Abstract
Background:
While right atrial (RA) enlargement is an established marker for adverse outcomes, the prognostic importance of RA dysfunction independent of RA size in pulmonary arterial hypertension (PAH) is not known.
Methods and Results:
Study subjects with PAH were prospectively enrolled from 2010 to 2014. RA function was measured using RA speckle-tracking longitudinal strain (LS) and strain rate (SR) during each phase of the cardiac cycle: (I) RA reservoir (peak longitudinal strain [PLS], peak systolic SR [PSSR]), (II) RA conduit (peak early diastolic SR [PEDSR]), and (III) RA active contraction (peak active contraction strain [PACS], peak contraction SR [PCSR]). The primary outcome was a composite of time to hospitalization or death assessed on follow up. A total of 63 subjects had complete echocardiographic data. Of these, 91% were females and the mean age was 58 ± 12 years. During the follow-up period (range:1–58 months), 39 were hospitalized or had died. After multivariable adjustment for age, gender and LA size, PLS, PACS, and PEDSR were significantly associated with increased risk of the composite outcome (p=0.0005, p=0.0167 and p=0.0054 respectively).
Conclusion:
Right atrial dysfunction independently predicts mortality and hospitalizations in patients with pulmonary arterial hypertension.
Keywords: right atrium, pulmonary hypertension, strain, right atrial function, right atrial strain method, right atrial speckle tracking strain, Echocardiography, Mortality/Survival
CLINICAL PERSPECTIVE
The right atrium (RA) is a simpler geometric structure and easier noninvasive measure than the right ventricle (RV). In this content we prospectively studied the feasibility and prognostic value of RA function using speckle tracking echocardiography (STE) in independent of its associations with RA size in patients with pulmonary hypertension. A total of 63 subjects had complete STE and included in the final analysis. After multivariable adjustment for age, gender and LA size, PLS, PACS, and PEDSR were significantly associated with increased risk of the composite outcome (p=0.0005, p=0.0167, and p=0.0054 respectively). RA dysfunction was associated with lower event-free survival in PAH. RA reservoir, conduit and active contraction function are an independent predictor of mortality and hospitalizations in PAH. Given these findings, RA STE may be an important tool to risk-stratify patients with PAH.
Introduction
Right atrial (RA) enlargement is a common finding in patients with pulmonary arterial hypertension (PAH).1, 2 Consistent with this, RA size has been demonstrated to be a predictor of mortality or transplantation in this group3–5 but atrial enlargement may not occur in a symmetrical uniform fashion. Atrial function is complex and has several components, consisting of a reservoir phase during atrial filling, a conduit phase during passive emptying of the atrium into the ventricle, and a pump phase during atrial systole.6 Coordination of these phasic functions plays an important role for the maintenance of overall cardiac function.7
In this context, there has been growing interest in markers of RA myocardial dysfunction that might help in early disease stratification prior to significant RA remodeling. 2D speckle tracking echocardiography (STE) has been shown to be feasible for investigating RA function.8 Recently, Querejeta Roca et al. found that RA function by STE is impaired in PAH independent of RA size or pressure.9 Therefore, the objective of our study was to explore the feasibility of strain-based measures of RA dysfunction and validate them by assessing their prognostic role, independent of RA size in study subjects with PAH.
Methods
Study Design
The data, methods used in the analysis, and materials used to conduct this study will be made available to any researcher for purposes of reproducing the results or replicating the procedure by emailing the corresponding author (S.R.). The study was designed as a prospective observational cohort study. We enrolled study subjects with known or suspected pulmonary hypertension (PH), referred from the Duke Pulmonary Vascular Disease Center to the Duke Cardiac Diagnostic Unit (CDU) for a clinically indicated transthoracic echocardiography (TTE).
Study Subjects and Data Collection
Study subjects were prospectively enrolled from 2010 to 2014. Inclusion criteria included mean pulmonary artery pressure (mPAP) ≥25 mmHg at rest and/or RV systolic pressure (RVSP) >40 mmHg and/or suspected diagnosis of PH. In addition, PH diagnosis was confirmed by right heart catheterization. Study subjects with significant arrhythmia were excluded from the study. The study protocol was approved by the institutional review board, and all patients provided written informed consent, which included consent for the TTE analysis.
Standard Echocardiography Methods
For RA STE analysis, all images were obtained at a frame rate of 50 to 60 fps, and three consecutive cardiac cycles were recorded. Right ventricle (RV) measures were performed, including tricuspid annular plane systolic excursion (TAPSE), tricuspid regurgitation (TR) severity, inferior vena cava (IVC) diameter, RV fractional area change (FAC) and RV global longitudinal strain (GLS). Left ventricular ejection fraction (LVEF) was also assessed using Simpson’s biplane method. All measurements were conducted in accordance with American Society of Echocardiography (ASE) recommendations.10
Speckle Tracking Strain and Strain Rate Analysis
Studies were uploaded to the vendor-independent TomTec image arena module (Munich, Germany: REF- Version 4.6 software). STE longitudinal strain (LS) and strain rate (SR) were performed offline in all studies with adequate image quality. RA STE inadequate quality was defined as poor visualization or poor tracking of >1 atrial segments, segment dropout, missing view, or significant foreshortening of the RV or RA (4 studies were excluded due to this). RV GLS was also measured, the endocardial border was manually traced in end systole and the software automatically traced a region of interest including the entire myocardium. The RV free wall and septal wall segments were averaged for RV GLS and adjusted to the pulmonary valve closure time.
To generate RA LS and SR curves, the RA endocardial border was manually traced at ventricular end systole in the apical 4-chamber view in each patient; the software divided the RA into 3 separate segments (RA lateral wall, RA roof, and RA septal wall). The RA was traced starting at the lateral tricuspid valve (TV) annulus, along the endocardial border of the RA lateral wall, RA roof, RA septal wall, and ending at the septal TV annulus. RA LS and SR curves were generated, tracking evaluated, and the region of interest generated was subsequently adjusted to include the full thickness of the RA myocardium. Therefore, in our study the R- wave (QRS complex) was the zero reference and all strain values were positive. (Figure 1)
Figure 1:
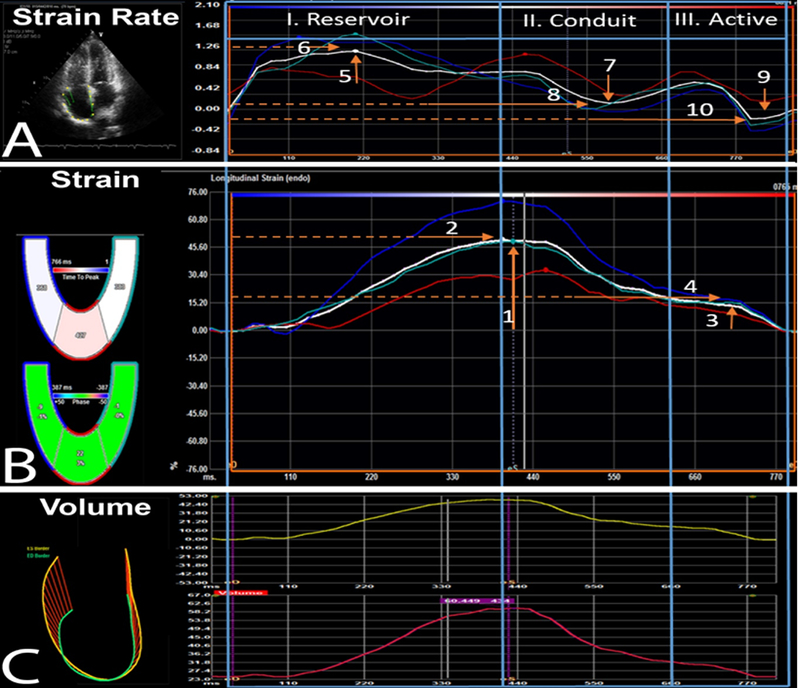
RA phasic function strain and strain rate speckle tracking strain analysis. Abbreviations: A. RA Strain Rate; B. RA Strain; C. RA Volumes: 1. Peak longitudinal strain [PLS]; 2. Time to PLS; 3. Peak active contraction strain [PACS]; 4. Time to PACS; 5. Peak systolic strain rate [PSSR]; 6. Time to PSSR; 7. Peak diastolic strain rate [PEDSR]; 8. Time to PEDSR; 9. Peak contraction strain rate [PCSR]; 10. Time to PCSR. R- wave (QRS complex) was the zero reference and all strain values were positive.
Using STE, the three phases of RA function were measured: (I) Reservoir function (Peak longitudinal strain [PLS], peak systolic strain rate [PSSR] with the time to PLS and to PSSR); (II) RA conduit function (Peak early diastolic SR [PEDSR], with time to PEDSR); and (III) RA active contraction function (peak active contraction strain [PACS], peak contraction strain rate [PCSR], with the time to PACS and to PCSR) (Figure 1). All measurements were performed by a single expert strain investigator (F.A.) blinded to clinical status. A second reader measured key LS and SR measurements on a randomly selected subset (20%) of the cohort to generate inter rater variability results. Table 1 represents key measurements and guidance related to STE LS and SR quantification of RA function.
Table 1:
Key measurements and guidance related to STE strain & SR quantification of RA function.
| Strain | Measurement | Strain Rate | Measurement |
|---|---|---|---|
| Reservoir | Reservoir | ||
| PLS | From the baseline to the first peak atrial strain curve wave. Corresponds to the ventricular systole. | PSSR | From the baseline to the first positive peak atrial strain rate wave. |
|
Time to PLS |
Time from beginning of R-wave (QRS complex) to the first strain peak wave. |
Time to PSSR |
Time from the beginning of the R-wave (QRS complex) to the first positive peak atrial strain wave. |
| Conduit | Conduit | ||
| PEDSR | From the baseline to the first negative peak atrial strain rate wave. | ||
| Time to PEDSR | Time from beginning of the R-wave (QRS complex) to the first negative peak atrial strain rate wave. | ||
| Active | Active | ||
| PACS | From the baseline to the second peak atrial strain curve wave. Corresponds to the ventricular diastole. | PCSR | From the baseline to the second negative peak atrial strain rate wave. |
|
Time to PACS |
Time from beginning of R-wave (QRS complex) to the second strain peak wave. |
Time to PCSR |
Time from beginning of the R-wave (QRS complex) to the second negative peak atrial strain wave. |
Peak longitudinal strain [PLS]; Peak active contraction strain [PACS]; Peak systolic strain rate [PSSR]; Peak diastolic SR [PEDSR]; Peak contraction strain rate [PCSR]
Clinical Data
For all study subjects enrolled in this study, we collected the following data: demographics, World Health Organization (WHO) functional class (FC), comorbidities, medications, vital signs, body mass index, and laboratory data including B-type natriuretic peptide (NT-proBNP), 6-minute walk distance (6MWD), death and hospitalizations. Study participants also underwent right heart catheterization; the following invasive hemodynamic measurements were included: cardiac output/Index (CI); LA pressure (LAP); mean pulmonary artery pressure (mPAP); pulmonary vascular resistance (PVR); RA pressure (RAP); and pulmonary capillary wedge pressure (PCWP).
Outcome
The primary endpoint was a combined outcome of composite of hospitalization or death. Date of last follow-up was defined as the date of death or last contact from Duke Medical Center administrative data sources. Mortality from the Social Security Death Master Files through October 2017 were incorporated into administrative data sources at Duke and accessed for ascertainment of the endpoint.
Statistical Analysis
To assess the strength of the absolute agreement between observers, the inter-class correlation coefficient (ICC) was used based on a two-way random effects analysis-of-variance model. High agreement is evidenced by a high ICC (close to 1). Baseline characteristics were described using mean, standard deviation, median and interquartile ranges for continuous variables and percentages for categorical variables. In our study PLS and PACS were dichotomized at cut off values of 25% and 13% respectively for baseline table stratification. P-values for differences in patient groups were generated using a Fisher Exact or Chi-Square test for categorical variables and Wilcoxon rank-sum testing for continuous variables, based on appropriateness. Cumulative event rates were calculated according to the Kaplan-Meier method, with all event or censoring times measured from the date of echocardiography. The significance of differences in the primary end point between groups was assessed with the use of the log-rank test. Cox proportional hazards models were examined to assess the relationship between RA function measures and the composite outcome, time to death or hospitalization. Linearity of continuous measures was examined using cubic spline polynomials. Variable transformations were determined when needed in order to satisfy the linearity assumption of the Cox models. Both unadjusted and adjusted relationships with outcome were examined for continuous RA measures. Stratification for Kaplan-Meier graphs are provided for illustrative purposes, stratifying risk at the median values for RA function (Figures 2A & 2B). Due to the limited number of events, adjusted covariates included only age, gender and RA size. Two-sided significance testing was used for all statistical tests, with a p-value <0.05 considered statistically significant. Analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC, USA). F.A., S.R., and Z.S. take responsibility for the raw data. L. S. takes responsibility for the statistical analyses.
Figure 2.
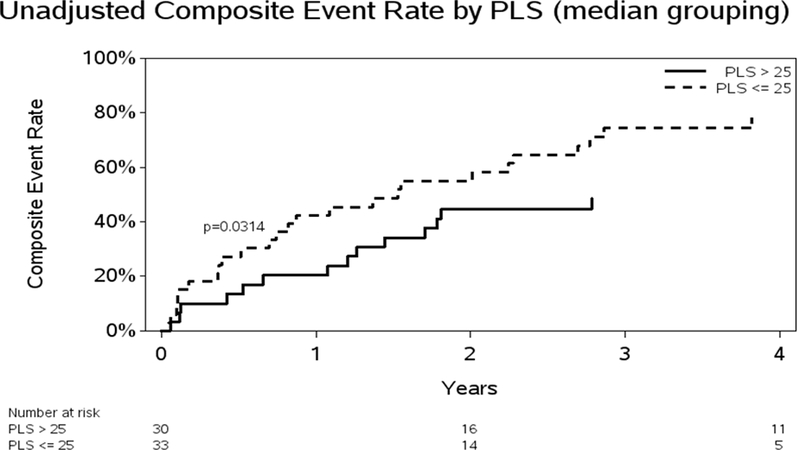
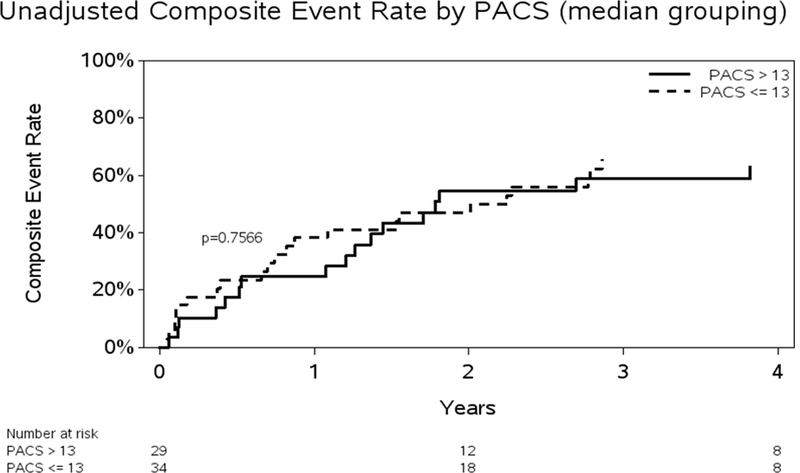
A. Unadjusted Kaplan-Meier curve for death or hospitalizations and PLS. Abbreviation: Peak longitudinal strain = PLS. B: Unadjusted Kaplan-Meier curve for death or hospitalizations and PACS. Abbreviation: Peak active contraction strain = PACS
Results
A total of 101 subjects were enrolled. One subject withdrew from the study, 2 were found to not have PAH and 31 were excluded because of having different causes of PH. Study results are based on 63 patients with significant precapillary pulmonary hypertension. Of those patients, 56 were WHO Group 1 and 7 were WHO Group 4.
Results for the total patient population were also generated (n=92), which included patients with combined pre- and post-capillary PH. In this larger cohort, significant unadjusted relationships with outcome were observed for PLS, PACS, PEDSR, time to PLS, and time to PEDSR. After multivariable adjustment, the relationships yielded similar results to the univariable models. Results appear in the supplemental tables and figures (Supplemental: Tables 1A &1B, 2A &2B, 3 and figures 1A &1B).
Feasibility & Reproducibility
Of the 67 study subjects with PAH, 4 were excluded from the final analysis because of inadequate image quality for STE analysis (feasibility of RA function assessment > 94%), resulting in 63 patients for outcome analyses. In 20 randomly selected study subjects, all the LS and SR measurements were assessed by two independent readers for assessment of variability using inter-class correlation analyses (ICC). Good reliability was determined with ICC of .701 for PLS and .656 for PACS. PSSR, PEDSR and PCSR and also had good reliability (.653, .667 and .728 respectively).
Baseline Characteristics
Tables 2A and2B summarize the overall baseline clinical and laboratory characteristics stratified by median PLS and PACS. The majority of study subjects (91%) were females; more than half (73%) were white, and the mean age was 58 ± 12 years. Consistent with the majority of the patients having significant precapillary PH, the average PVR was 10 ± 5.4 WU. Almost half the study subjects had at least WHO FC II symptoms; with a mean (standard deviation) 6MWD of 372 ±132 meters, and 87% were on PH medications and majority of the cohort had an abnormal PLS and PACS.
Table 2-A:
Baseline Characteristics of Population of PAH Patients - PLS stratification
| Characteristic | PLS≤25 [N=33] |
PLS>25 [N=30] |
Total [N= 63] |
P-value |
|---|---|---|---|---|
| Age (yrs.) | 0.788 | |||
| N | 33 | 30 | 63 | |
| Mean (S.D.) | 58.4 (11.54) | 57.8 (13.66) | 58.1 (12.49) | |
| Median (Q1, Q3) | 61.0 (50.0, 66.0) | 57.0 (46.0, 68.0) | 59.0 (48.0, 66.0) | |
| (Min., Max.) | (41, 80) | (35, 84) | (35, 84) | |
| Sex | 0.094 | |||
| Male | 1/33 (3.0%) | 5/30 (16.7%) | 6/63 (9.5%) | |
| Female | 32/33 (97.0%) | 25/30 (83.3%) | 57/63 (90.5%) | |
| Race | 0.872 | |||
| White | 24/32 (75.0%) | 20/28 (71.4%) | 44/60 (73.3%) | |
| Black | 7/32 (21.9%) | 7/28 (25.0%) | 14/60 (23.3%) | |
| Asian | 0/32 (0.0%) | 1/28 (3.6%) | 1/60 (1.7%) | |
| Other | 1/32 (3.1%) | 0/28 (0.0%) | 1/60 (1.7%) | |
| WHO Functional Class | 0.147 | |||
| 1 | 1/33 (3.0%) | 0/30 (0.0%) | 1/63 (1.6%) | |
| 2 | 16/33 (48.5%) | 22/30 (73.3%) | 38/63 (60.3%) | |
| 3 | 12/33 (36.4%) | 7/30 (23.3%) | 19/63 (30.2%) | |
| 4 | 4/33 (12.1%) | 1/30 (3.3%) | 5/63 (7.9%) | |
| Six-min. Walk Distance (m) | 0.313 | |||
| N | 31 | 30 | 61 | |
| Mean (S.D.) | 349.5 (149.55) | 394.3 (108.76) | 371.5 (131.95) | |
| Median (Q1, Q3) | 376.7 (236.0, 493.8) | 408.5 (302.7, 477.4) | 407.0 (263.0, 480.0) | |
| (Min., Max.) | (73.0, 575.0) | (152.0, 582.5) | (73.0, 582.5) | |
| PH Medications | 1.000 | |||
| 0 | 4/33 (12.1%) | 4/30 (13.3%) | 8/63 (12.7%) | |
| 1 | 29/33 (87.9%) | 26/30 (86.7%) | 55/63 (87.3%) | |
| RA Mean | 0.290 | |||
| N | 32 | 29 | 61 | |
| Mean (S.D.) | 10.9 (11.52) | 7.8 (3.79) | 9.4 (8.82) | |
| Median (Q1, Q3) | 8.5 (5.5, 13.0) | 7.0 (5.0, 9.0) | 8.0 (5.0, 11.0) | |
| (Min., Max.) | (1, 68) | (2, 17) | (1, 68) | |
| PA Mean | 0.725 | |||
| N | 33 | 30 | 63 | |
| Mean (S.D.) | 47.5 (14.96) | 47.0 (12.09) | 47.3 (13.56) | |
| Median (Q1, Q3) | 51.0 (38.0, 54.0) | 46.5 (40.0, 54.0) | 47.0 (40.0, 54.0) | |
| (Min., Max.) | (20, 80) | (27, 72) | (20, 80) | |
| PA systolic | 0.761 | |||
| N | 33 | 29 | 62 | |
| Mean (S.D.) | 73.3 (21.46) | 75.4 (18.54) | 74.3 (20.02) | |
| Median (Q1, Q3) | 75.0 (60.0, 86.0) | 74.0 (66.0, 90.0) | 75.0 (60.0, 90.0) | |
| (Min., Max.) | (35, 118) | (42, 110) | (35, 118) | |
| PA diastolic | 0.525 | |||
| N | 33 | 29 | 62 | |
| Mean (S.D.) | 31.5 (11.33) | 29.7 (9.67) | 30.6 (10.54) | |
| Median (Q1, Q3) | 32.0 (23.0, 39.0) | 31.0 (22.0, 35.0) | 31.5 (22.0, 38.0) | |
| (Min., Max.) | (10, 54) | (12, 48) | (10, 54) | |
| Cardiac Index (L/min/m2) | 0.923 | |||
| N | 33 | 30 | 63 | |
| Mean (S.D.) | 2.5 (0.81) | 2.6 (0.83) | 2.5 (0.81) | |
| Median (Q1, Q3) | 2.2 (2.1, 3.0) | 2.5 (1.8, 3.1) | 2.3 (1.9, 3.0) | |
| (Min., Max.) | (1.4, 4.8) | (1.5, 4.6) | (1.4, 4.8) | |
| PVR (WU) | 0.907 | |||
| N | 33 | 30 | 63 | |
| Mean (S.D.) | 10.0 (5.56) | 9.9 (5.42) | 10.0 (5.45) | |
| Median (Q1, Q3) | 9.9 (5.6, 13.3) | 8.7 (6.4, 13.9) | 9.6 (5.8, 13.4) | |
| (Min., Max.) | (1.6, 27.1) | (1.5, 20.3) | (1.5, 27.1) | |
| Reveal Risk Score | 0.013 | |||
| N | 33 | 30 | 63 | |
| Mean (S.D.) | 7.8 (3.20) | 5.8 (2.46) | 6.9 (3.03) | |
| Median (Q1, Q3) | 8.0 (5.0, 10.0) | 6.0 (4.0, 7.0) | 6.0 (5.0, 9.0) | |
| (Min., Max.) | (3, 14) | (2, 11) | (2, 14) |
PLS= Peak longitudinal strain, PH= Pulmonary Hypertension, WHO= World Health Organization
Table 2B:
Baseline Characteristics of Population of PAH Patients – PACS stratification
| Characteristic | PACS≤ 13 [N=34] |
PACS>13 [N=29] |
Total [N=63] |
P-value |
|---|---|---|---|---|
| Age (yrs.) | 0.473 | |||
| N | 34 | 29 | 63 | |
| Mean (S.D.) | 59.4 (13.15) | 56.7 (11.74) | 58.1 (12.49) | |
| Median (Q1, Q3) | 61.5 (46.0, 69.0) | 56.0 (48.0, 66.0) | 59.0 (48.0, 66.0) | |
| (Min., Max.) | (41, 84) | (35, 80) | (35, 84) | |
| Sex | 0.007 | |||
| Male | 0/34 (0.0%) | 6/29 (20.7%) | 6/63 (9.5%) | |
| Female | 34/34 (100.0%) | 23/29 (79.3%) | 57/63 (90.5%) | |
| Race | 0.855 | |||
| White | 25/34 (73.5%) | 19/26 (73.1%) | 44/60 (73.3%) | |
| Black | 8/34 (23.5%) | 6/26 (23.1%) | 14/60 (23.3%) | |
| Asian | 0/34 (0.0%) | 1/26 (3.8%) | 1/60 (1.7%) | |
| Other | 1/34 (2.9%) | 0/26 (0.0%) | 1/60 (1.7%) | |
| WHO Functional Class | 0.507 | |||
| 1 | 1/34 (2.9%) | 0/29 (0.0%) | 1/63 (1.6%) | |
| 2 | 20/34 (58.8%) | 18/29 (62.1%) | 38/63 (60.3%) | |
| 3 | 9/34 (26.5%) | 10/29 (34.5%) | 19/63 (30.2%) | |
| 4 | 4/34 (11.8%) | 1/29 (3.4%) | 5/63 (7.9%) | |
| PH Medications | 1.000 | |||
| 0 | 4/34 (11.8%) | 4/29 (13.8%) | 8/63 (12.7%) | |
| 1 | 30/34 (88.2%) | 25/29 (86.2%) | 55/63 (87.3%) | |
| Right Atrial Mean | 0.412 | |||
| N | 33 | 28 | 61 | |
| Mean (S.D.) | 8.4 (5.06) | 10.6 (11.83) | 9.4 (8.82) | |
| Median (Q1, Q3) | 6.0 (4.0, 11.0) | 8.0 (6.0, 11.0) | 8.0 (5.0, 11.0) | |
| (Min., Max.) | (1, 21) | (2, 68) | (1, 68) | |
| Pulmonary Artery Mean | 0.408 | |||
| N | 34 | 29 | 63 | |
| Mean (S.D.) | 45.4 (12.74) | 49.6 (14.35) | 47.3 (13.56) | |
| Median (Q1, Q3) | 47.5 (34.0, 52.0) | 47.0 (40.0, 60.0) | 47.0 (40.0, 54.0) | |
| (Min., Max.) | (20, 70) | (25, 80) | (20, 80) | |
| Pulmonary Arterial systolic | 0.369 | |||
| N | 34 | 28 | 62 | |
| Mean (S.D.) | 71.7 (18.76) | 77.5 (21.36) | 74.3 (20.02) | |
| Median (Q1, Q3) | 75.5 (56.0, 82.0) | 73.5 (62.5, 91.0) | 75.0 (60.0, 90.0) | |
| (Min., Max.) | (35, 102) | (42, 118) | (35, 118) | |
| Pulmonary Arterial diastolic | 0.420 | |||
| N | 34 | 28 | 62 | |
| Mean (S.D.) | 29.6 (10.14) | 31.9 (11.05) | 30.6 (10.54) | |
| Median (Q1, Q3) | 30.5 (20.0, 35.0) | 33.0 (23.0, 39.0) | 31.5 (22.0, 38.0) | |
| (Min., Max.) | (10, 50) | (12, 54) | (10, 54) | |
| Cardiac Index (L/min/m2) | 0.490 | |||
| N | 34 | 29 | 63 | |
| Mean (S.D.) | 2.5 (0.71) | 2.7 (0.92) | 2.5 (0.81) | |
| Median (Q1, Q3) | 2.3 (1.9, 2.9) | 2.6 (1.9, 3.1) | 2.3 (1.9, 3.0) | |
| (Min., Max.) | (1.4, 4.4) | (1.5, 4.8) | (1.4, 4.8) | |
| Pulmonary Vascular Resistance (WU) | 0.735 | |||
| N | 34 | 29 | 63 | |
| Mean (S.D.) | 9.5 (4.81) | 10.5 (6.16) | 10.0 (5.45) | |
| Median (Q1, Q3) | 9.6 (5.6, 13.3) | 9.6 (6.4, 13.6) | 9.6 (5.8, 13.4) | |
| (Min., Max.) | (1.6, 20.3) | (1.5, 27.1) | (1.5, 27.1) | |
| Reveal Risk Score | 0.961 | |||
| N | 34 | 29 | 63 | |
| Mean (S.D.) | 7.0 (3.47) | 6.7 (2.47) | 6.9 (3.03) | |
| Median (Q1, Q3) | 6.0 (4.0, 10.0) | 7.0 (5.0, 8.0) | 6.0 (5.0, 9.0) | |
| (Min., Max.) | (3, 14) | (2, 11) | (2, 14) |
PACS= Peak active contraction strain, PH= Pulmonary Hypertension, WHO= World Health Organization. Note: The median value of PACS is 12.5, (which is not technically the median, but a rounded up version of the median). There is 1 patient with 12.5, 1 patient with 12.8, and 1 patient with 13 for PACS.
Distribution of RA function variables
Tables 3A and3B provide echocardiographic characteristics and distributions for the RA function values, overall and by strata. PLS median was 25 (18.5, 34.0) (median (IQR)) and mean of 25.5 ±11.4 (mean ± SD). PACS median was 12.5 (9.7, 16.3) with mean of 13.8 ±6.8. Distributions for PSSR, PEDSR, PCSR and corresponding time to variables are provided in the Tables 3A and3B.
Table 3A:
Baseline Echocardiographic Variables for the Study Population of PAH Patients - PLS stratification
| Echo Parameter | PLS≤25 [N=33] |
PLS>25 [N=30] |
Total [N= 63] |
P-value |
|---|---|---|---|---|
| LV Ejection Fraction (%) | 0.228 | |||
| N | 30 | 28 | 58 | |
| Mean (S.D.) | 59.91 (6.99) | 62.19 (6.89) | 61.01 (6.98) | |
| Median (Q1, Q3) | 59.54 (53.65, 67.15) | 60.97 (57.49, 68.10) | 60.32 (56.23, 67.27) | |
| (Min., Max.) | (44.95, 70.59) | (48.77, 74.59) | (44.95, 74.59) | |
| Tricuspid Valve Regurgitation | 0.303 | |||
| Trivial | 12/33 (36.4%) | 13/30 (43.3%) | 25/63 (39.7%) | |
| Mild | 8/33 (24.2%) | 11/30 (36.7%) | 19/63 (30.2%) | |
| Moderate | 8/33 (24.2%) | 5/30 (16.7%) | 13/63 (20.6%) | |
| Severe | 5/33 (15.2%) | 1/30 (3.3%) | 6/63 (9.5%) | |
| Right Atrial Area (cm2) | 0.033 | |||
| N | 33 | 30 | 63 | |
| Mean (S.D.) | 22.2 (6.71) | 19.1 (4.92) | 20.8 (6.08) | |
| Median (Q1, Q3) | 21.1 (18.2, 25.9) | 18.2 (15.9, 20.5) | 20.2 (16.3, 23.7) | |
| (Min., Max.) | (12.7, 40.7) | (11.5, 30.3) | (11.5, 40.7) | |
| Right Atrial Volume (ml) | 0.044 | |||
| N | 32 | 30 | 62 | |
| Mean (S.D.) | 75.3 (36.10) | 58.8 (24.22) | 67.3 (31.79) | |
| Median (Q1, Q3) | 68.1 (50.0, 87.6) | 53.2 (42.2, 68.5) | 61.6 (43.4, 74.7) | |
| (Min., Max.) | (28.8, 176.3) | (26.2, 110.6) | (26.2, 176.3) | |
| RV FAC (%) | 0.055 | |||
| N | 32 | 30 | 62 | |
| Mean (S.D.) | 30.4 (8.23) | 34.5 (8.21) | 32.4 (8.40) | |
| Median (Q1, Q3) | 28.2 (24.6, 37.1) | 35.0 (29.1, 41.9) | 32.5 (25.8, 39.1) | |
| (Min., Max.) | (18.3, 46.5) | (17.5, 50.4) | (17.5, 50.4) | |
| RV GLS (%) | <0.001 | |||
| N | 31 | 30 | 61 | |
| Mean (S.D.) | −16.3 (4.99) | −20.5 (4.64) | −18.4 (5.23) | |
| Median (Q1, Q3) | −15.7 (−18.2, −13.3) | −20.1 (−22.2, −18.6) | −18.3 (−21.2, −14.2) | |
| (Min., Max.) | (−33.1, −7.8) | (−32.1, −8.9) | (−33.1, −7.8) | |
| (Min., Max.) | (−27.3, −14.0) | (−30.5, −10.3) | (−30.5, −10.3) | |
| LV GLS (%) | 0.514 | |||
| N | 23 | 27 | 50 | |
| Mean (S.D.) | −19.1 (2.91) | −18.4 (3.49) | −18.7 (3.22) | |
| Median (Q1, Q3) | −19.0 (−21.5, −16.5) | −18.4 (−21.7, −15.8) | −18.5 (−21.5, −16.2) | |
| (Min., Max.) | (−25.5, −14.6) | (−25.8, −11.2) | (−25.8, −11.2) | |
| TAPSE (cm) | <0.001 | |||
| N | 32 | 30 | 62 | |
| Mean (S.D.) | 1.7 (0.41) | 2.1 (0.45) | 1.9 (0.47) | |
| Median (Q1, Q3) | 1.7 (1.5, 1.9) | 2.2 (1.8, 2.4) | 1.9 (1.6, 2.2) | |
| (Min., Max.) | (0.9, 2.7) | (1.0, 2.8) | (0.9, 2.8) | |
| PLS % | <0.001 | |||
| N | 33 | 30 | 63 | |
| Mean (S.D.) | 17.26 (7.22) | 34.62 (7.54) | 25.53 (11.40) | |
| Median (Q1, Q3) | 19.80 (15.00, 23.00) | 34.00 (28.00, 40.10) | 25.00 (18.50, 34.00) | |
| (Min., Max.) | (0.02, 25.00) | (25.20, 52.00) | (0.02, 52.00) | |
| Time to PLS | 0.407 | |||
| N | 31 | 30 | 61 | |
| Mean (S.D.) | 384 (94) | 408 (78) | 396 (87) | |
| Median (Q1, Q3) | 389 (320, 430) | 398 (360, 431) | 395 (350, 430) | |
| (Min., Max.) | (200, 567) | (291, 620) | (200, 620) | |
| PACS % | <0.001 | |||
| N | 33 | 30 | 63 | |
| Mean (S.D.) | 10.19 (5.31) | 17.70 (6.07) | 13.77 (6.79) | |
| Median (Q1, Q3) | 10.00 (8.10, 11.90) | 15.85 (13.00, 21.30) | 12.50 (9.70, 16.30) | |
| (Min., Max.) | (0.01, 26.00) | (9.20, 31.00) | (0.01, 31.00) | |
| Time to PACS | 0.278 | |||
| N | 29 | 30 | 59 | |
| Mean (S.D.) | 693 (189) | 721 (167) | 707 (177) | |
| Median (Q1, Q3) | 635 (559, 774) | 687 (632, 790) | 675 (596, 783) | |
| (Min., Max.) | (446, 1333) | (478, 1334) | (446, 1334) | |
| PSSR | <0.001 | |||
| N | 31 | 30 | 61 | |
| Mean (S.D.) | 1.05 (0.31) | 1.35 (0.33) | 1.20 (0.35) | |
| Median (Q1, Q3) | 1.00 (0.90, 1.30) | 1.20 (1.10, 1.50) | 1.10 (1.00, 1.40) | |
| (Min., Max.) | (0.40, 1.70) | (1.00, 2.10) | (0.40, 2.10) | |
| Time to PSSR | 0.392 | |||
| N | 28 | 30 | 58 | |
| Mean (S.D.) | 174 (70) | 179 (40) | 177 (56) | |
| Median (Q1, Q3) | 176 (126, 196) | 172 (150, 206) | 175 (145, 200) | |
| (Min., Max.) | (76, 400) | (85, 261) | (76, 400) | |
| PEDSR | 0.003 | |||
| N | 24 | 25 | 49 | |
| Mean (S.D.) | 0.44 (0.34) | 0.74 (0.41) | 0.59 (0.40) | |
| Median (Q1, Q3) | 0.36 (0.30, 0.54) | 0.69 (0.42, 0.95) | 0.49 (0.34, 0.80) | |
| (Min., Max.) | (0.06, 1.70) | (0.04, 1.78) | (0.04, 1.78) | |
| Time to PEDSR | 0.112 | |||
| N | 18 | 22 | 40 | |
| Mean (S.D.) | 672 (143) | 762 (187) | 722 (173) | |
| Median (Q1, Q3) | 668 (557, 764) | 743 (659, 776) | 697 (600, 773) | |
| (Min., Max.) | (471, 922) | (500, 1379) | (471, 1379) | |
| PCSR | 0.010 | |||
| N | 22 | 22 | 44 | |
| Mean (S.D.) | 1.03 (0.55) | 1.52 (0.62) | 1.27 (0.63) | |
| Median (Q1, Q3) | 0.82 (0.58, 1.30) | 1.55 (1.10, 2.00) | 1.21 (0.74, 1.70) | |
| (Min., Max.) | (0.38, 2.30) | (0.54, 2.80) | (0.38, 2.80) | |
| Time to PCSR | 0.288 | |||
| N | 20 | 25 | 45 | |
| Mean (S.D.) | 483 (115) | 520 (109) | 504 (112) | |
| Median (Q1, Q3) | 526 (362, 561) | 533 (450, 590) | 533 (427, 581) | |
| (Min., Max.) | (277, 643) | (299, 700) | (277, 700) |
LV= Left ventricle, RV= Right ventricle, FAC= Fractional area change, GLS= Global longitudinal strain, TAPSE= Tricuspid annular plane systolic excursion, PLS= Peak longitudinal strain, PACS= Peak active contraction strain, PSSR= Peak systolic strain rate, PEDSR= Peak diastolic strain rate, PCSR= Peak contraction strain rate.
Table 3B:
Baseline Echocardiographic Variables for the Study Population of PAH Patients - PACS stratification
| Echo Parameter | PACS≤13 [N=34] |
PACS>13 [N= 29] |
Total [N= 63] |
P-value |
|---|---|---|---|---|
| LV Ejection Fraction (%) | 0.690 | |||
| N | 32 | 26 | 58 | |
| Mean (S.D.) | 60.80 (6.50) | 61.26 (7.64) | 61.01 (6.98) | |
| Median (Q1, Q3) | 60.32 (55.59, 67.21) | 60.63 (56.50, 68.73) | 60.32 (56.23, 67.27) | |
| (Min., Max.) | (50.32, 74.23) | (44.95, 74.59) | (44.95, 74.59) | |
| Tricuspid Valve Regurgitation | 0.807 | |||
| Trivial | 13/34 (38.2%) | 12/29 (41.4%) | 25/63 (39.7%) | |
| Mild | 9/34 (26.5%) | 10/29 (34.5%) | 19/63 (30.2%) | |
| Moderate | 8/34 (23.5%) | 5/29 (17.2%) | 13/63 (20.6%) | |
| Severe | 4/34 (11.8%) | 2/29 (6.9%) | 6/63 (9.5%) | |
| RA Area (cm2) | 0.159 | |||
| N | 34 | 29 | 63 | |
| Mean (S.D.) | 22.0 (7.15) | 19.3 (4.20) | 20.8 (6.08) | |
| Median (Q1, Q3) | 21.0 (15.7, 26.7) | 18.6 (16.7, 21.1) | 20.2 (16.3, 23.7) | |
| (Min., Max.) | (11.5, 40.7) | (12.9, 30.3) | (11.5, 40.7) | |
| RA Volume (ml) | 0.233 | |||
| N | 33 | 29 | 62 | |
| Mean (S.D.) | 73.8 (38.37) | 59.9 (20.28) | 67.3 (31.79) | |
| Median (Q1, Q3) | 63.0 (43.4, 100.1) | 58.2 (45.5, 68.5) | 61.6 (43.4, 74.7) | |
| (Min., Max.) | (26.2, 176.3) | (31.5, 110.6) | (26.2, 176.3) | |
| RV FAC (%) | 0.708 | |||
| N | 33 | 29 | 62 | |
| Mean (S.D.) | 31.9 (8.39) | 32.9 (8.54) | 32.4 (8.40) | |
| Median (Q1, Q3) | 30.1 (25.7, 38.8) | 33.2 (26.3, 39.1) | 32.5 (25.8, 39.1) | |
| (Min., Max.) | (18.3, 46.5) | (17.5, 50.4) | (17.5, 50.4) | |
| RV GLS (%) | 0.151 | |||
| N | 32 | 29 | 61 | |
| Mean (S.D.) | −17.7 (5.72) | −19.2 (4.59) | −18.4 (5.23) | |
| Median (Q1, Q3) | −18.0 (−20.1, −13.6) | −18.7 (−21.7, −16.1) | −18.3 (−21.2, −14.2) | |
| (Min., Max.) | (−33.1, −7.8) | (−31.5, −8.9) | (−33.1, −7.8) | |
| LV GLS (%) | 0.372 | |||
| N | 24 | 26 | 50 | |
| Mean (S.D.) | −19.1 (2.95) | −18.3 (3.46) | −18.7 (3.22) | |
| Median (Q1, Q3) | −18.8 (−21.9, −16.8) | −18.4 (−21.0, −15.9) | −18.5 (−21.5, −16.2) | |
| (Min., Max.) | (−25.5, −14.6) | (−25.8, −11.2) | (−25.8, −11.2) | |
| TAPSE (cm) | 0.003 | |||
| N | 34 | 28 | 62 | |
| Mean (S.D.) | 1.8 (0.43) | 2.1 (0.45) | 1.9 (0.47) | |
| Median (Q1, Q3) | 1.7 (1.6, 2.0) | 2.2 (1.8, 2.4) | 1.9 (1.6, 2.2) | |
| (Min., Max.) | (0.9, 2.8) | (1.0, 2.8) | (0.9, 2.8) | |
| PLS | <.001 | |||
| N | 34 | 29 | 63 | |
| Mean (S.D.) | 19.74 (9.67) | 32.31 (9.44) | 25.53 (11.40) | |
| Median (Q1, Q3) | 20.50 (15.00, 25.00) | 30.10 (25.20, 40.10) | 25.00 (18.50, 34.00) | |
| (Min., Max.) | (0.02, 38.00) | (15.00, 52.00) | (0.02, 52.00) | |
| Time to PLS | 0.897 | |||
| N | 32 | 29 | 61 | |
| Mean (S.D.) | 393.16 (86.93) | 398.62 (87.45) | 395.75 (86.49) | |
| Median (Q1, Q3) | 395.00 (351.50, 430.00) | 390.00 (347.00, 431.00) | 395.00 (350.00, 430.00) | |
| (Min., Max.) | (200.00, 567.00) | (229.00, 620.00) | (200.00, 620.00) | |
| PACS | <0.001 | |||
| N | 34 | 29 | 63 | |
| Mean (S.D.) | 8.93 (3.25) | 19.43 (5.29) | 13.77 (6.79) | |
| Median (Q1, Q3) | 10.00 (8.10, 11.00) | 17.00 (15.30, 24.00) | 12.50 (9.70, 16.30) | |
| (Min., Max.) | (0.01, 13.00) | (13.50, 31.00) | (0.01, 31.00) | |
| Time to PACS | 0.559 | |||
| N | 30 | 29 | 59 | |
| Mean (S.D.) | 720.77 (184.78) | 693.62 (170.55) | 707.42 (176.92) | |
| Median (Q1, Q3) | 715.00 (605.00, 827.00) | 660.00 (596.00, 774.00) | 675.00 (596.00, 783.00) | |
| (Min., Max.) | (446.00, 1334.0) | (478.00, 1333.0) | (446.00, 1334.0) | |
| PSSR | 0.109 | |||
| N | 32 | 29 | 61 | |
| Mean (S.D.) | 1.11 (0.31) | 1.29 (0.37) | 1.20 (0.35) | |
| Median (Q1, Q3) | 1.10 (0.97, 1.30) | 1.20 (1.00, 1.50) | 1.10 (1.00, 1.40) | |
| (Min., Max.) | (0.40, 1.70) | (0.70, 2.10) | (0.40, 2.10) | |
| Time to PSSR | 0.539 | |||
| N | 29 | 29 | 58 | |
| Mean (S.D.) | 182.72 (63.30) | 170.93 (49.07) | 176.83 (56.45) | |
| Median (Q1, Q3) | 177.00 (150.00, 200.00) | 169.00 (145.00, 193.00) | 174.50 (145.00, 200.00) | |
| (Min., Max.) | (82.00, 400.00) | (76.00, 298.00) | (76.00, 400.00) | |
| PEDSR | 0.191 | |||
| N | 27 | 22 | 49 | |
| Mean (S.D.) | 0.54 (0.39) | 0.66 (0.41) | 0.59 (0.40) | |
| Median (Q1, Q3) | 0.41 (0.33, 0.65) | 0.60 (0.34, 0.86) | 0.49 (0.34, 0.80) | |
| (Min., Max.) | (0.06, 1.70) | (0.04, 1.78) | (0.04, 1.78) | |
| Time to PEDSR | 0.724 | |||
| N | 18 | 22 | 40 | |
| Mean (S.D.) | 746.22 (217.55) | 701.27 (127.72) | 721.50 (172.99) | |
| Median (Q1, Q3) | 717.50 (654.00, 872.00) | 696.50 (597.00, 769.00) | 696.50 (599.50, 773.00) | |
| (Min., Max.) | (471.00, 1379.0) | (500.00, 1058.0) | (471.00, 1379.0) | |
| PCSR | <0.001 | |||
| N | 22 | 22 | 44 | |
| Mean (S.D.) | 0.90 (0.42) | 1.64 (0.58) | 1.27 (0.63) | |
| Median (Q1, Q3) | 0.78 (0.58, 1.20) | 1.60 (1.27, 2.00) | 1.21 (0.74, 1.70) | |
| (Min., Max.) | (0.38, 2.00) | (0.52, 2.80) | (0.38, 2.80) | |
| Time to PCSR | 0.318 | |||
| N | 23 | 22 | 45 | |
| Mean (S.D.) | 517.83 (118.09) | 488.86 (106.28) | 503.67 (112.15) | |
| Median (Q1, Q3) | 550.00 (458.00, 602.00) | 496.50 (413.00, 564.00) | 533.00 (427.00, 581.00) | |
| (Min., Max.) | (277.00, 700.00) | (299.00, 700.00) | (277.00, 700.00) |
LV= Left ventricle, RV= Right ventricle, FAC= Fractional area change, GLS= Global longitudinal strain, TAPSE= Tricuspid annular plane systolic excursion, PLS= Peak longitudinal strain, PACS= Peak active contraction strain, PSSR= Peak systolic strain rate, PEDSR= Peak diastolic strain rate, PCSR= Peak contraction strain rate. Note: The median value of PACS is 12.5, (which is not technically the median, but a rounded up version of the median). There is 1 patient with 12.5, 1 patient with 12.8, and 1 patient with 13 for PACS.
RA reservoir function
Compared with study subjects with higher PLS values, those with PLS values ≤ 25% had a higher proportion of females (97% vs 83%), however this difference was not statistically significant. Study subjects with lower PLS also had worse global and regional RV function as assessed by GLS and TAPSE (both p<0.001). Differences with other echocardiographic variables were observed. Stratified PLS results are provided in Table 3A.
RA active contraction function
Compared with subjects with higher PACS values, those with PACS values ≤ 13 were more likely to be female (p=0.007), had significantly lower TAPSE (p = 0.003) than study subjects with higher PACS. Differences with other echocardiographic parameters were observed. Stratified PACS results are provided in Table 3B.
RA reservoir, conduit and active contraction functions and outcomes
During the follow-up period (mean: 36, median: 44, range: 1–58 months); a two thirds of the cohort (n=39, 62%) were hospitalized or had died. A total of 24 patients expired, 15 of these were preceded with a hospitalization. Prior to implementation of the unadjusted and adjusted Cox proportional hazards modeling, tests for model linearity in the RA function variables revealed a violation only for PACS. PLS, PSSR, PEDSR and PCSR all satisfied the Cox model assumption of linearity. PACS truncated at a maximum value of 13 was shown to satisfy the linearity assumption and this transformation was used in subsequent modeling results. No significant change in risk was observed for PACS values increasing above 13. On univariate analyses both, PLS (p=0.0003) and PACS (p=0.0241) were associated with increased risk of subsequent events. PEDSR was also significant (p=0.0018). A 35% increase in risk was associated with a 5 unit drop in PLS (HR = 1.351; 95% CI = 1.141, 1.600). A 13% increase in risk was associated with a 1 unit drop in the transformed PACS variable (HR = 1.127; 95% CI = 1.024, 1.241). The risk associated with PACS values > 13 was the same as values of 13. In the subset of patients with data for PEDSR (n-=42), a significant association with increased risk was significant (HR for .1 decrease: 1.200; CI: 1.050, 1.371; p=−.0018. Time to PLS and PACS were also significant in the unadjusted models, with increased risk associated with lower values. Unadjusted Kaplan-Meier figures are provided, stratified at median values for PLS and PACS for further illustration of time to event results. PLS groups stratified at the median value were significantly different while PACS groups were not. (Figures 2A and 2B)
RA function variables were examined in the multivariable setting, adjusting for age, gender and RA area. In this setting, both PLS and PACS retained their significant association with time to death or rehospitalization, p=0.0005 and p=0.0167 respectively. In the subset with PEDSR data complete (n=42), PEDSR was significant, adjusted for age and gender. In the adjusted setting, an HR of 1.395 (CI: 1.158, 1.681) was observed for PLS (HR for 5-unit decrease) and a HR of 1.135 (1.023, 1.260) for PACS (1-unit decrease). The adjusted HR for PEDSR was 1.210 (CI: 1.058, 1.384), reflecting the change in risk for a .1 unit drop in PEDSR.
Discussion
In this prospective observational study, we found that RA dysfunction, as assessed by RA reservoir, conduit and active contraction, is an independent predictor of mortality and hospitalizations in PH. Both active contraction (PACS) and passive conduit function (PEDSR) were associated with outcomes. The early part of ventricular diastole is when passive filling of the ventricle occurs, dependent on RV relaxation properties11 without active energy expenditure by the atria.6 PH can increase RV diastolic pressure and thus create an unfavorable RA-RV pressure gradient. Changes in these parameters may be seen or perhaps prognostic in early stages of PH where RV diastolic dysfunction may precede systolic dysfunction. In late diastole, the RA behaves like a pump: RA pressure rises due to active atrial contraction, coinciding with the p wave on the EKG, and pushes the blood through the tricuspid valve toward the RV.6 In early disease stages the RA active contraction increases, likely as a compensatory mechanism for the reduced reservoir and passive conduit function.12As RV dysfunction progresses because of RV pressure overload, the preload reserve responds by the Frank-Starling mechanism and the RA compensates for ventricular dysfunction to maintain cardiac output by augmentation of RA active contraction function. However, when RV pressure overload progresses further and the preload reserve reaches its limit, RA compensation for the increased RV afterload is lost, leading to a decrease of cardiac output with the onset of severe RV failure and rapid deterioration until death occurs.13
We evaluated RA reservoir function using both LS and SR, and we showed that study subjects with lower PLS values had significantly lower event-free survival than those with higher values. The RA serves as a reservoir for systemic venous return when the tricuspid valve is closed, reflecting diastolic dysfunction of the RV.6 In patients with RV hypertrophy, such as in PH, the forceful RV contraction leads to an increased downward displacement of the tricuspid valve annulus, thereby increasing atrial filling as a compensatory mechanism in early disease stages; however due to chronic elevation in RV pressure, the RA wall is often stretched. Our study supports an important role of RA reservoir function in PAH, similar to that observed in other disease states, Padeletti et al. demonstrated that RA PLS significantly correlated with pulmonary pressure, they also showed that RA PLS could predict PH in patients with HF.14 Gaynor et al. reported that chronic RV pressure overload did not change RV systolic function, but deteriorated RV diastolic function, whereas the RA became more distensible, which contributed to maintain RV filling of the stiffened ventricle.15 This compensatory adaptation of the RA would postpone clinical failure in patients with chronic PH.15 Recently, a retrospective study of 37 patients with pulmonary arterial hypertension, found that reduced RA STE was associated with abnormal hemodynamics and adverse clinical outcomes analyses.16 The present prospective study establishes the prognostic utility of RA function in this patient group. Future studies are indicated to investigate longitudinal, serial measurements of RA strain on disease-specific therapy, and determine whether improved RA function contributes to meaningful clinical recovery.
Strengths and limitations
The strengths of the study include the prospective and standardized recruitment of PH study subjects, the number of subjects included in the final analysis (with high feasibility of STE) and the assessment of reproducibility. This study also used a dedicated RA oriented vendor-independent module. In addition, this is one of the first studies on RA function with outcomes independent of clinical variables and RA size in study subjects with PH. Limitations include that the results are from a single-center study. Another limitation is that the software package measures strain and strain rate at the endocardium with a lack of normative values to compare to previous studies using LV strain packages. Due to this lack of normative values, we chose a threshold for comparison of strain values as the median of the observed RA strains in this population. Also, a single-plane algorithm underestimates RA and RV volumes compared to 3D TTE. Lastly, the possibility of a Type I error exists when performing a large number of statistical tests on a small number of subjects.
Conclusions
We found that evaluating RA function with strain was feasible and that RA dysfunction was associated with lower event-free survival in in a cohort of patients with PAH. RA reservoir, conduit and active contraction functions were independent predictors of mortality and hospitalizations in PAH. Given these findings, RA STE may be a useful tool to risk-stratify patients with PAH.
Supplementary Material
Acknowledgments
Sources of Funding: This study was funded by a research grant from the American Society of Echocardiography (ZS & DR). This research was supported by NIH training grant # 5T32HL069749–13 (A. Mandawat).
Footnotes
Disclosures: None
References:
- 1.Cioffi G, de Simone G, Mureddu G, Tarantini L, Stefenelli C. Right atrial size and function in patients with pulmonary hypertension associated with disorders of respiratory system or hypoxemia. Eur J Echocardiogr. 2007;8:322–331. [DOI] [PubMed] [Google Scholar]
- 2.Do DH, Therrien J, Marelli A, Martucci G, Afilalo J, Sebag IA. Right atrial size relates to right ventricular end-diastolic pressure in an adult population with congenital heart disease. Echocardiography. 2011;28:109–116. [DOI] [PubMed] [Google Scholar]
- 3.D’Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Kernis JT, Levy PS, Pietra GG, Reid LM, Reeves JT, Rich S, Vreim CE, Williams GW, Wu M. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991;115:343–9. [DOI] [PubMed] [Google Scholar]
- 4.Raymond RJ, Hinderliter AL, Willis PW, Ralph D, Caldwell EJ, Williams W, Ettinger NA, Hill NS, Summer WR, de Boisblanc B, Schwartz T, Koch G, Clayton LM, Jobsis MM, Crow JW, Long W. Echocardiographic predictors of adverse outcomes in primary pulmonary hypertension. J Am Coll Cardiol. 2002;39:1214–1219. [DOI] [PubMed] [Google Scholar]
- 5.Bustamante-Labarta M, Perrone S, De La Fuente RL, Stutzbach P, De La Hoz RP, Torino A, Favaloro R. Right atrial size and tricuspid regurgitation severity predict mortality or transplantation in primary pulmonary hypertension. J Am Soc Echocardiogr. 2002;15:1160–1164. [DOI] [PubMed] [Google Scholar]
- 6.Blume GG, McLeod CJ, Barnes ME, Seward JB, Pellikka PA, Bastiansen PM, Tsang TS. Left atrial function: physiology, assessment, and clinical implications. Eur J Echocardiogr. 2011;12:421–430. [DOI] [PubMed] [Google Scholar]
- 7.Matsuda Y, Toma Y, Ogawa H, Matsuzaki M, Katayama K, Fujii T, Yoshino F, Moritani K, Kumada T, Kusukawa R. Importance of left atrial function in patients with myocardial infarction. Circulation. 1983;67:566–571. [DOI] [PubMed] [Google Scholar]
- 8.Padeletti M, Cameli M, Lisi M, Malandrino A, Zaca V, Mondillo S. Reference values of right atrial longitudinal strain imaging by two-dimensional speckle tracking. Echocardiography. 2012;29:147–52. [DOI] [PubMed] [Google Scholar]
- 9.Querejeta Roca G, Campbell P, Claggett B, Solomon SD, Shah AM. Right Atrial Function in Pulmonary Arterial Hypertension. Circ Cardiovasc Imaging. 2015;8:e003521; discussion e003521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713; quiz 786–8. [DOI] [PubMed] [Google Scholar]
- 11.Gaynor SL, Maniar HS, Prasad SM, Steendijk P, Moon MR. Reservoir and conduit function of right atrium: impact on right ventricular filling and cardiac output. Am J Physiol Heart Circ Physiol. 2005;288:H2140–H2145. [DOI] [PubMed] [Google Scholar]
- 12.Willens HJ, Fertel DP, Qin J, Labrador E, Lowery MH. Effects of age and pulmonary arterial hypertension on the different phases of right atrial function. Int J Cardiovasc Imaging. 2008;24:703–710. [DOI] [PubMed] [Google Scholar]
- 13.Bristow MR, Zisman LS, Lowes BD, Abraham WT, Badesch DB, Groves BM, Voelkel NF, Lynch DM, Quaife RA. The pressure-overloaded right ventricle in pulmonary hypertension. Chest. 1998;114:101S–106S. [DOI] [PubMed] [Google Scholar]
- 14.Padeletti M, Cameli M, Lisi M, Zaca V, Tsioulpas C, Bernazzali S, Maccherini M, Mondillo S. Right atrial speckle tracking analysis as a novel noninvasive method for pulmonary hemodynamics assessment in patients with chronic systolic heart failure. Echocardiography. 2011;28:658–664. [DOI] [PubMed] [Google Scholar]
- 15.Gaynor SL, Maniar HS, Bloch JB, Steendijk P, Moon MR. Right atrial and ventricular adaptation to chronic right ventricular pressure overload. Circulation. 2005;112:I212–I218. [DOI] [PubMed] [Google Scholar]
- 16.Bhave NM, Visovatti SH, Kulick B, Kolias TJ, McLaughlin VV. Right atrial strain is predictive of clinical outcomes and invasive hemodynamic data in group 1 pulmonary arterial hypertension. Int J Cardiovasc Imaging. 2017;33:847–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


