Abstract
Background:
Cigarette smoke contains compounds similar to coal tar, an ancient remedy of eczema. Some studies have reported protective effects of maternal gestational smoking on offspring eczema; however, others have shown no or increased risks. Similarly, studies linking breastfeeding duration and eczema have demonstrated contradictory findings. No study has yet investigated combined effects of these two factors on eczema.
Objective:
Since tobacco compounds can pass to offspring via breast milk, we investigated their combined effects on eczema development from childhood to adolescence.
Methods:
We obtained information regarding gestational smoking, exclusive breastfeeding duration, and eczema at ages 1-to-2, 4, 10, and 18 years from the Isle of Wight (IOW) birth cohort, UK. Using generalized estimating equations we assessed the interaction of gestational smoking and residual exclusive breastfeeding duration (Resid_BF_duration, obtained by regressing the latter on maternal smoking) on eczema over time adjusting for confounders. For the three transition periods of 1-or-2 to4 years, 4–10, and 10–18 years we estimated risks of persistent, incident, and remitting eczema associated with the interaction using repeated measurements.
Results:
If the mother smoked during gestation, longer Resid_BF_duration was associated with a lower risk of eczema, compared to if she did not smoke. The risk ratios (95% CI) if the mother smoked during gestation and exclusively breastfed for at least 3, 9, 15, 21 weeks are 0.7 (0.6, 1.7), 0.6 (0. 4, 0.9), 0.5 (0.3, 0.8), and 0.4 (0.2, 0. 8), respectively. Additionally, in all three transition periods, the risk of persistent eczema was lower with longer Resid_BF_duration if the mother smoked during gestation.
Conclusions and clinical relevance:
Results suggest a protective effect of gestational smoking combined with longer duration of exclusive breastfeeding on early onset persistent eczema. Future studies should examine underlying biological mechanisms. Prolonged breastfeeding should be encouraged even if the mother smoked during gestation.
Introduction
Eczema is a chronic relapsing inflammatory skin disease characterized by a disrupted epidermal barrier [1, 2] with a lifetime prevalence ranging from 16–20% in the UK and US [3, 4], which is further increased in the developing countries [5]. Characteristically, an early onset eczema often progresses to other allergic diseases [6–9], a phenomenon known as atopic march [8, 9]. In addition to a low quality of life [4, 10] of the affected individual, it also poses an economic burden on the families due to high cost of treatment [10, 11].
The association of two early life environmental exposures, maternal gestational smoking and breastfeeding duration, respectively has been reported in the literature [12–18]. Cigarette smoke exposure during the in utero period, a critical time window of development [19], influences several long-term health outcomes [20]. Cigarette smoke has a similar composition to coal tar [21], which is an ancient remedy of eczema [22]. Experimental studies suggest that coal tar may improve the skin barrier by inducing the AHR (aryl-hydrocarbon receptor) gene [22]. Interestingly, cigarette smoke also influences the methylation of AHRR (aryl-hydrocarbon receptor repressor) [23] indicating a possible link between biological pathways influenced by cigarette smoke and those related to eczema. Existing studies report a protective effect of maternal gestational smoking for eczema in offspring [16, 18], null effects [24] and increased risks [3, 25].
Regarding breastfeeding, several studies have explored its association with eczema in offspring and have reported contradictory findings. Elbert et al demonstrated that non-exclusive breastfeeding is associated with a weak increase in the risk of eczema (adjusted odds ratio 1.11, 95%CI: 1.01, 1.23) [26]. Ito et al [12] showed that longer breastfeeding is associated with an increased risk of atopic dermatitis in offspring whereas Lee et al have reported null effects [27]. Turati et al described that early weaning is protective of eczema [28]. Our group showed that in children with filaggrin loss of function mutation, a longer duration of breastfeeding reduced the risk of eczema at age 1-or-2 years [29]. Ramirez et al reported an increased risk of eczema at age 6 years associated with mixed mode of feeding (combination of direct feeding, pumping and feeding and formula feeding) compared to direct feeding at the breast [30]. Systematic reviews by Kramer et al and Lodge et al have reported a lower risk of eczema linked to exclusive breastfeeding [14, 15].
Although the above studies revealed contradictory findings regarding the association of smoking during pregnancy and breastfeeding with eczema when investigated separately, no study has yet investigated whether the combined effect of the two factors contribute towards the risk of eczema. It is known that if mother smokes during pregnancy, compounds of tobacco smoke may be stored in breast fat tissue [31]. These coal tar like compounds may then be passed to offspring during breastfeeding [32]. Thus, we hypothesize that combined effect of exclusive breastfeeding duration and maternal smoking on eczema lowers the risk of eczema in the offspring. To this end, we analyzed the interaction of duration of breastfeeding and maternal smoking during pregnancy on eczema from infancy to late adolescence (ages 1-or-2, 4, 10 and 18 years) in boys and girls using data from the Isle of Wight birth cohort.
Methods
Isle of Wight birth cohort
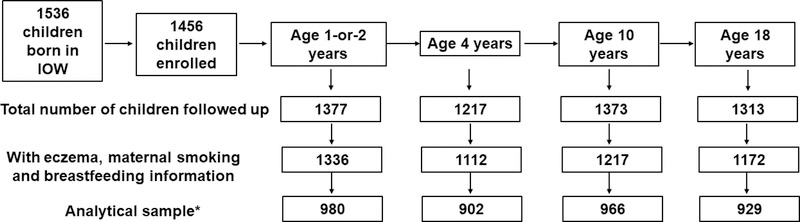
Isle of Wight birth cohort is a longitudinal cohort, established on the Isle of Wight, UK to prospectively study the natural history of allergic conditions. From January 1989 to February 1990 1,536 children were born on the Isle of Wight (IOW), among which, 1,456 mother-child pairs were enrolled into the cohort study after exclusion of perinatal deaths, adoptions, and refusals. Children were followed up at 1, 2, 4, 10, and 18 years of age (Figure 1). This study represents a dynamic cohort since some children did not participate at some assessments but rejoined in the next.
Figure 1:
Description of whole cohort participants followed up at each age.
*Analytical sample refers to the sample that was analyzed at each age with complete information for eczema, breastfeeding duration, maternal gestational smoking and all other covariates.
The research ethics committee on the IOW (NRES Committee South Central - Hampshire B, U.K.), and in Memphis (University of Memphis Institutional Review Board, FWA00006815) approved the study and informed written parental consent was obtained for all participants at recruitment and subsequently at each follow-up. The IOW birth cohort has been described in detail elsewhere [33–35].
Data collection
Information regarding eczema symptoms was acquired though detailed interviews and examinations at the follow-ups at 1, 2, 4, 10, and 18 years. A postal or telephone questionnaire was sent if a visit was not possible [36]. At age 1 and 2 years all children came for a clinical visit, whereas at age 10 and 18 years 88% and 75% were seen at clinical visits, respectively. Information regarding how many were seen at clinical visits at age 4 is not available in our records.
Eczema was defined using the Hanifin and Rajka criteria using the information obtained via questionnaires [37]. We ascertained all major features (itching, typical morphology and distribution, chronic or chronically relapsing itchiness and personal history of atopic dermatitis) and some minor features (early age of onset, anterior neck folds) as required by the Hanifin and Rajka criteria [38, 39]. Since the 1st year and 2nd year follow up was conducted in a small time window, we combined them for analytical purposes. Mothers were asked at child birth regarding ‘how many cigarettes were smoked during pregnancy?’. This information was used to construct a binary smoking during pregnancy variable with categories ‘Yes’ (If the mothers reported >=1 cigarettes) and ‘No’ (If the mothers reported 0 number of cigarettes). Regarding breastfeeding, the mother was asked at the 1- and 2-year follow-up whether the child was breastfed and the total duration of breastfeeding (in weeks). In addition, age of starting formula feeding (in weeks) was also ascertained. Duration of exclusive breastfeeding (weeks) was determined by comparing the total duration of breastfeeding with the age of initiating formula feeding or introduction of solid foods.
Socioeconomic status, maternal and paternal eczema, FLG loss of function genetic variants, and gender were included as confounders since they potentially associate with maternal smoking, breastfeeding duration, and eczema. Parental occupation was reported at birth, number of children in the index child’s bedroom was collected at age 4 years, and family income was ascertained at age 10 years. These three variables were clustered to produce a family social status variable used in our analyses [40]. Gender, and maternal and paternal eczema were determined at birth.
Genotyping
DNA samples were extracted from 1,150 cohort participants from blood or saliva and assessed using GoldenGate Genotyping Assays (Illumina, Inc, SanDiego, CA) on the beadXpressVeracode platform (Illumina, Inc, SanDiego, CA) per Illumina’s protocol. Data were analyzed using the genotyping module of the GenomeStudio Software package (Illumina, Inc, SanDiego, CA). Individuals carrying the minor allele for at least one of the FLG variants R501X, 2282del4, or S3247X were classified as having filaggrin haploinsufficiency [41].
Statistical analysis
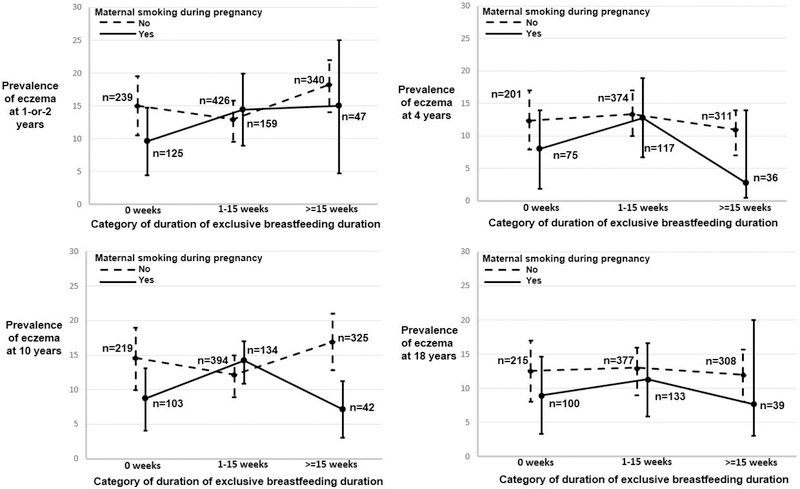
We plotted the proportion of eczema age 1-or-2, 4, 10, and 18 years against duration of exclusive breastfeeding categorized into none, 1–15 weeks and more than 15 weeks comparing those with and without maternal smoking to descriptively assess their interplay.
Since maternal gestational smoking itself may influence breastfeeding duration it is difficult to interpret their crude combined effect on eczema. To address this, we calculated residual breastfeeding with the following equation: residuals = actual duration of breastfeeding – estimated effect of breastfeeding due to smoking. These residuals are not explained by maternal smoking, making the two variables independent of each other. However, the residual exclusive breastfeeding duration (Resid-BF-duration) is correlated with exclusive breastfeeding duration (Spearman Correlation Coefficient: 0.93, p-value: <0.0001) and was used throughout the analysis.
To investigate the suggestive non-linear trends from the plots, we used repeated measures of eczema at ages 1-or-2, 4, 10, and 18 years as outcome and assessed the interaction of polynomial functions (cubic-B spline and quadratic) of Resid-BF-duration and age of eczema assessment using Generalized Linear Mixed Model (GLMM), Generalized Estimating Equation (GEE), and General Additive Models (GAM). The analyses were stratified by maternal gestational smoking. Thereafter, to explore linear associations we estimated the risk of eczema associated with the combined effect of maternal gestational smoking and duration of breastfeeding. The risks were assessed using log-linear models with repeated measures using GEE [42]. The log link function was used in the model to obtain risk ratios for the interaction of residual duration of exclusive breastfeeding and maternal gestational smoking [43].
Loss of function filaggrin genetic polymorphisms, gender, socioeconomic status of offspring at age 10, maternal and paternal eczema status, and gestational age were included in the model as potential confounders. We started with a model containing all main effects and the interaction effect of maternal gestational smoking and duration of exclusive breastfeeding on the risk of eczema at different ages. After evaluation of whether the interaction term was significant, we assessed confounding on the joint effect of duration of exclusive breastfeeding and maternal smoking, i.e., risk ratio (RR)= exp (β1×maternal smoking + β2× Resid-BF-duration +β3× (maternal smoking× Resid-BF-duration)).
Additionally, to further evaluate whether the effect of exclusive breastfeeding duration differs if the mother smokes during pregnancy vs. not, we performed an analysis stratified by maternal gestational smoking using model with repeated measures of eczema. A p-value ≤ 0.05 was used to indicate statistical significance. Statistical analyses were performed using the SAS statistical package (version 9.4; SAS Institute, Cary, NC, USA).
Next, for each of the three transition periods of ages 1-or-2 to 4 years, 4 to 10 years, and 10 to 18 years we defined persistence of eczema as having eczema at both time points. Similarly, we defined incidence if a person was eczema-free in the first time point but developed eczema in the second time point, and remission as a having eczema in the first time point and becoming eczema-free at the second time point. These criteria were used to classify participants into incidence, remission, persistence at all three transition periods. We classified participants without eczema across all time points as eczema-free. With persistent eczema vs. eczema-free as outcome we estimated the risks associated with the combined effect of gestational smoking and exclusive breastfeeding duration with repeated measurements over the three transitions. Similarly, we estimated the risk with incident eczema vs. eczema-free, and remitting vs. eczema-free as outcome across all the transition periods.
Results
The number of participants enrolled and followed up at each age is demonstrated in Figure 1. There was no significant difference in the prevalence of eczema, duration of exclusive breastfeeding, maternal smoking during pregnancy, FLG loss of function variants, gender, socioeconomic status, and maternal and paternal eczema status between the total cohort with offspring eczema information and the sample analyzed at each of the ages 1–2, 4, 10, and 18 years (Table 1 and 2).
Table 1:
Comparison of prevalence of eczema at ages 1–2 years, 4 years, 10 years, and 18 years between whole cohort and sample analyzed¥. In addition, number of participants analyzed with incident, persistent, remitting eczema in the three transitions* of age 1-or-2 to 4, age 4 to 10, and age 10 to 18 are also presented.
| Total cohort | Sample analyzed |
p- value |
||
|---|---|---|---|---|
| N (%) | n (%) | |||
| Age 1-or-2 years |
N=1377 | n=980 | ||
| Prevalence | Yes | 196 (14.2) | 153 (15.6) | 0.3 |
| No | 1181 (85.7) | 827 (84.4) | ||
| Missing | ||||
| Prevalence | Age 4 | N=1217 | n= 902 | 0.8 |
| Yes | 145 (11.9) | 105 (11.6) | ||
| No | 1067 (87.7) | 797 (88.4) | ||
| Missing | 5 (0.4) | |||
| N=1143# | n=902# | 0.8 | ||
| Transition | Incidence | 57/ 1143 (5.0) | 43/ 902 (4.7) | |
| from age 1–2 to 4 years | Persistence | 78/ 1143 (7.0) | 62/ 902 (7.0) | |
| Remission | 103/ 1143 (9.0) | 86/ 902 (9.5) | ||
| None | 905/ 1143 (79) | 711/ 902 (78.8) | ||
| Age 10 | N=1373 | n=966 | 0.5 | |
| Prevalence | Yes | 186 (13.6) | 142 (14.7) | |
| No | 1173 (85.4) | 824 (85.3) | ||
| 14 (1.0) | ||||
| N=1153# | n=888# | 0.7 | ||
| Transition | Incidence | 88/ 1153 (7.6) | 69/ 888 (7.7) | |
| from age 4 | Persistence | 72/ 1153 (6.2) | 58/ 888 (6.5) | |
| to 10 years | Remission | 56/ 1153 (4.9) | 44/ 888 (4.9) | |
| None | 937/ 1153 (81.3) | 717/ 888 (80.7) | ||
| Age 18 | N=1313 | n=929 | 0.7 | |
| Prevalence | Yes | 161 (12.3) | 110 (11.8) | |
| No | 1146 (87.3) | 819 (88.2) | ||
| Missing | 6 (0.5) | |||
| N=1230# | n=915# | 0.7 | ||
| Transition | Incidence | 73/ 1230 (5.9) | 54/ 915 (5.9) | |
| from age 10 | Persistence | 71/ 1230 (5.8) | 52/ 915 (5.6) | |
| to 18 years | Remission | 96/ 1230 (7.8) | 78/ 915 (8.5) | |
| None | 990/ 1230 (80.5) | 731/ 915 (80.0) | ||
Sample analyzed is sample with no missing for any of the covariates in the model.
Eczema in transition periods are defined as incident (present in the second time point but not in the first), persistent (present at both time points), remitting (present at first time point but not in the second).
Only participants with no missing eczema information at both time points are considered, e.g., 1153 participants had eczema information at both ages 4 and 10 years. Similarly, in the analyzed sample out of 888 had eczema information at both ages 4 and 10 years.
Table 2:
Distribution of potential risk factors and confounders in the birth cohort and the analytical sample.
| Covariate | Total cohort at Age 1/2 (N=1377) (n%) |
Sample at Age 1/2 (n=980) (n%) |
p* | Total cohort at Age 4 (N=1217) (n%) |
Sample at Age 4 (n=902) (n%) |
p* | Total cohort at Age 10 (N=1373) (n%) |
Sample at Age 10 (n=966) (n%) |
p* | Total cohort at Age 18 (N=1313) (n%) |
Sample at Age 18 (n=929) (n%) |
p* |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eczema | 0.3 | 0.8 | 0.5 | 0.7 | ||||||||
| Yes | 196 (14.2) | 153 (15.6) | 145 (11.9) | 105 (11.6) | 186 (13.6) | 824 (85.3) | 161 (12.3) | 110 (11.8) | ||||
| No | 1181 (85.8) | 827 (84.4) | 1067 (87.7) | 797 (88.4) | 1173 (85.4) | 142 (14.7) | 1146 (87.3) | 819 (88.2) | ||||
| Missing | 5 (0.41) | 14 (1.0) | 6 (0.4) | |||||||||
|
Maternal smoking |
0.1 | 0.8 | 0.3 | 0.1 | ||||||||
| Yes | 339 (24.6) | 214 (21.8) | 251 (20.6) | 184 (20.4) | 319 (23.2) | 757 (78.4) | 305 (23.2) | 192 (20.6) | ||||
| No | 1032 (75) | 766 (78.2) | 963 (79.1) | 718 (79.6) | 1048 (76.3) | 209 (21.6) | 1002 (76.3) | 737 (79.3) | ||||
| Missing | 6 (0.44) | 3 (0.2) | 6 (0.4) | 6 (0.5) | ||||||||
|
FLG LOF¥ variants |
0.9 | 0.9 | 0.8 | 0.9 | ||||||||
| Yes | 112 (8.1) | 102 (10.4) | 106 (8.7) | 95 (10.5) | 114 (8.3) | 101 (10.5) | 111 (8.4) | 834 (89.8) | ||||
| No | 964 (70) | 878 (89.6) | 902 (74.1) | 807 (89.5) | 1011 (73.6) | 865 (89.5) | 980 (74.6) | 95 (10.2) | ||||
| Missing | 301 (22) | 209 (17.2) | 248 (18.1) | 222 (17) | ||||||||
|
Maternal eczema |
0.9 | 0.9 | 0.7 | 0.8 | ||||||||
| Yes | 168 (12.2) | 121 (12.3) | 145 (11.9) | 109 (12.1) | 162 (11.8) | 120 (12.4) | 161 (12.3) | 118 (12.7) | ||||
| No | 1199 (87.1) | 859 (87.7) | 1062 (87.3) | 793 (87.9) | 1199 (87.3) | 846 (87.6) | 1141 (86.9) | 811 (87.3) | ||||
| Missing | 10 (0.7) | 10 (0.8) | 12 (0.8) | 11 (0.8) | ||||||||
|
Paternal eczema |
0.5 | 0.8 | 0.6 | 0.8 | ||||||||
| Yes | 87 (6.3) | 69 (7.0) | 78 (6.4) | 61 (6.7) | 89 (6.5) | 69 (7.1) | 90 (6.8) | 67 (7.2) | ||||
| No | 1273 (92.4) | 911 (93) | 1127 (92.6) | 841 (93.2) | 1266 (92.2) | 897 (92.9) | 1204 (91.7) | 862 (92.8) | ||||
| Missing | 17 (1.23) | 12 (0.9) | 18 (1.31) | 19 (1.5) | ||||||||
| SES# | 0.3 | 0.3 | 0.5 | 0.5 | ||||||||
| Low | 193 (14.02) | 139 (14.2) | 165 (13.6) | 108 (11.9) | 188 (13.7) | 133 (13.7) | 177 (13.5) | 125 (13.5) | ||||
| Mid | 967 (70.2) | 755 (77.0) | 943 (77.5) | 711 (78.8) | 1006 (73.3) | 747 (77.3) | 952 (72.5) | 721 (77.6) | ||||
| High | 100 (7.3) | 86 (8.8) | 108 (8.87) | 83 (9.2) | 109 (7.9) | 86 (8.9) | 103 (7.8) | 83 (8.9) | ||||
| Missing | 117 (8.5) | 1 (0.1) | 70 (5.1) | 81 (6.2) | ||||||||
| Gender | 0.2 | 0.5 | 0.3 | 0.3 | ||||||||
| Male | 700 (50.1) | 474 (48.4) | 620 (50.9) | 443 (49.1) | 697 (50.7) | 468 (48.4) | 653 (49.7) | 440 (47.4) | ||||
| Female | 677 (49.16) | 506 (51.6) | 597 (49.1) | 459 (50.9) | 676 (49.2) | 498 (51.5) | 660 (50.3) | 489 (52.6) | ||||
| Median (minimum, maximum) | ||||||||||||
| Exclusive breast feeding duration (weeks) |
6 (0, 80) | 6 (0, 80) | 0.8 | 6 (0, 80) | 6 (0, 80) | 0.8 | 6 (0, 80) | 6 (0, 80) | 0.5 | 6 (0, 76) | 6 (0, 76) | 0.8 |
| Weeks of gestation |
40 (30, 45) | 40 (28, 45) | 0.2 | 40 (28, 45) | 40 (28, 45) | 0.9 | 40.0 (28, 45) | 40.0 (30, 45) | 0.7 | 40 (28, 44) | 40 (30,44) | 0.8 |
p-value
FLG loss of function genetic variants
Socio economic cluster
We compared the prevalence of offspring eczema at ages 1-or-2, 4, 10, and 18 years between those with maternal gestational smoke exposure and those without (Figure 2). We have used three categories of exclusive breastfeeding duration (none, 1–15 weeks, and >= than 15 weeks) for this descriptive comparison. At all the four ages, the proportion of eczema is not significantly different between the two smoking groups at any category of breastfeeding duration (chi-square p-value>0.05). The plots suggest that the proportion of eczema at age 1-or-2, 4, 10, and 18 years may vary in a non-linear manner with longer actual breastfeeding (original variable) in the two maternal smoking groups. However, we found no significant non-linear relationship between Resid_BF_duration and eczema at ages 1-or-2, 4, 10, and 18 years in both the maternal smoking groups. To explore the linear association, we then analyzed the interaction effect between maternal gestational smoking and Resid-BF-duration, on repeated measurements of eczema at age 1 or 2, 4, 10, and 18 years. The interaction of Resid-BF-duration and maternal gestational smoking was significantly associated with eczema in offspring (p-value: 0.04) such that the risk was lower with increasing duration of breastfeeding in and showed a dose response relationship (Table 3). Risk ratios if the mother smoked during gestation and breastfed for at least 3, 9, 15, 21 weeks of breastfeeding were 0.7 (95%CI: 0.6, 1.1), 0.6 (95%CI: 0. 4,0.9), 0.5 (95%CI: 0.3, 0.8), and 0.4 (95%CI: 0.2, 0.8), respectively (Table 3, Figure 3). The interaction effect of gestational smoking and Resid_BF_duration was not significantly different at any age (i.e., the three-way interaction of maternal smoking*Resid_BF_duration*age was not significant). Nevertheless, the three-way interaction showed a diminished risk at all ages. Among the other covariates, FLG loss of function variants, weeks of gestation, maternal and paternal history of eczema, were significantly associated with eczema.
Figure 2:
Comparison of the prevalence of eczema over increasing duration of breastfeeding (categorized in 15 week interval) if with or without maternal smoking during pregnancy at ages 1–2 years, 4 years, 10 years and 18 years
Table 3:
Association of maternal gestational smoking and residual duration of exclusive breastfeeding¥ with eczema at ages 1-or-2, 4, 10, and 18 adjusted for covariates.
| Outcome: Eczema prevalence at all the time points (k=3777) |
Outcome: Eczema persistence at all three transitions (k=2331) |
|||||
|---|---|---|---|---|---|---|
| Parameter | Levels | Risk ratio (95% CI) |
p- value |
Risk ratio (95% CI) |
p- value |
|
| Maternal gestational smoking | 0.81 (0.6, 1.1) | 0.15 | 0.61 (0.36, 1.06) | 0.08 | ||
| Residual exclusive breastfeeding duration |
1.0 (0.9, 1.01) | 0.9 | 0.99 (0.98, 1.01) | 0.75 | ||
| Maternal gestational smoking × | ||||||
| Residual exclusive breastfeeding duration#* |
0 weeks | 0.8 (0.6, 1.1) | 0.15 | 0.6, (0.36, 1.06) | 0.08 | |
| ≥ 3 weeks | 0.7 (0.5, 1.0) | 0.06 | 0.5 (0.3, 0.9) | 0.04 | ||
| ≥ 9 weeks | 0.6 (0.4, 0.9) | 0.02 | 0.4 (0.2, 0.8) | 0.01 | ||
| ≥ 15 weeks | 0.5 (0.3, 0.8) | 0.02 | 0.3 (0.1, 0.8) | 0.01 | ||
| ≥ 21 weeks | 0.4 (0.2, 0.8) | 0.02 | 0.2 (0.08, 0.7) | 0.01 | ||
| FLG loss of function variants | 1.6 (1.2, 2.1) | 0.003 | 1.9 (1.19, 3.05) | 0.007 | ||
| Time | Age 1-or-2 | Ref. | Ref. | Transition 1 | Ref. | Ref. |
| (1-or-2 to 4 years) | ||||||
| Age 4 | 0.7 (0.62, 0.9) | 0.001 | Transition 2 | 1.02 (0.97, 1.1) | 0.4 | |
| (4 to 10 years) | ||||||
| Age 10 | 0.9 (0.8, 1.1) | 0.5 | Transition 3 | 0.89 (0.74, 1.1) | 0.2 | |
| (10 to 18 years) | ||||||
| Age 18 | 0.76 (0.6, 0.9) | 0.007 | ||||
| Weeks of gestation | 1.1 (1.0, 1.2) | 0.03 | 1.1 (0.9, 1.3) | 0.24 | ||
| Maternal eczema status | 1.3 (0.9, 1.8) | 0.06 | 1.2 (0.75, 2.1) | 0.4 | ||
| Paternal eczema status | 1.7 (1.3, 2.4) | 0.001 | 2.1 (1.2, 3.5) | 0.004 | ||
| Gender (Female is reference) | Male | 0.9 (0.7, 1.2) | 0.57 | 0.9 (0.7, 1.4) | 0.9 | |
| Socio economic status (high is reference) |
Mid | 0.9 (0.7, 1.4) | 0.9 | 1.02 (0.6, 1.9) | 0.9 | |
| Low | 0.9 (0.6, 1.4) | 0.7 | 0.8 (0.35, 1.8) | 0.6 | ||
Residuals are calculated by regressing exclusive breastfeeding duration on maternal gestational smoking.
Overall p-value is 0.04 when the outcome is eczema prevalence at ages 1-or-2, 4, 10, and 18 years.
Overall p-value is 0.03 when the outcome is persistent eczema for all transitions from age 1-or-2 to 4 years, age 4 to 10 years, age 10 to 18 years
Figure 3:
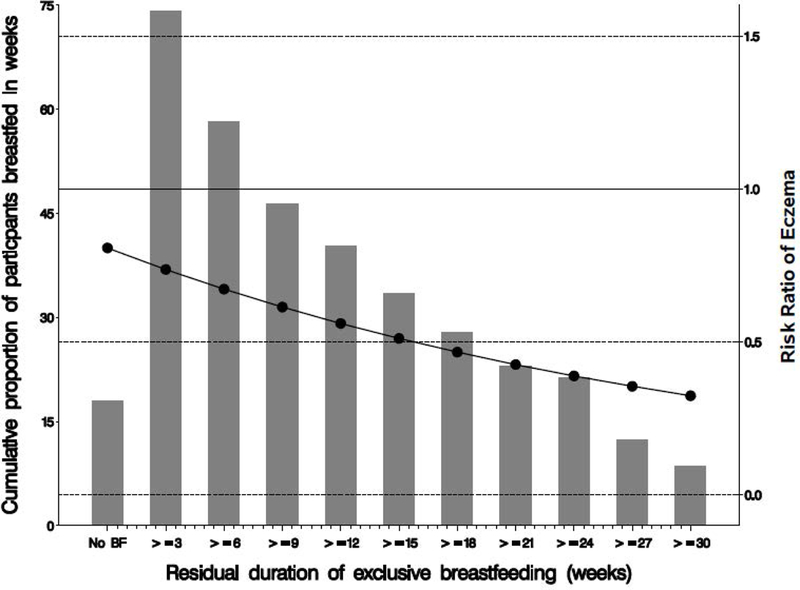
Risk of eczema in offspring with increasing residual duration of breastfeeding if the mothers smoked during pregnancy compared to non-smoking during pregnancy for repeated measurements of the prevalence eczema at ages 1–2 years, 4 years, 10 years and 18 years (n=3777).
We additionally explored the association of Resid-BF-duration with eczema stratified by maternal gestational smoking (Table 4). In the strata where the mothers did not smoke, Resid-BF-duration and eczema were not associated (overall RR in non-smokers: 1.00, 95%CI: 0.99, 1.01). However, in the strata with maternal smoking, the risk ratio of eczema was significantly lower in the offspring with increasing Resid-BF-duration. The risk ratios if the mother smoked and exclusively breastfed for at least 3, 9, 15, 21 weeks are 0.91 (95%CI: 0.84, 0.99), 0.76 (95%CI: 0.58, 0.98), 0.63 (95%CI: 0.41, 0.93), and 0.52 (95%CI: 0.28, 0.95), respectively (Table 4, Figure 3).
Table 4:
Association of residual exclusive breastfeeding duration¥ with prevalence of eczema (measured repeatedly at ages 1-or-2, 4, 10, and 18 years) stratified by maternal gestational smoking.
| Maternal smoking during pregnancy | |||||
|---|---|---|---|---|---|
| Parameter | Levels | NO (k=3011) |
YES (k=839) |
||
| Risk ratio (95% CI) |
p- value |
Risk ratio (95% CI) |
p- value |
||
| Residual duration of exclusive breastfeeding |
1.00 (0.99, 1.01) | 0.75 | 0.97 (0.94, 0.99) | 0.04 | |
| 0 weeks | Ref. | Ref. | |||
| ≥ 3 weeks | 1 (0.98, 1.03) | 0.75 | 0.91 (0.84, 0.99) | 0.04 | |
| ≥ 9 weeks | 1.01 (0.93, 1.1) | 0.75 | 0.76 (0.58, 0.98) | 0.04 | |
| ≥ 15 weeks | 1.02 (0.89, 1.2) | 0.75 | 0.63 (0.41, 0.97) | 0.04 | |
| ≥ 21 weeks | 1.03 (0.85, 1.3) | 0.75 | 0.52 (0.29, 0.96) | 0.04 | |
| FLG loss of function variants |
1.5 (1.06, 2.06) | 0.02 | 1.99 (1.02, 3.90) | 0.04 | |
| Time | Year 1/ 2 | Ref. | Ref. | Ref. | Ref. |
| Year 4 | 0.76 (0.63, 0.92) | 0.006 | 0.66 (0.4, 1.08) | 0.1 | |
| Year 10 | 0.94 (0.78, 1.13) | 0.51 | 0.98 (0.64, 1.48) | 0.9 | |
| Year 18 | 0.74 (0.60, 0.92) | 0.007 | 0.86 (0.54, 1.39) | 0.55 | |
| Weeks of gestation | 1.1 (1.0, 1.23) | 0.09 | 1.12 (0.96, 1.33) | 0.15 | |
| Maternal eczema status | 1.3 (0.97, 1.85) | 0.07 | 1.17 (0.61, 2.24) | 0.62 | |
| Paternal eczema status | 1.87 (1.32, 2.63) | 0.0004 | 1.24 (0.54, 2.86) | 0.61 | |
| Gender (Female is the reference) |
Male | 0.89 (0.70, 1.14) | 0.36 | 1.06 (0.64, 1.76) | 0.81 |
Residuals are calculated by regressing exclusive breastfeeding duration on maternal gestational smoking.
Note: Socio economic status not added in the model since it did not have enough variability in the smoking =‘Yes’ group
It is known that the effect of maternal gestational smoking on the offspring may extend to the first year of life or even longer [20]. We performed an additional analysis by comparing children without any smoke exposure to children who were exposed to smoke in utero or passively within 1-or-2 years after child birth (year in which the mother breastfed). We also found a significant protective effect on eczema with increasing Resid-BF-duration if the child was exposed to cigarette smoke in utero or during age 1-or-2 years (Supplementary table S1).
Next, we considered three transition periods: 1-or-2 to 4 years, 4–10 years, and 10–18 years and assessed the interaction of Resid-BF-duration and maternal gestational smoking on persistent eczema vs. eczema-free, incidence eczema vs. eczema-free, and remission vs. eczema-free as outcome using repeated measurements across all the transition periods. Results show that the risk of persistent eczema is lower with increasing Resid-BF-duration if the mother smoked during pregnancy (Table 3). The risk ratios if the mother smoked and exclusively breastfed for at least 0, 3, 9, 15, 21 weeks were 0.6 (95%CI: 0.36, 1.1), 0.5 (95%CI: 0.2, 08), 0. 4 (95%CI: 0.2, 0.8), 0.3 (95%CI: 0. 1, 0.8), and 0.2 (95%CI: 0.08, 0.7), respectively. Regarding the comparison of incidence eczema vs. eczema-free, and remission vs. eczema-free, we did not identify statistically significant interaction effects.
Discussion
In this longitudinal and prospective birth cohort study we explored risks of eczema associated with the interaction of duration of exclusive breastfeeding and maternal gestational smoking using repeated eczema measurements at 1-or-2, 4, 10, and 18 years. To make the two interacting variables independent of each other throughout the analyses we used residual exclusive breastfeeding duration (Resid-BF-duration) calculated by regressing exclusive breastfeeding duration on maternal smoking. We found that if the mother smoked during pregnancy, a longer exclusive breastfeeding duration is protective of eczema in offspring (p-value: 0.04, Table 3). The combined effect of longer Resid-BF-duration and higher number of cigarettes smoked was also significantly associated with a lower risk of eczema with longer duration of breastfeeding. Stratification by maternal or paternal eczema did not change these associations suggesting no effect modification by parental eczema. Excluding four participants with very long duration of breastfeeding (>= 60 weeks) also did not change the results. In agreement with this, the stratified the analysis by maternal gestational smoking showed that longer Resid-BF-duration was protective of eczema only in the strata where mothers smoked during pregnancy (Table 4). We additionally performed the analyses without taking into account the shared smoking-breastfeeding effect using the crude breastfeeding duration, which demonstrates similar results (Supplementary Table S2-S4).
Regarding persistent eczema determined from age 1-or-2 to 4 years, age 4 to 10 years, and age 10 to 18 years, the interaction of maternal smoking during pregnancy and Resid-BF-duration also showed a significant protective effect (Table 3).
There was no significant difference in the study characteristics of the whole cohort and the study participants at ages 1-or-2, 4, 10, and 18 years indicating that a selection bias is unlikely. The diagnosis of eczema was made using the questionnaire based on Hanifin and Rajka’s criteria [44]. The chances of misclassification are low since the participants developed rashes in typical locations (anticubital or popliteal fossae, ankles, face or neck) validating the eczema definition [44]. All questionnaires focused on eczema in the preceding 12 months to minimize the potential for recall bias that might occur if participants were asked to recall the entire period between assessments.
One of the limitations of this study is that maternal smoking status is self-reported. However, we have assessed the DNA methylation of a CpG (cytosine-phosphate-guanine) site cg05575921 of the AHRR (Aryl-Hydrocarbon Receptor Repressor) gene, a well-established epigenetic biomarker of smoking. Offspring whose mothers smoked during pregnancy had significantly lower cord blood methylation of this CpG site at birth (estimate: −0.43 and p-value: 0.009), which is in agreement with prior studies of prenatal smoke exposure and supports the validity of the smoking information [45]. Nevertheless, a misclassification of smoking exposure may have resulted in an underestimation of its risks. We also performed an additional analysis to check the interaction of residual duration of exclusive breastfeeding with number of cigarettes smoked, instead of a binary maternal gestational smoking. Again, this interaction was significantly associated with eczema (interaction term p-value: 0.01), showing a diminishing risk with combined increases in both duration of breastfeeding and number of cigarettes smoked.
Breastfeeding duration was determined retrospectively from the mothers at ages 1 year and 2 year follow up. It is unlikely to be affected by recall, since it has been shown that maternal recall within <=3 years provides a valid and reliable estimate of breastfeeding history [46]. However, self-reported breastfeeding duration may be influenced by digit preference of the participants to report number of weeks close to a month (e.g., 4 or 8 weeks). We avoided this by focusing on for instance 3 weeks, 9, 15 and 21 weeks of breastfeeding duration (Table 3, Figure 3).
Experimental studies indicate that breast milk contains proteins [47] and fatty acids [48] that may influence the immune response affecting eczema. Other studies propose that cigarette smoke exposure also changes the T lymphocyte system [49]. Interestingly, tobacco smoke contains compounds such as polycyclic aromatic hydrocarbons (PAHs) similar to coal tar [21], an ancient topical remedy of eczema [22]. Coal tar is shown to improve eczema by inducing the AHR and ARNT genes involved in detoxification the PAHs, which in turn, upregulates epidermal barrier forming proteins [50, 51]. Maternal gestational smoking is also known to influence the methylation of the AhR pathway genes in the offspring, suggesting a link in the underlying biological pathways affected by smoking and those involved in eczema [52].
Current epidemiological studies show inconsistent associations of breastfeeding duration, maternal gestational smoking with eczema, when investigated separately [3, 12, 14–18]. None of the studies have yet investigated the interaction of maternal gestational smoking and duration of breastfeeding in relation to eczema. Our results add to the literature that it is not smoking or breastfeeding alone, but the combined effect of longer duration of exclusive breastfeeding and maternal gestational smoking, which is protective of eczema, providing a potential explanation to the previously conflicting findings. If the mother smokes during pregnancy, the coal tar like PAH compounds in tobacco smoke [53], being lipophilic in nature, are stored in maternal breast fat tissue [31] and may be passed to the infant via breast milk [32] influencing the development of eczema in the offspring.
Smoking during pregnancy, however, is not recommended because the health hazards related to smoking are far greater than the suggestive benefit from our findings. However, mothers should be encouraged to exclusively breastfeed for the longest possible duration even they did not quit smoking during pregnancy. Most importantly, future studies should explore the composition of breastmilk of mothers who smoked during gestation and determine the underlying genetic, molecular, and epigenetic pathways so that alternative targeted therapy can be developed to prevent or alleviate eczema.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge the participation and cooperation of the children and parents of Isle of Wight and appreciate the hard work of Mrs. Sharon Matthews and the Isle of Wight research team in collecting data and Nikki Graham for technical support. We thank the High-Throughput Genomics Group at the Wellcome Trust Centre for Human Genetics (funded by Wellcome Trust grant reference 090532/Z/09/Z) for the generation of the methylation data.
Funding
This work has been supported by National Institute of Health [R01 AI091905 and R01HL132321 to W.K., R01 AI121226 to H.Z. and J.W.H.]; National Asthma Campaign, UK [364]; and the National Institutes of Health / National Heart, Lung, and Blood Institute [R01 HL082925 to S.H.A.]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, USA, and National Asthma Campaign, UK.
Footnotes
Conflicts of interests
None of the authors has any conflicts of interest to declare.
References
- 1.Boguniewicz M and Leung DY, Atopic dermatitis: a disease of altered skin barrier and immune dysregulation. Immunol Rev, 2011. 242(1): p. 233–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sohn A, et al. , Eczema. Mt Sinai J Med, 2011. 78(5): p. 730–9. [DOI] [PubMed] [Google Scholar]
- 3.Kim HB, et al. , Lifetime prevalence of childhood eczema and the effect of indoor environmental factors: Analysis in Hispanic and non-Hispanic white children. Allergy Asthma Proc, 2016. 37(1): p. 64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewis-Jones S, Quality of life and childhood atopic dermatitis: the misery of living with childhood eczema. Int J Clin Pract, 2006. 60(8): p. 984–92. [DOI] [PubMed] [Google Scholar]
- 5.Flohr C and Mann J, New insights into the epidemiology of childhood atopic dermatitis. Allergy, 2014. 69(1): p. 3–16. [DOI] [PubMed] [Google Scholar]
- 6.Bantz SK, Zhu Z, and Zheng T, The Atopic March: Progression from Atopic Dermatitis to Allergic Rhinitis and Asthma. J Clin Cell Immunol, 2014. 5(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pyun BY, Natural history and risk factors of atopic dermatitis in children. Allergy Asthma Immunol Res, 2015. 7(2): p. 101–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spergel JM and Paller AS, Atopic dermatitis and the atopic march. J Allergy Clin Immunol, 2003. 112(6 Suppl): p. S118–27. [DOI] [PubMed] [Google Scholar]
- 9.Zheng T, et al. , The atopic march: progression from atopic dermatitis to allergic rhinitis and asthma. Allergy Asthma Immunol Res, 2011. 3(2): p. 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silverberg JI, Health Care Utilization, Patient Costs, and Access to Care in US Adults With Eczema: A Population-Based Study. JAMA Dermatol, 2015. 151(7): p. 743–52. [DOI] [PubMed] [Google Scholar]
- 11.Lee BW and Detzel PR, Treatment of childhood atopic dermatitis and economic burden of illness in Asia Pacific countries. Ann Nutr Metab, 2015. 66 Suppl 1: p. 18–24. [DOI] [PubMed] [Google Scholar]
- 12.Ito J and Fujiwara T, Breastfeeding and risk of atopic dermatitis up to the age 42 months: a birth cohort study in Japan. Ann Epidemiol, 2014. 24(4): p. 267–72. [DOI] [PubMed] [Google Scholar]
- 13.Kim JH, Role of Breast-feeding in the Development of Atopic Dermatitis in Early Childhood. Allergy Asthma Immunol Res, 2017. 9(4): p. 285–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kramer MS, Breastfeeding and allergy: the evidence. Ann Nutr Metab, 2011. 59 Suppl 1: p. 20–6. [DOI] [PubMed] [Google Scholar]
- 15.Lodge CJ, et al. , Breastfeeding and asthma and allergies: a systematic review and meta-analysis. Acta Paediatr, 2015. 104(467): p. 38–53. [DOI] [PubMed] [Google Scholar]
- 16.Magnusson LL, et al. , Wheezing, asthma, hayfever, and atopic eczema in childhood following exposure to tobacco smoke in fetal life. Clin Exp Allergy, 2005. 35(12): p. 1550–6. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka K, et al. , Pre- and Postnatal Smoking Exposure and Risk of Atopic Eczema in Young Japanese Children: A Prospective Prebirth Cohort Study. Nicotine Tob Res, 2017. 19(7): p. 804–809. [DOI] [PubMed] [Google Scholar]
- 18.Taylor-Robinson DC, et al. , Do early-life exposures explain why more advantaged children get eczema? Findings from the U.K. Millennium Cohort Study. Br J Dermatol, 2016. 174(3): p. 569–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barker DJ and Clark PM, Fetal undernutrition and disease in later life. Rev Reprod, 1997. 2(2): p. 105–12. [DOI] [PubMed] [Google Scholar]
- 20.Knopik VS, et al. , The epigenetics of maternal cigarette smoking during pregnancy and effects on child development. Dev Psychopathol, 2012. 24(4): p. 1377–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodgman A, Smith CJ, and Perfetti TA, The composition of cigarette smoke: a retrospective, with emphasis on polycyclic components. Hum Exp Toxicol, 2000. 19(10): p. 573–95. [DOI] [PubMed] [Google Scholar]
- 22.van den Bogaard EH, et al. , Coal tar induces AHR-dependent skin barrier repair in atopic dermatitis. J Clin Invest, 2013. 123(2): p. 917–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vogel CFA and Haarmann-Stemmann T, The aryl hydrocarbon receptor repressor - More than a simple feedback inhibitor of AhR signaling: Clues for its role in inflammation and cancer. Curr Opin Toxicol, 2017. 2: p. 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kantor R, et al. , Association of atopic dermatitis with smoking: A systematic review and meta-analysis. J Am Acad Dermatol, 2016. [DOI] [PMC free article] [PubMed]
- 25.Tanaka K, et al. , Pre- and postnatal smoking exposure and risk of atopic eczema in young Japanese children: a prospective pre-birth cohort study. Nicotine Tob Res, 2016. [DOI] [PubMed]
- 26.Elbert NJ, et al. , Duration and exclusiveness of breastfeeding and risk of childhood atopic diseases. Allergy, 2017. [DOI] [PubMed]
- 27.Lee KS, et al. , Does Breast-feeding Relate to Development of Atopic Dermatitis in Young Korean Children?: Based on the Fourth and Fifth Korea National Health and Nutrition Examination Survey 2007–2012. Allergy Asthma Immunol Res, 2017. 9(4): p. 307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turati F, et al. , Early weaning is beneficial to prevent atopic dermatitis occurrence in young children. Allergy, 2016. 71(6): p. 878–88. [DOI] [PubMed] [Google Scholar]
- 29.Ziyab AH, et al. , Filaggrin gene loss-of-function variants modify the effect of breast-feeding on eczema risk in early childhood. Allergy, 2016. 71(9): p. 1371–3. [DOI] [PubMed] [Google Scholar]
- 30.Soto-Ramirez N, et al. , Infant feeding patterns and eczema in children in the first 6 years of life. Clin Exp Allergy, 2017. 47(10): p. 1285–1298. [DOI] [PubMed] [Google Scholar]
- 31.Terry PD and Rohan TE, Cigarette smoking and the risk of breast cancer in women: a review of the literature. Cancer Epidemiol Biomarkers Prev, 2002. 11(10 Pt 1): p. 953–71. [PubMed] [Google Scholar]
- 32.Madhavan ND and Naidu KA, Polycyclic aromatic hydrocarbons in placenta, maternal blood, umbilical cord blood and milk of Indian women. Hum Exp Toxicol, 1995. 14(6): p. 503–6. [DOI] [PubMed] [Google Scholar]
- 33.Arshad SH and Hide DW, Effect of environmental factors on the development of allergic disorders in infancy. J Allergy Clin Immunol, 1992. 90(2): p. 235–41. [DOI] [PubMed] [Google Scholar]
- 34.Arshad SH, Stevens M, and Hide DW, The effect of genetic and environmental factors on the prevalence of allergic disorders at the age of two years. Clin Exp Allergy, 1993. 23(6): p. 504–11. [DOI] [PubMed] [Google Scholar]
- 35.Scott M, et al. , Influence of atopy and asthma on exhaled nitric oxide in an unselected birth cohort study. Thorax, 2010. 65(3): p. 258–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soto-Ramirez N, et al. , Epidemiologic methods of assessing asthma and wheezing episodes in longitudinal studies: measures of change and stability. J Epidemiol, 2013. 23(6): p. 399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arshad SH, et al. , Cohort Profile: The Isle Of Wight Whole Population Birth Cohort (IOWBC). Int J Epidemiol, 2018. 47(4): p. 1043–1044i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deleuran M and Vestergaard C, Clinical heterogeneity and differential diagnosis of atopic dermatitis. Br J Dermatol, 2014. 170 Suppl 1: p. 2–6. [DOI] [PubMed] [Google Scholar]
- 39.Hanifin J, Rajka G, Diagnostic features of atopic eczema. . Acta Derm Venereol (Stockh), 1980. 92: p. 44–7. [Google Scholar]
- 40.Ogbuanu IU, et al. , Effect of breastfeeding duration on lung function at age 10 years: a prospective birth cohort study. Thorax, 2009. 64(1): p. 62–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ziyab AH, et al. , Interplay of filaggrin loss-of-function variants, allergic sensitization, and eczema in a longitudinal study covering infancy to 18 years of age. PLoS One, 2012. 7(3): p. e32721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeger SL and Liang KY, Longitudinal data analysis for discrete and continuous outcomes. Biometrics, 1986. 42(1): p. 121–30. [PubMed] [Google Scholar]
- 43.Zhang J and Yu KF, What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA, 1998. 280(19): p. 1690–1. [DOI] [PubMed] [Google Scholar]
- 44.Ziyab AH, et al. , Trends in eczema in the first 18 years of life: results from the Isle of Wight 1989 birth cohort study. Clin Exp Allergy, 2010. 40(12): p. 1776–84. [DOI] [PubMed] [Google Scholar]
- 45.Philibert RA, et al. , Changes in DNA methylation at the aryl hydrocarbon receptor repressor may be a new biomarker for smoking. Clin Epigenetics, 2013. 5(1): p. 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li R, Scanlon KS, and Serdula MK, The validity and reliability of maternal recall of breastfeeding practice. Nutr Rev, 2005. 63(4): p. 103–10. [DOI] [PubMed] [Google Scholar]
- 47.Bergmann RL, et al. , Socioeconomic status is a risk factor for allergy in parents but not in their children. Clin Exp Allergy, 2000. 30(12): p. 1740–5. [DOI] [PubMed] [Google Scholar]
- 48.Laitinen K, et al. , Breast milk fatty acids may link innate and adaptive immune regulation: analysis of soluble CD14, prostaglandin E2, and fatty acids. Pediatr Res, 2006. 59(5): p. 723–7. [DOI] [PubMed] [Google Scholar]
- 49.Holt PG, Immune and inflammatory function in cigarette smokers. Thorax, 1987. 42(4): p. 241–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Du L, Hoffman SM, and Keeney DS, Epidermal CYP2 family cytochromes P450. Toxicol Appl Pharmacol, 2004. 195(3): p. 278–87. [DOI] [PubMed] [Google Scholar]
- 51.Furue M, et al. , Role of AhR/ARNT system in skin homeostasis. Arch Dermatol Res, 2014. 306(9): p. 769–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Joubert BR, et al. , DNA Methylation in Newborns and Maternal Smoking in Pregnancy: Genome-wide Consortium Meta-analysis. Am J Hum Genet, 2016. 98(4): p. 680–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Niehoff N, et al. , Polycyclic aromatic hydrocarbons and postmenopausal breast cancer: An evaluation of effect measure modification by body mass index and weight change. Environ Res, 2017. 152: p. 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.