Abstract
BACKGROUND:
We recently reported anti-CD40 monoclonal antibody and rapamycin (aCD40/rapa) to be a reliable, nontoxic, immunosuppressive regimen for combined islet and kidney transplantation (CIKTx) in nonhuman primates (NHPs). In the current study, we attempted to induce allograft tolerance through the mixed chimerism approach using a conditioning regimen with aCD40 and belatacept (Bela).
METHODS:
Five CIKTx or kidney transplant (KTx) recipients were treated with aCD40/rapa for 4 months. All recipients then received a conditioning regimen including horse anti-thymocyte globulin (hATG) and aCD40/Bela. The results were compared with previous reports of recipients treated with Bela-based regimens.
RESULTS:
All 3 CIKTx recipients developed mixed chimerism, which was significantly superior to that observed in the previous Bela-based studies. Nevertheless, all CIKTx recipients in this study lost their islet and renal allografts as a result of cellular and humoral rejection on days 140, 89, and 84. The 2 KTx-alone recipients were treated with the same conditioning regimen and suffered rejection on days 127 and 116, despite the development of excellent chimerism. B lymphocyte reconstitution dominated by memory phenotypes was associated with early development of donor-specific antibodies in 4/5 recipients. In vitro assays showed no donor-specific regulatory T cell (Treg) expansion, which has been consistently observed in tolerant recipients with our mixed chimerism approach.
CONCLUSION:
Despite displaying excellent immunosuppressive efficacy, costimulatory blockade with anti-CD40 mAb (2C10R4) may inhibit the induction of renal or islet allograft tolerance via a mixed chimerism approach.
Keywords: anti-CD40 mAb, belatacept, CIKTx, dual CB, KTx, Mixed chimerism, NHP
INTRODUCTION
We previously developed a clinically applicable conditioning regimen for simultaneous kidney and bone marrow transplantation (SKBMT) in nonhuman primates (NHPs) using horse anti-thymocyte globulin (hATG) and belatacept (Bela/hATG)1. This protocol was subsequently modified for deceased donor transplantation by delaying donor bone marrow transplantation (DBMT) until 4 months after kidney transplantation (delayed DBMT)2. In the delayed tolerance protocol, more profound T cell depletion with rabbit ATG (rATG) in place of hATG was required to suppress memory T cell (TMEM) responses presumably induced by the renal allograft despite conventional immunosuppression administered until DBMT. However, when this delayed DBMT was applied to combined islet and kidney transplantation (CIKTx), a prominent inflammatory cytokine syndrome elicited by rATG3 resulted in loss of islet function. Therefore, we further revised the delayed DBMT regimen for CIKTx recipients by restoring hATG, expecting only moderate T cell depletion but less inflammatory responses. To suppress TMEM responses after DBMT, we instead added anti-CD40 monoclonal antibody (mAb), which has been demonstrated to have inhibitory effects on TMEM in NHP transplant models.3,4 Furthermore, the synergistic effect of anti-CD40 mAb and CTLA4Ig has been suggested in murine allograft models5 and in an NHP bone marrow transplant model6.
In the current study, we evaluated the efficacy of dual costimulatory blockade (CB) with anti-CD40 mAb and belatacept for induction of allograft tolerance via the mixed chimerism approach.
MATERIALS AND METHODS
Animals and pair selections
A total of 10 cynomolgus monkeys, including donor animals, weighing 3–8 kg were used for this study (Charles River Primates, Wilmington, MA). Donors and recipients were paired based on ABO compatibility and major histocompatibility complex (MHC) mismatching. MHC characterization was performed as previously described7,8. All surgical procedures as well as postoperative care of animals was performed in accordance with National Institutes of Health guidelines for the care and use of primates and approved by the Massachusetts General Hospital Institutional Animal Care and Use Committee.
Experimental design
The results from aCD40/Bela/hATG-treated animals were compared with results from previously reported recipients who received either Bela/hATG1 or Bela/rATG2.
Maintenance immunosuppression before DBMT:
Three cynomolgus monkeys received CIKTx and 2 received KTx alone from MHC-mismatched donors. All monkeys were treated with anti-CD40 mAb9 (NIH Nonhuman Primate Reagent Resource, Boston, MA, Cat# PR-4047, RRID:AB_2716325) (2C10R4: 20 mg/kg i.v. on days 0, 2, 5, 7, 9, and weekly at 10 mg/kg) plus daily rapamycin (LC laboratories, Woburn, MA) i.m. from day 0 to maintain trough levels at 10–15 ng/ml. Anti-inflammatory therapy, including tocilizumab (Chugai Pharm, Tokyo, Japan) (anti-IL-6R mAb: 10 mg/kg i.v. on days 0 and 5) and etanercept (Immunex, Seattle, WA) (TNF-alpha receptor fusion protein: 25 mg i.v. on days 0, 3, 7, and 10), was administered to all recipients (Fig. 1A)3.
Figure 1: The DBMT conditioning regimens with or without anti-CD40 mAb.
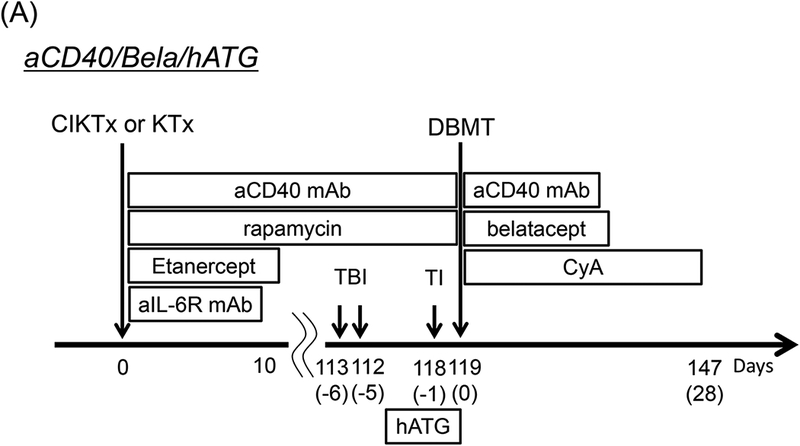
aCD40/Bela/hATG: Two to 3 weeks before combined islet and kidney transplantation (CIKTx), cynomolgus monkey recipients received streptozotocin (STZ: 75 mg/kg) for induction of diabetes. After transplantation, the CIKTx or kidney transplant (KTx)-alone recipients received the same immunosuppressive therapy with anti-CD40 mAb and rapamycin for 4 months. Anti-inflammatory therapies included anti-IL-6R mAb (10 mg/kg) and TNF-alpha receptor fusion protein (Etanercept: 25 mg) up to day 10 (A).3 At four months after transplantation, all recipients were conditioned with total body irradiation (TBI: 1.5 Gy × 2 on days −6 and −5, relative to DBMT), local thymic irradiation (TI: 7 Gy on day −1, relative to DBMT), and horse anti-thymocyte globulin (hATG: 50 mg/kg ×c 3 on days −2, −1 and 0, relative to DBMT). Following donor bone marrow transplantation (DBMT), the recipients were treated with dual costimulatory blockade (CB) with anti-CD40 mAb (20 mg/kg, on post-DBMT days 0, 2, 5, 7, 9, and 12) and belatacept (20 mg/kg, on post-DBMT days 0, 2, 5, and 15). Cyclosporine A (CyA) was administered for 28 days following DBMT, after which no immunosuppression was administered (A).
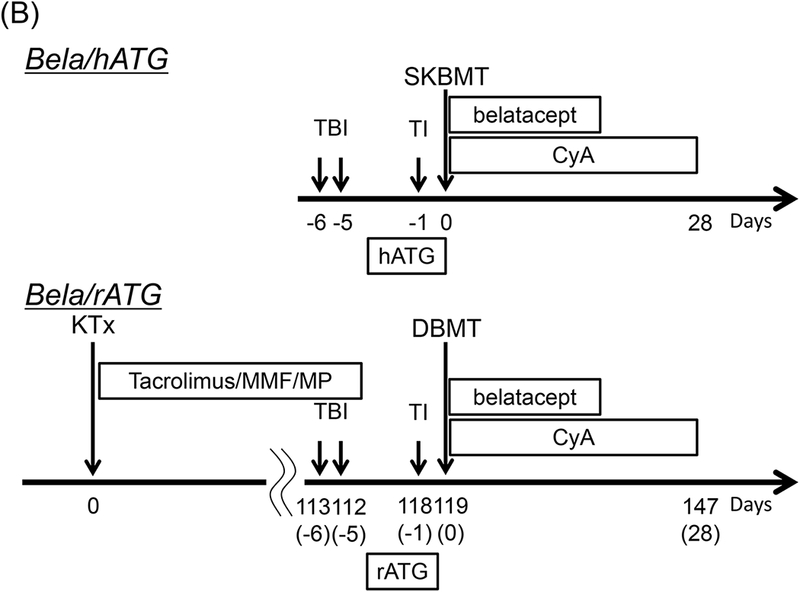
Bela/hATG: In a previous study, the conditioning regimen consisted of low-dose TBI (1.5 Gy × 2) on days −6 and −5 relative to simultaneous kidney and bone marrow transplantation (SKBMT), TI on day-1, hATG (50 mg/kg × 3 on days −2, −1, and 0) and belatacept (20 mg/kg × 4 on days 0, 2, 5, and 15). A 1-month course of CyA was administered to maintain therapeutic trough levels of 250–350 ng/ml (B).1
Bela/rATG: In the previous deceased donor study, all recipients initially underwent KTx alone with a conventional triple drug immunosuppressive regimen consisting of tacrolimus, mycophenolate mofetil, and prednisone (B). Four months after KTx alone, the recipients underwent conditioning and DBMT (delayed-DBMT). The conditioning regimen for DBMT is same as the Bela/hATG regimen, except the hATG was replaced with rATG (B).2
Conditioning regimen for DBMT used in this study:
aCD40/Bela/hATG (n=5):
Four months after CIKTx or KTx alone, all NHP recipients were conditioned with total body irradiation (TBI: 1.5 Gy on days −6 and −5, relative to the day of DBMT), local thymic irradiation (TI: 7 Gy on day −1), and hATG (Atgam: Pharmacia and Upjohn, Kalamazoo, MI: 50 mg/kg i.v. on days −2, −1 and 0). Following DBMT, the recipients were treated with anti-CD40 mAb (2C10R4: 20 mg/kg i.v. on days 0 and 2, 5, 7, 9 and 12 post-DBMT) and belatacept (Bela: provided by Bristol Meyer Squibb, New York, NY: 20 mg/kg i.v. on days 0, 2, 5, and 15 post-DBMT). Cyclosporine A (CyA: Novartis, Basel, Switzerland) was administered i.m. on days 1–28 post DBMT, after which no immunosuppression was administered (Fig. 1A).
Conditioning regiments from previous studies
Bela/hATG (n=5)1:
The conditioning regimen consisted of low-dose TBI (1.5 Gy × 2) on days −6 and −5 relative to simultaneous kidney and bone marrow transplantation (SKBMT), TI on day-1, hATG (50 mg/kg × 3 on days −2, −1, and 0, relative to SKBMT), and belatacept (20 mg/kg × 4 on days 0, 2, 5, and 15, relative to SKBMT). A 1-month course of CyA was administered to maintain therapeutic trough levels of 250–350 ng/ml (Fig. 1B)1.
Bela/rATG (n=4)2:
All recipients initially underwent KTx alone with a conventional triple drug immunosuppressive regimen consisting of tacrolimus (Astellas Pharma, Inc., Osaka, Japan) (starting with 0.1 mg/kg/day i.m. to maintain trough levels of 10–20 ng/dl), mycophenolate mofetil (Roche, Inc., Nutley, NJ) (200 mg/day), and prednisone (starting with 40 mg/day and tapering to a 1 mg/day maintenance dose in 2 weeks) (Fig. 1B). Four months after KTx alone, the recipients underwent conditioning and DBMT (delayed-DBMT). The conditioning regimen for DBMT was identical to the Bela/hATG regimen, with the exception of hATG, which was replaced by rATG (Thymoglobulin: Bridgewater, NJ: 10 mg/kg on day −2 and −1, relative to DBMT) (Fig. 1B)2.
Diabetes induction and management
As previously reported3, CIKTx recipients received streptozotocin (STZ) i.v. at a dose of 75 mg/kg (Zanosar, Teva Parenteral Medicines, Irvine, CA) 2 – 3 weeks before planned CIKTx after which blood glucose (BG) levels were monitored twice daily and measured by Accu-Check active II (Roche, Indianapolis, IN). After administration of STZ, all animals developed diabetes, defined as 3 consecutive fasting blood glucose (FBG) readings >250 mg/dL with C-peptide levels <50 pg/mL (C-peptide, Luminex, Merck Millipore, Billerica, MA). Regular insulin (Neutral Protamine Hagedorn and Humulin R, Eli Lilly Co., Indianapolis, IN) and Lantus (Eli Lilly Co.) were administered via a sliding scale to maintain BG levels <200 mg/dL.
Islet isolation and CIKTx
As previously reported3, the protocol for islet isolation was based on a modified human islet isolation protocol10. Under general anesthesia, monkeys underwent heterotopic KTx and bilateral native nephrectomies as previously described11, followed by islet allograft infusion into a branch of the inferior mesentery vein. The islet was gravity-infused slowly over a period of approximately 10–15 min.
Definition of islet rejection and BG control after rejection
The loss of islet function was diagnosed when the FBG level exceeded 250 mg/dL for 3 consecutive days. The date of graft rejection was defined as day 1 of the first 3 consecutive days of rejection. Subsequent to rejection, recipient animals were treated with exogenous insulin to maintain BG levels <200 mg/dL3.
Flow cytometric analyses
Peripheral blood mononuclear cells (PBMCs) were labeled with a combination of the following mAbs: CD3 (SP34–2), CD4 (L200), CD8 (SK1), CD21 (B-ly4), CD27 (M-T271), CD28 (CD28.2), CD95 (DX2), and IgM (G20–127) (BD Pharmingen, San Jose, CA), CD20 (2H7) (Biolegend, Inc., San Diego, CA) and FOXP3 (236A/E7) (eBioscience, Inc., San Diego, CA). Based on previous reports, which suggest that long-term allograft survival is associated with a predominance of immature B lymphocyte reconstitution,12,13 CD3-CD20+CD21+IgM+ or CD3-CD20+CD21-CD27+ B cells representing immature or mature B cell phenotypes, respectively, were monitored before and after conditioning. The fluorescence of the stained samples was analyzed using FACSverse (BD Biosciences, San Jose, CA) and Accuri flow cytometers (BD PharMingen), and FlowJo software (Tree Star, Inc., Ashland, OR).
Detection of chimerism
After standard water shock treatment, peripheral blood cells were stained with fluorescein isothiothianate (FITC)-conjugated mouse anti-human HLA class I (Bw6) mAb (Miltenyi Biotec, San Diego, CA), which cross-reacts with cynomolgus monkeys. The cells were incubated for 30 min at 4°C, and then washed and analyzed on FACScan (Becton Dickinson, Mountain View, CA). In all experiments, the percentage of cells stained with antibody was determined using a one-color fluorescence histogram and compared with those obtained from donor and pretreatment frozen recipient cells, which were used as positive and negative controls. The percentage of cells considered positive was determined using the fluorescence level at the beginning of the positive peak for the positive control stain as the cutoff value and by subtracting the percentage of cells stained with an isotype control. By using forward and 90° light scatter (FSC and SSC, respectively) dot plots, the lymphocyte (FSC- and SSC-low), granulocyte (SSC-high), and monocyte (FSC-high but SSC-low) populations were gated, and chimerism was determined separately for each population. Nonviable cells were excluded by propidium iodide (Thermo Fischer Scientific, Grand Island, NY) staining.
Mixed lymphocyte culture
T cells were purified from PBMCs by negative selection with a pan T cell isolation kit (Miltenyi Biotec, San Diego, CA). The isolated T cells were labeled with carboxyfluorescein succinimidyl ester (CFSE) (Life Technologies, Carlsbad, CA) at a concentration of 3 μM per 107 cells at 37°C for 5 min and cultured in 96-well V-bottom plates with irradiated PBMCs or T cell activation beads (αCD2/αCD3/αCD28 mAb) (Miltenyi Biotec, San Diego, CA). After 5 days, the cells were stained with antibodies and CFSE dilution was assessed by flow cytometry14. Results were compared with those of previously published tolerant recipients14.
Detection of donor specific antibody (DSA)
Anti-donor alloantibody was detected by flow cytometric analysis. Donor PBMCs were first incubated with recipient sera for 30 min at 4°C. After washing with FACS medium, FITCconjugated mouse anti-human IgG mAb was added and incubated for 30 min at 4°C and then washed. After washing, PBMCs were fixed with 2% paraformaldehyde. Cells were then acquired and analyzed with a cytometer. A positive reaction was defined as a shift greater than 10 channels in mean fluorescence intensity of donor lymphocytes when using test sera compared with a pretransplant serum control.
Statistical analyses
All measured outcomes are summarized as the mean ± standard deviation unless otherwise specified. Between-group pairwise comparison of means was performed by linear contrast in a random intercept longitudinal linear mixed effects model setting. Statistical analyses were performed with GraphPad PRISM 7.01 (GraphPad Software, Inc, San Diego, CA). We used Mantel-Cox Log-rank test to analyze the survival of different groups; P values lower than 0.05 have been considered statistically significant.
RESULTS
Nonmyeloablative conditioning with aCD40/Bela/hATG successfully induced multilineage mixed chimerism but failed to achieve tolerance in CIKTx.
Four months after CIKTx, all 3 recipients were treated with the conditioning regimen summarized in Fig. 1 followed by DBMT. After DBMT, recipients were treated with anti-CD40 mAb and belatacept and a 1-month course of CyA, before discontinuing all immunosuppression. All 3 CIKTx recipients successfully developed excellent multilineage mixed chimerism up to day 68 (Fig. 2). Nevertheless, all 3 CIKTx recipients rejected both islet and renal allografts by days 84–140 post-DBMT (Table 1). Kidney allografts at autopsy showed acute T cell mediated rejection (TCMR) as well as antibody mediated rejection (AMR) with positive C4d staining in all 3 CIKTx recipients (Figs. 3A, 3B, and 3C). Mononuclear cell infiltration was also observed in the transplanted islet (Fig. 3D).
Figure 2: Development of peripheral blood mixed chimerism after DBMT.
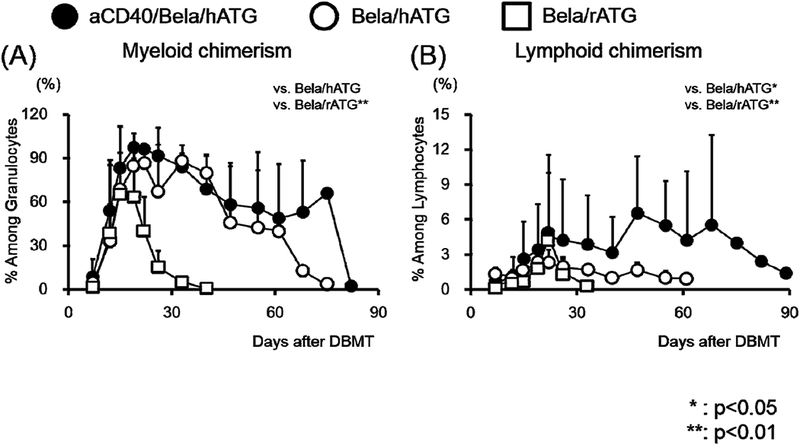
Excellent hematopoietic chimerism was induced in both myeloid (A) and lymphoid (B) lineages in all recipients treated with the aCD40/Bela/hATG regimen (closed circle). The levels of chimerism were significantly superior to those observed in previous studies of belatacept-based regimens (Bela/hATG: open circle1 or Bela/rATG: open square2). *: p<0.05, **: p<0.01.
Table 1:
Chimerism and allograft survival in belatacept-based regimens
Figure 3: Islet and kidney allograft histopathological findings.
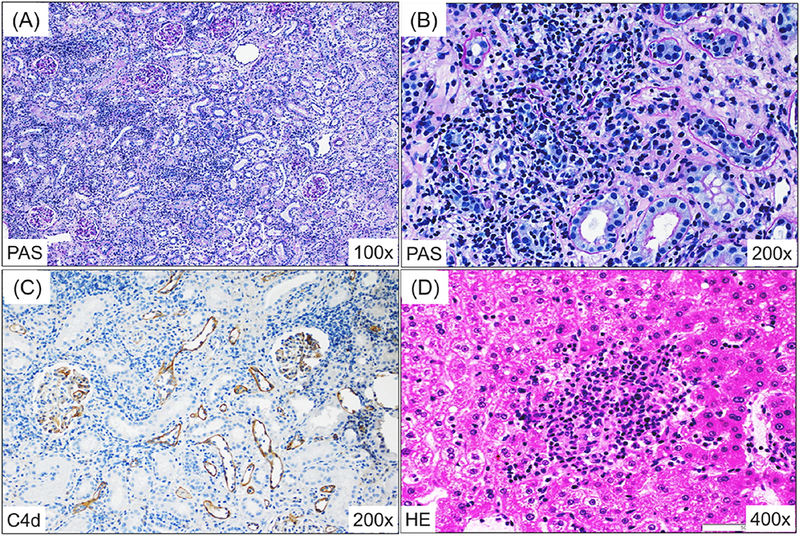
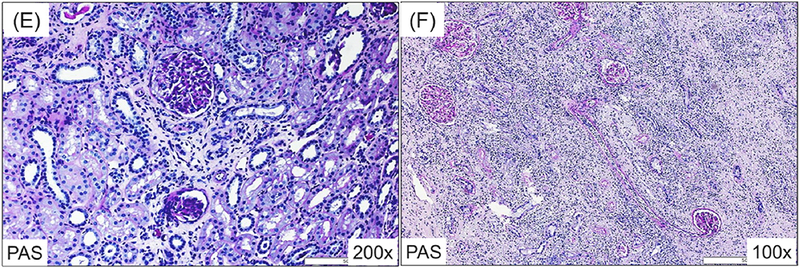
The histopathologic findings of the renal and islet allografts in recipients of aCD40/Bela/hATG. The kidney allografts of all 3 CIKTx recipients showed mononuclear cell infiltration (PAS, 100x) (A), dense tubulointerstitial infiltrates (PAS, 200x) (B), and C4d deposition (IHC C4d, 200x) (C) at autopsy. Mononuclear cell infiltration was also observed in the transplanted islet (HE, 400x) (D). A protocol kidney biopsy from an monkey undergoing KTx alone before conditioning showed minimal mononuclear cell infiltration and no evidence of rejection (PAS, 200x) (E); however, at autopsy (day 116), it had histologic features of antibody-mediated rejection with dense tubulointerstitial infiltrates (PAS, 100x) as well as transmural vasculitis (F) with C4d deposition (not shown).
Conditioning with aCD40/Bela/hATG also failed to induce tolerance in KTx alone recipients despite successful induction of chimerism.
The failure to induce allograft tolerance despite successful induction of chimerism in 3 CIKTx recipients prompted us to examine the same conditioning regimen in KTx alone recipients. Two cynomolgus monkey recipients underwent KTx with maintenance immunosuppression using aCD40/rapa. Both recipients did well without rejection for 4 months (Fig. 3E). At that point they received the same conditioning regimen used for the CIKTx, and then underwent DBMT. Both developed excellent mixed chimerism, and in one, the chimerism was prolonged lasting up to 3 months post-DBMT (Fig. 2). The levels and duration of chimerism in all 5 recipients treated with the aCD40/Bela/hATG regimen were in fact significantly superior to those observed in the previously reported recipients that achieved long-term immunosuppression-free survival with either Bela/rATG in the delayed-DBMT2 or Bela/hATG in the SKBMT protocol1. However, both KTx alone recipients treated with the aCD40/Bela/hATG regimen rejected their renal allografts on days 116 and 127 (Table 1, Fig. 3F).
Addition of anti-CD40 mAb therapy resulted in early B cell sensitization
There was no significant difference in overall CD3-CD20+ B cell reconstitution between the aCD40/Bela/hATG and Bela/rATG regimens (Fig. 4A). Subset analysis at 2 months after DBMT, however, revealed significantly more memory B cells (CD3-CD20+CD21-CD27+), but significantly fewer immature B cells (CD3-CD20+CD21+IgM+) in the aCD40/Bela/hATG recipients, compared with the Bela/rATG group (Fig. 4B). The memory phenotype dominant B cell subsets observed in the anti-CD40 mAb-treated recipients were consistently associated with early development of DSA, while DSA was suppressed in recipients treated with the Bela/rATG regimen (Fig. 4C).
Figure 4: Dual CB therapy induced early B cell sensitization.
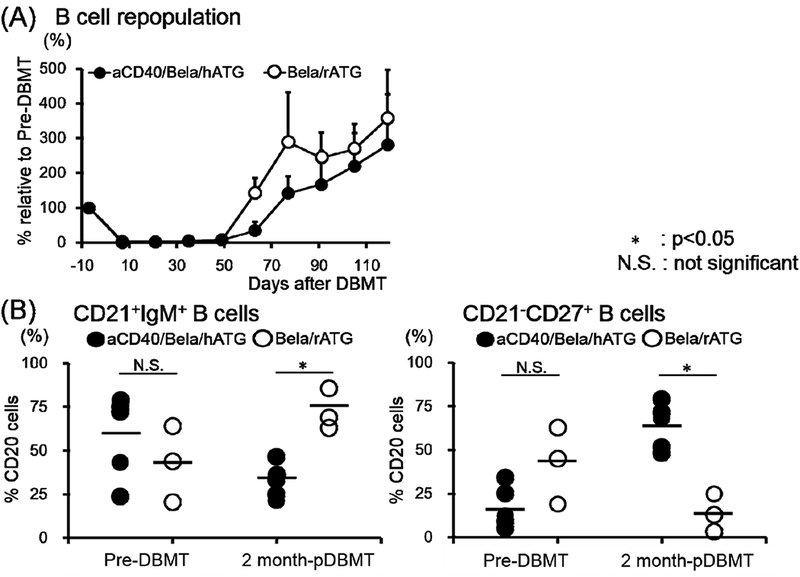
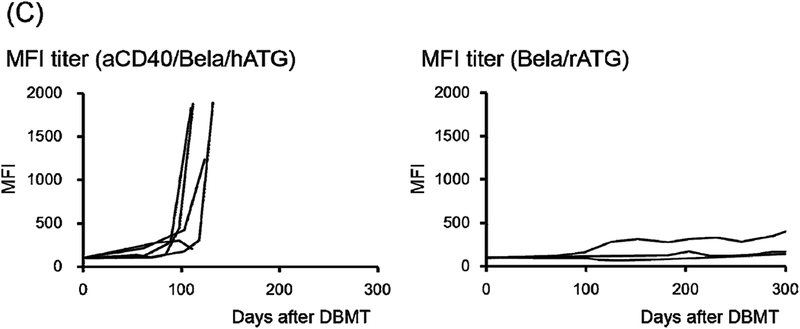
B cell (CD3-CD20+ cells) reconstitution, including immature (CD21+IgM+) and memory (CD21-CD27+) phenotypes, relative to those before DBMT conditioning were closely monitored. These values were compared with 3 previously reported “delayed-DBMT” KTx recipients who were conditioned with rabbit anti-thymocyte globulin and belatacept (rATG/Bela).2 (A) Peripheral blood B cell counts rapidly declined after the initiation of DBMT conditioning and then recovered by day 70 in both aCD40/Bela/hATG and Bela/rATG. There was no significant difference in B cell reconstitution between aCD40/Bela/hATG (n=5: closed circle with S.D.) and rATG/Bela (n=3: open circle with S.D.). (B) In rATG/Bela recipients (n=3), immature B cells (CD3-CD20+CD21+IgM+) were dominant at 2 months after DBMT (left panel, open circle). In contrast, B cells with memory phenotype (CD3-CD20+CD21-CD27+) were dominant at 2 months after DBMT in the aCD40/Bela/hATG recipients (n=5) (right panel, closed circle). *: p<0.05, N.S.: not significant. (C) Rapid development of DSA was observed in 4/5 aCD40/Bela/hATG treated recipients (left panel), while DSA development was suppressed in the Bela/rATG recipients (right panel).
Positive anti-donor CD8+ T cell responses and absence of donor-antigen-specific Treg expansion in vitro in recipients of the aCD40/Bela/hATG protocol.
A consistent immunologic feature observed in our previous studies included loss of anti-donor CD8+ T cell responses (Figs. 5A and 5B), but sustained anti-donor CD4+ T cell responses (Figs. 5C and 5D) with significant donor specific expansion of CD4+FOXP3+ regulatory T cells (Treg) in tolerant recipients (Figs. 5E and 5F) (data from previously published studies in 8 recipients that achieved renal allograft tolerance)14. In cynomolgus monkeys, peripheral blood Tregs comprised 1–2% of the CD4+ T cells, but expanded to 8 ± 5.4% after donor antigen stimulation in tolerant recipients. In contrast, sustained anti-donor responses were observed in both CD8+ (Figs. 5A and 5B) and CD4+ T cells (Figs. 5C and 5D), and no donor specific Treg expansion (Figs. 5E and 5F) was detected in any of the 5 CIKTx or KTx-alone recipients treated with the aCD40/Bela/hATG regimen.
Figure 5: Neither loss of anti-donor CD8+ responses nor donor-antigen-specific Treg expansion was observed in aCD40/Bela/hATG treated animals in vitro.
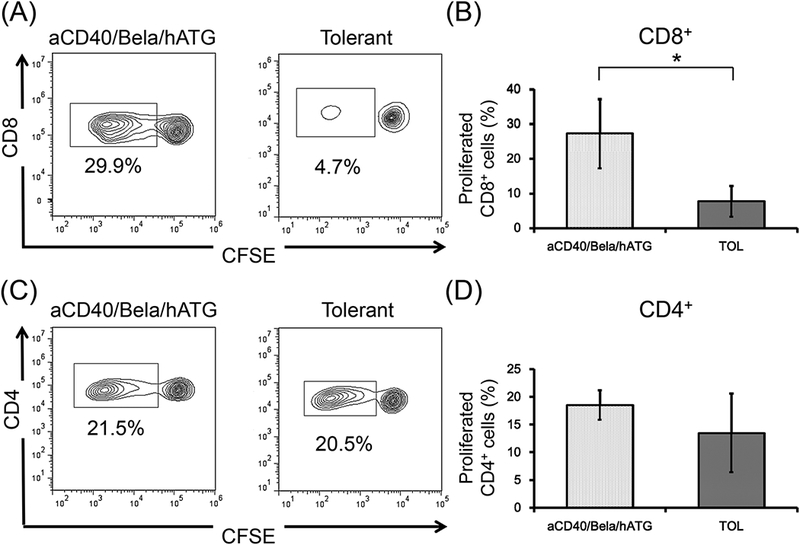
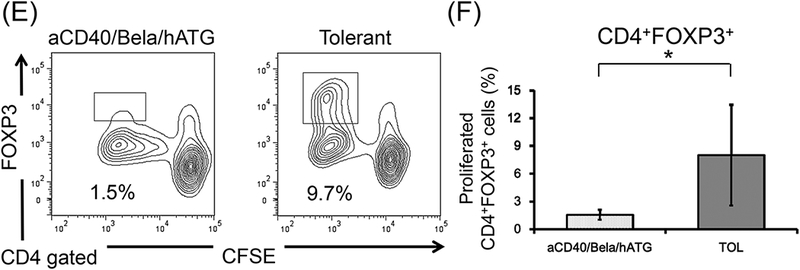
CD3+ T cells isolated from aCD40/Bela/hATG treated recipients were labeled CFSE and were cultured with irradiated self or donor peripheral mononuclear cells (PBMCs) for 5 days. Cultured cells were then stained for CD4, CD8, and FOXP3. Representative flow cytometric data (A, CD8; C, CD4; E, CD4 gated FOXP3) and mean % proliferation relative to the response to self antigens (B, CD8; D, CD4; F, CD4 gated FOXP3) are shown compared with those of previously published results in 8 tolerant recipients.14 The loss of anti-donor CD8+ T cell response was not observed in the aCD40/Bela/hATG treated recipients, which was significantly higher than those observed in the tolerant recipients (A and B). Substantial anti-donor CD4+ T cell proliferation was observed in the aCD40/Bela/hATG treated animals, comparable to that observed in tolerant recipients (C and D). Among these proliferated CD4+ cells after donor stimulation, a significant proportion of proliferating CD4+ cells were found in FOXP3 intolerant recipients, while such FOXP3+ cell proliferation was minimal in the aCD40/Bela/hATG treated animals (E and F). Proliferated cells (%) = Proliferated cells (%) with donor antigens - Proliferated cells (%) with self antigens. Data are presented as the mean ± SD. *P<0.05.
DISCUSSION
The CD40/CD154 pathway is pivotal to generating an effective immune response against foreign antigens.15,16 Blockade of this pathway with anti-CD154 mAb has been demonstrated to promote allograft tolerance in various animal models,17–19 including our NHP model using the mixed chimerism approach.20 However, the clinical application of this approach has been delayed because of the adverse thrombophilic effects of anti-CD154 mAb observed in NHPs21 and humans.22 Blocking its counterpart, CD40, with anti-CD40 mAb has therefore been tested with encouraging observations in various preclinical23,24 and clinical studies.25 We also recently reported that the combination of anti-CD40 mAb and rapamycin provides effective immunosuppression without toxicity in combined islet and kidney transplantation (CIKTx).3 Since the immunosuppressive effects of anti-CD40 mAb on TMEM have been demonstrated in previous studies,3,4 we added anti-CD40 mAb to induce mixed chimerism in the delayed-DBMT protocol. As expected, all 3 CIKTx recipients developed excellent chimerism (Fig. 2), which was superior to that observed in recipients who achieved long-term immunosuppression-free renal allograft survival with the belatacept-based regimens.1,2 Nevertheless, none of CIKTx recipients achieved long-term allograft survival and succumbed to acute antibody-mediated rejection 140 days after DBMT (Table 1). To exclude the possibility that the exceptionally high antigenic or inflammatory properties of the islet allografts induced strong alloimmune responses, which could have triggered rejection of both islet and renal allografts, we tested the same aCD40/Bela/hATG regimen in 2 KTx alone recipients. Both KTx recipients also failed to achieve long-term immunosuppression-free renal allograft survival despite successful induction of chimerism (Table 1). These findings rule out the notion that failure of tolerance induction with the aCD40/Bela/hATG regimen is due to the islet allograft. This was an unexpected outcome since renal allograft loss due to acute rejection is rare in recipients who successfully develop mixed chimerism with our conditioning regimen. As shown in Fig. 6 and Table 1, allograft survival was significantly shorter in the aCD40/Bela/hATG group compared to recipients in previous studies who successfully developed mixed chimerism following treatment with the Bela/hATG1 or Bela/rATG2 regimen.
Figure 6: Renal allograft survival curves in belatacept-based conditioning regimens.
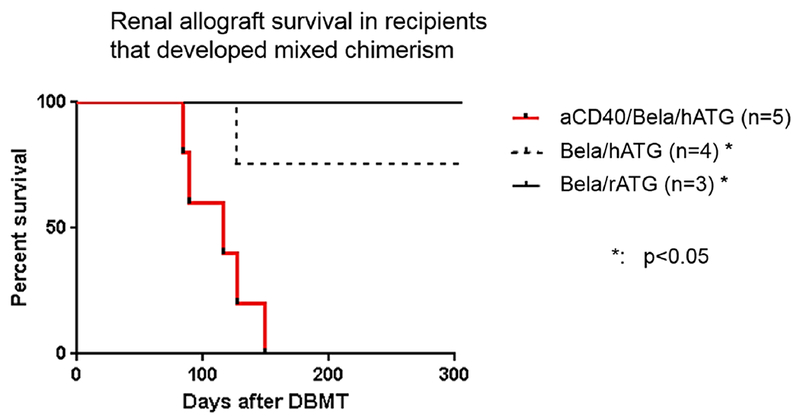
Renal allograft survival in recipients treated with aCD40/Bela/hATG (n=5, red line) was significantly shorter than that reported for either simultaneous kidney and bone marrow transplantation (SKBMT) recipients treated with the Bela/hATG regimen (n=4, black dotted line),1 or “delayed-DBMT” recipients treated with the Bela/rATG (n=3, black line)2 regimen. Mantel-Cox Log-rank test was used. *: p<0.05.
These observations have caused us to conclude that anti-CD40 mAb may inhibit the induction of allograft tolerance, at least in our mixed chimerism approach. Ramakrishnan et al26 also reported failure of renal allograft tolerance despite induction of chimerism in recipients treated with nonmyeloablative conditioning regimen with dual CD28/CD40 (3A8) costimulatory blockade. They attributed the failure of tolerance to the compartmentalized nature of the chimerism, which displayed poor lymphoid chimerism. Our studies have consistently shown that induction of donor chimerism, including the lymphoid lineage, is critical for induction of stable tolerance.1,20,27 In the current study, donor chimerism was achieved; therefore, this explanation does not account for our observations.
It is far more likely that the failure of tolerance induction in the aCD40/Bela/hATG recipients was due to an inadequate regulatory response. Since the major mechanism of renal allograft tolerance via transient mixed chimerism is considered regulatory rather than deletional,14,28 systemic delivery of donor antigens by donor chimeric lymphoid cells may be necessary to induce antigen-specific unresponsiveness. Pilat et al29 recently demonstrated that induction of mixed chimerism and skin allograft tolerance were not achieved by nonmyeloablative conditioning (low dose TBI, rapamycin with anti-CD4 or anti-CD8 mAb) with CTLA4Ig when a CD40−/− mouse is used either as a recipient or a donor. Since the CD40 signal is critical for activation and maturation of the antigen-presenting cells (APCs) required for full T cell activation,30 it is plausible that absence of CD40 signaling in either donor (direct pathway) or recipient (indirect pathway) APCs may have interfered with maturation of APCs in our NHP recipient, resulting in insufficient donor antigen presentation to the recipient regulatory cells. We hypothesize that such donor antigen presentation by donor chimeric cells is needed to induce the immunologic features of peripheral tolerance (donor-specific Treg expansion associated with the loss of anti-donor CD8+ T cell responses) observed in our tolerant recipients.14 In contrast, such immunologic features, especially donor-specific Treg expansion, which can promote robust tolerance to the donor,31 were not observed in those recipients treated with the aCD40/Bela/hATG regimen (Fig. 5). Although blockade of the CD154 molecule (CD40 ligand) by anti-CD154 mAb with or without donor-specific transfusion has been shown to generate donor-specific Tregs in murine transplant models32,33, blockade of its counter ligand, CD40, molecule may result in failure of Treg expansion and T cell tolerance. Pan et al have shown that CD40 is required for myeloid derived suppressor cell (MDSC) mediated T cell suppression and Treg expansion. In their murine tumor model, treatment with anti-CD40 reversed T cell tolerance and prevented tumor-associated expansion of Tregs34. Similar to their observation, treatment with anti-CD40 mAb failed to induce Treg expansion and allograft tolerance in our NHP model.
In the current study, we observed early DSA development associated with B cell reconstitution predominantly with memory-phenotype B cells in recipients treated with aCD40/Bela/ATG regimens (Fig. 4). Since significant memory-type B cell predominance was not observed in the recipients treated with the Bela/ATG regimen, the enhanced B cell differentiation may have been caused by anti-CD40 mAb. Although enhancement of B cell differentiation by the anti-CD40 mAb clone, 2C10R4, was not observed in vitro (data not shown), the agonistic effect of 2C10R4 on B cells was observed when it was used in vivo. Conversely, effective suppression of B cell differentiation by anti-CD40 mAb has been reported in NHPs. Kim et al13 showed that additional treatment with anti-CD40 mAb (2C10R4) or belatacept for 2 months consistently suppressed the DSA development in KTx recipients treated with anti-CD3 immunotoxin, tacrolimus, and alefacept in NHPs. This DSA suppression was associated with predominance of immature B cells and depressed memory B cells. Furthermore, Aoyagi et al24 reported that long-term (6 months) treatment with anti-CD40 mAb (ASKP1240) monotherapy effectively suppressed DSA development, while 2 weeks of treatment failed to suppress DSA in their NHP KTx model. These studies may indicate that B cell differentiation is effectively inhibited as long as anti-CD40 mAb is administered. Since anti-CD40 mAb was administered only up to day 12 post-DBMT in our conditioning regimen, the short duration of the antibody administration after DBMT may have resulted in homeostatic proliferation of memory B cells which led to early DSA development.
Although anti-CD40 mAb appears to be an excellent immunosuppressive agent, it may inhibit the induction of allograft tolerance by abrogating Treg expansion, in addition to its other agonistic effects on B cells. Although the outcome of the current study was negative, we think it is important to document these findings for future studies on tolerance induction involving costimulatory blockade.
ACKNOWLEDGMENTS
The present work was supported in part by the Canadian Foundation for Innovation and grant U19AI102405, part of the NIH NHP Transplantation Tolerance Cooperative Study Group sponsored by the National Institute of Allergy and Infectious Diseases and the National Institute of Diabetes and Digestive and Kidney Diseases. Anti-CD40 mAb (2C10R4) was provided by the Nonhuman Primate Reagent Resource (funded by NIH grants R24OD010976 and U24AI126683).
Founding sources: This work was supported by NIH grant U19 AI102405–01.
ABBREVIATIONS:
- AMR
antibody mediated rejection
- ATG
antithymocyte globulin
- CB
costimulatory blockade
- CIKTx
combined islet and kidney transplantation
- CyA
cyclosporine A
- DBMT
donor bone marrow transplantation
- KTx
kidney transplantation
- mAb
monoclonal antibody
- MHC
major histocompatibility complex
- SKBMT
simultaneous kidney and bone marrow transplantation
- TBI
total body irradiation
- TCMR
T cell mediated rejection
- TI
thymic irradiation
Footnotes
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by Transplantation. This manuscript was also not prepared or funded by any commercial organization.
REFERENCES
- 1.Yamada Y, Ochiai T, Boskovic S, et al. Use of CTLA4Ig for Induction of Mixed Chimerism and Renal Allograft Tolerance in Nonhuman Primates. Am J Transplant. 2014;14(12):2704–2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hotta K, Oura T, Dehnadi A, et al. Long-term Nonhuman Primate Renal Allograft Survival Without Ongoing Immunosuppression in Recipients of Delayed Donor Bone Marrow Transplantation. Transplantation. 2018;4(10):0000000000002078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oura T, Hotta K, Lei J, et al. Immunosuppression with CD40 Costimulatory Blockade plus Rapamycin for Simultaneous Islet and Kidney Transplantation in Nonhuman Primates. Am J Transplant. 2016;8(10):13999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oura T, Yamashita K, Suzuki T, et al. Long-term hepatic allograft acceptance based on CD40 blockade by ASKP1240 in nonhuman primates. Am J Transplant. 2012;12(7):1740–1754. [DOI] [PubMed] [Google Scholar]
- 5.Gilson CR, Milas Z, Gangappa S, et al. Anti-CD40 monoclonal antibody synergizes with CTLA4-Ig in promoting long-term graft survival in murine models of transplantation. J Immunol. 2009;183(3):1625–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Page A, Srinivasan S, Singh K, et al. CD40 blockade combines with CTLA4Ig and sirolimus to produce mixed chimerism in an MHC-defined rhesus macaque transplant model. Am J Transplant. 2012;12(1):115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Connor SL, Blasky AJ, Pendley CJ, et al. Comprehensive characterization of MHC class II haplotypes in Mauritian cynomolgus macaques. Immunogenetics. 2007;59(6):449–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pendley CJ, Becker EA, Karl JA, et al. MHC class I characterization of Indonesian cynomolgus macaques. Immunogenetics. 2008;60(7):339–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lowe M, Badell IR, Thompson P, et al. A novel monoclonal antibody to CD40 prolongs islet allograft survival. Am J Transplant. 2012;12(8):2079–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lei J, Kim JI, Shi S, et al. Pilot Study Evaluating Regulatory T Cell-Promoting Immunosuppression and Nonimmunogenic Donor Antigen Delivery in a Nonhuman Primate Islet Allotransplantation Model. Am J Transplant. 2015;15(10):2739–2749. [DOI] [PubMed] [Google Scholar]
- 11.Cosimi AB, Delmonico FL, Wright JK, et al. Prolonged survival of nonhuman primate renal allograft recipients treated only with anti-CD4 monoclonal antibody. Surgery. 1990;108(2):406–413; discussion 413–404. [PubMed] [Google Scholar]
- 12.Liu C, Noorchashm H, Sutter JA, et al. B lymphocyte-directed immunotherapy promotes long-term islet allograft survival in nonhuman primates. Nat Med. 2007;13(11):1295–1298. [DOI] [PubMed] [Google Scholar]
- 13.Kim EJ, Kwun J, Gibby AC, et al. Costimulation blockade alters germinal center responses and prevents antibody-mediated rejection. Am J Transplant. 2014;14(1):59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hotta K, Aoyama A, Oura T, et al. Induced regulatory T cells in allograft tolerance via transient mixed chimerism. JCI insight. 2016;1(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Kooten C, Banchereau J. CD40-CD40 ligand. J Leukoc Biol. 2000;67(1):2–17. [DOI] [PubMed] [Google Scholar]
- 16.Quezada SA, Jarvinen LZ, Lind EF, Noelle RJ. CD40/CD154 interactions at the interface of tolerance and immunity. Annu Rev Immunol. 2004;22:307–328. [DOI] [PubMed] [Google Scholar]
- 17.Kirk AD, Burkly LC, Batty DS, et al. Treatment with humanized monoclonal antibody against CD154 prevents acute renal allograft rejection in nonhuman primates. Nat Med. 1999;5(6):686–693. [DOI] [PubMed] [Google Scholar]
- 18.Montgomery SP, Xu H, Tadaki DK, et al. Combination induction therapy with monoclonal antibodies specific for CD80, CD86, and CD154 in nonhuman primate renal transplantation. Transplantation. 2002;74(10):1365–1369. [DOI] [PubMed] [Google Scholar]
- 19.Xu H, Tadaki DK, Elster EA, et al. Humanized anti-CD154 antibody therapy for the treatment of allograft rejection in nonhuman primates. Transplantation. 2002;74(7):940–943. [DOI] [PubMed] [Google Scholar]
- 20.Koyama I, Kawai T, Andrews D, et al. Thrombophilia associated with anti-CD154 monoclonal antibody treatment and its prophylaxis in nonhuman primates. Transplantation. 2004;77(3):460–462. [DOI] [PubMed] [Google Scholar]
- 21.Schmidtko J, Wang R, Wu CL, et al. Posttransplant lymphoproliferative disorder associated with an Epstein-Barr-related virus in cynomolgus monkeys. Transplantation. 2002;73(9):1431–1439. [DOI] [PubMed] [Google Scholar]
- 22.Kirk AD, Sollinger HW, Vincenti F, Stecher S, Nadeau K. Preliminary results of the use of humanized anti-CD154 in human renal allotransplantation. Am J Transplant. 2001;1(S191). [Google Scholar]
- 23.Imai A, Suzuki T, Sugitani A, et al. A novel fully human anti-CD40 monoclonal antibody, 4D11, for kidney transplantation in cynomolgus monkeys. Transplantation. 2007;84(8):1020–1028. [DOI] [PubMed] [Google Scholar]
- 24.Aoyagi T, Yamashita K, Suzuki T, et al. A human anti-CD40 monoclonal antibody, 4D11, for kidney transplantation in cynomolgus monkeys: induction and maintenance therapy. Am J Transplant. 2009;9(8):1732–1741. [DOI] [PubMed] [Google Scholar]
- 25.Goldwater R, Keirns J, Blahunka P, et al. A phase 1, randomized ascending single-dose study of antagonist anti-human CD40 ASKP1240 in healthy subjects. Am J Transplant. 2013;13(4):1040–1046. [DOI] [PubMed] [Google Scholar]
- 26.Ramakrishnan SK, Page A, Farris AB, et al. Evidence for kidney rejection after combined bone marrow and renal transplantation despite ongoing whole-blood chimerism in rhesus macaques. Am J Transplant. 2012;12(7):1755–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawai T, Cosimi AB, Colvin RB, et al. Mixed allogeneic chimerism and renal allograft tolerance in cynomolgus monkeys. Transplantation. 1995;59(2):256–262. [PubMed] [Google Scholar]
- 28.Yamada Y, Nadazdin O, Boskovic S, et al. Repeated Injections of IL-2 Break Renal Allograft Tolerance Induced via Mixed Hematopoietic Chimerism in Monkeys. Am J Transplant. 2015;15(12):3055–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pilat N, Klaus C, Schwarz C, et al. Rapamycin and CTLA4Ig synergize to induce stable mixed chimerism without the need for CD40 blockade. Am J Transplant. 2015;15(6):1568–1579. [DOI] [PubMed] [Google Scholar]
- 30.Xu H, Zhang X, Mannon RB, Kirk AD. Platelet-derived or soluble CD154 induces vascularized allograft rejection independent of cell-bound CD154. J Clin Invest. 2006;116(3):769–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duran-Struuck R, Sondermeijer HP, Buhler L, et al. Effect of Ex Vivo-Expanded Recipient Regulatory T Cells on Hematopoietic Chimerism and Kidney Allograft Tolerance Across MHC Barriers in Cynomolgus Macaques. Transplantation. 2017;101(2):274–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Maurik A, Herber M, Wood KJ, Jones ND. Cutting edge: CD4+CD25+ alloantigen-specific immunoregulatory cells that can prevent CD8+ T cell-mediated graft rejection: implications for anti-CD154 immunotherapy. J Immunol. 2002;169(10):5401–5404. [DOI] [PubMed] [Google Scholar]
- 33.Ferrer IR, Wagener ME, Song M, Kirk AD, Larsen CP, Ford ML. Antigen-specific induced Foxp3+ regulatory T cells are generated following CD40/CD154 blockade. Proc Natl Acad Sci U S A. 2011;108(51):20701–20706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pan PY, Ma G, Weber KJ, et al. Immune stimulatory receptor CD40 is required for T-cell suppression and T regulatory cell activation mediated by myeloid-derived suppressor cells in cancer. Cancer Res. 2010;70(1):99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]


