Abstract
Objective
We evaluated the biological effects of low-dose methotrexate (LDMTX) on 3 novel brachial artery grayscale ultrasound measures that may indicate subclinical arterial injury.
Approach and Results
Exploratory analysis from a clinical trial of people with HIV infection at increased cardiovascular disease risk who were randomly assigned to LDMTX (target dose 15 mg/week) or placebo. Brachial artery ultrasound grayscale median, gray level difference statistic texture contrast (GLDS-CON), and gray level texture entropy were measured at baseline and after 24 weeks of intervention. Findings from the intention-to-treat (N=148) and adequately dosed (N=118) populations were consistent, so the adequately dosed population results are presented. Participants were a median (Q1, Q3) age of 54 (50, 60) years. After 24 weeks, the LDMTX intervention was associated with a 25.4% (−18.1, 58.6; p=0.007) increase in GLDS-CON compared to 1.3% (−29.1, 44.7; p=0.97) with placebo (p=0.05) and a 0.10 u (−0.06, 0.23; p=0.026) increase in entropy compared to 0.02 u (−0.11, 0.14; p=0.54) with placebo (p=0.14). At week 24, changes in CD4+ T-cells correlated inversely with changes in GLDS-CON (ρ= −0.20, p=0.031), and entropy (ρ= −0.21, p=0.023). Changes in D-dimer levels, but no other inflammatory biomarkers, also correlated inversely with changes in GLDS-CON (ρ= −0.23, p=0.014) and entropy (ρ= −0.26, p=0.005).
Conclusions
Brachial artery GLDS-CON and entropy increased after 24 weeks of LDMTX, though the latter was not significantly different from placebo. Grayscale changes were associated with decreases in CD4+ T-cell and D-dimer concentrations and may indicate favorable arterial structure changes.
Keywords: Arteries, Cardiovascular Disease, Human Immunodeficiency Virus, Tissue characterization, Ultrasonic imaging
Introduction
Excess atherosclerotic cardiovascular disease (ASCVD) risk in people with human immunodeficiency virus (HIV) infection may be related to inflammation and disordered immune regulation that persist despite suppression of viral replication.1–7 Traditional techniques for imaging arterial injury in HIV have been limited to those that detect macroscopic changes such as carotid artery wall thickening, coronary artery calcification, carotid and coronary artery plaque formation, and brachial artery flow-mediated dilation.8 The conclusions of studies that used these imaging technologies generally agreed with epidemiological observations that HIV infection is associated with increased ASCVD risk in addition to traditional ASCVD risk factors, however the effects of antiretroviral and anti-inflammatory therapies on ASCVD risk and arterial injury are not known.1–4,8–10 Studies using 18F-fluorodeoxyglucose positron emission tomography/computed tomography imaging identified persistent arterial inflammation among treated and suppressed patients with HIV infection, which correlates with circulating inflammatory markers.5,11,12 In AIDS Clinical Trials Group Study A5314, anti-inflammatory treatment with low-dose methotrexate (LDMTX) did not improve brachial artery (BA) endothelial function or 8 inflammatory biomarkers in people with treated and suppressed HIV infection.13,14 However the impact of LDMTX on arterial structure remains unknown.
Recent advances in ultrasound arterial image analysis use statistical techniques to characterize grayscale pixel density, brightness, and variation.15–19 These measures can characterize tissue composition in the carotid arterial wall and in plaques. First-order statistics such as the grayscale median (GSM) and entropy are derived from the image pixel brightness histogram and can be used to characterize the overall echogenicity and randomness in the arterial wall.15,16,20,21 Gray-level difference statistics such as contrast (GLDS-CON) describe spatial relationships between two ultrasound pixels.15,16,20,21 When measured in the carotid arterial bed, these parameters have been associated with ASCVD risk factors and future ASCVD events.18,19,21–26 Carotid artery GSM levels have been associated with ASCVD risk factors in HIV-infected women26 and with circulating levels of inflammatory and oxidation biomarkers in older adults.23 Changes in carotid artery GSM have been described with statin therapy in the general population,27–29 however very little is known about these markers in the BA other than one cross-sectional study of ASCVD risk factors in older adults.30 They have not been studied in people living with HIV infection and their response to an anti-inflammatory intervention has never been assessed previously.
We performed an exploratory analysis to evaluate the biological effects of LDMTX on three novel BA grayscale ultrasound measures that may indicate subclinical arterial injury: BA GSM, entropy, and GLDS-CON. We also performed ultrasound phantom imaging to demonstrate the effects of ultrasound scatterer properties on these grayscale ultrasound measures.
Materials and Methods
Data, analytic methods, and study material are accessible by the public and other investigators using standard operating procedures from the National Institute of Health’s AIDS Clinical Trials Group (https://actgnetwork.org/clinical-trials/access-published-data).
Participants
The analysis population was comprised of participants in A5314 (NCT01949116), a phase II, randomized, multi-center, double-blind, placebo-controlled trial that evaluated the safety and efficacy of LDMTX among HIV-infected individuals at least 40 years old who had been virologically suppressed on continuous antiretroviral therapy for at least 24 weeks, who had a CD4+ T-cell count ≥400 cells/mm3, and who had documented ASCVD or who were at increased ASCVD risk.13 Participants started on 5 mg weekly of LDMTX or placebo at study entry and if tolerated after 1 week, the dose was increased to 10 mg weekly through week 12, then if tolerated, the dose was increased to 15 mg weekly until week 24. Folic acid, 1 mg daily, was prescribed to participants in both study groups. All participants provided informed consent and the study was approved of by the institutional review boards at each participating site and the Brachial Artery Ultrasound Reading Center in Madison, WI. The study design, biomarker measurement techniques, and main outcomes were presented previously.13,31
BA Ultrasound Imaging and Measurement of Flow and Diameters13,31,32–34
Participants were required to fast, not smoke, not drink caffeinated products, or exercise for at least 8 hours prior to testing. Using a linear array vascular ultrasound transducer, the right brachial artery was located above the elbow and scanned in longitudinal sections. After recording resting B-mode images of the brachial artery and spectral Doppler images of flow, the forearm cuff was inflated to 250 mmHg for 5 minutes to induce reactive hyperemia. Immediately after cuff deflation, spectral Doppler images were obtained to verify hyperemia and to determine peak brachial artery hyperemic flow velocity. All images were acquired by sonographers who were trained and certified by University of Wisconsin Atherosclerosis Imaging Research Program Laboratory. BA ultrasound images were sent electronically to the core lab for quality assessment and interpretation by a single, experienced technician using Access Point Web software (Freeland Systems, Alpharetta, GA). BA diameters and spectral Doppler flow velocities were measured in triplicate before and after reactive hyperemia at both time points. Flow-mediated dilation was defined as the ratio of the maximum BA diameter obtained within 90 seconds after reactive hyperemia to the resting diameter, expressed as a percent.
BA Grayscale Analysis (Figure 1)
Figure 1.
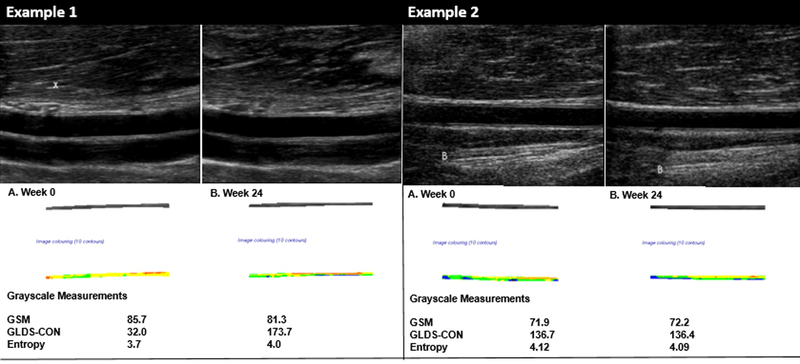
Examples of brachial artery grayscale measurements at week 0 and week 24.
Left Panel: Example 1 demonstrates changes in GLDS-CON and entropy values in a participant with minimal change in the GSM value between weeks 0 and 24. Note the increased contrast in the extracted grayscale image of the brachial artery wall and the difference in grayscale values depicted by more and varying colors in the arterial wall.
Right Panel: Example 2 demonstrates very little change in the GSM, GLDS-CON and entropy values in a participant between weeks 0 and 24. Panel A. Grayscale imaging of the brachial artery at week 0. The “B” demonstrates an extra-arterial landmark used to keep the image centered during brachial artery imaging and to help identify the same section of the brachial artery from visit to visit. Note the more homogenous appearance of the extracted grayscale image of the arterial wall and its uniform colorization.
Colorized segmentations correspond to the following grayscale values: 0–25 = black, 26–50=blue, 51–75=green, 76–100=yellow, 101–125=orange and 126–255=red
B and X in an image represent extra arterial landmarks used for image reproducibility.
GSM = Grayscale median
GLDS-CON = Gray level difference statistic – contrast
Resting DICOM images of the proximal BA at end-diastole were converted to bitmaps and normalized by assigning the blackest area of the blood a grayscale value of 0 and the whitest area of the middle two-fourths of the adventitia a grayscale value of 190, then were standardized to a uniform pixel density of 20/mm using LifeQ Medical Software (Nicosia, Cyprus).24,25,31,35 A reproducible 10 mm segment of the BA wall that was present in all scans within and between visits was segmented by placing the caliper on the blood-intima and media-adventitia interfaces. From this segmentation process, first order statistics from the image histogram were used to measure GSM (a measure of overall echogenicity of the pixels in the BA far wall) and entropy (a measure of randomness of the pixels in the BA far wall). The gray level difference statistic method was used to measure contrast (a measure of differences in grayscale values between pixels at different distances and directions in the BA far wall).25,31 Each grayscale measure is in arbitrary units.
Ultrasound Phantom Studies (Figure 2)
Figure 2. Ultrasound Phantom Images.
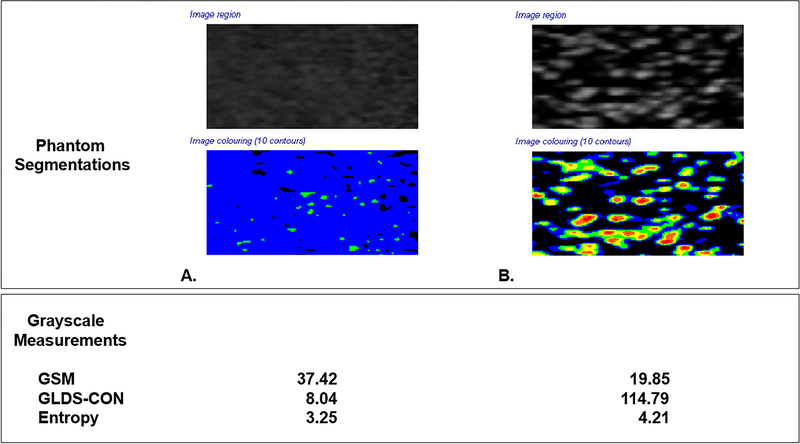
Grayscale segmentations from two different ultrasound imaging phantoms with different acoustic scattering properties. Phantom Images were obtained with the Acuson S2000 ultrasound system and 9L4 transducer (Siemens Medical Solutions, Malvern, PA). All instrumentation settings remained constant for both phantom images.
Panel A. Ultrasound phantom images (404GS precision small parts grayscale phantom, Gammex, Middleton, WI) with tissue-mimicking material at speed of sound 1540±10 m/s and attenuation coefficient 0.7±0.01 dB/cm-MHz showing scatterer properties sufficient to yield a fully developed speckle pattern which demonstrates higher GSM, lower GLDS-CON and lower entropy values.
Panel B. Ultrasound phantom images with 800 glass bead scatterers/cm3 demonstrating lower GSM, higher GLDS-CON, and higher entropy values. Note variation in grayscale appearance and corresponding colorized values in the segmented images.
Colorized segmentations correspond to the following grayscale values: 0–25 = black, 26–50=blue, 51–75=green, 76–100=yellow, 101–125=orange and 126–255=red
B and X in an image represent extra arterial landmarks used for image reproducibility.
GSM = Grayscale median
GLDS-CON = Gray level difference statistic – contrast
We acquired images from two unique ultrasound phantoms with different acoustic scattering properties. One phantom was custom built at the University of Wisconsin (Clear 800 7/29/99)36 with 800 glass bead scatterers/cm3, sound speed of 1542 m/s at 22°C) and one phantom is commercially available (404GS precision small parts grayscale phantom with tissue-mimicking material at speed of sound 1540±10 m/s and attenuation coefficient 0.7±0.01 dB/cm-MHz, Gammex, Middleton, WI). Images were acquired using the same ultrasound system, transducer, and instrumentation settings (Acuson S2000 ultrasound system, 9L4 transducer, Siemens Medical Solutions, Malvern, PA).
Statistical Analysis
Between-intervention group comparisons used the Wilcoxon rank sum test or Fisher’s exact test. Within-group changes in continuous outcomes used the Wilcoxon signed rank test. Correlations were assessed with the non-parametric Spearman’s correlation coefficient (ρ) and its associated p-value. All p-values are presented at their nominal alpha level of 0.05 with no formal adjustment for multiple comparisons. Because of site differences in ultrasound instrumentation, we performed sensitivity analyses using a mixed effects models for changes in GSM, GLDS-CON, and entropy that included main intervention and random site effects. Analyses also were performed on the log10 scale to represent relative rather than absolute effects of the intervention. Scan-rescan and measurement agreement between paired baseline measures were summarized by Bland-Altman plots and intra-class correlation coefficients. Because Bland-Altman plots revealed a cone-shape with increasing differences between values at higher values of GLDS-CON that were not seen with log10 transformation, these measurement values were analyzed on the log10 scale and back-transformed to percentage change values for presentation.
Findings from the intention-to-treat (N=176 participants randomized to study A5314, 148 with readable images at weeks 0 and 24) and adequately dosed (N=129 with continuous 24-week including ≥8 15 mg doses of LDMTX or placebo, 118 with readable images at weeks 0 and 24) populations were consistent. Since the primary goal of this exploratory analysis was to evaluate the biological effects of LDMTX, results from the adequately dosed population that would provide the maximum possible effect of the intervention are presented; the results of the intention-to-treat analyses are in the Supplementary Tables.
Results
Participant Characteristics and Correlations at Baseline, Week 0
Participants were 94% male, had a median (Q1-Q3) age of 54 (50–60) years, and a 10-year ASCVD risk of 8.7 (5.1–12.9)% (Table 1). Corresponding values for the ITT group participants are in Supplementary Table 1. At baseline, BA flow-mediated dilation correlated inversely with BA GLDS-CON (ρ= −0.20, p=0.026) and BA entropy (ρ= −0.20, p=0.026), but not GSM. None of the grayscale measures correlated significantly with any of the ASCVD risk factors in Table 1 except for GLDS-CON with glucose and GSM with CRP and D-Dimer (data not shown). However, CD4+T-cell concentrations were inversely correlated with GLDS-CON (ρ= −0.21, p=0.022) and entropy (ρ= −0.19, p=0.034) as were CD8+T-cells: GLDS-CON (ρ= −0.18, p=0.041) and entropy (ρ= −0.18, p=0.043) (Table 2). Similar correlations were not seen for GSM.
Table 1.
Baseline Participant Characteristics by Intervention Group (Adequately-Dosed Population)
| Intervention Group | ||||
|---|---|---|---|---|
| Characteristic | Total (N=118) |
LDMTX (N=55) |
Placebo (N=63) |
|
| Age (years) | Median (Q1, Q3) | 54 (50, 60) | 56 (51, 61) | 53 (49, 57) |
| Sex | M | 111 (94%) | 50 (91%) | 61 (97%) |
| F | 7 (6%) | 5 (9%) | 2 (3%) | |
| CD4+ T-cells (/mm³) | Median (Q1, Q3) | 686 (546, 891) | 678 (527, 838) | 728 (550, 942) |
| CD8+ T-cells (/mm³) | Median (Q1, Q3) | 788 (546, 1053) | 765 (527, 897) | 790 (582, 1123) |
| 10-year ASCVD risk (%) | Median (Q1, Q3) | 8.7 (5.1, 12.9) | 8.7 (4.9, 14.1) | 8.5 (5.1, 12.6) |
| Smoking history | Never Smoked | 48 (41%) | 25 (46%) | 23 (37%) |
| >1–5 years | 5 (4%) | 2 (4%) | 3 (5%) | |
| >5–10 years | 7 (6%) | 1 (2%) | 6 (10%) | |
| >10 years | 57 (49%) | 26 (48%) | 31 (49%) | |
| Weight (kg) | Median (Q1, Q3) | 85.0 (75.3, 97.0) | 88.0 (77.2, 98.8) | 84.2 (74.4, 93.3) |
| Total cholesterol (mg/dL) | Median (Q1, Q3) | 174 (156, 208) | 179 (158, 217) | 172 (156, 192) |
| High-density lipoprotein cholesterol (mg/dL) |
Median (Q1, Q3) | 45 (36, 56) | 52 (40, 59) | 42 (33, 55) |
| Glucose (mg/dL) | Median (Q1, Q3) | 93 (86, 102) | 95 (87, 104) | 92 (84, 101) |
| C-reactive protein (mg/L) | Median (Q1, Q3) | 2.2 (1.1, 4.3) | 2.4 (1.3, 5.0) | 2.0 (1.0, 4.1) |
| D-dimer (ng/ml) | Median (Q1, Q3) | 143.5 (98.8, 193.5) | 151.5 (111.2, 220.1) | 140.5 (90.4, 171.2) |
| Lipid-lowering medication use |
Yes | 75 (64%) | 35 (64%) | 40 (63%) |
| No | 43 (36%) | 20 (36%) | 23 (37%) | |
| Antihypertensive medication use |
Yes | 82 (69%) | 37 (67%) | 45 (71%) |
| No | 36 (31%) | 18 (33%) | 18 (29%) | |
| Hypoglycemic medication use |
Yes | 26 (22%) | 10 (18%) | 16 (25%) |
| No | 92 (78%) | 45 (82%) | 47 (75%) | |
| Brachial artery flow-mediated dilation (%) |
Median (Q1, Q3) | 3.4 (2.1, 4.8) | 3.5 (2.2, 5.0) | 3.0 (2.0, 4.6) |
| Brachial artery grayscale median (u) |
Median (Q1, Q3) | 74.8 (60.4, 88.2) | 73.7 (59.9, 85.2) | 77.4 (62.1, 91.8) |
| Brachial artery gray level difference statistic-contrast (u) |
Median (Q1, Q3) | 125.5 (85.1, 182.9) | 139.0 (83.0, 185.9) | 118.2 (85.1, 173.4) |
| Brachial artery entropy (u) | Median (Q1, Q3) | 4.2 (4.0, 4.4) | 4.2 (4.0, 4.4) | 4.2 (4.0, 4.3) |
Table 2.
Correlations between CD4+ and CD8+ T-Cells and Brachial Artery Grayscale Measures at Baseline and Changes at Week 24
| Spearman’s rho (p-value) | ||||
|---|---|---|---|---|
| GSM | Entropy | GldmCon | ||
| Week 0 (Overall) | CD4+ T-Cells (/mm³) | 0.03 (0.77) | −0.19 (0.034) | −0.21 (0.022) |
| CD8+ T-Cells (/mm³) | −0.03 (0.77) | −0.18 (0.043) | −0.18 (0.041) | |
| Δ to Week 24 (Overall) | CD4+ T-Cells (/mm³) | −0.07 (0.44) | −0.21 (0.023) | −0.20 (0.031) |
| CD8+ T-Cells (/mm³) | −0.08 (0.41) | −0.16 (0.08) | −0.16 (0.09) | |
Changes in Grayscale Markers Within- and Between-Groups from Week 0 to Week 24
After 24 weeks, GSM did not change within- or between-groups, however the LDMTX intervention was associated with a 25.4% (−18.1, 58.6; p=0.007) increase in GLDS-CON compared to 1.3% (−29.1, 44.7, p=0.97) with placebo (between-group p=0.05) and a 0.10 units (−0.06, 0.23; p=0.026) increase in entropy compared to 0.02 units (−0.11, 0.14; p=0.54) with placebo (between-group p=0.14) (Table 3). The within-group and between-arm differences for GLDS-CON and the within-group changes for entropy were robust in sensitivity analyses that included site adjustment and log10 transformation. Corresponding values for the ITT analysis are in Supplementary Table 2.
Table 3.
Brachial Artery Grayscale Measures by Intervention Group at Baseline and Changes at Week 24
| Total N = 129) |
LDMTX (N = 59) |
Placebo (N = 70) |
Group Difference |
||
|---|---|---|---|---|---|
| Grayscale median | |||||
| Week 0 | N | 124 | 59 | 65 | |
| Median (Q1, Q3) |
74.2 (59.0, 87.7) |
71.8 (55.1, 84.2) |
77.4 (62.1, 91.8) |
||
| Δ to Week 24 | N | 118 | 55 | 63 | |
| Median (Q1, Q3) |
−0.29 (−11.4, 10.5) |
1.54 (−11.1,10.5) |
−1.29 (−12.8, 10.5) |
||
| P-value | 0.54 | 0.93 | 0.42 | 0.55 | |
| Gray level difference statistic-contrast | |||||
| Week 0 | N | 124 | 59 | 65 | |
| Median (Q1, Q3) |
126.1 (84.0, 185.5) |
139.0 (81.8, 195.2) |
120.2 (93.0, 173.4) |
||
| % Δ to Week 24 | N | 118 | 55 | 63 | |
| Median (Q1, Q3) |
7.3% (−21.3, 52.7)% |
25.4% (−18.1, 58.6)% |
1.3% (−29.1, 44.7)% |
||
| P-value | 0.06 | 0.007 | 0.97 | 0.05 | |
| Entropy | |||||
| Week 0 | N | 124 | 59 | 65 | |
| Median (Q1, Q3) |
4.19 (4.03, 4.38) |
4.18 (4.00, 4.39) |
4.20 (4.04, 4.31) |
||
| Δ to Week 24 | N | 118 | 55 | 63 | |
| Median (Q1, Q3) |
0.04 (−0.11, 0.19) |
0.10 (−0.06, 0.23) |
0.02 (−0.11, 0.14) |
||
| P-value | 0.037 | 0.026 | 0.54 | 0.14 | |
At week 24, changes in CD4+ T-cells correlated inversely with changes in GLDS-CON (ρ= −0.20, p=0.031), and entropy (ρ= −0.21, p=0.023); directionally similar but not statically significant changes were noted for CD8+ T-cells (Table 2) and for CD4+ and CD8+ T-cells in analyses restricted to the LDMTX arm alone (data not shown). Changes in D-dimer levels correlated inversely with changes in GLDS-CON (ρ= −0.23, p=0.014), and entropy (ρ= −0.26, p=0.005). These correlations were similar in site-adjusted sensitivity analyses (data not shown). We did not find strong correlations between changes in any grayscale measure and changes in CRP, interleukin-6, or several other inflammatory biomarkers (i.e., fibrinogen, interferon-γ inducible protein-10, vascular cell adhesion molecule, soluble CD14, and soluble CD163) (data not shown). We did not find any associations between changes in flow-mediated dilation and changes in any BA grayscale measure (data not shown)
Ultrasound Phantom Measurements (Figure 2)
The ultrasound phantom with scatterer properties sufficient to produce a fully developed speckle pattern yielded images with a higher GSM value of 37.42 units, lower GLDS-CON value of 8.04 units, and lower entropy value of 3.25 units compared to a phantom constructed with 800 glass bead scatterers/cm3 that had a GSM value of 19.85 units, a GLDS-CON value of 114.79 units, and an entropy value of 4.21 units.
Grayscale Measurement Reproducibility
A single reader (JMW) measured images from 115 participants who were scanned twice at baseline, blinded to the first measurement. Intraclass correlation coefficients were 0.77 for GSM, 0.79 for GLDS-CON, and 0.69 for entropy.
Discussion
In this exploratory study of people with treated and suppressed HIV infection at increased ASCVD risk, two novel measures of BA grayscale texture – GLDS-CON and entropy – increased after 24 weeks of LDMTX, though the latter was not significantly different from placebo. These measures have not been described previously in the BA, though in the carotid artery, higher levels are associated with lower ASCVD risk and lower levels of ASCVD risk factors.21,25 Increases in all three grayscale markers correlated with reductions in CD4+ and CD8+ T-cells as well as D-Dimer, a marker of inflammation and coagulation that is associated with increased ASCVD risk and mortality in people with HIV3,39 and the general population.40,41 These findings may indicate that the grayscale changes observed in the LDMTX are related to less arterial inflammation.
There were no significant correlations between changes in grayscale markers and C-reactive protein, interleukin-6, or other inflammatory markers, in the primary analysis of study A5314. We also did not detect an effect of LDMTX on any of the 8 inflammatory biomarkers we measured despite seeing significant declines in CD4+ and CD8+ T-cell subsets after 26 weeks.13,37,38 We believe this was due to very high variability in the levels of each inflammatory marker and that the T-cell count changes more reliably reflect the anti-inflammatory effects of LDMTX in this population. Interestingly, inflammatory markers that predict CVD risk in the general population (like hsCRP and IL-6) do not reliably predict CVD risk in HIV-infected populations42 and when they do, their predictive power is less, possibly due to variation due to inflammation from persistent viremia, microbial translocation across the intestinal wall, infections, or immune activation due to HIV.7 It is not a surprise that in a smaller study like ours that we did not see an effect of LDMTX on inflammatory biomarkers, especially in the presence of declines in CD4+ and CD8+ T-cell counts and numerically more infections, which likely overwhelmed LDMX effects on these variable biomarkers.13,37,38
Atherosclerosis is common in the BA.43 BA ultrasound imaging primarily has been used to study conduit artery endothelial function since BA flow-mediated dilation predicts future ASCVD events44–47 and improves with lipid-lowering therapy and antiretroviral therapy in people with HIV infection.32,48,49 BA intima-media thickness also is related to ASCVD risk30,50, but to our knowledge, there only has been one report that described associations of BA GSM30 and none has evaluated the other gray scale texture markers in the BA that we reported here. In a study of 1016 older adults in Sweden, BA GSM was associated with several ASCVD risk factors and independently associated with two measures of oxidation and inflammatory stress (conjugated dienes of low-density lipoproteins and oxidized glutathione).30 We found that BA GSM was associated inversely with two inflammatory markers, CRP and D-Dimer at baseline and that decreases in D-Dimer levels in the LDMTX group were associated with increases in BA GSM, GLDS-CON, and entropy. We also found associations of baseline levels of CD4+ and CD8+ T-cells with baseline GLDS-CON and entropy as well as associations between their changes with LDMTX. These findings may indicate that BA grayscale measures reflect arterial injury due to inflammation or T-cell changes.
Our ultrasound phantom experiments demonstrate that the brachial artery grayscale measurements we reported differ based on the structure and scattering properties of the material that the ultrasound wave traverses. It is very likely that this is true with human tissue as well and that cellular changes in human tissue may lead to changes in their ultrasound scattering properties. Several studies that have examined ultrasound features of carotid plaques and histopathology from carotid endarterectomy specimens suggest that ultrasound grayscale features are strongly associated with tissue composition.35,51,52 Echogenicity of the carotid artery wall and plaques change with statin treatment.27–29 We recently demonstrated that improvements in walking function in people with peripheral arterial disease were associated with increases in BA GLDS-CON and entropy, therefore it is possible that the changes in grayscale texture features we observed in the LDMTX group represent changes in arterial wall composition associated with reduced arterial inflammation.53 This is consistent with our previous report that LDMTX may be associated with less aortic uptake using 18F-fluorodeoxyglucose positron emission tomography/computed tomography.14
These exploratory data underscore the importance of newer imaging techniques like ultrasound grayscale analysis of arterial texture and 18FDG PET/CT that may be able to detect arterial changes that are not evident in macroscopic measures of arterial size, thickness, or plaque burden. This study supports the value of advanced arterial ultrasound grayscale analyses to describe arterial pathophysiology and associations with clinical markers and interventions Although their clinical utility has not been established, we have demonstrated that in the carotid artery, higher GLDS-CON and entropy are associated with a significantly lower risk of coronary heart disease events in the Multi-Ethnic Study of Atherosclerosis.21 To put our findings in context, we estimated the potential clinical effect size of the changes in GLDS-CON and entropy we observed, assuming that (i) the signal related to GLDS-CON is similar in the carotid arteries and the brachial arteries, (ii) the longitudinal change in response to a drug intervention is similar to the cross-sectional effect size for predicting future coronary heart disease events, and (iii) that mean population changes reflect individual risk responses. Given the magnitude of the increase in brachial artery GLDS-CON we observed in the LDMTX group (+25.4%) from a baseline of 126 units, we expect this increase may be associated with an approximately 5–14% decrease in risk of future CHD events.
Limitations
Although we described novel findings from a carefully conducted randomized clinical trial of a novel anti-inflammatory intervention in people with stable HIV disease, our findings have limitations. The BA grayscale measures we reported were from exploratory analyses that were not pre-specified at the trial outset, so our findings should be considered hypothesis-generating with implications for BA imaging as a research tool and the effects of LDMTX on the BA, rather than its clinical effects. The BA markers explored in this study are highly novel, so the clinical relevance of their associations and changes we observed with LDMTX are uncertain, however they provide insights into the pathobiology of arterial injury in stable HIV disease and changes with an anti-inflammatory intervention that appears to reduce ASCVD event risk in patients with autoimmune diseases such as rheumatoid arthritis and psoriatic arthritis.54–57
Since the aim of this study was to investigate the biological effects of LDMTX on the BA (as opposed to its effect as a clinical intervention), we chose to report the adequately dosed group results, however the intention-to-treat results were very similar and are presented in the Supplementary Tables. Differences in ultrasound instrumentation at each site may have introduced variability into our measures, however our findings were consistent in two sensitivity analyses, one using a mixed-model that controlled for the site effect and another that evaluated relative changes on a participant level. Though both GLDS-CON and entropy improved significantly in the LDMTX group, the 24-week change in entropy with LDMTX compared to the change in the placebo group was not statistically significant. Similarly, some of the correlations of changes in CD4+ and CD8+ T-cells with changes in the gray scale markers after 24 weeks exceeded the nominal level of statistical significance, whereas others were just above it. It is possible that some of our findings may be due to chance given the relatively small size of this study and that some p-values are close to 0.05, however we believe that the consistency of the findings in this study (especially in regard to the CD4+ T-cell and D-Dimer associations) and between studies provides insights into the pathobiology of arterial injury in stable HIV disease, changes with an anti-inflammatory intervention, as well as the potential use of ultrasound grayscale imaging of the brachial artery as a research tool for investigating arterial biology and possibly cardiovascular disease risk. The modest, inverse baseline association of our measures with BA flow-mediated dilation are difficult to explain, especially since BA flow-mediated dilation did not change with LDMTX in A5314 and changes in flow-mediated dilation were not associated with changes in the gray scale measures.13,31
Conclusions
In a post hoc analysis of data from a randomized clinical trial of people with HIV infection, BA GLDS-CON and entropy increased after 24 weeks of LDMTX, though the latter was not significantly different from placebo. Arterial texture changes were associated with decreases in CD4+ T-cells and D-dimer levels and may indicate favorable changes in arterial structure. Advanced arterial ultrasound grayscale analysis is a promising tool to describe arterial pathophysiology and associations with clinical markers and interventions.
Supplementary Material
Highlights.
In people with suppressed HIV infection, low-dose methotrexate for 24 weeks was associated with significant increases in brachial artery contrast and entropy (the increase in contrast was greater than seen in the placebo arm)
Changes in brachial artery echolucency, contrast, and entropy were inversely associated with changes in CD4+ T-cells and D-dimer levels
There were no significant correlations between changes in grayscale markers and other inflammatory markers such C-reactive protein and interleukin-6
Ultrasound phantom experiments demonstrated that each grayscale measurement differed based on the structure and scattering properties of the material that the ultrasound wave traversed.
These grayscale arterial texture changes may indicate favorable changes in arterial structure
Acknowledgements
Participating AIDS Clinical Trials Units
The following ACTUs participated in this study: 101 - Massachusetts General Hospital CRS; 107 - Brigham and Women's Hospital Therapeutics CRS; 201 - Johns Hopkins University CRS; 601 - University of California, Los Angeles CARE Center CRS; 603 - Harbor University of California Los Angeles Center CRS; 701 - University of California, San Diego AntiViral Research Center CRS; 801 - University of California, San Francisco HIV/AIDS CRS; 1001 - University of Pittsburgh CRS; 1201 - University of Southern California CRS; 2101 - Washington University Therapeutics CRS; 2301 - Ohio State University CRS; 2401 - Cincinnati CRS; 2501 - Case Western Reserve University CRS; 2701 - Northwestern University CRS; 2951 - The Miriam Hospital (TMH) CRS; 3201 - Chapel Hill CRS; 3203 - Greensboro CRS; 3652 - Vanderbilt Therapeutics CRS; 6101 - University of Colorado Hospital CRS; 6201 - Penn Therapeutics CRS; 31473 - Houston AIDS Research Team CRS; 31786 - New Jersey Medical School Clinical Research Center CRS; 31788 - Alabama CRS
Other
The authors greatly appreciate the assistance of the AIDS Clinical Trials Group Statistical and Data Analysis Center and the AIDS Clinical Trials Group Optimization of Antiretroviral Therapy Committee, the clinical trials support from Social and Scientific Systems, Inc., and the efforts of the research participants.
Sources of Funding
The project described was supported by award numbers U01AI068636 and U01AI0686334 from the National Institute of Allergy and Infectious Diseases and HL1177131 from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases, the National Heart, Lung, and Blood Institute, or the National Institutes of Health.
Abbreviations
- ASCVD
atherosclerotic cardiovascular disease
- BA
brachial artery
- GLDS-CON
gray level difference statistic texture contrast
- GSM
grayscale median
- HIV
human immunodeficiency virus
- LDMTX
low-dose methotrexate
Footnotes
Disclosures
Regarding the content of this manuscript, there are no conflicts of interest to disclose.
References
- 1.Freiberg MS, Chang CC, Kuller LH, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med 2013;173:614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 2007;92:2506–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS medicine 2008;5:e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Triant VA, Meigs JB, Grinspoon SK. Association of C-reactive protein and HIV infection with acute myocardial infarction. Journal of acquired immune deficiency syndromes (1999) 2009;51:268–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Subramanian S, Tawakol A, Burdo TH, et al. Arterial inflammation in patients with HIV. JAMA 2012;308:379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stein JH, Hsue PY. Inflammation, immune activation, and CVD risk in individuals with HIV infection. JAMA 2012;308:405–406. [DOI] [PubMed] [Google Scholar]
- 7.Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet (London, England) 2013;382:1525–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stein JH, Currier JS, Hsue PY. Arterial disease in patients with human immunodeficiency virus infection: what has imaging taught us? J Am Coll Cardiol Cardiovasc Imaging 2014;7:515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Obel N, Thomsen HF, Kronborg G, et al. Ischemic heart disease in HIV-infected and HIV-uninfected individuals: a population-based cohort study. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America 2007;44:1625–1631. [DOI] [PubMed] [Google Scholar]
- 10.Strategies for Management of Antiretroviral Therapy Study Group, Emery S, Neuhaus JA, et al. Major clinical outcomes in antiretroviral therapy (ART)-naive participants and in those not receiving ART at baseline in the SMART study. J Infect Dis 2008;197:1133–1144. [DOI] [PubMed] [Google Scholar]
- 11.Yarasheski KE, Laciny E, Overton ET, et al. 18FDG PET-CT imaging detects arterial inflammation and early atherosclerosis in HIV-infected adults with cardiovascular disease risk factors. Journal of inflammation (London, England) 2012;9:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tawakol A, Ishai A, Li D, et al. Association of arterial and lymph node inflammation with distinct inflammatory pathways in human immunodeficiency virus infection. JAMA Cardiol 2017;2:163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsue P, Ribaudo H, Deeks SG, et al. Impact of LDMTX on immune activation and endothelial function in treated HIV. 25th Conference on Retroviruses and Opportunistic Infections (CROI); March 4–7 2018; Abstract: 79 Boston, MA. . [Google Scholar]
- 14.Tawakol A, Ribaudo H, Ishai AE, et al. Impact of LDMTX on arterial inflammation in persons with treated HIV. Conference on Retroviruses and Opportunistic Infections (CROI); March 4–7, 2018; Abstract: 684LB Boston, MA. . [Google Scholar]
- 15.Christodoulos IC, Kyriacoou E, Pattichis MS, Pattichis CS. Plaque feature extraction. In: Nicolaides A, Beach KW, Kyriacou E, Pattichis CS, eds. Ultrasound and carotid bifurcation atherosclerosis London ; New York: Springer; 2012:233–246. [Google Scholar]
- 16.Griffin M KE, Kakkos SK, Beach KW, Nicolaides A. Image normalization, plaque typing, and texture feature extraction. Ultrasound and carotid bifurction atherosclerosis London: Springer; 2012:193. [Google Scholar]
- 17.El-Barghouty NM, Levine T, Ladva S, et al. Histological verification of computerised carotid plaque characterisation. Eur J Vasc Endovasc Surg 1996;11:414–416. [DOI] [PubMed] [Google Scholar]
- 18.Loizou CP, Pantziaris M, Pattichis MS, et al. Ultrasound image texture analysis of the intima and media layers of the common carotid artery and its correlation with age and gender. Comput Med Imaging Graph 2009;33:317–324. [DOI] [PubMed] [Google Scholar]
- 19.Loizou CP, Georgiou N, Griffin M, et al. Texture analysis of the media-layer of the left and right common carotid artery. IEEE-Embs International Conference on Biomedical and Health Informatics (BHI) 2014:684–687.
- 20.Hall-Beyer M GLCM Texture: a tutorial v. 3.0 2017; http://hdl.handle.net/1880/51900, last accessed: 2017-04-04.
- 21.Mitchell C, Korcarz CE, Gepner AD, et al. Carotid artery echolucency, texture features, and incident cardiovascular disease events: the Multi-Ethnic Study of Atherosclerosis (MESA). Submitted for publication 2018.
- 22.Wohlin M, Sundstrom J, Andren B, et al. An echolucent carotid artery intima-media complex is a new and independent predictor of mortality in an elderly male cohort. Atherosclerosis 2009;205:486–491. [DOI] [PubMed] [Google Scholar]
- 23.Andersson J, Sundstrom J, Gustavsson T, et al. Echogenecity of the carotid intima-media complex is related to cardiovascular risk factors, dyslipidemia, oxidative stress and inflammation: the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study. Atherosclerosis 2009;204:612–618. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell C, Piper ME, Korcarz CE, et al. Echogenicity of the carotid arterial wall in active smokers. Journal of Diagnostic Medical Sonography 2017;34:161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitchell CC, Korcarz CE, Tattersall MC, et al. Carotid artery ultrasound texture, cardiovascular risk factors, and subclinical arterial disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Br J Radiol 2018;91:20170637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jung M, Parrinello CM, Xue X, et al. Echolucency of the carotid artery intima-media complex and intima-media thickness have different cardiovascular risk factor relationships: the Women’s Interagency HIV Study. Journal of the American Heart Association 2015;4: e001405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lind L, Peters SA, den Ruijter HM, et al. Effect of rosuvastatin on the echolucency of the common carotid intima-media in low-risk individuals: the METEOR trial. J Am Soc Echocardiogr 2012;25:1120–1127.e1121. [DOI] [PubMed] [Google Scholar]
- 28.Kadoglou NP, Gerasimidis T, Moumtzouoglou A, et al. Intensive lipid-lowering therapy ameliorates novel calcification markers and GSM score in patients with carotid stenosis. Eur J Vasc Endovasc Surg 2008;35:661–668. [DOI] [PubMed] [Google Scholar]
- 29.Della-Morte D, Moussa I, Elkind MS, et al. The short-term effect of atorvastatin on carotid plaque morphology assessed by computer-assisted gray-scale densitometry: a pilot study. Neurol Res 2011;33:991–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lind L, Andersson J, Ronn M, et al. Brachial artery intima-media thickness and echogenicity in relation to lipids and markers of oxidative stress in elderly subjects:--the prospective investigation of the vasculature in Uppsala Seniors (PIVUS) Study. Lipids 2008;43:133–141. [DOI] [PubMed] [Google Scholar]
- 31.LifeQMedical v 4.5. Carotid plaque texture analysis research software for ultrasonic arterial wall and atherosclerotic plaques measurements. Operation manual [computer program] Version 4.5 Cyprus; 2013. [Google Scholar]
- 32.Torriani FJ, Komarow L, Parker RA, et al. Endothelial function in human immunodeficiency virus-infected antiretroviral-naive subjects before and after starting potent antiretroviral therapy: The ACTG (AIDS Clinical Trials Group) Study 5152s. J Am Coll Cardiol 2008;52:569–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stein JH, Brown TT, Ribaudo HJ, et al. Ultrasonographic measures of cardiovascular disease risk in antiretroviral treatment-naive individuals with HIV infection. AIDS 2013;27:929–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 2002;39:257–265. [DOI] [PubMed] [Google Scholar]
- 35.Mitchell CC, Stein JH, Cook TD, et al. Histopathologic validation of grayscale carotid plaque characteristics related to plaque vulnerability. Ultrasound Med Biol 2017;43:129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson T In-vivo Frequency Dependent Backscatter Estimations in Liver In. [dissertation]: Medical Physics Department, University of Wisconsin, Madison; 2000:67. [Google Scholar]
- 37.Hsue PY RH, Deeks SG, Bell T, et al. Safety and Impact of Low-Dose Methotrexate on Endothelial Function and Inflammation in Individuals with Treated Human Immunodeficiency Virus: AIDS Clinical Trials Group Study A5314. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2018. (provisional acceptance). [DOI] [PMC free article] [PubMed]
- 38.NIoAaID. Safety and Effectiveness of Low-Dose Methotrexate for Reducing Inflammation in HIV-Infected Adults on ARV Medications Last update: January 10, 2018 2018; https://clinicaltrials.gov/ct2/show/NCT01949116.
- 39.Ford ES, Greenwald JH, Richterman AG, et al. Traditional risk factors and D-dimer predict incident cardiovascular disease events in chronic HIV infection. AIDS 2010;24:1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ridker PM, Hennekens CH, Cerskus A, Stampfer MJ. Plasma concentration of cross-linked fibrin degradation product (D-dimer) and the risk of future myocardial infarction among apparently healthy men. Circulation 1994;90:2236–2240. [DOI] [PubMed] [Google Scholar]
- 41.Empana JP, Canoui-Poitrine F, Luc G, et al. Contribution of novel biomarkers to incident stable angina and acute coronary syndrome: the PRIME Study. European heart journal 2008;29:1966–1974. [DOI] [PubMed] [Google Scholar]
- 42.Baker JV, Duprez D. Biomarkers and HIV-associated cardiovascular disease. Current opinion in HIV and AIDS 2010;5:511–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sorensen KE, Kristensen IB, Celermajer DS. Atherosclerosis in the human brachial artery. J Am Coll Cardiol 1997;29:318–322. [DOI] [PubMed] [Google Scholar]
- 44.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation 2007;115:1285–1295. [DOI] [PubMed] [Google Scholar]
- 45.Gokce N, Keaney JF Jr., Hunter LM, et al. Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol 2003;41:1769–1775. [DOI] [PubMed] [Google Scholar]
- 46.Modena MG, Bonetti L, Coppi F, et al. Prognostic role of reversible endothelial dysfunction in hypertensive postmenopausal women. J Am Coll Cardiol 2002;40:505–510. [DOI] [PubMed] [Google Scholar]
- 47.Neunteufl T, Heher S, Katzenschlager R, et al. Late prognostic value of flow-mediated dilation in the brachial artery of patients with chest pain. Am J Cardiol 2000;86:207–210. [DOI] [PubMed] [Google Scholar]
- 48.Stein JH, Merwood MA, Bellehumeur JL, et al. Effects of pravastatin on lipoproteins and endothelial function in patients receiving human immunodeficiency virus protease inhibitors. Am Heart J 2004;147:E18. [DOI] [PubMed] [Google Scholar]
- 49.Hurlimann D, Chenevard R, Ruschitzka F, et al. Effects of statins on endothelial function and lipid profile in HIV infected persons receiving protease inhibitor-containing anti-retroviral combination therapy: a randomised double blind crossover trial. Heart 2006;92:110–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iwamoto Y, Maruhashi T, Fujii Y, et al. Intima-media thickness of brachial artery, vascular function, and cardiovascular risk factors. Arterioscler Thromb Vasc Biol 2012;32:2295–2303. [DOI] [PubMed] [Google Scholar]
- 51.Doonan RJ, Gorgui J, Veinot JP, et al. Plaque echodensity and textural features are associated with histologic carotid plaque instability. J Vasc Surg 2016;64:671–677.e678. [DOI] [PubMed] [Google Scholar]
- 52.Sztajzel R, Momjian S, Momjian-Mayor I, et al. Stratified gray-scale median analysis and color mapping of the carotid plaque: correlation with endarterectomy specimen histology of 28 patients. Stroke 2005;36:741–745. [DOI] [PubMed] [Google Scholar]
- 53.Berroug JKC, Mitchell CKC, Weber JM., McDermott MDSJ. Effects of supervised walking exercise on brachial artery intima-media thickness, echogenicity, and grayscale texture in the PROPEL randomized clinical trial. Submitted for publication 2018.
- 54.van Halm VP, Nurmohamed MT, Twisk JW, et al. Disease-modifying antirheumatic drugs are associated with a reduced risk for cardiovascular disease in patients with rheumatoid arthritis: a case control study. Arthritis research & therapy 2006;8:R151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Prodanovich S, Ma F, Taylor JR, et al. Methotrexate reduces incidence of vascular diseases in veterans with psoriasis or rheumatoid arthritis. Journal of the American Academy of Dermatology 2005;52:262–267. [DOI] [PubMed] [Google Scholar]
- 56.Solomon DH, Avorn J, Katz JN, et al. Immunosuppressive medications and hospitalization for cardiovascular events in patients with rheumatoid arthritis. Arthritis and rheumatism 2006;54:3790–3798. [DOI] [PubMed] [Google Scholar]
- 57.Naranjo A, Sokka T, Descalzo MA, et al. Cardiovascular disease in patients with rheumatoid arthritis: results from the QUEST-RA study. Arthritis research & therapy 2008;10:R30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


