Abstract
Pervasive transcription of eukaryotic genomes generates a plethora of noncoding RNAs. In fission yeast, the heterochromatin factor Clr4/Suv39 methyltransferase facilitates RNA interference (RNAi)–mediated processing of centromeric transcripts into small interfering RNAs (siRNAs). Clr4 also mediates degradation of antisense RNAs at euchromatic loci, but the underlying mechanism has remained elusive. We show that Clr4 and the RNAi effector RITS (RNA-induced transcriptional silencing) interact with Mlo3, a protein related to mRNA quality control and export factors. Loss of Clr4 impairs RITS interaction with Mlo3, which is required for centromeric siRNA production and antisense suppression. Mlo3 also interacts with the RNA surveillance factor TRAMP, which suppresses antisense RNAs targeted by Clr4 and RNAi. These findings link Clr4 to RNA quality control machinery and suggest a pathway for processing potentially deleterious RNAs through the coordinated actions of RNAi and other RNA processing activities.
The widespread transcription of eukaryotic genomes necessitates elaborate quality control and surveillance mechanisms, which monitor RNA biogenesis to detect and destroy aberrant RNA (1–4). In Schizosaccharomyces pombe, methylation of histone H3 lysine 9 (H3K9me) by Clr4 provides binding sites for chromodomain proteins, including Chp1 subunit of an Argonaute (Ago1)–containing RNA-induced transcriptional silencing (RITS) complex required for the processing centromeric transcripts to small interfering RNAs (siRNAs) (5). Loss of Clr4 causes severe defects in centromeric siRNA production (6). However, siRNA can be detected in cells where H3K9 is mutated to unmethylatable (such as H3K9R) residues (7, 8), suggesting an additional role for Clr4. The Clr4 complex (ClrC) interacts with RITS (6, 8, 9), and in addition to their role at centromeres, these factors suppress antisense transcripts at euchromatic loci (10, 11).
A yeast two-hybrid screen using full-length Clr4 as the bait identified Mlo3 (12) as an interacting protein (table S1). Mlo3 is related to Saccharomyces cerevisiae Yra1 and mammalian Aly/REF (13) and is required for nuclear export of RNA (13). Immunoprecipitation analysis detected Mlo3 interacting with Clr4 (Fig. 1A) and another ClrC subunit, Rik1 (fig. S1). Moreover, recombinant Mlo3 bound Clr4, and this interaction was mediated by the amino-terminal (amino acids 1 to 55) and carboxy-terminal (amino acids 134 to 199) regions of Mlo3 (fig. S2), known to bind mRNA export machinery (13). Thus, Clr4 associates with Mlo3 in vitro and in vivo.
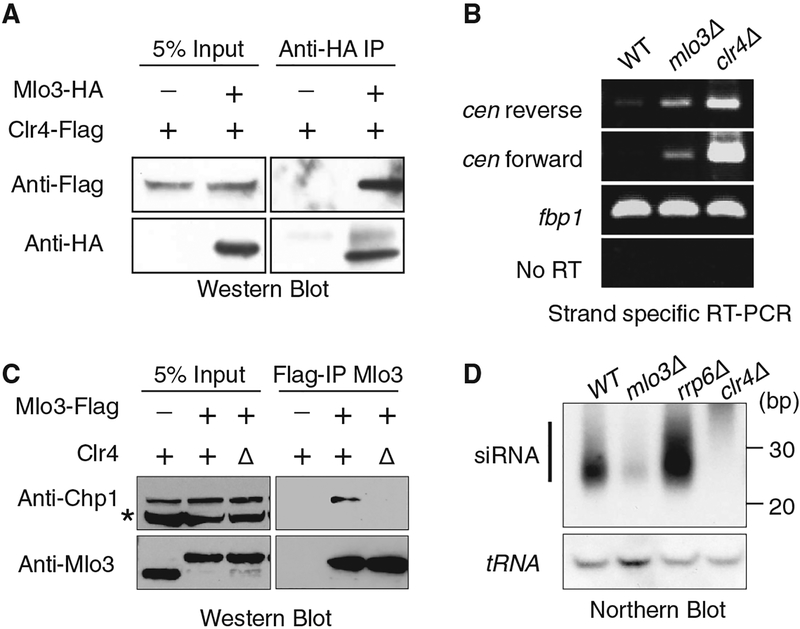
Fig. 1.
Clr4- and RITS-interacting protein Mlo3 is required for the production of centromeric siRNA. (A) Immunoprecipitation (IP) of Mlo3-hemagglutinin (HA) using antibody to HA was followed by Western blotting with antibody to Flag to detect Clr4-Flag. Less than 10% of total Mlo3 is associated with ClrC. (B) Strand-specific RT-PCR of RNA isolated from WT, mlo3Δ, and clr4Δ cells. (C) Clr4 facilitates Mlo3 interaction with Chp1. Mlo3-Flag immunoprecipitated fractions from indicated cells were subjected to Western blot analyses with antibody to Chp1. The asterisk indicates a nonspecific band. (D) siRNAs isolated from indicated strains were analyzed by Northern blot with probes specific for dg/dh centromeric repeats (upper) or for tRNA used as a loading control (bottom). bp, base pairs.
Given the role of Clr4 in heterochromatin assembly, we investigated whether Mlo3 affects heterochromatic silencing (5). Cells lacking Mlo3 maintain H3K9me and its interacting Swi6/HP1 protein at levels comparable to wild-type (WT) cells at major heterochromatic loci (fig. S3). However, mlo3Δ resulted in a considerable increase in the levels of centromeric repeat transcripts, although to a lesser extent than in clr4Δ (Fig. 1B). The accumulation of repeat transcripts in mlo3Δ was not linked to enhanced RNA polymerase II (Pol II) transcription. We therefore explored the possibility that Mlo3 mediates processing of repeat RNAs. Because Clr4 interacts with RNA processing complex RITS (6, 8, 9), we investigated whether Mlo3 also interact with RITS. We found that Mlo3 coimmunoprecipitated with Chp1, a subunit of RITS (Fig. 1C). This interaction was not sensitive to DNase I and RNase A treatment but was severely compromised upon loss of Clr4 (Fig. 1C), suggesting that Clr4 connects Mlo3 to RNA interference (RNAi). Indeed, mlo3Δ caused severe reduction in the levels of centromeric siRNAs (Fig. 1D). Thus, in addition to creating H3K9me binding sites for RITS, Clr4 physically and functionally links RITS to Mlo3 to mediate processing of centromeric transcripts.
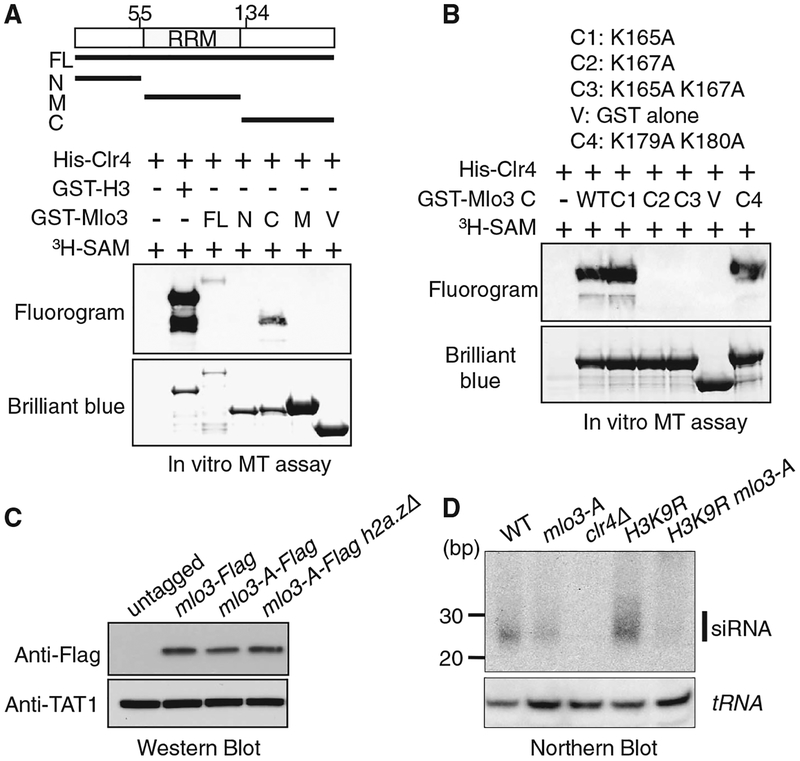
Because histone lysine methyltransferases can methylate nonhistone proteins (14–18), we investigated whether Clr4 methylates Mlo3. Recombinant Clr4 could methylate the carboxy-terminal region of Mlo3, but not the amino-terminal or middle region (Fig. 2A). Within the carboxy-terminal region, lysines 165 and 167 are in a sequence context that resembles H3K9. We mutated these and lysines 179 and 180 to alanine. A methylation assay using recombinant Mlo3 carrying single- or double-mutant combinations showed that Clr4 methylates K167 of Mlo3 in vitro (Fig. 2B). Furthermore, Western blot using an antibody generated against methylated Mlo3 peptide recognized Mlo3 purified from WT cells, and the signal was diminished in clr4Δ mutant (fig. S4). To explore the importance of these results, we generated Schizosaccharomyces pombe strain in which Lys165 and Lys167 residues located in close proximity were mutated simultaneously to alanine to generate the mlo3-A mutant. Mutant Mlo3 protein was expressed at WT levels (Fig. 2C). Interestingly, mlo3-A caused a decrease in levels of centromeric siRNA as compared to WT (Fig. 2D). Further reduction in siRNAs was observed in mlo3-A H3K9R double mutant (Fig. 2D), although a residual signal seemed to be present when compared to clr4Δ. Thus, in addition to Clr4 bridging the interaction between RITS and Mlo3, the methylation of Mlo3 might be important for siRNA production.
Fig. 2.
Clr4 methylates Mlo3 to facilitate centromeric siRNA production. (A and B) Methyltransferase assay, performed using recombinant Clr4 with glutathione S-transferase (GST)–Mlo3 as the substrate and S-adenosyl-[methyl-3H]-methionine as the methyl donor. Histone H3 fused to GST (GST-H3) was included as a positive control. Clr4 targets histone H3 more efficiently than Mlo3 in vitro. Fluorography indicates proteins that were methylated by Clr4. (A) Clr4 methylates the carboxy-terminal region of Mlo3. (B) Clr4 methylates Mlo3 at Lys167 in vitro. The GST fused carboxy-terminal region of Mlo3 carrying different mutations were used as the substrates. (C) Mlo3-A expresses at WT levels. WT and mutant Mlo3 were detected by Western analysis using antibody to Flag. Western blot with antibody to tubulin TAT1 was used as a control. (D) siRNAs in indicated strains were examined by Northern blot using a probe corresponding to dg/dh centromeric repeats (top). tRNA was used as a loading control (bottom).
In light of the results described above, we wondered whether Mlo3 also mediates Clr4-dependent suppression of antisense RNAs at euchromatic loci. As a prelude to addressing this question, we investigated Mlo3 localization across genome by chromatin immunoprecipitation coupled to microarrays (ChIP-chip). Mlo3 showed a broad distribution at euchromatic loci and a relative depletion at heterochromatic regions (figs. S5 and S6). clr4Δ, which causes enhanced Pol II occupancy at centromeric repeats (19, 20), resulted in increased Mlo3 binding at repeat loci (fig. S5), supporting the transcription-coupled loading of mRNA processing and export factors (21–24). At euchromatic loci, Mlo3 preferentially localized at the gene body, and its localization peaked near the 3′end of open reading frames (fig. S6). Expression profiling of mlo3Δ cells on both DNA strands showed dramatic accumulation of antisense RNAs at euchromatic loci (~23.5% of genes) (fig. S7), in particular at convergent genes (fig. S8). Strand-specific reverse transcription polymerase chain reaction (RT-PCR) and Northern blot analyses confirmed elevated levels of antisense RNAs corresponding to read-through transcripts in mlo3Δ cells (Fig. 3, A and B). Therefore, Mlo3 is required for the suppression of antisense RNAs at Pol II-transcribed genes.
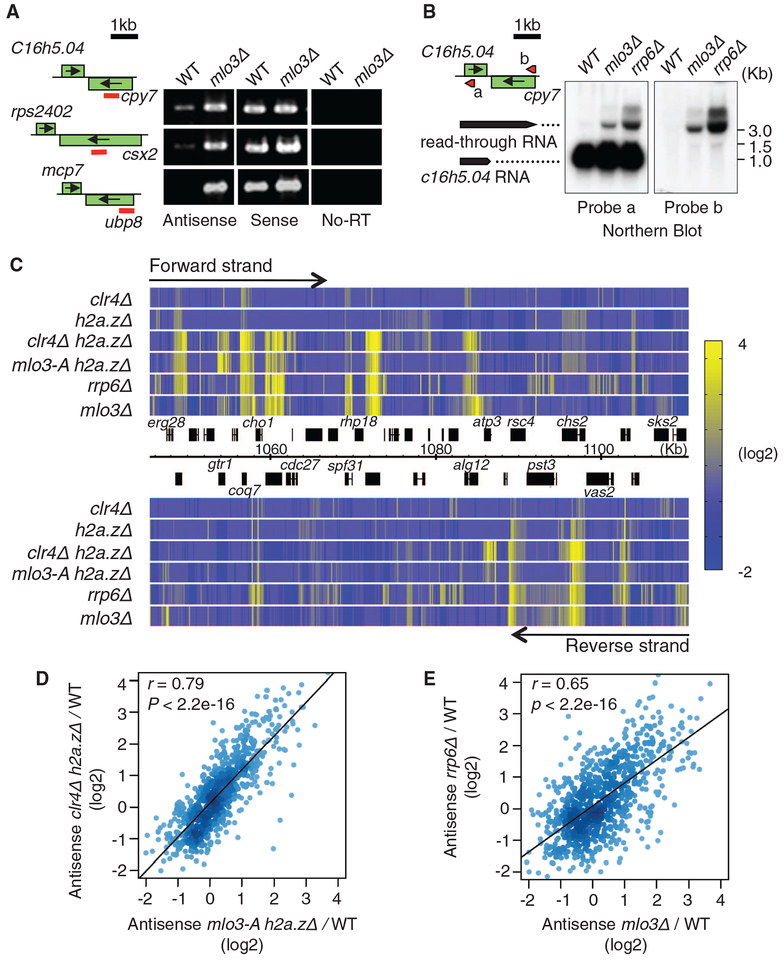
Fig. 3.
Mlo3 is required for suppression of antisense RNAs targeted by Clr4 and by the exosome. (A) Strand-specific RTPCR of RNA isolated from WT and mlo3Δ. (B) Northern blot analyses of RNA at SPBC16h5.04-cyp7. Probes complementary to sense (probe a) or antisense (probe b) strands of SPBC16h5.04 gene are shown by red arrows indicating the probe position and 5′ to 3′ direction. (C) Heat map showing up-regulation of antisense RNA in indicated strains at a representative region of chromosome 2. Transcriptome profiling was performed using tiling microarray. Forward-strand transcripts are shown on top, and reverse-strand transcripts are shown at the bottom. Relative expression values (mutant/WT) were converted into color gradient. (D and E) Density plot comparing changes in antisense RNA levels in clr4Δh2a.zΔ and mlo3-Ah2a.zΔ(D), or rrp6Δ and mlo3Δ (E). Median antisense ratios were calculated for 842 genes. Pearson’s correlation coefficient (r) and the P value of the linear regression are indicated.
We also tested the effects of the mlo3-A mutant on antisense RNA levels, in particular at loci targeted by Clr4 and RNAi (10). Because clr4Δ and ago1Δ enhance antisense RNA levels when combined with a variant histone h2a.zΔ (10), we examined the mlo3-A mutant transcriptome with or without H2A.Z. Like clr4Δ and ago1Δ, mlo3-A also showed weak up-regulation of antisense RNAs (4.7% genes) (fig. S9). However, the mlo3-A h2a.zΔ double mutant showed a synergistic increase in antisense RNAs (18.6% of genes) (Fig. 3C and figs. S8 and S9). mlo3-A caused antisense RNA up-regulation at the same genomic loci that were affected by clr4Δ or ago1Δ (Fig. 3, C and D, and fig. S8). Moreover, both clr4D and mlo3-A showed similar slight accumulation of poly (A)+ RNA signal in the nucleus (fig. S10), suggesting a further functional connection between Clr4 and Mlo3. Together with the results described above, these results suggest that Clr4 cooperates with Mlo3 and Ago1 to suppress antisense RNA.
Because antisense RNA levels in mlo3Δ were consistently higher than in clr4Δ and ago1Δ mutants, we wondered whether Mlo3 has an additional Clr4-independent role in antisense suppression. To address this, we performed immunoaffinity purification of functional, Flag-tagged Mlo3 (Mlo3-Flag)(fig. S11A). Mol3 copurified with a complex related to the Trf4p/Air2p/Mtr4p polyadenylation (TRAMP) (Fig. 4A and table S2), an exosome cofactor promoting degradation of aberrant RNA (2, 4, 25, 26). All TRAMP subunits, including Cid14, Mtr4 and Air1 (25), could be identified in the Mlo3-Flag purified fraction (Fig. 4A). When a Flag-tagged Cid14 subunit of TRAMP (Cid14-Flag) was purified (fig. S11B), a large number of peptides derived from Mlo3 together with the components of TRAMP were identified (Fig. 4A and table S3). The interaction between Cid14 and Mlo3 was insensitive to DNase I and RNase A treatment (Fig. 4B) and did not require Clr4 (fig. S12). Thus, Mlo3 physically associates with TRAMP, a complex involved in the surveillance and degradation of aberrant RNA by the exosome. In this regard, the antisense profile of mlo3Δ closely resembles that of rrp6Δ (Fig. 3, C and E, and fig. S8).
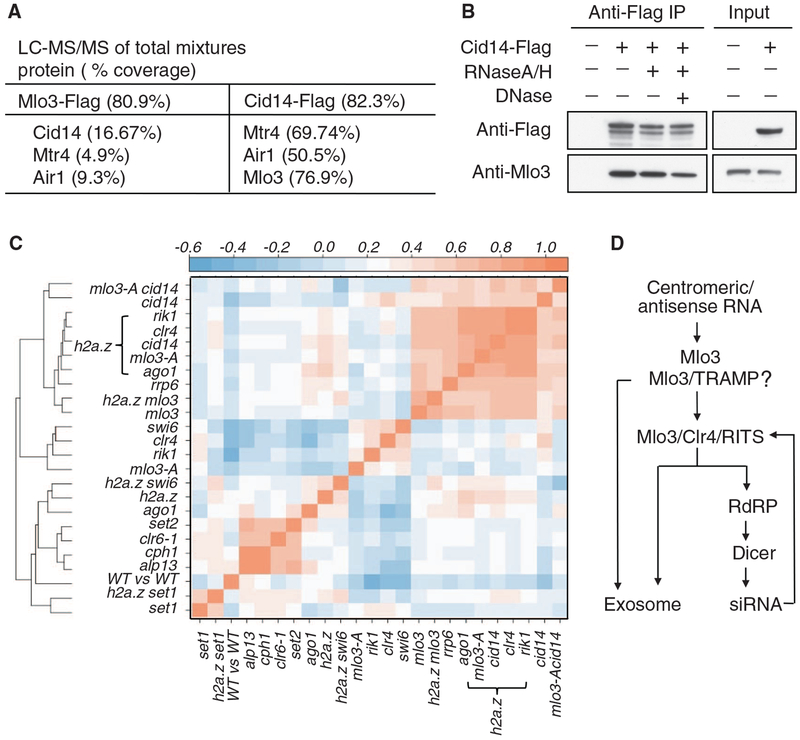
Fig. 4.
Mlo3 associates with the TRAMP complex, which is involved in antisense RNA suppression. (A) Mass spectrometry analysis of Mlo3-Flag or Cid14-Flag purified fractions. A detailed list of the proteins identified is provided in table S2 and S3. (B) TRAMP association with Mlo3 is independent of nucleic acids. IP of Cid14-Flag using antibody to Flag was followed by Western blotting with the indicated antibodies. (C) Hierarchical clustering of mutants on the basis of similarities of their antisense profiles. Pairwise comparisons of anti-sense profiles were performed by calculating the median antisense ratio (mutant/WT) for 842 genes. Pearson’s correlation coefficients were converted into color codes. Except for mlo3-A and clr6-1 mutants, deletion alleles of genes indicated were used. (D) Model showing processing of centromeric and antisense RNA by the coordinatedactionofMlo3,TRAMP, Clr4, RNAi, and the exosome (see also fig. S14). Because low levels of siRNAs are detected in mlo3Δ cells, it is likely that additional mechanisms also target RNAi to centromeric RNAs.
To explore the relationship between the factors described above, we compared the distribution of antisense RNA in various mutants. These analyses revealed a strong correlation between antisense up-regulation in clr4Δ ha2.zΔ, ago1Δ h2a.zΔ, and mlo3-A h2a.zΔ, consistent with Mlo3, Clr4, and RNAi cooperating to suppress antisense RNAs (Fig. 3D and fig. S13). In addition, Cid14 targets RNAs affected by Mlo3, Clr4, and Ago1 (fig. S13). Using correlation coefficients (r values) obtained from pairwise comparisons of antisense profiles of different mutants, we clustered mutants in a hierarchical manner according to the similarity of their antisense profiles (Fig. 4C) (10). As shown previously (10), components of the Clr6 histone deacetylase and Set2 form a distinct cluster, thereby confirming the functional connection between these factors in suppressing antisense RNAs produced from cryptic promoters (Fig. 4C). Double mutants carrying null alleles of either ClrC subunits (e.g., rik1Δ and clr4Δ), RNAi machinery (ago1Δ), TRAMP (cid14Δ), or mlo3-A mutant in combination with deletion of h2a.z form a tight cluster closely associated with rrp6Δ and mlo3Δ. In addition, the mlo3-A single mutant associated closely with clr4Δ and rik1Δ mutants (Fig. 4C). This analysis provides further support for these factors cooperating to suppress antisense RNA.
These analyses showing that Clr4 interacts with Mlo3 to promote the processing of centromeric and antisense RNAs suggest a mechanism for the immediate detection and processing of these noncoding RNAs. Mlo3 may determine the fate of the RNAs by either preparing them for nuclear export or facilitating their destruction (2, 27). Mlo3-associated RNA processing activities, RNAi and TRAMP, might collaborate to degrade RNA (Fig. 4D and fig. S14). Indeed, mlo3Δ (this study) or cid14Δ (25) impair centromeric siRNA production by the RNAi machinery. Although defects in siRNA production could result from sequestering of RNAi proteins by the RNAs accumulating in mlo3Δ and cid14Δ cells, this is unlikely because rrp6Δ, which mimics accumulation of RNAs observed in mlo3Δ and cid14Δ (Fig. 3, C and E, and Fig. 4C), does not reduce siRNA production (Fig. 1D). We postulate that Mlo3 and/or Mlo3/TRAMP serve as gatekeepers that channel RNAs into the exosome and/or RNAi pathways. Whereas RNAs targeted by Mlo3/TRAMP could be degraded directly by the exosome, Clr4-mediated interaction between Mlo3 and RITS channels these RNAs into the RNAi pathway (Fig. 4D and fig. S14). Methylation of Mlo3 might influence the recognition of aberrant RNA by factors such as RITS, which also binds H3K9me (6, 8, 9). Clr4 and Mlo3 may function as a hub that integrates heterochromatin formation with RNA processing through the cooperative action of RNAi, TRAMP, and exosome. Our results expand current views about the functions of the heterochromatin machinery, which not only modifies chromatin but also acts in RNA quality control and surveillance. This is important because the uncontrolled accumulation of noncoding RNA can adversely affect genome stability and modify the epigenetic profiles of genomes (5, 28, 29).
Supplementary Material
Acknowledgments
We thank R. Dhar and R. Allshire for antibody to Mlo3 and strains, E. Chen for helpful contributions, andF. Reyes-Turcu, N. Komissarova, and M. Lichten for comments on manuscripts and discussions. Microarray data are available at the National Center for Biotechnology Information’s Gene Expression Omnibus repository under accession numbers GSE26999 and GSE17271. This work is supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute.
Footnotes
Supporting Online Material
References and Notes
- 1.Moore MJ, Proudfoot NJ, Cell 136, 688 (2009). [DOI] [PubMed] [Google Scholar]
- 2.Houseley J, LaCava J, Tollervey D, Nat. Rev. Mol. Cell Biol. 7, 529 (2006). [DOI] [PubMed] [Google Scholar]
- 3.Kim VN, Dreyfuss G, Mol. Cells 12, 1 (2001). [PubMed] [Google Scholar]
- 4.Wyers F et al. , Cell 121, 725 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Grewal SI, Jia S, Nat. Rev. Genet 8, 35 (2007). [DOI] [PubMed] [Google Scholar]
- 6.Zhang K, Mosch K, Fischle W, Grewal SI, Nat. Struct. Mol. Biol 15, 381 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Djupedal I et al. , EMBO J. 28, 3832 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerace EL, Halic M, Moazed D, Mol. Cell 39, 360 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bayne EH et al. , Cell 140, 666 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zofall M et al. , Nature 461, 419 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gullerova M, Proudfoot NJ, Cell 132, 983 (2008). [DOI] [PubMed] [Google Scholar]
- 12.Javerzat JP, Cranston G, Allshire RC, Nucleic Acids Res. 24, 4676 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thakurta AG, Gopal G, Yoon JH, Kozak L, Dhar R, EMBO J. 24, 2512 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chuikov S et al. , Nature 432, 353 (2004). [DOI] [PubMed] [Google Scholar]
- 15.Sampath SC et al. , Mol. Cell 27, 596 (2007). [DOI] [PubMed] [Google Scholar]
- 16.Zhang K et al. , Cell 122, 723 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rathert P et al. , Nat. Chem. Biol 4, 344 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang J, Berger SL, Curr. Opin. Genet. Dev 18, 152 (2008). [DOI] [PubMed] [Google Scholar]
- 19.Chen ES et al. , Nature 451, 734 (2008). [DOI] [PubMed] [Google Scholar]
- 20.Fischer T et al. , Proc. Natl. Acad. Sci. U.S.A. 106, 8998 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Libri D et al. , Mol. Cell. Biol 22, 8254 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zenklusen D, Vinciguerra P, Wyss JC, Stutz F, Mol. Cell. Biol 22, 8241 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strässer K et al. , Nature 417, 304 (2002). [DOI] [PubMed] [Google Scholar]
- 24.Hieronymus H, Silver PA, Nat. Genet 33, 155 (2003). [DOI] [PubMed] [Google Scholar]
- 25.Bühler M, Haas W, Gygi SP, Moazed D, Cell 129, 707 (2007). [DOI] [PubMed] [Google Scholar]
- 26.LaCava J et al. , Cell 121, 713 (2005). [DOI] [PubMed] [Google Scholar]
- 27.Jensen TH, Rosbash M, Nat. Struct. Biol 10, 10 (2003). [DOI] [PubMed] [Google Scholar]
- 28.Li X, Manley JL, Genes Dev. 20, 1838 (2006). [DOI] [PubMed] [Google Scholar]
- 29.Huertas P, Aguilera A, Mol. Cell 12, 711 (2003). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.