Abstract
Objective:
Monocyte-derived foam cells are one of the key players in the formation of atherosclerotic plaques. Adenosine receptors and extracellular adenosine have been demonstrated to modulate foam cell formation. Adenosine kinase (ADK) is a major enzyme regulating intracellular adenosine levels, but its functional role in myeloid cells remains poorly understood. To enhance intracellular adenosine levels in myeloid cells, ADK was selectively deleted in novel transgenic mice using Cre-LoxP technology and foam cell formation and the development of atherosclerotic lesions were determined.
Approach and Results:
ADK was upregulated in macrophages upon ox-LDL treatment in vitro and was highly expressed in foam cells in atherosclerotic plaques. Atherosclerotic mice deficient in ADK in myeloid cells were generated by breeding floxed ADK (ADKF/F) mice with myeloid-specific Cre recombinase-expressing (LysM-Cre) mice and apolipoprotein E-deficient (ApoE−/−) mice. Mice absent ADK in myeloid cells exhibited much smaller atherosclerotic plaques compared with controls. In vitro assays showed that ADK deletion or inhibition resulted in increased intracellular adenosine and reduced DNA methylation of the ABCG1 gene. Loss of methylation was associated with ABCG1 upregulation, enhanced cholesterol efflux and eventually decreased foam cell formation.
Conclusions:
Augmentation of intracellular adenosine levels through ADK knockout in myeloid cells protects ApoE−/− mice against atherosclerosis by reducing foam cell formation via the epigenetic regulation of cholesterol trafficking. ADK inhibition is a promising approach for the treatment of atherosclerotic diseases.
Keywords: atherosclerosis, foam cell formation, cholesterol efflux, gene methylation, basic research, vascular disease
Introduction
Atherosclerosis is a chronic inflammatory vascular disease that encompasses the continuum from an early fatty streak, established lesion, vulnerable plaque to ruptured plaque1–3. In response to vascular injury induced by disturb flow, lipid accumulation and many other risk factors, circulating monocytes are recruited to the arterial vessel wall, differentiate into macrophages, and further turn into foam cells after phagocytosis of modified lipids1–3. Accumulation of foam cells in the sub- endothelial space leads to fatty streak formation, which is a critical stage in the initiation of atherosclerosis4–6. These changes do not go unopposed and macrophages and vascular cells also activate a variety of pathways to reduce macrophage lipid loading and suppress foam cell formation. For example, macrophages and vascular cells produce adenosine, an endogenous purine ribonucleoside, that binds to and activates the adenosine receptor 2A (A2AR) on foam cells, and consequently diminishes foam cell formation7, 8. This concept is in line with the many beneficial effects of adenosine in a number of vascular disorders. Previous studies on the effect of adenosine on foam cell formation have emphasized the effect of extracellular adenosine7, 8. However, adenosine is also generated intracellularly9, and the role of intracellular adenosine in regulating foam cell formation has not yet been appreciated.
The generation of intracellular adenosine occurs from either the dephosphorylation of adenosine monophosphate (AMP) by 5’-nucleotidase (> 70%) or the hydrolysis of S-adenosyl-homocysteine (SAH) via SAH-hydrolase (SAHH)9, 10(Supplemental Figure 4A). In addition, intracellular adenosine kinase (ADK) and adenosine deaminase (ADA) are the enzymes responsible for the clearance of intracellular adenosine9, 10. Given that the Km of ADK for adenosine (1 μM) is much lower than that of ADA (25–150 μM), ADK is regarded as the major enzyme regulating the levels of intracellular adenosine under both physiological and pathophysiological conditions9, 10(Supplemental Figure 4A). Blockade of ADK disrupts the balance of adenosine nucleotides and significantly enhances the intracellular adenosine concentration11. Elevated intracellular adenosine reverses SAHH-mediated hydrolysis and directs the reaction toward the formation of SAH, which is a potent end-product inhibitor of transmethylation reactions12, 13(Supplemental Figure 4A). Inhibition of the transmethylation pathway by SAHH inhibitors results in a reduction of immune and/or inflammatory responses in T cells and macrophages14, 15. It remains unclear whether increased intracellular adenosine by ADK deletion in macrophages modulates foam cell formation by altering flux through the transmethylation pathway.
In the current study, we have utilized multiple approaches, including in vitro assays with ADK-deficient bone marrow-derived macrophages, foam cell formation, as well as experiments in vivo in models of atherosclerosis in mice selectively deficient in ADK in myeloid cells to determine the role of myeloid ADK in regulating foam cell formation and the formation of atherosclerotic lesions in mice deficient in apolipoprotein E.
Materials and Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Mouse generation and breeding
ADK floxed (ADKF/F) mice were generated by insertion of loxP-sites on both sides of exon seven of the ADK gene. ADKF/F mice were then crossed with Lysm-cre mice, a mouse line in which Cre is selectively expressed in lysozyme-expressing myeloid cells (Stock Number: 004718, The Jackson Laboratory, Bar Harbor, ME). The resulting ADKf/f Lysmcre/cre (ADKMAC-KO) mice are ADK deficient selectively in myeloid cells. ADKMAC-KO mice in the C57BL/6J background were bred with ApoE−/− mice (C57BL/6J background, Stock Number: 002052, The Jackson Laboratory) to generate ApoE−/−/ADKMAC-KO mice and their littermate control (ApoE−/−/Lysmcre/cre, named as ApoE−/−/ADKWT mice in the manuscript). All mice were maintained on a 12:12-h light-dark cycle. All animal experiments and care were approved by the IACUC at Peking University (Shenzhen, China) and Augusta University (Augusta, GA, USA) in accordance with NIH guidelines.
Animal experiments
For the atherosclerosis studies, we used genetically-engineered male and female mice based on established protocols with a minor modification16, 17. ApoE−/−/ADKMAC-KO mice and their littermates ApoE−/−/ADKWT mice at 8 weeks of age were fed a western diet (TD.88137, ENVIGO, Indianapolis, IN, USA) for 16 weeks. At the end of the feeding regime, the mice were anesthetized, and the chest and abdominal cavities were opened carefully. Blood was drawn directly from the heart. The vasculature was perfused with PBS and 4% PFA successively. Whole aortas were isolated to examine atherosclerotic lesions by oil red O staining. The heart was extracted and cut transversally. The top part of heart was processed to analyze atherosclerotic plaque development in the aortic sinus. In additional experiments to assess early changes in atherosclerosis, ApoE−/−/ADKMAC-KO mice and their littermates ApoE−/−/ADKWT mice at 7 weeks of age were fed a Western diet for 8 weeks. The aortic sinuses and brachiocephalic arteries were collected and processed for analysis of lesion size and changes in cellularity. Flow cytometry analysis of inflammatory cells in atherosclerotic lesions was conducted according to published protocols18. Detailed materials and methods are available in the online-only data supplement.
Bone marrow-derived macrophage culture and treatments
Following euthanization of mice, femurs and tibias were collected and transected. Bone marrow cells were flushed from the femurs and tibias. The cell suspension was pipetted repeatedly to obtain a single-cell suspension, which was then filtered with a 70 μm cell strainer and centrifuged at 800 g for 8 minutes. The acquired cells were plated at a density of 2 × 106/mL and cultured in RPMI 1640 medium (SH30809.01B, HyClone, Logan, UT, USA) supplemented with 10% FBS (04–002-1A, Biological Industries, Beit Haemek, Israel), 15% L929 conditioned medium, and 1% penicillin-streptomycin for 6 days. In some experiments, 50 μg/mL or 100 μg/mL ox-LDL (YB-002, Yiyuan Biotech, Guangzhou, Guangdong, China), 20 μM ABT702 (2372, Tocris Bioscience, Bristol, UK), 5 μM ZM241385 (1036,Tocris Bioscience, Bristol, UK), 2 μM MRS1754 (2752,Tocris Bioscience, Bristol, UK), 2 μM 5-Aza-2’-deoxycytidine (5’-Aza-dc) (A3656, Sigma-Aldrich, Saint Louis, MO, USA) or 20 μM Adenosine periodate oxidized (adox) (A7154, Sigma-Aldrich, Saint Louis, MO, USA) was added to the culture medium to treat macrophages.
Quantitation of atherosclerotic lesions
Aortas were fixed with 4% PFA (as described above) and rinsed in distilled water (30 seconds) and 60% isopropyl alcohol (15 seconds) successively. The aortas were stained with oil red O (O0625, Sigma-Aldrich, Saint Louis, MO, USA) staining buffer for 30 min, rinsed in 60% isopropyl alcohol for 20 seconds to remove background staining, and then placed in PBS to remove the remaining adventitial tissue. Aortas were pinned and the interior exposed for photography. The images of oil red O stained aortas were analyzed by Image-Pro Plus software.
Oil red O staining of foam cells
After ox-LDL treatment, BMDM cultured in Chamber Slide™ System (154534PK, Nunc™ Lab-Tek™ II, Roskilde, Denmark) were first fixed with 4% PFA and stained with Oil red O (O0625, Sigma-Aldrich, Saint Louis, MO, USA) staining buffer for 10 min and counter-stained with hematoxylin (C0105, Beyotime, Shanghai, China) for 3 min. The slides were then mounted with Glycerol Jelly Mounting Medium (S2150, Solarbio, Beijing, China). The percentage of oil red O-stained area per cell was quantified using Image-J software.
Measurement of total cholesterol level in foam cells
After ox-LDL treatment, the total cholesterol in foam cells was extracted using hexane: isopropanol (3:2) and assayed by Amplex® Red Cholesterol Assay Kit (A12216, Invitrogen, Eugene, OR, USA). The fluorescence was measured in a fluorescence microplate reader using excitation at 560 nm and emission detection at 590 nm. The cholesterol content was adjusted to the protein concentration (μg/mg).
Dil-ox-LDL uptake
BMDMs were incubated with Dil-ox-LDL (YB-0010, Yiyuan Biotech, Guangzhou, Guangdong, China) for 6 h. For confocal microscopy, the cells cultured in Chamber Slide™ System (154534PK, Nunc™ Lab-Tek™ II, Roskilde, Denmark) were fixed with 4% PFA and mounted with antifade mountant with DAPI (S36938, Life Technologies, Eugene, OR, USA) and then fixed with nail polish. For flow cytometry analysis, the cells were trypsinized, washed with PBS and resuspended in 500 μL PBS for flow cytometry analysis.
Cholesterol efflux assay
BMDMs were first incubated with 300 μL BODIPY cholesterol (810255P, Avanti Polar Lipids, Alabaster, AL, USA) labeling medium for 1 hour. After washing three times with PBS, the cells were equilibrated with RPMI 1640 containing 0.2% BSA for 12 hours. The fluorescence in the cells at this time point was regarded as the total fluorescence. The cells were then incubated with 25 μg/mL human plasma apoA-I (SRP4693, Sigma-Aldrich, Saint Louis, MO, USA) or 50 μg/mL native human HDL (YB-003, Yiyuan Biotech, Guangzhou, Guangdong, China) for 12 hours. After incubation, the efflux medium was collected and centrifuged at 10,000 g, 4°C for 5 min, and the fluorescence intensity was measured (excitation 482 nm, emission 515 nm). The percentage of cholesterol efflux was calculated by dividing the fluorescence in the medium by the total fluorescence.
Methylation-specific PCR
Genomic DNA was extracted from mouse BMDM using the DNAiso reagent (9770Q, Takara, Kusatsu, Shiga, Japan). A Bisulfte Conversion Reaction (DP215, TIANGEN, Beijing, China) was then conducted to convert unmethylated C to U; whereas methylated C remains unchanged. Bisulfite-treated DNA was then used as a template for methylation specific PCR according to the manufacturer’s instructions (R100A, Takara, Kusatsu, Shiga, Japan). The MS-qPCR primers (M primer, U primer) were designed using MethPrimer 2.0 using a template 1000 bp upstream and downstream of the mouse ABCG1 transcription start site (TSS).
Bisulfite sequencing PCR
Genomic DNA was extracted from mouse BMDM and modified using the the EpiTect Fast DNA Bisulfite Kit (59824, QIAGEN, Dusseldorf, German). CpG islands in the ABCG1 promoter and ABCA1 promotor were amplified. The amplified PCR products were cloned into a linearized vector using T4 DNA Ligase (EL0014, Invitrogen, Carlsbad, CA, USA). Ten positive clones were picked for DNA sequencing analysis. The level of methylated CpG sites between −96 bp to +188 bp on the ABCG1 promotor were compared using genomic DNA from ADK KO and ADK WT BMDMs. The level of methylated CpG sites between −40 bp to +251 bp in the ABCA1 promotor were evaluated and compared between the ADK KO and ADK WT BMDMs.
Statistical analysis
Statistical analyses were performed with GraphPad Software. Data are presented as the mean ± SEM. Normal distribution and equality of variances of all data was evaluated using the Kolmogorov-Smirnov test and F test. Data that followed a normal distribution were analyzed by one-way ANOVA with Bonferroni’s post hoc tests or by two-tailed unpaired Student’s t test. Data that failed normal distribution were analyzed by Kruskal-Wallis test with post hoc tests or by non-parametric Mann-Whitney test. The null hypothesis was rejected at P < 0.05.
Results
Increased ADK expression in foam cells
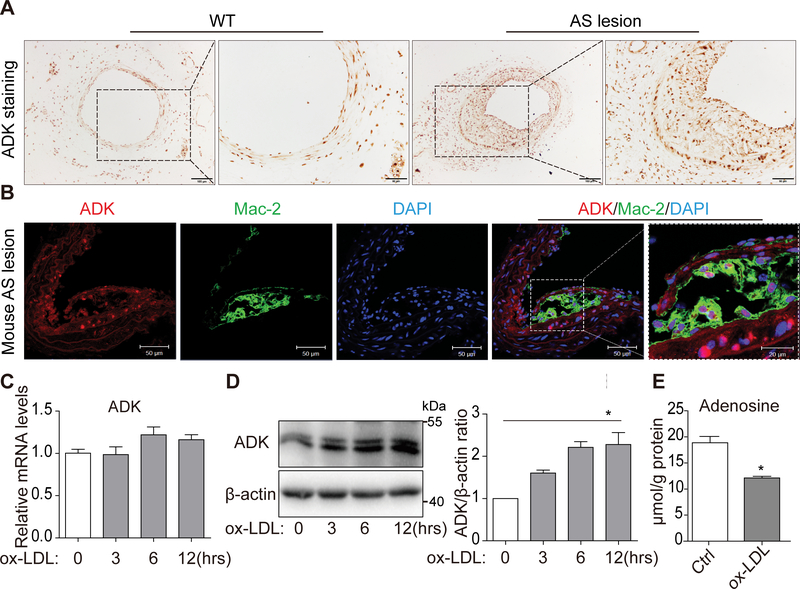
To determine the expression level of ADK in atherosclerotic arteries, we examined arteries of wild-type (WT) mice and apolipoprotein E-deficient (ApoE−/−) mice. Immunohistochemical staining of ADK showed that expression levels in atherosclerotic arteries were much higher than that in arteries from WT mice (Figure 1A). Furthermore, immunofluorescent double staining with Mac-2 and ADK antibodies showed that in sections from atherosclerotic arteries, cells in Mac-2-positive areas were ADK-positive (Figure 1B), indicating that indeed macrophages/foam cells express ADK.
Figure 1. ADK expression is upregulated in both aorta lesions and in vitro-induced foam cells.
A, Immunohistochemical analysis of ADK expression in atherosclerotic arteries of ApoE−/− and WT mice. Scale bars: 100 μm (1st and 3rd columns) and 50 μm (2nd and 4th columns). B, Double immunostaining of ADK and macrophage marker Mac-2 in aortic sinuses of ApoE−/− mice. Scale bars: 50 μm. C, Real-Time PCR analysis of ADK mRNA level in BMDMs treated with ox-LDL (50 μg/mL) for 0, 3, 6, 12 hrs. n = 6. D, Western blot analysis and densitometric quantification of ADK protein level in BMDMs treated with ox-LDL (50 μg/mL) for 0, 3, 6, 12 hrs. *P < 0.05. n = 3. E, HPLC analysis of intracellular adenosine in BMDMs treated with ox-LDL (50 μg/mL) for 12 h. *P < 0.05. n = 3. Data in bar graphs are expressed as mean ± SEM. Statistical significance was determined by Kruskal-Wallis test with post hoc tests (for D) and Mann Whitney test (for E).
To examine ADK expression in in vitro-induced foam cells, bone marrow-derived macrophages (BMDMs) were cultured and then challenged with oxidized low-density lipoprotein (ox-LDL). As shown in Figure 1C, the mRNA levels of ADK were not changed after ox-LDL treatment. However, the protein levels of ADK were increased in a time-dependent manner (Figure 1D), and this was accompanied by decreased levels of intracellular adenosine (Figure 1E).
Reduced atherosclerosis in mice deficient in myeloid ADK
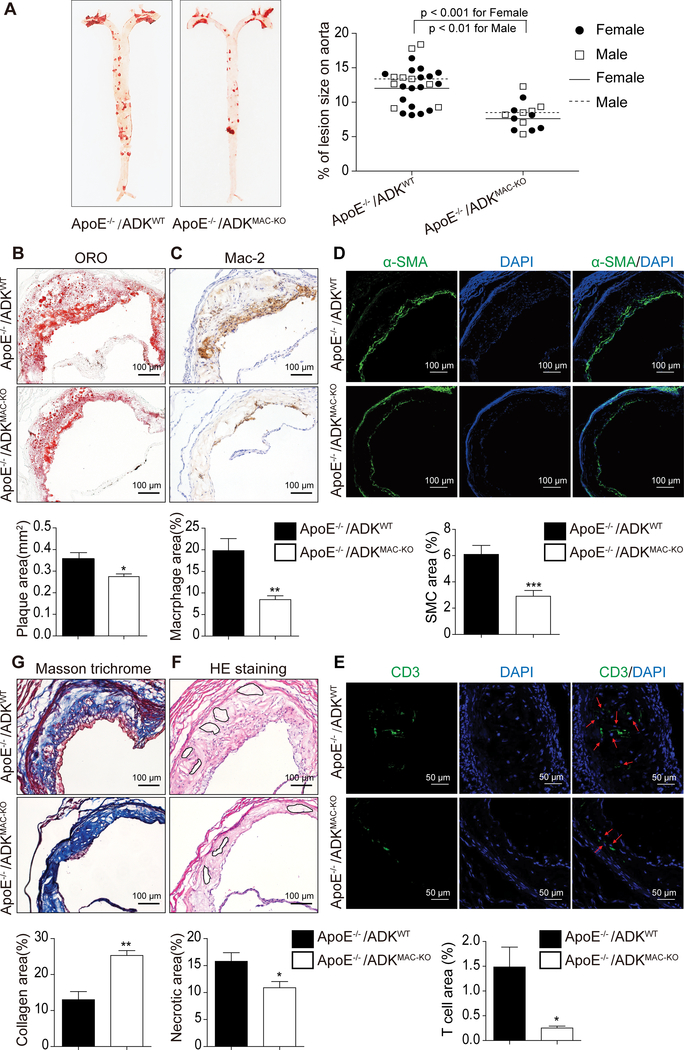
To determine whether macrophage ADK deficiency affects atherosclerosis development, ApoE−/−/ADKMAC-KO mice and their littermates ApoE−/−/ADKWT mice were fed a western diet for 16 weeks to induce atherogenesis. These mice exhibited no significant differences in the levels of blood cholesterol and triglycerides, the number of circulating leukocytes and their differentials (Supplemental Table I-II), whereas it was noted that female mice showed a modest decrease in blood glucose in ApoE−/−/ ADKMAC-KO mice as compared to ApoE−/−/ADKWT mice and a slight increase in the number of platelets in ApoE−/−/ADKMAC-KO mice compared with that in ApoE−/−/ADKWT mice (Supplemental Table I-II). Using FACS analysis, the percentage of Ly6Chi monocytes in circulating monocytes was determined and no difference was found in the numbers of Ly6Chi and Ly6Clo monocytes between ApoE−/−/ADKMAC-KO mice and ApoE−/−/ADKWT mice (Supplemental Figure 1A-B). The percentage of CD25+/FoxP3+ circulating CD4+ regulatory T cells was increased 2.5 fold in ApoE−/−/ADKMAC-KO mice as compared with that in ApoE−/−/ADKWT mice (Supplemental Figure 1D). Oil red O staining on aortae showed that the lesion area was 36.5% smaller in ApoE−/−/ADKMAC-KO mice compared to ApoE−/−/ADKWT mice (Figure 2A, 7.629 ± 0.721% in ApoE−/−/ ADKMAC-KO group vs 12.018 ± 0.662% in ApoE−/−/ADKWT group for female mice, P < 0.001; 8.500 ± 0.869% in ApoE−/−/ ADKMAC-KO group vs 13.389 ± 1.124% in ApoE−/−/ADKWT group for male mice, P < 0.01). Oil red O staining of the aortic sinus also revealed significantly smaller lesion areas in ApoE−/−/ADKMAC-KO mice than in ApoE−/− /ADKWT mice (Figure 2B, 0.274 ± 0.013 mm2 in ApoE−/−/ADKMAC-KO group vs 0.358 ± 0.028 mm2 in ApoE−/−/ADKWT group, P < 0.05). To further characterize the cellular composition of atherosclerotic lesions, we employed mac-2 staining to identify macrophages, α-SMA staining for smooth muscle cells and CD3 staining for T cells in the aortic sinus. Despite no significant difference in endothelial adhesion molecule expression between the two groups (Supplemental Figure 3C), the Mac-2-positive area was dramatically decreased in ApoE−/−/ADKMAC-KO mice as compared to that in ApoE−/−/ADKWT mice (Figure 2C, 8.459 ± 0.89% in ApoE−/−/ ADKMAC-KO group vs 19.80 ± 2.78% in ApoE−/−/ADKWT group, P < 0.01). The α-SMA-positive area was also significantly decreased in ApoE−/−/ADKMAC-KO mice compared to ApoE−/−/ADKWT mice (Figure 2D, 2.912 ± 0.439% in ApoE−/−/ ADKMAC-KO group vs 6.099 ± 0.684% in ApoE−/−/ADKWT group, P < 0.001). The CD3-positive area was reduced in ApoE−/−/ADKMAC-KO mice versus that in ApoE−/−/ADKWT mice (Figure 2E, 0.253 ± 0.039% in ApoE−/−/ ADKMAC-KO group vs 1.485 ± 0.403% in ApoE−/−/ADKWT group, P < 0.05), but there was no difference in the percentage of regulatory T cells in the aortic lesions (Supplemental Figure 1E). In addition, we also analyzed plaque stability using hematoxylin & eosin (HE) staining of necrotic cores and Masson trichrome staining of collagen in the aortic sinus. HE staining showed that the necrotic core area was slightly smaller in ApoE−/−/ADKMAC-KO mice than that in ApoE−/−/ADKWT mice (Figure 2F, 10.88 ± 1.16% in ApoE−/−/ADKMAC-KO group vs 15.78 ± 1.61% in ApoE−/−/ADKWT group, P < 0. 05). The aortic sinus in ApoE−/−/ADKMAC-KO mice had a higher collagen content than in ApoE−/−/ADKWT mice (Figure 2G, 24.36 ± 1.27% in ApoE−/−/ADKMAC-KO group vs 14.44 ± 2.25% in ApoE−/−/ADKWT group, P < 0. 01), which implies more stable plaques. Thus, myeloid deletion of ADK significantly decreased the atherosclerotic surface lesion area in the whole aorta and improved atherosclerosis plaque stability in mice subject to a high fat western diet for 16 weeks. Furthermore, we also investigated the effect of macrophage ADK deficiency on the formation of early atherosclerotic plaques in mice fed a western diet for 8 weeks. A reduction in lesion area and macrophage positive areas in both brachiocephalic arteries and aortic sinuses were found in ApoE−/−/ADKMAC-KO mice compared to those in ApoE−/−/ADKWT mice (Supplemental Figure 2A-D). Furthermore, atherosclerotic plaques in brachiocephalic arteries exhibited a smaller necrotic area in ApoE−/−/ADKMAC-KO mice than in ApoE−/−/ADKWT mice, implying that plaques in ApoE−/−/ADKMAC-KO mice are more stable than those in ApoE−/−/ADKWT mice, even though the collagen content in lesions of both groups of mice was comparable (Supplemental Figure 3A-B).
Figure 2.. Myeloid ADK deletion alleviates atherosclerosis in ApoE−/− mice feeding a western diet for 16 weeks.
A, Oil red O staining of en face aortas of ApoE−/−/ADKMAC-KO and ApoE−/−/ADKWT mice and percentage of aortic lesion area on total aortic surface area. n ≥ 14. B, Oil red O staining of aortic sinuses of ApoE−/−/ADKMAC-KO and ApoE−/−/ADKWT mice and quantification of the lesion areas. *P < 0.05. n = 10. C, Mac-2 staining of macrophages in aortic sinuses of ApoE−/−/ADKMAC-KO and ApoE−/−/ADKWT mice and percentages of mac-2 positive area. **P < 0.01. n = 8. D, α-SMA staining of smooth muscle cells in aortic sinuses of ApoE−/−/ADKMAC-KO and ApoE−/−/ADKWT mice and percentages of α-SMA positive area. ***P < 0.001. n = 9. E, CD3 staining of T cells in aortic sinuses of ApoE−/−/ADKMAC-KO and ApoE−/−/ADKWT mice and percentages of CD3 positive area. *P < 0.05. n = 6. F, HE staining of necrotic core area in aortic sinuses of ApoE−/−/ADKMAC-KO and ApoE−/−/ADKWT mice and percentages of necrotic core area. *P < 0.05. n = 8. G, Masson’s Trichrome staining of collagen in aortic sinuses of ApoE−/−/ADKMAC-KO and ApoE−/−/ADKWT mice and percentages of collagen area. **P < 0.01. n = 8. Data in bar graphs are expressed as mean ± SEM. Statistical significance was determined by unpaired Student’s t-test.
Decreased foam cell formation of ADK-deficient macrophages
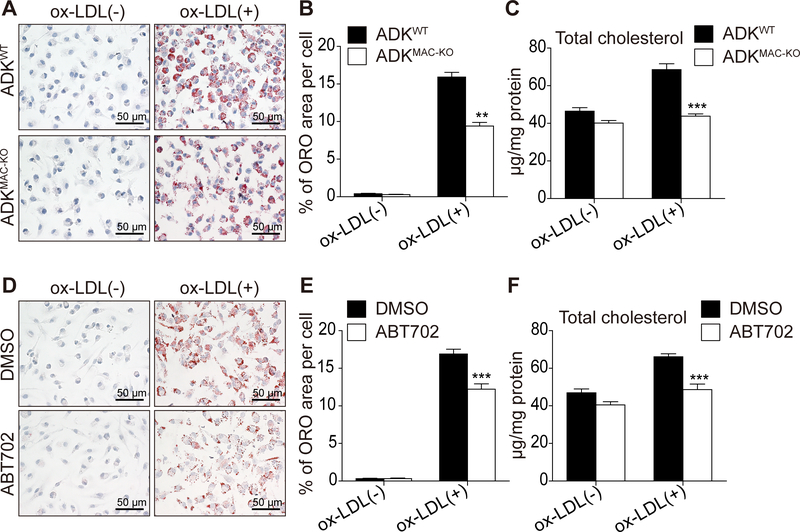
Foam cell formation plays a vital role in the development of atherosclerotic lesions4–6. For this reason, we investigated whether ADK deficiency impacts foam cell formation. Bone marrow cells from ADKWT and ADKMAC-KO mice were cultured and then differentiated into BMDMs, followed by treatment with ox-LDL for 24 h to induce foam cell formation. Oil red O staining (Figure 3A-B) revealed that ADK deficiency decreased foam cell formation by about 40% as compared to the control group, and total cholesterol levels were much lower in ADK-deficient foam cells than in WT foam cells (Figure 3C). BMDMs from WT mice were pre-treated with vehicle or the ADK inhibitor ABT702 and then incubated with ox-LDL for 24 h to induce foam cell formation. Oil red O staining (Figure 3D-E) and total cholesterol measurements (Figure 3F) also demonstrated that inhibition of ADK activity decreases foam cell formation. These results indicate that inactivation of ADK impairs the ability of ox-LDL to promote foam cell formation, a critical process in atherosclerosis.
Figure 3. ADK knockdown decreases ox-LDL-induced foam cell formation in vitro.
A and B, Oil red O staining of ox-LDL-induced foam cells in BMDMs from ADKWT and ADKMAC-KO mice incubated with ox-LDL (100 μg/mL) for 24 h and quantitative data. **P < 0.01. n = 6. C, Total cholesterol content of BMDMs from ADKWT and ADKMAC-KO mice incubated with ox-LDL (100 μg/mL) for 24 h. ***P < 0.001. n = 6–8. D and E, Oil red O staining of ox-LDL-induced foam cells in BMDMs pretreated with DMSO or ABT702 (20 μM) following incubation with ox-LDL (100 μg/mL) for 24 h and quantitative data. ***P < 0.001. n = 6. F, Total cholesterol content of BMDMs pretreated with DMSO or ABT702 (20 μM) following incubation with ox-LDL (100 μg/mL) for 24 h. ***P < 0.001. n = 6. Data in bar graphs are expressed as mean ± SEM. Statistical significance was determined by unpaired Student’s t-test (for B and E) and one-way ANOVA with Bonferroni’s post hoc tests (for C and F).
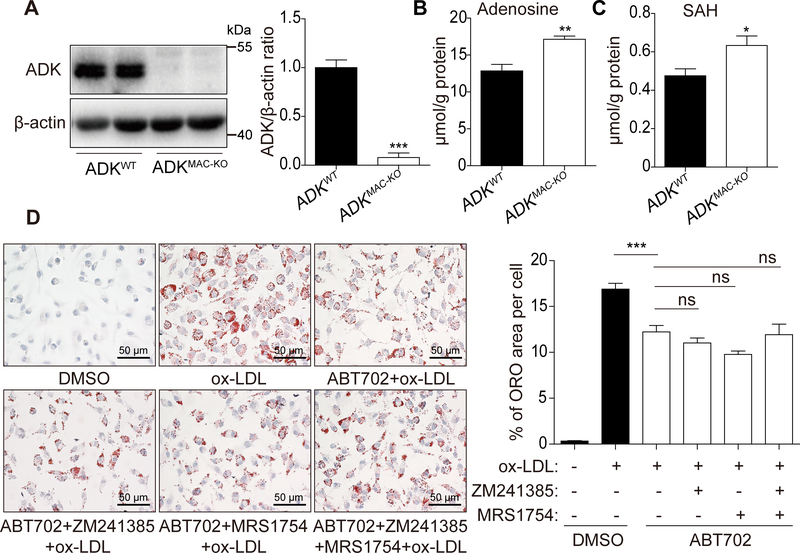
ADK deficiency increases both intracellular and extracellular adenosine levels in endothelial cells19. We found that loss of ADK elicited similar effects in BMDMs (Figure 4A-B). In addition, we also found that ADK deficiency decreased both intracellular and extracellular ATP/ADP/AMP levels in BMDMs (Supplemental Figure 4B-C). To determine whether adenosine reduces foam cell formation via activation of adenosine receptors, we next treated macrophages with the A2AR antagonist ZM241385, the A2BR antagonist MRS1754 or both in the presence of the ADK inhibitor ABT702, followed by incubation with ox-LDL for 24 h. Treatment of BMDMs with adenosine receptor antagonists did not affect ox-LDL-induced foam cell formation in ABT702-treated macrophages (Figure 4D), which indicates that decreased formation of foam cells in ADK-deficient macrophages is adenosine receptor-independent.
Figure 4. The repressed foam cell formation in ADK-deficient macrophages is adenosine receptor-independent.
A, Western blot analysis and densitometric quantification of ADK protein level in BMDMs from ADKWT and ADKMAC-KO mice. ***P < 0.001. n = 6. B, HPLC analysis of intracellular adenosine in BMDMs from ADKWT and ADKMAC-KO mice. **P < 0.01. n = 6. C, HPLC analysis of intracellular SAH in BMDMs from ADKWT and ADKMAC-KO mice. *P < 0.05. n = 6. D, Oil red O staining of BMDMs pre-treated with DMSO, ABT702 (20 μM), ABT702 (20 μM) and ZM241385 (5 μM), ABT702 (20 μM) and MRS1754 (2 μM), ABT702 (20 μM) and ZM241385 (5 μM) and MRS1754 (2 μM) following incubation with ox-LDL (100 μg/mL) for 24 h and quantitative data. ***P < 0.001. n = 6. Data in bar graphs are expressed as mean ± SEM. Statistical significance was determined by unpaired Student’s t-test (for A, B, C) and one-way ANOVA with Bonferroni’s post hoc tests (for D).
Augmented cholesterol efflux in ADK-deficient foam cells
Foam cell formation involves a balance between uptake of modified LDL and efflux of cholesterol in macrophages5. Therefore, decreased foam cell formation in ADK-deficient macrophages may result from compromised ox-LDL uptake or enhanced cholesterol efflux or both. To distinguish between these possibilities, we firstly compared ox-LDL uptake in macrophages of ADKWT and ADKMAC-KO mice using confocal microscopy and FACS analysis. BMDMs of ADKWT and ADKMAC-KO mice were incubated with fluorescent Dil-ox-LDL for 6 h. There was no significant difference in ox-LDL uptake between macrophages of ADKWT and ADKMAC-KO mice (Figure 5A-B). Cholesterol efflux was determined using BODIPY-cholesterol20, a fluorescent analog of free cholesterol. Cholesterol efflux to mature HDL was enhanced by 36% in the group of ADK-deficient foam cells compared to the control group (Figure 5D). No difference in efflux to nascent apoA-I was found between the two groups (Figure 5C). Thus, enhanced cholesterol efflux to mature HDL contributes to decreased foam cell formation for ADK-deficient macrophages.
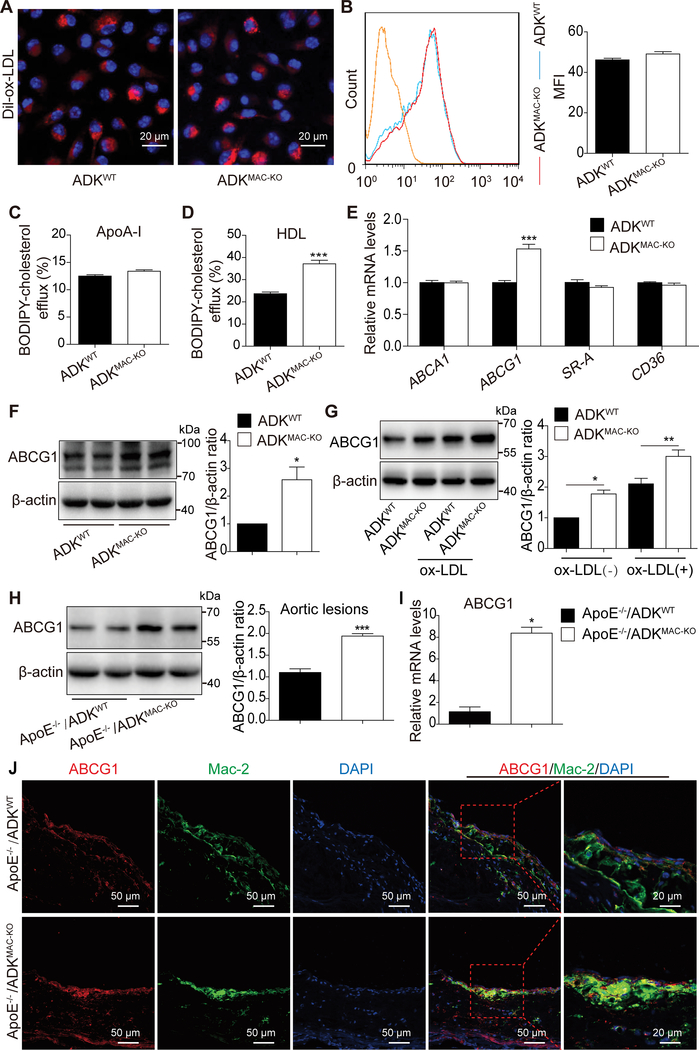
Figure 5. Cholesterol efflux is enhanced via ABCG1-mediated efflux to HDL in ADK-deficient BMDMs.
A, Representative images of Dil-ox-LDL uptake in BMDMs from ADKWT and ADKMAC-KO mice treated with fluorescence-labeled Dil-ox-LDL (20 μg/mL) for 6 h. B, Flow cytometry analysis of fluorescence intensity in BMDMs from ADKWT and ADKMAC-KO mice incubated with fluorescence-labeled Dil-ox-LDL (20 μg/mL) for 6 h and Mean Fluorescence Intensity (MFI). n = 3. C and D, Cholesterol efflux to human plasma apoA-I or human native HDL in BMDMs from ADKWT and ADKMAC-KO mice was examined using BODIPY-cholesterol as described in Materials and Methods (Supplemental Material). ***P < 0.001. n = 6. E, Real-time PCR analysis of the mRNA level of scavenger receptor SR-A and CD36 and cholesterol transporter ABCA1 and ABCG1 in BMDMs from ADKWT and ADKMAC-KO mice. ***P < 0.001. n = 6. F, Western blot analysis and densitometric quantification of protein level of ABCG1 in BMDMs from ADKWT and ADKMAC-KO mice. *P < 0.05. n = 5. G, Western blot analysis and densitometric quantification of protein level of ABCG1 in BMDMs from ADKWT and ADKMAC-KO mice treated with ox-LDL (100 μg/mL) for 12 h. *P < 0.05, **P < 0.01. n = 5. H, Western blot analysis and densitometric quantification of protein level of ABCG1 in aortic lesions from ApoE−/−/ADKWT and ApoE−/−/ADKMAC-KO mice. ***P < 0.001. n = 5. I, Real-time PCR analysis of the mRNA level of ABCG1 in plaque macrophages sorted from atherosclerotic arteries of ApoE−/−/ADKWT and ApoE−/−/ADKMAC-KO mice. *P < 0.05. n = 3. J, Double immunostaining of ABCG1 and macrophage marker Mac-2 in aortic sinus from ApoE−/−/ADKWT and ApoE−/−/ADKMAC-KO mice. Data in bar graphs are expressed as mean ± SEM. Statistical significance was determined by unpaired Student’s t-test (for D, E, F, H), one-way ANOVA with Bonferroni’s post hoc tests (for G) and Mann Whitney test (for I).
To investigate the molecular mechanisms underlying decreased foam cell formation, we next determined whether ADK deficiency alters the expression of scavenger receptors, SR-A and CD36, or the cholesterol transporters, ABCA1 and ABCG1. No difference was found in the mRNA levels of ABCA1, SR-A, and CD36 between the two groups (Figure 5E). However, the mRNA level of cholesterol transporter ABCG1 was increased by 1.5-fold in ADK-deficient macrophages as compared to WT macrophages (Figure 5E). We then examined the protein expression of ABCG1 in macrophages from ADKWT and ADKMAC-KO mice by immunoblotting. The protein level of ABCG1 was increased by 2.6 fold in ADK-deficient macrophages as compared to control macrophages (Figure 5F). Furthermore, we also examined the effect of ADK deficiency on ABCG1 expression in the presence of ox-LDL. The level of ABCG1 in ADK-deficient macrophages was much higher than that of WT macrophages following ox-LDL treatment (Figure 5G). We also found that the protein level of ABCG1 was increased about 1.9 fold in the aortic lesions of ApoE−/−/ADKMAC-KO mice versus that in ApoE−/−/ADKWT mice (Figure 5H). Moreover, using a published protocol21, we employed flow cytometry to isolate macrophages from atherosclerotic plaques (Supplemental Figure 5) and examined the mRNA level of ABCG1. The mRNA level of ABCG1 was increased about 8 fold in macrophages from plaques of ApoE−/−/ADKMAC-KO mice compared with that from ApoE−/−/ADKWT mice (Figure 5I). Immunofluorescent staining of sections of aortic sinuses using both mac-2 and ABCG1 antibodies showed that ABCG1 protein expression was also significantly upregulated in plaque macrophages of ApoE−/−/ADKMAC-KO mice versus that in ApoE−/−/ADKWT mice (Figure 5J). These results suggest that ADK-deficiency enhances cholesterol efflux in macrophages through ABCG1, but not ABCA1.
Decreased DNA methylation of ABCG1 in ADK-deficient macrophages
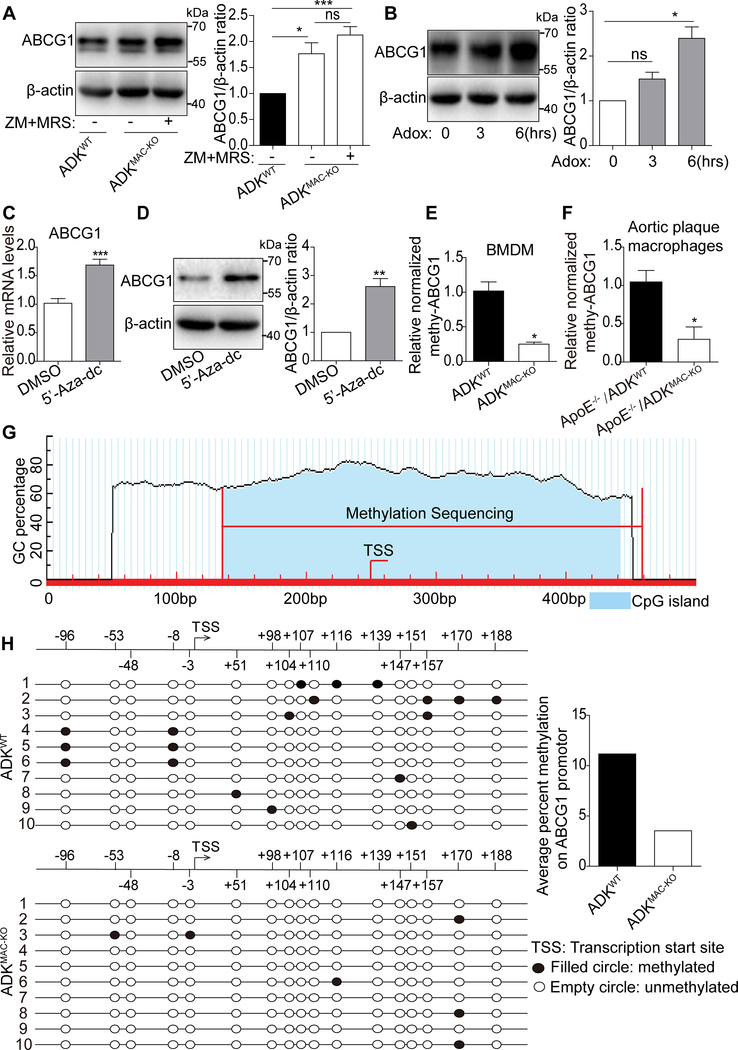
In neurons and endothelial cells, ADK deficiency enhances the levels of intracellular adenosine and SAH13, 19. We found that this also occurs in ADK-deficient BMDMs (Figure 4C). To test whether intracellular adenosine regulates ABCG1 expression via extracellular adenosine receptors after adenosine is transported out, ADK-deficient BMDMs were treated with the A2AR antagonist ZM241385 and the A2BR antagonist MRS1754. Neither treatment altered the increased expression level of ABCG1 in ADK-deficient macrophages (Figure 6A). Elevated intracellular adenosine levels have been shown to reduce DNA methylation through inhibition of SAHH activity13, 19. To test whether decreased SAHH activity and subsequent inhibition of DNA methylation are associated with the increased expression of ABCG1 in ADK-deficient macrophages, we treated BMDMs with the SAHH inhibitor, Adox or DNA methylation inhibitor, 5’-Aza-dc. As shown in Figure 6B-D, Adox upregulated ABCG1 at the protein level and 5’-Aza-dc increased the level of ABCG1 at both the mRNA and protein levels, suggesting that suppression of DNA methylation enhances ABCG1 expression in ADK-deficient macrophages. To assess changes in the methylation level of the ABCG1 gene, DNA from ADK-deficient and WT BMDMs was modified by bisulfite, and the levels of promoter methylation were quantified by RT–PCR. The level of methylated ABCG1 was much lower in ADK-deficient BMDMs than in the WT macrophages (Figure 6E). Furthermore, the level of methylated ABCG1 was also much lower in sorted macrophages from plaques of ApoE−/−/ADKMAC-KO mice than that from ApoE−/−/ADKWT mice (Figure 6F). To provide additional validation of the PCR data, bisulfite sequencing of ABCG1 CpG islands was performed (Figure 6G). The fragment between −96 bp to +188 bp of the ABCG1 promoter region was evaluated. The methylation rate of CpG elements in ADK-deficient BMDMs was reduced by 68% compared to that in control macrophages (Figure 6H). These results indicate that decreased DNA methylation in the ABCG1 promoter is at least one of the major factors underlying increased ABCG1 expression in ADK-deficient macrophages.
Figure 6. ABCG1 promotor hypomethylation accounts for enhanced ABCG1 expression in ADK-deficient macrophages.
A, Western blot analysis and densitometric quantification of protein level of ABCG1 in BMDMs from ADKMAC-KO mice treated with ZM241385 (5 μM) and MRS1754 (2 μM). *P < 0.05, ***P < 0.001. n = 5. B, Western blot analysis and densitometric quantification of protein level of ABCG1 in BMDMs treated with adox (20 μM) for 0, 3, 6 hrs. *P < 0.05. n = 3. C, Real-time PCR analysis of ABCG1 mRNA level in BMDMs treated with 5’-Aza (2 μM). ***P < 0.001. n = 6. D, Western blot analysis and densitometric quantification of protein level of ABCG1 in BMDMs treated with 5’-Aza (2 μM). **P < 0.01. n = 5. E, Methylation-specific PCR analysis of methylation status of ABCG1 promotor in BMDMs from ADKWT and ADKMAC-KO mice. *P < 0.05. n = 3. F, Methylation-specific PCR analysis of methylation status of ABCG1 promotor in plaque macrophages sorted from atherosclerotic arteries of ApoE−/−/ADKWT and ApoE−/−/ADKMAC-KO mice. *P < 0.05. n = 4–5. G, Schematic illustration of the predicted CpG islands within the promoter region of the ABCG1 gene. H, Bisulfite sequencing PCR analysis of the CpG site methylation status in ABCG1 CpG islands in BMDMs from ADKWT and ADKMAC-KO mice. Data in bar graphs are expressed as mean ± SEM. Statistical significance was determined by one-way ANOVA with Bonferroni’s post hoc tests (for A), Kruskal-Wallis test with post hoc tests (for B), unpaired Student’s t-test (for C and D) and Mann Whitney test (for E and F).
Discussion
In this study, we found that augmentation of intracellular adenosine levels through deletion or inhibition of ADK suppresses foam cell formation and subsequently inhibits atherosclerosis. Interestingly, we found that these effects are largely independent of purinergic receptor activation and instead occur through decreased DNA methylation of the ABCG1 gene leading to increased protein expression. These findings reveal a novel epigenetic pathway by which intracellular adenosine exerts anti-atherogenic effects in macrophages.
ADK regulates intracellular adenosine production in foam cells, which are a major cellular component of atherosclerotic lesions4–6. The cores of atherosclerotic lesions are hypoxic, as evidenced by the increased levels of hypoxia induced factors (HIFs) and positive pimonidazole staining 22–24. Pathways for adenosine accumulation are highly active under hypoxic conditions and subject to transcriptional regulation by HIFs25, 26. For example, the major adenosine-generating enzymes, such as CD73, 5’-nucleotidase, and adenosine-catabolizing enzymes such as ADK and ADA, are regulated by HIFs 25, 26. These changes presumably augment adenosine levels resulting in stimulation of adenosine receptors which are thought to mediate the beneficial effect of adenosine. Indeed, a few studies have demonstrated that adenosine and adenosine receptors are important for suppression of foam cell formation8. ADK is an intracellular enzyme that metabolizes intracellular adenosine and subsequently limits the export of adenosine into the extracellular environment9. We have shown that in response to hypoxia, endothelial ADK is down-regulated, resulting in an increase of both intracellular and extracellular adenosine. The enhancement of adenosine levels in endothelial cells accelerates angiogenesis19. In contrast, in macrophages treated with ox-LDL, ADK expression is increased and this is accompanied by a decrease in intracellular adenosine. Increased ADK levels in foam cells may decrease the overall level of adenosine in lesions and therefore oppose the beneficial effects of adenosine, indicating the necessity of inhibiting ADK to suppress foam cell formation.
We found that ADK deficiency in macrophages led to a reduction in ABCG1 gene methylation and reduced foam cell formation. Foam cell formation is the net result of lipid uptake versus efflux with each pathway subject to regulatory control by a range of molecules4–6. It is well established that ABCA1 and ABCG1 are major regulators of lipid efflux27. ABCG1 expression is subject to epigenetic control, and its expression level is inversely correlated with the level of gene methylation 28, 29. Clinical data show that the rates of diabetes and cardiovascular disorders are increased in patients with high levels of ABCG1 methylation30–32. We have found that ABCG1-mediated cholesterol efflux plays an important role in decreased foam cell formation in ADK-deficient macrophages. In ADK deficient macrophages, ABCG1 protein expression was not affected by antagonists of adenosine receptors suggesting other actions of adenosine. In contrast, the increased ABCG1 level in ADK-deficient macrophages is seen in macrophages treated with DNA methylation inhibitor. In endothelial cells, ADK deficiency leads to DNA hypomethylation19. In line with a previous study, both bisulfite sequencing and methylation-specific PCR of ABCG1 revealed a significant decrease in the levels of DNA methylation on the ABCG1 gene promoter in ADK-deficient macrophages. In contrast, no significant decrease in the levels of DNA methylation on the ABCA1 gene promotor was found in ADK-deficient macrophages (Supplemental Figure 6). This indicates that compared with ABCA1, the ABCG1 gene is more susceptible to epigenetic regulation by DNA methylation in the context of ADK deficiency.
Adenosine receptors do not critically participate in the decreased amount of foam cell formation of ADK-deficient macrophages. Involvement of A2AR in foam cell formation has been intensively studied over the past several years. A2AR occupancy inhibits foam cell formation via several pathways. First, A2AR agonists upregulate the reverse cholesterol transport proteins cholesterol 27-hydroxylase and ABCA1, both of which are important for transport of cholesterol out of macrophages, resulting in decreased foam cell formation33, 34. Secondly, lectin-like oxidized LDL receptor-1(LOX-1) is a membrane-bound receptor that is found at high concentration in human atherosclerotic lesions and cultured human macrophages35, 36. As a cell surface endocytosis receptor, macrophage LOX-1 binds and internalizes ox-LDL and therefore promotes foam cell formation. A2AR activation downregulates macrophage LOX-1 and inhibits foam cell formation8. In contrast to the above studies, blocking adenosine receptors, including A2AR and A2BR, did not reverse decreased foam cell formation in ADK-deficient macrophages, indicating that the ability of ADK-deficiency to regulate foam cell formation does not occur through adenosine receptors. ADK deletion increases intracellular adenosine levels and conceptually is thought to be transported to the extracellular compartment to activate adenosine receptors9, 19. It is likely that adenosine receptor occupancy secondary to increased extracellular adenosine may repress foam cell formation under different conditions. However, in macrophages with ADK deficiency, the intracellular epigenetic pathway appears to be the dominant effect. Under these conditions, the effect of adenosine receptors on anti-foam cell formation in ADK-deficient macrophages may not be as easily detected.
The ability of ADK-deficiency to mitigate macrophage inflammation may also contribute to attenuated atherosclerosis in ApoE−/−/ADKMAC-KO mice. ADK deficiency did not significantly affect the percentages of circulating Ly6Chi and Ly6Clo monocyte, the levels of major monocyte recruiting molecules such as PSGL-1, L-selectin, CCR2 and VLA-4 (Supplemental Figure 1C), as well as M1/M2 macrophage polarization in in vitro assays. However, in response to LPS stimulation, ADK-deficient macrophages released lower levels of proinflammatory cytokines such as TNFα, IL-6 and IL-1β (data not shown). The effect of adenosine receptor 2, especially A2AR, on cytokine production from activated macrophages has been shown to be complex. While a large body of studies have reported that the occupancy of A2AR with either exogenous adenosine or A2AR agonists suppresses production of proinflammatory cytokines 37–41, a recent study shows that A2AR-deficient macrophages exhibit a low level of constitutive inflammasome activation42. Previous studies have indicated that macrophage ABCG1 is critically involved in the production of proinflammatory cytokines43, 44. Therefore, in ADK-deficient macrophages, the suppression of inflammatory activity may be attributed to upregulation of ABCG1. The post-translational methylation of lysine 4 of histone 3 (H3K4) is associated with the transcription of proinflammatory cytokines45, 46. In endothelial cells, ADK deficiency decreases the levels of methylated H3K4 and further decreases endothelial inflammation47 . It is very likely that a similar signaling modulation also exists in macrophages and that the low level of methylated histone results in suppressed inflammation in ADK-deficient macrophages.
The importance of lymphocytes in atherosclerosis has been appreciated for decades and it has been demonstrated that regulatory T cells play a protective role in atherogenesis in mice with hyperlipidemia48–50. The percentage of blood regulatory T cells is elevated in myeloid ADK deficient mice compared with controls. Additionally, a slight increase of regulatory T cells in atherosclerotic lesions in myeloid ADK deficient mice was observed although the increase did not reach significance. Increased regulatory T cells in myeloid specific ADK deficient mice may also contribute to the decreased atherosclerosis observed in these mice, although it remains unclear and further study are needed to determine the mechanism by which the number of regulatory T cells is increased and establish cause and effect.
Collectively, we have demonstrated that ADK deletion in macrophages exerts an anti-atherosclerotic effect by decreasing foam cell formation through epigenetic modulation of DNA methylation of ABCG1, which enhances ABCG1 expression leading to increased lipid efflux from foam cells. We have recently shown an anti-inflammatory effect of ADK-deficiency on endothelial cells47. Therefore, targeting ADK, which can influence multiple cellular participants in the formation of atherosclerosis, is likely to be a promising target for the prevention and treatment of atherosclerosis.
Supplementary Material
Highlights.
Macrophages and foam cells express adenosine kinase (ADK), which regulates the level of intracellular adenosine.
Knockdown (KD) or inhibition of adenosine kinase suppresses foam cell formation and the development of atherosclerotic plaques.
Inhibitory effect of ADK knockdown or inhibition on foam cell formation is achieved by ABCG1-mediated increased lipid efflux.
Enhanced intracellular adenosine-mediated hypomethylation of the ABCG1 gene but not adenosine receptors plays a critical role in ADK KD-induced inhibitory effect on foam cell formation.
Acknowledgments
a) Acknowledgments: none
b) Sources of Funding: This work was supported by grants from the Shenzhen Science and Technology Innovation Committee (JCYJ20170412150405310, JCYJ20160525154531263, JCYJ20160506170316776, and JCYJ20170810163238384), Guangdong Natural Science Foundation (2014A030312004), American Heart Association (16GRNT30510010) and the National Institutes of Health (R01HL134934, R01DK095862 and R01 HL142097).
Nonstandard Abbreviations and Acronyms
- ADK
adenosine kinase
- ADA
adenosine deaminase
- SAH
S-adenosyl homocysteine
- AMP
Adenosine monophosphate
- SAHH
S-adenosyl homocysteine hydrolase
- A2AR
adenosine receptor 2A
- ApoE
apolipoprotein E
- BMDM
bone marrow-derived macrophage
- ox-LDL
oxidized low density lipoprotein
- SR-A
scavenger receptor A
- CD36
cluster of differentiation 36
- LOX-1
lectin-like oxidized LDL receptor-1
- ABCA1
ATP binding cassette transporter A1
- ABCG1
ATP binding cassette transporter G1
- ApoA-I
apolipoprotein A-I
- HDL
high density lipoprotein
- HIFs
hypoxia induced factors
Footnotes
c) Disclosures: none
References
- 1.Libby P Inflammation in atherosclerosis. Nature. 2002;420:868–874 [DOI] [PubMed] [Google Scholar]
- 2.Glass CK, Witztum JL. Atherosclerosis. The road ahead. Cell. 2001;104:503–516 [DOI] [PubMed] [Google Scholar]
- 3.Ross R Atherosclerosis--an inflammatory disease. The New England journal of medicine. 1999;340:115–126 [DOI] [PubMed] [Google Scholar]
- 4.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: A dynamic balance. Nat Rev Immunol. 2013;13:709–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tabas I, Bornfeldt KE. Macrophage phenotype and function in different stages of atherosclerosis. Circulation research. 2016;118:653–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Csoka B, Selmeczy Z, Koscso B, Nemeth ZH, Pacher P, Murray PJ, Kepka-Lenhart D, Morris SM Jr., Gause WC, Leibovich SJ, Hasko G. Adenosine promotes alternative macrophage activation via a2a and a2b receptors. Faseb j. 2012;26:376–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reiss AB, Cronstein BN. Regulation of foam cells by adenosine. Arteriosclerosis, thrombosis, and vascular biology. 2012;32:879–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boison D Adenosine kinase: Exploitation for therapeutic gain. Pharmacol Rev. 2013;65:906–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park JGR. Adenosine metabolism, adenosine kinase, and evolution. 2013. [Google Scholar]
- 11.Boison D, Scheurer L, Zumsteg V, Rulicke T, Litynski P, Fowler B, Brandner S, Mohler H. Neonatal hepatic steatosis by disruption of the adenosine kinase gene. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:6985–6990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moffatt BA, Stevens YY, Allen MS, Snider JD, Pereira LA, Todorova MI, Summers PS, Weretilnyk EA, Martin-McCaffrey L, Wagner C. Adenosine kinase deficiency is associated with developmental abnormalities and reduced transmethylation. Plant Physiol. 2002;128:812–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams-Karnesky RL, Sandau US, Lusardi TA, Lytle NK, Farrell JM, Pritchard EM, Kaplan DL, Boison D. Epigenetic changes induced by adenosine augmentation therapy prevent epileptogenesis. The Journal of clinical investigation. 2013;123:3552–3563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lawson BR, Manenkova Y, Ahamed J, Chen X, Zou JP, Baccala R, Theofilopoulos AN, Yuan C. Inhibition of transmethylation down-regulates cd4 t cell activation and curtails development of autoimmunity in a model system. J Immunol. 2007;178:5366–5374 [DOI] [PubMed] [Google Scholar]
- 15.Pike MC, Snyderman R. Transmethylation reactions regulate affinity and functional activity of chemotactic factor receptors on macrophages. Cell. 1982;28:107–114 [DOI] [PubMed] [Google Scholar]
- 16.Daugherty A, Tall AR, Daemen M, Falk E, Fisher EA, Garcia-Cardena G, Lusis AJ, Owens AP 3rd, Rosenfeld ME, Virmani R, American Heart Association Council on Arteriosclerosis T, Vascular B, Council on Basic Cardiovascular S. Recommendation on design, execution, and reporting of animal atherosclerosis studies: A scientific statement from the american heart association. Arteriosclerosis, thrombosis, and vascular biology. 2017;37:e131–e157 [DOI] [PubMed] [Google Scholar]
- 17.Robinet P, Milewicz DM, Cassis LA, Leeper NJ, Lu HS, Smith JD. Consideration of sex differences in design and reporting of experimental arterial pathology studies-statement from atvb council. Arteriosclerosis, thrombosis, and vascular biology. 2018;38:292–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindau A, Hardtner C, Hergeth SP, Blanz KD, Dufner B, Hoppe N, Anto-Michel N, Kornemann J, Zou J, Gerhardt LM, Heidt T, Willecke F, Geis S, Stachon P, Wolf D, Libby P, Swirski FK, Robbins CS, McPheat W, Hawley S, Braddock M, Gilsbach R, Hein L, von zur Muhlen C, Bode C, Zirlik A, Hilgendorf I. Atheroprotection through syk inhibition fails in established disease when local macrophage proliferation dominates lesion progression. Basic Res Cardiol. 2016;111:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu Y, Wang Y, Yan S, Zhou Y, Yang Q, Pan Y, Zeng X, An X, Liu Z, Wang L, Xu J, Cao Y, Fulton DJ, Weintraub NL, Bagi Z, Hoda MN, Wang X, Li Q, Hong M, Jiang X, Boison D, Weber C, Wu C, Huo Y. Intracellular adenosine regulates epigenetic programming in endothelial cells to promote angiogenesis. 2017;9:1263–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sankaranarayanan S, Kellner-Weibel G, de la Llera-Moya M, Phillips MC, Asztalos BF, Bittman R, Rothblat GH. A sensitive assay for abca1-mediated cholesterol efflux using bodipy-cholesterol. J Lipid Res. 2011;52:2332–2340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butcher MJ, Herre M, Ley K, Galkina E. Flow cytometry analysis of immune cells within murine aortas. J Vis Exp. 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sluimer JC, Gasc JM, van Wanroij JL, Kisters N, Groeneweg M, Sollewijn Gelpke MD, Cleutjens JP, van den Akker LH, Corvol P, Wouters BG, Daemen MJ, Bijnens AP. Hypoxia, hypoxia-inducible transcription factor, and macrophages in human atherosclerotic plaques are correlated with intraplaque angiogenesis. J Am Coll Cardiol. 2008;51:1258–1265 [DOI] [PubMed] [Google Scholar]
- 23.Silvola JM, Saraste A, Forsback S, Laine VJ, Saukko P, Heinonen SE, Yla-Herttuala S, Roivainen A, Knuuti J. Detection of hypoxia by [18f]ef5 in atherosclerotic plaques in mice. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:1011–1015 [DOI] [PubMed] [Google Scholar]
- 24.Aarup A, Pedersen TX, Junker N, Christoffersen C, Bartels ED, Madsen M, Nielsen CH, Nielsen LB. Hypoxia-inducible factor-1alpha expression in macrophages promotes development of atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2016;36:1782–1790 [DOI] [PubMed] [Google Scholar]
- 25.Karmouty-Quintana H, Xia Y, Blackburn MR. Adenosine signaling during acute and chronic disease states. J Mol Med (Berl). 2013;91:173–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bowser JL, Lee JW, Yuan X, Eltzschig HK. The hypoxia-adenosine link during inflammation. J Appl Physiol (1985). 2017;123:1303–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yvan-Charvet L, Wang N, Tall AR. Role of hdl, abca1, and abcg1 transporters in cholesterol efflux and immune responses. Arteriosclerosis, thrombosis, and vascular biology. 2010;30:139–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guay SP, Brisson D, Lamarche B, Gaudet D, Bouchard L. Epipolymorphisms within lipoprotein genes contribute independently to plasma lipid levels in familial hypercholesterolemia. Epigenetics. 2014;9:718–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pfeiffer L, Wahl S, Pilling LC, Reischl E, Sandling JK, Kunze S, Holdt LM, Kretschmer A, Schramm K, Adamski J, Klopp N, Illig T, Hedman AK, Roden M, Hernandez DG, Singleton AB, Thasler WE, Grallert H, Gieger C, Herder C, Teupser D, Meisinger C, Spector TD, Kronenberg F, Prokisch H, Melzer D, Peters A, Deloukas P, Ferrucci L, Waldenberger M. DNA methylation of lipid-related genes affects blood lipid levels. Circ Cardiovasc Genet. 2015;8:334–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dayeh T, Tuomi T, Almgren P, Perfilyev A, Jansson PA, de Mello VD, Pihlajamaki J, Vaag A, Groop L, Nilsson E, Ling C. DNA methylation of loci within abcg1 and phospho1 in blood DNA is associated with future type 2 diabetes risk. Epigenetics. 2016;11:482–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peng P, Wang L, Yang X, Huang X, Ba Y, Chen X, Guo J, Lian J, Zhou J. A preliminary study of the relationship between promoter methylation of the abcg1, galnt2 and hmgcr genes and coronary heart disease. PLoS One. 2014;9:e102265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hedman AK, Mendelson MM, Marioni RE, Gustafsson S, Joehanes R, Irvin MR, Zhi D, Sandling JK, Yao C, Liu C, Liang L, Huan T, McRae AF, Demissie S, Shah S, Starr JM, Cupples LA, Deloukas P, Spector TD, Sundstrom J, Krauss RM, Arnett DK, Deary IJ, Lind L, Levy D, Ingelsson E. Epigenetic patterns in blood associated with lipid traits predict incident coronary heart disease events and are enriched for results from genome-wide association studies. Circ Cardiovasc Genet. 2017;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reiss AB, Rahman MM, Chan ES, Montesinos MC, Awadallah NW, Cronstein BN. Adenosine a2a receptor occupancy stimulates expression of proteins involved in reverse cholesterol transport and inhibits foam cell formation in macrophages. J Leukoc Biol. 2004;76:727–734 [DOI] [PubMed] [Google Scholar]
- 34.Bingham TC, Fisher EA, Parathath S, Reiss AB, Chan ES, Cronstein BN. A2a adenosine receptor stimulation decreases foam cell formation by enhancing abca1-dependent cholesterol efflux. J Leukoc Biol. 2010;87:683–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kataoka H, Kume N, Miyamoto S, Minami M, Moriwaki H, Murase T, Sawamura T, Masaki T, Hashimoto N, Kita T. Expression of lectinlike oxidized low-density lipoprotein receptor-1 in human atherosclerotic lesions. Circulation. 1999;99:3110–3117 [DOI] [PubMed] [Google Scholar]
- 36.Kume N, Moriwaki H, Kataoka H, Minami M, Murase T, Sawamura T, Masaki T, Kita T. Inducible expression of lox-1, a novel receptor for oxidized ldl, in macrophages and vascular smooth muscle cells. Ann N Y Acad Sci. 2000;902:323–327 [DOI] [PubMed] [Google Scholar]
- 37.Hasko G, Szabo C, Nemeth ZH, Kvetan V, Pastores SM, Vizi ES. Adenosine receptor agonists differentially regulate il-10, tnf-alpha, and nitric oxide production in raw 264.7 macrophages and in endotoxemic mice. J Immunol. 1996;157:4634–4640 [PubMed] [Google Scholar]
- 38.Bshesh K, Zhao B, Spight D, Biaggioni I, Feokistov I, Denenberg A, Wong HR, Shanley TP. The a2a receptor mediates an endogenous regulatory pathway of cytokine expression in thp-1 cells. J Leukoc Biol. 2002;72:1027–1036 [PubMed] [Google Scholar]
- 39.Kreckler LM, Wan TC, Ge ZD, Auchampach JA. Adenosine inhibits tumor necrosis factor-alpha release from mouse peritoneal macrophages via a2a and a2b but not the a3 adenosine receptor. J Pharmacol Exp Ther. 2006;317:172–180 [DOI] [PubMed] [Google Scholar]
- 40.Ryzhov S, Zaynagetdinov R, Goldstein AE, Novitskiy SV, Blackburn MR, Biaggioni I, Feoktistov I. Effect of a2b adenosine receptor gene ablation on adenosine-dependent regulation of proinflammatory cytokines. J Pharmacol Exp Ther. 2008;324:694–700 [DOI] [PubMed] [Google Scholar]
- 41.Hasko G, Cronstein B. Regulation of inflammation by adenosine. Front Immunol. 2013;4:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ouyang X, Ghani A, Malik A, Wilder T, Colegio OR, Flavell RA, Cronstein BN, Mehal WZ. Adenosine is required for sustained inflammasome activation via the a(2)a receptor and the hif-1alpha pathway. Nature communications. 2013;4:2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baldan A, Gomes AV, Ping P, Edwards PA. Loss of abcg1 results in chronic pulmonary inflammation. J Immunol. 2008;180:3560–3568 [DOI] [PubMed] [Google Scholar]
- 44.Wojcik AJ, Skaflen MD, Srinivasan S, Hedrick CC. A critical role for abcg1 in macrophage inflammation and lung homeostasis. J Immunol. 2008;180:4273–4282 [DOI] [PubMed] [Google Scholar]
- 45.Foster SL, Hargreaves DC, Medzhitov R. Gene-specific control of inflammation by tlr-induced chromatin modifications. Nature. 2007;447:972–978 [DOI] [PubMed] [Google Scholar]
- 46.Medzhitov R, Horng T. Transcriptional control of the inflammatory response. Nat Rev Immunol. 2009;9:692–703 [DOI] [PubMed] [Google Scholar]
- 47.Xu Y, Wang Y, Yan S, Yang Q, Zhou Y, Zeng X, Liu Z, An X, Toque HA, Dong Z, Jiang X, Fulton DJ, Weintraub NL, Li Q, Bagi Z, Hong M, Boison D, Wu C. Regulation of endothelial intracellular adenosine via adenosine kinase epigenetically modulates vascular inflammation. 2017;8:943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Daugherty A, Pure E, Delfel-Butteiger D, Chen S, Leferovich J, Roselaar SE, Rader DJ. The effects of total lymphocyte deficiency on the extent of atherosclerosis in apolipoprotein e−/− mice. The Journal of clinical investigation. 1997;100:1575–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dansky HM, Charlton SA, Harper MM, Smith JD. T and b lymphocytes play a minor role in atherosclerotic plaque formation in the apolipoprotein e-deficient mouse. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:4642–4646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ait-Oufella H, Salomon BL, Potteaux S, Robertson AK, Gourdy P, Zoll J, Merval R, Esposito B, Cohen JL, Fisson S, Flavell RA, Hansson GK, Klatzmann D, Tedgui A, Mallat Z. Natural regulatory t cells control the development of atherosclerosis in mice. Nat Med. 2006;12:178–180 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.