Abstract
In this article, we examine evidence supporting the role of reproductive steroids in the regulation of mood and behavior in women and the nature of that role. In the first half of the article, we review evidence for the following: (i) the reproductive system is designed to regulate behavior; (ii) from the subcellular to cellular to circuit to behavior, reproductive steroids are powerful neuroregulators; (iii) affective disorders are disorders of behavioral state; and (iv) reproductive steroids affect virtually every system implicated in the pathophysiology of depression. In the second half of the article, we discuss the diagnosis of the three reproductive endocrine-related mood disorders (premenstrual dysphoric disorder, postpartum depression, and perimenopausal depression) and present evidence supporting the relevance of reproductive steroids to these conditions. Existing evidence suggests that changes in reproductive steroid levels during specific reproductive states (i.e., the premenstrual phase of the menstrual cycle, pregnancy, parturition, and the menopause transition) trigger affective dysregulation in susceptible women, thus suggesting the etiopathogenic relevance of these hormonal changes in reproductive mood disorders. Understanding the source of individual susceptibility is critical to both preventing the onset of illness and developing novel, individualized treatments for reproductive-related affective dysregulation.
Introduction
In this article, we pose and answer the following question: Is there evidence to support a role of reproductive steroids in the regulation of mood and behavior in women, and if so, what is the nature of that role? The primary female reproductive steroids, estrogen and progesterone, are derived from cholesterol, secreted by the ovaries, circulate in the bloodstream, and cross the blood-brain barrier. 17β-estradiol, the primary physiologic estrogen, varies over the menstrual cycle and powerfully affects behavior. More recent evidence suggests that estradiol and progesterone are not only capable of acute neuromodulatory effects (i.e., “neuroactive steroids”), but are actually synthesized in the brain, which has led to their inclusion in the group of “neurosteroid” compounds. In the first half of this article, we review evidence that the reproductive steroids estradiol and progesterone regulate biological systems and behaviors that are implicated in depression. This evidence is organized according to four major themes. First, the reproductive system powerfully regulates a variety of behaviors, including consummatory behavior (e.g., eating) and reproductive behavior (e.g., mate seeking, copulation). Second, both human and non-human animal studies demonstrate that reproductive steroids are potent neuroregulators from the subcellular to cellular level to neural circuits to behavior. Reproductive steroids organize and activate behavior across the lifespan, and their effects are observable across multiple units of analysis, from the most basic (e.g., cellular receptor function) to the most complex (e.g., cognition). Third, affective disorders are disorders of behavioral state. Depression, then, is not a collection of symptoms but rather represents a dysfunctional, self-organized, integrated ensemble of behaviors, perceptions, cognitions, and neurobiological characteristics (a “state”), the appearance of which reflects the activation or inhibition of specific brain cortical networks. The reproductive system is designed to powerfully regulate behavior states, as seen, for example, in the choreographing of sexual receptivity in concert with cyclic ovarian function. As such, changes in reproductive endocrine function have the capacity to precipitate a “switch” in behavioral state. Fourth, reproductive steroids affect virtually every system implicated in the pathophysiology of depression. For example, reproductive steroids regulate the neurotransmitters and neurosteroids implicated in depression as well as neuroplasticity, neuroprotection, neural circuit activity and synchronicity. They also regulate the HPA axis and immune function. Taken together, these lines of evidence suggest a role for reproductive steroids in the affective dysregulation that accompanies mood disorders in women.
In the second half of this article, we review the empirical literature on reproductive endocrine-related mood disorders, including premenstrual dysphoric disorder (PMDD), postpartum depression (PPD), and perimenopausal depression. Evidence supporting the relevance of reproductive steroids to these disorders is a focus of our review. This evidence comes from case-control studies of hormone changes and attendant mood symptoms and hormone manipulation, genetic, and neuroimaging studies. Despite accumulating evidence that reproductive steroids may trigger affective dysregulation in women susceptible to reproductive endocrine-related mood disorders, the source of individual susceptibility remains unclear. Future research in this area is critical to both preventing the onset of illness and developing novel, individualized treatments for affective disorders in women.
The Neuroregulatory Effects of Reproductive Steroid Hormones
Two key principles govern our understanding of the neuroregulatory effects of reproductive steroid hormones. The first principle is that behavior is a readout of central nervous system activity. Non-human animal studies have demonstrated that stimulation of specific brain regions and neurons affects behavior either directly or as an interactive process with other stimuli. For example, electrical stimulation of a key neuroendocrine region, the hypothalamus, in rats results in a number of hormone-mediated behaviors, including eating, drinking, and reproduction (25, 200, 212, 237), all reward-seeking behaviors. Electrical stimulation of the ventromedial nucleus of the hypothalamus facilitates the lordosis response in female rats outside the cycle phase when lordosis usually occurs (estrus) (212, 237). Another example of how changes in the activity of specific brain regions or neurons can be shown to influence behavior involves stimulation of the neural reward circuitry. In vivo photostimulation of specific neurons that project to the ventral tegmental reward circuitry increases avoidant behavior (i.e., avoiding a chamber where shock had been delivered previously) and decreases reward (sucrose) seeking in mice (140), behavioral phenotypes that are both relevant to depression (88, 270) and modulated by estrogen (166,336).
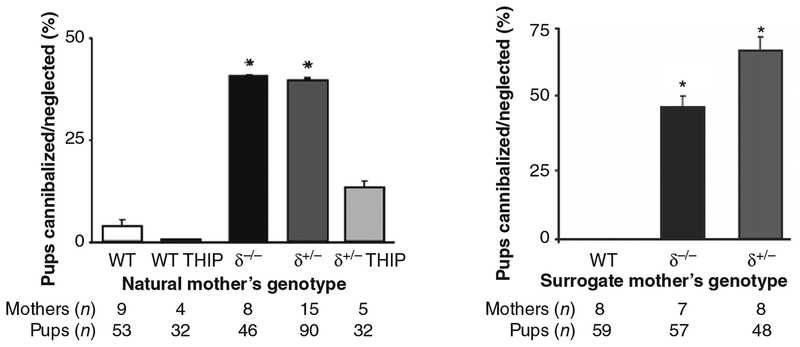
The second principle is that, just as with any physiologic stimulus, reproductive steroid-related behavioral and neuroregulatory effects are highly context dependent (259, 261). The organizational and activational effects of reproductive steroids are time sensitive and developmental stage dependent. For example, in one study, exposure of Chinook salmon eggs to an aromatase inhibitor to prevent estradiol exposure for only 2 h during development when the gonads were bipotent created a fully functional phenotypic male from a genotypic female (238). Another example involves the initiation of hormone replacement therapy relative to the time since menopause. Some studies suggest that early initiation of hormone-replacement therapy reduces the risk of coronary heart disease (124,257), while other studies have shown that hormone replacement therapy initiated in older women with a longer time since menopause is associated with either no change in risk (123) or increased risk of coronary heart disease (256). Many effects of reproductive steroids also are sex specific (19, 69, 192). Estradiol administration followed by progesterone triggers a gonadotropin-releasing hormone (GnRH)-stimulated luteinizing hormone (LH) surge in females but not in males, leading some to conclude that the responsible hypothalamic GnRH neurons are hard-wired differently in males and females (108). Independent of differences in “wiring,” however, cells isolated from males and females may show very different responses to reproductive steroids administered in vitro (Fig. 1) (19,339,364). The target cell and tissue provide a context—estradiol, for example, increases the MAP (mitogen-activated protein) kinase signaling protein ERK1 in neurons but decreases it in glial cells in the rat brain (364, 365). The environment also determines the effect of reproductive steroids on the brain and behavior: in rats, maternal behavior (i.e., licking and grooming) increases estrogen sensitivity in the medial preoptic area of adult female offspring, which, in turn, promotes maternal behavior of the offspring (47,48). Finally, the genome clearly provides a context that determines the effects of reproductive steroids on brain and behavior. Maguire and Mody, for example, demonstrated a γ-aminobutyric acid A (GABAA) receptor δ-subunit knockout mouse that displays no behavioral abnormalities until during and after pregnancy, at which time the dam exhibits depressive behavior and cannibalizes its offspring (181). Thus, hormonal states associated with reproduction may provoke affective dysregulation in the context of a genetic susceptibility. Careful attention to these contextual variables is essential for understanding the neurobiological sources of individual variation in behavior.
Figure 1.
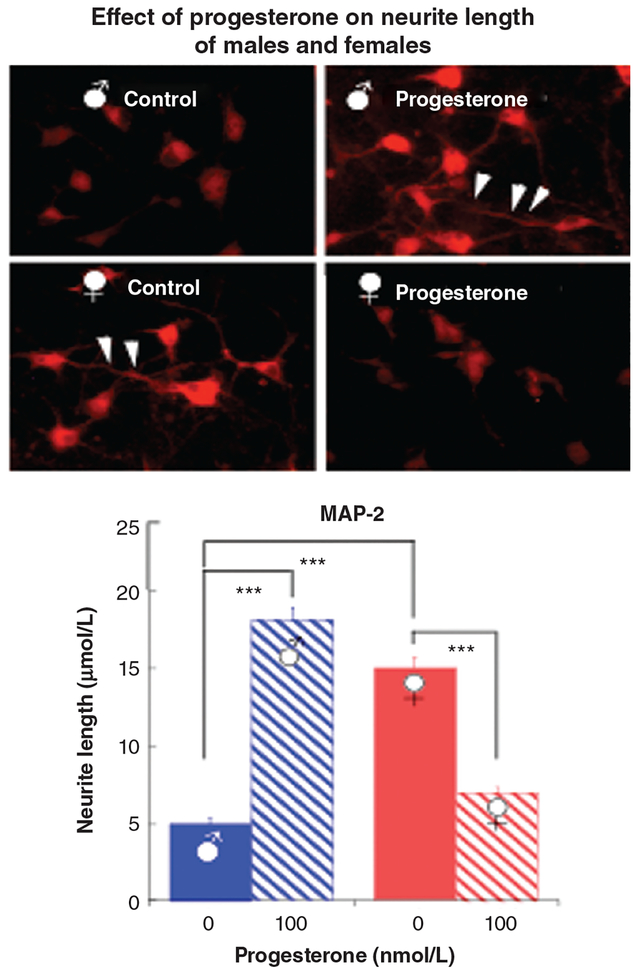
Seventy-two hours of culture of rat cortical neurons with 100 nm progesterone has opposite effects on neurite length [assessed after staining with microtubule associated protein-2 (MAP2)] in neurons from male and female animals. The top panel shows cortical neurons stained with MAP2 in male (top) and female (bottom) untreated control rats (left) and those treated with progesterone (right). The bottom panel is a bar graph showing neurite length in male (blue) and female (red) untreated control rats (solid) and those treated with progesterone (striped). Neurite length is greater in female animals compared with males in the absence of progesterone, whereas the opposite is true following exposure to progesterone (L. Zhang et al., unpublished data). ∗∗∗P < 0.001.
The reproductive system regulates behavior: from a teleological perspective, two systems that are critical for survival of the species are the appetitive (to obtain energy sources) and the reproductive systems. As such, it is critical that the behavioral repertoires for feeding and for sex be redundant, highly conserved, and compelling, as the associated behavioral outcomes are not optional (at the species level). As elegantly shown by Pfaff et al. (160, 236, 237), even “simple” reproductive behaviors like lordosis represent the complex integrative product of multiple signaling pathways, all with the intent of commandeering the attention, motivation, social behavior, and motor behavior of the female rodent to facilitate reproduction. Given the CNS systems affected and the powerful demand characteristics of reproductive hormonal signaling (sex literally and figuratively commands our attention), it is hardly surprising that reproductive steroids play a major role in the regulation of many nonreproductive behaviors. Examples too numerous to comprehensively cite include the following:
Cognition.
Levels of estradiol determine the mode of cognition in female rats, such that females use hippocampal-sensitive strategies (i.e., use spatial cues) under conditions of high estradiol and striatal-sensitive strategies (i.e., remembering what the rat did in the last run through the maze) under conditions of low estradiol (157). Similarly, estradiol levels are associated with cognitive performance in human females. During the menstrual cycle, women show increased verbal abilities and decreased visual-spatial abilities when estradiol and progesterone levels are high and the opposite pattern when estradiol and progesterone levels are low (127), and estradiol levels are significantly correlated with neural activity in frontal and parietal areas during visual-spatial processing (278) and the left inferior frontal gyrus during verbal processing (55). Cognitive function declines during menopause (116), irrespective of age, which may result from estrogen’s effects on synaptic organization of the prefrontal cortex, hippocampus, and dorsolateral prefrontal cortex (249, 319, 356, 357). Although support for estrogen’s role in menopause-related cognitive decline has not been well supported by human studies, a clear role for estrogen in cognition has been demonstrated in non-human primate studies. For example, in aged non-human primates, ovariectomy induces spatial memory deficits, which are reversed with cyclic, low-dose estrogen treatment (249).
Motivation.
The tendency for humans to choose immediate rewards over larger, delayed rewards, called the “now bias,” varies with frontal dopamine levels (294). Cyclic elevations in estradiol modulate dopamine levels in the prefrontal cortex, and as a result, impulsive behavior changes in response to changes in estrogen levels; as estrogen levels decrease, impulsive behavior increases (294).
Consummatory behavior.
Reproductive steroids regulate eating behavior. Food intake increases and locomotor activity decreases in certain female rodents following ovariectomy and the subsequent depletion of estradiol and progesterone, leading to eventual weight gain (354). However, estradiol replacement following ovariectomy can inhibit this weight gain (106,202,354). Moreover, eating behavior is regulated by cyclic changes in estrogen and progesterone levels in women (80,154).
Maternal behavior.
Estrogen and oxytocin modulate maternal licking and grooming behavior, pup gathering, and nest building in rodents (48). Late-follicular estrogen levels correlate with the reported ideal number of children, a measure of maternal tendency, in nulliparous women (167).
Motor behavior.
Reproductive steroids have both organizational and activational effects on motor behavior, and estrogen is necessary for the proper development of critical nervous system sensorimotor behaviors (214). Specifically, exogenous administration of estrogen to prenatal rodents results in increased spontaneous motor activity in adulthood (77), suggesting that estrogen has organizational effects on sensorimotor development. Estrogen also has activational effects on motor behavior. In rodents, locomotor activity increases during estrus as estrogen levels rise [see (297) for a review]. Exogenous estradiol administration increases locomotion (e.g., running wheel activity) in mice, and this effect is mediated by estrogen receptor alpha (ERα) in the medial preoptic area (218). Progesterone administration similarly increases motor behavior in mice (i.e., horizontal crossings and open field entries) (100). In women, cyclic increases in estrogen are associated with increased motor coordination (128), and in postmenopausal women with Parkinson’s disease, estrogen treatment improves motor abilities (327).
Sleep.
Sleep complaints are one of the most common symptoms related to the menopause transition (346). Declining estrogen levels that occur during the menopause transition are associated with more frequent awakenings and trouble falling asleep (161). Estrogen treatment, however, can improve the sleep disturbances, decrease the difficulty of initiating sleep, and decrease nocturnal restlessness and awakenings in peri- and postmenopausal women (240,320). Women who discontinue hormone therapy report an increase in the frequency of sleep problems once the therapy is suspended (326). However, further research may be needed to better understand the complex relationships between reproductive steroids and sleep, particularly during the menopause transition. Because the menopause transition is associated with numerous vasomotor symptoms, it is not clear whether the improvement in sleep quality is due to direct effects of estrogen therapy independent of the resolution of vasomotor symptoms. Additional evidence for the regulatory effects of hormones on sleep states can be found in animal studies. In ovariectomized rats, ovarian hormones affect recovery sleep differently than spontaneous sleep (66). When the rats are sleep-satiated, estradiol promotes arousal, but after sleep deprivation, both estradiol and progesterone facilitate rapid eye movement sleep (66).
Temperature regulation.
Estradiol and progesterone appear to have opposing effects on thermoregulation [see Charkoudian and Stachenfeld (49) for a comprehensive review]. In general, progesterone exposure increases and estradiol exposure decreases the thermoregulatory set point (305). Temperature regulation is perturbed during the transition to menopause. Perimenopausal estradiol withdrawal precipitates vasomotor symptoms (hot flushes) (255), and estradiol treatment alleviates these symptoms (217). Although these relationships have been well established, the mechanisms by which reproductive steroids influence thermoregulation are less clear (49).
From the subcellular to cellular to circuit to behavior, reproductive steroids are powerful neuroregulators. The classic mechanism of reproductive steroid action is genomic. Steroids are lipophilic and permeate the cell membrane to bind intracytoplasmic or intranuclear receptors. These receptors are transcription factors that can bind to response elements in the DNA and regulate gene expression, transcription, and protein synthesis. This classical view, while accurate, is hopelessly incomplete, because it is now clear that myriad other factors participate in the regulation and consequences of an activated receptor.
In addition to their intracellular location, steroid receptors exist on the cell membrane, where they rapidly regulate ion channel activity and activate signal transduction systems. Broadly labeled “nonclassical,” these activities were once regarded as of little consequence but are increasingly viewed as being of great physiologic importance, particularly with regard to understanding behavior. The particular relevance of the acute, nonclassical effects of estradiol and progesterone to behavior is twofold: First, reproductive steroids act like neurotransmitters in the brain, rapidly affecting brain function and behavior. Estradiol, for example, can be acutely and locally synthesized in nerve terminals and then released into the synapse where it can acutely mediate male rodent reproductive behavior (12). Second, estradiol and progesterone activate protein kinases that modify the steroid hormone receptor in such a way as to amplify its transcriptional effects upon further exposure or reexposure to the reproductive steroid (332, 333). As such, steroids sensitize targets to their own signal. Moreover, as a function of the role of intracellular signaling proteins in the activation of the steroid receptor, there is considerable cross talk between cellular growth factors, neurotransmitters, and steroid hormones. In some cases, neurotransmitters (e.g., dopamine) can directly activate the steroid receptor and mimic the effects of steroids even in their absence (241).
Effect of context
Adding another layer of complexity, the specific genomic and nonclassical actions of reproductive steroids are completely context-dependent. Steroid-activated receptors influence gene transcription by forming combinations with other intracellular proteins, including coregulators and cointegrators. Coregulators determine whether gene transcription is enhanced or suppressed by the activated receptor (176, 177, 223). Coregulators, of which over 350 have been identified, comprise coactivators, which associate with agonist bound receptors to stimulate gene transcription, and corepressors, which may associate with either unbound or antagonist bound receptors to inhibit gene transcription. Cointegrators allow activated receptors to regulate genomic expression through sites (e.g., activating protein [AP-1] binding site) other than the classical DNA hormone response elements by tethering the receptor to transcription factors (e.g., Jun, Fos, and ATF) that bind the DNA. Coregulators and cointegrators both contain enzymatic activity (e.g., histone acetylase) and recruit other coregulators and transcription factors to modify the chromatin structure and regulate transcription. Coregulators and cointegrators also exist in a tissue-specific fashion, and the availability of these proteins determines the transcriptional response to an activated hormone receptor, thus explaining how estrogen receptor modulators can act like agonists in some tissues and like antagonists in others (129). The diversity of hormone effects is further facilitated by the existence of different hormone receptor subtypes. The estrogen receptor, for example, has two major isoforms: ERα and estrogen receptor beta (ERβ). ERα and ERβ are encoded by separate genes, are found in different brain regions, and have different physiologic effects (133). Finally, as shown in Figure 2, reproductive steroids are synthesized from cholesterol, are highly homologous, and serve as precursors for one another. As a result, the presence and availability of enzymes that convert one steroid hormone to another markedly affect the amplitude and nature of the steroid signal. The effects of estrogen and progesterone are therefore dependent upon many factors that differ from cell to cell and tissue to tissue. These tissue-specific effects will become apparent in our discussion of the effects of reproductive steroids on brain function and network dynamics, where the effects are dependent on the specific brain region and reproductive stage (e.g., menstrual phase or menopausal status), as well as on several other factors, including receptor density, prior exposure, and neuroregulatory milieu. Our discussion of the effects of reproductive steroids on brain tissue will focus on their relevance to regulating affective states. For additional information about steroid receptor structure, function, and distribution in the brain, the interested reader is encouraged to see excellent, recent reviews of these topics (40,188,193,196,343).
Figure 2.
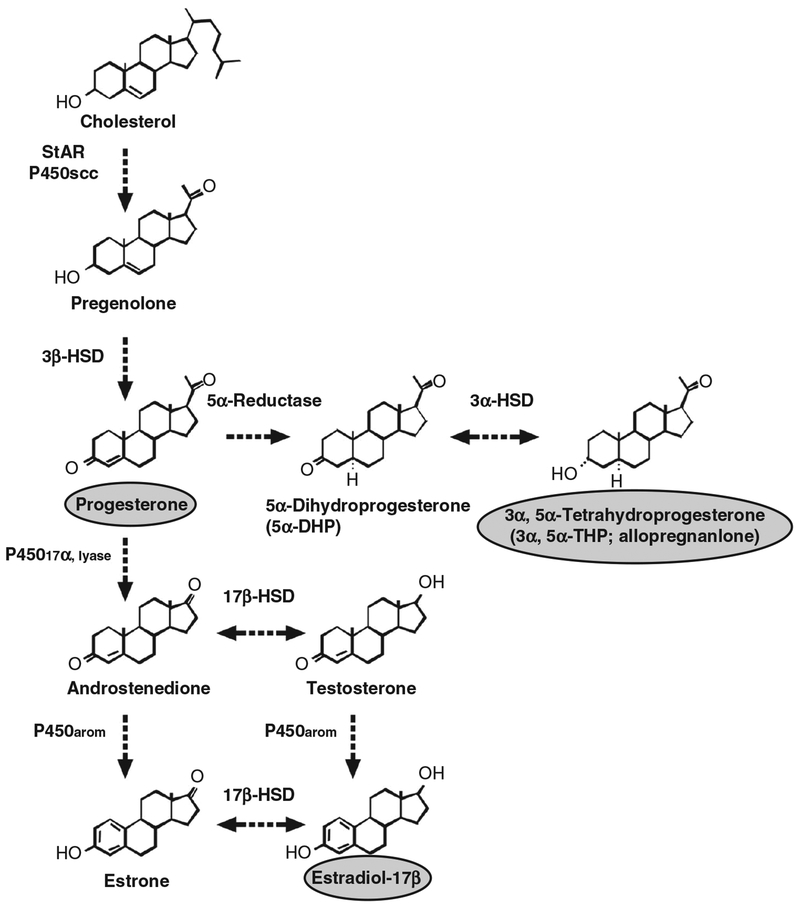
Synthetic pathways for the steroid hormones progesterone, estradiol, and allopregnanolone, reproduced with permission from (328). Note that the same enzymes have multiple actions in the hormone cascade.
Reproductive Steroids: Regulators of Affective State
Affective and cognitive dysfunction in depression
Depression is defined by the presence of affective, cognitive, behavioral, and neurovegetative symptoms. Depressed mood and anhedonia are prominent (5). Other core symptoms include feelings of worthlessness and guilt, impaired concentration and decision-making, suicidality, insomnia, lassitude, appetite problems (i.e., appetite loss or gain), psychomotor agitation or retardation, and irritability (341). Major depressive disorder (MDD) is diagnosed when symptoms are present most of the day, nearly every day, for at least two weeks (5). Depressive disorders have an episodic course: roughly 25% of major depressive episodes (MDEs) remit within 2 months, and over 80% remit within one year, with or without treatment (54). Of those who experience remission, approximately 60% will also experience recurrence (335). Although multiple etiological models of depression exist, the most promising models account for the episodic nature of the illness. Specifically, many individuals respond to triggering events with characteristic increases in negative affect (i.e., sadness and anxiety), but for individuals with depression, these affective states are more persistent. One might therefore conclude that depression is the result of a faulty affective switch that, once triggered, gets “stuck.” According to cognitive theories of depression, this faulty switch is the result of information-processing biases: the experience of negative emotions results in the activation of critical contextual and self-evaluative thoughts, and an inability to disengage from these negative thoughts results in a persistent, depressed state (158), characterized by both heightened negative affect and suppressed positive affect. Importantly, negative and positive affect are dimensions that vary independently: negative affect can be high when positive affect is low or high (340), and the various combinations of high and low negative and positive affect are associated with different psychiatric disorders (342).
Negative affectivity is an emotional dimension of distress and dissatisfaction that contains a number of specific negative emotions, including sadness, guilt, fear, anger, and disgust. Negative affect is common to both depression and anxiety and accounts for their substantial comorbidity and sequential relation (52). Depression and anxiety disorders coexist at a much higher rate than that expected based on chance, and anxiety precedes depression just as often as depression precedes anxiety (199). Family and twin studies have shown that depression and anxiety share a genetic susceptibility to such an extent that they are indistinguishable (258). Thus, for an etiological model of depression to be useful, it must also account for symptoms of anxiety, and as such, examining negative affectivity rather than sadness per se allows one to capture the range of emotional dysregulation that characterizes depression.
Anhedonia was first identified as a loss of the capacity to experience pleasure by Ribot in 1894. In the non-human animal literature, anhedonia is defined behaviorally as a deficit in hedonic behavior, including reduced intake in palatable solutions, reduced preference for palatable solutions, and reduced brain self-stimulation, which have been shown to respond to a variety of medications that treat depression in humans, including tricyclic antidepressants (e.g., desipramine) (205), selective serotonin reuptake inhibitors (SSRI) (e.g., citalopram) (310), and atypical antidepressants (e.g., mianserin) (204). In humans, anhedonia can be defined as an absence of positive affect (52), a notion that is more consistent with Ribot’s original conceptualization of anhedonia than any definition since. Positive affect includes a range of positive mood states, including joy, energy, enthusiasm, interest, alertness, and self-confidence, that are diminished in patients with depression (342), schizophrenia (32), and certain personality disorders (51). However, low positive affect in combination with high negative affect is uniquely related to depression (342), and measures of positive and negative affect are sensitive to daily mood changes. Moreover, in individuals with MDD, positive affect increases following treatment with SSRIs (e.g., paroxetine) and serotonin-norepinephrine (NE) reuptake inhibitors (e.g., venlafaxine) (67), which is similar to the effects of antidepressant medication on anhedonic behavior in non-human animals.
In addition to affective dysregulation, depression is characterized by myriad cognitive abnormalities. Early cognitive theories of depression suggested that biased cognitive processing accounts for the onset, maintenance, and recurrence of depressive episodes (17, 18). In particular, depression was thought to be associated with impairments in perception, attention, and memory. Recent research in cognitive neuroscience has largely supported these theories. Depression is associated with impaired short-term (working) memory and long-term memory. In depressed mood states, the accessibility of mood-congruent material is increased, enabling those with a depressed mood to have improved memory for negatively valenced emotional material, compared with neutral material. However, while depressed individuals tend to remember negatively valenced emotional material as well as nondepressed individuals, they demonstrate impaired memory for neutral material (207). Individuals with depression have difficulty removing irrelevant negative material from their working memory (139,143,207). Furthermore, autobiographical memory specificity, or the ability to recall specific autobiographical memories of events less than one day ago, is reduced in individuals with depression (59). There is a positive relationship between depressed mood and impaired autobiographical memory specificity suggesting that as a depressed mood increases, memory impairments worsen (59).
The negative memory bias in depression can be accounted for by attentional biases (33). Mood-congruent attentional biases have been found in individuals with depression and dysphoria (114, 142, 159, 226). Individuals with depression maintain attention for negative words and mood-congruent (sad) faces and have difficulty disengaging from that negative information (114, 142, 159). This attentional bias to negative information may contribute to the continuous processing of negative information, such as rumination (159). Rumination, defined as the tendency to perseverate on negative emotions, characterizes chronic, severe depression (216). Rumination is associated with increased interference from irrelevant negative material (143). This inability to dismiss irrelevant negative cognitions and memories may lead to difficulties with processing new information and a prolonged negative mood state.
In addition to memory and attention, depression may also influence problem solving, processing speed, reaction time, and decision-making. Depressive symptoms have been associated with impaired every-day problem-solving ability and less effective problem-solving solutions (71, 361). Induction of mild dysphoria reduces the problem-solving ability of formerly depressed individuals with a history of suicidal ideation (349). Increased depression severity is associated with reduced processing speed (191) and increased intraindividual reaction time variability (144). In addition, depressed individuals experience more decisional conflict (246) and score lower on decision-making skills (141) than those without depression. Overall, increased depressive symptoms have been associated with poor cognitive abilities and impaired executive function (15,72,191).
Effects of reproductive steroids on biological systems implicated in depression
Reproductive steroids affect virtually every system implicated in the pathophysiology of depression (260). Estradiol regulates the synthesis, metabolism, receptor concentration, and trafficking of neurotransmitters implicated in depression, including serotonin, dopamine, and NE. In animal studies, estradiol has been shown to inhibit serotonin transporter (SERT) mRNA (234), alter SERT protein levels and binding (26, 91, 194, 262, 312), increase serotonin (5-HT) 2A receptor binding and mRNA (313), facilitate imipramine-induced down regulation of 5-HT2 receptors (148), decrease activity of the 5-HT1A receptor, and increase the expression of 5-HT1A receptors (50, 323). In humans, prolactin increases following administration of the serotonergic agents meta-chlorophenylpiperazine (318) and buspirone (68) during the luteal phase compared with the follicular phase of the menstrual cycle, suggesting that reproductive hormones may modulate the serotonergic system. Further evidence comes from human imaging studies, where combined estrogen and progestin replacement is associated with increased 5-HT2A binding in the anterior cingulate cortex (ACC), dorsolateral prefrontal cortex, and lateral orbitofrontal cortex (209). For a review of the possible role of these interactions in affective regulation, see Rubinow et al. (262).
Estrogens influence the synthesis, release, and metabolism of dopamine and can modulate dopamine receptor expression and function (289). However, the effects are complicated, with different studies demonstrating significant variations in the direction of effects and brain regions impacted (164). Several studies have demonstrated that estradiol increases dopamine release and receptor density in the striatum. During the rat estrous cycle, the density of striatal dopamine uptake sites is highest during proestrus (206), which coincides with peak estradiol (184) and dopamine levels (206). Also in rodents, pregnancy and lactation are associated with increased levels of dopamine and its primary metabolite DOPAC in striatal tissue (45). Ovariectomy reduces striatal dopamine receptor density (37), extracellular dopamine levels, and behaviors mediated by the striatal dopaminergic system [for a review see Becker (20)]. Estrogen treatment increases striatal dopamine receptor density (227), dopamine uptake sites in the striatum (206), dopamine release, and striatal dopamine-mediated behaviors (20). Estradiol also modulates dopamine neuron activity in the ventral tegmental area (363), the effects of which are believed to modify the rewarding properties of reproductive states and facilitate reproductive success in female rats (20). In contrast, it is well established that estradiol exerts antidopaminergic effects on the anterior pituitary and hypothalamus. In the hypothalamus, estradiol inhibits the synthesis and activity of tyrosine hydroxylase (35, 230), the rate-limiting enzyme in dopamine synthesis, which results in decreased dopamine release to the anterior pituitary (57). Estradiol also inhibits anterior pituitary prolactin cell responsiveness to dopamine, which results in increased prolactin release (250). It has been suggested that the antidopaminergic effects of estrogen protect against schizophrenia in women of reproductive age, when the incidence is 40% lower in women than in men. Moreover, adjunctive treatment with estradiol has been shown to reduce positive and general symptoms of schizophrenia in regularly menstruating women (163).
Finally, reproductive steroids modulate noradrenergic system activity. Estradiol and progesterone influence NE synaptic communication in the hypothalamus, preoptic area, and cortex, although the effects are region-specific (89). In the hypothalamus and preoptic area, estrogen attenuates β-adrenergic and presynaptic α2-adrenergic inhibition of NE release (89, 145, 235). Estrogen promotes NE release in the hypothalamus by stabilizing α2-adrenenoceptor phosphorylation and uncoupling the receptor from G protein (330), rather than by regulating receptor density (89). In the cortex, estradiol regulates postsynaptic α2-adrenenoceptors. In one study, estradiol treatment reduced cortical α2D-adrenenoceptor messenger RNA by 50% compared with ovariectomized control animals, and this reduction was accompanied by a significant decrease in cortical α2-adrenenoceptor density (146). The noradrenergic effects of estrogen in the frontal cortex are thought to explain hormonal modulation of cognitive function (146). For example, in young, ovariectomized rats, estrogen replacement amplifies the effects of stress on working memory by inhibiting the stimulation of NE α2-adrenenoceptors (283), thus supporting a role for estrogen in both the affective and cognitive symptoms associated with depression.
Estrogen regulates the neuroplasticity processes implicated in depression. Brain-derived neurotrophic factor (BDNF) levels in the forebrain and hippocampus decrease following ovariectomy and increase with estradiol treatment (150, 302). Notably, BDNF levels decrease during periods of depression and stress and increase with antidepressant treatment (287). Estradiol treatment results in increased cyclic adenosine monophosphate response element-binding protein activity (365) and the neurotrophin receptor protein tyrosine kinase receptor type 1 (TrkA) (301) and type 2 (TrkB) (9), and it decreases glycogen synthase kinase-3 (GSK-3) beta activity (46) in rodents, similar to antidepressant medications. Estradiol increases hippocampal long-term potentiation and spine density (39), which contribute to synaptic plasticity. Of note, this regulation of hippocampal spine density is dependent on glutamate receptor (NMDA) signaling (358), which has also been implicated in the etiopathogenesis of depression (162, 183). In the amygdala, estradiol also increases BDNF, TrkB, and proline-rich receptor-like protein kinase 2 (pERK2), which is associated with decreased depressive-like behavior (101).
Estrogen plays a role in neuroprotection. Estrogen protects against oxidative damage, glutamate excess, and beta-amyloid toxicity (117,203,292,308) in hippocampal (190) and cortical neurons (43). Rapid neuroprotection by estradiol is partially mediated by G-protein-coupled receptor 30 (GPR30) and the subsequent downregulation of NMDA receptors (175). Interestingly, the neuroprotective effects of estradiol are sexually dimorphic and mediated by ERα (43). In a study of rat pups, pretreatment with 17β-estradiol protected female but not male cortical neurons from glutamate toxicity (43). Estradiol regulates cellular energetics by improving mitochondrial respiratory efficiency and prevents oxygen-free radicals that are thought to adversely affect mitochondrial energetics in depression (102, 103, 170). Estradiol can activate adenosine monophosphate kinase, an enzyme that plays a role in cellular regulation and homeostasis, through an ERβ-mediated effect (173). In mice, ovariectomy-induced loss of estrogen led to significant deficits in bioenergetics and accumulation of mitochondrial amyloid beta (Aβ), whereas estradiol treatment initiated at the time of ovariectomy prevented the deficits (360).
Depression is considered a stress-related disorder characterized by hypothalamic-pituitary-adrenal (HPA) axis hyper-activity (215). Estradiol modulates the HPA axis: estradiol regulates basal and stress-induced adrenocorticotropic hormone (ACTH) and cortisol in animals via its effects on the glucocorticoid receptor and corticotropin-releasing hormone (CRH) (266). As a result, it has been suggested that estrogen add-back therapy with the loss of ovarian function may help prevent potential HPA axis hyperactivity at menopause by decreasing ACTH and cortisol response to CRH (104). Nonetheless, unlike in rodents, it appears that progesterone rather than estradiol may be responsible for the increased HPA axis activity observed during the luteal phase of the menstrual cycle (251,252).
Depression is characterized by immune dysregulation and “inflammation.” Individuals with MDD have been shown to have higher circulating levels of proinflammatory cytokines, including tumor necrosis factor-α and interleukin (IL)-6 (73), and treatment with the cytokine interferon-alpha results in MDD in 50% of patients (245). As reviewed by Cunningham et al. (58), estradiol modulates immune system function. Estradiol regulates cytokine production and receptor expression, monocyte and macrophage function, activation of effector cells, and the number and function of dendritic cells and antigen presenting cells (58). Estradiol inhibits inflammation (178) and produces effects that are opposite to the proinflammatory immune alterations associated with depression (42,314). Further, estradiol protects against stress-induced increases in neuroinflammation in the hippocampus (244). Thus, irrespective of the molecular etiopathogenesis of depression, a possible role for reproductive steroids reasonably can be posited.
Network Neuroscience: Reproductive Steroids Regulate the Neural Networks Implicated in Depression
Affective disorders are characterized by dysregulation of mood, cognition, and behavior. There is no set of symptoms that universally and comprehensively identifies affective disorders, but certain disturbances appear with great frequency. As noted earlier, these include alterations in pleasure and in the assignment of affective “valence” to events, loss of flexibility in changing mood state, negative self-image, disturbed interpretation of social interactions, reduced appetitive behavior, and reduced motivation for social interactions. Depressed patients see both positive events as less positive and negative events as more negative. All three domains affected by depression can be considered to result from deficient information processing, which in turn may reflect disturbed activity in or regulation of the neural networks giving rise to the neural computations underlying emergent behavioral and perceptual processes. Both structural and functional imaging studies provide strong evidence for abnormal neural processing in depression.
Brain regions often implicated in depression include the ACC, dorsolateral prefrontal cortex, dorsomedial prefrontal cortex, amygdala, and hippocampus, which together form the frontolimbic “emotion processing” circuitry (92, 126, 155). For example, findings in depression include hypoactivity in the dorsolateral prefrontal cortex (62, 92) and hyperactivity in the subgenual ACC (75,186,187). Gray matter volumetric loss is observed in many of these same regions, including the rostral ACC and the posterior cingulate cortex, precuneus, prefrontal cortex (dorsolateral prefrontal cortex and dorsomedial prefrontal cortex), and orbitofrontal cortex (120). It is, however, the interactions of these and other brain regions that are the likely locus of dysfunction, and these interactions are described by structural, functional, and effective connectomics. Structural connectivity describes anatomical connections (usually of white matter and defined by diffusion tensor imaging), functional connectivity describes statistical patterns of dynamic interactions among brain regions, and effective connectivity refers to directed, causal effects. Brain regions that are functionally integrated in the service of responding to the environment constitute a network (229, 303), and these networks are degenerate; that is, one network can perform many functions, and one function can be performed by many networks (303). Examples of networks include dorsal and ventral attentional networks, frontosubcortical regulatory network [a mood regulation system (156)], central executive network (which regulates attention, decision making, working memory and involves the dorsolateral prefrontal cortex and the posterior parietal cortex) (198), mesolimbic reward circuitry, and resting state networks (60), including the default mode network (DMN) (93). Several of these networks have been described as dysfunctional in depression. First, the DMN is a resting state network that is active under nontask-driven conditions and involves the rostral ACC, posterior cingulate cortex, precuneus, medial prefrontal cortex, temporoparietal junction (inferior parietal and posterior temporal), plus, less frequently, hippocampus, medial temporal lobe, and lateral temporal cortex (93, 119, 185). The DMN has been reported to be upregulated in MDD (hyperactive during emotional processing, with increased glucose metabolism and cerebral blood flow) (119, 169, 284, 347). This upregulation would be consistent with impaired executive function and emotional regulation (seen in depression) and may be effectively targeted by deep brain stimulation, which is accompanied by symptom reduction and decreased subgenual ACC metabolism (187). The DMN is the substrate for reflective, self-referential associative thought (e.g., focus on feelings and thoughts, judging one’s own character, and rumination) (93). Increased connectivity has been observed in depression between the dorsomedial prefrontal cortex and regions of the DMN (ACC, posterior cingulate cortex/precuneus) as well as components of the Affective Network (ACC) and Cognitive Control Network (dorsolateral prefrontal cortex)—each of which is implicated in emotional dysregulation and depression (285). This increased connectivity may represent the pathological linking of networks, resulting in the attentional shifts, excessive self-focus, cognitive interference, and autonomic dysregulation that characterize depression. Of note, although resting-state functional connectivity between the subgenual ACC and the hippocampus is increased in depressed patients, structural activity is decreased, suggesting that the functional changes may be compensatory (165). Further, many of the regions in the DMN showing significant changes in activation in depressed patients (e.g., ACC, posterior cingulate cortex, middle frontal cortex, and precuneus) are highly connected hubs, the most influential nodes responsible for regulating information transfer within and between networks. In contrast to the increased functional connectivity within the DMN, the central executive network shows significantly decreased functional connectivity in depressed patients, and response to treatment has been associated with modulating the connectivity between these and other networks implicated as dysfunctional in depression (174). Finally, synchronized neuronal oscillations in the alpha frequency (8–12Hz), which coordinate cortical:cortical and cortical:subcortical connectivity (and specifically modulate connections among the dorsal ACC, anterior insula, anterior PFC, and thalamus (264)), are increased in depression, consistent with the increased functional connectivity seen in the DMN and other resting state networks (119,122,171,366).
Reproductive steroids regulate many of the networks implicated in depression. The majority of research in this area focuses on the influence of reproductive steroids on brain function in adult women, and that will be the focus of our review of neural networks. Aside from activational effects of reproductive steroids, however, there are also organizational effects, although they have been less studied in relation to mood disorders. The classical organizational-activational model of hormone effects on brain development and function is being replaced by a parallel model that includes multiple sex-specific signals and pathways that unfold across the lifespan (189). Permanent differentiating (organizational) effects of reproductive steroids on neural systems have been studied in mouse models. The majority of these studies have shown that in the perinatal period of hormone sensitivity estradiol is masculinizing (albeit estradiol derived locally in the brain from testosterone) and exerts region-specific effects that include neurogenesis, neuroprotection, and apoptosis (189). Permanent differentiating effects of reproductive steroids may also occur at puberty and contribute to mood disorders in women (293); however, because systematic study of these effects in humans is not possible, these effects are not well understood.
Positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) studies of euthymic women have demonstrated that reproductive hormones modulate the neural circuits involved in normal mood states. Not surprisingly, these studies, which in general have small sample sizes, differ in their results, potentially as a function of differences in study design, sample age and reproductive status, means of varying hormone exposure (menstrual cycle phases vs. exogenous administration), length of hormone exposure, nature of hormone evaluated, exact timing of imaging relative to reproductive condition, levels of hormones achieved, nature of the stimulus (e.g., cognitive vs. affective), imaging methods, nature of imaging analysis (e.g., activation vs. connectivity), etc. Nonetheless, one can say the following: (i) in regional activation studies across the menstrual cycle, reproductive steroids impact the reactivity of the same brain regions identified as dysfunctional in depression. For example, high-estrogen menstrual cycle phases have been associated with increased activation in the amygdala, medial orbitofrontal cortex, ACC (albeit inconsistently), medial frontal gyrus, inferior frontal gyrus, and hippocampus (7, 8, 16, 56, 111, 279, 345, 362). In a study by Protopopescu et al. (242), women showed increased activation of the medial orbitofrontal cortex and decreased activation of the lateral orbitofrontal cortex during the luteal phase compared with the follicular phase, which suggests the ability to evaluate behavioral responses on the basis of reward value may be regulated by reproductive hormones and impaired in the days prior to the onset of menses (81). Modulatory effects have also been observed during experimentally created hormonal states. A PET study of asymptomatic women showed decreased activation of the dorsolateral prefrontal cortex, inferior parietal lobule, and posterior inferior temporal cortex during leuprolide-induced hypogonadism, whereas the expected pattern of cortical activity returned following either estradiol or progesterone add-back (23). Studies of the effects of combined oral contraceptives have identified increased activity in the medial prefrontal cortex, ACC, dorsolateral prefrontal cortex, and inferior frontal gyrus (1,239,263), and decreased activity in posterior cingulate cortex, medial frontal gyrus, inferior frontal gyrus, and insula (1,109).
Effects of ovarian steroids on circuit function have been examined with studies of both neural functional connectivity and measurements of the coherence of network activation. Changes in connectivity on fMRI have been observed, again across the menstrual cycle or following hormonal intervention. Findings vary between studies, in part as a presumed consequence of differences in both probes (e.g., spatial vs. verbal tasks) and analytic methods. In comparisons between menstrual and luteal phases, functional connectivity between right temporal lobe and left inferior parietal lobe is higher in the luteal phase (344), while that between the right middle frontal gyrus and the left inferior parietal cortex is stronger (more negative; i.e. greater lateralization) during menses (322,345). Despite inconsistencies, data suggest that interactions within and between hemispheres and between cortical and subcortical regions vary as a function of menstrual cycle phase (322). These altered functional relationships may be reflected in the observation that alpha asymmetry, observed as disturbed in several mood disorders, is altered by menstrual cycle phase (although again contradictory results exist). Further, at a circuit level, reproductive hormones regulate neural reward circuitry in humans, with increased activity in the superior orbitofrontal cortex and amygdala during reward anticipation and in the midbrain, striatum, and left ventrolateral prefrontal cortex during reward delivery in the follicular phase (compared with the luteal phase) (74). Changes in connectivity following exogenous administration of reproductive steroids include the following: estradiol-induced increased effective connectivity between the right hippocampus and both the right superior and medial PFC (225) as well as between the amygdala and both temporal and prefrontal cortices (224); progesterone-induced decreased connectivity between the amygdala and fusiform gyrus and increased connectivity between the amygdala and the ACC (350); and estrogen-induced increased connectivity between the thalamus and basal ganglia (149).
While the canonical circuits described earlier are often investigated hemodynamically via blood-oxygen-level-dependent imaging (219), parallel electroencephalography (EEG) research aims to understand the temporal association and dissociation of circuits and their modulation by sex steroids. EEG is used to create a visual representation of the brain’s endogenous electric fields, which are dynamic in nature and influenced by circuit-level activity (99). Similar to fMRI, EEG is capable of implicating circuits in processes of cognition, emotion, and in the case of many mood disorders and pathological affective states (151, 213, 338). The best characterized patterns of activity, or oscillations, are within the alpha band, which spans the frequency of 8 to 12 Hertz (Hz) and is related to attentional modulation (153). Aberrant oscillations have been identified as potential biomarkers for affective disorders (321), and EEG-recorded cortical activity is dynamic with respect to ovarian hormonal milieu (41).
Though the data are far less abundant than that of fMRI, varying activation patterns across the menstrual cycle have also been observed using EEG (27, 41, 195, 253). Due likely to lack of hormonal confirmation of phase and variance of tasks, the results of these studies are somewhat conflicting. Nonetheless, cortical dynamics may be altered at specific time points in the menstrual cycle, as indicated by interhemispheric transfer time (131), attentional blink (138) and resting state alpha oscillation (41). Of these observations, the latter is particularly compelling, as hemispheric asymmetry in the alpha band has been identified in numerous psychiatric conditions, including depression (63, 134), social anxiety disorder (208), panic disorder (352), and PMDD (10, 172). A model by Davidson (61) describes this phenomenon as a result of lateralization of emotion processing, wherein positive emotions activate the left PFC, and negative emotions the right. In support of this model, both individuals with depression and those with PMDD show greater alpha oscillation power in the left hemisphere than the right (10, 134). The relationship between alpha oscillation and neural activation is inverse, such that the left-skew of power (greater alpha oscillations in the left hemisphere) in individuals with depression represents decreased activity in the left hemisphere, which has also been demonstrated via fMRI (121). Further, alpha asymmetry induced in healthy controls viewing footage of disaster returns quickly to baseline, but more pronounced asymmetry predicted mood deterioration during the following week (228). Alpha asymmetry can be used to differentiate those in remission from depression from those who were never depressed (4, 63, 113, 135, 307), suggesting its potential as a depression biomarker.
The ability of reproductive hormone changes to provoke depressive symptoms may occur as a result of the affect regulatory effects of neurosteroids. Neurosteroids are steroid hormone metabolites that are synthesized in the brain and modulate the inhibitory and excitatory neurotransmitters GABA and glutamate, respectively. Neurosteroids identified as most relevant to depression are dehydroepiandrosterone (DHEA) and allopregnanolone (ALLO). Blunted DHEA secretion is associated with MDD (112, 137, 197, 274, 359), and DHEA administration has antidepressant effects (271,355). ALLO, a progesterone metabolite, also has been implicated in depression and is particularly relevant to reproductive mood disorders. Several lines of evidence suggest a role for ALLO in triggering affective dysregulation. In rodents, ALLO regulates the GABA receptor (182) and has anxiolytic effects (29,30,348). ALLO withdrawal results in anxiety behaviors and insensitivity to benzodiazepines (298,299). In humans, depression is associated with decreased circulating ALLO levels, and successful antidepressant treatment is associated with increased ALLO (87,254,280,282,311,331). ALLO has been shown to regulate the basic neurobiological systems that are dysregulated in MDD, including the HPA axis (14,147,231,232), the immune system (132), and neuroprotection (70, 267). ALLO also modulates the limbic system (3,31), which, as described previously, has been implicated in MDD.
The Role of Gonadal Steroids in Reproductive Mood Disorders
Reproductive mood disorders are characterized by affective dysregulation and functional impairment that occur during specific reproductive states (e.g., the premenstrual phase of the menstrual cycle, pregnancy, parturition, and the transition to menopause). Dysregulated affect in reproductive mood disorders includes increased negative affect (i.e., irritability, anger, sadness, and anxiety), decreased positive affect (i.e., anhedonia), and affective lability (233,329), while functional impairment is defined by clinically significant distress or disability in social, occupational, or other important activities (6). Reproductive mood disorders include PMDD, PPD, and perimenopausal depression.
Premenstrual dysphoric disorder
Diagnosis
PMDD is a reproductive mood disorder that affects 2% to 5% of women and is characterized by a recurrent, predictable pattern of distressing emotional and somatic symptoms that begin during the mid- to late-luteal phase of the menstrual cycle, when estradiol and progesterone levels are relatively high, and remit after the onset of menses, when estradiol and progesterone levels are relatively low and stable (86). In research conducted by our team, PMDD diagnosis required prospective daily assessment of mood symptoms over the course of three consecutive menstrual cycles. PMDD was defined by a 30% increase (relative to the range of the scale employed) in mean negative mood symptoms during the week before menses compared with the week after menses, a more stringent criterion than that of the Fifth Edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) (5). For the purpose of this review, we will use the term PMDD to refer to both premenstrual dysphoria (the term used prior to DSM-5) and DSM-5 PMDD.
The effect of cyclic changes in gonadal steroids on mood in PMDD
The physiology of the hypothalamic-pituitary-gonadal (HPG) axis, which regulates the menstrual cycle, is well understood (94) and illustrated in Figure 3. In brief, under the control of the hypothalamic GnRH, both follicle-stimulating hormone (FSH) and LH are released from the pituitary. During the first “half” of the menstrual cycle—the follicular phase—FSH stimulates the progressive growth of ovarian follicles. One follicle becomes dominant and, toward the end of the follicular phase, releases dramatically increased levels of estradiol. This “spike” of estradiol exerts positive feedback on the pituitary and results in a peak of LH, which is followed by the release of an egg from the mature follicle, ovulation. Following ovulation, the remaining part of the mature follicle undergoes “luteinization” and, under control of LH, secretes progressively increased levels of progesterone and estradiol. About midway through the luteal phase, which lasts for 14 ± 2 days, the corpus luteum involutes, progesterone (and estradiol) levels drop, which signals the shedding of the endometrium (uterine lining) or menstruation, which lasts for approximately five days. By convention, the onset of menses is designated the first day of the menstrual cycle. (If fertilization occurs, the corpus luteum is sustained by human chorionic gonadotropin and menses does not occur.) Because PMDD symptoms occur in predictable patterns relative to menses (i.e., heightened symptoms during the week before menses compared with the week after menses), many have speculated that estrogen, progesterone, or their combination trigger mood symptoms. As a rule, basal hormone studies of PMDD, and of reproductive mood disorders in general, have been singularly unrevealing, with no consistently observed differences between patients and controls. Indeed, there is virtually no convincing evidence for a hormone abnormality in PMDD. Reproductive steroids can, nonetheless, be demonstrated to trigger affective dysregulation, albeit in the context of an antecedent susceptibility.
Figure 3.
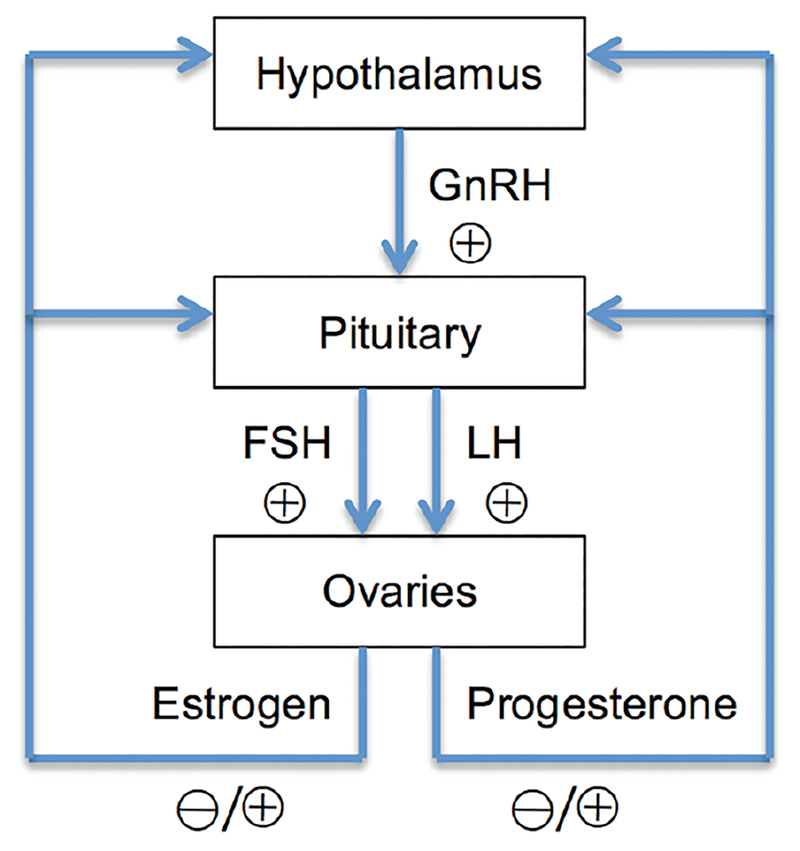
The hypothalamic-pituitary-gonadal (HPG) axis. The hypothalamus secretes GnRH, which acts on the pituitary gland. In response, the pituitary gland releases the gonadotropins LH and FSH. LH and FSH stimulate the ovaries to produce estrogen and progesterone. Depending on the menstrual cycle phase, estrogen and progesterone provide either positive or negative feedback to the hypothalamus and pituitary.
In a study employing GnRH agonist-induced ovarian suppression and hormone add-back, Schmidt et al. (276) demonstrated that elimination of the menstrual cycle eliminated PMDD symptoms, while estradiol and progesterone add-back precipitated symptom return. The same hormone manipulation had no mood effects in women lacking a history of PMDD (Fig. 4). Thus, ovarian steroids triggered PMDD symptoms, but only in a group of women that were otherwise susceptible to the mood destabilizing effects of estradiol and progesterone. Finally, in a recently completed study employing ovarian suppression and continuous hormone add-back for three months, Schmidt et al. (unpublished data) demonstrated that it was the change in hormone and not the hormone level itself that was the critical signal in precipitating affective symptoms in women with a history of PMDD. While the precise mechanisms by which the change in hormone levels provokes affective symptoms in PMDD remain unclear, studies in rodents demonstrate that dramatic behavioral changes can be precipitated by acute changes in levels of neurosteroids (286,300).
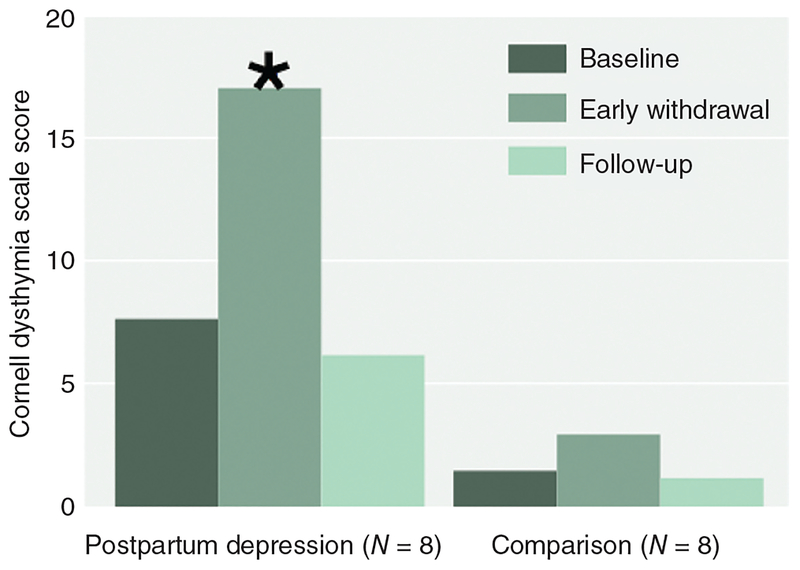
Figure 4.
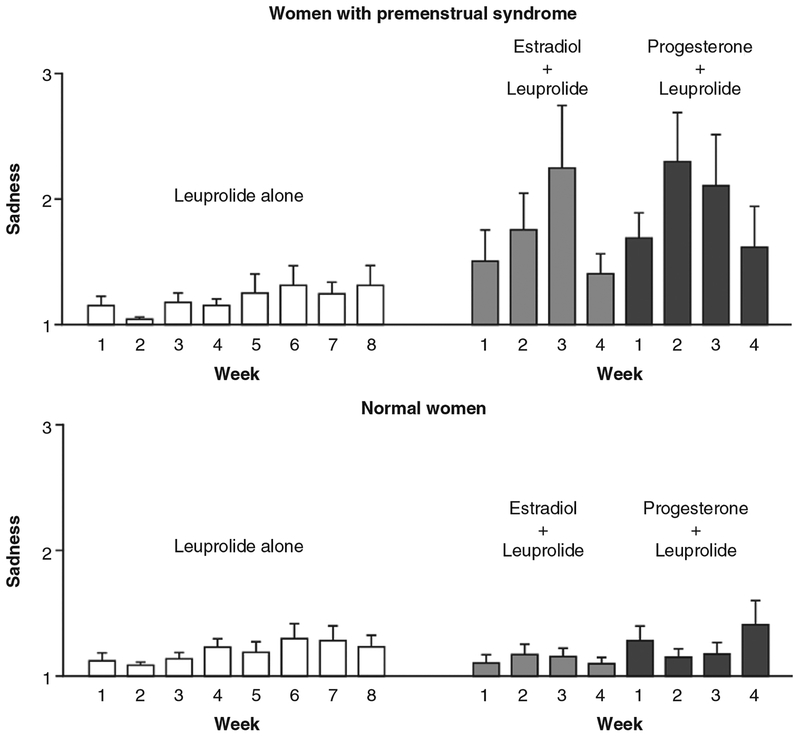
Recurrence of sadness in women with premenstrual syndrome during estradiol or progesterone add-back in the context of GnRH agonist-induced ovarian suppression, reproduced with permission from (276). Ten women with premenstrual syndrome and 15 control women had minimal mood symptoms while receiving leuprolide acetate (a GnRH agonist). In contrast, the women with premenstrual syndrome but not the controls had a significant increase in sadness during the administration of either estradiol or progesterone. Values are the means (±SE) of the seven daily scores on the sadness scale of the Daily Rating Form (82, 125) for each of the 8 weeks preceding hormone replacement (leuprolide alone) and during the 4 weeks of estradiol (plus leuprolide) and progesterone (plus leuprolide) replacement. A score of 1 indicates that they symptom was not present, and a score of 6 indicates that it was present in the extreme. P = 0.003 for interaction of treatment condition, diagnostic group, and week.
Neurosteroid regulation of mood in PMDD
The majority of neurosteroid research in PMDD has focused on the progesterone metabolite ALLO, a potent GABA receptor regulator. For comprehensive reviews of the role of neurosteroids and neuroactive steroids in affective disorders see (76) and (281). Researchers have examined abnormalities in circulating ALLO in PMDD with varying results. Although several investigators observed decreased serum ALLO levels in women with PMDD compared with controls on menstrual cycle day 26 (248), during the luteal phase only (201), or during the follicular phase only (28), women with PMDD in two of these studies (28, 201) had lower progesterone levels, which likely accounts for their lower ALLO levels. This explanation is supported by Girdler et al.’s (110) observation that women with PMDD had both higher progesterone and ALLO levels than controls during the luteal phase. Successful treatment with antidepressant medication was associated with reduced ALLO levels in a study by Freeman et al. (97), but it is unclear whether baseline differences in ALLO or progesterone may account for this finding. Other studies showed neither diagnosis-related differences in ALLO or pregnanolone (277,337) nor any difference in ALLO levels in women with PMDD before and after successful treatment with citalopram (315). The use of different assays, antibodies, and methods of extraction across studies may account for some the variability in findings. Nonetheless, there is no reliable evidence that peripheral ALLO levels distinguish those with PMDD from those without.
In the absence of consistent basal neurosteroid abnormalities in PMDD, researchers have examined the response to various challenges, including the administration of neurosteroids, benzodiazepines, and mental stress. One reliable neurophysiologic measure of GABAA receptor sensitivity is saccadic eye velocity, which characterizes the speed of eye movements that bring the retinal image being viewed onto the fovea. Saccadic eye velocity is correlated with self-reported sedation and suppressed by benzodiazepines (316). Women with PMDD show differences from controls in pregnanolone-modulated saccadic eye velocity and sedation in the luteal phase (316), although these differences may be attributable to saccadic eye velocity response to vehicle in those with PMDD and blunted sedation in controls during the follicular phase. Patients with severe PMDD show blunted saccadic eye velocity and sedation responses to the GABAA receptor modulators pregnanolone and midazolam compared with women with less severe PMDD (316, 316), which may indicate an altered profile of GABAA receptor subunits. Midazolam insensitivity, in particular, could suggest increased expression of the α-and/or δ-subunits (317). However, neurosteroid levels were not reported for women with more or less severe PMDD, making it difficult to draw conclusions about whether the effects may have been attributable to variation in baseline hormone levels, given the risk of increased sampling variability with small samples (n = 6). Although women with PMDD also show a blunted ALLO response to stress (110), as mentioned earlier, the lack of an expected increase in ALLO following stress may be attributed to elevated progesterone and ALLO levels at baseline in the women with PMDD compared with controls. Similarly, women with PMDD demonstrate altered metabolism of progesterone to ALLO (152), although women with PMDD had an elevated ALLO/progesterone ratio at baseline, and women with PMDD and controls showed the same pattern of results following progesterone administration (152). The concept of altered steroidogenesis conferring vulnerability to reproductive-related illness is supported by research in polycystic ovarian syndrome, which is characterized by enhanced peripheral 5α-reductase activity and results in hyperandrogenism (90) and attendant behavioral symptoms, including depression and anxiety (64). Steroid synthesis may be similarly altered in PMDD such that mood symptoms result from decreased enzymatic conversion of progesterone to ALLO by 5α-reductase and 3α-hydroxysteroid dehydrogenase (3α-HSD) (Fig. 1). One potential mechanism that could account for differential enzymatic metabolism of progesterone to ALLO is suggested by Borolato et al. (36). They demonstrated that early life stress reduces the expression of 5α-reductase isoforms in the rat nucleus accumbens and medial prefrontal cortex, thereby reducing the synthesis of ALLO from progesterone.
Finally, possible ALLO abnormalities in PMDD also have been examined through inspection of the timing of symptom onset or exacerbation relative to changes in ALLO levels. As with progesterone, circulating ALLO levels fluctuate over the course of the menstrual cycle because ALLO is a progesterone metabolite. As such, plasma ALLO concentrations are highest during the mid-luteal phase, decline to low levels in the premenstrual phase and follicular phase, and are correlated with progesterone levels in healthy women (107, 277). Because of the potent anxiolytic effects of ALLO, one would expect decreased mood symptoms during periods of elevated progesterone and ALLO levels (i.e., the mid-luteal phase of the menstrual cycle). However, PMDD symptoms emerge during the mid-luteal phase (during elevated ALLO concentrations) and remit after the onset of menses (during decreased ALLO concentrations) (233). Other hypothesis-contradicting observations include the following: progesterone administration is either ineffective or exacerbates affective symptoms in women with PMDD (95, 96, 265, 276, 324); and drugs that prevent ovulation, and thus prevent the subsequent increase in progesterone and ALLO levels, prevent PMDD symptoms (276).
Although the timing of symptom onset and response to progesterone administration in PMDD seemingly conflict with the literature on the anxiolytic effects of ALLO, an animal model of ALLO function in PMDD explains how increasing circulating progesterone levels may trigger affective dysregulation in susceptible women. Smith et al. (300) demonstrated that under certain hormonal and behavioral conditions ALLO exerts anxiogenic effects. ALLO withdrawal followed by ALLO administration results in anxiogenesis in response to an aversive stimulus. This effect is mediated by an increase in GABAA receptor α4 expression, which is associated with insensitivity to the benzodiazepine agonist lorazepam (300). Thus, ALLO withdrawal modifies the GABAA receptor such that the reintroduction of ALLO, in combination with an aversive stimulus, triggers hippocampal excitability rather than inhibition, resulting in an amplified stress response. One possible cellular mechanism for the abnormal response to ALLO administration after ALLO withdrawal is presented by Shen et al. (286), in which a particular configuration of subunits (α4β2δ) of GABAA receptors, a configuration induced by changes in ALLO levels, reverses the effects of ALLO from enhancing to inhibiting GABA-gated current. Thus, ALLO released as a result of an aversive stimulus during the luteal phase (and following the rise in ALLO post ovulation) may paradoxically increase negative mood symptoms. These findings may help explain the timing of affective symptom onset during the luteal phase of the menstrual cycle and during pregnancy, the negative mood effects of progesterone administration in both PMDD and PPD, and the salutary effects of ovulation suppression in women PMDD.
Consistent with these models, women with PMDD show abnormalities in cortical inhibition and GABA levels across the menstrual cycle. Cerebral cortical inhibition increases during the luteal phase in healthy control women, a presumed effect of increased ALLO levels and a finding that is absent in women with PMDD (295,296). Women with PMDD show reduced GABA levels in the occipital cortex during the follicular phase and increasing levels from the follicular to the luteal phase, whereas healthy controls show decreasing levels from the follicular to the luteal phase (84). Healthy control women also show a significant negative association between ALLO and cortical GABA levels, whereas women with PMDD do not (84), suggesting a luteal phase-specific reduction in GABA receptor function. Taken together, these findings are consistent with an abnormal response to ALLO in women with PMDD.
Reanalysis of existing data from our prior studies also supports the role of ALLO in PMDD. We examined ALLO levels and depressive symptoms in a previous study of ovarian suppression with depot leuprolide acetate (a GnRH agonist) and subsequent progesterone add-back in women with PMDD (276). During progesterone add-back (i.e., when progesterone levels were held steady), ALLO levels declined significantly in those with PMDD but not in controls (269). In addition, the change in ALLO was negatively associated with premenstrual symptoms in women with PMDD but not in controls, which suggests that declining levels of ALLO worsened symptoms in those with PMDD.
ALLO acts on GABAA receptors (29), which are present throughout the cortex and limbic system (38,136). Infusion of ALLO into the amygdala has antidepressant effects in rodents (83, 288). In healthy, euthymic women, progesterone administration increases amygdala connectivity to the dorsomedial prefrontal cortex similar to antidepressant medication (351). Pregnenolone administration (leading to increased ALLO levels) reduces amygdala and insula activity, increases dorsomedial prefrontal cortex activity, and increases connectivity between the amygdala and dorsomedial prefrontal cortex (304). However, the influence of ALLO on neural circuits implicated in depression has not been studied in women with PMDD, who, based on the ALLO findings presented earlier, may show the opposite pattern of results. Nonetheless, the existing research on reproductive steroid and neurosteroid regulation of mood provides important clues for understanding the role of neural networks in PMDD.
Neuroimaging in PMDD
Brain imaging (e.g., EEG, fMRI, or PET) has been employed to detect abnormalities that distinguish women with PMDD from healthy, regularly menstruating control women. Using EEG, Baehr et al. (10) demonstrated that women with PMDD had greater cerebral asymmetry during the luteal phase compared with the follicular phase, a pattern that was absent in control women. As described earlier, this result is consistent with prior EEG research linking cortical asymmetry, left frontal hypoactivity, behavioral approach deficits, and MDD (61, 325) and is also consistent with the temporal pattern of mood symptoms in PMDD, which are confined to the luteal phase and distinguish PMDD from MDD. In two independent fMRI studies, women with PMDD showed increased amygdala responses to negative stimuli when compared with healthy control women (109, 243), implicating the frontolimbic “emotion processing” circuitry in PMDD. Other studies have demonstrated that PMDD is characterized by decreased responsiveness to behavioral inhibition in the medial orbitofrontal cortex during the premenstrual phase (243), increased right cerebellar blood flow during the luteal phase compared with the follicular phase (247), and decreased activity of the left insula during the follicular phase (both compared with controls and with the luteal phase) (13). To directly assess whether reproductive steroids influence brain function in PMDD, Baller et al. (11) (Fig. 5) examined brain responsivity to a working memory task using fMRI and PET under conditions of experimentally induced hypogonadism, estradiol add-back, and progesterone add-back in women with and without PMDD. Across all hormone conditions, women with PMDD showed greater activation of the dorsolateral prefrontal cortex, medial prefrontal gyrus, and cerebellum. Among the women with PMDD, dorsolateral prefrontal cortex activation was associated with lower global assessment of functioning, earlier age of onset, and greater preintervention menses-related changes in negative affect (11). Taken together, these findings suggest that PMDD is characterized by a combination of stable, trait-like functional abnormalities in the dorsolateral prefrontal cortex and amygdala, areas responsible for cognitive, emotional, and social functions, as well as menstrual phase-specific abnormalities that may result from changing reproductive steroid levels. The focus of imaging studies thus far have largely relied on whole-brain and region-of-interest analyses designed to examine activity of individual brain areas. The future demand, however, will be on a network neuroscience approach to understanding how the neural networks implicated in depression are regulated by neurosteroids and neuroactive steroids in those with and without PMDD.
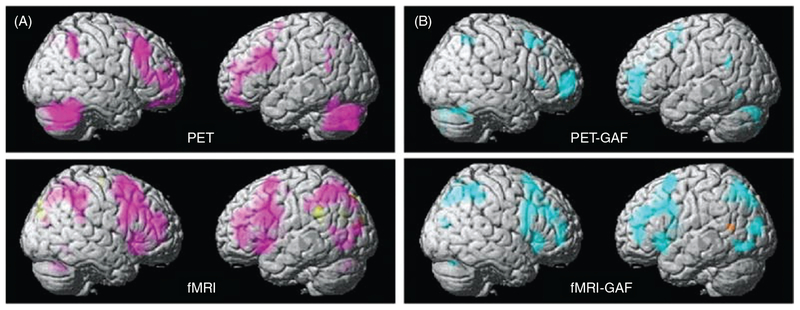
Figure 5.
PET and fMRI activation in a multimodal imaging study of women with PMDD and a comparison group of women, reproduced with permission from (11). Panel A shows the between-group differences in activation using PET and fMRI. Regions in which patients had greater activation than comparison subjects are shown in pink. Panel B shows the correlations between Global Assessment of Functioning Scale (GAF) scores and activation in patients using PET and fMRI. Regions in which these two measures were negatively correlated (the greater the overactivation, the more severe impairment indicated by GAF scores) are shown in blue.
Postpartum depression
Diagnosis
According to the DSM-5, PPD is defined as a MDE beginning either during pregnancy or within the first four weeks following delivery (6). Although PPD is only distinguished from MDE by the timing of onset (i.e., they share the diagnostic criteria of persistent sadness, anhedonia, sleep disturbance, appetite change, psychomotor agitation/retardation, lethargy, impaired cognition, feelings of worthlessness or guilt, and suicidal ideation) (6), psychomotor agitation and impaired concentration/decision-making have been shown to be prominent symptoms of PPD, whereas sadness, suicidal ideation, and anhedonia are prominent symptoms of non-perinatal MDD (24). PPD also is characterized by mood fluctuations and preoccupation with infant health. In addition, the majority of women with PPD have a comorbid anxiety disorder, and symptoms commonly associated with PPD include generalized anxiety symptoms, panic attacks, and rumination (353). Approximately 20% of women experience a clinically significant depressive episode (i.e., either a minor depressive episode or a MDE) and 7% meet full diagnostic criteria for a MDE within three months postpartum (105). Although nearly every study conducted to date suggests that PPD is not more common than nonpregnancy-related MDD (220), one large epidemiological study demonstrated that the postpartum period is associated with an increased risk of depression (334).
The effect of perinatal changes in gonadal steroids on mood in PPD
Many have hypothesized that reproductive steroids play an etiological role in PPD (221) because the precipitous drop in hormone concentrations that occur at childbirth are temporally associated with the reported onset of depressive symptoms in the majority of women with PPD (268, 309). Progesterone initially is produced by the corpus luteum, until about the tenth week of gestation when the placenta becomes the primary source of progesterone. Circulating progesterone, derived from maternal cholesterol, increases progressively from 10 ng/mL in early pregnancy to 100 to 200 ng/mL at term. ALLO also increases about 10-fold during pregnancy. Estrogen hormones, first derived from maternal then from fetal androgens, are synthesized by the placenta during pregnancy. Estradiol and estrone increase 100-fold during pregnancy, while estriol increases about one thousand-fold. Estradiol drops to prepregnancy, follicular phase levels immediately following delivery. Despite the dramatic changes in reproductive steroids during and after pregnancy and substantial interindividual differences in absolute circulating steroid levels, there is no reliable evidence that women who develop PPD have blunted postpartum hormone levels or experience greater or more rapid reductions in reproductive hormone levels at delivery than women without PPD.
Treatment studies involving the administration of exogenous estradiol to women with active PPD symptoms or those at high risk for PPD have suggested a role for reproductive hormones in the pathogenesis of PPD. Sichel et al. (290) conducted a prevention study in which they treated 11 women a history of PPD with Premarin, a conjugated equine estrogen, starting just after delivery to prevent the precipitous drop in estradiol and the onset of MDD. Almost all (10 out of 11) of the women remained free of depression in the first year post-partum (290). A couple of small treatment studies have been conducted in women with current PPD. Gregoire et al. (118) showed that those randomly assigned to receive transdermal estradiol treatment (n = 34) improved significantly more than those who received placebo (n = 27). However, it is worth noting that nearly half the participants received concurrent antidepressant treatment. Ahokas et al. (2) conducted a subsequent treatment study examining the effects of short-term (8 week) sublingual estradiol on depressive symptoms in 23 women with severe PPD. Many of the women in the study were considered treatment resistant and hypogonadal based on their serum estradiol concentrations. Depressive symptoms significantly decreased after the first week of treatment, and by the end of the eighth week, all of the participants had achieved remission based on their depressive symptom scores. Results of this study suggest that low or decreasing estradiol levels may contribute to or maintain depressive symptoms in susceptible women, while stable or increasing estradiol levels may ameliorate depressive symptoms. While these treatment studies support the notion that estradiol plays a role in PPD, they have low statistical power, lack control groups, and were conducted with a heterogeneous sample of women (i.e., those with and without exposure to antidepressant medication; those with depression onset before, during, or after pregnancy; those with and without “hypogonadism”). In addition, the stress, sleep deprivation, and homeostatic fluctuations that occur during pregnancy and parturition are important confounds that limit the ability to draw definitive conclusions about the role of estrogen in PPD based on these studies.
To isolate the effects of reproductive steroids on mood symptoms, Bloch et al. (34) mimicked the hormone profile of pregnancy and the postpartum in nonpregnant, asymptomatic women with a history of PPD and those without such a history by administering high-dose estradiol and progesterone during ovarian suppression followed by placebo only (to induce hormone withdrawal). Despite identical hormonal conditions, women with a history of PPD showed increased mood symptoms during estradiol and progesterone add-back and a further exacerbation of mood symptoms during hormone withdrawal, whereas those without a history of PPD showed no change in mood (Fig. 6). Similar to research in PMDD, the precise mechanism by which changing estradiol and progesterone trigger affective dysregulation is unclear.
Figure 6.
Mean scores on the Cornell Dysthymia Scale before and after estrogen and progesterone replacement in eight women with a history of postpartum depression and eight healthy comparison women, reproduced with permission from (34). The significant difference between baseline and hormone withdrawal periods in the group with a history of postpartum depression (P < 0.01) is denoted by the symbol (∗).
Neurosteroid regulation of mood in PPD
The neurosteroid ALLO may play a role in PPD, albeit a paradoxical one. PPD most commonly begins during pregnancy (when ALLO levels are relatively high) (115), and anxiety and depressive symptoms during pregnancy are the strongest predictors of PPD (222). Epperson et al. (85) demonstrated that the postpartum period is associated with decreased cortical GABA and ALLO compared with the follicular phase, and this effect was seen in postpartum women regardless of whether they experienced mood disturbance. Treatment with progestin (norethisterone enanthate, also known as the “mini pill”), which is thought to increase circulating ALLO levels, has been shown to increase depressive symptoms in the post-natal period (168). However, compared with the untreated control group, circulating progesterone levels were lower in those administered norethisterone (168), and thus, ALLO levels would have been lower as well. Progesterone administered in combination with estradiol precipitates depressive symptoms in women with a history of PPD (34), suggesting that ALLO may have role in provoking symptoms, albeit an indirect one. Although these findings run counter to the literature demonstrating that ALLO is a potent anxiolytic and antidepressant, animal models help explain the emergence of depressive symptoms in the context of reproductive steroid withdrawal.
As mentioned earlier, Maguire and Mody (181) demonstrated a GABAA receptor δ-subunit knockout mouse that displays no behavioral abnormalities until during and after pregnancy, at which point the dam exhibits depressive behavior and cannibalizes its offspring (181). Thus, in the context of a genetic susceptibility, hormonal states associated with reproduction may provoke affective dysregulation. Reproductive hormone changes that occur during pregnancy and parturition alter the GABAA receptor δ-subunit (179–181). As ALLO levels increase during pregnancy, GABAA receptor δ-subunit expression is downregulated, and as ALLO levels rapidly decrease after delivery, GABAA receptor δ-subunit is recovered (179). The knockout animal’s inability to regulate the GABAA receptor δ-subunit during pregnancy and the postpartum results in behavioral abnormalities consistent with PPD (Fig. 7). Thus, similar to women with PPD, GABAA receptor δ-subunit knock-out mice exhibit no behavioral abnormalities before pregnancy, but they are insensitive to ALLO during pregnancy and show behavioral abnormalities consistent with anxiety and depression after pregnancy (181). This model demonstrates that changes in circulating ALLO levels are capable of triggering depression in the context of a genetic susceptibility.
Figure 7.
Abnormal maternal behaviors in GABAA receptor δ-subunit-deficient mice, reproduced with permission from (181). Panel on right shows that decreased survival of pups born to GABAA receptor δ-subunit-deficient (Gabrd+/− and Gabrd−/−) mice is reduced by including a GABAA receptor δ-subunit-selective agonist (THIP) in drinking water. Panel on left shows that pups born to Gabrd+/− and Gabrd−/− mice but reared by a surrogate wild-type mother immediately following birth had an increase in survival compared with wild-type pups reared by Gabrd+/− and Gabrd−/− surrogate mothers immediately following birth.
Neuroimaging in PPD
PPD is associated with decreased resting state functional connectivity between the ACC, amygdala, hippocampus, and dorsolateral prefrontal cortex in the context of falling progesterone and ALLO levels in the postpartum period (65). Notably, PPD is characterized by functional abnormalities of the same brain areas seen in nonpuerperal MDD: the amygdala, insula, striatum, orbitofrontal cortex, and dorsomedial prefrontal cortex (210,211,291). PPD also is associated with decreased connectivity between the amygdala and prefrontal cortex, which suggests emotion processing (corticolimbic) network dysfunction is involved in the pathophysiology of PPD (211). The majority of neuroimaging studies of PPD have included women with histories of both PPD and nonpuerperal depression. As we make the case here, reproductive mood disorders like PPD may have neuroendocrine triggers that are distinct from the etiopathology of nonpuerperal depression. As such, results of prior imaging studies are difficult to interpret in the context of a hypothesized neuroendocrine trigger. As reviewed here, however, the human literature provides evidence of a hormone-sensitive PPD subtype that is characterized by behavioral (affective) and neural dysregulation present only during pregnancy and the postpartum period in the context of rapid changes in reproductive steroid concentrations. A future direction of this line of research is to examine the effects of reproductive steroid changes on the functional neural networks implicated in depression in women susceptible to PPD.
Perimenopausal depression
Diagnosis
Perimenopausal depression is not recognized formally in the diagnostic nomenclature. Our research team has defined perimenopausal depression as MDD with an onset temporally associated with menstrual cycle irregularity and elevated FSH in women age 45 or older. The risk of new onset depression increases twofold during the menopause transition (53, 98). Prior research suggests that women with a longer transition to menopause, vasomotor symptoms, stressful life events proximate to the menopause transition, histories of depression (i.e., MDD, PPD, or PMDD), poor physical health, histories of smoking, disrupted sleep, reduced parity, and those who are unmarried are at increased risk of developing perimenopausal depression.
The effect of perimenopausal changes in gonadal steroids on mood
The Stages of Reproductive Aging Workshop (STRAW) criteria (130) are used to define 10 discrete stages of the menopause transition (Fig. 8). During the early stages of reproductive aging (sometimes called the “premenopause”), the menstrual cycle is regular and FSH levels remain normal. As women approach the menopausal transition, they enter the “late reproductive stage” (STRAW stage-3a), which is characterized by subtle changes in menstrual flow and the length of the menstrual cycle and variable FSH levels. The menopause transition (the “perimenopause”) includes both the early and late stages. The early menopause transition (STRAW stage-2) occurs when a woman experiences a change of 7 or more days in the length of consecutive menstrual cycles and FSH levels begin to rise. Prior research suggests that FSH levels begin to rise and estradiol levels begin to fall about 2 years before the final menstrual period (44, 84), but the length of early menopause transition is variable (130). The late menopause transition (STRAW stage-1) is characterized by amenorrhea of 60 days or longer accompanied by an FSH level greater than 25 IU/L and lasts between one and three years. Women often begin experiencing vasomotor symptoms (hot flashes, night sweats) during this time. Menopause is defined as the permanent end of menstruation for 12 months accompanied by the loss of ovarian activity. The postmenopause occurs after the final menstrual period and involves continuous decline of ovarian hormones but steady, elevated levels of FSH. Given the reproductive steroid variability and withdrawal in the transition to menopause, some have hypothesized a role for estrogen and progesterone in perimenopausal depression.
Figure 8.
The STRAW +10 staging system for reproductive aging in women, reproduced with permission from (130). FMP, final menstrual period.
In a 5-year longitudinal study of 29 women, a 14-fold increase in MDD was observed in the 24 months surrounding menopause (273). In a larger cross sectional study (N = 116), depressive episodes were more likely during the late perimenopause compared with the pre-, early-, and postmenopause (306). Although estradiol levels are highly variable in regularly cycling women and during premenopause and early perimenopause, the average level of circulating estradiol declines dramatically during the transition from early to late perimenopause (44), which is accompanied by an increased incidence of depression (273, 306). In a large study of perimenopausal women, greater variability in estradiol concentrations, and not absolute estradiol concentrations per se, was associated with both elevated depression symptom severity as well as diagnosed MDD during the menopause transition (98). The role of estradiol in perimenopausal depression is further supported by hormone manipulation studies. Depressive symptoms decreased following 3 weeks of estradiol treatment in 34 women with perimenopausal depression compared with baseline and women taking placebo (275). Despite comparable ovarian hormone levels in women with perimenopausal depression and controls (273, 274), women with a history of perimenopausal depression (but not those lacking that history) rapidly experience depression recurrence when blindly withdrawn from estradiol (272). Thus, estradiol withdrawal appears to trigger perimenopausal depression in susceptible women, and estradiol treatment ameliorates these symptoms.
Neuroimaging of perimenopausal depression
Although several neuroimaging studies have attempted to identify hormonal mechanisms of cognitive dysfunction seen during the perimenopause (21,22,78,79), none has examined neural correlates of depression or the neural mechanisms of the antidepressant effects of estradiol. Existing imaging studies do, however, document abnormalities in neural networks responsible for cognition and memory in postmenopausal women, which are reversed by estrogen therapy (21,22,78,79, 149). Menopause is associated with reduced phonemic verbal fluency and increased activation of the bilateral inferior frontal cortex and left temporal pole (21). Short-term estrogen treatment of postmenopausal women is associated with increased prefrontal activation (78). Long-term estrogen treatment of postmenopausal women is associated with increased activation of the frontal and parietal cortices, insula, hippocampus, cingulate, putamen and raphe (22). In the context of an anti-cholinergic challenge, estrogen treatment buffered activation of the left medial frontal gyrus and increased activation of the precuneus (79). Thus, estradiol affects cholinergic system regulation of cognition-related brain activation in post-menopausal women. The effects of estrogen and progesterone on perimenopausal women have not been as well studied, and this represents an important area for future research given that the effects of estradiol are likely to be most beneficial when started during the menopause transition rather than in the postmenopause.
Conclusion
Reproductive steroids powerfully regulate a number of behaviors extending well beyond reproductive and maternal behavior to eating behavior, cognition, motor behavior, sleep, and impulse control. Reproductive steroid regulation of behavior is therefore essential to our survival as a species. Not surprisingly, then reproductive steroids are potent neuroregulators from the subcellular to cellular level to circuit to behavior. Reproductive steroid regulation of circuits and behaviors is relevant to mood disorders. In particular, mood disorders are characterized by affective, cognitive, behavioral, and neurovegetative symptoms, which suggests the presence of neural network dysfunction. The neural circuits and networks shown to be dysregulated in depression include the frontosubcortical regulatory network, central executive network, mesolimbic reward circuitry, and DMN. Activity in regions throughout these networks is modulated by estrogen and progesterone or their metabolites. Changes in reproductive steroid levels during specific reproductive states (i.e., the premenstrual phase of the menstrual cycle, pregnancy, parturition, and the menopause transition) have been shown to trigger affective dysregulation in susceptible women. In those with PMDD, fluctuations in estradiol and progesterone destabilize mood. Similarly, in PPD, both increases and decreases in estradiol and progesterone appear to trigger depressive symptoms in susceptible women. Finally, the twofold increase in depressive symptoms seen during the menopause transition is temporally coincident with the decrease in estradiol that occurs during the late perimenopause. Further, mood destabilization is associated with the degree of estradiol variability rather than absolute levels, suggesting once again that the change in hormone levels contributes to mood dysregulation in the context of an antecedent susceptibility. In addition, animal models suggest that ALLO may trigger mood symptoms as a function of GABAA receptor α4- and/or δ-subunit alterations that are heritable or consequent to ALLO withdrawal combined with stress (179–181, 286, 300). Thus, the source of susceptibility to reproductive mood disorders may involve estradiol, progesterone (mediated by ALLO), or both. Understanding the source of individual susceptibility is critical to both preventing the onset of illness and developing novel, individualized treatments for reproductive-related affective dys-regulation. The capacity of changes in reproductive steroids to dysregulate affect suggests a potentially important role of these hormones and their metabolites in reproductive mood disorders.
References
- 1.Abler B, Kumpfmüller D, Grön G, Walter M, Stingl J, Seeringer A. Neural correlates of erotic stimulation under different levels of female sexual hormones. PLoS One 8: e54447, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahokas A, Kaukoranta J, Wahlbeck K, Aito M. Estrogen deficiency in severe postpartum depression: Successful treatment with sublingual physiologic 17beta-estradiol: A preliminary study. J Clin Psychiatry 62: 332–336, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Akwa Y, Purdy RH, Koob GF, Britton KT. The amygdala mediates the anxiolytic-like effect of the neurosteroid allopregnanolone in rat. Behav Brain Res 106: 119–125, 1999. [DOI] [PubMed] [Google Scholar]
- 4.Allen JJB, Urry HL, Hitt SK, Coan JA. The stability of resting frontal electroencephalographic asymmetry in depression. Psychophysiology 41: 269–280, 2004. [DOI] [PubMed] [Google Scholar]
- 5.American Psychiatric Association, DSM-5 Task Force. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. Arlington, VA: American Psychiatric Association, 2013. [Google Scholar]
- 6.American Psychiatric Association, others. Diagnostic and Statistical Manual of Mental Disorders (5th ed.). Arlington, VA: American Psychiatric Publishing, 2013. [Google Scholar]
- 7.Amin Z, Epperson CN, Constable RT, Canli T. Effects of estrogen variation on neural correlates of emotional response inhibition. NeuroImage 32: 457–464, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Andreano JM, Cahill L. Menstrual cycle modulation of medial temporal activity evoked by negative emotion. NeuroImage 53: 1286–1293, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Babayan AH, Kramár EA. Rapid effects of oestrogen on synaptic plasticity: Interactions with actin and its signalling proteins. J Neuroendocrinol 25: 1163–1172, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baehr E, Rosenfeld P, Miller L, Baehr R. Premenstrual dysphoric disorder and changes in frontal alpha asymmetry. Int J Psychophysiol 52: 159–167, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Baller EB, Wei S-M, Kohn PD, Rubinow DR, Alarcón G, Schmidt PJ, Berman KF. Abnormalities of dorsolateral prefrontal function in women with premenstrual dysphoric disorder: A multimodal neuroimaging study. Am J Psychiatry 170: 305–314, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balthazart J, Ball GF. Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci 29: 241–249, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Bannbers E, Gingnell M, Engman J, Morell A, Comasco E, Kask K, Garavan H, Wikström J, Sundström Poromaa I. The effect of premenstrual dysphoric disorder and menstrual cycle phase on brain activity during response inhibition. J Affect Disord 142: 347–350, 2012. [DOI] [PubMed] [Google Scholar]
- 14.Barbaccia ML, Roscetti G, Trabucchi M, Purdy RH, Mostallino MC, Concas A, Biggio G. The effects of inhibitors of GABAergic transmission and stress on brain and plasma allopregnanolone concentrations. Br J Pharmacol 120: 1582–1588, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barnes DE, Alexopoulos GS, Lopez OL, Williamson JD, Yaffe K. Depressive symptoms, vascular disease, and mild cognitive impairment: Findings from the cardiovascular health study. Arch Gen Psychiatry 63: 273–279, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Bayer J, Bandurski P, Sommer T. Differential modulation of activity related to the anticipation of monetary gains and losses across the menstrual cycle. Eur J Neurosci 38: 3519–3526, 2013. [DOI] [PubMed] [Google Scholar]
- 17.Beck AT. Depression: Clinical, Experimental, and Theoretical Aspects. New York: Harper & Row, 1967. [Google Scholar]
- 18.Beck AT. Cognitive Therapy and the Emotional Disorders. New York, NY: Penguin, 1979. [Google Scholar]
- 19.Becker JB. Direct effect of 17β-estradiol on striatum: Sex differences in dopamine release. Synapse 5: 157–164, 1990. [DOI] [PubMed] [Google Scholar]
- 20.Becker JB. Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacol Biochem Behav 64: 803–812, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Berent-Spillson A, Persad CC, Love T, Sowers M, Randolph JF, Zubieta J-K, Smith YR. Hormonal environment affects cognition independent of age during the menopause transition. J Clin Endocrinol Metab 97: E1686–E1694, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berent-Spillson A, Persad CC, Love T, Tkaczyk A, Wang H, Reame NK, Frey KA, Zubieta JK, Smith YR. Early menopausal hormone use influences brain regions used for visual working memory. Menopause 17: 692–699, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berman KF, Schmidt PJ, Rubinow DR, Danaceau MA, Van Horn JD, Esposito G, Ostrem JL, Weinberger DR. Modulation of cognition-specific cortical activity by gonadal steroids: A positron-emission tomography study in women. Proc Natl Acad Sci U S A 94: 8836–8841, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bernstein IH, Rush AJ, Yonkers K, Carmody TJ, Woo A, McConnell K, Trivedi MH. Symptom features of postpartum depression: Are they distinct? Depress Anxiety 25: 20–26, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berridge KC, Valenstein ES. What psychological process mediates feeding evoked by electrical stimulation of the lateral hypothalamus? Behav Neurosci 105: 3–14, 1991. [DOI] [PubMed] [Google Scholar]
- 26.Bethea CL, Lu NZ, Gundlah C, Streicher JM. Diverse actions of ovarian steroids in the serotonin neural system. Front Neuroendocrinol 23: 41–100, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Bibawi D, Cherry B, Hellige JB. Fluctuations of perceptual asymmetry across time in women and men: Effects related to the menstrual cycle. Neuropsychologia 33: 131–138, 1995. [DOI] [PubMed] [Google Scholar]
- 28.Bičíková M, Dibbelt L, Hiill M, Hampl R, Stárka L. Allopregnanolone in women with premenstrual syndrome. Horm Metab Res 30: 227–229, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Bitran D, Hilvers RJ, Kellogg CK. Anxiolytic effects of 3α-hydroxy-5α[β]-pregnan-20-one: Endogenous metabolites of progesterone that are active at the GABAA receptor. Brain Res 561: 157–161, 1991. [DOI] [PubMed] [Google Scholar]
- 30.Bitran D, Purdy RH, Kellog CK. Anxiolytic effect of progesterone is associated with increases in cortical alloprenanolone and GABAA receptor function. Pharmacol Biochem Behav 45: 423–428, 1993. [DOI] [PubMed] [Google Scholar]
- 31.Bixo M, Andersson A, Winblad B, Purdy RH, Böckstrom T. Progesterone, 5α-pregnane-3,20-dione and 3α-hydroxy-5α-pregnane-20-one in specific regions of the human female brain in different endocrine states. Brain Res 764: 173–178, 1997. [DOI] [PubMed] [Google Scholar]
- 32.Blanchard JJ, Mueser KT, Bellack AS. Anhedonia, positive and negative affect, and social functioning in schizophrenia. Schizophr Bull 24: 413–424, 1998. [DOI] [PubMed] [Google Scholar]
- 33.Blaut A, Paulewicz B, Szastok M, Prochwicz K, Koster E. Are attentional bias and memory bias for negative words causally related? J Behav Ther Exp Psychiatry 44: 293–299, 2013. [DOI] [PubMed] [Google Scholar]
- 34.Bloch M, Schmidt PJ, Danaceau M, Murphy J, Nieman L, Rubinow DR. Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry 157: 924–930, 2000. [DOI] [PubMed] [Google Scholar]
- 35.Blum M, McEwen BS, Roberts JL. Transcriptional analysis of tyrosine hydroxylase gene expression in the tuberoinfundibular dopaminergic neurons of the rat arcuate nucleus after estrogen treatment. J Biol Chem 262: 817–821, 1987. [PubMed] [Google Scholar]
- 36.Bortolato M, Devoto P, Roncada P, Frau R, Flore G, Saba P, Pistritto G, Soggiu A, Pisanu S, Zappala A, Ristaldi MS, Tattoli M, Cuomo V, Marrosu F, Barbaccia ML. Isolation rearing-induced reduction of brain 5α-reductase expression: Relevance to dopaminergic impairments. Neuropharmacology 60: 1301–1308, 2011. [DOI] [PubMed] [Google Scholar]
- 37.Bossé R, Di Paolo T. Dopamine and GABAA receptor imbalance after ovariectomy in rats: Model of menopause. J Psychiatry Neurosci 20: 364–371, 1995. [PMC free article] [PubMed] [Google Scholar]
- 38.Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A 108: 16050–16055, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bredemann TM, McMahon LL. 17β Estradiol increases resilience and improves hippocampal synaptic function in helpless ovariectomized rats. Psychoneuroendocrinology 42: 77–88, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brinton RD, Thompson RF, Foy MR, Baudry M, Wang J, Finch CE, Morgan TE, Pike CJ, Mack WJ, Stanczyk FZ, Nilsen J. Progesterone receptors: Form and function in brain. Front Neuroendocrinol 29: 313–339, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brötzner CP, Klimesch W, Doppelmayr M, Zauner A, Kerschbaum HH. Resting state alpha frequency is associated with menstrual cycle phase, estradiol and use of oral contraceptives. Brain Res 1577: 36–44, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown CM, Mulcahey TA, Filipek NC, Wise PM. Production of proinflammatory cytokines and chemokines during neuroinflammation: Novel roles for estrogen receptors α and β. Endocrinology 151: 4916–4925, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bryant DN, Dorsa DM. Roles of estrogen receptors alpha and beta in sexually dimorphic neuroprotection against glutamate toxicity. Neuro-science 170: 1261–1269, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burger H. The menopausal transition—endocrinology. J Sex Med 5: 2266–2273, 2008. [DOI] [PubMed] [Google Scholar]
- 45.Byrnes EM, Byrnes JJ, Bridges RS. Increased sensitivity of dopamine systems following reproductive experience in rats. Pharmacol Biochem Behav 68: 481–489, 2001. [DOI] [PubMed] [Google Scholar]
- 46.Cardona-Gomez P, Perez M, Avila J, Garcia-Segura L, Wandosell F. Estradiol inhibits GSK3 and regulates interaction of estrogen receptors, GSK3, and beta-catenin in the hippocampus. Mol Cell Neurosci 25: 363–373, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Champagne FA, Weaver ICG, Diorio J, Dymov S, Szyf M, Meaney MJ. Maternal care associated with methylation of the estrogen receptor-α1b promoter and estrogen receptor-α expression in the medial preoptic area of female offspring. Endocrinology 147: 2909–2915, 2006. [DOI] [PubMed] [Google Scholar]
- 48.Champagne F, Diorio J, Sharma S, Meaney MJ. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc Natl Acad Sci U S A 98: 12736–12741, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Charkoudian N, Stachenfeld NS. Reproductive hormone influences on thermoregulation in women [Online] Comprehensive Physiology. John Wiley & Sons, Inc. http://onlinelibrary.wiley.com/doi/10.1002/cphy.c130029/abstract [31 Jul. 2015]. [DOI] [PubMed] [Google Scholar]
- 50.Clarke WP, Maayani S. Estrogen effects on 5-HT1A receptors in hippocampal membranes from ovariectomized rats: Functional and binding studies. Brain Res 518: 287–291, 1990. [DOI] [PubMed] [Google Scholar]
- 51.Clark LA. Temperament as a unifying basis for personality and psychopathology. J Abnorm Psychol 114: 505–521, 2005. [DOI] [PubMed] [Google Scholar]
- 52.Clark LA, Watson D. Tripartite model of anxiety and depression: psychometric evidence and taxonomic implications. J Abnorm Psychol 100: 316–336, 1991. [DOI] [PubMed] [Google Scholar]
- 53.Cohen LS, Soares CN, Vitonis AF, Otto MW, Harlow BL. Risk for new onset of depression during the menopausal transition: The Harvard Study of Moods and Cycles. Arch Gen Psychiatry 63: 385, 2006. [DOI] [PubMed] [Google Scholar]
- 54.Coryell W, Akiskal HS, Leon AC, Winokur G, Maser JD, Mueller TI, Keller MB. The time course of nonchronic major depressive disorder: Uniformity across episodes and samples. Arch Gen Psychiatry 51: 405–410, 1994. [DOI] [PubMed] [Google Scholar]
- 55.Craig MC, Fletcher PC, Daly EM, Rymer J, Brammer M, Giampietro V, Murphy DGM. Physiological variation in estradiol and brain function: a functional magnetic resonance imaging study of verbal memory across the follicular phase of the menstrual cycle. Horm Behav 53: 503–508, 2008. [DOI] [PubMed] [Google Scholar]
- 56.Craig MC, Murphy DG. Estrogen: Effects on normal brain function and neuropsychiatric disorders. Climacteric 10(Suppl 2): 97–104, 2007. [DOI] [PubMed] [Google Scholar]
- 57.Cramer OM, Parker CR, Porter JC. Estrogen inhibition of dopamine release into hypophysial portal blood. Endocrinology 104: 419–422, 1979. [DOI] [PubMed] [Google Scholar]
- 58.Cunningham M, Gilkeson G. Estrogen receptors in immunity and autoimmunity. Clin Rev Allergy Immunol 40: 66–73, 2011. [DOI] [PubMed] [Google Scholar]
- 59.Dalgleish T, Williams JMG, Golden A-MJ, Perkins N, Barrett LF, Barnard PJ, Yeung CA, Murphy V, Elward R, Tchanturia K, Watkins E. Reduced specificity of autobiographical memory and depression. J Exp Psychol Gen 136: 23–42, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Damoiseaux JS, Rombouts SARB, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A 103: 13848–13853, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Davidson RJ. Anterior electrophysiological asymmetries, emotion, and depression: Conceptual and methodological conundrums. Psychophysiology 35: 607–614, 1998. [DOI] [PubMed] [Google Scholar]
- 62.Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. Depression: Perspectives from affective neuroscience. Annu Rev Psychol 53: 545–574, 2002. [DOI] [PubMed] [Google Scholar]
- 63.Debener S, Beauducel A, Nessler D, Brocke B, Heilemann H, Kayser J. Is resting anterior EEG alpha asymmetry a trait marker for depression? Findings for healthy adults and clinically depressed patients. Neuropsychobiology 41: 31–37, 2000. [DOI] [PubMed] [Google Scholar]
- 64.Deeks AA, Gibson-Helm ME, Teede HJ. Anxiety and depression in polycystic ovary syndrome: A comprehensive investigation. Fertil Steril 93: 2421–2423, 2010. [DOI] [PubMed] [Google Scholar]
- 65.Deligiannidis KM, Sikoglu EM, Shaffer SA, Frederick B, Svenson AE, Kopoyan A, Kosma CA, Rothschild AJ, Moore CM. GABAergic neuroactive steroids and resting-state functional connectivity in postpartum depression: A preliminary study. J Psychiatr Res 47: 816–828, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Deurveilher S, Rusak B, Semba K. Estradiol and progesterone modulate spontaneous sleep patterns and recovery from sleep deprivation in ovariectomized rats. Sleep 32: 865–877, 2009. [PMC free article] [PubMed] [Google Scholar]
- 67.Dichter GS, Tomarken AJ, Freid CM, Addington S, Shelton RC. Do venlafaxine XR and paroxetine equally influence negative and positive affect? J Affect Disord 85: 333–339, 2005. [DOI] [PubMed] [Google Scholar]
- 68.Dinan TG, Barry S, Yatham LN, Mobayed M, O’Hanlon M. The reproducibility of the prolactin response to buspirone: Relationship to the menstrual cycle. Int Clin Psychopharmacol 5: 119–134, 1990. [DOI] [PubMed] [Google Scholar]
- 69.Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: A systematic review and meta-analysis. JAMA 295: 1288–1299, 2006. [DOI] [PubMed] [Google Scholar]
- 70.Djebaili M, Guo Q, Pettus EH, Hoffman SW, Stein DG. The neurosteroids progesterone and allopregnanolone reduce cell death, gliosis, and functional deficits after traumatic brain injury in rats. J Neuro-trauma 22: 106–118, 2005. [DOI] [PubMed] [Google Scholar]
- 71.Donaldson C, Lam D. Rumination, mood and social problem-solving in major depression. Psychol Med 34: 1309–1318, 2004. [DOI] [PubMed] [Google Scholar]
- 72.Dotson VM, Resnick SM, Zonderman AB. Differential association of concurrent, baseline, and average depressive symptoms with cognitive decline in older adults. Am J Geriatr Psychiatry 16: 318–330, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctôt KL. A meta-analysis of cytokines in major depression. Biol Psychiatry 67: 446–457, 2010. [DOI] [PubMed] [Google Scholar]
- 74.Dreher JC, Schmidt PJ, Kohn P, Furman D, Rubinow D, Berman KF. Menstrual cycle phase modulates reward-related neural function in women. Proc Natl Acad Sci U S A 104: 2465–2470, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr 13: 663–681, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dubrovsky B. Neurosteroids, neuroactive steroids, and symptoms of affective disorders. Pharmacol Biochem Behav 84: 644–655, 2006. [DOI] [PubMed] [Google Scholar]
- 77.Dugard ML, Tremblay-Leveau H, Mellier D, Caston J. Prenatal exposure to ethinylestradiol elicits behavioral abnormalities in the rat. Dev Brain Res 129: 189–199, 2001. [DOI] [PubMed] [Google Scholar]
- 78.Dumas JA, Kutz AM, Naylor MR, Johnson JV, Newhouse PA. Increased memory load-related frontal activation after estradiol treatment in post-menopausal women. Horm Behav 58: 929–935, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dumas JA, Kutz AM, Naylor MR, Johnson JV, Newhouse PA. Estradiol treatment altered anticholinergic-related brain activation during working memory in postmenopausal women. NeuroImage 60: 1394–1403, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Edler C, Lipson SF, Keel PK. Ovarian hormones and binge eating in bulimia nervosa. Psychol Med 37: 131–141, 2007. [DOI] [PubMed] [Google Scholar]
- 81.Elliott R, Dolan RJ, Frith CD. Dissociable functions in the medial and lateral orbitofrontal cortex: Evidence from human neuroimaging studies. Cereb Cortex 10: 308–317, 2000. [DOI] [PubMed] [Google Scholar]
- 82.Endicott J, Halbreich U. Retrospective report of premenstrual depressive changes: Factors affecting confirmation by daily ratings. Psychopharmacol Bull 18: 109–112, 1982. [Google Scholar]
- 83.Engin E, Treit D. The anxiolytic-like effects of allopregnanolone vary as a function of intracerebral microinfusion site: The amygdala, medial prefrontal cortex, or hippocampus. Behav Pharmacol 18: 461–470, 2007. [DOI] [PubMed] [Google Scholar]
- 84.Epperson CN, Haga K, Mason GF, Sellers E, Gueorguieva R, Zhang W, Weiss E, Rothman DL, Krystal JH. Cortical gamma-aminobutyric acid levels across the menstrual cycle in healthy women and those with premenstrual dysphoric disorder: A proton magnetic resonance spectroscopy study. Arch Gen Psychiatry 59: 851–858, 2002. [DOI] [PubMed] [Google Scholar]
- 85.Epperson CN, Gueorguieva R, Czarkowski KA, Stiklus S, Sellers E, Krystal JH, Rothman DL, Mason GF. Preliminary evidence of reduced occipital GABA concentrations in puerperal women: A 1H-MRS study. Psychopharmacology (Berl) 186: 425–433, 2006. [DOI] [PubMed] [Google Scholar]
- 86.Epperson CN, Steiner M, Hartlage SA, Eriksson E, Schmidt PJ, Jones I, Yonkers KA. Premenstrual dysphoric disorder: Evidence for a new category for DSM-5. Am J Psychiatry 169: 465–475, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Eser D, Schüle C, Baghai TC, Romeo E, Rupprecht R. Neuroactive steroids in depression and anxiety disorders: Clinical studies. Neuroendocrinology 84: 244–254, 2006. [DOI] [PubMed] [Google Scholar]
- 88.Eshel N, Roiser JP. Reward and punishment processing in depression. Biol Psychiatry 68: 118–124, 2010. [DOI] [PubMed] [Google Scholar]
- 89.Etgen AM, Ansonoff MA, Quesada A. Mechanisms of ovarian steroid regulation of norepinephrine receptor-mediated signal transduction in the hypothalamus: Implications for female reproductive physiology. Horm Behav 40: 169–177, 2001. [DOI] [PubMed] [Google Scholar]
- 90.Fassnacht M, Schlenz N, Schneider SB, Wudy SA, Allolio B, Arlt W. Beyond adrenal and ovarian androgen generation: Increased peripheral 5α-reductase activity in women with polycystic ovary syndrome. J Clin Endocrinol Metab 88: 2760–2766, 2003. [DOI] [PubMed] [Google Scholar]
- 91.Fink G, Sumner BEH. Oestrogen and mental state. Nature 383: 306–306, 1996. [DOI] [PubMed] [Google Scholar]
- 92.Fitzgerald PB, Oxley TJ, Laird AR, Kulkarni J, Egan GF, Daskalakis ZJ. An analysis of functional neuroimaging studies of dorsolateral pre-frontal cortical activity in depression. Psychiatry Res 148: 33–45, 2006. [DOI] [PubMed] [Google Scholar]
- 93.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8: 700–711, 2007. [DOI] [PubMed] [Google Scholar]
- 94.Franz WB. Basic review: Endocrinology of the normal menstrual cycle. Prim Care 15: 607–616, 1988. [PubMed] [Google Scholar]
- 95.Freeman E, Rickels K, Sondheimer S, Polansky M. Ineffectiveness of progesterone suppository treatment for premenstrual syndrome. JAMA 264: 349–353, 1990. [PubMed] [Google Scholar]
- 96.Freeman E, Rickels K, Sondheimer S, Polansky M. A double-blind trial of oral progesterone, alprazolam, and placebo in treatment of severe premenstrual syndrome. JAMA 274: 51–57, 1995. [PubMed] [Google Scholar]
- 97.Freeman EW, Frye CA, Rickels K, Martin PAG, Smith SS. Allopregnanolone levels and symptom improvement in severe premenstrual syndrome. J Clin Psychopharmacol 22: 516–520, 2002. [DOI] [PubMed] [Google Scholar]
- 98.Freeman EW, Sammel MD, Lin H, Nelson DB. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch Gen Psychiatry 63: 375–382, 2006. [DOI] [PubMed] [Google Scholar]
- 99.Fröhlich F. Endogenous and exogenous electric fields as modifiers of brain activity: Rational design of noninvasive brain stimulation with transcranial alternating current stimulation. Dialogues Clin Neurosci 16: 93–102, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Frye CA, Walf AA, Rhodes ME, Harney JP. Progesterone enhances motor, anxiolytic, analgesic, and antidepressive behavior of wild-type mice, but not those deficient in type 1 5 alpha-reductase. Brain Res 1004: 116–124, 2004. [DOI] [PubMed] [Google Scholar]
- 101.Furuta M, Numakawa T, Chiba S, Ninomiya M, Kajiyama Y, Adachi N, Akema T, Kunugi H. Estrogen, predominantly via estrogen receptor α, attenuates postpartum-induced anxiety- and depression-like behaviors in female rats. Endocrinology 154: 3807–3816, 2013. [DOI] [PubMed] [Google Scholar]
- 102.Gardner A, Boles RG. Beyond the serotonin hypothesis: Mitochondria, inflammation and neurodegeneration in major depression and affective spectrum disorders. Prog Neuropsychopharmacol Biol Psychiatry 35: 730–743, 2011. [DOI] [PubMed] [Google Scholar]
- 103.Gardner A, Johansson A, Wibom R, Nennesmo I, von Döbeln U, Hagen-feldt L, Hällström T. Alterations of mitochondrial function and correlations with personality traits in selected major depressive disorder patients. J Affect Disord 76: 55–68, 2003. [DOI] [PubMed] [Google Scholar]
- 104.Gavin KM, Gibbons E, Stavros A, Nakamura T, Villalon KL, Kohrt WM. Ovarian hormone suppression with estradiol add-back therapy in premenopausal women reduces dynamic HPA axis activity. Endocrine Reviews 34S: SUN-55, 2013. [Google Scholar]
- 105.Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression: A systematic review of prevalence and incidence. Obstet Gynecol 106: 1071–1083, 2005. [DOI] [PubMed] [Google Scholar]
- 106.Geary N, Trace D, McEwen B, Smith GP. Cyclic estradiol replacement increases the satiety effect of CCK-8 in ovariectomized rats. Physiol Behav 56: 281–289, 1994. [DOI] [PubMed] [Google Scholar]
- 107.Genazzani AR, Petraglia F, Bernardi F, Casarosa E, Salvestroni C, Tonetti A, Nappi RE, Luisi S, Palumbo M, Purdy RH, Luisi M. Circulating levels of allopregnanolone in humans: Gender, age, and endocrine influences. J Clin Endocrinol Metab 83: 2099–2103, 1998. [DOI] [PubMed] [Google Scholar]
- 108.Gillies GE, McArthur S. Estrogen actions in the brain and the basis for differential action in men and women: A case for sex-specific medicines. Pharmacol Rev 62: 155–198, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gingnell M, Morell A, Bannbers E, Wikström J, Sundström Poromaa I. Menstrual cycle effects on amygdala reactivity to emotional stimulation in premenstrual dysphoric disorder. Horm Behav 62: 400–406, 2012. [DOI] [PubMed] [Google Scholar]
- 110.Girdler SS, Straneva PA, Light KC, Pedersen CA, Morrow AL. Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biol Psychiatry 49: 788–797, 2001. [DOI] [PubMed] [Google Scholar]
- 111.Goldstein JM, Jerram M, Poldrack R, Ahern T, Kennedy DN, Seidman LJ, Makris N. Hormonal cycle modulates arousal circuitry in women using functional magnetic resonance imaging. J Neurosci Off J Soc Neurosci 25: 9309–9316, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Goodyer IM, Herbert J, Altham PM, Pearson J, Secher SM, Shiers HM. Adrenal secretion during major depression in 8- to 16-year-olds, I. Altered diurnal rhythms in salivary cortisol and dehydroepiandrosterone (DHEA) at presentation. Psychol Med 26: 245–256, 1996. [DOI] [PubMed] [Google Scholar]
- 113.Gotlib IH. EEG alpha asymmetry, depression, and cognitive functioning. Cogn Emot 12: 449–478, 1998. [Google Scholar]
- 114.Gotlib IH, Krasnoperova E, Yue DN, Joormann J. Attentional biases for negative interpersonal stimuli in clinical depression. J Abnorm Psychol 113: 127–135, 2004. [DOI] [PubMed] [Google Scholar]
- 115.Gotlib IH, Whiffen VE, Mount JH, Milne K, Cordy NI. Prevalence rates and demographic characteristics associated with depression in pregnancy and the postpartum. J Consult Clin Psychol 57: 269–274, 1989. [DOI] [PubMed] [Google Scholar]
- 116.Greendale GA, Huang M-H, Wight RG, Seeman T, Luetters C, Avis NE, Johnston J, Karlamangla AS. Effects of the menopause transition and hormone use on cognitive performance in midlife women. Neurology 72: 1850–1857, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Green PS, Gridley KE, Simpkins JW. Nuclear estrogen receptor-independent neuroprotection by estratrienes: A novel interaction with glutathione. Neuroscience 84: 7–10, 1998. [DOI] [PubMed] [Google Scholar]
- 118.Gregoire AJ, Kumar R, Everitt B, Henderson AF, Studd JW. Transdermal oestrogen for treatment of severe postnatal depression. Lancet 347: 930–933, 1996. [DOI] [PubMed] [Google Scholar]
- 119.Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF. Resting-state functional connectivity in major depression: Abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry 62: 429–437, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Grieve SM, Korgaonkar MS, Koslow SH, Gordon E, Williams LM. Widespread reductions in gray matter volume in depression. NeuroImage Clin 3: 332–339, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Grimm S, Beck J, Schuepbach D, Hell D, Boesiger P, Bermpohl F, Niehaus L, Boeker H, Northoff G. Imbalance between left and right dorsolateral prefrontal cortex in major depression is linked to negative emotional judgment: An fMRI study in severe major depressive disorder. Biol Psychiatry 63: 369–376, 2008. [DOI] [PubMed] [Google Scholar]
- 122.Grimm S, Boesiger P, Beck J, Schuepbach D, Bermpohl F, Walter M, Ernst J, Hell D, Boeker H, Northoff G. Altered negative BOLD responses in the default-mode network during emotion processing in depressed subjects. Neuropsychopharmacology 34: 932–943, 2009. [DOI] [PubMed] [Google Scholar]
- 123.Grodstein F, Lifford K, Resnick NM, Curhan GC. Postmenopausal hormone therapy and risk of developing urinary incontinence. Obstet Gynecol 103: 254–260, 2004. [DOI] [PubMed] [Google Scholar]
- 124.Grodstein F, Manson JE, Stampfer MJ. Hormone therapy and coronary heart disease: The role of time since menopause and age at hormone initiation. J Womens Health 15: 35–44, 2006. [DOI] [PubMed] [Google Scholar]
- 125.Halbreich U, Endicott J, Lesser J. The clinical diagnosis and classification of premenstrual changes. Can J Psychiatry Rev Can Psychiatr 30: 489–497, 1985. [DOI] [PubMed] [Google Scholar]
- 126.Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH. Functional neuroimaging of major depressive disorder: A meta-analysis and new integration of base line activation and neural response data. Am J Psychiatry 169: 693–703, 2012. [DOI] [PubMed] [Google Scholar]
- 127.Hampson E. Variations in sex-related cognitive abilities across the menstrual cycle. Brain Cogn 14: 26–43, 1990. [DOI] [PubMed] [Google Scholar]
- 128.Hampson E, Kimura D. Reciprocal effects of hormonal fluctuations on human motor and perceptual-spatial skills. Behav Neurosci 102: 456–459, 1988. [DOI] [PubMed] [Google Scholar]
- 129.Han SJ, DeMayo FJ, Xu J, Tsai SY, Tsai M-J, O’Malley BW. Steroid receptor coactivator (SRC)-1 and SRC-3 differentially modulate tissue-specific activation functions of the progesterone receptor. Mol Endocrinol 20: 45–55, 2006. [DOI] [PubMed] [Google Scholar]
- 130.Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, Sherman S, Sluss PM, de Villiers TJ. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. Fertil Steril 97: 843–851, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hausmann M, Hamm JP, Waldie KE, Kirk IJ. Sex hormonal modulation of interhemispheric transfer time. Neuropsychologia 51: 1734–1741, 2013. [DOI] [PubMed] [Google Scholar]
- 132.He J, Evans C-O, Hoffman SW, Oyesiku NM, Stein DG. Progesterone and allopregnanolone reduce inflammatory cytokines after traumatic brain injury. Exp Neurol 189: 404–412, 2004. [DOI] [PubMed] [Google Scholar]
- 133.Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Ström A, Treuter E, Warner M, Gustafsson J-Å. Estrogen receptors: How do they signal and what are their targets. Physiol Rev 87: 905–931, 2007. [DOI] [PubMed] [Google Scholar]
- 134.Henriques JB, Davidson RJ. Regional brain electrical asymmetries discriminate between previously depressed and healthy control subjects. J Abnorm Psychol 99: 22–31, 1990. [DOI] [PubMed] [Google Scholar]
- 135.Henriques JB, Davidson RJ. Left frontal hypoactivation in depression. J Abnorm Psychol 100: 535–545, 1991. [DOI] [PubMed] [Google Scholar]
- 136.Herman JP, Cullinan WE. Neurocircuitry of stress: Central control of the hypothalamo–pituitary–adrenocortical axis. Trends Neurosci 20: 78–84, 1997. [DOI] [PubMed] [Google Scholar]
- 137.Heuser I, Deuschle M, Luppa P, Schweiger U, Standhardt H, Weber B. Increased diurnal plasma concentrations of dehydroepiandrosterone in depressed patients. J Clin Endocrinol Metab 83: 3130–3133, 1998. [DOI] [PubMed] [Google Scholar]
- 138.Holländer A, Hausmann M, Hamm JP, Corballis MC. Sex hormonal modulation of hemispheric asymmetries in the attentional blink. J Int Neuropsychol Soc 11: 263–272, 2005. [DOI] [PubMed] [Google Scholar]
- 139.Howe ML, Malone C. Mood-congruent true and false memory: Effects of depression. Memory 19: 192–201, 2011. [DOI] [PubMed] [Google Scholar]
- 140.Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL, Stuber GD. Distinct extended amygdala circuits for divergent motivational states. Nature 496: 224–228, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jollant F, Bellivier F, Leboyer M, Astruc B, Torres S, Verdier R, Castelnau D, Malafosse A, Courtet P. Impaired decision making in suicide attempters. Am J Psychiatry 162: 304–310, 2005. [DOI] [PubMed] [Google Scholar]
- 142.Joormann J, Gotlib IH. Selective attention to emotional faces following recovery from depression. J Abnorm Psychol 116: 80–85, 2007. [DOI] [PubMed] [Google Scholar]
- 143.Joormann J, Gotlib IH. Updating the contents of working memory in depression: Interference from irrelevant negative material. J Abnorm Psychol 117: 182–192, 2008. [DOI] [PubMed] [Google Scholar]
- 144.Kaiser S, Roth A, Rentrop M, Friederich H-C, Bender S, Weisbrod M. Intra-individual reaction time variability in schizophrenia, depression and borderline personality disorder. Brain Cogn 66: 73–82, 2008. [DOI] [PubMed] [Google Scholar]
- 145.Karkanias GB, Etgen AM. Estradiol attenuates α2-adrenoceptor-mediated inhibition of hypothalamic norepinephrine release. J Neurosci 13: 3448–3455, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Karkanias GB, Li C-S, Etgen AM. Estradiol reduction of α2-adrenoceptor binding in female rat cortex is correlated with decreases in α2A/D-adrenoceptor messenger RNA. Neuroscience 81: 593–597, 1997. [DOI] [PubMed] [Google Scholar]
- 147.Kehoe P, Mallinson K, McCormick CM, Frye CA. Central allopregnanolone is increased in rat pups in response to repeated, short episodes of neonatal isolation. Dev Brain Res 124: 133–136, 2000. [DOI] [PubMed] [Google Scholar]
- 148.Kendall D, Stancel G, Enna S. Imipramine: Effect of ovarian steroids on modifications in serotonin receptor binding. Science 211: 1183–1185, 1981. [DOI] [PubMed] [Google Scholar]
- 149.Kenna HA, Rasgon NL, Geist C, Small G, Silverman D. Thalamo-basal ganglia connectivity in postmenopausal women receiving estrogen therapy. Neurochem Res 34: 234–237, 2009. [DOI] [PubMed] [Google Scholar]
- 150.Kiss Á, Delattre AM, Pereira SIR, Carolino RG, Szawka RE, Anselmo-Franci JA, Zanata SM, Ferraz AC. 17β-Estradiol replacement in young, adult and middle-aged female ovariectomized rats promotes improvement of spatial reference memory and an antidepressant effect and alters monoamines and BDNF levels in memory- and depression-related brain areas. Behav Brain Res 227: 100–108, 2012. [DOI] [PubMed] [Google Scholar]
- 151.Klasen M, Kreifelts B, Chen Y-H, Seubert J, Mathiak K. Neural processing of emotion in multimodal settings. Front Hum Neurosci 8: 822, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Klatzkin RR, Leslie Morrow A, Light KC, Pedersen CA, Girdler SS. Associations of histories of depression and PMDD diagnosis with allopregnanolone concentrations following the oral administration of micronized progesterone. Psychoneuroendocrinology 31: 1208–1219, 2006. [DOI] [PubMed] [Google Scholar]
- 153.Klimesch W. α-band oscillations, attention, and controlled access to stored information. Trends Cogn Sci 16: 606–617, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Klump KL, Keel PK, Culbert KM, Edler C. Ovarian hormones and binge eating: Exploring associations in community samples. Psychol Med 38: 1749–1757, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Koenigs M, Grafman J. The functional neuroanatomy of depression: Distinct roles for ventromedial and dorsolateral prefrontal cortex. Behav Brain Res 201: 239–243, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Korgaonkar MS, Fornito A, Williams LM, Grieve SM. Abnormal structural networks characterize major depressive disorder: A connectome analysis. Biol Psychiatry 76: 567–574, 2014. [DOI] [PubMed] [Google Scholar]
- 157.Korol DL. Role of estrogen in balancing contributions from multiple memory systems. Neurobiol Learn Mem 82: 309–323, 2004. [DOI] [PubMed] [Google Scholar]
- 158.Koster EHW, De Lissnyder E, Derakshan N, De Raedt R. Understanding depressive rumination from a cognitive science perspective: The impaired disengagement hypothesis. Clin Psychol Rev 31: 138–145, 2011. [DOI] [PubMed] [Google Scholar]
- 159.Koster EHW, De Raedt R, Goeleven E, Franck E, Crombez G. Mood-congruent attentional bias in dysphoria: Maintained attention to and impaired disengagement from negative information. Emot Wash DC 5: 446–455, 2005. [DOI] [PubMed] [Google Scholar]
- 160.Kow L-M, Pfaff DW. Mapping of neural and signal transduction pathways for lordosis in the search for estrogen actions on the central nervous system. Behav Brain Res 92: 169–180, 1998. [DOI] [PubMed] [Google Scholar]
- 161.Kravitz HM, Zhao X, Bromberger JT, Gold EB, Hall MH, Matthews KA, Sowers MFR. Sleep disturbance during the menopausal transition in a multi-ethnic community sample of women. Sleep 31: 979–990, 2008. [PMC free article] [PubMed] [Google Scholar]
- 162.Krystal JH, Sanacora G, Blumberg H, Anand A, Charney DS, Marek G, Epperson CN, Goddard A, Mason GF. Glutamate and GABA systems as targets for novel antidepressant and mood-stabilizing treatments. Mol Psychiatry 7: S71–S80, 2002. [DOI] [PubMed] [Google Scholar]
- 163.Kulkarni J, de Castella A, Fitzgerald PB, Gurvich CT, Bailey M, Bartholomeusz C, Burger H. Estrogen in severe mental illness: A potential new treatment approach. Arch Gen Psychiatry 65: 955–960, 2008. [DOI] [PubMed] [Google Scholar]
- 164.Kulkarni J, Gurvich C, Lee SJ, Gilbert H, Gavrilidis E, de Castella A, Berk M, Dodd S, Fitzgerald PB, Davis SR. Piloting the effective therapeutic dose of adjunctive selective estrogen receptor modulator treatment in postmenopausal women with schizophrenia. Psychoneuroendocrinology 35: 1142–1147, 2010. [DOI] [PubMed] [Google Scholar]
- 165.De Kwaasteniet B, Ruhe E, Caan M, Rive M, Olabarriaga S, Groefsema M, Heesink L, van Wingen G, Denys D. Relation between structural and functional connectivity in major depressive disorder. Biol Psychiatry 74: 40–47, 2013. [DOI] [PubMed] [Google Scholar]
- 166.Larson EB, Roth ME, Anker JJ, Carroll ME. Effect of short- vs. long-term estrogen on reinstatement of cocaine-seeking behavior in female rats. Pharmacol Biochem Behav 82: 98–108, 2005. [DOI] [PubMed] [Google Scholar]
- 167.Law Smith MJ, Deady DK, Moore FR, Jones BC, Cornwell RE, Stirrat M, Lawson JF, Feinberg DR, Perrett DI. Maternal tendencies in women are associated with estrogen levels and facial femininity. Horm Behav 61: 12–16, 2012. [DOI] [PubMed] [Google Scholar]
- 168.Lawrie TA, Hofmeyr GJ, De Jager M, Berk M, Paiker J, Viljoen E. A double-blind randomised placebo controlled trial of postnatal norethisterone enanthate: The effect on postnatal depression and serum hormones. Br J Obstet Gynaecol 105: 1082–1090, 1998. [DOI] [PubMed] [Google Scholar]
- 169.Lemogne C, le Bastard G, Mayberg H, Volle E, Bergouignan L, Lehéricy S, Allilaire J-F, Fossati P. In search of the depressive self: Extended medial prefrontal network during self-referential processing in major depression. Soc Cogn Affect Neurosci 4: 305–312, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Leonard BE. The immune system, depression and the action of antidepressants. Prog Neuropsychopharmacol Biol Psychiatry 25: 767–780, 2001. [DOI] [PubMed] [Google Scholar]
- 171.Leuchter AF, Cook IA, Jin Y, Phillips B. The relationship between brain oscillatory activity and therapeutic effectiveness of transcranial magnetic stimulation in the treatment of major depressive disorder. Front Hum Neurosci 7: 37, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lin I-M, Tsai Y-C, Peper E, Yen C-F. Depressive mood and frontal alpha asymmetry during the luteal phase in premenstrual dysphoric disorder. J Obstet Gynaecol Res 39: 998–1006, 2013. [DOI] [PubMed] [Google Scholar]
- 173.Lipovka Y, Konhilas JP. Estradiol activates AMPK through interaction with extrogen receptor beta (15.4). FASEB J 28: 15.4, 2014. [Google Scholar]
- 174.Liston C, Chen AC, Zebley BD, Drysdale AT, Gordon R, Leuchter B, Voss HU, Casey BJ, Etkin A, Dubin MJ. Default mode network mechanisms of transcranial magnetic stimulation in depression. Biol Psychiatry 76: 517–526, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Liu S, Zhang N, Guo Y, Zhao R, Shi T, Feng S, Wang S, Yang Q, Li X, Wu Y, Ma L, Hou Y, Xiong L, Zhang W, Zhao M. G-protein-coupled receptor 30 mediates rapid neuroprotective effects of Estrogen via depression of NR2B-containing NMDA receptors. J Neurosci 32: 4887–4900, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lonard DM, O’Malley BW. Nuclear receptor coregulators: Judges, juries, and executioners of cellular regulation. Mol Cell 27: 691–700, 2007. [DOI] [PubMed] [Google Scholar]
- 177.Lonard DM, O’Malley BW. Nuclear receptor coregulators: Modulators of pathology and therapeutic targets. Nat Rev Endocrinol 8: 598–604, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lu J, Xu Y, Sheng H, Tang Z, Ni X. 17β-Estradiol replacement to ovariectomized rats ameliorates depression-like behavior (876.2). FASEB J 28: 876.2, 2014. [Google Scholar]
- 179.Maguire J, Ferando I, Simonsen C, Mody I. Excitability changes related to GABAA receptor plasticity during pregnancy. J Neurosci 29: 9592–9601, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABA(A) receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci 8: 797–804, 2005. [DOI] [PubMed] [Google Scholar]
- 181.Maguire J, Mody I. GABAAR plasticity during pregnancy: Relevance to postpartum depression. Neuron 59: 207–213, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science 232: 1004–1007, 1986. [DOI] [PubMed] [Google Scholar]
- 183.Manji HK, Quiroz JA, Sporn J, Payne JL, Denicoff K, A Gray N, Zarate CA Jr., Charney DS. Enhancing neuronal plasticity and cellular resilience to develop novel, improved therapeutics for difficult-to-treat depression. Biol Psychiatry 53: 707–742, 2003. [DOI] [PubMed] [Google Scholar]
- 184.Marcondes FK, Miguel KJ, Melo LL, Spadari-Bratfisch RC. Estrous cycle influences the response of female rats in the elevated plus-maze test. Physiol Behav 74: 435–440, 2001. [DOI] [PubMed] [Google Scholar]
- 185.Mars RB, Neubert F-X, Noonan MP, Sallet J, Toni I, Rushworth MFS. On the relationship between the “default mode network” and the “social brain”. Front Hum Neurosci 6: 189, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Mayberg HS, Brannan SK, Tekell JL, Silva JA, Mahurin RK, McGinnis S, Jerabek PA. Regional metabolic effects of fluoxetine in major depression: Serial changes and relationship to clinical response. Biol Psychiatry 48: 830–843, 2000. [DOI] [PubMed] [Google Scholar]
- 187.Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron 45: 651–660, 2005. [DOI] [PubMed] [Google Scholar]
- 188.McCARTHY MM. Estradiol and the developing brain. Physiol Rev 88: 91–134, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.McCarthy MM, Arnold AP. Reframing sexual differentiation of the brain. Nat Neurosci 14: 677–683, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.McClure RES, Barha CK, Galea LAM. 17β-Estradiol, but not estrone, increases the survival and activation of new neurons in the hippocampus in response to spatial memory in adult female rats. Horm Behav 63: 144–157, 2013. [DOI] [PubMed] [Google Scholar]
- 191.McDermott LM, Ebmeier KP. A meta-analysis of depression severity and cognitive function. J Affect Disord 119: 1–8, 2009. [DOI] [PubMed] [Google Scholar]
- 192.McEwen BS. Invited Review: Estrogens effects on the brain: Multiple sites and molecular mechanisms. J Appl Physiol 91: 2785–2801, 2001. [DOI] [PubMed] [Google Scholar]
- 193.McEwen BS, Akama KT, Spencer-Segal JL, Milner TA, Waters EM. Estrogen effects on the brain: Actions beyond the hypothalamus via novel mechanisms. Behav Neurosci 126: 4–16, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.McQueen JK, Wilson H, Dow RC. Oestradiol-17β increases serotonin transporter (SERT) binding sites and SERT mRNA expression in discrete regions of female rat brain. J Physiol 495: 114, 1996. [Google Scholar]
- 195.Mead LA, Hampson E. A sex difference in turning bias in humans. Behav Brain Res 78: 73–79, 1996. [DOI] [PubMed] [Google Scholar]
- 196.Micevych P, Dominguez R. Membrane estradiol signaling in the brain. Front Neuroendocrinol 30: 315–327, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Michael A, Jenaway A, Paykel ES, Herbert J. Altered salivary dehydroepiandrosterone levels in major depression in adults. Biol Psychiatry 48: 989–995, 2000. [DOI] [PubMed] [Google Scholar]
- 198.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24: 167–202, 2001. [DOI] [PubMed] [Google Scholar]
- 199.Moffitt TE, Harrington H, Caspi A, Kim-Cohen J, Goldberg D, Gregory AM, Poulton R. Depression and generalized anxiety disorder: Cumulative and sequential comorbidity in a birth cohort followed prospectively to age 32 years. Arch Gen Psychiatry 64: 651–660, 2007. [DOI] [PubMed] [Google Scholar]
- 200.Mogenson GJ, Stevenson JAF. Drinking induced by electrical stimulation of the lateral hypothalamus. Exp Neurol 17: 119–127, 1967. [DOI] [PubMed] [Google Scholar]
- 201.Monteleone P, Luisi S, Tonetti A, Bernardi F, Genazzani AD, Luisi M, Petraglia F, Genazzani AR. Allopregnanolone concentrations and premenstrual syndrome. Eur J Endocrinol Eur Fed Endocr Soc 142: 269–273, 2000. [DOI] [PubMed] [Google Scholar]
- 202.Mook DG, Kenney NJ, Roberts S, Nussbaum AI, Rodier WII. Ovarian-adrenal interactions in regulation of body weight by female rats. J Comp Physiol Psychol 81: 198–211, 1972. [DOI] [PubMed] [Google Scholar]
- 203.Mooradian AD. Antioxidant properties of steroids. J Steroid Biochem Mol Biol 45: 509–511, 1993. [DOI] [PubMed] [Google Scholar]
- 204.Moreau JL, Bourson A, Jenck F, Martin JR, Mortas P. Curative effects of the atypical antidepressant mianserin in the chronic mild stress-induced anhedonia model of depression. J Psychiatry Neurosci 19: 51–56, 1994. [PMC free article] [PubMed] [Google Scholar]
- 205.Moreau JL, Jenck F, Martin JR, Mortas P, Haefely WE. Antidepressant treatment prevents chronic unpredictable mild stress-induced anhedonia as assessed by ventral tegmentum self-stimulation behavior in rats. Eur Neuropsychopharmacol 2: 43–49, 1992. [DOI] [PubMed] [Google Scholar]
- 206.Morissette M, Di Paolo T. Sex and estrous cycle variations of rat striatal dopamine uptake sites. Neuroendocrinology 58: 16–22, 1993. [DOI] [PubMed] [Google Scholar]
- 207.Moritz S, Gläscher J, Brassen S. Investigation of mood-congruent false and true memory recognition in depression. Depress Anxiety 21: 9–17, 2005. [DOI] [PubMed] [Google Scholar]
- 208.Moscovitch DA, Santesso DL, Miskovic V, McCabe RE, Antony MM, Schmidt LA. Frontal EEG asymmetry and symptom response to cognitive behavioral therapy in patients with social anxiety disorder. Biol Psychol 87: 379–385, 2011. [DOI] [PubMed] [Google Scholar]
- 209.Moses EL, Drevets WC, Smith G, Mathis CA, Kalro BN, Butters MA, Leondires MP, Greer PJ, Lopresti B, Loucks TL, Berga SL. Effects of estradiol and progesterone administration on human serotonin 2A receptor binding: A PET study. Biol Psychiatry 48: 854–860, 2000. [DOI] [PubMed] [Google Scholar]
- 210.Moses-Kolko EL, Fraser D, Wisner KL, James JA, Saul AT, Fiez JA, Phillips ML. Rapid habituation of ventral striatal response to reward receipt in postpartum depression. Biol Psychiatry 70: 395–399, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Moses-Kolko EL, Perlman SB, Wisner KL, James J, Saul AT, Phillips ML. Abnormally reduced dorsomedial prefrontal cortical activity and effective connectivity with amygdala in response to negative emotional faces in postpartum depression. Am J Psychiatry 167: 1373–1380, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Napoli A, Powers JB, Valenstein ES. Hormonal induction of behavioral estrus modified by electrical stimulation of hypothalamus. Physiol Behav 9: 115–117, 1972. [DOI] [PubMed] [Google Scholar]
- 213.Nelson BD, McGowan SK, Sarapas C, Robison-Andrew EJ, Altman SE, Campbell ML, Gorka SM, Katz AC, Shankman SA. Biomarkers of threat and reward sensitivity demonstrate unique associations with risk for psychopathology. J Abnorm Psychol 122: 662–671, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Nelson BP, Henriet RP, Holt AW, Bopp KC, Houser AP, Allgood OE, Turner JE. The role of estrogen in the developmental appearance of sensory-motor behaviors in the zebrafish (Danio rerio): The characterization of the “listless” model. Brain Res 1222: 118–128, 2008. [DOI] [PubMed] [Google Scholar]
- 215.Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron 34: 13–25, 2002. [DOI] [PubMed] [Google Scholar]
- 216.Nolen-Hoeksema S. The role of rumination in depressive disorders and mixed anxiety/depressive symptoms. J Abnorm Psychol 109: 504–511, 2000. [PubMed] [Google Scholar]
- 217.Notelovitz M, Lenihan JP Jr, McDermott M, Kerber IJ, Nanavati N, Arce J-C. Initial 17β-estradiol dose for treating vasomotor symptoms1. Obstet Gynecol 95: 726–731, 2000. [DOI] [PubMed] [Google Scholar]
- 218.Ogawa S, Chan J, Gustafsson J-Å, Korach KS, Pfaff DW. Estrogen increases locomotor activity in mice through estrogen receptor α: Specificity for the type of activity. Endocrinology 144: 230–239, 2003. [DOI] [PubMed] [Google Scholar]
- 219.Ogawa S, Lee TM, Kay AR, Tank DW. Brain Magnetic Resonance Imaging with Contrast Dependent on Blood Oxygenation. Proceedings of the National Academy of Sciences 87(24): 9868–9872, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.O’Hara MW. Postpartum Depression: Causes and Consequences. New York: Springer-Verlag, 1995. [Google Scholar]
- 221.O’Hara MW, Schlechte JA, Lewis DA, Varner MW. Controlled prospective study of postpartum mood disorders: Psychological, environmental, and hormonal variables. J Abnorm Psychol 100: 63–73, 1991. [DOI] [PubMed] [Google Scholar]
- 222.O’Hara MW, Swain AM. Rates and risk of postpartum depression—A meta-analysis. Int Rev Psychiatry 8: 37–54, 1996. [Google Scholar]
- 223.O’Malley BW. The “fourth dimension” of gene transcription. Mol Endocrinol 23: 587–589, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ottowitz WE, Derro D, Dougherty DD, Lindquist MA, Fischman AJ, Hall JE. FDG-PET analysis of amygdalar-cortical network covariance during pre- versus post-menopausal estrogen levels: Potential relevance to resting state networks, mood, and cognition. Neuro Endocrinol Lett 29: 467–474, 2008. [PMC free article] [PubMed] [Google Scholar]
- 225.Ottowitz WE, Siedlecki KL, Lindquist MA, Dougherty DD, Fischman AJ, Hall JE. Evaluation of prefrontal-hippocampal effective connectivity following 24 hours of estrogen infusion: an FDG-PET study. Psychoneuroendocrinology 33: 1419–1425, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Paelecke-Habermann Y, Pohl J, Leplow B. Attention and executive functions in remitted major depression patients. J Affect Disord 89: 125–135, 2005. [DOI] [PubMed] [Google Scholar]
- 227.Di Paolo T, Poyet P, Labrie F. Prolactin and estradiol increase striatal dopamine receptor density in intact, castrated and hypophysectomized rats. Prog Neuropsychopharmacol Biol Psychiatry 6: 377–382, 1982. [DOI] [PubMed] [Google Scholar]
- 228.Papousek I, Weiss EM, Schulter G, Fink A, Reiser EM, Lackner HK. Prefrontal EEG alpha asymmetry changes while observing disaster happening to other people: Cardiac correlates and prediction of emotional impact. Biol Psychol 103: 189–194, 2014. [DOI] [PubMed] [Google Scholar]
- 229.Park H-J, Friston K. Structural and functional brain networks: From connections to cognition. Science 342: 1238411, 2013. [DOI] [PubMed] [Google Scholar]
- 230.Pasqualini C, Leviel V, Guibert B, Faucon-Biguet N, Kerdelhué B. Inhibitory actions of acute estradiol treatment on the activity and quantity of tyrosine hydroxylase in the median eminence of ovariectomized rats. J Neuroendocrinol 3: 575–580, 1991. [DOI] [PubMed] [Google Scholar]
- 231.Patchev VK, Hassan AHS, Holsboer F, Almeida OFX. The neurosteroid tetrahydroprogesterone attenuates the endocrine response to stress and exerts glucocorticoid-like effects on vasopressin gene transcription in the rat hypothalamus. Neuropsychopharmacology 15: 533–540, 1996. [DOI] [PubMed] [Google Scholar]
- 232.Patchev VK, Shoaib M, Holsboer F, Almeida OFX. The neurosteroid tetrahydroprogesterone counteracts corticotropin-releasing hormone-induced anxiety and alters the release and gene expression of corticotropin-releasing hormone in the rat hypothalamus. Neuroscience 62: 265–271, 1994. [DOI] [PubMed] [Google Scholar]
- 233.Pearlstein T, Yonkers KA, Fayyad R, Gillespie JA. Pretreatment pattern of symptom expression in premenstrual dysphoric disorder. J Affect Disord 85: 275–282, 2005. [DOI] [PubMed] [Google Scholar]
- 234.Pecins-Thompson M, Brown NA, Bethea CL. Regulation of serotonin re-uptake transporter mRNA expression by ovarian steroids in rhesus macaques. Mol Brain Res 53: 120–129, 1998. [DOI] [PubMed] [Google Scholar]
- 235.Petitti N, Etgen AM. Alpha 1-adrenoceptor augmentation of beta-stimulated cAMP formation is enhanced by estrogen and reduced by progesterone in rat hypothalamic slices. J Neurosci 10: 2842–2849, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Pfaff DW. Luteinizing hormone-releasing factor potentiates lordosis behavior in hypophysectomized ovariectomized female rats. Science 182: 1148–1149, 1973. [DOI] [PubMed] [Google Scholar]
- 237.Pfaff DW, Sakuma Y. Facilitation of the lordosis reflex of female rats from the ventromedial nucleus of the hypothalamus. J Physiol 288: 189–202, 1979. [PMC free article] [PubMed] [Google Scholar]
- 238.Piferrer F, Baker IJ, Donaldson EM. Effects of natural, synthetic, aromatizable, and nonaromatizable androgens in inducing male sex differentiation in genotypic female Chinook Salmon (Oncorhynchus tshawytscha). Gen Comp Endocrinol 91: 59–65, 1993. [DOI] [PubMed] [Google Scholar]
- 239.Pletzer BA, Kerschbaum HH. 50 years of hormonal contraception—time to find out, what it does to our brain. Front Neurosci 8: 256, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Polo-Kantola P, Erkkola R, Helenius H, Irjala K, Polo O. When does estrogen replacement therapy improve sleep quality? Am J Obstet Gynecol 178: 1002–1009, 1998. [DOI] [PubMed] [Google Scholar]
- 241.Power RF, Mani SK, Codina J, Conneely OM, O’Malley BW. Dopaminergic and ligand-independent activation of steroid hormone receptors. Science 254: 1636–1639, 1991. [DOI] [PubMed] [Google Scholar]
- 242.Protopopescu X, Pan H, Altemus M, Tuescher O, Polanecsky M, McEwen B, Silbersweig D, Stern E. Orbitofrontal cortex activity related to emotional processing changes across the menstrual cycle. Proc Natl Acad Sci U S A 102: 16060–16065, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Protopopescu X, Tuescher O, Pan H, Epstein J, Root J, Chang L, Altemus M, Polanecsky M, McEwen B, Stern E, Silbersweig D. Toward a functional neuroanatomy of premenstrual dysphoric disorder. J Affect Disord 108: 87–94, 2008. [DOI] [PubMed] [Google Scholar]
- 244.Pyter LM, Kelly SD, Harrell CS, Neigh GN. Sex differences in the effects of adolescent stress on adult brain inflammatory markers in rats. Brain Behav Immun 30: 88–94, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trends Immunol 27: 24–31, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Van Randenborgh A, de Jong-Meyer R, Hüffmeier J. Decision making in depression: Differences in decisional conflict between healthy and depressed individuals. Clin Psychol Psychother 17: 285–298, 2010. [DOI] [PubMed] [Google Scholar]
- 247.Rapkin AJ, Berman SM, Mandelkern MA, Silverman DHS, Morgan M, London ED. Neuroimaging evidence of cerebellar involvement in premenstrual dysphoric disorder. Biol Psychiatry 69: 374–380, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Rapkin AJ, Morgan M, Goldman L, Brann DW, Simone D, Mahesh VB. Progesterone metabolite allopregnanolone in women with premenstrual syndrome. Obstet Gynecol 90: 709–714, 1997. [DOI] [PubMed] [Google Scholar]
- 249.Rapp PR, Morrison JH, Roberts JA. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J Neurosci 23: 5708–5714, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Raymond V, Beaulieu M, Labrie F, Boissier J. Potent antidopaminergic activity of estradiol at the pituitary level on prolactin release. Science 200: 1173–1175, 1978. [DOI] [PubMed] [Google Scholar]
- 251.Roca CA, Schmidt PJ, Altemus M, Deuster P, Danaceau MA, Putnam K, Rubinow DR. Differential menstrual cycle regulation of hypothalamic-pituitary-adrenal axis in women with premenstrual syndrome and controls. J Clin Endocrinol Metab 88: 3057–3063, 2003. [DOI] [PubMed] [Google Scholar]
- 252.Roca CA, Schmidt PJ, Deuster PA, Danaceau MA, Altemus M, Putnam K, Chrousos GP, Nieman LK, Rubinow DR. Sex-related differences in stimulated hypothalamic-pituitary-adrenal axis during induced gonadal suppression. J Clin Endocrinol Metab 90: 4224–4231, 2005. [DOI] [PubMed] [Google Scholar]
- 253.Rode C, Wagner M, Güntürkün O. Menstrual cycle affects functional cerebral asymmetries. Neuropsychologia 33: 855–865, 1995. [DOI] [PubMed] [Google Scholar]
- 254.Romeo E, Ströhle A, Spalletta G, di Michele F, Hermann B, Holsboer F, Pasini A, Rupprecht R. Effects of antidepressant treatment on neuroactive steroids in major depression. Am J Psychiatry 155: 910–913, 1998. [DOI] [PubMed] [Google Scholar]
- 255.Rossmanith WG, Ruebberdt W. What causes hot flushes? The neuroendocrine origin of vasomotor symptoms in the menopause. Gynecol Endocrinol 25: 303–314, 2009. [DOI] [PubMed] [Google Scholar]
- 256.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SAA, Howard BV, Johnson KC, Kotchen JM, Ockene J, Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results From the Women’s Health Initiative randomized controlled trial. JAMA 288: 321–333, 2002. [DOI] [PubMed] [Google Scholar]
- 257.Rossouw JE, Prentice RL, Manson JE, Wu L, Barad D, Barnabei VM, Ko M, LaCroix AZ, Margolis KL, Stefanick ML. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA 297: 1465–1477, 2007. [DOI] [PubMed] [Google Scholar]
- 258.Roy M-A, Neale MC, Pedersen NL, Mathé AA, Kendler KS. A twin study of generalized anxiety disorder and major depression. Psychol Med 25: 1037–1049, 1995. [DOI] [PubMed] [Google Scholar]
- 259.Rubinow DR. Reproductive steroids in context. Arch Women’s Ment Health 8: 1–5, 2005. [DOI] [PubMed] [Google Scholar]
- 260.Rubinow DR, Girdler SS. Hormones, heart disease, and health: Individualized medicine versus throwing the baby out with the bathwater. Depress Anxiety 28: 282–296, 2011. [DOI] [PubMed] [Google Scholar]
- 261.Rubinow DR, Schmidt PJ. Gonadal steroids, brain, and behavior: Role of context. Dialogues Clin Neurosci 4: 123–137, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Rubinow DR, Schmidt PJ, Roca CA. Estrogen–serotonin interactions: Implications for affective regulation. Biol Psychiatry 44: 839–850, 1998. [DOI] [PubMed] [Google Scholar]
- 263.Rumberg B, Baars A, Fiebach J, Ladd ME, Forsting M, Senf W, Gizewski ER. Cycle and gender-specific cerebral activation during a verb generation task using fMRI: Comparison of women in different cycle phases, under oral contraception, and men. Neurosci Res 66: 366–371, 2010. [DOI] [PubMed] [Google Scholar]
- 264.Sadaghiani S, Scheeringa R, Lehongre K, Morillon B, Giraud A-L, Kleinschmidt A. Intrinsic connectivity networks, alpha oscillations, and tonic alertness: A simultaneous electroencephalography/functional magnetic resonance imaging study. J Neurosci 30: 10243–10250, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Sampson GA. Premenstrual syndrome: A double-blind controlled trial of progesterone and placebo. Br J Psychiatry 135: 209–215, 1979. [DOI] [PubMed] [Google Scholar]
- 266.Sato K, Akaishi T, Matsuki N, Ohno Y, Nakazawa K. beta-Estradiol induces synaptogenesis in the hippocampus by enhancing brain-derived neurotrophic factor release from dentate gyrus granule cells. Brain Res 1150: 108–120, 2007. [DOI] [PubMed] [Google Scholar]
- 267.Sayeed I, Parvez S, Wali B, Siemen D, Stein DG. Direct inhibition of the mitochondrial permeability transition pore: A possible mechanism for better neuroprotective effects of allopregnanolone over progesterone. Brain Res 1263: 165–173, 2009. [DOI] [PubMed] [Google Scholar]
- 268.Schiller C. The hormone withdrawal hypothesis of postpartum depression: A translational approach [Online]. Theses Diss. http://ir.uiowa.edu/etd/1261. [Google Scholar]
- 269.Schiller CE, Schmidt PJ, Rubinow DR. Allopregnanolone as a mediator of affective switching in reproductive mood disorders. Psychopharmacology (Berl) 231: 3557–3567, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Schlaepfer TE, Cohen MX, Frick C, Kosel M, Brodesser D, Axmacher N, Joe AY, Kreft M, Lenartz D, Sturm V. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology 33: 368–377, 2008. [DOI] [PubMed] [Google Scholar]
- 271.Schmidt P, Daly RC, Bloch M, Smith MJ, Danaceau MA, St Clair LS, Murphy JH, Haq N, Rubinow DR. Dehydroepiandrosterone monotherapy in midlife-onset major and minor depression. Arch Gen Psychiatry 62: 154–162, 2005. [DOI] [PubMed] [Google Scholar]
- 272.Schmidt PJ, Ben Dor R, Martinez PE, Guerrieri GM, Harsh VL, Thompson K, Koziol DE, Nieman LK, Rubinow DR. Effects of estradiol withdrawal on mood in women with past perimenopausal depression: A randomized clinical trial. JAMA Psychiatry 72: 714–726, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Schmidt PJ, Haq N, Rubinow DR. A longitudinal rvaluation of the relationship between reproductive status and mood in perimenopausal women. Am J Psychiatry 161: 2238–2244, 2004. [DOI] [PubMed] [Google Scholar]
- 274.Schmidt PJ, Murphy JH, Haq N, Danaceau MA, St Clair L. Basal plasma hormone levels in depressed perimenopausal women. Psychoneuroendocrinology 27: 907–920, 2002. [DOI] [PubMed] [Google Scholar]
- 275.Schmidt PJ, Nieman L, Danaceau MA, Tobin MB, Roca CA, Murphy JH, Rubinow DR. Estrogen replacement in perimenopause-related depression: A preliminary report. Am J Obstet Gynecol 183: 414–420, 2000. [DOI] [PubMed] [Google Scholar]
- 276.Schmidt PJ, Nieman LK, Danaceau MA, Adams LF, Rubinow DR. Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. N Engl J Med 338: 209–216, 1998. [DOI] [PubMed] [Google Scholar]
- 277.Schmidt PJ, Purdy RH, Moore PH, Paul SM, Rubinow DR. Circulating levels of anxiolytic steroids in the luteal phase in women with premenstrual syndrome and in control subjects. J Clin Endocrinol Metab 79: 1256–1260, 1994. [DOI] [PubMed] [Google Scholar]
- 278.Schöning S, Engelien A, Kugel H, Schäfer S, Schiffbauer H, Zwitserlood P, Pletziger E, Beizai P, Kersting A, Ohrmann P, Greb RR, Lehmann W, Heindel W, Arolt V, Konrad C. Functional anatomy of visuo-spatial working memory during mental rotation is influenced by sex, menstrual cycle, and sex steroid hormones. Neuropsychologia 45: 3203–3214, 2007. [DOI] [PubMed] [Google Scholar]
- 279.Schoning S, Zwitserlood P, Engelien A, Behnken A, Kugel H, Schiff-bauer H, Lipina K, Pachur C, Kersting A, Dannlowski U, Baune BT, Zwanzger P, Reker T, Heindel W, Arolt V, Konrad C. Working-memory fMRI reveals cingulate hyperactivation in euthymic major depression. Hum Brain Mapp 30: 2746–2756, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Schüle C, Baghai TC, di Michele F, Eser D, Pasini A, Schwarz M, Rupprecht R, Romeo E. Effects of combination treatment with mood stabilizers and mirtazapine on plasma concentrations of neuroactive steroids in depressed patients. Psychoneuroendocrinology 32: 669–680, 2007. [DOI] [PubMed] [Google Scholar]
- 281.Schüle C, Eser D, Baghai TC, Nothdurfter C, Kessler JS, Rupprecht R. Neuroactive steroids in affective disorders: Target for novel antidepressant or anxiolytic drugs? Neuroscience 191: 55–77, 2011. [DOI] [PubMed] [Google Scholar]
- 282.Schüle C, Romeo E, Uzunov DP, Eser D, di Michele F, Baghai TC, Pasini A, Schwarz M, Kempter H, Rupprecht R. Influence of mirtaza-pine on plasma concentrations of neuroactive steroids in major depression and on 3α-hydroxysteroid dehydrogenase activity. Mol Psychiatry 11: 261–272, 2005. [DOI] [PubMed] [Google Scholar]
- 283.Shansky RM, Bender G, Arnsten AFT. Estrogen prevents norepinephrine alpha-2a receptor reversal of stress-induced working memory impairment. Stress 12: 457–463, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, Mintun MA, Wang S, Coalson RS, Raichle ME. The default mode network and self-referential processes in depression. Proc Natl Acad Sci U S A 106: 1942–1947, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci U S A 107: 11020–11025, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Shen H, Gong QH, Aoki C, Yuan M, Ruderman Y, Dattilo M, Williams K, Smith SS. Reversal of neurosteroid effects at α4β2δ GABAA receptors triggers anxiety at puberty. Nat Neurosci 10: 469–477, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Shimizu E, Hashimoto K, Okamura N, Koike K, Komatsu N, Kumakiri C, Nakazato M, Watanabe H, Shinoda N, Okada S, Iyo M. Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol Psychiatry 54: 70–75, 2003. [DOI] [PubMed] [Google Scholar]
- 288.Shirayama Y, Muneoka K, Fukumoto M, Tadokoro S, Fukami G, Hashimoto K, Iyo M. Infusions of allopregnanolone into the hippocampus and amygdala, but not into the nucleus accumbens and medial prefrontal cortex, produce antidepressant effects on the learned helplessness rats. Hippocampus 21: 1105–1113, 2011. [DOI] [PubMed] [Google Scholar]
- 289.Shulman LM. Is there a connection between estrogen and Parkinson’s disease? Parkinsonism Relat Disord 8: 289–295, 2002. [DOI] [PubMed] [Google Scholar]
- 290.Sichel DA, Cohen LS, Robertson LM, Ruttenberg A, Rosenbaum JF. Prophylactic estrogen in recurrent postpartum affective disorder. Biol Psychiatry 38: 814–818, 1995. [DOI] [PubMed] [Google Scholar]
- 291.Silverman ME, Loudon H, Safier M, Protopopescu X, Leiter G, Liu X, Goldstein M. Neural dysfunction in postpartum depression: An fMRI pilot study. CNS Spectr 12: 853–862, 2007. [DOI] [PubMed] [Google Scholar]
- 292.Singer CA, Rogers KL, Strickland TM, Dorsa DM. Estrogen protects primary cortical neurons from glutamate toxicity. Neurosci Lett 212: 13–16, 1996. [DOI] [PubMed] [Google Scholar]
- 293.Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol 26: 163–174, 2005. [DOI] [PubMed] [Google Scholar]
- 294.Smith CT, Sierra Y, Oppler SH, Boettiger CA. Ovarian cycle effects on immediate reward selection bias in humans: A role for estradiol. J Neurosci 34: 5468–5476, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Smith MJ, Adams LF, Schmidt PJ, Rubinow DR, Wassermann EM. Effects of ovarian hormones on human cortical excitability. Ann Neurol 51: 599–603, 2002. [DOI] [PubMed] [Google Scholar]
- 296.Smith MJ, Adams LF, Schmidt PJ, Rubinow DR, Wassermann EM. Abnormal luteal phase excitability of the motor cortex in women with premenstrual syndrome. Biol Psychiatry 54: 757–762, 2003. [DOI] [PubMed] [Google Scholar]
- 297.Smith SS. Female sex steroid hormones: From receptors to networks to performance—Actions on the sensorimotor system. Prog Neurobiol 44: 55–86, 1994. [DOI] [PubMed] [Google Scholar]
- 298.Smith SS, Gong QH, Hsu FC, Markowitz RS, French-Mullen JM, Li X. GABA(A) receptor alpha4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature 392: 926–930, 1998. [DOI] [PubMed] [Google Scholar]
- 299.Smith SS, Gong QH, Li X, Moran MH, Bitran D, Frye CA, Hsu F-C. Withdrawal from 3α-OH-5α-Pregnan-20-One using a pseudopregnancy model alters the kinetics of hippocampal GABAA-gated current and increases the GABAAreceptor α4 subunit in association with increased anxiety. J Neurosci 18: 5275–5284, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Smith SS, Ruderman Y, Frye C, Homanics G, Yuan M. Steroid withdrawal in the mouse results in anxiogenic effects of 3alpha,5beta-THP: A possible model of premenstrual dysphoric disorder. Psychopharmacology (Berl) 186: 323–333, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Sohrabji F, Greene LA, Miranda RC, Toran-Allerand CD. Reciprocal regulation of estrogen and NGF receptors by their ligands in PC12 cells. J Neurobiol 25: 974–988, 1994. [DOI] [PubMed] [Google Scholar]
- 302.Sohrabji F, Miranda RC, Toran-Allerand CD. Estrogen differentially regulates estrogen and nerve growth factor receptor mRNAs in adult sensory neurons. J Neurosci 14: 459–471, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Sporns O. Structure and function of complex brain networks. Dialogues Clin Neurosci 15: 247–262, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Sripada RK, Marx CE, King AP, Rampton JC, Ho SS, Liberzon I. Allopregnanolone elevations following pregnenolone administration are associated with enhanced activation of emotion regulation neurocircuits. Biol Psychiatry 73: 1045–1053, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Stachenfeld NS, Silva C, Keefe DL. Estrogen modifies the temperature effects of progesterone. J Appl Physiol 88: 1643–1649, 2000. [DOI] [PubMed] [Google Scholar]
- 306.Steinberg EM, Rubinow DR, Bartko JJ, Fortinsky PM, Haq N, Thompson K, Schmidt PJ. A cross-sectional evaluation of perimenopausal depression. J Clin Psychiatry 69: 973–980, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Stewart JL, Bismark AW, Towers DN, Coan JA, Allen JJB. Resting frontal EEG asymmetry as an endophenotype for depression risk: Sexspecific patterns of frontal brain asymmetry. J Abnorm Psychol 119: 502–512, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Stirone C, Duckles SP, Krause DN, Procaccio V. Estrogen increases mitochondrial efficiency and reduces oxidative stress in cerebral blood vessels. Mol Pharmacol 68: 959–965, 2005. [DOI] [PubMed] [Google Scholar]
- 309.Stowe ZN, Hostetter AL, Newport DJ. The onset of postpartum depression: Implications for clinical screening in obstetrical and primary care. Am J Obstet Gynecol 192: 522–526, 2005. [DOI] [PubMed] [Google Scholar]
- 310.Strekalova T, Gorenkova N, Schunk E, Dolgov O, Bartsch D. Selective effects of citalopram in a mouse model of stress-induced anhedonia with a control for chronic stress. Behav Pharmacol 17: 271–287, 2006. [DOI] [PubMed] [Google Scholar]
- 311.Ströhle A, Romeo E, Hermann B, Pasini A, Spalletta G, di Michele F, Holsboer F, Rupprecht R. Concentrations of 3α-reduced neuroactive steroids and their precursors in plasma of patients with major depression and after clinical recovery. Biol Psychiatry 45: 274–277, 1999. [DOI] [PubMed] [Google Scholar]
- 312.Sumner BE, Grant KE, Rosie R, Hegele-Hartung C, Fritzemeier KH, Fink G. Effects of tamoxifen on serotonin transporter and 5-hydroxytryptamine2A receptor binding sites and mRNA levels in the brain of ovariectomized rats with or without acute estradiol replacement. Mol Brain Res 73: 119–128, 1999. [DOI] [PubMed] [Google Scholar]
- 313.Sumner BEH, Fink G. Estrogen increases the density of 5-Hydroxytryptamine2A receptors in cerebral cortex and nucleus accumbens in the female rat. J Steroid Biochem Mol Biol 54: 15–20, 1995. [DOI] [PubMed] [Google Scholar]
- 314.Sunday L, Tran MM, Krause DN, Duckles SP. Estrogen and progestagens differentially modulate vascular proinflammatory factors. Am J Physiol Endocrinol Metab 291: E261–E267, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Sundström I, Bäckstrom T. Citalopram increases pregnanolone sensitivity in patients with premenstrual syndrome: An open trial. Psychoneuroendocrinology 23: 73–88, 1998. [DOI] [PubMed] [Google Scholar]
- 316.Sundström I, Nyberg S, Bäckström T. Patients with premenstrual syndrome have reduced sensitivity to midazolam compared to control subjects. Neuropsychopharmacology 17: 370–381, 1997. [DOI] [PubMed] [Google Scholar]
- 317.Sundström Poromaa I, Smith S, Gulinello M. GABA receptors, progesterone and premenstrual dysphoric disorder. Arch Womens Ment Health 6: 23–41, 2003. [DOI] [PubMed] [Google Scholar]
- 318.Su TP, Schmidt PJ, Danaceau M, Murphy DL, Rubinow DR. Effect of menstrual cycle phase on neuroendocrine and behavioral responses to the serotonin agonist m-chlorophenylpiperazine in women with premenstrual syndrome and controls. J Clin Endocrinol Metab 82: 1220–1228, 1997. [DOI] [PubMed] [Google Scholar]
- 319.Tang Y, Janssen WGM, Hao J, Roberts JA, McKay H, Lasley B, Allen PB, Greengard P, Rapp PR, Kordower JH, Hof PR, Morrison JH. Estrogen replacement increases spinophilin-immunoreactive spine number in the prefrontal cortex of female rhesus monkeys. Cereb Cortex 14: 215–223, 2004. [DOI] [PubMed] [Google Scholar]
- 320.Terauchi M, Obayashi S, Akiyoshi M, Kato K, Matsushima E, Kubota T. Effects of oral estrogen and hypnotics on Japanese peri- and post-menopausal women with sleep disturbance. J Obstet Gynaecol Res 37: 741–749, 2011. [DOI] [PubMed] [Google Scholar]
- 321.Thibodeau R, Jorgensen RS, Kim S. Depression, anxiety, and resting frontal EEG asymmetry: A meta-analytic review. J Abnorm Psychol 115: 715–729, 2006. [DOI] [PubMed] [Google Scholar]
- 322.Thimm M, Weis S, Hausmann M, Sturm W. Menstrual cycle effects on selective attention and its underlying cortical networks. Neuroscience 258: 307–317, 2014. [DOI] [PubMed] [Google Scholar]
- 323.Thomas M, Bland DA, Clarke CH, Cunningham KA. Estrogen regulation of serotonin (5-HT) transporter and 5-HT1A receptor mRNA in female rat brain. Abstr Soc Neurosci 23: 1501, 1997. [Google Scholar]
- 324.Tiemstra JD, Patel K. Hormonal therapy in the management of premenstrual syndrome. J Am Board Fam Pract 11: 378–381, 1998. [DOI] [PubMed] [Google Scholar]
- 325.Tomarken AJ, Dichter GS, Garber J, Simien C. Resting frontal brain activity: Linkages to maternal depression and socio-economic status among adolescents. Biol Psychol 67: 77–102, 2004. [DOI] [PubMed] [Google Scholar]
- 326.Tom SE, Anderson ML, Landis CA, Aiello Bowles EJ, Woods NF, Reed SD, Newton KM, Buist DSM. Sleep problems after short-term hormone therapy suspension: Secondary analysis of a randomized trial. Menopause N Y N 18: 1184–1190, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Tsang K-L, Ho S-L, Lo S-K. Estrogen improves motor disability in parkinsonian postmenopausal women with motor fluctuations. Neurology 54: 2292–2298, 2000. [DOI] [PubMed] [Google Scholar]
- 328.Tsutsui K, Ukena K, Sakamoto H, Okuyama S-I, Haraguchi S. Biosyn-thesis, mode of action, and functional significance of neurosteroids in the Purkinje cell. Front Endocrinol 2: 61, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Tuohy A, McVey C. Subscales measuring symptoms of non-specific depression, anhedonia, and anxiety in the Edinburgh Postnatal Depression Scale. Br J Clin Psychol 47: 153–169, 2008. [DOI] [PubMed] [Google Scholar]
- 330.Ungar S, Makman MH, Morris SA, Etgen AM. Estrogen uncouples beta-adrenergic receptor from the stimulatory guanine nucleotide-binding protein in female rat hypothalamus. Endocrinology 133: 2818–2826, 1993. [DOI] [PubMed] [Google Scholar]
- 331.Uzunova V, Sheline Y, Davis JM, Rasmusson A, Uzunov DP, Costa E, Guidotti A. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc Natl Acad Sci U S A 95: 3239–3244, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Vasudevan N, Kow L-M, Pfaff DW. Early membrane estrogenic effects required for full expression of slower genomic actions in a nerve cell line. Proc Natl Acad Sci U S A 98: 12267–12271, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Vasudevan N, Pfaff DW. Non-genomic actions of estrogens and their interaction with genomic actions in the brain. Front Neuroendocrinol 29: 238–257, 2008. [DOI] [PubMed] [Google Scholar]
- 334.Vesga-Lopez O, Blanco C, Keyes K, Olfson M, Grant BF, Hasin DS. Psychiatric disorders in pregnant and postpartum women in the United States. Arch Gen Psychiatry 65: 805–815, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Wainwright NWJ, Surtees PG. Childhood adversity, gender and depression over the life-course. J Affect Disord 72: 33–44, 2002. [DOI] [PubMed] [Google Scholar]
- 336.Walf AA, Frye CA. ERβ-selective estrogen receptor modulators produce antianxiety behavior when administered systemically to ovariectomized rats. Neuropsychopharmacology 30: 1598–1609, 2005. [DOI] [PubMed] [Google Scholar]
- 337.Wang M, Seippel L, Purdy RH, Bãckström T. Relationship between symptom severity and steroid variation in women with premenstrual syndrome: study on serum pregnenolone, pregnenolone sulfate, 5 alpha-pregnane-3,20-dione and 3 alpha-hydroxy-5 alpha-pregnan-20-one. J Clin Endocrinol Metab 81: 1076–1082, 1996. [DOI] [PubMed] [Google Scholar]
- 338.Wang X-J. Neurophysiological and computational principles of cortical rhythms in cognition. Physiol Rev 90: 1195–1268, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Waters EM, Simerly RB. Estrogen induces caspase-dependent cell death during hypothalamic development. J Neurosci 29: 9714–9718, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J Pers Soc Psychol 54: 1063–1070, 1988. [DOI] [PubMed] [Google Scholar]
- 341.Watson D, O’Hara MW, Simms LJ, Kotov R, Chmielewski M, McDade-Montez EA, Gamez W, Stuart S. Development and validation of the Inventory of Depression and Anxiety Symptoms (IDAS). Psychol Assess 19: 253–268, 2007. [DOI] [PubMed] [Google Scholar]
- 342.Watson D, Weber K, Assenheimer JS, Clark LA, Strauss ME, McCormick RA. Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. J Abnorm Psychol 104: 3–14, 1995. [DOI] [PubMed] [Google Scholar]
- 343.Weiser MJ, Foradori CD, Handa RJ. Estrogen receptor beta in the brain: From form to function. Brain Res Rev 57: 309–320, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Weis S, Hausmann M, Stoffers B, Sturm W. Dynamic changes in functional cerebral connectivity of spatial cognition during the menstrual cycle. Hum Brain Mapp 32: 1544–1556, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Weis S, Hausmann M, Stoffers B, Vohn R, Kellermann T, Sturm W. Estradiol modulates functional brain organization during the menstrual cycle: An analysis of interhemispheric inhibition. J Neurosci 28: 13401–13410, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Wesström J, Nilsson S, Sundström-Poromaa I, Ulfberg J. Restless legs syndrome among women: Prevalence, co-morbidity and possible relationship to menopause. Climacteric 11: 422–428, 2008. [DOI] [PubMed] [Google Scholar]
- 347.Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol 8: 49–76, 2012. [DOI] [PubMed] [Google Scholar]
- 348.Wieland S, Lan NC, Mirasedeghi S, Gee KW. Anxiolytic activity of the progesterone metabolite 5α-pregnan-3α-ol-20-one. Brain Res 565: 263–268, 1991. [DOI] [PubMed] [Google Scholar]
- 349.Williams JMG, Barnhofer T, Crane C, Beck AT. Problem solving deteriorates following mood challenge in formerly depressed patients with a history of suicidal ideation. J Abnorm Psychol 114: 421–431, 2005. [DOI] [PubMed] [Google Scholar]
- 350.Van Wingen GA, van Broekhoven F, Verkes RJ, Petersson KM, Back-strom T, Buitelaar JK, Fernandez G. Progesterone selectively increases amygdala reactivity in women. Mol Psychiatry 13: 325–333, 2008. [DOI] [PubMed] [Google Scholar]
- 351.Van Wingen GA, Ossewaarde L, Bäckström T, Hermans EJ, Fernández G. Gonadal hormone regulation of the emotion circuitry in humans. Neuroscience 191: 38–45, 2011. [DOI] [PubMed] [Google Scholar]
- 352.Wise V, McFarlane AC, Clark CR, Battersby M. An integrative assessment of brain and body function “at rest” in panic disorder: A combined quantitative EEG/autonomic function study. Int J Psychophysiol 79: 155–165, 2011. [DOI] [PubMed] [Google Scholar]
- 353.Wisner KL, Sit DY, McShea MC, Rizzo DM, Zoretich RA, Hughes CL, Eng HF, Luther JF, Wisniewski SR, Costantino ML, Confer AL, Moses-Kolko EL, Famy CS, Hanusa BH. Onset timing, thoughts of self-harm, and diagnoses in postpartum women with screen-positive depression findings. JAMA Psychiatry 70: 490–498, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Witte MM, Resuehr D, Chandler AR, Mehle AK, Overton JM. Female mice and rats exhibit species-specific metabolic and behavioral responses to ovariectomy. Gen Comp Endocrinol 166: 520–528, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Wolkowitz OM, Reus VI, Keebler A, Nelson N, Friedland M, Brizen-dine L, Roberts E. Double-blind treatment of major depression with dehydroepiandrosterone. Am J Psychiatry 156: 646–649, 1999. [DOI] [PubMed] [Google Scholar]
- 356.Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat [published erratum appears in J Neurosci 1992 Oct; 12(10): following table of contents]. J Neurosci 12: 2549–2554, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol 336: 293–306, 1993. [DOI] [PubMed] [Google Scholar]
- 358.Woolley CS, McEwen BS. Estradiol regulates hippocampal dendritic spine density via an N-methyl-d-aspartate receptor-dependent mechanism. J Neurosci 14: 7680–7687, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Yaffe K, Ettinger B, Pressman A, Seeley D, Whooley M, Schaefer C, Cummings S. Neuropsychiatric function and dehydroepiandrosterone sulfate in elderly women: A prospective study. Biol Psychiatry 43: 694–700, 1998. [DOI] [PubMed] [Google Scholar]
- 360.Yao J, Irwin R, Chen S, Hamilton R, Cadenas E, Brinton RD. Ovarian hormone loss induces bioenergetic deficits and mitochondrial β-amyloid. Neurobiol Aging 33: 1507–1521, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Yen Y-C, Rebok GW, Gallo JJ, Jones RN, Tennstedt SL. Depressive symptoms impair everyday problem-solving ability through cognitive abilities in late life. Am J Geriatr Psychiatry 19: 142–150, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Zeidan MA, Igoe SA, Linnman C, Vitalo A, Levine JB, Klibanski A, Goldstein JM, Milad MR. Estradiol modulates medial prefrontal cortex and amygdala activity during fear extinction in women and female rats. Biol Psychiatry 70: 920–927, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Zhang D, Yang S, Yang C, Jin G, Zhen X. Estrogen regulates responses of dopamine neurons in the ventral tegmental area to cocaine. Psychopharmacology (Berl) 199: 625–635, 2008. [DOI] [PubMed] [Google Scholar]
- 364.Zhang L, Li Bs, Zhao W, Chang YH, Ma W, Dragan M, Barker JL, Hu Q, Rubinow DR. Sex-related differences in MAPKs activation in rat astrocytes: Effects of estrogen on cell death. Brain Res Mol Brain Res 103: 1–11, 2002. [PubMed] [Google Scholar]
- 365.Zhou Y, Watters JJ, Dorsa DM. Estrogen rapidly induces the phosphorylation of the cAMP response element binding protein in rat brain. Endocrinology 137: 2163–2166, 1996. [DOI] [PubMed] [Google Scholar]
- 366.Zhou Y, Yu C, Zheng H, Liu Y, Song M, Qin W, Li K, Jiang T. Increased neural resources recruitment in the intrinsic organization in major depression. J Affect Disord 121: 220–230, 2010. [DOI] [PubMed] [Google Scholar]