Abstract
Genetic and pharmacological inhibition of the phosphoinositide 3-kinase γ (PI3Kγ) exerts anti-inflammatory and protective effects in a number of inflammatory and autoimmune diseases. Spontaneously hypertensive rats subjected to embolic middle cerebral occlusion were treated with AS605240 (30 mg/kg) at 2 or 4 hours, tissue plasminogen activator (tPA, 10 mg/kg) at 2 or 6 hours, or AS605240 at 4 hours plus tPA at 6 hours. Infarct volume, brain hemorrhage, neurological function, microvascular thrombosis, and cerebral microvessel patency were examined. We found that treatment with AS605240 alone at 2 hours or the combination treatment with AS605240 at 4 hours and tPA at 6 hours significantly reduced infarct volume and neurological deficits at 3 days after stroke compared with ischemic rats treated with saline, AS605240 alone at 4 hours, and tPA alone at 6 hours. Moreover, the combination treatment effectively prevented the delayed tPA-induced cerebral hemorrhage. These protective effects are associated with reduced disruption of the blood brain barrier (BBB), reduced downstream microvascular thrombosis, and improved microvascular patency by AS605240. Inhibition of the nuclear transcription factor-kB (NF-kB)-dependent MMP-9 (matrix metalloproteinase-9) and PAI-1 (plasminogen activator inhibitor-1) in the ischemic brain endothelium may underlie the neurovascular protective effect of AS605240. In addition, the combination treatment significantly reduced circulating platelet P-selectin expression and platelet-leukocyte aggregation compared with ischemic rats treated with saline or tPA alone at 6 hours. In conclusion, inhibition of PI3Kγ with AS605240 reduces delayed tPA-induced intracerebral hemorrhage and improves microvascular patency, which likely contributes to neuroprotective effect of the combination treatment.
Keywords: PI3-kinase gamma, tissue plasminogen activator, microvascular patency, brain hemorrhage, ischemic stroke
Introduction
Stroke is a leading cause of morbidity and mortality worldwide. To date, intravenous tissue plasminogen activator (IV tPA) remains the only FDA-approved drug therapy for acute ischemic stroke (AIS) [1]. However, only 3% to 5% of all patients with AIS in the United States receive IV tPA [2]. This is mainly related to increased risks of intracerebral hemorrhage with delayed IV tPA. It is well known that IV tPA beyond 3 hours of stroke onset significantly increases intracerebral bleeding although it could be extended up to 4.5 hours in selected patients [3,4]. Thus, there is a great need for developing an adjuvant agent that safely extends the therapeutic time window for tPA and would make the thrombolytic therapy accessible to more stroke patients.
Phosphoinositide 3-kinase gamma (PI3Kγ), the only class IB PI3K isoform, has emerged as a key regulator of immunity and inflammation. PI3Kγ expressed on different cell types including leukocytes, platelets, and endothelial cells acts as a crucial signal molecule controlling a wide range of immune and inflammatory responses and cardiovascular functions [5]. Genetic deletion and pharmacological inhibition of PI3Kγ display protective effects in animal models of human diseases, such as systemic lupus, rheumatoid arthritis, autoimmune diabetes, atherosclerosis, lung disease, sepsis, and colitis [6–11]. Moreover, PI3Kγ has also been shown to play a critical role in the regulation of neuroinflammation in several neurovascular diseases including focal cerebral ischemia, surgical brain injury, and Alzheimer’s disease [12–15]. However, the therapeutic potential of PI3Kγ inhibition in experimental stroke has not been investigated.
Thrombolytic therapy with tPA restores cerebral blood flow through its desirable fibrinolytic action. However, clinical use of tPA is limited by narrow time window for thrombolysis, which is related to exacerbated BBB injury and increased risks of brain hemorrhage induced by delayed tPA treatment [16]. Matrix metalloproteinase-9 (MMP-9) is a key regulator of these tPA-associated complications [16]. Studies in both experimental models and stroke patients suggest that infiltrated neutrophils represent a major source of increased MMP-9 in the ischemic brain, and delayed tPA treatment may further increase MMP-9 particularly in the ischemic brain endothelium [17–20]. Our previous study has shown that PI3Kγ deficiency protects against ischemia/reperfusion-induced disruption of the blood-brain barrier (BBB) via inhibiting NF-kB-dependent neuroinflammation [13]. High blood pressure, also known as hypertension, is the single most important risk factor for stroke. Hypertension causes about 50% of ischemic strokes and also increases the risk of hemorrhagic transformation. In this study, we investigated the neurovascular protection of AS605240, a PI3Kγ selective inhibitor, alone and in combination with thrombolytic therapy in a hypertensive rat model of embolic stroke.
Methods
Data supporting the findings of this study are available from the corresponding author on reasonable request. This manuscript adheres to the AHA Journals’ implementation of the Transparency and Openness Promotion (TOP) Guidelines. Animal protocols were approved by the Institutional Animal Care and Use Committee at Penn State University College of Medicine. A detailed Methods section is provided in the Online Supplement.
Animals and Stroke Model
Spontaneously hypertensive rats (SHRs, male, 12–15 week-old, weighing 270–310g, from Charles River Laboratories) were subjected to embolic middle cerebral artery occlusion (MCAO) according to the standard operating procedure in our laboratory [18, 21].
Experimental protocols
A diagram of the experimental design and animal groups is shown in Figure 1A. Ischemic rats (SHRs) were randomly assigned to the following treatment or control groups: treated with AS605240 alone at 2 or 4 hours, or the combination treatment with AS605240 at 4 hours and tPA at 6 hours. Control groups consisted of ischemic rats treated with 0.9% saline, tPA alone at 2 hours or 6 hours after stroke. For randomization, the web tool www.randomizer.org was used. Animals were randomly assigned to each group via random numbers generated on an Excel spreadsheet. Recombinant human tPA (Alteplase, Genentech, Inc., San Francisco, CA) was intravenously administered at a “rat dose” of 10 mg/kg (10% as a bolus and 90% as a 30 min infusion) using a syringe infusion pump (Harvard Apparatus, Holliston, MA, USA). The PI3Kγ-selective inhibitor AS605240 (Cayman Chemical, purity >99%; molecular weight 257.27 Da) was dissolved in the solvent: 0.5% carboxymethylcellulose /0.25%Tween- 20 [6,7] and given orally at 30 mg/kg per day initiated at 2 h or 4 h after the onset of ischemia. The dose of 30 mg/kg/day for AS605240 was selected based on the dose-response assessment (Figure S1A). All outcome measures were performed by investigators blinded to group assignment. The number of animals used in each experimental group and the total number of animals used in this study is summarized in Table S1 in the Online Supplement.
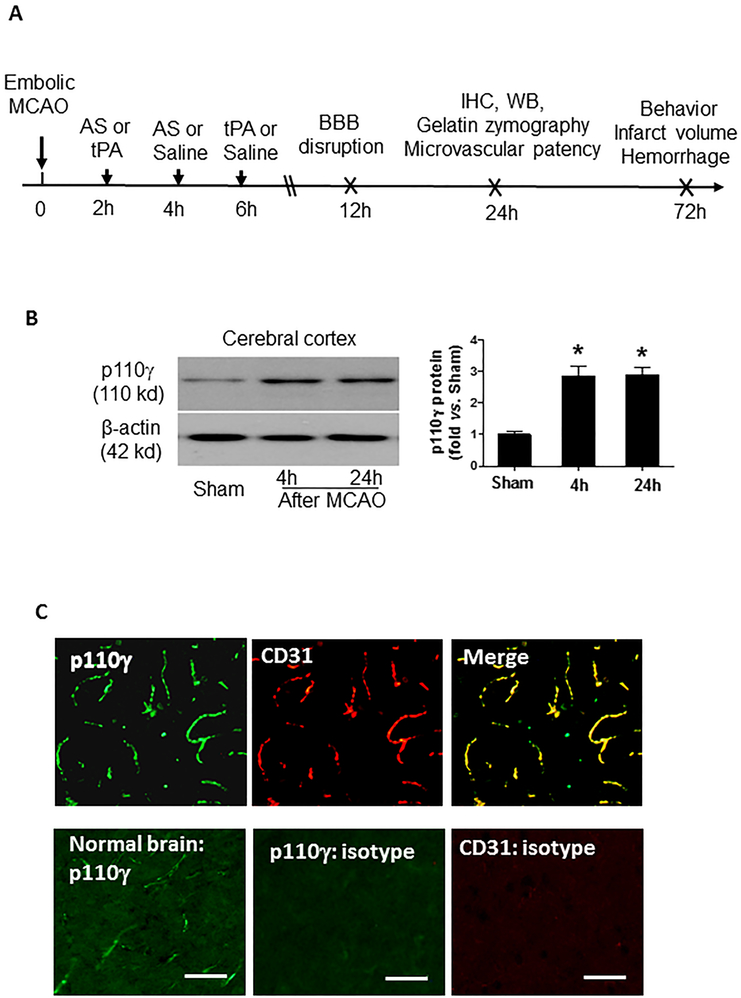
Figure 1.
A, Experimental design. MCAO, middle cerebral artery occlusion; AS, AS605240, a PI3Kγ-selective inhibitor); tPA, tissue plasminogen activator; WB, Western blot; IHC, immunohistochemistry. Experimental groups: Sham, MCAO+ saline, MCAO+ AS at 2h or 4h, MCAO + tPA at 2h or 6h; MCAO + AS at 4h + tPA at 6h. B, Representative images of Western blots showing PI3K-p110γ protein levels detected in the cerebral cortex in sham and MCAO at 4h and 24h after surgery. Semi-quantitation of immunoblots was analyzed by densitometry. Data are expressed as mean ± SEM from three independent experiments. *P<0.05 vs. Sham. C, Representative images of double immunostaining showing the colocalization of PI3K-p110γ (green) with CD31 (endothelial marker, red) at 24 hours after MCAO. n=5 rats per group. Images were acquired from the peri-infarct cortex. Negative control stained with isotype-matched control antibody did not show detectable labeling. Bar=50 μm.
Infarct volume, intracerebral hemorrhage, BBB permeability, and neurological deficits
Infarct volume and brain swelling were measured in TTC-stained coronal sections on day 3 after MCAO [18]. Hemoglobin levels were measured in the TTC-stained sections by a spectrophotometric assay using Drabkin reagent (Sigma-Aldrich) [22]. It has been shown that hemoglobin measure can be simultaneously performed on TTC-stained sections [23]. The modified Bederson score [24] and foot-fault test [25] were performed by a blinded investigator. In a separate set of experiment, the blood-brain barrier (BBB) permeability was determined by measuring the extravasation of Evans blue dye (Sigma-Aldrich) into the brain tissue at 12h after MCAO [18].
Western blot and gelatin zymography
These assays were performed as we described previously [18]. Protein extracts were obtained from the cerebral cortices (bregma +1.0 to −2.0 mm).
Immunohistochemistry
Immunohistochemistry was performed as we described previously [18]. The brains were removed and 10-μm-thick frozen coronal sections (bregma −0.4 to −1.4 mm) were obtained for immunohistochamical analysis (for details please see Online Supplement/Methods). Cerebral microvascular patency was assessed using the FITC-labeled dextran method [18].
Analysis of platelet activation and platelet-leukocyte aggregates in whole blood
Blood (500 μL) was collected into heparinized tubes via retro-orbital bleeding under deep anesthesia at 24 hours after sham or MCAO. Blood samples were run on a BD Accuri™ C6 Flow Cytometry and data analyzed by FlowJo software. Platelets in whole blood were identified by their characteristic light scattering and membrane expression of the platelet specific glycoprotein V (CD42d). Platelet surface P-selectin expression and platelet-leukocyte aggregates were analyzed as described previously [26].
Statistical Analysis
Data are expressed as mean ± standard error of the mean. The GraphPad Prism 5 software package was used for statistical analysis. The normality of data were assessed with the D’Agostino-Pearson omnibus test. For normally distributed variables, one-way analysis of variance (ANOVA) followed by the Bonferroni post hoc test were used to assess differences between groups. If only 2 groups were compared, an unpaired, 2-tailed Student t test, was applied. The Kruskal-Wallis and Mann-Whitney U tests were used to explore differences between groups in non-normally distributed variables. Nonparametric functional outcome scores were compared by Kruskal-Wallis test with post hoc Dunn corrections. Sample size calculation (power = 0.8, α= 0.05) was performed using an online calculator (http://www.lasec.cuhk.edu.hk/sample-size-calculation.html). Based on our previous infarct volume data using the same stroke model [18] estimated 6 animals per group would be required to detect an infarct equivalent to 25% of the uninjured hemisphere. For comparison of survival data, the log-rank test was used. p<0.05 was considered statistically significant.
Results
Focal cerebral ischemia increases PI3Kγ expression in ischemic brain microvessels
Western blot analysis showed that PI3K-p110γ protein expression in the cerebral cortex significantly increased as early as 4 hours and remained elevated 24 hours after stroke (Figure 1B). Immunohistochemistry was performed to detect the expression and distribution of PI3Kγ in the ischemic cerebral cortex 24 hours after MCAO. The expression pattern of PI3Kγ was similar in SHRs (Figure 1C) and normotensive Sprague-Dawley (SD) rats (Figure S2A). In normal rat brain PI3Kγ was detectable at low levels and restricted to vascular-like structures. MCAO robustly induced PI3Kγ expression that was predominantly expressed in cerebral microvessels. These data suggest that brain microvascular endothelial cells are the major cellular source of increased brain PI3Kγ expression in the acute phase of ischemic stroke.
Effects of PI3Kγ inhibition on tPA-associated hemorrhage and acute stroke outcome
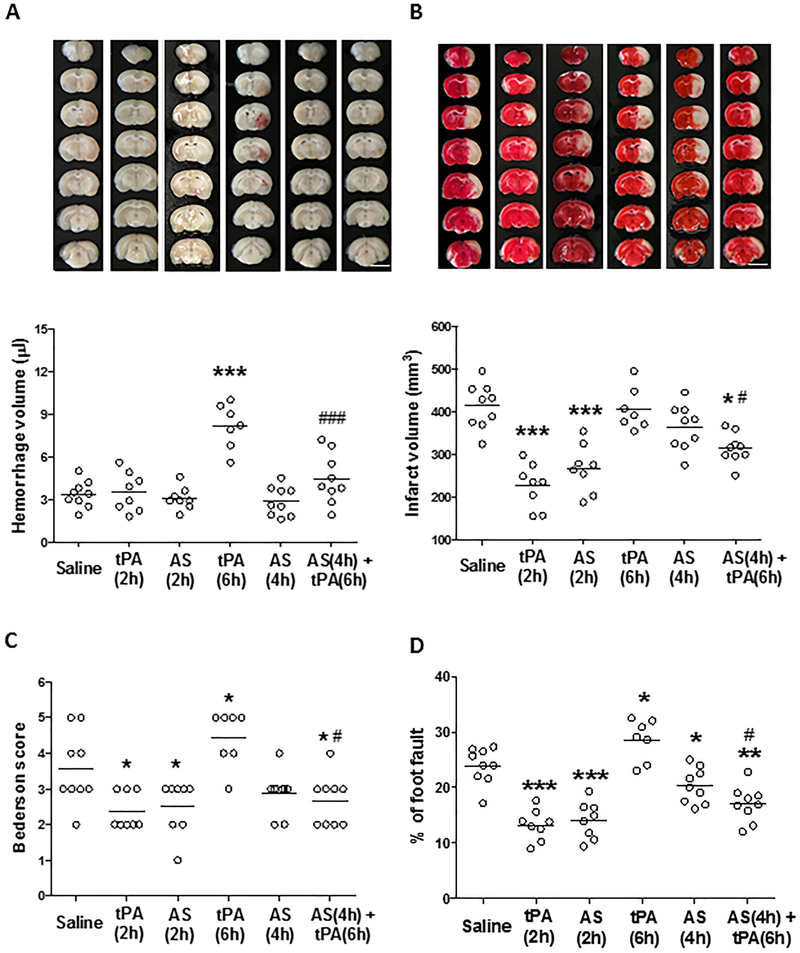
Intracerebral hemorrhage (ICH) and acute stroke outcome were assessed on day 3 after stroke in SHRs. IV tPA at 2 hours reduced infarct volume without increasing ICH. In contrast, IV tPA at 6 hours had no effect on infarct volume but increased ICH (Figure 2A and2B). PI3Kγ inhibition with AS605240 alone initiated at 2 h significantly reduced infarct volume and improved neurological function compared with the saline group, while starting its treatment at 4h provided only minimal protective effects. However, the combination treatment with AS605240 at 4h plus tPA at 6 h not only reduced the tPA-induced ICH (Figure 2A) but also significantly reduced infarct volume (Figure 2B) and brain swelling (Figure S3A), and improved neurological function (Figure 2C and2D). Physiological parameters were not altered by AS605240 (Table S2).
Figure 2.
Effect of PI3Kγ inhibition on tPA-induced intracerebral hemorrhage and acute stroke outcome. A and B, Representative images (chosen from the median animal each group) of unstained coronal sections (A, top) showing intracerebral hemorrhage (red color) and TTC-stained coronal sections (B, top) showing tissue infarction (white color) in the indicated groups 72 hours after stroke. Quantitative analysis of hemorrhage volume (A, bottom) and infarct volume (B, bottom). C and D, Neurological deficits were assessed by Bederson score (C) and foot-fault test (D). n= 7–9 rats per group. AS: 605240. *P<0.05, **P<0.01, and ***P<0.001 vs. Saline; #P<0.05 and ###P<0.001 vs. tPA (6h).
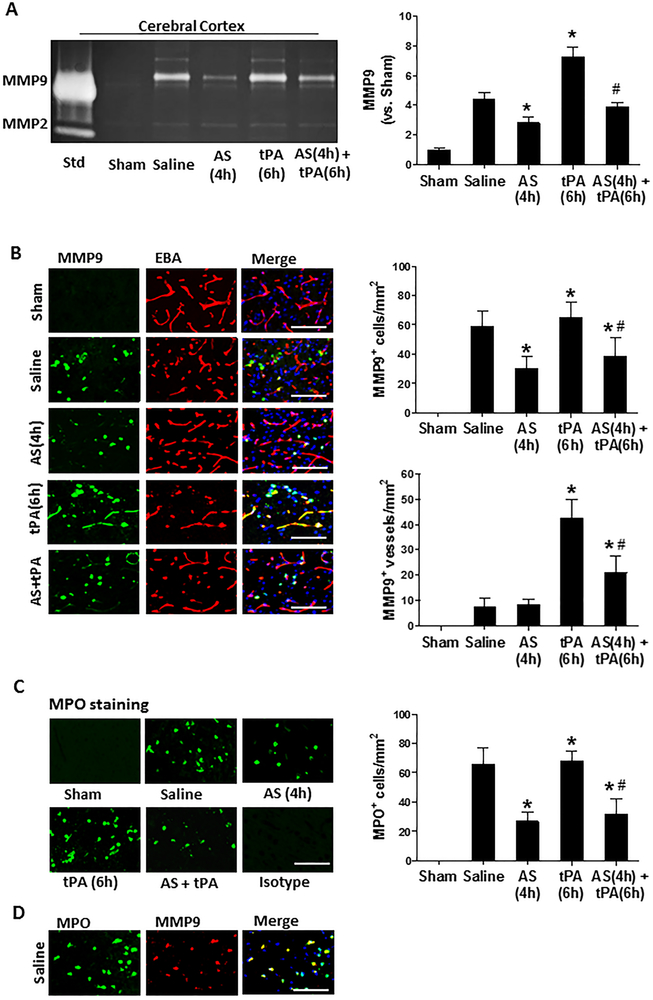
Effects of PI3Kγ inhibition on the ischemia-induced and delayed tPA-enhanced MMP-9 in the ischemic brain
Matrix metalloproteinases (MMPs), in particular MMP-9, plays a pivotal role in the BBB disruption and tPA-associated hemorrhage after stroke [22]. Gelatin zymography was performed to assess MMP-2/MMP-9 activities in the cerebral cortex at 24 hours after stroke (Figure 3A). As expected, both enzyme activities were low at baseline in sham control. MMP-9 activity was significantly increased by ischemia and further enhanced by delayed tPA, and these increases were significantly inhibited by AS605240, whereas MMP-2 activity was not altered by either ischemia, tPA, or AS605240. Immunohistochemistry was performed to determine MMP-9 expression (Figure 3B) and infiltrating neutrophils (marked by MPO staining, Figure 3C) in the brain at 24h after stroke. As expected, both MMP-9 and MPO immunoreactivity were absent in the sham-operated rats and markedly increased in stroke rats. It is worth to note that focal cerebral ischemia (MCAO only, without tPA) significantly increased MMP-9 expression almost exclusively in individual cells but rarely in cerebral vessels, whereas delayed tPA at 6 hours caused further increase in MMP-9 expression prominently in brain microvessels (marked by EBA staining). Double immunostaining indicated that the MMP-9-positive cells were almost exclusively myeloperoxidase (MPO)-positive neutrophils (Figure 3D). Importantly, the ischemia-induced infiltrating neutrophils and neutrophil-derived MMP-9 as well as the tPA-enhanced brain microvascular MMP-9 expression were reduced by AS605240 treatment.
Figure 3.
Effect of PI3Kγ inhibition on the ischemia-induced and tPA-enhanced MMP-9 expression in the ischemic brain 24 hours after stroke. A, Representative images of gelatin zymography for MMP-2/−9 activity in the cerebral cortex in the indicated groups. Semi-quantitation of MMP-9 bands was analyzed by densitometry. Std: standard human MMP-2/−9 mixed marker. Data are mean ± SEM from 3 independent experiments. *P<0.05 vs. Saline; #P<0.05 vs tPA (6h). B, Representative images of double immunofluorescence staining for MMP-9 with the endothelial barrier antigen (EBA) in the indicated groups. C, Representative images of brain-infiltrating neutrophils immunostained by anti-myeloperoxidase (MPO) antibody in the indicated groups. D, Representative images of double immunofluorescence staining for MPO and MMP-9 in the saline-treated MCAO group, indicating that MMP-9 positive cells are almost MPO-positive infiltrated neutrophils in the ischemic brain. Negative stain control without primary antibody did not show detectable labeling. B-D, Images were acquired from peri-infarct cortex. Bar= 50 μm. MMP-9+ cells, MMP-9+ vessels, and MPO+ neutrophils were counted as described in the Method section. n=5 rats per group. *P<0.05 vs. Saline, and #P<0.05 vs tPA (6h).
Effects of PI3Kγ inhibition on microvascular thrombosis and cerebral vascular patency
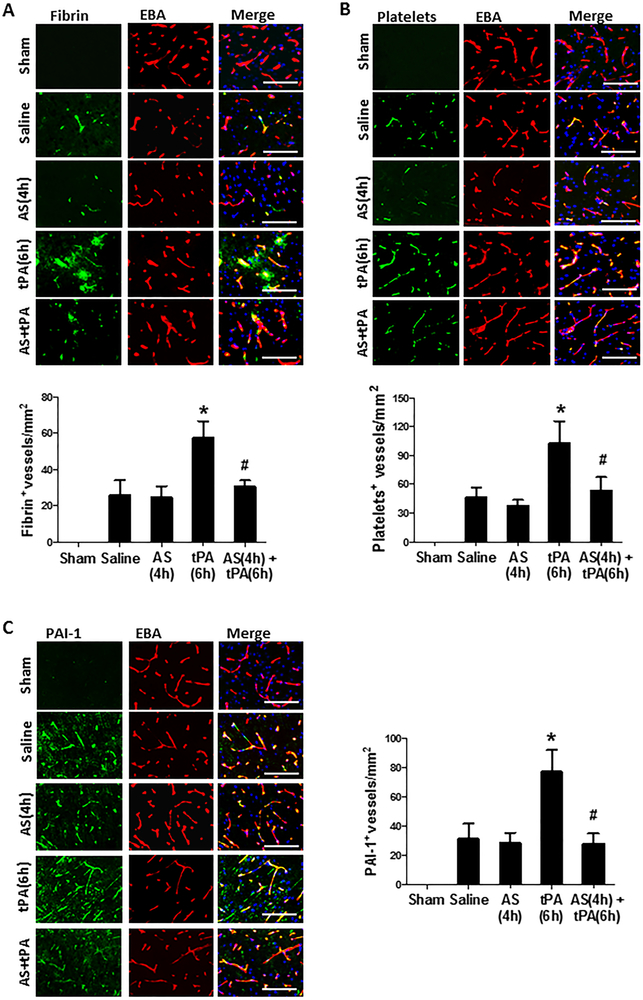
Intravascular fibrin/fibrinogen deposition and platelet accumulation substantially contribute to secondary microvascular thrombosis after ischemic stroke [27]. Double immunofluorescence staining showed that intravascular fibrin/fibrinogen deposition (Figure 4A) and platelet accumulation (Figure 4B) were rarely detected in sham-operated, non-ischemic rats, and at a relatively low level detected in the saline-treated stroke rats possibly due to low cerebral perfusion in downstream microvessels without tPA thrombolysis in this stroke model induced by blood clots. However, both the fibrin/fibrinogen and platelets deposited in downstream microvessels were markedly increased in stroke rats with delayed tPA, and these increases were profoundly inhibited by combination treatment with AS605240 (Figure 4A and4B). NF-kB-dependent upregulation of PAI-1 (plasminogen activator inhibitor-1) in ischemic brain endothelium may foster intravascular fibrin/fibrinogen deposition after acute focal cerebral ischemia. Immunohistochemistry showed that combination treatment with AS60524 significantly reduced ischemia-induced and delayed tPA-enhanced NF-kB p65 phosphorylation (Figure S2B) and PAI-1 expression in brain endothelial cells observed in the peri-ischemic area of the cerebral cortex (Figure 4C).
Figure 4.
Effect of PI3K γ inhibition on microvascular thrombosis and PAI-1 expression 24 hours after stroke. A and B, Representative images of double immunofluorescence staining showing fibrin (A, top) or thrombocytes (B, top) deposited in brain microvessels (marked by EBA staining). The number of fibrin-positive (A, bottom) and thrombocyte-positive (B, bottom) vessels was counted as described in the Methods section. C, Representative images of double immunofluorescence staining for PAI-1 (green) with EBA (red) in indicated groups. The number of PAI-1-positive vessels was counted as described in the Methods section. A through C: Images were acquired from peri-infarct cortex; Bar=50 μm; n=5 per group; *P<0.05 vs. Saline, and #P<0.05 vs tPA (6h).
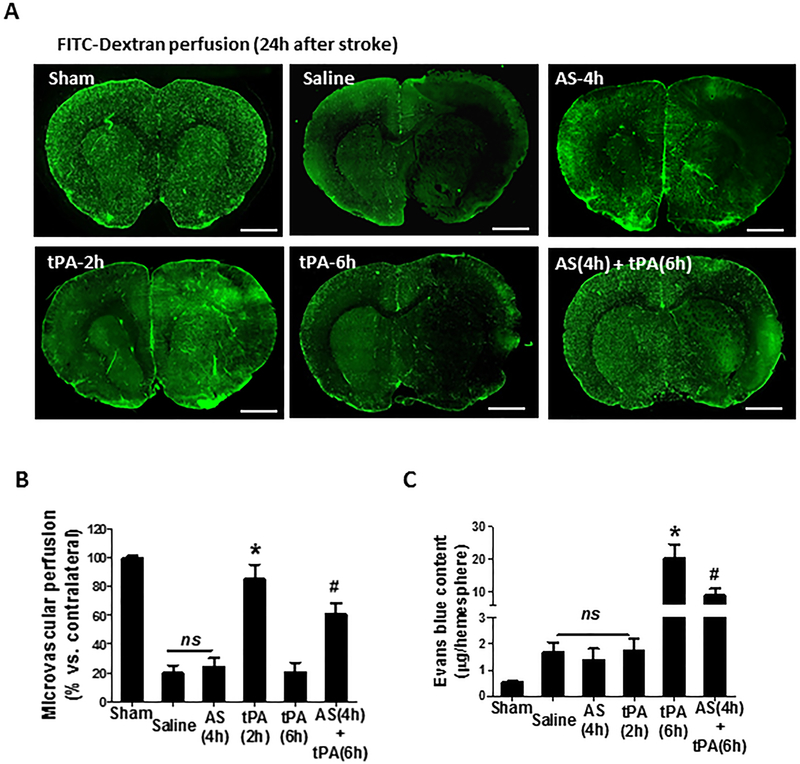
Next, we used the FITC-dextran perfusion method to assess microvascular patency (Figure 5A and5B). Microvascular patency (determined by cerebral area perfused with FITC-dextran) was reduced by >80% in the saline-treated MCAO group compared with sham control. As expected, early IV tPA (at 2h) successfully restored the cerebral perfusion to 85% of the baseline but AS605240 (at 4h) alone and delayed IV tPA (at 6h) were ineffective. Encouragingly, the combination treatment with AS605240 plus delayed tPA restored the cerebral perfusion to 60% of the baseline. Early BBB disruption after thrombolytic therapy predicts brain hemorrhage in patients with acute stroke [28]. BBB permeability was assessed by Evans blue extravasation into the brain parenchyma at 12 hours after stroke onset. Evans blue extravasation moderately increased (<2-fold) in the saline-, AS605240-, and early tPA (2h)-treated groups but dramatically increased (> 20-fold) in the delayed tPA (6h) group, however, the delayed tPA-enhanced BBB damage was significantly attenuated by the combination treatment with AS605240 (Figure 5C).
Figure 5.
Effect of PI3Kγ inhibition on microvascular perfusion and BBB permeability. A, Representative images showing microvascular FITC-dextran perfusion in the indicated groups at 24h after stroke. Scale bars: 200 um. B, Data are expressed as the percentage of the microvascular area perfused with FITC-dextran in the ipsilateral versus contralateral hemisphere. n=5 per group. *P<0.05 vs. Saline, and #P<0.05 vs. tPA (6h). C, Evans blue extravasation into ischemic brain tissue in the indicated groups 12 hours after stroke. n=6 per group. *P<0.05 vs. Saline, and #P<0.05 vs. tPA (6h). ns: no significant differences.
Effects of PI3Kγ inhibition on platelet activation and platelet-leukocyte aggregation in vivo
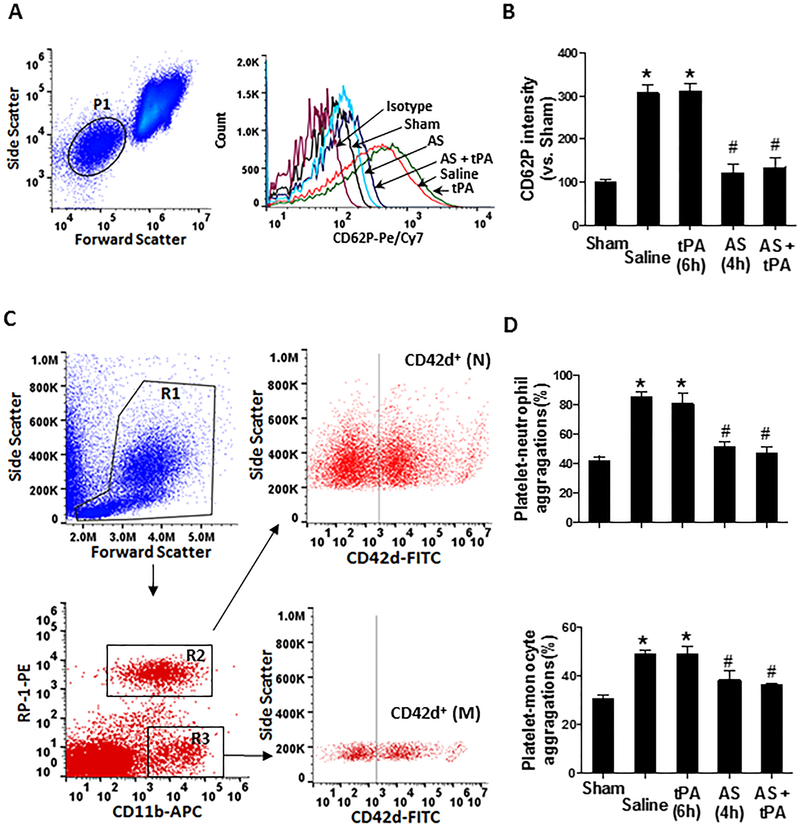
Elevated levels of circulating platelet activation and platelet-leukocyte aggregates contribute importantly to inflammatory and thromboembolic events in ischemic stroke [29]. We used flow cytometry to analyze platelet activation and platelet-leukocyte aggregates in whole blood at 24h after ischemia onset (Figure 6). Increased platelet activation (determined by P-selectin expression) and increased platelet-monocyte aggregates and platelet-granulocyte aggregates were found in the saline-treated stroke rats but IV tPA had no additional effect. However, PI3Kγ inhibition with AS605240 (at 4h) significantly reduced the ischemia-induced platelet P-selectin expression and the platelet-leukocyte aggregation at 24h after stroke.
Figure 6.
Effect of PI3Kγ inhibition on circulating platelet activation and platelet-leukocyte aggregation in vivo. Flow cytometry was performed on whole blood collected via retro-orbital plexus at 24 hours after stroke. A, (left) Forward vs. side scatter density plot of whole blood, in which the platelet population is gated by P1, and (right) Representative histogram of flow cytometry showing P-selectin (CD62P) expression on platelets in the indicated groups. B, Quantitative analysis of platelet P-selectin. Data are expressed as mean fluorescence intensity (MFI). C, Gating strategy for flow cytometric analysis of total leukocytes (circle area in R1) in whole blood: neutrophils (N; R2) and monocytes (M; R3) were defined by their differential expression of CD11b and RP-1 (specific for rat granulocytes). FITC-labeled CD42d mAb was used as a specific platelet marker to identify platelet neutrophil/monocyte complexes. D, Quantitative analysis of platelet-leukocyte aggregates: CD42d+neutrophils and CD41+monocytes. n = 5 rats per group. *p<0.05 vs. Sham and #p<0.05 vs. tPA (6h).
Discussion
The present study, for the first time, demonstrates that therapeutic inhibition of PI3Kγ with its selective inhibitor AS605240 alone initiated at 2 hours after MCAO or the combination treatment with AS605240 at 4 hours plus tPA at 6 hours after stroke substantially reduced infarct volume and neurological deficits. The observed neuroprotective effects by AS605240 are likely attributed to reduced intracerebral hemorrhage, reduced BBB damage, and improved cerebral microvascular patency.
In the present study, we chose to work with hypertensive rats since hypertension is a major modifiable risk factor for stroke and influences clinical outcome. Compared with normotensive rats, SHRs displayed (1) similarly reproducible but larger infarct size, and (2) a similar therapeutic time window for IV tPA but higher incidence of hemorrhagic transformation and higher mortality after ischemic stroke [30,31]. Normotensive Wistar-Kyoto (WKY) rats, the closest genetic control for the SHRs, are rarely used for focal ischemia models in literature. A previous study had compared tPA thrombolytic therapy in SHRs and WKY rats using the same embolic stroke model [23]. In SHRs, delayed IV tPA at 6 hours significantly increased hemorrhage and worsened neurological deficits whereas in WKY rats IV tPA at 6 hours did not significantly increase hemorrhage and unexpectedly reduced infarct volume and neurological deficits. Thus, the WKY embolic model is not suitable for preclinical translational research of thrombolytic therapy. However, other normotensive rats including Sprague Dawley (SD) rats [32–34] are often used in literature for stroke research with thrombolytic therapy.
In the present study, we investigated whether and how inhibition of PI3Kγ extends the therapeutic time window for tPA, using combined treatment with AS605240 at 4 hours and IV tPA at 6 hours after embolic MCAO in the rat. This combination modality is appropriate and has been previously established to extend the therapeutic window for tPA to 6 hours after embolic MCAO in rats with other adjunct drugs, such as taurine, atorvastatin, minocycline, and the proteasome inhibitor ps-519 [18, 35–37]. The therapeutic window of tPA is 2 to 3 hours after embolic MCAO in rats. It has been shown that the standard rodent dose of tPA (10 mg/kg) lost its effectiveness beyond 4 hours after embolic MCAO in rats and instead increased infarct volume, as well as hemorrhagic transformation [32]. Adjunct treatment before IV tPA could block ischemic core enlargement and salvage the penumbra as early as possible and thus might extend the thrombolytic time window for tPA beyond 3–4.5 hours after stroke onset.
PI3Kγ is a multifaceted protein, expressed in many different types of immune and non-immune cells. Increased expression of PI3Kγ in platelets, leukocytes, and endothelial cells has been implicated in inflammation and cardiovascular disease. Genetic and pharmacological targeting of PI3Kγ has yielded encouraging therapeutic benefits in a number of experimental models of human diseases [6–15]. We have previously demonstrated that PI3Kγ deficiency significantly reduced BBB injury, tissue infarction, and neurological deficits after focal cerebral ischemia/reperfusion in mice [13]. We further found that PI3Kγ deficiency blocked tPA-induced brain hemorrhage when tPA was administered at 6 hours after onset of ischemia in mice (Figure S4). These findings prompted us to investigate the therapeutic potential of targeting PI3Kγ in experimental stroke. The pharmacokinetics of the AS605240 compound (t1/2: 2.2 hours) have been well documented in the literature [7]. Oral administration of AS605240 (5 to 50 mg/kg, once daily) has been shown to be effective in several animal disease models [6–11]. Using a high performance liquid chromatography mass spectrometry (HPLC/MS) we measured the AS605240 concentration in rat cerebrospinal fluid (CSF) and serum. Our unpublished data indicated that AS605240 is able to cross the intact BBB in the normal brain and more readily cross the damaged BBB in the ischemic brain. In this study, treatment with AS605240 alone significantly reduced infarct volume when administered at 2 hours after stroke onset, but was minimally effective when administered at 4 hours after stroke onset. Besides its effects on infarction, more importantly, we found that AS605240 may profoundly ameliorate BBB disruption and intracerebral hemorrhage. Thrombolysis with delayed tPA at 6 hours exacerbated early BBB disruption and significantly increased hemorrhage volume compared with saline and early (2-hour) tPA. Encouragingly, these complications associated with delayed tPA thrombolysis were significantly decreased by the combination treatment with AS605240. In addition, the combination treatment with AS605240 may also have ameliorated the high rates of 3-day mortality in SHRs, compared with either the saline- or delayed tPA alone treated group, although it did not reach statistical significance due to the small animal numbers tested (Table S3). Collectively, our data suggest that PI3Kγ inhibition with AS605240 may extend the tPA’s therapeutic time window to 6 hours after ischemia onset.
The brain endothelium is a major target for the tPA-induced BBB breakdown and brain hemorrhage in acute stroke. It is well documented that delayed IV tPA induces brain hemorrhage through activating NF-kB (nuclear factor kB)-dependent upregulation of MMP-9 in ischemic brain endothelium after acute stroke [38]. Our previous study has shown that PI3Kγ deficiency suppressed ischemia/reperfusion-induced NF-kB activation and MMP-9 (derived from either infiltrated neutrophils or ischemic brain endothelium) in mice [13]. In the present study, we found that administration of AS605240 effectively prevented tPA-induced hemorrhage primarily by targeting PI3Kγ in the brain endothelium. This is supported by the following findings: (1) PI3Kγ expression was robustly induced in the ischemic brain endothelium in the acute phase (4–24h) after stroke, (2) PI3Kγ inhibition with AS605240 at 4h suppressed tPA-enhanced NF-kB activation and reduced MMP-9 expression in the ischemic brain endothelium, and profoundly attenuated the delayed tPA-enhanced (over 20-fold) BBB permeability early (at 12h) after ischemia onset. Early disruption of the BBB after thrombolysis with tPA predicts fatal hemorrhage in patients with acute ischemia stroke [28]. Collectively, our results reveal a previously undescribed role for PI3Kγ in tPA-induced brain hemorrhage by driving MMP-9 activation in ischemic brain endothelium after stroke that can be therapeutically targeted by administration of AS605240.
Secondary microvascular thrombosis is an obstacle hindering the efficacy of reperfusion therapies (IV tPA, mechanical thrombectomy, or both) for patients with acute ischemic stroke (AIS) [39]. A subset of stroke patients, despite successful tPA thrombolysis, still exhibit progressive neurological deterioration and this is likely related to incomplete microvascular reperfusion [27, 40]. While mechanical thrombectomy is becoming a highly efficacious therapy in stroke patients with proximal large vessel occlusions, about one-third of thrombectomy recipients did not recover to functional independence despite fast and successful recanalization by acute mechanical thrombectomy and this may also partially be explained by incomplete microvascular reperfusion [41, 42]. Using light/electron microscopy and intravital imaging, experimental studies have shown that microvascular lumina were obstructed with platelets, leukocytes, and fibrin-rich aggregates during early focal ischemia and reperfusion [43–48]. Thus, preventing secondary microvascular thrombosis might improve the success rate of reperfusion therapies in stroke [27,49]. In the present study, early thrombolysis with tPA (at 2h) restored (≈85%) microvascular patency but delayed tPA (at 6h) was ineffective. However, the combined treatment with AS605240 plus 6-hour tPA significantly restored (≈60%) microvascular patency compared with saline and delayed tPA alone. The observed anti-thrombotic effects by AS605240 are likely attributed to reduced fibrin/fibrinogen and platelets deposited in downstream microvessels, which in turn reduces downstream microvascular thrombosis and improves microvascular patency. Although some antithrombotics, e.g., antiplatelet agents (aspirin, clopidogrel) and heparin, may be able to restore microvascular reperfusion, antithrombotics may also increase the risk of symptomatic intracranial hemorrhage in stroke patients. It has been shown that PI3Kγ deficiency did not influence bleeding time in mice [50]. Here, we further found that AS605240 treatment did not prolong tail bleeding time in both normal and MCAO rats (Figure S1B). These results suggest that inhibition of PI3Kγ with AS605240 would be beneficial for reperfusion therapy (IV tPA and/or endovascular thrombectomy) by reducing secondary downstream microvascular thrombosis and thereby ameliorating microvascular reperfusion.
PI3Kγ is known to be key mediators of inflammation and thrombosis, 2 closely intertwined processes. Increased platelet activation and platelet-leukocyte interactions link inflammatory and thromboembolic events in ischemic stroke [29]. An intravital imaging study by Desilles et al showed that MCAO induced rapid and profound leukocyte margination that fostered fibrinogen deposition and thrombosis in downstream microvessels [51]. We recently demonstrated that genetic deletion of PI3Kγ in platelets inhibited platelet activation and interaction with leukocytes and endothelial cells after vascular injury in mice [52]. In the present study, we found that inhibition of PI3Kγ with AS605240 profoundly inhibited circulating platelet activation and platelet-leukocyte (neutrophil, monocyte) aggregation elicited by MCAO. Our findings suggest that the microvascular protection by inhibition of PI3Kγ probably involves a combined antithrombotic and anti-inflammatory mechanism.
Perspectives
The present study provides the first evidence that the combination treatment with AS605240 may extend the therapeutic window for tPA to 6 h after ischemic stroke. Therapeutic potential with multiple mechanisms of action of PI3Kγ inhibition suggests that PI3Kγ represents a promising target for treating ischemic stroke, and therefore merits further preclinical investigation and evaluation. A limitation in the present study was only using young adult male rats. It should be noted that stroke is a sexually dimorphic disease, with differences between males and females in both animals and humans. Females generally have strokes at older ages than males and, therefore, have a worse stroke outcome. The therapeutic effects of AS605240, alone and in combination with tPA, in aged rats in both sexes are currently under investigation in our laboratory.
Supplementary Material
Novelty and Significance
What Is New?
The upregulated PI3Kγ on brain microvascular endothelial cells contributes to ischemic brain injury and may be a novel therapeutic target in ischemic stroke.
Therapeutic inhibition of PI3Kγ with its selective inhibitor AS605240 effectively blocks tPA-induced brain hemorrhage and extends the tPA’s therapeutic time window to 6 hours after ischemia onset in spontaneously hypertensive rats.
The neuroprotective mechanisms of action of PI3Kγ inhibition by AS605240 are associated with profound inhibition of the ischemia-induced and delayed (6-hour) tPA-enhanced expression of MMP-9 and PAI-1 in the ischemic brain endothelium, leading to reduced BBB disruption and hemorrhagic transformation and improved microvascular patency.
What Is Relevant?
Clinical use of tPA for thrombolysis is limited by narrow time windows mainly due to increased risks of brain hemorrhage.
Incomplete microvascular reperfusion resulting from secondary downstream microvascular thrombosis hinders the efficacy of tPA thrombolytic therapy in ischemic stroke.
Hypertension is a major modifiable risk factor for stroke and influences clinical outcome.
Summary
We demonstrate that therapeutic targeting of PI3Kγ may be a novel approach to block tPA-induced brain hemorrhage and improve microvascular reperfusion in acute ischemic stroke.
Acknowledgments
Sources of Funding
This work was supported by the National Institutes of Health grants NS089991 and NS088719 (Dr. Li).
Footnotes
Disclosures
None
Contributor Information
Rong Jin, Department of Neurosurgery and Neuroscience Institute, Penn State Hershey Medical Center, Hershey.
Adam Y Xiao, Molecular and Cellular Physiology, Louisiana State University Health Sciences Center, Shreveport..
Jarvis Li, Hershey High School, Louisiana State University Health Sciences Center, Shreveport..
Min Wang, Department of Neurosurgery and Neuroscience Institute, Penn State Hershey Medical Center, Hershey.
Guohong Li, Department of Neurosurgery and Neuroscience Institute, Penn State Hershey Medical Center, Hershey.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart Disease and Stroke Statistics-2017 Update: a report from the American Heart Association. Circulation. 2017;135: e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adeoye O, Hornung R, Khatri P, Kleindorfer D. Recombinant tissue-type plasminogen activator use for ischemic stroke in the United States: a doubling of treatment rates over the course of 5 years. Stroke. 2011; 42:1952–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng NT, Kim AS. Intravenous thrombolysis for acute ischemic stroke within 3 hours versus between 3 and 4.5 hours of symptom onset. The Neurohospitalist. 2015; 5:101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008; 359:1317–1329. [DOI] [PubMed] [Google Scholar]
- 5.Hirsch E, Lembo G, Montrucchio G, Rommel C, Costa C, Barberis L. Signaling through PI3Kgamma: a common platform for leukocyte, platelet and cardiovascular stress sensing. Thromb Haemost. 2006; 95:29–35. [PubMed] [Google Scholar]
- 6.Barber DF, Bartolomé A, Hernandez C, Flores JM, Redondo C, Fernandez-Arias C, et al. PI3Kgamma inhibition blocks glomerulonephritis and extends lifespan in a mouse model of systemic lupus. Nat Med 2005;11: 933–935. [DOI] [PubMed] [Google Scholar]
- 7.Camps M, Ruckle T, Ji H, Ardissone V, Rintelen F, Shaw J, et al. Blockade of pi3kgamma suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nat Med 2005;11: 936–943. [DOI] [PubMed] [Google Scholar]
- 8.Azzi J, Moore RF, Elyaman W, Mounayar M, El Haddad N, Yang S, et al. The novel therapeutic effect of phosphoinositide 3-kinase-γ inhibitor AS605240 in autoimmune diabetes. Diabetes. 2012; 6:1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fougerat A, Gayral S, Gourdy P, Schambourg A, Rückle T, Schwarz MK, et al. Genetic and pharmacological targeting of phosphoinositide 3-kinase-gamma reduces atherosclerosis and favors plaque stability by modulating inflammatory processes. Circulation. 2008;117:1310–1317. [DOI] [PubMed] [Google Scholar]
- 10.Kim DI, Kim SR, Kim HJ, Lee SJ, Lee HB, Park SJ, et al. PI3K-gamma inhibition ameliorates acute lung injury through regulation of IκBα/NF-κB pathway and innate immune responses. J Clin Immunol 2012; 32:340–351. [DOI] [PubMed] [Google Scholar]
- 11.van Dop WA, Marengo S, te Velde AA, Ciraolo E, Franco I, ten Kate FJ, et al. The absence of functional PI3Kgamma prevents leukocyte recruitment and ameliorates DSS-induced colitis in mice. Immunol Lett 2010; 131:33–39. [DOI] [PubMed] [Google Scholar]
- 12.Jin R, Yu S, Song Z, Quillin JW, Deasis DP, Penninger JM, et al. Phosphoinositide 3-kinase-gamma expression is upregulated in brain microglia and contributes to ischemia-induced microglial activation in acute experimental stroke. Biochem Biophys Res Comm 2010; 399:458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin R, Song Z, Yu S, Piazza A, Nanda A, Penninger JM, et al. Phosphatidylinositol-3-kinase gamma plays a central role in blood-brain barrier dysfunction in acute experimental stroke. Stroke. 2011; 42:2033–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang L, Sherchan P, Wang Y, Reis C, Applegate RL, Tang J, et al. Phosphoinositide 3-kinase gamma contributes to neuroinflammation in a rat model of surgical brain injury. J Neurosci 2015;35:10390–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Passos GF, Figueiredo CP, Prediger RD, Silva KA, Siqueira JM, Duarte FS, et al. Involvement of phosphoinositide 3-kinase gamma in the neuro-inflammatory response and cognitive impairments induced by beta-amyloid 1–40 peptide in mice. Brain Behav Immun 2010; 24:493–501. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Tsuji K, Lee SR, Ning M, Furie KL, Buchan AM, et al. Mechanisms of hemorrhagic transformation after tissue plasminogen activator reperfusion therapy for ischemic stroke. Stroke. 2004;35:2726–2730. [DOI] [PubMed] [Google Scholar]
- 17.Cheng T, Petraglia AL, Li Z, Thiyagarajan M, Zhong Z, Wu Z, et al. Activated protein C inhibits tissue plasminogen activator-induced brain hemorrhage. Nat Med 2006;12:1278–1285. [DOI] [PubMed] [Google Scholar]
- 18.Jin R, Xiao AY, Liu S, Wang M, Li G. Taurine reduces tissue plasminogen activator-induced hemorrhage and microvascular thrombosis after embolic stroke in rat. Stroke. 2018;49:1708–1718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barr TL, Latour LL, Lee KY, Schaewe TJ, Luby M, Chang GS, et al. Blood–brain barrier disruption in humans is independently associated with increased matrix metalloproteinase-9. Stroke. 2010;41:e123–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosell A, Cuadrado E, Ortega-Aznar A, Hernández-Guillamon M, Lo EH, Montaner J. MMP-9-positive neutrophil infiltration is associated to blood–brain barrier breakdown and basal lamina type IV collagen degradation during hemorrhagic transformation after human ischemic stroke. Stroke. 2008;39:1121–1126 [DOI] [PubMed] [Google Scholar]
- 21.Jin R, Zhu X, Li G. Embolic middle cerebral artery occlusion (MCAO) for ischemic stroke with homologous blood clots in rats. J Vis Exp 2014;91:51956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sumii T, Lo EH. Involvement of matrix metalloproteinase in thrombolysis-associated hemorrhagic transformation after embolic focal ischemia in rats. Stroke. 2002;33:831–836. [DOI] [PubMed] [Google Scholar]
- 23.Asahi M, Asahi K, Wang X, Lo EH. Reduction of tissue plasminogen activator-induced hemorrhage and brain injury by free radical spin trapping after embolic focal cerebral ischemia in rats. J Cereb Blood Flow Metab 2000;20:452–457. [DOI] [PubMed] [Google Scholar]
- 24.Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. [DOI] [PubMed] [Google Scholar]
- 25.Hernandez TD, Schallert T. Seizures and recovery from experimental brain damage. Exp Neurol 1998;102:318–324. [DOI] [PubMed] [Google Scholar]
- 26.Jin R, Xiao AY, Song Z, Yu S, Li J, Cui MZ, et al. Platelet CD40 mediates leukocyte recruitment and neointima formation after arterial denudation injury in atherosclerosis-prone mice. Am J Pathol 2018;188:252–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Meyer SF, Denorme F, Langhauser F, Geuss E, Fluri F, Kleinschnitz C. Thromboinflammation in stroke brain damage. Stroke. 2016;47:1165–1172. [DOI] [PubMed] [Google Scholar]
- 28.Kastrup A, Gröschel K, Ringer TM, Redecker C, Cordesmeyer R, Witte OW, et al. Early disruption of the blood-brain barrier after thrombolytic therapy predicts hemorrhage in patients with acute stroke. Stroke. 2008;39:2385–2387. [DOI] [PubMed] [Google Scholar]
- 29.Franks ZG, Campbell RA, Weyrich AS, Rondina MT. Platelet-leukocyte interactions link inflammatory and thromboembolic events in ischemic stroke. Ann N Y Acad Sci 2010; 1207: 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Collins VE, Donnan GA, Macleod MR, Howells DW. Hypertension and experimental stroke therapies. J Cereb Blood Flow Metab 2013;33:1141–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fluri F, Schuhmann MK, Kleinschnitz C. Animal models of ischemic stroke and their application in clinical research. Drug Des Devel Ther 2015;9:3445–3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kano T, Katayama Y, Tejima E, Lo EH. Hemorrhagic transformation after fibrinolytic therapy with tissue plasminogen activator in a rat thromboembolic model of stroke. Brain Res 2000;854:245–248. [DOI] [PubMed] [Google Scholar]
- 33.Li M, Chen S, Shi X, Lyu C, Zhang Y, Tan M, et al. Cell permeable HMGB1-binding heptamer peptide ameliorates neurovascular complications associated with thrombolytic therapy in rats with transient ischemic stroke. J Neuroinflammation. 2018;15:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Q, Han X, Lan X, Hong X, Li Q, Gao Y, et al. Inhibition of tPA-induced hemorrhagic transformation involves adenosine A2b receptor activation after cerebral ischemia. Neurobiol Dis 2017;108:173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang L, Chopp M, Jia L, Cui Y, Lu M, Zhang ZG. Atorvastatin extends the therapeutic window for tPA to 6 h after the onset of embolic stroke in rats. J Cereb Blood Flow Metab 2009;29:1816–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murata Y, Rosell A, Scannevin RH, Rhodes KJ, Wang X, Lo EH. Extension of the thrombolytic time window with minocycline in experimental stroke. Stroke. 2008;39:3372–3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang L, Zhang ZG, Zhang RL, Lu M, Adams J, Elliott PJ, Chopp M. Postischemic (6-hour) treatment with recombinant human tissue plasminogen activator and proteasome inhibitor ps-519 reduces infarction in a rat model of embolic focal cerebral ischemia. Stroke. 2001;32:2926–2931. [DOI] [PubMed] [Google Scholar]
- 38.Harari Olivier A. and Liao James K.. NF-κB and innate immunity in ischemic stroke. Ann N Y Acad Sci 2010; 1207: 32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kehrel BE, Fender AC. Resolving Thromboinflammation in the Brain After Ischemic Stroke? Circulation. 2016. ;133:2128–2131. [DOI] [PubMed] [Google Scholar]
- 40.Alexandrov AV, Hall CE, Labiche LA, Wojner AW, Grotta JC. Ischemic stunning of the brain: early recanalization without immediate clinical improvement in acute ischemic stroke. Stroke. 2004;35:449–452. [DOI] [PubMed] [Google Scholar]
- 41.Ganesh A, Goyal M. Thrombectomy for Acute Ischemic Stroke: Recent Insights and Future Directions. Curr Neurol Neurosci Rep 2018;18:59. [DOI] [PubMed] [Google Scholar]
- 42.Goyal M, Menon BK, van Zwam WH, Dippel DWJ, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723–1731. [DOI] [PubMed] [Google Scholar]
- 43.Okada Y, Copeland BR, Fitridge R, Koziol JA, del Zoppo GJ. Fibrin contributes to microvascular obstructions and parenchymal changes during early focal cerebral ischemia and reperfusion. Stroke. 1994;25:1847–1853. [DOI] [PubMed] [Google Scholar]
- 44.Zhang ZG, Zhang L, Tsang W, Goussev A, Powers C, Ho KL, et al. Dynamic platelet accumulation at the site of the occluded middle cerebral artery and in downstream microvessels is associated with loss of microvascular integrity after embolic middle cerebral artery occlusion. Brain Res 2001;912:181–194. [DOI] [PubMed] [Google Scholar]
- 45.del Zoppo GJ, Schmid-Schönbein GW, Mori E, Copeland BR, Chang CM. Polymorphonuclear leukocytes occlude capillaries following middle cerebral artery occlusion and reperfusion in baboons. Stroke. 1991;22:1276–1283. [DOI] [PubMed] [Google Scholar]
- 46.Zhang ZG, Chopp M, Goussev A, Lu D, Morris D, Tsang W, et al. Cerebral microvascular obstruction by fibrin is associated with upregulation of PAI-1 acutely after onset of focal embolic ischemia in rats. J Neurosci 1999;19:10898–10907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ishikawa M, Cooper D, Arumugam TV, Zhang JH, Nanda A, Granger DN. Platelet-leukocyte-endothelial cell interactions after middle cerebral artery occlusion and reperfusion. J Cereb Blood Flow Metab 2004;24:907–915. [DOI] [PubMed] [Google Scholar]
- 48.Ritter LS, Orozco JA, Coull BM, McDonagh PF, Rosenblum WI. Leukocyte accumulation and hemodynamic changes in the cerebral microcirculation during early reperfusion after stroke. Stroke. 2000;31:1153–1161. [DOI] [PubMed] [Google Scholar]
- 49.Gursoy-Ozdemir Y, Yemisci M, Dalkara T. Microvascular protection is essential for successful neuroprotection in stroke. J Neurochem 2012;123 Suppl 2:2–11. [DOI] [PubMed] [Google Scholar]
- 50.Hirsch E, Bosco O, Tropel P, Laffargue M, Calvez R, Altruda F, et al. Resistance to thromboembolism in PI3Kgamma-deficient mice. FASEB J 2001;15:2019–2021. [DOI] [PubMed] [Google Scholar]
- 51.Desilles JP, Loyau S, Syvannarath V, Gonzalez-Valcarcel J, Cantier M, Louedec L, et al. Alteplase reduces downstream microvascular thrombosis and improves the benefit of large artery recanalization in stroke. Stroke. 2015;46:3241–3248. [DOI] [PubMed] [Google Scholar]
- 52.Wang C, Jin R, Nanda A, Yan J, Li G. Platelet PI3Kγ contributes to carotid intima-media thickening under severely reduced flow conditions. PLoS One. 2015;10:e0129265. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.