Abstract
Background:
Merkel cell polyomavirus (MCV) was discovered by digital transcriptome subtraction as a monoclonal infection of Merkel cell carcinoma (MCC) tumors. Subsequent studies have repeatedly confirmed that MCV is the likely cause for most MCC. Polymerase chain reaction—based detection of the virus in other nonmelanoma skin cancers, however, has been inconsistent and controversial.
Objective:
We sought to directly assay for MCV infection in squamous cell carcinoma (SCC) or basal cell carcinoma (BCC) tumor cells by immunostaining for viral antigen.
Methods:
CM2B4, a monoclonal antibody to exon 2 peptides of MCV T antigen, was used to examine tumors from 20 patients with MCC with and without secondary SCC or BCC tumors.
Results:
MCV T antigen was readily detected in 15 (75%) of 20 MCC tumors including 11 MCC tumors from patients with secondary SCC or BCC. In contrast to MCC, none of these secondary BCC or SCC was MCV T- antigen positive.
Limitations:
A limitation was the small study size with antigen detection including only the MCV large T and 57kT proteins.
Conclusions:
MCV T antigen is generally not expressed in BCC or SCC tumors from a population favored to have MCV infection, ie, those persons already given the diagnosis of MCV-positive MCC. This suggests that episodic polymerase chain reaction detection of MCV genome in BCC or SCC tumors may represent coincidental rather than causal infection, and that these tumors share other noninfectious risk factors. (J Am Acad Dermatol 2010;63:400–3.)
Keywords: basal cell carcinoma, CM2B4, Merkel cell polyomavirus, squamous cell carcinoma, T antigen
Merkel cell polyomavirus (MCV) is monoclonally integrated into the genome of most Merkel cell carcinoma (MCC),1 an aggressive neuroendocrine skin cancer. Ultraviolet and ionizing radiation exposure have been postulated to increase the risk of MCC, and MCC has been reported to occur together with other skin cancers including basal cell carcinoma (BCC) and squamous cell carcinoma (SCC).2 Intriguingly, tumor-specific mutations are found in MCV genomes isolated from tumor tissues but not healthy tissues, leading to speculation that sun exposure may increase risk for MCC by direct mutagenic effects on the virus itself.3 Multiple surveys have confirmed that MCV is present in approximately 70% to 80% of MCC tumors from a variety of settings but also show that non-MCC tissues have infection rates of 0% to 16% by polymerase chain reaction (PCR).4–6 Rates of MCV detection in other nonmelanoma skin cancers vary widely between different studies, leaving the issue unresolved of whether this virus could be causal for other skin cancers besides MCC.
To determine if MCV plays a role in other nonmelanoma skin cancers, we examined secondary BCC and SCC tumors from immunocompetent patients having a preceding primary MCC tumor. Our rationale for choosing this population was that if MCV plays a role in causing BCC or SCC, the virus would be most readily seen in tumors arising in persons who already developed an MCV-infected MCC.
METHODS
Institutional review board approval was obtained and 20 patients were identified as having a pathologic diagnosis of MCC between 1996 and 2008 at Southern California Kaiser Permanente in the Woodland Hills and Kern County service regions. Archival MCC tumor blocks and secondary BCC or SCC tumor blocks were retrieved and patient charts were reviewed. All MCC tumors were confirmed by positive cytokeratin 20 staining.
To assess infection with MCV, archival paraffin- embedded tissue blocks were stained with a monoclonal antibody, CM2B4 (IgG2b isotype), raised against exon 2 of the MCV T antigen.7 Immunohistochemistry methods are described elsewhere in detail.7 Briefly, epitope retrieval was performed using EDTA antigen retrieval buffer (Dako, Glostrup, Denmark) after de- paraffinization and hydrogen peroxide treatment. After blocking with Protein Block (Dako), samples were reacted overnight to antibody CM2B4 (1:10–1:50 hybridoma supernatant). After washing, samples were incubated with Mouse Envision Polymer (Dako) for subsequent deaminobenzidine reaction. Immunostaining readily detects uniform tumor cell MCV infection in MCC tumors with an 83% sensitivity compared with PCR7 and tumor tissues were processed together with known MCV-positive and MCV-negative control samples. The antibody does not cross-react with other human and primate polyomavirus T antigens, including JC virus, BK virus, WU virus, and Simian Vacuolating virus 40.7
RESULTS
All 20 patients with MCC were Caucasian with a 3:1 male predominance and a median age of 73 years (range 54–83 years). Extent of MCC disease at presentation included 11 (55%) with local disease only, 7 (35%) with nodal disease, and 2 (10%) with distant metastatic disease. The majority of MCC tumors were in sun-exposed sites including head/neck (40%), trunk (20%), and upper limb (25%). The remaining 15% were in relatively sun-protected sites including lower limb (10%) and buttocks (5%). According to chart review, none of the patients were immunosuppressed. Twelve (60%) of these patients developed secondary BCC or SCC subsequent to their MCC diagnosis.
Fifteen (75%) of 20 MCC tumors in our cohort stained positive for MCV T antigen by CM2B4 immunostaining. MCC tumors positive for CM2B4 staining revealed antigen expression limited to tumor cells and not present in healthy surrounding tissue cells. For those MCC tumors having CM2B4 positivity, the majority of tumor cells stained positive for viral antigen. There was concordance in MCV positivity for 3 primary MCC tumors in which disseminated lymph node or metastatic tissue sections were available. Twelve of these patients with MCC developed a subsequent nonmelanoma skin cancer, including 9 BCC and 3 SCC tumors. Although 11 (92%) of the 12 MCC tumors from patients with secondary tumors were positive for MCV T antigen, none of the secondary BCC (Fig 1) or SCC (Fig 2) showed evidence for CM2B4 reactivity.
Fig 1.
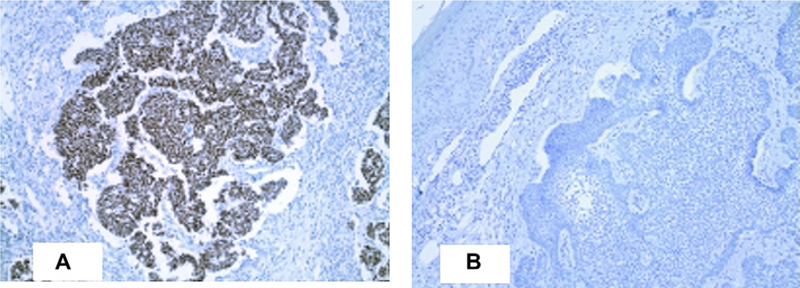
Representative patient with paired Merkel cell carcinoma (A) and subsequent basal cell carcinoma (B) immunostaining of Merkel cell polyomavirus T antigen with CM2B4. (Original magnification: ×100.)
Fig 2.
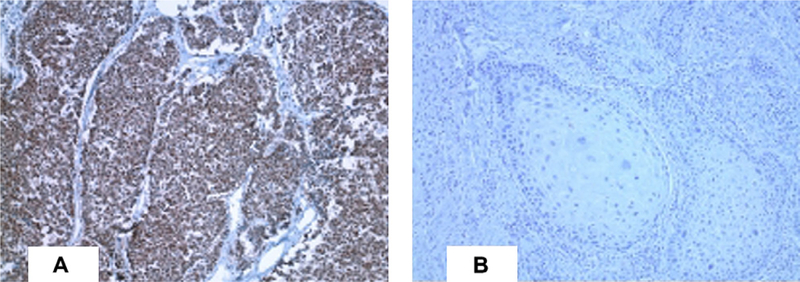
Representative patient with paired Merkel cell carcinoma (A) and subsequent squamous cell carcinoma (B) immunostaining of Merkel cell polyomavirs T antigen with CM2B4. (Original magnification: ×100.)
DISCUSSION
Both tissue surveys and serologic studies suggest that a substantial fraction of adult human beings may be asymptomatically infected with MCV.8,9 Viral genome can be found by PCR at low copy number in peripheral blood, skin, and gut tissues as an incidental infection.1 Using real-time PCR, Becker et al5 found 38 (85%) of 45 European primary MCC tumors positive for MCV with concordance in metastatic tissue. These authors also examined 24 BCC from patients and found a 12.5% MCV infection rate in these tumors. This is comparable with skin tissue detection rates found by others and is most consistent with incidental carriage of infection in these tumors. In contrast, Kassem et al10 recently reported detecting MCV by PCR in 62% of SCC, BCC, and Bowen disease tissues from immunosuppressed patients and in 32% of these cancers from immunocompetent patients. In contrast, Ridd et al11 examined 156 nonmelanoma skin cancers from patients with transplantation, including BCC, SCC, keratoacanthomas, and Bowen disease tumors, only one of which (a keratoacanthoma) was positive for MCV DNA. None of the patients from these studies had a preceding MCC. These conflicting studies used PCR to detect MCV DNA in bulk tissue which cannot resolve incidental infection from causal infection of BCC and SCC tumors.
To resolve these controversial findings, we used CM2B4 antibody staining because it allows direct detection of MCV protein expression in tumor cells. We examined matched pairs of MCC and BCC or SCC tumors from 12 patients to optimize the opportunity to detect MCV infection in the BCC and SCC tumors. Instead, none of the non-MCC tumors were positive for viral protein expression. This suggests that although nonmelanoma skin cancers may share some risk factors with MCC, such as excessive sun exposure, they are not likely to be caused by MCV infection. Detection of MCV DNA PCR positivity in BCC and SCC tumors from immunosuppressed persons is likely to represent loss of immune control over viral replication, leading to enhanced coinci0dental detection. Evidence that MCV infection is specific to MCC tumor cells and is not commonly found in other skin cancers lends additional weight to the conclusion that MCV is the causal factor for MCV-infected MCC tumors.
CAPUSLE SUMMARY.
Merkel cell polyomavirus is a newly discovered virus that is the likely cause of most Merkel cell carcinomas.
Conflicting evidence has been published suggesting that Merkel cell polyomavirus may also contribute to other nonmelanoma skin cancers.
Using a new monoclonal antibody we show that most secondary squamous and basal cell carcinomas from patients with Merkel cell carcinoma are negative for the virus, indicating that when the virus is found in these tumors it is most likely a coincidental infection.
Acknowledgments
Supported by National Institutes of Health CA136363, CA120726; Al Copeland Foundation; University of Pittsburgh EXPLORER grant; and American Cancer Society Professorship Grants to Drs Chang and Moore.
Abbreviations used:
- BCC:
basal cell carcinoma
- MCC:
Merkel cell carcinoma
- MCV:
Merkel cell polyomavirus
- PCR:
polymerase chain reaction
- SCC:
squamous cell carcinoma
Footnotes
Disclosure: The University of Pittsburgh has submitted patents that may be related to this work.
REFERENCES
- 1.Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science 2008; 319:1096–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ball NJ, Tanhuanco-Kho G. Merkel cell carcinoma frequently shows histologic features of basal cell carcinoma: a study of 30 cases. J Cutan Pathol 2007;34:612–9. [DOI] [PubMed] [Google Scholar]
- 3.Shuda M, Feng H, Kwun HJ, Rosen ST, Gjoerup O, Moore PS, et al. T antigen mutations are a human tumor-specific signature for Merkel cell polyomavirus. Proc Natl Acad Sci U S A 2008;105:16272–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kassem A, Schopflin A, Diaz C, Weyers W, Stickeler E, Werner M, et al. Frequent detection of Merkel cell polyomavirus in human Merkel cell carcinomas and identification of a unique deletion in the VP1 gene. Cancer Res 2008;68: 5009–13. [DOI] [PubMed] [Google Scholar]
- 5.Becker JC, Houben R, Ugurel S, Trefzer U, Pfohler C, Schrama D. Merkel cell polyomavirus is frequently present in Merkel cell carcinoma of European patients. J Invest Dermatol 2009;129: 248–50. [DOI] [PubMed] [Google Scholar]
- 6.Foulongne V, Kluger N, Dereure O, Brieu N, Guillot B, Segondy M. Merkel cell polyomavirus and Merkel cell carcinoma, France. Emerg Infect Dis 2008;14:1491–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shuda M, Arora R, Kwun HJ, Feng H, Sarid R, Fernandez-Figueras MT, et al. Human Merkel cell polyomavirus infection I: MCV T antigen expression in Merkel cell carcinoma, lymphoid tissues and lymphoid tumors. Int J Cancer 2009;125:1243–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kean JM, Rao S, Wang M, Garcea RL. Seroepidemiology of human polyomaviruses. PLoS Pathog 2009;5:e1000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tolstov YL, Pastrana DV, Feng H, Becker JC, Jenkins FJ, Moschos S, et al. Human Merkel cell polyomavirus infection II: MCV is a common human infection that can be detected by conformational capsid epitope immunoassays. Int J Cancer 2009;125:1250–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kassem A, Technau K, Kurz AK, Pantulu D, Loning M, Kayser G, et al. Merkel cell polyomavirus sequences are frequently detected in nonmelanoma skin cancer of immunosuppressed patients. Int J Cancer 2009;125:356–61. [DOI] [PubMed] [Google Scholar]
- 11.Ridd K, Yu S, Bastian BC. The presence of polyomavirus in non-melanoma skin cancer in organ transplant recipients is rare. J Invest Dermatol 2009;129:250–2. [DOI] [PMC free article] [PubMed] [Google Scholar]


