Abstract
Rationale:
The mechanisms driving athero-thrombotic risk in individuals with JAK2V617F (Jak2VF) positive clonal hematopoiesis (CH) or myeloproliferative neoplasms (MPN) are poorly understood.
Objective:
The goal of this study was to assess atherosclerosis and underlying mechanisms in hypercholesterolemic mice with hematopoietic Jak2VF expression.
Methods and Results:
Irradiated low-density lipoprotein receptor knockout (Ldlr−/−) mice were transplanted with bone marrow from WT or Jak2VF mice and fed a high fat high cholesterol Western diet (WD). Hematopoietic functions and atherosclerosis were characterized. After 7 weeks of WD Jak2VF mice showed increased atherosclerosis. Early atherosclerotic lesions showed increased neutrophil adhesion and content, correlating with lesion size. After 12 weeks of WD Jak2VF lesions showed increased complexity, with larger necrotic cores, defective efferocytosis, prominent iron deposition and co-staining of erythrocytes and macrophages suggesting erythrophagocytosis. Jak2VF erythrocytes were more susceptible to phagocytosis by WT macrophages and showed decreased surface expression of CD47, a “don’t eat me” signal. Human JAK2VF erythrocytes were also more susceptible to erythrophagocytosis. Jak2VF macrophages displayed increased expression and production of pro-inflammatory cytokines and chemokines, prominent inflammasome activation, increased p38 MAP kinase signaling and reduced levels of MerTK, a key molecule mediating efferocytosis. Increased erythrophagocytosis also suppressed efferocytosis.
Conclusions:
Hematopoietic Jak2VF expression promotes early lesion formation and increased complexity in advanced atherosclerosis. In addition to increasing hematopoiesis and neutrophil infiltration in early lesions, Jak2VF caused cellular defects in erythrocytes and macrophages, leading to increased erythrophagocytosis but defective efferocytosis. These changes promote accumulation of iron in plaques and increased necrotic core formation which, together with exacerbated pro-inflammatory responses, likely contribute to plaque instability.
Keywords: Atherosclerosis, efferocytosis, erythrophagocytosis, inflammasome activation, JAK2V617F, myeloproliferation, hypercholesterolemia, coronary heart disease, clonal hematopoiesis
INTRODUCTION
Myeloproliferative neoplasms (MPNs) including essential thrombocytosis, polycythemia vera (PV) and primary myelofibrosis, present as clonal expansions of one or more myeloid lineages.1 In 2005 several groups identified somatic JAK2V617F (JAK2VF) mutations in ≈95% of PV patients and in ≈50–60% of essential thrombocytosis and primary myelofibrosis patients.2–6 The mutation activates JAK2 (Janus kinase 2) and downstream signaling pathways7, 8 leading to proliferation of hematopoietic stem and progenitor cells (HSPCs). MPN patients are at significantly increased risk of athero-thrombotic events, including cardiac ischemic events and thrombotic stroke.9 More recently, DNA sequencing of subjects in the general population has shown that more than 10% of people aged 70 or more have clones of blood cells bearing mutations that have been associated with hematological malignancies, primarily loss of function variants in epigenetic modifiers TET2, ASXL1, DNTM3A, as well as JAK2VF. While clonal hematopoiesis (CH) was associated with an increased risk of hematological malignancies, unexpectedly there was also a 2–3 fold increase in the risk of atherosclerotic cardiovascular disease (CVD), identifying CH as a major risk factor for CVD in the elderly. Moreover, the prevalence of CH increases from age 40 onward and CH mutations increase the risk of early onset myocardial infarction (<50 years old) by 4 fold. Although less common than the epigenetic modifier variants, the increase in risk appears to be strongest for the JAK2VF variant (12-fold increase in CVD). While the association of CH with atherosclerosis in human populations could be confounded by aging, a causal relationship between TET2 deficiency and atherosclerosis was shown in mouse models with pan-hematopoietic or myeloid TET2 deficiency, and increased macrophage inflammation was implicated as an underlying mechanism.10, 11 Whether the same or different atherogenic mechanisms are involved in the effects of other CH mutations is not known. In this study we have assessed atherosclerosis in mice with hematopoietic Jak2VF expression and explored the underlying mechanisms.
METHODS
The authors declare that all supporting data, analytical methods and materials developed from this group within the article and its online supplementary files are available.
A detailed description of methods and materials is provided in the Online Data Supplement.
RESULTS
Increased atherosclerosis in Jak2VF mice.
To assess the impact of hematopoietic Jak2VF expression on atherosclerosis, sub-lethally irradiated WT or Ldlr−/− mice were transplanted with WT or Jak2VF expressing bone marrow (BM) cells.12 To assess a possible interaction of the mutation with hypercholesterolemia, WT recipients were fed a chow diet (CD) and Ldlr−/− recipients were fed WD. While WT recipients fed the chow diet remained normocholesterolemic, WD feeding caused progressive hypercholesterolemia in Ldlr−/− recipients (Online Figure IA). However, the increase in plasma cholesterol caused by the WD was less pronounced in the Ldlr−/− mice receiving Jak2VF BM (≈30% lower after 7 weeks, p<0.01), reflecting reduced VLDL+LDL cholesterol levels as shown elsewhere13, which may reflect increased uptake of LDL by expanded myeloid cells14 or inflammatory cytokine effects on hepatic production.15 Plasma HDL cholesterol in WD-fed mice (Online Figure IB) or plasma total cholesterol in CD-fed mice (Online Figure IC) showed no change. Plasma triglyceride (TG) levels were also decreased in WD-fed Jak2VF recipients (Online Figure ID). Relative to the WT recipients, Jak2VF recipients displayed expansion of HSPCs, erythrocyte (ERP) and megakaryocyte (MKP) progenitors in BM (Online Figure IE) and marked erythrocytosis, thrombocytosis and neutrophilia (Online Figure IF through IJ), as reported12, on both chow and WD diets. There was marked erythrocyte microcytosis and anisocytosis (Online Figure IIA and IIB). Jak2VF also markedly increased platelet/monocyte and platelet/neutrophil aggregates (Online Figure IIC and IID), likely reflecting increased platelet and leukocyte counts and increased platelet activation as evidenced by increased surface P selectin presentation in the basal or PAR4 agonist (AYPGKF)-stimulated state (Online Figure IIE and IIF). The increases in HSPC counts (Online Figure IE), neutrophilia (Online Figure IJ), platelet/monocyte and platelet/neutrophil aggregates (Online Figure IIC and IID), platelet surface P-selectin (Online Figure IIE) in Jak2VF were significantly more pronounced on the WD (p<0.05) as assessed by 2-way ANOVA and Sidak’s post-hoc test for multiple comparisons.
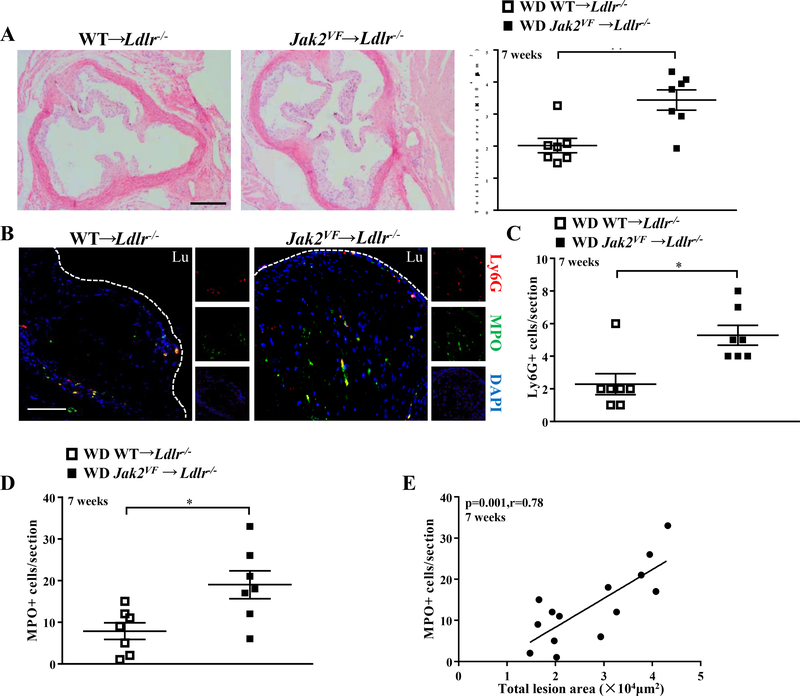
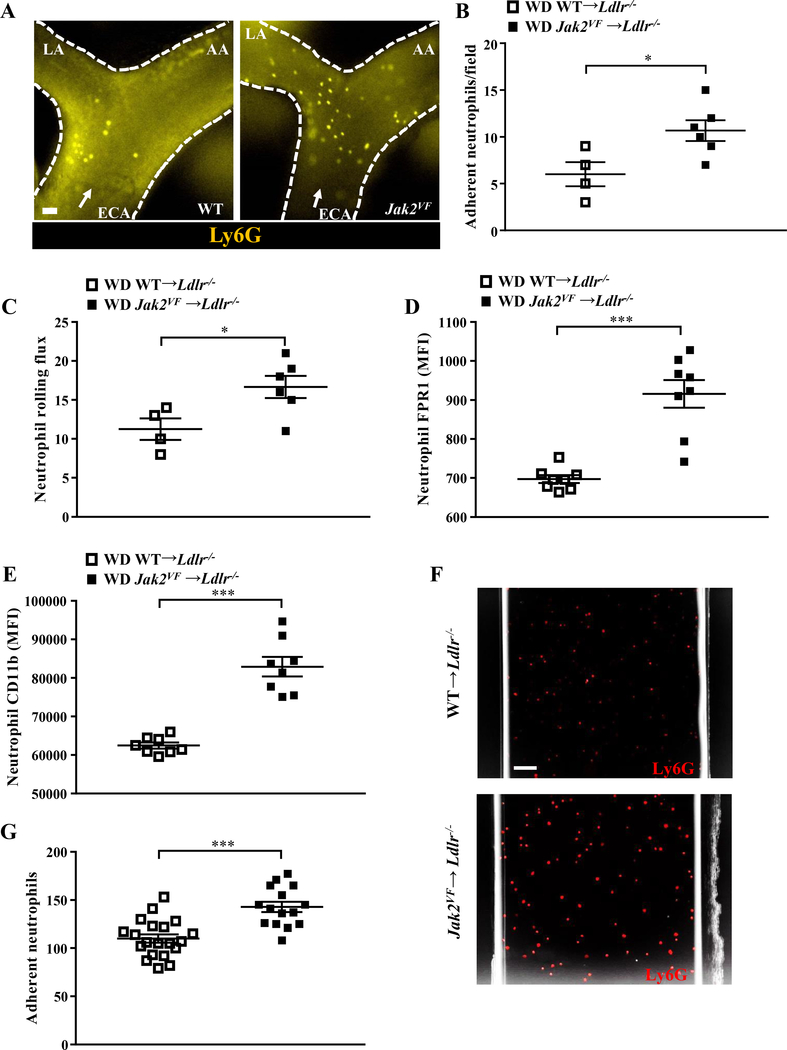
Despite the lower plasma cholesterol levels, atherosclerotic lesion size in the aortic root was increased by 1.6 fold in Jak2VF recipients fed WD for 7 weeks (Figure 1A), indicating a potent pro-atherogenic impact of Jak2VF. Consistent with the pronounced neutrophilia, there was a marked increase in neutrophils in early lesions of Jak2VF recipients (shown by Ly6G staining; Figure 1B and 1C; Online Figure IIIA) while macrophage content was unchanged (Online Figure IIIB). Lesional MPO, another neutrophil marker which largely overlapped with the Ly6G marker in lesional cells (Figure 1B), was also markedly increased in early lesions of Jak2VF recipients (Figure 1D) and correlated with lesion size (Figure 1E), consistent with a pro-atherogenic role of neutrophils in early atherogenesis.16 Intravital fluorescence microscopy showed a marked increase in neutrophil rolling and firm adhesion on early carotid artery lesions in Jak2VF recipients (Figure 2A through 2C); monocyte rolling but not adhesion was significantly increased (Online Figure IIIC and IIID). Neutrophils from WD-fed Jak2VF recipients showed evidence of activation with increased expression of formyl peptide receptor 1 (FPR1) and adhesion molecule CD11b (Figure 2D and 2E). Neutrophils displayed increased adhesion to recombinant cell adhesion molecules (Figure 2F and 2G). Thus, in addition to neutrophilia, neutrophil activation likely contributed to increased adhesion and entry of neutrophils into early atherosclerotic plaques.
Figure 1: Increased early atherosclerotic lesions and neutrophil infiltration in Jak2VF mice.
(A) Representative H&E-stained aortic root lesions and quantification of total lesion area of female Ldlr−/− recipients after 7 weeks of WD. Mann-Whitney U test. Scale bar, 500μm. (B) Representative immunofluorescence images of MPO (Green) and Ly6G (Red) with DAPI (Blue) of aortic root lesions from female mice fed WD for 7 weeks. Mann-Whitney U test. Scale bar,100μm. (C) Quantification of Ly6G positive cells (Mann-Whitney U test) and (D) MPO positive cells in the lesions. Unpaired t test. (E) Correlation between MPO+ neutrophils and total lesion size after 7 weeks of WD. *p<0.05, **p<0.01. Spearman correlation test.
Figure 2: Increased rolling and adhesion of neutrophils in Jak2VF mice.
Female Ldlr−/− recipients were fed WD for 5 weeks. (A) Representative image of epifluorescence intravital microscopy of the carotid artery showing interaction of Ly6G-stained neutrophils with the arterial vessel wall. ECA, external carotid artery; LA, lingual artery; AA, auricular artery. Arrow indicates flow direction. Scale bar, 50μm. (B) Quantification of Ly6G-stained neutrophil adhesion in the carotid artery by intravital microscopy. Mann-Whitney U test. (C) Neutrophil rolling flux was assessed by intravital microscopy. Expression of (D) FPR1 and (E) CD11b MFI on neutrophils were measured by flow cytometry. Mann-Whitney U test. (F,G) Flow chamber assays for neutrophil adhesion using equal number of neutrophils. (F) Representative images and (G) quantification of adhesion. Mann-Whitney U test. Scale bar, 50μm. *p<0.05, ***p<0.001.
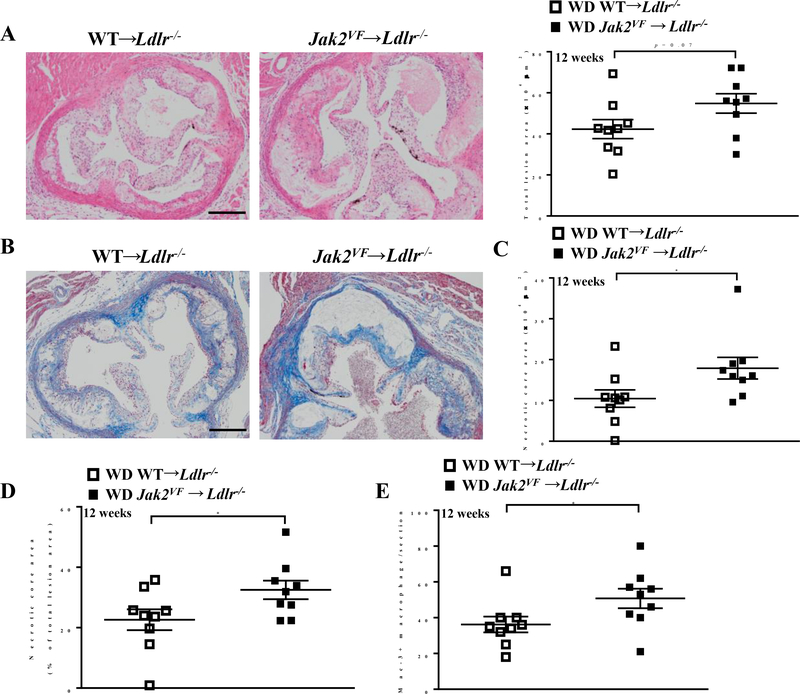
In mice fed the WD for 12 weeks, plasma total cholesterol and TG levels were ≈50% lower in Jak2VF recipients (Online Figure IVA and IVB). Despite the markedly reduced cholesterol and TG levels, lesion size showed a trend to be increased (1.3 fold, p=0.07) in the Jak2VF recipients (Figure 3A). Necrotic core area, a well-established index of plaque instability17, was significantly increased, both in absolute terms (area of necrotic core per section, 1.7 fold increase) or relative to lesion size (% of total lesion area, 1.4 fold increase) (Figure 3B though 3D) in Jak2VF recipients compared to controls. Lesional macrophages (Figure 3E) but not neutrophils (Online Figure IVC) were increased in advanced lesions of Jak2VF recipients. Unlike in early lesions, lesional neutrophil count showed no significant correlation with lesion area in advanced lesions (Online Figure IVD). Collagen content and fibrous cap thickness did not show differences between the genotypes in advanced lesions (Online Figure IVE).
Figure 3: Increased necrotic core in advanced lesions of Jak2VF mice.
(A) Representative H&E-stained aortic root lesions and quantification of total lesion area of female Ldlr−/− recipients after 12 weeks of WD. Unpaired t test. Scale bar, 500μm. (B) Representative massion trichrome stain images of lesions. Scale bar, 500μm. (C) Quantification of necrotic core area and (D) as a percentage of total lesion area of female Ldlr−/− recipients after 12 weeks WD. Mann-Whitney U test. (E) Quantification of Mac-3+ macrophage in the lesions of female mice WD-fed for 12 weeks. Mann-Whitney U test. *p<0.05.
Jak2VF increases lesional erythrophagocytosis in advanced atherosclerosis.
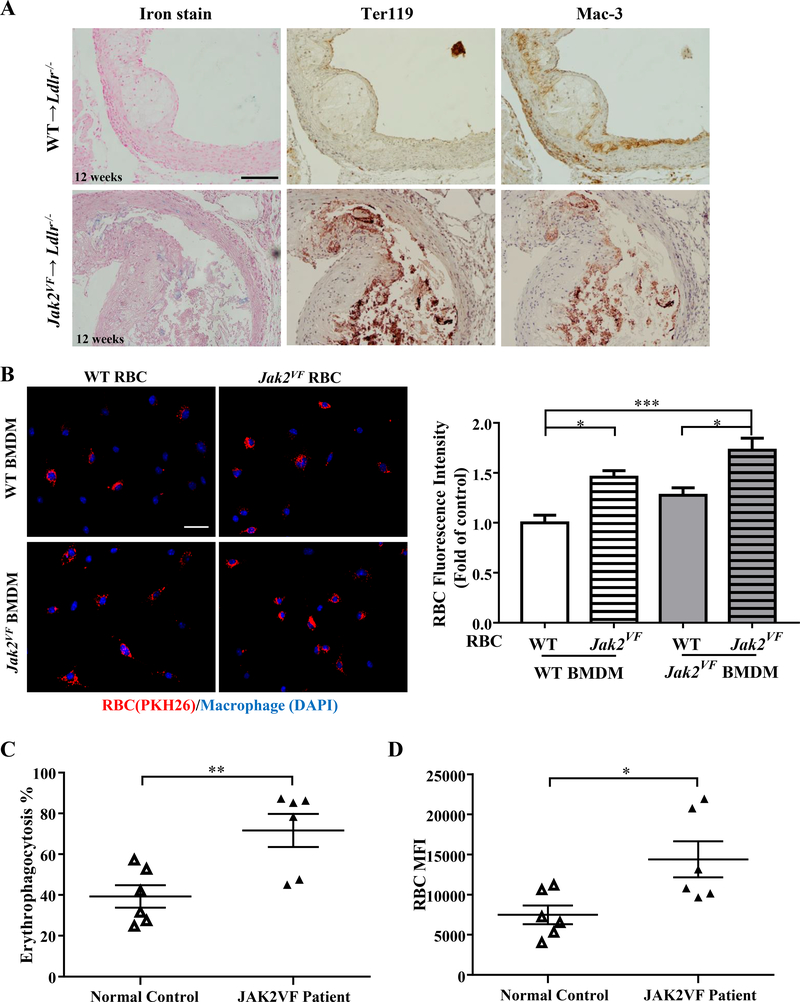
Increased hematocrit as well as abnormalities in RBC morphology in Jak2VF mice (Online Figure IF; Online Figure IIA and IIB) suggested a possible role of erythrocytes in atherosclerosis.18 To assess this, we stained lesions for iron and erythrocytes. While WT recipients showed little lesional iron deposition, iron staining was clearly identified in the majority of Jak2VF recipients in advanced lesions (Figure 4A, Online Figure IVF, p<0.05, Chi-Squared Test). Staining of Ter119, a specific erythrocyte marker, was also increased in advanced lesions of Jak2VF recipients (Figure 4A, Online Figure IVG, p<0.05, Chi-Squared Test). Interestingly, when the Ter119 positive sections were stained with antibodies against the macrophage marker Mac-3, the two markers were largely co-localized (Figure 4A), suggesting erythrophagocytosis. Notably, macrophages containing erythrocyte markers were primarily found on the periphery of necrotic cores (Figure 4A), particularly in 7 week early lesions (Online Figure VA).
Figure 4: Jak2VF mice displayed marked increase in erythrophagocytosis.
(A) Representative images of iron staining, immunohistochemistry staining of red blood cell marker Ter119 and macrophage marker Mac-3 in lesions of female Ldlr−/− recipients after 12 weeks WD. Scale bar, 100μm. (B) Bone marrow derived macrophages (DAPI, Blue) were incubated with 2 million PKH26-labeled erythrocytes (Red) overnight and quantification of relative red blood cells fluorescence intensity. Cells from both male and female mice were used for the assays. Pooled data from 5 independent experiments were used for analysis. Scale bar, 50μm. 2-way ANOVA. (C) Erythrophagocytosis rate (Mann-Whitney U test) and (D) red blood cells MFI of human normal control and JAK2VF patients were measured by flow cytometry. Unpaired t test. *p<0.05, **p<0.01,***p<0.001.
To assess potential pathways for entry of RBCs into lesions, we assessed Von Willebrand Factor staining. However, there was little staining in advanced lesions and no difference between Jak2VF and WT recipients (not shown). We did detect a marked increase in erythrocyte/neutrophil and erythrocyte/monocyte complexes in the circulation in Jak2VF recipients (Online Figure VB), suggesting the possibility that erythrocyte/leukocyte complex formation facilitates RBC entry via the luminal surface of plaques.
Jak2VF erythrocytes are more susceptible to erythrophagocytosis.
To gain insights into the mechanism of erythrophagocytosis in lesions, we incubated WT or Jak2VF erythrocytes with WT or Jak2VF macrophages and assessed erythrophagocytosis by fluorescence microscopy. Jak2VF erythrocytes showed increased uptake by either WT or Jak2VF macrophages (Figure 4B). Erythrophagocytosis rather than surface binding of erythrocytes by phagocytes was confirmed by reconstructed 3D images obtained by fluorescence confocal microscopy as well as overlay on bright field images (Online Figure VIA and VIB). To assess whether the findings of aberrant erythrophagocytosis with mouse Jak2VF erythrocytes could be recapitulated with human erythrocytes, we obtained human blood samples from JAK2VF positive MPN patients and matched control human subjects. These patients were newly identified, non-treated or being treated with aspirin or phlebotomy but not with hydroxyurea or ruxolitinib. Incubation of human erythrocytes with human macrophages derived from peripheral blood mononuclear cells of healthy human subjects also resulted in robust erythrophagocytosis (Online Figure VIC). Quantification of erythrophagocytosis by flow cytometry indicated increased erythrophagocytosis of JAK2VF erythrocytes compared to control erythrocytes (Figure 4C and 4D).
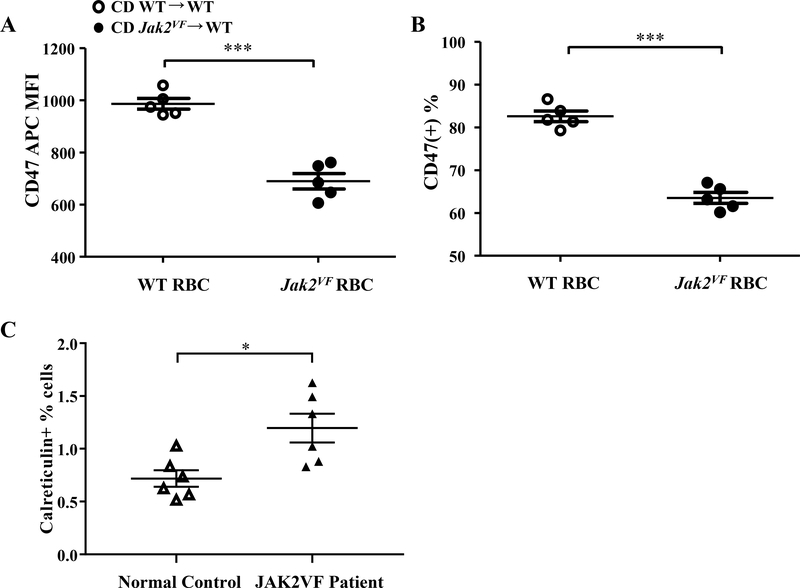
Decreased CD47, a “don’t eat me signal” or increased calreticulin, an “eat me” signal, in Jak2VF erythrocytes.
Accelerated erythrocyte aging has been proposed to explain increased erythrophagocytosis in some erythrocytosis models.19 Increased erythrocyte band 4.1a/4.1b ratio has been used as a marker for erythrocyte senescence.19 Band 4.1a/4.1b ratio in mouse Jak2VF erythrocytes was not altered relative to the WT erythrocytes (Online Figure VID), suggesting no change of erythrocyte senescence. However, we noticed that band 4.2 was markedly decreased in Jak2VF erythrocytes (Online Figure VID and VIE). Band 4.2 deficiency has been linked to accelerated erythrocyte clearance and marked reduction of CD4720,21, a molecule protecting erythrocytes from fortuitous phagocytosis by macrophages.22 We assessed erythrocyte surface CD47 levels by flow cytometry and found a significant reduction in Jak2VF erythrocytes (Figure 5A and 5B). In contrast, human Jak2VF erythrocytes did not show a decrease in surface CD47 (not shown) but rather displayed increased surface calreticulin (Figure 5C), consistent with a recent report.23 Surface calreticulin counteracts CD47 signaling and promotes phagocytosis of erythrocytes.24 These results suggest distinct mechanisms promoting erythrophagocytosis of human versus mouse Jak2VF erythrocytes.
Figure 5: Jak2VF mice showed reduced surface CD47 expression in erythrocytes.
Erythrocytes were from chow-fed female mice. (A) Mean fluorescence intensity of anti-CD47 antibody bound to erythrocytes (Unpaired t test) and (B) percentage of CD47hi erythrocytes by flow cytometry. Unpaired t test. (C) Percentage of calreticulin positive erythrocytes in human normal controls and JAK2VF patients. Unpaired t test.*p<0.05, ***p<0.001.
Defective efferocytosis in advanced lesions in Jak2VF mice.
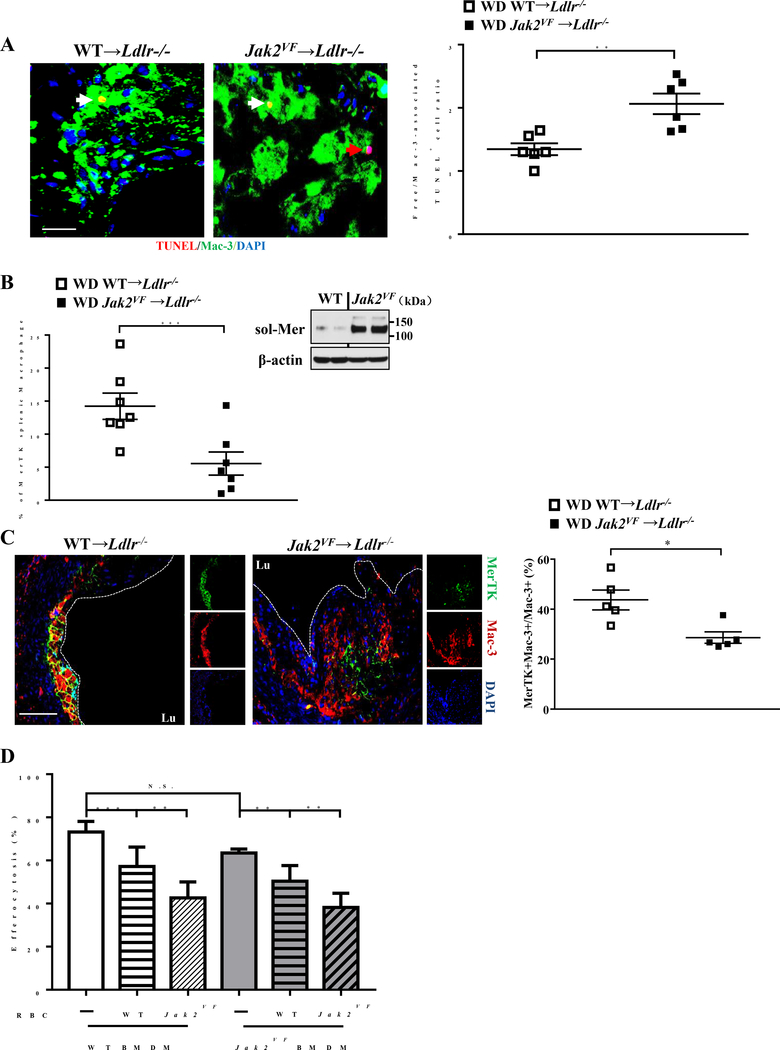
A body of work indicates that efficient efferocytosis of apoptotic cells by lesional macrophages is a key event limiting necrotic core formation in advanced atherosclerosis.25–27 The increased necrotic core in advanced lesions of Jak2VF mice led us to assess lesional efferocytosis. The ratio of free vs macrophage-associated apoptotic cells was markedly increased in advanced lesions of Jak2VF mice (Figure 6A), indicating defective efferocytosis. Macrophage c-Mer tyrosine kinase (MerTK) serves as a cell surface receptor and signaling molecule mediating efferocytosis, and MerTK has a central role in promoting efferocytosis and decreasing necrotic core formation in atherosclerotic lesions.25,28 We thus assessed surface MerTK levels in vivo in splenic macrophages and levels of soluble MerTK (sol-Mer), the product of surface MerTK cleavage, generated from the cultured macrophages of WT and Jak2VF mice. This showed increased cleavage (inset, Figure 6B) and markedly decreased cell surface MerTK levels (Figure 6B) in Jak2VF compared to WT macrophages, while plasma sol-Mer levels showed no change (Online Figure VIF). Importantly, MerTK levels in lesional macrophages were markedly decreased in advanced lesions of Jak2VF mice (Figure 6C; Online Figure VIG and VIH). Next, we assessed the potential impact of erythrophagocytosis on efferocytosis ex vivo. Co-incubation of WT or Jak2VF macrophages with erythrocytes and apoptotic Jurkat cells led to suppression of efferocytosis of Jurkat cells relative to incubation with Jurkat cells alone (Figure 6D). WT and Jak2VF macrophages did not show difference in efferocytosis. In contrast, Jak2VF erythrocytes caused a more pronounced suppression of efferocytosis in both WT and Jak2VF macrophages (Figure 6D), likely reflecting the increased susceptibility of Jak2VF erythrocytes to erythrophagocytosis. Together, these findings suggest that increased uptake of erythrocytes in combination with decreased uptake of apoptotic cells by lesional macrophages contributes to advanced lesion complexity including increased necrotic core formation in Jak2VF mice.
Figure 6: Defective efferocytosis was associated with decreased macrophage surface MerTK in Jak2VF mice.
(A) Representative images of advanced lesions (12 weeks WD-fed) in which apoptotic cells were stained by TUNEL (Red), macrophages by Mac-3 (Green) and nuclei by DAPI (Blue). Efferocytosis was assessed as the ratio of free to macrophage-associated TUNEL positive cells. The red arrow depicts free apoptotic cells and the white arrow depicts macrophage associated apoptotic cells. Scale bar, 20μm. Unpaired t test. (B) Percentage of MerTK postive macrophages in total spleen cells as determined by flow cytometry and western blot of soluble MerTK levels in cultured media of splenic macrophages. Unpaired t test. (C) Representative single (small panel) or merged fluorescence images (large panel) of Mac-3 (Red), MerTK (Green) or DAPI (Blue) and quantification of the ratio of MerTK/Mac-3 co-positive to Mac-3 positive macrophages. Scale bar, 100μm. Mann-Whitney U test. (D) Bone marrow derived WT or Jak2VF macrophages were treated with or without 5 million WT or Jak2VF erythrocytes in the presence of apoptotic Jurkat cells for 20 hours to assess efferocytosis by fluorescence microscope. Data were the representative of 5 independent experiments. 2-way ANOVA. *p<0.05, **p<0.01, ***p<0.001.
Increased inflammatory activation of Jak2VF macrophage.
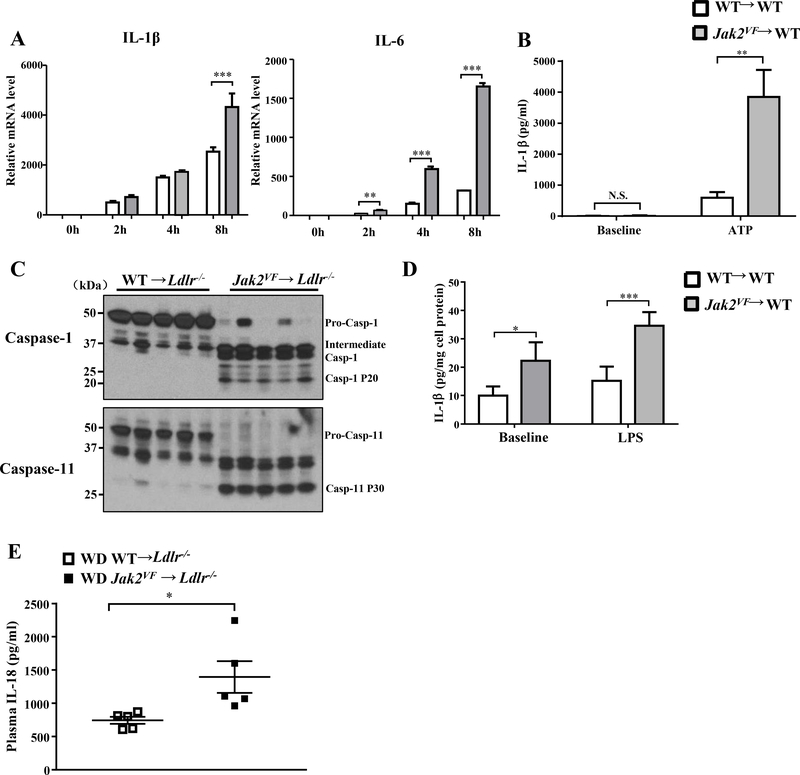
To assess inflammatory responses we challenged WT or Jak2VF macrophages with LPS, a stimulus relevant to toll-like receptor/Myeloid differentiation primary response 88 (TLR4/MyD88) and toll-like receptor/TIR-domain-containing adapter-inducing interferon-β (TLR4/TRIF) signaling pathways that are known to promote atherogenesis.29,30 Jak2VF macrophages showed increased expression of pro-inflammatory cytokines and chemokines i.e. IL-1β, IL-6, iNOS, Tnf-α and MCP-1 (Figure 7A; Online Figure IX; Online Figure XB; Online Figure XI). While IL-6 secretion from Jak2VF macrophages was markedly increased, the increase in IL-1β secretion following 8 hour LPS stimulation was only moderate (Online Figure VIIA and VIIB), and less pronounced than the increase in IL-1β mRNA levels (Figure 7A). Prominent IL-1β secretion requires inflammasome activation.31 Indeed, ATP-stimulated IL-1β production from Jak2VF macrophages was increased more pronouncedly relative to wild type cells (Figure 7B), consistent with inflammasome activation. To evaluate the relevance in vivo, we examined inflammasome activation by assessing caspase 1 and caspase 11 cleavage in splenic CD11b+ or CD11b- cells. This was markedly increased in Jak2VF CD11b+ (Figure 7C) but not WT CD11b+ (Figure 7C) or Jak2VF or WT CD11b- cells (not shown), suggesting activation of both NLRP3 and non-canonical, Caspase-11 dependent macrophage inflammasomes.32 Markedly increased IL-1β production from the freshly isolated splenic Jak2VF CD11b+ cells, particularly in response to LPS, was also consistent with inflammasome activation in vivo (Figure 7D). Additional evidence showing inflammasome activation came from the finding that plasma levels of IL-18, which production depends on and is considered as a marker of inflammasome activation in vivo33, were markedly increased in Jak2VF mice (Figure 7E).
Figure 7: Jak2VF myeloid cells displayed enhance inflammasome activation.
(A) Con-A induced peritoneal macrophages were challenged with or without 10ng/ml LPS for the indicated time and qPCR analysis of mRNA level of IL-1β and IL-6. Data were from 5 independent experiments. 1-way ANOVA. (B) ELISA of IL-1β in cultured medium of bone marrow derived macrophage challenged with 10ng/ml LPS for 1 hour followed by 1mM ATP for 3 hours. Baseline was LPS (10ng/ml) only for 4 hours. 1-way ANOVA. (C) Western blot of caspase-1 and caspase-11 cleavage in splenic CD11b+ cells from female recipients WD-fed for 7 weeks. (D) ELISA of IL-1β in cultured medium of CD11b+ cells from female recipients WD-fed for 7 weeks. Cells were treated with or without 1μg/ml LPS for 8h. Data were the representative from 4 independent experiments. 2-way ANOVA. (E) ELISA of plasma IL-18 in female recipients WD-fed for 8 weeks. Unpaired t test. *p<0.05, **p<0.01, ***p<0.001.
While increased pro-inflammatory responses to LPS were consistently detected in briefly cultured concanavalin (ConA)-elicited mouse peritoneal macrophages (Figure 7A; Online Figure VIIC), the responses were less prominent in bone marrow derived macrophages (BMDM) cultured for 7 days (Online Figure VIID). ConA is known to induce T cell proliferation and interferon-γ (IFNγ) responses in vivo34 and JAK2 has an essential role in mediating IFNγ signaling.35 To explore the possibility that macrophages were primed for increased signaling in ConA-treated Jak2VF mice, we assessed multiple molecules mediating IFNγ and JAK2 signaling. Levels of phosphorylated p38, JNK and AKT in the non-LPS treated basal state were significantly increased in ConA-elicited Jak2VF macrophages, following a brief 6-hour culture in vitro (Online Figure VIIIA) and this was largely reversed after 48 hours in culture (Online Figure VIIIB). Total and phosphorylated STAT1 were markedly decreased, a finding consistent with the negative feedback regulation of STAT1 by IFNγ signaling.36 P38 and JNK are critical in mediating TLR4 initiated pro-inflammatory responses in macrophage.37,38 Inhibition of p38, JNK or combined inhibition of p38 and JNK partially or completely reversed the LPS-induced pro-inflammatory responses of ConA-elicited Jak2VF macrophages (Online Figure IX), suggesting that increased priming had a major role in the enhanced inflammatory response to LPS. We also assessed the potential impact of altered endoplasmic reticulum (ER) stress or autophagy, which are known to regulate pro-inflammatory activation of macrophages.39,40 CCAAT-enhancer-binding protein homologous protein (CHOP) expression, a marker of ER stress, showed no difference between WT and Jak2VF macrophages either in the basal or tunicamycin-induced ER stress state (Online Figure XA). Rapamycin, an inducer of autophagy, decreased the expression of some cytokines in response to LPS but the effect was proportionate for WT and Jak2VF macrophages (Online Figure XB). JAK1/2 inhibitor such as ruxolitinib has been approved as a treatment for JAK2VF positive MPN patients.41 Notably, ruxolitinib reversed the increase in pro-inflammatory cytokine and chemokine expression in Jak2VF macrophages, except for Tnf-α (Online Figure XI). In contrast, ruxolitinib failed to reverse the increased susceptibility of Jak2VF erythrocytes to erythrophagocytosis (Online Figure XC).
DISCUSSION
Our study demonstrates that hematopoietic Jak2VF expression in hypercholesterolemic mice results in accelerated atherosclerosis with features of plaque instability, consistent with the increase in athero-thrombotic cardiovascular disease seen in patients with JAK2VF-associated MPN or CH.10,11,13,42 Increased neutrophil infiltration due to neutrophilia and neutrophil activation likely accounts for accelerated early lesion formation. In contrast, advanced atherosclerotic lesions displayed increased necrotic cores, increased macrophages, iron deposition and evidence of erythrophagocytosis: similar features have been associated with atherosclerotic plaque instability in humans.18
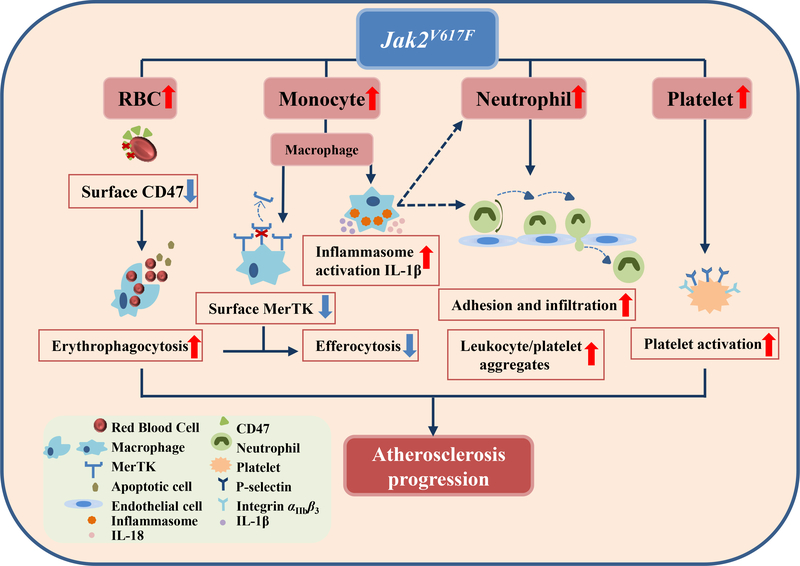
On a mechanistic level, Jak2VF macrophages displayed increased cleavage and reduced surface levels of MerTK in association with defective efferocytosis in advanced lesions, likely contributing to increased necrotic core formation (Figure 8).43,44 Jak2VF macrophages showed increased inflammatory responses including p38 map kinase activation likely promoting MerTK cleavage.45 There was marked inflammasome activation in Jak2VF macrophages leading to increased IL-1β secretion and increased IL-18 plasma levels. Increased production of macrophage inflammatory cytokines could contribute to increased neutrophil production and activation and entry of leukocytes into lesions.46,47 Augmented phagocytosis of RBC by macrophages likely reflected both increased RBC production as well as intrinsic RBC defects which were seen in both mice and humans. Erythrophagocytosis was shown to suppress efferocytosis, suggesting a mechanistic link between these two processes. Thus our studies demonstrate that the mechanisms underlying the pro-atherogenic effect of Jak2VF are multifaceted, involving different hematopoietic lineages and their interactions (Figure 8).
Figure 8: Schematic model.
Mechanisms underlying increased atherosclerosis in Jak2VF mice.
Aberrant hematopoiesis and neutrophil infiltration of lesions were associated with increased early atherosclerosis in Jak2VF mice, as reported in other models of neutrophil overproduction.16 Consistent with a major role of neutrophils, increased rolling and firm adhesion of neutrophils was shown by intravital microscopy of carotid arteries in Jak2VF mice. Platelet activation, neutrophil activation, platelet/neutrophil and platelet/monocyte aggregates which were prominently increased in Jak2VF mice are known to promote recruitment of inflammatory leukocytes into lesions.48,49 Importantly, several of these atherogenic propensities, such as basal platelet P-selectin exposure and platelet-monocyte aggregates, were augmented by an interaction of hypercholesterolemia with the Jak2VF mutation. In addition, hypercholesterolemia interacted with the Jak2VF to synergistically increase the bone marrow hematopoietic stem cell population, possibly reflecting cross-talk between JAK2VF signaling with signaling pathways that are activated by cholesterol accumulation in HSPCs.50 This raises the possibility that hypercholesterolemia could promote the evolution of CH.
Erythophagocytosis has been described as a prominent feature in complex human atherosclerotic lesions and proposed to promote macrophage foam cell formation and lesional necrotic core formation.18 Erythrocyte and macrophage markers co-localize in or around lesional necrotic cores in advanced human atherosclerotic lesions, suggesting that erythrophagocytosis may contribute to plaque instability.18 Increased erythrophagocytosis appeared to involve different mechanisms in mouse versus human RBCs – with decreased levels of CD47, a don’t eat me signal in mice and increased levels of calreticulin, a pro-phagocytic signal in human RBCs. The mechanisms responsible for increased RBC entry into lesions are uncertain but could involve the observed increase in formation of RBC-leukocyte aggregates which could carry RBCs from the arterial lumen into the subendothelial space. One limitation of the study is use of female Ldlr−/− mice only as recipients for atherosclerosis studies. Nevertheless, both male and female mice were used for in vitro assays of erythrophagocytosis, indicating that the altered erythrophagocytosis was not limited to females.
The elevated expression of multiple pro-inflammatory cytokines and chemokines in Jak2VF macrophage in response to LPS stimulation suggests that heightened inflammation also contributes to accelerated atherosclerosis in Jak2VF mice. Increased lesional inflammation could trigger cleavage of cell surface MerTK in macrophages in Jak2VF mice, leading to defective efferocytosis and increased necrotic core formation.25 Cleavage of macrophage cell surface MerTK is primarily mediated by ADAM17 and this process can be up-regulated by Toll-like receptor 4 and p38 MAP kinase signaling45 which was increased in Jak2VF macrophages. Therefore, pro-inflammatory macrophage activation in Jak2VF mice could exacerbate atherosclerosis by impaired efferocytosis via p38 MAP kinase and by pro-inflammatory cytokine and chemokine production. As previously reported32 there was no detectable inflammasome activation in Western diet fed Ldlr−/− mice transplanted with wild type bone marrow. However, there was prominent inflammasome activation in splenic CD11b+ cells which includes macrophages in Jak2VF mice. The increased production of IL-1β and possibly IL-18 could also contribute to neutrophilia and neutrophil infiltration detected in Jak2VF mice.46,47,51
Another shortcoming of our study is that it involved pan-hematopoietic Jak2VF and thus abnormalities in hematopoiesis may have contributed more prominently to atherogenesis than in a true CH model.11 Although prospectively studied CH subjects did not have abnormal blood cell counts at baseline52, qualitative changes in blood cell function, as well as development of myelo-proliferation in some patients53, appear likely. In humans carrying mutations that cause CH and increased CVD risk, the single abnormality in blood cell phenotypes was an increase in erythrocyte anisocytosis52 which was also prominent in Jak2VF mice and is a known CVD risk factor in the general population.54,55 We speculate that anisocytosis may be a marker of aberrant erythrocyte properties, possibly reflecting increased erythrocyte production or interactions between inflammatory myeloid cells and erythroblasts in the bone marrow56, that predisposes to atherosclerosis, for example by stimulation of erythhrophagocytosis in atherosclerotic lesions.
The recommended treatment for low risk PV patients includes phlebotomy and low dose aspirin.57,58 Current recommendations suggest that the hematocrit should be maintained below 45%42, as higher hematocrits are associated with increased cardiovascular death and major thrombosis in PV patients.42 Our findings suggest that Jak2VF erythrocytes may have a direct role in promoting advanced atherosclerosis and plaque instability, raising the possibility that even lower levels of hematocrit may be desirable. Finally, our findings highlight the importance of increased myelopoiesis, pro-inflammatory macrophage activation, platelet activation and PLA formation in atherogenesis, suggesting the need for effective anti-platelet and cytoreductive therapies in MPNs and perhaps in CH. Since many of the underlying atherogenic mechanisms were aggravated by hypercholesterolemia in Jak2VF mice, control of LDL cholesterol via statins and proprotein convertase subtilisin/kexin type 9 (PCSK9) mAbs may be particularly important in patients with JAK2VF-associated MPN or CH. Finally, the recent demonstration in the CANTOS trial that IL-1β antibodies reduced coronary heart disease opens a new vista on anti-inflammatory therapies as a treatment for atherosclerosis59, and our findings suggest that patients with CH or MPN may particularly benefit from this or other anti-inflammatory therapies.
Supplementary Material
NOVELTY AND SIGNIFICANCE
What Is Known?
Acquired activating mutations of JAK2 notably JAK2V617F (JAK2VF) drive development of clonal hematopoiesis (CH) and myeloproliferative neoplasms (MPN).
JAK2VF positive CH and MPN are associated with increased athero-thrombotic risk, but the underlying mechanisms are poorly understood.
What New Information Does This Article Contribute?
Hematopoietic Jak2VF expression in Ldlr−/− mice promotes neutrophil-enriched early lesion formation.
Advanced lesions show increased necrotic cores, defective efferocytosis and prominent erythrophagocytosis
Jak2VF macrophages displayed increased expression of pro-inflammatory cytokines and inflammasome activation, possibly driving neutrophil entry into lesions.
Jak2VF erythrocytes undergo increased uptake by macrophages (erythrophagocytosis), leading to impaired uptake of apoptotic cells (efferocytosis), which together with increased cleavage of MerTK, promotes necrotic core formation and plaque instability.
Jak2VF, a gain of function mutation that is commonly found in elderly patients with myeloproliferative neoplasms or clonal hematopoiesis, is associated with increased risk of athero-thrombotic diseases. In Ldlr−/− mice expressing Jak2VF in hematopoietic tissues, we showed accelerated early atherosclerosis and increased complexity of advanced lesions, despite lower levels of LDL cholesterol. Early lesions showed increased binding of neutrophils to endothelium and increased numbers of neutrophils in plaques. More advanced lesions showed increased necrotic cores, defective efferocytosis and erythrophagocytosis. Erythrophagocytosis refected a Jak2VF intrinsic red cell defect. Defective efferocytosis was linked to macrophage infammation and MerTK cleavage and to competition between red cells and apoptotic cells for macrophage uptake. These studies provide direct evidence that Jak2VF increases atherogenesis, involving different hematopoietic lineages and their interactions.
Acknowledgments
SOURCES OF FUNDING
This study was supported by the National Institutes of Health Grant RO1 HL107653 (to A. R. Tall) and RO1 HL118567 (to N. Wang). The CNIC is supported by the MCIU and the Pro CNIC Foundation, and is a Severo Ochoa Center of Excellence (MEIC award SEV-2015–0505). The Columbia University CCTI and Diabetes Research Center Flow Cytometry Cores, supported in part by the Office of the Director, NIH, under awards S10RR027050, S10OD020056 and 5P30DK063608, were used for this study.
Nonstandard Abbreviations and Acronyms:
- BM
Bone marrow
- BMDM
Bone marrow-derived macrophage
- CH
Clonal hematopoiesis
- ConA
Concanavalin A
- DAPI
4′,6-diamidino-2-phenylindole
- HSPC
hematopoietic stem and progenitor cells
- IFNγ
Interferon gamma
- JAK
Janus kinase
- LPS
Lipopolysaccharide
- MAPK
Mitogen-activated protein kinase
- MerTK
c-Mer tyrosine kinase
- MPN
Myeloproliferative neoplasms
- RBC
Red blood cell
- WD
Western diet
- WT
White type
Footnotes
DISCLOSURE
The authors disclose no conflict of interest.
REFERENCES
- 1.Campbell PJ and Green AR. The myeloproliferative disorders. The New England journal of medicine. 2006;355:2452–66. [DOI] [PubMed] [Google Scholar]
- 2.James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C, Garcon L, Raslova H, Berger R, Bennaceur-Griscelli A, Villeval JL, Constantinescu SN, Casadevall N and Vainchenker W. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–8. [DOI] [PubMed] [Google Scholar]
- 3.Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, Vassiliou GS, Bench AJ, Boyd EM, Curtin N, Scott MA, Erber WN, Green AR and Cancer Genome P. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–61. [DOI] [PubMed] [Google Scholar]
- 4.Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, Tichelli A, Cazzola M and Skoda RC. A gain-of-function mutation of JAK2 in myeloproliferative disorders. The New England journal of medicine. 2005;352:1779–90. [DOI] [PubMed] [Google Scholar]
- 5.Zhao R, Xing S, Li Z, Fu X, Li Q, Krantz SB and Zhao ZJ. Identification of an acquired JAK2 mutation in polycythemia vera. The Journal of biological chemistry. 2005;280:22788–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ, Boggon TJ, Wlodarska I, Clark JJ, Moore S, Adelsperger J, Koo S, Lee JC, Gabriel S, Mercher T, D’Andrea A, Frohling S, Dohner K, Marynen P, Vandenberghe P, Mesa RA, Tefferi A, Griffin JD, Eck MJ, Sellers WR, Meyerson M, Golub TR, Lee SJ and Gilliland DG. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer cell. 2005;7:387–97. [DOI] [PubMed] [Google Scholar]
- 7.James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C, Garcon L, Raslova H, Berger R, Bennaceur-Griscelli A, Villeval JL, Constantinescu SN, Casadevall N and Vainchenker W. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005. [DOI] [PubMed] [Google Scholar]
- 8.Lu X, Levine R, Tong W, Wernig G, Pikman Y, Zarnegar S, Gilliland DG and Lodish H. Expression of a homodimeric type I cytokine receptor is required for JAK2V617F-mediated transformation. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:18962–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Landolfi R, Marchioli R, Kutti J, Gisslinger H, Tognoni G, Patrono C, Barbui T and European Collaboration on Low-Dose Aspirin in Polycythemia Vera I. Efficacy and safety of low-dose aspirin in polycythemia vera. The New England journal of medicine. 2004;350:114–24. [DOI] [PubMed] [Google Scholar]
- 10.Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, McConkey M, Gupta N, Gabriel S, Ardissino D, Baber U, Mehran R, Fuster V, Danesh J, Frossard P, Saleheen D, Melander O, Sukhova GK, Neuberg D, Libby P, Kathiresan S and Ebert BL. Clonal Hematopoiesis and Risk of Atherosclerotic Cardiovascular Disease. N Engl J Med. 2017;377:111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuster JJ, MacLauchlan S, Zuriaga MA, Polackal MN, Ostriker AC, Chakraborty R, Wu CL, Sano S, Muralidharan S, Rius C, Vuong J, Jacob S, Muralidhar V, Robertson AA, Cooper MA, Andres V, Hirschi KK, Martin KA and Walsh K. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science. 2017;355:842–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mullally A, Lane SW, Ball B, Megerdichian C, Okabe R, Al-Shahrour F, Paktinat M, Haydu JE, Housman E, Lord AM, Wernig G, Kharas MG, Mercher T, Kutok JL, Gilliland DG and Ebert BL. Physiological Jak2V617F expression causes a lethal myeloproliferative neoplasm with differential effects on hematopoietic stem and progenitor cells. Cancer Cell. 2010;17:584–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu DJ, Peloso GM, Yu H, Butterworth AS, Wang X, Mahajan A, Saleheen D, Emdin C, Alam D, Alves AC, Amouyel P, Di Angelantonio E, Arveiler D, Assimes TL, Auer PL, Baber U, Ballantyne CM, Bang LE, Benn M, Bis JC, Boehnke M, Boerwinkle E, Bork-Jensen J, Bottinger EP, Brandslund I, Brown M, Busonero F, Caulfield MJ, Chambers JC, Chasman DI, Chen YE, Chen YI, Chowdhury R, Christensen C, Chu AY, Connell JM, Cucca F, Cupples LA, Damrauer SM, Davies G, Deary IJ, Dedoussis G, Denny JC, Dominiczak A, Dube MP, Ebeling T, Eiriksdottir G, Esko T, Farmaki AE, Feitosa MF, Ferrario M, Ferrieres J, Ford I, Fornage M, Franks PW, Frayling TM, Frikke-Schmidt R, Fritsche LG, Frossard P, Fuster V, Ganesh SK, Gao W, Garcia ME, Gieger C, Giulianini F, Goodarzi MO, Grallert H, Grarup N, Groop L, Grove ML, Gudnason V, Hansen T, Harris TB, Hayward C, Hirschhorn JN, Holmen OL, Huffman J, Huo Y, Hveem K, Jabeen S, Jackson AU, Jakobsdottir J, Jarvelin MR, Jensen GB, Jorgensen ME, Jukema JW, Justesen JM, Kamstrup PR, Kanoni S, Karpe F, Kee F, Khera AV, Klarin D, Koistinen HA, Kooner JS, Kooperberg C, Kuulasmaa K, Kuusisto J, Laakso M, Lakka T, Langenberg C, Langsted A, Launer LJ, Lauritzen T, Liewald DCM, Lin LA, Linneberg A, Loos RJF, Lu Y, Lu X, Magi R, Malarstig A, Manichaikul A, Manning AK, Mantyselka P, Marouli E, Masca NGD, Maschio A, Meigs JB, Melander O, Metspalu A, Morris AP, Morrison AC, Mulas A, Muller-Nurasyid M, Munroe PB, Neville MJ, Nielsen JB, Nielsen SF, Nordestgaard BG, Ordovas JM, Mehran R, O’Donnell CJ, Orho-Melander M, Molony CM, Muntendam P, Padmanabhan S, Palmer CNA, Pasko D, Patel AP, Pedersen O, Perola M, Peters A, Pisinger C, Pistis G, Polasek O, Poulter N, Psaty BM, Rader DJ, Rasheed A, Rauramaa R, Reilly DF, Reiner AP, Renstrom F, Rich SS, Ridker PM, Rioux JD, Robertson NR, Roden DM, Rotter JI, Rudan I, Salomaa V, Samani NJ, Sanna S, Sattar N, Schmidt EM, Scott RA, Sever P, Sevilla RS, Shaffer CM, Sim X, Sivapalaratnam S, Small KS, Smith AV, Smith BH, Somayajula S, Southam L, Spector TD, Speliotes EK, Starr JM, Stirrups KE, Stitziel N, Strauch K, Stringham HM, Surendran P, Tada H, Tall AR, Tang H, Tardif JC, Taylor KD, Trompet S, Tsao PS, Tuomilehto J, Tybjaerg-Hansen A, van Zuydam NR, Varbo A, Varga TV, Virtamo J, Waldenberger M, Wang N, Wareham NJ, Warren HR, Weeke PE, Weinstock J, Wessel J, Wilson JG, Wilson PWF, Xu M, Yaghootkar H, Young R, Zeggini E, Zhang H, Zheng NS, Zhang W, Zhang Y, Zhou W, Zhou Y, Zoledziewska M, Charge Diabetes Working G, Consortium EP-I, Consortium E-C, Consortium G, Program VAMV, Howson JMM, Danesh J, McCarthy MI, Cowan CA, Abecasis G, Deloukas P, Musunuru K, Willer CJand Kathiresan S. Exome-wide association study of plasma lipids in >300,000 individuals. Nat Genet. 2017;49:1758–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ginsberg H, Gilbert HS, Gibson JC, Le NA and Brown WV. Increased low-density-lipoprotein catabolism in myeloproliferative disorders. Ann Intern Med. 1982;96:311–6. [DOI] [PubMed] [Google Scholar]
- 15.Robertson J, Peters MJ, McInnes IB and Sattar N. Changes in lipid levels with inflammation and therapy in RA: a maturing paradigm. Nat Rev Rheumatol. 2013;9:513–23. [DOI] [PubMed] [Google Scholar]
- 16.Drechsler M, Megens RT, van Zandvoort M, Weber C and Soehnlein O. Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation. 2010;122:1837–45. [DOI] [PubMed] [Google Scholar]
- 17.Thorp E, Li G, Seimon TA, Kuriakose G, Ron D and Tabas I. Reduced apoptosis and plaque necrosis in advanced atherosclerotic lesions of Apoe−/− and Ldlr−/− mice lacking CHOP. Cell Metab. 2009;9:474–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kolodgie FD, Gold HK, Burke AP, Fowler DR, Kruth HS, Weber DK, Farb A, Guerrero LJ, Hayase M, Kutys R, Narula J, Finn AV and Virmani R. Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med. 2003;349:2316–25. [DOI] [PubMed] [Google Scholar]
- 19.Bogdanova A, Mihov D, Lutz H, Saam B, Gassmann M and Vogel J. Enhanced erythro-phagocytosis in polycythemic mice overexpressing erythropoietin. Blood. 2007;110:762–9. [DOI] [PubMed] [Google Scholar]
- 20.Bruce LJ, Ghosh S, King MJ, Layton DM, Mawby WJ, Stewart GW, Oldenborg PA, Delaunay J and Tanner MJ. Absence of CD47 in protein 4.2-deficient hereditary spherocytosis in man: an interaction between the Rh complex and the band 3 complex. Blood. 2002;100:1878–85. [DOI] [PubMed] [Google Scholar]
- 21.Mouro-Chanteloup I, Delaunay J, Gane P, Nicolas V, Johansen M, Brown EJ, Peters LL, Van Kim CL, Cartron JP and Colin Y. Evidence that the red cell skeleton protein 4.2 interacts with the Rh membrane complex member CD47. Blood. 2003;101:338–44. [DOI] [PubMed] [Google Scholar]
- 22.Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD and Lindberg FP. Role of CD47 as a marker of self on red blood cells. Science. 2000;288:2051–4. [DOI] [PubMed] [Google Scholar]
- 23.Brusson M, Cochet S, Leduc M, Guillonneau F, Mayeux P, Peyrard T, Chomienne C, Le Van Kim C, Cassinat B, Kiladjian JJ and El Nemer W. Enhanced calreticulin expression in red cells of polycythemia vera patients harboring the JAK2(V617F) mutation. Haematologica. 2017;102:e241–e244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gardai SJ, McPhillips KA, Frasch SC, Janssen WJ, Starefeldt A, Murphy-Ullrich JE, Bratton DL, Oldenborg PA, Michalak M and Henson PM. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123:321–34. [DOI] [PubMed] [Google Scholar]
- 25.Tabas I, Garcia-Cardena G and Owens GK. Recent insights into the cellular biology of atherosclerosis. J Cell Biol. 2015;209:13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kojima Y, Weissman IL and Leeper NJ. The Role of Efferocytosis in Atherosclerosis. Circulation. 2017;135:476–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tabas I Heart disease: Death-defying plaque cells. Nature. 2016;536:32–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thorp E and Tabas I. Mechanisms and consequences of efferocytosis in advanced atherosclerosis. J Leukoc Biol. 2009;86:1089–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM, Akira S, Rajavashisth TB and Arditi M. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci U S A. 2004;101:10679–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richards MR, Black AS, Bonnet DJ, Barish GD, Woo CW, Tabas I, Curtiss LK and Tobias PS. The LPS2 mutation in TRIF is atheroprotective in hyperlipidemic low density lipoprotein receptor knockout mice. Innate Immun. 2013;19:20–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lamkanfi M and Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157:1013–22. [DOI] [PubMed] [Google Scholar]
- 32.Westerterp M, Fotakis P, Ouimet M, Bochem AE, Zhang H, Molusky MM, Wang W, Abramowicz S, la Bastide-van Gemert S, Wang N, Welch CL, Reilly MP, Stroes ES, Moore KJ and Tall AR. Cholesterol Efflux Pathways Suppress Inflammasome Activation, NETosis and Atherogenesis. Circulation. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Youm YH, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, D’Agostino D, Planavsky N, Lupfer C, Kanneganti TD, Kang S, Horvath TL, Fahmy TM, Crawford PA, Biragyn A, Alnemri E and Dixit VD. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kusters S, Gantner F, Kunstle G and Tiegs G. Interferon gamma plays a critical role in T cell-dependent liver injury in mice initiated by concanavalin A. Gastroenterology. 1996;111:462–71. [DOI] [PubMed] [Google Scholar]
- 35.Parganas E, Wang D, Stravopodis D, Topham DJ, Marine JC, Teglund S, Vanin EF, Bodner S, Colamonici OR, van Deursen JM, Grosveld G and Ihle JN. Jak2 is essential for signaling through a variety of cytokine receptors. Cell. 1998;93:385–95. [DOI] [PubMed] [Google Scholar]
- 36.Yuan C, Qi J, Zhao X and Gao C. Smurf1 protein negatively regulates interferon-gamma signaling through promoting STAT1 protein ubiquitination and degradation. J Biol Chem. 2012;287:17006–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsuzawa A, Saegusa K, Noguchi T, Sadamitsu C, Nishitoh H, Nagai S, Koyasu S, Matsumoto K, Takeda K and Ichijo H. ROS-dependent activation of the TRAF6-ASK1-p38 pathway is selectively required for TLR4-mediated innate immunity. Nat Immunol. 2005;6:587–92. [DOI] [PubMed] [Google Scholar]
- 38.Han MS, Jung DY, Morel C, Lakhani SA, Kim JK, Flavell RA and Davis RJ. JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science. 2013;339:218–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martinez J, Cunha LD, Park S, Yang M, Lu Q, Orchard R, Li QZ, Yan M, Janke L, Guy C, Linkermann A, Virgin HW and Green DR. Noncanonical autophagy inhibits the autoinflammatory, lupus-like response to dying cells. Nature. 2016;533:115–9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Bronner DN, Abuaita BH, Chen X, Fitzgerald KA, Nunez G, He Y, Yin XM and O’Riordan MX. Endoplasmic Reticulum Stress Activates the Inflammasome via NLRP3- and Caspase-2-Driven Mitochondrial Damage. Immunity. 2015;43:451–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Verstovsek S, Kantarjian H, Mesa RA, Pardanani AD, Cortes-Franco J, Thomas DA, Estrov Z, Fridman JS, Bradley EC, Erickson-Viitanen S, Vaddi K, Levy R and Tefferi A. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med. 2010;363:1117–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marchioli R, Finazzi G, Specchia G, Cacciola R, Cavazzina R, Cilloni D, De Stefano V, Elli E, Iurlo A, Latagliata R, Lunghi F, Lunghi M, Marfisi RM, Musto P, Masciulli A, Musolino C, Cascavilla N, Quarta G, Randi ML, Rapezzi D, Ruggeri M, Rumi E, Scortechini AR, Santini S, Scarano M, Siragusa S, Spadea A, Tieghi A, Angelucci E, Visani G, Vannucchi AM, Barbui T and Group C-PC. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med. 2013;368:22–33. [DOI] [PubMed] [Google Scholar]
- 43.Cai B, Thorp EB, Doran AC, Sansbury BE, Daemen MJ, Dorweiler B, Spite M, Fredman G and Tabas I. MerTK receptor cleavage promotes plaque necrosis and defective resolution in atherosclerosis. J Clin Invest. 2017;127:564–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thorp E, Cui D, Schrijvers DM, Kuriakose G and Tabas I. Mertk receptor mutation reduces efferocytosis efficiency and promotes apoptotic cell accumulation and plaque necrosis in atherosclerotic lesions of apoe−/− mice. Arterioscler Thromb Vasc Biol. 2008;28:1421–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thorp E, Vaisar T, Subramanian M, Mautner L, Blobel C and Tabas I. Shedding of the Mer tyrosine kinase receptor is mediated by ADAM17 protein through a pathway involving reactive oxygen species, protein kinase Cdelta, and p38 mitogen-activated protein kinase (MAPK). J Biol Chem. 2011;286:33335–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hsu LC, Enzler T, Seita J, Timmer AM, Lee CY, Lai TY, Yu GY, Lai LC, Temkin V, Sinzig U, Aung T, Nizet V, Weissman IL and Karin M. IL-1beta-driven neutrophilia preserves antibacterial defense in the absence of the kinase IKKbeta. Nat Immunol. 2011;12:144–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miller LS, O’Connell RM, Gutierrez MA, Pietras EM, Shahangian A, Gross CE, Thirumala A, Cheung AL, Cheng G and Modlin RL. MyD88 mediates neutrophil recruitment initiated by IL-1R but not TLR2 activation in immunity against Staphylococcus aureus. Immunity. 2006;24:79–91. [DOI] [PubMed] [Google Scholar]
- 48.Huo Y, Schober A, Forlow SB, Smith DF, Hyman MC, Jung S, Littman DR, Weber C and Ley K. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat Med. 2003;9:61–7. [DOI] [PubMed] [Google Scholar]
- 49.Sreeramkumar V, Adrover JM, Ballesteros I, Cuartero MI, Rossaint J, Bilbao I, Nacher M, Pitaval C, Radovanovic I, Fukui Y, McEver RP, Filippi MD, Lizasoain I, Ruiz-Cabello J, Zarbock A, Moro MA and Hidalgo A. Neutrophils scan for activated platelets to initiate inflammation. Science. 2014;346:1234–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yvan-Charvet L, Pagler T, Gautier EL, Avagyan S, Siry RL, Han S, Welch CL, Wang N, Randolph GJ, Snoeck HW and Tall AR. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science. 2010;328:1689–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jorgensen I, Lopez JP, Laufer SA and Miao EA. IL-1beta, IL-18, and eicosanoids promote neutrophil recruitment to pore-induced intracellular traps following pyroptosis. Eur J Immunol. 2016;46:2761–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, Lindsley RC, Mermel CH, Burtt N, Chavez A, Higgins JM, Moltchanov V, Kuo FC, Kluk MJ, Henderson B, Kinnunen L, Koistinen HA, Ladenvall C, Getz G, Correa A, Banahan BF, Gabriel S, Kathiresan S, Stringham HM, McCarthy MI, Boehnke M, Tuomilehto J, Haiman C, Groop L, Atzmon G, Wilson JG, Neuberg D, Altshuler D and Ebert BL. Age-related clonal hematopoiesis associated with adverse outcomes. The New England journal of medicine. 2014;371:2488–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Genovese G, Kahler AK, Handsaker RE, Lindberg J, Rose SA, Bakhoum SF, Chambert K, Mick E, Neale BM, Fromer M, Purcell SM, Svantesson O, Landen M, Hoglund M, Lehmann S, Gabriel SB, Moran JL, Lander ES, Sullivan PF, Sklar P, Gronberg H, Hultman CM and McCarroll SA. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. The New England journal of medicine. 2014;371:2477–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wen Y High red blood cell distribution width is closely associated with risk of carotid artery atherosclerosis in patients with hypertension. Exp Clin Cardiol. 2010;15:37–40. [PMC free article] [PubMed] [Google Scholar]
- 55.Danese E, Lippi G and Montagnana M. Red blood cell distribution width and cardiovascular diseases. J Thorac Dis. 2015;7:E402–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ramos P, Casu C, Gardenghi S, Breda L, Crielaard BJ, Guy E, Marongiu MF, Gupta R, Levine RL, Abdel-Wahab O, Ebert BL, Van Rooijen N, Ghaffari S, Grady RW, Giardina PJ and Rivella S. Macrophages support pathological erythropoiesis in polycythemia vera and beta-thalassemia. Nat Med. 2013;19:437–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tefferi A and Barbui T. Polycythemia vera and essential thrombocythemia: 2015 update on diagnosis, risk-stratification and management. Am J Hematol. 2015;90:162–73. [DOI] [PubMed] [Google Scholar]
- 58.Vannucchi AM. How I treat polycythemia vera. Blood. 2014;124:3212–20. [DOI] [PubMed] [Google Scholar]
- 59.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ and Group CT. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.