Abstract
Chronic neuropathic pain may be caused, in part, by loss of inhibition in spinal pain processing pathways due to attenuation of local GABAergic tone. Nociception and nocifensive behaviors are reduced after enhancement of tonically activated extrasynaptic GABAAR mediated currents by agonist ligands for δ subunit-containing GABAARs. However, typical ligands that target δ subunit-containing GABAARs are limited due to sedative effects at higher doses. We used the spinal nerve ligation (SNL) and gp120 models of experimental neuropathic pain to evaluate compound 2–261, a non-benzodiazepine (BZ) site positive allosteric modulator (PAM) of α4β3δ GABAARs optimized to be non-sedative by selective activation of β2/3 subunit-containing GABAARs over receptor subtypes incorporating β1 subunits. Similar levels of 2–261 were detected in the brain and plasma following intraperitoneal administration. While systemic 2–261 did not alter sensory thresholds in sham-operated animals, it significantly reversed SNL-induced thermal and tactile hypersensitivity in a GABAAR dependent fashion. Intrathecal 2–261 produced conditioned place preference (CPP) and elevated dopamine levels in the nucleus accumbens of nerve-injured, but not sham-operated rats. Additionally, systemic pretreatment with 2–261 blocked CPP from spinal clonidine in SNL rats. Moreover, 2–261 reversed thermal hyperalgesia and partially reversed tactile allodynia in the gp120 model of HIV-related neuropathic pain. The effects of 2–261 likely required interaction with the α4β3δ GABAAR since 2–301, a close structural analog of 2–261 with limited extrasynaptic receptor efficacy, was not active. Thus, 2–261 may produce pain relief with diminished side-effects through selective modulation of β2/3 subunit-containing extrasynaptic GABAARs.
Keywords: GABAA receptors, positive allosteric modulation, β2/3 subunits, neuropathic pain, conditioned place preference, dopamine release
Summary
Compound 2–261, a non-benzodiazepine (BZ) site positive allosteric modulator (PAM) of α4β3δ GABAARs was effective in reducing affective and sensory qualities of experimental neuropathic pain.
INTRODUCTION
Few therapies with novel mechanisms of action have been introduced to clinical practice for pain [45]. Poor overall efficacy of available medications is reflected by high numbers-needed-to-treat values (i.e., NNT) [14,42,46,65]. Non-steroidal anti-inflammatory agents (NSAIDs) often have limited efficacy in chronic pain [13,37]. Opioid use for the treatment of chronic non-malignant pain remains uncertain due to questionable efficacy [37] and serious toxicity including lethal respiratory depression, somnolence, constipation, hyperalgesia, tolerance, dependence and addiction that collectively diminish quality of life [21]. Thus, targeting a non-opioid receptor intimately involved in pain with decreased abuse and addiction liability would be highly significant [19].
GABAARs have long been hypothesized to represent targets for pain therapeutics [28]. GABAARs are complex heteropentameric proteins formed by two α, two β and one γ subunit [61,63] that function to hyperpolarize neurons and inhibit neurotransmission through chloride ion influx. Diminished synaptic and/or extrasynaptic inhibition at spinal GABA receptors promotes enhanced network excitability that sustains chronic pain [26,27,69]. It has been reported that the expression of GABAAR δ subunits and the GABAergic tonic current decreased in substantia geletinosa neurons following chronic constriction injury in mice and that enhancement of δ subunits-containing GABAARs in the spinal cord dorsal horn neurons attenuated acute nociception, blocked long-term potentiation and inhibited formalin-induced spontaneous pain [27,41]. Recently, de Koninck and colleagues demonstrated that down-regulation of spinal KCC2 potassium chloride co-transporters results in loss of the electrochemical chloride gradient in states of experimental chronic pain [9]. Therefore, restoration of spinal inhibitory control is hypothesized to be analgesic [16,74].
Thalamic GABAAR isoforms are also associated with the processing of pain signals [44,51,62]. The thalamus exhibits both phasic and tonic GABAergic inhibitory mechanisms via GABA projections to sensory relay neurons, originating sensory nuclei or the thalamic reticular nucleus (Rt) [44,62]. Microinjection of the GABAAR antagonist picrotoxin into the ventrobasal thalamus (VB) complex in rats results in pain-like behavior [51]. Immunohistological studies indicate robust expression of α4, β2, and δ GABAAR subunits in the thalamus [30,53]. Gaboxadol, a α4βδ subtype-selective agonist, is analgesic in animals and humans [1,18,36,38,39,57,58,59], suggesting that activation of α4βδ GABAARs may contribute to the reduction in pain.
We previously reported a series of non-benzodiazepine site enaminone positive allosteric modulators (PAMs) that show preference for β2/3 expressing GABAARs as promising therapies for anxiety [17,24]. The unique GABA mechanism of β2/3 preferring compounds suggests that these compounds could modulate pain states in a therapeutically superior manner compared to currently prescribed GABAAR PAMs (i.e., benzodiazepines, BZs). Here, we report 2–261, a non-steroidal small molecule β2/3 subunit preferring PAM, evokes robust positive modulation at BZ-insensitive α4β3δ GABAARs to mitigate chronic pain states.
METHODS
Two electrode voltage-clamp electrophysiology.
Two-electrode voltage clamp recordings were made 3–14 days following mRNA injections of isoforms of human GABAAR αβ or αβδ subunits at a holding voltage of −70 mV using electrophysiological procedures that were previously described [24]. The GABA recordings were performed in standard Ringer and drug/wash solutions were applied via a microcapillary “linear array” that enables sub-second application of agonists. Currents were recorded on a PC-based computer (PClamp 9.0, Molecular Devices, Sunnyvale, CA). All compounds were tested with a 30 second pretreatment prior to co-application with EC10 GABA (concentration of GABA that evokes 10% of the maximum response) for the control response. The GABA EC10 was determined in each individual oocyte expressing the receptor subtype of interest. Percent modulation of the GABA EC10 by the modulator was calculated as: (((I GABA EC10 + modulator / I GABA EC10) X 100) – 100). A four-parameter logistic equation (GraphPad Software, San Diego, CA) was used to fit concentration-effect data.
Animals
Adult, male Sprague-Dawley rats (250–350 g, Envigo, Indianapolis, IN) were used. The experiments followed the guidelines of the Committee for Research and Ethical Issues of IASP. All procedures were approved by the University of Arizona Institutional Animal Care and Use Committee. Rats were housed three per cage on a 12 hour light-dark cycle (lights on at 7 am) with food and water ad libitum. After intrathecal (i.th.) catheterization or intracranial cannulation, animals were housed individually. All behavioral studies were performed by experimenters blinded to the experimental groups and treatments.
L5/L6 Spinal nerve ligation (L5/L6 SNL)
Lumbar 5 and 6 spinal nerve were ligated according to [34]. Briefly, 5 and 2 % isoflurane delivered in air at 1 L/min were used for initiation and maintenance of anesthesia. L5/L6 were tightly ligated with 4–0 silk suture using aseptic procedure. The animals were returned to their home cages for recovery for 12–14 days. Sham surgeries were done similarly without ligation of the spinal nerves. The health of the animals was monitored closely and any that demonstrated motor deficits or failed to develop tactile or thermal hypersensitivity after nerve injury were excluded from further testing; 6–8 animals were used per group.
HIV-related neuropathic pain
HIV-related neuropathic pain was induced with spinal administration of the HIV envelope protein gp120 (300 ng/20 μl/rat, i.th.) injected every other day for 3 days (i.e., at days 1, 3 & 5) according to published reports [72]; 5–6 animals were used per group.
Nucleus accumbens (NAc) cannulation
Stereotaxic surgeries were performed as previously published [67]. Rats were anesthetized with ketamine/xylazine 80/12 mg/kg, i.p. (dissolved in normal saline, Western Medical Supply, Arcadia, CA/Sigma, St. Louis, MO). A single guide cannula (AG-8, EICOM, San Diego, CA) was implanted into the left NAc shell using the co-ordinates determined by a rat brain atlas (Paxinos and Watson, 6th Edition): anteroposterior: bregma +1.7 mm; medial lateral: midline −1.0 mm; dorsoventral: skull−6.0 mm. Rats were allowed to recover for 7 days individually after surgery. Data from animals with incorrect cannula placements were discarded by post hoc histological verification.
Intrathecal (i.th.) catheterization
The animals were anesthetized ketamine/xylazine (80/12 mg/kg, i.p.) anesthesia, and i.th. catheters (polyethylene 10, 7.5 cm) were inserted through the atlanto-occipital membrane with the catheter tip positioned slightly rostral to the lumbar spinal cord as described previously [67]. Animals were housed individually afterwards and allowed to recover for at least 7 days. Rats with motor deficits were euthanized. Intrathecl injection volumes were a 5 (drug) or 20 (gp120) μl treatment solution plus a 0.5 μl air bubble and a 10 μl saline flush.
Evaluation of tactile thresholds and thermal latencies
The paw withdrawal threshold was evaluated using calibrated von Frey filaments (Stoelting, Wood Dale, IL) ranging from 0.41 to 15 g (4–150 N) to probe the plantar surface perpendicularly in a Dixon “up and down” fasion as described previously [6,11,35,68]. Antiallodynic effects were calculated as (Post-dose threshold – Pre-dose threshold)/(Baseline – Pre-dose threshold) x 100. For thermal hyperalgesia, paw withdrawal latency was evaluated using Hargreaves’ analgesia meter with an infrared radiant heat source as previously described [5,48,66]. Rats were acclimated to the Plexiglas chambers for 30 min and the left hindpaws were tested. The intensity of the heat stimulus was determined initially to set the baseline latencies at approximately 20 sec. A maximal cutoff of 33 sec was used to prevent tissue damage. Antihyperalgesic effects were calculated as (Post-dose latency – Pre-dose latency)/(Baseline – Pre-dose Latency) x 100.
Conditioned Place Preference (CPP) Procedures
We used the single trial conditioning protocol for CPP as previously described [35,40,48]. Briefly, on baseline (Day 1) and testing (Day 3) days, rats were allowed to explore all three CPP chambers that had distinct olfactory, tactile and visual cues for 15 min. Anymaze (Stoelting, USA) were used to record the time spent in each chamber. The animals were counterbalanced based on their baseline results to prevent pre-conditioning bias. Two pairings were done on conditioning day (Day 2, 14 days post-SNL). In the morning, rats received a vehicle injection (5 μl, i.th.) and paired with the assigned chamber for 30 min. Four hours later, rats received 2–261 (0.2 μg/5 μl, i.th. injection) and were paired with the opposite chamber for 30 min. Difference scores were calculated as test time subtracted by baseline time in the drug-paired chamber. Animals with biased baseline (i.e., <20% or >80% of time spent in one chamber on Day 1) were excluded from further test. Additionally, data from animals that showed no, or low, levels of exploration on test day (<10 sec spent in two out of the three chambers on Day 3) were excluded from the data analysis.
Separate groups of rats were tested for the blockade of i.th. clonidine (10 μg)-indued CPP by pretreatment of systemic 2–261 (10 mg/kg, i.p.) in SNL rats. The procedures were as described above except that during the conditioning day, the animals were pretreated with vehicle (1 ml/kg, i.p.) followed 30 min later by i.th. injection of saline (5 μl) and then immediately paired with one chamber. In the afternoon, the animals received 2–261 (10 mg/kg, i.p.) followed 30 min later by i.th. clonidine (10 μg/5 μl) administration and then were immediately paired with the other chamber. 9–16 animals were used for SNL and sham-operated groups.
In vivo microdialysis and HPLC quantification of dopamine (DA)
Microdialysis was done in awake, freely moving animals as described previously [48,67]. The microdialysis probe (AI-8–2, EICOM, San Diego, CA) with semipermeable membrane (MW cutoff: 20 kDa) was inserted into the NAc through the guide cannula with a 2 mm protrusion to pass beyond scar tissue. Artificial cerebrospinal fluid (aCSF: 147.0 mM NaCl, 2.8 mM KCl, 1.2 mM MgCl2 and 1.2 mM CaCl2) was perfused at 1.25 μl/min and 30 min fractions were collected into cold (4ºC) amber Eppendorf tubes pre-filled with 1.0 μl 40× antioxidant solution (6.0 mM L-cysteine, 2.0 mM oxalic acid and 1.3% w/v glacial acetic acid) [25]. After a 90 min washout period, 2 baseline and 4 treatment fractions (30 min/fraction) plus 2 positive control fractions after cocaine (20 mg/kg, i.p.) injection were collected.
All fractions were analyzed using liquid chromatography (Agilent 1100 HPLC equipped with a 5020 guard cell (set at 350 mV) and a MD-150 column) with an electrochemical detector (Coulochem III 5014B, Electrode1 set at −150 mV, and Electrode2 at 250 mV; Thermofisher, USA) at ambient temperature. The limit of detection (LOD) and limit of quantification (LOQ) were calculated to be 0.286 and 0.868 pg, respectively, from 6 serial dilutions of DA standard (1.25 – 40 pg). The integration of the DA peaks from HPLC chromatograms was performed by an experimenter blinded to the treatment groups.
In order to compare the results from different groups of animals, we calculated Percent Change from Baseline values. The accumulated area under the time effect curve (AUC) was also calculated. Data from rats with extremely low basal DA levels (below LOQ), misplaced cannula, uneven baselines (>50% difference in DA concentrations between the two baseline fractions), or lack of sufficient increase of extracellular DA after cocaine injections (<100% over baseline) were excluded from data analysis. The group size for Sham/Vehicle, Sham/2–261, SNL/Vehicle and SNL/2–261 was 4, 7, 10 and 15, respectively.
Drug administration
2–261 and analogs were synthesized as previously described [24] and dissolved in 10% DMSO and 90% saline. Allopregnanolone was synthesized as previously described [55]. Gabazine was purchased from Sigma Aldrich (St. Louis, MO). Clonidine hydrochloride (Tocris, Ellisville, MO) and cocaine (obtained from NIDA) were dissolved in saline (0.9% NaCl). Gp120 (SPEED BioSystems, Gaithersburg, MD) was dissolved in 0.1% BSA in 0.1 M PBS at 15 ng/μl final concentration [72]. The dose volume for i.p. injections was 1 ml/kg, unless specified otherwise.
Pharmacokinetic studies of 2–261
PK analysis was performed as previously described [70]. Briefly halothane-euthanized animals received cardiac puncture at various timepoints after 2–261 administration. Plasma in blood samples was separated by centrifugation at 1000 × g for 6 min. Rat brains were removed after perfusion with saline. Brain and plasma samples were stored at −20°C until processed for extraction and analysis. Brain samples (~0.1 g) were combined with 1 mL phosphate-buffered saline (pH 7.4) and homogenized and combined with 1 μL 1–029 [33] (10 μg/mL) as internal standard. 0.5 mL ethyl acetate (EtOAc) was used to extract brain samples. 0.5 mL acetonitrile (ACN) was used to extract plasma samples also combined with 1 μL 1–029 (10 μg/mL). Brain and plasma extract/solvent mixtures were spun 1000 × g for 10 min. The decanted plasma and brain extraction supernatants were evaporated to dryness. Samples were resuspended in 100 μL of ACN, vortexed and pipetted to sample tubes. Plasma and brain samples were acquired through a Shimadzu SIL-HTc autosampler, run through a C18 column (Poroshell 120 EC-C18 4.6 × 50 mm, 2.7 μM; Agilent Technologies, Santa Clara CA, USA) using a Shimadzu LC-10ADvp HPLC via a 90% ACN / 10% H2O mobile phase and multiple reaction monitoring on a Quattro Premier (Waters, Milford, MA, USA) using MassLynx v4.0 software.
Statistics
All results are expressed as mean ± SEM. GraphPad Prism 7 and JFlashcalc (developed by Dr. Michael Ossipov) are used for statistics analyses. Unpaired t-test (two-tailed, for two groups), One-Way ANOVA post-hoc Tukey’s multiple comparisons test or repeated measure Two-Way ANOVA post-hoc Sidak’s, Tukey’s or Fisher LSD multiple comparisons test are used for more than 2 groups comparison. Paired t-test (two-tailed) is used for CPP data analysis. Significance is set at P<0.05.
RESULTS
2–261 is a potent and efficacious modulator of GABAA α4β3δ receptors.
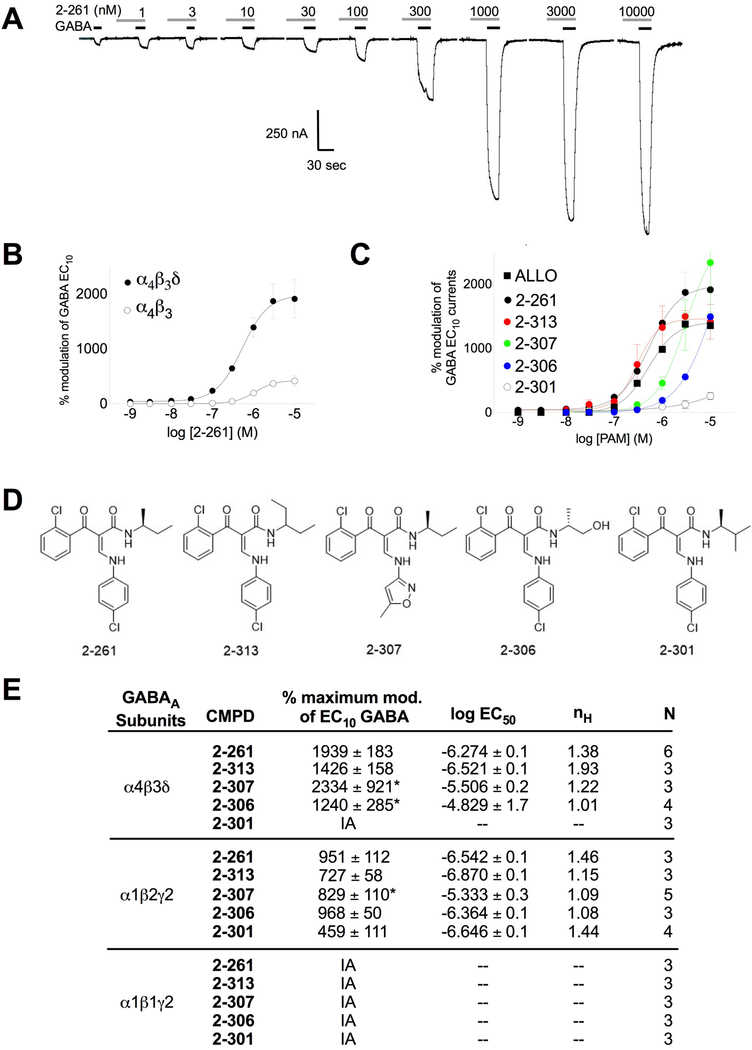
2–261 showed high-efficacy (>1000%) positive allosteric modulation of GABA-evoked EC10 currents in oocytes expressing GABAARs without significant direct effects up to a concentration of 10,000 nM (Fig. 1A). 2–261 elicited maximum modulation of GABA-evoked EC10 currents in human α4β3δ GABAARs of ~1600% (EC50 = 265 nM) versus ~400% (EC50 = 1000 nM) in human α4β3 GABAARs (Fig. 1B). 2–261 exceeded the neurosteroid allopregnanolone (ALLO) for positive modulation of α4β3δ GABAARs in potency (520 nM) and maximum efficacy (~1400%) (Fig. 1C). We evaluated a series of close 2–261 analogs with limited efficacy at β1-subunit containing GABAARs (similar to 2–261) and demonstrated differential potency and efficacy for positive modulation of α4β3δ GABAARs (Fig. 1C, 1D and 1E). These 2–261 analogs also exhibited robust maximum modulation of β2/3-subunit containing GABAARs (Fig. 1E). Structure-activity analysis shows the position of methyl addition to 2–261 either maintains (2–313) or significantly reduces (2–301) efficacy as a modulator of α4β3δ GABAARs (Fig. 1C, 1D and 1E). This suggests strict steric requirements for α4β3δ GABAAR modulation on the amide moiety of the enaminone template. Compounds like 2–307, where a methylisoxazole replaces the 4-chorophenyl group, retain large amounts of efficacy at α4β3δ GABAARs (Fig. 1C, 1D and 1E). This suggests that heteroaryl groups can replace aryl groups on the amine moiety of the enaminone. Replacement of a methyl group on the amide of 2–261 with a hydroxyl gives 2–306. While 2–306 is still selective for β2/3 over β1-subunit containing GABAARs, it is much less potent than 2–261 at α4β3δ receptors (EC50 >10 μM) (Fig. 1E) indicating that polar groups on the amide moiety reduce activity.
Figure 1.
Effect of 2–261 and analogs on extransynaptic δ-subunit containing GABAA receptors. (A) Representative current tracings of the 2–261 dose response from two-electrode voltage-clamp recordings in X. laevis oocytes expressing human α4β3δ GABAA receptors. (B) Dose response of 2–261 on GABA EC10 – evoked currents in oocytes expressing human GABAA α4β3 (open circles) or α4β3δ (closed circles) receptors. (C) Dose response of 2–261 and various 2–261 analogs on GABA EC10 – evoked currents in oocytes expressing human GABAA α4β3δ receptors. (D) Chemical structures of 2–261 analogs tested. (E) Summary table of 2–261 and analogs on synaptic and extrasynaptic GABAA receptors. Data represent the mean ± S.E.M. (n = 3–6), * represents modulation at 10,000 nM rather than calculated maximum response due to insolubility of compounds beyond this concentration.
Spinal administration of 2–261 reverses spinal nerve ligation (SNL) induced tactile and thermal hypersensitivity in rats.
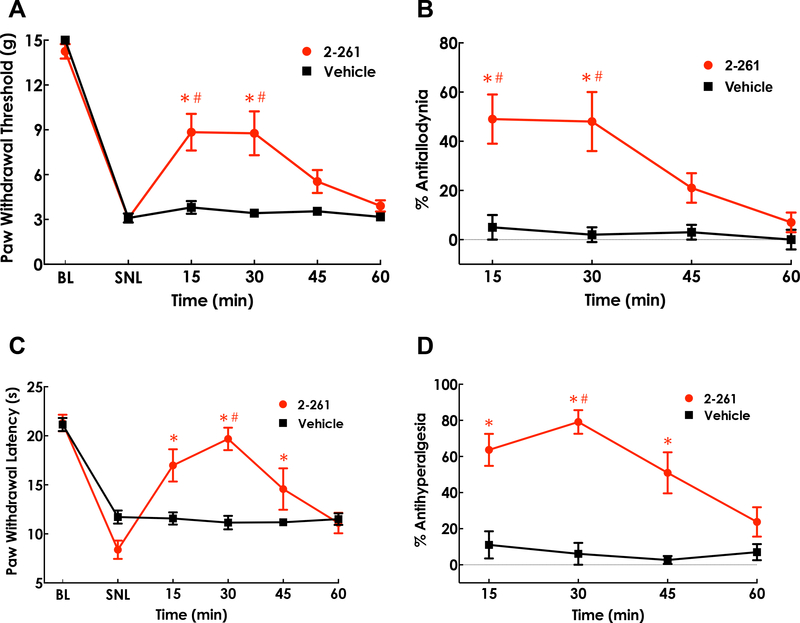
Based on its in vitro receptor subtype selectivity and potency/efficacy profile, 2–261 was used as the prototype α4β3δ-active, β2/3 preferring GABAAR PAM for evaluation of possible effects on the modulation of chronic neuropathic pain. Intrathecal administration of 2–261 (0.2 μg/5 μl) (Fig. 2) was evaluated 14 days following ligation of the L5/L6 spinal nerves. Nerve-injured animals exhibited the expected signs of neuropathic dysesthesias of the affected paw. Administration of 2–261 produced significant reversal (~50%) of tactile thresholds within 15 min in SNL rats (Fig. 2A, 2B) and diminished quickly after 30 min (P<0.001 at 15 and 30 min compared to pre-dose SNL-baseline or vehicle treatment at the same time point). Almost full reversal of thermal hypersensitivity was observed without thermal response thresholds elevating above pre-injury baselines at similar time points post-dose (Fig. 2C, 2D, P<0.001 at 15, 30 and 45 min compared to pre-dose SNL-baseline; P<0.001 at 30 min compared to vehicle treatment at the same time point).
Figure 2.
Antiallodynic and antihyperalgesic effects of intrathecal 2–261 in SNL rats. Paw withdrawal threshold (A) to the innocuous tactile stimulus via calibrated von Frey filaments and paw withdrawal latency (C) to the noxious thermal stimulus via Hargreaves apparatus were assessed prior to SNL surgeries (baseline, BL), at 14 days post-SNL (SNL), and 15, 30, 45, 60, 75, 90 min after drug treatment. %Antiallodynia (B) and %Antihyperalgesia (D) was calculated as (Post dose – Pre dose)/(BL – Pre dose) x 100. Spinal 2–261 (0.2 μg in 5 μl, i.th.) produced statistically significant antiallodynic effect at 15 and 30 min and antihyperalgesic effect at 15, 30 and 45 min post-dose. * P<0.05 compared to pre-dose (post-SNL) baseline; # P<0.05 compared to the vehicle group at the same time point. Repeated measure Two-Way ANOVA post hoc Sidak’s multiple comparison test was used. N=6–8/group.
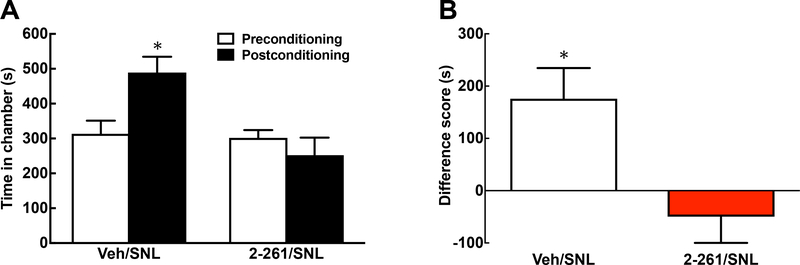
Spinal 2–261 alleviates nerve injury-induced ongoing pain and elicits enhanced release of DA from nucleus accumbens (NAc).
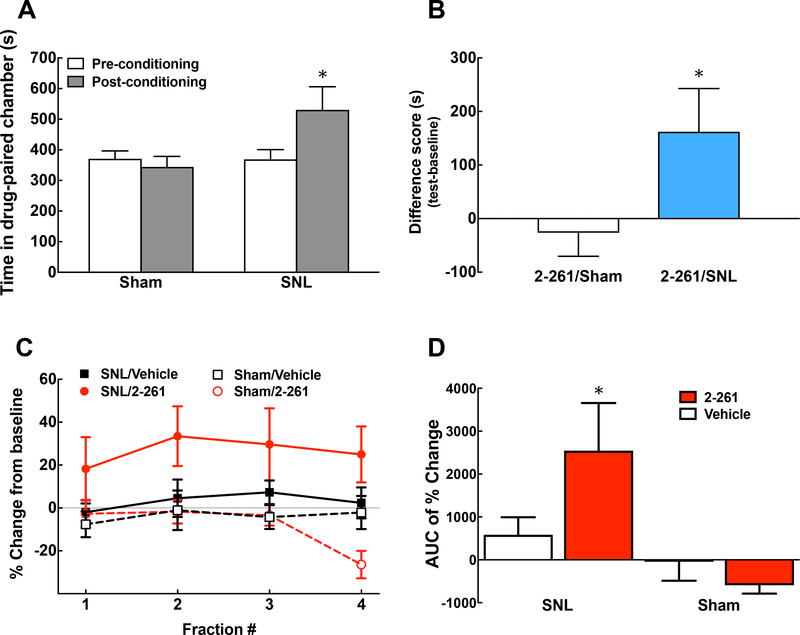
We further evaluated the potential effect of spinal 2–261 (0.2 μg/0.5 μl, i.th.) on inhibiting ongoing pain using the conditioned place preference (CPP) assay as well as measurement of in vivo DA release from the NAc (Fig. 3). Rats received either vehicle or 2–261 treatment paired with a specific chamber at day 14 post-SNL or sham surgeries. The rats with nerve injury preferred the 2–261-paired chamber, suggesting that 2–261 provides sufficient relief of ongoing pain to promote learning (Fig. 3A, 3B, P<0.05 pre-conditioning vs. post-conditioning). No preference was observed in sham-operated animals following 2–261 treatment. The lack of intrinsic reward or addictive liabilities of this compound is consistent with our previous results in naïve animals [70]. The mitigation of pain aversiveness by 2–261 also results in enhanced DA release to the interstital fluid in the NAc selectively in SNL animals (Fig 3C, 3D). The release of NAc DA increased from baseline throughout the entire collection period (4 fractions of 30 min each, total 2 h, Fig. 3C) and peaked at the 2nd fraction. The baseline DA levels among SNL and sham-operated animals were not significantly different (0.35 ± 0.021 and 0.35 ± 0.02 pg/μl, or 0.882 ± 0.135 and 0.885 ± 0.190 nM, respectively), in agreement with our previous findings [67]. We converted the data from each time point to Percent Change from Baseline in order to normalize the data within each individual animal. The accumulated change of DA release across two hours following 2–261 administration is illustrated as area under the time effect curve (AUC) showing significantly higher DA release in SNL/2–261 group than that of SNL/Vehicle or Sham/2–261 group (Fig. 3D, P<0.05 compared to the SNL/vehicle or Sham/2–261 group). In line with the CPP results, NAc DA levels did not change following drug administration in sham-operated rats.
Figure 3.
Spinal 2–261 relieves spontaneous pain and activates the reward pathway in SNL rats. Conditioned place preference (CPP) was performed at days 13–15 post-SNL or sham surgeries. Time Spent in the Drug (2–261, 0.2 μg in 5 μl, i.th.)-Paired Chamber before and after the conditioning (A) as well as the Difference Score between these two time points (B) are demonstrated. The animals clearly prefer 2–261-paired chamber after nerve injury (P<0.05 Pre-vs. Post-conditioning in A, Paired t-test), which is not observed in the sham-operated group (P<0.05 SNL vs. Sham groups in B, Unpaired t-test). N = 10 and 16 for SNL and sham, respectively. The release of DA from the nucleus accumbens shell in the microdialysis fractions (30 min/fraction) following administration of 2–261 (0.2 μg in 5 μl, i.th.) are determined in rats with SNL or Sham injuries (C). %Change from Baseline was used to normalize samples from individual animals. The area under the time effect curve (AUC) was calculated to compare the effect of each treatment (D). * P<0.05 compared to SNL/Vehicle or Sham/2–261. One-Way ANOVA post hoc Fisher’s LSD test was used to analyze the data. N = 4, 7, 10 and 15 for Sham/Vehicle, Sham/2–261, SNL/Vehicle and SNL/2–261, respectively.
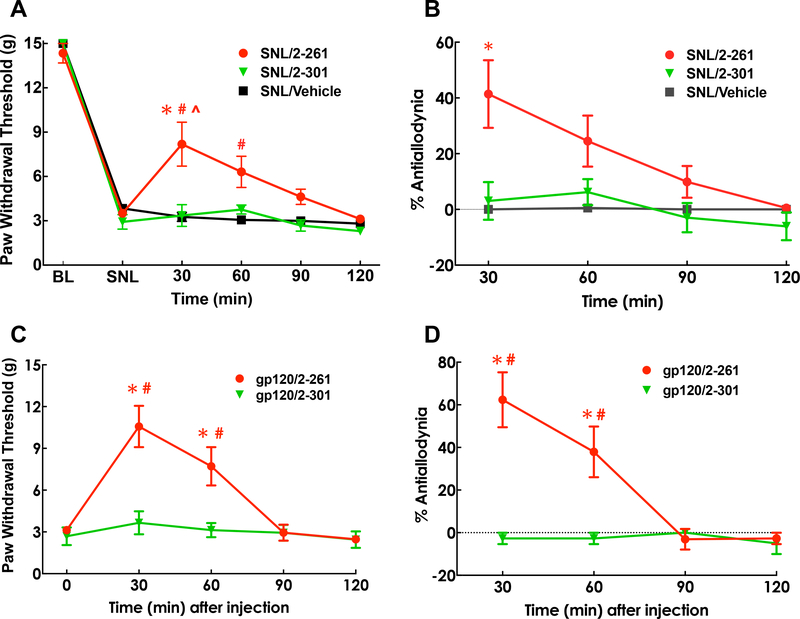
Systemic administration of 2–261 reverses SNL-induced tactile and thermal hypersensitivity in rats.
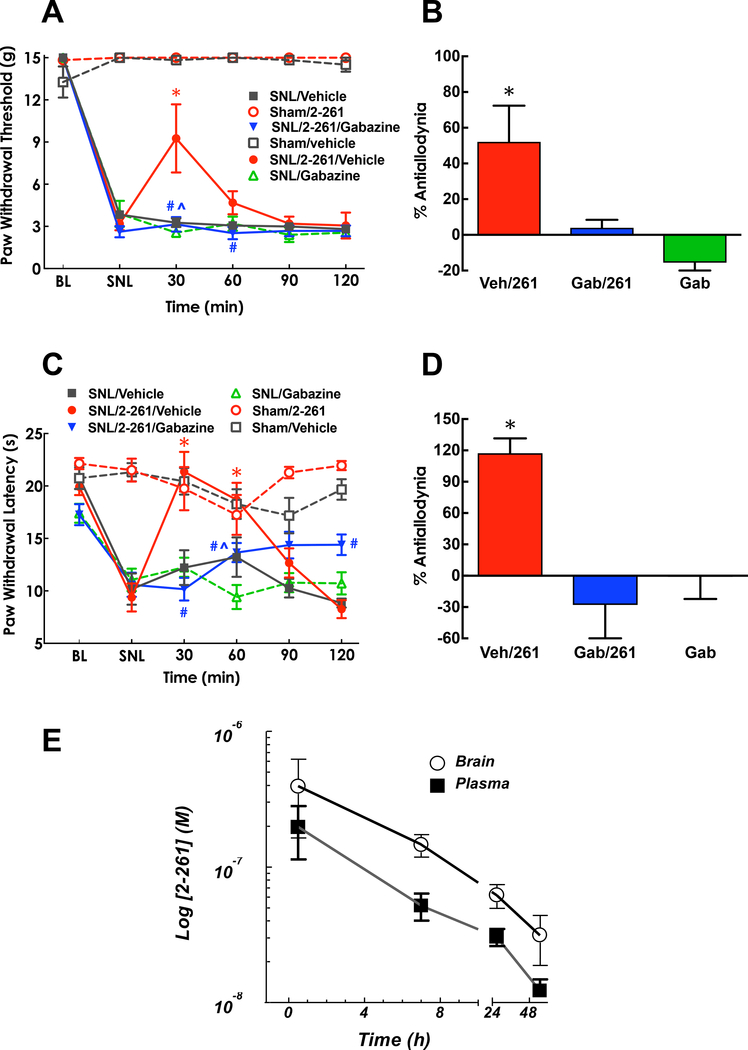
We tested the efficacy of systemic 2–261 in alleviating nerve-injury induced hypersensitivity by administering 2–261 at a dose previously demonstrated to produce anxiolytic actions (i.e., 10 mg/kg, i.p.) [17]. No change in baseline tactile or thermal thresholds was observed when 2–261 was administered in sham-operated controls. 2–261 significantly reversed the expected signs of neuropathic dysesthesias including tactile allodynia and thermal hyperalgesia of the affected paw in animals with SNL injury. Systemic 2–261 produced significant, albeit partial, reversal of tactile thresholds (Fig. 4A, P<0.001 at 30 and 60 min post-dose compared to pre-dose (SNL) baseline or vehicle treatment at the same time point) in SNL rats and full reversal of thermal hypersensitivity (Fig. 4C, P<0.001 at 30 and 60 min post-dose compared to pre-dose (SNL) baseline or vehicle treatment at the same time point). Note that thermal response thresholds were not elevated above pre-injury baselines suggesting that 2–261 normalized hypersensitivity but did not inhibit physiological thermal nociception. The activity of 2–261 was completely abolished by systemic pretreatment of the GABAAR antagonist gabazine at 1 mg/kg i.p., indicating that 2–261 exerts its effects by potentiating GABAAR function (Fig. 4B and 4D, P<0.05 and P<0.01 SNL/Gabazine/2–261 vs. SNL/Vehicle/2–261 treatment for tactile and thermal hypersensitivity, respectively). The long duration and equivalent brain to plasma ratio of 2–261 concentrations after 10 mg/kg i.p. administration (Fig. 4E, [2–261]brain vs [2–261]plasma not statistically different by Repeated measure Two-Way ANOVA post hoc Sidak’s multiple comparison test) also suggests that supraspinal loci may be engaged to facilitate relief of pain.
Figure 4.
Antiallodynic and antihyperalgesic effects of systemic administration of 2–261 in SNL rats. Paw withdrawal threshold (A) to the innocuous tactile stimulus via calibrated von Frey filaments and paw withdrawal latency (C) to the noxious thermal stimulus via Hargreaves apparatus were assessed prior to SNL surgeries (baseline, BL), at 14 days post-SNL (SNL), and 30, 60, 9, 120 min after drug treatment. %Antiallodynia (B) and %Antihyperalgesia (D) was shown at 30 min post dose and calculated as (Post dose – Pre dose)/(BL – Pre dose) x 100. Systemic 2–261 (10 mg/kg, i.p.) produced statistically significant antiallodynic effect at 30 min and antihyperalgesic effect at 30 and 60 min post-dose. Both effects were blocked by gabazine (1 mg/kg, s.c.) given at 30 min prior to 2–261. * P<0.05 compared to pre-dose (post-SNL) baseline; # P<0.05 compared to the SNL/2–261/vehicle group at the same time point; ^ P<0.05 compared to the SNL/Gabazine group at the same time point. Repeated measure Two-Way (A, C) or One-Way (B, D) ANOVA post hoc Tukey’s multiple comparison test was used. N=5–6/group. 2–261 (10 mg/kg, i.p.) reached peak concentration within 30 min post-dose and quickly dissipated (E). The concentrations in the brain were not statistically different than those of plasma at all time points tested using Repeated measure Two-Way ANOVA post hoc Sidak’s multiple comparison test.
Pretreatment of systemic 2–261 blocked spinal clonidine-induced CPP in SNL rats.
In addition to sensory thresholds, systemic 2–261 treatment was evaluated in the CPP assay for modulation of pain aversiveness. CPP is a measure of learning in animals to associate a context with a treatment. For this reason, rapid pharmacokinetics of drug administration is important in promoting single trial learning for pain relief. We have shown that systemic treatments that abolish aversive states of pain can block the CPP that is produced with rapidly acting treatments (e.g., i.th. clonidine, an α-adrenergic agonist) [35]. Here, 2–261 blocked the CPP resulting from spinal clonidine in SNL rats such that the post-conditioning with clonidine does not occur (Fig. 5, P<0.05 pre-conditioning vs. post-conditioning in SNL/Vehicle group for the Time Spent in Clonidine-Paired Chamber; P<0.05 SNL/Vehicle vs. SNL/2–261 group for the Difference Score). This result suggests that systemic 2–261 produces relief of the aversive state of ongoing pain that is sufficient to abolish motivational drive to seek a context associated with spinal clonidine.
Figure 5.
Systemic 2–261 relieves spontaneous pain in SNL rats. Conditioned place preference (CPP) was performed at days 13–15 post-SNL surgeries. The conditioning was done by pairing one chamber with spinal clonidine (10 μg/5 μl, i.th.) and the opposite chamber with saline administration. 2–261 (10 mg/kg, i.p.) or vehicle was given 30 min prior to each spinal treatments. Time Spent in the clonidine-paired chambers before and after the conditioning (A) as well as the Difference Score between these two time points (B) are demonstrated. The animals receiving vehicle pretreatment prefer clonidine-paired chamber after nerve injury (P<0.05 Pre-vs. Post-conditioning in A, Paired t-test). 2–261 pre-treatment blocked clonidine-induced CPP. This was shown in Difference Score as well (P<0.05 vehicle vs. 2–261 groups in B, Unpaired t-test). N=9 and 11 in vehicle- and 2–261-pretreated group, respectively.
Relief of chronic pain by 2–261 depends on activity at α4β3δ GABAARs.
We tested 2–301, a compound that retains activity at β2/3 subunits, but has very limited activity at α4β3δ subunits (see Fig. 1C and 1E) [24]. 2–301 (20 mg/kg i.p.) did not show activity against tactile hypersensitivity caused by SNL at twice the dose of 2–261 following i.p. administration (Fig. 6A, 6B, P<0.001 at 30 and 60 min post-dose compared to pre-dose (SNL) baseline or vehicle or 2–301 treatment at the same time point), consistent with the hypothesis that activity at α4β3δ is essential for antihyperalgesic activity. The lack of analgesic activity of 2–301 due to limited brain penetration is unlikely since 2–301 has been previously reported to exhibit robust anxiolytic activity at 15% of the dose used in the current study via the same route of administration [71].
Figure 6.
Comparison of the effects of 2–261 and 2–301 in rats with neuropathic pain induced by nerve injury (SNL, A, B) or HIV envelope protein gp120 (C, D). Paw withdrawal threshold (A, C) to the innocuous tactile stimulus via calibrated von Frey filaments was assessed. %Antiallodynia (B, D) was calculated as (Post dose – Pre dose)/(BL – Pre dose) x 100. Systemic 2–261 (10 mg/kg, i.p.) produced statistically significant antiallodynic effect at 30 and 60 min post-dose at 14 days post-SNL (A, B) or 7 days post-gp120 (C, D). 2–301 (20 mg/kg, i.p.) did not alter the allodynia in either model. * P<0.05 compared to pre-dose (post-SNL or gp120) baseline; # P<0.05 compared to 2–301 treatment at the same time point; ^ P<0.05 compared to the vehicle group at the same time point. Repeated measure Two-Way ANOVA post hoc Tukey’s multiple comparison test was used. N=5–6/group.
Effects in HIV-related neuropathic pain.
In addition to physical trauma, neuropathic pain can also result from infections, including from the human immunodeficiency virus (HIV). HIV related neuropathic pain is very difficult to treat and clinical trials demonstrate that reuptake blockers are ineffective and gabapentinoids are marginally effective [47]. We therefore tested 2–261 (10 mg/kg, i.p.) in a model of HIV-related neuropathic pain in order to determine whether the extrasynaptic δ-subunit hypothesis applied in other models of chronic pain. The HIV envelope protein, gp120 (300 ng/20 μl, i.th.), was injected every other day for 3 days via lumbar puncture. Long-lasting tactile allodynia was developed after 7 days post-dose. Similar to its effect in the SNL trauma-induced neuropathic pain model, systemic 2–261 significantly reversed tactile allodynia in gp120 pretreated rats (Fig. 6C, 6D, P<0.01 or P<0.05 at 30 and 60 min post-dose compared to pre-dose (SNL) baseline or 2–301 treatment at the same time point, respectively), an observation that indicates its potential utility in the treatment of chronic pain states. As in the SNL model, we found that 2–301 (20 mg/kg i.p.) was devoid of activity in the gp120 neuropathic pain model at twice the effective dose of 2–261.
DISCUSSION
It is not currently known whether chronic non-malignant pain can be treated by modulators of GABAARs. Compounds that are currently available show (a) no evidence of effectiveness clinically (e.g., BZs); (b) are not used due to intolerable side-effects (for review, see [60]); and (c) lack of activity at α4β3δ GABAAR subtypes that may be essential for analgesia [4,52]. BZs have been suggested to modulate acute pain (e.g., the tail-flick test) in uninjured animals [49,54,73] based on evaluation of sensory thresholds, but, this observation is not consistent with clinical experience since virtually no clinical evidence for analgesic effects of BZs exists [12,20,43]. One reason for this disconnect may be that preclinical studies emphasizing sensory withdrawal thresholds are complicated by subtle effects related to sedation and motor function. Additionally, such reflexive measures are unlikely to capture dimensions of pain that matter most to humans (i.e., pain aversiveness) [15]. We have demonstrated in multiple pain models that ongoing pain can be unmasked through behavioral assessment using conditioned place preference (CPP), a procedure that pairs a context with a treatment that effectively alleviates ongoing pain [10,35,40,48,50,56]. Relief of aversive states (e.g., thirst, hunger, cold) is a natural reward that evokes DA release within the NAc as seen in animal and human imaging studies [2,3].
We have synthesized and previously characterized the in vitro and in vivo pharmacology of the enaminone 2–261, a GABAAR subtype selective PAM with anxiolytic activity in mice and rats [17,24]. Repeated systemic administration of 2–261 was demonstrated to be devoid of reinforcing properties, lacked effects on learning and memory at doses well in excess of anxiolytic doses, did not potentiate the depressant effects of ethanol and did not induce tolerance to its anxiolytic effects [70]. In the current study, 2–261 and its analogs were shown to have potent and robust effects on extrasynaptic α4β3δ GABAARs comparable to ALLO, the prototypical α4β3δ GABAAR neurosteroid PAM. Extrasynaptic α4β3δ GABAARs-mediated tonic inhibition have been suggested to play important roles in promoting central sensitization and pathological pain. While δ-subunit null (Gabrd−/−) mice have similar responses to acute nociception and formalin phase 1 responses, phase 2 formalin responses are greatly potentiated in these animals [4]. Compound 2–261 is therefore representative of a unique class of non-steroidal small molecules that are orally bioavailable, have robust and potent activity as α4β3δ GABAAR PAMs and are selective for β2/3 subunit-containing receptor subtypes, a profile that may contribute to a benign side-effect profile. Other small molecule PAMs have been reported to have selective actions at α4β3δ GABAAR subtypes [29,64] but none with the degree of receptor subtype selectivity demonstrated by the enaminone series that mitigates potential side effects. Activity of 2–261 in pain states associated with central sensitization supports the possibility of actions at extrasynaptic α4β3δ GABAARs though further experimentation will be required before drawing definitive conclusions.
We evaluated the potential effects of 2–261 in assays that can capture affective (aversive) dimensions of pain, using pain motivated behaviors, a measure that may be viewed as “analogous” to human self-report in non-verbal animals. Additionally, we have previously shown that in multiple rodent pain models that extracellular DA levels in NAc increase in response to pain alleviating drugs [10,48,67] i.e., relief of a pain-induced aversive state is rewarding. In the current study we used NAc DA efflux as an objective neurochemical correlate of behavior to establish efficacy of novel compounds in diminishing the aversive dimension of chronic pain. Spinal and systemic administration of 2–261 was effective in modulation of both sensory and affective (aversive) outcome measures, indicating that actions at α4β3δ and selectivity for β2/3 subunit containing GABAARs have utility in promoting pain relief. This notion is further reinforced by the observation that a close structural analog of 2–261 which lacks activity at α4β3δ (2–301) does not induce pain relief in the models tested.
Preclinically, much attention has been focused on restoring GABA-mediated inhibition in spinal networks that may be diminished in experimental models of neuropathic pain as a way to develop new pain therapeutics. Selective reduction of protein expression of spinal GABAA δ subunits has been shown after peripheral nerve injury [27]. Moreover, enhanced GABAA δ subunit activity blunts the initiation and maintenance of spinal LTP to ameliorate central sensitization of nociceptive behaviors [41]. However, significant pain-mitigating effects may be achieved by enhancing GABAA δ subunit activity in regions of the nervous system beyond the spinal cord and must be considered in the evaluation of the potential of this target for treatment of pain. In this regard, Heinricher and colleagues have administered non-subunit selective GABAAR agonists within the rostral ventromedial medulla (RVM), a major site of descending pain modulation that is engaged by many clinically effective pain drugs (e.g., opiates, reuptake blockers), and showed hyperalgesic effects [22]. Hyperalgesia from direct application of GABAAR agonists onto the RVM may result from specific inhibition of OFF-cells normally responsible for feedback loops that buffer pain signals through the RVM [7,8,23].
While this confound may initially appear as a limitation of the utility of compounds administered by the systemic route, our data indicate that systemic administration of 2–261 does not produce hyperalgesia at doses associated with pain relief. Additionally, increased inhibition within the rostral anterior cingulate cortex (rACC), an area thought to integrate the aversive features of pain [31,32,56], produces CPP selectively in injured rats [56]. These observations are consistent with the systemic activity of 2–261 in producing CPP in neuropathic rats suggesting that modulation of pain aversiveness within supraspinal networks in other brain regions that express α4, β2, and δ GABAA subunits such as the thalamus may additionally contribute to a non-spinal site of action of β2/3 subunit selective GABAAR PAMs [30,53].
Additionally, we found that spinal 2–261 reversed evoked hyperalgesia in SNL rats without promoting increases in sensory thresholds in sham-operated rats (i.e., did not elicit analgesia). The antihyperalgesic actions of spinal 2–261 were accompanied by an increase in NAc DA release selectively in rats with neuropathic pain suggesting the reward of pain relief. The lack of CPP or DA efflux after 2–261 treatment in sham-operated rats indicates that spinal 2–261 is not intrinsically rewarding. Thus the mechanism associated with 2–261 appears unlikely to be associated with abuse potential of available analgesics such as opioids. Additionally, the high brain penetration feature of molecules similar to 2–261 may allow for actions at both supraspinal and spinal levels. Activity at both supraspinal and spinal sites of these molecules might therefore result in site-site synergy, a possibility that requires further experimentation. If true, such site-site synergy would potentially allow their use for treatment of pain at doses that are increasingly separable from those producing side-effects (i.e., increased therapeutic index).
Chronic pain patients often suffer from pain co-morbid with anxiety. The development of novel α4β3δ-active, β2/3 subunit preferring GABAAR PAMs that avoid the side-effects of the traditional agonist BZs suggests an innovative profile of compounds that may produce pain modulation together with anti-anxiety effects. Thus, combined pain relief and anxiolytic actions within a single molecule may be a highly desirable mechanism that may provide clinical benefit.
Finally, we note that the successful association of drug-induced pain relief with the paired chamber in injured rats suggests that spinal 2–261 does not lead to detectable impairment of learning and memory which is consistent with our earlier observations on coginitive function [70]. Our data collectively indicate that GABAA β2/3 subtype-selective PAMs with corresponding efficacy at α4β3δ GABAARs and a benign side effect profile represent a promising new target for the development of novel analgesics to treat chronic pain.
Acknowledgments:
This work was supported by grants from the National Institutes on Drug Abuse (DA041307, F.P.; DA041454, K.W.G.).
Footnotes
The authors of the manuscript have no conflict of interest to report.
REFERENCES
- 1.Asiedu MN, Mejia G, Ossipov MK, Malan TP, Kaila K, Price TJ. Modulation of spinal GABAergic analgesia by inhibition of chloride extrusion capacity in mice. J Pain 2012;13:546–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baliki MN, Geha PY, Fields HL, Apkarian AV. Predicting value of pain and analgesia: nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron 2010;66:149–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becerra L, Navratilova E, Porreca F, Borsook D. Analogous responses in the nucleus accumbens and cingulate cortex to pain onset (aversion) and offset (relief) in rats and humans. J Neurophysiol 2013;110:1221–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonin RP, Labrakakis C, Eng DG, Whissell PD, De Koninck Y, Orser BA. Pharmacological enhancement of delta-subunit-containing GABA(A) receptors that generate a tonic inhibitory conductance in spinal neurons attenuates acute nociception in mice. Pain 2011;152:1317–1326. [DOI] [PubMed] [Google Scholar]
- 5.Burgess SE, Gardell LR, Ossipov MH, Malan TP Jr, Vanderah TW, Lai J, Porreca F. Time-dependent descending facilitation from the rostral ventromedial medulla maintains, but does not initiate, neuropathic pain. J Neurosci 2002;22:5129–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994;53:55–63. [DOI] [PubMed] [Google Scholar]
- 7.Chen Q, Roeder Z, Li MH, Zhang Y, Ingram SL, Heinricher MM. Optogenetic Evidence for a Direct Circuit Linking Nociceptive Transmission through the Parabrachial Complex with Pain-Modulating Neurons of the Rostral Ventromedial Medulla (RVM). eNeuro 2017;4:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cleary DR, Neubert MJ, Heinricher MM. Are opioid-sensitive neurons in the rostral ventromedial medulla inhibitory interneurons? Neurosci 2008;151:564–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coull JA, Boudreau D, Bachand K, Prescott SA, Nault F, Sík A, De Koninck P, De Koninck Y. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature 2003;424:938–42. [DOI] [PubMed] [Google Scholar]
- 10.De Felice M, Eyde N, Dodick D, Dussor GO, Ossipov MH, Fields HL, Porreca F. Capturing the aversive state of cephalic pain preclinically. Ann Neurol 2013;74:257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol 1980;20:441–62. [DOI] [PubMed] [Google Scholar]
- 12.Dundee JW, Haslett WH. The benzodiazepines. A review of their actions and uses relative to anaesthetic practice. Br J Anaesth 1970;42:217–34. [DOI] [PubMed] [Google Scholar]
- 13.Dworkin RH, O’Connor AB, Audette J, Baron R, Gourlay GK, Haanpää ML, Kent JL, Krane EJ, Lebel AA, Levy RM, Mackey SC, Mayer J, Miaskowski C, Raja SN, Rice AS, Schmader KE, Stacey B, Stanos S, Treede RD, Turk DC, Walco GA, Wells CD. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc 2010;85:S3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ebell M, Kripke C. Gabapentin for pain: balancing benefit and harm. Am Fam Physician 2006;73:435. [PubMed] [Google Scholar]
- 15.Fields HL. Pain: an unpleasant topic. Pain 1999;Suppl 6:S61–9. [DOI] [PubMed] [Google Scholar]
- 16.Gagnon M, Bergeron MJ, Lavertu G, Castonguay A, Tripathy S, Bonin RP, Perez-Sanchez J, Boudreau D, Wang B, Dumas L, Valade I, Bachand K, Jacob-Wagner M, Tardif C, Kianicka I, Isenring P, Attardo G, Coull JA, De Koninck Y. Chloride extrusion enhancers as novel therapeutics for neurological diseases. Nat Med 2013;19:1524–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gee KW, Tran MB, Hogenkamp DJ, Johnstone TB, Bagnera RE, Yoshimura RF, Huang JC, Belluzzi JD, Whittemore ER. Limiting activity at beta1-subunit-containing GABAA receptor subtypes reduces ataxia. J Pharmacol Exp Ther 2010;332:1040–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grognet A, Hertz F, DeFeudis FV. Comparison of the analgesic actions of THIP and morphine. Gen Pharmacol. 1983;14:585–9. [DOI] [PubMed] [Google Scholar]
- 19.Guerrini G, Ciciani G, Bruni F, Selleri S, Martini C, Daniele S, Ghelardini C, Di Cesare Mannelli L, Costanzo A. Development of ligands at gamma-aminobutyrric acid type A (GABAA) receptor subtype as new agents for pain relief. Bioorg Med Chem 2011;19:7441–52. [DOI] [PubMed] [Google Scholar]
- 20.Haslett WH, Dundee JW. Studies of drugs given before anaesthesia. XIV. Two benzodiazepine derivatives--chlordiazepoxide and diazepam. Br J Anaesth 1968;40:250–8. [DOI] [PubMed] [Google Scholar]
- 21.Hayes CJ,Li X,Li C, Shah A, Kathe N, Bhandari NR,Payakachat N. Health-Related Quality of Life among Chronic Opioid Users, Nonchronic Opioid Users, and Nonopioid Users with Chronic Noncancer Pain. Health Serv Res 2018. February 25. doi: 10.1111/1475-6773.12836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heinricher MM, Kaplan HJ. GABA-mediated inhibition in rostral ventromedial medulla: role in nociceptive modulation in the lightly anesthetized rat. Pain 1991;47:105–13. [DOI] [PubMed] [Google Scholar]
- 23.Heinricher MM, McGaraughty S. Analysis of excitatory amino acid transmission within the rostral ventromedial medulla: implications for circuitry. Pain 1998;75:247–255. [DOI] [PubMed] [Google Scholar]
- 24.Hogenkamp DJ, Johnstone TB, Huang JC, Li WY, Tran M, Whittemore ER, Bagnera RE, Gee KW. Enaminone amides as novel orally active GABAA receptor modulators. J Med Chem 2007;50:3369–79. [DOI] [PubMed] [Google Scholar]
- 25.Hubbard KE, Wells A, Owsens TS, Tagen M, Fraga CH, Stewart CF. Determination of dopamine, serotonin, and their metabolites in pediatric cerebrospinal fluid by isocratic high performance liquid chromatography coupled with electrochemical detection. Biomed Chromatogr 2010;24:626–31. [DOI] [PubMed] [Google Scholar]
- 26.Hwang JH, Yaksh TL. The effect of spinal GABA receptor agonists on tactile allodynia in a surgically-induced neuropathic pain model in the rat. Pain 1997;70:15–22. [DOI] [PubMed] [Google Scholar]
- 27.Iura A, Takahashi A, Hakata S, Mashimo T, Fujino Y. Reductions in tonic GABAergic current in substantia gelatinosa neurons and GABAA receptor δ subunit expression after chronic constriction injury of the sciatic nerve in mice. Eur J Pain 2016;20:1678–1688. [DOI] [PubMed] [Google Scholar]
- 28.Jacob TC, Moss SJ, Jurd R. GABA(A) receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat Rev Neurosci 2008;9:331–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jensen ML, Wafford KA, Brown AR, Belelli D, Lambert JJ, Mirza NR. A study of subunit selectivity, mechanism and site of action of the delta selective compound 2 (DS2) at human recombinant and rodent native GABA(A) receptors. Br J Pharmacol 2013;168:1118–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jia F, Pignataro L, Harrison NL. GABAA receptors in the thalamus: alpha4 subunit expression and alcohol sensitivity. Alcohol 2007,41:177–85. [DOI] [PubMed] [Google Scholar]
- 31.Johansen JP, Fields HL, Manning BH. The affective component of pain in rodents: direct evidence for a contribution of the anterior cingulate cortex. Proc Natl Acad Sci U S A 2001;98:8077–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johansen JP, Fields HL. Glutamatergic activation of anterior cingulate cortex produces an aversive teaching signal. Nat Neurosci 2004;7:398–403. [DOI] [PubMed] [Google Scholar]
- 33.Johnstone TB, Hogenkamp DJ, Coyne L, Su J, Halliwell RF, Tran MB, Yoshimura RF, Li WY, Wang J, Gee KW. Modifying quinolone antibiotics yields new anxiolytics. Nat Med 2004;10:31–2. [DOI] [PubMed] [Google Scholar]
- 34.Kim SH and Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain 1992;50:355–63. [DOI] [PubMed] [Google Scholar]
- 35.King T, Vera-Portocarrero L, Gutierrez T, Vanderah TW, Dussor G, Lai J, Fields HL, Porreca F. Unmasking the tonic-aversive state in neuropathic pain. Nat Neurosci 2009;12:1364–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kjaer M, Nielsen H. The analgesic effect of the GABA-agonist THIP in patients with chronic pain of malignant origin. A phase-1–2 study. Br J Clin Pharmacol 1983;16:477–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krebs EE, Gravely A, Nugent S, Jensen AC, DeRonne B, Goldsmith ES, Kroenke K, Bair MJ, Noorbaloochi S. Effect of opioid vs non-opioid medications on pain-related function in patients with chronic back pain or hipor knee osteoarthritis pain: The SPACE randomized clinical trial. JAMA 2018;319:872–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krosgaard-Larsen P, Frølund B, Liljefors T, Ebert B. GABA(A) agonists and partial agonists: THIP (Gaboxadol) as a non-opioid analgesic and a novel type of hypnotic. Biochem Pharmacol 2004;68:1573–80. [DOI] [PubMed] [Google Scholar]
- 39.Lindeburg T, Folsgard S, Sillesen H, Jacobsen E, Kehlet H. Analgesic, respiratory and endocrine responses in normal man to THIP, a GABA-agonist. Acta Anaesthesiol Scand 1983;27:10–2. [DOI] [PubMed] [Google Scholar]
- 40.Liu P, Okun A, Ren J, Guo RC, Ossipov MH, Xie J, King T, Porreca F. Ongoing pain in the MIA model of osteoarthritis. Neurosci Lett 2011;493:72–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu JP, He YT, Duan XL, Suo ZW, Yang X, Hu XD. Enhanced Activities of δ Subunit-containing GABAA Receptors Blocked Spinal Long-term Potentiation and Attenuated Formalin-induced Spontaneous Pain. Neuroscience 2018;371:155–165. [DOI] [PubMed] [Google Scholar]
- 42.Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy or chronic pain. Cochrane Database Syst Rev 2009(4):CD007115. [DOI] [PubMed] [Google Scholar]
- 43.McClish A Diazepam as an intravenous induction agent for general anaesthesia. Can Anaesth Soc J 1966;13:562–75. [DOI] [PubMed] [Google Scholar]
- 44.McCormick DA. Neurotransmitter actions in the thalamus and cerebral cortex. J Clin Neurophysiol 1992;9:212–23. [DOI] [PubMed] [Google Scholar]
- 45.Mogil JS, Davis KD, Derbyshire SW. The necessity of animal models in pain research. Pain 2010;151:12–7. [DOI] [PubMed] [Google Scholar]
- 46.Moore RA, Derry S, Aldington D, Cole P, Wiffen PJ. Amitriptyline for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev 2012(12):CD008242. [DOI] [PubMed] [Google Scholar]
- 47.Murray EA, Wise SP, Rhodes SEV. What Can Different Brains Do with Reward? In: Gottfried JA, editor. Neurobiology of Sensation and Reward; Boca Raton (FL)2011. [PubMed] [Google Scholar]
- 48.Navratilova E, Xie JY, Okun A, Qu C, Eyde N, Ci S, Ossipov MH, King T, Fields HL, Porreca F. Pain relief produces negative reinforcement through activation of mesolimbic reward-valuation circuitry. Proc Natl Acad Sci U S A 2012;109:20709–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Niv D, Davidovich S, Geller E, Urca G. Analgesic and hyperalgesic effects of midazolam: dependence on route of administration. Anesth Analg 1988;67:1169–73. [PubMed] [Google Scholar]
- 50.Okun A, DeFelice M, Eyde N, Ren J, Mercado R, King T, Porreca F. Transient inflammation-induced ongoing pain is driven by TRPV1 sensitive afferents. Mol Pain 2011;7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oliveras JL, Montagne-Clavel J. The GABAA receptor antagonist picrotoxin induces a ‘pain-like’ behavior when administered into the thalamic reticular nucleus of the behaving rat: a possible model for ‘central’ pain? Neurosci Lett 1994;179:21–4. [DOI] [PubMed] [Google Scholar]
- 52.Peng HY, Chen GD, Lee SD, Lai CY, Chiu CH, Cheng CL, Chang YS, Hsieh MC, Tung KC, Lin TB. Neuroactive steroids inhibit spinal reflex potentiation by selectively enhancing specific spinal GABA(A) receptor subtypes. Pain 143: 12–20, 2009. [DOI] [PubMed] [Google Scholar]
- 53.Persohn E, Malherbe P, Richards JG. Comparative molecular neuroanatomy of cloned GABAA receptor subunits in the rat CNS. J Comp Neurol 1992;326:193–216. [DOI] [PubMed] [Google Scholar]
- 54.Pomeranz B, Nguyen P. Intrathecal diazepam suppresses nociceptive reflexes and potentiates electroacupuncture effects in pentobarbital-anesthetized rats. Neurosci Lett 1987;77:316–20. [DOI] [PubMed] [Google Scholar]
- 55.Purdy RH, Morrow AL, Blinn JR, Paul SM. Synthesis, metabolism, and pharmacological activity of 3-alpha-hydroxy steroids which potentiate GABA-receptor-mediated chloride ion uptake in rat cerebral cortical synaptoneurosomes. J Med Chem 1990;33:1572–81. [DOI] [PubMed] [Google Scholar]
- 56.Qu C, King T, Okun A, Lai J, Fields HL, Porreca F. Lesion of the rostral anterior cingulate cortex eliminates the aversiveness of spontaneous neuropathic pain following partial or complete axotomy. Pain 2011;152:1641–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reyes-Vazquez C, Dafny N. Microiontophoretically applied THIP effects upon nociceptive responses of neurons in medial thalamus. Appl Neurophysiol 1983;46:254–60. [DOI] [PubMed] [Google Scholar]
- 58.Rode F, Jensen DG, Blackburn-Munro G, Bjerrum OJ. Centrally-mediated antinociceptive actions of GABA(A) receptor agonists in the rat spared nerve injury model of neuropathic pain. Eur J Pharmacol 2005;516:131–8. [DOI] [PubMed] [Google Scholar]
- 59.Rode F, Thomsen M, Broløs T, Jensen DG, Blackburn-Munro G, Bjerrum OJ. The importance of genetic background on pain behaviours and pharmacological sensitivity in the rat sparedserve injury model of peripheral neuropathic pain. Eur J Pharmacol 2007;564:103–11. [DOI] [PubMed] [Google Scholar]
- 60.Rudolph U, Knoflach F. Beyond classical benzodiazepines: novel therapeutic potential of GABAA receptor subtypes. Nat Rev Drug Discov 2011;10:685–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schofield PR, Darlison MG, Fujita N, Burt DR, Stephenson FA, Rodriguez H, Rhee LM, Ramachandran J, Reale V, Glencorse TA, Seeburg PH, Barnard EA. Sequence and functional expression of the GABA A receptor shows a ligand-gated receptor super-family. Nature 1987;328:221–7. [DOI] [PubMed] [Google Scholar]
- 62.Steriade M, Deschenes M. The thalamus as a neuronal oscillator. Brain Res 1984;320:1–63. [DOI] [PubMed] [Google Scholar]
- 63.Tsang SY, Ng SK, Xu Z, Xue H. The evolution of GABAA receptor-like genes. Mol Biol Evol 2007;24:599–610. [DOI] [PubMed] [Google Scholar]
- 64.Wafford KA, van Niel MB, Ma QP, Horridge E, Herd MB, Peden DR, Belelli D, Lambert JJ. Novel compounds selectively enhance delta subunit containing GABA A receptors and increase tonic currents in thalamus. Neuropharmacology 2009;56:182–189. [DOI] [PubMed] [Google Scholar]
- 65.Wiffen P, Collins S, McQuay H, Carroll D, Jadad A, Moore A. Anticonvulsant drugs for acute and chronic pain. Cochrane Database Syst Rev. 2005(3):CD001133. [DOI] [PubMed] [Google Scholar]
- 66.Xie JY, Herman DS, Stiller CO, Gardell LR, Ossipov MH, Lai J, Porreca F, Vanderah TW. Cholecystokinin in the rostral ventromedial medulla mediates opioid-induced hyperalgesia and antinociceptive tolerance. J Neurosci 2005;25:409–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xie JY, Qu C, Patwardhan A, Patwardhan A, Ossipov MH, Navratilova E, Becerra L, Borsook D, Porreca F. Activation of mesocorticolimbic reward circuits for assessment of relief of ongoing pain: a potential biomarker of efficacy. Pain 2014;155:1659–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yaksh TL, Stevens CW. Simple catheter preparation for permitting bolus intrathecal administration during chronic intrathecal infusion. Pharmacol Biochem Bull 1986;25:483–5. [DOI] [PubMed] [Google Scholar]
- 69.Yamamoto T, Yaksh TL. Effects of intrathecal strychnine and bicuculline on nerve compression-induced thermal hyperalgesia and selective antagonism by MK-801. Pain 1993;54:79–84. [DOI] [PubMed] [Google Scholar]
- 70.Yoshimura RF, Tran MB, Hogenkamp DJ, Johnstone TB, Xie JY, Porreca F, Gee KW. Limited central side effects of a β-subunit subtype-selective GABAA receptor allosteric modulator. J Psychopharmacol 2014;28:472–8. [DOI] [PubMed] [Google Scholar]
- 71.Yoshimura RF, Tran MB, Hogenkamp DJ, Ayala NL, Johnstone T, Dunnigan AJ, Gee TK, Gee KW. Allosteric modulation of nicotinic and GABAA receptor subtypes differentially modify autism-like behaviors in the BTBR mouse model. Neuropharmacology 2017;126:38–47. [DOI] [PubMed] [Google Scholar]
- 72.Yuan SB, Shi Y, Chen J, Zhou X, Li G, Gelman BB, Lisinicchia JG, Carlton SM, Ferguson MR, Tan A, Sarna SK, Tang SJ. Gp120 in the pathogenesis of human immunodeficiency virus-associated pain. Ann Neurol 2014;75:837–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zambotti F, Zonta N, Tammiso R, Conci F, Hafner B, Zecca L, Ferrario P, Mantegazza P. Effects of diazepam on nociception in rats. Naunyn Schmiedebergs Arch Pharmacol 1991;344:84–9. [DOI] [PubMed] [Google Scholar]
- 74.Zeilhofer HU, Benke D, Yevenes GE. Chronic pain states: pharmacological strategies to restore diminished inhibitory spinal pain control. Annu Rev Pharmacol Toxicol 2012;52:111–33. [DOI] [PubMed] [Google Scholar]