Introduction
One in six children meets body mass index (BMI) criteria for obesity.1 Children with obesity are more likely to suffer gastroesophageal reflux disease (GERD)2, a condition increasingly recognized as chronic, and one that frequently requires long-term acid-suppression therapy with proton pump inhibitors (PPIs).3 Neither clinical guidelines, nor labeling from the U.S. Food and Drug Administration (FDA), are available for dosing PPIs for children with obesity, as children with obesity are traditionally excluded from clinical trials, leaving prescribers guessing the initial drug dose and, often, increasing doses empirically, to compensate for increased body size.
In the only study of PPI pharmacokinetics in children with obesity, we recently demonstrated decreased apparent oral drug clearance (CL/F) for the PPI pantoprazole, compared to historical, pediatric or adult values, previously reported for individuals without obesity.4 The observed decrease in pantoprazole CL/F translated to higher systemic drug exposures (area under the concentration-time curve (AUC0–inf) and maximum drug concentration (Cmax)) for children with obesity, for every milligram-per-kilogram of drug received, compared to historical pediatric controls without obesity.
Growing concerns emerge regarding the long-term consequences of systemic PPI overexposure in children (e.g., pneumonia, enteric infections, vitamin/mineral deficiencies)5, with certain adverse events (e.g., osteopenia, fractures) postulated to be specifically related to high drug AUC0–inf and/or Cmax over time.6 To mitigate the risk of systemic PPI overexposure, in this follow-up, single-center, open-label investigation, we aimed to prospectively evaluate lean-body-weight-based (LBW) dosing of pantoprazole for children meeting BMI criteria for normal-weight, overweight and obese.
Methods
After an overnight fast, sixty-two children (6–17 years of age) with GERD, normal liver function, no significant comorbidities, abstaining from drugs known to induce/inhibit hepatic cytochrome P450 (CYP) 2C19 responsible for pantoprazole clearance, received a single 1.2 mg/kg oral dose of C13-pantoprazole-sodium in bicarbonate solution (Cambridge Isotopes Laboratories Inc.) based on lean body weight (LBW). C13 labeled drug was used to aid with secondary metabolite analyses (data not shown). LBW was calculated using a validated equation, the Janmahasatian equation.7 Plasma pantoprazole concentrations were measured from an indwelling venous catheter, using high performance liquid chromatography with ultraviolet detection (HPLC-UV) as previously described for PPIs8, before pantoprazole administration and at 10 time-points, over 8 hours, after pantoprazole administration. HPLC-UV Analyses were run in duplicate; lower limit of detection 0.025 uM pantoprazole. Pantoprazole pharmacokinetic (PK) profiles were generated using standard non-compartmental analysis (Kinetica v5), and PK parameters compared between groups, using Spearmann’s Correlation, Chi-Square and ANOVA (SPSS v23; α=0.05). To control for genetic variation, all participants were genotyped for CYP2C19 *2, *3, *4, and *17 alleles, using TaqMan techniques, and assigned genotype-based phenotypes as poor-metabolizer (PM; two *2,*3, or *4 no-function alleles), intermediate-metabolizer (IM; one no-function allele), normal-metabolizer (NM; two functional alleles), or ultra-rapid-metabolizer (UM; two *17 increased-function alleles).
Results/Discussion
Sixty-two children completed the study (clinicaltrials.gov NCT01887743); two participants were excluded from analysis due to incomplete sample collection. Twenty-nine children (48%) met BMI criteria for normal-weight, 16 (27%) for overweight (85–94th BMI percentile for age) and 15 (25%) for obese (≥95th percentile for age). BMI cohorts were comparable in terms of age, sex, ethnicity and CYP2C19 phenotype (Table 1). In line with population prevalence studies of CYP2C19 pharmacogenetics9, most children had CYP2C19 NM phenotypes (71%), 23% IM, 3% PM and 2% UM. As expected, PK parameters for children with PM/UM phenotypes were significantly different from the majority (95%) of the population (data not shown); therefore, children with PM/UM phenotypes were excluded from PK comparisons between BMI cohorts.
Table 1.
Demographic and pharmacokinetic (PK) characteristics for 57 children with the most commonly encountered CYP2C19 phenotypes, normal-metabolizer (NM) and intermediate- metabolizer (IM).
| Normal BMI (n=28) | Overweight BMI (n=16) | Obese BMI (n=13) | p-value* | |
|---|---|---|---|---|
| Age (years) | 13.7 ± 3.8 | 14.9 ± 2.6 | 12.7 ± 3.0 | 0.2 |
| BMI percentile for age | 62.0 ± 18.2 | 89.3 ± 2.5 | 97.7 ± 1.8 | 0.0001 |
| BMI z-score | 0.3 ± 0.5 | 1.3 ± 0.2 | 2.1 ± 0.4 | 0.0001 |
| Total Body Weight (TBW; kg) | 51.2 ± 17.5 | 65.9 ± 11.8 | 77.5 ± 28.7 | 0.0001 |
| Lean Body Weight (LBW; kg) | 38.8 ± 13.5 | 44.0 ± 9.6 | 48.1 ± 16.1 | 0.1 |
| Female | 50% | 69% | 60% | 0.2 |
| CYP2C19 NM | 82% | 75% | 69% | 0.3 |
| CYP2C19 IM | 18% | 25% | 31% | 0.3 |
| Ethnicity | ||||
| Caucasian | 72% | 88% | 53% | 0.2 |
| African American | 21% | 6% | 20% | 0.2 |
| Hispanic | 7% | 0 | 20% | 0.2 |
| Mixed | 0 | 6% | 7% | NA |
| Observed PK Parameters | ||||
| t1/2 (h) | 0.87 ± 0.3 | 1.04 ± 0.37 | 0.95 ± 0.32 | 0.7 |
| Tmax (h) | 0.73 ± 0.29 | 0.75 ± 0.26 | 0.54 ± 0.14 | 0.07 |
| Cmax (mcg/mL) | 1.81 ± 0.72 | 1.94 ± 0.71 | 2.68 ± 0.98 | 0.005 |
| AUC0–inf (mcg* h/mL) | 3.03 ± 1.92 | 3.25 ± 1.63 | 4.33 ± 2.61 | 0.2 |
| CL/F (L/h) | 20.4 ± 14.2 | 18.7 ± 7.43 | 16.8 ± 8.55 | 0.6 |
| Vd/F (L) | 30.1 ± 21.5 | 30.2 ± 12.3 | 22.8 ± 10.1 | 0.4 |
| Adjusted PK Parameters | ||||
| adjCmax (mcg/mL per mg/kg TBW) | 2.03 ± 0.92 | 2.46 ± 0.93 | 3.52 ± 1.13 | 0.0001 |
| adjAUC0–inf (mcg* h/mL per mg/kg TBW) | 3.45 ± 2.48 | 4.23± 2.61 | 5.82 ± 3.86 | 0.04 |
| adjCL/F (L/h/kg TBW) | 0.42 ± 0.27 | 0.29 ± 0.12 | 0.23 ± 0.13 | 0.03 |
| adjVd/F (L/kg TBW) | 0.59 ± 0.36 | 0.46 ± 0.2 | 0.3 ± 0.1 | 0.008 |
PK parameters are reported for single oral dose administration of pantoprazole, 1.2mg/kg lean body weight (LBW), as values unadjusted (i.e., raw/observed) and adjusted (adj) for total body weight (TBW);
p-values provided for comparisons between children with normal weight vs. obesity.
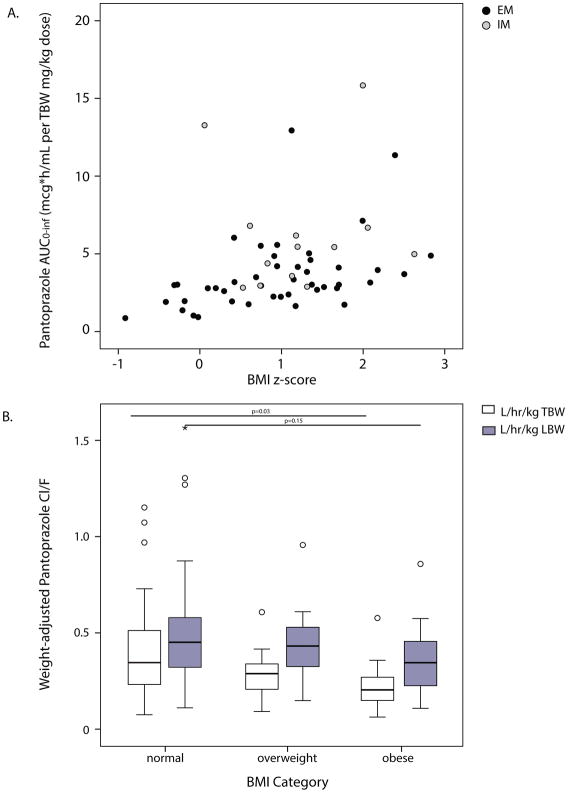
A statistically significant, positive correlation was observed between pantoprazole AUC0–inf and BMI (r=0.44, p=0.01; Figure 1A). Adjusted for milligrams of drug received per kilogram total body weight (TBW), children with higher BMIs achieved higher systemic exposures to pantoprazole and slower drug CL/F, with TBW-adjusted CL/F reduced 50% in children with obesity vs. children with normal BMIs for age (0.23±0.13 vs. 0.42±0.27 L/h/kg TBW, p=0.03; Figure 1B). LBW-dosing compensated for this reduction in CL/F (p=0.15; Figure 1B), achieving comparable systemic pantoprazole exposures (AUC0–inf) for children with normal, overweight and obese BMIs (Table 1). Differences in pantoprazole Cmax remained, using LBW-dosing (Table 1); however, this likely reflects a limitation of our sampling strategy, which did not capture the true Tmax or Cmax of the immediate-release pantoprazole formulation for children with normal BMI’s nor those with BMI’s meeting criteria for obesity/overweight.
Figure 1.
Pantoprazole systemic exposure (AUC0–inf; A) and apparent oral drug clearance (CL/F; B), normalized for drug dose received and total body weight (TBW) or lean body weight (LBW), in children (n=57) with the most commonly-encountered CYP2C19 phenotypes, normal metabolizer (NM) and intermediate metabolizer (IM); weight status defined by body mass index (BMI) for age. (A) Spearmann’s correlation (r=0.44, p=0.01); (B) p-values provided for comparison between children with normal weight vs. obesity; open circles represent outliers 1.5x the interquartile range (IQR), asterisk 3x IQR.
To achieve comparable systemic PPI exposures for children with and without obesity, we recommend using LBW, rather than TBW-based dosing for pantoprazole. Based on our findings, we are using population-based pharmacokinetic modeling approaches to evaluate whether the current, FDA-approved, weight-tiered pantoprazole dosing indications, which are based on TBW (e.g., 20 mg oral pantoprazole daily if TBW 15–40kg and 40mg if TBW >40kg), are appropriate for children with obesity.
Acknowledgments
VS, JSL and GLK conceived the study. VS, JW, RP and AG carried out the study experiments. VS and SAR analyzed the data. VS, SAR, CF, JSL and GLK interpreted the data. All authors were involved in writing the manuscript and had final approval of the submitted and published versions.
The authors gratefully acknowledge Anil Modak, PhD (Cambridge Isotopes Laboratories; Cambridge, MA) for providing the study drug.
Footnotes
Conflicts of Interest:
This study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Training Program in Pediatric/Developmental Clinical Pharmacology (1T32HD069038-05; GLK, PI) and a Marion Merrell Dow Clinical Scholar Award from The Children’s Mercy Hospital (VS and CF, co-PIs).
VS, JW and GLK received salary support from the NICHD Pediatric Trials Network (NICHD-2012-PAN01).
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States. JAMA Pediatr. 2013;311:06–14. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koebnick C, Getahun D, Smith N, Porter AH, Der-Sarkissian JK, Jacobsen SJ. Extreme childhood obesity is associated with increased risk for gastroesophageal reflux disease in a large population-based study. Int J Pediatr Obes. 2011;6:e267–63. doi: 10.3109/17477166.2010.491118. [DOI] [PubMed] [Google Scholar]
- 3.Gold B. Gastroesophageal reflux disease: could intervention in childhood reduce the risk of later complications? Am J Med. 2004;5(suppl 1):23–29. doi: 10.1016/j.amjmed.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 4.Shakhnovich V, Smith PB, Guptill JT, et al. Obese children require lower doses of pantoprazole than nonobese peers to achieve equal systemic drug exposures. J Pediatr. 2018;193:102–8. doi: 10.1016/j.jpeds.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stark CM, Cade MN. Side effects and complications of proton pump inhibitors: a pediatric perspective. J Pediatr. 2016;168:16–22. doi: 10.1016/j.jpeds.2015.08.064. [DOI] [PubMed] [Google Scholar]
- 6.Dubcenco E, Beers-Block PM, Kim L, et al. A proton pump inhibitor in the reformulation setting: bioequivalence and potential implications for long-term safety. Clin Transl Sci. 2017;10:387–94. doi: 10.1111/cts.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janmahasatian S, Duffull SB, Ash S, Ward LC, Byrne NM, Green B. Quantification of lean bodyweight. Clin Pharmacokinet. 2005;44:1051–65. doi: 10.2165/00003088-200544100-00004. [DOI] [PubMed] [Google Scholar]
- 8.Aoki I, Okummura M, Yashiki T. High-performance liquid chromatographic determination of lansoprazole and its metabolite in human serum and urine. J Chromatogr A. 1991;571:283–90. doi: 10.1016/0378-4347(91)80457-n. [DOI] [PubMed] [Google Scholar]
- 9.PharmGKB and CPIC. Stanford University; 2017. [11 Jan 2017]. Gene-specific information tables for CYP2C19. https://www.pharmgkb.org/page/cyp2c19RefMaterials. [Google Scholar]