Abstract
Objective
AIO-PK0104 investigated two treatment strategies in advanced pancreatic cancer (PC): a reference sequence of gemcitabine/erlotinib followed by 2nd-line capecitabine was compared with a reverse experimental sequence of capecitabine/erlotinib followed by gemcitabine.
Methods
281 patients with PC were randomly assigned to 1st-line treatment with either gemcitabine plus erlotinib or capecitabine plus erlotinib. In case of treatment failure (eg, disease progression or toxicity), patients were allocated to 2nd-line treatment with the comparator cytostatic drug without erlotinib. The primary study endpoint was time to treatment failure (TTF) after 1st- and 2nd-line therapy (TTF2; non-inferiority design). KRAS exon 2 mutations were analysed in archival tumour tissue from 173 of the randomised patients.
Results
Of the 274 eligible patients, 43 had locally advanced and 231 had metastatic disease; 140 (51%) received 2nd-line chemotherapy. Median TTF2 was estimated with 4.2 months in both arms; median overall survival was 6.2 months with gemcitabine/erlotinib followed by capecitabine and 6.9 months with capecitabine/erlotinib followed by gemcitabine, respectively (HR 1.02, p=0.90). TTF for 1st-line therapy (TTF1) was significantly prolonged with gemcitabine/ erlotinib compared to capecitabine/erlotinib (3.2 vs 2.2 months; HR 0.69, p=0.0034). Skin rash was associated with both TTF2 (rash grade 0/1/2–4:2.9/4.3/ 6.7 months, p<0.0001) and survival (3.4/7.0/ 9.6 months, p<0.0001). Each arm showed a safe and manageable toxicity profile during 1st- and 2nd-line therapy. A KRAS wild-type status (52/173 patients, 30%) was associated with an improved overall survival (HR 1.68, p=0.005).
Conclusion
Both treatment strategies are feasible and demonstrated comparable efficacy; KRAS may serve as biomarker in patients with advanced PC treated with erlotinib.
INTRODUCTION
Exocrine pancreatic cancer (PC) remains a global health problem: in 2008, an estimated number of 165 100 new cases were diagnosed worldwide in developed countries, with a nearly identical number of annual PC deaths (161 800).1 For more than a decade, the nucleoside analogue gemcitabine has been regarded as a standard of care for patients with advanced disease, providing clinical benefit and a moderate improvement in survival.2,3 Several randomised phase 3 trials have failed to show a survival benefit for gemcitabine-based combination chemotherapy; however, data from meta-analyses suggest a possible survival benefit for the use of platinum analogues or fluoropyrimidines in combination with gemcitabine in selected patients (eg, those with metastatic disease and a good performance status).4–9 Based on the results of a randomised trial conducted by Moore et al, the combination of gemcitabine with the novel anti-EGFR tyrosine kinase inhibitor erlotinib (100 mg/day) received US regulatory approval from the FDA in November 2005 for 1st-line treatment of advanced PC. The observed survival benefit in this unselected patient population (n=569) was statistically significant, but clinically rather modest (5.9 vs 6.2 months; HR 0.82, p=0.038).10 Within the pivotal PA.3 study, a small subgroup of patients (n=23) was treated with an increased dose level of erlotinib (150 mg/day): as 11 patients (48%) of this cohort required protocol-prescribed dose reductions for toxicity, the authors recommended a daily dose of erlotinib 100 mg for the indication advanced PC.10 In contrast, a phase 1b clinical trial in patients with non-resectable PC and other advanced solid malignancies found the combination of standard gemcitabine and 150 mg erlotinib daily to be tolerated well.11
Preclinical and early clinical data support the investigation of erlotinib also in combination with the oral fluoropyrimidine capecitabine.12,13 A 2nd-line phase 2 study in gemcitabine pretreated patients with advanced PC found the combination of capecitabine together with a daily dose of 150 mg erlotinib safe and feasible.13 However, up to now, no internationally accepted standard approach for salvage chemotherapy after failure of 1st-line gemcitabine has been established in PC. Nevertheless, increasing evidence exists that 2nd-line chemotherapy may improve survival in selected patients after gemcitabine failure, and a fluoropyrimidine-based therapeutic approach seems rational in this patient population.14–16 Thus, the prospective inclusion of predefined 2nd-line treatment strategiesdalso within the setting of randomised phase 3 1st-line clinical trialsdappears consistent. With the use of a sequential trial design, a prospective evaluation of therapeutic strategies using two successive lines of systemic treatment can be investigated.17 Validated molecular prognostic or even predictive biomarkers for efficacy of anti-EGFR agents like erlotinib or cetuximab are still lacking in PC. Recently only the authors of the erlotinib pivotal PA.3 trial (n=569) reported a biomarker analysis on KRAS mutation (n=117) and EGFR gene copy number (n=107) in a small subset of their study patients.18 Within some retrospective single-centre studies, the presence of a KRAS codon 12 mutation was found to be a negative prognostic factor in PC patients not receiving anti-EGFR treatment.19
The main objectives of this multicentre, randomised AIO phase 3 trial were: first, to investigate the efficacy and safety of erlotinib (150 mg/day) in combination with either gemcitabine or capecitabine as 1st-line treatment; second, to assess the feasibility of a prospectively predefined 2nd-line chemotherapy after failure of the 1st-line regimen; and third, to prospectively correlate skin rash during erlotinib treatment with efficacy outcome parameters. Additionally (within a post-hoc translational sub-study), archival formalin fixed paraffin embedded (FFPE) tumour tissue obtained from trial participants was analysed centrally for KRAS mutation status.
PATIENTS AND METHODS
Patient population and study design
Adult patients between 18 and 75 years of age with a histologically or cytologically confirmed diagnosis of treatment-naïve advanced exocrine PC (stage III and IV) and adequate organ function were eligible. No previous chemotherapy or radio-therapy was allowed and a Karnofsky performance status (KPS) of at least 60% was required. The study had approval of the ethical committees in all participating German centres and each patient gave written informed consent prior to any study specific procedure. This study was conducted according to GCP/ ICH guidelines and according to the Declaration of Helsinki. Details on the included patient population, study design and treatment for this trial have already been published previously in the context of an interim safety analysis.20 The primary study objective was a non-inferiority comparison of the two treatment arms with regard to time-to-treatment failure after 1st- and 2nd-line therapy (TTF2). Secondary endpoints included time to treatment failure after 1st-line therapy (TTF1), objective response by imaging (according to RECIST version 1.0), overall survival (OS) and toxicity. This trial was registered at http://www.clinicaltrials.gov (trial identifier: NCT00440167).
Randomisation
For this prospective, multicentre, two-arm, AIO phase 3 trial, patients were stratified according to stage (locally advanced vs metastatic disease) and centre; randomisation was performed centrally by fax in a 1:1 ratio. Patients and investigators were not blinded to treatment assignments.
Treatment procedures
Within a reference arm, patients received 1st-line chemotherapy with gemcitabine (1000 mg/m2 intravenously over 30 min weekly × 7 followed by 1 week rest, then weekly × 3 every 4 weeks, according to the Burris regimen3) in combination with erlotinib (150 mg daily); in case of treatment failure, 2nd-line therapy with single-agent capecitabine (1000 mg/m2 twice daily for two weeks, followed by 1 week rest) was initiated. Treatment failure was defined by the occurrence of disease progression, unacceptable toxicity, patient refusal to continue the current treatment (for any reason) or death from any cause. In the experimental arm, 1st-line therapy consisted of oral capecitabine (1000 mg/m2 twice daily for 2 weeks, followed by 1 week rest) and erlotinib (150 mg daily); in case of treatment failure, 2nd-line therapy with single-agent gemcitabine (1000 mg/m2 intravenously over 30 min weekly × 7 followed by 1 week rest, then weekly × 3 every 4 weeks, according to the Burris regimen3) was recommended to the participating patients. Treatment continued until disease progression or unacceptable toxicity. If necessary, protocol-defined dose reductions were performed according to clinical and laboratory parameters. Supportive treatment (eg, antiemetic therapy) was administered according to local standards of the participating centres. Unique, study-specific recommendations for therapy of treatment-associated skin rash and diarrhoea were included in the study protocol and the participating centres were advised to follow these recommendations for optimal supportive rash and diarrhoea management.20
Efficacy and safety evaluation
Pretreatment evaluation included complete history and physical examination, assessment of vital signs, KPS, disease symptoms/ quality of life, and a CT scan of the abdomen. Regularly performed laboratory tests included complete blood counts, creatinine, liver enzymes and total bilirubin. CA 19–9 was assessed locally at baseline and at day 1 of each cycle. Response evaluations according to RECIST (version 1.0) were performed locally for the first time after 8 weeks in gemcitabine arms (after the first cycle) and subsequently after every other treatment cycle (8-week interval). For capecitabine arms, the first CT staging was performed after 9 weeks (after the first three cycles), and subsequently after every other treatment cycle (6-week interval). If not stated otherwise, all statistical analyses for the efficacy endpoints TTF and OS were done on an ‘intention-to-treat’ basis (‘ITT population’ consisting of all eligible patients randomised according to the protocol inclusion and exclusion criteria). Additionally, a second statistical analysis containing patients treated per protocol only (‘PP population’) was conducted. For the PP analysis, all study patients that received at least two cycles of the allocated treatment and who did not show early disease progression within that timeframe were eligible. Toxicity analyses were carried out for each patient who received at least one dose of the study drugs according to the protocol (‘safety population’). Toxicity was assessed on day 1 of each treatment cycle and was classified according to the National Cancer Institute Common Toxicity Criteria (NCI-CTC), version 2.0.
Statistical analyses
This multicentre AIO phase 3 trial was designed as a non inferiority study on two treatment sequences with a ‘cross-over’ of the chemotherapy drugs, with TTF2 being the primary endpoint. TTF was defined as the time from random assignment until disease progression, death from any cause, patient refusal or unacceptable toxicity. Assuming a TTF2 of 6 months in the reference arm (gemcitabine plus erlotinib followed by capecitabine), a non-inferiority margin of Δ=7 weeks for the experimental arm (capecitabine plus erlotinib followed by gemcitabine), corresponding to an HR of 1.37 was to be excluded by a 95% CI. Based on a power of 80% and a type I error rate of 5%, a total population of 270 analysable patients (135 in each arm) was required. All time-to-event curves for TTF and OS were estimated according to the Kaplane-Meier method, and differences between groups were analysed using the HR with CI and the log-rank test, with a p value of <0.05 being regarded as statistically significant. All reported p values are two-sided.
KRAS mutation analyses
Archival FFPE tumour tissue (obtained during routine procedures for histological confirmation of the PC diagnosis) was requested retrospectively from the participating centres/pathologists for KRAS analysis. Cytological specimens were not included. All KRAS mutation analyses were performed centrally at the University of Munich, Department of Pathology (Max-Borst Laboratory for Cancer Research) by AJ. KRAS mutations in codons 12 and 13 were investigated by established routine pyrosequencing using KRAS exon 2 specific primers and Pyro-Mark Gold kits (Qiagen, Hilden, Germany). Pyrosequencing was performed on a Pyromark Q24 device (Qiagen) as reported previously.21
RESULTS
Patient characteristics
Overall, 281 PC patients from 46 German centres were randomized between May 2006 and December 2008. The trial flow is summarised within the CONSORT diagram in figure 1. Seven patients were classified as non-eligible due to violation of inclusion criteria and 16 randomised patients did not start study treatment. Clinical baseline characteristics of the 274 eligible patients (ITT population) are summarised in table 1. At the time of final trial analysis in December 2010, 245 of the 274 eligible patients (89%) had died. The two treatment groups were well balanced with regard to age, stage of disease and KPS. The majority of the included patients suffered from pancreatic adenocarcinoma (96%) and in patients with distant metastases at study entry, the most frequently involved organ was the liver (71%).
Figure 1.
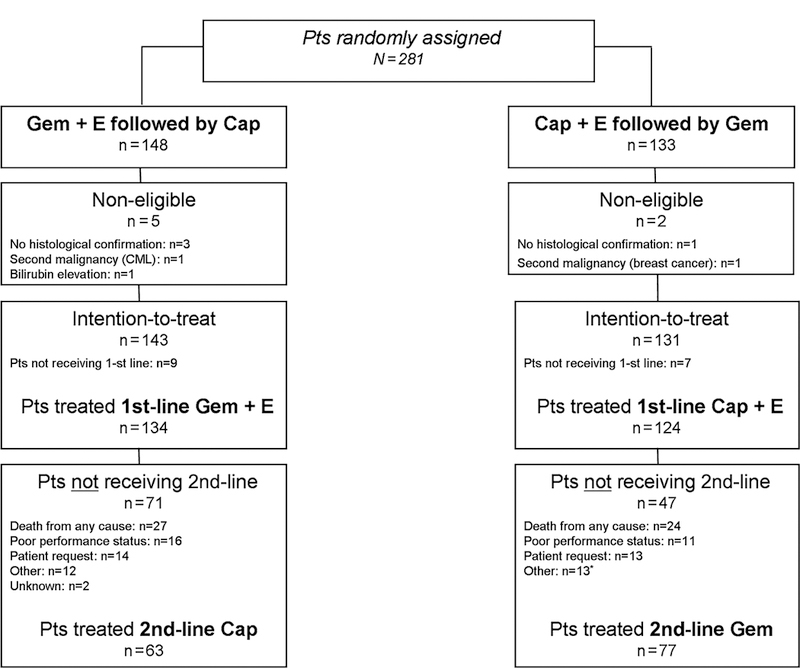
CONSORT diagram, Cap, Capecitabine; E, Erlotinib; Gem, Gemcitabine; Pts, Patients.
Table 1.
Baseline patient characteristics (intention-to-treat population, n = 274)
| Gem+E/Cap (n=143) |
Cap+E/Gem (n=131) |
|||
|---|---|---|---|---|
| Parameter | N | % | N | % |
| Age (years) | ||||
| Median | 65 | 63 | ||
| Range | 32–78 | 38–75 | ||
| Gender | ||||
| Male | 82 | 57 | 83 | 63 |
| Female | 61 | 43 | 48 | 37 |
| Stage of disease | ||||
| Locally advanced | 21 | 15 | 22 | 17 |
| Metastatic | 122 | 85 | 109 | 83 |
| Performance status | ||||
| KPS 60–80% | 50 | 35 | 49 | 37 |
| KPS 90–100% | 85 | 59 | 79 | 60 |
| Missing | 8 | 6 | 3 | 2 |
| Previous surgery | 8 | 6 | 17 | 13 |
| BMI at randomisation | ||||
| Median | 24.4 | 23.8 | ||
| Range | 16–37.6 | 16.2–37.6 | ||
| Weight loss during 3 months before randomisation (kg) | ||||
| Median | 5 | 7 | ||
| Range | 0–47 | 0–45 | ||
| Baseline CA 19–9 (U/ml)* | ||||
| Median | 1999 | 1756 | ||
| Range | 1–700 000 | 1–1000 000 | ||
n=245/274.
BMI, body mass index; Cap, capecitabine; E, erlotinib; Gem, gemcitabine; KPS, Karnofsky performance status.
Treatment
The median number of treatment cycles (1st- and 2nd-line therapy) was 5 in both arms (range 0‒26). Overall, 1198 treatment cycles were applied during 1st-line therapy and 446 cycles were administrated as 2nd-line treatment. The main reasons for termination of 1st-line study treatment (both arms) were confirmed disease progression (62%), tumour-related death (14%), patient refusal (9%) and toxicity (7%); 140 out of the 274 eligible patients (51%) received the predefined 2nd-line chemotherapy. During 2nd-line treatment (both arms) most patients discontinued chemotherapy because of confirmed progressive disease (57%), followed by decline in performance status (15%), tumour-related death (11%) and patient refusal (9%); 3 out of 140 patients (2%) discontinued 2nd-line chemotherapy due to unacceptable toxicity. A detailed analysis of treatment delays and dose reductions of the study medication (separately analysed with regard to cytotoxic agents vs erlotinib, 1st- vs 2nd-line therapy) is summarised in table 2. Erlotinib dose reductions were performed in 11% of patients receiving 1st-line capecitabine/erlotinib and in 27% of patients treated with front-line gemcitabine/erlotinib, respectively.
Table 2.
Treatment administration
| Gem+E/Cap (n=143) |
Cap+E/Gem (n=131) |
|||
|---|---|---|---|---|
| Parameter | N | % | N | % |
| Duration of 1st-line treatment (days) | ||||
| Median | 92 | 64 | ||
| Range | 1–743 | 2–583 | ||
| Duration of 2nd-line treatment (days) | ||||
| Median | 36 | 44 | ||
| Range | 1–253 | 1–392 | ||
| No. of treatment cycles per patient: 1st-line therapy | ||||
| Median | 3 | 3 | ||
| Range | 0–22 | 0–24 | ||
| No. of treatment cycles per patient: 2nd-line therapy | ||||
| Median | 2 | 2 | ||
| Range | 1–12 | 1–14 | ||
| No. of evaluable treatment cycles: 1st-line therapy | 642 | 556 | ||
| Cycles with treatment delay | 147 | 23 | 65 | 12 |
| Cycles with dose reduction of Chemotherapy | 219 | 34 | 87 | 16 |
| Cycles with dose reduction of erlotinib | 128 | 20 | 39 | 7 |
| No. of evaluable treatment cycles: 2nd-line therapy | 174 | 272 | ||
| Cycles with treatment delay | 35 | 20 | 65 | 24 |
| Cycles with dose reduction of chemotherapy | 19 | 11 | 91 | 33 |
Cap, capecitabine; E, erlotinib; Gem, gemcitabine.
Efficacy results
Median TTF2, the primary study endpoint, was estimated at 4.2 months in both arms (HR 1.00, 95% CI 0.78 to 1.28; p=1.0). The 95% CI testing non-inferiority had a limit of 1.23, clearly excluding the predefined inferiority margin of 1.37. The objective response rate during 1st-line treatment was 16% for gemcitabine plus erlotinib and 5% for capecitabine plus erlotinib; corresponding disease control rates (objective response rate plus stable disease) were 51% and 38%, respectively (table 3). With the use of 2nd-line chemotherapy, a further objective disease control was achieved in 22% of patients receiving capecitabine and in 36% of patients treated with gemcitabine. Results for the secondary study endpoints TTF1 and OS are summarised in table 4 and in figure 2: TTF1 was significantly prolonged in the gemcitabine/erlotinib arm (3.2 vs 2.2 months), but this advantage did not translate into a difference in TTF2 (4.2 vs 4.2 months) or OS (6.2 vs 6.9 months). The 1-year OS rate was 22% (95% CI 0.16% to 0.30%) in the gemcitabine/erlotinib followed by capecitabine arm and 23% (95% CI 0.17% to 0.32%) in the capecitabine/erlotinib followed by gemcitabine arm, respectively.
Table 3.
Treatment efficacy: response by imaging during 1st- and 2nd-line therapy
| Gem+E/Cap |
Cap+E/Gem |
|||
|---|---|---|---|---|
| Parameter | N | % | N | % |
| Evaluable 1st-line patients (ITT) | 143 | 131 | ||
| Complete remission | 1 | 1 | 0 | 0 |
| Partial remission | 21 | 15 | 7 | 5 |
| Stable disease | 51 | 36 | 43 | 33 |
| Progressive disease | 43 | 30 | 60 | 46 |
| Not assessable | 27 | 19 | 21 | 16 |
| Evaluable 2nd-line patients (ITT) | 63 | 77 | ||
| Complete remission | 0 | 0 | 0 | 0 |
| Partial remission | 2 | 3 | 5 | 6 |
| Stable disease | 12 | 19 | 23 | 30 |
| Progressive disease | 37 | 59 | 38 | 49 |
| Not assessable | 12 | 19 | 11 | 14 |
Cap, capecitabine; E, erlotinib; Gem, gemcitabine; ITT, intention-to-treat analysis.
Table 4.
Treatment efficacy: time-to-event endpoints (ITT)
| Gem+E/Cap (n=143) | Cap+E/Gem (n=131) | |||
|---|---|---|---|---|
| Parameter | Median (months) | Median (months) | HR (95% CI) | p Value |
| TTF2 | 4.2 | 4.2 | 1.00 (0.78 to 1.28) | 1.0 |
| TTF1 | 3.2 | 2.2 | 0.69 (0.54 to 0.89) | 0.0034 |
| TTFc* † | 2.0 | 2.5 | 1.87 (1.31 to 2.66) | 0.00047 |
| OS | 6.2 | 6.9 | 1.02 (0.79 to 1.31) | 0.90 |
| OSc* † | 3.2 | 5.0 | 1.56 (1.09 to 2.22) | 0.014 |
HR (with Cap+E/Gem sequence as reference throughout all comparisons).
Exploratory analysis
n=63/77.
Cap, capecitabine; E, erlotinib; Gem, gemcitabine; ITT, intention-to-treat analysis; OS, overall survival; OSc, overall survival after start of cross-over 2nd-line therapy; TTF1: time-to-treatment failure after 1st-line therapy; TTF2: time-to-treatment failure after 1st- and 2nd-line therapy; TTFc: time-to-treatment failure after start of cross-over 2nd-line therapy.
Figure 2.
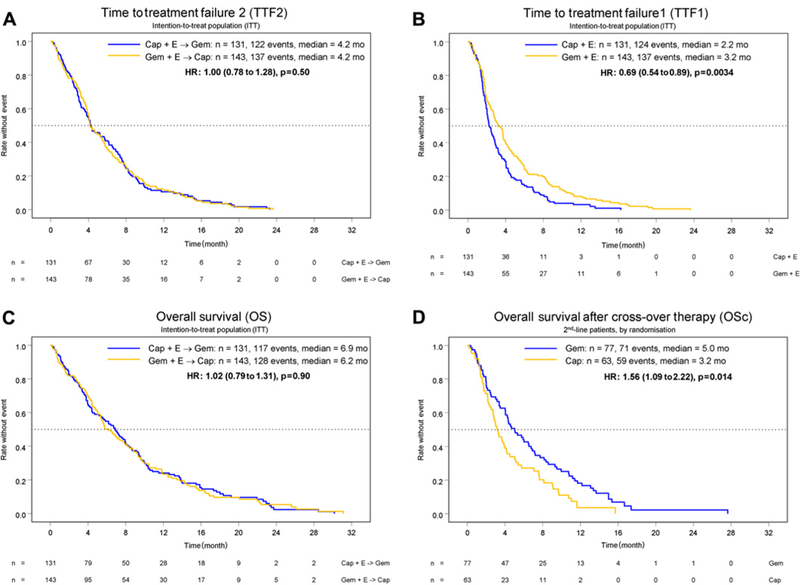
Time-to-treatment failure (TTF) and overall survival (OS). (A) TTF2, (B) TTF1, (C) OS. Exploratory analysis on overall survival (OSc) after start of 2nd-line chemotherapy (‘cross-over patient population’, n=140). (D) OSc.
Based on the PP analysis (n=239), TTF2 was estimated at 4.7 months in the gemcitabine/erlotinib followed by capecitabine arm and at 4.4 months in the capecitabine/erlotinib followed by gemcitabine arm (HR 0.99, 95% CI 0.76 to 1.28; p=0.46). The secondary endpoint TTF1 also favoured the gemcitabine/erlotinib arm in the PP analysis (3.7 vs 2.5 months; HR 0.66, 95% CI 0.51 to 0.86; p=0.002) and median OS for PP patients was nearly identical between the two arms (7.0 vs 6.9 months; HR 0.98, 95% CI 0.75 to 1.28; p=0.88).
The investigators additionally performed a non-predefined exploratory statistical analysis in order to test the hypothesis that the use of 2nd-line gemcitabine equals a possible superiority of gemcitabine compared to capecitabine during 1st-line treatment: when analysing 2nd-line patients only (n=140; figure 1), time-to-treatment failure (2.5 vs 2.0 months) as well as the overall survival (5.0 vs 3.2 months) in the ‘cross-over’ 2nd-line population (TTFc, OSc; calculated from the start of 2nd-line chemotherapy) both favoured gemcitabine over capecitabine (see table 4 and figure 2).
Subgroup analyses
Figure 3 illustrates the pre-planned subgroup analyses for a correlation of skin rash with TTF2 and OS in erlotinib-treated patients (n=255). Patients without skin rash had a significantly worse outcome than patients with skin rash of grade 2 or above with regard to TTF2 (2.9 vs 6.7 months) and OS (3.4 vs 9.6 months). Stage of disease at randomisation (locally advanced vs metastatic) was also associated with TTF2 (8.0 vs 4.1 months; HR 1.73, 95% CI 1.24 to 2.42; p=0.0011) and OS (11.9 vs 5.7 months; HR 1.85, 95% CI 1.30 to 2.63; p=0.00047). As expected, the OS of patients that received both random assigned lines of therapy (1st- and 2nd-line treatment) was longer compared to patients that terminated study treatment after 1st-line therapy (8.8 vs 3.6 months).
Figure 3.
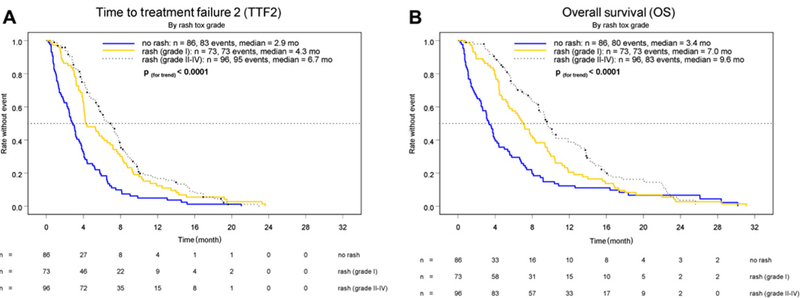
Time-to-treatment failure 2 (TTF2, A) and overall survival (OS, B) grouped by intensity of skin rash (grade 0‒4, according to NCI-CTCv2.0).
Safety results
Toxicity during 1st-line therapy
Haematological and non-haematological toxicity data for both 1st-line arms are summarised in table 5. Haematological toxicity was more frequent in the gemcitabine-containing arm (grade 3/4: <15%), whereas stomatitis and hand‒foot syndrome occurred more often in the capecitabine/erlotinib arm. Skin toxicity and diarrhoea were comparable between both 1st-line regimens. A trend for increased infectious complications was observed for the gemcitabine/erlotinib arm (grade 3/4: 18% vs 13%). A pneumonitis syndrome was diagnosed in two patients (2%) in the gemcitabine/erlotinib arm (both grade 3) and in none of the patients treated with capecitabine/erlotinib.
Table 5.
Toxicity events during 1st-line therapy (NCI-CTCv2.0)
| Percentage of patients |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gem+E (n=132)* |
Cap+E (n=124)* |
|||||||||
| Grade |
Grade |
|||||||||
| Toxicity | 1 | 2 | 3 | 4 | All | 1 | 2 | 3 | 4 | All |
| Leucocytopenia | 23 | 29 | 7 | 1 | 60 | 7 | 3 | 0 | 0 | 10 |
| Thrombocytopenia | 20 | 14 | 8 | 3 | 45 | 3 | 1 | 2 | 0 | 6 |
| Anaemia | 21 | 35 | 11 | 2 | 69 | 21 | 15 | 5 | 0 | 41 |
| Infection | 9 | 22 | 14 | 4 | 49 | 6 | 12 | 12 | 1 | 31 |
| Diarrhoea | 23 | 23 | 6 | 1 | 53 | 29 | 20 | 9 | 3 | 61 |
| Nausea | 30 | 30 | 8 | 0 | 68 | 24 | 26 | 5 | 0 | 55 |
| Vomiting | 21 | 18 | 4 | 1 | 44 | 13 | 17 | 3 | 1 | 34 |
| Stomatitis | 9 | 8 | 2 | 0 | 19 | 22 | 9 | 3 | 0 | 34 |
| Skin rash | 25 | 32 | 9 | 1 | 67 | 31 | 23 | 6 | 1 | 61 |
| Handefoot syndrome | 8 | 2 | 0 | 0 | 10 | 19 | 13 | 6 | 0 | 38 |
| Pneumonitis | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 0 |
Cap, capecitabine; E, erlotinib; Gem, gemcitabine.
Safety population (n=256).
Toxicity during 2nd-line therapy
The safety profiles of gemcitabine and capecitabine during 2nd-line chemotherapy were comparable to those assessed during front-line treatment, and toxicity was manageable in both arms. The only grade 3/4 toxicities occurring in >10% of patients were anaemia (11%) and infection (17%), both in the gemcitabine arm (table 6).
Table 6.
Toxicity events during 2nd-line therapy (NCI-CTCv2.0)
| Percentage of patients |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cap (n=62)* |
Gem (n=77)* |
|||||||||
| Grade |
Grade |
|||||||||
| Toxicity | 1 | 2 | 3 | 4 | All | 1 | 2 | 3 | 4 | All |
| Leucocytopenia | 10 | 6 | 2 | 0 | 18 | 21 | 30 | 4 | 0 | 55 |
| Thrombocytopenia | 3 | 2 | 2 | 0 | 7 | 14 | 12 | 5 | 0 | 31 |
| Anaemia | 29 | 16 | 5 | 0 | 50 | 23 | 39 | 8 | 3 | 73 |
| Infection | 6 | 6 | 5 | 2 | 19 | 12 | 10 | 17 | 0 | 39 |
| Diarrhoea | 14 | 2 | 0 | 0 | 16 | 19 | 13 | 3 | 0 | 35 |
| Nausea | 21 | 19 | 2 | 2 | 44 | 27 | 22 | 6 | 1 | 56 |
| Vomiting | 10 | 6 | 3 | 0 | 19 | 14 | 16 | 3 | 1 | 34 |
| Stomatitis | 3 | 3 | 3 | 0 | 9 | 10 | 0 | 0 | 0 | 10 |
| Skin rash | 16 | 5 | 0 | 0 | 21 | 27 | 6 | 0 | 1 | 34 |
| Handefoot syndrome | 13 | 3 | 5 | 0 | 21 | 13 | 3 | 1 | 0 | 17 |
Cap, capecitabine; Gem, gemcitabine.
Safety population (n=139).
KRAS analyses
FFPE tumour blocks were available from 208 of the 281 randomised patients (74%) and KRAS mutation analysis was technically successful in 173 cases. A KRAS wild-type status was found in 52 of these 173 FFPE tumour samples (30%); all detected KRAS mutations (121/173, 70%) were within codon 12, with c.35G>A-p.G12D (82/121, 68%) being the most frequent one. The KRAS status was significantly correlated with OS in a univariate analysis: median OS was estimated with 7.9 months within the KRAS wild-type group, whereas median OS was 5.7 months in the KRAS mutation group (HR 1.68, 95% CI 1.17 to 2.41; p=0.005). No statistically significant correlation of KRAS status with either stage of disease (locally advanced vs metastatic), baseline performance status, treatment arm and other efficacy endpoints like TTF or objective response was detected (data not shown).
DISCUSSION
The randomised AIO-PK0104 trial indicated that both investigated sequential therapeutic strategies were equally effective (regarding TTF2 and OS) and safe in treatment-naive patients with advanced PC. TTF2 was selected as the primary study objective as this composite endpoint reflects a summary of efficacy-related and patient-relevant outcome parameters for a palliative treatment regimen. Furthermore, TTF2 also could serve as an indicator for the failure of a prospectively predefined treatment strategy within the setting of a sequential trial design. TTF1, a secondary trial endpoint, was significantly prolonged with gemcitabine/erlotinib, suggesting a potential superiority of gemcitabine over capecitabine in this clinical context. Referring to data from the adjuvant setting, neither the large ESPAC-3v2 nor the RTOG 97–04 study found clear evidence for the superiority of a fluoropyrimidine compared to gemcitabine.22,23 A head-to-head comparison of gemcitabine to capecitabine in the (adjuvant or palliative) treatment of PC is still lacking; however, our exploratory data on TTFc and OSc in the 2nd-line population at least suggest a possible superiority of gemcitabine (table 4). Toxicity data obtained from this trial compare well with the gemcitabine/erlotinib arms in the PA.3 and AViTA study, although AIO-PK0104 investigated a higher dose of erlotinib (150 mg/day compared to 100 mg/day).10,24 Furthermore, the rate of erlotinib dose reductions during 1st-line treatment with gemcitabine plus erlotinib (150 mg/day) was markedly lower in AIO-PK0104 patients compared with the small subgroup of PA.3 patients that also received erlotinib 150 mg/day (27% vs 48%).10 Of note, the rate of skin rash (all grades: about 70%) as well as the survival data (median OS 6.2 months, 1-year OS rate 22%) were nearly identical for the gemcitabine/erlotinib arm in AIO-PK0104 and in PA.3.10 Despite the higher erlotinib dose (150 mg/day) during 1st-line treatment, no increase in non haematological toxicity was observed based on cross-trial comparisons for gemcitabine/erlotinib, and also no increase in potentially overlapping skin and gastrointestinal toxicities was found for the combination of capecitabine with erlotinib.10,13,24 Within a prospectively defined subgroup analysis of this AIO phase 3 study, skin rash could be confirmed as an important and clinically relevant surrogate parameter for treatment efficacy (regarding both TTF2 and OS) in our study population.10,24,25
AIO-PK0104 was the first PC phase 3 trial that added a prospectively predefined 2nd-line treatment after failure of a 1st-line erlotinib-containing therapy: 51% of patients were able to receive the allocated salvage chemotherapy, and the potential of disease control combined with a manageable tolerability for selected patients was confirmed for such an approach. Based on other randomised data (eg, from the CONKO-003 study15) a further controlled clinical investigation of 2nd-line chemotherapy is thus strongly recommended in future advanced PC trials.14,17 The currently available (although limited) data on 2nd-line treatment thereby suggest that the combination of a fluoropyrimidine with a platinum compound (or with irinotecan) could be regarded as the most effective treatment regimen.14,15 Specifically in such a context of palliative chemotherapy trials, a profound evaluation of quality of life endpoints should also be included.
The clinical value of biologicals in the treatment of advanced PC still remains controversial: specifically for agents targeting the VEGF pathway and its receptors (eg, bevacizumab and axitinib), negative survival data were recently published from large international phase 3 trials.26–28 Cetuximab, an anti-EGFR monoclonal antibody, also did not add therapeutic efficacy to standard gemcitabine in an unselected patient population treated within the SWOG S0205 study.29 In contrast, recent data from prospective clinical trials have provided valid evidence for an intensification of combination chemotherapy in order to improve survival outcome (eg, by use of the FOLFIRINOX regimen).30,31 Thus, novel treatment strategies are urgently awaited and future preclinical and clinical research efforts should focus, for example, on the targeting of different pathways as well as on the improvement of translational research in order to identify and validate relevant targets and molecular pathways in PC.32,33 In contrast to the (preliminary) biomarker results of the PA.3 study, a higher rate of KRAS wild-type patients within our study cohort was observed (30% vs 21%), and the KRAS wild type status was associated with an improved OS in our patient population (of which FFPE tissue was available).18 Whether the favourable survival prognosis of KRAS wild-type patients in our cohort is thus a prognostic phenomenon (eg, independent of erlotinib treatment) or a predictive marker for erlotinib efficacy could not be defined since erlotinib was applied in both trial arms. While recent data from a retrospective non-randomised single-centre analysis suggest that KRAS may rather be a predictive marker for erlotinib efficacy than a prognostic factor, this information needs to be verified by a prospective study.34
In conclusion, AIO-PK0104 is the first phase 3 clinical trial in advanced PC that investigated a prospectively predefined sequential 1st- and 2nd-line treatment strategy including an anti-EGFR targeted biological agent; both treatment arms were tolerated well and clinical efficacy was comparable for TTF2 and OS. A sequential trial design is feasible within a multicentre context, and future clinical studies should also focus on 2nd-line therapy in patients with advanced PC. Furthermore, the KRAS proto-oncogene may also serve as a biomarker in patients with advanced PC treated with anti-EGFR agents; whether this correlation is prognostic or predictive remains to be defined.
Significance of this study.
What is already known on this subject?
-
▸
Gemcitabine-based chemotherapy remains an international standard of care for patients with non-resectable, advanced pancreatic cancer (PC).
-
▸
Anti-EGFR treatment with the tyrosine kinase inhibitor erlotinib, as well as chemotherapy intensification by application of the FOLFIRINOX regimen, both significantly improved overall survival in randomised phase 3 trials.
-
▸
The optimal (sequential) regimen for the use of gemcitabine, erlotinib and the oral fluoropyrimidine capecitabine remains unclear in advanced PC.
-
▸
Molecular predictors for the efficacy of anti-EGFR treatments in PC have not been defined up to now.
What are the new findings?
-
▸
The sequential use of gemcitabine, erlotinib and capecitabine is safe and equally effective in PC; gemcitabine appears to be more effective in 1st-and 2nd-line therapy than capecitabine and therefore remains the preferred combination partner for erlotinib.
-
▸
Skin rash is strongly correlated with efficacy outcome measures in PC patients treated with erlotinib.
-
▸
KRAS wild-type status appears to be associated with improved overall survival in patients treated with erlotinib in this AIO study.
How might it impact on clinical practice in the foreseeable future?
-
▸
The benefit of adding erlotinib to chemotherapy is restricted to patients that experience skin rash during treatment; non-rash patients are characterised by a very poor outcome and need to be offered novel treatment strategies.
-
▸
Second-line salvage chemotherapy is effective and safe in selected PC patients.
-
▸
KRAS could serve as the first biomarker for improved survival in erlotinib-treated patients; the predictive value of KRAS for erlotinib efficacy remains to be defined prospectively.
Acknowledgements
The authors wish to thank all patients and their families, nurses, study coordinators and investigators for their active participation in this AIO study.
This study was registered at ClinicalTrials.gov, number NCT00440167.
Funding This work was supported by Hoffmann-La Roche, Germany.
VH: Roche (consultant, honoraria for scientific presentations, research funding). UV-K: none. DW: none. EK: none. AM: none. CW: none. SK: none. GK: none. TCG: none. LFvW: none. MRC: Roche (honoraria for scientific presentations, research funding, travel grants). MG: none. TFG: none. SH-B: none. OR: none. GB: none. TH: none. YDK: none. AJ: none. SN: none. SB: Roche (honoraria for scientific presentations, research funding, travel grants).
Footnotes
Ethics approval This study was conducted with the approval of the ethics committee of the Ludwig-Maximilians University of Munich, Germany.
Provenance and peer review Not commissioned; externally peer reviewed.
REFERENCES
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69–90. [DOI] [PubMed] [Google Scholar]
- 2.Hidalgo M Pancreatic cancer. N Engl J Med 2010;362:1605–17. [DOI] [PubMed] [Google Scholar]
- 3.Burris HA 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997;15:2403–13. [DOI] [PubMed] [Google Scholar]
- 4.Heinemann V, Quietzsch D, Gieseler F, et al. Randomized phase III trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancer. J Clin Oncol 2006;24:3946–52. [DOI] [PubMed] [Google Scholar]
- 5.Heinemann V, Labianca R, Hinke A, et al. Increased survival using platinum analog combined with gemcitabine as compared to single-agent gemcitabine in advanced pancreatic cancer: pooled analysis of two randomized trials, the GERCOR/ GISCAD intergroup study and a German multicenter study. Ann Oncol 2007;18:1652–9. [DOI] [PubMed] [Google Scholar]
- 6.Herrmann R, Bodoky G, Ruhstaller T, et al. ; Swiss Group for Clinical Cancer Research, Central European Cooperative Oncology Group. Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: a randomized, multicenter, phase III trial of the Swiss Group for Clinical Cancer Research and the Central European Cooperative Oncology Group. J Clin Oncol 2007;25:2212–17. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham D, Chau I, Stocken DD, et al. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J Clin Oncol 2009;27:5513–18. [DOI] [PubMed] [Google Scholar]
- 8.Boeck S, Hoehler T, Seipelt G, et al. Capecitabine plus oxaliplatin (CapOx) versus capecitabine plus gemcitabine (CapGem) versus gemcitabine plus oxaliplatin (mGemOx): final results of a multicenter randomized phase II trial in advanced pancreatic cancer. Ann Oncol 2008;19:340–7. [DOI] [PubMed] [Google Scholar]
- 9.Heinemann V, Boeck S, Hinke A, et al. Meta-analysis of randomized trials: evaluation of benefit from gemcitabine-based combination chemotherapy applied in advanced pancreatic cancer. BMC Cancer 2008;8:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007;25:1960–6. [DOI] [PubMed] [Google Scholar]
- 11.Dragovich T, Huberman M, von Hoff DD, et al. Erlotinib plus gemcitabine in patients with unresectable pancreatic cancer and other solid tumors: a phase IB trial. Cancer Chemother Pharmacol 2007;60:295–303. [DOI] [PubMed] [Google Scholar]
- 12.Ouchi KF, Yanagisawa M, Sekiguchi F, et al. Antitumor activity of erlotinib in combination with capecitabine in human tumor xenograft models. Cancer Chemother Pharmacol 2006;57:693–702. [DOI] [PubMed] [Google Scholar]
- 13.Kulke MH, Blaszkowsky LS, Ryan DR, et al. Capecitabine plus erlotinib in gemcitabine-refractory advanced pancreatic cancer. J Clin Oncol 2007;25:4787–92. [DOI] [PubMed] [Google Scholar]
- 14.Boeck S, Heinemann V. The role of second-line chemotherapy after gemcitabine failure in patients with advanced pancreatic cancer. Future Oncol 2008;4:41–50. [DOI] [PubMed] [Google Scholar]
- 15.Pelzer U, Kubica K, Stieler J, et al. A randomized trial in patients with gemcitabine refractory pancreatic cancer. Final results of the CONKO 003 study. J Clin Oncol 2008;26:abstract #4508 (Suppl). [Google Scholar]
- 16.Boeck S, Wilkowski R, Bruns CJ, et al. Oral capecitabine in gemcitabine-pretreated patients with advanced pancreatic cancer. Oncology 2007;73:221–7. [DOI] [PubMed] [Google Scholar]
- 17.Dahan L, Bonnetain F, Ychou M, et al. Combination 5-fluorouracil, folinic acid and cisplatin (LV5FU2-CDDP) followed by gemcitabine or the reverse sequence in metastatic pancreatic cancer: final results of a randomised strategic phase III trial (FFCD 0301). Gut 2010;59:1527–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Da Cunha Santos G, Dhani N, Tu D, et al. Molecular predictors of outcome in a phase 3 study of gemcitabine and erlotinib therapy in patients with advanced pancreatic cancer: National Cancer Institute of Canada Clinical Trials Group Study PA.3. Cancer 2010;116:5599–607. [DOI] [PubMed] [Google Scholar]
- 19.Lee J, Jang KT, Ki CS, et al. Impact of epidermal growth factor receptor (EGFR) kinase mutations, EGFR gene amplifications, and KRAS mutations on survival of pancreatic adenocarcinoma. Cancer 2007;109:1561–9. [DOI] [PubMed] [Google Scholar]
- 20.Boeck S, Vehling-Kaiser U, Waldschmidt D, et al. Erlotinib 150 mg daily plus chemotherapy in advanced pancreatic cancer: an interim safety analysis of a multicenter, randomized, cross-over phase III trial of the ‘Arbeitsgemeinschaft Internistische Onkologie’. Anticancer Drugs 2010;21:94–100. [DOI] [PubMed] [Google Scholar]
- 21.Neumann J, Zeindl-Eberhart E, Kirchner T, et al. Frequency and type of KRAS mutations in routine diagnostic analysis of metastatic colorectal cancer. Pathol Res Pract 2009;205:858–62. [DOI] [PubMed] [Google Scholar]
- 22.Neoptolemos JP, Stocken DD, Bassi C, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA 2010;304:1073–81. [DOI] [PubMed] [Google Scholar]
- 23.Regine WF, Winter KA, Abrams RA, et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA 2008;299:1019–26. [DOI] [PubMed] [Google Scholar]
- 24.Van Cutsem E, Vervenne WL, Bennouna J, et al. Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J Clin Oncol 2009;27:2231–7. [DOI] [PubMed] [Google Scholar]
- 25.Verslype C, Vervenne W, Bennouna J, et al. Rash as a marker for the efficacy of gemcitabine plus erlotinib-based therapy in pancreatic cancer: results from the AViTA Study. J Clin Oncol 2009;27:abstract #4532 (Suppl). [Google Scholar]
- 26.Burris H 3rd, Rocha-Lima C. New therapeutic directions for advanced pancreatic cancer: targeting the epidermal growth factor and vascular endothelial growth factor pathways. Oncologist 2008;13:289–98. [DOI] [PubMed] [Google Scholar]
- 27.Kindler HL, Niedzwiecki D, Hollis D, et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J Clin Oncol 2010;28:3617–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kindler HL, Ioka T, Richel DJ, et al. Axitinib plus gemcitabine versus placebo plus gemcitabine in patients with advanced pancreatic adenocarcinoma: a double-blind randomised phase 3 study. Lancet Oncol 2011;12:256–62. [DOI] [PubMed] [Google Scholar]
- 29.Philip PA, Benedetti J, Corless CL, et al. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-directed intergroup trial S0205. J Clin Oncol 2010;28:3605–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817–25. [DOI] [PubMed] [Google Scholar]
- 31.Hess V, Pratsch S, Potthast S, et al. Combining gemcitabine, oxaliplatin and capecitabine (GEMOXEL) for patients with advanced pancreatic carcinoma (APC): a phase I/II trial. Ann Oncol 2010;21:2390–5. [DOI] [PubMed] [Google Scholar]
- 32.Mazur PK, Siveke JT. Genetically engineered mouse models of pancreatic cancer: unravelling tumour biology and progressing translational oncology. Gut. Published Online First: 26 August 2011. doi: 10.1136/gutjnl-2011-300756. [DOI] [PubMed] [Google Scholar]
- 33.Collisson EA, Sadanandam A, Olson P, et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat Med 2011;17:500–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim ST, Lim do H, Jang KT, et al. Impact of KRAS mutations on clinical outcomes in pancreatic cancer patients treated with first-line gemcitabine-based chemotherapy. Mol Cancer Ther 2011;10:1993–9. [DOI] [PubMed] [Google Scholar]


