Abstract
Introduction
The primary goal in the management of acute ST segment elevation myocardial infarction (STEMI) is to open the occluded artery at an early stage. The development of no-reflow is multifactorial, and the etiology is not fully understood. There is accumulating evidence that anemia is related to a series of severe complications in cardiovascular disease (CVD) such as thromboembolic events, bleeding complications, uncontrolled hypertension, and inflammation characterized by elevated levels of inflammatory cytokines.
Aim
We investigated the relationship between hemoglobin level and the no-reflow of infarct-related artery (IRA) in patients with STEMI undergoing primary percutaneous coronary intervention (PPCI).
Material and methods
A total of 3804 patients with acute STEMI who underwent PPCI were enrolled. The patients were divided into two groups according to thrombolysis in myocardial infarction (TIMI) flow grades after PPCI. Hematological parameters were measured on admission. Univariate and multivariate logistic regression analyses were conducted to assess the association between hemoglobin level and no-reflow.
Results
In the current study, 471 (12.4%) patients presented with no-reflow after PPCI. The patients in the no-reflow group had a significantly lower hemoglobin level (12.1 ±1.9 g/dl vs. 13.8 ±1.8 g/dl, p < 0.001). The multivariate logistic regression models revealed that hemoglobin level (OR = 0.564, 95% CI: 0.526–0.605; p < 0.001) was an independent predictor of development of no-reflow. The cutoff value for hemoglobin level was 11.5 g/dl with sensitivity of 83.0% and specificity of 80.0% (AUC = 0.844, 95% CI: 0.821–0.867; p < 0.001).
Conclusions
Our results suggest that hemoglobin level showed a moderate diagnostic performance regarding the prediction of no-reflow in patients with STEMI undergoing PPCI.
Keywords: myocardial infarction, no-reflow, hemoglobin level
Summary
The primary goal in the management of acute ST segment elevation myocardial infarction (STEMI) is to open the occluded artery at an early stage. The development of no-reflow is multifactorial, and the etiology is not fully understood. There is accumulating evidence that anemia is related to a series of severe complications in cardiovascular disease (CVD) such as thromboembolic events, bleeding complications, uncontrolled hypertension, and inflammation characterized by elevated levels of inflammatory cytokines. We investigated the relationship between hemoglobin level and the no-reflow of infarct-related artery (IRA) in patients with STEMI undergoing primary percutaneous coronary intervention (PPCI). A total 3804 patients with acute STEMI who underwent PPCI were enrolled. The patients were divided into two groups according to thrombolysis in myocardial infarction (TIMI) flow grades after PPCI. The multivariate logistic regression models revealed that hemoglobin level (OR = 0.564, 95% CI: 0.526–0.605; p < 0.001) was an independent predictor of development of no-reflow. Our results suggest that hemoglobin level showed a moderate diagnostic performance regarding the prediction of no-reflow in patients with STEMI undergoing PPCI.
Introduction
The primary goal in the management of acute ST segment elevation myocardial infarction (STEMI) is to open the occluded artery at an early stage. Primary percutaneous coronary intervention (PPCI) is currently the best available reperfusion therapy in the case of acute STEMI [1]. Up to 95% of occluded coronary arteries can be reopened in the setting of STEMI [2, 3]. The coronary no-reflow (NR) phenomenon is observed if cardiac tissue cannot be perfused normally despite opening of the occluded vessel in absence of spasm, dissection, or distal macro-embolus [4, 5]. No-reflow may develop in 2–30% of acute STEMI cases after PPCI [6, 7]. Several factors may contribute to development of no-reflow including distal embolization, ischemia-reperfusion injury resulting from oxygen free radical production, microvascular damage, myocardial necrosis and stunning, release of active tissue factor from the dissected plaque, and vasoconstriction secondary to α adrenergic tone, thromboxane A2 or serotonin released from platelets [4, 8–10].
The major function of erythrocytes is to transport hemoglobin, which in turn carries oxygen from the lungs to the tissues. Anemia is a frequent comorbidity in cardiovascular disease and is associated with higher mortality as well as increased hospitalization after myocardial infarction, even under mild anemia [11–14]. There is accumulating evidence that anemia is related to a series of severe complications in cardiovascular diseases (CVD) such as thromboembolic events, bleeding complications, uncontrolled hypertension, and inflammation characterized by elevated levels of inflammatory cytokines [15–17].
The development of no-reflow is multifactorial, and the etiology has not been understood clearly. The association between hemoglobin level and the no-reflow of the infarct-related artery (IRA) in patients with STEMI remains unknown.
Aim
In this study, we investigated the relationship between hemoglobin level and the no-reflow of IRA in patients with STEMI undergoing PPCI.
Material and methods
This retrospective study was conducted between January 2012 and June 2016. A total of 3804 subjects who presented with acute STEMI within 12 h from the symptom onset were included in this study. Clinical, demographic, historical, angiographic, treatment and laboratory data were obtained from the hospital’s medical database. The STEMI was defined based on criteria by the European Society of Cardiology [18]. Exclusion criteria were patients with hemoglobin level of less than 10 g/dl, inflammatory disease, hematological disorders, acute anemia, any active bleeding, polycythemia, end-stage renal and liver failure, coagulopathy, malignancy, unstable angina pectoris, non-ST elevation myocardial infarction, elective PCI, venous graft-related infarcts, non-cardiogenic shock or history of cardiac arrest on admission. Coronary angiography was performed in 90 min following the admission. All patients received dual antiplatelet therapy with aspirin (162–325-mg), and a clopidogrel (300 mg for patients < 75 years of age, 75 mg for patients > 75 years of age) loading dose or ticagrelor (180 mg) loading dose. Preprocedural anticoagulation consisted of intravenous unfractionated heparin (35–70 IU/kg) in all cases. PPCI with stent implantation was performed according to the current guidelines [19]. Anterograde coronary flow in the responsible vessel was graded according to the thrombolysis in myocardial infarction (TIMI) scale [20]. To evaluate the intracoronary thrombus burden, we applied the TIMI thrombus scale [21]. We preliminarily calculated the intracoronary thrombus burden after crossing the occluded site with a 0.014-inch guide wire and/or non-inflated balloon catheter. The low-thrombus burden group was defined as a thrombus grade of 0 to 2, and high-thrombus burden was defined as a thrombus grade of at least 3.
The TIMI flow grades were evaluated by the consensus of two interventional cardiologists blinded to the clinical and laboratory data by using the quantitative cardiovascular angiographic software. Coronary no-reflow was defined as TIMI grade < 3 after vessel recanalization with the absence of angiographic stenosis, spasm, dissection, or thrombosis. Any major coronary vessels with a diameter narrowed by 50% or more were defined as significant stenosis. If there was stenosis of > 50% diameter affecting more than two epicardial coronary arteries, it was defined as multivessel disease. Successful primary PCI procedure was defined as obtaining residual stenosis of < 10% with TIMI-3 flow in IRA by visual evaluation. In addition, quantitative angiographic analysis of the lesion length and reference vessel diameter was performed using a digital edge-detection algorithm and by selecting end-diastolic frames demonstrating the stenosis in its most severe and non-foreshortened projection.
Patients underwent standard 2-dimensional echocardiography with a digital ultrasonic device system (iE33; Philips, Netherlands) while lying in left lateral decubitus position before discharge. Left ventricular ejection fraction (LVEF) was measured by the Simpson method in the 2-dimensional echocardiographic apical 4-chamber view. Patients were divided into 2 groups according to TIMI flow grades after PPCI.
In all patients, blood samples were collected from the antecubital vein in the emergency room prior to the administration of aspirin, clopidogrel, ticagrelor and an unfractionated heparin bolus for laboratory analysis. Complete blood count (CBC) parameters were measured by an ABX Pentra DX 120 hematology analyzer immediately after sampling. Biochemical parameters were measured by the Roche Cobas Integra 800 (Roche Diagnostics Limited, Switzerland). Informed consent of each subject and approval of the Local Ethics Committee were obtained.
Statistical analysis
Continuous variables were presented as mean (standard deviation) and categorical variables as number (percentage). The distributions of the continuous variables across the study groups were tested with the Kolmogorov-Smirnov test. Continuous variables were compared using Student’s t test. Categorical data were compared using the χ2 or Fisher’s exact tests, when needed. Receiver operating characteristic (ROC) curve was used to assign the sensitivity and specificity of hemoglobin level and the optimal cut-off value for predicting no-reflow in patients with STEMI. Univariate and multivariate logistic regression analyses were conducted to assess the association of hemoglobin level and no-reflow, and in-hospital mortality. In stepwise multivariate regression analysis (Backward, Wald), effect size was adjusted for all variables with a univariate significance level of < 0.2. Adjusted odds ratios (OR), along with their 95% CIs were presented. A 2-tailed p-value of < 0.05 was considered statistically significant. All statistical analyses were performed using the IBM SPSS software (IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp.)
Results
Our study included 3804 patients (678 female; mean age of 57.2 ±11.3 years) with acute STEMI who underwent PPCI within 12 h from symptom onset. In the current study, 471 (12.4%) subjects developed no-reflow after PPCI. The baseline demographic, clinical, laboratory findings and in-hospital mortality rates of the no-reflow and normal reflow groups are summarized in Table I. In sub-analysis of the patients with hemoglobin level between 10 to 12 g/dl, we found that 327 (9.8%) patients had a normal reflow pattern while 163 (34.6%) patients had a no-reflow pattern (p < 0.001).
Table I.
Baseline characteristics
| Parameter | Normal reflow (n = 3333) | No-reflow (n = 471) | P-value |
|---|---|---|---|
| Age [years] | 57.5 ±11.5 | 65.8 ±12.7 | < 0.001 |
| Male (%) | 2748 (82.4) | 378 (80.2) | 0.185 |
| Hypertension (%) | 1286 (38.6) | 204 (43.3) | < 0.001 |
| Diabetes mellitus (%) | 897 (26.9) | 141 (30) | 0.003 |
| History of smoking (%) | 750 (22.5) | 99 (21) | 0.484 |
| Hyperlipidemia (%) | 787 (23.6) | 107 (22.7) | 0.650 |
| Family history of coronary artery disease (%) | 295 (8.8) | 40 (8.5) | 0.510 |
| Previous myocardial infarction (%) | 731 (21.9) | 117 (24.8) | 0.120 |
| Previous percutaneous coronary intervention (%) | 575 (17.3) | 84 (17.8) | 0.790 |
| Previous coronary artery bypass grafting (%) | 183 (4.1) | 51 (10.8) | < 0.001 |
| Heart rate [beat/min] | 77 ±14 | 83 ±21 | 0.002 |
| Time from symptoms onset to PCI [h] | 7.2 ±12.6 | 9.6 ±10.5 | < 0.001 |
| Door to balloon time [min] | 19.3 ±10.2 | 19.5 ±10.5 | 0.756 |
| Use of antiaggregant (clopidogrel/ticagrelor) (%) | 2243 (67)/1090 (33) | 306 (65)/165 (35) | 0.510 |
| Left ventricular ejection fraction (%) | 48.7 ±10.5 | 40.6 ±11.6 | < 0.001 |
| Cardiac function Killip grade (%): | < 0.001 | ||
| 1 | 2777 (83.3) | 312 (66.2) | |
| 2 | 328 (9.8) | 60 (12.7) | |
| 3 | 120 (3.7) | 45 (9.6) | |
| 4 | 108 (3.2) | 54 (11.5) | |
| Admission of glucose level [mg/dl] | 153.2 ±73.7 | 182.8 ±99.7 | < 0.001 |
| Admission of creatinin kinase – MB [U/l] | 90.5 ±115.2 | 112.3 ±130.4 | < 0.001 |
| Peak creatinin kinase-MB [U/l] | 140.3 ±146.3 | 192.4 ±174.2 | < 0.001 |
| Preoperative systolic blood pressure [mm Hg] | 13 5 ±35 | 127 ±30 | < 0.001 |
| Preoperative diastolic blood pressure [mm Hg] | 72 ±13 | 78 ±15 | < 0.001 |
| Red blood cell count [× 106/ml] | 4.3 ±0.6 | 3.8 ±0.7 | < 0.001 |
| Hematocrit (%) | 41.6 ±6.7 | 36.2 ±5.5 | < 0.001 |
| Hemoglobin [g/l] | 13.8 ±1.8 | 12.1 ±1.9 | < 0.001 |
| Platelet count [× 103/ml] | 240 ±72 | 260 ±100 | < 0.001 |
| White blood cell count [× 103/ml] | 10.7 ±4.6 | 12.0 ±4.9 | < 0.001 |
| Red cell distrubition width (%) | 13.6 ±1.6 | 14.8 ±2.2 | 0.001 |
| Mean platelet volume [fl] | 8.3 ±1.1 | 9.6 ±1.1 | 0.002 |
| In-hospital mortality (%) | 156 (4.7) | 75 (15.9) | < 0.001 |
Data are presented as mean.
Additionally, the average Hb level of patients with coronary artery bypass grafting (CABG) was 12.8 ±2.0 g/dl while the patients without CABG had an average hemoglobin level of 13.4 ±1.9 g/dl (p < 0.001).
The normal reflow and no-reflow groups were similar with respect to gender, hyperlipidemia, history of smoking, family history of coronary artery disease, history of infarction, history of PCI, glomerular filtration rate, door to balloon time and use of antiaggregants. Compared with the normal reflow group, the no-reflow group was older (65.8 ±12.7 vs. 57.5 ±11.5 years, p < 0.001). The no-reflow group had more patients with hypertension (43.3% vs. 38.6%, p < 0.001), more diabetes mellitus (30% vs. 26.9%, p < 0.005), more previous CABG (10.8% vs. 4.1%, p < 0.001), higher heart rate (83 ±21 vs. 77 ±21 bpm, p < 0.005), longer symptom onset to balloon time (9.6 ±10.5 vs. 7.2 ±12.6 h, p < 0.001), higher glucose level on admission (182.8 ±99.7 vs. 153.2 ±73.7 mg/dl, p < 0.001), higher admission creatine kinase-MB (112.3 ±130.4 vs. 90.5 ±115.2 U/l, p < 0.001), higher peak creatinin kinase-MB (192.4 ±174.2 vs. 140.3 ±146.3 U/l, p < 0.001), higher Killip grade (≥ 2; 33.8% vs. 16.7%, p < 0.001), but lower pre-PPCI systolic pressure (127 ±30 vs. 135 ±35 mm Hg, p < 0.001), lower pre-PPCI diastolic pressure (66 ±15 vs. 72 ±13 mm Hg, p < 0.001), and lower ejection fraction (40.6 ±11.6% vs. 48.7 ±10.5%, p < 0.001).
In comparison of hematological parameters on admission, the patients in the no-reflow group had a significantly higher white blood cell count (12.0 ±4.9 vs. 10.7 ±4.6, p < 0.001), platelet count (260 ±100 vs. 240 ±72, p < 0.001), red cell distribution width (RDW) (14.8 ±2.2 vs. 13.6 ±1.6, p < 0.001), and mean platelet volume (MPV) (9.6 ±1.1 vs. 8.3 ±1.1, p < 0.001), but lower red blood cell (RBC) count (3.8 ±0.7 vs. 4.3 ±0.6, p < 0.001), hemoglobin count (12.1 ±1.9 vs. 13.8 ±1.8 g/dl, p < 0.001), and hematocrit count (36.2 ±5.5% vs. 41.6 ±6.7%, p < 0.001). The patients in the no-reflow group had a significantly higher in-hospital mortality rate (15.95 vs. 4.7%, p < 0.001).
The angiographic and procedural characteristics of the groups are presented in Table II. The normal reflow and no-reflow groups were similar with respect to infarct-related coronary artery and use of thrombus aspiration. The patients in the no-reflow group had more multivessel disease (59.2% vs. 41%, p < 0.001), more low-grade pre-TIMI flow (< 1; 75.7% vs. 69.8%, p < 0.001), more thrombus burden (57.5% vs. 43.8%, p < 0.001) and more frequent use of tirofiban (46.5% vs. 25.6%, p < 0.001) compared to the patients in the normal reflow group. The patients in the no-reflow group had a significantly smaller reference vessel diameter (2.6 ±0.8 vs. 3.2 ±1.2 mm, p < 0.001), but longer lesion length (19.3 ±8.6 vs. 13.5 ±12.2 mm, p < 0.001) compared to the normal reflow patients. There was a significant difference with respect to frequency of the reperfusion method (p < 0.001). Balloon dilatation alone without stenting was more frequently used in the no-reflow group. However, stent implantation following pre-dilatation and direct stent implantation were more frequently used in the normal reflow group.
Table II.
Angiographic finding and primary percutaneous coronary intervention
| Parameter | Normal reflow (n = 3333) | No-reflow (n = 471) | P-value |
|---|---|---|---|
| Multivessel disease (%) | 1365 (41) | 279 (59.2) | < 0.001 |
| Infarct related coronary artery (%): | 0.925 | ||
| Left main artery | 48 (1.4) | 6 (1.3) | |
| Left ascending artery | 1586 (47.6) | 220 (46.7) | |
| Left circumflex artery | 1129 (33.9) | 162 (34.4) | |
| Right artery | 570 (17.1) | 83 (17.6) | |
| Preintervention TIMI-flow (%): | < 0.001 | ||
| 0 | 2325 (69.8) | 357 (75.7) | |
| 1 | 356 (10.7) | 72 (15.4) | |
| 2 | 497 (14.9) | 34 (7.2) | |
| 3 | 155 (4.6) | 8 (1.7) | |
| Thrombus burden (%): | < 0.001 | ||
| Low thrombus burden | 1873 (56.2) | 200 (42.5) | |
| High trombus burden | 1460 (43.8) | 271 (57.5) | |
| Lenght of target lesion [mm] | 13.5 ±12.2 | 19.3 ±8.6 | < 0.001 |
| Reference vessel diameter [mm] | 3.2 ±1.2 | 2.6 ±0.8 | < 0.001 |
| Reperfusion method (%): | < 0.001 | ||
| Balloon dilation | 396 (11.9) | 65 (13.8) | |
| Balloon predilation following stent implantation | 2395 (71.9) | 368 (78.2) | |
| Stent implantation | 542 (16.2) | 38 (8) | |
| Thrombus aspiraton (%) | 137 (4.1) | 27 (5.7) | 0.170 |
| Use of tirofiban (%) | 854 (25.6) | 219 (46.5) | < 0.001 |
Data are presented as mean. TIMI – thrombolysis in myocardial infarction.
The multivariate logistic regression models revealed that hemoglobin level (odds ratio (OR) = 0.564, 95% confidence interval (CI): 0.526–0.605; p < 0.001), age (OR = 1.043, 95% CI: 1.032–1.054; p < 0.001), diabetes mellitus (OR = 1.528, 95% CI: 1.018–2.856; p = 0.03), multi-vessel disease (OR = 1.574, 95% CI: 1.177–2.105; p = 0.002), reference vessel diameter (OR = 0.412, 95% CI: 0.289–0.587; p < 0.001), white blood cell count (OR = 1.066, 95% CI: 1.025–1.108; p = 0.001), pain to balloon time (OR = 1.030, 95% CI: 1.011–1.049; p = 0.002), Killip grade (≥ 2; OR = 2.161, 95% CI: 1.599–2.922; p < 0.001), pre-TIMI (OR = 0.505, 95% CI: 0.341–0.748; p < 0.001), thrombus burden (OR = 1.438, 95% CI: 1.131–1.828, p < 0.001), and reperfusion method with stent implantation following pre-dilatation (OR = 1.464, 95% CI: 1.017–2.103; p = 0.04) were found to be independent factors predicting development of no-reflow in patients undergoing PPCI after adjustment for gender, history of hypertension, previous CABG, systolic blood pressure, diastolic blood pressure, length of target vessel, glucose level, platelet count, RDW, MPV heart rate on admission and use of antiaggregant, as shown in Table III.
Table III.
Univariate and multivariable logistic regression analysis for prediction of no-reflow
| Variable | Univariate | Multivariable | ||||
|---|---|---|---|---|---|---|
| Unadjusted OR | 95% CI | P-value | Adjusted OR | 95% Cl | P-value | |
| Age | 1.061 | 1.052–1.071 | < 0.001 | 1.043 | 1.032–1.054 | < 0.001 |
| Gender (male) | 0.699 | 0.463–1.057 | 0.09 | |||
| Hypertension | 1.761 | 1.383–2.242 | < 0.001 | |||
| Diabetus mellitus | 1.354 | 1.074–1.707 | 0.003 | 1.528 | 1.018–2.856 | 0.03 |
| Previous coronary by-pass grafting | 2.803 | 1.986–3.956 | < 0.001 | |||
| Preoperative systolic pressure [mm Hg] | 0.988 | 0.984–0.991 | < 0.001 | |||
| Preoperative diastolic pressure [mm Hg] | 0.974 | 0.967–0.981 | < 0.001 | |||
| Multivessel disease | 1.945 | 1.592–2.377 | < 0.001 | 1.574 | 1.177–2.105 | 0.002 |
| Reference vessel diameter [mm] | 0.419 | 0.383–0.458 | < 0.001 | 0.412 | 0.289–0.587 | < 0.001 |
| Length of target lesion [mm] | 1.110 | 1.095–1.125 | < 0.001 | |||
| Admission of glucose level [mg/dl] | 1.004 | 1.003–1.008 | < 0.001 | |||
| White blood cell [×103/ml] | 1.014 | 1.008–1.045 | < 0.001 | 1.066 | 1.025–1.108 | 0.001 |
| Hemoglobin [g/l] | 0.523 | 0.490–0.558 | < 0.001 | 0.564 | 0.526–0.605 | < 0.001 |
| Platelet count [× 103/ml] | 1.003 | 1.001–1.004 | < 0.001 | |||
| Red cell distrubition width (%) | 1.302 | 1.233–1.374 | < 0.001 | |||
| Mean platelet volume [fl] | 1.174 | 1.067–1.291 | < 0.001 | |||
| Pain to ballon time [h] | 1.120 | 1.105–1.132 | < 0.001 | 1.030 | 1.011–1.049 | 0.002 |
| Heart rate [beat/min] | 1.010 | 1.003–1.016 | 0.002 | |||
| Killip grade (≥ 2) | 2.506 | 2.225–2.823 | < 0.001 | 2.161 | 1.599–2.922 | < 0.001 |
| Preintervention TIMI-flow | 0.687 | 0.613–0.771 | < 0.001 | 0.505 | 0.341–0.748 | < 0.001 |
| Thrombus burden | 1.304 | 1.121–1.517 | < 0.001 | 1.438 | 1.131–1.828 | 0.003 |
| Use of antiaggregant (ticagrelor) | 1.325 | 0.570–3.090 | 0.510 | |||
| Reperfusion method (balloon predilatation following stent implantation) | 2.920 | 2.544–3.352 | < 0.001 | 1.464 | 1.017–2.103 | 0.04 |
OR – odds ratio, Cl – confidence interval, TIMI – thrombolysis in myocardial infarction.
Similarly, multivariate logistic regression analysis was performed to detect whether hemoglobin level was an independent factor predicting incidence of in-hospital mortality. We found that hemoglobin level was an independent factor predicting in-hospital mortality (OR = 0.850, 95% CI: 0.765–0.945; p < 0.005).
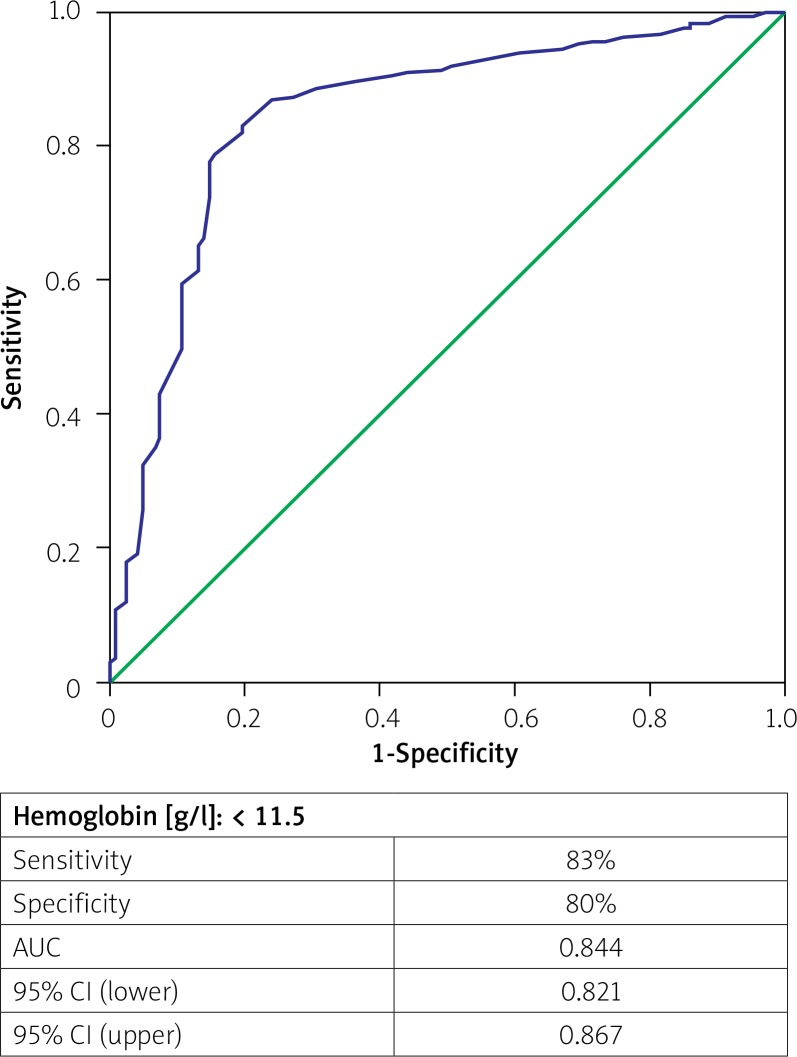
ROC curve analysis was performed to determine the cutoff value for blood hemoglobin level to detect no-reflow in patients with acute STEMI who underwent PPCI (Figure 1). The cutoff value for hemoglobin was 11.5 g/dl with sensitivity of 83.0% and specificity of 80.0% (AUC = 0.844, 95% CI: 0.821–0.867; p < 0.001).
Figure 1.
Receiver operating characteristic curve of hemoglobin for predicting no reflow
AUC – area under curve.
Two blinded interventional cardiologists assessed the data of 100 randomly selected patients for the interobserver and intraobserver agreements for the final coronary flow decision as reflow or no-reflow. The weighted k value between two observers was 0.64 (0.46–0.83). The data were reassessed by the same interventional cardiologist 2 weeks later and the weighted k value was found to be 0.80 (0.66–0.94).
Discussion
The present study results showed that low hemoglobin level was an independent predictor of no-reflow in patients with STEMI who underwent PPCI. For the first time in the literature we have observed that presence of anemia was a predictor of no-reflow. Among patients with acute STEMI, there was a significant increase in no-reflow as the baseline hemoglobin dropped below 11.5 g/dl.
Normally, anemia increases myocardial oxygen demand since a higher stroke volume and heart rate are required to maintain adequate systemic oxygen delivery. However, among the patients with STEMI, the heart is not capable of pumping much greater quantities of blood due to ongoing loss of myocardial tissue. Further, the coronary circulation is likely to be decreased due to low coronary flow in patients with anemia [22]. Also, during myocardial infarction, lower quantities of oxygen are delivered to the peripheral tissues among the patients with a low hemoglobin level. All accompanying compensatory mechanisms, such as increased sympathetic activity, will lead to increased coronary artery microvascular resistance resulting in microvascular spasm and dysfunction.
Anemia has also been linked to inflammatory responses including cytokine and erythropoietin release, which cause endothelial dysfunction, accelerate atherosclerosis, trigger plaque instability, and create a pro-coagulant state [23, 24]. Anemia subsequently promotes erythropoietin release, which causes platelet activation and induction of plasminogen activator inhibitor-1, a pro-coagulant cytokine [23]. This cascade of adverse reactions could accelerate the no-reflow.
Depending on the decrease in hemoglobin level, changes in viscosity may be correlated with changes in deformability or changes in RBC-mediated nitric oxide (NO) metabolism. Effects of blood viscosity on shear stress-induced activation of endothelial NO synthase were also proposed [25]. Therefore, a decrease in blood viscosity may also reduce endothelial-derived NO production and decrease NO bioavailability. Also, NO is a potent inhibitor of platelet aggregation, acting via activation of soluble guanylyl cyclase in platelets [26, 27]. Therefore, one possible explanation for increased no-reflow frequency with a low hemoglobin level may be the fact that fewer RBCs in patients with STEMI may decrease endothelial-derived NO production and decrease NO bioavailability. Another possible coexisting mechanism involved in pro-thrombotic processes may be altered PLT-RBC interaction. There is compelling evidence that RBCs and platelets from patients with different types of anemia such as sickle cell disease, β-thalassemia, and myelodysplastic syndrome show dysregulation of redox systems and altered PLT-RBC interactions [28–30]. The combination of these processes may explain the pathophysiology of the underlying no-reflow phenomenon observed in patients with STEMI with a lower baseline hemoglobin level. We believe that a low hemoglobin level may accelerate the no-reflow phenomenon by affecting the functions of other hematologic parameters.
Previous studies have revealed that anemia is associated with in-hospital and long-term mortality in patients with myocardial infarction [31–34]. The pathophysiologic link between anemia and mortality in patients with MI is not clear but increased sympathetic tonus, tendency for bleeding, deterioration of the ischemia and cardiogenic shock have been speculated as potential mechanisms [22, 33–37].
We found that low hemoglobin level showed a moderate diagnostic performance regarding the prediction of no-reflow in patients with STEMI undergoing PPCI. Anemia may be a predictor of coronary no-reflow, which is associated with high mortality and morbidity in patients with myocardial infarction.
Limitations
The present study had several important limitations. The cause of anemia in patients with a low baseline hemoglobin concentration in the study was unknown, although patients with recent bleeding, known bleeding diathesis, or significant hematologic-oncological or renal diseases (all important potential confounders) were excluded from the study. Another limitation to the study was that erythropoietin levels were not measured in these patients.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Guarini G, Huqi A, Morrone D, et al. Pharmacological approaches to coronary microvascular dysfunction. Pharmacol Ther. 2014;144:283–302. doi: 10.1016/j.pharmthera.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 2.Morishima I, Sone T, Mokuno S, et al. Clinical significance of no-reflow phenomenon observed on angiography after successful treatment of acute myocardial infarction with percutaneous transluminal coronary angioplasty. Am Heart J. 1995;130:239–43. doi: 10.1016/0002-8703(95)90434-4. [DOI] [PubMed] [Google Scholar]
- 3.Gibson CM, Schomig A. Coronary and myocardial angiography: angiographic assessment of both epicardial and myocardial perfusion. Circulation. 2004;109:3096–105. doi: 10.1161/01.CIR.0000134278.50359.CB. [DOI] [PubMed] [Google Scholar]
- 4.Rezkalla SH, Kloner RA. Coronary no-reflow phenomenon. Curr Treat Options Cardiovasc Med. 2005;7:75–80. doi: 10.1007/s11936-005-0008-0. [DOI] [PubMed] [Google Scholar]
- 5.Eeckhout E, Kern MJ. The coronary no-reflow phenomenon: a review of mechanisms and therapies. Eur Heart J. 2001;22:729–39. doi: 10.1053/euhj.2000.2172. [DOI] [PubMed] [Google Scholar]
- 6.Butler MJ, Chan W, Taylor AJ, et al. Management of the no-reflow phenomenon. Pharmacol Ther. 2011;132:72–85. doi: 10.1016/j.pharmthera.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 7.Chan W, Stub D, Clark DJ, et al. Usefulness of transient and persistent no reflow to predict adverse clinical outcomes following percutaneous coronary intervention. Am J Cardiol. 2012;109:478–85. doi: 10.1016/j.amjcard.2011.09.037. [DOI] [PubMed] [Google Scholar]
- 8.Hearse DJ, Bolli R. Reperfusion induced injury: manifestations, mechanisms, and clinical relevance. Cardiovasc Res. 1992;26:101–8. doi: 10.1093/cvr/26.2.101. [DOI] [PubMed] [Google Scholar]
- 9.Bonderman D, Teml A, Jakowitsch J, et al. Coronary no-reflow is caused by shedding of active tissue factor from dissected atherosclerotic plaque. Blood. 2002;99:2794–800. doi: 10.1182/blood.v99.8.2794. [DOI] [PubMed] [Google Scholar]
- 10.Gregorini L, Marco J, Kozakova M, et al. Alpha-adrenergic blockade improves recovery of myocardial perfusion and function after coronary stenting in patients with acute myocardial infarction. Circulation. 1999;99:482–90. doi: 10.1161/01.cir.99.4.482. [DOI] [PubMed] [Google Scholar]
- 11.Ducrocq G, Puymirat E, Steg PG, et al. Blood transfusion, bleeding, anemia, and survival in patients with acute myocardial infarction: FAST-MI registry. Am Heart J. 2015;170:726–34 e2. doi: 10.1016/j.ahj.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Jolicoeur EM, O’Neill WW, Hellkamp A, et al. Transfusion and mortality in patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. Eur Heart J. 2009;30:2575–83. doi: 10.1093/eurheartj/ehp279. [DOI] [PubMed] [Google Scholar]
- 13.Bassand JP, Afzal R, Eikelboom J, et al. Relationship between baseline haemoglobin and major bleeding complications in acute coronary syndromes. Eur Heart J. 2010;31:50–8. doi: 10.1093/eurheartj/ehp401. [DOI] [PubMed] [Google Scholar]
- 14.Rousseau M, Yan RT, Tan M, et al. Relation between hemoglobin level and recurrent myocardial ischemia in acute coronary syndromes detected by continuous electrocardiographic monitoring. Am J Cardiol. 2010;106:1417–22. doi: 10.1016/j.amjcard.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 15.Westenbrink BD, Alings M, Connolly SJ, et al. Anemia predicts thromboembolic events, bleeding complications and mortality in patients with atrial fibrillation: insights from the RE-LY trial. J Thromb Haemost. 2015;13:699–707. doi: 10.1111/jth.12874. [DOI] [PubMed] [Google Scholar]
- 16.Marketou M, Patrianakos A, Parthenakis F, et al. Systemic blood pressure profile in hypertensive patients with low hemoglobin concentrations. Int J Cardiol. 2010;142:95–6. doi: 10.1016/j.ijcard.2008.11.096. [DOI] [PubMed] [Google Scholar]
- 17.Stenvinkel P, Barany P. Anaemia, rHuEPO resistance, and cardiovascular disease in end-stage renal failure; links to inflammation and oxidative stress. Nephrol Dial Transplant. 2002;17(Suppl 5):32–7. doi: 10.1093/ndt/17.suppl_5.32. [DOI] [PubMed] [Google Scholar]
- 18.Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2018;39:119–77. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 19.Windecker S, Kolh P, Alfonso F, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI) Eur Heart J. 2014;35:2541–619. doi: 10.1093/eurheartj/ehu278. [DOI] [PubMed] [Google Scholar]
- 20.The Thrombolysis in Myocardial Infarction (TIMI) trial Phase I findings. N Engl J Med. 1985;312:932–6. doi: 10.1056/NEJM198504043121437. [DOI] [PubMed] [Google Scholar]
- 21.Sianos G, Papafaklis MI, Serruys PW. Angiographic thrombus burden classification in patients with ST-segment elevation myocardial infarction treated with percutaneous coronary intervention. J Invasive Cardiol. 2010;22:6B–14B. [PubMed] [Google Scholar]
- 22.Most AS, Ruocco NA Jr, Gewirtz H. Effect of a reduction in blood viscosity on maximal myocardial oxygen delivery distal to a moderate coronary stenosis. Circulation. 1986;74:1085–92. doi: 10.1161/01.cir.74.5.1085. [DOI] [PubMed] [Google Scholar]
- 23.Twomley KM, Rao SV, Becker RC. Proinflammatory, immunomodulating, and prothrombotic properties of anemia and red blood cell transfusions. J Thromb Thrombolysis. 2006;21:167–74. doi: 10.1007/s11239-006-5206-4. [DOI] [PubMed] [Google Scholar]
- 24.Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 25.Martini J, Carpentier B, Negrete AC, et al. Paradoxical hypotension following increased hematocrit and blood viscosity. Am J Physiol Heart Circ Physiol. 2005;289:H2136–43. doi: 10.1152/ajpheart.00490.2005. [DOI] [PubMed] [Google Scholar]
- 26.Gambaryan S, Geiger J, Schwarz UR, et al. Potent inhibition of human platelets by cGMP analogs independent of cGMP-dependent protein kinase. Blood. 2004;103:2593–600. doi: 10.1182/blood-2003-09-3349. [DOI] [PubMed] [Google Scholar]
- 27.Gambaryan S, Kobsar A, Hartmann S, et al. NO-synthase-/NO-independent regulation of human and murine platelet soluble guanylyl cyclase activity. J Thromb Haemost. 2008;6:1376–84. doi: 10.1111/j.1538-7836.2008.03014.x. [DOI] [PubMed] [Google Scholar]
- 28.Amer J, Fibach E. Oxidative status of platelets in normal and thalassemic blood. Thromb Haemost. 2004;92:1052–9. doi: 10.1160/TH04-04-0234. [DOI] [PubMed] [Google Scholar]
- 29.Amer J, Ghoti H, Rachmilewitz E, et al. Red blood cells, platelets and polymorphonuclear neutrophils of patients with sickle cell disease exhibit oxidative stress that can be ameliorated by antioxidants. Br J Haematol. 2006;132:108–13. doi: 10.1111/j.1365-2141.2005.05834.x. [DOI] [PubMed] [Google Scholar]
- 30.Ghoti H, Amer J, Winder A, et al. Oxidative stress in red blood cells, platelets and polymorphonuclear leukocytes from patients with myelodysplastic syndrome. Eur J Haematol. 2007;79:463–7. doi: 10.1111/j.1600-0609.2007.00972.x. [DOI] [PubMed] [Google Scholar]
- 31.Nikolsky E, Aymong ED, Halkin A, et al. Impact of anemia in patients with acute myocardial infarction undergoing primary percutaneous coronary intervention: analysis from the Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications (CADILLAC) Trial. J Am Coll Cardiol. 2004;44:547–53. doi: 10.1016/j.jacc.2004.03.080. [DOI] [PubMed] [Google Scholar]
- 32.Sabatine MS, Morrow DA, Giugliano RP, et al. Association of hemoglobin levels with clinical outcomes in acute coronary syndromes. Circulation. 2005;111:2042–9. doi: 10.1161/01.CIR.0000162477.70955.5F. [DOI] [PubMed] [Google Scholar]
- 33.Spencer FA, Moscucci M, Granger CB, et al. Does comorbidity account for the excess mortality in patients with major bleeding in acute myocardial infarction? Circulation. 2007;116:2793–801. doi: 10.1161/CIRCULATIONAHA.107.694273. [DOI] [PubMed] [Google Scholar]
- 34.Dundar C, Oduncu V, Erkol A, et al. In-hospital prognostic value of hemoglobin levels on admission in patients with acute ST segment elevation myocardial infarction undergoing primary angioplasty. Clin Res Cardiol. 2012;101:37–44. doi: 10.1007/s00392-011-0361-9. [DOI] [PubMed] [Google Scholar]
- 35.McKechnie RS, Smith D, Montoye C, et al. Prognostic implication of anemia on in-hospital outcomes after percutaneous coronary intervention. Circulation. 2004;110:271–7. doi: 10.1161/01.CIR.0000134964.01697.C7. [DOI] [PubMed] [Google Scholar]
- 36.Levy PS, Kim SJ, Eckel PK, et al. Limit to cardiac compensation during acute isovolemic hemodilution: influence of coronary stenosis. Am J Physiol. 1993;265:H340–9. doi: 10.1152/ajpheart.1993.265.1.H340. [DOI] [PubMed] [Google Scholar]
- 37.Dauerman HL, Goldberg RJ, White K, et al. Revascularization, stenting, and outcomes of patients with acute myocardial infarction complicated by cardiogenic shock. Am J Cardiol. 2002;90:838–42. doi: 10.1016/s0002-9149(02)02704-2. [DOI] [PubMed] [Google Scholar]