Abstract
Patients with autonomic failure are characterized by disabling orthostatic hypotension (OH) due to impaired sympathetic activity, but even severely affected patients have residual sympathetic tone which can be harnessed for their treatment. For example, norepinephrine transporter (NET) blockade with atomoxetine raises blood pressure (BP) in autonomic failure patients by increasing synaptic norepinephrine concentrations; acetylcholinesterase inhibition with pyridostigmine increases BP by facilitating ganglionic cholinergic neurotransmission to increase sympathetic outflow. We tested the hypothesis that pyridostigmine will potentiate the pressor effect of atomoxetine and improve orthostatic tolerance and symptoms in patients with severe autonomic failure. Twelve patients received a single oral dose of either placebo, pyridostigmine 60 mg, atomoxetine 18 mg or the combination on separate days in a single blind, crossover study. BP was assessed seated and standing before and 1-hour postdrug. In these severely affected patients, neither pyridostigmine nor atomoxetine improved BP or orthostatic tolerance compared to placebo. The combination, however, significantly increased seated BP in a synergistic manner (133±9/80±4 mmHg vs. 107±6/66±4 mmHg for placebo, 105±5/67±3 mmHg for atomoxetine and 99±6/64±4 mmHg for pyridostigmine; P<0.001); the maximal increase in seated BP with the combination was 33±8/18±3 mmHg at 60 minutes postdrug. Only the combination showed a significant improvement of orthostatic tolerance and symptoms. In conclusion, the combination pyridostigmine and atomoxetine had a synergistic effect on seated BP which was associated with improvement in orthostatic tolerance and symptoms. This pharmacologic approach could be useful in patients with severe autonomic failure but further safety and long-term efficacy studies are needed.
Keywords: autonomic failure, orthostatic hypotension, atomoxetine, pyridostigmine, orthostatic tolerance
Orthostatic hypotension (OH) dominates the clinical picture in patients with autonomic failure. It is the cause of significant disability and its treatment can be challenging. OH is particular severe in patients with primary neurodegenerative disorders of either central autonomic pathways (multiple system atrophy [MSA]) or peripheral autonomic fibers (pure autonomic failure [PAF] or Parkinson disease [PD]). Even severely affected patients can have some residual sympathetic tone.1 We and others have recently explored the therapeutic approach of harnessing this residual sympathetic tone to treat OH. For example, blockade of the synaptic reuptake of norepinephrine with the selective norepinephrine transporter (NET) inhibitor atomoxetine increases blood pressure (BP) and improves OH in autonomic failure patients.2,3 Similarly, peripheral acetylcholinesterase inhibition with pyridostigmine preferentially increases upright BP presumably by enhancing nicotinic neurotransmission at the level of the sympathetic ganglia.4,5
Not all patients, however, respond to these treatments. Atomoxetine is more effective in patients with MSA, afflicted by central neurodegeneration of autonomic pathways and intact efferent sympathetic fibers, but may not be effective in patients with severe peripheral autonomic failure (PAF or PD with OH), in whom the primary neurodegenerative disorder resides in efferent sympathetic fibers.6 Similarly, pyridostigmine is not as potent as other pressor agents, and may be ineffective in severely affected patients.7
In this study, we hypothesized that the combination of pyridostigmine and atomoxetine would have a greater pressor effect than each drug alone in severe autonomic failure patients; pyridostigmine would increase efferent sympathetic activity by enhancing sympathetic ganglia neurotransmission and ultimately increase norepinephrine release, while atomoxetine would potentiate this effect by inhibiting the reuptake of synaptic norepinephrine (Figure 1). In addition, we evaluated whether this pressor effect of the combination on seated BP and orthostatic tolerance is synergistic and whether this interaction would translate into improvement of orthostatic symptoms.
Figure 1.
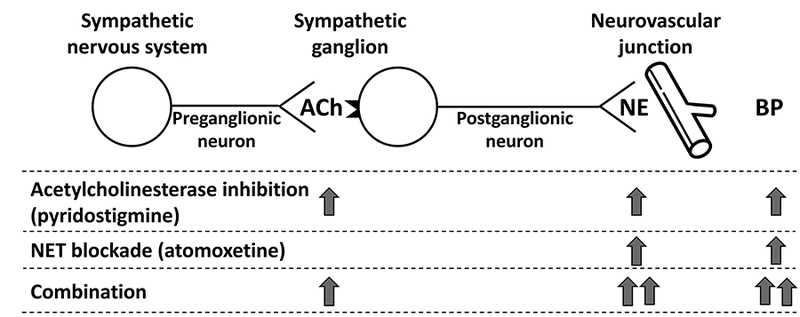
Proposed mechanism of the synergistic pressor effect of the combination atomoxetine and pyridostigmine. See text for details. ACh, acetylcholine; NE, norepinephrine; BP, blood pressure.
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request.
Subjects
A total of 12 patients with neurogenic OH and severe autonomic failure (5 with pure autonomic failure [PAF], 3 with probable multiple system atrophy [MSA], 2 with Parkinson disease, 1 with amyloidosis and 1 with autonomic failure of unknown pathogenesis) were recruited from referrals to Vanderbilt University Autonomic Dysfunction Center. Clinical diagnoses were defined using current diagnostic criteria.8–10 OH was defined as ≥20-mmHg decrease in systolic BP (SBP) or ≥10 mmHg of diastolic BP (DBP) within 3 minutes on standing.11 The neurogenic nature of their orthostatic hypotension was documented by an abnormal response to Valsalva maneuver. Patients were excluded if they were bedridden, or if they had contraindications to administration of pressor agents (e.g. coronary artery disease). The Vanderbilt University Investigational Review Board approved this study, and written informed consent was obtained from each subject before initiating the study (http://ClinicalTrials.gov NCT00223691).
Screening Procedures
Patients were admitted to the Clinical Research Center at Vanderbilt University Medical Center for the duration of their study participation. and were fed a low-monoamine, caffeine-free diet containing 150-mEq sodium and 70-mEq potassium per day. Medications affecting BP, blood volume and the autonomic nervous system were withheld for ≥5 half-lives before testing. The screening consisted of a medical history, physical examination, 12-lead ECG, laboratory assessments, and standardized autonomic function including orthostatic stress test, Valsalva maneuver, hyperventilation, cold pressor test, isometric handgrip and sinus arrhythmia.12 BP and heart rate (HR) were obtained using an automated oscillometric sphygmomanometer (Dinamap ProCare, GE Healthcare), finger photoplethysmography (Finometer, FMS, or Nexfin, BMEYE), and continuous ECG. During the orthostatic test, blood samples were obtained for norepinephrine while patients were supine and upright, as described previously.13 Plasma norepinephrine was measured by high-performance liquid chromatography with electrochemical detection.14
General Protocol
Acute medication trials were conducted in a post-void state and ≥2.5 hours after meals to avoid any confounding effects from postprandial hypotension. Patients were seated on a chair with their feet on the floor. BP and HR were recorded every 5 minutes with an automated brachial BP cuff (Dinamap ProCare, GE Healthcare). After 30 minutes of baseline measurements, patients were asked to stand for 10 minutes or until they developed symptoms of presyncope. BP and HR were measured at 1, 3, 5 and 10 minutes of standing (or as tolerated). The amount of time patients were able to stand was recorded by the study nurse using a timer. The study medication was given immediately after sitting. BP and HR were measured for the following 60 minutes, and the assessment of orthostatic tolerance was repeated at the end of this period, as described above. We assessed BP at 60min post-drug as it corresponds to the peak pressor effects of atomoxetine and pyridostigmine in autonomic failure.4,7,15 Patients were asked to rate the severity of their orthostatic symptoms immediately after the orthostatic stress tests using the Orthostatic Hypotension Symptom Assessment (OHSA) score.16 The questionnaire consisted of 6 items, including the following: (1) lightheadedness, dizziness, feeling faint or like passing out; (2) blurring vision, seeing spots, tunnel vision; (3) trouble concentrating; (4) weakness; (5) fatigue; and (6) head, neck or shoulder discomfort. Each item was scored on a 0–10 scale (with 0 reflecting absence of symptoms), and the total scores (range 0–60) before and after treatment were used as a measure of symptom burden.
Patients were given a single oral dose of placebo, atomoxetine 18 mg (Eli Lilly pharmaceuticals, Indianapolis, Ind.), pyridostigmine bromide 60 mg (Valeant Pharmaceuticals North America LLC, Bridgewater, NJ) or the combination of atomoxetine 18 mg and pyridostigmine bromide 60 mg in a single-blind, crossover fashion. The doses of atomoxetine and pyridostigmine have been previously shown to elicit pressor responses in autonomic failure patients.2–5,7,15Medication trials with placebo, atomoxetine and pyridostigmine were done on separate days in a random order, either on consecutive days or 1 day apart. For safety reasons the combination was given after the study days with active medications due to the concern that patients who had a large pressor response to either atomoxetine or pyridostigmine alone could have a larger and unsafe pressor response to the combination.
Statistical Methods
We hypothesized that the combination had a greater effect on seated BP compared to each drug alone, and that the combination had a synergistic effect on seated BP compared to the sum of pressor effects of the 2 drugs individually. The primary outcome was defined a priori as the seated SBP during the 60-minute postdrug period, as most patients with severe autonomic failure are only able to stand for a few seconds to minutes due to disabling presyncopal symptoms. The seated position provides a well-tolerated orthostatic stress in these patients that allows for evaluation over prolonged time periods, and in previous studies we have found that the acute pressor response on the seated position is a reasonable predictor of that on standing.2,3,7,15 Overall differences among treatment groups were analyzed by two-way repeated-measures ANOVA. If a significant overall treatment difference was found, paired comparisons between the combination and each drug alone (placebo, atomoxetine and pyridostigmine) were performed using paired t tests with Bonferroni correction as post hoc test. A similar approach was used to test whether there is any difference in seated DBP and HR between treatment groups. The synergistic pressor effect of the combination was assessed by comparing the change from baseline in seated SBP (ΔSBP) during the combination with the sum of the pressor responses to the two drugs individually during the postdrug period using the same approach.
Secondary outcomes included orthostatic tolerance and orthostatic symptom score. The orthostatic tolerance was defined as the area under the curve of standing SBP calculated by the trapezoidal rule (AUCSBP; upright SBP multiplied by standing time). This is a composite score that integrates both the standing time and the upright SBP.15 Wilcoxon signed-rank test was used to test whether each treatment decreased the secondary outcomes compared with their baselines, and whether the change from baseline in orthostatic tolerance (AUCSBP) after the combination was greater than the sum of effects after atomoxetine and pyridostigmine alone. Comparisons were made only for patients who could stand after all active medications. Power calculation was based on preliminary data from 3 patients. The standard deviation of the difference in seated SBP among treatment groups 1-hour postdrug was 16 mmHg. An increase in seated blood pressure of 20 mmHg would be a clinically meaningful difference, representing the approximate magnitude of response achieved with other vasoconstrictor drugs.17 Based on these data, a sample size of 12 patients would have 97% power to detect a difference in means among treatments with an α level of 0.05 using paired t test analysis (PS Dupont, version 3.0.34). Data are presented as mean ± SEM unless otherwise noted. All of the tests were 2-tailed, and a P value of <0.05 was considered significant. Analyses were performed with SPSS version 23.0 (IBM Corp).
Results
Patient Characteristics and Autonomic Testing
We studied 12 patients with severe autonomic failure (7 men, 69±3 years, BMI 25±1 Kg/m2). Patient clinical and autonomic characteristics are shown in Tables 1 and 2. Severe autonomic failure was evidenced by a profound decrease in SBP on standing (−62±7 mmHg), without an adequate compensatory increase in HR (11±2 bpm), and by impaired autonomic reflexes. Respiratory sinus arrhythmia was markedly reduced in all patients, suggesting parasympathetic dysfunction. Evidence of sympathetic dysfunction included an exaggerated decrease in SBP during phase II and absence of BP overshoot during phase IV of the Valsalva maneuver, and blunted pressor responses to isometric handgrip and cold pressor tests.
Table 1. Patient Characteristics.
| Patient | Diagnosis | Gender | Age (y) | Body Weight (Kg) |
Systolic BP (mmHg) | Heart rate (bpm) | Norepinephrine (pg/mL) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Supine | Upright | Supine | Upright | Supine | Upright | |||||
| 1 | Amyloidosis | M | 73 | 75 | 110 | 69 | 80 | 91 | 111 | 206 |
| 2 | PAF | M | 65 | 88 | 154 | 88 | 57 | 62 | 32 | 79 |
| 3 | AF | F | 74 | 62 | 173 | 114 | 82 | 97 | 127 | 241 |
| 4 | PD+OH | M | 61 | 88 | 127 | 83 | 78 | 101 | 214 | 422 |
| 5 | PAF | M | 73 | 72 | 111 | 69 | 70 | 75 | 60 | 60 |
| 6 | PD+OH | M | 79 | 91 | 172 | 74 | 71 | 69 | 144 | 421 |
| 7 | PAF | M | 60 | 64 | 93 | 47 | 80 | 89 | 74 | 94 |
| 8 | PAF | M | 78 | 101 | 148 | 84 | 72 | 82 | 246 | 398 |
| 9 | MSA | F | 56 | 69 | 152 | 78 | 73 | 97 | 127 | 169 |
| 10 | PAF | F | 78 | 61 | 190 | 71 | 69 | 77 | 56 | 104 |
| 11 | MSA | F | 58 | 61 | 153 | 99 | 69 | 82 | 178 | 233 |
| 12 | MSA | F | 49 | 68 | 124 | 92 | 63 | 75 | 97 | 242 |
| Mean±SEM | 69±3 | 75±4 | 142±9 | 81±5 | 72±2 | 83±4 | 122±19 | 222±38 | ||
Data are presented as mean±SEM. PAF, pure autonomic failure; AF, autonomic failure of unknown etiology; PD+OH, Parkinson disease with neurogenic orthostatic hypotension; MSA, multiple system atrophy; M, male; F, female. Data were obtained at Screening.
Table 2. Autonomic Function Tests and Orthostatic Stress Test.
| Parameters (Unit) | Patients | Normals* | ||
|---|---|---|---|---|
| Orthostatic change in systolic BP, mmHg | −62 ± 7 | ≤ 20 | ||
| Orthostatic change in heart rate, bpm | 11 ± 2 | 5–10 | ||
| Sinus arrhythmia ratio | 1.05 ± 0 | 1.2 ± 0.1 | ||
| Depressor response to Valsalva in phase II, mmHg | −68 ± 8 | ≤ 20 | ||
| BP response to Valsalva phase IV, mm Hg† | −40 ± 5 | >20 | ||
| Valsalva ratio | 1.08 ± 0 | 1.5 ± 0.2 | ||
| Depressor response to hyperventilation, mmHg | −21 ± 4 | −5 ± 6.3 | ||
| Pressor response to cold pressor, mmHg | 4 ± 3 | 24 ± 13 | ||
| Pressor response to handgrip, mmHg | −3 ± 4 | 16 ± 6 | ||
Values are expressed as mean±SEM. Pressor responses are given as changes in systolic BP.
Normal values are from the Autonomic Dysfunction Database at Vanderbilt University Medical Center.
A negative value for phase IV of the Valsalva maneuver indicates that the blood pressure overshoot was absent.
Pressor Effect of Drugs
All participants (n=12) completed the four treatment arms. Average baseline seated SBP and DBP were similar among placebo (95±6/60±3 mmHg), atomoxetine (92±7/61±4 mmHg), pyridostigmine (105±8/65±4 mmHg) and the combination groups (100±7/61±4 mmHg; P=0.160 for SBP and P=0.639 for DBP by repeated-measures ANOVA), suggesting that no significant carryover effects were present between study days. The combination significantly increased seated SBP and DBP compared to placebo and to each drug alone (Figure 2A and 2B; P<0.001 for drug*time interaction for comparisons in SBP and DBP; two-way repeated measures ANOVA). The maximal increase in SBP and DBP was seen 60 minutes after the combination (33±8/18±3 mmHg), with an average BP of 133±9/80±4 mmHg. At this timepoint, the seated BP was significantly higher with the combination than with placebo (107±6/66±4 mmHg; P<0.001), atomoxetine (105±5/67±3 mmHg; P<0.001) and pyridostigmine (99±6/64±4 mmHg; P<0.001). There was no significant difference in seated SBP or DBP between placebo vs. atomoxetine, placebo vs. pyridostigmine or between the two drugs. We found that the change from baseline in seated SBP with the combination was significantly greater than the sum of the SBP changes produced by the two drugs individually (Figure 2C; P=0.019 for main treatment effect, two-way repeated measures ANOVA), suggesting a synergistic, rather than an additive, pressor effect. A similar trend was observed for DBP (P=0.014). BP changes were not accompanied by significant changes from baseline in HR (placebo: −2±1 bpm; atomoxetine: −4±2 bpm; pyridostigmine: −1±1 bpm; and the combination:−4±2 bpm; all comparisons: P>0.05).
Figure 2.
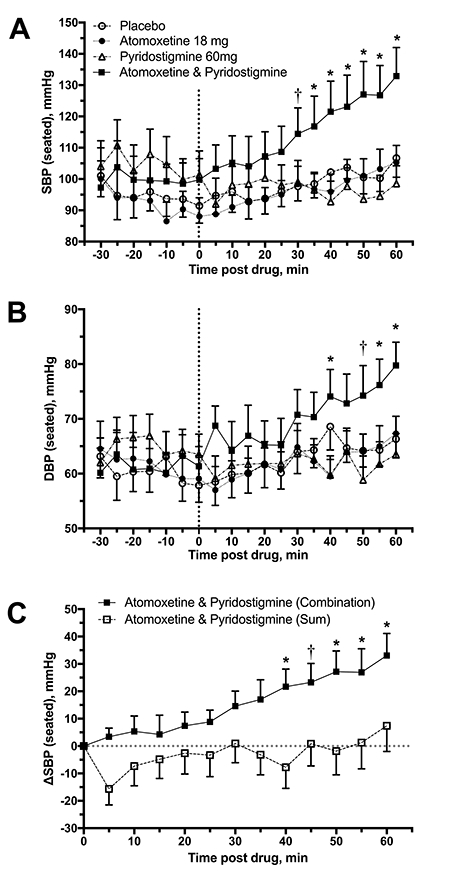
Seated systolic (SBP, A) and diastolic (DBP, B) blood pressures before and after drug administration (discontinued line). The combination significantly increased seated SBP and DBP compared to placebo and to each drug alone. There was no significant difference between placebo vs. atomoxetine, placebo vs. pyridostigmine or between the two drugs. Panel C shows the changes from baseline in seated SBP (ΔSBP) during 60 minutes postdrug. The increase in SBP with the combination was significantly greater than the sum of the SBP changes produced by the two drugs individually. Values are expressed as mean±SEM. Overall differences were analyzed by two-way repeated measures ANOVA. *P<0.001 and †P<0.05, adjusted for multiple comparisons using Bonferroni correction.
Orthostatic Tolerance and Symptoms
Of the 12 patients studied, 10 were able to stand during all treatment arms and were included in the analysis of orthostatic tolerance. The change from baseline in 1-minute standing BP was −2±4/1±5 mmHg with placebo, 14±6/6±5 mmHg with atomoxetine, −1±5/1±4 mmHg with pyridostigmine and 20±9/10±3 mmHg with the combination. Only the combination showed an improvement in orthostatic tolerance, as indicated by a significantly higher standing AUCSBP at 60 minutes postdrug compared with that at baseline (779±173 versus 551±136, respectively; P<0.001; Figure 3A). In contrast, the AUCSBP did not increase significantly after atomoxetine (619±145 versus 463±136 at baseline; P=0.106), pyridostigmine (466±110 versus 438±109 at baseline; P=0.557) or placebo (546±118 versus 424±112 at baseline; P=0.131). This represents an increase in AUCSBP of 228 for the combination group versus 156 for atomoxetine, 28 for pyridostigmine and 122 for placebo. The standing AUCDBP followed a similar trend. The AUCDBP at 60 minutes postdrug significantly increased with the combination (460±104 versus 356±89, respectively; P=0.01), but not with pyridostigmine (321±74 versus 289±75 at baseline; P=0.232) or placebo (360±80 versus 290±82 at baseline; P=0.193). Atomoxetine, however, tended to increase AUCDBP but the difference did not reach statistical significance (421±102 versus 332±106 at baseline; P=0.084). The increase from baseline in standing AUCSBP and AUCDBP produced by the combination was not statistically different than the sum of that produced by the two drugs individually (AUCSBP 228±64 versus 184±85 for the sum, and AUCDBP 105±31 versus 120±7 for the sum; P=0.625 and P=0.695, respectively).
Figure 3.
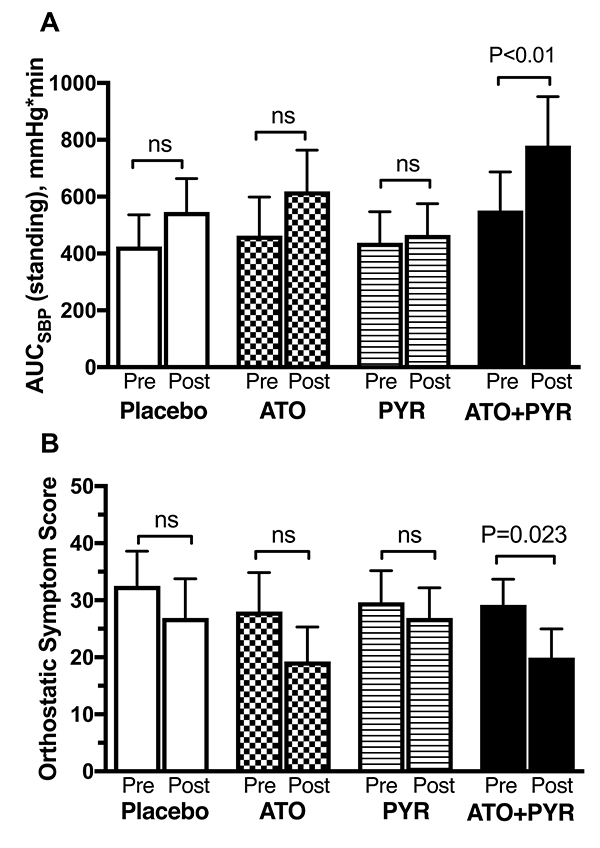
Area under the curve of standing SBP (ΔAUCSBP, A) and orthostatic symptom score (B) at baseline (pre) and after 1-hour postdrug (post). The total score ranges from 0–60, with lower scores reflecting lower symptom burden. Values are expressed as mean±SEM. The P values were generated by Wilcoxon signed-rank test.
Eight patients completed orthostatic symptom scores for all treatment arms. Two patients had incomplete (n=1) or missing questionnaires (n=1). The total orthostatic symptom burden after 1-hour postdrug significantly improved with the combination (lower scores) as compared to baseline (19.9±5.0 vs. 29.2±4.5, respectively; P=0.023; Figure 3B). Atomoxetine produced a similar improvement in the orthostatic symptom score but the difference did not reach statistical significance (19.3±6.1 vs. 28.0±6.8 at baseline; P=0.086). In contrast, the total symptom burden did not improve after pyridostigmine (26.9±5.3 vs. 29.6±5.6 at baseline; P=0.273).
In order to determine predictors of a good therapeutic response, we assessed the association between plasma norepinephrine levels measured at Screening and BP response. No significant correlation was found between supine or upright plasma norepinephrine levels and the seated or upright BP (AUC of SBP or DBP) response.
Discussion
The main finding of this study was that the combination of peripheral acetylcholinesterase inhibition with pyridostigmine and norepinephrine reuptake blockade with atomoxetine acted synergistically to increase seated BP in autonomic failure patients that were unresponsive to either medication alone. Furthermore, this synergistic effect of the combination was associated with improvement in orthostatic tolerance and symptoms. We propose that this interaction may be explained by enhanced cholinergic sympathetic ganglionic transmission by acetylcholinesterase inhibition, resulting in increased residual sympathetic tone, combined with increased synaptic norepinephrine concentrations by NET blockade. This pharmacologic approach may be a useful therapeutic alternative in autonomic failure patients who do not respond to either drug alone.
Norepinephrine reuptake inhibition with atomoxetine increases the synaptic concentrations of norepinephrine and enhances the activation of pre- and post-synaptic adrenoreceptors. In the periphery, NET blockade would lead to increases in BP and HR.18 This effect, however, appears to be partly counteracted by a central clonidine-like sympatholytic effect mediated by activation of α−2 adrenoreceptors in the brain.19–22 This central sympatholytic effect is likely to be significant in subjects with intact autonomic function, and probably accounts for the observation that NET inhibitors result in only minimal, if any, increases in BP. Thus, the overall pressor effect of systemic NET inhibition seems to depend on the balance between peripheral sympathetic stimulation and central sympathetic inhibition.18 Consistent with this, we previously reported that atomoxetine preferentially increased BP in patients with central autonomic failure (MSA), who lack of central autonomic modulation but have intact peripheral sympathetic fibers and residual sympathetic tone, while having less of an effect in those with peripheral autonomic failure (PAF and PD with OH), who have low sympathetic tone due to peripheral sympathetic denervation.2,15 The range of BP responses to atomoxetine, however, was wide with significant overlap between groups, suggesting that the pressor effect mainly depends on the presence of residual sympathetic tone with norepinephrine release from peripheral sympathetic nerves. In this study, we found no effect on seated BP with atomoxetine in our group of patients. This could be explained partly by the fact that 67% (n=8) of patients were severe peripheral forms of autonomic failure, whereas 25% (n=3) were MSA. The MSA patients, however, had a similar effect on seated BP with atomoxetine to that of patients with peripheral autonomic failure (ΔSBP 11±8 mmHg and 12±9 mmHg, respectively). The cause for the poor response to atomoxetine in the MSA patients is not apparent from our studies.
Acetylcholinesterase inhibition with pyridostigmine potentiates transmission of impulses from cholinergic neurons across the synaptic cleft. Because pre-ganglionic autonomic neurons are cholinergic, pyridostigmine is thought to amplify sympathetic ganglionic neurotransmission. Traffic to the autonomic ganglia is normally reduced while supine but maximally activated during upright posture, this medication offers the theoretical advantage of preferentially increasing sympathetic neurotransmission and upright BP in patients with autonomic failure, and in proportion to their orthostatic needs.4 Indeed, pyridostigmine preferentially prevented the orthostatic fall in BP without worsening supine hypertension in central and peripheral autonomic failure patients.5 The pressor effect of pyridostigmine, however, is rather modest; in published studies upright SBP was only 4 mmHg higher in the pyridostigmine group compared with the placebo group.5 Moreover, a greater pressor response to pyridostigmine was seen in patients with relatively preserved baroreflex function,4 suggesting that the pyridostigmine response is related to the degree of residual sympathetic function. In support of this concept, we found that, in our cohort of patients severely affected by autonomic impairment, pyridostigmine had no effect on seated BP or orthostatic tolerance, which is in agreement with our previous studies.7
Given that both pyridostigmine and atomoxetine exert their pressor effects through activation of residual sympathetic tone, it is not surprising that they have little if any effect in patients with severe autonomic failure, particularly those with degeneration of postganglionic efferent noradrenergic fibers. This was exemplified by the results of the present study. Neither pyridostigmine nor atomoxetine had any significant pressor effect on seated BP. Co-administration of both medications, however, significantly increased seated BP. Moreover, the magnitude of the pressor response to the combination was higher than the sum of the responses to each drug alone, suggesting a synergistic pressor response (Figure 2C). We propose that pyridostigmine and atomoxetine act synergistically at two distinct and complementary levels to enhance residual sympathetic tone (Figure 1). In the sympathetic ganglia, acetylcholinesterase inhibition would facilitate sympathetic ganglionic neurotransmission and increase postganglionic sympathetic nerve traffic. In the neurovascular junction, norepinephrine concentrations would be further increased through reduced NE clearance by the NET blockade. Our findings support the hypothesis that residual sympathetic efferent fibers can be pharmacologically engaged to treat orthostatic hypotension in autonomic failure patients, even in those with severe peripheral autonomic failure, given that the loss of efferent sympathetic function is incomplete in many patients.
Supine hypertension is present in ~50% of patients with autonomic failure, and often complicates the management of OH.23 In such patients, the goal of treatment is to preferentially improve upright BP, without increasing supine or seated BP. Currently, no pharmacologic treatment achieves this goal. Midodrine and other pressor agents can induce or worsen supine hypertension, given that these drugs increase both supine and standing BP, so that OH (the difference between supine and standing BP) is often not selectively improved. Pyridostigmine, on the other hand, has been shown to preferentially improve upright BP, but its effects are modest.4,5 Atomoxetine may also preferentially improve upright BP compared to midodrine.3 Consistent with this, we found that atomoxetine tended to increase orthostatic tolerance (AUCBP) despite the lack of response on seated BP. We did not find, however, that the combination had a preferential greater improvement in upright BP compared to seated BP (Figure 4). Further research is needed to determine if this will be the case in less severe patients. The combination significantly improved orthostatic tolerance but we were not able to document a synergistic effect. The AUCSBP tended to be greater than the sum of that produced by the two drugs individually, but the difference did not reach statistical significance possibly due to the variability of the response in these severely affected patients.
Figure 4.
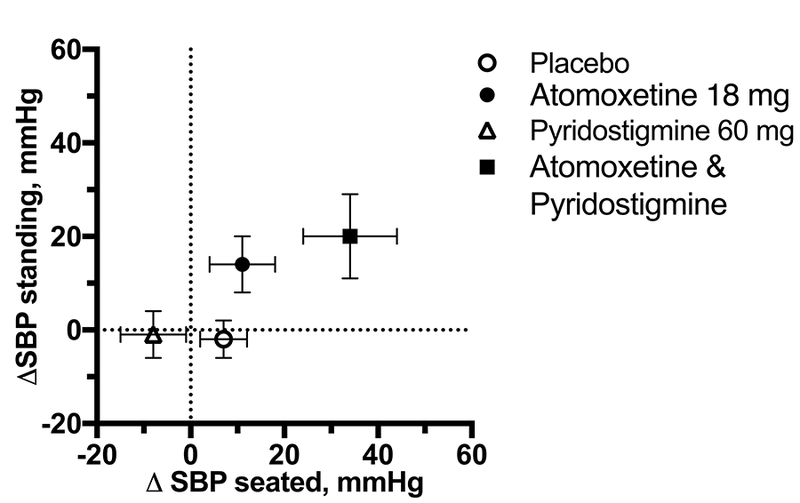
Changes from baseline in seated SBP (ΔSBP) and standing SBP (1-min standing) after 1 h postdrug (postdrug minus baseline SBP). Neither atomoxetine nor pyridostigmine, alone or in combination, produced selective increase in upright SBP. Values are expressed as mean±SEM.
Potential limitations to this study included a relatively small sample of patients. Our study was powered to detect differences in seated SBP, the primary outcome. We cannot rule out, however, that secondary outcomes including orthostatic tolerance or symptoms may have reached significance with additional patients. In addition, we did not monitor BP beyond 1 hour after drug administration. Although we predicted that the peak pressor response to the combination would occur at 1 hour postdrug based on our results and previous studies,4,7,15 it is possible that some patients may have larger pressor responses to the combination at longer time points, particularly those who are poor metabolizers of cytochrome P450 CYP2D6 (~7% of whites and 2% of blacks), which metabolizes atomoxetine.24 Furthermore, it is possible that the combination induces new or worsens preexistent supine hypertension in these patients, as seen with most pressor agents; we did not assess supine BP because we designed our study to comply with standard of care in patients with autonomic failure in whom we recommend as a routine clinical practice to avoid the supine position for 4 to 5 hours after drug administration and omit a dose if supine or sitting BP is >180/110 mm Hg. The effects on long-term outcomes such as frequency of falls, long-term safety and efficacy was not assessed in our study. Further research is required to assess the long-term effects of the combination. Finally, diagnosis of pure autonomic failure was made clinically, and pathological confirmation, which requires an autopsy, was lacking. We cannot rule out that the PAF patients may later prove to have other disorders such as MSA, PD, dementia with Lewy bodies or autoimmune autonomic ganglionopathy.25,26
Perspectives
Autonomic failure provides a unique human model to explore cardiovascular pharmacology, given that the hemodynamic effects of drugs are magnified or even “unmasked” in these patients because of the extreme sensitivity they have to any pressor or depressor stimuli. Medications that enhance sympathetic activity can produce significant pressor responses in autonomic failure patients depending on the degree of residual sympathetic function. Our results showed that the combination of atomoxetine and pyridostigmine elicited a profound and synergistic effect on seated BP in severe autonomic failure patients, despite the lack of response to each drug alone. This supports the hypothesis that residual sympathetic efferent fibers can be pharmacologically engaged even in patients with severe peripheral autonomic failure, given that the loss of efferent sympathetic function is incomplete in many patients. This synergistic interaction can be exploited in the treatment of orthostatic hypotension in patients who do not respond to these drugs individually. Further research is required to assess the long-term safety and efficacy of the combination and its pressor effects in less severe patients.
Novelty and Significance:
1. What is New:
The combination of peripheral acetylcholinesterase inhibition with pyridostigmine and norepinephrine reuptake blockade with atomoxetine acted synergistically to increase seated BP in autonomic failure patients that were unresponsive to either medication alone.
This drug combination was associated with improvement in orthostatic tolerance and symptoms.
2. What is Relevant:
Autonomic failure provides a unique human model to understand cardiovascular pharmacology given the lack of baroreflex buffering.
In these patients, residual sympathetic efferent fibers can be pharmacologically engaged, even in those with severe peripheral autonomic denervation given that the loss of efferent sympathetic function is incomplete in many patients.
The synergistic effect of the combination can be exploited in the treatment of orthostatic hypotension in patients who do not respond to these drugs individually.
3. Summary:
In severe autonomic failure patients, the combination pyridostigmine and atomoxetine had a synergistic effect on seated BP and improved orthostatic tolerance and symptoms. Future safety and long-term efficacy studies are required to address the clinical usefulness of this approach.
Acknowledgements
We acknowledge the patients who volunteered for these studies and the Clinical Research Center nurses who made this study possible.
Source of Funding
This work was supported by National Institutes of Health grants (NIH) PO1 HL56693, U54 NS065736, R01 HL122847, and UL1 TR002243 (National Center for Advancing Translational Sciences). Additional support was provided by American Heart Association grant 14CRP20380211 (L.O.). C.A.S. was supported by Doris Duke Foundation Career Development Award and by FDA grant R01 FD04778–02. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Advancing Translational Sciences or the NIH.
Footnotes
Conflicts of Interest/Disclosures
None.
References
- 1.Jordan J, Shannon JR, Black BK, Lance RH, Squillante MD, Costa F, Robertson D. N(N)-nicotinic blockade as an acute human model of autonomic failure. Hypertension. 1998;31:1178–1184. [DOI] [PubMed] [Google Scholar]
- 2.Shibao C, Raj SR, Gamboa A, Diedrich A, Choi L, Black BK, Robertson D, Biaggioni I. Norepinephrine transporter blockade with atomoxetine induces hypertension in patients with impaired autonomic function. Hypertension. 2007;50:47–53. [DOI] [PubMed] [Google Scholar]
- 3.Ramirez CE, Okamoto LE, Okamoto LE, Arnold AC, Gamboa A, Gamboa A, Diedrich A, Diedrich A, Choi L, Choi L, Raj SR, Raj SR, Robertson D, Robertson D, Biaggioni I, Biaggioni I, Shibao CA. Efficacy of atomoxetine versus midodrine for the treatment of orthostatic hypotension in autonomic failure. Hypertension. 2014;64:1235–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singer W, Opfer-Gehrking TL, McPhee BR, Hilz MJ, Bharucha AE, Low PA. Acetylcholinesterase inhibition: a novel approach in the treatment of neurogenic orthostatic hypotension. J Neurol Neurosurg Psychiatr 2003;74:1294–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singer W, Sandroni P, Opfer-Gehrking TL, Suarez GA, Klein CM, Hines S, O’Brien PC, Slezak J, Low PA. Pyridostigmine treatment trial in neurogenic orthostatic hypotension. Arch Neurol 2006;63:513–518. [DOI] [PubMed] [Google Scholar]
- 6.Shibao C, Okamoto LE, Biaggioni I. Pharmacotherapy of autonomic failure. Pharmacol Ther 2012;134:279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shibao C, Okamoto LE, Gamboa A, Yu C, Diedrich A, Raj SR, Robertson D, Biaggioni I. Comparative efficacy of yohimbine against pyridostigmine for the treatment of orthostatic hypotension in autonomic failure. Hypertension. 2010;56:847–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, Wood NW, Colosimo C, Dürr A, Fowler CJ, Kaufmann H, Klockgether T, Lees A, Poewe W, Quinn N, Revesz T, Robertson D, Sandroni P, Seppi K, Vidailhet M. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71:670–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaufmann H Consensus statement on the definition of orthostatic hypotension, pure autonomic failure and multiple system atrophy. Clin Auton Res 1996;6:125–126. [DOI] [PubMed] [Google Scholar]
- 10.Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, Obeso J, Marek K, Litvan I, Lang AE, Halliday G, Goetz CG, Gasser T, Dubois B, Chan P, Bloem BR, Adler CH, Deuschl G. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 2015;30:1591–1601. [DOI] [PubMed] [Google Scholar]
- 11.Gibbons CH, Schmidt P, Biaggioni I, Frazier-Mills C, Freeman R, Isaacson S, Karabin B, Kuritzky L, Lew M, Low P, Mehdirad A, Raj SR, Vernino S, Kaufmann H. The recommendations of a consensus panel for the screening, diagnosis, and treatment of neurogenic orthostatic hypotension and associated supine hy… J Neurol 2017;18:8–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mosqueda-Garcia R Evaluation of autonomic failure Disorders of the Autonomic Nervous System Luxembourg: Harwood Academic Publishers GmbH; 1995;25:59. [Google Scholar]
- 13.Arnold AC, Okamoto LE, Gamboa A, Shibao C, Raj SR, Robertson D, Biaggioni I. Angiotensin II, Independent of Plasma Renin Activity, Contributes to the Hypertension of Autonomic Failure. Hypertension. 2013;61:701–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldstein DS, Eisenhofer G, Stull R, Folio CJ, Keiser HR, Kopin IJ. Plasma dihydroxyphenylglycol and the intraneuronal disposition of norepinephrine in humans. J Clin Invest 1988;81:213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okamoto LE, Shibao C, Gamboa A, Choi L, Diedrich A, Raj SR, Black BK, Robertson D, Biaggioni I. Synergistic Effect of Norepinephrine Transporter Blockade and α−2 Antagonism on Blood Pressure in Autonomic Failure. Hypertension. 2012;59:650–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaufmann H, Malamut R, Norcliffe-Kaufmann L, Rosa K, Freeman R. The Orthostatic Hypotension Questionnaire (OHQ): validation of a novel symptom assessment scale. Clin Auton Res 2012;22:79–90. [DOI] [PubMed] [Google Scholar]
- 17.Jordan J, Shannon JR, Biaggioni I, Norman R, Black BK, Robertson D. Contrasting actions of pressor agents in severe autonomic failure. Am J Med 1998;105:116–124. [DOI] [PubMed] [Google Scholar]
- 18.Mayer AF, Schroeder C, Heusser K, Tank J, Diedrich A, Schmieder RE, Luft FC, Jordan J. Influences of norepinephrine transporter function on the distribution of sympathetic activity in humans. Hypertension. 2006;48:120–126. [DOI] [PubMed] [Google Scholar]
- 19.Esler MD, Wallin G, Dorward PK, Eisenhofer G, Westerman R, Meredith I, Lambert G, Cox HS, Jennings G. Effects of desipramine on sympathetic nerve firing and norepinephrine spillover to plasma in humans. Am J Physiol 1991;260:R817–23. [DOI] [PubMed] [Google Scholar]
- 20.Birkenfeld AL, Schroeder C, Boschmann M, Tank J, Franke G, Luft FC, Biaggioni I, Sharma AM, Jordan J. Paradoxical effect of sibutramine on autonomic cardiovascular regulation. Circulation. 2002;106:2459–2465. [DOI] [PubMed] [Google Scholar]
- 21.Eisenhofer G, Saigusa T, Esler MD, Cox HS, Angus JA, Dorward PK. Central sympathoinhibition and peripheral neuronal uptake blockade after desipramine in rabbits. Am J Physiol 1991;260:R824–32. [DOI] [PubMed] [Google Scholar]
- 22.Tank J, Schroeder C, Diedrich A, Szczech E, Haertter S, Sharma AM, Luft FC, Jordan J. Selective impairment in sympathetic vasomotor control with norepinephrine transporter inhibition. Circulation. 2003;107:2949–2954. [DOI] [PubMed] [Google Scholar]
- 23.Shannon J, Jordan J, Costa F, Robertson RM, Biaggioni I. The hypertension of autonomic failure and its treatment. Hypertension. 1997;30:1062–1067. [DOI] [PubMed] [Google Scholar]
- 24.Sauer J-M, Ring BJ, Witcher JW. Clinical pharmacokinetics of atomoxetine. Clin Pharmacokinet 2005;44:571–590. [DOI] [PubMed] [Google Scholar]
- 25.Kaufmann H, Norcliffe-Kaufmann L, Palma JA, Biaggioni I, Low PA, Singer W, Goldstein DS, Peltier AC, Shibao CA, Gibbons CH, Freeman R, Robertson D, Autonomic Disorders Consortium. Natural history of pure autonomic failure: A United States prospective cohort. Ann Neurol 2017;81:287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barbic F, Dipaola F, Andreetta F, Brunetta E, Dalla Vecchia L, Mantegazza R, Furlan R, Antozzi C. Long-term cardiovascular autonomic and clinical changes after immunoglobulin G immunoadsorption therapy in autoimmune autonomic ganglionopathy. J Hypertens 2017;35:1513–1520. [DOI] [PubMed] [Google Scholar]


