolysaccharide-induced footpad model and dextran sodium sulfate-induced murine colitis, while the function of SPARC in regulating inflammatory processes depends on its cellular origin (Ng et al., 2013; Rempel et al., 2007; Sangaletti et al., 2011). Plasma levels of SPARC positively correlate with hypersensitive C-reactive protein and white blood cell numbers in gestational diabetes, indicating that SPARC is upregulated in the setting of inflammation (Xu et al., 2013).
In the heart, SPARC is detected primarily in fibroblasts and, at lower levels, in endothelial cells, cardiomyocytes, and macrophages (McCurdy et al., 2011; Ridinger et al., 2009). SPARC deletion delays age-related increases in macrophage infiltration and proinflammatory marker expression. Age-related increases in markers of M1 macrophage polarization and decreases in a marker of M2 macrophage polarization are blunted in SPARC-null murine hearts, indicating SPARC acts as a mediator of age-related cardiac inflammation by facilitating M1 > M2 polarization (Toba et al., 2015).
There is no direct study investigating the role of SPARC in renal aging, and only one report shows that SPARC mRNA decreases with age (Table 2) (Hultstrom et al., 2012). However, there are many reports demonstrating pro-inflammatory and fibrogenic roles of SPARC in renal diseases. In addition, SPARC treatment increases M1 > M2 polarization in isolated peritoneal macrophages, and this direct effect of SPARC to macrophages suggests that SPARC may induce inflammaging by M1 polarization not only in the hearts but also the kidney (Toba et al., 2015). Serum SPARC levels are elevated in patients with fibrotic renal injury (Kanauchi, Nishioka, & Dohi, 2000). In subtotal nephrectomized renal injury model, induced by right subcapsular nephrectomy and ligation of approximately two-thirds of the left kidney, SPARC expression is increased in both the sclerotic glomerulus and damaged tubulointerstitium (Wu et al., 1997). SPARC gene deletion reduces the expression of MCP-1 and IL-1ß, and TGF-ß, as well as reduces macrophages and collagen deposition in the perivascular and tubulointerstitial regions and leads to significant functional improvement in angiotensin II-induced hypertensive mice (Socha, Manhiani, Said, Imig, & Motamed, 2007). The decrease in SPARC mRNA levels in the aged kidney may be due to negative feedback.
In addition to studies showing angiotensin II treatment induces SPARC expression in mesangial cells and in animals, ACE inhibition or AT1 receptor blockade reduces renal SPARC expression in subtotal nephrectomized rats (Socha et al., 2007; Wu et al., 1997).
ADAMTS1 is expressed during kidney development, and its expression becomes lower after birth (Thai & Iruela-Arispe, 2002). Lipopolyssacharide up-regulates renal ADAMTS1 expression, suggesting a significant role for ADAMTS1 in inflammation (Kuno et al., 1997; Thai & Iruela-Arispe, 2002). In rats, ischemia/reperfusion injury induces ADAMTS1 expression and reduces VEGF in proximal tubules in the first week after reperfusion (Basile, Fredrich, Chelladurai, Leonard, & Parrish, 2008). In the UUO kidney, ADAMTS1 mRNA is induced in the renal tubular epithelial cells in the outer stripe of the outer medulla, localized to the area damaged by the acute kidney injury (Nakamura, Sakai, Ohata, & Komurasaki, 2007). ADAMTS1 may accelerate acute injury in the aging kidney by inducing inflammation, vascular rarefaction, and fibrosis.
6. Aging effects on MMPs in the heart and kidney
There are 25 members of reported mammalian MMPs, which are classified into collegenases, gelatinases, stromelysins, matrilysins, membrane-type MMPs, and other MMPs (Iyer, Patterson, Fields, & Lindsey, 2012). MMPs are endogenously inhibited by the tissue inhibitors of metalloproteinases (TIMPs: TIMP1–4). All TIMPs inhibit pro-and active MMPs with relatively low selectivity, by forming tight non-covalent 1:1 complexes. MMPs are important key regulators of ECM turnover by remodeling and degrading ECM components. Age-related changes in MMPs and TIMPs are summarized in Table 3.
Table 3.
Summary of Age-related Changes in MMPs in Heart and Kidney.
| Change with age | Sample type | Species | References | ||
|---|---|---|---|---|---|
| Heart | MMP-1 | ↓ | Protein | SD rat, FBNF1 rat | (Kwak et al, 2011; Thomas et al., 1998) |
| ↓ | mRNA | Rat | (Robert et al., 1997) | ||
| MMP-2 | ↓ | Protein | FBNF1 rat | (Kwak et al., 2011) | |
| ↓ | mRNA | rat | (Robert et al., 1997) | ||
| ↑ | Protein | Sheep | (Horn et al., 2012) | ||
| ↑ | mRNA | FBN rat, mouse, C57BL/6 | (Batkai et al., 2007; Toba et al., 2016; Wang, Zhang, et al., 2010) | ||
| ↑ | Protein (plasma) | Human | (Bonnema et al., 2007) | ||
| ↔ | Protein (soluble, insoluble) | CB6F1 | (Lindsey et al., 2005) | ||
| MMP-3 | ↑ | Protein (soluble) | FBNF1 rat | (Kwak et al., 2011) | |
| ↔ | Protein | FBNF1 rat | (Kwak et al., 2011) | ||
| ↑ | mRNA | C57BL/6 | (Toba et al., 2016) | ||
| ↓ | Protein (soluble) | CB6F1 | (Lindsey et al., 2005) | ||
| ↑ | Protein (insoluble) | CB6F1 | (Lindsey et al., 2005) | ||
| MMP-7 | ↑ | Protein (plasma) | Human | (Bonnema et al., 2007) | |
| ↔ | Protein (soluble) | CB6F1 | (Lindsey et al., 2005) | ||
| MMP-8 | ↔ | Protein (soluble) | CB6F1 | (Lindsey et al., 2005) | |
| ↑ | Protein (insoluble) | CB6F1 | (Lindsey et al., 2005) | ||
| MMP-9 | ↑ | mRNA | mouse, C57BL/6 | (Batkai et al., 2007; Toba et al., 2016) | |
| ↓ | Protein (plasma) | Human | (Bonnema et al., 2007) | ||
| ↑ | Protein (plasma) | C57BL6/J | (Chiao et al., 2011) | ||
| ↑ | Protein | C57BL6/J | (Chiao et al., 2012) | ||
| ↑ | mRNA | C57BL6/J | (Chiao et al., 2012) | ||
| MMP-12 | ↓ | Protein (soluble) | CB6F1 | (Lindsey et al., 2005) | |
| MMP-13 | ↓ | mRNA | C57BL/6 | (Toba et al., 2016) | |
| ↓ | Protein (soluble) | CB6F1 | (Lindsey et al., 2005) | ||
| MMP-14 | ↓ | Protein | FBNF1 rat | (Kwak et al., 2011) | |
| ↑ | mRNA | FBN rat | (Wang, Zhang, et al., 2010) | ||
| ↓ | Protein (soluble) | CB6F1 | (Lindsey et al., 2005) | ||
| ↑ | Protein (insoluble) | CB6F1 | (Lindsey et al., 2005) | ||
| MMP-15 | ↓ | mRNA | C57BL/6 | (Toba et al., 2016) | |
| MMP-28 | ↓ | Protein | C57BL/6J | (Ma et al., 2012) | |
| TIMP-1 | ↑ | Protein | FBNF1 rat | (Kwak et al., 2011) | |
| ↔ | Protein | Sheep | (Horn et al., 2012) | ||
| ↑ | Protein (plasma) | Human, C57BL6/J | (Bonnema et al., 2007; Chiao et al., 2011) | ||
| TIMP-2 | ↔ | Protein | FBNF1 rat, sheep | (Horn et al., 2012; Kwak et al., 2011) | |
| ↓ | mRNA | FBN rat | (Wang, Zhang, et al., 2010) | ||
| ↑ | Plasma | Human | (Bonnema et al., 2007) | ||
| ↔ | Protein (soluble) | CB6F1 | (Lindsey et al., 2005) | ||
| TIMP-3 | ↔ | Protein | Sheep | (Horn et al., 2012) | |
| ↔ | Protein (soluble) | CB6F1 | (Lindsey et al., 2005) | ||
| ↓ | Protein (insoluble) | CB6F1 | (Lindsey et al., 2005) | ||
| TIMP-4 | ↔ | Protein | Sheep | (Horn et al., 2012) | |
| ↑ | Protein (plasma) | Human | (Bonnema et al., 2007) | ||
| ↔ | Protein (soluble) | CB6F1 | (Lindsey et al., 2005) | ||
| Kidney | MMP-1 | ↓ | Protein | SDrat | (Gagliano et al., 2000) |
| MMP-2 | ↔ | Protein | SDrat | (Gagliano et al., 2000) | |
| ↓ | mRNA, protein | NIH mouse | (Zhang et al., 2006) | ||
| ↑ | mRNA | Fischer rat | (Oelusarz et al., 2013) | ||
| MMP-3 | ↓ | mRNA | Fischer rat | (Oelusarz et al., 2013) | |
| MMP-7 | ↑ | mRNA, protein | Fischer rat | (Chen et al., 2007) | |
| ↑ | mRNA | Fischer rat | (Oelusarz et al., 2013) | ||
| MMP-9 | ↓ | Protein | DS rat, SD rat | (Maric et al., 2004; Satoh et al., 2013) | |
| ↑ | mRNA, protein | Wistar rat | (Zhao et al., 2016) | ||
| ↓ | mRNA, protein | NIH mouse | (Zhang et al., 2006) | ||
| ↑ | mRNA | Fischer rat | (Oelusarz et al., 2013) | ||
| ↓ | mRNA | Fischer rat | (Sangaralingham et al., 2016) | ||
| MMP-12 | ↑ | mRNA | Fischer rat | (Oelusarz et al., 2013) | |
| MMP-13 | ↑ | Protein | C57BL/6J | (Dasgupta et al., 2009) | |
| MMP-14 | ↑ | mRNA | Fischer rat | (Oelusarz et al., 2013) | |
| MMP-16,17,19,20,23,25 | ↑ | mRNA | Fischer rat | (Oelusarz et al., 2013) | |
| TIMP-1 | ↑ | mRNA, protein | NIH mouse | (Zhang et al., 2006) | |
| ↑ | mRNA | Fischer rat | (Oelusarz et al., 2013; Sangaralingham et al., 2016) | ||
| TIMP-2 | ↑ | mRNA, protein | NIH mouse | (Zhang et al., 2006) | |
| TIMP-3 | ↑ | Protein | human | (Macgregor et al., 2009) | |
| ↓ | Protein | C57BL/6 | (Kassiri et al., 2009) |
↑ increased, ↓ decreased, ↔ unchanged, MMP matrix metalloproteinase, TIMP tissue inhibitor of metalloproteinases, SD Sprague-Dawley, DS Dahl-salt sensitive.
6.1. MMPs in the aging heart
Among the MMPs, collagenases (e.g. MMP-1 and MMP-13), gelatinases (e.g. MMP-2 and MMP-9), stromelysin (e.g. MMP-3), matrilysin (e.g. MMP-7), metalloelastase (MMP-12), and the membrane-type MMP (e.g. MT1-MMP; MMP-14) are highly related to myocardial remodeling and consequently cardiac function (Kwak, 2013; Lindsey & Zamilpa, 2012).
MMP-1 and MMP-2 are abundant in normal LV (Iyer et al., 2012; Lindsey & Zamilpa, 2012). Abundant expression of MMP-1 and MMP-2 suggests a homeostatic role of these MMPs in the heart. Cardiac pro-MMP-1 and MMP-2 activity in rats decrease with age by 45% and 40%, respectively (Robert et al., 1997). MMP-14 (MT1-MMP) is detected in normal LV (Iyer et al., 2012; Lindsey & Zamilpa, 2012). In sedentary old (31 months old) rats displaying fibrillar collagen deposition, active MMP-1, MMP-2, and MMP-14 in the ECM fraction of the LVs are reduced, and TIMP-1 and TGF-β elevated compared to young (3 months old) group (Kwak et al., 2011). Exercise training reduces these age-related changes in collagen contents and imbalance of MMPs and TIMPs (Kwak et al., 2011). Cardiomyocyte restricted overexpression of human MMP-1 in mice results in a marked deterioration of systolic and diastolic function at older age associated with loss of cardiac interstitial collagen and compensatory cardiac hypertrophy (Kim et al., 2000). At physiological levels, MMP-1, MMP-2, and MMP-14 are anti-fibrotic factors. With age, MMP-2 and MMP-14 activity increases, along with NADPH oxidase, inflammatory cytokines, and collagen content (Batkai et al., 2007; Horn et al., 2012; Wang, Zhang, et al., 2010).
In humans with no evidence of CVD, circulating levels of MMP-2, MMP-7, TIMP-1, TIMP-2, and TIMP-4 increase with age, and the levels of MMP-7, TIMP-1, and TIMP-4 correlate with the decline in early to late (E/A) LV filling ratio (Bonnema et al., 2007). Upregulation of MMP-7 and TIMPs contributes to diastolic dysfunction in elderly populations. We found that MMP-3, MMP-8, MMP-9, MMP-12, and MMP-14 increase in the insoluble protein fraction of old (23 months old) mouse LV compared with young (3 months old) and/or middle-aged (15 months old) mouse LV, suggesting enhanced ECM degradative and pro-fibrotic capacity in the aging LV (Lindsey et al., 2005). Indeed, cardiomyocyte-restricted overexpression of MMP-14, yielding approximately a 200% increase in MMP-14 expression, induces fibrillar collagen in young (3 month old) LV and exacerbates the age-related increase in LV fibrillar collagen deposition by >2-fold by middle age (14 months) (Spinale et al., 2009). These mice also display LV dilation and systolic dysfunction. A greater activation of TGF-β and its signaling, displayed with increased expression of low molecular weight latency-associated TGF-binding protein and phosphorylated Smad2 in the middle-aged LV, is further enhanced by MMP-14 overexpression. Age-related enhancement of MMP expression and activity actually leads to elevated collagen deposition and consequent LV stiffness in the aging heart.
MMP-9 is predominantly expressed in leucocytes and also produced by cardiomyocytes, fibroblasts, vascular smooth muscle, endothelial cells, neutrophils, and mast cells (Lindsey & Zamilpa, 2012). The normal LV septum and free wall express MMP-9, but at very low levels compared to injury LV such as post-myocardial infarction (Iyer et al., 2012; Lindsey & Zamilpa, 2012). Aged mice exhibit elevated circulating MMP-9 levels, which positively correlate with plasma MCP-1 and enddiastolic dimension (Chiao et al., 2011). MMP-9 mRNA increases after the age of 12–15 months in the murine LV, and MMP-9 protein from the age of 26–34 months (Chiao et al., 2012). MMP-9 may be an indicator of accelerated cardiac aging.
6.2. MMPs in the aging kidney
Ten members of MMPs (MMP-1, MMP-2, MMP-3, MMP-9, MMP-13, MMP-14, MMP-24, MMP-25, MMP-27, MMP-28) and three members of TIMPs (TIMP-1, TIMP-2, TIMP-3) are expressed in the kidney (Catania, Chen, & Parrish, 2007; Tan & Liu, 2012). Under basal conditions, MMPs are at low levels and are tightly regulated. Since a high rate of ECM turnover is required during kidney development, MMPs are highly expressed in the developing kidney, playing important roles in nephron formation. Among MMPs expressed in adult kidneys, MMP-1, MMP-2, MMP-3, MMP-9, MMP-13, and MMP-14 have been extensively studied (Giannandrea & Parks, 2014; Tan & Liu, 2012).
In 2, 6, 12, and 19 month old Sprague Dawley rats, MMP-1 protein decreases and the collagen I protein, not mRNA, increases in the renal cortex with age, which is associated with age-related fibrosis and glomerular and tubular histological injury. Collagen III protein decreases with age, potentially due to the progressive replacement with collagen I. The adverse accumulation of collagen I, due to insufficient degradation, is caused by age-related decrease in MMP-1 (Gagliano et al., 2000). MMP-13, a murine interstitial collagenase, is produced from fibroblasts and degrades collagen I, II, and III. Different from MMP-1, renal MMP-13 protein expression increases with age (Dasgupta, Kar, Van Remmen, & Melendez, 2009). MMP-1 and MMP-13 are redox-sensitive enzymes, and Cu/Zn-super oxide dismutase deletion exacerbates age-related increase in MMP-13 and adverse loss of collagen in the Bowman’s capsule, which is crucial for filtration (Dasgupta et al., 2009). Age-related oxidative stress contributes to excessive and adverse collagen degradation through MAP kinase signaling and MMP-13 over-expression. MMP-1 increases in renal fibrosis in the settings of dialysis, kidney transplants, and progressive kidney scarring. Fibroblasts from patients with various premature aging diseases release high levels of MMP-1.
MMP-7 is not detected in normal human kidney, but is upregulated in distal tubular epithelium in various pathological conditions including polycystic kidney disease in humans and UUO in mice, and acute folic acid nephropathy in mice (Catania et al., 2007). Due to its inducible expression, MMP-7 has been proposed as a new screening marker for kidney damage. The correlation between fibrotic changes and MMP-7 activation are reported in the kidney, as well as in the lung and liver (Bauer et al., 2017; Huang et al., 2005). Aging increases renal expression of MMP-7, along with elevated renal and urinary kidney injury molecule-1 (Chen et al., 2007). Susceptibility to ischemic injury is increased in kidneys with age, characterized by elevated lactate dehydrogenase release and exacerbated structural damage and corrected by caloric restriction (Chen et al., 2007). Caloric restriction inhibits age-related MMP-7 upregulation and fibrotic collagen deposition, suggesting a significant role for MMP-7 induction in age-related renal fibrosis (Oelusarz et al., 2013). MMP-7-overexpressed in kidney cells in vitro show increases in collagen Ia2 and collagen IIIa1 transcription mediated by phosphoinositide 3-kinase, p38, ERK, Src, and protein kinase A signaling, independently of inflammation (Oelusarz et al., 2013). Interestingly, these effects may be caused by pro-MMP-7, because the authors detect no active form of MMP-7 in the aging kidney. MMP-7 activates MMP-2 and MMP-9, both of which are well-reported to contribute to renal pathogenesis and fibrosis.
TIMP-3 protein expression in renal parenchymal tissues at autopsy correlates with advancing age, not gender, ethnicity, diabetes, hyper-tension, or smoking status. Renal TIMP-3 is not detected in subjects under 20 years old and becomes strongly induced in the 40s. TIMP-3 is expressed around small and intermediate-sized arterioles and occasionally the afferent arterioles of the glomerulus (Macgregor, Eberhart, Fraig, Lu, & Halushka, 2009). In contrast, TIMP-3 expression in the renal medulla is lower in old mice (2 years old) than the young mice (12 weeks old) (Kassiri et al., 2009).
The renal TIMP-1 levels increase with age, and transgenic overexpression of TIMP-1 accelerates age-related downregulation of MMP-2 and MMP-9 expression and activity, collagen III and IV accumulation, fibrosis, and renal dysfunction (Zhang et al., 2006). An imbalance in MMPs and TIMPs causes ECM accumulation in the aging kidney (Sangaralingham et al., 2016). Because TIMP-1 overexpression also promotes age-related increases in ICAM-1, macrophage infiltration, and TGF-ß, increases in TIMP-1 mediates age-related renal fibrosis at least in part by enhancing inflammation through ICAM-1 upregulation (Zhang et al., 2006).
MMP-2 and MMP-9 are produced from mesangial cells, glomerular cells, epithelial cells, endothelial cells, tubular cells of collecting ducts, fibroblasts, and macrophages in the kidney (Tan & Liu, 2012). Renal MMP-2 and MMP-9 are at low levels in the absence of pathology. Advancing age causes decreases in MMP-2 and MMP-9 mRNA, protein, and activity and increases TIMP-1, which contribute to the decline in fibrillar collagen degradation (Oelusarz et al., 2013). Likewise, old (12 month old) female Dahl-salt sensitive rats receiving low (0.1%) salt chow exhibit an aging physiological and structural phenotype, with increased TGF-β and reduced activity of MMP-2 and MMP-9 (Maric et al., 2004). MMP-2 and MMP-9 increases with age can be inhibited by caloric restriction (Oelusarz et al., 2013). Kidneys from old rats (20 month old) express higher levels of MMP-9 protein compared to younger rats, and atorvastatin improves age-related pathological changes by reducing MMP-9 (Zhao et al., 2016).
7. MMP-9 as a potential therapeutic target for cardiorenal aging
MMP-9, first known as neutrophil gelatinase or gelatinase b, processes a variety of ECM substrates such as collagens, elastin, entactin, fibronectin, gelatin, laminin, and proteoglycans and non-ECM substrates such as endothelin-1, IL-1β, IL-6, IL-8, osteopontin, latent TGF-β, TNF-α, VEGF, and even other MMPs (MMP-2 and MMP-13) and itself. Because of the wide range of substrates, MMP-9 plays important roles in the process of cardiorenal aging and the pathogenesis of CVD and CKD. MMP-9 also has strong association with common mechanisms of cardiac and renal aging. For example, angiotensin II, RAGE, and oxidative stress are reported to induce upregulation of MMP-9, and physiological levels of NO produced by eNOS decreases MMP-9 (Cipollone et al., 2003; O’Sullivan, Medina, Ledwidge, Radomski, & Gilmer, 2014; Pushpakumar et al., 2013; Ridnour et al., 2007; Stacy, Yu, Horak, & Larson, 2007).
7.1. MMP-9 in the aging heart
In the setting of myocardial infarction, older mice show a more drastic increase in LV MMP-9 and a higher risk of cardiac rupture despite a greater extent of collagen content and crosslinking than the young mice (Yang et al., 2008). MMP-9 deletion improves survival and blunts age-related LV dilation post-myocardial infarction (Yabluchanskiy et al., 2016). Macrophages isolated from MMP-9-null infarct LVs demonstrate enhanced expression of M2 markers, including TGF-ß1. Promoted macrophage M2 polarization may contribute to the increased collagen accumulation, which results in a stiffer infarct region and limits LV dilation and further adverse remodeling in MMP-9-null aged mice (Voorhees et al., 2015). Hence, inhibition of MMP-9 is expected to be a promised therapeutic strategy against myocardial infarction in the elderly.
Cardiac MMP-9 expression and activity increases with age, while plasma MMP-9 increases in mice and decreases in humans. The role of MMP-9 as an age accelerator is supported by evidence that global MMP-9 gene deletion attenuates age-related LV diastolic dysfunction and fibrosis (Chiao et al., 2012). MMP-9 expression in healthy murine LV doubles with age (Chiao et al., 2012; Yabluchanskiy et al., 2014). In senescent mice (>23 months of age), MMP-9 co-localizes with macrophages (Chiao et al., 2011). MMP-9 in cardiac fibroblasts actually declines beginning at middle-age in mice, supporting that macrophages are major source of MMP-9 in the aging LV (Lindsey et al., 2005).
MMP-9 deletion leads to increased angiogenic factors in the plasma (von Willebrand factor) and the LV (VEGF and integrin αv), which support an increase in the number of vessel numbers in the aging LV (Yabluchanskiy et al., 2014). In MMP-9 null LV, vascular permeability is also attenuated, indicating that deleterious effects of MMP-9 on cardiac capillaries include both promoting vessel rarefaction and vascular leakiness (Yabluchanskiy et al., 2014).
Age-related increases in the expression of pro-inflammatory genes, including Ccr7, Ccr10, IL-1f8, IL-13, and IL-20, are blunted by MMP-9 gene deletion (Yabluchanskiy et al., 2014). While macrophages produce MMP-9, MMP-9 also affects macrophage polarization. With advancing age, wild type mice show a linear increase in cardiac M1 macrophages and decrease in cardiac M2 macrophages; in MMP-9-null mice, these linear changes are abolished. Aged LVs from MMP-9 null mice have a higher cardiac M2 macrophage ratio than age-matched wild type LVs, indicating that MMP-9 facilitates macrophage M2 to M1 polarization. MMP-9 stimulated young peritoneal macrophages show an M1/M2 mid-transition polarization state (Ma et al., 2015). MMP-9 promotes cardiac inflammaging by changing macrophage polarization without affecting the number of macrophages.
MMP-9 deletion inhibits fibrillar collagen deposition by inhibiting TGF-β signaling, characterized by decreased TGF-β-induced protein (Tgfbi) and Smad2 phosphorylation, and its downstream pro-fibrotic periostin and connective tissue growth factor (Chiao et al., 2012). Fibro-blasts play principal roles in controlling ECM homeostasis, and MMP-9 expression in fibroblasts from middle-aged and old murine LVs decreases compared to the young (Lindsey et al., 2005). MMP-9 may play important roles in maintaining physiological function of fibroblasts when produced locally and at a physiological amount.
Elastin microfibril interface-located protein-1 (EMILIN-1), an adhesive glycoprotein, is secreted by myocytes, endothelial cells, vascular smooth muscle cells, and adventitial fibroblasts. Myocardial EMILIN-1 expression decreases with age, and global gene deletion of MMP-9 attenuates age-related EMILIN-1 downregulation (Padmanabhan Iyer et al., 2016). EMILIN-1 deficiency is associated with vascular stiffening and hypertension (Zacchigna et al., 2006). Thus, MMP-9-induced cardiac aging and stiffness may be mediated by EMILIN-1 reduction.
MMP-9 cleaves osteopontin, and MMP-9-cleaved osteopontin fragments increase fibroblast migration in vitro (Lindsey, Zouein, Tian, Padmanabhan Iyer, & de Castro Bras, 2015). Though the total levels of osteopontin expression does not show age-related increase in the normal heart (Singh et al., 2010), MMP-9-cleaved osteopontin fragment may increase with age and play important roles in cardiac fibrosis by migrating fibroblasts.
Citrate synthase, a rate limiting enzyme of tricarboxylic acid cycle and a marker of mitochondrial function, is a MMP-9 substrate in vivo and in vitro (de Castro Bras et al., 2014). The citrate synthase activity decreases in the infarct LV of wild type mice, but not in MMP-9-null mice, suggesting that MMP-9 promotes mitochondrial dysfunction after myocardial infarction (de Castro Bras et al., 2014). Because mitochondrial dysfunction is a strong cell senescent factor, MMP-9 may contribute to cardiac aging by cleaving citrate synthase.
7.2. MMP-9 in the aging kidney
MMP-9 is expressed in the developing metanephric mesenchyme and is decreased in the adult murine kidney, with very low to no expression in collecting duct epithelial cells and glomeruli. MMP-9 null mice display no significant differences in renal phenotype in the absence of injury (Andrews et al., 2000). MMP-9 is elevated in a variety of kidney disease models and humans, including patients with IgA nephritis, Henoch-Schönlein nephritis, non-IgA mesangial proliferative glomerulonephritis, lupus nephritis, and Alport syndrome (Urushihara, Kagami, Kuhara, Tamaki, & Kuroda, 2002). In serum of polycystic kidney disease patients, MMP-9 and collagen IV are elevated. In allograft rejection after kidney transplantation, MMP-9 is increased and the immunosuppressive agent rapamycin decreases MMP-9 (Tan & Liu, 2012).
MMP-9 is colocalized in scattered neutrophils within diseased glomeruli in acute poststreptococcal glomerulonephritis (Urushihara et al., 2002). In UUO mice, renal MMP-9 activity increases, and the major sources of MMP-9 are tubular epithelial cells, myofibroblasts, and macrophages. Peritubular endothelial cells also express MMP-9, which has potential to induce endothelial-to-mesenchymal transition (EndMT) (Abadir et al., 2011; Catania et al., 2007; Tan et al., 2013; Zhao et al., 2013; Zhao et al., 2017). MMP-9-induced EMT in UUO rats is STAT3-dependent, and mesenchymal stem cells protect against renal fibrosis by suppressing the STAT3-MMP-9-EMT axis (Matsui et al., 2017). A neutralizing antibody for MMP-9 given at early- and late-stages reduces MMP-9-cleaved osteopontin, macrophage infiltration, tubular cell EMT, and fibrosis in the kidney (Tan et al., 2013).
MMP-9 mRNA, protein, and activity increase or decrease with age in the rat kidney, depending on the strain evaluated. Even in the same strain, renal MMP-9 mRNA increases or decreases depending on the studies. In a mouse model of anti-glomerular basement membrane nephritis, MMP-9 null mice exhibit severe renal dysfunction and fibrin deposition, suggesting a protective role of MMP-9 (Lelongt et al., 2001). TGF-ß-induced Wnt/ß-catenin signaling, the fibrogenic pathway, induces MMP-9 transcription, indicating a link between MMP-9 expression and fibrogenic signals (Wang, Dai, Li, & Liu, 2011). In renal fibroblasts, tissue-plasminogen activator induces MMP-9 gene and protein expression (Hu et al., 2006). As a posttranslational regulation, the interaction between MMP-9 and neutrophil gelatinase-associated lipocalin (NGAL), a renal injury marker, prevents MMP-9 degradation, suggesting prolonged MMP-9 action in renal pathology.
The anti-angiogenic factor angiostatin is produced by plasminogen cleavage by several MMPs, including MMP-9 (Patterson & Sang, 1997). In the arteries of diabetic CKD patients, upregulation of MMP-9 and angiostatin correlate with impaired angiogenesis, endothelial dysfunction, and vessel stiffening (Chung et al., 2009). In acute kidney injury, renal angiostatin is generated due to MMP-9 activation (Basile, Fredrich, Weihrauch, Hattan, & Chilian, 2004). Age-related increases in angiostatin with decreased MMP-9 expression and activity have also been reported in the aging rat kidney (Satoh et al., 2013).
In wild type mice with nephritis, the macrophage number per kidney is 2000, in MMP-9 null mice with nephritis, this number decreases to 500 (Kluger et al., 2013). Bone marrow transplantation of wild type-derived donor cells to MMP-9-deficient mice prior to the induction of nephritis cancels the suppression of renal tissue injury and macrophage recruitment, suggesting a crucial role of leucocyte-derived MMP-9 in recruiting pro-inflammatory macrophages (Kluger et al., 2013). MMP-9 cleaves osteopontin, and cleavage enhances macrophage migration in vitro, suggesting MMP-9 is essential for inflammatory cell migration directed by osteopontin cleavage (Tan et al., 2013). Among the leucocyte subpopulations, activated macrophages are reported to be the major source of MMP-9 (Kluger et al., 2013). Because macrophage number increases with age in the kidney, the macrophage is presumed to be a major source of MMP-9 in aging kidney. The fact that MMP-9 polarizes M1 > M2 macrophages in vitro and that M1 > M2 polarization increases in aging kidney suggest MMP-9 may play roles in renal inflammaging.
Tubular epithelial cells that are cultured with activated macrophage-conditioned media undergo EMT, which is suppressed by a MMP-2/9 inhibitor or MMP-9 knockout, suggesting a significant role of macrophage-derived MMP-9 in EMT (Tan et al., 2010). Since less EMT occurs in MMP-9-ablated tubular epithelial cells than WT cells, tubular epithelial cells are also the origins of MMP-9.
MMP-9 causes fibrosis as a consequence of interactions with the inflammatory process and also by direct signaling. In addition to catalyzing pro-TGF-β to its active form, MMP-9 directly mediates EMT in renal cells downstream and independent of TGF-β (Wang et al., 2010). For example, MMP-9 deletion decreases EMT and collagen deposition without affecting TGF-β. In vitro, even without TGF-β stimulation, increased MMP-9 can promote EMT by digesting matrigel that mimics native tubular basement membrane, suggesting that MMP-9 promotes EMT by tubular basement membrane destruction (Wang, Zhou, et al., 2010).
TGF-ß-induced EndMT and concomitant production of collagen I and fibronectin in cultured mouse renal peritubular endothelial cells is suppressed by MMP-9 deletion, showing that MMP-9 promotes renal fibrosis through EndMT (Zhao et al., 2017). In the same study, MMP-9-mediated EndMT is demonstrated to be mediated by Notch signaling.
8. Potential therapeutic targets for CVD and CKD in the elderly
Both physiological aging and pathological premature aging in the heart and kidney induce inflammation and subsequent fibrosis. However, in pathological states, more numbers of macrophages are detected in the heart and the kidney compared to what is seen with physiological aging. For example, macrophage numbers increase approximately 10 times in hypertensive heart failure and 100 times in myocardial infarction (days 3–5 post-myocardial infarction) compared to non-diseased controls (Halade et al., 2013; Lindsey et al., 2006; Xia et al., 2009). In addition to macrophages, various inflammatory cells contribute to disease progression. While macrophages play roles in hypertensive heart failure, neutrophils and macrophages increase after the onset of myocardial infarction (Halade et al., 2013; Lindsey et al., 2006). In diseased kidney, myocytes, macrophages (hypertensive nephrosclerosis, diabetic nephropathy, and acute kidney injury), lymphocytes (diabetic nephropathy), and neutrophils (acute kidney injury) participate in the pathogenesis (Duran-Salgado & Rubio-Guerra, 2014; Ferenbach & Bonventre, 2015; Meyrier, 2015).
8.1. For CVD
In hypertensive heart failure, TGF-β is induced at day 7, when macrophage numbers start to decline, suggesting that TGF-β may bridge the inflammatory and reparative phases (Xia et al., 2009). As pressure overload is not associated with significant cardiomyocyte loss, TGF-β-induced matrix deposition results in fibrotic remodeling (Kuwahara et al., 2004; Xia et al., 2009). Aged mice show worse outcome after pressure overload, while there is also a study reporting that aged mice exhibit less LV remodeling and higher survival than young mice following size-appropriate transverse aortic constriction (Geng et al., 2017).
Macrophage activation after coronary artery occlusion is biphasic with both M1 and M2 macrophages being present. While M1 macrophages promote ECM degradation, M2 macrophages contribute to scar formation by releasing TGF-β, IL-10, and VEGF (Nahrendorf, Pittet, & Swirski, 2010). Fibrillar collagen content increases approximately 10 to 20-fold on days 7–15 post-myocardial infarction (Cleutjens, Verluyten, Smiths, & Daemen, 1995). Increased collagen content and collagen crosslinking during the maturation phage leads to LV stiffness and heart failure. Insufficient fibrillar collagen formation, in contrast, results in inadequate wound healing and scar formation and can lead to excessive wall thinning and cardiac rupture.
Transverse aortic contraction induces an approximate 4-fold increase in SPARC expression (Bradshaw et al., 2009). In SPARC-null mice, pressure overload-induced increases in fibrillar collagen content and myocardial stiffness are attenuated (Bradshaw et al., 2009). In a mouse model of myocardial infarction, SPARC expression increases in the LV, colocalizing with myofibroblasts and leukocytes (Schellings et al., 2009). SPARC deletion preserves LV systolic function at the early stage (day 3) after coronary ligation (McCurdy et al., 2011). In contrast, SPARC deletion results in increased mortality from an increased incidence of cardiac rupture and failure day 7 post-myocardial infarction due to immature collagen formation (Schellings et al., 2009). Suppression of SPARC may not be beneficial but rather deleterious for the long term treatment in the settings of myocardial infarction.
MMP-9 deletion attenuates LV remodeling and dysfunction after transverse aortic constriction (Heymans et al., 2005). The adverse effect of MMP-9 in the pressure-overloaded heart is strengthened by a recent study that demonstrates that angiotensin II facilitates cardiac hypertrophy through MMP-9 activation (Wang et al., 2017).
In a murine myocardial infarction model, a robust increase in MMP-9 activity is corresponding with infiltration of neutrophils (day 1) and macrophages (day 4) (Halade et al., 2013). Robustly increased MMP-9 from inflammatory cells raises the possibility that MMP-9 facilitates early LV injury post-myocardial infarction (Yabluchanskiy, Ma, Iyer, Hall, & Lindsey, 2013). Whereas MMP-9 gene deletion attenuates LV dilation in both young and old murine models of myocardial infarction, pharmacological inhibition of MMP-9, decreasing MMP-9 activity by 30%, prevents neutrophil apoptosis, which leads to prolonged inflammation, insufficient scar formation and enhanced matrix degradation, suggesting a critical role of MMP-9 in inflammatory process for a subsequent adaptive scar formation (Iyer et al., 2016).
Restricted overexpression of human MMP-9 in aged mice exacerbates age-related hypertrophy, vessel rarefaction, inflammation, and fibrosis (Toba et al., 2017). Overexpression of macrophage MMP-9 in aged mice superimposed on myocardial infarction causes decreased angiogenesis and increased macrophage infiltration with M2 macrophage phenotype. While collagen content after myocardial infarction is greater in transgenic mice than wild type mice, overexpression of macrophage MMP-9 results in reduced collagen crosslinking, attenuated cardiac stiffness, and improved diastolic function (Zamilpa et al., 2012). One interpretation is that the diverse actions of macrophage MMP-9 in aged hearts depend on the physiological or pathological context, the concentration of MMP-9, and the indirect downstream effects on vascular cells, inflammatory cells, and fibroblasts.
8.2. For CKD
Histopathological features of hypertensive nephrosclerosis include microvascular changes with hyalinesis of the preglomerular vessel walls and thickening of the intima and reduplication of the internal elastic lamina of the arcuate and interlobar arteries, leading to glomerulosclerosis and patchy tubular atrophy, which are associated with inflammation and tubulointerstitial fibrosis (Hill, 2008; Klanke et al., 2008; Meyrier, 2015). Aging kidneys display lesions close to hyper-tensive nephrosclerosis even without hypertension (Marin, Gorostidi, Fernandez-Vega, & Alvarez-Navascues, 2005). Animal models of hypertensive nephrosclerosis demonstrate that angiotensin II plays major roles in creating microvascular lesions and stimulating inflammatory cascades that end in renal fibrosis (Mezzano et al., 2001). Comorbidities of metabolic disorders such as metabolic syndrome and obesity increase oxidative stress and inflammation directly and through an increase of angiotensin II. Since angiotensin II plays pivotal roles in renal aging, high levels of age-related angiotensin II in the kidney may contribute to the susceptibility to hypertensive renal injury in the elderly populations.
Diabetic nephropathy is characterized with mesangial expansion, thickening of basement, tubular, and glomerular membrane, and ECM accumulation in these membranes that eventually cause tubular atrophy, tubulointerstitial and glomerular fibrosis, and glomerulosclerosis (Duran-Salgado & Rubio-Guerra, 2014; Li et al., 2008). Metabolic changes due to hyperglycemia lead to AGE generation, poly pathway activation, abnormal PKC activation, and oxidative stress. Among various cytokines, chemokines, and adhesion molecules, which are involved in the inflammatory response in diabetic nephropathy, circulating MCP-1 and vascular cell adhesion molecule-1 and renal TNF-α correlate with the severity of albuminuria in diabetic patients and animals. TGF-β is one of the principle mediators of fibrosis in diabetic nephropathy, and its concentration is higher in diabetic patients with albuminuria (Duran-Salgado & Rubio-Guerra, 2014; Li et al., 2008).
In many cases, progression of renal failure is due to a secondary insult (e.g. shock, sepsis, surgery, and administration of nephrotoxic agents), associated with transient intrarenal hypoperfusion (acute kidney injury) rather than worsening of primary renal disease (Ferenbach & Bonventre, 2015). Ischemia-reperfusion injury particularly affects the outer stripe of the outer medulla where baseline hypoxia exists. Following insult, the kidney has the ability to return to normal function, whereas functional recovery potential declines with age (Schmitt & Cantley, 2008). Vascular injury remains for several days, and injured vasculatures show impaired NO generation, adhesion molecules expression, and high permeability. Within the first 24 h, resident immune cells release a burst of chemotactic signals, and injured tubular and endothelial cells promote neutrophil migration, followed by monocyte recruitment, which differentiations predominantly M1 macrophages. Day 5, macrophage polarization shifts to M2 subsets, which are necessary for renal repair by releasing various factors including TGF-β. Depending on the background (e.g. aging, hypertension, and diabetes) and the extent and frequency of injury, acute kidney injury leads to being arrested in dedifferentiated tubular cells with production of profibrogenic factors, resulting in maladaptive repair and developing CKD. In maladaptive repair, increased numbers of myofibroblasts are key players in the collagen deposition (Ferenbach & Bonventre, 2015). Pathological stimulus results in more prominent injury in the elderly people who potentially have levels of basal inflammation and fibrosis. Despite the cause of injury, inhibiting maladaptive repair which results in excessive fibrosis is the common and critical target for renal aging and CKD.
Inhibitors for MMPs suppress proteinuria and glomerular injury with or without anti-hypertensive action (Williams et al., 2011). In spontaneously hypertensive rats, which show proteinuria a fibrillar collagen deposition, renal MMP-9 levels are higher compared to normotensive control, while spontaneously hypertensive stroke-prone rats shows progressive inflammatory cell infiltration and severe fibrosis with decreased levels of MMP-9 (Camp, Smiley, Hayden, & Tyagi, 2003; Gianella et al., 2007).
Type 2 diabetic Goto-Kakizaki rats demonstrate high expression of MMP-9, collagen I, collagen III, and fibronectin during the early stages before functional and histological pathology emerge (Portik-Dobos et al., 2006). MMP-9 deletion attenuates nephropathy in the streptozotocin-induced type 1 diabetic model (Li et al., 2014). These reports highlight the exacerbating roles of MMP-9 in diabetic nephropathy. Indeed, administration of the MMP inhibitor doxycycline to patients with diabetic nephropathy receiving ACE inhibitors and angiotensin II receptor blockers significantly reduce proteinuria for the short duration of 3 months (Aggarwal et al., 2010).
As described above, MMP-9 contributes to decreases in renal capillary density after ischemia by generating angiostatin (Basile et al., 2004). MMP-9 deletion stabilizes microvascular density after ischemia/reperfusion by preserving renal VEGF (Lee et al., 2011). In addition to vessel rarefaction, ischemia-induced degradation of the tight junction protein zonula occludens-1 in glomerulus is associated to the increased MMP-9 activity (Caron, Desrosiers, & Beliveau, 2005).
9. Sex differences in cardiorenal aging
Sex-differences in cardiorenal aging have been under-investigated. The age-related progression of cardiovascular and renal diseases is slower in premenopausal women compared with men (Keller & Howlett, 2016; Perry 3rd., 1999; Reckelhoff, 2001; Silbiger & Neugarten, 2003).
In the heart, the number of LV myocytes declines through apoptosis with age in male human and animals but not in the female (Olivetti et al., 1995; Olivetti, Melissari, Capasso, & Anversa, 1991; Zhang et al., 2007). In addition to myocyte apoptosis, the decrease in peak Ca2+ transients/contractions in myocytes from aged male animals but not female control may be one of the reasons why heart failure are seen more in older men than older women (Dibb, Rueckschloss, Eisner, Isenberg, & Trafford, 2004; Dunlay & Roger, 2012; Feridooni, Dibb, & Howlett, 2015). Risk factors for heart failure differ between genders with myocardial ischemia playing a critical role in men and hypertension being important in women (Greiten, Holditch, Arunachalam, & Miller, 2014).
In the healthy population, the age-related decline in GFR starts from approximately the age of 30 in men, while GFR remains stable beyond the age of 50 in women. Renal plasma flow is also preserved in aging women, whereas it falls with age in men (Berg, 2006; Kielstein et al., 2003). Plasma ADMA levels increase with age and correlate with renal plasma flow declines in healthy aging men (Kielstein et al., 2003). In women, age-related increases in ADMA delay until approximately 50 years old and correlate with endothelial dysfunction (Celermajer et al., 1994; Schulze et al., 2005).
Angiotensin II-overexpressed aged male myocytes maintain contractile function but exert the increased vulnerability to arrhythmic activity. In contrast, female myocytes from intracardiac angiotensin II-transgenic mice are more susceptible to age-related contractile deficit and exhibit a low level of spontaneous activity in the pressure of prolonged Ca2+ release, suggesting compensatory mechanisms to prevent spontaneity in the pro-arrhythmogenic setting (Mellor et al., 2014). Despite the controversy, the majority of reports support that ACE inhibitors have lower efficacy in women than men, and ARBs should be chosen for women (Sullivan, 2008). Males have greater levels of AT1 receptor expression in the kidney and higher AT1 receptor bindings in glomeruli (Sullivan, 2008).
There is accumulating evidence that estrogens protect cardiovascular tissue (Baylis, 2012; Zheng et al., 2003). In addition to subsequent renal protection by improving cardiovascular function, estrogens exert direct kidney protection. Though some studies demonstrate that estrogens induce worse renal pathology, estrogens inhibit ECM accumulation and vascular smooth muscle and mesangial cell growth (Baylis, 2012). Estrogen receptor α gene deletion accelerates age-related albuminuria and glomerular damage and develops age-related hypertension (Elliot et al., 2007; Zhu et al., 2002). Estrogens inhibit RAS by various mechanisms, whereas androgens stimulate it (Diz, 2008; Komukai, Mochizuki, & Yoshimura, 2010). Estrogens exert NO stimulatory effects by direct and indirect actions (Baylis, 2012). The effects of sex hormones on the cardiac and renal RAS and NO bioavailability may influence the sex differences in aging.
In 699 Framingham Study participants free of heart failure and previous myocardial infarction (mean age, 57 years, 58% women), plasma MMP-9 associates with increased LV diastolic dimensions, increased wall thickness, and higher LV mass in men but not in women (Sundstrom et al., 2004). In 832 consecutive outpatients with chest pain (mean age 53.7, 516 men, 346 women), serum MMP-9 levels independently correlates with the diagnosis of non-calcified and mixed plaques in women but not in men (Gu et al., 2017).
17β-estradiol treatment increases MMP-9 expression and activation in mesangial cells (Potier et al., 2001). In the aged (12 months old) rat kidney, ovariectomy increases TGF-β expression and decreases cortical MMP-9 activity, which are reversed by 17β-estradiol replacement (Maric et al., 2004). Age-related and postmenopausal renal dysfunction may be induced by insufficient ECM degradation by MMP-9, but the relationship between sex difference and MMP-9 in renal aging is not clarified.
Further investigation into the sex-specific variation in aging and pathological mechanisms, including MMP-9, will be needed for adequate and effective therapeutic strategy against cardiorenal aging and CVD and CKD in the elderly population.
10. Conclusions
Common mechanisms of cardiac and renal aging that affect each other include RAS activation and stimulation of the AGE-RAGE axis to induce oxidative stress, which decreases NO bioavailability. These mechanisms all have strong bidirectional relationships with MMP-9, and a major conclusion of this review is that macrophage-derived MMP-9 promotes cardiorenal aging by vessel rarefaction, inflammation, and fibrosis. In terms of ECM, SPARC and osteopontin interact closely with MMP-9 may be a therapeutic target for cardiorenal aging. The wide MMP-9 network provides strong rationale for examining MMP-9 as a comprehensive therapeutic cross-organ target (Figs. 3 and 4).
Fig. 3.
Mechanistic diagram showing effects of macrophage-derived matrix metalloproteinase (MMP)-9 in cardiorenal aging. In addition to reported common aging mechanisms such as oxidative stress, renin-angiotensin system (RAS), nitric oxide (NO) deficiency, and advanced glycation end product (AGE), all of which cause cellular senescence, and extracellular matrix (ECM) components including osteopontin and secreted protein acidic and rich in cysteine (SPARC), macrophage-derived MMP-9 causes inflammation and fibrosis dependent and independent vascular rarefaction in the heart and kidney. Age-related systemic changes and comorbidities of risk factors (hypertension, diabetes, dyslipidemia) facilitates cardiorenal aging. Cardiovascular disease (CVD) and chronic kidney disease (CKD) interact each other, exacerbating their dysfunctional changes.
Fig. 4.
Suggested roles of matrix metalloproteinase (MMP)-9 in inflammaging and fibrosis in the heart and the kidney. MMP-9, produced from recruited macrophages in the aging heart, promotes inflammaging by increasing macrophage M1 polarization, not macrophage recruitment. Macrophage-derived MMP-9 in the aging kidney induces both macrophage M1 polarization and leucocyte mobilization. MMP-9 converts latent TGF-β to active form, which leads to fibrosis. Epithelial-mesenchymal transition (EMT) in the kidney is both TGF-β-dependent and independent. EndMT; endothelial-to-mesenchymal transition.
Although we conclude that MMP-9 inhibition is critical for age-related CVD and CKD, no MMP-9-specific inhibitor has not become in clinical use yet and in fact it may be inhibition of specific MMP-9 substrate targets will be more effective. Until clinically available selective MMP-9 inhibitors have been developed, alternative MMP-9 inhibition with clinically available agents would be helpful. For example, peroxisome proliferator-activated receptors α and γ ligands downregulate the MMP-9 expression in lipopolysaccharide-treated human monocytic THP-1 cells (Shu et al., 2000). Since angiotensin II induces MMP-9 expression and angiotensin II-induced cardiomyocyte hypertrophy is mediated by MMP-9, tissue protective actions by RAS inhibitors may be at least in part by MMP-9 inhibition (Wang, Cheng, et al., 2017). In addition to these agents, suppression of inflammation by lifestyle modification and treatment of diseases results in the decline in inflammatory cells, which are the major source of MMP-9.
Future studies to determine the extent and timing of MMP-9 inhibition and the background which divides the effects of MMP-9 and to develop clinically available selective MMP-9 inhibitors will provide further possibility of macrophage-derived MMP-9.
Acknowledgments
This work was supported by the National Institutes of Health (NIH) HL075360, HL129823, and GM114833 to MLL, and by HL051971, GM104357, and GM115428; and the Biomedical Laboratory Research and Development Service of the Veterans Affairs Office of Research and Development Award 5I01BX000505 to MLL; and grant-in-aids for scientific research from Kyoto Pharmaceutical University 16–05 and Hoansha Foundation to HT. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Institutes of Health or the U.S. Department of Veterans Affairs.
Abbreviations:
- CVD
cardiovascular disease
- CKD
chronic kidney disease
- MMP
matrix metalloproteinase
- ECM
extracellular matrix
- GFR
glomerular filtration rate
- SASP
senescence-associated secretory phenotype
- LV
left ventricle
- VEGF
vascular endothelial cell growth factor
- NO
nitric oxide
- eNOS
endothelial NO synthase
- ROS
reactive oxygen species
- IL
interleukin
- TNF
tumor necrosis factor
- Ccl
chemokine (C-C motif) ligand
- Ccr
CC chemokine receptor
- Mif
macrophage migration inhibitory factor
- MCP
monocyte chemoattractant protein
- TGF
transforming growth factor
- EMT
epithelial-mesenchymal transition
- ICAM
intracellular adhesion molecule
- PDGF
platelet-derived growth factor
- MAP
p38 mitogen-activated protein
- UUO
unilateral ureteral obstruction
- ROS
reactive oxygen species
- RAS
renin-angiotensin system
- ACE
angiotensin converting enzyme
- ADMA
asymmetric dimethylarginine
- cGMP
cyclic guanosine monophosphate
- AGE
advanced glycation end product
- RAGE
receptor of AGE
- SPARC
secreted protein acidic and rich in cysteine
- ADAMTS
a disintegrin and metalloproteinase with thrombospondin-like motifs
- TIMP
tissue inhibitors of metalloproteinase
- EMILIN-1
elastin microfibril interface-located protein-1
- SD
Sprague-Dawley
- DS
Dahl salt-sensitive
Footnotes
Conflict of interest statement
The authors declare that there are no conflicts of interests.
References
- Abadir PM, Foster DB, Crow M, Cooke CA, Rucker JJ, Jain A, … Walston JD. (2011). Identification and characterization of a functional mitochondrial angiotensin system. Proceedings of the National Academy of Sciences of the United States of America 108, 14849–14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Kader K, & Palevsky PM (2009). Acute kidney injury in the elderly. Clinics inGeriatric Medicine 25, 331–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta JC, Banito A, Wuestefeld T, Georgilis A, Janich P, Morton JP, … Gil J. (2013). A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nature Cell Biology 15, 978–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal HK, Jain D, Talapatra P, Yadav RK, Gupta T, & Kathuria KL (2010). Evaluation of role of doxycycline (a matrix metalloproteinase inhibitor) on renal functions in patients of diabetic nephropathy. Renal Failure 32, 941–946. [DOI] [PubMed] [Google Scholar]
- Ago T, Matsushima S, Kuroda J, Zablocki D, Kitazono T, & Sadoshima J (2010). The NADPH oxidase Nox4 and aging in the heart. Aging (Albany NY) 2, 1012–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfaras I, Di Germanio C, Bernier M, Csiszar A, Ungvari Z, Lakatta EG, & de Cabo R (2016). Pharmacological Strategies to Retard Cardiovascular Aging. Circulation Research 118, 1626–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altin SE, & Schulze PC (2011). Fractalkine: A novel cardiac chemokine? CardiovascularResearch 92, 361–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews KL, Betsuyaku T, Rogers S, Shipley JM, Senior RM, & Miner JH (2000). Gelatinase B (MMP-9) is not essential in the normal kidney and does not influence progression of renal disease in a mouse model of Alport syndrome. The American Journal of Pathology 157, 303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annoni G, Luvara G, Arosio B, Gagliano N, Fiordaliso F, Santambrogio D, … Masson S. (1998). Age-dependent expression of fibrosis-related genes and collagen deposition in the rat myocardium. Mechanisms of Ageing and Development 101, 57–72. [DOI] [PubMed] [Google Scholar]
- Baltaci SB, Mogulkoc R, & Baltaci AK (2016). Resveratrol and exercise. BiomedicalReports 5, 525–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltanas A, Solesio ME, Zalba G, Galindo MF, Fortuno A, & Jordan J (2013). The senescence-accelerated mouse prone-8 (SAM-P8) oxidative stress is associated with upregulation of renal NADPH oxidase system. Journal of Physiology and Biochemistry 69, 927–935. [DOI] [PubMed] [Google Scholar]
- Basile DP, Fredrich K, Chelladurai B, Leonard EC, & Parrish AR (2008). Renal ischemia reperfusion inhibits VEGF expression and induces ADAMTS-1, a novel VEGF inhibitor. American Journal of Physiology. Renal Physiology 294, F928–F936. [DOI] [PubMed] [Google Scholar]
- Basile DP, Fredrich K, Weihrauch D, Hattan N, & Chilian WM (2004). Angiostatin and matrix metalloprotease expression following ischemic acute renal failure. American Journal of Physiology. Renal Physiology 286, F893–F902. [DOI] [PubMed] [Google Scholar]
- Bastard JP, Jardel C, Bruckert E, Blondy P, Capeau J, Laville M, … Hainque B. (2000). Elevated levels of interleukin 6 are reduced in serum and subcutaneous adipose tissue of obese women after weight loss. The Journal of Clinical Endocrinology and Metabolism 85, 3338–3342. [DOI] [PubMed] [Google Scholar]
- Batkai S, Rajesh M, Mukhopadhyay P, Hasko G, Liaudet L, Cravatt BF, … Pacher P. (2007). Decreased age-related cardiac dysfunction, myocardial nitrative stress, in-flammatory gene expression, and apoptosis in mice lacking fatty acid amide hydro-lase. American Journal of Physiology. Heart and Circulatory Physiology 293, H909–H918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer Y, White ES, de Bernard S, Cornelisse P, Leconte I, Morganti A, … Nayler O. (2017). MMP-7 is a predictive biomarker of disease progression in patients with idiopathic pulmonary fibrosis. ERJ Open Research, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylis C (2012). Sexual dimorphism: The aging kidney, involvement of nitric oxide deficiency, and angiotensin II overactivity. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 67, 1365–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benigni A, Cassis P, & Remuzzi G (2010). Angiotensin II revisited: New roles in inflammation, immunology and aging. EMBO Molecular Medicine 2, 247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benigni A, Corna D, Zoja C, Sonzogni A, Latini R, Salio M, … Remuzzi G. (2009). Disruption of the Ang II type 1 receptor promotes longevity in mice. The Journal of Clinical Investigation 119, 524–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg UB (2006). Differences in decline in GFR with age between males and females. Reference data on clearances of inulin and PAH in potential kidney donors. Nephrology, Dialysis, Transplantation 21, 2577–2582. [DOI] [PubMed] [Google Scholar]
- Berger MM (2005). Can oxidative damage be treated nutritionally? Clinical Nutrition 24,172–183. [DOI] [PubMed] [Google Scholar]
- Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, … Liu PP. (2006). Outcome of heart failure with preserved ejection fraction in a population-based study. The New England Journal of Medicine 355, 260–269. [DOI] [PubMed] [Google Scholar]
- Biernacka A, & Frangogiannis NG (2011). Aging and cardiac fibrosis. Aging and Disease 2, 158–173. [PMC free article] [PubMed] [Google Scholar]
- Blair SN, Kohl HW 3rd, Paffenbarger RS Jr., Clark DG, Cooper KH, & Gibbons LW (1989). Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA 262, 2395–2401. [DOI] [PubMed] [Google Scholar]
- Bock JS, & Gottlieb SS (2010). Cardiorenal syndrome: New perspectives. Circulation 121, 2592–2600. [DOI] [PubMed] [Google Scholar]
- Bolignano D, Mattace-Raso F, Sijbrands EJ, & Zoccali C (2014). The aging kidney revisited: A systematic review. Ageing Research Reviews 14, 65–80. [DOI] [PubMed] [Google Scholar]
- Bolton WK, & Sturgill BC (1980). Spontaneous glomerular sclerosis in aging Sprague-Dawley rats. II. Ultrastructural studies. The American Journal of Pathology 98, 339–356. [PMC free article] [PubMed] [Google Scholar]
- Bonnema DD, Webb CS, Pennington WR, Stroud RE, Leonardi AE, Clark LL, … Zile MR. (2007). Effects of age on plasma matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinases (TIMPs). Journal of Cardiac Failure 13, 530–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borlaug BA (2016). Cardiac aging and the fountain of youth. European Journal of Heart Failure 18, 611–612. [DOI] [PubMed] [Google Scholar]
- Bornstein P, & Sage EH (2002). Matricellular proteins: Extracellular modulators of cell function. Current Opinion in Cell Biology 14, 608–616. [DOI] [PubMed] [Google Scholar]
- Boudoulas KD, Triposkiadis F, Parissis J, Butler J, & Boudoulas H (2017). The Cardio-Renal Interrelationship. Progress in Cardiovascular Diseases 59, 636–648. [DOI] [PubMed] [Google Scholar]
- Bouzid MA, Hammouda O, Matran R, Robin S, & Fabre C (2014). Changes in oxidative stress markers and biological markers of muscle injury with aging at rest and in response to an exhaustive exercise. PLoS One 9, e90420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw AD, Baicu CF, Rentz TJ, Van Laer AO, Boggs J, Lacy JM, & Zile MR (2009). Pressure overload-induced alterations in fibrillar collagen content and myocardial diastolic function: Role of secreted protein acidic and rich in cysteine (SPARC) in post-synthetic procollagen processing. Circulation 119, 269–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw AD, Baicu CF, Rentz TJ, Van Laer AO, Bonnema DD, & Zile MR (2010). Age-dependent alterations in fibrillar collagen content and myocardial diastolic function: Role of SPARC in post-synthetic procollagen processing. American Journal of Physiology. Heart and Circulatory Physiology 298, H614–H622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw AD, & Sage EH (2001). SPARC, a matricellular protein that functions in cellular differentiation and tissue response to injury. The Journal of Clinical Investigation 107, 1049–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brekken RA, & Sage EH (2000). SPARC, a matricellular protein: At the crossroads of cell-matrix. Matrix Biology 19, 569–580. [DOI] [PubMed] [Google Scholar]
- Bruunsgaard H, & Pedersen BK (2003). Age-related inflammatory cytokines and disease. Immunology and Allergy Clinics of North America 23, 15–39. [DOI] [PubMed] [Google Scholar]
- Bujak M, Kweon HJ, Chatila K, Li N, Taffet G, & Frangogiannis NG (2008). Aging-related defects are associated with adverse cardiac remodeling in a mouse model of reperfused myocardial infarction. Journal of the American College of Cardiology 51, 1384–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H, Yuan Z, Fei Q, & Zhao J (2012). Investigation of thrombospondin-1 and transforming growth factor-beta expression in the heart of aging mice. Experimental and Therapeutic Medicine 3, 433–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camelliti P, Borg TK, & Kohl P (2005). Structural and functional characterisation of cardiac fibroblasts. Cardiovascular Research 65, 40–51. [DOI] [PubMed] [Google Scholar]
- Camp TM, Smiley LM, Hayden MR, & Tyagi SC (2003). Mechanism of matrix accumulation and glomerulosclerosis in spontaneously hypertensive rats. Journal of Hypertension 21, 1719–1727. [DOI] [PubMed] [Google Scholar]
- Campbell DJ, Somaratne JB, Jenkins AJ, Prior DL, Yii M, Kenny JF, … Kelly DJ. (2012). Diastolic dysfunction of aging is independent of myocardial structure but associated with plasma advanced glycation end-product levels. PLoS One 7, e49813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannata A, Camparini L, Sinagra G, Giacca M, & Loffredo FS (2016). Pathways for salvage and protection of the heart under stress: Novel routes for cardiac rejuvenation. Cardiovascular Research 111, 142–153. [DOI] [PubMed] [Google Scholar]
- Caron A, Desrosiers RR, & Beliveau R (2005). Ischemia injury alters endothelial cell properties of kidney cortex: Stimulation of MMP-9. Experimental Cell Research 310, 105–116. [DOI] [PubMed] [Google Scholar]
- de Castro Bras LE, Cates CA, DeLeon-Pennell KY, Ma Y, Iyer RP, Halade GV, … Lindsey ML. (2014). Citrate synthase is a novel in vivo matrix metalloproteinase-9 substrate that regulates mitochondrial function in the postmyocardial infarction left ventricle. Antioxidants & Redox Signaling 21, 1974–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catania JM, Chen G, & Parrish AR (2007). Role of matrix metalloproteinases in renal pathophysiologies. American Journal of Physiology. Renal Physiology 292, F905–F911. [DOI] [PubMed] [Google Scholar]
- Cau SB, Carneiro FS, & Tostes RC (2012). Differential modulation of nitric oxide synthases in aging: Therapeutic opportunities. Frontiers in Physiology 3, 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cavanagh EM, Inserra F, & Ferder L (2015). Angiotensin II blockade: How its molecular targets may signal to mitochondria and slow aging. Coincidences with calorie restriction and mTOR inhibition. American Journal of Physiology. Heart and Circulatory Physiology 309, H15–H44. [DOI] [PubMed] [Google Scholar]
- de Cavanagh EM, Piotrkowski B, Basso N, Stella I, Inserra F, Ferder L, & Fraga CG (2003). Enalapril and losartan attenuate mitochondrial dysfunction in aged rats. The FASEB Journal 17, 1096–1098. [DOI] [PubMed] [Google Scholar]
- Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, & Deanfield JE (1994). Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. Journal of the American College of Cardiology 24, 471–476. [DOI] [PubMed] [Google Scholar]
- Chen G, Bridenbaugh EA, Akintola AD, Catania JM, Vaidya VS, Bonventre JV, … Parrish AR. (2007). Increased susceptibility of aging kidney to ischemic injury: Identification of candidate genes changed during aging, but corrected by caloric restriction. American Journal of Physiology. Renal Physiology 293, F1272–F1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao YA, Dai Q, Zhang J, Lin J, Lopez EF, Ahuja SS, … Jin YF. (2011). Multi-analyte profiling reveals matrix metalloproteinase-9 and monocyte chemotactic protein-1 as plasma biomarkers of cardiac aging. Circulation. Cardiovascular Genetics 4, 455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao YA, & Rabinovitch PS (2015). The aging heart. Cold Spring Harbor Perspectives in Medicine 5, a025148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao YA, Ramirez TA, Zamilpa R, Okoronkwo SM, Dai Q, Zhang J, … Lindsey ML. (2012). Matrix metalloproteinase-9 deletion attenuates myocardial fibrosis and diastolic dysfunction in ageing mice. Cardiovascular Research 96, 444–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung AW, Yang HH, Sigrist MK, Brin G, Chum E, Gourlay WA, & Levin A (2009). Matrix metalloproteinase-2 and −9 exacerbate arterial stiffening and angiogenesis in diabetes and chronic kidney disease. Cardiovascular Research 84, 494–504. [DOI] [PubMed] [Google Scholar]
- Cieslik KA, Taffet GE, Carlson S, Hermosillo J, Trial J, & Entman ML (2011). Immune-inflammatory dysregulation modulates the incidence of progressive fibrosis and diastolic stiffness in the aging heart. Journal of Molecular and Cellular Cardiology 50, 248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipollone F, Iezzi A, Fazia M, Zucchelli M, Pini B, Cuccurullo C, … Mezzetti A. (2003). The receptor RAGE as a progression factor amplifying arachidonate-dependent in-flammatory and proteolytic response in human atherosclerotic plaques: Role of glycemic control. Circulation 108, 1070–1077. [DOI] [PubMed] [Google Scholar]
- Cleutjens JP, Verluyten MJ, Smiths JF, & Daemen MJ (1995). Collagen remodeling after myocardial infarction in the rat heart. The American Journal of Pathology 147, 325–338. [PMC free article] [PubMed] [Google Scholar]
- Coppe JP, Desprez PY, Krtolica A, & Campisi J (2010). The senescence-associated secretory phenotype: The dark side of tumor suppression. Annual Review of Pathology 5, 99–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai DF, Chen T, Johnson SC, Szeto H, & Rabinovitch PS (2012). Cardiac aging: From molecular mechanisms to significance in human health and disease. Antioxidants & Redox Signaling 16, 1492–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai DF, Chen T, Wanagat J, Laflamme M, Marcinek DJ, Emond MJ, … Rabinovitch PS. (2010). Age-dependent cardiomyopathy in mitochondrial mutator mice is attenuated by overexpression of catalase targeted to mitochondria. Aging Cell 9, 536–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai DF, & Rabinovitch PS (2009). Cardiac aging in mice and humans: The role of mitochondrial oxidative stress. Trends in Cardiovascular Medicine 19, 213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai DF, Rabinovitch PS, & Ungvari Z (2012). Mitochondria and cardiovascular aging.Circulation Research 110, 1109–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta J, Kar S, Van Remmen H, & Melendez JA (2009). Age-dependent increases in interstitial collagenase and MAP Kinase levels are exacerbated by superoxide dismutase deficiencies. Experimental Gerontology 44, 503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson J, Miltz W, Mir AK, & Wiessner C (2003). Targeting monocyte chemoattractant protein-1 signalling in disease. Expert Opinion on Therapeutic Targets 7, 35–48. [DOI] [PubMed] [Google Scholar]
- Denic A, Glassock RJ, & Rule AD (2016). Structural and functional changes with the aging kidney. Advances in Chronic Kidney Disease 23, 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibb KM, Rueckschloss U, Eisner DA, Isenberg G, & Trafford AW (2004). Mechanisms underlying enhanced cardiac excitation contraction coupling observed in the senescent sheep myocardium. Journal of Molecular and Cellular Cardiology 37, 1171–1181. [DOI] [PubMed] [Google Scholar]
- Diz DI (2008). Lewis K. Dahl memorial lecture: The renin-angiotensin system and aging.Hypertension 52, 37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlay SM, & Roger VL (2012). Gender differences in the pathophysiology, clinical presentation, and outcomes of ischemic heart failure. Current Heart Failure Reports 9, 267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran-Salgado MB, & Rubio-Guerra AF (2014). Diabetic nephropathy and inflammation. World Journal of Diabetes 5, 393–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert N, Jakob O, Gaedeke J, van der Giet M, Kuhlmann MK, Martus P, … Schaeffner ES. (2017). Prevalence of reduced kidney function and albuminuria in older adults: The Berlin Initiative Study. Nephrology, Dialysis, Transplantation 32, 997–1005. [DOI] [PubMed] [Google Scholar]
- Eikmans M, Baelde HJ, de Heer E, & Bruijn JA (2001). Effect of age and biopsy site on extracellular matrix mRNA and protein levels in human kidney biopsies. Kidney International 60, 974–981. [DOI] [PubMed] [Google Scholar]
- Eirin A, Lerman A, & Lerman LO (2017). The Emerging Role of Mitochondrial Targeting in Kidney Disease. Handbook of Experimental Pharmacology 240, 229–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliot SJ, Berho M, Korach K, Doublier S, Lupia E, Striker GE, & Karl M (2007). Gender-specific effects of endogenous testosterone: Female alpha-estrogen receptor-de-ficient C57Bl/6J mice develop glomerulosclerosis. Kidney International 72, 464–472. [DOI] [PubMed] [Google Scholar]
- Emamian SA, Nielsen MB, Pedersen JF, & Ytte L (1993). Kidney dimensions at sonography: Correlation with age, sex, and habitus in 665 adult volunteers. AJR. American Journal of Roentgenology 160, 83–86. [DOI] [PubMed] [Google Scholar]
- Fan YY, Kohno M, Hitomi H, Kitada K, Fujisawa Y, Yatabe J, … Nakano D. (2011). Aldosterone/Mineralocorticoid receptor stimulation induces cellular senescence in the kidney. Endocrinology 152, 680–688. [DOI] [PubMed] [Google Scholar]
- Federico C (2010). Natriuretic peptide system and cardiovascular disease. Heart Views 11, 10–15. [PMC free article] [PubMed] [Google Scholar]
- Feng X, Wang L, & Li Y (2011). Change of telomere length in angiotensin II-induced human glomerular mesangial cell senescence and the protective role of losartan. Molecular Medicine Reports 4, 255–260. [DOI] [PubMed] [Google Scholar]
- Ferder L, Romano LA, Ercole LB, Stella I, & Inserra F (1998). Biomolecular changes in the aging myocardium: The effect of enalapril. American Journal of Hypertension 11, 1297–1304. [DOI] [PubMed] [Google Scholar]
- Ferenbach DA, & Bonventre JV (2015). Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nature Reviews. Nephrology 11, 264–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenbach DA, Nkejabega NC, McKay J, Choudhary AK, Vernon MA, Beesley MF, … Hughes J. (2011). The induction of macrophage hemeoxygenase-1 is protective during acute kidney injury in aging mice. Kidney International 79, 966–976. [DOI] [PubMed] [Google Scholar]
- Feridooni HA, Dibb KM, & Howlett SE (2015). How cardiomyocyte excitation, calcium release and contraction become altered with age. Journal of Molecular and Cellular Cardiology 83, 62–72. [DOI] [PubMed] [Google Scholar]
- Ferrandi C, Ardissone V, Ferro P, Ruckle T, Zaratin P, Ammannati E, … Cirillo R. (2007). Phosphoinositide 3-kinase gamma inhibition plays a crucial role in early steps of inflammation by blocking neutrophil recruitment. The Journal of Pharmacology and Experimental Therapeutics 322, 923–930. [DOI] [PubMed] [Google Scholar]
- Ferrari AU, Radaelli A, & Centola M (2003). Invited review: Aging and the cardiovascular system. Journal of Applied Physiology 95, 2591–2597. [DOI] [PubMed] [Google Scholar]
- Ferrario CM (2016). Cardiac remodelling and RAS inhibition. Therapeutic Advances in Cardiovascular Disease 10, 162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer D, Rossa S, Landmesser U, Spiekermann S, Engberding N, Hornig B, & Drexler H (2005). Endothelial dysfunction in patients with chronic heart failure is independently associated with increased incidence of hospitalization, cardiac transplantation, or death. European Heart Journal 26, 65–69. [DOI] [PubMed] [Google Scholar]
- Fliser D, Franek E, Joest M, Block S, Mutschler E, & Ritz E (1997). Renal function in the elderly: Impact of hypertension and cardiac function. Kidney International 51, 1196–1204. [DOI] [PubMed] [Google Scholar]
- Fliser D, Zeier M, Nowack R, & Ritz E (1993). Renal functional reserve in healthy elderly subjects. Journal of the American Society of Nephrology 3, 1371–1377. [DOI] [PubMed] [Google Scholar]
- Forman DE, Butler J, Wang Y, Abraham WT, O’Connor CM, Gottlieb SS, … Krumholz HM. (2004). Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. Journal of the American College of Cardiology 43, 61–67. [DOI] [PubMed] [Google Scholar]
- de Freitas EV, Batlouni M, & Gamarsky R (2012). Heart failure in the elderly. Journal of Geriatric Cardiology 9, 101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froogh G, Pinto JT, Le Y, Kandhi S, Aleligne Y, Huang A, & Sun D (2017). Chymase-dependent production of angiotensin II: An old enzyme in old hearts. American Journal of Physiology. Heart and Circulatory Physiology 312, H223–H231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliano N, Arosio B, Santambrogio D, Balestrieri MR, Padoani G, Tagliabue J, … Annoni G. (2000). Age-dependent expression of fibrosis-related genes and collagen deposition in rat kidney cortex. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 55, B365–B372. [DOI] [PubMed] [Google Scholar]
- Gazoti Debessa CR, Mesiano Maifrino LB, & Rodrigues de Souza R (2001). Age related changes of the collagen network of the human heart. Mechanisms of Ageing and Development 122, 1049–1058. [DOI] [PubMed] [Google Scholar]
- Gekle M (2017). Kidney and aging - A narrative review. Experimental Gerontology 87,153–155. [DOI] [PubMed] [Google Scholar]
- Geng X, Hwang J, Ye J, Shih H, Coulter B, Naudin C, … Boyle AJ. (2017). Aging is protective against pressure overload cardiomyopathy via adaptive extracellular matrix remodeling. American Journal of Cardiovascular Disease 7, 72–82. [PMC free article] [PubMed] [Google Scholar]
- Ghebre YT, Yakubov E, Wong WT, Krishnamurthy P, Sayed N, Sikora AG, & Bonnen MD (2016). Vascular aging: Implications for cardiovascular disease and therapy. Translational Medicine (Sunnyvale), 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh AK, Bradham WS, Gleaves LA, De Taeye B, Murphy SB, Covington JW, & Vaughan DE (2010). Genetic deficiency of plasminogen activator inhibitor-1 promotes cardiac fibrosis in aged mice: Involvement of constitutive transforming growth factor-beta signaling and endothelial-to-mesenchymal transition. Circulation 122, 1200–1209. [DOI] [PubMed] [Google Scholar]
- Gianella A, Nobili E, Abbate M, Zoja C, Gelosa P, Mussoni L, … Sironi L. (2007). Rosuvastatin treatment prevents progressive kidney inflammation and fibrosis in stroke-prone rats. The American Journal of Pathology 170, 1165–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannandrea M, & Parks WC (2014). Diverse functions of matrix metalloproteinases during fibrosis. Disease Models & Mechanisms 7, 193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golbidi S, Badran M, & Laher I (2012). Antioxidant and anti-inflammatory effects of exercise in diabetic patients. Experimental Diabetes Research 2012, 941868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg TH, & Finkelstein MS (1987). Difficulties in estimating glomerular filtration rate in the elderly. Archives of Internal Medicine 147, 1430–1433. [PubMed] [Google Scholar]
- Gottlieb SS, Abraham W, Butler J, Forman DE, Loh E, Massie BM, … Krumholz HM. (2002). The prognostic importance of different definitions of worsening renal function in congestive heart failure. Journal of Cardiac Failure 8, 136–141. [DOI] [PubMed] [Google Scholar]
- Gourtsoyiannis N, Prassopoulos P, Cavouras D, & Pantelidis N (1990). The thickness of the renal parenchyma decreases with age: A CT study of 360 patients. AJR. American Journal of Roentgenology 155, 541–544. [DOI] [PubMed] [Google Scholar]
- Graf K, Do YS, Ashizawa N, Meehan WP, Giachelli CM, Marboe CC, … Hsueh WA. (1997). Myocardial osteopontin expression is associated with left ventricular hyper-trophy. Circulation 96, 3063–3071. [DOI] [PubMed] [Google Scholar]
- Greiten LE, Holditch SJ, Arunachalam SP, & Miller VM (2014). Should there be sexspecific criteria for the diagnosis and treatment of heart failure? Journal of Cardiovascular Translational Research 7, 139–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu C, Wang F, Hou Z, Lv B, Wang Y, Cong X, & Chen X (2017). Sex-related differences in serum matrix metalloproteinase-9 screening non-calcified and mixed coronary atherosclerotic plaques in outpatients with chest pain. Heart and Vessels 32, 1424–1431. [DOI] [PubMed] [Google Scholar]
- Haigis MC, & Sinclair DA (2010). Mammalian sirtuins: Biological insights and disease relevance. Annual Review of Pathology 5, 253–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halade GV, Jin YF, & Lindsey ML (2013). Matrix metalloproteinase (MMP)-9: A proximal biomarker for cardiac remodeling and a distal biomarker for inflammation. Pharmacology & Therapeutics 139, 32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L, Li M, & Liu X (2013). Effects of long-term atorvastatin treatment on cardiac aging. Experimental and Therapeutic Medicine 6, 721–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman D (1956). Aging: A theory based on free radical and radiation chemistry. Journal of Gerontology 11, 298–300. [DOI] [PubMed] [Google Scholar]
- Heidland A, Sebekova K, & Schinzel R (2001). Advanced glycation end products and the progressive course of renal disease. American Journal of Kidney Diseases 38, S100–S106. [DOI] [PubMed] [Google Scholar]
- Heilbronn LK, Noakes M, & Clifton PM (2001). Energy restriction and weight loss on very-low-fat diets reduce C-reactive protein concentrations in obese, healthy women. Arteriosclerosis, Thrombosis, and Vascular Biology 21, 968–970. [DOI] [PubMed] [Google Scholar]
- Heilbronn LK, & Ravussin E (2003). Calorie restriction and aging: Review of the literature and implications for studies in humans. The American Journal of Clinical Nutrition 78, 361–369. [DOI] [PubMed] [Google Scholar]
- Heitzer T, Baldus S, von Kodolitsch Y, Rudolph V, & Meinertz T (2005). Systemic endothelial dysfunction as an early predictor of adverse outcome in heart failure. Arteriosclerosis, Thrombosis, and Vascular Biology 25, 1174–1179. [DOI] [PubMed] [Google Scholar]
- Hewitson TD (2012). Fibrosis in the kidney: Is a problem shared a problem halved. Fibrogenesis & Tissue Repair 5, S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymans S, Lupu F, Terclavers S, Vanwetswinkel B, Herbert JM, Baker A, … Moons L. (2005). Loss or inhibition of uPA or MMP-9 attenuates LV remodeling and dysfunction after acute pressure overload in mice. The American Journal of Pathology 166, 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill GS (2008). Hypertensive nephrosclerosis. Current Opinion in Nephrology and Hypertension 17, 266–270. [DOI] [PubMed] [Google Scholar]
- Hillege HL, Girbes AR, de Kam PJ, Boomsma F, de Zeeuw D, Charlesworth A, … van Veldhuisen DJ. (2000). Renal function, neurohormonal activation, and survival in patients with chronic heart failure. Circulation 102, 203–210. [DOI] [PubMed] [Google Scholar]
- Hillege HL, Nitsch D, Pfeffer MA, Swedberg K, McMurray JJ, Yusuf S, … van Veldhuisen DJ. (2006). Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation 113, 671–678. [DOI] [PubMed] [Google Scholar]
- Holloszy JO (1997). Mortality rate and longevity of food-restricted exercising male rats:A reevaluation. Journal of Applied Physiology 82, 399–403. [DOI] [PubMed] [Google Scholar]
- Holloszy JO, Smith EK, Vining M, & Adams S (1985). Effect of voluntary exercise on longevity of rats. Journal of Applied Physiology 59, 826–831. [DOI] [PubMed] [Google Scholar]
- Horn MA, Graham HK, Richards MA, Clarke JD, Greensmith DJ, Briston SJ, … Trafford AW. (2012). Age-related divergent remodeling of the cardiac extracellular matrix in heart failure: Collagen accumulation in the young and loss in the aged. Journal of Molecular and Cellular Cardiology 53, 82–90. [DOI] [PubMed] [Google Scholar]
- Horn MA, & Trafford AW (2016). Aging and the cardiac collagen matrix: Novel mediators of fibrotic remodelling. Journal of Molecular and Cellular Cardiology 93, 175–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou CL, Wang MJ, Sun C, Huang Y, Jin S, Mu XP, … Zhu YC. (2016). Protective effects of hydrogen sulfide in the ageing kidney. Oxidative Medicine and Cellular Longevity 2016, 7570489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houard X, Touat Z, Ollivier V, Louedec L, Philippe M, Sebbag U, … Michel JB. (2009). Mediators of neutrophil recruitment in human abdominal aortic aneurysms. Cardiovascular Research 82, 532–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K, Yang J, Tanaka S, Gonias SL, Mars WM, & Liu Y (2006). Tissue-type plasminogen activator acts as a cytokine that triggers intracellular signal transduction and induces matrix metalloproteinase-9 gene expression. The Journal of Biological Chemistry 281, 2120–2127. [DOI] [PubMed] [Google Scholar]
- Huang CC, Chuang JH, Chou MH, Wu CL, Chen CM, Wang CC, … Tai MH. (2005). Matrilysin (MMP-7) is a major matrix metalloproteinase upregulated in biliary atresia-associated liver fibrosis. Modern Pathology 18, 941–950. [DOI] [PubMed] [Google Scholar]
- Hultstrom M, Leh S, Paliege A, Bachmann S, Skogstrand T, & Iversen BM (2012). Collagen-binding proteins in age-dependent changes in renal collagen turnover: Microarray analysis of mRNA expression. Physiological Genomics 44, 576–586. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Inagi R, Miyata T, Nagai R, Arai M, Miyashita M, … Nangaku M. (2011). Glyoxalase I retards renal senescence. The American Journal of Pathology 179, 2810–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagi R (2016). RAGE and glyoxalase in kidney disease. Glycoconjugate Journal 33,619–626. [DOI] [PubMed] [Google Scholar]
- Irita J, Okura T, Jotoku M, Nagao T, Enomoto D, Kurata M, … Higaki J. (2011). Osteopontin deficiency protects against aldosterone-induced inflammation, oxidative stress, and interstitial fibrosis in the kidney. American Journal of Physiology. Renal Physiology 301, F833–F844. [DOI] [PubMed] [Google Scholar]
- Ito N, Ohishi M, Yamamoto K, Tatara Y, Shiota A, Hayashi N, … Ogihara T. (2007). Renin-angiotensin inhibition reverses advanced cardiac remodeling in aging spontaneously hypertensive rats. American Journal of Hypertension 20, 792–799. [DOI] [PubMed] [Google Scholar]
- Iyer RP, de Castro Bras LE, Patterson NL, Bhowmick M, Flynn ER, Asher M, … Lindsey ML. (2016). Early matrix metalloproteinase-9 inhibition post-myocardial infarction worsens cardiac dysfunction by delaying inflammation resolution. Journal of Molecular and Cellular Cardiology 100, 109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer RP, Patterson NL, Fields GB, & Lindsey ML (2012). The history of matrix metal-loproteinases: Milestones, myths, and misperceptions. American Journal of Physiology. Heart and Circulatory Physiology 303, H919–H930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jugdutt BI, Palaniyappan A, Uwiera RR, & Idikio H (2009). Role of healing-specificmatricellular proteins and matrix metalloproteinases in age-related enhanced early remodeling after reperfused STEMI in dogs. Molecular and Cellular Biochemistry 322, 25–36. [DOI] [PubMed] [Google Scholar]
- Kanauchi M, Nishioka M, & Dohi K (2000). Secreted protein acidic and rich in cysteine (SPARC) in patients with diabetic nephropathy and tubulointerstitial injury. Diabetologia 43, 1076–1077. [DOI] [PubMed] [Google Scholar]
- Kang DH, Anderson S, Kim YG, Mazzalli M, Suga S, Jefferson JA, … Johnson RJ. (2001). Impaired angiogenesis in the aging kidney: Vascular endothelial growth factor and thrombospondin-1 in renal disease. American Journal of Kidney Diseases 37, 601–611. [DOI] [PubMed] [Google Scholar]
- Kaplan C, Pasternack B, Shah H, & Gallo G (1975). Age-related incidence of sclerotic glomeruli in human kidneys. The American Journal of Pathology 80, 227–234. [PMC free article] [PubMed] [Google Scholar]
- Kassiri Z, Oudit GY, Kandalam V, Awad A, Wang X, Ziou X, … Scholey JW. (2009). Loss of TIMP3 enhances interstitial nephritis and fibrosis. Journal of the American Society of Nephrology 20, 1223–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima S, & Yokoyama M (2004). Dysfunction of endothelial nitric oxide synthase and atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology 24, 998–1005. [DOI] [PubMed] [Google Scholar]
- Keller KM, & Howlett SE (2016). Sex Differences in the Biology and Pathology of the Aging Heart. The Canadian Journal of Cardiology 32, 1065–1073. [DOI] [PubMed] [Google Scholar]
- Kielstein JT, Bode-Boger SM, Frolich JC, Ritz E, Haller H, & Fliser D (2003). Asymmetric dimethylarginine, blood pressure, and renal perfusion in elderly subjects. Circulation 107, 1891–1895. [DOI] [PubMed] [Google Scholar]
- Kim HE, Dalal SS, Young E, Legato MJ, Weisfeldt ML, & D’Armiento J (2000). Disruption of the myocardial extracellular matrix leads to cardiac dysfunction. The Journal of Clinical Investigation 106, 857–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klanke B, Cordasic N, Hartner A, Schmieder RE, Veelken R, & Hilgers KF (2008). Blood pressure versus direct mineralocorticoid effects on kidney inflammation and fibrosis in DOCA-salt hypertension. Nephrology, Dialysis, Transplantation 23, 3456–3463. [DOI] [PubMed] [Google Scholar]
- Kluger MA, Zahner G, Paust HJ, Schaper M, Magnus T, Panzer U, & Stahl RA (2013). Leukocyte-derived MMP9 is crucial for the recruitment of proinflammatory macrophages in experimental glomerulonephritis. Kidney International 83, 865–877. [DOI] [PubMed] [Google Scholar]
- Koga K, Kenessey A, Powell SR, Sison CP, Miller EJ, & Ojamaa K (2011). Macrophage migration inhibitory factor provides cardioprotection during ischemia/reper-fusion by reducing oxidative stress. Antioxidants & Redox Signaling 14, 1191–1202. [DOI] [PubMed] [Google Scholar]
- Komukai K, Mochizuki S, & Yoshimura M (2010). Gender and the renin-angiotensinaldosterone system. Fundamental & Clinical Pharmacology 24, 687–698. [DOI] [PubMed] [Google Scholar]
- Kuilman T, & Peeper DS (2009). Senescence-messaging secretome: SMS-ing cellular stress. Nature Reviews. Cancer 9, 81–94. [DOI] [PubMed] [Google Scholar]
- Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, … Prolla TA. (2005). Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 309, 481–484. [DOI] [PubMed] [Google Scholar]
- Kuno K, Kanada N, Nakashima E, Fujiki F, Ichimura F, & Matsushima K (1997). Molecular cloning of a gene encoding a new type of metalloproteinase-disintegrin family protein with thrombospondin motifs as an inflammation associated gene. The Journal of Biological Chemistry 272, 556–562. [DOI] [PubMed] [Google Scholar]
- Kuwahara F, Kai H, Tokuda K, Takeya M, Takeshita A, Egashira K, & Imaizumi T (2004). Hypertensive myocardial fibrosis and diastolic dysfunction: Another model of inflammation? Hypertension 43, 739–745. [DOI] [PubMed] [Google Scholar]
- Kwak HB (2013). Aging, exercise, and extracellular matrix in the heart. Journal ofExercise Rehabilitation 9, 338–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak HB, Kim JH, Joshi K, Yeh A, Martinez DA, & Lawler JM (2011). Exercise training reduces fibrosis and matrix metalloproteinase dysregulation in the aging rat heart. The FASEB Journal 25, 1106–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laederach K, & Weidmann P (1987). Plasma and urinary catecholamines as related to renal function in man. Kidney International 31, 107–111. [DOI] [PubMed] [Google Scholar]
- Lakatta EG (2003). Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part III: Cellular and molecular clues to heart and arterial aging. Circulation 107, 490–497. [DOI] [PubMed] [Google Scholar]
- Lakatta EG (2015). So! What’s aging? Is cardiovascular aging a disease? Journal ofMolecular and Cellular Cardiology 83, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatta EG, & Levy D (2003). Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part II: The aging heart in health: Links to heart disease. Circulation 107, 346–354. [DOI] [PubMed] [Google Scholar]
- Lawler JM, & Powers SK (1998). Oxidative stress, antioxidant status, and the contracting diaphragm. Canadian Journal of Applied Physiology 23, 23–55. [DOI] [PubMed] [Google Scholar]
- Lawson J, Elliott J, Wheeler-Jones C, Syme H, & Jepson R (2015). Renal fibrosis in feline chronic kidney disease: Known mediators and mechanisms of injury. Veterinary Journal 203, 18–26. [DOI] [PubMed] [Google Scholar]
- Lee DW, & Yu BP (1990). Modulation of free radicals and superoxide dismutases by age and dietary restriction. Aging (Milano) 2, 357–362. [DOI] [PubMed] [Google Scholar]
- Lee HY, Zeeshan HMA, Kim HR, & Chae HJ (2017). Nox4 regulates the eNOS uncoupling process in aging endothelial cells. Free Radical Biology & Medicine 113, 26–35. [DOI] [PubMed] [Google Scholar]
- Lee SY, Horbelt M, Mang HE, Knipe NL, Bacallao RL, Sado Y, & Sutton TA (2011). MMP-9 gene deletion mitigates microvascular loss in a model of ischemic acute kidney injury. American Journal of Physiology. Renal Physiology 301, F101–F109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Kwak HB, Hord J, Kim JH, & Lawler JM (2015). Exercise training attenuates age-dependent elevation of angiotensin II type 1 receptor and Nox2 signaling in the rat heart. Experimental Gerontology 70, 163–173. [DOI] [PubMed] [Google Scholar]
- Leeuwenburgh C, & Heinecke JW (2001). Oxidative stress and antioxidants in exercise.Current Medicinal Chemistry 8, 829–838. [DOI] [PubMed] [Google Scholar]
- Lekawanvijit S, Kompa AR, Wang BH, Kelly DJ, & Krum H (2012). Cardiorenal syndrome: The emerging role of protein-bound uremic toxins. Circulation Research 111, 1470–1483. [DOI] [PubMed] [Google Scholar]
- Lelongt B, Bengatta S, Delauche M, Lund LR, Werb Z, & Ronco PM (2001). Matrix metalloproteinase 9 protects mice from anti-glomerular basement membrane nephritis through its fibrinolytic activity. The Journal of Experimental Medicine 193, 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D, Ehret GB, Rice K, Verwoert GC, Launer LJ, Dehghan A, … van Duijn CM. (2009). Genome-wide association study of blood pressure and hypertension. Nature Genetics 41, 677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SY, Huang PH, Yang AH, Tarng DC, Yang WC, Lin CC, … Lin SJ. (2014). Matrix metalloproteinase-9 deficiency attenuates diabetic nephropathy by modulation of podocyte functions and dedifferentiation. Kidney International 86, 358–369. [DOI] [PubMed] [Google Scholar]
- Li Y, Qi Y, Kim MS, Xu KZ, Huang TH, Rong X, … Yamahara J. (2008). Increased renal collagen cross-linking and lipid accumulation in nephropathy of Zucker diabetic fatty rats. Diabetes/Metabolism Research and Reviews 24, 498–506. [DOI] [PubMed] [Google Scholar]
- Li YF, Cao XJ, Bai XY, Lin SP, & Shi ST (2010). Change of expression of renal alpha1-adrenergic receptor and angiotensin II receptor subtypes with aging in rats. Aging Clinical and Experimental Research 22, 123–128. [DOI] [PubMed] [Google Scholar]
- Liang CT, & Barnes J (1995). Renal expression of osteopontin and alkaline phosphatase correlates with BUN levels in aged rats. The American Journal of Physiology 269, F398–F404. [DOI] [PubMed] [Google Scholar]
- Lim JH, Kim EN, Kim MY, Chung S, Shin SJ, Kim HW, … Choi BS. (2012). Age-associated molecular changes in the kidney in aged mice. Oxidative Medicine and Cellular Longevity 2012, 171383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Chen J, Zhang Z, Johnson GV, Cooper AJ, Feola J, … Goligorsky MS. (2016). Endostatin and transglutaminase 2 are involved in fibrosis of the aging kidney. Kidney International 89, 1281–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Chen J, Ziman B, Marshall S, Maizel J, & Goligorsky MS (2014). Endostatin and kidney fibrosis in aging: A case for antagonistic pleiotropy? American Journal of Physiology. Heart and Circulatory Physiology 306, H1692–H1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey ML, Escobar GP, Dobrucki LW, Goshorn DK, Bouges S, Mingoia JT, … Spinale FG. (2006). Matrix metalloproteinase-9 gene deletion facilitates angiogenesis after myocardial infarction. American Journal of Physiology. Heart and Circulatory Physiology 290, H232–H239. [DOI] [PubMed] [Google Scholar]
- Lindsey ML, Goshorn DK, Squires CE, Escobar GP, Hendrick JW, Mingoia JT, … Spinale FG. (2005). Age-dependent changes in myocardial matrix metalloproteinase/tissue inhibitor of metalloproteinase profiles and fibroblast function. Cardiovascular Research 66, 410–419. [DOI] [PubMed] [Google Scholar]
- Lindsey ML, & Zamilpa R (2012). Temporal and spatial expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases following myocardial infarction. Cardiovascular Therapeutics 30, 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey ML, Zouein FA, Tian Y, Padmanabhan Iyer R, & de Castro Bras LE (2015). Osteopontin is proteolytically processed by matrix metalloproteinase 9. Canadian Journal of Physiology and Pharmacology 93, 879–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lithgow GJ, & Kirkwood TB (1996). Mechanisms and evolution of aging. Science 273, 80. [DOI] [PubMed] [Google Scholar]
- Long DA, Newaz MA, Prabhakar SS, Price KL, Truong LD, Feng L, … Johnson RJ. (2005). Loss of nitric oxide and endothelial-derived hyperpolarizing factor-mediated responses in aging. Kidney International 68, 2154–2163. [DOI] [PubMed] [Google Scholar]
- Lu C, He JC, Cai W, Liu H, Zhu L, & Vlassara H (2004). Advanced glycation endproduct (AGE) receptor 1 is a negative regulator of the inflammatory response to AGE in mesangial cells. Proceedings of the National Academy of Sciences of the United States of America 101, 11767–11772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Li X, Li L, Li C, & Wang H (1996). Variation of intrarenal angiotensin II and angiotensin II receptors by acute renal ischemia in the aged rat. Renal Failure 18, 19–29. [DOI] [PubMed] [Google Scholar]
- Ma L, Wang K, Shang J, Cao C, Zhen P, Liu X, … Liu H. (2014). Anti-peroxynitrite treatment ameliorated vasorelaxation of resistance arteries in aging rats: Involvement with NO-sGC-cGKs pathway. PLoS One 9, e104788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Chiao YA, Clark R, Flynn ER, Yabluchanskiy A, Ghasemi O, … Jin YF. (2015). Deriving a cardiac ageing signature to reveal MMP-9-dependent inflammatory signalling in senescence. Cardiovascular Research 106, 421–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Chiao YA, Zhang J, Manicone AM, Jin YF, & Lindsey ML (2012). Matrix metalloproteinase-28 deletion amplifies inflammatory and extracellular matrix responses to cardiac aging. Microscopy and Microanalysis 18, 81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macgregor AM, Eberhart CG, Fraig M, Lu J, & Halushka MK (2009). Tissue inhibitor of matrix metalloproteinase-3 levels in the extracellular matrix of lung, kidney, and eye increase with age. The Journal of Histochemistry and Cytochemistry 57, 207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield AS, Nevala WK, Dronca RS, Leontovich AA, Shuster L, & Markovic SN (2012). Normal ageing is associated with an increase in Th2 cells, MCP-1 (CCL1) and RANTES (CCL5), with differences in sCD40L and PDGF-AA between sexes. Clinical and Experimental Immunology 170, 186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maric C, Sandberg K, & Hinojosa-Laborde C (2004). Glomerulosclerosis and tubulointerstitial fibrosis are attenuated with 17beta-estradiol in the aging Dahl salt sensitive rat. Journal of the American Society of Nephrology 15, 1546–1556. [DOI] [PubMed] [Google Scholar]
- Marin R, Gorostidi M, Fernandez-Vega F, & Alvarez-Navascues R (2005). Systemic and glomerular hypertension and progression of chronic renal disease: The dilemma of nephrosclerosis. Kidney International. Supplement, S52–S56. [DOI] [PubMed] [Google Scholar]
- Matsui F, Babitz SA, Rhee A, Hile KL, Zhang H, & Meldrum KK (2017). Mesenchymal stem cells protect against obstruction-induced renal fibrosis by decreasing STAT3 activation and STAT3-dependent MMP-9 production. American Journal of Physiology. Renal Physiology 312, F25–F32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavri A, Alessi MC, Bastelica D, Geel-Georgelin O, Fina F, Sentocnik JT, … Juhan-Vague I. (2001). Subcutaneous abdominal, but not femoral fat expression of plasminogen activator inhibitor-1 (PAI-1) is related to plasma PAI-1 levels and insulin resistance and decreases after weight loss. Diabetologia 44, 2025–2031. [DOI] [PubMed] [Google Scholar]
- Mays PK, McAnulty RJ, Campa JS, & Laurent GJ (1991). Age-related changes in collagen synthesis and degradation in rat tissues. Importance of degradation of newly synthesized collagen in regulating collagen production. The Biochemical Journal 276 (Pt 2), 307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurdy SM, Dai Q, Zhang J, Zamilpa R, Ramirez TA, Dayah T, … Lindsey ML. (2011). SPARC mediates early extracellular matrix remodeling following myocardial infarction. American Journal of Physiology. Heart and Circulatory Physiology 301, H497–H505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh D, & Gil J (2018). Senescence and aging: Causes, consequences, and therapeutic avenues. The Journal of Cell Biology 217, 65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei C, & Zheng F (2009). Chronic inflammation potentiates kidney aging. Seminars inNephrology 29, 555–568. [DOI] [PubMed] [Google Scholar]
- Mellor KM, Curl CL, Chandramouli C, Pedrazzini T, Wendt IR, & Delbridge LM (2014). Ageing-related cardiomyocyte functional decline is sex and angiotensin II dependent. Age (Dordrecht, Netherlands) 36, 9630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes AB, Ferro M, Rodrigues B, Souza MR, Araujo RC, & Souza RR (2012). Quantification of left ventricular myocardial collagen system in children, young adults, and the elderly. Medicina (B Aires) 72, 216–220. [PubMed] [Google Scholar]
- Meschiari CA, Ero OK, Pan H, Finkel T, & Lindsey ML (2017). The impact of aging on cardiac extracellular matrix. Geroscience 39, 7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer B, Mortl D, Strecker K, Hulsmann M, Kulemann V, Neunteufl T, … Berger R. (2005). Flow-mediated vasodilation predicts outcome in patients with chronic heart failure: Comparison with B-type natriuretic peptide. Journal of the American College of Cardiology 46, 1011–1018. [DOI] [PubMed] [Google Scholar]
- Meyrier A (2015). Nephrosclerosis: Update on a centenarian. Nephrology, Dialysis,Transplantation 30, 1833–1841. [DOI] [PubMed] [Google Scholar]
- Mezzano SA, Ruiz-Ortega M, & Egido J (2001). Angiotensin II and renal fibrosis.Hypertension 38, 635–638. [DOI] [PubMed] [Google Scholar]
- Miyazaki M, & Takai S (2006). Tissue angiotensin II generating system by angiotensin-converting enzyme and chymase. Journal of Pharmacological Sciences 100, 391–397. [DOI] [PubMed] [Google Scholar]
- Mora JC, & Valencia WM (2018). Exercise and Older Adults. Clinics in Geriatric Medicine 34, 145–162. [DOI] [PubMed] [Google Scholar]
- Mount PF, & Power DA (2006). Nitric oxide in the kidney: Functions and regulation of synthesis. Acta Physiologica (Oxford, England) 187, 433–446. [DOI] [PubMed] [Google Scholar]
- Mullens W, Abrahams Z, Skouri HN, Francis GS, Taylor DO, Starling RC, … Tang WH. (2008). Elevated intra-abdominal pressure in acute decompensated heart failure: A potential contributor to worsening renal function? Journal of the American College of Cardiology 51, 300–306. [DOI] [PubMed] [Google Scholar]
- Nahrendorf M, Pittet MJ, & Swirski FK (2010). Monocytes: Protagonists of infarct in-flammation and repair after myocardial infarction. Circulation 121, 2437–2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura A, Sakai Y, Ohata C, & Komurasaki T (2007). Expression and significance of a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS)-1 in an animal model of renal interstitial fibrosis induced by unilateral ureteral obstruction. Experimental and Toxicologic Pathology 59, 1–7. [DOI] [PubMed] [Google Scholar]
- Neviere R, Yu Y, Wang L, Tessier F, & Boulanger E (2016). Implication of advanced glycation end products (Ages) and their receptor (Rage) on myocardial contractile and mitochondrial functions. Glycoconjugate Journal 33, 607–617. [DOI] [PubMed] [Google Scholar]
- Ng YL, Klopcic B, Lloyd F, Forrest C, Greene W, & Lawrance IC (2013). Secreted protein acidic and rich in cysteine (SPARC) exacerbates colonic inflammatory symptoms in dextran sodium sulphate-induced murine colitis. PLoS One 8, e77575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohria A, Hasselblad V, Stebbins A, Pauly DF, Fonarow GC, Shah M, … Hill JA. (2008). Cardiorenal interactions: Insights from the ESCAPE trial. Journal of the American College of Cardiology 51, 1268–1274. [DOI] [PubMed] [Google Scholar]
- North BJ, & Sinclair DA (2012). The intersection between aging and cardiovascular disease. Circulation Research 110, 1097–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley R, & Tharakan B (2014). Vascular hyperpermeability and aging. Aging and Disease 5, 114–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelusarz A, Nichols LA, Grunz-Borgmann EA, Chen G, Akintola AD, Catania JM, … Parrish AR. (2013). Overexpression of MMP-7 Increases Collagen 1A2 in the Aging Kidney. Physiological Reports 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi Y, & Manabe I (2016). Macrophages in age-related chronic inflammatory diseases. NPJ Aging and Mechanisms of Disease 2, 16018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivetti G, Giordano G, Corradi D, Melissari M, Lagrasta C, Gambert SR, & Anversa P (1995). Gender differences and aging: Effects on the human heart. Journal of the American College of Cardiology 26, 1068–1079. [DOI] [PubMed] [Google Scholar]
- Olivetti G, Melissari M, Capasso JM, & Anversa P (1991). Cardiomyopathy of the aging human heart. Myocyte loss and reactive cellular hypertrophy. Circulation Research 68, 1560–1568. [DOI] [PubMed] [Google Scholar]
- O’Rourke MF, & Hashimoto J (2007). Mechanical factors in arterial aging: A clinical perspective. Journal of the American College of Cardiology 50, 1–13. [DOI] [PubMed] [Google Scholar]
- O’Rourke MF, Safar ME, & Dzau V (2010). The cardiovascular continuum extended: Aging effects on the aorta and microvasculature. Vascular Medicine 15, 461–468. [DOI] [PubMed] [Google Scholar]
- Ortmann J, Amann K, Brandes RP, Kretzler M, Munter K, Parekh N, … Barton M. (2004). Role of podocytes for reversal of glomerulosclerosis and proteinuria in the aging kidney after endothelin inhibition. Hypertension 44, 974–981. [DOI] [PubMed] [Google Scholar]
- O’Sullivan S, Medina C, Ledwidge M, Radomski MW, & Gilmer JF (2014). Nitric oxide-matrix metaloproteinase-9 interactions: Biological and pharmacological significance—NO and MMP-9 interactions. Biochimica et Biophysica Acta 1843, 603–617. [DOI] [PubMed] [Google Scholar]
- Padmanabhan Iyer R, Chiao YA, Flynn ER, Hakala K, Cates CA, Weintraub ST, & de Castro Bras LE (2016). Matrix metalloproteinase-9-dependent mechanisms of reduced contractility and increased stiffness in the aging heart. Proteomics. Clinical Applications 10, 92–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardali E, Sanchez-Duffhues G, Gomez-Puerto MC, & Ten Dijke P (2017). TGF-beta-induced endothelial-mesenchymal transition in fibrotic diseases. International Journal of Molecular Sciences, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson BC, & Sang QA (1997). Angiostatin-converting enzyme activities of human matrilysin (MMP-7) and gelatinase B/type IV collagenase (MMP-9). The Journal of Biological Chemistry 272, 28823–28825. [DOI] [PubMed] [Google Scholar]
- Pedersen BK (2011). Muscles and their myokines. The Journal of Experimental Biology 214, 337–346. [DOI] [PubMed] [Google Scholar]
- Perry HM 3rd. (1999). The endocrinology of aging. Clinical Chemistry 45, 1369–1376. [PubMed] [Google Scholar]
- Pichler RH, Hugo C, Shankland SJ, Reed MJ, Bassuk JA, Andoh TF, … Couser WG. (1996). SPARC is expressed in renal interstitial fibrosis and in renal vascular injury. Kidney International 50, 1978–1989. [DOI] [PubMed] [Google Scholar]
- Pinto AR, Paolicelli R, Salimova E, Gospocic J, Slonimsky E, Bilbao-Cortes D, … Rosenthal NA. (2012). An abundant tissue macrophage population in the adult murine heart with a distinct alternatively-activated macrophage profile. PLoS One 7, e36814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portik-Dobos V, Harris AK, Song W, Hutchinson J, Johnson MH, Imig JD, … Ergul A. (2006). Endothelin antagonism prevents early EGFR transactivation but not increased matrix metalloproteinase activity in diabetes. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 290, R435–R441. [DOI] [PubMed] [Google Scholar]
- Potier M, Elliot SJ, Tack I, Lenz O, Striker GE, Striker LJ, & Karl M (2001). Expression and regulation of estrogen receptors in mesangial cells: Influence on matrix metalloproteinase-9. Journal of the American Society of Nephrology 12, 241–251. [DOI] [PubMed] [Google Scholar]
- Preisser L, Teillet L, Aliotti S, Gobin R, Berthonaud V, Chevalier J, … Verbavatz JM. (2000). Downregulation of aquaporin-2 and −3 in aging kidney is independent of V(2) vasopressin receptor. American Journal of Physiology. Renal Physiology 279, F144–F152. [DOI] [PubMed] [Google Scholar]
- Pushpakumar S, Kundu S, Pryor T, Givvimani S, Lederer E, Tyagi SC, & Sen U (2013). Angiotensin-II induced hypertension and renovascular remodelling in tissue inhibitor of metalloproteinase 2 knockout mice. Journal of Hypertension 31, 2270–2281 (discussion 2281). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy R, & Schmidt AM (2012). Receptor for advanced glycation end products (RAGE) and implications for the pathophysiology of heart failure. Current Heart Failure Reports 9, 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rammos C, Hendgen-Cotta UB, Pohl J, Totzeck M, Luedike P, Schulze VT, … Rassaf T. (2014). Modulation of circulating macrophage migration inhibitory factor in the elderly. BioMed Research International 2014, 582586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rammos C, Hendgen-Cotta UB, Totzeck M, Pohl J, Ludike P, Flogel U, … Rassaf T. (2016). Impact of dietary nitrate on age-related diastolic dysfunction. European Journal of Heart Failure 18, 599–610. [DOI] [PubMed] [Google Scholar]
- Reckelhoff JF (2001). Gender differences in the regulation of blood pressure.Hypertension 37, 1199–1208. [DOI] [PubMed] [Google Scholar]
- Rempel SA, Hawley RC, Gutierrez JA, Mouzon E, Bobbitt KR, Lemke N, … Miller CG. (2007). Splenic and immune alterations of the Sparc-null mouse accompany a lack of immune response. Genes and Immunity 8, 262–274. [DOI] [PubMed] [Google Scholar]
- Ren J, Dong F, Cai GJ, Zhao P, Nunn JM, Wold LE, & Pei J (2010). Interaction between age and obesity on cardiomyocyte contractile function: Role of leptin and stress signaling. PLoS One 5, e10085. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ridinger H, Rutenberg C, Lutz D, Buness A, Petersen I, Amann K, & Maercker C (2009). Expression and tissue localization of beta-catenin, alpha-actinin and chondroitin sulfate proteoglycan 6 is modulated during rat and human left ventricular hypertrophy. Experimental and Molecular Pathology 86, 23–31. [DOI] [PubMed] [Google Scholar]
- Ridnour LA, Windhausen AN, Isenberg JS, Yeung N, Thomas DD, Vitek MP, … Wink DA. (2007). Nitric oxide regulates matrix metalloproteinase-9 activity by guanylyl-cyclase-dependent and -independent pathways. Proceedings of the National Academy of Sciences of the United States of America 104, 16898–16903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert V, Besse S, Sabri A, Silvestre JS, Assayag P, Nguyen VT, … Delcayre C. (1997). Differential regulation of matrix metalloproteinases associated with aging and hyper-tension in the rat heart. Laboratory Investigation 76, 729–738. [PubMed] [Google Scholar]
- Roh J, Rhee J, Chaudhari V, & Rosenzweig A (2016). The role of exercise in cardiac aging: From physiology to molecular mechanisms. Circulation Research 118, 279–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruge T, Carlsson AC, Larsson TE, Carrero JJ, Larsson A, Lind L, & Arnlov J (2014). Endostatin level is associated with kidney injury in the elderly: Findings from two community-based cohorts. American Journal of Nephrology 40, 417–424. [DOI] [PubMed] [Google Scholar]
- Rule AD, Amer H, Cornell LD, Taler SJ, Cosio FG, Kremers WK, … Stegall MD. (2010). The association between age and nephrosclerosis on renal biopsy among healthy adults. Annals of Internal Medicine 152, 561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbatini M, Sansone G, Uccello F, De Nicola L, Giliberti A, Sepe V, … Andreucci VE. (1994). Functional versus structural changes in the pathophysiology of acute ischemic renal failure in aging rats. Kidney International 45, 1355–1361. [DOI] [PubMed] [Google Scholar]
- Sahoo S, Meijles DN, & Pagano PJ (2016). NADPH oxidases: Key modulators in aging and age-related cardiovascular diseases? Clinical Science (London, England) 130, 317–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangaletti S, Tripodo C, Cappetti B, Casalini P, Chiodoni C, Piconese S, … Colombo MP. (2011). SPARC oppositely regulates inflammation and fibrosis in bleomycin-induced lung damage. The American Journal of Pathology 179, 3000–3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangaralingham SJ, Wang BH, Huang L, Kumfu S, Ichiki T, Krum H, & Burnett JC Jr. (2016). Cardiorenal fibrosis and dysfunction in aging: Imbalance in mediators and regulators of collagen. Peptides 76, 108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargento L, Simoes AV, Longo S, Lousada N, & Dos Reis RP (2016). Treatment with optimal dose angiotensin-converting enzyme inhibitors/angiotensin receptor blockers has a positive effect on long-term survival in older individuals (aged N70 years) and octogenarians with systolic heart failure. Drugs & Aging 33, 675–683. [DOI] [PubMed] [Google Scholar]
- Satoh M, Fujimoto S, Horike H, Ozeki M, Nagasu H, Tomita N, … Kashihara N. (2011). Mitochondrial damage-induced impairment of angiogenesis in the aging rat kidney. Laboratory Investigation 91, 190–202. [DOI] [PubMed] [Google Scholar]
- Satoh M, Kidokoro K, Ozeki M, Nagasu H, Nishi Y, Ihoriya C, … Kashihara N. (2013). Angiostatin production increases in response to decreased nitric oxide in aging rat kidney. Laboratory Investigation 93, 334–343. [DOI] [PubMed] [Google Scholar]
- Schaeffner ES, Ebert N, Delanaye P, Frei U, Gaedeke J, Jakob O, … Martus P. (2012). Two novel equations to estimate kidney function in persons aged 70 years or older. Annals of Internal Medicine 157, 471–481. [DOI] [PubMed] [Google Scholar]
- Schellings MW, Vanhoutte D, Swinnen M, Cleutjens JP, Debets J, van Leeuwen RE, … Heymans S. (2009). Absence of SPARC results in increased cardiac rupture and dysfunction after acute myocardial infarction. The Journal of Experimental Medicine 206, 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmer SH, Sayed MM, Reil JC, Ukena C, Linz D, Kindermann M, … Bohm M. (2014). Improvements in left ventricular hypertrophy and diastolic function following renal denervation: Effects beyond blood pressure and heart rate reduction. Journal of the American College of Cardiology 63, 1916–1923. [DOI] [PubMed] [Google Scholar]
- Schmitt R, & Cantley LG (2008). The impact of aging on kidney repair. American Journal of Physiology. Renal Physiology 294, F1265–F1272. [DOI] [PubMed] [Google Scholar]
- Schneider RR, Eng DG, Kutz JN, Sweetwyne MT, Pippin JW, & Shankland SJ (2017). Compound effects of aging and experimental FSGS on glomerular epithelial cells. Aging (Albany NY) 9, 524–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman IH, Zhou MS, Treuer AV, Chadipiralla K, Hare JM, & Raij L (2010). Altered renal expression of angiotensin II receptors, renin receptor, and ACE-2 precede the development of renal fibrosis in aging rats. American Journal of Nephrology 32, 249–261. [DOI] [PubMed] [Google Scholar]
- Schulze F, Maas R, Freese R, Schwedhelm E, Silberhorn E, & Boger RH (2005). Determination of a reference value for N(G), N(G)-dimethyl-L-arginine in 500 subjects. European Journal of Clinical Investigation 35, 622–626. [DOI] [PubMed] [Google Scholar]
- Seals DR, Brunt VE, & Rossman MJ (2018). Strategies for Optimal Cardiovascular Aging. American Journal of Physiology. Heart and Circulatory Physiology 315(2), H183–H188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S, Nagase M, Yoshida S, Kawachi H, & Fujita T (2007). Podocyte as the target for aldosterone: Roles of oxidative stress and Sgk1. Hypertension 49, 355–364. [DOI] [PubMed] [Google Scholar]
- Shimizu MH, Araujo M, Borges SM, de Tolosa EM, & Seguro AC (2004). Influence of age and vitamin E on post-ischemic acute renal failure. Experimental Gerontology 39, 825–830. [DOI] [PubMed] [Google Scholar]
- Shioi T, & Inuzuka Y (2012). Aging as a substrate of heart failure. Journal of Cardiology 60, 423–428. [DOI] [PubMed] [Google Scholar]
- Shu H, Wong B, Zhou G, Li Y, Berger J, Woods JW, … Cai TQ. (2000). Activation of PPARalpha or gamma reduces secretion of matrix metalloproteinase 9 but not inter-leukin 8 from human monocytic THP-1 cells. Biochemical and Biophysical Research Communications 267, 345–349. [DOI] [PubMed] [Google Scholar]
- Silbiger SR, & Neugarten J (2003). The role of gender in the progression of renal disease. Advances in Renal Replacement Therapy 10, 3–14. [DOI] [PubMed] [Google Scholar]
- Simao S, Gomes P, Pinto V, Silva E, Amaral JS, Igreja B, … Soares-da-Silva P. (2011). Age-related changes in renal expression of oxidant and antioxidant enzymes and oxidative stress markers in male SHR and WKY rats. Experimental Gerontology 46, 468–474. [DOI] [PubMed] [Google Scholar]
- Simioni C, Zauli G, Martelli AM, Vitale M, Sacchetti G, Gonelli A, & Neri LM (2018). Oxidative stress: Role of physical exercise and antioxidant nutraceuticals in adulthood and aging. Oncotarget 9, 17181–17198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simm A, Muller B, Nass N, Hofmann B, Bushnaq H, Silber RE, & Bartling B (2015). Protein glycation - Between tissue aging and protection. Experimental Gerontology 68, 71–75. [DOI] [PubMed] [Google Scholar]
- Singh M, Foster CR, Dalal S, & Singh K (2010). Role of osteopontin in heart failure associated with aging. Heart Failure Reviews 15, 487–494. [DOI] [PubMed] [Google Scholar]
- Socha MJ, Manhiani M, Said N, Imig JD, & Motamed K (2007). Secreted protein acidic and rich in cysteine deficiency ameliorates renal inflammation and fibrosis in angiotensin hypertension. The American Journal of Pathology 171, 1104–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal RS, & Weindruch R (1996). Oxidative stress, caloric restriction, and aging. Science 273, 59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son M, Chung WJ, Oh S, Ahn H, Choi CH, Hong S, … Byun K. (2017). Age dependent accumulation patterns of advanced glycation end product receptor (RAGE) ligands and binding intensities between RAGE and its ligands differ in the liver, kidney, and skeletal muscle. Immunity & Ageing 14, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spannella F, Giulietti F, Balietti P, Cocci G, Landi L, Lombardi FE, … Sarzani R. (2018). Renin-Angiotensin system blockers and statins are associated with lower in-hospital mortality in very elderly hypertensives. Journal of the American Medical Directors Association 19(4), 342–347. [DOI] [PubMed] [Google Scholar]
- Spinale FG, Escobar GP, Mukherjee R, Zavadzkas JA, Saunders SM, Jeffords LB, … Stroud RE. (2009). Cardiac-restricted overexpression of membrane type-1 matrix metalloproteinase in mice: Effects on myocardial remodeling with aging. Circulation. Heart Failure 2, 351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacy LB, Yu Q, Horak K, & Larson DF (2007). Effect of angiotensin II on primary cardiac fibroblast matrix metalloproteinase activities. Perfusion 22, 51–55. [DOI] [PubMed] [Google Scholar]
- Steenman M, & Lande G (2017). Cardiac aging and heart disease in humans. BiophysicalReviews 9, 131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinghen AE, Massy ZA, Vlassara H, Striker GE, & Boullier A (2016). Uremic Toxicity of Advanced Glycation End Products in CKD. Journal of the American Society of Nephrology 27, 354–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JC (2008). Sex and the renin-angiotensin system: Inequality between the sexes in response to RAS stimulation and inhibition. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 294, R1220–R1226. [DOI] [PubMed] [Google Scholar]
- Sun R, Zhu B, Xiong K, Sun Y, Shi D, Chen L, … Xue L. (2017). Senescence as a novel mechanism involved in beta-adrenergic receptor mediated cardiac hypertrophy. PLoS One 12, e0182668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrom J, Evans JC, Benjamin EJ, Levy D, Larson MG, Sawyer DB, … Vasan RS. (2004). Relations of plasma matrix metalloproteinase-9 to clinical cardiovascular risk factors and echocardiographic left ventricular measures: The Framingham Heart Study. Circulation 109, 2850–2856. [DOI] [PubMed] [Google Scholar]
- Sverdlov AL, Ngo DT, Chan WP, Chirkov YY, & Horowitz JD (2014). Aging of the nitric oxide system: Are we as old as our NO? Journal of the American Heart Association, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai S, Jin D, & Miyazaki M (2010). New approaches to blockade of the renin-angiotensin-aldosterone system: Chymase as an important target to prevent organ damage. Journal of Pharmacological Sciences 113, 301–309. [DOI] [PubMed] [Google Scholar]
- Takai S, Jin D, & Miyazaki M (2012). Multiple mechanisms for the action of chymase inhibitors. Journal of Pharmacological Sciences 118, 311–316. [DOI] [PubMed] [Google Scholar]
- Takazakura E, Sawabu N, Handa A, Takada A, Shinoda A, & Takeuchi J (1972). Intrarenal vascular changes with age and disease. Kidney International 2, 224–230. [DOI] [PubMed] [Google Scholar]
- Tan RJ, & Liu Y (2012). Matrix metalloproteinases in kidney homeostasis and diseases. American Journal of Physiology. Renal Physiology 302, F1351–F1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan TK, Zheng G, Hsu TT, Lee SR, Zhang J, Zhao Y, … Harris DC. (2013). Matrix metalloproteinase-9 of tubular and macrophage origin contributes to the pathogenesis of renal fibrosis via macrophage recruitment through osteopontin cleavage. Laboratory Investigation 93, 434–449. [DOI] [PubMed] [Google Scholar]
- Tan TK, Zheng G, Hsu TT, Wang Y, Lee VW, Tian X, … Harris DC. (2010). Macrophage matrix metalloproteinase-9 mediates epithelial-mesenchymal transition in vitro in murine renal tubular cells. The American Journal of Pathology 176, 1256–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanskanen M, Peuralinna T, Polvikoski T, Notkola IL, Sulkava R, Hardy J, … Myllykangas L. (2008). Senile systemic amyloidosis affects 25% of the very aged and associates with genetic variation in alpha2-macroglobulin and tau: A population-based autopsy study. Annals of Medicine 40, 232–239. [DOI] [PubMed] [Google Scholar]
- Terman A, & Brunk UT (1998). Lipofuscin: Mechanisms of formation and increase with age. APMIS 106, 265–276. [DOI] [PubMed] [Google Scholar]
- Thai SN, & Iruela-Arispe ML (2002). Expression of ADAMTS1 during murine development. Mechanisms of Development 115, 181–185. [DOI] [PubMed] [Google Scholar]
- Thomas CV, Coker ML, Zellner JL, Handy JR, Crumbley AJ 3rd, & Spinale FG (1998). Increased matrix metalloproteinase activity and selective upregulation in LV myocardium from patients with end-stage dilated cardiomyopathy. Circulation 97, 1708–1715. [DOI] [PubMed] [Google Scholar]
- Thomas DP, Cotter TA, Li X, McCormick RJ, & Gosselin LE (2001). Exercise training attenuates aging-associated increases in collagen and collagen crosslinking of the left but not the right ventricle in the rat. European Journal of Applied Physiology 85, 164–169. [DOI] [PubMed] [Google Scholar]
- Thomas DP, Zimmerman SD, Hansen TR, Martin DT, & McCormick RJ (2000). Collagen gene expression in rat left ventricle: Interactive effect of age and exercise training. Journal of Applied Physiology 89, 1462–1468. [DOI] [PubMed] [Google Scholar]
- Tian Y, Riazi S, Khan O, Klein JD, Sugimura Y, Verbalis JG, & Ecelbarger CA (2006). Renal ENaC subunit, Na-K-2Cl and Na-Cl cotransporter abundances in aged, water-restricted F344 × Brown Norway rats. Kidney International 69, 304–312. [DOI] [PubMed] [Google Scholar]
- Toba H, Cannon PL, Yabluchanskiy A, Iyer RP, D’Armiento J, & Lindsey ML (2017). Transgenic overexpression of macrophage matrix metalloproteinase-9 exacerbates age-related cardiac hypertrophy, vessel rarefaction, inflammation, and fibrosis. American Journal of Physiology. Heart and Circulatory Physiology 312, H375–H383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toba H, de Castro Bras LE, Baicu CF, Zile MR, Lindsey ML, & Bradshaw AD (2015). Secreted protein acidic and rich in cysteine facilitates age-related cardiac inflammation and macrophage M1 polarization. American Journal of Physiology. Cell Physiology 308, C972–C982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toba H, de Castro Bras LE, Baicu CF, Zile MR, Lindsey ML, & Bradshaw AD (2016). Increased ADAMTS1 mediates SPARC-dependent collagen deposition in the aging myocardium. American Journal of Physiology. Endocrinology and Metabolism 310, E1027–E1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toba H, Kojima Y, Wang J, Noda K, Tian W, Kobara M, & Nakata T (2012). Erythropoietin attenuated vascular dysfunction and inflammation by inhibiting NADPH oxidase-derived superoxide production in nitric oxide synthase-inhibited hypertensive rat aorta. European Journal of Pharmacology 691, 190–197. [DOI] [PubMed] [Google Scholar]
- Toba H, Morishita M, Tojo C, Nakano A, Oshima Y, Kojima Y, … Nakata T. (2011). Recombinant human erythropoietin ameliorated endothelial dysfunction and macrophage infiltration by increasing nitric oxide in hypertensive 5/6 nephrectomized rat aorta. European Journal of Pharmacology 656, 81–87. [DOI] [PubMed] [Google Scholar]
- Toba H, Nakashima K, Oshima Y, Kojima Y, Tojo C, Nakano A, … Nakata T. (2010). Erythropoietin prevents vascular inflammation and oxidative stress in subtotal nephrectomized rat aorta beyond haematopoiesis. Clinical and Experimental Pharmacology & Physiology 37, 1139–1146. [DOI] [PubMed] [Google Scholar]
- Toba H, Sawai N, Morishita M, Murata S, Yoshida M, Nakashima K, … Nakata T. (2009). Chronic treatment with recombinant human erythropoietin exerts renoprotective effects beyond hematopoiesis in streptozotocin-induced diabetic rat. European Journal of Pharmacology 612, 106–114. [DOI] [PubMed] [Google Scholar]
- Tomiyama H, & Yamashina A (2010). Non-invasive vascular function tests: Their path-ophysiological background and clinical application. Circulation Journal 74, 24–33. [DOI] [PubMed] [Google Scholar]
- Tomiyama H, & Yamashina A (2015). Vascular dysfunction: A key player in chronic cardio-renal syndrome. Internal Medicine 54, 1465–1472. [DOI] [PubMed] [Google Scholar]
- Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, Fok M, … Garg AX. (2006). Chronic kidney disease and mortality risk: A systematic review. Journal of the American Society of Nephrology 17, 2034–2047. [DOI] [PubMed] [Google Scholar]
- Trinity JD, Wray DW, Witman MA, Layec G, Barrett-O’Keefe Z, Ives SJ, … Richardson RS. (2016). Ascorbic acid improves brachial artery vasodilation during progressive handgrip exercise in the elderly through a nitric oxide-mediated mechanism. American Journal of Physiology. Heart and Circulatory Physiology 310, H765–H774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoporis JN, Izhar S, Leong-Poi H, Desjardins JF, Huttunen HJ, & Parker TG (2010). S100B interaction with the receptor for advanced glycation end products (RAGE): A novel receptor-mediated mechanism for myocyte apoptosis postinfarction. Circulation Research 106, 93–101. [DOI] [PubMed] [Google Scholar]
- Uribarri J, Cai W, Peppa M, Goodman S, Ferrucci L, Striker G, & Vlassara H (2007). Circulating glycotoxins and dietary advanced glycation endproducts: Two links to in-flammatory response, oxidative stress, and aging. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 62, 427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urushihara M, Kagami S, Kuhara T, Tamaki T, & Kuroda Y (2002). Glomerular distribution and gelatinolytic activity of matrix metalloproteinases in human glomerulonephritis. Nephrology, Dialysis, Transplantation 17, 1189–1196. [DOI] [PubMed] [Google Scholar]
- Vallance P, & Leiper J (2004). Cardiovascular biology of the asymmetric dimethylarginine:dimethylarginine dimethylaminohydrolase pathway. Arteriosclerosis, Thrombosis, and Vascular Biology 24, 1023–1030. [DOI] [PubMed] [Google Scholar]
- Velthuis-te Wierik EJ, Meijer P, Kluft C, & van den Berg H (1995). Beneficial effect of a moderately energy-restricted diet on fibrinolytic factors in non-obese men. Metabolism 44, 1548–1552. [DOI] [PubMed] [Google Scholar]
- Vlassara H, Uribarri J, Ferrucci L, Cai W, Torreggiani M, Post JB, … Striker GE. (2009). Identifying advanced glycation end products as a major source of oxidants in aging: Implications for the management and/or prevention of reduced renal function in elderly persons. Seminars in Nephrology 29, 594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorhees AP, DeLeon-Pennell KY, Ma Y, Halade GV, Yabluchanskiy A, Iyer RP, … Han HC. (2015). Building a better infarct: Modulation of collagen cross-linking to increase infarct stiffness and reduce left ventricular dilation post-myocardial infarction. Journal of Molecular and Cellular Cardiology 85, 229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wali RK, Wang GS, Gottlieb SS, Bellumkonda L, Hansalia R, Ramos E, … Weir MR. (2005). Effect of kidney transplantation on left ventricular systolic dysfunction and congestive heart failure in patients with end-stage renal disease. Journal of the American College of Cardiology 45, 1051–1060. [DOI] [PubMed] [Google Scholar]
- Wang D, Dai C, Li Y, & Liu Y (2011). Canonical Wnt/beta-catenin signaling mediates transforming growth factor-beta1-driven podocyte injury and proteinuria. Kidney International 80, 1159–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Toba H, Morita Y, Nakashima K, Noda K, Tian W, … Nakata T. (2013). Endothelial dysfunction, macrophage infiltration and NADPH oxidase-dependent superoxide production were attenuated by erythropoietin in streptozotocin-induced diabetic rat aorta. Pharmacology 91, 48–58. [DOI] [PubMed] [Google Scholar]
- Wang M, & Shah AM (2015). Age-associated pro-inflammatory remodeling and functional phenotype in the heart and large arteries. Journal of Molecular and Cellular Cardiology 83, 101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Zhang J, Walker SJ, Dworakowski R, Lakatta EG, & Shah AM (2010). Involvement of NADPH oxidase in age-associated cardiac remodeling. Journal of Molecular and Cellular Cardiology 48, 765–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Cheng M, Hu Z, Hu S, Zou Q, Lai X, … Wu G. (2017). Angiotensin II facilitates matrix metalloproteinase-9-mediated myosin light chain kinase degradation in pressure overload-induced cardiac hypertrophy. Cellular Physiology and Biochemistry 44, 2281–2295. [DOI] [PubMed] [Google Scholar]
- Wang WJ, Cai GY, & Chen XM (2017). Cellular senescence, senescence-associated secretory phenotype, and chronic kidney disease. Oncotarget 8, 64520–64533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Bonventre JV, & Parrish AR (2014). The aging kidney: Increased susceptibility to nephrotoxicity. International Journal of Molecular Sciences 15, 15358–15376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhou Y, Tan R, Xiong M, He W, Fang L, … Yang J. (2010). Mice lacking the matrix metalloproteinase-9 gene reduce renal interstitial fibrosis in obstructive nephropathy. American Journal of Physiology. Renal Physiology 299, F973–F982. [DOI] [PubMed] [Google Scholar]
- Wetzels JF, Kiemeney LA, Swinkels DW, Willems HL, & den Heijer M (2007). Ageand gender-specific reference values of estimated GFR in Caucasians: The Nijmegen Biomedical Study. Kidney International 72, 632–637. [DOI] [PubMed] [Google Scholar]
- Wiggins J (2009). Podocytes and glomerular function with aging. Seminars in Nephrology 29, 587–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JM, Zhang J, North P, Lacy S, Yakes M, Dahly-Vernon A, & Roman RJ (2011). Evaluation of metalloprotease inhibitors on hypertension and diabetic nephropathy. American Journal of Physiology. Renal Physiology 300, F983–F998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winton FR (1931). The influence of venous pressure on the isolated mammalian kidney. The Journal of Physiology 72, 49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LL, Cox A, Roe CJ, Dziadek M, Cooper ME, & Gilbert RE (1997). Secreted protein acidic and rich in cysteine expression after subtotal nephrectomy and blockade of the renin-angiotensin system. Journal of the American Society of Nephrology 8, 1373–1382. [DOI] [PubMed] [Google Scholar]
- Wynn TA (2008). Cellular and molecular mechanisms of fibrosis. The Journal of Pathology 214, 199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Lee K, Li N, Corbett D, Mendoza L, & Frangogiannis NG (2009). Characterization of the inflammatory and fibrotic response in a mouse model of cardiac pressure overload. Histochemistry and Cell Biology 131, 471–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Sakatsume M, Nishi S, Narita I, Arakawa M, & Gejyo F (2001). Expression, roles, receptors, and regulation of osteopontin in the kidney. Kidney International 60, 1645–1657. [DOI] [PubMed] [Google Scholar]
- Xu B, Chibber R, Ruggiero D, Kohner E, Ritter J, & Ferro A (2003). Impairment of vascular endothelial nitric oxide synthase activity by advanced glycation end products. The FASEB Journal 17, 1289–1291. [DOI] [PubMed] [Google Scholar]
- Xu L, Ping F, Yin J, Xiao X, Xiang H, Ballantyne CM, … Li M. (2013). Elevated plasma SPARC levels are associated with insulin resistance, dyslipidemia, and inflammation in gestational diabetes mellitus. PLoS One 8, e81615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabluchanskiy A, Ma Y, Chiao YA, Lopez EF, Voorhees AP, Toba H, … Jin YF. (2014). Cardiac aging is initiated by matrix metalloproteinase-9-mediated endothelial dys-function. American Journal of Physiology. Heart and Circulatory Physiology 306, H1398–H1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabluchanskiy A, Ma Y, DeLeon-Pennell KY, Altara R, Halade GV, Voorhees AP, … Lindsey ML. (2016). Myocardial Infarction superimposed on aging: MMP-9 deletion promotes M2 macrophage polarization. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 71, 475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabluchanskiy A, Ma Y, Iyer RP, Hall ME, & Lindsey ML (2013). Matrix metalloproteinase-9: Many shades of function in cardiovascular disease. Physiology (Bethesda) 28, 391–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakes FM, & Van Houten B (1997). Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proceedings of the National Academy of Sciences of the United States of America 94, 514–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HC, Rossini M, Ma LJ, Zuo Y, Ma J, & Fogo AB (2011). Cells derived from young bone marrow alleviate renal aging. Journal of the American Society of Nephrology 22, 2028–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Ma Y, Han W, Li J, Xiang Y, Liu F, … Gao XM. (2008). Age-related differences in postinfarct left ventricular rupture and remodeling. American Journal of Physiology. Heart and Circulatory Physiology 294, H1815–H1822. [DOI] [PubMed] [Google Scholar]
- Zacchigna L, Vecchione C, Notte A, Cordenonsi M, Dupont S, Maretto S, … Bressan GM. (2006). Emilin1 links TGF-beta maturation to blood pressure homeostasis. Cell 124, 929–942. [DOI] [PubMed] [Google Scholar]
- Zamilpa R, Ibarra J, de Castro Bras LE, Ramirez TA, Nguyen N, Halade GV, … Lindsey ML. (2012). Transgenic overexpression of matrix metalloproteinase-9 in macrophages attenuates the inflammatory response and improves left ventricular function post-myocardial infarction. Journal of Molecular and Cellular Cardiology 53, 599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamilpa R, Navarro MM, Flores I, & Griffey S (2014). Stem cell mechanisms during left ventricular remodeling post-myocardial infarction: Repair and regeneration. World Journal of Cardiology 6, 610–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zgheib NK, Sleiman F, Nasreddine L, Nasrallah M, Nakhoul N, Isma’eel H, & Tamim H (2018). Short Telomere Length is Associated with Aging, Central Obesity, Poor Sleep and Hypertension in Lebanese Individuals. Aging and Disease 9, 77–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Chen X, Hong Q, Lin H, Zhu H, Liu Q, … Yin Z. (2006). TIMP-1 promotes age-related renal fibrosis through upregulating ICAM-1 in human TIMP-1 transgenic mice. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 61, 1130–1143. [DOI] [PubMed] [Google Scholar]
- Zhang XP, Vatner SF, Shen YT, Rossi F, Tian Y, Peppas A, … Vatner DE. (2007). Increased apoptosis and myocyte enlargement with decreased cardiac mass; distinctive features of the aging male, but not female, monkey heart. Journal of Molecular and Cellular Cardiology 43, 487–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Dong Y, Tian X, Tan TK, Liu Z, Zhao Y, … Zheng G. (2013). Matrix metalloproteinases contribute to kidney fibrosis in chronic kidney diseases. World Journal of Nephrology 2, 84–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Cheng Q, Ye P, Yang G, Liu S, Ao Q, … Hu Y. (2016). Atorvastatin improves pathological changes in the aged kidney by upregulating peroxisome proliferator-activated receptor expression and reducing matrix metalloproteinase-9 and transforming growth factor-beta1 levels. Experimental Gerontology 74, 37–42. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Qiao X, Tan TK, Zhao H, Zhang Y, Liu L, … Zheng G. (2017). Matrix metalloproteinase 9-dependent Notch signaling contributes to kidney fibrosis through peritubular endothelial-mesenchymal transition. Nephrology, Dialysis, Transplantation 32, 781–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng F, Plati AR, Potier M, Schulman Y, Berho M, Banerjee A, … Striker GE. (2003). Resistance to glomerulosclerosis in B6 mice disappears after menopause. The American Journal of Pathology 162, 1339–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou XJ, Rakheja D, Yu X, Saxena R, Vaziri ND, & Silva FG (2008). The aging kidney.Kidney International 74, 710–720. [DOI] [PubMed] [Google Scholar]
- Zhou XJ, Saxena R, Liu Z, Vaziri ND, & Silva FG (2008). Renal senescence in 2008:Progress and challenges. International Urology and Nephrology 40, 823–839. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Bian Z, Lu P, Karas RH, Bao L, Cox D, … Mendelsohn ME. (2002). Abnormal vascular function and hypertension in mice deficient in estrogen receptor beta. Science 295, 505–508. [DOI] [PubMed] [Google Scholar]
- Zieman SJ, Melenovsky V, & Kass DA (2005). Mechanisms, pathophysiology, and therapy of arterial stiffness. Arteriosclerosis, Thrombosis, and Vascular Biology 25, 932–943. [DOI] [PubMed] [Google Scholar]
- Zoccali C (2006). Asymmetric dimethylarginine (ADMA): A cardiovascular and renal risk factor on the move. Journal of Hypertension 24, 611–619. [DOI] [PubMed] [Google Scholar]