Abstract
Background:
Regulatory T cell (Treg)-based immunotherapies have been studied as potential cell-based modalities for promoting transplant survival. However, the efficacy of local delivery of Tregs in corneal transplantation has not been fully elucidated. Herein, we investigated the kinetics of migration of subconjunctivally injected Tregs and their role in promoting corneal allograft survival.
Methods:
GFP+CD4+CD25+Foxp3+ Tregs were isolated from draining lymph nodes (DLNs) of GFP transgenic mice and were subconjunctivally injected to corneal allograft recipients. Next, Tregs, conventional T cells (Tconv) or a combination of both was locally injected to graft recipients, and graft survival was determined by evaluating opacity scores for 10 weeks. Transplanted mice without treatment served as controls. The frequencies of MHC-II+CD11b+ antigen presenting cells (APCs), IFNγ+CD4+ Th1 cells, and CD45+ cells in the DLNs and cornea were evaluated at week 2 posttransplantation using flow cytometry. Expression of IFNγ, IL-10 and TGF-β in the grafts were assessed using RT-PCR and ELISA.
Results:
GFP+ Tregs were detected in the ipsilateral cornea and DLNs of recipients 6 hours after injection. Subconjunctival injection of Tregs significantly decreased the frequencies of mature APCs in the graft and DLNs, suppressed Th1 frequencies in DLNs, and inhibited CD45+ cell infiltration to the graft. Finally, locally delivered Tregs significantly reduced the expression of IFN-γ, enhanced the levels of IL-10 and TGF-β in the graft, and promoted long-term allograft survival.
Conclusions:
Our study elucidates the kinetics of migration of locally delivered Tregs and shows their role in suppressing host immune response against the allograft.
INTRODUCTION
Corneal transplantation is the most commonly performed form of solid tissue transplantation worldwide, with nearly 40 000 cases of penetrating keratoplasty performed annually in the U.S. alone. 1,2 Despite the remarkably high success rates of corneal grafts, largely due to the immune privileged status of the cornea, immune-mediated rejection still remains the major cause of corneal graft failure.3 In addition, currently available immunosuppressive drugs are associated with several adverse side effects. It is, therefore, imperative to develop safer and more effective immunomodulatory strategies to improve corneal allograft survival.
Regulatory T cells (Tregs) play a critical role in immune homeostasis and induction of tolerance towards self- and alloantigens.4 As a promising strategy, Treg-based immunotherapies have been investigated in treatment of autoimmune diseases, such as multiple sclerosis 5 and uveitis 6 as well as in promoting survival of grafts in several organs, including skin,7 islet,8 heart 9 and liver.10 These therapies have mostly relied on either in vitro polyclonal expansion of Tregs followed by their adoptive transfer to graft recipients prior to transplantation, or using molecular therapies to reconstitute Tregs while suppressing pathogenic effector T cells 11,12. The relevance of Tregs in induction of tolerance against corneal allografts has been well established. The functional status of Tregs, primarily their expression of Foxp3, has been correlated with their ability to promote corneal graft survival.13 Our group has previously shown that intravenous injection of allograft acceptor-derived Tregs or ex vivo-conditioned CCR7high Tregs significantly reduces corneal allograft rejection rates.13,14 Reports from other groups have also demonstrated that adoptive transfer of in vitro expanded Tregs to allograft recipients promotes corneal graft survival.15
Despite the promising results of these studies, however, a major obstacle in translating adoptive transfer approaches into clinical treatments is the relatively low frequencies of Tregs, which necessitates in vitro expansion of Tregs prior to adoptive transfer in order to yield sufficient number of cells to reach the cornea and effectively target alloreactive effector cells.16 Prolonged in vitro expansion of Tregs itself can accentuate loss of Foxp3 expression by Tregs and thus decrease their suppressive function.17 A therapeutic alternative to overcome these limitations would consist of local delivery of Tregs to graft recipients via subconjunctival injection. However, little is known about the effect of local delivery of Tregs in regulating the alloimmune response towards the graft, and the dynamics of distribution of Tregs upon subconjunctival injection have not yet been fully elucidated.
Herein we show for the first time that locally administered Tregs specifically migrate from the conjunctivae to the ipsilateral cornea and draining lymph nodes of graft recipients, where they promote allograft survival by suppressing APC maturation, Th1 cell activation, and inflammatory cell infiltration at the ocular surface.
MATERIALS AND METHODS
Animals
8- to 10-week-old male BALB/c and C57BL/6 mice were purchased from Charles River Laboratories, Wilmington, MA. 8-week-old Green fluorescent protein (GFP) transgenic BALB/cByJ male mice (CByJ.B6-Tg(UBC-GFP)30Scha/J) were obtained from The Jackson Laboratory, Bar Harbor, ME. The use of GFP transgenic mice was approved by the Committee on Microbiological Safety at Harvard Medical School. Animals were housed in a pathogen-free environment at the Schepens Eye Research Institute Animal Care Facility. Animals were treated according to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Visual Research, and all animal experiments were approved by the Animal Care and Use Committee of Schepens Eye Research Institute. Mice were anesthetized with intraperitoneal injections of 120mg/kg ketamine and 20mg/kg xylazine for surgical procedures.
Orthotopic corneal transplantation
Corneal transplantation was performed using C57BL/6 mice as donors and BALB/c mice as graft recipients, as described previously.18 Briefly, the central cornea of donor C57BL/6 mice was marked with a 2 mm diameter trephine and excised with Vannas scissors (Storz Instruments Company, San Damis, CA). Recipient graft beds were prepared by excising a 1.5 mm diameter area in the central cornea of BALB/c mice. Donor buttons were transplanted and secured into host beds with 8 interrupted 11–0 nylon sutures. Eyelids were closed for 24 hours with 8–0 nylon sutures. Corneal sutures were removed at day 7 after transplantation.
Evaluation of graft survival
Transplanted corneas were examined weekly in a masked fashion for 10 weeks using a slit-lamp microscope. A standardized opacity grading system was used to define rejection.19 Grafts with opacity scores higher than 2 for at least 2 consecutive weeks were considered as immune-rejected.20 Eyes with complications such as hemorrhage, cataract, or infection were excluded from the analysis.
Cell sorting
CD4+CD25+ Tregs were isolated from lymph nodes and spleen of naïve BALB/c mice and GFP transgenic BALB/cByJ mice by magnetic-assisted cell sorting (MACS) using mouse Treg isolation kits (Miltenyi Biotec, Bergisch-Gladbach, Germany). CD4+CD25+ cells were collected as Tregs, and the remaining cells (CD4+CD25−) were collected as conventional T cells (Tconv). The purity of sorted Tregs (frequencies of Foxp3+ cells) was >90%, as determined by flow cytometry.
Subconjunctival injection of Tregs to allograft recipients
1×105 Tregs isolated from naïve BALB/c mice or GFP transgenic BALB/cByJ mice, CD4+CD25− Tconv or combination of both were suspended in 10µl PBS and injected subconjunctivally to mice using a 33-gauge metal needle and 100µl syringe (Hamilton Company, Reno, NV) immediately after corneal transplantation. Transplanted mice without receiving a subconjunctival injection served as the control.
Cornea and conjunctivae digestion and lymph node cell preparation
Excised corneas and conjunctivae were digested in RPMI media (Lonza, Walkersville, MD) containing 2 mg/mL DNase I (Roche, Mannheim, Germany) and 4 mg/mL Collagenase D (Roche, Mannheim, Germany) for 60 minutes in 37 °C and filtered through a 70-µm cell strainer. Ipsilateral draining submandibular and cervical lymph nodes were collected after transplantation and single cell suspensions were prepared. Spleen single cell suspensions were made and red blood cell lysis was performed using red blood cell lysis buffer (Sigma-Aldrich, St. Louis, MO).
Flow cytometry
All cell suspensions were incubated with an Fc receptor blocking antibody (R&D Systems, Minneapolis, MN) for 10 minutes to avoid nonspecific staining, and were subsequently stained with the following antibodies: anti-CD11b (M1/70), antimouse I-A/I-E (major histocompatibility complex (MHC) II, M5/114.15.2), anti-CD4 (RM4–5) and anti-IFNγ (XMG1.2) (Biolegend, San Diego, CA). For intracellular IFNγ staining, cells were stimulated with phorbol 12-myristate 13-acetate (PMA; 50 ng/mL; Sigma-Aldrich) and Ionomycin (500 ng/mL; Sigma-Aldrich) overnight in the presence of Golgistop (0.7 µL/100 µL media; BD Biosciences, San Jose, CA), and were fixed and permeabilized with appropriate buffers (eBioscience). Proper isotype controls were used for all antibodies. Stained cells were analyzed using the LSRII flow cytometer (BD Biosciences, Franklin Lakes, NJ) and the results were analyzed using FlowJo software (FlowJo LLC, Ashland, OR).
Enzyme-linked immunosorbent assay
Corneas were collected in PBS, homogenized on ice and centrifuged. The supernatant was assayed using commercial ELISA kits for total protein (Pierce BCA Protein Assay Kit; Thermo scientific, IL), IL-10, TGF-β1, and IFNγ (eBioscience) according to the manufacturers’ instructions.
Real Time-PCR
Corneas were collected in Trizol (Ambion, Life Technologies, Grand Island, NY), and then homogenized on ice. Briefly, the RNA was extracted and purified by using RNeasy Micro kit (Qiagen, Valencia, CA). Total RNA was reverse transcripted using a Superscript III kit (Invitrogen, Carlsbad, CA). Real-time PCR was performed using TaqMan Universal PCR Mastermix (Applied Biosystems, Foster City, CA) and preformulated primers for IFNγ (Mm01168134_m1), and GAPDH (Mm99999915_gl). The results were analyzed using the CalQplex threshold and normalized using GAPDH as an internal control.
Statistical analysis
The Kaplan-Meier survival curve was used to determine graft survival and the log-rank test was used to compare survival rates between the groups. Student’s t test or Mann–Whitney U test were used for comparison of quantitative data between each 2 groups, when appropriate. In case of zero value in 1 group, 1-sample t test was employed for such comparison. Results are presented as mean ± standard error of mean (SEM). P < 0.05 was considered statistically significant.
RESULTS
Tregs migrate to the ipsilateral cornea and draining lymph nodes of graft recipients after subconjunctival injection
In order to determine the kinetics of Treg migration after subconjunctival injection, 1×105 Green Fluorescent Protein (GFP)+ Tregs were injected into graft recipients immediately after performing allogeneic corneal transplantation. Ipsilateral conjunctivae, cornea, and cervical and submandibular DLN were harvested at 6 hours, 48 hours, and 7 days posttransplantation, and the absolute and percentage frequencies of GFP+CD4+CD25+ Tregs were determined using flow cytometry. In the ipsilateral conjunctivae, Tregs were predominantly detected 6 hours after injection and decreased gradually in number by day 7 (Figure 1). In ipsilateral corneas, Tregs could be detected in low frequencies at 6 hours postinjection, but reached their maximum frequencies after 48 hours. In ipsilateral DLNs, Tregs were observed 6 hours after injection, slightly increased in number after 48 hours, and were nearly undetectable on day 7 postinjection (Figure 1).
Figure 1. Dynamic distribution of GFP+ Tregs injected subconjunctivally after corneal transplantation.

GFP+ Tregs were isolated and subconjunctivally injected to allograft recipients immediately after transplantation. Ipsilateral conjunctivae, corneas, draining lymph nodes (DLN), and spleen were harvested at different time points and analyzed using flow cytometry. Zero hour (0 hr) data is derived from transplanted mice not receiving GFP+ Treg injection. Representative flow cytometric dot plots show frequencies of GFP + Tregs in different tissues at different time points after injection. In ipsilateral conjunctivae, Tregs were predominantly detected at 6h, and decreased gradually from 6h to day 7. In ipsilateral corneas, Tregs were detected at 6h postinjection, but reached their maximum frequencies after 48 hours. In ipsilateral DLN, Tregs were detected at 6h, slightly increased in frequencies at 48h, and were undetectable on day 7. Ipsi, ipsilateral; Conj, conjunctivae; DLN, draining lymph node; preinj., preinjection; postinj., postinjection. Data represents mean ± SEM of 2 independent experiments and each time point consists of 4 mice.
Subconjunctivally injected Tregs suppress corneal and lymphoid antigen-presenting cell maturation
During inflammation, corneal APCs undergo maturation and express high levels of MHC class II and costimulatory molecules.21,22 These cells play a fundamental role in mediating the adaptive immune responses, as they migrate to draining lymph nodes via afferent lymphatics and present alloantigens to naïve T cells.23,24 We thus investigated whether subconjunctivally injected Tregs can inhibit the maturation of APCs in the cornea and draining lymph nodes. 1×105 Tregs, Tconv or combination of both was delivered via subconjunctival injection to mice immediately after allogeneic corneal transplantation. Transplanted mice not receiving subconjunctival treatment served as controls. The ipsilateral corneas and DLNs were harvested on day 14 after surgery. Previous work from our group has showed day 14 to be the optimal time point for evaluation of the immune response to allograft prior to the onset of graft rejection at week 3.13,20,25 The absolute and percentage frequencies of CD11b+ MHC II+ mature APCs and expression of MHC II by these cells in the cornea and DLNs were investigated. Our flow cytometry data demonstrated that the absolute frequencies of mature APCs were significantly reduced in the cornea of mice treated with Tregs alone, and not with combination of Tregs and conventional T cells, compared to Tconv-treated (p=0.024) and untreated transplanted mice (p=0.037) by 45% and 43%, respectively (Figure 2A & B). In addition, corneal APCs of graft recipients treated with Tregs showed significantly lower levels of MHC class II (as assessed by mean fluorescence intensity [MFI]) compared to Tconv-treated (p=0.004) and untreated controls (p=0.008) (Figure 2C). In DLN, the absolute frequencies of CD11b+ MHC II+ mature APCs were significantly higher in untreated controls (p<0.0001) and graft recipients treated with Tconv (p=0.0004) compared to uninflamed corneas of naïve nontransplanted mice. However, transplanted mice receiving injection of Tregs alone, and not Tregs plus conventional T cells, displayed a significant decrease in the absolute frequencies of mature APCs and APC expression of MHC class II compared to Tconv-treated (p<0.001 and p<0.001, respectively) and untreated graft recipients (p=0.002 and p=0.002, respectively) (Figure 2D-F).
Figure 2. Subconjunctivally injected Tregs suppress corneal and lymphoid antigen-presenting cell (APC) maturation.
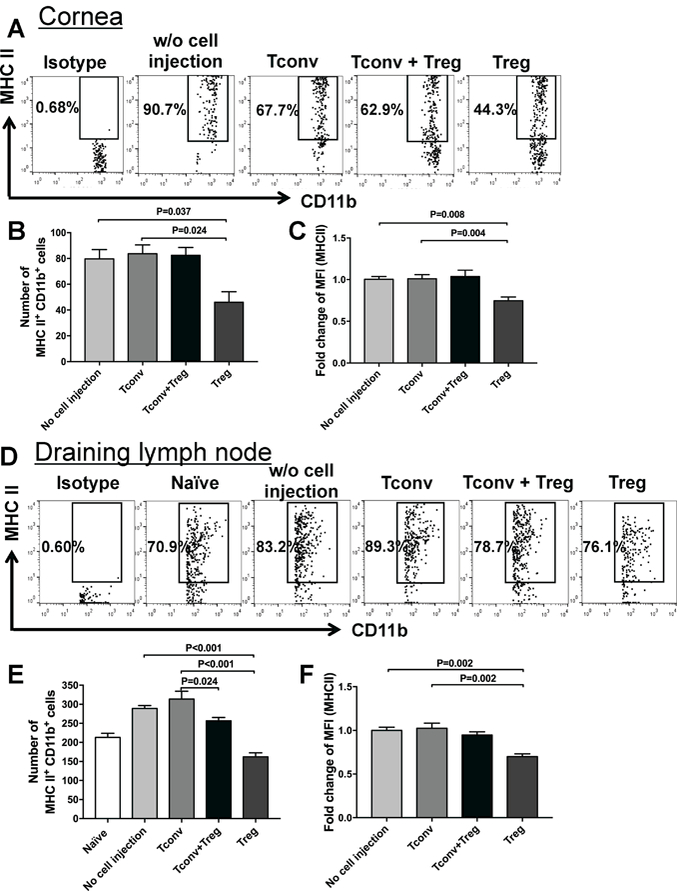
Representative flow cytometry dot plots and bar graphs showing percent and absolute frequencies of CD11b+ MHC II+ mature APCs in the (A & B) cornea and (D & E) draining lymph nodes (DLN) of transplanted mice treated with subconjunctival injection of Tregs, Tconv, Treg plus Tconv and untreated controls. (C & F) Fold change of mean fluorescence intensity (MFI) of MHC II in CD11b+ cells was assessed in cornea and DLN by normalizing the values in each group to the mean MFI of transplanted uninjected controls. Each group in panels A-C consists of 4–6 samples, and every sample contains 2 ipsilateral corneas from 2 different mice. Each group in panels D-F consists of 6 lymph nodes from 6 different mice. Data represent mean ± SEM of 2 independent experiments.
Subconjunctival injection of Tregs decreases activation of IFNγ+ CD4+ Th1 cells in the draining lymph nodes
APC-primed T cells undergo clonal expansion in draining lymph nodes and differentiate into IFNγ-secreting CD4+ Th1 cells, which are considered the predominant effector cells mediating corneal allograft rejection.26–28 Therefore, we next assessed the frequencies of IFNγ+ CD4+ Th1 cells in the ipsilateral DLNs of allograft recipients 2 weeks after transplantation. Flow cytometry analysis showed significantly higher frequencies of Th1 cells in untreated controls (p=0.006) and graft recipients treated with Tconv (p=0.003) compared to naïve nontransplanted mice. However, with Treg treatment, the frequencies of IFNγ+ CD4+ Th1 cells decreased significantly compared to both untreated and Tconv-treated graft recipients (p=0.048 and p=0.017, respectively) (Figures 3A and B). Next, we quantified mRNA and protein expression levels of proinflammatory cytokine IFNγ in the ipsilateral cornea at day 14 after transplantation using real-time PCR and ELISA, respectively. Graft recipients treated with Tregs demonstrated significantly lower IFNγ mRNA levels compared to Tconv-treated controls (p=0.029) (Figure 3C). IFNγ protein levels were undetectable in naïve corneas or Treg-treated corneas, but were significantly higher in corneas of Tconv-treated mice (p=0.003) and untreated controls (p=0.001) (Figure 3D).
Figure 3. Subconjunctival injection of Tregs decreases activation of IFNγ + CD4+ Th1 cells in the draining lymph node.
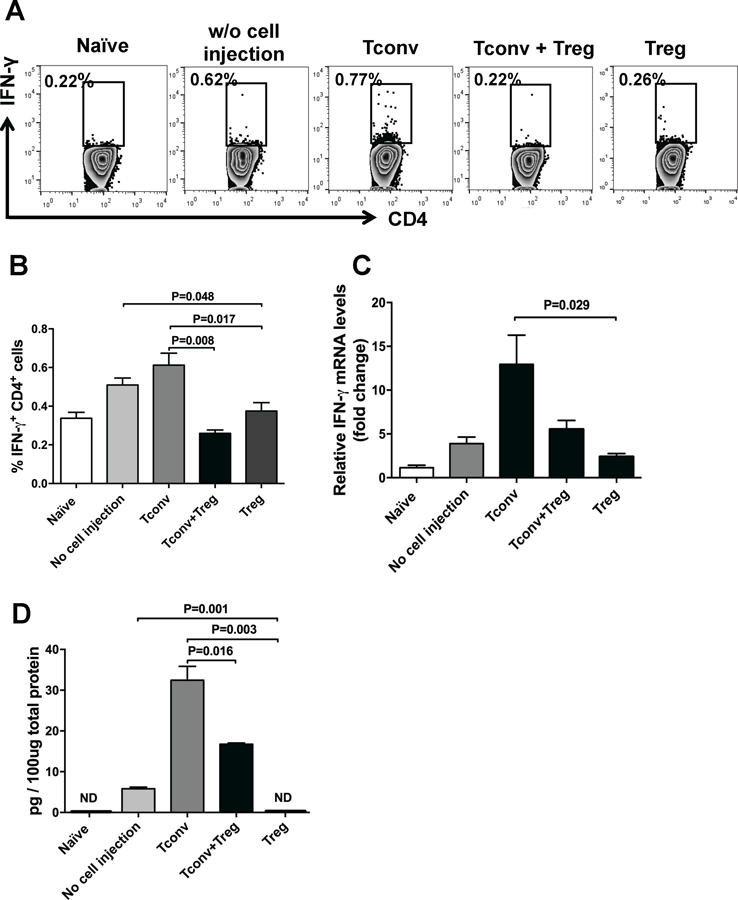
(A) Representative flow cytometric dot plots showing IFNγ-expressing CD4+ T cells in the ipsilateral draining lymph nodes of naïve mice, transplanted mice treated with subconjunctival injection of CD4+CD25+ Tregs, CD4+CD25− conventional T cells (Tconv), Treg plus Tconv and untreated controls at week 2 posttransplantation. (B) Bar graph showing frequencies of IFN-γ+ CD4+ Th1 cells in different treatment groups. (C) Relative changes in mRNA levels of IFN-γ in cornea 2 weeks after transplantation were measured using real-time PCR normalized to values in naïve mice. Levels of IFN-γ were significantly lower in the cornea of graft recipients treated with Tregs compared with Tconv-treated controls. (D) The protein levels of IFN-γ in the cornea of different treatment groups were analyzed using ELISA. IFNγ levels were undetectable in naïve corneas or Treg-treated corneas, but were significantly higher in corneas of Tconv-treated and untreated controls. Data represents mean ± SEM of 2 independent experiments and each group consists of 6 mice. ND, not detectable.
Treg injection suppresses CD45+ leukocyte infiltration into the cornea and increases expression of antiinflammatory cytokines
To determine whether local injection of Tregs could suppress ocular inflammation, we examined the frequencies of CD45+ leukocytes infiltrating the grafted corneas using flow cytometry. The frequencies of CD45+ inflammatory cells were significantly decreased in Treg-treated graft recipients compared to Tconv-treated and untreated controls (p=0.016 and p=0.028, respectively), indicating suppressed corneal inflammation with subconjunctival Treg treatment (Figures 4A and B). Given that one of the principal mechanisms by which Tregs suppress effector immune responses is through secretion of IL-10 and TGF-β 29,30, we next used ELISA to analyze the protein expression levels of these antiinflammatory cytokines in ipsilateral corneas of grafted mice at day 14 after transplantation. As shown in Figures 4C and 4D, Treg treatment was associated with 4.7-fold and 1.4-fold increase in levels of IL-10 and TGF-β in the ipsilateral cornea of graft recipients compared to untreated controls (p=0.03 and p=0.031, respectively). We observed a similar trend in IL-10 and TGF-β levels comparing Treg-treated mice with Tconv-treated controls (p=0.029 and p=0.057, respectively).
Figure 4. Treg injection suppresses CD45+ leukocyte infiltration and increases expression of antiinflammatory cytokines in the cornea.
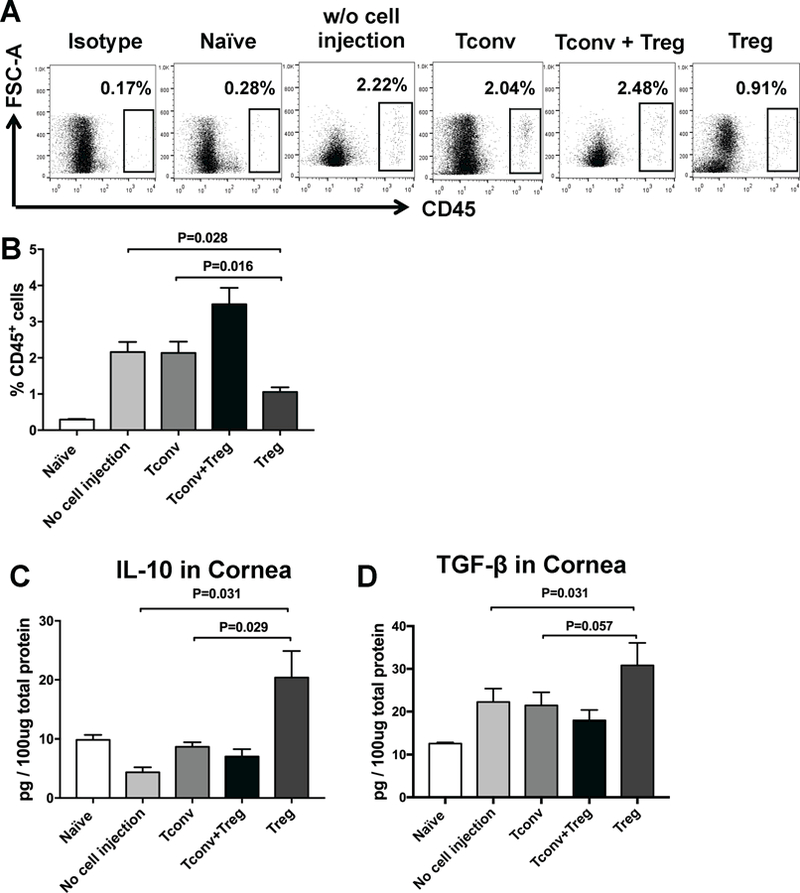
(A) Representative flow cytometric dot plots and (B) Bar graph showing frequencies of CD45+ leukocytes in naïve mice, transplanted mice treated with subconjunctival injection of CD4+CD25+ Tregs, CD4+CD25− conventional T cells (Tconv), Treg plus Tconv and untreated controls at week 2 posttransplantation. (C) The expression levels of IL-10 and TGF-β in cornea after transplantation were evaluated using ELISA. IL-10 and TGF-β levels were significantly higher in Treg-treated graft recipients compared to T conv-treated mice and untreated controls. Data were obtained from n = 6 mice/group and represent mean ± SEM of 2 independent experiments.
Subconjunctival injection of Tregs improves corneal allograft survival and decreases graft opacity scores
Finally, to evaluate the effect of locally delivered Tregs on long-term allograft survival, graft recipients treated with subconjunctival Tregs, Tconv or combination of both were examined weekly for graft opacity until week 10 posttransplantation. Transplanted mice not receiving subconjunctival treatment served as controls. Our results revealed significantly lower graft opacity scores in Treg-treated group compared with Tconv-treated group and untreated controls (p=0.017 and p=0.033 at week 10 after transplantation, respectively) (Figure 5A). Allograft recipients who were treated with Tregs demonstrated significantly higher survival rates (75%) during weeks 3 to 8 after transplantation compared to Tconv-treated (33%, p=0.033) and untreated control groups (30%, p=0.029) (Figure 5B). These data collectively indicate that Tregs, but not their effector counterparts, ie, CD4+ CD25− Tconv or total CD4+ cells (Treg+ Tconv), are the cellular component that suppress the effector alloimmune response and improve graft survival.
Figure 5. Subconjunctival injection of Tregs improves corneal allograft survival and decreases graft opacity scores.
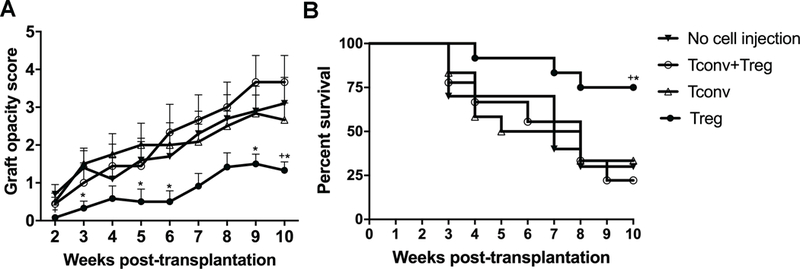
Allogeneic corneal transplantation was performed followed by immediate subconjunctival injection of 1×105 of CD4+CD25+ Tregs, CD4+CD25− conventional T cells (Tconv), or Treg plus Tconv. Untreated transplanted mice served as the control. Graft survival was monitored for up to 10 weeks posttransplantation. (A) Graft opacity scores were significantly lower in Treg-treated group compared with Tconv-treated mice and untreated controls. (B) Weekly examination of grafts revealed a significant increase in graft survival in Treg-treated mice compared with Tconv-treated (75% vs. 33%) and untreated controls (75% vs. 30%) during weeks 3 to 8 after transplantation. Data are combined from 2 independent experiments (n=12). Data shown in panel A represent mean ± SEM. Kaplan-Meier curve and Log-rank test were used to determine graft survival in panel B. * p<0.05 for difference between Treg- and Tconv-treated groups, and + p<0.05 for difference between Treg-treated group and untreated transplanted controls.
DISCUSSION
Tregs-based therapies have been recognized as promising cell-based strategies for induction of tolerance to allografts.11 The efficacy of Treg immunotherapies in restoring immune tolerance has been investigated in several clinical trials for graft-versus-host disease,31,32 liver transplantation,33 type 1 diabetes,34 and kidney transplantation.4 The encouraging early results from these trials support further investigations on the role of Tregs in other organ transplants such as the cornea. Previous studies, including prior investigations from our group, have shown the functional relevance of adoptive transfer of in vitro-expanded Tregs in promoting corneal allograft survival13–15. In this study we define the mechanism by which local administration of freshly isolated Tregs alter the host immune response against the allograft. We demonstrate that Tregs migrate to the cornea and draining lymph nodes of grafted mice after subconjunctival injection. Specifically, locally delivered Tregs inhibit APC maturation, suppress T cell activation, and curb the ocular surface inflammation by upregulating the expression of immunosuppressive cytokines and decreasing leukocyte infiltration to the graft.
Tregs primarily act in secondary lymphoid tissues to regulate the alloimmune responses.35 Studies have shown that Tregs also migrate to the site of graft, where they regulate the ongoing local immune response mediated by alloreactive effector T cells.36,37 Herein, we show the dynamics of distribution of Tregs after subconjunctival injection. Our results demonstrate that locally delivered Tregs migrate to the ipsilateral draining lymph nodes and cornea of grafted mice; they are detected in DLNs of graft recipients as early as 6 hours after injection and reach their maximum frequencies in the cornea after 48 hours. The route of Treg administration plays a central role in tissue distribution of Tregs. It has been reported that after intravenous injection, Tregs are mainly detected in blood, spleen, and DLN, with only a small number reaching the site of corneal graft.14,15 This could explain why previous reports have failed to show any beneficial effects from intravenous injection of natural Tregs on graft survival.14,15 The conjunctiva is highly vascularized and is rich in lymphatic vessels, which allows locally delivered Tregs to easily home to the draining lymph nodes.38 Subsequent migration of Tregs to the cornea is dependent on newly formed corneal blood and lymphatic vessels developed after transplantation.39
Tregs induce tolerance through different mechanisms, such as direct T cell cytolysis, interleukin-2 deprivation, secretion of inhibitory cytokines IL-10 and IL-35, and APC downregulation.40 Here we show that locally delivered Tregs prevent the induction phase of the alloimmune response in draining lymph nodes of graft recipients through inhibiting APC maturation and Th1 effector cell activation. We further demonstrate that injected Tregs regulate the ongoing local immune response at the graft site through upregulating the expression of immunosuppressive cytokines, IL-10 and TGF-β at the ocular surface and by decreasing CD45+ inflammatory cell infiltration into the graft. In a study by Hildebrand et al, the investigators demonstrate that subconjunctival injection of unprimed CD4+CD25+ Tregs to baby rats undergoing keratoplasty significantly improves corneal allograft survival, while systemic administration of Tregs fail to provide such protection against graft rejection41. Although these findings corroborate the results of our study in showing the efficacy of local delivery of unprimed Tregs in improving corneal allograft survival, their work did not address the kinetics of locally delivered Tregs or the mechanism by which these cells prolong long-term allograft survival. Our study fully elucidates the dynamics of distribution of Tregs after subconjunctival injection and demonstrates that locally delivered Tregs regulate the alloimmune response towards the graft both in the draining lymph nodes and at the site of graft. In addition, our data show that injection of Tregs’ effector counterparts, ie, CD4+ CD25− Tconv or the combination of Tregs and Tconv fail to suppress the effector immune responses towards the allograft, indicating that Tregs are the key cellular component improving graft outcomes. Abrogated immunoregulatory function of Tregs injected in combination with T conv, as evident by their reduced ability to suppress APC maturation and T cell activation, could be due to Tconv-induced impairment in Treg function, Tconv resistance to Treg suppression, or a combination of both. Various studies have investigated mechanisms by which conventional T cells overcome Treg suppression42,43. High levels of inflammatory cytokines released by injected conventional T cells in addition to effector T cells that have infiltrated the graft after transplantation could abrogate the suppressive function of Tregs. In addition, injection of conventional T cells skews the balance between Tconv and Tregs in favor of conventional T cells and creates an inflammatory milieu where Tregs demonstrate lower suppressive potential. Given that IL-10 and TGF-β are the major cytokines associated with suppressive capacity of Tregs, this effect was reflected in our results as secretion of significantly lower levels of IL-10 and TGF-β by Tregs injected in combination with Tconv compared to Tregs injected alone.
We found that the frequencies of injected Tregs progressively decreased with time, and only a small number of Tregs could be detected in the conjunctivae by day 7. In regards to the limited life span of Tregs,6,44 a consideration is if a single injection would be sufficient or whether a second dose of injection is required to further improve graft survival. Guo et al found that adoptive transfer of a second dose of in vitro-activated Tregs at day 15 after transplantation does not improve graft survival.15 Cunnusamy et al proposed that Treg-mediated suppression is critical for corneal allograft survival during the initial sensitization phase of the alloimmune response, and it is not required after day 30 posttransplantation.45 The endogenous Tregs can increase at later time points, serving as a negative feedback loop to prevent excessive damage to the graft.46 This is consistent with our observation that the immunosuppressive effects of subconjunctivally injected Tregs last up to day 14 despite the fact that very few Tregs are detectable on day 7 after transplantation. However, whether a second injection of Tregs after day 14 will confer additional benefits to graft recipients should be further evaluated.
In summary, our study is the first report to our knowledge to show the dynamics of distribution of Tregs after subconjunctival injection, and define the mechanism by which locally delivered Tregs promote allograft survival. Our data demonstrates the efficacy of local delivery of Tregs in promoting graft survival without requiring prior in vitro expansion, which could potentially make the application of Treg-based therapies significantly more feasible in the clinic. These findings have direct implications in transplant immunology and can provide a solid basis for future clinical translational studies using autologous Tregs to promote allograft survival in human corneal transplantation.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Susanne Eiglmeier for her helpful discussions regarding the project. The first author (CS) would like to thank China Scholarship Council for their support.
Funding: This study was supported by the National Institutes of Health/National Eye Institute Grant R01 EY012963 to RD.
Abbreviations:
- APC
Antigen presenting cell
- DLN
Draining lymph node
- GFP
Green Fluorescent Protein
- IFN-γ
Interferon-γ
- IL-10
Interleukin-10
- MACS
magnetic-assisted cell sorting
- MFI
mean fluorescence intensity
- SEM
standard error of mean
- Tconv
Conventional T cells
- TGF-β
Transforming Growth Factor-β
- Th1
T helper 1
- Treg
Regulatory T cells
Footnotes
Disclosure: The authors have no financial conflicts of interest.
Contributor Information
Chunyi Shao, Schepens Eye Research Institute, Massachusetts Eye & Ear Infirmary, Department of Ophthalmology, Harvard Medical School, 20 Staniford Street, Boston, 02114, MA, USA.
Yihe Chen, Schepens Eye Research Institute, Massachusetts Eye & Ear Infirmary, Department of Ophthalmology, Harvard Medical School, 20 Staniford Street, Boston, 02114, MA, USA.
Takeshi Nakao, Schepens Eye Research Institute, Massachusetts Eye & Ear Infirmary, Department of Ophthalmology, Harvard Medical School, 20 Staniford Street, Boston, 02114, MA, USA.
Afsaneh Amouzegar, Schepens Eye Research Institute, Massachusetts Eye & Ear Infirmary, Department of Ophthalmology, Harvard Medical School, 20 Staniford Street, Boston, 02114, MA, USA.
Jia Yin, Schepens Eye Research Institute, Massachusetts Eye & Ear Infirmary, Department of Ophthalmology, Harvard Medical School, 20 Staniford Street, Boston, 02114, MA, USA.
Maryam Tahvildari, Schepens Eye Research Institute, Massachusetts Eye & Ear Infirmary, Department of Ophthalmology, Harvard Medical School, 20 Staniford Street, Boston, 02114, MA, USA.
Zala Lužnik, Schepens Eye Research Institute, Massachusetts Eye & Ear Infirmary, Department of Ophthalmology, Harvard Medical School, 20 Staniford Street, Boston, 02114, MA, USA.
Sunil K. Chauhan, Schepens Eye Research Institute, Massachusetts Eye & Ear Infirmary, Department of Ophthalmology, Harvard Medical School, 20 Staniford Street, Boston, 02114, MA, USA
Reza Dana, Schepens Eye Research Institute, Massachusetts Eye & Ear Infirmary, Department of Ophthalmology, Harvard Medical School, 20 Staniford Street, Boston, 02114, MA, USA.
REFERENCES
- 1.Chong EM, Dana MR. Graft failure IV. Immunologic mechanisms of corneal transplant rejection. Int Ophthalmol 2008;28:209–222. [DOI] [PubMed] [Google Scholar]
- 2.Bank Association of America. 2015. Eye Banking Statistical Report http://www.restoresight.org/wp-content/uploads/2016/03/2015-Statistical-Report.pdf. Published 2016.
- 3.Thompson RW Jr, Price MO, Bowers PJ, Price FW Jr. Long-term graft survival after penetrating keratoplasty. Ophthalmology 2003;110:1396–1402. [DOI] [PubMed] [Google Scholar]
- 4.Khan MA. T regulatory cell mediated immunotherapy for solid organ transplantation: A clinical perspective. Mol Med 2016;22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eliseeva DD, Lifshits GV, Lokhonina AV, Zhdanov DD, Zavalishin IA, Bykovskaya SN. The treatment by expanded ex vivo autologous regulatory T-cells CD4+CD25+FoxP3+CD127low restores the balance of immune system in patients with remitting-relapsing multiple sclerosis. Zh Nevrol Psikhiatr Im S S Korsakova 2016;116:54–62. [DOI] [PubMed] [Google Scholar]
- 6.Gregoire S, Terrada C, Martin GH, et al. Treatment of Uveitis by In Situ Administration of Ex Vivo-Activated Polyclonal Regulatory T Cells. J Immunol 2016;196:2109–2118. [DOI] [PubMed] [Google Scholar]
- 7.Noyan F, Zimmermann K, Hardtke-Wolenski M, et al. Prevention of Allograft Rejection by Use of Regulatory T Cells With an MHC-Specific Chimeric Antigen Receptor. Am J Transplant 2017;17:917–930. [DOI] [PubMed] [Google Scholar]
- 8.Lee K, Nguyen V, Lee KM, Kang SM, Tang Q. Attenuation of donor-reactive T cells allows effective control of allograft rejection using regulatory T cell therapy. Am J Transplant 2014;14:27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xia G, He J, Leventhal JR. Ex vivo-expanded natural CD4+CD25+ regulatory T cells synergize with host T-cell depletion to promote long-term survival of allografts. Am J Transplant 2008;8:298–306. [DOI] [PubMed] [Google Scholar]
- 10.Ma B, Yang JY, Song WJ, et al. Combining Exosomes Derived from Immature DCs with Donor Antigen-Specific Treg Cells Induces Tolerance in a Rat Liver Allograft Model. Sci Rep 2016;6:32971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lam AJ, Hoeppli RE, Levings MK. Harnessing Advances in T Regulatory Cell Biology for Cellular Therapy in Transplantation. Transplantation 2017;101:2277–2287. [DOI] [PubMed] [Google Scholar]
- 12.Tkachev V, Furlan SN, Watkins B, et al. Combined OX40L and mTOR blockade controls effector T cell activation while preserving Treg reconstitution after transplant. Sci Transl Med 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chauhan SK, Saban DR, Lee HK, Dana R. Levels of Foxp3 in regulatory T cells reflect their functional status in transplantation. J Immunol 2009;182:148–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chauhan SK, Saban DR, Dohlman TH, Dana R. CCL-21 conditioned regulatory T cells induce allotolerance through enhanced homing to lymphoid tissue. J Immunol 2014;192:817–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo X, Jie Y, Ren D, et al. In vitro-expanded CD4(+)CD25(high)Foxp3(+) regulatory T cells controls corneal allograft rejection. Hum Immunol 2012;73:1061–1067. [DOI] [PubMed] [Google Scholar]
- 16.Safinia N, Scotta C, Vaikunthanathan T, Lechler RI, Lombardi G. Regulatory T Cells: Serious Contenders in the Promise for Immunological Tolerance in Transplantation. Front Immunol 2015;6:438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chai JG, Coe D, Chen D, Simpson E, Dyson J, Scott D. In vitro expansion improves in vivo regulation by CD4+CD25+ regulatory T cells. J Immunol 2008;180:858–869. [DOI] [PubMed] [Google Scholar]
- 18.Inomata T, Mashaghi A, Di Zazzo A, Dana R. Ocular surgical models for immune and angiogenic responses. J Biol Methods 2015;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sonoda Y, Streilein JW. Orthotopic corneal transplantation in mice--evidence that the immunogenetic rules of rejection do not apply. Transplantation 1992;54:694–704. [DOI] [PubMed] [Google Scholar]
- 20.Tahvildari M, Omoto M, Chen Y, et al. In Vivo Expansion of Regulatory T Cells by Low-Dose Interleukin-2 Treatment Increases Allograft Survival in Corneal Transplantation. Transplantation 2016;100:525–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamrah P, Liu Y, Zhang Q, Dana MR. Alterations in corneal stromal dendritic cell phenotype and distribution in inflammation. Arch Ophthalmol 2003;121:1132–1140. [DOI] [PubMed] [Google Scholar]
- 22.Huq S, Liu Y, Benichou G, Dana MR. Relevance of the direct pathway of sensitization in corneal transplantation is dictated by the graft bed microenvironment. J Immunol 2004;173:4464–4469. [DOI] [PubMed] [Google Scholar]
- 23.Dana MR. Corneal antigen-presenting cells: diversity, plasticity, and disguise: the Cogan lecture. Invest Ophthalmol Vis Sci 2004;45:722–727; 721. [DOI] [PubMed] [Google Scholar]
- 24.Amouzegar A, Chauhan SK, Dana R. Alloimmunity and Tolerance in Corneal Transplantation. J Immunol 2016;196:3983–3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hua J, Inomata T, Chen Y, et al. Pathological conversion of regulatory T cells is associated with loss of allotolerance. Sci Rep 2018;8:7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cunnusamy K, Niederkorn JY. IFN-gamma blocks CD4+CD25+ Tregs and abolishes immune privilege of minor histocompatibility mismatched corneal allografts. Am J Transplant 2013;13:3076–3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hegde S, Beauregard C, Mayhew E, Niederkorn JY. CD4(+) T-cell-mediated mechanisms of corneal allograft rejection: role of Fas-induced apoptosis. Transplantation 2005;79:23–31. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Hamrah P, Zhang Q, Taylor AW, Dana MR. Draining lymph nodes of corneal transplant hosts exhibit evidence for donor major histocompatibility complex (MHC) class II-positive dendritic cells derived from MHC class II-negative grafts. J Exp Med 2002;195:259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamaguchi T, Wing JB, Sakaguchi S. Two modes of immune suppression by Foxp3(+) regulatory T cells under inflammatory or non-inflammatory conditions. Semin Immunol 2011;23:424–430. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med 2001;194:629–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Theil A, Tuve S, Oelschlagel U, et al. Adoptive transfer of allogeneic regulatory T cells into patients with chronic graft-versus-host disease. Cytotherapy 2015;17:473–486. [DOI] [PubMed] [Google Scholar]
- 32.Brunstein CG, Miller JS, McKenna DH, et al. Umbilical cord blood-derived T regulatory cells to prevent GVHD: kinetics, toxicity profile, and clinical effect. Blood 2016;127:1044–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Todo S, Yamashita K, Goto R, et al. A pilot study of operational tolerance with a regulatory T-cell-based cell therapy in living donor liver transplantation. Hepatology 2016;64:632–643. [DOI] [PubMed] [Google Scholar]
- 34.Bluestone JA, Buckner JH, Fitch M, et al. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci Transl Med 2015;7:315ra189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siegmund K, Feuerer M, Siewert C, et al. Migration matters: regulatory T-cell compartmentalization determines suppressive activity in vivo. Blood 2005;106:3097–3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dijke IE, Weimar W, Baan CC. Regulatory T cells after organ transplantation: where does their action take place? Hum Immunol 2008;69:389–398. [DOI] [PubMed] [Google Scholar]
- 37.Graca L, Cobbold SP, Waldmann H. Identification of regulatory T cells in tolerated allografts. J Exp Med 2002;195:1641–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakao S, Hafezi-Moghadam A, Ishibashi T. Lymphatics and lymphangiogenesis in the eye. J Ophthalmol 2012;2012:783163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cursiefen C, Cao J, Chen L, et al. Inhibition of hemangiogenesis and lymphangiogenesis after normal-risk corneal transplantation by neutralizing VEGF promotes graft survival. Invest Ophthalmol Vis Sci 2004;45:2666–2673. [DOI] [PubMed] [Google Scholar]
- 40.Noval Rivas M, Chatila TA. Regulatory T cells in allergic diseases. J Allergy Clin Immunol 2016;138:639–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hildebrand A, Jarsch C, Kern Y, Bohringer D, Reinhard T, Schwartzkopff J. Subconjunctivally applied naive Tregs support corneal graft survival in baby rats. Mol Vis 2014;20:1749–1757. [PMC free article] [PubMed] [Google Scholar]
- 42.Mercadante ER, Lorenz UM. Breaking Free of Control: How Conventional T Cells Overcome Regulatory T Cell Suppression. Front Immunol 2016;7:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walker LS. Regulatory T cells overturned: the effectors fight back. Immunology 2009;126:466–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ding Y, Shen S, Lino AC, Curotto de Lafaille MA, Lafaille JJ. Beta-catenin stabilization extends regulatory T cell survival and induces anergy in nonregulatory T cells. Nat Med 2008;14:162–169. [DOI] [PubMed] [Google Scholar]
- 45.Cunnusamy K, Chen PW, Niederkorn JY. IL-17A-dependent CD4+CD25+ regulatory T cells promote immune privilege of corneal allografts. J Immunol 2011;186:6737–6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang Q, Bluestone JA, Kang SM. CD4(+)Foxp3(+) regulatory T cell therapy in transplantation. J Mol Cell Biol 2012;4:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]


