Abstract
Purpose:
Sleep following training can enhance motor skill memory consolidation while chronic sleep disruption can have the converse effect. The aim of this investigation was to explore the relationship between sleep measured by wrist actigraphy, motor skill consolidation and primary motor cortex excitability in young, healthy individuals.
Methods:
Training was a visuospatial finger-tracking task. Dependent measures included tracking skill performance, cortical excitability, measures of sleep, and level of arousal. Assessments occurred pre-training, post-training and at 12 h and 24 h retention. An activity monitor was worn on the wrist during the nights preceding and following training.
Results:
Results indicate that sleep during the night following training was predictive of 1) offline skill consolidation following training (R2 = 0.34) and 2) cortical excitability at 24 h follow-up (R2 = 0.35) with less time spent awake associated with better skill performance and lower cortical excitability at 24 h follow-up. No difference in measures of sleep was observed between nights of sleep (p > 0.05). Sleep the night before training did not influence skill performance, skill acquisition during training, nor measures of cortical excitability at pre-training assessment.
Conclusions:
These findings suggest a relationship between motor skill development, cortical excitability and sleep following training. These results invite further investigation into the utility of actigraphy as a low-cost, easy-to-administer alternative to polysomnography for short and long-term evaluation of the relationship between sleep, cortical excitability and motor skill learning in healthy and patient populations.
Keywords: Actigraphy, sleep, motor learning, cortical excitability, TMS, memory, consolidation, enhancement
1. Introduction
There is growing evidence that sleep is an important factor to consider in investigations of motor learning and performance in healthy individuals as well as impaired populations (Walker and Stickgold, 2006; Siengsukon and Boyd, 2009b). Underlying the process of motor learning is memory formation. Skill memories are initially acquired and encoded during exposure to a task. These newly acquired memories then undergo a process of consolidation where the encoded memory becomes resistant to interference from competing information and skill performance level is retained at follow-up testing (Brashers-Krug et al., 1996; Karni et al., 1998). Consolidation of certain skills or task information may be dependent on sleep in healthy individuals (Walker et al., 2003; Robertson et al., 2004; Cohen and Robertson, 2007) and patients with chronic stroke (Siengsukon and Boyd, 2009a; Siengsukon and Boyd, 2009c). After visuomotor tracking skill training, performance can also be enhanced evidenced by improved skill performance without further training exposure (Borich et al., 2011). But, it is unclear if sleep following tracking training is associated with this type of motor skill consolidation.
It is possible to evaluate sleep on multiple levels, from subjective report to sophisticated objective measurement. Standardized subjective methods have been widely administered as valid and reliable identifiers of certain sleep disorders and are also used to measure response to intervention (Buysse et al., 2008). These instruments are easy to administer and provide quantitative data regarding sleep-wake function. However, these data are not reliably related to objective measures of sleep (Buysse et al., 2008). The gold standard for the measurement of sleep is polysomnography (PSG) (Kushida et al., 2005). Although PSG provides excellent direct electrophysiological assessment of sleep, it is expensive, time-consuming, labor intensive and difficult to evaluate long-term sleep function in natural environments (Zollman et al., 2010). It also is often impractical to conduct PSG in research investigations or use it as a clinical screening tool (Buysse et al., 2008).
A convenient, low-cost, easy to administer alternative to PSG for sleep measurement is actigraphy (Acebo and LeBourgeois, 2006). This methodology involves affixing an activity monitor, an actigraph, to the extremity or trunk of an individual. The monitor uses an accelerometer to measure movement over time that can be transduced into a digital representation that is subjected to a computer-based algorithm to determine sleep-wake activity (Acebo and LeBourgeois, 2006). This approach to assessing sleep-wake function is inherently limited by the indirect nature of the measurement but can provide useful data to allow for considered inferences of sleep quantity and quality (Morgenthaler et al., 2007).
Actigraphy has been shown to be strongly correlated with PSG in normal healthy adults (Acebo and LeBourgeois, 2006; Weiss et al., 2010) and is considered a valid method to assist in determining sleep patterns in normal individuals and certain sleep disorder populations (Morgenthaler et al., 2007). Advances in technology, increased research applications, and uniform study guidelines have made actigraphy a viable alternative to PSG to objectively quantify parameters of sleep patterning including time awake, time asleep, sleep efficiency and number of awakenings.
Currently, there is a paucity of research evaluating the relationship between sleep measured across multiple nights and motor skill development. Typically, a sleep diary is the only assessment of sleep in studies involving learning and PSG across multiple nights is rare secondary to cost and logistical concerns associated with repeated administrations. It is also not possible to objectively quantify the relationship between sleep and learning using subjective sleep diary in isolation. Actigraphy offers improved quantification of sleep parameters versus subjective report and greater feasibility compared to PSG in certain research applications. Pairing it with non-invasive techniques to probe neural activity may also augment the utility of actigraphy.
One such technique is transcranial magnetic stimulation (TMS). Used in isolation, cortical excitability can be inferred through measurement of peripheral muscle responses to TMS non-invasively applied to the contralateral primary motor cortex (M1) (Barker, 1999). Excitability of M1 has been shown to be transiently modulated by various types of motor skill training (Ziemann et al., 2001; Perez et al., 2004; Gallasch et al., 2009; Borich et al., 2011) and these changes have been shown to be predictive of offline skill development across a twenty-four epoch including a sleep episode following training (Borich et al., 2011). A limitation to these studies is that sleep is often not objectively measured (Walker et al., 2003; Robertson et al., 2004; Cohen and Robertson, 2007), therefore the contributions of sleep to changes in motor skill in response to training and associated modulation of cortical excitability remain unknown. Additionally, the association between actigraphic sleep quantification and cortical excitability has not been previously investigated. The primary purpose of this study was to investigate the relationships between sleep measured by wrist actigraphy, visuospatial motor skill learning and measures of cortical excitability in response to one training episode in young, healthy individuals.
2. Materials and methods
2.1. Experimental design
A single-blind design was employed whereby subjects (n = 20, mean age ± SD: 23.2 ± 3.9y, 10F) participated in three visits, each separated by twelve hours. Recruitment was conducted with University and local community advertisement. Subjects were screened for potential sleep disorders and seizure history involving self or first-degree relative prior to inclusion. Right-handedness was confirmed by the Edinburgh Handedness Inventory (Oldfield, 1971). Each subject was naïve to the motor skill tracking apparatus. All subjects gave written and informed consent according to the Declaration of Helsinki prior to the first visit.
The first visit occurred in the morning (8 am ± 1 h) where skill performance and cortical excitability were assessed before and after the skill training session. After a 12 h interval consisting of normal waking activity, each subject returned for follow-up assessment of skill and cortical excitability without further training. After another 12 h interval containing sleep, subjects returned for a second follow-up skill assessment, 24 h after t raining (Fig. 1A). During the 12 h wake interval, subjects were instructed to refrain from napping. During the 12 h interval containing sleep, subjects were instructed to refrain from alcohol intake or stimulant use to avoid changes in sleep quality and/or architecture associated with altered memory consolidation.
Fig. 1.
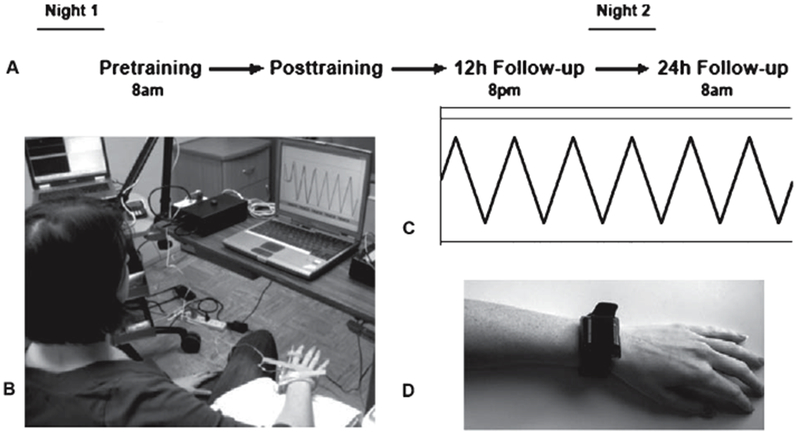
A) Schematic of experimental design. B) Finger-tracking apparatus. C) Target waveform used during finger tracking performance assessments. D) Tri-axial activity monitor attached to the wrist.
2.2. Visuospatial motor skill assessment
The behavioral task employed has been described in detail elsewhere (Carey et al., 1994). Briefly, subjects were required to continuously track a visual target using flexion/extension movements of their right index finger (Fig. 1B). During each finger-tracking assessment, five 10 s trials were presented consecutively. Target pattern (Fig. 1C) and subject response were displayed throughout each trial. Accuracy was quantified by root-mean square error (RMSE) indexed (AI) to participants’ range of motion expressed as a percentage (Carey et al., 1994). The training protocol, which consisted of 105 trials separated into four (5 min) training blocks with 1 min rest periods between each block, began immediately following completion of the first finger-tracking assessment. A host of training protocols, created by varying tracking conditions and target parameters, were used to encourage skill acquisition. The assessment waveform was not presented during training. The post-training assessment was completed directly following the end of the training protocol.
2.3. Cortical excitability assessment
To measure levels of excitability thresholds for the target muscle, right first dorsal interosseus (FDI), were determined using single pulse TMS targeted to the primary motor cortex (M1) contralateral to the test muscle. Resting (RMT) and active (AMT) motor threshold were determined to probe neuronal membrane activity, primarily of sodium-gated membrane channels (Ziemann et al., 1996). Silver/silver chloride disposable electrodes were affixed in a belly-tendon montage of the right FDI and electromyographic signals were collected for TMS-elicited motor evoked potentials (MEPs) using a Cadwell Sierra amplifier (Cadwell Laboratory, Washington) (sampling rate: 2560 kHz, sensitivity: 100 μv/div, filter: 20–2000 Hz). To find the optimal position for eliciting MEPs from FDI, a 70-mm figure-eight TMS coil connected to a Magstim rapid magnetic stimulator (Magstim Co Ltd. Dyfed, UK) was used. The coil was held tangentially to the skull with the handle directed 45° posterolateral to the midsagittal line of the skull over the approximate area of Ml inducing a posterior-anterior current flow in the brain (Sakai et al., 1997).
To determine RMT, the arm was placed in a relaxed, comfortable position to minimize muscle activation in the limb. Single-pulse magnetic stimuli were delivered manually to the scalp location overlying the presupposed Ml locus of FDI representation at approximately 0.1 Hz starting at an intensity of 50% of the maximum stimulator output (MSO). Intensity level was adjusted systematically until the RMT was found, defined as the minimum intensity required to elicit MEPs >50 μv peak-to-peak in at least 5 of 10 trials of FDI at rest (Rossini et al., 1999). For AMT determination, subjects were asked to actively abduct the second digit (5–10% of maximum voluntary contraction of FDI) (Rosler et al., 2002) against a strain gauge coupled to a load cell. For AMT, MEPs elicited had to exceed 200μV peak-to-peak in at least 5 of 10 trials (Rossini et al., 1999). Thresholds were represented as percentage of MSO (%MSO). This threshold identification procedure was performed in the same manner at all four assessments.
2.4. Actigraphic assessment of sleep
To quantify sleep, subjects were given an actigraphy monitor the day before the initial visit (ActiSleep sleep monitor, ActiGraph™, Pensacola, FL). Subjects were instructed to wear the monitor on their dominant wrist during time in bed the night preceding training and the night following training to objectively measure parameters of sleep (time awake, time asleep, sleep efficiency, number of awakenings and sleep latency) (Table 1) (Morgenthaler et al., 2007). Movement data was collected, summated over 60 s epochs and converted to digital activity counts at 60 Hz. Sleep scoring was conducted using the ActiLife software package (v. 4.4.1, ActiGraph™, Pensacola, FL). An adapted scoring algorithm was used to determine the sleep state for each recorded epoch across a sliding 11 min window (Sadeh et al., 1994). Sleep diary entry was used to determine time into and out of bed.
Table 1.
Actigraphic measurements of sleep quality
| Measure | Description | Night 1 (mean ± SD) | Night 2 (mean ± SD) | Night 2 − Night 1 (mean ± SD) | t-score | p-value |
|---|---|---|---|---|---|---|
| Time asleep (min) | Time between sleep onset and final awake time | 350.73 ± 78.19 | 358.2 ± 75.29 | 7.48 ± 69.94 | −0.453 | 0.656 |
| Sleep latency (min) | Time between time into be and sleep onset | 3.89 ± 5.50 | 3.55 ± 6.78 | −0.33 ± 4.70 | 0.301 | 0.767 |
| Awakenings (#) | Number of separate epochs awake | 17.89 ± 8.47 | 17.83 ± 7.85 | −0.06 ± 8.02 | 0.029 | 0.977 |
| Time awake (min) | Total time awake during the sleep interval | 62.44 ± 28.75 | 64.39 ± 47.46 | 1.94 ± 49.15 | −0.168 | 0.869 |
| Average time awake (min) | Time awake/awakenings | 4.07 ± 2.98 | 4.68 ± 4.95 | 0.61 ± 5.37 | −0.482 | 0.636 |
| Sleep efficiency (%) | Time “asleep’/total time in bed*100 | 84.41 ± 7.59 | 84.72 ± 11.67 | 0.31 ± 11.37 | −0.115 | 0.909 |
2.5. Level of arousal and sleep characteristics
Level of arousal at each assessment was evaluated using the Stanford Sleepiness Scale (SSS) (Hoddes et al., 1973). The Pittsburgh Sleep Quality Index (PSQI) (Buysse et al., 1989) and Epworth Sleepiness Scale (ESS) (Johns, 1991) were administered at the beginning of the first visit to quantify individual sleep characteristics and daytime sleepiness respectively.
2.6. Statistical analysis
Data processing and quantification of all dependent measures were conducted by an investigator blinded to assessment. Normality and heterogeneity of variance were assessed for each dependent measure. Paired t-tests were conducted for each measure of sleep between nights of sleep and tracking accuracy change from pre-training to post-training assessment. A one-sample t-test was then applied to change scores across 24 h post-training interval to evaluate offline skill development. One-way ANOVAs (assessment) were conducted for TMS thresholds (RMT, AMT) and SSS data. Two-tailed post-hoc pairwise comparisons with Bonferroni correction for multiple comparisons (adjusted p-value<0.05) were conducted for significant results.
Hierarchical multiple regression analyses were conducted to evaluate the predictive capacity of sleep parameters on tracking performance and change in performance from post-training to each follow-up assessment. Additionally, the association between TMS thresholds and preceding night sleep was evaluated in the same manner. For these analyses, the preceding night of sleep was used in the model. For example, at 12 h follow-up, actigraphy data from Night 1 was used; while at 24 h follow-up, data from Night 2 was evaluated. For each regression analysis, correlations between predictors and dependent measures were also measured. Durbin-Watson diagnostics were provided to ensure independence of the residuals from the regression analysis. Casewise diagnostics were used to determine extreme outliers to exclude from the model (>2SD). The final model for each analysis was based on overall significance of each model (p <0.05) and the significance level of the R2 change when adding a new predictor to the model.
3. Results
The primary results indicated a significant association between time awake the night after training with (1) offline motor skill memory consolidation and (2) M1 excitability at 24 h retention assessment.
3.1. Visuospatial motor skill
Significant skill improvement was observed immediately following training (mean AI change ± SD: 9.2 ± 10.4%, t(19) = 3.97, p = 0.001). Motor skill consolidation, measured bychanges in tracking performance from post-training to 24 h retention assessment, was found to be significant (mean AI change ± SD: 3.0 ± 5.6%, t(18) = 2.326, p = 0.032) (Fig. 2A). Time awake the night following training was a significant predictor of this skill consolidation after training with less time awake associated with greater change in skill performance (R2 = 0.336, β = −0.580, p <0.012) (Fig. 2B). Sleep the night before training was not significantly correlated with AI at pre-training assessment (Table 2). At 12 h follow-up, a trend for time awake and time asleep as significant predictors of skill performance (R2 = 0.300, βs = −0.547, −0.470, p = 0.059) but not change in level of skill during the first 12 h interval was observed.
Fig. 2.
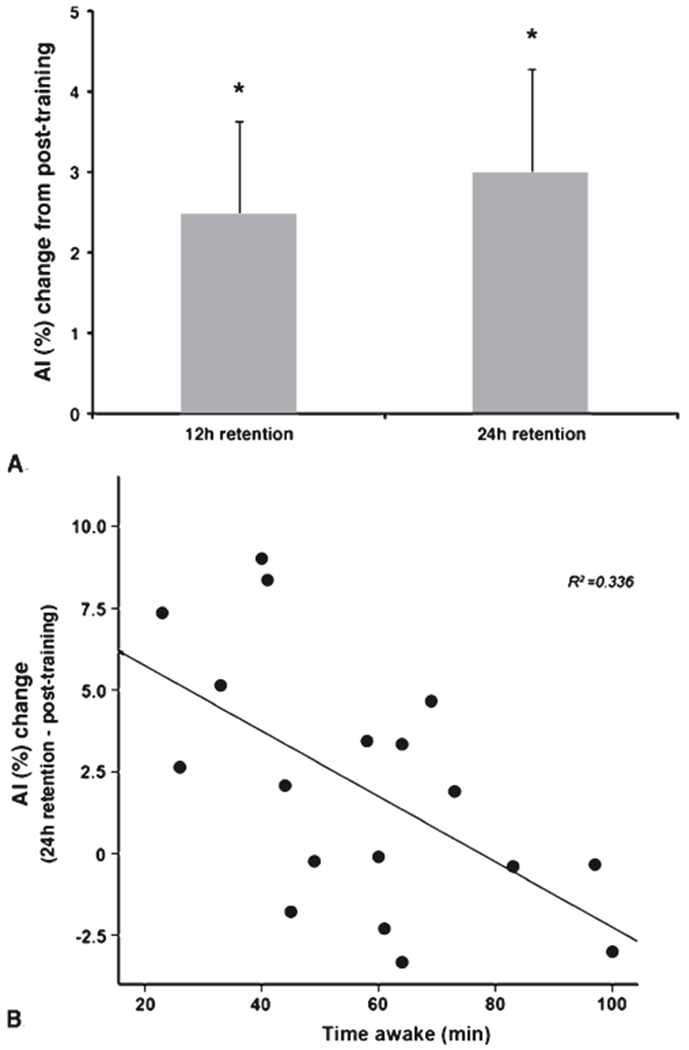
A) Offline skill development after training. Without further training exposure, accuracy index (AI) values, representing level of skill, were increased at 12 h retention and (*p < 0.05) and remained stable for at least one day. B) Relationship between skill development after training and sleep the night following training. The amount of time spent awake during the night following training was associated with the degree of skill development during the 24 h epoch following skill training (p < 0.012). Less time awake was associated with greater skill development after training.
Table 2.
Correlation maxtrix between sleep the night before training, baseline skill performance and cortical excitability
| Al | rMT | aMT | Sleep efficiency | Time asleep | Awakenings | Time awake | Avg. time awake | Sleep latency | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Al | Pearson r | 1 | 0.317 | 0.186 | 0.234 | −0.143 | −0.363 | −0.382 | −0.108 | −0.061 |
| Sig. (2-tailed) | 0.185 | 0.447 | 0.335 | 0.548 | 0.126 | 0.107 | 0.66 | 0.803 | ||
| N | 20 | 19 | 19 | 19 | 20 | 19 | 19 | 19 | 19 | |
| RMT | Pearson r | 0.317 | 1 | 0.885** | −0.013 | −0.12 | 0.169 | 0 | −0.198 | −0.258 |
| Sig. (2-tailed) | 0.185 | 0 | 0.959 | 0.625 | 0.503 | 0.999 | 0.431 | 0.301 | ||
| N | 19 | 19 | 19 | 18 | 19 | 18 | 18 | 18 | 18 | |
| AMT | Pearson r | 0.186 | 0.885** | 1 | −0.018 | −0.01 | 0.137 | 0.032 | −0.152 | −0.262 |
| Sig. (2-tailed) | 0.447 | 0 | 0.945 | 0.968 | 0.587 | 0.9 | 0.546 | 0.295 | ||
| N | 19 | 19 | 19 | 18 | 19 | 18 | 18 | 18 | 18 |
AI: accuracy index, RMT: resting motor threshold, AMT: active motor threshold.
p <0.01.
3.2. Measures of cortical excitability
There were no differences in TMS thresholds across assessments (RMT: F(3,69) = 0.463, p = 0.709, AMT: F(3,69) = 0.022, p = 0.995) (Fig. 3A). Time awake during the second night of sleep was predictive of AMT at 24 h assessment (R2 = 0.347, β = 0.589, p<0.016) where greater time awake was associated with higher AMT values, indicating lower cortical excitability (Fig. 3B). Night 1 sleep was not correlated significantly with TMS thresholds at pre-training assessment (Table 2).
Fig. 3.
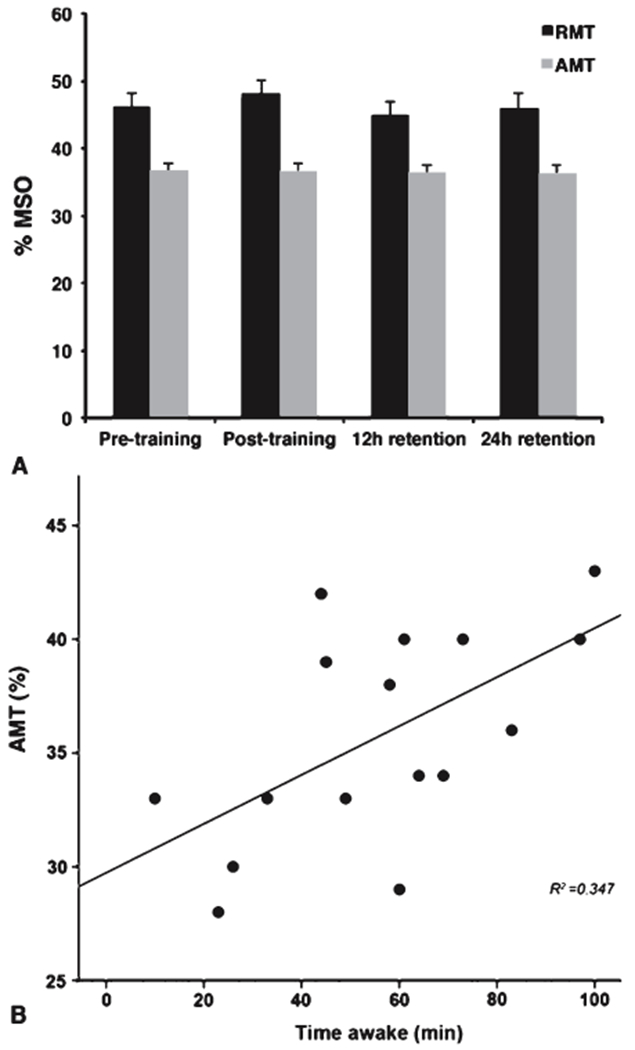
A) No changes in TMS-based motor thresholds, expressed as a percentage of maximum stimulator output (MSO), were observed between assessments. Both resting (RMT) and active (AMT) motor thresholds within the left primary motor cortex were consistent throughout assessments. B) Sleep during the night following motor skill training was associated with cortical excitability at 24 h followup assessment. Increasing time spent awake was associated with higher AMT values, indicating lower primary motor cortex excitability, the following morning (p = 0.016).
A significant association between skill performance and TMS thresholds was not demonstrated at any assessment. A trend for an association between skill performance changes from post-training to 24 h follow-up assessment with AMT at 24 h follow-up assessment (R2 = 0.222, β = −0.471, p < 0.076) was observed.
3.3. Level of arousal and sleep quality assessments
Level of arousal (SSS score) was not different across assessments (F(3,74) = 0.266, p = 0.850). Sleep parameters were significantly (p < 0.05) correlated to subjective measures of sleep quality, daytime sleepiness and level of arousal (PSQI, ESS, SSS) (Pearson’s r range: 0.230–0.462) (Table 3).
Table 3.
Correlation matrix for subjective measures of sleep quality, daytime sleepiness, level of arousal and actigraphic measures of sleep
| PSQI | ESS | SSS | Sleep efficiency | Time asleep | Awakenings | Time awake | Avg. time awake | Sleep latency | ||
|---|---|---|---|---|---|---|---|---|---|---|
| PSQI | Pearson r | 1 | 0.250* | 0.293** | −0.305** | −0.198 | −0.233* | 0.211 | 0.462** | −0.003 |
| Sig. (2-tailed) | 0.025 | 0.009 | 0.007 | 0.078 | 0.041 | 0.066 | <0.005 | 0.978 | ||
| N | 80 | 80 | 78 | 77 | 80 | 77 | 77 | 77 | 76 | |
| ESS | Pearson r | 0.250* | 1 | 0.273* | 0.108 | −0.287** | −0.369** | −0.228* | 0.156 | 0.004 |
| Sig. (2-tailed) | 0.025 | 0.015 | 0.35 | 0.01 | 0.001 | 0.046 | 0.176 | 0.973 | ||
| N | 80 | 80 | 78 | 77 | 80 | 77 | 77 | 77 | 76 | |
| SSS | Pearson r | 0.293** | 0.273* | 1 | 0 | −0.098 | −0.201 | −0.131 | 0.072 | 0.249* |
| Sig. (2-tailed) | 0.009 | 0.015 | 0.998 | 0.391 | 0.084 | 0.263 | 0.54 | 0.032 | ||
| N | 78 | 78 | 78 | 75 | 78 | 75 | 75 | 75 | 74 |
PSQI: Pittsburgh Sleep Quality Index, ESS: Epworth Sleepiness Scale, SSS: Stanford Sleepiness Scale.
p<0.01,
p<0.05.
4. Discussion
Sleep may uniquely benefit to motor skill memory formation following task exposure (Walker and Stickgold 2006; Siengsukon and Boyd 2009b). Our findings demonstrate that the level of offline memory consolidation observed one day after training was associated with amount of time spent awake following one training episode. Additionally, time awake was predictive of the level of M1 cortical excitability at retention testing. These preliminary findings are the first to suggest a relationship between motor skill memory consolidation, actigraphic measurement of sleep and cortical excitability in young, healthy individuals.
Here, actigraphic assessment of sleep did not demonstrate differences in sleep between the night before and the night after motor skill training. However, one measure of sleep, time awake, was shown to be predictive of level of skill development one day after training. Time spent awake during this night was also associated with levels of excitability that may be related to performance changes observed. These findings are supported by work using high-density EEG during sleep the night after skill training (Huber et al., 2004). Utilizing the high spatial resolution of this imaging technique, Huber and colleagues (Huber et al., 2004) demonstrated that overall sleep architecture does not change following motor skill training, rather, local changes in slow wave sleep activity during stage 3/4 sleep in the primary sensorimotor cortices contralateral to the trained hand were observed. The current results compliment these findings by demonstrating a significant predictive relationship between sleep quantified by actigraphy, TMS-evoked measures of excitability and motor skill learning.
Prior to training, actigraphic measures of sleep were not associated with novel finger-tracking performance. After a twelve-hour interval of waking activity following training, a trend for a relationship between sleep and tracking skill performance was observed. This positive relationship between sleep the night prior to training and tracking performance at 12 h follow-up assessment during the following evening may have been related to level of arousal and alertness since there was no observable relationship between this sleep epoch and the change in skill performance from post-training to follow-up. Yet, subjective level of arousal was not different at this time point. The research paradigm attempted to account for time of day effects in skill performance over time and the results suggest that these confounds were adequately controlled. These data demonstrate a beneficial link between sleep measured by actigraphy and subsequent finger tracking performance after training.
Although sleep was not significantly related to immediate offline skill improvements, it was predictive of the change in performance in tracking accuracy one day after training. These data may indicate performance improvements due to improved alertness and arousal levels secondary to improved sleep the previous night. This is unlikely, however, as measures of sleep were not significantly different between nights, nor was arousal level changed at 24 h follow-up assessment. Additionally, skill performance at this assessment was not significantly related to sleep; rather, the change in skill performance over the preceding 24 h interval was associated with sleep indicating that the relationship between sleep and motor skill consolidation is unlikely due to skill performance-related effects.
Although an expected relationship between sleep and motor skill consolidation was observed, interpretation is cautious due to the inherent limitations of sleep assessment using actigraphic sleep monitoring. Actigraphy lacks spatial specificity of cortical processes that occur during sleep (Acebo and LeBourgeois, 2006; Weiss et al., 2010). It also has poor resolution to measure changes in specific sleep stages. Therefore, the neural substrates for the current findings remain speculative.
Although the neural mechanisms underlying the relationship between skill development and sleep after training remain unknown, TMS-based evaluation of cortical excitability may provide insight into these mechanisms. Here, the greater time spent awake the night after training was associated with reduced cortical excitability indicated by increased AMT. Time awake the night before training was not, however, associated with motor thresholds and there was no difference in motor threshold between pre-training and 24 h retention assessment. Therefore, it is difficult from these data to interpret the relationship between actigraphic measurement of sleep and cortical excitability within skill learning paradigms. To clarify this potential relationship, future work should monitor sleep and cortical excitability for a greater number of nights both pre- and post-training. Additional measures assessing corticospinal (single-pulse MEP) and intra-cortical (paired-pulse TMS and cortical silent period) excitability should also be collected. Also experimental manipulations of sleep duration and fragmentation could be applied to further elucidate the relationships between sleep and cortical excitability within the context of motor skill learning.
Previous work has shown that experimentally-induced sleep deprivation can lead to reduced cortical excitability (Civardi et al., 2001; Manganotti et al., 2006). Also, in patients with chronic sleep disorders, increased thresholds and reduced intracortical inhibition have been observed (Oliviero et al., 2005; Joo et al., 2010; Nardone et al., 2010). In humans, sleep deprivation has been shown to reduce memory consolidation (Gais et al., 2006) and memory acquisition (Yoo et al., 2007) for declarative learning. It appears that memory consolidation is more susceptible to sleep fragmentation than memory acquisition for spatial learning in a rodent model of obstructive sleep apnea (OSA)(Ward et al., 2009). Also, deficits in memory consolidation and learning have been shown for multiple chronic sleep disorder patient populations (Cipolli et al., 2009; Kloepfer et al., 2009; Nissen et al., 2011). Here, we observed a non-significant trend between skill development and cortical excitability at 24 h retention. This test was found to be underpowered statistically therefore interpretation is limited. Although taken together, these findings may suggest that the relationship between sleep and skill development after one training episode is modulated by neural activity in the trained M1.
Although interpretation of the relationship between learning following training and sleep is guarded, these preliminary findings suggest that sleep the night following training may be an important factor to consider in the evaluation of motor skill development following skill training. This relationship may also be associated with cortical excitability of the trained M1. Previous work has established a relationship between PSG and actigraphy (Acebo and LeBourgeois, 2006; Morgenthaler et al., 2007) but definitive statements regarding how sleep after training influences or is associated with skill development will require experiments utilizing actigraphy in conjunction with PSG. These results also invite follow-up investigation using other types of motor skills to comprehensively evaluate the relationship between sleep and motor skill learning.
5. Conclusions
The conclusions drawn from this work provide a substantive foundation to further evaluate the relationship between actigraphic sleep quantification and the time course of motor skill learning in healthy individuals and individuals following neurologic insult. This line of inquiry will contribute to a comprehensive evaluation of the utility of actigraphy as a tool to help inform future rehabilitation paradigms by understanding the relationship between sleep and (re)learning during recovery from neurologic injury.
Acknowledgments
This study was supported by a Doctoral Dissertation Fellowship from the University of Minnesota Graduate School (M.B.). This publication was made possible by support from the National Center for Research Resources’ (NCRR) grant M01 RR00400, a component of the National Institutes of Health. Its contents are solely the responsibility of the authors, and do not necessarily represent the official views of NIH or NCRR.
Footnotes
Copyright of Restorative Neurology & Neuroscience is the property of IOS Press and its content may not be copied or emailed to multiple sites or posted to a listserv without the copyright holder’s express written permission. However, users may print, download, or email articles for individual use.
References
- Acebo C & LeBourgeois MK (2006). Actigraphy. Respir Care Clin N Am, 12, 23–30. [DOI] [PubMed] [Google Scholar]
- Barker AT (1999). The history and basic principles of magnetic nerve stimulation. Electroen Clin Neurophysiol – Suppl, 51, 3–21. [PubMed] [Google Scholar]
- Borich M, Furlong M, Holsman D & Kimberley TJ (2011). Goal-directed visuomotor skill learning: Off-line enhancement and the importance of the primary motor cortex. Restor Neurol Neurosci, 29, 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brashers-Krug T, Shadmehr R & Bizzi E (1996). Consolidation in human motor memory. Nature, 382, 252–255. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Hall ML & Strollo PJ, et al. (2008). Relationships between the Pittsburgh Sleep Quality Index (PSQI), Epworth Sleepiness Scale (ESS), and clinical/polysomnographic measures in a community sample. J Clin Sleep Med, 4, 563–571. [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR & Kupfer DJ (1989). The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiat Res, 28, 193–213. [DOI] [PubMed] [Google Scholar]
- Carey J, Bogard C, King B & Suman V (1994). Finger-movement tracking scores in healthy subjects. Percept Mot Skills, 79, 563–576. [DOI] [PubMed] [Google Scholar]
- Cipolli C, Campana G, Campi C, et al. (2009). Sleep and time course of consolidation of visual discrimination skills in patients with narcolepsy-cataplexy. J Sleep Res, 18, 209–220. [DOI] [PubMed] [Google Scholar]
- Civardi C, Boccagni C, Vicentini R, et al. (2001). Cortical excitability and sleep deprivation: Atranscranial magnetic stimulation study. J Neurol Neurosurg Psychiatry, 71, 809–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen DA & Robertson EM (2007). Motor sequence consolidation: Constrained by critical time windows or competing components. Exp Brain Res, 177, 440–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gais S, Lucas B & Born J (2006). Sleep after learning aids memory recall. Learn Mem, 13, 259–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallasch E, Christova M, Krenn M, Kossev A & Rafolt D (2009). Changes in motor cortex excitability following training of a novel goal-directed motor task. Eur J Appl Physi, 105, 47–54. [DOI] [PubMed] [Google Scholar]
- Hoddes E, Zarcone V, Smythe H, Phillips R & Dement WC (1973). Quantification of sleepiness: A new approach. Psychophysiology, 10, 431–436. [DOI] [PubMed] [Google Scholar]
- Huber R, Ghilardi MF, Massimini M & Tononi G (2004). Local sleep and learning. Nature, 430, 78–81. [DOI] [PubMed] [Google Scholar]
- Johns MW (1991). A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep, 14, 540–545. [DOI] [PubMed] [Google Scholar]
- Joo EY, Kim HJ, Lim YH, Koo DL & Hong SB (2010). Altered cortical excitability in patients with untreated obstructive sleep apnea syndrome. Sleep Med, 11, 857–861. [DOI] [PubMed] [Google Scholar]
- Karni A, Meyer G, Rey-Hipolito C, Jezzard P, Adams MM, Turner R & Ungerleider LG (1998). The acquisition of skilled motor performance: Fast and slow experience-driven changes in primary motor cortex. Proc Natl Acad Sci USA, 95, 861–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloepfer C, Riemann D, Nofzinger EA, et al. (2009). Memory before and after sleep in patients with moderate obstructive sleep apnea. J Clin Sleep Med, 5, 540–548. [PMC free article] [PubMed] [Google Scholar]
- Kushida CA, Littner MR, Morgenthaler T, et al. (2005). Practice parameters for the indications for polysomnography and related procedures: An update for 2005. Sleep, 28, 499–521. [DOI] [PubMed] [Google Scholar]
- Manganotti P, Bongiovanni LG, Fuggetta G, Zanette G & Fiaschi A (2006). Effects of sleep deprivation on cortical excitability in patients affected by juvenile myoclonic epilepsy: A combined transcranial magnetic stimulation and EEG study. J Neurol Neurosurg Psychiatry, 77, 56–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenthaler T, Alessi C, Friedman L, et al. (2007). Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: An update for 2007. Sleep, 30, 519–529. [DOI] [PubMed] [Google Scholar]
- Nardone R, Bergmann J, Lochner P, et al. (2010). Modafinil reverses hypoexcitability of the motor cortex in narcoleptic patients: ATMS study. Sleep Med, 11, 870–875. [DOI] [PubMed] [Google Scholar]
- Nissen C, Kloepfer C, Feige B, Piosczyk H, Spiegelhalder K, Voderholzer U & Riemann D (2011). Sleep-related memory consolidation in primary insomnia. J Sleep Res, 20, 129–136. [DOI] [PubMed] [Google Scholar]
- Oldfield R (1971). The assessment and analysis ofhandedness: The Edinburgh inventory. Neuropsychologia, 9(1), 97–113. [DOI] [PubMed] [Google Scholar]
- Oliviero A, Della Marca G, Tonali PA, et al. (2005). Functional involvement of cerebral cortex in human narcolepsy. J Neurol, 252,56–61. [DOI] [PubMed] [Google Scholar]
- Perez MA, Lungholt BK, Nyborg K & Nielsen JB (2004). Motor skill training induces changes in the excitability of the leg cortical area in healthy humans. Exp Brain Res, 159, 197–205. [DOI] [PubMed] [Google Scholar]
- Robertson EM, Pascual-Leone A & Press DZ (2004). Awareness modifies the skill-learning benefits of sleep. CurrBiol, 14, 208–212. [DOI] [PubMed] [Google Scholar]
- Rosler KM, Petrow E, Mathis J, Aranyi Z, Hess CW & Magistris MR (2002). Effect of discharge desynchronization on the size of motor evoked potentials: An analysis. Clin Neurophysiol, 113, 1680–1687. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Berardelli A, Deuschl G, Hallett M, Maertens de Noordhout AM, Paulus W & Pauri F (1999). Applications of magnetic cortical stimulation. The International Federation of Clinical Neurophysiology. Electroen Clin Neurophysiol - Supp, 52, 171–185. [PubMed] [Google Scholar]
- Sadeh A, Sharkey KM & Carskadon MA (1994). Activity-based sleep-wake identification: An empirical test of methodological issues. Sleep, 17, 201–207. [DOI] [PubMed] [Google Scholar]
- Sakai K, Ugawa Y, Terao Y, et al. (1997). Preferential activation of different I waves by transcranial magnetic stimulation with a figure-of-eight-shaped coil. Exp Brain Res, 113, 24–32. [DOI] [PubMed] [Google Scholar]
- Siengsukon C & Boyd LA (2009a). Sleep enhances off-line spatial and temporal motor learning after stroke. Neurorehab Neural Re, 23, 327–335. [DOI] [PubMed] [Google Scholar]
- Siengsukon CF & Boyd LA (2009b). Does sleep promote motor learning? Implications for physical rehabilitation. Phys Ther, 89, 370–383. [DOI] [PubMed] [Google Scholar]
- Siengsukon CF & Boyd LA (2009c). Sleep to learn after stroke: Implicit and explicit off-line motor learning. Neurosci Lett, 451, 1–5. [DOI] [PubMed] [Google Scholar]
- Walker MP, Brakefield T, Hobson JA & Stickgold R (2003). Dissociable stages of human memory consolidation and reconsolidation. Nature, 425, 616–620. [DOI] [PubMed] [Google Scholar]
- Walker MP & Stickgold R (2006). Sleep, memory, and plasticity. Annu Rev Psychol, 57, 139–166. [DOI] [PubMed] [Google Scholar]
- Ward CP, McCoy JG, McKenna JT, Connolly NP, McCarley RW & Strecker RE (2009). Spatial learning and memory deficits following exposure to 24 h of sleep fragmentation or intermittent hypoxia in a rat model of obstructive sleep apnea. Brain Res, 1294, 128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss AR, Johnson NL, Berger NA, et al. (2010). Validity of activity-based devices to estimate sleep. J Clin Sleep Med, 6, 336–342. [PMC free article] [PubMed] [Google Scholar]
- Yoo SS, Hu PT, Gujar N, Jolesz FA & Walker MP (2007). A deficit in the ability to form new human memories without sleep. Nat Neurosci, 10, 385–392. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Lonnecker S, Steinhoff BJ & Paulus W (1996). Effects of antiepileptic drugs on motor cortex excitability in humans: A transcranial magneticstimulation study. Ann Neurol, 40, 367–378. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Muellbacher W, Hallett M & Cohen LG (2001). Modulation of practice-dependent plasticity in human motor cortex. Brain, 124, 1171–1181. [DOI] [PubMed] [Google Scholar]
- Zollman FS, Cyborski C, Duraski SA, Zollman FS, Cyborski C & Duraski SA (2010). Actigraphy for assessment of sleep in traumatic brain injury: Case series, review of the literature and proposed criteria for use. Brain Injury, 24, 748–754. [DOI] [PubMed] [Google Scholar]


