Abstract
Recently, mathematical models of human integrative physiology, derived from Guyton’s classic 1972 model of the circulation, have been used to investigate potential mechanistic abnormalities mediating salt-sensitivity and salt-induced hypertension. We performed validation testing of two of the most evolved derivatives of Guyton’s 1972 model, QCP-2005 and HumMod-3.0.4, to determine if the models accurately predict sodium balance and hemodynamic responses of normal subjects to increases in salt intake within the real-life range of salt intake in humans. Neither model, nor the 1972 Guyton model, accurately predicts the usual changes in sodium balance, cardiac output, and systemic vascular resistance that normally occur in response to clinically realistic increases in salt intake. Furthermore, although both contemporary models are extensions of the 1972 Guyton model, testing revealed major inconsistencies between model predictions with respect to sodium balance and hemodynamic responses of normal subjects to short-term and long-term salt loading. These results demonstrate significant limitations with the hypotheses inherent in Guyton models regarding the usual regulation of sodium balance, cardiac output and vascular resistance in response to increased salt intake in normal salt-resistant humans. Accurate understanding of the normal responses to salt loading is a prerequisite for accurately establishing abnormal responses to salt loading. Accordingly, the present results raise concerns about the interpretation of studies of salt sensitivity with the various Guyton models. These findings indicate a need for continuing development of alternative models that incorporate mechanistic concepts of blood pressure regulation fundamentally different from those in the 1972 Guyton model and its contemporary derivatives.
Keywords: sodium chloride, dietary, salt, hypertension, blood pressure, kidney
Introduction
Major advances in computer hardware and software technology have enabled scientists to develop large scale mathematical models which simulate various complex physiologic functions of the human body.1 The most useful computer-based models of integrative physiology are mathematical formulations of hypotheses about principles and mechanisms that govern physiologic variables and their interaction2, e.g., variables such as sodium balance, cardiac output, blood pressure, etc. Testing the validity of mathematical models, by assessing their ability to predict experimental outcomes, provides a useful way of determining the soundness of the mechanistic hypotheses inherent in the models.2 In addition to validation testing, it is possible to compare the outputs of separate computer models to identify inconsistencies between the models, and thereby identify discrepancies between the hypotheses/concepts embodied in the different mathematical formulations.
The development of mathematical models for exploring hypotheses regarding mechanisms regulating cardiovascular function was pioneered by Guyton, Coleman, and colleagues at the University of Mississippi.3–6 In 1972, Guyton and colleagues published a model of the cardiovascular system4 that is said to incorporate more than 150 variables.1, 7 This model, which was derived from an earlier version developed by Guyton and Coleman,8 is referred to as “Guyton’s large circulatory model,”9 or the “1972 Guyton model,”1 and is said by Hall to be “the first large-scale model of the entire cardiovascular system.”10 As noted by investigators at the University of Mississippi including Dr. Thomas Coleman, one of the main developers of the 1972 model, the model was used to test a variety of hypotheses “mainly focusing on acute and chronic blood pressure control and the role of the kidney in the long term regulation of blood pressure.”1
The 1972 Guyton model was revised and expanded by Guyton and colleagues over decades and has served as the foundation for development of much more complex models of human physiology that can be readily run on personal computers with Windows-based operating systems.1 The most evolved derivative of the 1972 Guyton model is a large, multi-scale model called “HumMod” that includes a Windows-based graphical user interface.11, 12 HumMod version 3.0.4 is said to contain over 8000 independent variables13 and according to its developers from the University of Mississippi, is “the best, most complete, mathematical model of human physiology ever created.”14
In reviewing the literature on the 1972 Guyton model and the most evolved derivatives of the Guyton model, we could not find validation studies testing the capacity of the models to accurately predict sodium balance, cardiac output, and vascular resistance responses of normal subjects to increases in dietary salt within the real life range of salt intake in humans. Accurate quantitative characterization of the physiologic responses to salt loading in normal control subjects is a prerequisite for accurately determining which physiologic responses to salt loading are abnormal in salt sensitive subjects.
In the present studies, we performed validation testing of the most highly evolved version of the Guyton model, HumMod 3.0.4, and one of its closely related predecessors, Quantitative Cardiovascular Physiology (QCP 2005), with respect to their capacity to predict the usual sodium balance and hemodynamic responses of normal humans to increases in dietary salt within the real life range of salt intake in humans (clinically realistic increases in salt intake). We also compared simulations performed with the historical 1972 Guyton model to those performed with HumMod 3.0.4. Testing demonstrated that none of these models accurately predicts the usual changes in sodium balance, cardiac output, and systemic vascular resistance that occur in response to increased salt intake in normal salt resistant humans. Furthermore, although the contemporary models are held to be extensions of the same 1972 Guyton model,1 testing revealed major inconsistencies between the predictions made by these models with respect to cardiovascular and sodium metabolic responses to acute and chronic increases in salt intake. These findings raise major questions about the validity of the hypotheses inherent in Guytonian models that pertain to the regulation of sodium balance and hemodynamic responses to changes in salt intake within the real-life range of salt intake in humans. The findings have implications for understanding the normal mechanisms usually involved in mediating salt resistance, and the abnormalities usually involved in the initiation and maintenance of salt-induced hypertension.
Methods
The data supporting the findings of this study are available within the article and its Online Supplement, and from the corresponding author on appropriate request.
Details on study design, definitions of terms, sources of computer modeling software, methods of running the computer simulations, and the benchmark human studies used in the validation testing are provided in the methods section of the Online Supplement.
Statistical Analysis
For validation testing, the predictions of the computer models were plotted against the human experimental data. A model was considered to fail validation testing when the salt-induced change predicted by the model fell outside the 95% confidence limits of the mean of the salt-induced changes observed in human studies. When comparing predictions between models, we applied criteria of +/−25% to the changes that occur with salt loading. Specifically, the predictions of HumMod 3.0.4 were considered to agree with those of another model when the changes predicted by the HumMod simulation were within ± 25% of those predicted by the other model.
Results
Contemporary Computer Models Fail to Accurately Predict Sodium Balance Responses to Short-Term Salt Loading in Normal Humans
With respect to predicting usual sodium balance responses to short-term increases in salt intake in normal humans (normotensive, salt resistant subjects), both computer models (HumMod 3.0.4 and QCP 2005) showed poor agreement with the results of the human studies and both models failed validation testing. Figure 1 shows that both model simulations vastly underestimate the cumulative amount of sodium that is usually retained in response to switching from a very low NaCl intake of ~ 30 mmol/70 kg body weight/day to a high salt intake of ~ 250 mmol/70 kg body weight/day in normal subjects (normotensive salt resistant subjects in the salt-loading protocol of Schmidlin et al).15 Figure 2 shows that HumMod 3.0.4 also vastly underestimates the cumulative amount of sodium that is usually retained when switching from a moderately low NaCl intake of ~ 100 mmol/day to a high salt intake of ~ 275 mmol/day in normal subjects (salt-loading protocol of Ishii et al).16 The QCP model provided a reasonable approximation of the usual amount of sodium retained in response to switching from a moderately low NaCl diet to a high NaCl diet for 5 days (Figure 2). Despite being extensions of the same 1972 Guyton model, the HumMod 3.0.4 and QCP 2005 models showed poor agreement with each other regarding the predicted trend in cumulative sodium balance over time as well as the predicted magnitude of sodium retention (Figures 1 and 2).
Figure 1.
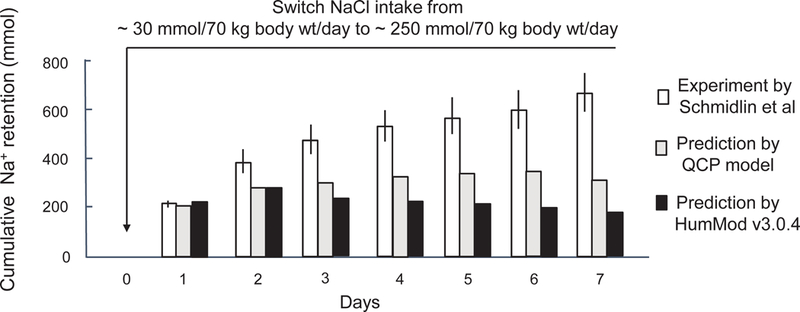
Experimental and predicted short-term effects on cumulative sodium balance induced by switching from a very low salt diet to a high salt diet. This figure shows cumulative sodium retention induced by switching NaCl intake from ~ 30 mmol/70 kg body weight/day to ~ 250 mmol/70 kg body weight/day according to the studies of Schmidlin and colleagues in normotensive salt resistant humans,15 and according to the modeling predictions of HumMod 3.0.4 and QCP 2005. These results for both the human study and the model simulations were determined without including non-renal losses of sodium in the balance calculations (see online Data Supplement for details). Results of studies in humans are presented as means and 95% confidence intervals.
Figure 2.
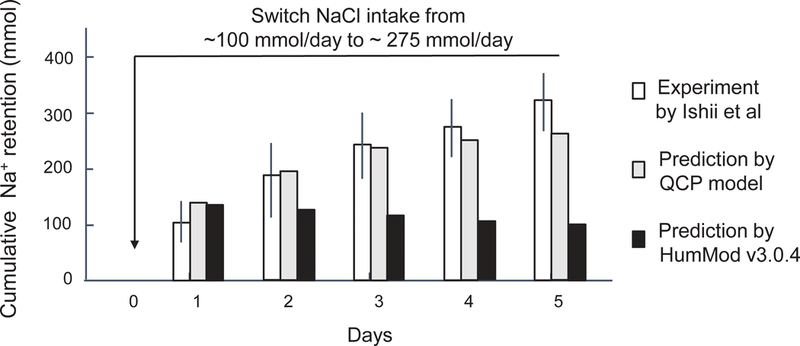
Experimental and predicted short-term effects on cumulative sodium balance induced by switching from a moderately low salt diet to a high salt diet. This figure shows cumulative sodium retention induced by switching NaCl intake from ~ 100 mmol/day to ~ 275 mmol/day according to the studies of Ishii and colleagues in normotensive salt resistant humans,16 and according to the predictions of HumMod 3.0.4 and QCP 2005. These results for both the human study and the model simulations were determined without including non-renal losses of sodium in the balance calculations. Results of studies in humans are presented as means and 95% confidence intervals.
Computer Model Predictions of Arterial Blood Pressure Responses to Short-Term Salt Loading in Normal Humans
In the study of Schmidlin et al,15 switching from a very low salt diet to a high salt diet induced a 5% decrease in mean arterial pressure during the first 12 to 48 hours of salt loading, followed by a return of mean arterial pressure to baseline by the 5th day of salt loading (Figure 3). Neither QCP nor HumMod accurately predicted this reduction in mean arterial pressure that is transiently induced upon switching normal subjects from a very low salt diet (~ 30 mmol NaCl/70 kg body weight/day) to a high salt diet (~250 mmol NaCl/70 kg body weight/day) (Figure 3). Both models accurately predicted that in normal subjects, switching from a very low salt diet to a high salt diet for 5 to 7 days causes minimal increases in blood pressure above baseline (Figure 3). Both models also accurately predicted the results from the study of Ishii et al which showed that in normal subjects,16 switching from a moderately low salt diet (~100 mmol NaCl/day) to a high salt diet (~275 mmol/day) for 5 days causes little or no increase in blood pressure. (data not shown).
Figure 3.
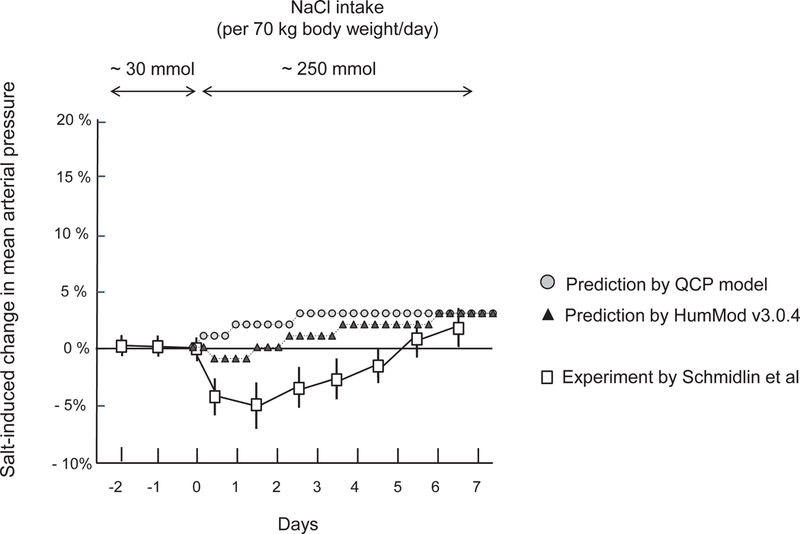
Experimental and predicted short-term changes in blood pressure induced by switching from a very low salt diet to a high salt diet. This figure shows percent changes in mean arterial pressure induced by switching NaCl intake from ~ 30 mmol NaCl/ kg body weight/day to ~ 250 mmol/70 kg body weight/day according to the studies of Schmidlin and colleagues in normotensive salt resistant humans,15 and according to the predictions of HumMod 3.0.4 and QCP 2005. Results of studies in humans are presented as means and 95% confidence intervals.
Contemporary Computer Models Fail to Accurately Predict Cardiac Output and Vascular Resistance Responses to Short-Term Salt Loading in Normal Humans
In model simulations of the changes in cardiac output and systemic vascular resistance that occur in response to short-term increases in salt intake, the predictions by both QCP and HumMod showed poor agreement with the experimental results and both models failed validation testing. The simulation results from HumMod 3.0.4 markedly overestimate the usual salt-induced increases in cardiac output (Figure 4), and markedly overestimate the usual salt-induced decreases in systemic vascular resistance (Figure 5). The simulation results from the QCP model significantly underestimate the usual salt-induced increases in cardiac output and salt-induced decreases in systemic vascular resistance (Figures 4 and 5).
Figure 4.
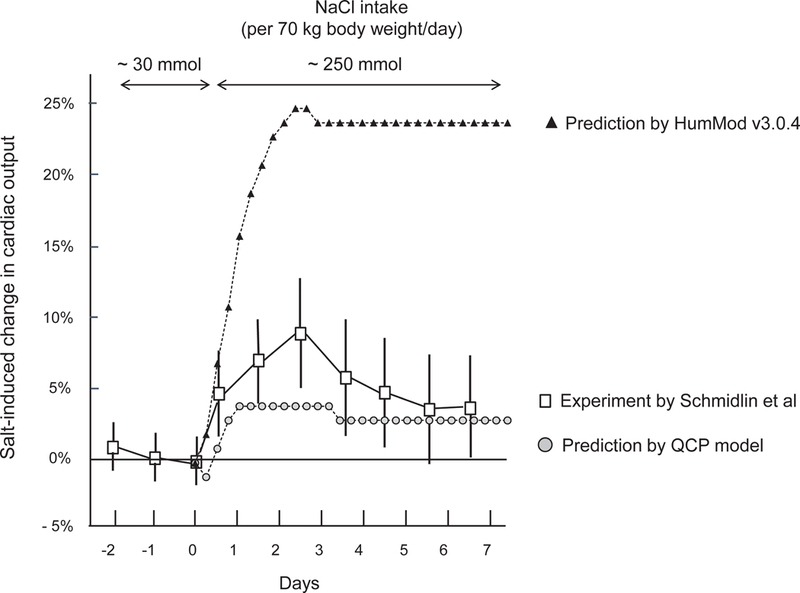
Experimental and predicted short-term changes in cardiac output induced by switching from a very low salt diet to a high salt diet. This figure shows percent changes in cardiac output induced by switching NaCl intake from ~ 30 mmol/70 kg body weight/day to ~ 250 mmol/70 kg body weight/day according to the studies of Schmidlin and colleagues in normotensive salt resistant humans,15 and according to the predictions of HumMod 3.0.4 and QCP 2005. Results of studies in humans are presented as means and 95% confidence intervals.
Figure 5.
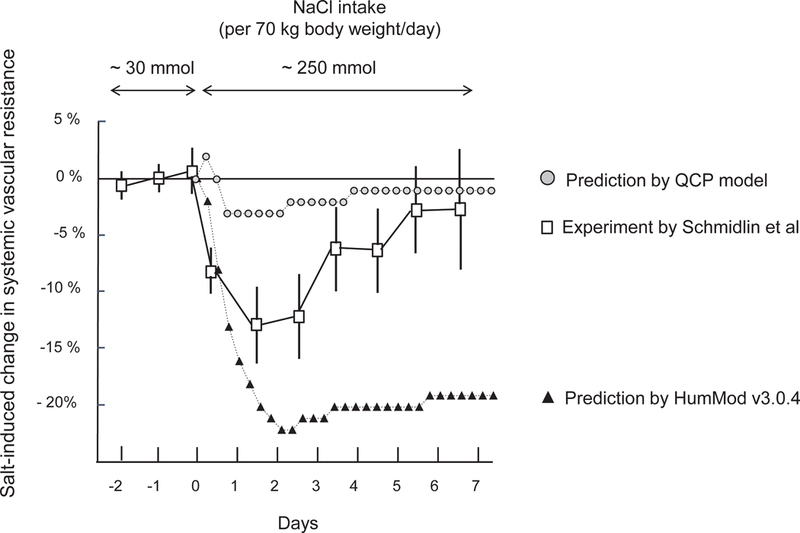
Experimental and predicted short-term changes in systemic vascular resistance induced by switching from a very low salt diet to a high salt diet. This figure shows percent changes in systemic vascular resistance induced by switching NaCl intake from ~ 30 mmol NaCl/70 kg body weight /day to ~ 250 mmol/70 kg body weight/day according to the studies of Schmidlin and colleagues in normotensive salt resistant humans,15 and according to the predictions of HumMod 3.0.4 and QCP 2005. Results of human studies are presented as means and 95% confidence intervals.
While both models fail to accurately estimate the magnitude of the changes in cardiac output and systemic vascular resistance usually induced by clinically realistic degrees of salt loading in normal subjects, both models appropriately reflect the general trends in these variables that normally occur in response to physiologic salt loading. Specifically, both models show that in normal subjects, non-extreme salt loading induces relatively little or no increase in blood pressure because normal subjects usually undergo robust vasodilation and reduce systemic vascular resistance sufficiently to offset the potential pressor effects of substantial salt-induced increases in cardiac output. These observations are consistent with the results of salt loading studies in normal salt resistant humans and in animals,15, 17 These observations are also consistent with the vasodysfunction theory of salt sensitivity18, 19 which holds that in response to salt loading, normal subjects undergo substantial decreases in systemic vascular resistance that offset potential pressor effects of salt-induced increases in cardiac output whereas salt sensitive subjects do not.
Comparing Predictions of HumMod 3.0.4 To Predictions of the Historical Guyton Model
In simulations of subjects with normal kidney function, switching from a very low NaCl diet to a high salt diet induces far greater short-term and long-term increases in blood pressure in the original 1972 Guyton model than in HumMod 3.0.4 (Figure 6 and Figure S7). The 1972 Guyton model clearly overestimates the extent to which arterial pressure increases in response to salt loading in HumMod 3.0.4 and in normal humans (Figures S7 and S8). Thus, the default subject with normal renal function simulated by the 1972 Guyton model is not normal and is salt sensitive according to criteria recommended for identifying salt sensitivity by the expert panel of the American Heart Association, and by other scientists with experience investigating salt sensitivity in humans.20, 21
Figure 6.
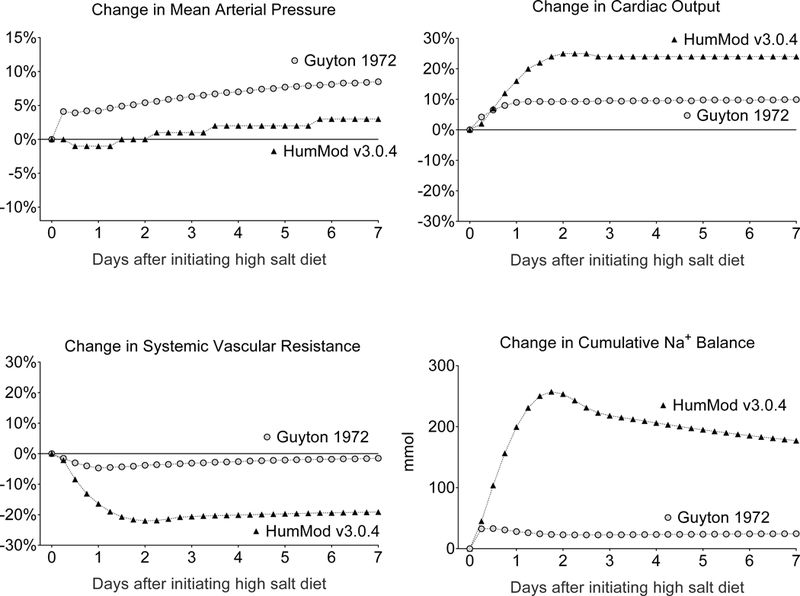
Short-term sodium metabolic and hemodynamic responses to salt loading predicted to occur by HumMod 3.0.4 and the 1972 Guyton model in subjects with normal renal function. This figure shows the percent changes in mean arterial pressure, cardiac output, and total peripheral resistance (systemic vascular resistance), and the changes in cumulative sodium balance, predicted to occur in response to switching from a low NaCl intake of 30 mmol/day to a high NaCl intake of 270 mmol/day for 7 days. The changes predicted to occur with long-term salt loading are shown in Figure S7.
Throughout both the initiation and maintenance phases of salt loading, the greater salt sensitivity in the 1972 Guyton model simulation than in the HumMod simulation is a consequence of much greater levels of systemic vascular resistance in the 1972 Guyton model than in HumMod 3.0.4 (see Figures 6, Figure S7, and raw data in the online supplement). Throughout salt loading, the salt-induced increases in cardiac output and sodium balance are much smaller in the 1972 Guyton model than in HumMod 3.0.4, yet the salt-induced increases in blood pressure are much greater in the 1972 Guyton model (Figures 6, S7). The greater salt sensitivity in the 1972 Guyton model than in HumMod or normal humans is not initiated by greater levels of sodium retention and cardiac output (Figures 6, S9, S10), but rather by greater levels of systemic vascular resistance (Figures 6, S11).
Computer Simulations of Long-Term Salt Loading: Conflicting Results Between Contemporary Models
Computer simulations of hemodynamic, sodium balance, and fluid volume responses to long-term salt loading also produced conflicting results between the contemporary models (see results in online data supplement). In addition, HumMod simulations of female subjects yielded striking differences from those of male subjects (see results in online data supplement).
Discussion
Arthur Clifton Guyton and colleagues pioneered the use of large-scale computer models to investigate potential physiologic abnormalities involved in the initiation and maintenance of salt-induced hypertension.4, 22 It is axiomatic that accurate identification of abnormal physiologic responses to a high salt diet depends on an accurate understanding of the normal physiologic responses to a high salt diet. In the present study, two large-scale, contemporary mathematical models of human integrative physiology, HumMod 3.0.412 and QCP 200523, both derivatives of the historical 1972 Guyton model of the circulation,4 failed validation testing with respect to accurately predicting the changes in sodium balance, cardiac output, and vascular resistance that usually occur in normal subjects in response to clinically realistic increases in dietary intake of salt. Depending on the different variables and model tested, the responses predicted were outside the 95% confidence intervals of the means of actual experimental responses, or were not directionally appropriate, or both. In addition, the 1972 Guyton model greatly underestimates the levels of sodium balance, and greatly overestimates the levels of blood pressure and systemic vascular resistance that usually occur with initiation of clinically realistic degrees of salt loading in normal humans (salt resistant normotensive humans).
The lack of published validation studies of the historical Guyton model, or of its contemporary derivatives, with respect to predicting normal sodium balance and hemodynamic responses to salt loading is surprising and prompted the present investigation. Despite the lack of model validation studies in salt loaded normal controls, these models have been used for many years to probe hypotheses about the possible role of abnormalities in sodium excretion, cardiac output, and vascular resistance in mediating pressor responses to both short-term and long-term salt loading.5, 6, 13, 24, 25 Also surprisingly,18, 19, 26 neither Guyton and his colleagues, nor most other investigators searching for abnormalities mediating salt sensitivity, published studies of sodium balance, cardiac output, and systemic vascular resistance responses to salt-loading in normal humans or animals. Despite the lack of such studies in normal controls, many investigators advocate the view that abnormally large increases in renal sodium retention and in cardiac output are usually involved in the pathogenesis of salt sensitivity.27–33 While increases in sodium balance and cardiac output occur in response to acute salt loading, the increases that occur in salt sensitive subjects are not greater than those that occur in salt resistant normal controls.18, 19, 26
In a recent effort to “test hypotheses of salt sensitivity,” Clemmer and colleagues at the University of Mississippi used HumMod 3.0.4 to simulate responses of cardiovascular, renal, and neurohormonal variables to massive degrees of salt-loading in normal subjects, and in subjects with various experimental manipulations.13 However, these investigators did not perform or cite any validation studies that tested the ability of HumMod to accurately predict the responses of normal humans to the extreme increases in salt intake that were used to define salt sensitivity in their simulation studies. In the simulation studies performed by Clemmer and colleagues, salt sensitivity was defined according to blood pressure responses to massive, clinically unrealistic increases in salt intake (> 30 fold increase in salt intake to 1000 mmol/day).13 Thus, even if simulations with HumMod 3.0.4 were to accurately predict the sodium balance and hemodynamic responses of normal subjects to such extreme degrees of salt loading, the relevance of such findings to the usual human responses to clinically realistic degrees of salt loading would be questionable.
Inconsistent Predictions Between Two Closely Related Derivatives of the 1972 Guyton Model
In addition to failing validation testing, the HumMod and QCP models predict vastly different results with respect to acute and chronic salt-induced changes in cardiac output, blood volume, interstitial fluid volume, plasma protein concentrations, and systemic vascular resistance. This is surprising because both models are viewed as “extensions” or “derivatives” of the same 1972 Guyton model of the circulation,1 and both “are due in large parts to the efforts of Dr. Thomas Coleman, a primary contributor to the 1972 Guyton model.”1 In the testing of HumMod, we also observed strikingly different results between simulations of salt loading in male subjects versus those in female subjects.
Comparing Predictions of the Original 1972 Guyton Model With Those of HumMod 3.0.4
In subjects with normal renal function, the original 1972 Guyton model predicts the occurrence of much greater salt-induced increases in blood pressure than those predicted by HumMod 3.0.4, the most recent extension of the Guyton model. This raises the question: what hemodynamic mechanism accounts for greater salt sensitivity in the 1972 Guyton model than in HumMod 3.0.4? In simulations with the 1972 Guyton model, the salt-induced increases in cardiac output and sodium balance are much smaller than those in simulations with HumMod 3.0.4 (Figure 6 and Figure S7). The greater acute and chronic salt sensitivity in the 1972 Guyton model than in HumMod 3.0.4 is caused by strikingly greater levels of vascular resistance with salt loading in the 1972 model than in HumMod 3.0.4, not by greater levels of sodium retention and cardiac output (Figure 6, Figure S7, and raw data in online-only supplement). In the 1972 Guyton model, acute and chronic salt loading causes only a modest decrease in systemic vascular resistance, whereas in HumMod 3.0.4, salt loading causes very large decreases in systemic vascular resistance, acutely and chronically (Figure S7). These findings raise an additional question: During the derivation of HumMod 3.0.4 from the 1972 Guyton model, what changes were made that account for the much greater decreases in systemic vascular resistance, and the much greater increases in sodium balance and cardiac output, that occur with salt loading in HumMod 3.0.4 than in the 1972 Guyton model? While extensive follow-up studies will be required to answer this question in detail, a brief discussion of some key points related to this issue is provided below.
Do Modern Models of Blood Pressure Regulation Include the Main “Core” Features of the Original Guyton Model?
According to a 2009 publication by Montani and Van Vliet, as the Guyton model evolved into more elaborate versions over the years, “the core of the model and the basic concepts remained untouched.”9 Specifically, Montani and Van Vliet noted that the “pressure-natriuresis relationship” and “blood flow autoregulation” are main features of the 1972 Guyton model that “remained as core concepts” as the model evolved.9 However, it appears that neither one of these “core concepts” of the 1972 Guyton model is retained in HumMod 3.0.4.
The Phenomenon of “Blood Flow Autoregulation” Does Not Appear to be Retained in the Most Evolved Version of the 1972 Guyton Model
According to the recent Guyton and Hall Textbook of Medical Physiology, the term “autoregulation” “means simply regulation of blood flow by the tissue itself. When increased blood volume increases the cardiac output, the blood flow increases in all tissues of the body, so this autoregulation mechanism constricts blood vessels all over the body, which in turn increases the total peripheral resistance.”32 However, in HumMod simulations of subjects with normal renal function, salt loading causes very large, sustained increases in blood volume (Figure S4) and cardiac output (Figure S7) that are associated with almost equally large, sustained decreases in total peripheral resistance (systemic vascular resistance), not sustained increases in total peripheral resistance (Figure S7). This type of vascular resistance response to salt-induced increases in cardiac output is opposite to the type of vascular resistance response expected to occur with “blood flow autoregulation.”
In simulations with the 1972 Guyton model, switching from a low salt diet to a high salt diet, induced a greater than 50% increase in the variable “ARM” defined as “the vasoconstrictor effects of all types of autoregulation” (for the ARM data, see online data supplement file labeled “Guyton_1972_Fortran_output”). In the 1972 Guyton model, this increase in the “vasoconstrictor effects of all types of autoregulation” could contribute to the failure of systemic vascular resistance to normally decrease in response to salt-induced increases in cardiac output. The large, sustained decreases in total peripheral resistance in the HumMod simulations suggest that the “core” feature of “blood flow autoregulation” in the 1972 Guyton model, has not been retained in HumMod 3.0.4 as a determinant of the systemic vascular resistance response to clinically realistic increases in salt intake in subjects with normal renal function (see the online supplemental material for a further discussion of autoregulation in the 1972 Guyton model).
This raises the question: has the core feature of early Guyton models that is held to mediate sustained salt-induced hypertension, i.e., the “pressure natriuresis relationship,” been retained in HumMod 3.0.4 ? This is a critical question because Guyton and colleagues have contended that for any given level of salt intake, the renal function curve (“pressure natriuresis relationship”) is the overriding determinant of long-term blood pressure control.5, 32, 34
The Pressure Natriuresis Relationship Is Not Retained as a Determinant of Blood Pressure in the Most Evolved Version of the 1972 Guyton Model
Although the “pressure natriuresis relationship” was incorporated as a critical determinant of blood pressure in early Guyton models of the circulation, investigators involved in the development of HumMod have recently stated that “In HumMod, there is no equation that describes the renal function curve or pressure natriuresis relationship per se.”13 In addition, the investigators emphasize that in HumMod, renal function curves (pressure natriuresis curves) “are not artificially injected into the model but are the end result of the simulations.”13 These comments indicate that the renal function curve (pressure natriuresis relationship), the main “core” determinant of long-term blood pressure control in the early Guyton models, has not been retained in HumMod 3.0.4 as a driver of blood pressure outcomes in the simulations. Further, Osborn and colleagues35–37 and Beard and colleagues38 pointedly chose not to include the pressure natriuresis curve as the main controller of blood pressure in their modern models of arterial pressure regulation. It should also be noted that the Guytonian view on the dominant role of the pressure-natriuresis relationship in determining the chronic level of blood pressure, appears to be an expression of the three tautological “laws of long-term arterial pressure regulation” stated by Guyton and Coleman many years ago.39–42 The omission of the pressure-natriuresis relationship as a driver of blood pressure outcomes in HumMod, and in other contemporary models,35–38 agrees with Beard’s admonition to stop teaching and promoting physiologic concepts that are based on tautological thinking.39
Which Mathematical Calculations and Equations in the 1972 Guyton Model Have Been Retained in Its Contemporary Derivatives ?
Because of the various ways in which the 1972 Guyton model and its derivatives are presented, it is difficult to discern how many of the mathematical calculations/equations from the early Guyton models are retained in the recent derivatives such as QCP and HumMod 3.0.4. As pointed out by Kofranek and colleagues, the 1972 Guyton model was originally presented as a diagram that appeared as some sort of “electrotechnical device,” the accompanying explicatory comments and reasoning behind the given formulas were “very brief,” and the model was disseminated in FORTRAN code.43 With respect to the QCP model, the mathematical background of the model is hidden in source code written in C++.44 With respect to HumMod, Kofranek and colleagues have noted that “the model description has been divided into thousands of XML files and more than a thousand directories” and “the entire structure of the model and following links and references are not easily identifiable.”44 However, with the reimplementation of HumMod and other models in newer, more comprehensible software simulation environments like that provided by the Modelica modeling language,44–46 it is hoped that opportunities for understanding differences in equations and structures between different models will improve.
Study Limitations
One of the main goals of the current study was to perform validation testing of the capacity of large scale models of human integrative physiology to accurately predict sodium balance and hemodynamic responses to increases in salt intake. The current studies were limited because we could identify only two rigorously controlled studies for testing the capacity of the models to accurately predict the usual sodium balance responses to salt loading in normal salt resistant subjects, and only one study for testing accuracy in predicting the usual cardiac output responses to salt loading. Thus, the current study only tested the validity of the models in predicting responses to changes in salt intake under a small number of experimental conditions. However, none of these limitations undermines the main conclusions of the current study.
Perspectives and Conclusions
In a discussion of the relevance of the 1972 Guyton model to modern cardiovascular physiology, Montani and Van Vliet state that “As Guyton pointed out, the most helpful contribution of the model is when it failed to correctly predict an empirical outcome, since that clearly indicated a limitation in our understanding of the system.”9 Indeed, the results of the present studies indicate that the 1972 Guyton model, and its contemporary derivatives HumMod 3.0.4 and QCP 2005, reflect an incomplete understanding of the systems regulating sodium balance, cardiac output, and systemic vascular resistance in response to clinically realistic increases in salt intake. According to Dr. S. Randall Thomas and colleagues, the models developed by Guyton and his many collaborators led them to “profoundly reorient our understanding of the causes of hypertension.”7 However, in light of the results of the current studies with the 1972 Guyton model and its derivatives, together with those of previous experimental studies in animals47–50 and humans,15, 18, 51, 52 the use of the models by Guyton and colleagues to “reorient understanding” of the causes of hypertension appears to have been overreaching.
The present findings underscore the value of validation testing of mathematical models to assess the accuracy of mechanistic hypotheses inherent in the models. It is hoped that these findings will motivate further revision of HumMod, and the continued development of alternative models36, 53 that incorporate concepts of blood pressure control mechanisms different from those in the Guyton models. For example, we support the view of Osborn and colleagues35–37 that future models include pathways involved in regulating vascular tone that are missing from, or inadequately developed in, the early Guyton model and its derivatives, e.g., pathways involved in regulating vascular resistance responses to changes in salt intake such as nitric oxide related pathways54 and various neural-hormonal pathways.36, 38, 53, 55–57 We believe that future models should follow the lead of HumMod 3.0.4 and other models35–38 by not incorporating the tautological concept in the early Guyton models which holds that, for a given level of salt intake, the pressure-natriuresis relationship (curve) determines the chronic level of arterial pressure.39, 41 We suggest that future models also avoid incorporating the concept embedded in Guyton models4, 22 which holds that virtually all retained sodium distributes in the extracellular fluid volume. This concept has been open to question for many years,58–61 and mounting evidence from the work of Heer and colleagues62 and Titze and colleagues63, 64 has cast further doubt on the concept. Finally, we would encourage investigators to develop new models, and evolve existing models, in open source, more comprehensible object-oriented modeling environments like Modelica, and make the code readily available so that other investigators can work freely to investigate and improve the models in a cooperative scientific fashion.
Supplementary Material
Novelty and Significance
What Is New?
Guyton’s 1972 model of the circulation, and two large-scale models of integrative physiology recently derived from the 1972 Guyton model, HumMod 3.0.4 and QCP 2005, fail to accurately predict, or agree on, the usual changes in sodium balance, cardiac output, and systemic vascular resistance that occur in response to clinically realistic increases in salt intake in normal humans.
What Is Relevant?
These findings are relevant for understanding the mechanisms that normally regulate blood pressure responses to clinically realistic increases in salt intake, and for recognizing the hemodynamic and renal abnormalities involved in the pathogenesis of salt-induced hypertension.
Summary
The 1972 Guyton model of the circulation, and its contemporary derivatives, do not accurately represent the mechanisms normally involved in regulating blood pressure and sodium metabolic responses to realistic degrees of salt loading in humans.
Acknowledgments
Sources of funding
National Center for Research Resources, M0 RR-00079, US Public Health Service; National Institutes of Health/National Heart, Lung and Blood Institute grant RO1-HL64230; Praemium Academiae award of the Czech Academy of Sciences to MP; and gifts from the Saw Island Foundation, the Antel Foundation, and the Maier Family Foundation.
Footnotes
Disclosures
None
REFERENCES
- 1.Hester RL, Iliescu R, Summers R, Coleman TG. Systems biology and integrative physiological modelling. J Physiol 2011;589(Pt 5): 1053–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beard DA, Bassingthwaighte JB, Greene AS. Computational modeling of physiological systems. Physiol Genomics 2005;23(1):1–3; discussion 4. [DOI] [PubMed] [Google Scholar]
- 3.Guyton AC, Coleman TG. Quantitative analysis of the pathophysiology of hypertension. Circ Res 1969;XXIV(5)(Supplement 1):1–19. [PubMed] [Google Scholar]
- 4.Guyton AC, Coleman TG, Granger HJ. Circulation: overall regulation. Annu Rev Physiol 1972;34:13–46. [DOI] [PubMed] [Google Scholar]
- 5.Guyton AC, Coleman TG, Cowley AW, Liard J-F, Norman RA, Manning RD. Systems analysis of arterial pressure regulation and hypertension. Annals of Biomedical Engineering 1972;1(2):254–281. [DOI] [PubMed] [Google Scholar]
- 6.Guyton AC, Montani JP, Hall JE, Manning RD Jr., Computer models for designing hypertension experiments and studying concepts. Am J Med Sci 1988;295(4):320–326. [DOI] [PubMed] [Google Scholar]
- 7.Thomas SR, Baconnier P, Fontecave J, Francoise JP, Guillaud F, Hannaert P, Hernandez A, Le Rolle V, Maziere P, Tahi F, White RJ. SAPHIR: a physiome core model of body fluid homeostasis and blood pressure regulation. Philos Trans A Math Phys Eng Sci 2008;366(1878):3175–3197. [DOI] [PubMed] [Google Scholar]
- 8.Guyton AC, Coleman TG. Long-term regulation of the circulation: interrelationships with body fluid volumes. In: Reeve EB, Guyton AC, eds. Physical bases of circulatory transport: regulation and exchange Philadelphia,: Saunders; 1967:179–201. [Google Scholar]
- 9.Montani JP, Van Vliet BN. Understanding the contribution of Guyton’s large circulatory model to long-term control of arterial pressure. Exp Physiol 2009;94(4):382–388. [DOI] [PubMed] [Google Scholar]
- 10.Hall JE. The pioneering use of systems analysis to study cardiac output regulation. Am J Physiol Regul Integr Comp Physiol 2004;287(5):R1009–1011. [DOI] [PubMed] [Google Scholar]
- 11.HC Simulation LLC. HumMod Available at: www.hummod.org. Accessed 01/11/2018, 2018.
- 12.Hester RL, Brown AJ, Husband L, Iliescu R, Pruett D, Summers R, Coleman TG. HumMod: A Modeling Environment for the Simulation of Integrative Human Physiology. Front Physiol 2011;2:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clemmer JS, Pruett WA, Coleman TG, Hall JE, Hester RL. Mechanisms of Blood Pressure Salt Sensitivity: New Insights from Mathematical Modeling. Am J Physiol Regul Integr Comp Physiol 2017;312:R451–R456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.HC Simulation LLC. HumMod origin story Available at: http://hummod.org/origin-story/. Accessed 2/10/2018, 2018.
- 15.Schmidlin O, Forman A, Leone A, Sebastian A, Morris RC Jr. Salt sensitivity in blacks: evidence that the initial pressor effect of NaCl involves inhibition of vasodilatation by asymmetrical dimethylarginine. Hypertension 2011;58(3):380–385. [DOI] [PubMed] [Google Scholar]
- 16.Ishii M, Atarashi K, Ikeda T, Hirata Y, Igari T, Uehara Y, Takagi M, Matsuoka H, Takeda T, Murao S. Role of the aldosterone system in the salt-sensitivity of patients with benign essential hypertension. Jpn Heart J 1983;24:79–89. [DOI] [PubMed] [Google Scholar]
- 17.Kurtz TW, DiCarlo SE, Pravenec M, Schmidlin O, Tanaka M, Morris RC. An alternative hypothesis to the widely held view that renal excretion of sodium accounts for resistance to salt-induced hypertension. Kidney Int 2016;90(5):965–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morris RC, Schmidlin O, Sebastian A, Tanaka M, Kurtz TW. Vasodysfunction that involves renal vasodysfunction, not abnormally increased renal retention of sodium, accounts for the initiation of salt-induced hypertension. Circulation 2016;133:881–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurtz TW, DiCarlo SE, Pravenec M, Morris RC Jr. The American Heart Association Scientific Statement on Salt Sensitivity of Blood Pressure: Prompting consideration of alternative conceptual frameworks for the pathogenesis of salt sensitivity ? Journal of Hypertension 2017;25:2214–2225. [DOI] [PubMed] [Google Scholar]
- 20.Elijovich F, Weinberger MH, Anderson CA, Appel LJ, Bursztyn M, Cook NR, Dart RA, Newton-Cheh CH, Sacks FM, Laffer CL. Salt Sensitivity of Blood Pressure: A Scientific Statement From the American Heart Association. Hypertension 2016;68(3):e7–e46. [DOI] [PubMed] [Google Scholar]
- 21.Kurtz TW, DiCarlo SE, Pravenec M, Morris RC. An Appraisal of Methods Recently Recommended for Testing Salt Sensitivity of Blood Pressure. Journal of the American Heart Association Vol 6:e005653; 2017:6:e005653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guyton AC, Coleman TG, Cowley AW Jr., Manning RD Jr., Norman RA Jr., Ferguson JD. A systems analysis approach to understanding long-range arterial blood pressure control and hypertension. Circulation Research 1974;35(2):159–176. [Google Scholar]
- 23.Abram SR, Hodnett BL, Summers RL, Coleman TG, Hester RL. Quantitative Circulatory Physiology: an integrative mathematical model of human physiology for medical education. Adv Physiol Educ 2007;31(2):202–210. [DOI] [PubMed] [Google Scholar]
- 24.Van Vliet BN, Montani JP. Circulation and fluid volume control. In: Walz W, ed. Integrative physiology in the proteomics and post-genomics agen Totowa, N.J.: Humana Press; 2005:43–64. [Google Scholar]
- 25.Clemmer JS, Hester RL, Pruett WA. Simulating a virtual population’s sensitivity to salt and uninephrectomy. Interface focus 2018;8(1):20160134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurtz TW, Dominiczak AF, DiCarlo SE, Pravenec M, Morris RC. Molecular based mechanisms of Mendelian forms of salt-dependent hypertension: Questioning the prevailing theory. Hypertension 2015;65:932–941. [DOI] [PubMed] [Google Scholar]
- 27.Guyton AC, ed. Arterial Pressure and Hypertension. Philadelphia: W.B. Saunders; 1980. Circulatory Physiology III. [Google Scholar]
- 28.Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell 2001;104(4):545–556. [DOI] [PubMed] [Google Scholar]
- 29.Scholl UI, Lifton RP. Inherited disorders of renal salt homeostasis: Insights from molecular genetics studies. In: Alpern RJ, Moe OW, Caplan M, eds. Seldin and Giebisch’s The Kidney Vol 1 (III). 5 ed. London: Elsevier; 2013:1213–1240. [Google Scholar]
- 30.Ando K, Fujita T. Pathophysiology of salt sensitivity hypertension. Ann Med 2012;44 Suppl 1:S119–126. [DOI] [PubMed] [Google Scholar]
- 31.Rossier BC, Staub O, Hummler E. Genetic dissection of sodium and potassium transport along the aldosterone-sensitive distal nephron: importance in the control of blood pressure and hypertension. FEBS Lett 2013;587(13):1929–1941. [DOI] [PubMed] [Google Scholar]
- 32.Hall JE. Guyton and Hall Textbook of Medical Physiology 13th ed. Philadelphia: Elsevier; 2015. [Google Scholar]
- 33.Hall JE. Renal dysfunction, rather than non-renal vascular dysfunction, mediates salt-induced hypertension. Circulation 2016;133:894–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guyton AC, Hall JE, Lohmeier TE, Manning RD Jr., Jackson TE. Position paper: The concept of whole body autoregulation and the dominant role of the kidneys for long-term blood pressure regulation. In: Laragh JH, Buhler FR, Seldin DW, eds. Frontiers in Hypertension Research New York: Springer-Verlag; 1981:125–134. [Google Scholar]
- 35.Osborn JW, Averina VA, Fink GD. Current computational models do not reveal the importance of the nervous system in long-term control of arterial pressure. Exp Physiol 2009;94(4):389–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Averina VA, Othmer HG, Fink GD, Osborn JW. A mathematical model of salt-sensitive hypertension: the neurogenic hypothesis. J Physiol 2015;593:3065–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Averina VA, Othmer HG, Fink GD, Osborn JW. A new conceptual paradigm for the haemodynamics of salt-sensitive hypertension: a mathematical modelling approach. J Physiol 2012;590(Pt 23):5975–5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beard DA, Pettersen KH, Carlson BE, Omholt SW, Bugenhagen SM. A computational analysis of the long-term regulation of arterial pressure. F1000Res 2013;2:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beard DA. Tautology vs. physiology in the etiology of hypertension. Physiology (Bethesda) 2013;28(5):270–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beard DA. Tautological Nature of Guyton’s Theory of Blood Pressure Control. Am J Hypertens 2017;30:e5. [DOI] [PubMed] [Google Scholar]
- 41.Kurtz TW, DiCarlo SE, Morris RC. Logical issues with the pressure natriuresis theory of chronic hypertension. Am.J.Hypertens 2016;29(12):1325–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kurtz TW, DiCarlo SE, Morris RC. Response to tautological nature of Guyton’s theory of blood pressure control. Am.J.Hypertens 2017;30:e6. [DOI] [PubMed] [Google Scholar]
- 43.Kofranek J, Rusz J. Restoration of Guyton’s diagram for regulation of the circulation as a basis for quantitative physiological model development. Physiol Res 2010;59(6):897–908. [DOI] [PubMed] [Google Scholar]
- 44.Kofranek J, Matejak M, Privitzer P. HumMod - Large Scale Physiological Models in Modelica. Proceedings of the 8th International Modelica Conference; March 20th-22nd; Technical University; Dresden; Germany: Linköping University Electronic Press; Linköpings universitet; 2011:713–724. [Google Scholar]
- 45.Fritzson PA. Principles of object oriented modeling and simulation with Modelica 3.3 : a cyber-physical approach. Second edition ed. Piscataway, New Jersey: IEEE Press/Wiley; 2015. [Google Scholar]
- 46.Matejak M, Kofranek J. Physiomodel - an integrative physiology in Modelica. Conf Proc IEEE Eng Med Biol Soc 2015;2015:1464–1467. [DOI] [PubMed] [Google Scholar]
- 47.Ganguli M, Tobian L, Iwai J. Cardiac output and peripheral resistance in strains of rats sensitive and resistant to NaCl hypertension. Hypertension 1979;1:3–7. [DOI] [PubMed] [Google Scholar]
- 48.Simchon S, Manger WM, Carlin RD, Peeters LL, Rodriguez J, Batista D, Brown T, Merchant NB, Jan K-M, Chien S. Salt-induced hypertension in Dahl salt-sensitive rats: Hemodynamics and renal responses. Hypertension 1989;13:612–621. [DOI] [PubMed] [Google Scholar]
- 49.Greene AS, Yu ZY, Roman RJ, Cowley AW Jr. Role of blood volume expansion in Dahl rat model of hypertension. Am J Physiol 1990;258:H508–H514. [DOI] [PubMed] [Google Scholar]
- 50.Krieger JE, Liard JF, Cowley AW Jr. Hemodynamics, fluid volume, and hormonal responses to chronic high-salt intake in dogs. Am J Physiol 1990;259(6 Pt 2):H1629–1636. [DOI] [PubMed] [Google Scholar]
- 51.Sullivan JM, Prewitt RL, Ratts TE, Josephs JA, Connor MJ. Hemodynamic characteristics of sodium-sensitive human subjects. Hypertension 1987;9:398–406. [DOI] [PubMed] [Google Scholar]
- 52.Schmidlin O, Sebastian AF, Morris RC Jr. What initiates the pressor effect of salt in salt-sensitive humans? Observations in normotensive blacks. Hypertension 2007;49(5):1032–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pettersen KH, Bugenhagen SM, Nauman J, Beard DA, Omholt SW. Arterial stiffening provides sufficient explanation for primary hypertension. PLoS Comput Biol 2014;10(5):e1003634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Feng W, Dell’Italia LJ, Sanders PW. Novel Paradigms of Salt and Hypertension. J Am Soc Nephrol 2017;28:1362–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leenen FH. The central role of the brain aldosterone-”ouabain” pathway in salt-sensitive hypertension. Biochim Biophys Acta 2010;1802(12):1132–1139. [DOI] [PubMed] [Google Scholar]
- 56.Blaustein MP, Chen L, Hamlyn JM, Leenen FH, Lingrel JB, Wier WG, Zhang J. Pivotal role of alpha2 Na+ pumps and their high affinity ouabain binding site in cardiovascular health and disease. J Physiol 2016;594:6079–6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Prager-Khoutorsky M, Choe KY, Levi DI, Bourque CW. Role of Vasopressin in Rat Models of Salt-Dependent Hypertension. Curr Hypertens Rep 2017;19(5):42. [DOI] [PubMed] [Google Scholar]
- 58.Cannon WB. Organization for physiological homeostasis. Physiol.Rev 1929;9:399–431. [Google Scholar]
- 59.Greene RW, Sapirstein LA. Total body sodium, potassium and nitrogen in rats made hypertensive by subtotal nephrectomy. Am J Physiol 1952;169(2):343–349. [DOI] [PubMed] [Google Scholar]
- 60.Ezrow L, Sapirstein LA. Excretion of sodium and water in rats made hypertensive by subtotal nephrectomy. Am J Physiol 1958;194(2):436–440. [DOI] [PubMed] [Google Scholar]
- 61.Wiig H, Luft FC, Titze JM. The interstitium conducts extrarenal storage of sodium and represents a third compartment essential for extracellular volume and blood pressure homeostasis. Acta Physiol (Oxf) 2018;222(3). [DOI] [PubMed] [Google Scholar]
- 62.Heer M, Baisch F, Kropp J, Gerzer R, Drummer C. High dietary sodium chloride consumption may not induce body fluid retention in humans. Am J Physiol Renal Physiol 2000;278(4):F585–595. [DOI] [PubMed] [Google Scholar]
- 63.Titze J, Muller DN, Luft FC. Taking another “look” at sodium. Can J Cardiol 2014;30(5):473–475. [DOI] [PubMed] [Google Scholar]
- 64.Titze J Water-free Na+ retention: interaction with hypertension and tissue hydration. Blood Purif 2008;26(1):95–99. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


