Abstract
Masked uncontrolled hypertension (MUCH) is defined as controlled automated office BP (AOBP <135/85 mmHg) in clinic in patients receiving antihypertensive medication(s), but uncontrolled BP out-of-clinic by 24-hour ambulatory blood pressure monitoring (ABPM; awake ≥135/85 mmHg).We hypothesized that MUCH patients have greater out-of-clinic sympathetic activity compared to true controlled hypertensives.
Patients being treated for hypertension were prospectively recruited after three or more consecutive clinic visits. All patients were evaluated by in-clinic AOBP, plasma catecholamines and spot-urine/plasma metanephrines. In addition, out-of-clinic 24-hr ABPM, 24-hr urinary for catecholamines and metanephrines was done.
Out of 237 patients recruited, 169 patients had controlled in-clinic BP of which 156 patients had completed ABPM. Seventy-four were true controlled hypertensives, i.e. controlled by clinic AOBP and by out-of-clinic ABPM. The remaining 82 were controlled by clinic AOBP, but uncontrolled during out-of-clinic ABPM, indicative of MUCH. After exclusion of 4 patients because of inadequate or lack of 24-hr urinary collections, 72 true controlled hypertensive and 80 MUCH patients were analyzed. MUCH patients had significantly higher out-of-clinic BP variability and lower heart rate variability compared to true controlled hypertensives as well as higher levels of out-of-clinic urinary catecholamines and metanephrines levels consistent with higher out-of-clinic sympathetic activity. In contrast, there was no difference in in-clinic plasma catecholamines and spot-urine/plasma levels of metanephrines between the two groups, consistent with similar levels of sympathetic activity while in clinic.
MUCH patients have evidence of heightened out-of-clinic sympathetic activity compared to true controlled hypertensives, which may contribute to the development of MUCH.
Keywords: masked uncontrolled hypertension, heightened sympathetic activity, catecholamines, metanephrines, blood pressure variability, heart rate variability
Summary
Patients with MUCH have evidence of heightened out-of-clinic sympathetic activity compared to true controlled hypertensive patients.
1. Introduction
Masked hypertension in untreated hypertensive patients or masked uncontrolled hypertension (MUCH) in treated hypertensive patients is defined as controlled blood pressure (BP) in clinic measured by office BP (< 130/80 mmHg) or automated office BP monitor (AOBP; < 135/85 mmHg); but uncontrolled BP out-of-clinic as measured by 24-hour (24-hr) ambulatory BP monitoring (ABPM; overall ≥ 130/80 or awake ≥ 135/85 mmHg) or home BP ≥ 130/80 mmHg 1.
The prevalence of masked hypertension/MUCH is higher in prehypertensives 2, African Americans 3, 4, elderly, 5 and in patients with diabetes 6-8, chronic kidney disease (CKD) 7, 9-12. In addition prevalence of masked hypertension/MUCH is reported to be higher in pediatric renal transplant recipients 13, 14 and adult renal transplant recipients 15.
The prevalence of masked hypertension is higher in patients receiving antihypertensive treatment 6, 16, 17 with prevalence of MUCH reported between 30 - 50% 6, 7, 17, 18. In addition, MUCH has also been shown to be a precursor to sustained hypertension 19.
Masked hypertension/MUCH patients have shown to have higher levels of albuminuria 20, 21 and increased rates of diastolic dysfunction 22. MUCH has shown to have greater all-cause and cardiovascular mortality compared to controlled hypertension and sustained uncontrolled hypertension treated on medications 23.
Patients with masked hypertension/MUCH have been reported to have evidence of higher sympathetic tone assessed by microneurography while in-clinic compared to patients with controlled hypertension 10, 24. No studies have assessed sympathetic activity in MUCH patients while out-of-clinic. Such an assessment would be important from a mechanistic standpoint as it is uncontrolled out-of-clinic BP that distinguishes the MUCH phenotype from true controlled hypertension which is, controlled BP both in- and out-of-clinic. We hypothesized that MUCH patients have greater out-of-clinic sympathetic activity compared to patients with true controlled hypertension, implicating heightened out-of-clinic sympathetic tone as a cause of MUCH. To test this hypothesis, we prospectively compared indices of in-clinic sympathetic activity plasma catecholamines and spot urine/plasma metanephrines in patients with confirmed MUCH with true controlled hypertensive patients. Indices of out-of-clinic sympathetic activity includes 24-hr ambulatory BP 25 and heart rate (HR) variability 26; 24-hr urinary catecholamines and metanephrines which are compared in MUCH and true controlled hypertensive patients.
2. Methods
Data will be made available upon request. It will be made available 1 year after completion of funding grant, so April 2020
A. Study population
Consecutive hypertensive patients on medications referred to the University of Alabama at Birmingham Hypertension Clinic were prospectively recruited between April 2014 and June 2018. Patients were enrolled after having been seen by a hypertension specialist for a minimum of three follow-up visits. All study patients were evaluated for secondary causes of hypertension including hyperaldosteronism, pheochromocytoma, and renal artery stenosis, as medically indicated. Pregnant patients and patients with CKD stage 4 or 5 (eGFR <30 ml/min/1.73m2), patients suspected of non-adherence, based on self-report or low medication refill rates, were excluded. The study was approved by the UAB Institutional Review Board and written informed consent was obtained from all participants.
B. BP measurement
I. Clinic automated office BP measurement (AOBP)
The clinic AOBP was measured using the BpTRU device, which automatically obtains 6 serial BP readings, one minute apart, before displaying the average of the last 5 readings. AOBP was measured after at least 5 minutes of quiet rest in a sitting position with the back supported and the arm supported at heart level 27. These assessments were unattended, i.e., unobserved in clinic 28-32. An appropriate sized cuff was used with the cuff bladder encircling at least 80% of the arm 32, 33. A BP cutoff of ≥ 135/85 mmHg for elevated BP was used based on literature validating automated BP devices 16, 34.
II. Out-of-clinic 24-hr ambulatory BP monitoring (ABPM)
Study patients had an ABPM done using an automated, noninvasive, oscillometric device (Oscar 2; Suntech Medical Inc, Morrisville, NC) 1, 35. ABPM measurements were done every 20 minutes during the daytime (awake) and every 30 minutes during the nighttime (asleep). Awake and asleep times were determined by patient self-report. ABPM was determined to be valid if >80% of measurements were successful. All patients were counselled to take all antihypertensive medications and not to exercise while during their 24-hr ABPM period. Controlled ambulatory BP was defined as mean daytime (awake) BP <135/80 mmHg 1, 35. Awake, asleep, and 24-hr ambulatory BP (systolic and diastolic) and HR variability were calculated 36.
C. Complete 24-hr urine collection
The creatinine excretion rate was used to determine the completeness of urine collections. Urine samples with a creatinine excretion rate of 15-25 mg/kg/day for men and 10-20 mg/kg/day for women were considered complete 37. In order to account for extremes in body sizes, incomplete collections were further evaluated. Measured creatinine clearance was compared to an expected creatinine clearance using the Cockcroft-gault formula with adjustments for body surface area. After review of incomplete urine collections, participants which had a measured creatinine clearance within 10% of the expected clearance, were included back in the analysis data.
D. Biochemical testing
I. Renal function panel, serum aldosterone, plasma renin activity
Serum electrolyes, blood urea nitrogen, serum creatinine, serum aldosterone and plasma renin activity were done according to routine laboratory methods. Serum aldosterone was analyzed by liquid chromatography-tandem mass spectrometry using multiple reaction monitoring in the negative mode 38 and plasma renin activity is measured by HPLC electrospray-tandem mass spectrometry 39 (Mayo Medical Laboratories, Rochester, MN).
II. Catecholamines and metanephrines
a. Catecholamines and metanephrines tested in clinic
i. Urine metanephrines in clinic
A spot urine collection was provided by all study participants in-clinic on the same day of completion of 24-hour urine collection. The first urine was voided and subsequent urine was collected while patients were in the clinic. The spot urine was used for determination of metanephrines (metanephrine, normetanephrine and total metanephrines) levels by reverse phase liquid chromatography-tandem mass spectrometry (LC-MS/MS) stable isotope dilution analysis (Mayo Medical Laboratories, Rochester, MN)40, 41. Values are reported in microgram (mcg) of metanephrine or normetanephrine per gram (g) of urine creatinine.
ii. Plasma catecholamines and metanephrines in clinic
Blood samples were collected from all study patients during the same clinic visit as spot urine collection. These samples were used for determination of plasma catecholamines (epinephrine, norepinephrine, total catecholamines) and metanephrines (metanephrine and normetanephrine). Plasma catecholamines were detected by high-performance liquid chromatography with electrochemical detection 42 and plasma metanephrines levels were determined by liquid chromatography-tandem mass spectometry (LC-MS/MS) (Mayo Medical Laboratories, Rochester, MN)40.
b. Out-of-clinic 24-hr urine catecholamines and metanephrines
Study participants completed an out-of-clinic 24-hr urine collection for determination of catecholamines (epinephrine, norepinephrine, total catecholamines) and metanephrines (metanephrine, normetanephrine, total metanephrines). Urinary catecholamines were determined by liquid chromatography with amperometric detection 43. Urinary metanephrines were determined by reverse phase LC-MS/MS stable isotope dilution analysis (Mayo Medical Laboratories, Rochester, MN) 40, 41 with values reported as mcg per 24 hour.
E. Statistical analysis
Descriptive analyses were performed to summarize the demographics, comorbidities and biochemical characteristics of study participants. Two sample t-tests were used to detect the difference in antihypertensive medications, BP values and biochemical (Urine/plasma catecholamines and metanephrines) variables between the true controlled hypertension and MUCH groups.
Linear mixed models were fitted to the BP and HR data, separately, to determine BP and HR variability, respectively 44, 45. Each model incorporated subject-specific random intercepts. BP and HR variance were computed for participants with true controlled vs. masked uncontrolled hypertension. Statistical significance of difference between these variance estimates was assessed 36.
Multivariable linear regression models were used to assess the relationship between BP and HR variability; in-clinic and out-of-clinic urinary/plasma catecholamines and metanephrines in hypertension groups adjusted for diabetes, BMI, smoking and use of calcium channel blockers, β-blockers, αβ-blockers and α2-agonists.
All analyses were performed using SAS version 9.4 and R version 3.5.0. A p-value less than 0.05 was considered statistically significant for two-sided tests.
3. Results
Two-hundred and thirty-seven hypertensive patients were prospectively recruited after three or more consecutive clinic visits. Of these patients, 169 had controlled in-clinic BP while receiving antihypertensive medications (Figure 1).
Figure 1.
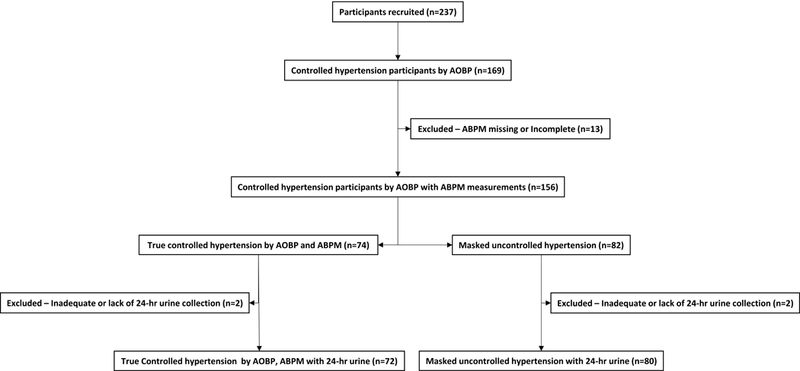
Schematic of enrolled study participants
A. Masked uncontrolled hypertension prevalence
Of the 169 patients with controlled in-clinic BP (AOBP <135/85 mmHg) on antihypertensive medications, 156 had adequate ABPM readings. Of these, 74 (47.4%) had controlled out-of-clinic ambulatory BP (ABPM; awake <135/85 mmHg), indicating true controlled hypertension. The remaining 82 (52.6%) were controlled in clinic (AOBP <135/85 mmHg), but had uncontrolled out-of-clinic ambulatory BP (ABPM; awake ≥135/85 mmHg), diagnostic of MUCH (Figure 1).
B. Completeness of 24-hr urine collections
Out of the 74 patients with true controlled hypertension and 82 patients with confirmed MUCH, 4 patients were excluded because of inadequate or lack of 24-hr urine collections, such that the final analysis included 72 patients with true controlled hypertension and 80 MUCH patients. (Figure 1).
C. Patient characteristics
I. Demographics and Comorbidities
The mean age of true controlled hypertensive and MUCH patients was 60.4±11.0 and 58.9±10.3 years, respectively. Among true controlled hypertensive patients, 44.8% were female and 50.7% were African American, while among patients with MUCH, 41.8% were female and 48.1% were African American. The median BMI was similar in the two groups, 33.1±7.5 kg/m2 in true controlled hypertensive and 33.9±5.9 kg/m2 in MUCH patients.
Diabetes was more common in MUCH patients (41.8%) compared to the true controlled hypertensive patients (22.4%, p=0.013) (Table 1).
Table 1:
Demographics, comorbidities, biochemistry and antihypertensive medications of patients with controlled and masked uncontrolled hypertension
| Variables | True controlled hypertension (n=72) |
Masked uncontrolled hypertension (n=80) |
p-value |
|---|---|---|---|
| Demographics | |||
| Age (years) | 60.4 ± 11.0 | 58.9 ± 10.3 | 0.404 |
| Female | 30 (44.8%) | 33 (41.8%) | 0.715 |
| African American | 34 (50.7%) | 38 (48.1%) | 0.750 |
| Comorbidities | |||
| Current smoker | 3 (4.5%) | 10 (12.7%) | 0.084 |
| Dyslipidemia | 43 (64.2%) | 53 (67.1%) | 0.712 |
| Congestive heart failure | 4 (6.0%) | 6 (7.6%) | 0.699 |
| Coronary artery disease | 10 (14.9%) | 9 (11.4%) | 0.527 |
| Peripheral vascular disease | 3 (4.5%) | 5 (6.3%) | 0.727 |
| Diabetes | 15 (22.4%) | 33 (41.8%) | 0.013 |
| Thyroid disorder | 7 (10.4%) | 13 (16.5%) | 0.293 |
| Prior stroke/transient ischemic attack | 10 (14.9%) | 13 (16.5%) | 0.800 |
| Gout | 10 (14.9%) | 9 (11.4%) | 0.527 |
| Body mass index (kg/m2) | 33.1 ± 7.5 | 33.9 ± 5.9 | 0.480 |
| Biochemistry | |||
| Sodium (mMol/L) | 138.3 ± 2.8 | 137.8 ± 3.4 | 0.457 |
| Potassium (mMol/L) | 4.0 ± 0.4 | 4.0 ± 0.4 | 0.826 |
| Bicarbonate (mMol/L) | 27.8 ± 3.1 | 28.4 ± 2.9 | 0.324 |
| Blood urea nitrogen (mg/dL) | 19.1 ± 8.4 | 17.9 ± 7.1 | 0.454 |
| Creatinine (mg/dL) | 1.1 ± 0.5 | 1.0 ± 0.3 | 0.305 |
| Serum aldosterone (ng/dL) | 8.7 ± 5.4 | 11.2 ± 8.7 | 0.054 |
| Plasma renin activity (ng/mL/hr) | 10.8 ± 22.1 | 11.7 ± 28.0 | 0.846 |
| Antihypertensive medications | |||
| Angiotensin converting enzyme inhibitors | 29 (43.3%) | 34 (43.0%) | 0.976 |
| Angiotensinogen receptor blockers | 29 (43.3%) | 30 (38.0%) | 0.515 |
| Calcium channel blockers | 44 (65.7%) | 61 (77.2%) | 0.122 |
| Thiazide diuretics | 49 (73.1%) | 63 (79.7%) | 0.346 |
| Loop diuretics | 2 (3.0%) | 4 (5.1%) | 0.688 |
| Endothelial sodium channel blocker | 2 (3.0%) | 0 | 0.209 |
| Mineralocorticoid receptor antagonists | 26 (38.8%) | 27 (34.2%) | 0.562 |
| α blockers | 2 (3.0%) | 3 (3.8%) | 1.000 |
| β blockers | 20 (29.9%) | 19 (24.1%) | 0.430 |
| αβ blockers | 9 (13.4%) | 22 (27.8%) | 0.034 |
| α2 agonists | 8 (11.9%) | 12 (15.2%) | 0.569 |
| Nitric oxide vasodilators | 0 | 4 (5.1%) | 0.125 |
| Potassium channel openers | 0 | 2 (2.5%) | 0.500 |
| Total antihypertensive medications | 3.3 ± 1.2 | 3.6 ± 1.2 | 0.156 |
II. Renal Function Panel, Serum Aldosterone, Plasma Renin Activity
There was no difference in serum electrolytes, blood urea nitrogen, serum creatinine, serum aldosterone and plasma renin activity in true controlled hypertensive versus MUCH patients (Table1).
D. Antihypertensive medications
The number of prescribed antihypertensive medications was similar for both groups (3.3±1.2 for patients with true controlled hypertensive and 3.6±1.2 for MUCH patients). There was no significant difference between the types of antihypertensive medication classes between true controlled and MUCH groups except that MUCH patients (27.8%) were on a significantly higher number of α-β-blockers (carvedilol and labetalol) than the true controlled hypertensive patients (13.4%; p=0.034) (Table 1).
E. BP measurements in- and out-of-clinic
I. Clinic AOBP measurement
The mean in-clinic BP readings were 113.8±10.3 / 70.4±7.8 mmHg for patients with true controlled hypertension versus 120.8±8.2 / 73.4±7.7 mmHg for patients with MUCH (p < 0.001 and p = 0.022) (Table 2).
Table 2:
Blood pressure, heart rate, blood pressure variability, heart rate variability, catecholamines and metanephrines values of patients with controlled and masked uncontrolled hypertension in and out of clinic
| Mean ± SD | ||||||
|---|---|---|---|---|---|---|
| Variables | True controlled hypertension (n=72) |
Masked uncontrolled hypertension (n=80) |
p-value | True controlled hypertension (n=72) |
Masked uncontrolled hypertension (n=80) |
p-value |
| Blood Pressure/Heart Rate | In-Clinic - AOBP * | Out-of-clinic - ABPM † | ||||
| Awake (Day-time) | ||||||
| Systolic BP ‡ (mmHg) | 113.8 ± 10.3 | 120.8 ± 8.2 | <0.001 | 124.2 ± 7.5 | 148.3 ± 11.4 | <0.001 |
| Diastolic BP ‡ (mmHg) | 70.4 ± 7.8 | 73.4 ± 7.7 | 0.022 | 70.8 ± 7.2 | 82.0 ± 8.0 | <0.001 |
| Mean arterial pressure (mmHg) | 84.9 ± 7.4 | 89.2 ± 6.4 | <0.001 | 88.0 ± 8.5 | 104.1 ± 7.5 | <0.001 |
| Pulse Pressure (mmHg) | 43.4 ± 9.8 | 47.5 ± 9.5 | 0.012 | 54.2 ± 9.4 | 66.4 ± 11.6 | <0.001 |
| Heart rate (beats/minute) | 71.6 ± 12.4 | 73.6 ± 11.5 | 0.308 | 72.6 ± 11.6 | 75.1 ± 11.3 | 0.190 |
| Systolic BP ‡ variability § (mmHg) | 168.0 (159.1, 177.3) | 281.2 (260.9, 303.2) | <0.001 | |||
| Diastolic BP ‡ variability § (mmHg) | 101.0 (95.7, 106.6) | 149.8 (139.0, 161.5) | <0.001 | |||
| Heart rate variability (beats/minute) | 76.2 (72.2, 80.4) | 66.8 (62.0, 71.9) | <0.001 | |||
| Asleep (Night-time) | ||||||
| Systolic BP ‡ (mmHg) | 115.2 ± 12.1 | 138.8 ± 19.9 | <0.001 | |||
| Diastolic BP ‡ (mmHg) | 62.7 ± 8.2 | 73.2 ± 11.8 | <0.001 | |||
| Mean arterial pressure (mmHg) | 79.7 ± 9.3 | 95.0 ± 13.2 | <0.001 | |||
| Pulse Pressure (mmHg) | 53.3 ± 10.5 | 65.7 ± 14.8 | <0.001 | |||
| Heart rate (beats/minute) | 66.8 ± 10.8 | 69.1 ± 11.6 | 0.213 | |||
| Systolic BP ‡ variability § (mmHg) | 135.5 (122.7, 149.7) | 279.2 (242.6, 321.3) | <0.001 | |||
| Diastolic BP ‡ variability § (mmHg) | 83.0 (75.2, 91.7) | 143.9 (125.0, 165.6) | <0.001 | |||
| Heart rate variability (beats/minute) | 38.2 (34.6, 42.2) | 29.3 (25.5, 33.7) | <0.001 | |||
| 24-hr (Overall) | ||||||
| Systolic BP ‡ (mmHg) | 122.1 ± 7.5 | 145.9 ± 11.9 | <0.001 | |||
| Diastolic BP ‡ (mmHg) | 68.9 ± 6.9 | 79.9 ± 8.3 | <0.001 | |||
| Mean arterial pressure (mmHg) | 86.6 ± 6.0 | 102.0 ± 8.0 | <0.001 | |||
| Pulse Pressure (mmHg) | 53.3 ± 8.1 | 66.0 ± 11.4 | <0.001 | |||
| Heart rate (beats/minute) | 71.2 ± 11.2 | 73.7 ± 11.2 | 0.188 | |||
| Systolic BP ‡ variability § (mmHg) | 188.6 (179.9, 197.7) | 328.5 (307.7, 350.7) | <0.001 | |||
| Diastolic BP ‡ variability § (mmHg) | 114.1 (108.9, 119.6) | 173.3 (162.3, 185.0) | <0.001 | |||
| Heart rate variability (beats/minute) | 77.9 (74.2, 81.7) | 70.0 (65.4, 74.9) | 0.001 | |||
| Catecholamines/Metanephrines | In-Clinic Random (/g Creatinine) | Out-of-clinic (/24-hours) | ||||
| Epinephrine | ||||||
| - Urinary (mcg) | 7.50 ± 3.72 | 7.11 ± 3.12 | 0.693 | |||
| - Plasma (pg/mL) | 51.36 ± 23.23 | 44.86 ± 20.65 | 0.297 | |||
| Norepinephrine | ||||||
| - Urinary (mcg) | 49.36 ± 25.88 | 59.47 ± 29.52 | 0.032 | |||
| - Plasma (pg/mL) | 697.58 ± 442.60 | 609.43 ± 266.85 | 0.282 | |||
|
Total Catecholamines
(Epinephrine & Norepinephrine) |
||||||
| - Urinary (mcg) | 51.64 ± 26.35 | 61.99 ± 29.85 | 0.030 | |||
| - Plasma (pg/mL) | 725.83 ± 455.27 | 640.40 ± 274.72 | 0.310 | |||
| Metanephrine | ||||||
| - Urinary (mcg) | 82.57 ± 43.15 | 85.79 ± 60.29 | 0.776 | 109.37 ± 59.66 | 133.96 ± 71.95 | 0.030 |
| - Plasma (nMol/L) | 0.21 ± 0.03 | 0.22 ± 0.05 | 0.741 | |||
| Normetanephrine | ||||||
| - Urinary (mcg) | 251.38 ± 139.67 | 251.72 ± 116.87 | 0.990 | 366.63 ± 149.42 | 434.41 ± 181.76 | 0.017 |
| - Plasma (nMol/L) | 0.75 ± 0.38 | 0.81 ± 0.40 | 0.407 | |||
|
Total Metanephrines
(Metanephrine & Normetanephrine) |
||||||
| - Urinary (mcg) | 333.95 ± 166.87 | 337.51 ± 153.45 | 0.919 | 476.00 ± 180.97 | 566.67 ± 218.13 | 0.008 |
AOBP, automated office blood pressure
ABPM, ambulatory blood pressure monitoring
BP, blood pressure
P-values and variance estimates are from linear mixed models with between-group heterogeneity
II. Out-of-Clinic BP measurements by ABPM
The mean awake (daytime) ambulatory BP for true controlled hypertensive patients was 124.2±7.5 / 70.8±7.2 compared to 148.3±11.4 / 82.0±8.0 mmHg for MUCH patients (p < 0.001). The mean asleep (nighttime) ambulatory BP was 115.2±12.1 / 62.7±8.2 for true controlled hypertensive patients versus 138.8±19.9 / 73.2±11.8 mmHg for MUCH patients (p < 0.001). The mean 24-hr ambulatory BP was 122.1±7.5 / 68.9±6.9 for true controlled hypertensive patients versus 145.9±11.9 / 79.9±8.3 mmHg for MUCH patients (p < 0.001) (Table 2).
MUCH patients had significantly higher out-of-clinic awake, asleep and 24-hr ambulatory BP (systolic and diastolic) variability and lower HR variability compared to true controlled hypertensive patients (Table 2). These differences persisted after multiple linear regression adjustment for diabetes, BMI, smoking, and use of calcium channel blockers, β-blockers, α-β-blockers and α2-agonists (Table 3).
Table 3:
Linear regression analysis of blood pressure variability, heart rate variability, catecholamines and metanephrines adjusted for factors affecting sympathetic activity in patients with controlled and masked uncontrolled hypertension in- and out-of- clinic
| p-value | ||||||
|---|---|---|---|---|---|---|
| In-Clinic | Out-of-clinic | |||||
| Variables | Unadjusted | Adjusted for diabetes & αβ-blockers |
Multivariable adjusted * |
Unadjusted | Adjusted for diabetes & αβ-blockers |
Multivariable adjusted * |
| Blood Pressure/Heart Rate | ||||||
| Awake (Day-time) | ||||||
| Systolic BP † variability (mmHg) | <0.001 | <0.001 | <0.001 | |||
| Diastolic BP † variability (mmHg) | <0.001 | <0.001 | <0.001 | |||
| Heart rate variability (beats/minute) | <0.001 | <0.001 | <0.001 | |||
| Asleep (Night-time) | ||||||
| Systolic BP † variability (mmHg) | <0.001 | <0.001 | <0.001 | |||
| Diastolic BP † variability (mmHg) | <0.001 | <0.001 | <0.001 | |||
| Heart rate variability (beats/minute) | <0.001 | <0.001 | <0.001 | |||
| 24-hr (Overall) | ||||||
| Systolic BP † variability (mmHg) | <0.001 | <0.001 | <0.001 | |||
| Diastolic BP † variability (mmHg) | <0.001 | <0.001 | <0.001 | |||
| Heart rate variability (beats/minute) | 0.001 | 0.001 | 0.001 | |||
| Catechoamines/Metanephrines | ||||||
| Epinephrine | ||||||
| - Urine | 0.693 | 0.671 | 0.571 | |||
| - Plasma | 0.297 | 0.500 | 0.983 | |||
| Norepinephrine | ||||||
| - Urinary | 0.032 | 0.031 | 0.048 | |||
| - Plasma | 0.275 | 0.319 | 0.365 | |||
|
Total Catecholamines
(Epinephrine & Norepinephrine) |
||||||
| - Urinary | 0.030 | 0.030 | 0.049 | |||
| - Plasma | 0.304 | 0.348 | 0.411 | |||
| Metanephrine | ||||||
| - Urinary | 0.785 | 0.914 | 0.886 | 0.030 | 0.017 | 0.017 |
| - Plasma | 0.748 | 0.497 | 0.642 | |||
| Normetanephrine | ||||||
| - Urinary | 0.990 | 0.938 | 0.846 | 0.017 | 0.008 | 0.009 |
| - Plasma | 0.408 | 0.239 | 0.329 | |||
|
Total Metanephrines
(Metanephrine & Normetanephrine) |
||||||
| - Urinary | 0.919 | 0.921 | 0.838 | 0.008 | 0.004 | 0.004 |
Multivariable adjusted for diabetes, BMI, smoking, calcium channel blockers, β-blockers, αβ-blockers and α2-agonist
BP, blood pressure
F. Biochemical evaluation - In-clinic and out-of-clinic catecholamines and metanephrines levels
I. In-clinic catecholamines and metanephrines levels
There was no evidence of difference between the in-clinic spot urinary metanephrine, normetanephrine, and total metanephrines levels in true controlled hypertensive and MUCH study groups. In-clinic plasma epinephrine, norepinephrine, total catecholamines, metanephrine and normetanephrine levels were also similar in both study groups (Table 2, Figure 2). An absence of significant differences persisted between the groups after multiple linear regression adjustment for diabetes, BMI, smoking, and use of calcium channel blockers, β-blockers, α-β-blockers and α2-agonists (Table 3).
Figure 2.
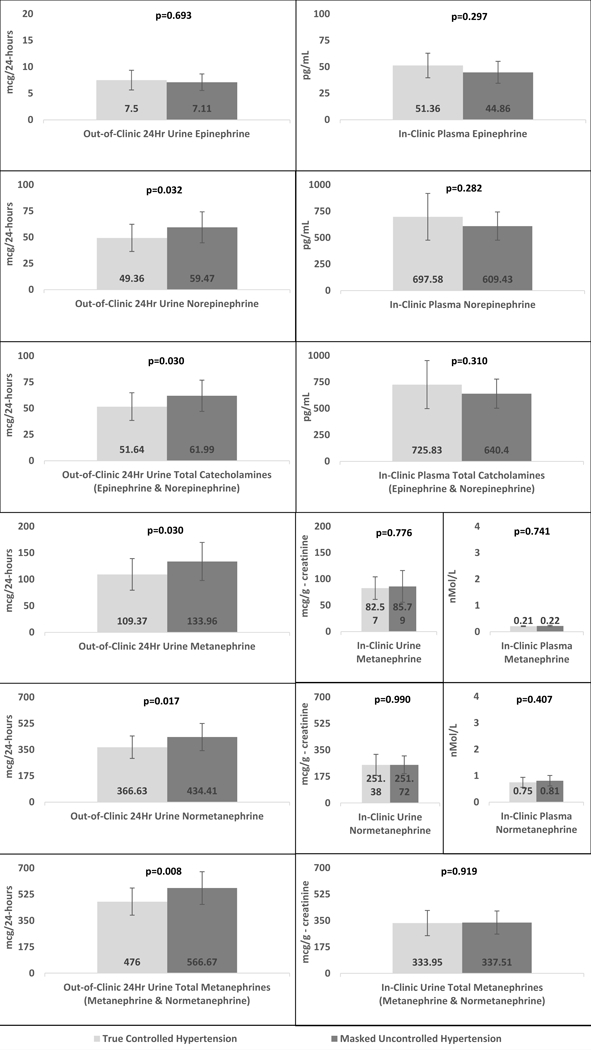
Catecholamines (Epinephrine, norepinephrine and total catecholamines) and Metanephrines (Metanephrine, normetanephrine and total metanephrines) level comparison between controlled and masked uncontrolled hypertension
II. Out-of-clinic catecholamines and metanephrine levels
Patients with MUCH had significantly higher levels of out-of-clinic 24-hr urinary excretion rates of norepinephrine (p=0.032), total catecholamines (p=0.030), metanephrine (p=0.030), normetaneprhine (p=0.017) and total metanephrines (p=0.008) compared to true controlled hypertensive patients (Table 2, Figure 2). These differences persisted after multiple linear regression adjustment for diabetes, BMI, smoking, and use of calcium channel blockers, β-blockers, α-β-blockers and α2-agonists (Table 3).
3. Discussion
This is the first study to prospectively compare in- and out-of-clinic sympathetic activity in patients with MUCH versus a comparator group of true controlled hypertensive patients. The results provide evidence of higher out-of-clinic sympathetic activity in MUCH patients compared to patients with true controlled hypertension.
Grassi et al. measured muscle sympathetic nerve activity (MSNA) from the peroneal nerve with use of microneurography and beat-to-beat arterial pressure at rest and during baroreceptor deactivation and activation in masked hypertensive and normotensive individuals in clinic 24. Resting MSNA in masked hypertensive patients was greater than in normotensive control individuals. Compared with normotensive subjects, baroreflex control of HR was significantly attenuated in masked hypertensive patients, whereas baroreflex-sympathetic control was unaffected. Homeostasis model assessment index was increased in patients in masked hypertension in direct relation with resting sympathetic nerve traffic, suggesting that masked hypertension is characterized by baseline sympathetic hyperactivity when assessed in the clinic setting. Plasma norepinephrine values obtained in clinic were not significantly different between the two groups 24.
Agarwal et al. assessed sympathetic tone in CKD patients with MUCH by measuring BP during graded symptom-limited exercise with a cycle ergometer and 7 min of recovery post-exercise 10. During recovery, the healthy control group had a 5.9% decline in systolic BP per minute, while MUCH patients had only a 3.3% per minute reduction in systolic BP. This difference was interpreted to indicate less withdrawal of sympathetic tone upon termination of exercise, resulting in vasoconstriction and delayed systolic BP recovery in the patients with MUCH, suggesting increased sympathetic tone a cause of MUCH 10. Thus the findings of Grassi et al. and Agarwal et al. provide evidence that patients with masked hypertension/MUCH have greater sympathetic activity while in the clinic (or the laboratory) compared to controlled hypertensive patients. However, in neither study was sympathetic activity assessed while study patients were out-of-clinic, when BP levels are by definition higher, consistent with the MUCH phenotype 10, 24.
The current study was designed to compare both in-clinic and out-of-clinic sympathetic activity in patients with MUCH versus a comparator group of patients with true controlled hypertension. In-clinic sympathetic tone was indexed by plasma catecholamines and urinary/plasma metanephrines levels, which were similar in the two study groups. In contrast, higher out-of-clinic sympathetic tone as evidenced by higher BP variability and lower HR variability in MUCH patients; Similarly, sympathetic tone indexed by 24-hr urinary catecholamines and metanephrines levels were significantly higher in the MUCH patients compared to true controlled hypertension.
Obesity and diabetes have been shown to increase sympathetic output 46-50. In the current study, there was no significant difference in BMI between the MUCH and controlled hypertensive groups. Diabetes, however, was more common in the MUCH patients compared to controls (41.8% vs 22.4%, respectively). Smoking, antihypertensive medication classes like calcium channel blockers, β-blockers, αβ-blockers and α2-agonists (clonidine and guafacine) affect sympathetic output. MUCH patients were on significantly more αβ-blockers than patients with true controlled hypertension. While smoking, antihypertensive medication classes, including calcium channel blockers, β-blockers and α2-agonists (clonidine and guafacine) were similar in both the groups.
Multiple linear regression adjustment for diabetes, BMI, smoking and use of calcium channel blockers, β-blockers, αβ-blockers and α2-agonist show significantly higher out-of-clinic BP variability, lower out-of-clinic HR variability (Table 3) and higher catecholamines/metanephrines levels in MUCH patients compared to controlled hypertensive patients (Table 3), suggesting persistent increases in sympathetic tone unrelated to smoking, obesity, diabetes and sympatholytic medications. Multiple linear regression adjustment for diabetes, BMI, smoking and use of calcium channel blockers, β-blockers, αβ-blockers and α2-agonists showed similar in-clinic catecholamines and metanephrines levels in MUCH patients compared to controlled hypertensive patients (Table 3).
These findings provide for the first time evidence of increased out-of-clinic sympathetic output as an important cause of MUCH (Figure 3). If confirmed, these findings suggest that therapeutic strategies, including centrally-acting agents that specifically target sympathetic output, might be beneficial in blunting or reversing the masked effect.
Figure 3.
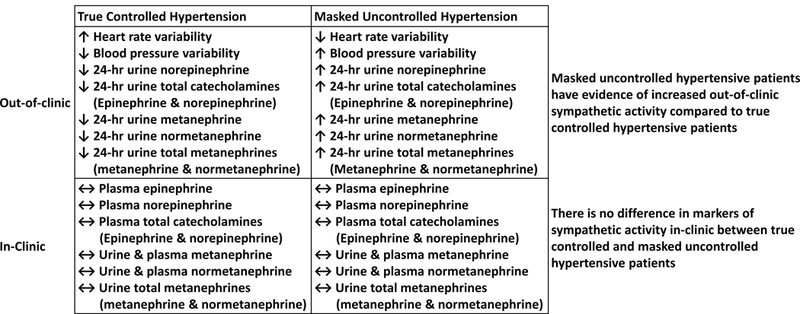
Schematic representation of out-of-clinic heightened sympathetic activity in masked uncontrolled hypertension.
Strengths of the current study include its prospective design; inclusion of a diverse and relatively large cohort; rigorous definition of MUCH and true controlled hypertension; comparison of MUCH patients to a comparator group of true controlled hypertension; and measurement of both in- and out-of-clinic BP levels and sympathetic activity.
Study weaknesses include indexing sympathetic tone indirectly by measurement of catecholamines and metanephrines levels as opposed to a more direct method such as with microneurography. Microneurography, however, is limited to the in-clinic setting.
Patients with MUCH have evidence of heightened out-of-clinic sympathetic activity compared to true controlled hypertensive patients. These findings suggest that heightened out-of-clinic sympathetic activity contributes to development of MUCH. If so, such patients may preferentially benefit from medications or interventional procedures that target sympathetic output.
Perspectives
Patients with MUCH have evidence of heightened out-of-clinic sympathetic activity compared to true controlled hypertensive patients suggesting that heightened out-of-clinic sympathetic activity contributes to development of MUCH. If so, such patients may preferentially benefit from medications or interventional procedures that target sympathetic output and help in management of MUCH and prevent increased cardiovascular, renal, and cerebrovascular risk associated with it.
Novelty and Significance.
What is new: This is the first study to evaluate the mechanism of masked uncontrolled hypertension (MUCH) while study patients were out-of-clinic, when BP levels are by definition higher, consistent with the MUCH phenotype.
What is relevent: This study shows that patients with MUCH have evidence of heightened out-of-clinic sympathetic activity compared to true controlled hypertensive patients suggesting that heightened out-of-clinic sympathetic activity contributes to development of MUCH. If so, such patients may preferentially benefit from medications or interventional procedures that target sympathetic output and help in management of MUCH and prevent increased cardiovascular, renal, and cerebrovascular risk associated with it.
Acknowledgments
Sources of funding
The National Institutes of Health (NIH R01 HL113004 and 2T32HL007457-36A1), the American Heart Association Strategically Focused Research Network (AHA 5SFRN2390002), and the National Institutes of Diabetes, Digestive, and Kidney Disease (K23 DK102660 to E.J.) supported this research.
Footnotes
Disclosures: None
References
- 1.O’Brien E, Parati G, Stergiou G, Asmar R, Beilin L, Bilo G, Clement D, de la Sierra A, de Leeuw P, Dolan E, Fagard R, Graves J, Head GA, Imai Y, Kario K, Lurbe E, Mallion JM, Mancia G, Mengden T, Myers M, Ogedegbe G, Ohkubo T, Omboni S, Palatini P, Redon J, Ruilope LM, Shennan A, Staessen JA, vanMontfrans G, Verdecchia P, Waeber B, Wang J, Zanchetti A, Zhang Y and European Society of Hypertension Working Group on Blood Pressure M. European Society of Hypertension position paper on ambulatory blood pressure monitoring. Journal of hypertension. 2013;31:1731–68. [DOI] [PubMed] [Google Scholar]
- 2.Franklin SS and Wong ND. The complexity of masked hypertension: diagnostic and management challenges. Curr Hypertens Rep. 2014;16:474. [DOI] [PubMed] [Google Scholar]
- 3.Veerabhadrappa P, Diaz KM, Feairheller DL, Sturgeon KM, Williamson ST, Crabbe DL, Kashem AM and Brown MD. Endothelial-dependent flow-mediated dilation in African Americans with masked-hypertension. American journal of hypertension. 2011;24:1102–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mezick EJ, Hall M and Matthews KA. Sleep duration and ambulatory blood pressure in black and white adolescents. Hypertension. 2012;59:747–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alwan H, Pruijm M, Ponte B, Ackermann D, Guessous I, Ehret G, Staessen JA, Asayama K, Vuistiner P, Younes SE, Paccaud F, Wuerzner G, Pechere-Bertschi A, Mohaupt M, Vogt B, Martin PY, Burnier M and Bochud M. Epidemiology of masked and white-coat hypertension: the family-based SKIPOGH study. PloS one. 2014;9:e92522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banegas JR, Ruilope LM, de la Sierra A, de la Cruz JJ, Gorostidi M, Segura J, Martell N, Garcia-Puig J, Deanfield J and Williams B. High prevalence of masked uncontrolled hypertension in people with treated hypertension. European heart journal. 2014;35:3304–12. [DOI] [PubMed] [Google Scholar]
- 7.Lehmann MV, Zeymer U, Dechend R, Kaiser E, Hagedorn I, Deeg E, Senges J and Schmieder RE. Ambulatory blood pressure monitoring: is it mandatory for blood pressure control in treated hypertensive patients?: prospective observational study. Int J Cardiol. 2013;168:2255–63. [DOI] [PubMed] [Google Scholar]
- 8.Franklin SS, Thijs L, Li Y, Hansen TW, Boggia J, Liu Y, Asayama K, Bjorklund-Bodegard K, Ohkubo T, Jeppesen J, Torp-Pedersen C, Dolan E, Kuznetsova T, Stolarz-Skrzypek K, Tikhonoff V, Malyutina S, Casiglia E, Nikitin Y, Lind L, Sandoya E, Kawecka-Jaszcz K, Filipovsky J, Imai Y, Wang J, Ibsen H, O’Brien E, Staessen JA and International Database on Ambulatory blood pressure in Relation to Cardiovascular Outcomes I. Masked hypertension in diabetes mellitus: treatment implications for clinical practice. Hypertension. 2013;61:964–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agarwal R, Pappas MK and Sinha AD. Masked Uncontrolled Hypertension in CKD. Journal of the American Society of Nephrology : JASN. 2016;27:924–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agarwal R and Pappas MK. Delayed systolic blood pressure recovery following exercise as a mechanism of masked uncontrolled hypertension in chronic kidney disease. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2016;32(10):1710–1717. [DOI] [PubMed] [Google Scholar]
- 11.Cha RH, Lee H, Lee JP, Kang E, Song YR, Kim YS and Kim SG. Changes of blood pressure patterns and target organ damage in patients with chronic kidney disease: results of the APrODiTe-2 study. Journal of hypertension. 2017;35:593–601. [DOI] [PubMed] [Google Scholar]
- 12.Pogue V, Rahman M, Lipkowitz M, Toto R, Miller E, Faulkner M, Rostand S, Hiremath L, Sika M, Kendrick C, Hu B, Greene T, Appel L, Phillips RA, African American Study of Kidney D and Hypertension Collaborative Research G. Disparate estimates of hypertension control from ambulatory and clinic blood pressure measurements in hypertensive kidney disease. Hypertension. 2009;53:20–7. [DOI] [PubMed] [Google Scholar]
- 13.Giordano U, Matteucci MC, Calzolari A, Turchetta A, Rizzoni G and Alpert BS. Ambulatory blood pressure monitoring in children with aortic coarctation and kidney transplantation. J Pediatr. 2000;136:520–3. [DOI] [PubMed] [Google Scholar]
- 14.Ferraris JR, Ghezzi L, Waisman G and Krmar RT. ABPM vs office blood pressure to define blood pressure control in treated hypertensive paediatric renal transplant recipients. Pediatr Transplant. 2007;11:24–30. [DOI] [PubMed] [Google Scholar]
- 15.Sberro-Soussan R, Rabant M, Snanoudj R, Zuber J, Bererhi L, Mamzer MF, Legendre C and Thervet E. Home and office blood pressure monitoring in renal transplant recipients. J Transplant. 2012;2012:702316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wohlfahrt P, Cifkova R, Movsisyan N, Kunzova S, Lesovsky J, Homolka M, Soska V, Bauerova H, Lopez-Jimenez F and Sochor O. Threshold for diagnosing hypertension by automated office blood pressure using random sample population data. Journal of hypertension. 2016;34:2180–6. [DOI] [PubMed] [Google Scholar]
- 17.Ohkubo T, Kikuya M, Metoki H, Asayama K, Obara T, Hashimoto J, Totsune K, Hoshi H, Satoh H and Imai Y. Prognosis of “masked” hypertension and “white-coat” hypertension detected by 24-h ambulatory blood pressure monitoring 10-year follow-up from the Ohasama study. J Am Coll Cardiol. 2005;46:508–15. [DOI] [PubMed] [Google Scholar]
- 18.Bobrie G, Clerson P, Menard J, Postel-Vinay N, Chatellier G and Plouin PF. Masked hypertension: a systematic review. Journal of hypertension. 2008;26:1715–25. [DOI] [PubMed] [Google Scholar]
- 19.Lurbe E, Thijs L, Torro MI, Alvarez J, Staessen JA and Redon J. Sexual dimorphism in the transition from masked to sustained hypertension in healthy youths. Hypertension. 2013;62:410–4. [DOI] [PubMed] [Google Scholar]
- 20.Albuminuria Agarwal R. and masked uncontrolled hypertension in chronic kidney disease. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2017;32(12):2058–2065. [DOI] [PubMed] [Google Scholar]
- 21.Ushigome E, Fukui M, Sakabe K, Tanaka M, Inada S, Omoto A, Tanaka T, Fukuda W, Atsuta H, Ohnishi M, Mogami S, Kitagawa Y, Oda Y, Yamazaki M, Hasegawa G and Nakamura N. Uncontrolled home blood pressure in the morning is associated with nephropathy in Japanese type 2 diabetes. Heart Vessels. 2011;26:609–15. [DOI] [PubMed] [Google Scholar]
- 22.Komori T, Eguchi K, Kabutoya T, Ishikawa J, Hoshide S and Kario K. Left ventricular diastolic function evaluated by the E/e’ ratio is impaired in patients with masked uncontrolled hypertension. Clin Exp Hypertens. 2014;36:538–44. [DOI] [PubMed] [Google Scholar]
- 23.Banegas JR, Ruilope LM, de la Sierra A, Vinyoles E, Gorostidi M, de la Cruz JJ, Ruiz-Hurtado G, Segura J, Rodriguez-Artalejo F and Williams B. Relationship between Clinic and Ambulatory Blood-Pressure Measurements and Mortality. N Engl J Med. 2018;378:1509–1520. [DOI] [PubMed] [Google Scholar]
- 24.Grassi G, Seravalle G, Trevano FQ, Dell’oro R, Bolla G, Cuspidi C, Arenare F and Mancia G. Neurogenic abnormalities in masked hypertension. Hypertension. 2007;50:537–42. [DOI] [PubMed] [Google Scholar]
- 25.Coulson JM. The relationship between blood pressure variability and catecholamine metabolites: a pilot study. J Hum Hypertens. 2015;29:50–2. [DOI] [PubMed] [Google Scholar]
- 26.Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. European heart journal. 1996;17:354–81. [PubMed] [Google Scholar]
- 27.Terent A and Breig-Asberg E. Epidemiological perspective of body position and arm level in blood pressure measurement. Blood pressure. 1994;3:156–63. [DOI] [PubMed] [Google Scholar]
- 28.Beckett L and Godwin M. The BpTRU automatic blood pressure monitor compared to 24 hour ambulatory blood pressure monitoring in the assessment of blood pressure in patients with hypertension. BMC cardiovascular disorders. 2005;5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wright JM, Mattu GS, Perry TL Jr, Gelferc ME, Strange KD, Zorn A and Chen Y. Validation of a new algorithm for the BPM-100 electronic oscillometric office blood pressure monitor. Blood pressure monitoring. 2001;6:161–5. [DOI] [PubMed] [Google Scholar]
- 30.Mattu GS, Heran BS and Wright JM. Overall accuracy of the BpTRU--an automated electronic blood pressure device. Blood pressure monitoring. 2004;9:47–52. [DOI] [PubMed] [Google Scholar]
- 31.Culleton BF, McKay DW and Campbell NR. Performance of the automated BpTRU measurement device in the assessment of white-coat hypertension and white-coat effect. Blood pressure monitoring. 2006;11:37–42. [DOI] [PubMed] [Google Scholar]
- 32.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG and Roccella EJ. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111:697–716. [DOI] [PubMed] [Google Scholar]
- 33.Manning DM, Kuchirka C and Kaminski J. Miscuffing: inappropriate blood pressure cuff application. Circulation. 1983;68:763–6. [DOI] [PubMed] [Google Scholar]
- 34.Myers MG, Kaczorowski J, Paterson JM, Dolovich L and Tu K. Thresholds for Diagnosing Hypertension Based on Automated Office Blood Pressure Measurements and Cardiovascular Risk. Hypertension. 2015;66:489–95. [DOI] [PubMed] [Google Scholar]
- 35.Pickering T Recommendations for the use of home (self) and ambulatory blood pressure monitoring. American Society of Hypertension Ad Hoc Panel. American journal of hypertension. 1996;9:1–11. [DOI] [PubMed] [Google Scholar]
- 36.Greven SC CM; Küchenhoff H; Peters A Restricted Likelihood Ratio Testing for Zero Variance Components in Linear Mixed Models Journal of Computational and Graphical Statistics. 2008;17:870–891. [Google Scholar]
- 37.Wielgosz A, Robinson C, Mao Y, Jiang Y, Campbell NR, Muthuri S and Morrison H. The Impact of Using Different Methods to Assess Completeness of 24-Hour Urine Collection on Estimating Dietary Sodium. Journal of clinical hypertension. 2016;18:581–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fredline VF, Taylor PJ, Dodds HM and Johnson AG. A reference method for the analysis of aldosterone in blood by high-performance liquid chromatography-atmospheric pressure chemical ionization-tandem mass spectrometry. Anal Biochem. 1997;252:308–13. [DOI] [PubMed] [Google Scholar]
- 39.Fredline VF, Kovacs EM, Taylor PJ and Johnson AG. Measurement of plasma renin activity with use of HPLC-electrospray-tandem mass spectrometry. Clin Chem. 1999;45:659–64. [PubMed] [Google Scholar]
- 40.Taylor RL and Singh RJ. Validation of liquid chromatography-tandem mass spectrometry method for analysis of urinary conjugated metanephrine and normetanephrine for screening of pheochromocytoma. Clin Chem. 2002;48:533–9. [PubMed] [Google Scholar]
- 41.Roden M, Raffesberg W, Raber W, Bernroider E, Niederle B, Waldhausl W and Gasic S. Quantification of unconjugated metanephrines in human plasma without interference by acetaminophen. Clin Chem. 2001;47:1061–7. [PubMed] [Google Scholar]
- 42.Jiang NS, Machacek D and Wadel OP. Further study on the two-column plasma catecholamine assay. Mayo Clinic proceedings. 1976;51:112–6. [PubMed] [Google Scholar]
- 43.Moyer TP, Jiang NS, Tyce GM and Sheps SG. Analysis for urinary catecholamines by liquid chromatography with amperometric detection: methodology and clinical interpretation of results. Clin Chem. 1979;25:256–63. [PubMed] [Google Scholar]
- 44.Fitzmaurice GML, N M; Ware JH. Applied Longitudinal Analysis. 2nd Edition ed: Wiley; 2011. [Google Scholar]
- 45.Edwards LJ and Simpson SL. An analysis of 24-h ambulatory blood pressure monitoring data using orthonormal polynomials in the linear mixed model. Blood pressure monitoring. 2014;19:153–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Esler M, Straznicky N, Eikelis N, Masuo K, Lambert G and Lambert E. Mechanisms of sympathetic activation in obesity-related hypertension. Hypertension. 2006;48:787–96. [DOI] [PubMed] [Google Scholar]
- 47.Facchini FS, Stoohs RA and Reaven GM. Enhanced sympathetic nervous system activity. The linchpin between insulin resistance, hyperinsulinemia, and heart rate. American journal of hypertension. 1996;9:1013–7. [DOI] [PubMed] [Google Scholar]
- 48.Lembo G, Napoli R, Capaldo B, Rendina V, Iaccarino G, Volpe M, Trimarco B and Sacca L. Abnormal sympathetic overactivity evoked by insulin in the skeletal muscle of patients with essential hypertension. The Journal of clinical investigation. 1992;90:24–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spraul M, Ravussin E and Baron AD. Lack of relationship between muscle sympathetic nerve activity and skeletal muscle vasodilation in response to insulin infusion. Diabetologia. 1996;39:91–6. [DOI] [PubMed] [Google Scholar]
- 50.Anderson EA and Mark AL. The vasodilator action of insulin. Implications for the insulin hypothesis of hypertension. Hypertension. 1993;21:136–41. [DOI] [PubMed] [Google Scholar]


