Abstract
Introduction:
The eighth edition of the tumor, node, and metastasis (TNM) staging system included the proposal that the T descriptor be determined according to the invasive component, excluding lepidic component, for nonmucinous lung adenocarcinomas. We sought to conduct a clinicopathologic comparative analysis of the newly proposed classification using invasive size versus total tumor size.
Methods:
Patients who underwent lung resection for primary lung adenocarcinoma with pathologic stage (p-Stage) I-IIA (based on total size [t]) were reviewed (n=1704). Pathologic invasive size was measured, and tumors were reclassified using invasive size (i). Cumulative incidence of recurrence (CIR) and lung cancer–specific cumulative incidence of death (LC-CID) were analyzed using a competing-risks approach. Prognostic discrimination by p-Stage(t) and p-Stage(i) was evaluated using a concordance index (C-index).
Results:
The use of invasive size resulted in downstaging in 377 of 1704 patients (22%), with twice as many patients with p-Stage IA1 (IA1[i] vs. IA1[t]: 389 [23%] vs. 195 [11%]). However, outcomes were similar between the two groups (IA1[i] vs. IA1[t]: 5-year-CIR, 11% vs. 13%; 5-year LC-CID, 5% vs. 7%). Prognostic discrimination by p-Stage(i) was better than by p-Stage(t) (C-index for p-Stage[i] vs. p-Stage[t]: recurrence, 0.614 vs. 0.593; lung cancer–specific death, 0.634 vs. 0.621).
Conclusions:
When invasive size, rather than total size, was used for the T descriptor, a larger number of patients were classified with a favorable prognosis (p-Stage IA1) and better prognostic discrimination of p-Stage I-IIA nonmucinous lung adenocarcinomas was achieved.
Keywords: Lung adenocarcinoma, invasive tumor size, consolidation tumor size, lung cancer-specific death, recurrence
Introduction
Following the results of the National Lung Screening Trial, which showed that screening with low-dose computed tomography (CT) reduces mortality from lung cancer, the detection of early-stage lung cancer has been expected to increase.1 In 2016, using survival data from a multinational cohort of patients with non-small cell lung cancer (NSCLC), the International Association for the Study of Lung Cancer (IASLC) refined their classification of early-stage node-negative lung cancers ≤5 cm as follows: IA (≤3 cm) and IB (>3 to ≤5 cm) in the seventh edition of the tumor, node, and metastasis (TNM) classification became IA1 (≤1 cm), IA2 (>1 to ≤2 cm), IA3 (>2 to ≤3 cm), IB (>3 to ≤4 cm), and IIA (>4 to ≤5 cm) in the eighth edition.2 Furthermore, the eighth edition addressed the correlation between radiologic part-solid nodules and the histologic components of nonmucinous lung adenocarcinomas (ADCs) and proposed the use of invasive size, rather than total size, for the T descriptor for nonmucinous lung ADCs with a lepidic component.3
These changes follow previous efforts to achieve better prognostic stratification in lung ADC, the most common histologic subtype of NSCLC. In 2011, a multidisciplinary group of experts from the IASLC, American Thoracic Society (ATS), and European Respiratory Society (ERS) proposed a new classification of lung ADC that included two new subtypes: ADC in situ (AIS) and minimally invasive ADC (MIA).4 The authors proposed that histologic patterns should be recorded in 5% increments and, on this basis, the predominant histologic pattern (lepidic, acinar, papillary, micropapillary, or solid) determined. In 2013, the Fleischner Society largely based their recommendations for the management of subsolid pulmonary nodules detected by CT scan on this histologic classification system.5 In 2015, the World Health Organization (WHO) adopted the histologic classification system following validation of independent cohorts.6–9
The TNM staging system remains the most useful tool for determining treatment options for patients with lung cancer.2 Tumor size is a key element of TNM staging,10 and the available evidence suggests that, for lung ADC tumors, invasive size is a better predictor of outcomes than total size.6, 11, 12 Recently, Aokage and colleagues analyzed a cohort of patients with resected stage I-IV NSCLC using both the seventh and eighth editions of the TNM classification.13 For staging of nonmucinous lung ADCs with a lepidic component, the T descriptor was determined by total size when the seventh edition was used and invasive size when the eighth was used; comparison revealed good correlation between solid size on CT and invasive size on pathologic assessment. In addition, concordance probability estimates and Akaike’s information criterion values for overall survival were higher for the eighth edition than the seventh edition.
To date, no study has investigated the impact of the changes to the definitions of T1 (a, b, and c) and T2 nonmucinous lung ADCs or compared the effect on prognosis of using invasive versus total size for the T descriptor. In this study, we performed a clinicopathologic comparative analysis of total versus invasive size in nonmucinous lung ADCs, with the goal of determining whether the use of invasive size improves prognostic discrimination when using the eighth edition of the TNM classification.
Methods
Patient Cohort
This retrospective study was approved by the Institutional Review Board at Memorial Sloan Kettering Cancer Center (MSK). All patients diagnosed with solitary lung ADC who had undergone surgical resection at MSK between January 2000 and December 2014 were reviewed. Patients who had undergone lung resection for primary lung ADC with pathologic stage (p-Stage) I-IIA (based on total tumor size) were included. Patients who had received induction therapy, had multiple nodules, had lung cancer within the preceding two years, had positive surgical margins, or had concurrent disease progression were excluded from analysis, as were patients with a histologic diagnosis of AIS, MIA, invasive mucinous ADC, or colloid predominant ADC (Supplementary Figure S1). Patient demographic information was obtained from the MSK Thoracic Surgery Service’s prospectively maintained lung cancer database. Data on clinical variables and follow-up information were obtained by reviewing patient medical records.
Recurrence and Lung Cancer–Specific Death as Endpoints
Postoperative lung cancer surveillance was performed in accordance with National Comprehensive Cancer Network (NCCN) guidelines.14 During the first 2 years after surgery, each patient received a physical examination, interval history review, and chest/upper abdomen CT scan, with or without contrast, every 6 to 12 months. Follow-up visits and surveillance CT scans were performed yearly after the first 2 years.
The study endpoints were recurrence and lung cancer–specific death. All recurrences were confirmed by clinical, radiologic, and pathologic assessment.15 In cases where a new tumor developed in the lung or pleura and a biopsy specimen was available, the histologic profile was reviewed to determine whether the new tumor was a metachronous primary tumor, a recurrence, or a metastasis; this was completed in accordance with the method developed by our group.16 Lung cancer–specific death was defined as death due to recurrent disease associated with resected lung cancer.17
Histologic Evaluation
All available hematoxylin and eosin–stained tumor slides were reviewed by at least two pathologists (S.L., K.K., and W.D.T.), who were blinded to patient clinical outcomes, using an Olympus BX51 microscope (Olympus, Tokyo, Japan) with a standard 22-mm diameter eyepiece. Any discrepancies between the pathologists during assignment of predominant subtypes were later resolved via consensus using a multihead microscope. The percentage of each histologic pattern was recorded in 5% increments. Tumors were classified in accordance with the 2011 IASLC/ATS/ERS classification and the 2015 WHO classification.4, 8 Tumors were grouped by histologic grading as low (lepidic), intermediate (papillary or acinar), or high (micropapillary or solid).18 Visceral pleural invasion (VPI), lymphovascular invasion (LVI), necrosis, and tumor spread through air spaces19 were also investigated.
Pathologic Assessment of Total and Invasive Tumor Size
Pathologic total tumor size was defined as the maximum diameter of the tumor. Pathologic tumor size was initially assessed by gross measurement using a ruler placed along the tumor in the gross specimen before or after cross-section of the tumor. The initial gross measurement was then reevaluated by microscopic evaluation to ultimately determine pathologic total tumor size.
Pathologic invasive tumor size was defined as the size of invasive components, excluding lepidic component, on microscopic examination. Although in some cases invasive size could be measured with a ruler in a single focus, in most cases this was not possible, owing to multiple foci of invasion or the presence of invasive areas on multiple slides. Therefore, as proposed by our group in 201420 and included in the 2015 WHO classification,8 for all cases in this study pathologic invasive tumor size was estimated using the following equation: pathologic invasive size = pathologic total size × percentage of invasive components/100. p-Stages for all tumors based on total size (t) were reclassified on the basis of invasive size (i).
Statistical Analysis
The outcomes of interest—recurrence and lung cancer–specific death—were analyzed in a competing-risk framework. For recurrence, death from any cause without recurrence was considered a competing event. For lung cancer–specific death, death from causes other than lung cancer or from unknown causes was considered a competing event. The cumulative incidence of recurrence (CIR) and the lung cancer–specific cumulative incidence of death (LC-CID) were used to estimate the probability of recurrence or lung cancer–specific death following surgical resection with curative intent.21 Patients who did not experience recurrence or die during the study period were censored at the time of the last available follow-up (assessed in April 2017). Differences in CIR or LC-CID between groups were tested using the Gray method.22 The concordance index (C-index) was assessed to evaluate prognostic discrimination by TNM classification. Statistical analyses were performed using R 3.1.1 (R Development Core Team, Vienna, Austria); the “survival” and “cmprsk” software packages were used in the analyses. All P values were two-sided, and significance was set at 5%.
Results
Number and Proportion of Patients with Each p-Stage
The use of invasive size resulted in downstaging in 377 patients (22%). In particular, twice as many patients were classified as the lowest stage (p-Stage IA1) when invasive size was used (IA1[i] vs. IA1[t]: 389 [23%] vs. 195 [11%]), secondary to downstaging in 194 patients (downstaged from: IA2[t], 164 [42%]; IA-3[t], 26 [7%]; IB[t] 4 [1%]). The proportion and number of patients who were classified with the same stage or downstaged when invasive size was used for staging are shown in Figure 1. Of the 195 patients with p-Stage IA1(t) according to the eighth edition of the TNM classification by total size, all were classified with the same stage according to the eighth edition TNM classification by invasive size (p-Stage IA1[i]). Of the 685 patients with p-Stage IA2(t), 164 (24%) were downstaged to IA1(i). Of the 336 patients with p-Stage IA3(t), 134 (40%) were downstaged to IA2(i) (n=108 [32%]) or IA1(i) (n=26 [8%]). Of the 133 patients with p-Stage IB(t) without VPI, 49 (37%) were downstaged to IA3(i) (n=32 [24%]), IA2(i) (n=13 [10%]), or IA1(i) (n=4 [3%]). All 260 patients with p-Stage IB(t) with VPI were classified with the same stage (p-Stage IB[i]) because of the presence of VPI, regardless of differences between total and invasive sizes. Of the 95 patients with p-Stage IIA(t), 30 (32%) were downstaged to IB(i) (n=23 [24%]), IA3(i) (n=5 [5%]), or IA2(i) (n=2 [2%]).
Figure 1.
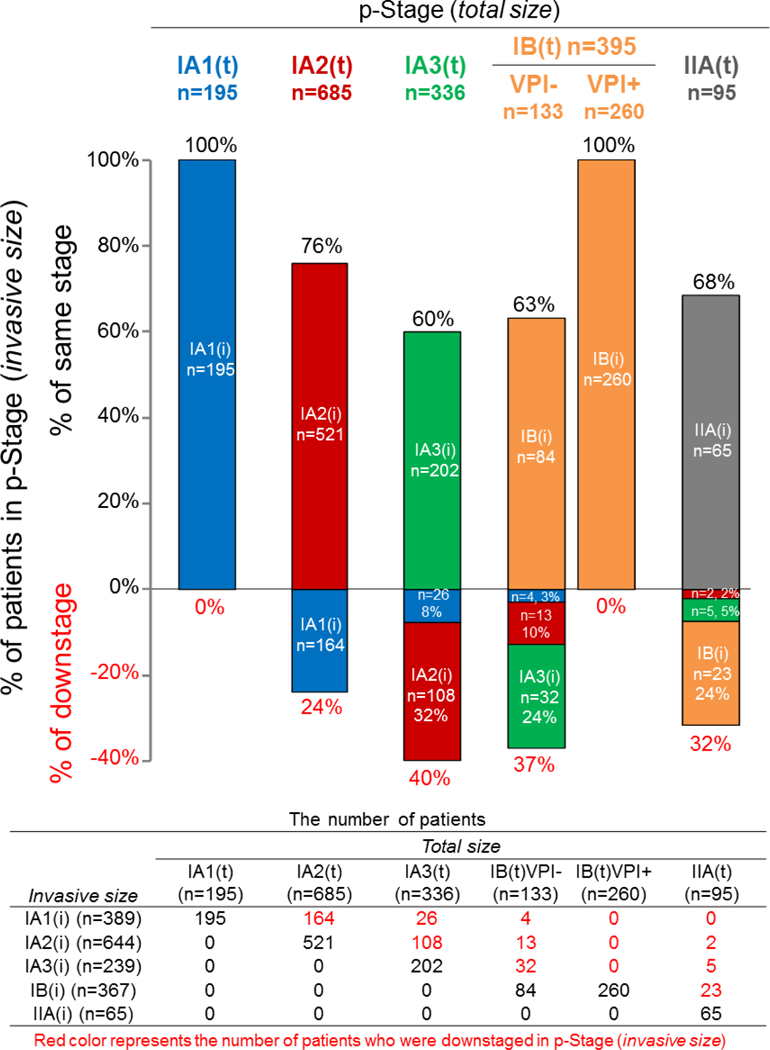
Proportion and number of patients who were classified as the same stage or downstaged when invasive size (i) was used, rather than total size (t), for each pathologic stage (p-Stage). VPI, visceral pleural invasion.
Clinicopathologic Characteristics by p-Stage(t) and p-Stage(i)
Table 1 lists clinicopathologic characteristics stratified by p-Stage(t) and p-Stage(i). Figure 2 shows the incidence of prognostic pathological factors by p-Stage(t) and p-Stage(i). When invasive size was used, better stepwise stratification of all factors, except low histologic grade, was achieved (lower incidence among the lowest stage [IA1] and higher incidence among the highest stage [IIA]). On the other hand, the use of invasive size resulted in a larger distribution of low-grade tumors in the p-Stage IA1 group (% of low-grade histologic subtype in p-Stage IA[i] vs. IA[t], 34% vs. 5%).
Table 1.
Clinicopathologic Characteristics and Outcomes by p-Stage(t) and p-Stage(i)
| p-Stage(t) | p-Stage(i) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IA1(t) n=195 | IA2(t) n=685 | IA3(t) n=336 | IB(t) n=393 | IIA(t) n=95 | IA1(i) n=389 | IA2(i) n=644 | IA3(i) n=239 | IB(i) n=367 | IIA(i) n=65 | |
| Age, years, median (25th–75th percentile) | 67 (61–72) | 69 (62–75) | 71 (65–77) | 70 (62–76) | 71(63–76) | 68 (62–74) | 70 (62–76) | 71 (63–78) | 69 (62–76) | 72 (63–78) |
| Sex | ||||||||||
| Female | 134 (69) | 428 (62) | 210 (63) | 235 (59) | 54 (57) | 261 (67) | 398 (62) | 152 (64) | 214 (58) | 36 (55) |
| Male | 61 (31) | 257 (38) | 126 (38) | 158 (40) | 41 (43) | 128 (33) | 246 (38) | 87 (36) | 153 (42) | 29 (45) |
| Smoking status | ||||||||||
| Never | 28 (14) | 123 (18) | 72 (21) | 69 (17) | 17 (18) | 71 (18) | 115 (18) | 49 (21) | 67 (18) | 7 (11) |
| Former | 138 (71) | 472 (69) | 235 (70) | 272 (69) | 69 (73) | 268 (69) | 454 (70) | 166 (69) | 249 (68) | 49 (75) |
| Current | 29 (15) | 90 (13) | 29 (9) | 52 (13) | 9 (9) | 50 (13) | 75 (12) | 24 (10) | 51 (14) | 9 (14) |
| Type of resection | ||||||||||
| Lobectomya | 87 (45) | 436 (64) | 278 (83) | 289 (73) | 90 (95) | 199 (51) | 437 (68) | 213 (89) | 269 (73) | 62 (95) |
| Sublobar | 108 (55) | 249 (36) | 58 (17) | 104 (26) | 5 (5) | 190 (49) | 207 (32) | 26 (11) | 98 (27) | 3 (5) |
| LVI | ||||||||||
| Absent | 146 (75) | 424 (62) | 206 (61) | 148 (37) | 35 (37) | 310 (80) | 386 (60) | 122 (51) | 125 (34) | 16 (25) |
| Present | 49 (25) | 261 (38) | 130 (39) | 245 (62) | 60 (63) | 79 (20) | 258 (40) | 117 (49) | 242 (66) | 49 (75) |
| VPI | ||||||||||
| Absent | 195 (100) | 685 (100) | 336 (100) | 133 (34) | 70 (74) | 389 (100) | 644 (100) | 239 (100) | 101 (28) | 46 (71) |
| Present | 0 (0) | 0 (0) | 0 (0) | 260 (66) | 25 (26) | 0 (0) | 0 (0) | 0 (0) | 266 (72) | 19 (29) |
| Necrosis (n=1519) | ||||||||||
| Absent | 165 (93) | 568 (91) | 261 (87) | 246 (74) | 52 (61) | 332 (95) | 535 (91) | 170 (82) | 227 (72) | 28 (50) |
| Present | 12 (7) | 57 (9) | 39 (13) | 86 (26) | 33 (39) | 16 (5) | 56 (9) | 38 (18) | 89 (28) | 28 (50) |
| STAS (n=1461) | ||||||||||
| Absent | 102 (56) | 363 (58) | 164 (59) | 149 (48) | 28 (42) | 239 (68) | 331 (56) | 90 (48) | 130 (45) | 16 (37) |
| Present | 79 (44) | 267 (42) | 112 (41) | 159 (52) | 38 (58) | 111 (32) | 261 (44) | 97 (52) | 159 (55) | 27 (63) |
| Predominant subtype | ||||||||||
| Lepidic | 10 (5) | 97 (14) | 46 (14) | 24 (6) | 5 (5) | 131 (34) | 38 (6) | 2 (1) | 11 (3) | 0 (0) |
| Acinar | 114 (58) | 326 (48) | 145 (43) | 185 (47) | 37 (39) | 162 (42) | 336 (52) | 115 (48) | 173 (47) | 21 (32) |
| Papillary | 36 (18) | 123 (18) | 83 (25) | 64 (16) | 22 (23) | 54 (14) | 133 (21) | 64 (27) | 63 (17) | 14 (22) |
| Micropapillary | 8 (4) | 39 (6) | 22 (7) | 21 (5) | 5 (5) | 10 (3) | 40 (6) | 19 (8) | 22 (6) | 4 (6) |
| Solid | 27 (14) | 100 (15) | 40 (12) | 99 (25) | 26 (27) | 32 (8) | 97 (15) | 39 (16) | 98 (27) | 26 (40) |
| Histologic grade | ||||||||||
| Low | 10 (5) | 97 (14) | 46 (14) | 24 (6) | 5 (5) | 131 (34) | 38 (6) | 2 (1) | 11 (3) | 0 (0) |
| Intermediate | 150 (77) | 449 (66) | 228 (68) | 249 (63) | 59 (62) | 216 (56) | 469 (73) | 179 (75) | 236 (64) | 35 (54) |
| High | 35 (18) | 139 (20) | 62 (18) | 120 (30) | 31 (33) | 42 (11) | 137 (21) | 58 (24) | 120 (33) | 30 (46) |
| Mutation (n=1385) | ||||||||||
| Wild type | 78 (50) | 289 (52) | 163 (53) | 166 (56) | 36 (56) | 162 (51) | 272 (51) | 113 (53) | 159 (57) | 26 (63) |
| EGFR | 25 (16) | 116 (21) | 64 (21) | 61 (21) | 11 (17) | 61 (19) | 116 (22) | 41 (19) | 55 (20) | 4 (10) |
| KRAS | 54 (34) | 155 (28) | 83 (27) | 67 (23) | 17 (27) | 95 (30) | 148 (28) | 58 (27) | 64 (23) | 11 (27) |
| Outcomes, % (95% confidence interval; lower, upper) | ||||||||||
| 5-year CIR | 13 (9, 20) | 15 (13,19) | 18 (14, 23) | 29 (24, 34) | 33 (24, 45) | 11 (8, 16) | 16 (13, 19) | 21 (16, 27) | 29 (24, 35) | 41 (30, 57) |
| 5-year LC–CID | 7(4, 12) | 6 (4, 8) | 7 (5, 11) | 15 (12, 20) | 20 (12, 31) | 5 (3, 9) | 6 (4, 9) | 9 (5, 14) | 16 (12, 20) | 25 (16, 41) |
| 5-year OS | 86 (80, 92) | 82 (79, 86) | 78(73, 83) | 72 (67, 77) | 63 (53, 76) | 84 (80, 89) | 81 (78, 85) | 79 (73, 85) | 71 (66, 77) | 51 (38, 68) |
Data are shown as no. (%), unless otherwise noted. CIR, cumulative incidence of recurrence; LC-CID, lung cancer-specific cumulative incidence of death; LVI, lymphovascular invasion; OS, overall survival; p-Stage(i), pathologic stage based on invasive size; p-Stage(t), pathologic stage based on total size; STAS, spread through air spaces; SUVmax, maximum standardized uptake value; VPI, visceral pleural invasion.
Included pneumonectomy or bilobectomy.
Figure 2.
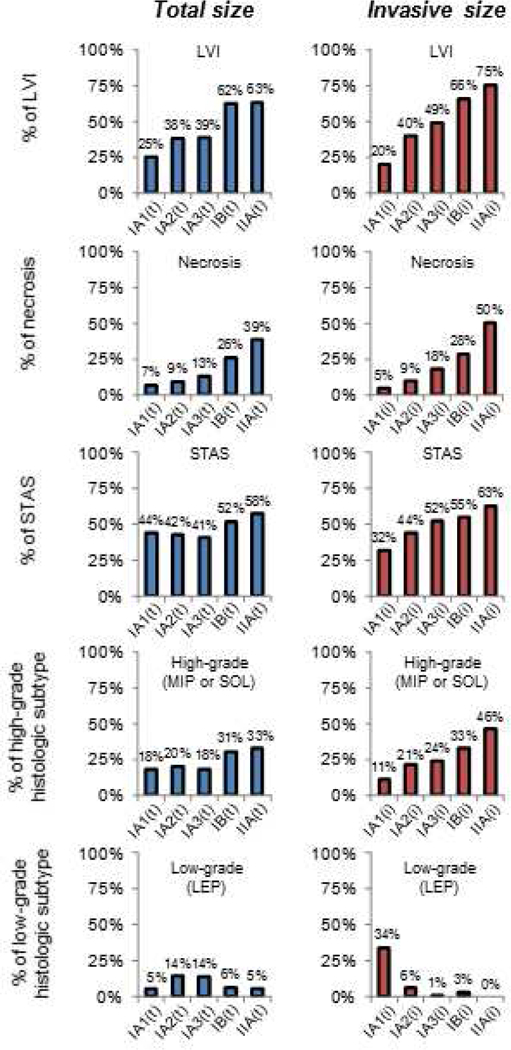
Incidence of prognostic pathologic factors stratified by pathologic stage based on invasive (i) size and pathologic stage based on total (t) size. LEP, lepidic; LVI, lymphovascular invasion; MIP, micropapillary; SOL, solid; STAS, spread through air spaces.
CIR by Same Stage or Downstaged
Figure 3 shows a comparison of CIR between patients who were classified as the same stage and those who were downstaged when invasive size was used. As shown in the figure, for all stages, patients who were downstaged had a lower risk of recurrence than those whose stage remained the same.
Figure 3.

Cumulative incidence of recurrence between patients who were classified as the same stage and those who were downstaged when invasive size (i) was used, rather than total size (t), for the T descriptor. CIR, cumulative incidence of recurrence; p-Stage, pathologic stage.
CIR and LC-CID by p-Stage(t) and p-Stage(i)
Figure 4 shows CIR and LC-CID curves stratified by p-Stage(t) and p-Stage(i). Although the p-Stage IA1(i) group (n=389) included 194 patients (50%) who were downstaged, risk of recurrence and lung cancer–specific death were slightly better in this group than in the p-Stage IA1(t) group (n=195) (IA1[i] vs. IA1[t]: 5-year CIR, 11% vs. 13%; 5-year LC-CID, 5% vs. 7%). On the other hand, 30 of 95 patients with p-Stage IIA(t) were downstaged, resulting in the higher risk of recurrence and lung cancer–specific death in the p-Stage IIA(i) group (n=65) (IIA[i] vs. IIA[t]: 5-year CIR, 41% vs. 32%; 5-year LC-CID, 25% vs. 20%). To ensure that patients who underwent sublobar resection did not skew the results, in Supplementary Figure S2, we show the results of only patients who underwent lobectomy—the results are similar and did not affect the statistical analysis.
Figure 4.
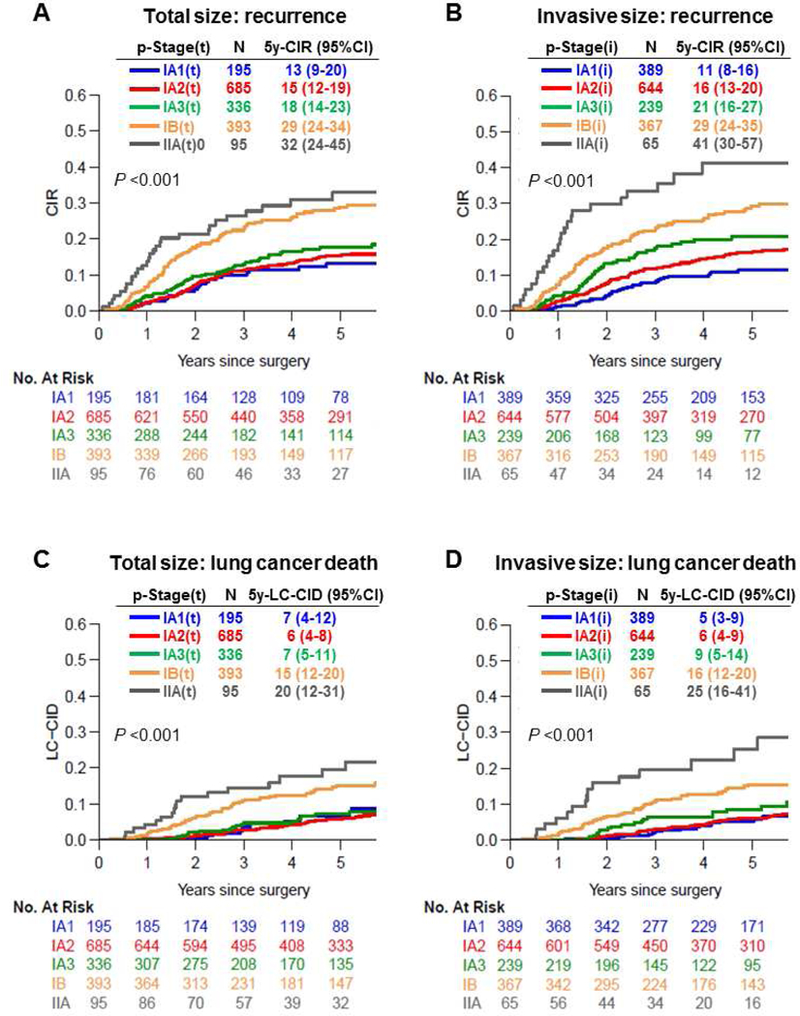
Cumulative incidence of recurrence and lung cancer–specific death by pathologic stage based on invasive size (p-Stage[i]) and pathologic stage based on total size (p-Stage[t]). CI, confidence interval; CIR, cumulative incidence of recurrence; LC-CID, lung cancer-specific cumulative incidence of death; p-Stage, pathologic stage; p-Stage based on invasive size; p-Stage(t), p-Stage based on total size.
Prognostic Discrimination (C-index) by p-Stage(t) and p-Stage(i)
Competing-risk regression models and C-indices for recurrence and lung cancer–specific death by p-Stage(t) and p-Stage(i) are summarized in Table 2. A higher C-index indicates better prognostic discrimination. Prognostic discrimination for predicting recurrence was better by p-Stage(i) than p-Stage(t); for predicting lung cancer–specific death, p-Stage(i) was better than p-Stage(t).
Table 2.
Competing-Risk Regression Model and Concordance Index for Recurrence and Lung Cancer-Specific Death
| p-Stage | Recurrence | Lung cancer-specific death | ||||||
|---|---|---|---|---|---|---|---|---|
| Total Size | Invasive Size | Total Size | Invasive Size | |||||
| SHR (95% CI) | P | SHR (95% CI) | P | SHR (95% CI) | P | SHR (95% CI) | P | |
| IA1 | ref. | ref. | ref. | ref. | ||||
| IA2 | 1.14 (0.73–1.80) | 0.6 | 1.53 (1.05–2.22) | 0.026 | 0.85 (0.47–1.54) | 0.6 | 1.18 (0.71–1.98) | 0.5 |
| IA3 | 1.39 (0.85–2.27) | 0.19 | 2.02 (1.31–3.12) | 0.002 | 0.98 (0.50–1.89) | 0.9 | 1.69 (0.93–3.08) | 0.088 |
| IB | 2.44 (1.55–3.83) | <0.001 | 2.97 (2.04–4.32) | <0.001 | 2.14 (1.19–3.82) | 0.011 | 2.87 (1.74–4.74) | <0.001 |
| IIA | 3.15 (1.82–5.46) | <0.001 | 5.43 (3.26–9.06) | <0.001 | 3.25 (1.64–6.43) | 0.001 | 5.84 (3.06–11.13) | <0.001 |
| C-index (95% CI) | 0.593 (0.579–0.642) | 0.614 (0.598–0.662) | 0.621 (0.603–0.687) | 0.634 (0.608–0.695) | ||||
CI, confidence interval; C-index, concordance index; p-Stage, pathologic stage; SHR, subhazard ratio.
Discussion
Our findings in this study validate the use of invasive size, rather than total size, for the T descriptor in nonmucinous lung ADCs with a lepidic component. The strengths of our study are as follows: (1) this is the first study to validate the use of invasive tumor size in a large, uniform cohort of patients with p-Stage I-IIA lung ADC; (2) our investigation revealed that the use of invasive size with the newly proposed staging system provides better stratification of lung cancer–specific outcomes (recurrence and lung cancer–specific death) and prognostic pathologic factors (such as presence of LVI and high-grade subtypes) according to increasing stage; and (3) our prognostic analysis used a competing-risks approach to address the large number of competing events among patients with early-stage lung cancer.17
When invasive size was used for the T descriptor, >20% of patients were downstaged to the most favorable stage (p-Stage IA1); furthermore, the IA1(i) group (n=389) had a slightly better prognosis than the IA1(t) group (n=195) (5-year CIR, 11% vs. 13%; 5-year LC-CID, 5% vs. 7%). Another important finding of our study was the better prognostic stratification when invasive size was used—that is, patients who were downstaged when invasive size was used had fewer recurrences than patients who were not downstaged—especially among patients with p-Stage IIA tumors. In p-Stage I-IIA lung ADCs following margin-negative (R0) resection, the recent NCCN guidelines do not recommend adjuvant chemotherapy, except for those with stage IB (T2aN0) or IIA (T2bN0) tumors with high-risk factors such as >4 cm tumor size, vascular or pleural involvement, wedge resection, or no lymph node evaluation.14 On the basis of the findings of the present study, we suspect that invasive size may be the important factor for consideration of adjuvant chemotherapy, owing to the significant prognostic difference between tumors >4 cm and 3–4 cm in invasive size. However, this requires further investigation in large clinical studies.
Previous studies investigated radiologic-pathologic correlation in lung nodules.23–26 Lee and colleagues investigated 58 lung ADCs using ≤1.25-mm-section CT images with three-dimensional (3-D) and two-dimensional (2-D) assessment. The authors observed a strong correlation between radiologic consolidation size and pathologic invasive size (correlation coefficient: 3-D, 0.82–0.87; 2-D, 0.72–0.88), with excellent interobserver reproducibility for radiologic consolidation size assessment (intraclass correlation coefficient, 0.92–0.98).26 A recent study investigated radiologic and pathologic correlation in 1792 patients with resected stage I-IV NSCLC and found a strong correlation between consolidation size on thin-section CT and pathologic invasive size (correlation coefficient, 0.83).13 Consolidation size on preoperative CT may be used to predict pathologic invasive size in clinical practice; however, further investigation (such as a prospective study) is warranted.
One of the potential challenges of using invasive size for staging is reproducibility of the distinction between lepidic and invasive patterns, the kappa value of which was reported to be 0.55 in typical cases and 0.08 in difficult cases.27 Specifically, it has been reported that it is difficult to differentiate lepidic pattern from papillary or acinar patterns.28 However, in another study, agreement in the distinction between AIS/MIA and invasive ADC was good, with a kappa of 0.65.29 In another study, the concordance (using intraclass correlation coefficients) between lepidic predominant subtype and other subtypes was 0.94.30 In addition, training may increase concordance in histologic subtyping.28, 31 Although these previous studies that distinguished lepidic “predominant” subtypes (or AIS/MIA) from other invasive morphologic pattern- predominant subtypes showed good results, the reproducibility of determining percentage of lepidic pattern might be more challenging.
It is recommended that mucinous ADCs be measured using total size, regardless of the extent of lepidic component, owing to the lack of validated data supporting the use of invasive size for these tumors3; therefore, we did not include patients diagnosed with invasive mucinous ADC or colloid tumors. In addition, mucinous ADCs usually display a consolidation area on CT scan, and radiologic-pathologic correlations between ground-glass and solid patterns on CT and lepidic and invasive patterns on histologic analysis have not been established4—although specific radiologic characteristics of mucinous ADC on thin-section CT were reported to be prognostic.32 More investigation of these tumors is needed.
For pathologic analysis in the present study, we estimated invasive size by multiplying the total tumor size by the percentage of the invasive component. Since, for many tumors, it is impossible to find a single focus of invasion on a single histologic slide, it is essential to have an alternative method. Our results suggest that the approach used in this study is significantly aligned with prognosis. Further investigation is warranted to compare the two methods for determining invasive size—(1) multiplying the total size by the percentage of the invasive component and (2) measuring the greatest diameter of the largest invasive focus using a ruler—especially in cases with multiple areas of invasion.
In conclusion, as proposed in the eighth edition of the TNM staging system, using invasive size, rather than total size, for the T descriptor achieves better prognostic discrimination by identifying a larger number of patients with a favorable prognosis (p-Stage IA1)—patients who were overstaged using total size. The use of invasive size also provides better stratification of prognoses, which may be useful for determining the most appropriate treatment for patients with early-stage nonmucinous lung ADCs.
Supplementary Material
Acknowledgments
We thank David B. Sewell of the MSK Thoracic Surgery Service for his editorial assistance.
Funding Support
P.S.A.’s laboratory work is supported by grants from the National Institutes of Health (P30 CA008748), the U.S. Department of Defense (CA170630, BC132124 and LC160212), the Joanne and John DallePezze Foundation, the Derfner Foundation, and the Mr. William H. Goodwin and Alice Goodwin, the Commonwealth Foundation for Cancer Research, and the Experimental Therapeutics Center of Memorial Sloan Kettering Cancer Center.
Abbreviations:
- 2-D
two-dimensional
- 3-D
three-dimensional
- ADC
adenocarcinoma
- AIS
adenocarcinoma in situ
- ATS
American Thoracic Society
- C-index
concordance index
- CIR
cumulative incidence of recurrence
- CT
computed tomography
- ETS
European Respiratory Society
- i
invasive size
- IASLC
International Association for the Study of Lung Cancer
- LC-CID
lung cancer–specific cumulative incidence of death
- LVI
lymphovascular invasion
- MIA
minimally invasive adenocarcinoma
- MSK
Memorial Sloan Kettering Cancer Center
- NSCLC
non-small cell lung cancer
- p-Stage
pathologic stage
- t
total size
- TNM
tumor, node, and metastasis
- WHO
World Health Organization
- VPI
visceral pleural invasion
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures of potential conflicts of interest: The authors have no potential conflicts of interest.
REFERENCES
- 1.Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. The New England journal of medicine 2011;365:395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2016;11:39–51. [DOI] [PubMed] [Google Scholar]
- 3.Travis WD, Asamura H, Bankier AA, et al. The IASLC Lung Cancer Staging Project: Proposals for Coding T Categories for Subsolid Nodules and Assessment of Tumor Size in Part-Solid Tumors in the Forthcoming Eighth Edition of the TNM Classification of Lung Cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2016. [DOI] [PubMed] [Google Scholar]
- 4.Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2011;6:244–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naidich DP, Bankier AA, MacMahon H, et al. Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology 2013;266:304–317. [DOI] [PubMed] [Google Scholar]
- 6.Yoshizawa A, Motoi N, Riely GJ, et al. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc 2011;24:653–664. [DOI] [PubMed] [Google Scholar]
- 7.Hung JJ, Yeh YC, Jeng WJ, et al. Predictive Value of the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society Classification of Lung Adenocarcinoma in Tumor Recurrence and Patient Survival. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2014. [DOI] [PubMed] [Google Scholar]
- 8.Travis WD, Brambilla E, Burke AP, et al. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. Lyon, France: International Agency for Research on Cancer; 2015. [DOI] [PubMed] [Google Scholar]
- 9.Warth A, Muley T, Meister M, et al. The novel histologic International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification system of lung adenocarcinoma is a stage-independent predictor of survival. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2012;30:1438–1446. [DOI] [PubMed] [Google Scholar]
- 10.Rami-Porta R, Bolejack V, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for the Revisions of the T Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2015;10:990–1003. [DOI] [PubMed] [Google Scholar]
- 11.Tsutani Y, Miyata Y, Mimae T, et al. The prognostic role of pathologic invasive component size, excluding lepidic growth, in stage I lung adenocarcinoma. The Journal of thoracic and cardiovascular surgery 2013;146:580–585. [DOI] [PubMed] [Google Scholar]
- 12.Borczuk AC, Qian F, Kazeros A, et al. Invasive size is an independent predictor of survival in pulmonary adenocarcinoma. The American journal of surgical pathology 2009;33:462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aokage K, Miyoshi T, Ishii G, et al. Clinical and Pathological Staging Validation in the Eighth Edition of the TNM Classification for Lung Cancer: Correlation between Solid Size on Thin-Section Computed Tomography and Invasive Size in Pathological Findings in the New T Classification. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2017;12:1403–1412. [DOI] [PubMed] [Google Scholar]
- 14.National Comprehensive Cancer Centers: NCCN clinical practice guidelines in oncology (NCCN Guidelines): Non-small cell lung cancer v4. 2018. Available at http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Accessed June 25 2018.
- 15.Donington J, Ferguson M, Mazzone P, et al. American College of Chest Physicians and Society of Thoracic Surgeons consensus statement for evaluation and management for high-risk patients with stage I non-small cell lung cancer. Chest 2012;142:1620–1635. [DOI] [PubMed] [Google Scholar]
- 16.Girard N, Deshpande C, Lau C, et al. Comprehensive histologic assessment helps to differentiate multiple lung primary nonsmall cell carcinomas from metastases. The American journal of surgical pathology 2009;33:1752–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eguchi T, Bains S, Lee MC, et al. Impact of Increasing Age on Cause-Specific Mortality and Morbidity in Patients With Stage I Non-Small-Cell Lung Cancer: A Competing Risks Analysis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2017;35:281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kadota K, Suzuki K, Kachala SS, et al. A grading system combining architectural features and mitotic count predicts recurrence in stage I lung adenocarcinoma. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc 2012;25:1117–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kadota K, Nitadori J, Sima CS, et al. Tumor Spread through Air Spaces is an Important Pattern of Invasion and Impacts the Frequency and Location of Recurrences after Limited Resection for Small Stage I Lung Adenocarcinomas. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2015;10:806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kadota K, Villena-Vargas J, Yoshizawa A, et al. Prognostic significance of adenocarcinoma in situ, minimally invasive adenocarcinoma, and nonmucinous lepidic predominant invasive adenocarcinoma of the lung in patients with stage I disease. The American journal of surgical pathology 2014;38:448–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dignam JJ, Zhang Q, Kocherginsky M. The use and interpretation of competing risks regression models. Clinical cancer research : an official journal of the American Association for Cancer Research 2012;18:2301–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gray RJ. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. The Annals of Statistics 1988;16:1141–1154. [Google Scholar]
- 23.Suzuki K, Koike T, Asakawa T, et al. A prospective radiological study of thin-section computed tomography to predict pathological noninvasiveness in peripheral clinical IA lung cancer (Japan Clinical Oncology Group 0201). Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2011;6:751–756. [DOI] [PubMed] [Google Scholar]
- 24.Asamura H, Hishida T, Suzuki K, et al. Radiographically determined noninvasive adenocarcinoma of the lung: survival outcomes of Japan Clinical Oncology Group 0201. The Journal of thoracic and cardiovascular surgery 2013;146:24–30. [DOI] [PubMed] [Google Scholar]
- 25.Okada M, Nishio W, Sakamoto T, et al. Correlation between computed tomographic findings, bronchioloalveolar carcinoma component, and biologic behavior of small-sized lung adenocarcinomas. The Journal of thoracic and cardiovascular surgery 2004;127:857–861. [DOI] [PubMed] [Google Scholar]
- 26.Lee G, Lee HY, Jeong JY, et al. Clinical impact of minimal micropapillary pattern in invasive lung adenocarcinoma: prognostic significance and survival outcomes. The American journal of surgical pathology 2015;39:660–666. [DOI] [PubMed] [Google Scholar]
- 27.Thunnissen E, Beasley MB, Borczuk AC, et al. Reproducibility of histopathological subtypes and invasion in pulmonary adenocarcinoma. An international interobserver study. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc 2012;25:1574–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Warth A, Cortis J, Fink L, et al. Training increases concordance in classifying pulmonary adenocarcinomas according to the novel IASLC/ATS/ERS classification. Virchows Archiv : an international journal of pathology 2012;461:185–193. [DOI] [PubMed] [Google Scholar]
- 29.Boland JM, Froemming AT, Wampfler JA, et al. Adenocarcinoma in situ, minimally invasive adenocarcinoma, and invasive pulmonary adenocarcinoma--analysis of interobserver agreement, survival, radiographic characteristics, and gross pathology in 296 nodules. Human pathology 2016;51:41–50. [DOI] [PubMed] [Google Scholar]
- 30.Duhig EE, Dettrick A, Godbolt DB, et al. Mitosis trumps T stage and proposed international association for the study of lung cancer/american thoracic society/european respiratory society classification for prognostic value in resected stage 1 lung adenocarcinoma. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2015;10:673–681. [DOI] [PubMed] [Google Scholar]
- 31.Warth A, Stenzinger A, von Brunneck AC, et al. Interobserver variability in the application of the novel IASLC/ATS/ERS classification for pulmonary adenocarcinomas. Eur Respir J 2012;40:1221–1227. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe H, Saito H, Yokose T, et al. Relation between thin-section computed tomography and clinical findings of mucinous adenocarcinoma. The Annals of thoracic surgery 2015;99:975–981. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


