Abstract
Preeclampsia (PE) is a pregnancy-specific disorder of new-onset hypertension linked to placental ischemia. While obesity is a major risk factor for PE, not all obese pregnant women develop pregnancy-induced hypertension or PE. Previously, we reported that placental ischemia-induced hypertension is dependent upon intact signaling of the sympathetic nervous system (SNS). Moreover, in various models of obesity, blockade of melanocortin-4 receptor (MC4R) signaling protects against the development of hypertension via suppression of the SNS. Less is known regarding this pathway during obese pregnancy. Although blockade of MC4R may lead to increased body weight during pregnancy, we tested the hypothesis that placental ischemia-induced hypertension is attenuated in obese MC4R-deficient pregnant rats. On gestational day (GD) 14, MC4R wild type (WT) or heterozygous-deficient (MC4R-def) rats were subjected to chronic placental ischemia via the reduced uterine perfusion pressure (RUPP) procedure or Sham surgery then examined on GD19. In Sham MC4R-def vs. Sham WT pregnant rats, there was increased body weight, fat mass, and circulating leptin levels but they had similar fetus weights. RUPP reduced fetus weights in both strains. RUPP increased blood pressure in WT rats but this response was significantly attenuated in MC4R-def rats, even though blood pressure was elevated in Sham MC4R-def over Sham WT. These data indicate that while obese MC4R-def pregnant rats have higher blood pressure during pregnancy, placental ischemia-induced hypertension is attenuated in obese MC4R-def pregnant rats. Thus, obese women with abnormal MC4R signaling may be less susceptible to the development of placental ischemia-induced hypertension.
Keywords: Blood Pressure, MC4R, Obesity, Preeclampsia, Women’s Health
Introduction
Hypertensive disorders of pregnancy are a leading cause of maternal and fetal morbidity and mortality throughout the world 1. These disorders include gestational hypertension, chronic hypertension, preeclampsia (PE), and chronic hypertension with superimposed PE. PE is particularly known for the complexity of its clinical presentation. It is diagnosed by new-onset hypertension occurring during the second half of pregnancy along with a range of cardiovascular, renal, visual, or neural alterations 2. As removal of the placenta is currently the only steadfast intervention to limit progression of maternal symptoms 3 and the retained placenta is associated with postpartum PE 4, it is believed that the ischemic placenta plays a central role in the pathogenesis of PE. In support of a role for placental ischemia in the development of hypertension in PE, several experimental models of surgical- or environment-induced placental ischemia/hypoxia have vascular dysfunction, hypertension, and other neurohumoral abnormalities routinely seen in women with PE 5.
While human and experimental animal studies have focused on placental ischemic disease in the pathogenesis of PE, less is known about how established risk factors impact this hypertensive pathway. This includes overweight and obesity 6–8. The number of overweight and obese women of reproductive age is rising 9–12; this is alarming because the incidence of PE progressively climbs with increasing pre-pregnancy body mass index (BMI = kg/m2). Indeed, the incidence of PE was shown to be 3% in normal weight gravidas (BMI = 18.5–24.9), 7% in class I obesity (BMI = 30–34.9), 9% in class II (BMI = 35–39.9), 11% with class III obesity (BMI = 40–49.9), and 13% in super-obese women (BMI =50) without chronic hypertension 8. Although these observational studies have established an association between obesity and PE, less is understood regarding how obesity influences the development of placental ischemia-induced hypertension. It has been suggested that obesity increases the risk of developing PE by increasing the susceptibility of the maternal cardiovascular-renal system to hypertension produced by placental ischemic and hypoxic stimuli 13, 14.
The increase in blood pressure associated with obesity is believed to be partly mediated by increases in sympathetic nervous system (SNS) activity as a result of melanocortin-4 receptor (MC4R) activation. This principle stems from studies showing that MC4R plays a dual role in cardio-metabolic function by regulating food intake and blood pressure. Obese humans and experimental animals having genetic deficiency or pharmacological antagonism of this receptor system have increased food intake 15, 16. This results from an attenuated ability of the satiety hormone, leptin, to signal to MC4R in the central nervous system to regulate food intake 17. Even though reduced MC4R signaling promotes obesity, studies in males show this increased body weight and fat mass is not accompanied by hypertension via suppression of the SNS 18. Interestingly, emerging evidence suggests a role for the SNS in modulating placental ischemia-induced hypertension. Studies in humans and experimental animals have reported that activity of the SNS is increased during PE 19, 20. Moreover, we recently found that adrenergic receptor blockade attenuated the development of placental ischemia-induced hypertension in rats with reduced uterine perfusion pressure (RUPP) 21. In anesthetized rats, antagonism of MC3/4R in the brain reduced blood pressure and SNS activity more so in pregnant versus non-pregnant groups, suggesting increased blood pressure dependency on this system during gestation 22. Investigators have also shown that obese MC4R-deficient offspring generated from breeding MC4R heterozygous-deficient parents are protected against maternal insults whereby they have attenuated hypertension following exposure to an in-utero high-fat diet or placental ischemia 23, 24. However, these studies did not assess the impact of MC4R-deficiency on placental ischemia-induced hypertension. While it is thought that obesity may exacerbate the blood pressure response to placental ischemia, increases in body weight associated with MC4R deficiency may not have this predicted effect because MC4R deficiency also attenuates activity of the SNS. Therefore, we tested the hypothesis that placental ischemia-induced hypertension is attenuated in MC4R-deficient obese pregnant rats by comparing the effects of RUPP on blood pressure between MC4R+/+ (wild type) and MC4R+/K314X (heterozygous-deficient) pregnant rats. We utilized the RUPP model of placental ischemia as a hypertensive second-hit to delineate whether MC4R-deficiency attenuates placental ischemia-induced hypertension even though they have elevated body weight, fat mass, and obesity-related metabolic factors during pregnancy. This would suggest that MC4R signaling is important for the development of placental ischemia-induced hypertension in obese pregnancies.
Methods
According to the Transparency and Openness Promotion (TOP) Guidelines, the data that support the findings of this study are available from the corresponding author upon reasonable request.
Animals
All animal experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals with all animal-use protocols approved by The University of Mississippi Medical Center’s Institutional Animal Care and Use Committee. Male and female MC4R+/K314X (heterozygous-deficient, MC4R-def) were obtained from Charles River Laboratories (Wilmington, MA, USA) and used as founders for our colony. The colony was propagated by non-brother-sister mating of heterozygous-def breeding pairs to preserve heterozygosity 25. Experimental MC4R heterozygous-def and WT rats were produced from ≥ 3 different heterozygous breeding pairs. All rats were maintained on standard chow diet (Teklad 7013/NIH31; Indianapolis, IN, USA) and water ad libitum. At weaning (3 weeks), all offspring were placed on NIH31 diet and tails biopsied for genotyping. A timeline illustrating the details of rat production (Supplemental Figure S1) and experimental protocols (Supplemental Figure S2) can be found in the online supplementary materials.
MC4R genotyping
The MC4R-deficient rat model was originally developed by an ENU-induced mutation in Wistar Hannover rats, which resulted in a truncated form of the receptor with the inability to be expressed in the plasma membrane 26. The mutation introduced an additional Ddel restriction site (CTNAG) within the MC4R gene. Thus, genotyping was performed using a restriction enzyme-based PCR assay utilizing the primers: Forward: TGATCATGTGTAACGCTGTCAT, Reverse: TGACAAATTCTGCAGGTCTCT to amplify 177 bp fragment of Mc4r gene. The PCR product was subjected to DdeI enzyme restriction and genotyping was performed using Qiagen QIAxcel Advanced capillary electrophoresis system. For wild-type rats, Ddel treated PCR fragment generated 2 bands at 120 and 57 bases and homozygous MC4R deficient rats gave 3 fragments at 106, 56, and 15 (unobserved).
Timed pregnancies and RUPP procedure
At ~13 weeks old, WT or MC4R heterozygous-def females were mated with WT or MC4R heterozygous-def males, respectively, for the generation of timed-pregnant experimental rats. The observation of sperm in vaginal smears was indicative of gestational day (GD) 0. On GD 14, the RUPP procedure was performed, as previously described 27. Briefly, a silver clip (0.203 mm, internal diameter) was placed around the sub-renal abdominal aorta above the uterine arteries along with clips (0.1 mm, internal diameter) on braches of the ovarian artery in both uterine horns. The normal pregnant control group consisted of a surgery with similar abdominal incision and suturing without clip placement.
Fat mass assessments
On GD 18, total body fat mass and total lean mass were examined using Echo-MRI-700 equipment (Echo Medical Systems, Houston, TX, USA). For this, rats were placed in restraint cages and the average of two scans recorded for each rat. On GD 19, retroperitoneal and peri-renal fat were collected and wet weights recorded to assess visceral fat mass.
Food intake
Food intake was assessed between GD 17 – 18 following recovery from surgeries on GD 14 and before conducting catheterizations for blood pressure measurements on GD 18.
Maternal blood pressure assessment
On GD 18, indwelling catheters were implanted in the left carotid artery and exposed at the nape of the neck. Catheters consisted of V/1 tubing attached to V/3 tubing (Scientific Commodities, Lake Havasu City, AZ, USA). Approximately 2.5 cm of the V/3 end of the catheter was inserted into the carotid. Catheters were filled with sterile heparin-0.9% saline solution (300 mg/mL; Pfizer, New York City, NY, USA) and stoppered with a stainless-steel catheter plug (SP22/12; Instech Laboratories, Plymouth Meeting, PA, USA) to maintain patency. On GD 19, conscious mean arterial blood pressure and heart rates were measured, as described previously 28. For this purpose, rats were placed in restrainers (Kent Scientific Corp, Torrington, CT, USA) and catheters connected to pressure transducers (MLT0699; ADInstruments, Colorado Springs, CO, USA) coupled to a computerized data acquisition system (PowerLab, ADInstruments). Readings were calibrated on every rat then data acquired at 1k/s. Once blood pressure readings stabilized (~1h), ~10 min of mean arterial blood pressure (MAP) and heart rate data were collected and averaged. The % increase in MAP in response to RUPP was calculated for each strain by: % increase = ((individual RUPP MAP - average normal pregnant MAP)/(average normal pregnant MAP))*100.
Blood collection and pregnancy biometrics
On GD 19, a midline incision was made and uterine horns with fetuses exteriorized. Blood was collected from the abdominal aorta into Vacutainer K2EDTA tubes (BD, Franklin Lakes, NJ, USA), spun at 2500 rpm for 12 min at 4°C, and plasma stored at −20°C. It was ensured that each fetus and matching placenta were weighed and recorded as individual fetal-placental units. Average fetal and placental weights were calculated per rat then averaged for each experimental group. Total viable or resorbed fetuses were noted. Percent fetal resorption = (number of resorbed fetuses/total number of fetuses)*100. Placental sufficiency = average viable fetal weight/average placental weight for each dam, as a surrogate measure of the nurturing capacity of the placenta, as previously described in humans 29.
Biochemistry
Plasma levels of the adipokines leptin and adiponectin were examined using Quantikine enzyme-linked immunosorbent assay (ELISA) kits from R&D Systems (Minneapolis, MN, USA - MOB00 and RRP300, respectively). As reduced bioavailability of placental growth factor (PlGF) mediates the development of placental ischemia-induced hypertension 30, plasma PlGF levels were also quantified by an ELISA kit from R&D Systems detecting mouse PlGF-2 (Minneapolis, MN, USA - MP200). Utilizing NCBI Protein Basic Local Alignment Search Tool (BLAST) software, it was found that mouse and rat PlGF-2 have 91% alignment (https://blast.ncbi.nlm.nih.gov/Blast.cgi).
Statistical analysis
Data were graphed and analyzed using GraphPad Prism version 7.0 (La Jolla, CA, USA). Data are presented as mean ± standard error of the mean (SEM). Statistically significant differences were defined as P<0.05. Two-way analysis of variance (ANOVA) tests were conducted followed by Tukey’s multiple comparisons tests and the results are inset in each graph. Symbols are included in these insets to highlight the differences between factors if a statistical interaction was not detected.
Results
Impact of MC4R deficiency on placental ischemia-induced hypertension
At GD 19, normal pregnant MC4R-def rats had greater MAP levels than their normal pregnant WT counterparts (Figure 1A). Placental ischemia, as induced by the RUPP procedure, produced frank hypertension in WT rats compared to normal pregnant WT control rats by GD 19 (Figure 1A). In contrast, placental ischemia-induced hypertension was significantly attenuated in the MC4R-def rats (Figure 1A). Indeed, this attenuated hypertensive response was highlighted by examining the % change in MAP, which further revealed that RUPP produced a greater increase in blood pressure in WT rats and a lesser response in MC4R-def rats (Figure 1B).
Figure 1.
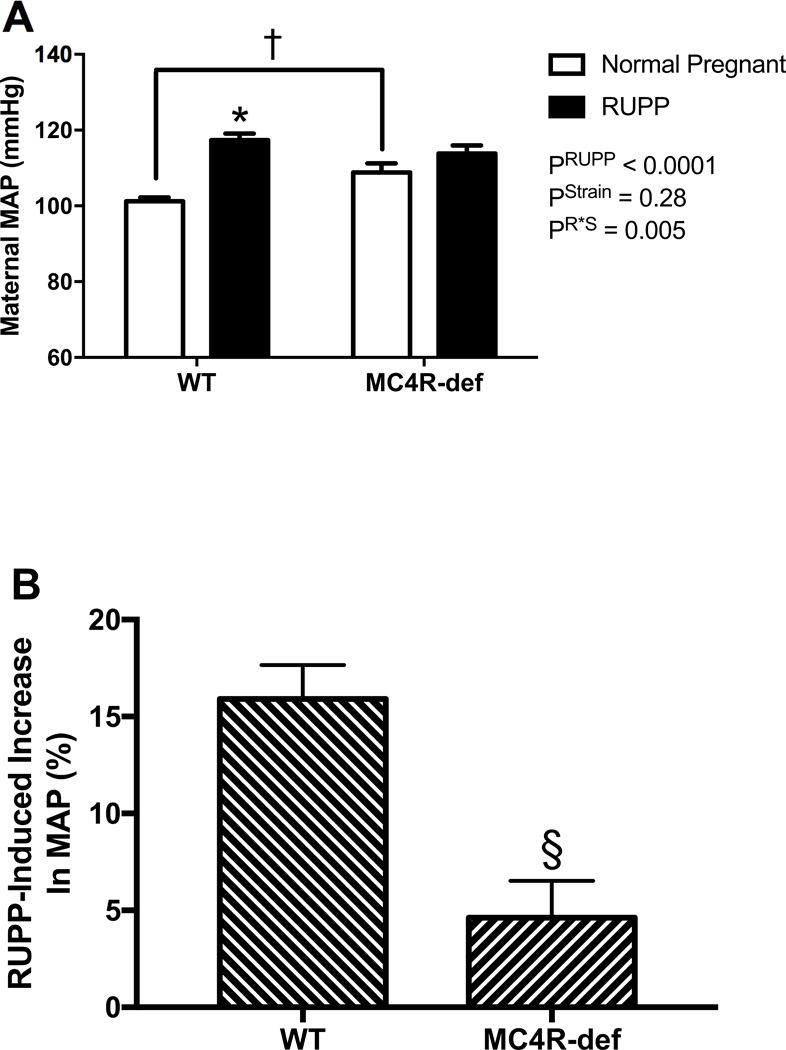
A: maternal mean arterial blood pressure (MAP), B: % increase in MAP at GD 19 in melanocortin-4 receptor (MC4R) wild type (WT) and deficient (MC4R-def) rats under normal pregnant or reduced uterine perfusion pressure (RUPP) conditions. Normal pregnant: WT, N = 21 and MC4R-def, N = 19. RUPP: WT, N = 14 and MC4R-def, N = 15. *P < 0.0001 vs. normal pregnant WT, †P = 0.02 vs. normal pregnant WT, §P = 0.0002 vs. WT.
Heart rate was not significantly different (P = 0.56) between normal pregnant WT (425 ± 7 bpm) or MC4R-def pregnant rats (430 ± 6 bpm) and RUPP did not impact this parameter in WT (443 ± 6 bpm) or MC4R-def pregnant rats (434 ± 11 bpm) (P = 0.10). Heart weight-to-body weight ratio was also not different (P = 0.08) between the normal pregnant groups (WT: 0.002 ± 0.00007 vs. MC4R-def: 0.002 ± 0.00005) but RUPP increased (P = 0.0003) this measure similarly in both WT (0.003 ± 0.0001) and MC4R-def rats (0.002 ± 0.0001).
Maternal biometrics
Figure 2 shows the impact of MC4R-heterozygous deficiency on maternal body weights by GD 19. When compared to normal pregnant WT rats, MC4R-def pregnant rats had significantly greater body weight by ~30 g (Figure 2A). The RUPP procedure reduced maternal body weight in both WT and MC4R-def rats likely due to reduced fetal weights and resorptions, but body weight remained greater in the latter RUPP group (Figure 2A). Analogous differences were found for total maternal fat mass (Figure 2B) and isolated visceral fat (Figure 2C).
Figure 2.
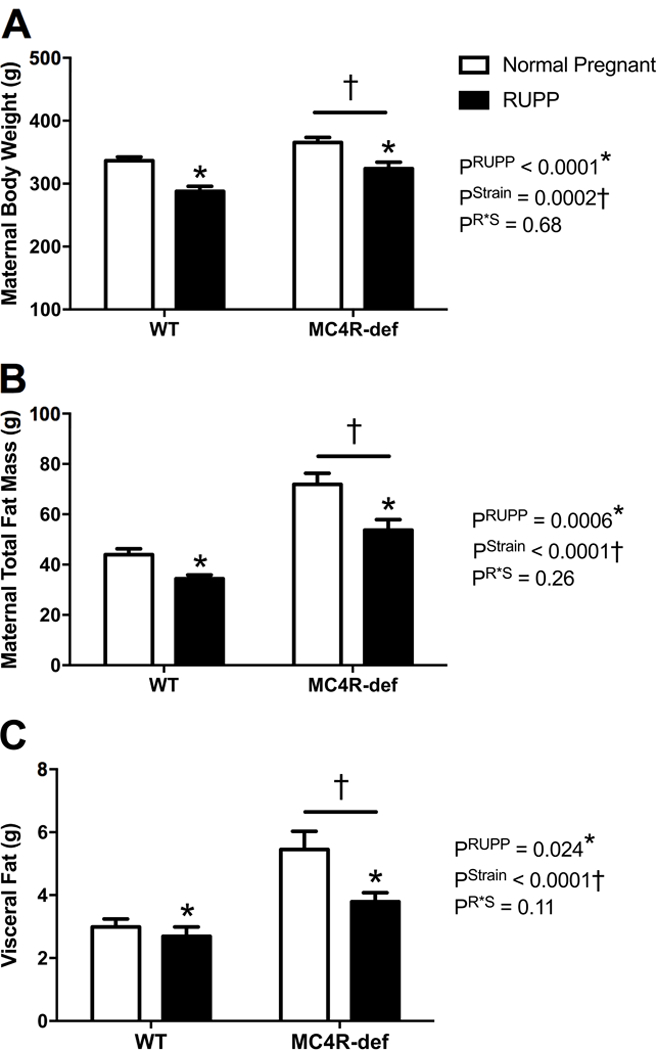
A: maternal body mass, B: maternal total body fat mass by Echo-MRI, and C: maternal visceral white adipose tissue mass isolated from the retroperitoneum and around the kidneys at the end of pregnancy in melanocortin-4 receptor (MC4R) wild type (WT) and deficient (MC4R-def) rats under normal pregnant or reduced uterine perfusion pressure (RUPP) conditions. Normal pregnant: WT, N = 16 and MC4R-def, N = 19. RUPP: WT, N = 13 and MC4R-def, N = 15. *P < 0.05 vs. normal pregnant counterparts, †P < 0.05 for MC4R-def vs. WT strain effect.
Echo-MRI revealed that maternal lean body mass was similar between normal pregnant groups (WT: 266 ± 3 g vs. MC4R-def: 272 ± 4 g, P = 0.010) and was reduced in both strains following RUPP (WT: 229 ± 7 g vs. MC4R-def: 248 ± 5 g, P < 0.0001). Food intake was assessed between GD 17 and 18, and this parameter was similar between normal pregnant groups (WT: 18 ± 1 g vs. MC4R-def: 21 ± 2 g, P = 0.27) but was reduced to a greater extent (P = 0.037) in RUPP MC4R-def rats (13 ± 2 g) than RUPP WT rats (19 ± 2 g).
Circulating obesity-related metabolic factors
Leptin levels, overall, were significantly elevated in the MC4R-def groups versus the WT groups and RUPP did not modify these levels (Figure 3A). Adiponectin levels were similar in the MC4R-def versus WT rats and were reduced in parallel by RUPP in both groups (Figure 3B). Insulin levels, overall, were significantly elevated in the MC4R-def groups versus the WT groups and RUPP did not modify these levels (Figure 3C).
Figure 3.
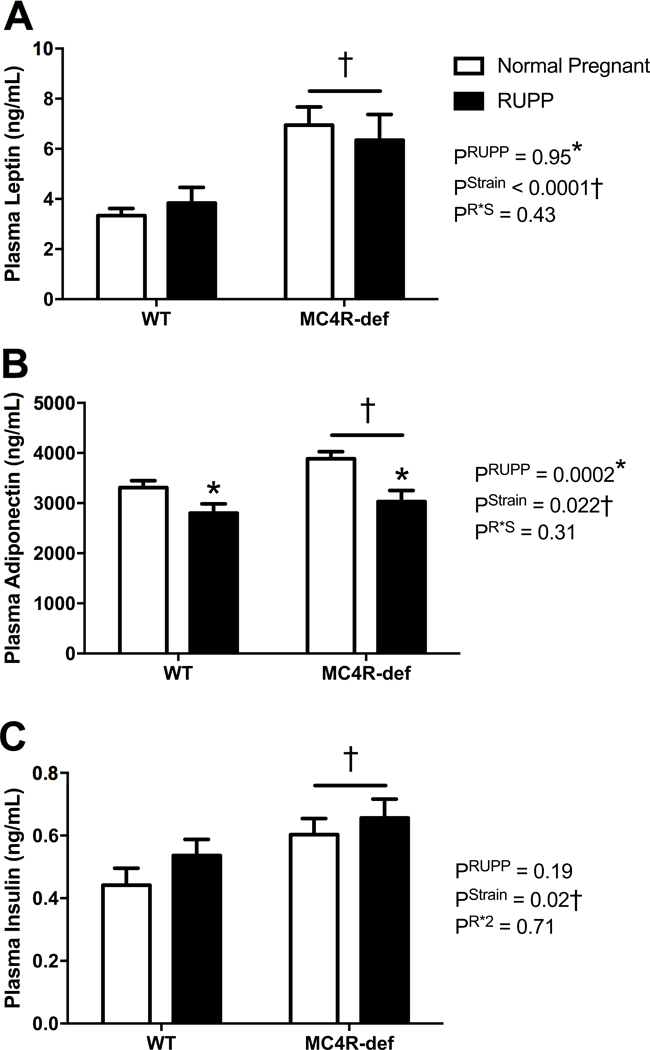
A: Plasma leptin, B: adiponectin, and C: insulin at GD 19 in melanocortin-4 receptor (MC4R) wild type (WT) and deficient (MC4R-def) rats under normal pregnant or reduced uterine perfusion pressure (RUPP) conditions. Normal pregnant: WT, N = 15 and MC4R-def, N = 15. RUPP: WT, N = 13 and MC4R-def, N = 12. *P < 0.05 vs. normal pregnant counterparts, †P < 0.05 for MC4R-def vs. WT strain effect.
Fetal and placental biometrics
Figure 4 presents fetal and placental biometrics in the 4 experimental groups at GD19. Average fetal weights were comparable between normal pregnant WT and MC4R-def rats (Figure 4A). The RUPP procedure produced IUGR similarly in both WT and MC4R-def groups (Figure 4A). Similar trends were found for total fetal weights between WT (26 ± 1 g) and MC4R-def (24 ± 1 g) normal pregnant rats and RUPP significantly (P < 0.05) reducing these values in WT (11 ± 1 g) and MC4R-def (12 ± 1 g) pregnant rats. There was no difference in the number of live fetuses in normal pregnant WT (14 ± 1) versus MC4R-def rats (13 ± 1) and these numbers were similarly reduced by RUPP in WT (7 ± 1) and MC4R-def rats (7 ± 1). Thus, RUPP-induced fetal resorption rates were similar between both genotypes (Figure 4B).
Figure 4.
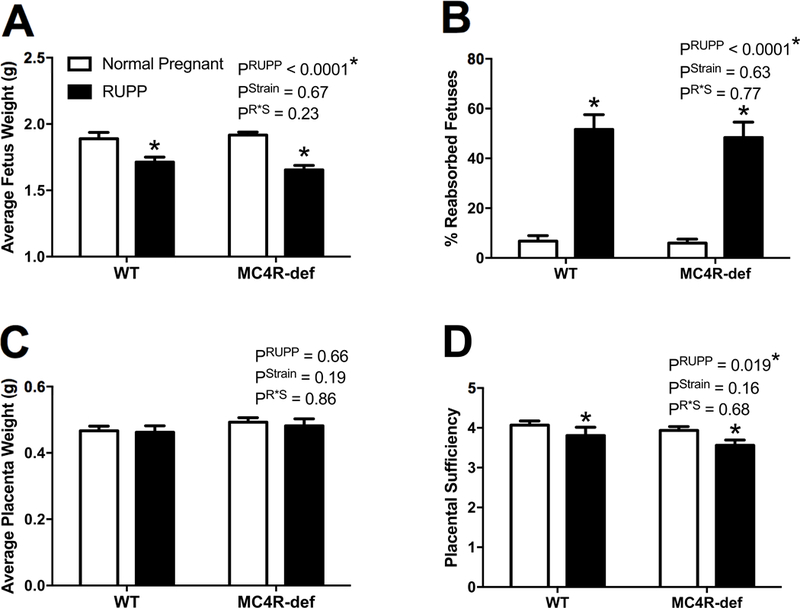
A: Average fetus weights, B: % resorbed fetuses, C: average placenta weights, and D: placental sufficiency at GD 19 in melanocortin-4 receptor (MC4R) wild type (WT) and deficient (MC4R-def) rats under normal pregnant or reduced uterine perfusion pressure (RUPP) conditions. Normal pregnant: WT, N = 16 and MC4R-def, N = 19. RUPP: WT, N = 13 and MC4R-def, N = 15. *P < 0.05 vs. normal pregnant counterparts.
Average placental weights were comparable between normal pregnant WT and MC4R-def rats at GD 19 and were not affected by RUPP (Figure 4C). Similar trends were found for total placental weights between WT (6.4 ± 0.3 g) and MC4R-def (6.2 ± 0.2 g) pregnant rats and RUPP significantly (P < 0.05) reducing these values in WT (2.9 ± 0.4 g) and MC4R-def (3.3 ± 0.4 g) pregnant rats. As for placental sufficiency, which is as a surrogate marker of the nutritive capacity of the placenta, there was no difference detected between either normal pregnant strains, but was reduced to similar values among both genotypes following RUPP (Figure 4D).
Bioavailable PlGF levels
Circulating levels of PlGF were comparable between normal pregnant WT and MC4R-def strains but were significantly reduced by similar magnitudes by RUPP in both strains (Figure 5).
Figure 5.
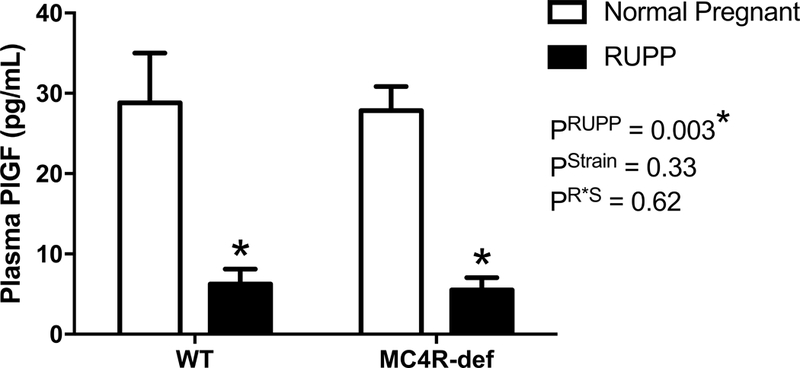
Placental growth factor (PlGF) levels in plasma at GD 19 in melanocortin-4 receptor (MC4R) wild type (WT) and deficient (MC4R-def) rats under normal pregnant or reduced uterine perfusion pressure (RUPP) conditions. Normal pregnant: WT, N = 12 and MC4R-def, N = 14. RUPP: WT, N = 12 and MC4R-def, N = 12. *P < 0.05 vs. normal pregnant counterparts.
Discussion
The novel finding of the present study is that obese pregnant rats due to MC4R deficiency have attenuated placental ischemia-induced hypertension. Our data indicate that while MC4R-def rats compared to WT rats have higher blood pressure during normal pregnancy, placental ischemia-induced hypertension is attenuated in MC4R-def pregnant rats. This attenuated blood pressure response occurred despite elevations in the obesity-related metabolic factors leptin and insulin in the obese pregnant rats and similar decreases in adiponectin and PlGF levels in response to RUPP between both MC4R-def and WT pregnant rats. Thus, obese pregnancies associated with abnormal MC4R signaling may be less susceptible to the development of placental ischemia-induced hypertension.
While human and experimental animal studies have focused on placental ischemic disease in the pathogenesis of PE, less is known about how established risk factors such as obesity impact the link between placental ischemia and blood pressure regulation during pregnancy. Several epidemiological studies have established a strong association between obesity and PE 6, 8, 31, 32. Ours is the first and only study comparing maternal blood pressure under normal pregnant conditions and the hypertensive response to placental ischemia in a model of obesity. We utilized a genetically obese pregnant rat model having heterozygous MC4R deficiency. The purpose for using a genetic model of obesity in rats was based on our previous studies where there were no dramatic changes in body weight or fat mass following high-fat diet feeding in female pregnant rats 33, 34. Other studies suggest that high-fat diet increases central MC4R signaling 35, but it has not been examined whether high fat feeding or obesity alters placental ischemia-induced hypertension via MC4R signaling. In the current study, we set forth to explore the importance of MC4R in control of blood pressure during normal pregnancy and in response to RUPP. In MC4R-def pregnant rats, we previously showed and confirmed here that they have elevated body weight, fat mass, and blood pressure under normal pregnant conditions 34. Moreover, our novel finding is that MC4R-def pregnant rats have attenuated placental ischemia-induced hypertension. The fact that not all obese pregnant women develop PE 32 suggests that obesity increases the susceptibility of the maternal cardiovascular system to hypertension produced by placental ischemic and hypoxic stimuli in only a portion of women, for instance those possibly having greater expression of MC4R. The epidemiological information regarding rates of altered expression of MC4R in obese women is meager. These are important studies requiring investigation, as our data suggest that obese pregnant women with MC4R deficiency have attenuated placental ischemia-induced hypertension in PE. Therefore, it is possible that certain forms of obesity associated with reduced MC4R signaling in the SNS may have an attenuated response to ischemic and hypoxic stimuli to the placenta and explain why, even though obesity is thought to be a major risk factor for PE, some obese pregnant women do not develop severe PE or accompanying PE-related adverse outcomes 36. This contention is based on previous reports that obese humans and experimental animals having genetic deficiency or pharmacological antagonism of MC4R activation are protected against the development of hypertension via suppression of the SNS 37. However, there has been a lack of human or experimental animal studies focused on assessing whether altered MC4R expression impacts the development of hypertension during pregnancy.
We tested the hypothesis that placental ischemia-induced hypertension is attenuated in MC4R-def pregnant rats by comparing the effects of RUPP on blood pressure between MC4R WT and MC4R heterozygous-def pregnant rats. MC4R heterozygous-def rats were utilized because of the low success rate of obtaining pregnant MC4R homozygous-def rats. Heterozygosity of the MC4R gene in humans is associated with increased food intake and is one of the most common forms of monogenetic obesity in humans 38. Sex differences have not been a focus of these genetic studies. The majority of studies using MC4R-def rats and mice have been conducted in males, where it was demonstrated that this receptor mediates the blood pressure response to hypertensive stimuli, including increased circulating levels of the adipokine, leptin, via activation of the SNS 17, 39–42. Leptin crosses the blood-brain barrier to target neurons expressing the leptin receptor to stimulate the release of alpha-melanocyte-stimulating hormone (α-MSH), which acts via MC4R expressed on second-order neurons to elicit sympathetic nerve activity (SNA) toward the periphery. Fewer studies have focused on the importance of MC4R in blood pressure regulation in females, especially during normal pregnancy and PE.
MC4R-def rats have increased blood pressure during normal pregnancy. On the surface, our blood pressure data in normal pregnant MC4R-def rats seem to contrast what has been demonstrated with MC4R deficiency in male rodents whereby increases in circulating levels of the obesity-related metabolic factor, leptin, led to downstream activation of central MC4R to promote SNA and hypertension 18. However, it is important to note that our pregnancy studies utilized heterozygous MC4R-def rats that were not completely deficient in the receptor. Therefore, based on findings from others suggesting that SNS activity is increased during normal pregnancy in humans 43, 44; that MC4R control of blood pressure and SNS activity are increased in pregnant versus non-pregnant rats 22; a vast amount of data that central MC4R signaling elicits sympathetic outflow; and leptin levels are increased in our obese hypertensive pregnant rats, we propose that obesity-related metabolic factors target central MC4R to chronically stimulate SNA and increase blood pressure in our obese hypertensive pregnant rats under normal pregnant conditions.
The MC4R-def rats in this study had a global deficiency in the receptor. Although this might be a limitation of the present study, it is not evident that MC4R is expressed in placental tissue 45 or that MC4R in the periphery controls blood pressure. Whereas, a body of evidence using brain-specific knockout mice and intracerebroventricular administration of antagonists to this receptor system, suggests that MC4R mediates its impact on blood pressure via elegant neural pathways 23, 40, 46, 47. Our study was not designed to examine brain-specific deficiency of MC4R on SNS outflow and downstream blood pressure regulation during pregnancy. Nor did this study delve into the timeframe for the development of hypertension in our obese pregnant model. We previously published that pre-pregnancy body weight is greater in MC4R-def versus that WT rats 34. In the current study, our focus was on the impact of MC4R deficiency on placental ischemia-induced hypertension towards the end of pregnancy. We did not assess body weight or blood pressure before pregnancy in the group of rats included in this study. As there are human data to suggest that elevated pre-pregnancy body weight is an independent risk factor for hypertension during pregnancy 48, future studies involving caloric restriction and biotelemetry blood pressure recordings should be conducted to deduce the importance of increased body weight, fat mass, and obesity-related metabolic factors on chronic blood pressure regulation in obese MC4R-def rats throughout pregnancy.
We previously observed that chronic infusion of the obesity factors leptin or insulin into once normotensive pregnant Sprague-Dawley rats elicited increases in blood pressure 49, 50. The levels of these factors were not altered by RUPP in MC4R-def pregnant rats; thus, they remained elevated following the procedure compared to WT pregnant rats. Even though the RUPP procedure reduced body weight and fat mass in both MC4R-def and WT pregnant rats, it is important to note that adiposity was still higher in RUPP MC4R-def over RUPP WT levels. As a result, we found a combination of elevated obesity-related metabolic factors coupled to a significant reduction in circulating PlGF. Placental ischemia and PE in humans and experimental animal models is marked by altered circulating levels of angiogenic factors, like PlGF 21, 51. Placental ischemia elicits the release of anti-angiogenic factors from the placenta; this includes the soluble fms-like tyrosine kinase-1 (sFlt-1) 52. Once in the circulation, sFlt-1 binds and reduces bioavailable PlGF and VEGF 53. Angiogenic factors, like PlGF and VEGF, are important for maintenance of maternal vascular health. PlGF plays an increased role in promoting vasorelaxation during normal pregnancy via action on its receptor, VEGFR1, whereas VEGF isoforms largely bind to VEGF2 to induce relaxation via stimulation of nitric oxide synthase (NOS) 54. We previously showed that RUPP rats have reduced levels of PlGF and administration of this factor abolishes their hypertension 30. These data suggest that RUPP-induced reductions in PlGF mediate this hypertensive pathology. In the current study, RUPP significantly reduced plasma levels of PlGF in WT rats, which is indicative of vascular dysfunction and hypertension. Interestingly, PlGF levels were similarly reduced in RUPP WT versus MC4R-def rats. Even so, MC4R-def pregnant rats presented with an attenuated placental ischemia-induced hypertensive response. Our data are reminiscent to those of da Silva et al who showed in male rats that chronic pharmacological blockade of these receptors directly in the brain attenuates the hypertensive response to systemic vascular dysfunction following NOS inhibition, which was produced by intravenous infusion of the non-selective NOS inhibitor, L-NAME 40. Collectively, these data suggest that, even in the face of reduced PlGF bioavailability, reduced NOS, and vascular dysfunction, MC4R deficiency attenuates the maternal hemodynamic response to placental ischemia produced by the RUPP procedure. Regardless of this combination of placental ischemic and obesity milieus that would be hypothesized to exaggerate blood pressure in MC4R-def RUPP rats, we did not find exaggeration of RUPP hypertension in MC4R-def pregnant rats. These data suggest that deficiency of MC4R attenuates the development of placental ischemia-induced hypertension using the RUPP model.
Our current research focused on the impact of MC4R deficiency on maternal blood pressure response to RUPP by using obese MC4R-def versus lean WT dams, but the consequences of this procedure on the health of the fetus from obese compared to lean dams remains to be elucidated. Frias et al found in non-human primates that 4 years of high-fat feeding elicited maternal obesity associated with reductions in uterine artery and placental volume blood flow 55. However, they did not observe a change in fetal weights between the dietary groups. They did not examine maternal blood pressure. We found no differences in fetal weights under normal pregnant conditions and RUPP similarly reduced these values in our obese versus lean pregnant model. It is likely that the development of maternal hypertension in response to surgically-induced RUPP is important to compensate for the reduced blood flow beyond the permanent clip placement during the surgery. The increased blood pressure would attempt to meet the metabolic demands of the fetal-placental unit and explain why there is not 100% fetal demise in the RUPP model. Interestingly, we found an attenuated blood pressure response to the RUPP procedure in MC4R-def versus WT rats, even though there were no differences in the number of fetuses, fetal weights, or fetal resorptions between RUPP MC4R-def and RUPP WT rats by gestational day 19. It is unknown whether the lack of a rise in blood pressure in RUPP MC4R-def compared to WT rats leads to exaggerations in adverse fetal development at birth in offspring from RUPP MC4R-def versus RUPP WT dams. Follow-up studies should assess utero-placental hemodynamics and outcomes in these offspring at birth by examining birthweights, at which time crown-rump length and head-to-abdomen circumference will be measured. It has been found in human studies that a postnatal head circumference that is larger than abdominal circumference is indicative of intrauterine growth restriction (IUGR) 56.
RUPP similarly reduced fetal weight and increased fetal resorption rates in both MC4R-def and WT pregnant rats. Furthermore, there were parallel reductions in circulating levels of PlGF in both groups, which is indicative of comparable levels of placental ischemic disease. Therefore, we believe that the difference is blood pressure response to RUPP was not dependent on different levels of placental ischemia or hypoxia, but due to reduced expression of the MC4R in the deficient pregnant rat model. We propose that, during normal pregnancy, the available amount MC4R in the obese heterozygous MC4R-def pregnant rats is suitable to elevate blood pressure due to increased circulating levels of obesity-related metabolic factors that activate this receptor system to elicit SNS activity and increases in blood pressure. With regards to placental ischemia-induced hypertension, we previously reported that adrenergic receptor blockade attenuates the blood pressure response to RUPP 21. Others found in non-pregnant rats that the pressor response to chronic central administration of an MC3/4R agonist was mediated by adrenergic activity 57. We currently found that hypertension following RUPP was attenuated in MC4R-def pregnant rats. Collectively, these results implicate that a fully intact SNS is required to facilitate the maternal blood pressure response to a hypertensive second-hit of placental ischemia.
Hypertension in PE is associated with increases in SNA. Reyes et al reviewed this topic at length by mentioning that such increases are a repeated observation in patients with PE 19. Others have also detected increased markers of SNA in surgical- and environment-induced experimental models of PE. For example, in RUPP dams or administration of lipopolysaccharide (LPS) to pregnant rats, there was an enrichment of genes related to increased neuronal signaling and vasoconstriction in aortic tissue 58 and increased adrenergic receptor-induced vasoconstriction in systemic and renal circulations 59. Moreover, cold-induced stress reduced mechanisms of placentation and elicited PE symptoms in rats 60. Chronic hypoxia by placement of dams in hypobaric chambers programs for increased SNA in offspring as adults 61. It is important to note that these are correlative findings without interventions to probe the contribution of the SNS in the maintenance of hypertension in PE. Whereas, we recently reported that RUPP rats have an enhanced effect to the blood pressure lowering effects of adrenergic receptor blockade 21. Our findings suggest that MC4R and SNS signaling play a permissive role in the pathogenesis of placental ischemia-induced hypertension. Future studies should confirm whether MC4R plays a role in hypertension found in other experimental models of PE.
Supplementary Material
Perspectives.
The prevalence of PE, a pregnancy-specific disorder of new-onset hypertension linked to placental ischemia, has increased over the past decades. This increase in prevalence is most likely related to an increase in a number of risk factors including obesity. While obesity is a major risk factor for PE, not all obese pregnant women develop pregnancy-induced hypertension or PE. Our data indicate that while obese MC4R-def pregnant rats have higher blood pressure during pregnancy, placental ischemia-induced hypertension is attenuated in obese MC4R-def pregnant rats. Thus, obese women with abnormal MC4R signaling may be less susceptible to the development of placental ischemia-induced hypertension.
Novelty and Significance.
What Is New? This is one of the first studies utilizing genetic models, specifically MC4R deficiency, to probe the impact of obesity on placental ischemic-induced hypertension.
What Is Relevant? While obesity is a major risk factor for PE, not all obese pregnant women develop pregnancy-induced hypertension or PE. Our data indicate that while obese MC4R-def pregnant rats have higher blood pressure during pregnancy, placental ischemia-induced hypertension is attenuated in obese MC4R-def pregnant rats. Thus, obese women with abnormal MC4R signaling may be less susceptible to the development of placental ischemia-induced hypertension.
Summary. MC4R deficiency attenuates placental ischemia-induced hypertension by utilizing the RUPP rat model.
Acknowledgments
We would like to thank Marietta Arany and Barbara Wilson for their expert laboratory assistance.
Sources of Funding
This study was supported by grants from NHLBI (R00HL130577 to FTS, T32HL105324–01 to FTS, and P01HL051971 to JPG) and NIGMS (P20GM104357 to FTS). Genotyping work was performed through the UMMC Molecular and Genomics Facility and is supported, in part, by funds from the NIGMS, including Mississippi INBRE (P20GM103476), Center for Psychiatric Neuroscience (CPN)-COBRE (P30GM103328), Obesity, Cardiorenal and Metabolic Diseases-COBRE (P20GM104357), and Mississippi Center of Excellence in Perinatal Research (MS-CEPR)-COBRE (P20GM121334).
Footnotes
Conflicts of Interest
We have nothing to disclose.
References
- 1.Ghulmiyyah L and Sibai B. Maternal mortality from preeclampsia/eclampsia. Semin Perinatol. 2012;36:56–9. [DOI] [PubMed] [Google Scholar]
- 2.American College of O, Gynecologists and Task Force on Hypertension in P. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122:1122–31. [DOI] [PubMed] [Google Scholar]
- 3.Turner JA. Diagnosis and management of pre-eclampsia: an update. Int J Womens Health. 2010;2:327–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Endler M, Saltvedt S, Cnattingius S, Stephansson O and Wikstrom AK. Retained placenta is associated with pre-eclampsia, stillbirth, giving birth to a small-for-gestational-age infant, and spontaneous preterm birth: a national register-based study. BJOG : an international journal of obstetrics and gynaecology. 2014;121:1462–70. [DOI] [PubMed] [Google Scholar]
- 5.LaMarca B, Amaral LM, Harmon AC, Cornelius DC, Faulkner JL and Cunningham MW Jr Placental Ischemia and Resultant Phenotype in Animal Models of Preeclampsia. Curr Hypertens Rep. 2016;18:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Persson M, Cnattingius S, Wikstrom AK and Johansson S. Maternal overweight and obesity and risk of pre-eclampsia in women with type 1 diabetes or type 2 diabetes. Diabetologia. 2016;59:2099–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poorolajal J and Jenabi E. The association between body mass index and preeclampsia: a meta-analysis. J Matern Fetal Neonatal Med. 2016;29:3670–6. [DOI] [PubMed] [Google Scholar]
- 8.Mbah AK, Kornosky JL, Kristensen S, August EM, Alio AP, Marty PJ, Belogolovkin V, Bruder K and Salihu HM. Super-obesity and risk for early and late pre-eclampsia. BJOG. 2010;117:997–1004. [DOI] [PubMed] [Google Scholar]
- 9.Biswas T, Uddin MJ, Mamun AA, Pervin S and S PG. Increasing prevalence of overweight and obesity in Bangladeshi women of reproductive age: Findings from 2004 to 2014. PLoS One. 2017;12:e0181080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanguru L, McCaw-Binns A, Bell J, Yonger-Coleman N, Wilks R and Hussein J. The burden of obesity in women of reproductive age and in pregnancy in a middle-income setting: A population based study from Jamaica. PLoS One. 2017;12:e0188677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amugsi DA, Dimbuene ZT, Mberu B, Muthuri S and Ezeh AC. Prevalence and time trends in overweight and obesity among urban women: an analysis of demographic and health surveys data from 24 African countries, 1991–2014. BMJ Open. 2017;7:e017344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD and Ogden CL. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA. 2016;315:2284–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li HP, Chen X and Li MQ. Gestational diabetes induces chronic hypoxia stress and excessive inflammatory response in murine placenta. Int J Clin Exp Pathol. 2013;6:650–9. [PMC free article] [PubMed] [Google Scholar]
- 14.Spradley FT, Palei AC and Granger JP. Increased risk for the development of preeclampsia in obese pregnancies: weighing in on the mechanisms. Am J Physiol Regul Integr Comp Physiol. 2015;309:R1326–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collet TH, Dubern B, Mokrosinski J, Connors H, Keogh JM, Mendes de Oliveira E, Henning E, Poitou-Bernert C, Oppert JM, Tounian P, Marchelli F, Alili R, Le Beyec J, Pepin D, Lacorte JM, Gottesdiener A, Bounds R, Sharma S, Folster C, Henderson B, O’Rahilly S, Stoner E, Gottesdiener K, Panaro BL, Cone RD, Clement K, Farooqi IS and Van der Ploeg LHT. Evaluation of a melanocortin-4 receptor (MC4R) agonist (Setmelanotide) in MC4R deficiency. Mol Metab. 2017;6:1321–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ju SH, Cho GB and Sohn JW. Understanding melanocortin-4 receptor control of neuronal circuits: Toward novel therapeutics for obesity syndrome. Pharmacol Res. 2018;129:10–19. [DOI] [PubMed] [Google Scholar]
- 17.da Silva AA, Spradley FT, Granger JP, Hall JE and do Carmo JM. Brain-mediated antidiabetic, anorexic, and cardiovascular actions of leptin require melanocortin-4 receptor signaling. J Neurophysiol. 2015;113:2786–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.da Silva AA, do Carmo JM, Wang Z and Hall JE. The brain melanocortin system, sympathetic control, and obesity hypertension. Physiology (Bethesda). 2014;29:196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reyes LM, Usselman CW, Davenport MH and Steinback CD. Sympathetic Nervous System Regulation in Human Normotensive and Hypertensive Pregnancies. Hypertension. 2018;71:793–803. [DOI] [PubMed] [Google Scholar]
- 20.Logue OC, George EM and Bidwell GL, 3rd. Preeclampsia and the brain: neural control of cardiovascular changes during pregnancy and neurological outcomes of preeclampsia. Clin Sci (Lond). 2016;130:1417–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spradley FT, Ge Y, Haynes BP, Granger JP and Anderson CD. Adrenergic receptor blockade attenuates placental ischemia-induced hypertension. Physiological reports. 2018;6:e13814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi Z, Cassaglia PA, Gotthardt LC and Brooks VL. Hypothalamic Paraventricular and Arcuate Nuclei Contribute to Elevated Sympathetic Nerve Activity in Pregnant Rats: Roles of Neuropeptide Y and alpha-Melanocyte-Stimulating Hormone. Hypertension. 2015;66:1191–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samuelsson AS, Mullier A, Maicas N, Oosterhuis NR, Eun Bae S, Novoselova TV, Chan LF, Pombo JM, Taylor PD, Joles JA, Coen CW, Balthasar N and Poston L. Central role for melanocortin-4 receptors in offspring hypertension arising from maternal obesity. Proc Natl Acad Sci U S A. 2016;113:12298–12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leptin Intapad S., Melanocortin 4 receptor and Renal Nerves Play a Role in High Blood Pressure Programmed by Intrauterine Growth Restriction in Mouse. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2016;30:1214.4. [Google Scholar]
- 25.Powell JR and Evans BR. How Much Does Inbreeding Reduce Heterozygosity? Empirical Results from Aedes aegypti. Am J Trop Med Hyg. 2017;96:157–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mul JD, van Boxtel R, Bergen DJ, Brans MA, Brakkee JH, Toonen PW, Garner KM, Adan RA and Cuppen E. Melanocortin receptor 4 deficiency affects body weight regulation, grooming behavior, and substrate preference in the rat. Obesity (Silver Spring). 2012;20:612–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alexander BT, Kassab SE, Miller MT, Abram SR, Reckelhoff JF, Bennett WA and Granger JP. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension. 2001;37:1191–5. [DOI] [PubMed] [Google Scholar]
- 28.Spradley FT, Palei AC and Granger JP. Obese melanocortin-4 receptor-deficient rats exhibit augmented angiogenic balance and vasorelaxation during pregnancy. Physiol Rep. 2013;1:e00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hunt K, Kennedy SH and Vatish M. Definitions and reporting of placental insufficiency in biomedical journals: a review of the literature. Eur J Obstet Gynecol Reprod Biol. 2016;205:146–9. [DOI] [PubMed] [Google Scholar]
- 30.Spradley FT, Tan AY, Joo WS, Daniels G, Kussie P, Karumanchi SA and Granger JP. Placental Growth Factor Administration Abolishes Placental Ischemia-Induced Hypertension. Hypertension. 2016;67:740–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeyabalan A Epidemiology of preeclampsia: impact of obesity. Nutr Rev. 2013;71 Suppl 1:S18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roberts JM, Bodnar LM, Patrick TE and Powers RW. The Role of Obesity in Preeclampsia. Pregnancy Hypertens. 2011;1:6–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palei AC, Spradley FT and Granger JP. Role of Nitric Oxide Synthase on Blood Pressure Regulation and Vascular Function in Pregnant Rats on a High-Fat Diet. Am J Hypertens. 2017;30:240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spradley FT, Palei AC and Granger JP. Differential body weight, blood pressure and placental inflammatory responses to normal versus high-fat diet in melanocortin-4 receptor-deficient pregnant rats. J Hypertens. 2016;34:1998–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Widiker S, Karst S, Wagener A and Brockmann GA. High-fat diet leads to a decreased methylation of the Mc4r gene in the obese BFMI and the lean B6 mouse lines. J Appl Genet. 2010;51:193–7. [DOI] [PubMed] [Google Scholar]
- 36.Durst JK, Tuuli MG, Stout MJ, Macones GA and Cahill AG. Degree of obesity at delivery and risk of preeclampsia with severe features. Am J Obstet Gynecol. 2016;214:651e1–5. [DOI] [PubMed] [Google Scholar]
- 37.do Carmo JM, da Silva AA, Wang Z, Fang T, Aberdein N, Perez de Lara CE and Hall JE. Role of the brain melanocortins in blood pressure regulation. Biochim Biophys Acta. 2017;1863:2508–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farooqi IS, Keogh JM, Yeo GS, Lank EJ, Cheetham T and O’Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med. 2003;348:1085–95. [DOI] [PubMed] [Google Scholar]
- 39.Maranon R, Lima R, Spradley FT, do Carmo JM, Zhang H, Smith AD, Bui E, Thomas RL, Moulana M, Hall JE, Granger JP and Reckelhoff JF. Roles for the sympathetic nervous system, renal nerves, and CNS melanocortin-4 receptor in the elevated blood pressure in hyperandrogenemic female rats. Am J Physiol Regul Integr Comp Physiol. 2015;308:R708–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.da Silva AA, do Carmo JM, Dubinion JH, Bassi M, Mokhtarpouriani K, Hamza SM and Hall JE. Chronic central nervous system MC3/4R blockade attenuates hypertension induced by nitric oxide synthase inhibition but not by angiotensin II infusion. Hypertension. 2015;65:171–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ni XP, Butler AA, Cone RD and Humphreys MH. Central receptors mediating the cardiovascular actions of melanocyte stimulating hormones. J Hypertens. 2006;24:2239–46. [DOI] [PubMed] [Google Scholar]
- 42.Rahmouni K, Haynes WG, Morgan DA and Mark AL. Role of melanocortin-4 receptors in mediating renal sympathoactivation to leptin and insulin. J Neurosci. 2003;23:5998–6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jarvis SS, Shibata S, Bivens TB, Okada Y, Casey BM, Levine BD and Fu Q. Sympathetic activation during early pregnancy in humans. J Physiol. 2012;590:3535–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greenwood JP, Scott EM, Stoker JB, Walker JJ and Mary DA. Sympathetic neural mechanisms in normal and hypertensive pregnancy in humans. Circulation. 2001;104:2200–4. [DOI] [PubMed] [Google Scholar]
- 45.Simamura E, Shimada H, Shoji H, Otani H and Hatta T. Effects of melanocortins on fetal development. Congenit Anom (Kyoto). 2011;51:47–54. [DOI] [PubMed] [Google Scholar]
- 46.da Silva AA, Kuo JJ and Hall JE. Role of hypothalamic melanocortin 3/4-receptors in mediating chronic cardiovascular, renal, and metabolic actions of leptin. Hypertension. 2004;43:1312–7. [DOI] [PubMed] [Google Scholar]
- 47.do Carmo JM, da Silva AA, Rushing JS and Hall JE. Activation of the central melanocortin system contributes to the increased arterial pressure in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol. 2012;302:R561–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Savitri AI, Zuithoff P, Browne JL, Amelia D, Baharuddin M, Grobbee DE and Uiterwaal CS. Does pre-pregnancy BMI determine blood pressure during pregnancy? A prospective cohort study. BMJ Open. 2016;6:e011626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palei AC, Spradley FT and Granger JP. Chronic hyperleptinemia results in the development of hypertension in pregnant rats. Am J Physiol Regul Integr Comp Physiol. 2015;308:R855–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Palei AC, Spradley FT and Granger JP. Euglycemic hyperinsulinemia increases blood pressure in pregnant rats independent of placental antiangiogenic and inflammatory factors. Am J Hypertens. 2013;26:1445–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weel IC, Baergen RN, Romao-Veiga M, Borges VT, Ribeiro VR, Witkin SS, Bannwart-Castro C, Peracoli JC, De Oliveira L and Peracoli MT. Association between Placental Lesions, Cytokines and Angiogenic Factors in Pregnant Women with Preeclampsia. PLoS One. 2016;11:e0157584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murphy SR, LaMarca BB, Parrish M, Cockrell K and Granger JP. Control of soluble fms-like tyrosine-1 (sFlt-1) production response to placental ischemia/hypoxia: role of tumor necrosis factor-alpha. Am J Physiol Regul Integr Comp Physiol. 2013;304:R130–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eddy AC, Bidwell GL 3rd, and George EM. Pro-angiogenic therapeutics for preeclampsia. Biol Sex Differ. 2018;9:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Osol G, Celia G, Gokina N, Barron C, Chien E, Mandala M, Luksha L and Kublickiene K. Placental growth factor is a potent vasodilator of rat and human resistance arteries. Am J Physiol Heart Circ Physiol. 2008;294:H1381–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Frias AE, Morgan TK, Evans AE, Rasanen J, Oh KY, Thornburg KL and Grove KL. Maternal high-fat diet disturbs uteroplacental hemodynamics and increases the frequency of stillbirth in a nonhuman primate model of excess nutrition. Endocrinology. 2011;152:2456–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sharma D, Shastri S and Sharma P. Intrauterine Growth Restriction: Antenatal and Postnatal Aspects. Clin Med Insights Pediatr. 2016;10:67–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuo JJ, da Silva AA, Tallam LS and Hall JE. Role of adrenergic activity in pressor responses to chronic melanocortin receptor activation. Hypertension. 2004;43:370–5. [DOI] [PubMed] [Google Scholar]
- 58.Lip SV, van der Graaf AM, Wiegman MJ, Scherjon SA, Boekschoten MV, Plosch T and Faas MM. Experimental preeclampsia in rats affects vascular gene expression patterns. Sci Rep. 2017;7:14807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spradley FT. Placental ischemia-induced hypertension is abolished by adrenergic receptor blockade. FASEB J. 2018;32:729.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kanayama N, Tsujimura R, She L, Maehara K and Terao T. Cold-induced stress stimulates the sympathetic nervous system, causing hypertension and proteinuria in rats. J Hypertens. 1997;15:383–9. [DOI] [PubMed] [Google Scholar]
- 61.Rook W, Johnson CD, Coney AM and Marshall JM. Prenatal hypoxia leads to increased muscle sympathetic nerve activity, sympathetic hyperinnervation, premature blunting of neuropeptide Y signaling, and hypertension in adult life. Hypertension. 2014;64:1321–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


