Abstract
Background:
The Surgical Treatment for Ischemic Heart Failure (STICH) trial demonstrated a survival benefit of coronary artery bypass grafting (CABG) in patients with ischemic cardiomyopathy and left ventricular dysfunction (LVD). The Surgical Thoracic Society (STS) and the EuroSCORE-2 (ES2) are used for risk-assessment in cardiac surgery, with little information available regarding their accuracy in patients with LVD. We assessed the ability of the STS and ES2 to evaluate 30-day post-operative mortality risk in STICH and a contemporary cohort (CC) of patients with a LV ejection fraction (LVEF)≤35% undergoing CABG outside of a trial setting.
Methods and Results:
The STS and ES2 were calculated for 814 STICH patients and 1246 consecutive patients in a CC. There were marked variations in 30-day post-operative mortality risk from one patient to another. The STS consistently calculated lower risk scores than ES2 (1.5 vs. 2.9 for the CC and 0.9 vs. 2.4 for the STICH cohort), and underestimated post-operative mortality risk. The STS and ES2 scores had moderately good C-statistics: CC (0.727, 95%CI:0.650,0.803 for STS, and 0.707, 95%CI:0.620,0.795 for ES2); STICH (0.744, 95%CI:0.677,0.812, for STS and 0.736, 95%CI:0.665,0.808 for ES2). Despite the CC patients having higher STS and ES2 score than STICH patients, mortality (3.5%) was lower than that of STICH (4.8%), suggesting a possible decrease in post-operative mortality over the last decade.
Conclusions:
The 30-day post-operative mortality risk of CABG in patients with LVD varies markedly. Both the STS and ES2 are effective in evaluating risk, although the STS tend to underestimate risk.
Keywords: Revascularization, Cardiovascular Surgery, Heart Failure
Introduction
Thirty-day mortality for patients undergoing surgical myocardial revascularization is usually assessed with standard risk scores, such as the Society of Thoracic Surgeons score (STS) (1) or the EuroSCORE-2 (2) and these have proven effective in assessing 30-day post-operative risk for a wide spectrum of patients undergoing cardiac surgery (3–6). However, these scores included a relatively small number of patients with severely reduced left ventricular ejection fraction (LVEF) when being developed, and to our knowledge, no data have been published with regards to their relative efficacy in assessing 30-day post-operative risk in this population. Also, in evaluating the pre- and perioperative variables associated with 30-day postoperative mortality in STICH, a number of variables not included in either the EuroSCORE-2 or the STS were strongly associated with outcomes raising the possibility that the influence of these variables could reduce the accuracy of the STS and EuroSCORE-2 in these patients (7). Finally, although the EuroSCORE-2 has excellent follow-up until hospital discharge, its follow-up 30-day post operation is as low as 56.6%, such that deaths between hospital discharge and 30 days post-operation were not captured in all patients (2). Analyses of the STS would suggest that up to 10% of post-operative deaths occur from the time of hospital discharge to 30 days post-operation, a percentage that could be expected to be greater in high-risk patients (8). Due to the growing number of patients undergoing CABG with coexisting severe left ventricular dysfunction, and in order to more optimally individualize risk prediction, and thus improve assessment of the risk/benefit of CABG for such patients, evaluating the utility of 30-day post-operative mortality risk assessment with standard risk scores, specifically among patients with ischemic cardiomyopathy, is required.
Patients were enrolled in the STICH trial from 2002 to 2007. Their operative mortality was comparable to that reported at that time (9–11). Since then, several reports suggest that mortality with CABG is decreasing despite increasing patient complexity (12, 13) such that the 30-day post-operative mortality risk associated with CABG in patients with a LVEF ≤ 35% may be less than that reported in STICH. Accordingly, the performance of these risk scores in STICH trial patients should be accompanied by an evaluation in STICH-like patients treated outside of a trial in a more contemporary time period. The development of such a contemporary cohort would also permit the assessment of whether there is evidence of improvement in operative mortality since the STICH trial was performed in this high-risk population.
The objectives of this study were thus to assess and compare the ability of the STS and EuroSCORE-2 to evaluate the risk of 30-day post-operative mortality in STICH patients and in a contemporary cohort of patients with a LVEF ≤ 35% undergoing CABG outside of a trial setting.
Methods
STICH CABG Cohort
The rationale and design of the STICH program of trials have been published previously (14). STICH was a prospective, multicenter, randomized trial sponsored by the National Heart, Lung, and Blood Institute (NHLBI) (NCT00023595) that recruited 2136 patients with coronary artery disease (CAD) and a LVEF of ≤ 35% between 2002 and 2007 from 127 centers in 26 countries (15). Two hypotheses were tested. Hypothesis 1 compared CABG plus optimal medical therapy (MED) vs. MED alone in patients with a LVEF ≤ 35% that were amenable to CABG. It found CABG plus MED to reduce all-cause mortality compared to MED alone (16, 17). Hypothesis 2 compared CABG with and without surgical ventricular reconstruction (SVR) in patients with a LVEF ≤ 35% and dominant akinesia or dyskinesia of the anterior wall requiring CABG. It found that SVR did not improve outcomes in patients undergoing CABG (18). The inclusion and exclusion criteria and the requirements for ensuring high quality surgical revascularization have also been detailed previously (14). Briefly, patients were required to have coronary anatomy amenable to CABG, and were excluded if they had left main CAD obstruction ≥50% or CCS ≥3. Patients with cardiogenic shock or with a recent MI thought to be an important cause of LV dysfunction were excluded from the trial (14). The NHLBI and the ethics committee at each recruiting institution approved the study protocol.
Of the 1534 patients in the STICH trial randomized to CABG with or without SVR, 814 had CABG without concomitant procedures (SVR or mitral valve procedure) or preoperative inotropes, and constituted the STICH CABG cohort. Follow-up was performed at the time of hospital discharge or at 30 days following surgery if the patient remained hospitalized for ≥ 30 days, and at 4-month intervals for the first year of follow-up, and thereafter at 6-month intervals over the entire follow-up period (14). There was 100% follow-up at 30 days post-operation.
Contemporary cohort of non-trial patients with a LVEF ≤35% having CABG
A contemporary cohort was established for the specific purpose of the present analysis. A total of 1246 consecutive patients with a LVEF ≤ 35% who underwent CABG without concomitant procedures were recruited from five medical centers (the Montreal Heart Institute, Montreal, Canada, n=719 (2010 to 2017); Jena Medical Centre, Jena, Germany, n=241 (2010 to 2017); the Golden Jubilee National Hospital, Glasgow, UK, n=115 (2008 to 2017); La Pitié-Salpêtrière Hospital, Paris, France, n=92 (2016 to 2017), and Georges Pompidou European Hospital, Paris, France, n=79 (2016 to 2017)). Patients with emergent or salvage procedures, critical/shock/resuscitation/inotrope dependent/acute MI were excluded from the analysis (n=2160) (Figure 1). Of these, three of the participating centres (Montreal, Jena, and Glasgow) were initially involved in the STICH trial. Baseline clinical and biological characteristics were obtained from each site’s computerized medical charts; values used being the closest to the date of surgery. Pre- intra- and post-operative management was based on an individual case-by-case analysis by each site’s heart team. Follow-up was obtained either from individual follow-up or from an administrative database after appropriate IRB approval. Data were securely sent on line as spreadsheets and centrally analyzed by the Duke Clinical Research Institute (Durham, NC). Follow-up was complete at 30 days in 1239 patients, the other 7 patients having been discharged stable to referring hospitals an average of 15 days (min 6 and max 28 days) days post-operation, and were not considered for the present analysis. The ethics committee at each recruiting institution approved the study protocol.
Figure 1:
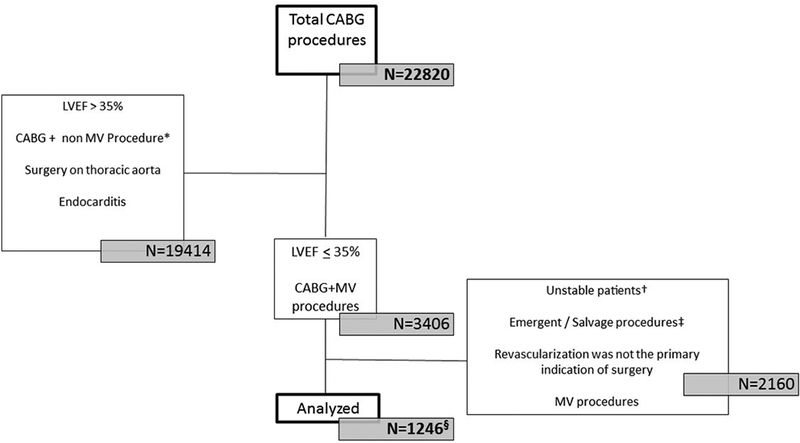
Inclusion process for patients in the contemporary cohort that had isolated CABG for ischemic LV dysfunction. §Seven patients were lost to follow-up within the first 30 days following surgery. *Non-MV procedure refers to any procedure combined with CABG other than repair/replacement of the mitral valve (SVR, aortic valve/ tricuspid valve repair or replacement, left atrial appendage closure, septal defect repair, tumor resection, and surgery on thoracic aorta). †Unstable refers to: Any cardiopulmonary resuscitation or mechanical ventilation before the start of the procedure; Pre-operative shock, peripheral hypoperfusion or end-organ damage; Critical pre-operative state; Surgery during the acute phase of myocardial infarction; Any sustained ventricular arrhythmia or aborted sudden cardiac death. ‡ Emergent refers to operation before the beginning of the next working day after decision to operate; Salvage refers to patients requiring cardiopulmonary resuscitation prior to induction of anaesthesia. §Seven patients were lost to follow-up within the first 30 days following surgery.
Primary endpoint
In the present study, the primary endpoint was 30-day postoperative mortality. Mortality was defined as any death within 30 days occurring after surgical procedure in any location: death from all-cause before discharge in the same hospital / facility, death after discharge to any other hospital / facility, or death after discharge at home.
Data analysis
Patient characteristics were summarized by patient cohort (STICH and contemporary) and by quintile of risk predicted by the STS and EuroSCORE-2. Unless otherwise noted, continuous variables were summarized as median (25th, 75th percentiles) and categorical variables were summarized as count (percentage). Differences in baseline characteristics across quintiles of risk are tested with chi-square tests for categorical variables and Kruskal Wallis tests or ANOVA for continuous variables. Cohorts are compared with chi-square tests for categorical variables and with Wilcoxon rank-sum or two-sample t-tests for continuous variables. Model discrimination was evaluated with the area under the ROC curve (C-statistic) and its 95% confidence interval from logistic regression (19). Differences in predicted risk score between STS and EURO2 for STICH and the contemporary cohorts were depicted in a mountain plot (20). Mountain plot is simply an empirical distribution function curve folded at 50th percentile (i.e. median).
STS and EuroSCORE-2 scoring
The definitions used to calculate the STS and EuroSCORE-2 are included in Supplemental Table 1. An imputed dataset was created using PROC MI (multiple imputation) in SAS v9.4 (SAS Institute, Inc., Cary, NC) with the method of fully conditional specification. Imputation carried out separately for the STICH and contemporary cohorts using all available baseline and preoperative variables to inform the imputation. For STICH, patients in both Hypotheses (H1 and H2) were included when imputing and in each cohort imputation was conducted before applying the exclusion criteria. In the contemporary cohort, apart from pulmonary artery systolic pressure (PASP), there were few missing values for the calculation of both the STS and EuroSCORE-2, thus the scoring of the EuroSCORE-2 was repeated without the inclusion of PASP for a sensitivity analysis. In the contemporary cohort, imputed values included PASP (N=302), NYHA class (N=88), chronic lung disease severity (N=50), timing of past MI (N=29), pre operative IABP (N=54), type of AF (paroxysmal vs. chronic, N=36), severity of mitral regurgitation (N=18), height and weight (N=7), peripheral vascular disease (N=3), cerebrovascular disease (N=2), diabetes (N=3), number of previous cardiac surgeries (N=1) and creatinine (N=1). Variables that needed to be imputed in STICH patients included mitral regurgitation severity (N=3), mobility (N=3), and acuteness of operation (N=1). Given the large percentage of missing PASP data in the STICH cohort, PASP was not imputed but assumed to be in the normal range when not available (N=660). Chronic lung disease was not documented in STICH and was assumed to be absent. Due to small percent of missing data, single random imputation rather than multiple imputation was conducted. Patient characteristics were manually entered into the online risk calculators for 15% of the contemporary cohort to confirm that the scores matched those calculated programmatically.
In order to assess whether missing values would significantly lead to underestimation of 30-day post-operative risk, an exploratory analysis in which all missing values were awarded the most severe abnormality (worst case scenario) was performed. For example, all missing NYHA values were coded a class 4, all missing COPD values were awarded severe COPD, and so on.
Evaluating Predictive value of STS and EuroSCORE-2
Logistic regression models were fit for mortality 30 days following CABG separately for the STICH and contemporary cohorts. The independent variable of interest was either STS or EuroSCORE-2. Model calibration was assessed with Hosmer-Lemeshow goodness-of-fit tests (5 groups) and by plotting the predicted probability of 30-day death with the observed mortality rate in quintiles of predicted risk. The overall event rate is shown with a horizontal reference line.
Comparing mortality rates in cohorts
To test whether the STICH and contemporary cohorts had different mortality rates after accounting for differences in risk score, a logistic model for 30-day mortality was built using “cohort” as an indicator variable and the (log-transformed) STS and EuroSCORE-2 scores as adjustment covariates. This model was used to estimate the odds ratio (95% CI) associated with being in the STICH cohort.
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Results
Baseline Characteristics and outcomes
Baseline characteristics of both STICH (n=814) and contemporary (n=1246) cohorts are displayed in Table 1. Briefly, patients in the contemporary cohort had slightly higher STS (median 1.5 vs. 0.9) and EuroSCORE-2 (median 2.9 vs. 2.4) scores. They were older (67 vs. 61 years), had more diabetes (45% vs. 39%), hypertension (77% vs. 59%), extra-cardiac arteriopathy (27% vs. 20%), more extensive CAD, more unstable angina (8% vs. 5%), had more IABP inserted pre-operatively (13% vs. 4%), and were more frequently considered urgent operations (27% vs. 9%). Patients in the STICH cohort had a lower LVEF (28% vs. 30%), more often a history of MI (80% vs. 54%), more advanced NYHA class, more severity of MR.
Table 1:
Baseline patient characteristics and 30-day mortality by cohort for patients with isolated CABG
| All patients (N=2060) | STICH cohort (N=814) | Contemporary cohort (N=1246) |
p-value | |
|---|---|---|---|---|
| 30-day postoperative mortality | 82 (4.0%) | 39 (4.8%) | 43 (3.5%) | 0.135 |
| Age (years) | 65 (57, 72) | 61 (54, 68) | 67 (59, 73) | <0.001 |
| Female sex | 269 (13.1%) | 105 (12.9%) | 164 (13.2%) | 0.863 |
| Height (cm) | 170 (165, 176) | 170 (165, 176) | 170 (165, 176) | 0.986 |
| Weight (kg) | 80 (71, 91) | 80 (70, 90) | 81 (71, 92) | 0.016 |
| Body mass index (kg/m2)* | 27.5 (24.6, 31.0) | 27.2 (24.4, 30.4) | 27.7 (24.7, 31.3) | 0.005 |
| Body surface area (m2)* | 1.92 (1.79, 2.06) | 1.92 (1.78, 2.05) | 1.93 (1.80, 2.07) | 0.067 |
| Creatinine (mg/dL)* | 1.05 (0.90, 1.27) | 1.10 (0.92, 1.27) | 1.03 (0.87, 1.28) | 0.017 |
| Cockcroft-Gault creatinine clearance (mL/min/1.73m2)* | 77 (59, 100) | 79 (61, 98) | 76 (56, 101) | 0.140 |
| Ejection fraction (%) | 30 (25, 35) | 28 (23, 35) | 30 (25, 35) | 0.001 |
| Diabetes | 879 (42.7%) | 317 (38.9%) | 562 (45.1%) | 0.006 |
| Non-insulin dependent | 531 (25.8%) | 194 (23.8%) | 337 (27.0%) | 0.103 |
| Insulin dependent | 348 (16.9%) | 123 (15.1%) | 225 (18.1%) | 0.081 |
| Hypertension | 1443 (70.0%) | 482 (59.2%) | 961 (77.1%) | <0.001 |
| Atrial fibrillation or flutter | 231 (11.2%) | 76 (9.3%) | 155 (12.4%) | 0.029 |
| Myocardial infarction (MI) | 1318 (64.0%) | 650 (79.9%) | 668 (53.6%) | <0.001 |
| Extracardiac arteriopathy (PVD or stroke) | 506 (24.6%) | 166 (20.4%) | 340 (27.3%) | <0.001 |
| PVD | 469 (22.8%) | 130 (16.0%) | 339 (27.2%) | <0.001 |
| Cerebrovascular disease/stroke† | 221 (10.7%) | 58 (7.1%) | 163 (13.1%) | <0.001 |
| IABP | 188 (9.1%) | 29 (3.6%) | 159 (12.8%) | <0.001 |
| Number of diseased vessels (50%) | <0.001 | |||
| 0 | 3 (0.1%) | 0 | 3 (0.2%) | |
| 1 | 75 (3.6%) | 56 (6.9%) | 19 (1.5%) | |
| 2 | 483 (23.4%) | 228 (28.0%) | 255 (20.5%) | |
| 3 | 1499 (72.8%) | 530 (65.1%) | 969 (77.8%) | |
| Proximal LAD stenosis ≥75% | 1383 (67.1%) | 587 (72.1%) | 796 (63.9%) | <0.001 |
| Prior cardiac surgery | 66 (3.2%) | 27 (3.3%) | 39 (3.1%) | 0.814 |
| Current NYHA class | <0.001 | |||
| I | 449 (21.8%) | 85 (10.4%) | 364 (29.2%) | |
| II | 834 (40.5%) | 401 (49.3%) | 433 (34.8%) | |
| III | 690 (33.5%) | 297 (36.5%) | 393 (31.5%) | |
| IV | 87 (4.2%) | 31 (3.8%) | 56 (4.5%) | |
| Unstable angina | 147 (7.1%) | 42 (5.2%) | 105 (8.4%) | 0.005 |
| Mitral regurgitation severity | <0.001 | |||
| None or trace | 1202 (58.3%) | 328 (40.3%) | 874 (70.1%) | |
| Mild (≤2+) | 736 (35.7%) | 420 (51.6%) | 316 (25.4%) | |
| Moderate (3+) | 114 (5.5%) | 62 (7.6%) | 52 (4.2%) | |
| Severe (4+) | 8 (0.4%) | 4 (0.5%) | 4 (0.3%) | |
| Moderate or severe tricuspid regurgitation | 43 (2.1%) | 13 (1.6%) | 30 (2.4%) | 0.208 |
| Pulmonary Artery Systolic Pressure (PASP)‡ | 34 (30, 43) | 37 (30, 46) | 30 (30, 40) | <0.001 |
| Any degree of aortic regurgitation | 373 (18.1%) | 113 (13.9%) | 260 (20.9%) | <0.001 |
| Poor mobility | 198 (9.6%) | 180 (22.1%) | 18 (1.4%) | <0.001 |
| Urgent operation | 409 (19.9%) | 70 (8.6%) | 339 (27.2%) | <0.001 |
| STS score | 1.2 (0.7, 2.3) | 0.9 (0.6, 1.6) | 1.5 (0.8, 2.8) | <0.001 |
| Euro-2 score | 2.6 (1.7, 4.5) | 2.4 (1.5, 3.8) | 2.9 (1.8, 4.8) | <0.001 |
In the contemporary cohort, height and weight are missing for 7 and creatinine is missing for 1.
Stroke was only documented for patients in the STICH cohort.
PASP is missing for 302 patients in the contemporary and 660 patients in the STICH cohort.
Mortality at 30 days in the STICH cohort was 4.8 % (N=39) and in the contemporary cohort was 3.5 % (N=43), with no significant difference in mortality across centers (p=0.17). After adjusting for higher STS and EuroSCORE-2 in the contemporary cohort, there was a greater risk of 30-day post-operative death in STICH as compared to the contemporary cohort (odds ratio: 2.21, 95% CI (1.35, 3.61); p=0.002).
EuroSCORE-2 and the STS in patients with ischemic HF
The distribution of predicted risk for the STICH cohort was largely below 2% for the STS and below 4% for the EuroSCORE-2 (Figure 2). The distribution of patient risk for the contemporary cohort was largely below 3% for the STS, and below 5% for the EuroSCORE-2. The overall median value of the STS score for the two cohorts was significantly lower than the EuroSCORE-2 (1.23 vs. 2.63, p<0.0001), with the most striking difference being past the 50th percentile (Figure 3).
Figure 2:
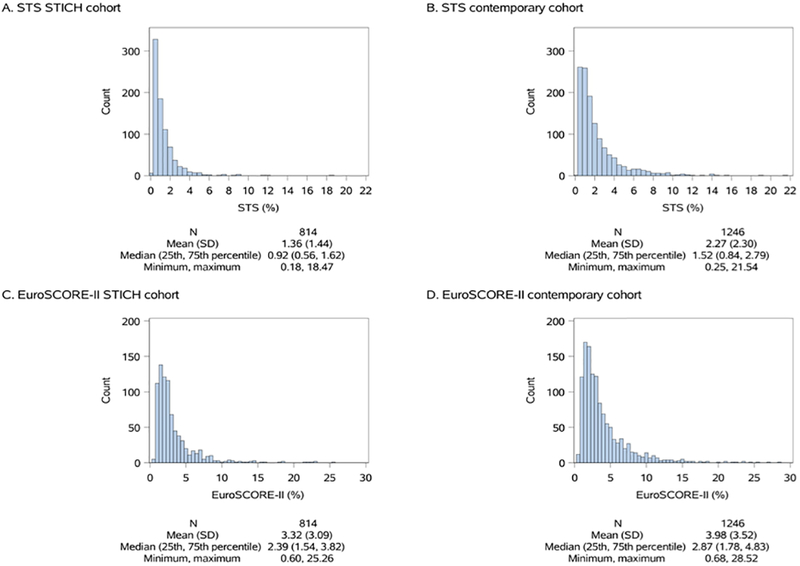
Distribution of STS and EuroSCORE-2 risk scores across the STICH (left) and the contemporary cohorts (right) for patients with isolated CABG.
Figure 3:
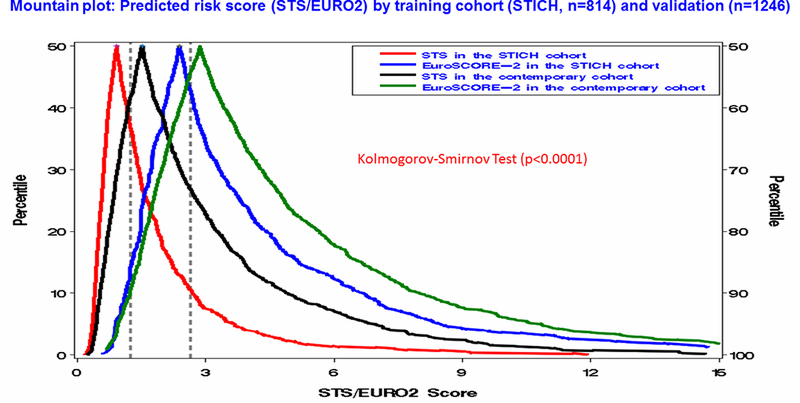
Cumulative distribution of the predicted risk of operative mortality assessed using the STS and the EuroSCORE-2 in both cohorts in patients with isolated CABG. Red: the STS score in STICH patients, Blue: the EuroSCORE-2 in STICH patients, Black: the STS in contemporary patients, Green: the EuroSCORE-2 in contemporary patients. Vertical dashed lines refer to median values for overall STS (1.23) and EuroSCORE-2 (2.63).
The C-statistic for the STS and the EuroSCORE-2 in predicting 30-day mortality in STICH patients and in the contemporary cohort were similar (Table 2). In the STICH cohort, the STS C-statistic was 0.744 (95% CI: 0.677, 0.812), and the EuroSCORE-2 C-statistic was 0.736 (95% CI: 0.665, 0.808). In the contemporary cohort the STS C-statistic 0.727 (95% CI: 0.650, 0.803) was similar to that of the EuroSCORE-2 C-statistic 0.707 (95% CI: 0.620, 0.795). Including or excluding pulmonary systolic arterial pressure (PASP) in the EuroSCORE-2 did not alter the C-statistic. Attributing worst case scenario values for missing variables did not significantly modify the C-statistic of either the STS or the EuroSCORE-2 in either cohort (STICH cohort: C-statistic for the STS: 0.762 (0.694, 0.830), and for the EuroSCORE-2: 0.749 (0.675, 0.822); and contemporary cohort: C-statistic for the STS: 0.733 (0.656, 0.810), and for the EuroSCORE-2: 0.706 (0.614, 0.797)).
Table 2:
The STS and the EuroSCORE-2 C-index for predicting 30-day mortality in STICH patients and in the contemporary cohort
| Score | C-index (95% CI) for STICH patients | C-index (95% CI) for contemporary cohort |
|---|---|---|
| EuroSCORE-2 (without PASP) | 0.734 (0.663, 0.805) | 0.710 (0.626, 0.793) |
| STS | 0.744 (0.677, 0.812) | 0.727 (0.650, 0.803) |
| EuroSCORE-2 | 0.736 (0.665, 0.808) | 0.707 (0.620, 0.795) |
The predicted vs. observed mortality rate for both the STS and EuroSCORE-2 appeared to be better for the contemporary cohort than for the STICH cohort (Figure 4). In the STICH cohort, the predicted mortality with both the STS and EuroSCORE-2 underestimated the observed mortality. In the contemporary cohort, the mortality predicted by the EuroSCORE-2 showed a good fit to the observed mortality, while the mortality predicted by the STS still underestimating observed mortality, but less than with the STICH cohort. Attributing worst case scenario for missing values did not completely correct the underestimation of actual 30-day post-operative risk with the STS (Supplement Figure 1). In both the primary and the sensitivity analyses, the underestimate of risk with the STS in the STICH cohort is supported by significant p-values from Hosmer-Lemeshow goodness-of-fit tests (p=0.021 and p=0.046, respectively).
Figure 4:
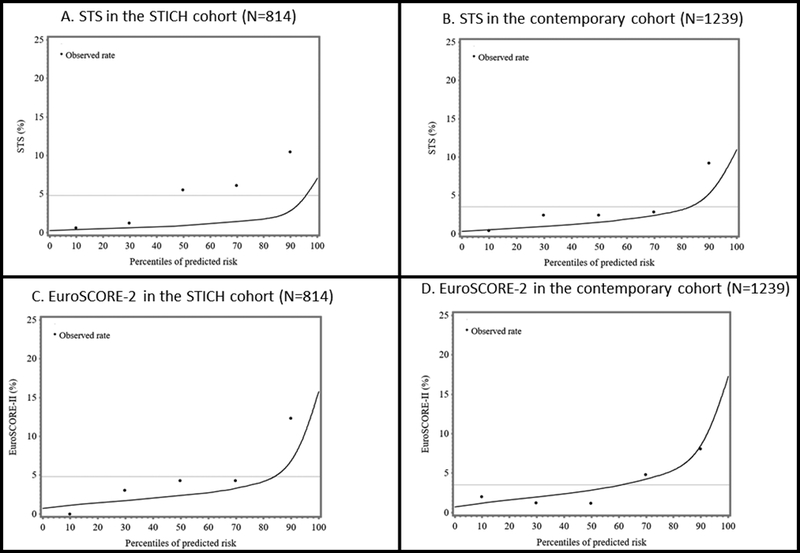
Actual vs. predicted 30-day postoperative mortality using the EuroSCORE-2 and the STS models in both cohorts (STICH patients (N=814), left and contemporary patients (N=1239, 7 patients were lost to follow-up), right) in patients with isolated CABG.
Separating the contemporary cohort patients by quintiles of risk according to the STS score (Table 3), or EuroSCORE-2 (Table 4), identified patients with greatly varying 30-day mortality risks, the lowest quintile of risk having a mortality of under 1%, and the highest quintile having mortality close to 8% or more.
Table 3:
Baseline characteristics and outcomes of patients according to quintiles* of risk for the STS
| Quintile 1 (N=412) | Quintile 2 (N=412) | Quintile 3 (N=412) | Quintile 4 (N=412) | Quintile 5 (N=412) | p-value | |
|---|---|---|---|---|---|---|
| 30-day postoperative mortality | 2 (0.5%) | 9 (2.2%) | 17 (4.1%) | 20 (4.9%) | 34 (8.3%) | <0.001 |
| Age (years) | 54 (49, 58) | 60 (56, 64) | 66 (60, 70) | 70 (65, 74) | 75 (70, 78) | <0.001 |
| Female sex | 16 (3.9%) | 37 (9.0%) | 41 (10.0%) | 70 (17.0%) | 105 (25.5%) | <0.001 |
| Height (cm) | 173 (168, 177) | 172 (167, 177) | 171 (165, 176) | 170 (164, 175) | 167 (160, 173) | <0.001 |
| Weight (kg) | 86 (78, 95) | 83 (74, 95) | 79 (70, 90) | 78 (69, 88) | 74 (65, 85) | <0.001 |
| Body mass index (kg/m2) | 29.0 (26.3, 31.8) | 28.4 (25.4, 31.7) | 26.8 (24.2, 30.6) | 27.1 (24.3, 30.3) | 26.4 (23.8, 29.8) | <0.001 |
| Body surface area (m2) | 2.00 (1.89, 2.11) | 1.96 (1.85, 2.10) | 1.92 (1.79, 2.05) | 1.89 (1.76, 2.03) | 1.83 (1.69, 1.97) | <0.001 |
| Creatinine (mg/dL) | 0.95 (0.82, 1.08) | 1.00 (0.88, 1.13) | 1.04 (0.90, 1.23) | 1.15 (0.95, 1.40) | 1.30 (1.01, 1.58) | <0.001 |
| Cockcroft-Gault creatinine clearance (mL/min/1.73m2) | 108 (92, 126) | 91 (75, 110) | 76 (65, 92) | 63 (53, 77) | 50 (38, 63) | <0.001 |
| Ejection fraction (%) | 30 (26, 35) | 30 (24, 35) | 29 (25, 35) | 30 (25, 35) | 30 (25, 33) | <0.001 |
| Diabetes | 134 (32.5%) | 169 (41.0%) | 178 (43.2%) | 203 (49.3%) | 195 (47.3%) | <0.001 |
| Non-insulin dependent | 106 (25.7%) | 108 (26.2%) | 107 (26.0%) | 117 (28.4%) | 93 (22.6%) | 0.545 |
| Insulin dependent | 28 (6.8%) | 61 (14.8%) | 71 (17.2%) | 86 (20.9%) | 102 (24.8%) | <0.001 |
| Hypertension | 249 (60.4%) | 278 (67.5%) | 286 (69.4%) | 304 (73.8%) | 326 (79.1%) | <0.001 |
| Atrial fibrillation or flutter | 25 (6.1%) | 30 (7.3%) | 43 (10.4%) | 55 (13.3%) | 78 (18.9%) | <0.001 |
| Myocardial infarction | 274 (66.5%) | 261 (63.3%) | 257 (62.4%) | 245 (59.5%) | 281 (68.2%) | 0.948 |
| Extracardiac arteriopathy (PVD or stroke) | 26 (6.3%) | 53 (12.9%) | 105 (25.5%) | 132 (32.0%) | 190 (46.1%) | <0.001 |
| PVD | 17 (4.1%) | 46 (11.2%) | 93 (22.6%) | 126 (30.6%) | 187 (45.4%) | <0.001 |
| Cerebrovascular disease/stroke† | 14 (3.4%) | 23 (5.6%) | 37 (9.0%) | 46 (11.2%) | 101 (24.5%) | <0.001 |
| IABP | 4 (1.0%) | 30 (7.3%) | 36 (8.7%) | 46 (11.2%) | 72 (17.5%) | <0.001 |
| Number of diseased vessels (50%) | <0.001 | |||||
| 0 | 0 (0.0%) | 1 (0.2%) | 1 (0.2%) | 0 (0.0%) | 1 (0.2%) | |
| 1 | 35 (8.5%) | 13 (3.2%) | 9 (2.2%) | 9 (2.2%) | 9 (2.2%) | |
| 2 | 133 (32.3%) | 104 (25.2%) | 102 (24.8%) | 73 (17.7%) | 71 (17.2%) | |
| 3 | 244 (59.2%) | 294 (71.4%) | 300 (72.8%) | 330 (80.1%) | 331 (80.3%) | |
| Proximal LAD stenosis ≥75% | 285 (69.2%) | 275 (66.7%) | 286 (69.4%) | 264 (64.1%) | 273 (66.3%) | 0.246 |
| Prior cardiac surgery | 0 (0.0%) | 1 (0.2%) | 3 (0.7%) | 14 (3.4%) | 48 (11.7%) | <0.001 |
| Current NYHA class | <0.001 | |||||
| I | 124 (30.1%) | 92 (22.3%) | 96 (23.3%) | 69 (16.7%) | 68 (16.5%) | |
| II | 181 (43.9%) | 182 (44.2%) | 172 (41.7%) | 156 (37.9%) | 143 (34.7%) | |
| III | 103 (25.0%) | 127 (30.8%) | 128 (31.1%) | 165 (40.0%) | 167 (40.5%) | |
| IV | 4 (1.0%) | 11 (2.7%) | 16 (3.9%) | 22 (5.3%) | 34 (8.3%) | |
| Unstable angina | 12 (2.9%) | 18 (4.4%) | 39 (9.5%) | 34 (8.3%) | 44 (10.7%) | <0.001 |
| Chronic pulmonary disease‡ | 8 (1.9%) | 25 (6.1%) | 37 (9.0%) | 48 (11.7%) | 99 (24.0%) | <0.001 |
| Mitral regurgitation severity | 0.086 | |||||
| None or trace | 258 (62.6%) | 225 (54.6%) | 244 (59.2%) | 237 (57.5%) | 238 (57.8%) | |
| Mild (≤2+) | 146 (35.4%) | 171 (41.5%) | 137 (33.3%) | 143 (34.7%) | 139 (33.7%) | |
| Moderate (3+) | 7 (1.7%) | 16 (3.9%) | 27 (6.6%) | 30 (7.3%) | 34 (8.3%) | |
| Severe (4+) | 1 (0.2%) | 0 | 4 (1.0%) | 2 (0.5%) | 1 (0.2%) | |
| Moderate or severe tricuspid regurgitation | 3 (0.7%) | 6 (1.5%) | 9 (2.2%) | 11 (2.7%) | 14 (3.4%) | 0.003 |
| Pulmonary Artery Systolic Pressure (PASP) | 32 (29, 41) | 34 (30, 43) | 33 (30, 43) | 33 (30, 44) | 35 (30, 45) | <0.001 |
| Any degree of aortic regurgitation | 54 (13.1%) | 60 (14.6%) | 73 (17.7%) | 90 (21.8%) | 96 (23.3%) | <0.001 |
| Poor mobility | 36 (8.7%) | 33 (8.0%) | 52 (12.6%) | 33 (8.0%) | 44 (10.7%) | 0.398 |
| Urgent operation | 26 (6.3%) | 64 (15.5%) | 80 (19.4%) | 101 (24.5%) | 138 (33.5%) | <0.001 |
| STS score | 0.5 (0.4, 0.5) | 0.8 (0.7, 0.9) | 1.2 (1.1, 1.4) | 2.0 (1.7, 2.3) | 4.0 (3.2, 6.0) | <0.001 |
| EuroSCORE-2 | 1.3 (1.0, 1.7) | 1.9 (1.5, 2.4) | 2.7 (2.1, 3.4) | 3.7 (2.7, 4.9) | 6.8 (4.9, 9.9) | <0.001 |
Quintiles are based on the scores in the combined dataset of contemporary and STICH cohorts. Quintile cut-points are 0.609, 0.972, 1.55, and 2.685.
Stroke was only documented for patients in the STICH cohort.
Chronic pulmonary disease was only documented for patients in the contemporary cohort.
IABP: intra-aortic balloon pump. LAD: left anterior descending artery. PVD: peripheral vascular disease.
Table 4:
Baseline characteristics and outcomes of patients according to quintiles* of risk for the EuroSCORE-2
| Quintile 1 (N=412) | Quintile 2 (N=412) | Quintile 3 (N=412) | Quintile 4 (N=412) | Quintile 5 (N=412) | p-value | |
|---|---|---|---|---|---|---|
| 30-day postoperative mortality | 4 (1.0%) | 10 (2.4%) | 12 (2.9%) | 18 (4.4%) | 38 (9.2%) | <0.001 |
| Age (years) | 58 (52, 63) | 60 (54, 68) | 65 (57, 70) | 70 (62, 74) | 72 (67, 77) | <0.001 |
| Female sex | 15 (3.6%) | 31 (7.5%) | 50 (12.1%) | 70 (17.0%) | 103 (25.0%) | <0.001 |
| Height (cm) | 172 (167, 177) | 172 (167, 177) | 171 (165, 176) | 170 (165, 176) | 168 (162, 174) | <0.001 |
| Weight (kg) | 86 (76, 95) | 84 (73, 96) | 80 (71, 92) | 78 (69, 86) | 75 (65, 85) | <0.001 |
| Body mass index (kg/m2) | 29.0 (25.9, 32.0) | 28.4 (25.3, 31.9) | 27.5 (24.6, 31.0) | 26.8 (24.2, 29.8) | 26.4 (23.8, 29.9) | <0.001 |
| Body surface area (m2) | 1.99 (1.87, 2.11) | 1.97 (1.83, 2.10) | 1.92 (1.79, 2.06) | 1.89 (1.77, 2.00) | 1.85 (1.71, 1.98) | <0.001 |
| Creatinine (mg/dL) | 0.97 (0.83, 1.10) | 0.98 (0.84, 1.14) | 1.06 (0.90, 1.25) | 1.10 (0.92, 1.34) | 1.30 (1.02, 1.60) | <0.001 |
| Cockcroft-Gault creatinine clearance (mL/min/1.73m2) | 100 (88, 117) | 92 (75, 111) | 75 (61, 96) | 67 (54, 81) | 51 (39, 69) | <0.001 |
| Ejection fraction (%) | 34 (30, 35) | 30 (25, 35) | 28 (25, 33) | 29 (24, 33) | 28 (22, 30) | <0.001 |
| Diabetes | 130 (31.6%) | 166 (40.3%) | 167 (40.5%) | 193 (46.8%) | 223 (54.1%) | <0.001 |
| Non-insulin dependent | 110 (26.7%) | 109 (26.5%) | 103 (25.0%) | 117 (28.4%) | 92 (22.3%) | 0.319 |
| Insulin dependent | 20 (4.9%) | 57 (13.8%) | 64 (15.5%) | 76 (18.4%) | 131 (31.8%) | <0.001 |
| Hypertension | 256 (62.1%) | 273 (66.3%) | 286 (69.4%) | 303 (73.5%) | 325 (78.9%) | <0.001 |
| Atrial fibrillation or flutter | 37 (9.0%) | 34 (8.3%) | 38 (9.2%) | 53 (12.9%) | 69 (16.7%) | <0.001 |
| Myocardial infarction | 248 (60.2%) | 265 (64.3%) | 253 (61.4%) | 268 (65.0%) | 284 (68.9%) | 0.015 |
| Extracardiac arteriopathy (PVD or stroke) | 6 (1.5%) | 53 (12.9%) | 85 (20.6%) | 132 (32.0%) | 230 (55.8%) | <0.001 |
| PVD | 6 (1.5%) | 51 (12.4%) | 78 (18.9%) | 118 (28.6%) | 216 (52.4%) | <0.001 |
| Cerebrovascular disease/stroke† | 1 (0.2%) | 23 (5.6%) | 33 (8.0%) | 51 (12.4%) | 113 (27.4%) | <0.001 |
| IABP | 16 (3.9%) | 31 (7.5%) | 33 (8.0%) | 45 (10.9%) | 63 (15.3%) | <0.001 |
| Number of diseased vessels (50%) | <0.001 | |||||
| 0 | 0 (0%) | 1 (0.2%) | 1 (0.2%) | 0 (0.0%) | 1 (0.2%) | |
| 1 | 22 (5.3%) | 13 (3.2%) | 12 (2.9%) | 13 (3.2%) | 15 (3.6%) | |
| 2 | 104 (25.2%) | 126 (30.6%) | 96 (23.3%) | 77 (18.7%) | 80 (19.4%) | |
| 3 | 286 (69.4%) | 272 (66.0%) | 303 (73.5%) | 322 (78.2%) | 316 (76.7%) | |
| Proximal LAD stenosis ≥75% | 274 (66.5%) | 276 (67.0%) | 284 (68.9%) | 278 (67.5%) | 271 (65.8%) | 0.894 |
| Prior cardiac surgery | 0 (0%) | 1 (0.2%) | 3 (0.7%) | 6 (1.5%) | 56 (13.6%) | <0.001 |
| Current NYHA class | <0.001 | |||||
| I | 134 (32.5%) | 111 (26.9%) | 89 (21.6%) | 64 (15.5%) | 51 (12.4%) | |
| II | 216 (52.4%) | 183 (44.4%) | 174 (42.2%) | 149 (36.2%) | 112 (27.2%) | |
| III | 62 (15.0%) | 113 (27.4%) | 140 (34.0%) | 174 (42.2%) | 201 (48.8%) | |
| IV | 0 (0%) | 5 (1.2%) | 9 (2.2%) | 25 (6.1%) | 48 (11.7%) | |
| Unstable angina | 4 (1.0%) | 25 (6.1%) | 30 (7.3%) | 38 (9.2%) | 50 (12.1%) | <0.001 |
| Chronic pulmonary disease‡ | 12 (2.9%) | 26 (6.3%) | 34 (8.3%) | 72 (17.5%) | 73 (17.7%) | <0.001 |
| Mitral regurgitation severity | <0.001 | |||||
| None or trace | 277 (67.2%) | 253 (61.4%) | 223 (54.1%) | 227 (55.1%) | 222 (53.9%) | |
| Mild (≤2+) | 128 (31.1%) | 141 (34.2%) | 152 (36.9%) | 153 (37.1%) | 162 (39.3%) | |
| Moderate (3+) | 7 (1.7%) | 15 (3.6%) | 34 (8.3%) | 31 (7.5%) | 27 (6.6%) | |
| Severe (4+) | 0 (0%) | 3 (0.7%) | 3 (0.7%) | 1 (0.2%) | 1 (0.2%) | |
| Moderate or severe tricuspid regurgitation | 2 (0.5%) | 6 (1.5%) | 8 (1.9%) | 10 (2.4%) | 17 (4.1%) | <0.001 |
| Pulmonary Artery Systolic Pressure (PASP) | 30.0 (26.6, 37.0) | 32.0 (29.0, 42.0) | 35.0 (30.0, 44.5) | 35.0 (30.0, 44.0) | 36.2 (30.0, 48.0) | <0.001 |
| Any degree of aortic regurgitation | 43 (10.4%) | 58 (14.1%) | 82 (19.9%) | 94 (22.8%) | 96 (23.3%) | <0.001 |
| Poor mobility | 9 (2.2%) | 34 (8.3%) | 44 (10.7%) | 47 (11.4%) | 64 (15.5%) | <0.001 |
| Urgent operation | 23 (5.6%) | 52 (12.6%) | 72 (17.5%) | 112 (27.2%) | 150 (36.4%) | <0.001 |
| STS score | 0.5 (0.4, 0.7) | 0.8 (0.6, 1.2) | 1.2 (0.9, 1.6) | 1.9 (1.4, 2.6) | 3.7 (2.6, 5.9) | <0.001 |
| EuroSCORE-2 | 1.2 (0.9, 1.4) | 1.8 (1.7, 2.0) | 2.6 (2.4, 2.9) | 3.9 (3.5, 4.5) | 7.5 (6.2, 10.2) | <0.001 |
Quintiles are based on the scores in the combined dataset of contemporary and STICH cohorts. Quintile cut-points are 1.506, 2.2305, 3.11, and 5.066.
Stroke was only documented for patients in the STICH cohort.
Chronic pulmonary disease was only documented for patients in the contemporary cohort.
IABP: intra-aortic balloon pump. LAD: left anterior descending artery. PVD: peripheral vascular disease.
Discussion
This study demonstrates that the operative risk of 30-day postoperative mortality after CABG in patients with a LVEF ≤ 35% varies substantially from one patient to the next, being as low as 1% in nearly 20% of patients, but as high as 8% in close to 20% of patients. Both the STS and EuroSCORE-2 are moderately effective in assessing this risk, but their performance is somewhat less predictive than that reported for the overall cardiac surgical population, with the STS more consistently underestimating the risk than the EuroSCORE-2. In the same patients, the EuroSCORE-2 consistently calculated significantly higher risk scores than the STS, with its values more closely approximating the observed mortality. In a contemporary cohort of patients with a LVEF ≤ 35% undergoing CABG, the predicted 30-day postoperative mortality (using either the STS or the EuroSCORE-2) were higher than those of patients in the STICH trial, nevertheless, their observed mortality was less than that in STICH suggesting that operative mortality in such patients may have decreased since the STICH trial. Thus, assessing the risk of 30-day postoperative mortality following CABG with the use of the STS or EuroSCORE-2 allows for more informed decisions, and should encourage more patients at lowest risk to proceed with CABG, and those at highest risk to consider alternate therapies. It should also facilitate benchmarking surgical outcomes in these patients and may also facilitate the choice of patients for the much needed trial comparing CABG to PCI in patients with severe LV dysfunction.
The ability of the STS and EuroSCORE-2 to identify risk of CABG in patients with a LVEF ≤ 35%
Risk-assessment is mandatory for a tailored approach at the time of surgery in patients with CAD and low EF (21). As such, risk-stratification using specific tools has become the rule in cardiac surgery (22, 23); the STS and the EuroSCORE-2 being two common multivariable models used in this setting (1, 2). The STS model is a complex (> 50 demographic and operative variables), and continuously updated model that allows for the prediction of post-operative mortality but also morbidity (such as the risk of renal failure or stroke). Although not updated on a regular basis, the EuroSCORE-2 is a more parsimonious model using only 18 variables, making it easier to calculate. Both the STS and the EuroSCORE-2 have been widely validated in external populations (3–6), generally yielding similar risk scores (24) and their relative calibration and discrimination performances recently proved to be similar on large samples of patients undergoing various cardiac procedures (25). Moreover, they both appear to also predict long-term outcomes (26–28).
In this study, both the STS and the EuroSCORE-2 performed moderately well in a population that has not previously been specifically addressed by either score, but with a C-index somewhat inferior to that reported for overall cardiac surgical populations (where their C-statistic is > 0.80). The EuroSCORE-2 has previously been shown to be less accurate in terms of risk prediction (29) when applied to specific high-risk populations, and the results of the present analyses would suggest that in this population of patients with a low EF, this is also true for the STS. Although methodological considerations may partly help explain less accuracy in high-risk patients (30), one might hypothesize that these models were not built to accurately capture surgical risk of mortality in specific sub-groups where certain risk factors carry an unusually large proportion of the risk (29). Also, it may be that the exclusion of patients with variables of instability, such as those having emergent or salvage procedures, or with pre-operative shock/resuscitation/inotrope dependence/acute MI may have had an impact.
The STS appeared to more consistently underestimate risk as compared with the EuroSCORE-2, but both the STS and EuroSCORE-2 significantly underestimated mortality in the STICH cohort, more than in the contemporary cohort. This did not appear to be the result of the exclusion of important risk factors or imputation of missing values as an exploratory analysis where all missing values were attributed the worst possible score did not significantly modify the C-statistic of either score in either cohort, and did not fully correct the underestimation of mortality by the STS. Although pulmonary artery systolic pressures is known to be a risk factor for CABG, the inclusion or exclusion of it in the EuroSCORE-2 did not appear to modify the C-statistic, perhaps because patients with very high values were largely excluded from surgery and thus does not appear to explain the difference in scores (31). These limitations notwithstanding, in the contemporary cohort, the EuroSCORE-2 appeared to more accurately estimate risk, and may be better than the STS for benchmarking 30-day post-operative mortality in these high-risk patients.
Variable and Changing Risk of CABG in patients with a LVEF≤35%
The overall 30-day post-operative mortality of STICH patients undergoing CABG (4.8%) was similar to or better than that reported by others in patients with HF and reduced LVEF (9–11). Mortality in the contemporary cohort for patients undergoing CABG (3.5%) was lower than that of STICH patients despite having a higher STS (1.5 vs. 0.9) and EuroSCORE-2 (2.9 vs. 2.4). After adjusting for the 30-day post-operative mortality risk scores, patients in the STICH cohort (surgeries in 2002–2007) had a significantly higher post-operative mortality (odds ratio: 2.21, 95% CI (1.35, 3.61); p=0.002)) suggesting that for the same risk score, operative mortality has decreased since the STICH trial. This may also suggest that patients at higher risk are now sent for CABG as compared with ten years earlier. Consistent with these findings is the reported decrease in mortality with CABG from 4.2% to 3.0% (29% reduction) in the STS database between the years 2002 to 2012 (13), and a consistent 3% mortality despite an increasing risk profile of patients undergoing CABG in Germany over a similar time period (12). These differences are difficult to explain as in the field of cardiac surgery there have been no major anaesthetic or technical advancements, and no major changes in cardioplegia types or delivery methods or other specific advancements that could explain the improvements in mortality. These changes may simply be the result of small improvements in patient selection, better timing and preparation of patients for surgery, greater use of arterial conduits, and better intra-operative and post-operative management (13).
The probability that operative mortality has decreased in this high-risk population, and the ability to accurately assess the operative mortality risk of patients with a LVEF ≤ 35% has multiple significant implications for patients and the field. For patients at very low risk it should encourage the use of CABG, and for higher risk patients, particularly those with significant angina, PCI or other options, rather than CABG, should seriously be considered despite registries suggesting that patients with a reduced LVEF that require revascularization generally fair better with CABG than with PCI (32–36). A definitive recommendation should await a randomized comparative trial of CABG vs. PCI in low EF patients (37). The apparent reduction in operative mortality in the contemporary cohort, and the ability to assess individual patient risk with the STS and EuroSCORE-2 should facilitate the much needed trial of CABG vs. PCI, which has also seen significant recent advances in this patient population (38).
Limitations:
Although this is the largest report of 30-day post-operative mortality risk assessment in patients with a reduced LVEF, the total number of events remains limited for both STICH and the contemporary cohorts. This notwithstanding, the similar C-statistic for the STS and EuroSCORE-2 in both cohorts suggests consistency across populations.
The STICH and contemporary cohorts excluded very unstable patients and patients undergoing a second procedure such that variables that assess acuity or a second procedure did not come into play and thus the present findings cannot be reliably applied to unstable patients or patients having a second procedure.
Finally, the information captured in the STICH trial did not include certain significant variables, such as the presence of paroxysmal atrial fibrillation (AF) (AF regardless of type was included), and the presence of chronic obstructive pulmonary disease (COPD), so the true STS and EuroSCORE-2 scores may have been underestimated. This limitation notwithstanding, COPD and other potential variables not documented in STICH were considered in the contemporary cohort (17.4% patients with COPD, N=217), and their inclusion had little impact on the predictive value of the different scores, perhaps because in such high-risk patients, those with significant COPD were excluded from CABG. Also an exploratory analysis that attributed the worse score possible for missing values did not significantly modify the C-statistics or fully correct for the underestimation of mortality risk with the STS, supporting our conclusions.
Conclusions:
The 30-day post-operative mortality risk of patients undergoing CABG with a LVEF ≤ 35% varies markedly from one patient to the next, and is consistently calculated to be lower with the STS than the EuroSCORE-2. Nevertheless, both the STS and EuroSCORE-2 are moderately effective in evaluating risk, although the STS tends to underestimate risk.
Supplementary Material
Clinical perspective
What is new?
In patients with severe LV dysfunction (LVEF ≤35%) undergoing coronary artery bypass grafting (CABG), both the STS and EuroSCORE-2 are moderately effective in assessing individual 30-day post-operative mortality risk, but their predictive accuracy is somewhat less than that reported for the overall cardiac surgical population. Also, the STS tends to underestimate risk. The lower 30-day post-operative mortality in a contemporary cohort of patients with a LVEF ≤35% undergoing CABG as compared with the STICH cohort (2002–2007), despite higher STS and EuroSCORE-2 scores, would suggest that 30-day post-operative mortality may be decreasing in such patients.
Clinical implications
The STS and EuroSCORE-2 are moderately effective in assessing the 30-day post-operative mortality of patients with severe LV dysfunction (LVEF≤35%) undergoing CABG, although the STS tends to underestimate risk somewhat. Both scores can be used by surgical programs to benchmark their CABG 30-day postoperative mortality outcomes in this population, but because observed mortality most closely mimicked EuroSCORE-2 it may be superior to the STS for benchmarking. The suggestion that 30-day post-operative mortality risk in such patients has decreased over the last decade should encourage a greater use of CABG in appropriate patients with severe LV dysfunction and coronary artery disease.
Acknowledgement
We thank Seanna Horan and Vanessa Moore for their valuable input, preparation and editing of this manuscript.
Source of Funding
This work was supported by grants U01HL69015, U01HL69013, and RO1HL105853 from the National Institutes of Health/National Heart, Lung, and Blood Institute. This work is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or National Heart, Lung, and Blood Institute.
Footnotes
Disclosures
Dr. Velazquez disclosed relationships with NHLBI, Alnylam Pharmaceuticals, Amgen, Expert Exchange, Merck, Novartis, and Pfizer. Dr Rouleau is consultant for Novartis, Astra Zeneca and Bayer.
Clinical Trial Registration: URL: http://www.clinicaltrials.gov. Unique identifier: http://clinicaltrials.gov/show/NCT00023595
References:
- 1.Shahian DM, O’Brien SM, Filardo G, Ferraris VA, Haan CK, Rich JB, Normand SL, DeLong ER, Shewan CM, Dokholyan RS, Peterson ED, Edwards FH, Anderson RP; Society of Thoracic Surgeons Quality Measurement Task Force. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 1--coronary artery bypass grafting surgery. Ann Thorac Surg. 2009; 88:S2–22. [DOI] [PubMed] [Google Scholar]
- 2.Nashef SA, Roques F, Sharples LD, Nilsson J, Smith C, Goldstone AR, Lockowandt U. EuroSCORE II. Eur J Cardiothorac Surg. 2012; 41:734–44; discussion 744–5. [DOI] [PubMed] [Google Scholar]
- 3.Nilsson J, Algotsson L, Höglund P, Lührs C, Brandt J. Comparison of 19 pre-operative risk stratification models in open-heart surgery. Eur Heart J. 2006; 27:867–74. [DOI] [PubMed] [Google Scholar]
- 4.Nilsson J, Algotsson L, Höglund P, Lührs C, Brandt J. Early mortality in coronary bypass surgery: the EuroSCORE versus The Society of Thoracic Surgeons risk algorithm. Ann Thorac Surg. 2004; 77:1235–9; discussion 1239–40. [DOI] [PubMed] [Google Scholar]
- 5.Chalmers J, Pullan M, Fabri B, McShane J, Shaw M, Mediratta N, Poullis M. Validation of EuroSCORE II in a modern cohort of patients undergoing cardiac surgery. Eur J Cardiothorac Surg. 2013; 43:688–94. [DOI] [PubMed] [Google Scholar]
- 6.Di Dedda U, Pelissero G, Agnelli B, De Vincentiis C, Castelvecchio S, Ranucci M. Accuracy, calibration and clinical performance of the new EuroSCORE II risk stratification system. Eur J Cardiothorac Surg. 2013; 43:27–32. [DOI] [PubMed] [Google Scholar]
- 7.Wrobel K, Stevens SR, Jones RH, Selzman CH, Lamy A, Beaver TM, Djokovic LT, Wang N, Velazquez EJ, Sopko G, Kron IL, DiMaio JM, Michler RE, Lee KL, Yii M, Leng CY, Zembala M, Rouleau JL, Daly RC, Al-Khalidi HR. Influence of Baseline Characteristics, Operative Conduct, and Postoperative Course on 30-Day Outcomes of Coronary Artery Bypass Grafting Among Patients With Left Ventricular Dysfunction: Results From the Surgical Treatment for Ischemic Heart Failure (STICH) Trial. Circulation. 2015; 132:720–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ring WS, Edgerton JR, Herbert M, Prince S, Knoff C, Jenkins KM, Jessen ME, Hamman BL. I mpact of Accurate 30-Day Status on Operative Mortality: Wanted Dead or Alive, Not Unknown. Ann Thorac Surg. 2017; 104:1987–1993. [DOI] [PubMed] [Google Scholar]
- 9.Ascione R, Narayan P, Rogers CA, Lim KH, Capoun R, Angelini GD. Early and midterm clinical outcome in patients with severe left ventricular dysfunction undergoing coronary artery surgery. Ann Thorac Surg. 2003; 76:793–9. [DOI] [PubMed] [Google Scholar]
- 10.Toumpoulis IK, Anagnostopoulos CE, DeRose JJ, Swistel DG. Early and midterm outcome after off-pump coronary artery bypass grafting in patients with left ventricular dysfunction. Heart Surg Forum. 2004; 7:E539–45; discussion E539–45. [DOI] [PubMed] [Google Scholar]
- 11.Ahmed WA, Tully PJ, Baker RA, Knight JL. Survival after isolated coronary artery bypass grafting in patients with severe left ventricular dysfunction. Ann Thorac Surg. 2009; 87:1106–12. [DOI] [PubMed] [Google Scholar]
- 12.Doenst T, Essa Y, Jacoub K, Moschovas A, Gonzalez-Lopez D, Kirov H, Diab M, Bargenda S, Faerber G. Cardiac surgery 2016 reviewed. Clin Res Cardiol. 2017; 106:851–867. [DOI] [PubMed] [Google Scholar]
- 13.McNeely C, Markwell S, Vassileva C. Trends in Patient Characteristics and Outcomes of Coronary Artery Bypass Grafting in the 2000 to 2012 Medicare Population. Ann Thorac Surg. 2016; 102:132–8. [DOI] [PubMed] [Google Scholar]
- 14.Velazquez EJ, Lee KL, O’Connor CM, Oh JK, Bonow RO, Pohost GM, Feldman AM, Mark DB, Panza JA, Sopko G, Rouleau JL, Jones RH; STICH Investigators. The rationale and design of the Surgical Treatment for Ischemic Heart Failure (STICH) trial. J Thorac Cardiovasc Surg. 2007; 134:1540–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones RH, White H, Velazquez EJ, Shaw LK, Pietrobon R, Panza JA, Bonow RO, Sopko G, O’Connor CM, Rouleau JL. STICH (Surgical Treatment for Ischemic Heart Failure) trial enrollment. J Am Coll Cardiol. 2010; 56:490–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Velazquez EJ, Lee KL, Deja MA, Jain A, Sopko G, Marchenko A, Ali IS, Pohost G, Gradinac S, Abraham WT, Yii M, Prabhakaran D, Szwed H, Ferrazzi P, Petrie MC, O’Connor CM, Panchavinnin P, She L, Bonow RO, Rankin GR, Jones RH, Rouleau JL; STICH Investigators. Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med. 2011; 364:1607–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Velazquez EJ, Lee KL, Jones RH, Al-Khalidi HR, Hill JA, Panza JA, Michler RE, Bonow RO, Doenst T, Petrie MC, Oh JK, She L, Moore VL, Desvigne-Nickens P, Sopko G, Rouleau JL; STICHES Investigators. Coronary-Artery Bypass Surgery in Patients with Ischemic Cardiomyopathy. N Engl J Med. 2016; 374:1511–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones RH, Velazquez EJ, Michler RE, Sopko G, Oh JK, O’Connor CM, Hill JA, Menicanti L, Sadowski Z, Desvigne-Nickens P, Rouleau JL, Lee KL; STICH Hypothesis 2 Investigators. Coronary bypass surgery with or without surgical ventricular reconstruction. N Engl J Med. 2009; 360:1705–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrell FE Jr. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis Springer series in statistics. New York: Springer-Verlag, 2001: pp 109–115. [Google Scholar]
- 20.Monti KL. Folded Empirical Distribution Function Curves-Mountain Plots. The American Statistician 1995; 49: 342–345. [Google Scholar]
- 21.Rouleau JL, Bonow RO. An approach to the rational use of revascularization in heart failure patients. Can J Cardiol. 2014; 30:281–7. [DOI] [PubMed] [Google Scholar]
- 22.Parsonnet V Risk stratification in cardiac surgery: is it worthwhile? J Card Surg. 1995; 10:690–8. [DOI] [PubMed] [Google Scholar]
- 23.Kolh P Importance of risk stratification models in cardiac surgery. Eur Heart J. 2006; 27:768–9. [DOI] [PubMed] [Google Scholar]
- 24.Ad N, Holmes SD, Patel J, Pritchard G, Shuman DJ, Halpin L. Comparison of EuroSCORE II, Original EuroSCORE, and The Society of Thoracic Surgeons Risk Score in Cardiac Surgery Patients. Ann Thorac Surg. 2016; 102:573–9. [DOI] [PubMed] [Google Scholar]
- 25.Sullivan PG, Wallach JD, Ioannidis JP. Meta-Analysis Comparing Established Risk Prediction Models (EuroSCORE II, STS Score, and ACEF Score) for Perioperative Mortality During Cardiac Surgery. Am J Cardiol. 2016; 118:1574–1582. [DOI] [PubMed] [Google Scholar]
- 26.O’Boyle F, Mediratta N, Fabri B, Pullan M, Chalmers J, McShane J, Shaw M, Poullis M. Long-term survival after coronary artery bypass surgery stratified by EuroSCORE. Eur J Cardiothorac Surg. 2012; 42:101–6. [DOI] [PubMed] [Google Scholar]
- 27.Lancaster TS, Schill MR, Greenberg JW, Ruaengsri C, Schuessler RB, Lawton JS, Maniar HS, Pasque MK, Moon MR, Damiano RJ Jr, Melby SJ. Long-Term Survival Prediction for CABG: Validation of the ASCERT Model with Comparison to STS PROM. Ann Thorac Surg. 2018; 105:1336–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klein LW, Edwards FH, DeLong ER, Ritzenthaler L, Dangas GD, Weintraub WS. ASCERT: the American College of Cardiology Foundation--the Society of Thoracic Surgeons Collaboration on the comparative effectiveness of revascularization strategies. JACC Cardiovasc Interv. 2010; 3:124–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Howell NJ, Head SJ, Freemantle N, van der Meulen TA, Senanayake E, Menon A, Kappetein AP, Pagano D. The new EuroSCORE II does not improve prediction of mortality in high-risk patients undergoing cardiac surgery: a collaborative analysis of two European centres. Eur J Cardiothorac Surg. 2013; 44:1006–11; discussion 1011. [DOI] [PubMed] [Google Scholar]
- 30.Nashef SA, Sharples LD. Editorial comment: Pride without prejudice: EuroSCORE II, the STS score and the high-risk patient subset. Eur J Cardiothorac Surg. 2013; 44:1012. [DOI] [PubMed] [Google Scholar]
- 31.Denault A, Deschamps A, Tardif JC, Lambert J, Perrault L. Pulmonary hypertension in cardiac surgery. Curr Cardiol Rev. 2010; 6:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolff G, Dimitroulis D, Andreotti F, Kołodziejczak M, Jung C, Scicchitano P, Devito F, Zito A, Occhipinti M, Castiglioni B, Calveri G, Maisano F, Ciccone MM, De Servi S, Navarese EP. Survival Benefits of Invasive Versus Conservative Strategies in Heart Failure in Patients With Reduced Ejection Fraction and Coronary Artery Disease: A Meta-Analysis. Circ Heart Fail. 2017; 10 pii: e003255. [DOI] [PubMed] [Google Scholar]
- 33.Marui A, Kimura T, Nishiwaki N, Mitsudo K, Komiya T, Hanyu M, Shiomi H, Tanaka S, Sakata R; CREDO-Kyoto PCI/CABG Registry Cohort-2 Investigators. Comparison of five-year outcomes of coronary artery bypass grafting versus percutaneous coronary intervention in patients with left ventricular ejection fractions≤50% versus >50% (from the CREDO-Kyoto PCI/CABG Registry Cohort-2). Am J Cardiol. 2014; 114:988–96. [DOI] [PubMed] [Google Scholar]
- 34.Deb S, Wijeysundera HC, Ko DT, Tsubota H, Hill S, Fremes SE. Coronary artery bypass graft surgery vs percutaneous interventions in coronary revascularization: a systematic review. JAMA. 2013; 310:2086–95. [DOI] [PubMed] [Google Scholar]
- 35.Hannan EL, Racz MJ, Walford G, Jones RH, Ryan TJ, Bennett E, Culliford AT, Isom OW, Gold JP, Rose EA. Long-term outcomes of coronary-artery bypass grafting versus stent implantation. N Engl J Med. 2005; 352:2174–83. [DOI] [PubMed] [Google Scholar]
- 36.Nagendran J, Bozso SJ, Norris CM, McAlister FA, Appoo JJ, Moon MC, Freed DH, Nagendran J. Coronary Artery Bypass Surgery Improves Outcomes in Patients With Diabetes and Left Ventricular Dysfunction. J Am Coll Cardiol. 2018; 71:819–827. [DOI] [PubMed] [Google Scholar]
- 37.Velazquez EJ, Petrie MC. CABG or PCI for Diabetic Patients With Left Ventricular Dysfunction: Closing in on the Truth? J Am Coll Cardiol. 2018; 71:828–831. [DOI] [PubMed] [Google Scholar]
- 38.Bangalore S, Guo Y, Samadashvili Z, Blecker S, Hannan EL. Revascularization in Patients With Multivessel Coronary Artery Disease and Severe Left Ventricular Systolic Dysfunction: Everolimus-Eluting Stents Versus Coronary Artery Bypass Graft Surgery. Circulation. 2016; 133:2132–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


