Abstract
Background/Aim:
To determine if early passage tumor cells obtained from patients with mesothelioma continue to express the tumor differentiation antigen mesothelin and their sensitivity to the anti-mesothelin immunotoxin SS1P.
Materials and Methods:
Cell cultures were established from ascites or pleural effusion of 6 peritoneal and 3 pleural mesothelioma patients, respectively. These cells were evaluated for mesothelin expression by immunohistochemistry and flow cytometry.
Results:
Although mesothelin was highly expressed in tumor biopsies of all patients, only 3 out of 9 malignant effusions from these patients when grown in short-term culture showed strong mesothelin positivity by IHC. By flow cytometry, the number of mesothelin sites per cell was variable ranging from 580 to 210,000 sites/cell. Cells with strong mesothelin expression by IHC and increased number of mesothelin sites/cell were sensitive to SS1P.
Conclusions:
Most mesothelioma tumors loose mesothelin when grown in vitro and the sensitivity of these cells to SS1P is dependent on the number of mesothelin sites/cell.
Keywords: Mesothelin, MPF, SS1P, pleural mesothelioma, peritoneal mesothelioma
Malignant mesothelioma is an asbestos-related cancer that arises from the mesothelial cells lining the pleural and peritoneal cavities and approximately 3,000 to 4,000 new cases are diagnosed in the United States each year (1, 2). The prognosis of patients with pleural and peritoneal mesotheliomas is different with pleural disease having the worst prognosis with a median overall survival of 12 months for patients with advanced disease (3). Some patients with peritoneal mesothelioma who have aggressive surgical resection of their tumors, can have a median overall survival greater than 5 years (4, 5). Development of more effective treatments is needed to improve the outcome of these patients and one such strategy involves targeting tumor differentiation antigens such as mesothelin.
Mesothelin is a glycosyl-phosphatidylinositol–anchored cell surface glycoprotein (6). The full-length mesothelin gene encodes a 71-kDa precursor protein that is processed to a 31-kDa shed fragment called megakaryocyte potentiating factor (MPF) and a 40-kDa membrane-bound protein termed mesothelin (7). Normal expression of mesothelin in human tissues is limited to the mesothelial cells of the pleura, peritoneum and pericardium (8). However, many cancers such as mesothelioma, ovarian adenocarcinoma, lung adeno-carcinoma and pancreatic cancer highly express mesothelin (9–12). This differential expression of mesothelin in tumors with limited expression on normal tissues makes it a good target for cancer therapy. The normal biological function of mesothelin is not known but recent studies have shown that mesothelin is the receptor for the mucin MUC16 and this interaction may play a role in tumor metastasis (13, 14). MPF was initially isolated from a pancreatic cancer cell line and was named so because in mouse bone marrow cultures it potentiated the megakaryocyte-potentiating activity of interleukin-3 (15). However, the biological function of MPF in humans is also not known, although a recent report suggested that it may play a role in tumor growth (16).
Both mesothelin and MPF can be detected in the serum of some patients with mesothelioma and are currently being evaluated as diagnostic tools for this disease (17–20). Although previous reports have shown that mesothelin is also present in the pleural fluid of some patients with pleural mesothelioma these studies have not correlated mesothelin expression in tumors and serum mesothelin with pleural fluid mesothelin (21). In addition, there is lack of data regarding mesothelin concentration in serum and ascites of patients with peritoneal mesothelioma.
There are several mesothelin-targeting agents in clinical trials for the treatment of mesothelin-expressing cancers (22). Clinical benefit, including disease stabilization and reduction of ascites, was observed in a phase I clinical trial of the anti-mesothelin immunotoxin SS1P in patients who had failed standard therapies (23). SS1P is currently in clinical trials for the treatment of patients with mesothelioma (24). Better understanding of factors that determine the efficacy of SS1P is needed to improve the clinical outcome. In the case of antibody-based therapies, cell surface receptor expression is an important determinant of antitumor activity as has been shown in the case of trastuzumab for treatment of HER-2-expressing breast cancers (25). The aim of this study was to isolate mesothelioma cells from ascites and pleural effusions, to quantitate their mesothelin expression and to correlate it with sensitivity to SS1P.
Material and Methods
Establishment of early-passage mesothelioma cells.
Early passage mesothelioma cells were established from the ascites or pleural fluid obtained from patients with mesothelioma seen at the National Cancer Institute on Institutional Review Board-approved protocols. We obtained ascites from six patients with peritoneal mesothelioma and pleural fluid from three patients with pleural mesothelioma. The ascites or pleural fluid (100–1000 mL) was centrifuged at 1000 rpm at room temperature for 3 min; the cell pellets were washed twice with phosphate buffered saline (PBS) and the red blood cells were removed by BD Pharm Lyse™-Lysing Buffer kit (BD Bioscience, Franklin Lakes, NJ), according to the manufacturer’s instructions, followed by washing twice with PBS. The cells were then resuspended in RPMI 1640 (Invitrogen, Carlsbad, CA) supplemented with 20% fetal bovine serum (FBS) (Lonza, Walkersville, MD) 2 mM glutamine (Invitrogen), units penicillin-streptomycin (Invitrogen) and 1 mM sodium pyruvate (Invitrogen). The cells were seeded into 175 mL culture flasks at a density of 2.5–4.0×105 cells/ml. After 24 h of incubation at 37˚C in a humidified, 5% CO2 atmosphere overnight, the medium containing non-adherent cells was replaced with fresh medium. The cultures were maintained by changing the medium twice weekly.
Cytological examination of the primary cell cultures.
The primary cell cultures were evaluated by a cytopathologist (A.F.), experienced in the cytologic evaluation of mesothelioma, to determine if these cultures were composed of mesothelial cells and therefore representing mesothelioma cell cultures. Cell blocks were made from these primary cultures and 5 micron sections were stained with hematoxylin and eosin (H&E) and with a panel of antibodies using the Ventana Automated Nexus Immunostainer (Ventana Medical Systems, Tucson, AZ). The antibody panel included antibodies to calretinin (Invitrogen), CD163 (Leica/Novocastra, Buffalo Grove, IL) and leukocyte common antigen (LCA, CD45) (Dako, Carpinteria, CA). Mesothelin staining was performed using the anti-mesothelin mAb 5B2 (Novocastra Laboratories, Ltd., Newcastle-on-Tyne, UK).
These primary cell cultures were classified as consistent with mesothelioma cells, suggestive of mesothelioma cells or cannot be determined if they are mesothelioma cells, based on cytomorphological and immunocytochemical expression findings. Cell cultures were designated as consistent with mesothelioma cells if expected cytomorphological features of mesothelioma cells were observed and immunoexpression of either calretinin or mesothelin seen in at least 5–50% of the cells. Also designated as consistent with mesothelioma cells, were cell cultures showing atypical cytomorphological features of mesothelioma cells but showing immunoexpression of either calretinin or mesothelin in >50% of the cells. Cell cultures were designated as suggestive of mesothelioma cells if expected cytomorphological features of mesothelioma cells were identified and either no immunoreactivity or immunoreactivity for calretinin or mesothelin was seen in ≤5% of the cells. Cell cultures that had atypical cytomorphological features of mesothelioma cells and showed ≤50% expression of calretinin and mesothelin were categorized as cannot be determined if they are mesothelioma cells.
Pathological examination of the original tumor specimens.
The original tumor samples obtained from patients at the time of their original diagnosis or at time of their debulking surgery were evaluated by a pathologist (M.M.) to establish the diagnosis and characterize the subtype of mesothelioma.
Immunohistochemistry for mesothelin expression on tumor biopsies and early-passage mesothelioma cells.
The original tumor biopsies from patients whose ascites/pleural effusion was used to establish short -term culture were evaluated for mesothelin expression by immunohistochemistry (IHC) using the anti-mesothelin mAb 5B2 (Novocastra Laboratories, Ltd.). To evaluate mesothelin expression in tumor cells grown in short-term culture formalin-fixed paraffin embedded cell blocks were prepared using the thrombin clot method and 5-micron sections were mounted on charged microscope slides. IHC for mesothelin expression was also performed using the anti-mesothelin mAb 5B2. Each slide was scored for the percent of tumor cells that were positive for mesothelin expression and the intensity of staining (+, mild; ++, moderate; and +++, intense).
Quantitation of mesothelin binding sites per cell on early-passage mesothelioma cells.
Early-passage mesothelioma cells were evaluated for mesothelin expression by flow cytometry. A minimum of 20,000 live cells per sample were analyzed on FACS Calibur (BD Bioscience) using CellQuest software (BD Bioscience). Data were analyzed using FlowJo software version 8.8.6 (Tree Star, Inc., Ashland, OR). To quantitate cell surface mesothelin expression the mouse anti-mesothelin antibody, MN (Rockland Immunochemicals Inc., Gilbertsville, PA) was conjugated with R-PE using R-PE Antibody All-In-One Conjugation Kit (Solulink, San Diego, CA), following the manufacturer’s instructions. Early-passage mesothelioma cells were then grown until confluent, trypsinized, washed and resuspended in FACS buffer (PBS with 5% FBS and 0.1% sodium azide) and incubated with MN antibody conjugated with R-PE. An isotype-matched antibody was used as a control. Geometric means were chosen as mean fluorescence intensity (MFI). The MFI of cells was compared with the MFI from a standard curve of PE-conjugate calibration beads (BD QuantiBRITE™ PE quantitation kit, BD Bioscience) and the number of mesothelin sites per cell was estimated.
Cytotoxicity of SS1P against early passage mesothelioma tumor cells.
The cytotoxicity of SS1P was evaluated using cells that were within 1–3 passages after plating the ascites/pleural effusion. Tumor cells (5×104/well) were seeded in a 24-well plate. The following immunotoxins were added at different concentrations: SS1P (0, 0.1, 1, 10 and 100 ng/mL), BL22, an anti-CD22 immunotoxin, as a negative control (0, 0.1, 1, 10 and 100 ng/mL) and HB21, an anti-transferrin receptor immunotoxin, as a positive control at 10 ng/mL (26, 27). The cells were incubated for 96 hr, then washed twice with PBS; fixed with 10% neutral buffered formalin solution (Sigma, St. Louis, MO) at room temperature for 5 min, and crystal violet dye, at a concentration of 1 mg/mL, was added and incubated for 5 min at room temperature. The cells were then washed, dried and destained in 1% acetic acid. Color intensity as determined by OD at 595 nm was measured using a Versamax microplate reader (Molecular Device, Sunnyvale, CA).
Evaluation of mesothelin and MPF levels in serum and ascites/pleural effusion.
Mesothelin concentration in patients’ serum and ascites/pleural effusion was determined using the commercially available MESOMARK™ (Fujirebio Diagnostics, Inc., Malvern, PA) ELISA kit and following manufacturer’s instructions. MPF levels in patients’ serum and ascites/pleural effusion were analyzed using the Ab-Match Assembly Human MPF kit (Medical & Biological Laboratories Co., Ltd., Japan) according to manufacturer’s instructions. For both mesothelin and MPF ELISA, samples were analyzed in duplicate.
Statistical analyses.
Statistical analysis was performed with Prism (version 5) for Mac (GraphPad software). A Mann Whitney test was used for statistical comparison. Correlation data were calculated using Spearman’s the correlation co-efficient. p<0.05 was considered statistically significant.
Results
Establishment of early-passage mesothelioma cells.
Nine early-passage malignant mesothelioma cell cultures (NCI-M-02, NCI-M-03, NCI-M-05, NCI-M-07, NCI-M-09, NCI-M-10, NCI-M-11, NCI-M-15 and NCI-M-16) were established from ascites or pleural effusion obtained from patients with mesothelioma except NCI-M-07, which was derived from tumor tissue. For the purposes of this study early-passage mesothelioma cells were defined as cells within 3 passages after the ascites or pleural effusion was plated in tissue culture flasks. The clinical characteristics of the patients from whom these samples were obtained are listed in Table I. The median age of the patients was 53 years (range 20–79 years). Six of the patients had peritoneal mesothelioma while three patients had pleural mesothelioma. The original tissue biopsies obtained at the time of diagnosis from these patients showed that all had epithelial malignant mesothelioma (Figure 1A).
Table I.
Characteristics of patients used to establish early-passage mesothelioma cell cultures.
| Patient |
Age (Yrs) |
Gender |
Mesothelioma |
| NCI-M-02 | 52 | Female | Pleural |
| NCI-M-03 | 32 | Male | Peritoneal |
| NCI-M-05 | 51 | Male | Peritoneal |
| NCI-M-07 | 20 | Female | Peritoneal |
| MCI-M-09 | 70 | Male | Peritoneal |
| NCI-M-10 | 79 | Male | Peritoneal |
| NCI-M-11 | 53 | Male | Peritoneal |
| NCI-M-15 | 69 | Male | Pleural |
| NCI-M-16 | 72 | Male | Pleural |
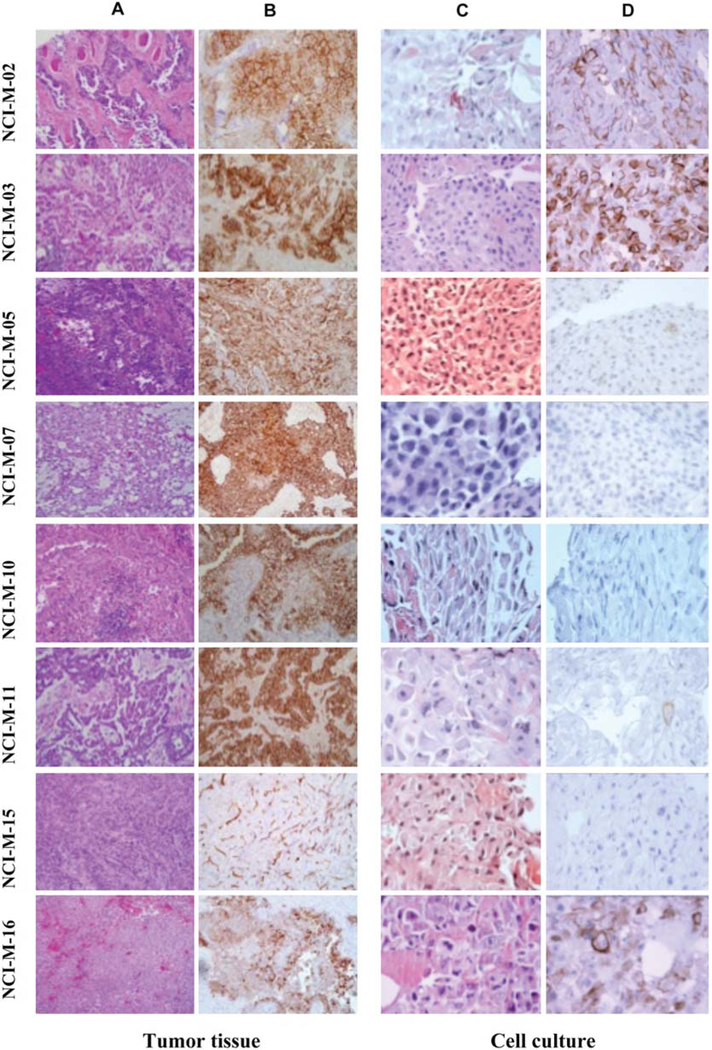
Figure 1.
Characterization of patients’ tissue biopsy and early-passage mesothelioma cells. H&E staining of the patients’ original tissue biopsy (A) and mesothelin staining of original tissue biopsy using the anti-mesothelin mAb 5B2 (B). Morphological features of the sections made from the cell blocks of patient tumor cells grown in short-term culture (C) as well as mesothelin staining using the anti-mesothelin mAb 5B2 (D).
Most early-passage cell cultures retain characteristics of mesothelioma.
All the early-passage mesothelioma cell cultures were evaluated by a cytopathologist, experienced in clinical diagnosis of mesothelioma to determine if the cultures retained mesothelioma characteristics. In addition to the mesothelioma markers mesothelin and calretinin, the cell cultures were also evaluated for LCA and CD163 to exclude contamination by leucocytes or macrophages, respectively. As shown in Table II all the early-passage cell cultures did not express leukocyte common antigen or CD163. The criteria used to categorize the cultures as consistent with mesothelioma cells, suggestive of mesothelioma cells or cannot be determined if they are mesothelioma cells (as described in Material and Methods), are similar to those used by the cytopathologist to make a diagnosis of mesothelioma in the ascites or pleural effusion obtained from patients. Using these criteria, five cultures (NCI-M-02, NCI-M-03, NCI-M-09, NCI-M-11 and NCI-M-16) were consistent with mesothelioma cells, two (NCI-M-7, and NCI-M-15) suggestive of mesothelioma cells and in two cases (NCI-M-05 and NCI-M-10) it could not be determined whether the cells in culture were mesothelioma cells.
Table II.
Morphological and IHC characteristics of early-passage mesothelioma cell cultures.
| Cell culture | Mesothelioma morphology |
Calretinin | Mesothelin | Leukocyte common antigen |
CD163 | Commentsa |
|
NCI-M-02 |
Typical |
+ (5–50%) |
+ (>50%) |
-b |
- |
Consistent with mesothelioma cells |
| NCI-M-03 | Typical | - | + (>50%) | - | - | Consistent with mesothelioma cells |
| NCI-M-05 | Atypical | + (<5%) | + (<5%) | - | - | Cannot be determined if they are mesothelioma cells |
| NCI-M-07 | Typical | - | - | - | - | Suggestive of mesothelioma cells |
| NCI-M-09 | Atypical | + (>50%) | + (<5%) | - | - | Consistent with mesothelioma cells |
| NCI-M-10 | Atypical | + (5–50%) | - | - | - | Cannot be determined if they are mesothelioma cells |
| NCI-M-11 | Typical | + (5–50%) | + (<5%) | - | - | Consistent with mesothelioma cells |
| NCI-M-15 | Typical | + (<5%) | - | - | - | Suggestive of mesothelioma cells |
| NCI-M-16 | Atypical | + (>50%) | + (>50%) | - | - | Consistent with mesothelioma cells |
These primary cell cultures were classified as consistent with mesothelioma cells, suggestive of mesothelioma cells or cannot be determined if they are mesothelioma cells, based on cytomorphological and immunocytochemical expression findings. The cell cultures were designated as consistent with mesothelioma cells if the typical cytomorphological features of mesothelioma cells were observed and immunoexpression of either calretinin or mesothelin was seen in at least 5–50% of the cells or if cells had atypical cytomorphological features of mesothelioma cells but immunoexpression of calretinin or mesothelin was present in >50% of the cells. Cell cultures were designated as suggestive of mesothelioma cells if expected cytomorphological features of mesothelioma cells were identified and either no immunoreactivity or immunoreactivity for calretinin or mesothelin was seen in <5% of the cells. Cell cultures that had atypical cytomorphological features of mesothelioma cells and had ≤50% expression of calretinin and mesothelin were categorized as cannot be determined if they are mesothelioma cells.
Negative.
Mesothelin expression is uniformly present in tumor biopsies of patients.
The tumor biopsies of patients that were used to establish the diagnosis were evaluated for mesothelin expression using IHC. Tumor specimens in all eight patients that were evaluated showed mesothelin expression (Figure 1B). The percent of tumor cells that were positive for mesothelin expression ranged from 70–100% and the intensity of staining ranged from ++ to +++. These results are consistent with the published data on mesothelin expression in epithelial mesothelioma (9).
Many mesothelioma tumor cells lose mesothelin expression when grown in short-term culture.
Cell blocks were prepared from the early-passage (within 3 passages) mesothelioma cells and tissue slides of these blocks were evaluated by H&E, as well as for mesothelin expression by IHC. By morphological examination of H&E slides 5/9 samples had the typical morphological features of mesothelioma while in four cases the morphology was suggestive of atypical mesothelial cells (Figure 1C). Mesothelin was highly expressed in only 3/9 samples by IHC (Figure 1D). As described in Table II, there was variability in the degree of mesothelin expression among the different cell cultures. In NCI-M-02, NCI-M-03 and NCI-M-16 mesothelin positivity was present in greater than 50% of the tumor cells, but was present in less than 5% of the tumor cells in NCI-M-05, NCI-M-09 and NCI-M-11. No mesothelin expression was noted in NCI-M-07, NCI-M-10 and NCI-M-15, although the original tumors of these patients highly expressed mesothelin (Figure 1A). These results suggest that some primary mesothelioma cells maintained in tissue culture may quickly loose mesothelin expression.
Quantitation of mesothelin expression by flow cytometry shows wide variability in mesothelin binding sites/cell.
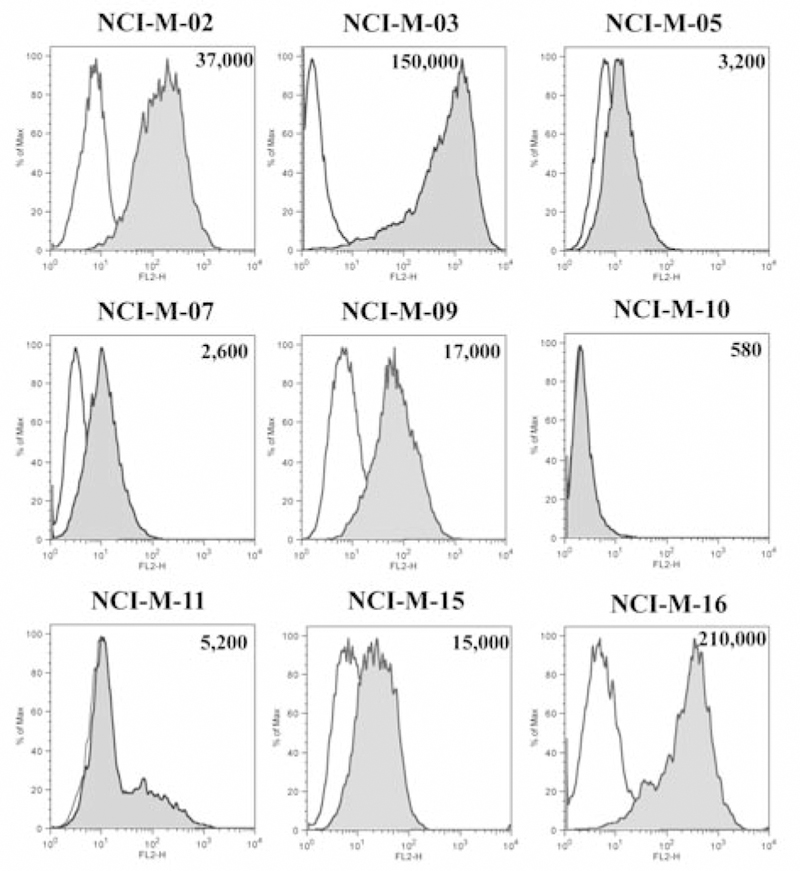
The cell surface level of mesothelin could be directly relevant to the tumor’s sensitivity to anti-mesothelin drug therapy. To better quantitate mesothelin expression we measured the average mesothelin binding sites of these early-passage mesothelioma cells with flow cytometry. As shown in Figure 2, some degree of mesothelin expression was present in all the cell lines but there was a wide variability in the degree of mesothelin positivity. Only NCI-M-02, NCI-M-03 and NCI-M-16 had high mesothelin expression with more than 30,000 mesothelin binding sites/cell. There was some correlation between the results from flow cytometry and IHC assays in regard to mesothelin expression. NCI-M-02, NCI-M-03 and NCI-M-16, which had >50% mesothelin expression by IHC, all had very high mesothelin expression by flow cytometry ranging from 37,000–210,000 sites/cell. In other cell cultures the correlation between mesothelin expression by flow cytometry and IHC was variable.
Figure 2.
FACS analysis for mesothelin expression of patients’ tumor cells grown in short-term culture. Cells were incubated with the anti-mesothelin mAb MN, conjugated with R-PE or isotype control antibody. Results are shown in terms of histogram plots for each cell line where the shaded area depicts the binding of MN antibody and the clear area shows the binding of isotype control antibody. The number of mesothelin sites/cell for each of the early-passage mesothelioma cell cultures is shown in each histograph.
The sensitivity of early-passage mesothelioma cells to SS1P is partially dependent on cell surface mesothelin level.
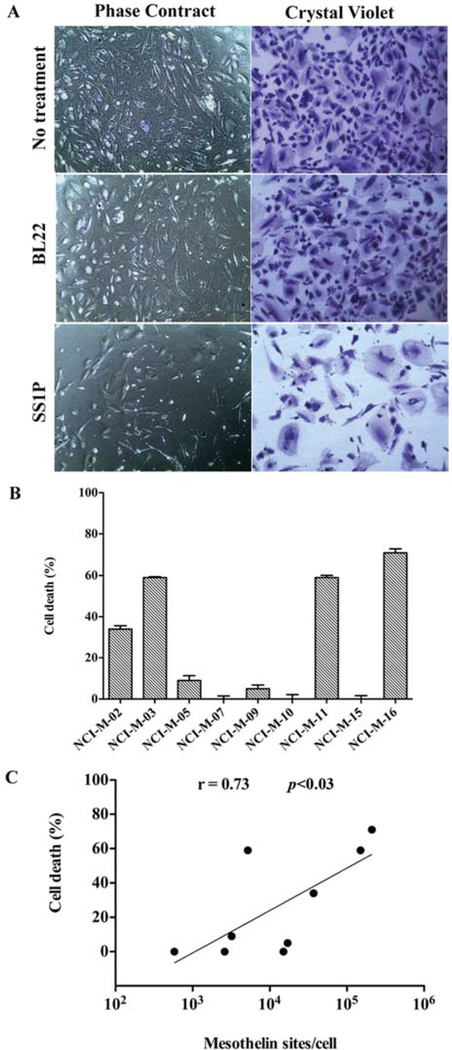
After characterizing the mesothelin expression of these early-passage mesothelioma cells we evaluated their sensitivity to the anti-mesothelin immunotoxin SS1P, a recombinant immunotoxin composed of an anti-mesothelin Fv fused to a truncated form of Pseudomonas exotoxin (PE38) that mediates cell killing (23). In addition to SS1P, these early-passage mesothelioma cells were also treated with HB21PE40, an immunotoxin targeting the transferin receptor which is universally expressed on the cell surface of most human cells, that caused >90% cell killing in all cells tested. As a negative control the CD22-targeting immunotoxin BL22 resulted in <5% cell killing, since this antigen is absent in solid tumors. As an example, the effect of these different immunotoxins on NCI-M-02 is shown in Figure 3A. While BL22 had very little effect on killing tumor cells, treatment with SS1P killed the majority of tumor cells. However, there are some remaining viable cells which are most likely contaminating fibroblasts or other cells in the primary culture that do not express mesothelin. In contrast, treatment with HB21PE40 killed all cells (data not shown).
Figure 3.
Cytotoxicity of SS1P against early-passage mesothelioma cells. (A) NCI-M-02 cells (5×104) were seeded in a 24 well plate and treated with 10 ng/ml of SS1P or BL22. After 96 h of incubation the cells were washed, fixed and cell viability was assessed by the crystal violet assay. (B) Effect of SS1P (10 ng/ml) on the different early-passage mesothelioma cells using the crystal violet assay. The y-axis represents percent cell death as compared to control untreated cells. Data represents the mean±SD of triplicate samples. (C) Spearman correlation analysis was performed using Graphpad to show the correlation between SS1P cytotoxicity and mesothelin sites/cell expressed by the early-passage mesothelioma cells.
SS1P at 10 ng/ml showed >30% (range 30–70%) death of cells obtained from patients NCI-M-02, NCI-M-03, NCI-M-11 and NCI-M-16 (Figure 3B). Although NCI-M-11 cells had modest mesothelin expression, by IHC or flow cytometry, compared to the other three sensitive cell lines, they were still sensitive to SS1P. Cells from the other five patients were resistant to killing by SS1P. In summary, there is some correlation between cell surface mesothelin level (mesothelin binding sites/cell) and cell’s sensitivity to SS1P (Spearman’s correlation r=0.71; p=0.0161) but other mechanisms may also play an important role (Figure 3C).
Ascites/pleural effusion mesothelin and MPF levels are significantly higher than in the serum.
As the early passage cell cultures were established from ascites/pleural effusion rather than tumor biopsies we wanted to determine if the concentration of mesothelin and MPF were elevated in these specimens that would be further suggestive of malignant effusion in addition to the cytological characteristics. Our results show that both mesothelin and MPF levels were elevated in the ascites/pleural effusion and were much higher than the concentration in the serum (Table III). The concentration of mesothelin in the ascites/pleural effusion was 5- to 26-fold higher than in the serum (median concentration in serum 9.5 nmol/L versus 170 nmol/L in ascites/pleural effusion; p=0.0003). Similar to mesothelin, the concentration of MPF in ascites/pleural effusion was 9 to 75-fold higher than the serum concentration (median serum concentration 4.9 nmol/L versus 143 nmol/L in ascites/pleural effusion; p<0.0001). There was good correlation between the serum mesothelin and ascites/pleural effusion mesothelin (r=0.86; p<0.02) but not between serum MPF concentration and ascites/pleural effusion MPF (r=0.57; p<0.15). However, the lack of correlation between serum and ascites/pleural effusion MPF could be due to the limited number of samples analyzed.
Table III.
Mesothelin and MPF levels in the serum and ascites/pleural effusion of patients used to establish early-passage mesothelioma cell cultures.
| Patient | Mesothelin (nmol/L) | MPF (nmol/L) |
||
| Serum | Ascites/Pleural effusion |
Serum | Ascites/Pleural effusion |
|
| NCI-M-02 | 9.5 | 95 | 13 | 115 |
| NCI-M-03 | 14.5 | 316 | 2.9 | 146 |
| NCI-M-05 | 38.5 | 284 | 14.1 | 234 |
| NCI-M-07 | 8 | n/d | 1 | n/d |
| NCI-M-09 | 4.4 | 70 | 4.9 | 126 |
| NCI-M-10 | 3.6 | 17 | 2.1 | 43 |
| NCI-M-11 | 24.2 | 240 | 15.8 | 372 |
| NCI-M-15 | 3.9 | 101 | 1.9 | 140 |
| NCI-M-16 | 12 | 239 | 8.3 | 293 |
n/d, Not determined.
Discussion
Using ascites or pleural effusion fluid from patients with peritoneal and pleural mesothelioma we established early-passage tumor cells and characterized them for mesothelin expression and for their sensitivity to the anti-mesothelin immunotoxin SS1P. In addition, we correlated mesothelin expression in these early-passage tumor cells with mesothelin expression in the original tumor.
The tumor specimens used in this study were obtained from patients with the epitheloid type of malignant mesothelioma. Previous studies have shown that almost all epitheloid mesotheliomas express mesothelin while the sarcomatous type of mesothelioma are mesothelin-negative (9, 28). In keeping with published data, tumor samples obtained from all patients either at the time of initial diagnosis or subsequent surgical resection, all showed strong mesothelin expression. However, when tumor cells obtained from the ascites or pleural effusion of these patients were grown in short-term culture and valuated for mesothelin expression many of these tumor cells were negative or had decreased mesothelin expression by IHC. It is unlikely that the cells in culture are non-malignant contaminating cells, such as macrophages or fibroblasts, since by morphological examination, they appeared to be epithelial mesothelial cells and were negative for leukocyte or macrophage markers. Seven of the nine primary cultures were characterized as consistent with or suggestive of mesothelioma, based on detailed pathological examination combining cytological features and IHC. In two cases it was difficult to determine if the cells in culture were of mesothelial origin. It is unlikely that this loss of mesothelin expression is due to problems related to formalin fixation and antigen retrieval, since by flow cytometry most of the cell cultures had decreased mesothelin sites/cell. However, the three early-passage cells that were strongly-positive for mesothelin expression by IHC also had the highest number of mesothelin sites/cells.
The sensitivity of these tumor cells grown in short-term culture to the anti-mesothelin immunotoxin SS1P was variable and dependent on mesothelin expression by the tumor cells. Cells that highly expressed mesothelin by IHC or flow cytometry showed greater than 30% growth inhibition at SS1P concentration of 10 ng/ml, which is significantly less than the blood levels obtained when patients are treated with SS1P (23). These results demonstrate that the tumor cells obtained from patients that continue to have high mesothelin expression when grown in short-term culture are sensitive to SS1P.
Some of the cell-bound mesothelin, as well as MPF, are shed into the serum and their levels are elevated in the majority of patients at the time of diagnosis (17–20). To get a better understanding of the kinetics of these biomarkers in patients with mesothelioma we evaluated the serum, as well as ascites/pleural effusion concentration of mesothelin and MPF, in patients whose tumor cells were used to establish short-term culture. Serum mesothelin levels were elevated in all the patients but there was a wide range, most likely reflecting tumor burden. However, mesothelin concentration in ascites/pleural effusion was in general 5- to 26-fold higher than the serum concentration. This is not surprising since mesothelioma is a tumor of the lining of the pleural and peritoneal cavities and the tumors shed mesothelin into these cavities. MPF, which is a cleaved product of the mesothelin precursor protein, was also elevated in the serum of all patients and its levels in ascites/pleural effusion were significantly higher than those in the serum.
Although, the mechanism of loss of mesothelin expression by mesothelioma tumor cells is not known, our results show that it is important to characterize mesothelin expression in mesothelioma cells lines used for in vitro and in vivo studies of mesothelin-targeted agents since mesothelin expression may be lost during in vitro cell culture. Also, since MPF has been suggested to play a role in tumor aggressiveness it will be important, in future studies, to evaluate the prognostic significance of serum and pleural effusion/ascites MPF in patients with mesothelioma.
Acknowledgements
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
References
- 1.Robinson BWS and Lake RA: Advances in malignant mesothelioma. N Engl J Med 353: 1591–1603, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Hassan R and Alexander R: Non-pleural mesotheliomas: mesothelioma of the peritoneum, tumica vaginalis and pericardium. Hematol Oncol Clin North Am 19: 1067–1087, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, Gatzemeier U, Boyer M, Emri S, Manegold C, Niyikiza C and Paoletti P: Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 21: 2636–2644, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Feldman AL, Libutti SK, Pingpank JF, Bartlett DL, Beresnev TH, Mavroukakis SM, Steinberg SM, Liewehr DJ, Kleiner DE and Alexander HR: Analysis of factors associated with outcome in patients with malignant peritoneal mesothelioma undergoing surgical debulking and intraperitoneal chemotherapy. J Clin Oncol 21: 4560–4567, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Yan TD, Deraco M, Baratti D, Kusamura S, Elias D, Glehen O, Gilly FN, Levine EA, Shen P, Mohamed F, Moran BJ, Morris DL, Chua TC, Piso P and Sugarbaker PH: Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: multi-institutional experience. J Clin Oncol 27: 6237–6242, 2009. [DOI] [PubMed] [Google Scholar]
- 6.Hassan R, Bera T and Pastan I: Mesothelin: a new target for immunotherapy. Clin Cancer Res 10: 3937–3942, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Chang K and Pastan I: Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc Natl Acad Sci USA 93: 136–140, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang K, Pastan I and Willingham MC: Isolation and characterization of a monoclonal antibody, K1, reactive with ovarian cancers and normal mesothelium. Int J Cancer 50: 373–381, 1992. [DOI] [PubMed] [Google Scholar]
- 9.Ordóñez NG: Value of mesothelin immunostaining in the diagnosis of mesothelioma. Mod Pathol 16: 192–197, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Hassan R, Kreitman RJ, Pastan I and Willingham MC: Localization of mesothelin in epithelial ovarian cancer. Appl Immunohistochem Mol Morphol 13: 243–247, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Argani P, Iacobuzio-Donahue C, Ryu B, Rosty C, Goggins M, Wilentz RE, Murugesan SR, Leach SD, Jaffee E, Yeo CJ, Cameron JL, Kern SE and Hruban RH: Mesothelin is overexpressed in the vast majority of ductal adenocarcinomas of the pancreas. Clin Cancer Res 27: 3862–3868, 2001. [PubMed] [Google Scholar]
- 12.Ordóñez NG: Application of mesothelin immunostaining in tumor diagnosis. Am J Surg Pathol 27: 1418–1428, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Rump A, Morikawa Y, Tanaka M, Minami S, Umesaki N, Takeuchi M and Miyajima A: Binding of ovarian cancer antigen CA125/MUC16 to mesothelin mediates cell adhesion. J Biol Chem 279: 9190–9198, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Gubbels JA, Belisle J, Onda M, Rancourt C, Migneault M, Ho M, Bera TK, Connor J, Sathyanarayana BK, Lee B, Pastan I and Patankar MS: Mesothelin-MUC16 binding is a high affinity, N-glycan dependent interaction that facilitates peritoneal metastasis of ovarian tumors. Mol Cancer 5: 50–65, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamaguchi N, Hattori K, Oh-eda M, Kojima T, Imai N and Ochi N: A novel cytokine exhibiting megakaryocyte potentiating activity from a human pancreatic tumor cell line HPC-Y5. J Biol Chem 269: 805–808, 1994. [PubMed] [Google Scholar]
- 16.Wang T, Kajino K, Abe M, Tan K, Maruo M, Sun G, Hagiwara Y, Maeda M and Hino O: Suppression of cell death by the secretory form of N-terminal ERC/mesothelin. Int J Mol Med 26: 185–191, 2010. [DOI] [PubMed] [Google Scholar]
- 17.Robinson BW, Creaney J, Lake R, Nowak A, Musk AW, de Klerk N, Winzell P, Hellstrom KE and Hellstrom I: Mesothelin-family proteins and diagnosis of mesothelioma. Lancet 362: 1612–1616, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Hassan R, Remaley AT, Sampson ML, Zhang J, Cox DD, Pingpank J, Alexander R, Willingham M, Pastan I and Onda M: Detection and quantitation of serum mesothelin, a tumor marker for patients with mesothelioma and ovarian cancer. Clin Cancer Res 12: 447–453, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Iwahori K, Osaki T, Serada S, Fujimoto M, Suzuki H, Kishi Y, Yokoyama A, Hamada H, Fujii Y, Yamaguchi K, Hirashima T, Matsui K, Tachibana I, Nakamura Y, Kawase I and Naka T: Megakaryocyte potentiating factor as a tumor marker of malignant pleural mesothelioma: evaluation in comparison with mesothelin. Lung Cancer 62: 45–54, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Hollevoet K, Nackaerts K, Thimpont J, Germonpré P, Bosquée L, De Vuyst P, Legrand C, Kellen E, Kishi Y, Delanghe JR and van Meerbeeck JP: Diagnostic performance of soluble mesothelin and megakaryocyte potentiating factor in mesothelioma. Am J Respir Crit Care Med 181: 620–625, 2010. [DOI] [PubMed] [Google Scholar]
- 21.Davies HE, Sadler RS, Bielsa S, Maskell NA, Rahman NM, Davies RJ, Ferry BL and Lee YC: Clinical impact and reliability of pleural fluid mesothelin in undiagnosed pleural effusions. Am J Respir Crit Care Med 180: 437–444, 2009. [DOI] [PubMed] [Google Scholar]
- 22.Hassan R and Ho M: Mesothelin targeted cancer immunotherapy. Eur J Cancer 44: 46–53, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hassan R, Bullock S, Premkumar A, Kreitman RJ, Kindler H, Willingham M and Pastan I: Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus intravenous infusion to patients with mesothelin expressing mesotheliomas, ovarian and pancreatic cancer. Clin Cancer Res 13: 5144–5149, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Hassan R, Sharon E, Schuler B, Mallory Y, Zhang J, Ling A and Pastan I: Antitumor activity of SS1P with pemetrexed and cisplatin for front-line treatment of pleural mesothelioma and utility of serum mesothelin as a marker of tumor response. J Clin Oncol 29(suppl): abstract 7026, 2011. [Google Scholar]
- 25.Lottner C, Schwarz, Diermeier S, Hartmann A, Knuechel R, Hofstaedter F and Brockhoff G: Simultaneous detection of HER2/neu gene amplification and protein overexpression in paraffin-embedded breast cancer. J Pathol 205: 577–584, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Kreitman RJ, Stetler-Stevenson M, Margulies I, Noel P, Fitzgerald DJ, Wilson WH and Pastan I: Phase II trial of recombinant immunotoxin RFB(dsFv)-PE38 (BL22) in patients with hairy cell leukemia. J Clin Oncol 27: 2983–2990, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shinohara H, Fan D, Ozawa S, Yano S, Van Arsdell M, Viner JL, Beers R, Pastan I and Fidler IJ: Site-specific expression of transferrin receptor by human colon cancer cells directly correlates with eradication by antitransferrin recombinant immunotoxin. Int J Oncol 17: 643–651, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Chang PK, Pai LH, Pass H, Pogrebniak HW, Tsao MS, Pastan I and Willingham MC: Monoclonal antibody K1 reacts with epithelial mesothelioma but not with lung adenocarcinoma. Am J Surg Pathol 16: 259–268, 1992. [DOI] [PubMed] [Google Scholar]