A 69-year-old man with persistent atrial fibrillation (AF) and a CHA2DS2-VAS score of 4 for age, congestive heart failure, arterial hypertension, and vascular disease underwent left atrial appendage (LAA) closure with a LARIAT (SentreHEART, Inc., Redwood City, CA) device in August 2013 due to contraindications for oral anticoagulation. The LARIAT device was applied using a standard transseptal and subxiphoid pericardial approach with general endotracheal anesthesia with no intraoperative complications. Transesophageal echocardiography (TEE) confirmed complete LAA closure with only a small leak. The patient was discharged on aspirin (1 × 75 mg/day) and ibuprofen (3 × 200 mg/day for 7 days). Three years later, he was readmitted because of unstable angina. Coronary angiography showed multivessel disease and the patient was qualified for coronary artery bypass grafting (CABG). On admission, the patient was still on aspirin and had AF with no history of thromboembolic events. Transesophageal echocardiography showed a diagnostically ambiguous, hypoechogenic mass, located on the lower part of the left atrium (Figure 1 A), small transseptal leak and complete LAA closure with no evidence of residual communication.
Figure 1.
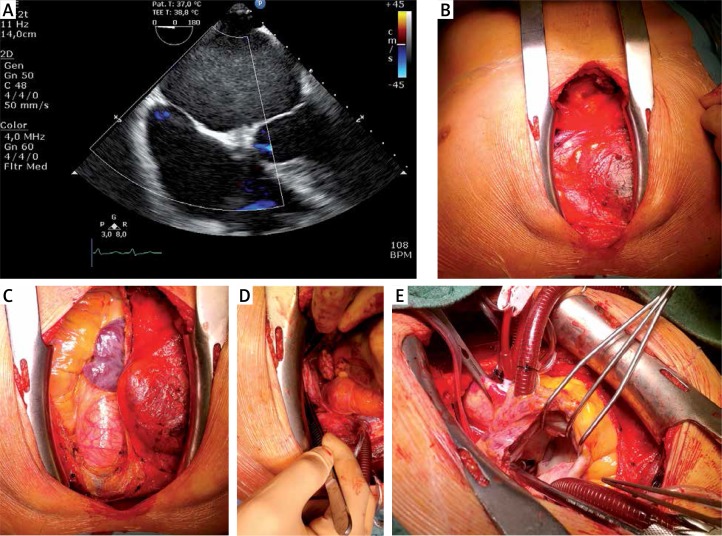
A – Transesophageal echocardiography (TEE) performed 3 years after LARIAT procedure. Hypoechogenic mass in the left atrium and no residual leak from left atrial appendage (LAA) to left atrium, B – Intraoperative picture during coronary artery bypass grafting. Anterior mediastinum without adhesions and signs of any intervention after pericardial access, C – no adhesions in pericardium, D – fully excluded LAA with LARIAT ligation loop. Shrunk and with different color due to necrosis remodeling, E – left atrium with no thrombus or remaining fibrous cap
The CABG was performed with standard techniques. Interestingly, there was no presence of adhesions across the entire surface of the anterior mediastinum and no signs of any intervention in that region (Figure 1 B). There were also no adhesions in the pericardium (Figure 1 C). Intraoperative examination revealed the presence of LARIAT suture tightened around the LAA, which was shrunk and remodeled due to postprocedural necrosis (Figure 1 D). The left atrium was opened to exclude the presence of thrombus. Intraoperative examination showed properly closed LAA with no pathological mass (Figure 1 E).
This is the first report to describe CABG with full median sternotomy following the LARIAT procedure. In contrast to endocardial approaches such as Watchman, Amplatzer or the LAmbre delivery system, the LARIAT device allows the percutaneous ligation of the LAA through the delivery of a suture via a combined epicardial and epicardial approach [1, 2]. Despite the high effectiveness of LAA closure with LARIAT [3, 4], there is a concern that the epicardial approach with pericardium puncture using the LARIAT delivery system may cause pericardial adhesion after the procedure. Importantly, pericardial adhesions may have consequences for the patient in case of the need for cardiac surgery. Therefore, patients often require anti-inflammatory therapy after the LARIAT procedure. Luckily, modifications of initial technique and the use of micropuncture needle and prophylactic colchicine has improved the safety profile of this device dramatically.
Our case suggests that anti-inflammatory therapy after the LARIAT procedure may prevent pericardial adhesion after epicardial access to the LAA. However, further studies are required to define the optimal dosage of ibuprofen and treatment duration to avoid inflammation in the pericardium.
Funding sources
Research grant No. UMO-2015/17/B/NZ5/00125 funded by the National Science Centre in Poland.
Conflict of interest
K. Bartus is a consultant to SentreHEART, Inc. R. Lee is a consultant and equity ownership for SentreHEART, Inc. Remaining authors declare no conflict of interest.
References
- 1.Bartus K, Gafoor S, Tschopp D, et al. Left atrial appendage ligation with the next generation LARIAT(+) suture delivery device: early clinical experience. Int J Cardiol. 2016;215:244–7. doi: 10.1016/j.ijcard.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Bartus K, Podolec J, Lee RJ, et al. Atrial natriuretic peptide and brain natriuretic peptide changes after epicardial percutaneous left atrial appendage suture ligation using LARIAT device. J Physiol Pharmacol. 2017;68:117–23. [PubMed] [Google Scholar]
- 3.Litwinowicz R, Bartus M, Ceranowicz P, et al. Stroke risk reduction after left atrial appendage occlusion in elderly patients with atrial fibrillation: long-term results. Pol Arch Intern Med. 2018;128:327–9. doi: 10.20452/pamw.4264. [DOI] [PubMed] [Google Scholar]
- 4.Litwinowicz R, Bartus M, Ceranowicz P, et al. Left atrial appendage occlusion for stroke prevention in diabetes mellitus patients with atrial fibrillation: long-term results. J Diabetes. 2018 Jul 12; doi: 10.1111/1753-0407.12824. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]