Abstract
Endometriosis is a chronic inflammatory disease characterized by the growth of endometrial glands and stroma outside of the uterus. The disease affects approximately 10–15% of women of reproductive age and presents with clinical symptoms of pelvic pain and infertility. Changes in the leukocyte populations within the ectopic tissue and eutopic endometrium have been reported, and data suggest these alterations contribute to the pathology and symptoms of the disease. In this review, we discussed differences when comparing uterine NK cells and regulatory T cells within the eutopic endometrium between patients with endometriosis and healthy patients, and how these differences relate to implantation failure and/or decreased clearance of menstrual tissue in patients with the disease. The data demonstrate a critical need to examine endometrium and menstrual tissue in patients with endometriosis excluded from studies examining unknown causes of infertility and heavy menstrual bleeding. The information gathered from excluded patients will further enhance our understanding of how the immune system contributes to the pathophysiology of endometriosis and help to identify biomarkers for patients at higher risk for developing endometriosis-associated infertility.
Keywords: Endometriosis, implantation failure, menstruation, NK cells, regulatory T cells
Introduction
Endometriosis is a chronic inflammatory disease characterized by the growth of endometrial glands and stroma outside of the uterus.1 The disease affects approximately 10–15% of women of reproductive age,2 and the pathogenesis is due to multiple factors that vary among patients.3 The primary clinical symptoms of endometriosis are infertility,4–7 poor oocyte development,8 dysmorphology of the Fallopian tubes, and intense abdominal pain from ectopic lesion growth.9,10 Sampson’s theory of retrograde menstruation is currently the most widely accepted theory for the initiation of endometriosis.11 However, additional mechanisms have also been proposed to explain endometriotic lesions in locations other than the peritoneal cavity such as coelomic metaplasia and metastasis of endometrial cells through the lymphatic system.12,13 Sampson’s theory of retrograde menstruation is supported by animal models,14 including the baboon model that we have used extensively in our laboratory.15 Ectopic lesions, with similar histology to those reported in human cases, develop in the animals following inoculation of menstrual tissue into the peritoneal cavity.
Leukocytes within the ectopic tissues in patients with endometriosis contribute to the survival and growth of the lesions. Studies examining ectopic endometrium have reported presence of regulatory T cells16,17 and M2 macrophages,18 low phagocytic activity of macrophages,19 high ratios of Th2:Th1 cells,20 and absence of NK cells21 in the lesions. This combination of leukocytes contributes to a ‘pro-growth’ and ‘pro-survival’ environment, rather than destruction, for ectopic endometrial tissue. In addition, the leukocyte populations within the ectopic tissues of patients with endometriosis may induce changes in the leukocyte populations of the eutopic endometrium. The leukocytes within the eutopic endometrium contribute to the stromal microenvironment of the uterus through multiple mechanisms, as reviewed below. Thus, endometriosis-induced changes in the eutopic endometrial leukocyte populations may directly affect the ability of the uterus to function.
The main function of the uterus is to provide an environment for implantation and growth of the developing embryo and the fetus. Each month, under the control of ovarian hormones, the stratum functionalis of the endometrium proliferates and the endometrium then differentiates in response to luteal phase progesterone in preparation for implantation. If implantation does not occur, the endometrium must be shed and the shed fragments must be eliminated to allow for the cycle to continue. Both of these stages in the monthly life cycle of the uterus, rebuilding the endometrium and shedding and eliminating the stratum functionalis in the absence of implantation, involve orchestration of endometrial leukocytes. When the endometrial leukocyte populations are dysregulated in number and/or function, the ability of the uterus to correctly regenerate the endometrium and/or eliminate shed endometrial cells is likely to be dysregulated as well. This review will focus on differences regarding the leukocytes populations within the eutopic endometrium of patients with endometriosis compared to patients without endometriosis. We will also discuss how these differences relate to implantation failure and/or decreased clearance of menstrual tissue in patients with the disease.
Differences in uterine NK cells associated with implantation failure
Uterine natural killer (uNK) cells are the predominant leukocyte population in the normal human endometrium.22 The level of NK cells varies during the menstrual cycle, representing 40% of the total leukocyte population during the proliferative phase, and increasing to 60% by mid-secretory phase.23 Approximately 70–80% of uNK cells are characterized as CD56brightCD16-.24 Activated uNK cells can produce angiogenic factors (VEGF, ANG2) that promote spiral artery remodeling and secrete cytokines (GM-CSF, CSF-1, TNFα, INFγ, TGFβ, LIF, IL2, CXCL10, CXL12) which direct the migration and invasion of the trophoblast.25–27 The activity of uNK cells is controlled by activating receptors, including the natural cytotoxicity receptors (NKp30 and NKp46),28 as well as inhibitory receptors, including the killer immunoglobulin-like receptor (KIR) and immunoglobulin-like transcript-2 (ILT2)26. In addition, the expression of CD16 on uNK cells coincides with increased cytotoxic activity.29
Dysregulation of uNK number and/or function could lead to implantation failure in patients with endometriosis, as reviewed by Thiruchelvam et al.30 In studies examining patients without endometriosis, a higher concentration of CD56+ uNK cells was reported in women with unexplained recurrent pregnancy loss compared to fertile women.31–34 However, other studies have reported no difference between the patients suffering from recurrent pregnancy loss and fertile patients35–38 Likewise, the data from studies examining uNK cells in patients with endometriosis are also variable. Some laboratories have reported a lower percentage of CD56+ NK cells and a defect in NK activity in the eutopic endometrium of women with endometriosis.39–41 Studies from our laboratory did not observe a difference in the percentage of CD56+ uNK cells when comparing patients with endometriosis to healthy controls. However, we did report a higher percentage of CD16+ cells (associated with cytotoxicity) and NKp46+ NK cells in the endometrium of patients with endometriosis who were infertile or experienced recurrent pregnancy loss, compared to fertile patients with endometriosis.42 These data suggest that increased activity of uNK cells, as controlled by specific receptors, may contribute to a stromal microenvironment that is less receptive to embryo implantation. In support of this hypothesis, a recent report by Nowak et al.43 demonstrated a significant risk for endometriosis associated with the presence of specific KIR receptors and their MHC I ligands (KIR2DS4del and HLA-C C2) and a lower risk of endometriosis in patients with different KIR receptors (KIR2DS5). Thus, additional studies are needed to elucidate the differences between uNK cells in patients with endometriosis versus patients without endometriosis and determine which changes increase the risk of implantation failure in these patients.
Differences in uterine NK cells associated with reduced clearance and/or increased viability of shed endometrial tissue
In the absence of implantation, granzyme+ perforin+ NK cells disperse throughout the stratum functionalis during the secretory phase.44 Recruitment of granzyme B+ perforin+ NK cells to secretory endometrium coincides with apoptosis of glandular epithelium.45 Perforin and granzymes are released from the granules in NK cells undergoing exocytosis. Perforin is a protein that polymerizes to form pores in the membranes of cells targeted by NK cells, allowing granzymes to enter into the cytosol of the target cells. Granzymes then initiate apoptosis of the target cells, such as the glandular epithelial cells, by activating caspases.46 As menstruation ensues, the uNK cells also undergo apoptosis.47 Decreased perforin and granzyme expression by uNK cells and/or decreased apoptosis of NK cells could lead to increased survival of endometrial fragments during menstruation. This, in turn, may increase the amount of tissue entering the peritoneal cavity following retrograde menstruation. Studies by Berbic et al.48 reported changes in uNK cells in patients with heavy menstrual bleeding, and a recent study by Biswas Shivhare et al.49 specifically reported a reduction of uNK cells in the late secretory phase of patients with heavy menstrual bleeding. Unfortunately, although heavy menstrual bleeding is a symptom in many patients with endometriosis, patients with endometriosis were excluded from these studies. Future studies measuring uNK cell numbers and granzyme/perforin levels at the onset of menstruation are needed to determine whether changes in the uNK cell populations that coincide with menstruation are dysregulated in patients with endometriosis.
Differences in uterine regulatory T cells associated with implantation failure
T cells are a diverse population of lymphocytes identified by absence/presence of specific CD markers, transcription factors, cytokine production, and cytotoxic capacity.50 Like uNK cells, the levels of uterine T cells (CD3+) vary throughout the menstrual cycle, representing 50% of the leukocyte population during the proliferative phase, and decreasing to <10% of the leukocytes population by the late secretory phase.51 In contrast to CD3+ T cells in peripheral blood, uterine CD3+ T cells consist of a larger proportion of CD8+ cells (66%) and smaller proportion of CD4+ cells (33%).51 The CD4+ T cell population includes Th1, Th2, regulatory T cells (Tregs), and Th17 cells, each of which secretes specific cytokines with wide-ranging effects.52 Tregs, often identified by expression of the transcription factor forkhead box P3 (FoxP3), secrete immunosuppressive cytokines that promote a state of immune tolerance.53 Data suggest that the immune tolerance created by Tregs is required for successful embryo implantation, as reduced levels of Foxp3 mRNA have been reported in patients with primary infertility compared to fertile patients.54 These findings lead our laboratory to examine Tregs in the baboon model of endometriosis. A decrease was observed in FoxP3+ cells and FoxP3 mRNA in the endometrium following induction of endometriosis in baboons.17 In contrast, the FoxP3+ cells and FoxP3 mRNA are maximum in the ectopic endometrium, similar to studies by Basta et al.55 that reported higher levels of FoxP3+ cells in ectopic endometrium compared to eutopic endometrium from healthy patients. Although these findings contradict the increased levels of FoxP3+ cells and FoxP3 mRNA reported in endometrium of women with endometriosis,16,56 the data from the baboon model and human patients suggest that endometriosis alters the level of Tregs within the endometrium. Additional studies are therefore needed to assess the number and function of uterine Tregs in more patients with endometriosis, particularly in those patients with endometriosis who are infertile. Likewise, the Treg:Th17 ratio within the endometrium of patients with endometriosis needs to be assessed and compared to the ratios recently reported in studies examining these ratios in endometrium from healthy patients.20 Disruptions in Treg/Th17 ratios have been associated with recurrent pregnancy loss and pre-eclampsia57 and thus may also play a role in endometriosis-associated infertility. More information regarding changes in uterine Tregs will assist in identifying targets for future endometriosis therapy.
Differences in uterine regulatory T cells associated with reduced clearance and/or increased viability of shed endometrial tissue
Minimal information currently exists regarding the role of Tregs in menstruation and the viability of shed endometrial tissue. As proposed by Berbic et al.,58 macrophages, mast cells, dendritic cells, neutrophils, and eosinophils all play a role in menstruation and their functions are affected by regulatory T cells. If the functions of uterine regulatory T cells are altered by endometriosis, this may promote increased survival of shed endometrial fragments, contributing to the progression of the disease. Additional studies examining uterine Treg activity and leukocytes in the menstrual tissue from patients with endometriosis are needed to test this hypothesis.
Concluding statement
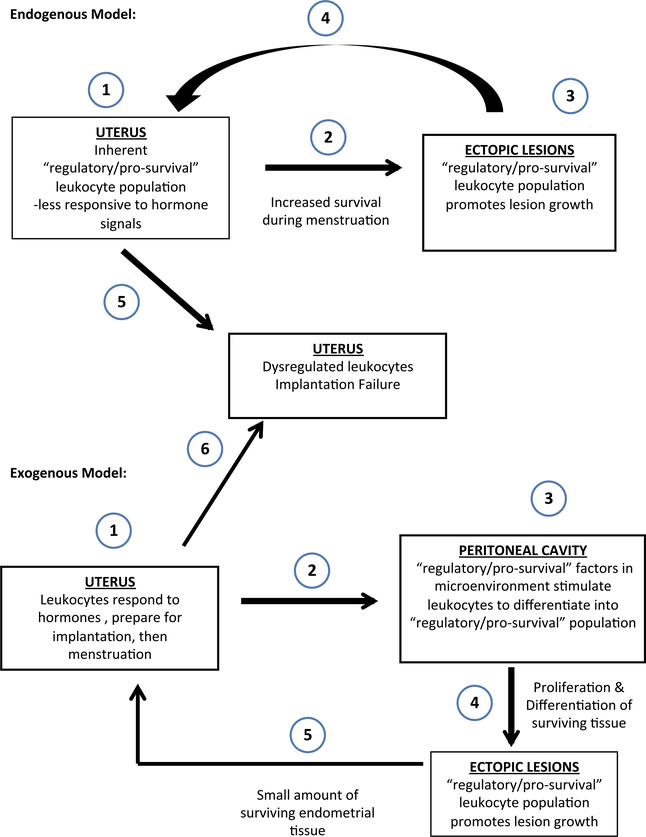
Endometriosis is a complex, systemic disease. The two models presented in Fig. 1 demonstrate how the ‘pro-growth/pro-survival,’ indicated as ‘regulatory’ leukocyte populations, may arise in the ectopic lesions. As shown in the endogenous model, it is possible that some patients may inherit factors that promote development of more ‘regulatory’ leukocytes within their eutopic endometrium. Following retrograde menstruation, these ‘regulatory’ leukocytes promote the survival of the lesions once the eutopic endometrial tissue enters the peritoneal cavity. On the other hand, as shown in the exogenous model, it is possible that other patients may have ‘non-regulatory’ leukocytes in the ectopic endometrium. However, following retrograde menstruation, these leukocytes may respond to factors in the peritoneal microenvironment and differentiate into a ‘regulatory’ phenotype. Both pathophysiologies are possible and are difficult to dissect in human patients, as the average delay in diagnosis of endometriosis is 8–10 years from the onset of the disease.59 In either case, the cells within the ectopic lesions survive, differentiate, and invade the serosa, resulting in the activation of an inflammatory response. The models in Fig. 1 also portray how the ectopic lesions, developed through either the ‘endogenous’ or ‘exogenous’ mechanism, may eventually affect the leukocyte populations in the eutopic endometrium. This could easily occur when cells and/or secreted factors derived from the ectopic sites circulate through the mesenteric lymphatic drainage and systemic blood that eventually recirculates through uterine blood vessels.
Fig. 1.
Models for development of ectopic lesions and their effect on eutopic endometrium. Inherited factors may lead to development of a regulatory environment in the eutopic endometrium (endogenous model) or a regulatory environment in the peritoneal cavity (exogenous model). Both result in survival of shed endometrial tissue and development of ectopic lesions. Cells and soluble factors from the lesions may then traffic to the eutopic endometrium and promote an environment that leads to implantation failure and continues development of the disease.
To further enhance our understanding of the relationship between the ectopic and eutopic endometrium, results comparing the ratios of leukocytes within the ectopic endometrium, peritoneal cavity, peripheral blood, eutopic endometrium, and menstrual tissue are needed. Determining the ratios of leukocytes between these tissues within individual patients may provide an internal control for comparisons. Likewise, it is possible that comparing ratios between patients may reduce the variability in the data reported among different studies. In addition, samples from patients with endometriosis are often excluded from studies involving patients with unexplained recurrent pregnancy loss, infertility, or heavy menstrual bleeding. However, the data reviewed above demonstrate a critical need to examine the tissue in these excluded patients to understand the infertility associated with endometriosis. The information gathered from excluded group of patients will further enhance our understanding of how the immune system contributes to the pathophysiology of endometriosis and help to identify biomarkers for patients at higher risk for developing endometriosis-associated infertility. Finally, the changes in the eutopic endometrium that relate to implantation defects and/or ineffective clearance of menstrual tissue may serve as targets for future treatments.
Acknowledgements
This research was supported by the Eunice Kennedy Shriver NICHD/NIH through cooperative agreement [U54 HD 40093 ATF and NICHD/NIH R01 HD067721—BAL & SLY] as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research.
References
- 1.Clement PB: The pathology of endometriosis: a survey of the many faces of a common disease emphasizing diagnostic pitfalls and unusual and newly appreciated aspects. Adv Anat Pathol 2007; 14:241–260. [DOI] [PubMed] [Google Scholar]
- 2.Eskenazi B, Warner ML: Epidemiology of endometriosis. Obstet Gynecol Clin North Am 1997; 24:235–258. [DOI] [PubMed] [Google Scholar]
- 3.Burney RO, Giudice LC: Pathogenesis and pathophysiology of endometriosis. Fertil Steril 2012; 98:511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sherwin JRA, Sharkey AM, Mihalyi A, Simsa P, Catalano RD, D’Hooghe TM: Global gene analysis of late secretory phase, eutopic endometrium does not provide the basis for a minimally invasive test of endometriosis. Hum Reprod 2008; 23:1063–1068. [DOI] [PubMed] [Google Scholar]
- 5.Jones CJP, Inuwa IM, Nardo LG, Litta P, Fazleabas AT: Eutopic endometrium from women with endometriosis shows altered ultrastructure and glycosylation compared to that from healthy controls–a pilot observational study. Reprod Sci 2009; 16:559–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Umezawa M, Tanaka N, Tainaka H, Takeda K, Ihara T, Sugamata M: Microarray analysis provides insight into the early steps of pathophysiology of mouse endometriosis model induced by autotransplantation of endometrium. Life Sci 2009; 84:832–837. [DOI] [PubMed] [Google Scholar]
- 7.Donaghay M, Lessey BA: Uterine receptivity: alterations associated with benign gynecological disease. Semin Reprod Med 2007; 25:461–475. [DOI] [PubMed] [Google Scholar]
- 8.Barnhart K, Dunsmoor-Su R, Coutifaris C: Effect of endometriosis on in vitro fertilization. Fertil Steril 2002; 77:1148–1155. [DOI] [PubMed] [Google Scholar]
- 9.Medina MG, Lebovic DI: Endometriosis-associated nerve fibers and pain. Acta Obstet Gynecol Scand 2009; 88:968–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang G, Tokushige N, Russell P, Dubinovsky S, Markham R, Fraser IS: Hyperinnervation in intestinal deep infiltrating endometriosis. J Minim Invasive Gynecol 2009; 16:713–719. [DOI] [PubMed] [Google Scholar]
- 11.Sampson JA: Metastatic or embolic endometriosis, due to the menstrual dissemination of endometrial tissue into the venous circulation. Am J Pathol 1927; 3:93–110.43. [PMC free article] [PubMed] [Google Scholar]
- 12.Gruenwald P: Origin of endometriosis form the mesenchyme of the celomic walls. Am J Obstet Gynecol 1942; 44:470–474. [Google Scholar]
- 13.Jubanyik KJ, Comite F: Extrapelvic endometriosis. Obstet Gynecol Clin North Am 1997; 24:411–440. [DOI] [PubMed] [Google Scholar]
- 14.Grümmer R: Translational animal models to study endometriosis-associated infertility. Semin Reprod Med 2013; 31:125–132. [DOI] [PubMed] [Google Scholar]
- 15.Braundmeier AG, Fazleabas AT: The non-human primate model of endometriosis: research and implications for fecundity. Mol Hum Reprod 2009; 15:577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berbic M, Hey-Cunningham AJ, Ng C, Tokushige N, Ganewatta S, Markham R, Russell P, Fraser IS: The role of Foxp3+ regulatory T-cells in endometriosis: a potential controlling mechanism for a complex, chronic immunological condition. Hum Reprod 2010; 25:900–907. [DOI] [PubMed] [Google Scholar]
- 17.Braundmeier A, Jackson K, Hastings J, Koehler J, Nowak R, Fazleabas A: Induction of endometriosis alters the peripheral and endometrial regulatory T cell population in the non-human primate. Hum Reprod 2012; 27:1712–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bacci M, Capobianco A, Monno A, Cottone L, Di Puppo F, Camisa B, Mariani M, Brignole C, Ponzoni M, Ferrari S, Panina-Bordignon P, Manfredi AA, Rovere-Querini P: Macrophages are alternatively activated in patients with endometriosis and required for growth and vascularization of lesions in a mouse model of disease. Am J Pathol 2009; 175:547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chuang P-C, Wu M-H, Shoji Y, Tsai S-J: Downregulation of CD36 results in reduced phagocytic ability of peritoneal macrophages of women with endometriosis. J Pathol 2009; 219:232–241. [DOI] [PubMed] [Google Scholar]
- 20.Takamura M, Koga K, Izumi G, Hirata T, Harada M, Hirota Y, Hiraike O, Fujii T, Osuga Y: Simultaneous detection and evaluation of four subsets of CD4+ T lymphocyte in lesions and peripheral blood in endometriosis. Am J Reprod Immunol 2015; 74:480–486. [DOI] [PubMed] [Google Scholar]
- 21.Jones RK, Bulmer JN, Searle RF: Phenotypic and functional studies of leukocytes in human endometrium and endometriosis. Hum Reprod Update 1998; 4:702–709. [DOI] [PubMed] [Google Scholar]
- 22.Nagler A, Lanier LL, Cwirla S, Phillips JH: Comparative studies of human FcRIII-positive and negative natural killer cells. J Immunol 1989; 143:3183–3191. [PubMed] [Google Scholar]
- 23.Tang AW, Alfirevic Z, Quenby S: Natural killer cells and pregnancy outcomes in women with recurrent miscarriage and infertility: a systematic review. Hum Reprod 2011; 26:1971–1980. [DOI] [PubMed] [Google Scholar]
- 24.King A, Loke YW: On the nature and function of human uterine granular lymphocytes. Immunol Today 1991; 12:432–435. [DOI] [PubMed] [Google Scholar]
- 25.Jokhi PP, King A, Sharkey AM, Smith SK, Loke YW: Screening for cytokine messenger ribonucleic acids in purified human decidual lymphocyte populations by the reverse-transcriptase polymerase chain reaction. J Immunol 1994; 153:4427–4435. [PubMed] [Google Scholar]
- 26.Dosiou C, Giudice LC: Natural killer cells in pregnancy and recurrent pregnancy loss: endocrine and immunologic perspectives. Endocr Rev 2005; 26:44–62. [DOI] [PubMed] [Google Scholar]
- 27.Kalkunte SS, Mselle TF, Norris WE, Wira CR, Sentman CL, Sharma S: Vascular endothelial growth factor C facilitates immune tolerance and endovascular activity of human uterine NK cells at the maternal-fetal interface. J Immunol 2009; 182:4085–4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vacca P, Moretta L, Moretta A, Mingari MC: Origin, phenotype and function of human natural killer cells in pregnancy. Trends Immunol 2011; 32:517–523. [DOI] [PubMed] [Google Scholar]
- 29.Verma S, Hiby SE, Loke YW, King A: Human decidual natural killer cells express the receptor for and respond to the cytokine interleukin 15. Biol Reprod 2000; 62:959–968. [DOI] [PubMed] [Google Scholar]
- 30.Thiruchelvam U, Wingfield M, OˊFarrelly C: Natural killer cells: key players in endometriosis. Am J Reprod Immunol 2015; 74:291–301. [DOI] [PubMed] [Google Scholar]
- 31.Clifford K, Flanagan AM, Regan L: Endometrial CD56+ natural killer cells in women with recurrent miscarriage: a histomorphometric study. Hum Reprod 1999; 14:2727–2730. [DOI] [PubMed] [Google Scholar]
- 32.Lachapelle MH, Miron P, Hemmings R, Roy DC: Endometrial T, B, and NK cells in patients with recurrent spontaneous abortion. Altered profile and pregnancy outcome. J Immunol 1996; 156:4027–4034. [PubMed] [Google Scholar]
- 33.Tuckerman E, Laird SM, Prakash A, Li TC: Prognostic value of the measurement of uterine natural killer cells in the endometrium of women with recurrent miscarriage. Hum Reprod 2007; 22:2208–2213. [DOI] [PubMed] [Google Scholar]
- 34.Quenby S, Bates M, Doig T, Brewster J, Lewis-Jones DI, Johnson PM, Vince G: Pre-implantation endometrial leukocytes in women with recurrent miscarriage. Hum Reprod 1999; 14:2386–2391. [DOI] [PubMed] [Google Scholar]
- 35.Michimata T, Ogasawara MS, Tsuda H, Suzumori K, Aoki K, Sakai M, Fujimura M, Nagata K, Nakamura M, Saito S: Distributions of endometrial NK cells, B cells, T cells, and Th2/Tc2 cells fail to predict pregnancy outcome following recurrent abortion. Am J Reprod Immunol 2002; 47:196–202. [DOI] [PubMed] [Google Scholar]
- 36.Fukui K, Yoshimoto I, Matsubara K, Hori R, Ochi H, Ito M: Leukocyte function-associated antigen-1 expression on decidual natural killer cells in patients with early pregnancy loss. Mol Hum Reprod 1999; 5:1083–1088. [DOI] [PubMed] [Google Scholar]
- 37.Matteo MG, Greco P, Rosenberg P, Mestice A, Baldini D, Falagario T, Martino V, Santodirocco M, Massenzio F, Castellana L, Specchia G, Liso A: Normal percentage of CD56bright natural killer cells in young patients with a history of repeated unexplained implantation failure after in vitro fertilization cycles. Fertil Steril 2007; 88:990–993. [DOI] [PubMed] [Google Scholar]
- 38.Tuckerman E, Mariee N, Prakash A, Li TC, Laird S: Uterine natural killer cells in peri-implantation endometrium from women with repeated implantation failure after IVF. J Reprod Immunol 2010; 87:60–66. [DOI] [PubMed] [Google Scholar]
- 39.Klentzeris LD, Bulmer JN, Liu DT, Morrison L: Endometrial leukocyte subpopulations in women with endometriosis. Eur J Obstet Gynecol Reprod Biol 1995; 63:41–47. [DOI] [PubMed] [Google Scholar]
- 40.Fernández-Shaw S, Clarke MT, Hicks B, Naish CE, Barlow DH, Starkey PM: Bone marrow-derived cell populations in uterine and ectopic endometrium. Hum Reprod 1995; 10:2285–2289. [DOI] [PubMed] [Google Scholar]
- 41.Jones RK, Bulmer JN, Searle RF: Immunohistochemical characterization of stromal leukocytes in ovarian endometriosis: comparison of eutopic and ectopic endometrium with normal endometrium. Fertil Steril 1996; 66:81–89. [DOI] [PubMed] [Google Scholar]
- 42.Giuliani E, Parkin KL, Lessey BA, Young SL, Fazleabas AT: Characterization of uterine NK cells in women with infertility or recurrent pregnancy loss and associated endometriosis. Am J Reprod Immunol 2014; 72:262–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nowak I, Płoski R, Barcz E, Dziunycz P, Kamiński P, Kostrzewa G, Milewski Ł, Roszkowski PI, Senitzer D, Malejczyk J, Kuśnierczyk P: KIR2DS5 in the presence of HLA-C C2 protects against endometriosis. Immunogenetics 2015; 67:203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tian X, Zhang L, Zhang X: Lymphocytes and perforin expression in endometrium during the menstrual cycle. Chin Med J (Engl) 2000; 113:930–933. [PubMed] [Google Scholar]
- 45.Igarashi T, Konno R, Okamoto S, Moriya T, Satoh S, Yajima A: Involvement of granule-mediated apoptosis in the cyclic changes of the normal human endometrium. Tohoku J Exp Med 2001; 193:13–25. [DOI] [PubMed] [Google Scholar]
- 46.Pipkin ME, Lieberman J: Delivering the kiss of death: progress on understanding how perforin works. Curr Opin Immunol 2007; 19:301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.King A, Wellings V, Gardner L, Loke YW: Immunocytochemical characterization of the unusual large granular lymphocytes in human endometrium throughout the menstrual cycle. Hum Immunol 1989; 24:195–205. [DOI] [PubMed] [Google Scholar]
- 48.Berbic M, Fraser IS: Immunology of normal and abnormal menstruation. Womens Health (Lond Engl) 2013; 9:387–395. [DOI] [PubMed] [Google Scholar]
- 49.Biswas Shivhare S, Bulmer JN, Innes BA, Hapangama DK, Lash GE: Menstrual cycle distribution of uterine natural killer cells is altered in heavy menstrual bleeding. J Reprod Immunol 2015; 112:88–94. [DOI] [PubMed] [Google Scholar]
- 50.Smith-Garvin JE, Koretzky GA, Jordan MS: T cell activation. Annu Rev Immunol 2009; 27:591–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Flynn L, Byrne B, Carton J, Kelehan P, OˊHerlihy C, OˊFarrelly C: Menstrual cycle dependent fluctuations in NK and T-lymphocyte subsets from non-pregnant human endometrium. Am J Reprod Immunol 2000; 43:209–217. [DOI] [PubMed] [Google Scholar]
- 52.Raphael I, Nalawade S, Eagar TN, Forsthuber TG: T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine 2015; 74:5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sakaguchi S, Miyara M, Costantino CM, Hafler DA: FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol 2010; 10:490–500. [DOI] [PubMed] [Google Scholar]
- 54.Jasper MJ, Tremellen KP, Robertson SA: Primary unexplained infertility is associated with reduced expression of the T-regulatory cell transcription factor Foxp3 in endometrial tissue. Mol Hum Reprod 2006; 12:301–308. [DOI] [PubMed] [Google Scholar]
- 55.Basta P, Majka M, Jozwicki W, Lukaszewska E, Knafel A, Grabiec M, Stasienko E, Wicherek L: The frequency of CD25+CD4+ and FOXP3+ regulatory T cells in ectopic endometrium and ectopic decidua. Reprod Biol Endocrinol 2010; 8:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen S, Zhang J, Huang C, Lu W, Liang Y, Wan X: Expression of the T regulatory cell transcription factor FoxP3 in peri-implantation phase endometrium in infertile women with endometriosis. Reprod Biol Endocrinol 2012; 10:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fu B, Tian Z, Wei H: TH17 cells in human recurrent pregnancy loss and pre-eclampsia. Cell Mol Immunol 2014; 11:564–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Berbic M, Ng CHM, Fraser IS: Inflammation and endometrial bleeding. Climacteric J Int Menopause Soc 2014; 17(Suppl 2):47–53. [DOI] [PubMed] [Google Scholar]
- 59.Arruda MS, Petta CA, Abrão MS, Benetti-Pinto CL: Time elapsed from onset of symptoms to diagnosis of endometriosis in a cohort study of Brazilian women. Hum Reprod 2003; 18:756–759. [DOI] [PubMed] [Google Scholar]