SUMMARY
Tie1 is a mechanistically poorly characterized endothelial cell (EC)-specific orphan receptor. Yet, Tie1 deletion is embryonic lethal and Tie1 has been implicated in critical vascular pathologies, including atherosclerosis and tumor angiogenesis. Here, we show that Tie1 does not function independently but exerts context-dependent effects on the related receptor Tie2. Tie1 was identified as an EC activation marker that is expressed during angiogenesis by a subset of angiogenic tip and remodeling stalk cells and downregulated in the adult quiescent vasculature. Functionally, Tie1 expression by angiogenic EC contributes to shaping the tip cell phenotype by negatively regulating Tie2 surface presentation. In contrast, Tie1 acts in remodeling stalk cells cooperatively to sustain Tie2 signaling. Collectively, our data support an interactive model of Tie1 and Tie2 function, in which dynamically regulated Tie1 versus Tie2 expression determines the net positive or negative effect of Tie1 on Tie2 signaling.
Graphical Abstract
In Brief
Using endothelial-specific conditional knockout mice, Savant et al. demonstrate a context-dependent modulatory function of Tie1 on Tie2 signaling. Tie1 is dynamically expressed by subset of endothelial cells in the postnatal retina. Dynamic regulation of Tie1 and Tie2 is required during angiogenesis and vascular remodeling.
INTRODUCTION
Blood vessel formation and patterning during angiogenesis is a multistep process that requires the precisely coordinated engagement of different signaling pathways in endothelial cells (ECs) (Herbert and Stainier, 2011). The vascular endothelial growth factor (VEGF)/VEGFR and Delta/Notch pathways act in concert to shape the properties of ECs during sprouting angiogenesis (Hellström et al., 2007; Phng and Gerhardt, 2009; Potente et al., 2011). Sprouting tip cells, which extend filopodia and migrate toward angiogenic stimuli, are followed by so-called stalk cells that proliferate to extend the sprout (Gerhardt and Betsholtz, 2005; Gerhardt et al., 2003). ECs of newly formed sprouts recruit pericytes, which leads to vessel maturation with ECs acquiring the so-called quiescent phalanx phenotype of resting blood vessels (Gerald et al., 2013; Mazzone et al., 2009). Although ECs acquire specific phenotypes during the individual steps of the angiogenic cascade, several studies have demonstrated dynamic rearrangements and plasticity of the tip, stalk, and phalanx cell phenotypes (Arima et al., 2011; Bentley et al., 2014).
The angiopoietin (Ang)/Tie-signaling pathway is essential for vessel remodeling and maturation (Augustin et al., 2009). Tie2 serves as the primary receptor of the Ang/Tie axis, transducing Ang1-mediated EC survival and maturation signals. In turn, Ang2 serves as context-dependent partial Tie2 agonist destabilizing ECs in the presence of Ang1 and activating Tie2 in the absence of the primary agonistic ligand Ang1 (Daly et al., 2013; Yuan et al., 2009). In contrast to the increasingly well understood Ang1/Ang2/Tie2 axis, the signaling mechanisms of the second Tie receptor, Tie1, remain largely unknown (Fukuhara et al., 2008; Saharinen et al., 2005, 2008; Seegar et al., 2010; Yuan et al., 2007). Despite extensive research, Tie1 continues to be an orphan receptor that does not serve as high-affinity angiopoietin receptor. Nevertheless, the late embryonic lethal phenotype of Tie1-deficient mice is an unambiguous demonstration of the essential requirement of Tie1 for normal vascular development and function (Puri et al., 1995; Sato et al., 1995). Mice lacking Tie1 die between embryonic day (E) 13.5 and birth from widespread edema due to perturbed microvessel integrity and lymphatic defects (D’Amico et al., 2010; Qu et al., 2010). Moreover, recent work has established that Tie1 is not just involved in embryonic vascular remodeling but also exerts critical functions in pathological adult vasculature, regulating tumor angiogenesis and atherosclerotic progression (D’Amico et al., 2014; Woo et al., 2011). Tie1 has even been proposed to be involved in the pathogenesis of Ebola virus infection (Rasmussen et al., 2014). Correspondingly, Tie1 expression is induced upon endothelial activation by hypoxia and VEGF as well as by disturbed blood flow at vessel bifurcations (McCarthy et al., 1998; Porat et al., 2004). This is in intriguing contrast to Tie2, which is transcriptionally downregulated upon EC activation notably in the angiogenic tip cells but is uniformly expressed in stalk and phalanx cells (del Toro et al., 2010; Felcht et al., 2012).
Tie1 has been proposed to serve as an endothelial mechanosensor because its expression is regulated by hemodynamic shear stress (Chen-Konak et al., 2003; Porat et al., 2004; Woo et al., 2011). This could suggest a potential role of Tie1 in blood-flow-regulated vascular pruning as it occurs during late angiogenic vascular remodeling (Potente et al., 2011). Moreover, Tie1-Tie2 interactions have been implicated in the regulation of Ang1-induced Tie2 signal transduction (Saharinen et al., 2005; Seegar et al., 2010), indicating ligand-independent functions of Tie1. Taking into consideration (1) the essential role of Tie1 during embryonic development, (2) the apparent differential expression of Tie1 and Tie2, (3) the demonstrated interaction of Tie1 and Tie2, and (4) the absence of a cognate Tie1 ligand, we hypothesized that Tie1 may exert its vascular-specific functions by acting as a dynamically regulated co-receptor of Tie2. To address this hypothesis, we studied Tie1 expression, signaling, and function in relation to Tie2 in cellular loss-of-function and gain-of-function experiments as well as in genetic in vivo models. The experiments identified Tie1 as a context-dependent modulator of Tie2 exerting negative effects on Tie2 in sprouting tip cells and positive effects on Tie2 in remodeling stalk cells. Quiescent phalanx cells were identified as Tie1-low, being mainly under the control of Tie2.
RESULTS
Tie1 Is Expressed by a Subset of Angiogenic and Remodeling ECs in the Postnatal Mouse Retinal Vasculature
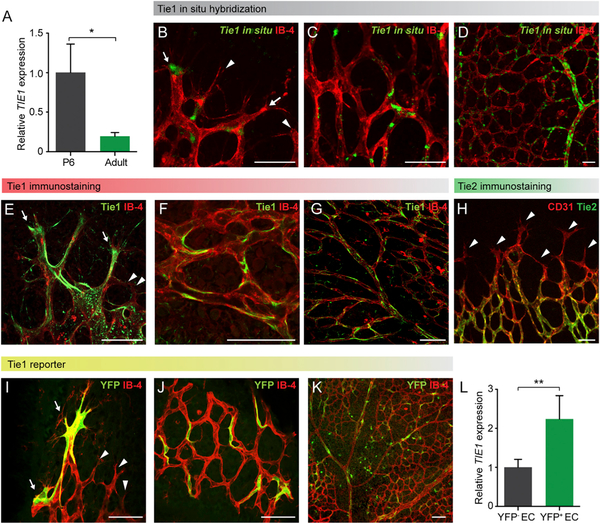
Previous studies have demonstrated the ubiquitous pan-endothelial expression of Tie1 during mouse embryonic development using transgenic mice expressing either Cre recombinase or reporters such as GFP and LacZ under the control of a minimal Tie1 promoter region (Gustafsson et al., 2001; Iljin et al., 2002; Korhonen et al., 1995). In adult mice, however, contradictory data for Tie1 expression have been reported using different reporter systems (Gustafsson et al., 2001; Iljin et al., 2002). To unambiguously clarify Tie1 expression in developing and quiescent vasculature, we isolated ECs from the lungs of postnatal day (P) 6 mice as well as adult mice by fluorescence-activated cell sorting (FACS) (Korn et al., 2014) and assessed Tie1 expression by qRT-PCR. Whereas EC from P6 mice prominently expressed Tie1, Tie1 expression in adult ECs was strongly downregulated (Figure 1A). Next, we analyzed Tie1 mRNA expression by in situ hybridization (ISH) of P6 whole-mounted retinas. Staining for the anti-sense probe revealed Tie1 expression in a subset of tip cells (Figure 1B) and a subset of stalk cells in the plexus region (Figures 1C and 1D). Hybridization with a sense probe confirmed the specificity of anti-sense ISH stainings (Figure S1A).
Figure 1. Tie1 Is Expressed by a Subset of Tip and Stalk Cells.
(A) TIE1 expression analyses of FACS-sorted lung ECs showing significant downregulation in adult mice compared to 6-day-old (P6) pups (n = 4 mice/group).
(B–D) Representative images showing ISH against Tie1 mRNA and blood vessels stained with isolectinB-4 (IB-4) at the sprouting front (B; arrows indicate stained tip cells, whereas arrowheads indicate unstained tip cells) and in the central plexus region (C and D) of whole-mounted P6 retina.
(E–G) Representative images of Tie1 immunostaining and blood vessels stained with IB-4 in the sprouting front region (E; arrows indicate Tie1-stained tip cells; arrowheads indicate unstained tip cells) and in the central plexus region (F) of whole-mounted P6 retina. (G) Overview image of the retinal vasculature showing mosaic pattern of Tie1 immunostaining is shown.
(H) Representative image of Tie2 immunostaining and blood vessels (CD31) showing Tie2-negative tip cells (arrowheads) and homogenous Tie2 staining in the plexus in P6 retina.
(I–K) Representative images of Tie1MiCreM/+ knockin-Rosa26YFP reporter mouse retina after tamoxifen treatment showing YFP expression (arrows) in a subset of tip cells (I; arrowheads indicate YFP-negative tip cells) and in a subset of stalk cells in the plexus region (J). (K) Overview of the retinal vasculature of reporter mice illustrating mosaic YFP expression pattern is shown.
(L) Relative TIE1 expression in YFP+ EC compared to YFP−EC of P6 reporter mice post-tamoxifen (EC from five to seven littermates pooled; n = 4 independent experiments).
*p < 0.05; **p < 0.01. The scale bar represents 50 μm. See also Figures S1 and S2.
In order to confirm the Tie1 ISH data on the protein level, we generated a novel monoclonal antibody (mAb) that specifically recognized the intracellular domain of murine Tie1 (designated 5F5). The specificity of mAb 5F5 immunostaining for Tie1 was validated on retinal tissue of mice with conditional EC-specific deletion of Tie1, ruling out any cross-reactivity with other molecules including Tie2 (Figure S1B). In P6 wild-type mice, mAb 5F5 detected a subset of tip cells in the angiogenic front (Figure 1E). Likewise, in the remodeling plexus region, 5F5 detected only a subset of ECs (Figure 1F). An overview of the arterial branch demonstrated the mosaic pattern of 5F5 immunoreactivity in the developing vasculature (Figure 1G). Conversely, we confirmed that Tie2 is downregulated in tip cells and homogenously expressed in stalk cells (Figure 1H) (del Toro et al., 2010; Felcht et al., 2012).
Because both ISH and immunostaining revealed a rather unusual mosaic pattern of Tie1 expression in tip and stalk cells, we performed genetic reporter staining as a third approach to stain for Tie1 expression. We generated toward this end Tie1-MeriCreMer (Tie1MiCreM/+) knockin mice, in which a tamoxifeninducible form of Cre recombinase was expressed under the control of the endogenous Tie1 promoter (Figures S1C–S1F). To detect Cre activity, Tie1MiCreM/+ mice were bred to Rosa26 yellow fluorescent protein (YFP) reporter mice (Srinivas et al., 2001). Upon single-dose tamoxifen treatment (P3 or P5), Tie1 promoter-driven YFP expression allowed the detection of Tie1-expressing cells (Figure S2A). Analysis of P6 retinas identified YFP+ cells, reflecting Tie1 promoter activity, in a subset of angiogenic tip cells (Figure 1I) as well as in a subset of ECs in the plexus region (Figures 1J and 1K). Thus, YFP+ EC showed a mosaic Tie1 expression pattern compatible with the Tie1 ISH and immunostaining data. Moreover, flow cytometric analyses revealed that YFP+ EC comprised around 2% of the total ECs in the lungs of P6 mice (Figure S2B). We subsequently isolated the YFP+EC and YFPEC populations using FACS and analyzed gene expression. No significant difference in PECAM1 expression, a pan-EC marker, was observed in the two EC populations (Figure S2C). Yet, TIE1 expression was more than 2-fold upregulated in YFP+EC compared to YFP–EC (Figure 1L), suggesting a correlation between Tie1 promoter activity and Tie1 transcript levels in this subset of ECs.
Tie1 Counter-Regulates Tie2 Cell Surface Presentation in Tip Cells but Does Not Signal Independently of Tie2
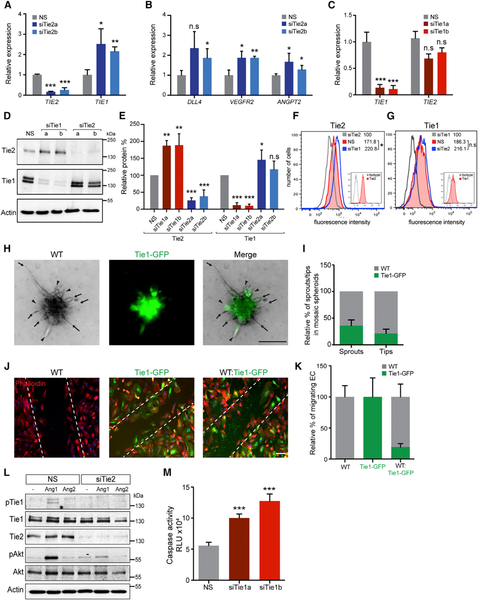
To determine the role of Tie1 in tip cells, we recapitulated tip cell-like conditions in vitro by depleting Tie2 in sub-confluent HUVEC. qRT-PCR analysis validated a significant decrease in TIE2 expression by two independent siRNAs (si-Tie2a and siTie2b) compared to non-silenced (NS) control siRNA (Figure 2A). Tie2 silencing resulted in the transcriptional upregulation of TIE1 as well as the expression of known tip cell-enriched genes such as DLL4, VEGFR2, and ANGPT2 (Figures 2A and 2B), demonstrating that the forced downregulation of Tie2 was sufficient to drive the tip cell program. Conversely, we also studied the effect of siRNA-mediated Tie1 silencing (siTie1a and siTie1b) on Tie2 and vice versa in sub-confluent HUVEC. Tie1 silencing did not significantly alter TIE2 transcript levels in sub-confluent cells (Figure 2C), but Tie2 protein was increased (Figures 2D and 2E). Tie2 depletion in turn resulted in a modest increase in Tie1 protein (Figures 2D and 2E). Flow cytometric analysis of non-permeabilized HUVEC showed that the surface pool of Tie2 receptor was increased upon loss of Tie1 (Figure 2F). Tie2 knockdown, however, weakly affected the surface pool of Tie1 (Figure 2G). Taken together, the experiments revealed that Tie1 counterbalanced Tie2 cell surface presentation in tip-like ECs.
Figure 2. Tie1 Counter-Balances Tie2 Expression in Tip Cells.
(A and B) Mimicking tip cell conditions in vitro by siRNA-mediated knockdown of Tie2 in sub-confluent HUVEC caused significant upregulation (A) of TIE1 and (B) of known tip cell-enriched genes like DLL4, VEGFR2, and ANGPT2 (NS, non-silenced control; siTie2a and siTie2b, two independent Tie2 siRNAs; n = 3).
(C) Tie1 silencing under similar conditions did not alter TIE2 expression (siTie1a and siTie1b, two independent Tie1 siRNAs; n = 3; n.s., not significant).
(D and E) Western blot analyses and densitometric quantification demonstrating an increase in Tie2 protein upon Tie1 silencing and vice versa (n = 3).
(F and G) Flow cytometric analyses of siTie1 compared to control, non-permeabilized HUVEC showing a significant increase in surface presented pool of Tie2 upon Tie1 depletion. Tie2 silencing did not affect Tie1 surface presentation compared to control. Insets show isotype controls, and numbers indicate geometric mean fluorescence intensity.
(H and I) Mosaic spheroid competition sprouting assay with 1:1 mixture of wild-type (WT), and Tie1-overexpressing (Tie1-GFP, green) HUVEC showing reduced contribution of Tie1-GFP cells in sprouts and tips compared to WT cells. Arrows indicate WT tips, whereas arrowheads show Tie1-GFP-positive tips.
(J) Phalloidin staining (red) of WT, Tie1-GFP (green), and 1:1 mixture of WT:Tie1-GFP HUVEC subjected to scratch wound assay (dotted lines).
(K) Quantification of migrated cells in the wound area showing decreased migration of Tie1-GFP cells in competition with WT EC.
(L) Control and siTie2 HUVEC were stimulated with Ang1 or Ang2 (400 ng/ml) for 20 min. Cell lysates were either immunoprecipitated with anti-Tie1 and immunoblotted sequentially with a pan-phosphotyrosine antibody (pTie1) and Tie1 or probed with antibodies against Tie2, pAkt, total Akt, and actin.
(M) Apoptosis assay after overnight serum deprivation showing increased caspase activity in Tie1-silenced HUVEC compared to control (n = 3).
*p < 0.05; **p < 0.01; ***p < 0.001. The scale bars represent 50 μm (H) and 100 μm (J). See also Figure S3.
Because only a subset of tip cells expressed Tie1, we assessed the potential of Tie1-overexpressing HUVEC (Tie1-GFP) in competition with wild-type (WT) HUVEC to acquire the tip cell position by performing mosaic spheroid sprouting assays. Toward this end, a 1:1 mixture of WT and Tie1-GFP expressing HUVEC was subjected to VEGF-induced sprouting in a 3D collagen matrix (Figure 2H). Tie1-GFP cells were under-represented both in the sprouts (35%) and even more so in the tip cell position (21%) compared to WT cells (Figure 2I). Similarly, when 1:1 mixtures of monolayer-cultured WT:Tie1-GFP cells were allowed to migrate in a scratch wound assay, Tie1-GFPexpressing ECs comprised only 19% of the total ECs at the migrating front (Figures 2J and 2K). Thus, these mosaic competition experiments imply that Tie1 expression did not functionally favor the tip cell position, even though a subset of tip cells upregulated Tie1.
Next, we asked whether Tie1 exerted functions in Tie2-low EC. In cultured EC, recombinant Ang1, but not Ang2, induced Tie2 phosphorylation as well as Akt and Erk activation (Figure S3A). Tie1 was weakly phosphorylated upon Ang1 stimulation (Figure 2L), confirming previously reported findings (Saharinen et al., 2005). Yet, Ang1 did not phosphorylate Tie1 in the absence of Tie2 (Figure 2L), suggesting that Tie1 phosphorylation was Tie2-dependent. It has previously been shown that Tie1 activates phosphoinositide 3-kinase (PI3K)-mediated Akt survival signaling (Kontos et al., 2002). Accordingly, Tie1 knockdown increased EC apoptosis in response to serum-deprivation stress (Figure 2M) but did not affect EC proliferation (Figure S3B). Taken together, EC activation upregulated Tie1 expression, which countered Tie2 cell surface presentation to possibly suppress Tie2 in tip cells. However, the Tie1 expression in tip cells could be transient as Tie1 cannot function independently of Tie2, and therefore, a mosaic tip cell expression pattern was observed.
Endothelial Tie1 Deletion Impairs Stalk Cell Function and Augments Vessel Regression
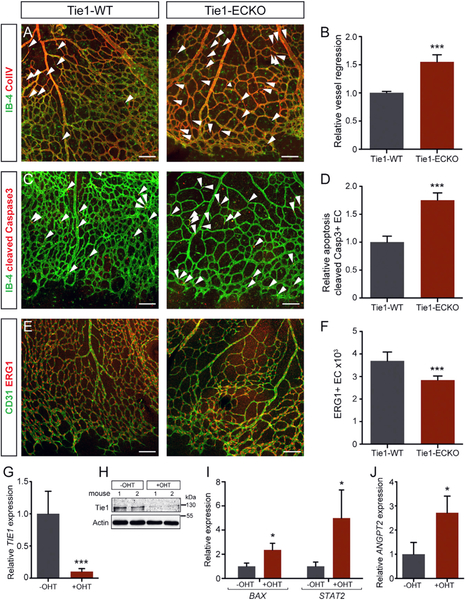
Given the mosaic expression of Tie1 in the postnatal retina, we asked whether Tie1 was required for retinal angiogenesis. Tie1flox/flox mice (Qu et al., 2010) were crossed with endothelial-specific Cdh5CreERT2 driver mice (Wang et al., 2010) to delete Tie1 in a temporally defined manner in EC (hereafter Tie1-ECKO). TIE1 expression was reduced by 85% in P6 Tie1-ECKO mice compared to Tie1flox/flox littermates (hereafter Tie1-WT; Figure S4G). Confirming and extending previously published data (D’Amico et al., 2014), whole-mount analyses of isolectinB-4 (IB-4)-stained P6 retinal vasculature revealed an impaired retinal vascularization in Tie1-ECKO mice, causing a significant decrease in the radial vessel outgrowth and vascular density compared to Tie1-WT (Figures S4A–S4C). The number of tip cells was reduced (Figures S4D and S4E), as was the number of branches (Figure S4F).
Because loss of Tie1 during embryonic development perturbs vascular remodeling and integrity (Dumont et al., 1994; Sato et al., 1995), we next assessed whether the reduced vascularization in Tie1-ECKO retinas was due to altered stalk cell remodeling. Postnatal EC deletion of Tie1 resulted in excessive regression of vessels in the plexus region as evidenced by increased numbers of collagen IV (ColIV)-positive and IB4-negative (ColIV+IB-4–) empty sleeves (Figures 3A and 3B). Enhanced vessel regression in Tie1-ECKO mice was accompanied by increased EC apoptosis marked by an elevated number of cleaved caspase3+ EC in the retina (Figures 3C and 3D). Moreover, CD31 co-staining of retinal vessels with the EC-specific transcription factor, ERG1 (Ets-related gene1) (Korn et al., 2014), identified a significant reduction of total number of ECs per retina area in Tie1-ECKO mice (Figures 3E and 3F).
Figure 3. Tie1 Deletion Increases Vessel Regression and EC Apoptosis in the Retinal Vasculature.
(A and B) Representative images of Tie1-WT and Tie1-ECKO retina from P6 mice post-tamoxifen treatment co-stained with (A) IB-4 and collagen IV (ColIV). Arrowheads indicate IB-4−ColIV+ empty sleeves quantified in (B) (n = 7–11 mice/group).
(C and D) IB-4 and cleaved caspase3 (C). Arrow-heads indicate cleaved caspase3+; IB-4+ EC quantified in (D) (n = 8–10 mice/group).
(E and F) Quantitation of CD31 and ERG1 to count the total number of EC in the retinal vasculature (E); ERG1+ nuclei quantified in (F) (n = 7 mice/group).
(G–J) Murine lung ECs isolated from Tie1flox/flox-Cdh5CreERT2 P6 mice and cultured without treatment (–OHT) or with active tamoxifen metabolite (+OHT) to induce Tie1 deletion in vitro. (G) qRTPCR and (H) western blot analyses showing significant downregulation of Tie1 upon treatment are shown. (I) Gene expression analyses demonstrating an increase in apoptosis-related genes (BAX and STAT2) and (J) ANGPT2 expression after OHT treatment (n = 3) are shown.
*p < 0.05; ***p < 0.001. The scale bar represents 100 μm. Images (A), (C), and (E) are composite of tiled and automatically stitched images. See also Figure S4.
To further analyze EC autonomous effects of Tie1 deletion, pulmonary EC from Tie1flox/flox-Cdh5CreERT2 mice were isolated and treated in culture with 4-hydroxytamoxifen (OHT), the active metabolite of tamoxifen, to induce Cre activity and, hence, Tie1 deletion (Figures 3G and 3H). Corresponding to the apoptosis phenotype observed in Tie1-ECKO retinas, Tie1 deletion in cultured ECs led to significant upregulation of apoptosis-related genes such as BAX and STAT2 (Figure 3I). Interestingly, ANGPT2 expression was also upregulated upon Tie1 deletion (Figure 3J), reflecting EC destabilization as it, for example, occurs during physiological vascular regression in the cyclic ovary (Goede et al., 1998).
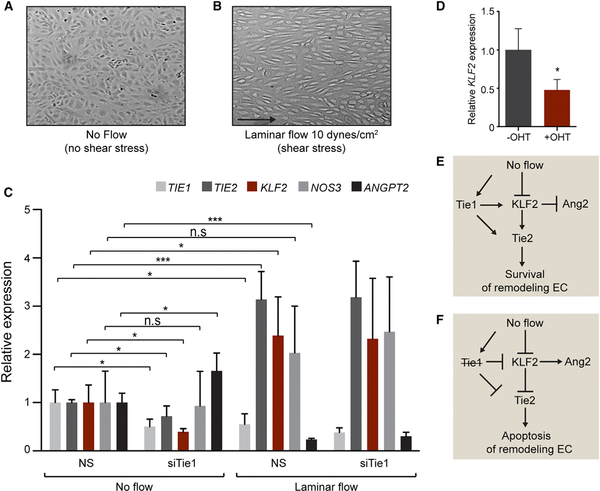
Tie1 Expression under Low Shear Stress Regulates EC Survival
Tie1 is upregulated in vascular regions exposed to disturbed flow acting as a mechanosensor transducing changes in shear forces (Porat et al., 2004; Woo et al., 2011). Based on the increased vessel regression observed in Tie1-ECKO mice, we comparatively analyzed gene expression in Tie1-silenced (siTie1) and non-silenced ECs (NS) cultured under static conditions or at 10 dynes/cm2 laminar flow. ECs exposed to laminar flow aligned in the direction of flow within 48 hr (Figures 4A and 4B). Laminar shear stress led to the induction of the established shearstress-regulated transcription factor KLF2 (Parmar et al., 2006) and the KLF2-regulated genes TIE2 and NOS3 as well as reduced ANGPT2 in control cells (Figure 4C). Laminar flow also led to downregulation of TIE1 expression, confirming previous findings of the negative regulation of Tie1 by laminar flow (Chen-Konak et al., 2003; Woo et al., 2011). Accordingly, forced silencing of Tie1 did not affect TIE2, KLF2, NOS3, and ANGPT2 expression under flow conditions compared to control cells. In turn, Tie1 deletion under static conditions caused a significant decrease in KLF2 and TIE2 expression, whereas upregulating ANGPT2 (Figure 4C). Additionally, KLF2 was significantly downregulated upon Tie1 deletion in pulmonary ECs isolated from Tie1flox/flox-Cdh5CreERT2 mice and treated in culture with 4-OHT compared to untreated cells (Figure 4D). Thus, induction of TIE1 expression under conditions of low shear stress or no blood flow such as in the regressing endothelium regulates KLF2 and TIE2 expression, thereby maintaining EC survival during the process of vessel remodeling (Figure 4E). In contrast, absence of Tie1 in remodeling EC exposed to no blood flow conditions reduced the expression of pro-survival factors such as KLF2 and TIE2, resulting in EC apoptosis (Figure 4F).
Figure 4. Low-Shear-Stress-Induced Tie1 Expression Regulates KLF2 and TIE2 Expression.
(A and B) Representative images showing HUVEC alignment upon shear stress after 48 hr of laminar flow at 10 dynes/cm2 compared to cells cultured under static flow conditions without shear stress.
(C) Gene expression analyses of Tie1-silenced (siTie1) and control (NS) HUVEC with no flow compared to siTie1 and control cells exposed to laminar flow. Relative TIE1, TIE2, KLF2, NOS3, and ANGPT2 expression normalized to respective gene expression in control, no flow condition is shown (n = 4).
(D) qRT-PCR analysis showing downregulation of KLF2 expression in murine lung ECs upon Tie1 deletion in vitro by treatment with active tamoxifen metabolite (+OHT) compared to untreated cells (–OHT; n = 3).
(E) Tie1 expression under no flow conditions regulates KLF2-mediated EC survival by regulating Tie2 and repressing Ang2.
(F) Loss of endothelial Tie1 under no flow conditions causes downregulation of KLF2 and Tie2 survival genes and increase in Ang2 expression causing EC apoptosis.
*p < 0.05; ***p < 0.001.
Tie1 Sustains Ang1-Tie2 Signaling
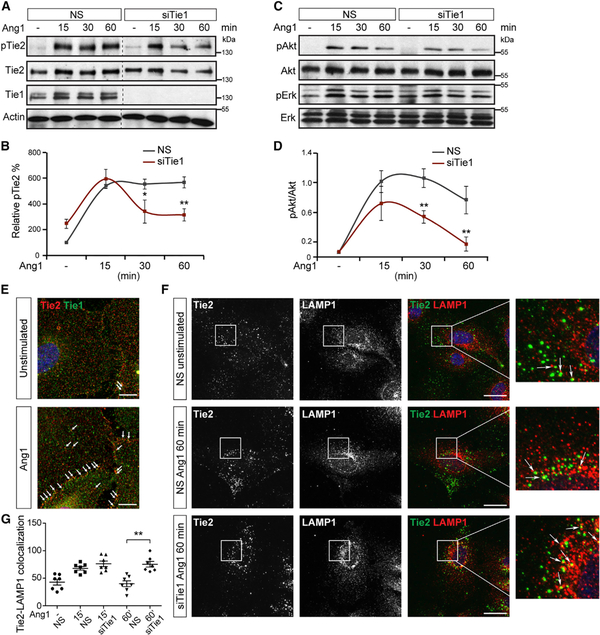
Pronounced vessel regression of stalk cells in Tie1-ECKO mice and the mechanosensory role of Tie1 in the remodeling vasculature pointed toward potential signaling roles of Tie1 during vessel remodeling. To assess the contribution of Tie1 to Ang1-Tie2 signaling during remodeling and maturation, we performed Ang1 stimulation experiments in confluent Tie1-silenced ECs. Prolonged Ang1 stimulation of Tie1-silenced confluent ECs led to reduced total Tie2 protein and Tie2 phosphorylation (pTie2) (Figure 5A). Plotting pTie2 signal intensity over time of Ang1 stimulation demonstrated sustained Ang1-induced pTie2 levels in control cells (Figure 5B). Although loss of Tie1 induced phosphorylation similarly in both cell populations, pTie2 levels declined significantly faster in Tie1-silenced cells compared to control cells (Figure 5B). Correspondingly, Ang1-mediated Akt activation downstream of pTie2 was downregulated in the absence of Tie1 (Figures 5C and 5D). Tie1 loss did not, however, alter Erk activation (Figure 5C). Furthermore, as total Tie2 protein was affected upon Tie1 silencing, we studied Tie2 protein turnover by inducing Ang1-mediated Tie2 internalization in Tie1-silenced cells compared to control cells. Accelerated loss of total Tie2 was observed in siTie1 cells compared to NS, indicating that Tie1 deletion accelerated Tie2 degradation (Figure S5).
Figure 5. Tie1 Sustains Ang1-Tie2 Signaling in Stalk Cells.
(A) Effect of Tie1 knockdown in confluent HUVEC on Ang1-induced Tie2 phosphorylation at different time points of Ang1 stimulation (15, 30, and 60 min). Cell lysates were either immunoprecipitated with anti-Tie2 and immunoblotted sequentially with pan-phosphotyrosine (pTie2) and Tie2 antibodies or probed with antibodies against Tie1 and actin loading control.
(B) Densitometric analysis of pTie2 showing declining pTie2 levels in siTie1 cells compared to sustained pTie2 levels in control cells (n = 3).
(C) Immunoblots showing the effect of Tie1 silencing on Ang1-mediated activation of Akt and Erk survival pathways.
(D) Densitometric quantification of pAkt to Akt ratio demonstrating a significant decrease in pAkt upon siTie1 compared to corresponding controls (n = 3).
(E) Immunofluorescence staining of Tie1 and Tie2 in HUVEC monolayers showing increased co-localization of Tie1 and Tie2 upon Ang1 stimulation compared to unstimulated control.
(F) Tie1-silenced and control ECs were incubated with an anti-Tie2 antibody to label the surface-presented Tie2 and stimulated with 400 ng/ml Ang1 for ligand-induced Tie2 internalization. Immunofluorescence staining of internalized Tie2 and lysosomal marker, LAMP1, showing Tie2 localization in the lysosomal compartment (arrows in higher magnification of boxed areas) is shown.
(G) Tie2-LAMP1 co-localization analyses showing significant increase of Tie2 in the lysosomes upon Tie1 silencing compared to control at the 60 min time point (n = 7 or 8 microscopic fields/condition).
*p < 0.05; **p < 0.01. The scale bars represent 10 μm (E) and 20 μm (F). See also Figure S5.
To investigate how Tie1 sustained Ang1-Tie2 signaling in confluent ECs, we analyzed Tie1 localization in relation to Tie2 in Ang1-stimulated EC monolayers. Confirming previous reports (Saharinen et al., 2005), there was increased co-localization of Tie1 and Tie2 especially at cell-cell contacts upon Ang1 stimulation compared to unstimulated ECs (Figure 5E), suggesting that Tie1-Tie2 heterodimers served as a signaling-incompetent (Hansen et al., 2010) reservoir for sustained Tie2 signaling.
Because loss of Tie1 affected Tie2 protein turnover in the presence of Ang1, we traced the fate of internalized Tie2 in Tie1-silenced and control ECs using an antibody uptake assay. In this assay, the surface-presented pool of Tie2 was labeled with a Tie2-specific antibody binding to its extracellular domain followed by Ang1 stimulation, causing internalization of labeled receptors. The internalized fraction of Tie2 co-localized at similar abundance with LAMP1, a marker of the lysosomal compartment, within 15 min of Ang1 stimulation in both cell populations. However, a significant increase in co-localization was observed in Tie1-silenced cells after 60 min of Ang1 stimulation compared to control, suggesting increased trafficking over time of Tie2 to the lysosomal pathway in the absence of Tie1 (Figures 5F and 5G).
Double Deficiency of Tie1 and Tie2 Suppresses Tip Cell Sprouting and Impairs Stalk Cell Remodeling
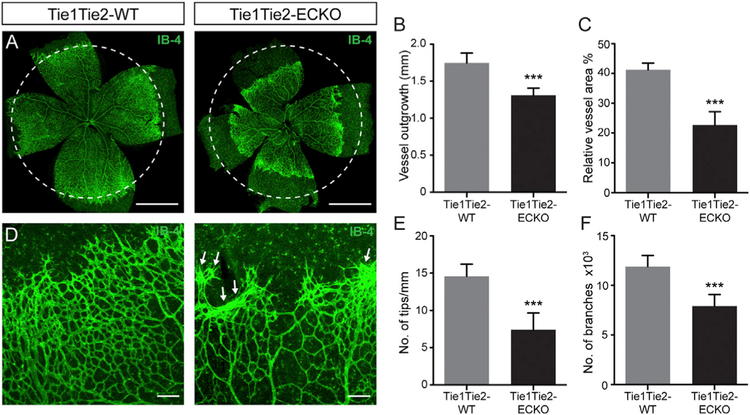
To examine the role of Tie1-Tie2 interaction during angiogenesis in vivo, we generated a conditional knockout mouse model targeting both Tie1 (Tie1flox/flox) and Tie2 (Tie2flox/flox) and bred these mice to endothelial-specific Cdh5CreERT2 driver mice (Tie1Tie2ECKO). Postnatal tamoxifen induction resulted in 80% downregulation of TIE1 expression and 55% reduction of TIE2 expression in these triple-transgenic pups (Figure S6). Analysis of the P6 whole-mount retinal vasculature labeled with IB-4 demonstrated a significant reduction in radial vessel outgrowth and vascularized area in the Tie1Tie2-ECKO retinal vasculature compared to Tie1flox/flox/Tie2flox/flox (hereafter Tie1Tie2-WT) littermate control mice (Figures 6A–6C). The number of tips and branches was also reduced in double-deficient retinas (Figures 6D–6F). Abnormal clustering of ECs at the sprouting front was observed in Tie1Tie2-ECKO retina (Figure 6D, arrows), which was not observed in Tie1-ECKO (Figure S4) or in Tie2-ECKO (Figure S7) mice.
Figure 6. Suppressed Angiogenesis in Tie1 and Tie2 Double-Deficient Mice.
(A) Representative images of IB-4-labeled P6 retinal vasculature of Tie1Tie2-WT and Tie1Tie2-ECKO mice post-tamoxifen treatment.
(B and C) Vascular analyses showing significant decrease in (B) vascular outgrowth and (C) relative vessel area in Tie1Tie2-ECKO mice compared to WT littermates (n = 10 or 11 mice/group).
(D) Vascular front of Tie1Tie2-ECKO mice showing unusual clustering of ECs (arrows).
(E and F) Number of tips/mm vascular front (E) and number of branches (F) were also reduced in Tie1Tie2-ECKO mice (n = 10 or 11 mice/group).
***p < 0.001. The scale bars represent 1 mm (A) and 100 μm (D). Images (A) and (D) are composite of tiled and automatically stitched images. See also Figures S6 and S7.
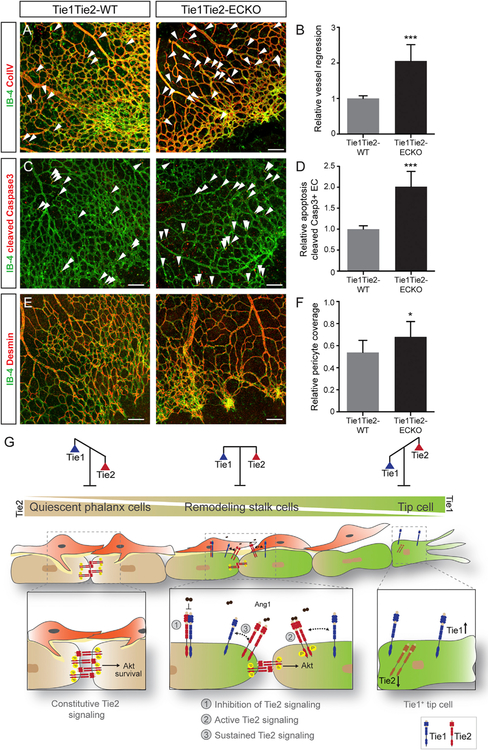
To determine whether Tie1 and Tie2 together play a role in the remodeling stalk cell vasculature, we analyzed ColIV+IB-4 empty basement sleeves in Tie1Tie2-WT and Tie1Tie2-ECKO mice. Genetic deletion of both receptors resulted in a significant increase in vessel regression in the proximal remodeling plexus region of P6 retina (Figures 7A and 7B). This increase was accompanied by enhanced EC apoptosis, which was assessed by cleaved caspase3+ EC staining (Figures 7C and 7D). The remodeling defects observed in Tie1Tie2-ECKO mice were more pronounced compared to Tie1-ECKO (Figure 3) mice, indicating cooperative functions of Tie1 and Tie2, particularly during vascular remodeling. Interestingly, the pericyte coverage of retinal vasculature analyzed by desmin staining was increased in Tie1Tie2-ECKO mice compared to Tie1Tie2-WT (Figures 7E and 7F), reflecting the preferential regression of immature, non-pericyte-covered microvessels in Tie1Tie2-ECKO mice.
Figure 7. Loss of Tie1 and Tie2 Causes Enhanced Vessel Regression in the Retina.
(A–D) Representative images of Tie1Tie2-WT and Tie1Tie2-ECKO P6 retinal vasculature post-tamoxifen treatment, labeled with IB-4 and co-stained with (A) collagen IV (ColIV); arrowheads indicate IB-4−ColIV+ empty sleeves quantified in (B) (n = 9 or 10 mice/group) or (C) cleaved caspase3; arrowheads indicate cleaved caspase3+; IB-4+ EC quantified in (D) (n = 8 or 9 mice/group).
(E and F) Representative images showing (E) desmin-positive pericyte coverage of blood vessels (IB-4) in Tie1Tie2-WT and Tie1Tie2-ECKO retina and quantification (F; n = 10 mice/group).
(G) Model demonstrating the contextual role of Tie1 counter-regulating Tie2 cell surface presentation in tip cells and sustaining Ang1-Tie2 signaling in the stalk cells by forming heterocomplexes.
*p < 0.05, ***p < 0.001. The scale bar represents 100 μm. Images (A), (C), and (E) are composite of tiled and automatically stitched images.
DISCUSSION
The molecules and mechanisms controlling sprouting angiogenesis have been unraveled in substantial molecular detail. In contrast, the mechanisms of vessel remodeling and maturation are much-less well understood. The Ang/Tie system is a prototypic regulator of vessel maturation and remodeling. The agonistic ligand Ang1 activates Tie2-transducing survival and quiescence signals including the PI3K/Akt pathway. The partial agonist Ang2 acts as a context-dependent weak stimulator or inhibitor of Tie2. Tie1, on the other hand, may be phosphorylated upon Ang1 or Ang4 stimulation (Saharinen et al., 2005). Likewise, phosphorylation of a chimeric form of Tie1 has been shown to activate the PI3K/Akt pathway (Kontos et al., 2002). Yet, it has not been established whether this may occur as a direct consequence of Tie1 phosphorylation or as a result of a crosstalk with Tie2.
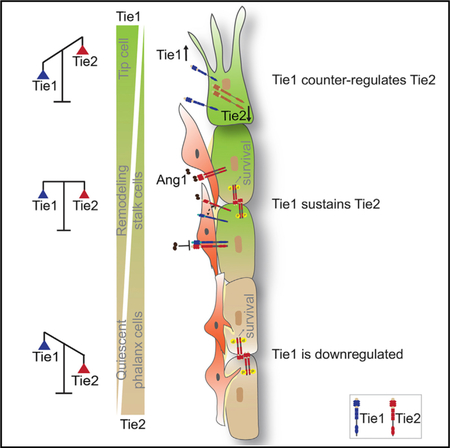
This study was aimed at systematically studying Tie1 expression and function during angiogenesis and vascular remodeling. Employing novel Tie1-specific tools (mAb 5F5, Tie1MiCreM/+ knockin-driven reporter mice, and Tie1Tie2 double-ECKO mice) and performing functional assays in vitro and in vivo, we show that (1) TIE1 expression is upregulated in a subset of angiogenic and remodeling ECs but downregulated in the quiescent vasculature in the adult; (2) Tie1 counter-regulates Tie2 cell surface presentation in tip cells; (3) low shear stress-dependent TIE1 expression regulates TIE2 and KLF2, enabling efficient vascular remodeling; (4) Tie1 sustains Ang1-Tie2 signaling in remodeling stalk cells, promoting EC survival; and (5) the functional synergism of Tie1 and Tie2 is essential for efficient angiogenesis and remodeling. Collectively, the data establish that Tie1 functions by negatively and positively affecting Tie2 expression and function in a context-dependent manner to fine-tune Tie2 during sprouting angiogenesis (negative regulation of Tie2 surface presentation) and vascular remodeling (sustaining Tie2 signaling; Figure 7G).
Tie1 has previously been shown to be dynamically expressed upon exogenous activation. Induction by VEGF, turbulent shear stress, and hypoxia all hinted at a positive role of Tie1 during sprouting angiogenesis (Chen-Konak et al., 2003; Porat et al., 2004). Conversely, the negative transcriptional regulation of Tie1 under conditions of laminar flow suggested reduced Tie1 expression in quiescent ECs (Woo et al., 2011). Indeed, employing quantitative FACS, we observed the strong downregulation of endothelial Tie1 expression in adult mice compared to P6 mice, suggesting a possible role of Tie1 during active angiogenesis. To further dissect Tie1 function during postnatal angiogenesis, we studied its positional expression in the tip and stalk cell context. Employing three independent approaches including ISH, immunostaining, and an inducible reporter mouse, we identified a mosaic expression pattern of Tie1 in the angiogenic and remodeling postnatal retinal vasculature. Using b-gal staining in Tie1+/lacZ mice, a recent study has suggested homogenous Tie1 expression in the postnatal retina (D’Amico et al., 2014). Yet, our study revealed a more-complex pattern of Tie1 expression in the retina vasculature. The observed mosaic pattern clearly demonstrated the transient and dynamic nature of Tie1 expression in the retinal vasculature. Moreover, the downregulation of Tie1 in the adult vasculature validated Tie1 as an EC activation marker and suggested that Tie1 may therapeutically be an attractive target molecule.
Tie1 expression in a subset of tip cells supports a role of Tie1 during sprouting angiogenesis. Indeed, postnatal deletion of Tie1 in ECs led to reduced tip cells and defective stalk cell remodeling. However, cellular competition experiments revealed no preference of Tie1-overexpressing cells for acquiring the tip cell position. Subsequent experiments showed that upregulation of Tie1 in tip-like cells served to negatively regulate Tie2 surface presentation, which was strongly downregulated during sprouting angiogenesis. In fact, we observed that the forced downregulation of Tie2 was sufficient to trigger the tip cell phenotype, suggesting that downregulated Tie2 is indeed an important determinant of tip cell function. To relate mosaic tip cell Tie1 expression to its function, time-lapse imaging studies of the sprouting endothelium are required. Such studies have previously identified the dynamic positional shuffling of tip and stalk cells, depending on constant re-evaluation of VEGF/VEGFR and Delta/Notch pathways (Jakobsson et al., 2010). Along these lines, a potential crosstalk between Tie1 and Delta/Notch system has been implicated because the anti-angiogenic effects of Tie1 deletion correlate with upregulation of the Notch pathway (D’Amico et al., 2014). Thus, our findings may guide future studies focusing on dynamic cell rearrangements of Tie1-expressing ECs and crosstalk between the Ang/Tie and Delta/Notch pathways. Interestingly, factors like VEGF and phorbol esters that cause EC activation and upregulation of Tie1 expression are also known to reduce the endothelial Tie1 surface pool by inducing proteolytic shedding of its extracellular domain, arguing for a transient role of Tie1 in tip cells (Singh et al., 2012; Tsiamis et al., 2002; Yabkowitz et al., 1999).
Tie2 signaling serves as an important regulator of vascular remodeling and maturation (Augustin et al., 2009). This study revealed an essential role of Tie1 in remodeling stalk cells as a modulator of Tie2 signaling. EC-specific deletion of Tie1 resulted in enhanced vessel regression in the retina due to EC apoptosisinduced pruning of the vasculature. Endothelial apoptosis and vessel pruning are driven by low shear stress in constricted, hypoperfused vessels (Korn and Augustin, 2015; Potente et al., 2011). Low shear-stress-induced Tie1 expression was associated with the expression of the shear-stress-responsive transcription factor KLF2. Increased expression of KLF2 is known to induce TIE2, whereas potently suppressing ANGPT2 to promote EC survival (Parmar et al., 2006). Tie1 loss also resulted in transcriptional upregulation of ANGPT2 both in vitro as well as in Tie1-ECKO mice. Ang2 overexpression promotes EC death and vessel regression particularly in the absence of VEGF (Cao et al., 2007; Lobov et al., 2002). As such, increased vessel regression in Tie1-ECKO mice could be attributed to the loss of KLF2-mediated Tie2 expression and increased Ang2, leading to EC apoptosis. Thus, the mechanosensory function of Tie1 in the remodeling ECs at sites of low blood flow is essential for EC survival and efficient remodeling.
During vascular maturation, the Ang1-Tie2-signaling axis is active in remodeling stalk cells, which recruit pericytes to achieve stability. Furthermore, activation of membrane receptors by ligands enhances endocytosis, causing downregulation of receptor abundance on the cell surface (Goh and Sorkin, 2013). We show that Tie1 sustains Ang1-Tie2 signaling potentially by forming heteromeric Tie1-Tie2 complexes that prevent Tie2 from being rapidly internalized and perpetuate Tie2 signaling. Tie1 thereby promotes the anti-apoptotic effects of Ang1 by enhancing Akt activation and EC survival. This functional cooperativity between Tie1 and Tie2 suggested by cellular experiments could be confirmed in vivo using EC-specific double-Tie1-Tie2 knockout mice. Postnatal vascularization was similarly impaired in Tie1Tie2-ECKO mice as in Tie1-ECKO mice except for the formation of vascular tufts at the sprouting front in Tie1Tie2-ECKO mice. However, the defects in vascular remodeling were more pronounced upon double deletion, highlighting the rate-limiting role of Tie1 and Tie2 interactions during the process of vascular remodeling.
The comparison of inducible Tie1-ECKO and Tie1Tie2-ECKO mice validated cooperative functions of Tie1 and Tie2 in a definite genetic setting. Yet, we could not readily compare these findings to inducible Tie2-ECKO mice, because recombination of inducible Tie2-ECKO was consistently low (30% and lower; Figure S7), whereas it was significantly higher in Tie1Tie2-ECKO mice. This unusual and surprising interdependence of Tie1Tie2 genetic recombination is subject of ongoing studies. For the purpose of the present study, the comparison of Tie1-ECKO and Tie1Tie2-ECKO mice was useful to show that Tie1 acts to modulate Tie2 function.
In summary, this study has mechanistically unraveled the complexity of the context-dependent modulatory role that the orphan receptor Tie1 exerts on Tie2 signaling. Tie1 expression by sprouting tip cells and remodeling stalk cells contextually regulates Tie2 expression, cell surface presentation, and signaling in a ligand-independent manner. The prominent downregulation of Tie1 in adult resting ECs and the strict activation-dependent transcriptional upregulation make Tie1 thereby an attractive target for therapeutic intervention aimed at interfering with the activated EC phenotype. For example, Tie1 has recently been proposed as a target for anti-angiogenic combination therapies (D’Amico et al., 2014). The findings of this study may thereby contribute to the mechanism-guided development of novel combination therapies with optimized balance of triggering vascular regression and promoting vascular normalization.
EXPERIMENTAL PROCEDURES
Mice and Tamoxifen Injections
Generation of Mutant Tie1MiCreM/+ Mice
The cDNA of a tamoxifen-inducible codon-improved Cre recombinase (iCre) (Shimshek et al., 2002) flanked by two mutated estrogen receptor sites (MeriCreMer) was inserted into the first exon of the Tie1 locus in embryonic stem cells to express the fusion protein MeriCreMer under the control of the endogenous Tie1 promoter (Figure S1C). The knockin allele was maintained on the C57BL/6 background. For tracing Tie1 expression, Tie1MiCreM/+ mice were crossed to Rosa26YFP (Gt(ROSA)26Sortm1.1(EYFP)Cos) reporter mice (Srinivas et al., 2001) and injected with 100 mg of tamoxifen (Sigma; T5648) in 50 μl peanut oil/5% ethanol on P3 or P5.
Tie1 and Tie2 Floxed Mice
Generation of Tie1 floxed mice has been described previously (D’Amico et al., 2010; Qu et al., 2010), and Tie2 floxed mice were generated by the Ingenious Targeting Laboratories (Figure S7). Tie1flox/flox and Tie2flox/flox mice were crossed with Tg(Cdh5-cre/ERT2)1Rha (Cdh5CreERT2) mice (Wang et al., 2010) to specifically delete endothelial Tie1 and Tie2 expression. Postnatal Cre recombination was induced by injecting 100 μg tamoxifen on P1–3 and P5. All animal experiments were approved by the local regulatory committee Bezirksregierung Karlsruhe, Germany (G60/13, G39/11, and G22/14). For further details, see the Supplemental Experimental Procedures.
Additional Procedures
Please refer to the Supplemental Experimental Procedures for details on whole-mount retina staining and image analysis, in situ hybridization, lung EC isolation, siRNA transfection, cell culture assays, RNA isolation, qRTPCR, immunoprecipitation, and western blotting.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism (GraphPad Software) and Excel (Microsoft Office). Data are expressed as mean ± SD. Statistical significance was determined by two-tailed Student’s t test. A p value of less than 0.05 was considered statistically significant (*p < 0.05; **p < 0.01; ***p < 0.001). “n” represents either the number of independent experiments or number of mice analyzed per group.
Supplementary Material
Highlights.
Tie1 is expressed by a subset of angiogenic and remodeling endothelial cells
In tip cells, Tie1 counter-regulates Tie2 cell surface presentation
In remodeling endothelial cells, Tie1 sustains Tie2 signaling and cell survival
The functional coordination of Tie1-Tie2 is required for angiogenesis and remodeling
ACKNOWLEDGMENTS
The authors would like to acknowledge the excellent technical support of Carleen Spegg, the DKFZ Laboratory Animal Facility, the DKFZ Transgenic Service, and the DKFZ Light Microscopy Core Facilities. We wish to thank Claudia Tessmer from the DKFZ monoclonal antibody facility for helping to generate mAb 5F5. We thank Katharina Ruhnke for technical support to generate Tie1MiCreM/+ embryonic stem cells. We also thank Hugo Marti (Heidelberg University) for providing the mouse Tie1 plasmid. This work was supported by grants from the SFB-TR23 Vascular Differentiation and Remodeling (project A3 to H.G.A.), the SFB873 Maintenance and Differentiation of Stem Cells in Development and Disease (project B6 to H.G.A. and project B11 to H.-R.R.), the Leducq Transatlantic Network of Excellence Lymph Vessels in Obesity and Cardiovascular Disease (to H.G.A.), the Helmholtz Alliance Preclinical Comprehensive Cancer Center (to H.G.A. and H.-R.R.), and an ERC Advanced Grant (233074 to H.-R.R.). H.G.A. is supported by an endowed chair from the Aventis Foundation.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes Supplemental Experimental Procedures, seven figures, and two tables and can be found with this article online at http://dx.doi.org/10.1016/j.celrep.2015.08.024.
REFERENCES
- Arima S, Nishiyama K, Ko T, Arima Y, Hakozaki Y, Sugihara K, Koseki H, Uchijima Y, Kurihara Y, and Kurihara H (2011). Angiogenic morphogenesis driven by dynamic and heterogeneous collective endothelial cell movement. Development 138, 4763–4776. [DOI] [PubMed] [Google Scholar]
- Augustin HG, Koh GY, Thurston G, and Alitalo K (2009). Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat. Rev. Mol. Cell Biol 10, 165–177. [DOI] [PubMed] [Google Scholar]
- Bentley K, Franco CA, Philippides A, Blanco R, Dierkes M, Gebala V, Stanchi F, Jones M, Aspalter IM, Cagna G, et al. (2014). The role of differential VE-cadherin dynamics in cell rearrangement during angiogenesis. Nat. Cell Biol 16, 309–321. [DOI] [PubMed] [Google Scholar]
- Cao Y, Sonveaux P, Liu S, Zhao Y, Mi J, Clary BM, Li CY, Kontos CD, and Dewhirst MW (2007). Systemic overexpression of angiopoietin-2 promotes tumor microvessel regression and inhibits angiogenesis and tumor growth. Cancer Res 67, 3835–3844. [DOI] [PubMed] [Google Scholar]
- Chen-Konak L, Guetta-Shubin Y, Yahav H, Shay-Salit A, Zilberman M, Binah O, and Resnick N (2003). Transcriptional and post-translation regulation of the Tie1 receptor by fluid shear stress changes in vascular endothelial cells. FASEB J 17, 2121–2123. [DOI] [PubMed] [Google Scholar]
- D’Amico G, Korhonen EA, Waltari M, Saharinen P, Laakkonen P, and Alitalo K (2010). Loss of endothelial Tie1 receptor impairs lymphatic vessel development-brief report. Arterioscler. Thromb. Vasc. Biol 30, 207–209. [DOI] [PubMed] [Google Scholar]
- D’Amico G, Korhonen EA, Anisimov A, Zarkada G, Holopainen T, Hägerling R, Kiefer F, Eklund L, Sormunen R, Elamaa H, et al. (2014). Tie1 deletion inhibits tumor growth and improves angiopoietin antagonist therapy. J. Clin. Invest 124, 824–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly C, Eichten A, Castanaro C, Pasnikowski E, Adler A, Lalani AS, Papadopoulos N, Kyle AH, Minchinton AI, Yancopoulos GD, and Thurston G (2013). Angiopoietin-2 functions as a Tie2 agonist in tumor models, where it limits the effects of VEGF inhibition. Cancer Res 73, 108–118. [DOI] [PubMed] [Google Scholar]
- del Toro R, Prahst C, Mathivet T, Siegfried G, Kaminker JS, Larrivee B, Breant C, Duarte A, Takakura N, Fukamizu A, et al. (2010). Identification and functional analysis of endothelial tip cell-enriched genes. Blood 116, 4025–4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont DJ, Gradwohl G, Fong GH, Puri MC, Gertsenstein M, Auerbach A, and Breitman ML (1994). Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes Dev 8, 1897–1909. [DOI] [PubMed] [Google Scholar]
- Felcht M, Luck R, Schering A, Seidel P, Srivastava K, Hu J, Bartol A, Kienast Y, Vettel C, Loos EK, et al. (2012). Angiopoietin-2 differentially regulates angiogenesis through TIE2 and integrin signaling. J. Clin. Invest 122, 1991–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara S, Sako K, Minami T, Noda K, Kim HZ, Kodama T, Shibuya M, Takakura N, Koh GY, and Mochizuki N (2008). Differential function of Tie2 at cell-cell contacts and cell-substratum contacts regulated by angiopoietin-1. Nat. Cell Biol 10, 513–526. [DOI] [PubMed] [Google Scholar]
- Gerald D, Chintharlapalli S, Augustin HG, and Benjamin LE (2013). Angiopoietin-2: an attractive target for improved antiangiogenic tumor therapy. Cancer Res 73, 1649–1657. [DOI] [PubMed] [Google Scholar]
- Gerhardt H, and Betsholtz C (2005). How do endothelial cells orientate? EXS 2005, 3–15. [DOI] [PubMed] [Google Scholar]
- Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, and Betsholtz C (2003). VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol 161, 1163–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goede V, Schmidt T, Kimmina S, Kozian D, and Augustin HG (1998). Analysis of blood vessel maturation processes during cyclic ovarian angiogenesis. Lab. Invest 78, 1385–1394. [PubMed] [Google Scholar]
- Goh LK, and Sorkin A (2013). Endocytosis of receptor tyrosine kinases. Cold Spring Harb. Perspect. Biol 5, a017459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson E, Brakebusch C, Hietanen K, and Fässler R (2001). Tie-1directed expression of Cre recombinase in endothelial cells of embryoid bodies and transgenic mice. J. Cell Sci 114, 671–676. [DOI] [PubMed] [Google Scholar]
- Hansen TM, Singh H, Tahir TA, and Brindle NP (2010). Effects of angiopoietins-1 and −2 on the receptor tyrosine kinase Tie2 are differentially regulated at the endothelial cell surface. Cell. Signal 22, 527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellström M, Phng LK, and Gerhardt H (2007). VEGF and Notch signaling: the yin and yang of angiogenic sprouting. Cell Adhes. Migr 1, 133–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert SP, and Stainier DY (2011). Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat. Rev. Mol. Cell Biol 12, 551–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iljin K, Petrova TV, Veikkola T, Kumar V, Poutanen M, and Alitalo K (2002). A fluorescent Tie1 reporter allows monitoring of vascular development and endothelial cell isolation from transgenic mouse embryos. FASEB J 16, 1764–1774. [DOI] [PubMed] [Google Scholar]
- Jakobsson L, Franco CA, Bentley K, Collins RT, Ponsioen B, Aspalter IM, Rosewell I, Busse M, Thurston G, Medvinsky A, et al. (2010). Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat. Cell Biol 12, 943–953. [DOI] [PubMed] [Google Scholar]
- Kontos CD, Cha EH, York JD, and Peters KG (2002). The endothelial receptor tyrosine kinase Tie1 activates phosphatidylinositol 3-kinase and Akt to inhibit apoptosis. Mol. Cell. Biol 22, 1704–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen J, Lahtinen I, Halmekytö M, Alhonen L, Jänne J, Dumont D, and Alitalo K (1995). Endothelial-specific gene expression directed by the tie gene promoter in vivo. Blood 86, 1828–1835. [PubMed] [Google Scholar]
- Korn C, and Augustin HG (2015). Mechanisms of vessel pruning and regression. Dev. Cell 34, 5–17. [DOI] [PubMed] [Google Scholar]
- Korn C, Scholz B, Hu J, Srivastava K, Wojtarowicz J, Arnsperger T, Adams RH, Boutros M, Augustin HG, and Augustin I (2014). Endothelial cell-derived non-canonical Wnt ligands control vascular pruning in angiogenesis. Development 141, 1757–1766. [DOI] [PubMed] [Google Scholar]
- Lobov IB, Brooks PC, and Lang RA (2002). Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc. Natl. Acad. Sci. USA 99, 11205–11210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzone M, Dettori D, Leite de Oliveira R, Loges S, Schmidt T, Jonckx B, Tian YM, Lanahan AA, Pollard P, Ruiz de Almodovar C, et al. (2009). Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Cell 136, 839–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MJ, Crowther M, Bell PR, and Brindle NP (1998). The endothelial receptor tyrosine kinase tie-1 is upregulated by hypoxia and vascular endothelial growth factor. FEBS Lett 423, 334–338. [DOI] [PubMed] [Google Scholar]
- Parmar KM, Larman HB, Dai G, Zhang Y, Wang ET, Moorthy SN, Kratz JR, Lin Z, Jain MK, Gimbrone MA Jr., and García-Cardeña G (2006). Integration of flow-dependent endothelial phenotypes by Kruppellike factor 2. J. Clin. Invest 116, 49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phng LK, and Gerhardt H (2009). Angiogenesis: a team effort coordinated by notch. Dev. Cell 16, 196–208. [DOI] [PubMed] [Google Scholar]
- Porat RM, Grunewald M, Globerman A, Itin A, Barshtein G, Alhonen L, Alitalo K, and Keshet E (2004). Specific induction of tie1 promoter by disturbed flow in atherosclerosis-prone vascular niches and flow-obstructing pathologies. Circ. Res 94, 394–401. [DOI] [PubMed] [Google Scholar]
- Potente M, Gerhardt H, and Carmeliet P (2011). Basic and therapeutic aspects of angiogenesis. Cell 146, 873–887. [DOI] [PubMed] [Google Scholar]
- Puri MC, Rossant J, Alitalo K, Bernstein A, and Partanen J (1995). The receptor tyrosine kinase TIE is required for integrity and survival of vascular endothelial cells. EMBO J 14, 5884–5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu X, Tompkins K, Batts LE, Puri M, and Baldwin HS (2010). Abnormal embryonic lymphatic vessel development in Tie1 hypomorphic mice. Development 137, 1285–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen AL, Okumura A, Ferris MT, Green R, Feldmann F, Kelly SM, Scott DP, Safronetz D, Haddock E, LaCasse R, et al. (2014). Host genetic diversity enables Ebola hemorrhagic fever pathogenesis and resistance. Science 346, 987–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saharinen P, Kerkelä K, Ekman N, Marron M, Brindle N, Lee GM, Augustin H, Koh GY, and Alitalo K (2005). Multiple angiopoietin recombinant proteins activate the Tie1 receptor tyrosine kinase and promote its interaction with Tie2. J. Cell Biol 169, 239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saharinen P, Eklund L, Miettinen J, Wirkkala R, Anisimov A, Winderlich M, Nottebaum A, Vestweber D, Deutsch U, Koh GY, et al. (2008). Angiopoietins assemble distinct Tie2 signalling complexes in endothelial cell-cell and cell-matrix contacts. Nat. Cell Biol 10, 527–537. [DOI] [PubMed] [Google Scholar]
- Sato TN, Tozawa Y, Deutsch U, Wolburg-Buchholz K, Fujiwara Y, Gendron-Maguire M, Gridley T, Wolburg H, Risau W, and Qin Y (1995). Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature 376, 70–74. [DOI] [PubMed] [Google Scholar]
- Seegar TC, Eller B, Tzvetkova-Robev D, Kolev MV, Henderson SC, Nikolov DB, and Barton WA (2010). Tie1-Tie2 interactions mediate functional differences between angiopoietin ligands. Mol. Cell 37, 643–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimshek DR, Kim J, Hübner MR, Spergel DJ, Buchholz F, Casanova E, Stewart AF, Seeburg PH, and Sprengel R (2002). Codon-improved Cre recombinase (iCre) expression in the mouse. Genesis 32, 19–26. [DOI] [PubMed] [Google Scholar]
- Singh H, Hansen TM, Patel N, and Brindle NP (2012). The molecular balance between receptor tyrosine kinases Tie1 and Tie2 is dynamically controlled by VEGF and TNFa and regulates angiopoietin signalling. PLoS ONE 7, e29319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, and Costantini F (2001). Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol 1, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiamis AC, Morris PN, Marron MB, and Brindle NP (2002). Vascular endothelial growth factor modulates the Tie-2:Tie-1 receptor complex. Microvasc. Res 63, 149–158. [DOI] [PubMed] [Google Scholar]
- Wang Y, Nakayama M, Pitulescu ME, Schmidt TS, Bochenek ML, Sakakibara A, Adams S, Davy A, Deutsch U, Lüthi U, et al. (2010). EphrinB2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature 465, 483–486. [DOI] [PubMed] [Google Scholar]
- Woo KV, Qu X, Babaev VR, Linton MF, Guzman RJ, Fazio S, and Baldwin HS (2011). Tie1 attenuation reduces murine atherosclerosis in a dose-dependent and shear stress-specific manner. J. Clin. Invest 121, 1624–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabkowitz R, Meyer S, Black T, Elliott G, Merewether LA, and Yamane HK (1999). Inflammatory cytokines and vascular endothelial growth factor stimulate the release of soluble tie receptor from human endothelial cells via metalloprotease activation. Blood 93, 1969–1979. [PubMed] [Google Scholar]
- Yuan HT, Venkatesha S, Chan B, Deutsch U, Mammoto T, Sukhatme VP, Woolf AS, and Karumanchi SA (2007). Activation of the orphan endothelial receptor Tie1 modifies Tie2-mediated intracellular signaling and cell survival. FASEB J 21, 3171–3183. [DOI] [PubMed] [Google Scholar]
- Yuan HT, Khankin EV, Karumanchi SA, and Parikh SM (2009). Angiopoietin 2 is a partial agonist/antagonist of Tie2 signaling in the endothelium. Mol. Cell. Biol 29, 2011–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.