Modern reperfusion treatment for the early stages of acute myocardial infarction—which is really quite miraculous, if you pause to think about it—arose from a fundamental understanding of coronary thrombosis. Might we someday also be able to restore function for patients who come in days, or even years, after a myocardial infarction? If we will ever develop biologic treatments that restore cardiac function through tissue regeneration, we need to understand the basics. Thus, how the cardiomyocytes of the heart replenish themselves is more than an academic curiosity, because this fundamental understanding will likely be crucial to building successful regenerative strategies for failing hearts.
In this issue of Circulation, five groups of investigators tackled the same biological question, and all reached the same conclusion: that cells in the heart expressing the Sca-1 cell surface antigen do not become cardiomyocytes to any meaningful degree and instead become endothelial cells. The papers add to the growing body of evidence that in adult mammals, our new cardiomyocytes arise from pre-existing cardiomyocytes and rarely (if at all) from adult cardiac stem cells1. However, these new papers don’t just provide a piece of the puzzle of myocardial biology; together, the papers also provide perspective on how science advances over time to benefit all of us. To explain this point, it’s worth considering how the concept of adult cardiac stem cells began.
Adult stem cells are cells that can self-renew and also differentiate to two or more different cell types. There are well-established examples of adult stem cells in, for example, the hematopoietic system, the intestinal epithelium, and the hair follicle.2 Around 15 years ago, adult stem cells were exciting for all areas of mammalian biology, for good reasons.3 The use of embryonic stem cells was highly controversial on ethical and religious grounds, hence adult stem cells appeared to be the solution to regenerating human tissues. George Walker Bush was President of the United States, and he supported adult stem cell research but opposed embryonic stem cell research 4. Adult stem cells could potentially be isolated from an existing tissue (possibly even a small biopsy or a sample of blood), expanded, and then used to create unlimited numbers of a patient’s own differentiated cells. No matter what the tissue of interest, investigators wanted to identify and isolate that tissue’s putative endogenous adult stem cells.
The concept of resident adult cardiac stem cells was therefore extremely attractive, because isolating and exploiting such a cell could theoretically generate new autologous cells to reconstitute damaged hearts. Essentially, every patient could be their own cardiomyocyte donor. The challenge for cardiovascular investigators was finding adult cardiac stem cells; without a cell surface marker to identify the cells, it was like looking for a needle in a haystack without knowing what a needle looks like. Thus, numerous investigators naturally turned to the adult hematopoietic system, in which well-characterized cell surface molecules like c-kit and Sca-1 had already been identified on stem cells. (The c-kit story has taken on a life of its own and will not be discussed in detail in this brief editorial.)
Sca-1 is a member of the Ly6 protein superfamily; there are at least 35 human and 61 mouse Ly6 proteins.5 The function of the mouse Sca-1 cell surface protein is still unknown, and there is no clear human counterpart (ortholog) of the mouse Sca-1 protein; the region of the mouse genome that encodes Sca-1 and several other Ly6 proteins is absent in the human genome.5 In 2003, Oh et al. reported isolating Sca-1+ cells from mouse myocardium; these cells could be cultured and steered toward apparent cardiomyocytes in the laboratory. In addition, when injected into injured mouse hearts, Sca-1+ cells appeared to fuse with cardiomyocytes and/or differentiate into cardiomyocytes.6 However, transplantation of cells may not reflect their endogenous natural role, and thus Uchida and colleagues studied a mouse that expressed a fluorescent protein on cells that at some point expressed Sca-1.7 Using mice genetically engineered to mark cells expressing a gene allows one to follow those cells over time, a technique in developmental biology called “lineage-mapping”. Uchida et al. concluded that non-cardiomyocytes that expressed Sca-1 continuously generate cardiomyocytes throughout life at a high rate.7
The concept of Sca-1+ cells as adult cardiac stem cells has, until now, not been fully examined by the broader scientific community. In this issue of Circulation, two original basic research articles and three research letters address this concept with new mouse genetic engineering technology. The study of Neidig et al. describes introduction of a drug-inducible (tamoxifen, widely used for lineage mapping) recombinase into the mouse genome at the Sca-1 locus. By turning on the recombinase with tamoxifen and marking Sca-1+ cells, they determined that Sca-1+ cells became endothelial cells, with very few cardiomyocytes marked.8 Vagnozzi et al. used the inducible recombinase method and also generated a constitutive (always on) recombinase at the Sca-1 locus; they found that Sca-1+ cells generated cardiac vasculature throughout development, during aging and following injury, with trivial contribution to the cardiomyocyte population.9 A third study, by Zhang et al., engineered a series of genetically altered mice to identify and track Sca-1+ cells in the heart; they found that Sca-1+ cells are only of the endothelial lineage.10 A fourth study by Tang et al. generated a new mouse with the inducible Cre recombinase made together with the Sca-1 protein and with a self-cleaving peptide sequence between the Cre protein and the endogenous Sca-1 protein; this strategy does not disrupt the endogenous production of the Sca-1 protein. Tang et al. found that no cardiomyocytes arose from the Sca-1+ cells, with Sca-1+ cells generating predominantly endothelial cells and fibroblasts.11 Finally, a fifth study by Soonpaa et al. isolated Sca-1+ cells from mice that expressed a fluorescent reporter protein and a second reporter that marked cardiomyocyte nuclei; this allowed the investigators to transplant Sca-1+ cells into injured heart cells and determine if the cells became cardiomyocytes. Soonpaa found no cardiomyocytes arising from the transplanted Sca-1+ cells.12
Thus, using many different techniques in many different laboratories, these five studies show that Sca-1+ cells in the heart rarely become cardiomyocytes, and the dominant fate of a Sca-1+ cardiac cell is to become an endothelial cell (Figure 1). In retrospect, we should not be surprised that Sca-1+ cells as well as c-kit+ cells13 become predominantly endothelial cells in the heart, as these markers were described in hematopoietic stem cells. Endothelial cells and hematopoietic stem cells share many markers as well as developmental origins.
Figure 1. Significant numbers of cardiomyocytes do not arise from Sca-1+ cells.
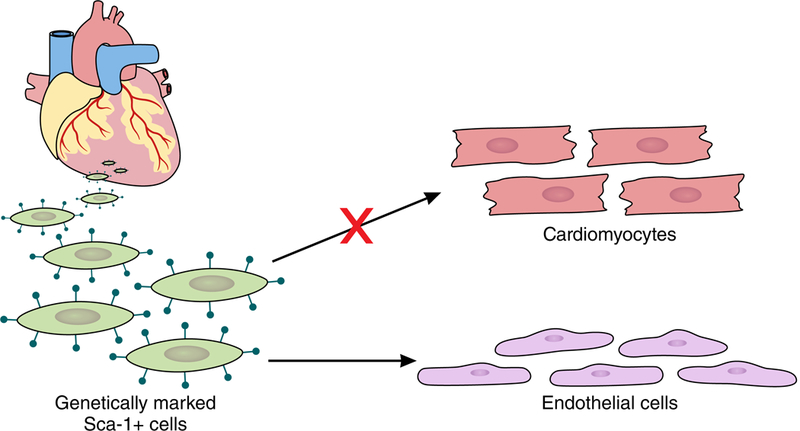
One theory of the mammalian heart has been that cardiomyocytes arose from adult stem cells marked by the cell surface protein Sca-1. In this issue of Circulation, five reports from different laboratories using different techniques reveal that Sca-1+ cells become primarily endothelial cells and don’t appreciably contribute to the adult cardiomyocyte pool.
Did the investigators who initially proposed Sca-1+ cells as adult cardiac stem cells do anything wrong? Absolutely not. These are investigators who have conducted outstanding science throughout their distinguished careers. These previous studies utilized the best techniques at that time and reported observations that are still valid for the approaches employed. However, the question as to the role of endogenous Sca-1+ cells was lacking and that awaited the lineage-mapping approaches that are discussed herein. As an example of how new techniques can change conclusions, my own laboratory performed studies a decade ago that suggested the importance of adult cardiac stem cells 14, only to discover later with more advanced technology that existing cardiomyocytes are the primary source for new myocyte generation in the adult mammalian heart 15. Furthermore, while the studies in this issue of Circulation rigorously eliminate the concept that Sca-1+ cells generate significant cardiomyocytes in the mouse, they do not eliminate the possibility that cardiac Sca-1+ cells can be manipulated productively in the laboratory. Finally, the expansion of the Sca-1+ endothelial pool may provide new insights into angiogenesis, and therefore Sca-1+ cells should continue as an investigative area.
In summary, this collection of new data indicates that endogenous Sca-1+ cells are not an important source of cardiomyocytes in adult mammals, adding to concept that cardiomyocytes themselves are the cells that generate new cardiomyocytes in the adult heart 16. Is this a case of one group of investigators proving another group of investigators to be wrong, or is it new techniques leading to a change in interpretation? The latter is the case here, as this is inherently the very process of science itself, working towards a consensus understanding of a concept with iterations that apply the best technology available at the time. And for that, we should applaud the investigators who pursued the adult stem cell studies in this issue of Circulation, as well as the investigators who started the adventure. In the long run, science is more of a team sport than an individual one.
Footnotes
Disclosures.
None.
References
- 1.Li Y, He L, Huang X, Issa Bhaloo S, Zhao H, Zhang S, Pu W, Tian X, Li Y, Liu Q, Yu W, Zhang L, Liu X, Liu K, Tang J, Zhang H, Cai D, Adams RH, Xu Q, Lui KO and Zhou B. Genetic Lineage Tracing of Non-Myocyte Population by Dual Recombinases. Circulation. 2018. pii: CIRCULATIONAHA.118.034250. doi: 10.1161/CIRCULATIONAHA.118.034250. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Clevers H and Watt FM. Defining Adult Stem Cells by Function, not by Phenotype. Annu Rev Biochem. 2018;87:1015–1027. [DOI] [PubMed] [Google Scholar]
- 3.Perin EC, Geng YJ and Willerson JT. Adult stem cell therapy in perspective. Circulation. 2003;107:935–938. [DOI] [PubMed] [Google Scholar]
- 4.Parker GC. Embryonic stem cell research: the state of the union of “those who create the lines” and “those who draw the lines”. Stem Cells Dev. 2006;15:623–629. [DOI] [PubMed] [Google Scholar]
- 5.Loughner CL, Bruford EA, McAndrews MS, Delp EE, Swamynathan S and Swamynathan SK. Organization, evolution and functions of the human and mouse Ly6/uPAR family genes. Hum Genomics. 2016;10:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, Pocius J, Michael LH, Behringer RR, Garry DJ, Entman ML and Schneider MD. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci U S A. 2003;100:12313–12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uchida S, De Gaspari P, Kostin S, Jenniches K, Kilic A, Izumiya Y, Shiojima I, Grosse Kreymborg K, Renz H, Walsh K and Braun T. Sca1-derived cells are a source of myocardial renewal in the murine adult heart. Stem Cell Reports. 2013;1:397–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neidig LE, Weinberger F, Palpant NJ, Mignone J, Martinson AM, Bender I, Nemoto N, Reinecke H, Pabon L, Molkentin JD, Murry CE and van Berlo JH. Evidence for minimal cardiogeneic potential of Sca-1 positive cells in the adult mouse heart. Circulation. 2018;XXX:XXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vagnozzi RJ, Sargent MA, Lin SJ, Palpant NJ, Murry CE and Molkentin JD. Genetic Lineage Tracing of Sca-1+ Cells Reveals Endothelial but Not Myogenic Contribution to the Murine Heart. Circulation. 2018;XXX:XXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang L, Sultana N, Yan J, Yang F, Chen F, Chepurko E, Yang F, Du Q, Zangi L, Xu M, Bu L and Cai C. Cardiac Sca-1+ cells are not intrinsic stem cells for myocardial development, renewal and repair. Circulation. 2018;XXX:XXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang J, Li Y, Huang X, He L, Zhang L, Wang H, Yu W, Tian X, Nie Y, Hu S, Wang Q, Lui K, Pu W and Zhou B. Fate Mapping of Sca1+ Cardiac Progenitor Cells in the Adult Mouse Heart. Circulation. 2018;XXX:XXX. [DOI] [PubMed] [Google Scholar]
- 12.Soonpaa MH, Lafontant PJ, Reuter S, Scherschel JA, Srour EF, Zaruba M, Rubart-von der Lohe M and Field LJ. Absence of cardiomyocyte differentiation of adult cardiac resident Sca-1+ cells into infarcted mouse hearts. Circulation. 2018;XXX:XXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Berlo JH, Kanisicak O, Maillet M, Vagnozzi RJ, Karch J, Lin SC, Middleton RC, Marban E and Molkentin JD. c-kit+ cells minimally contribute cardiomyocytes to the heart. Nature. 2014;509:337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsieh PC, Segers VF, Davis ME, MacGillivray C, Gannon J, Molkentin JD, Robbins J and Lee RT. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med. 2007;13:970–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, Wu TD, Guerquin-Kern JL, Lechene CP and Lee RT. Mammalian heart renewal by pre-existing cardiomyocytes. Nature. 2013;493:433–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eschenhagen T, Bolli R, Braun T, Field LJ, Fleischmann BK, Frisen J, Giacca M, Hare JM, Houser S, Lee RT, Marban E, Martin JF, Molkentin JD, Murry CE, Riley PR, Ruiz-Lozano P, Sadek HA, Sussman MA and Hill JA. Cardiomyocyte Regeneration: A Consensus Statement. Circulation. 2017;136:680–686. [DOI] [PMC free article] [PubMed] [Google Scholar]


