Abstract
Purpose of review:
Recent basic studies have yielded important new insights into the molecular mechanisms that regulate growth locally. Simultaneously clinical studies have identified new molecular defects that cause growth failure and overgrowth, and genome-wide association studies have elucidated the genetic basis for normal human height variation.
Recent findings:
The Hippo pathway has emerged as one of the major mechanisms controlling organ size. In addition, an extensive genetic program has been described that allows rapid body growth in the fetus and infant but then causes growth to slow with age in multiple tissues. In human genome-wide association studies, hundreds of loci associated with adult stature have been identified; many appear to involve genes that function locally in the growth plate. Clinical genetic studies have identified a gene, GPR101, that is responsible for growth hormone excess, and a second gene, DNMT3A, in which mutations cause an overgrowth syndrome through epigenetic mechanisms.
Summary:
These recent advances in our understanding of somatic growth not only provide insight into childhood growth disorders but also have broader medical applications because disruption of these regulatory systems contributes to oncogenesis.
Keywords: body size, proliferation, endocrine, genetic program, growth plate
Introduction
Body growth is the cumulative sum of cell proliferation and cell enlargement which occurs in multiple tissues. Growth can be influenced by genetic, nutritional, environmental, and hormonal factors, but it usually proceeds in a remarkably predictable pattern – growth is rapid in early life and gradually decelerates with age until it eventually ceases when the organism achieves its final size (1). In humans, this decline in growth rate is briefly interrupted by the pubertal growth spurt, but then the decline in growth rate resumes, causing somatic growth to cease by late adolescence (2). Similar patterns of growth occur in other mammals, except that the pubertal growth spurt only occurs in humans. However, the time course varies markedly, such that, in general, the period of juvenile growth is more prolonged in larger mammals (3).
Longitudinal bone growth, the process by which the body length increases, occurs at the growth plate by a process called endochondral ossification. The growth plate is a cartilaginous structure at the ends of long bones and vertebral bodies. It consists of chondrocytes arranged in three morphologically distinct layers: the resting, proliferative, and hypertrophic zones. Chondrocytes in the resting zone behave as progenitor cells (4), giving rise to clones of rapidly proliferating chondrocytes, which are arranged in columns in the proliferative zone. Farther towards the center of the bone, the chondrocytes stop proliferating and undergo hypertrophic differentiation. The newly formed cartilage is then remodeled into bone. These processes result in the progressive formation of new bone tissue underneath the growth plate and consequently, bone elongation.
Endocrine Regulation of Growth: the GH-IGF-I axis
GH and IGFs are potent stimulators of growth. GH excess caused by pituitary adenomas in childhood leads to gigantism. Conversely, GH deficiency or GH insensitivity caused by defects in the GH receptor or downstream pathways markedly impairs postnatal growth (5).
GH-secreting pituitary adenomas can be hereditary or sporadic. Mutations in AIP, encoding aryl-hydrocarbon receptor-interacting protein, are most frequently observed in familial isolated pituitary adenomas (FIPA) and can also occur in sporadic cases (6). A number of syndromes can result in GH-secreting pituitary adenomas and subsequent overgrowth and other endocrinopathies including MEN1, Carney complex, and McCune-Albright syndrome.
Recently, a microduplication of Xq26.3 was identified as a cause of early-onset gigantism and GH hypersecretion (7). This pediatric disorder was termed X-linked acrogigantism (X-LAG). GPR101, one of the four genes in the duplicated region that also includes CD40LG, ARHGEF6, and RBMX, encodes an orphan G-protein coupled receptor and is proposed to be the gene responsible for development of the GH excess. Clinically, X-LAG patients can exhibit overgrowth as young as 2–3 months of age, markedly elevated GH, IGF-I and prolactin, acromegalic features, increased appetite, and hyperinsulinemia (8).
GH has a minor role in fetal growth, despite the presence of its receptor (GHR) in embryos (9). Unlike GH, both IGF-I and -II are essential for fetal growth and their production in utero is independent of GH. Mutations of the IGF-I receptor gene in humans lead to severe IUGR (10;11). In appropriate for gestational age (AGA) newborns, cord serum concentration of IGF-I and -II are positively correlated with birth weight (12;13). IGF-I is also critical for postnatal growth and mutations in the gene or the receptor gene result in severe postnatal growth retardation (14).
Estrogen and Androgen
Another hormone that plays an important role in growth regulation is estrogen. The pubertal growth spurt is primarily driven by estrogen, as patients with androgen insensitivity show a near-normal growth spurt (15), while patients with aromatase deficiency, who are thus unable to convert androgen to estrogen, show little or no growth spurt (16). The effect of estrogen on the growth spurt is mediated in large part through the GH-IGF-I axis (17), but estrogen may also have direct, local effects on the growth plate. Human growth plate chondrocytes, which express both estrogen receptor (ER) α and β (18), when placed in primary culture, respond to stimulation by estrogen (19). Apart from inducing the pubertal growth spurt, estrogen also induces fusion of the epiphysis during puberty (20). Premature exposure to estrogen in precocious puberty results in accelerated skeletal maturation, premature epiphyseal fusion and decreased final stature (21). Conversely, lack of estrogen due to hypogonadism is associated with delayed epiphyseal fusion and tall stature. In a man with ER α mutations, and thus estrogen resistance, longitudinal bone growth persisted well into adulthood (22), and ossification of the growth plate finally occurred at 35 years of age (23). Recently, a woman with estrogen resistance due to homozygous loss-of-function mutations in ER α has been described (24). She had no apparent growth spurt. At the age of 17 years 8 months, she had a height of 162.6 cm, bone age of 13.5 years, and unfused radial and ulnar epiphyses. This report confirms the importance of ER α in mediating the effects of estrogen on human skeletal growth. This phenomenon has been exploited pharmacologically using aromatase inhibitors to block estrogen production and thus slow skeletal maturation in boys with constitutional delay of growth (25) and idiopathic short stature (26).
Evidence from animal studies suggests that estrogen does not promote epiphyseal fusion by directly stimulating ossification of the growth plate. Instead estrogen appears to promote the development process of growth plate senescence, causing chondrocyte proliferation to stop earlier, which secondarily triggers earlier epiphyseal fusion (20;27). A recent study suggests that estrogen accelerates growth plate senescence by irreversibly depleting progenitor cells in the resting zone of the growth plate (28).
Nutrition
Nutritional status strongly influences body growth. Overnutrition may lead to rapid linear growth and, in females, early pubertal onset, but does not lead to increased adult height. Conversely, malnutrition can delay juvenile growth and permanently impact final adult height (29;30).
Chronic malnutrition induces a state of GH resistance in humans, with increased GH levels but decreased circulating IGF-1. (31). This resistance to GH is due primarily to decreased GH receptor (GHR) mRNA expression in the liver (32;33), and in some cases, end-organ IGF-1 resistance (34). In addition, undernutrition increases cortisol levels (35), decreases thyroid hormone levels (36), and, decreases androgen and estrogen production (37), which may all contribute to the slowing of growth.
Recent studies have helped elucidate the mechanisms by which malnutrition causes GH resistance. Normally, GH is secreted from the anterior pituitary and binds to a receptor in hepatocytes which activates JAK2. Activation of JAK2 leads to phosphorylation of STAT5, which translocates to the nucleus to bind regulatory elements of target genes including IGF1. Simultaneously, GH stimulates suppressor of cytokine signaling 2 (SOCS2) which is an important negative regulator of the GH signaling cascade. Recent evidence suggests that GH resistance in states of malnutrition may be mediated by fibroblast growth factor 21 (FGF21) and Sirtuin 1 (SIRT1). Starvation induces hepatic production of FGF21 leading to increased fatty acid oxidation, ketogenesis, and gluconeogenesis. In FGF21 transgenic mice, there is decreased STAT 5 phosphorylation and increased Socs2 protein resulting in GH resistance (38;39). SIRT1 is a histone deacetylase that, like FGF21, promotes gluconeogenesis and fatty acid oxidation during fasting. After 48-hr of fasting, SIRT1 knockdown mice showed higher levels of IGF1 protein and Igf1 mRNA in the liver compared with WT mice. The same study showed that SIRT1 deacetylates lysine residues on STAT5, resulting in decreased STAT5 phosphorylation and subsequent GH resistance (40).
However, the attenuated growth seen in chronic malnutrition may not be due primarily to GH resistance at the liver, but rather at the growth plate. Linear growth is not affected in mice with liver-specific deletion of GHR despite almost complete hepatic GH resistance (41). In contrast, chronic malnutrition leads to GH insensitivity directly in chondrocytes through FGF21-induced upregulation of LEPROT and LEPROTL1 (42), both of which are thought to negatively regulate GHR trafficking to the cell membrane.
Interestingly, one recent study showed that GH resistance in preterm infants (<32 weeks gestation) may also be mediated by elevated FGF21. In these preterm infants, circulating FGF21 levels were negatively associated with change in SD score for length during the first 5 weeks of life (43). The same study also showed that FGF21 induced expression of SOCS2 and inhibited GH-induced STAT5 phosphorylation in primary human chondrocytes, confirming previous mechanistic findings in mice (44).
Evidence that non-endocrine factors are responsible for growth deceleration
All the aforementioned endocrine systems are important regulators of growth, and yet curiously, the gradual deceleration of body growth with age does not seem to be driven primarily by hormonal changes. Circulating IGF-I, which reflects GH action on the liver, increases from infancy through adolescence, as growth is slowing. Interestingly, levels of GH and IGF-I remain considerably high after growth has essentially ceased after the second decade of life,. Furthermore, organs from young animals that are transplanted into adult recipients continue to grow rapidly, suggesting that growth deceleration is an intrinsic property of the growing tissues rather than the systemic environment.
Hippo signaling pathway: from Drosophila to Mammals
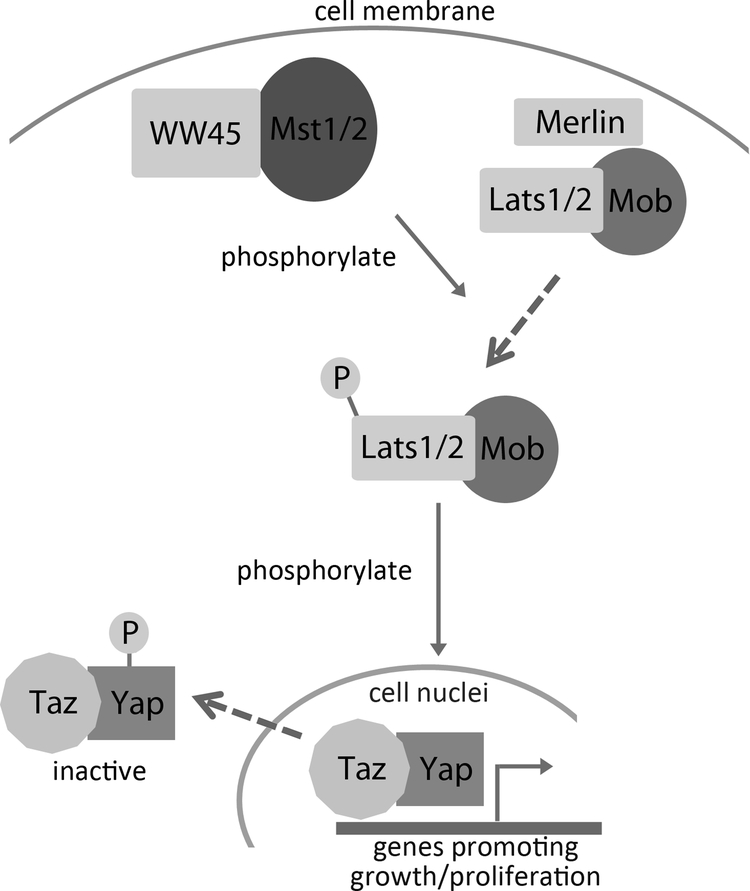
Recently, the Hippo pathway has emerged as one of the major mechanisms controlling organ size and organ growth. This pathway was first discovered in Drosophila using mosaic genetic screening; mutations of components of the Hippo signaling pathway led to robust cell-autonomous overgrowth of the imaginal disc, which is part of the Drosophila larva that later develops into part of the exterior of the adult insect (45;46). This pathway was later found to be highly conserved between Drosophila and mammals (47;48) (Fig.1), and strongly implicated in cancers (49). Many upstream members of the pathway, including MST1/2 (50), LATS1/2 (51) and Merlin (52), were identified to be tumor suppressors, while YAP and TAZ are oncogenes found to be elevated in many human cancers (53), including breast cancer (54), colon cancer (55), and liver cancer (56). The role of the Hippo pathway in organ size regulation is demonstrated by mice with liver-specific YAP overexpression, which show excessive liver enlargement (48). Similarly, tissue-specific knockout of MST1/2 or WW45 has been reported to induce liver, stomach, heart, and spleen enlargements (57;58), but not in kidney, lung, and intestine (57). Evidence suggests that the Hippo signaling pathway regulates organ growth and organ size by modulating cell proliferation (57;58) and progenitor cell differentiation (59;60). The role of the Hippo pathway in human linear growth is yet unknown. One exciting aspect worth exploring in the future is the function of Hippo pathway in the growth plate, and how, or if, the Hippo pathway regulates normal juvenile growth and growth deceleration, as it remains unclear whether the Hippo pathway activity changes with age.
Fig 1. Mammalian Hippo signaling pathway regulates organ growth.
The mammalian homologues of Drosophila Hippo (Hpo) are called MST1 kinase and MST2 kinase. Close to the cytoplasmic membrane, MST1/2 forms an active complex with WW repeat scaffolding protein WW45 (or Salvador in Drosophila). MST/WW45 complex then phosphorylates nuclear DBF-2-related kinase LATS1/2 (or Wts in Drosophila) with the help of another tumor suppressor Merlin/NF2, which is required for the recruitment of LATS1/2 to the cytoplasmic membrane. LATS in turn interacts with MOB to phosphorylate transcriptional coactivators YAP and TAZ (or Yorkie in Drosophila), causing inactivation excluion from the nucleus, and downregulation of genes important for cell cycle progressive, proliferation and organ growth.
Multi-organ growth-limiting genetic program
As described above, somatic growth is rapid in early life and gradually decelerates with age as the organism approaches its adult size. This growth deceleration is primarily caused by declining proliferation in multiple organs. We hypothesized that this growth deceleration is driven by a local growth-regulating genetic program that is common among different organs. Consistent with this hypothesis, expression microarray identified an extensive genetic program that occurs during growth deceleration, is common to kidney, lung, and heart, and is conserved among mice, rats, and sheep (61–63). More limited data suggest that this program occurs also in liver (64). This common program involves the downregulation of hundreds of genes with age. For many of these genes, knockdown of expression in vitro inhibits cell proliferation and knockout in vivo inhibits body growth, suggesting that they normally promote growth (64). These findings suggest that high levels of local expression of these growth-promoting genes support the rapid proliferation of juvenile organs, but, as organs approach their adult sizes, these genes are gradually downregulated, causing growth to slow and cease. Because these changes are common to multiple organs, it helps explain how growth in different organs is coordinated and slows concordantly, therefore maintaining body proportions. Interestingly, the program plays out more gradually in sheep than in mice, providing a possible explanation for the more prolonged growth and therefore greater adult body size of large mammals (63). Many genes in the program are oncogenes, suggesting that overexpression of or failure to downregulate these genes leads to tumorigenesis (65–67).
More recently, we showed evidence that transcription factor E2F3 serves as a master regulator, orchestrating at least a subset of this genetic program (68). In mice, expression of E2f3 is downregulated with age in multiple organs, and overexpression of E2f3 in hepatocytes isolated from late juvenile mice led to upregulation of many genes in the program, including Igf2 (68). Expression of E2F3 and IGF2 in humans is similarly downregulated with age, and interestingly, upregulated and positively correlated in bladder cancer and prostate cancer (68), suggesting that failure of this genetic program might be involved in the pathogenesis of these cancers.
One interesting property of this growth-limiting genetic program is that it seems to be regulated by growth itself (69). When newborn rats were temporarily placed on a tryptophan deficient diet (which suppresses appetite and induces malnutrition) to inhibit growth (64) and then subsequently switched to a regular diet, all major organs showed catch-up growth (64). In these rats, we found that the growth-limiting genetic program had been significantly delayed (64). These findings suggest that mammalian body growth is limited, at least in part, by a negative feedback loop. Growth causes the downregulation of a large set of growth-promoting genes, which in turn causes body growth to progressively slow and eventually cease, thus setting a fundamental limit on adult body size (69).
What we learned from GWAS of height variations
The process of longitudinal bone growth is governed by a complex interplay of endocrine signals, including GH, IGFs, thyroid hormone, and sex steroids (70), which act on growth plate cartilage. However, bone growth is also regulated by the expression of many other genes acting locally in the growth plate. It is estimated that 70–90% of adult stature is determined genetically, and various studies using genome-wide association (GWA) have identified many genes that contribute, albeit with a small effect size, to the normal variation in height and thus growth (71–77). In 2010, a GWA meta-analysis by the GIANT Consortium identified close to 200 loci that contribute about 10% of the normal variation in adult height (78). Interestingly, apart from several genes that participate in the GH-IGF-I system, most of these 200 loci did not include genes that are known to affect other hormonal systems.
To help identify more causative genes in these height-associated GWA loci, we recently performed a bioinformatic analysis of these GWAS data (79). We reasoned that many of the causative genes within these loci influence height because they are expressed in and play important functions in the growth plate. Therefore, we used expression microarray studies of mouse and rat growth plate, human disease databases, and a mouse knockout phenotype database to identify genes within the GWAS loci that are likely required for normal growth plate function. Each of these approaches identified significantly more genes within the GWA height loci than at random genomic locations, and the combined analysis together identified 78 genes important for growth plate function, including multiple genes that participate in PTHrP-IHH, BMP and CNP signaling, but also many genes that have not been implicated in any known endocrine or paracrine system (Table 1) (79). Our findings therefore suggest that human stature is determined by a large number of genetic factors that locally affect growth plate function. This conclusion is further supported by the most recent analysis by the GIANT Consortium, reporting over 400 height-associated loci, with cartilage being the most strongly implicated organ/tissue system (Table 1 lists 32 of these 78 genes) (80).
Table 1.
List of genes associated with adult stature by genome-wide association studies (GWAS) for which expression microarray studies suggest a role in growth plate biology.
| SNPa | Candidate geneb,c | GP Spatial Regulationd | GP Temporal Regulatione |
|---|---|---|---|
| rs3764419 | ADAP2 | high in HZ | |
| rs2145272 | BMP2 | ↑R-P-H* | |
| rs7864648 | BNC2 | low in HZ | |
| rs4282339 | CCDC99 | low in HZ | |
| rs17511102 | CDC42EP3 | ↑R-P-H | |
| rs1490384 | CENPW | low in HZ | |
| rs2665838 | DDX42 | low in HZ | |
| rs3791675 | EFEMP1 | high in RZ | |
| rs310405 | FAM46A | high in RZ | |
| rs10748128 | FRS2 | low in HZ | |
| rs6879260 | GFPT2 | low in HZ | |
| rs2166898 | GLI2 | high in RZ | |
| rs720390 | IGF2BP2 | ↓3–12w | |
| rs12470505 | IHH | ↑R-P-H | ↓3–12w |
| rs10037512 | MEF2C | high in HZ | ↓3–12w |
| rs2279008 | MYO9B | high in HZ | |
| rs12470505 | NHEJ1 | ↑R-P-H | |
| rs9969804 | OMD | ↓R-P-H | |
| rs9459531 | PDE10A | high in PZ | |
| rs11958779 | PPAP2A | high in HZ | |
| rs788867 | PRKG2 | low in RZ | |
| rs1490384 | RSPO3 | high in HZ | |
| rs9472414 | RUNX2 | ↑R-P-H | ↓3–12w |
| rs2247341 | SLBP | low in HZ | |
| rs3812163 | SNRNP48 | ↓3–12w | |
| rs2247341 | TACC3 | low in HZ | |
| rs9428104 | TBX15 | ↓R-P-H | |
| rs528045 | TOP2A | low in HZ | |
| rs4738736 | TOX | ↓R-P-H | |
| rs4470914 | TWIST1 | ↑3–12w | |
| rs2718423 | ZBTB20 | high in RZ | |
| rs7027110 | ZNF462 | low in HZ |
SNP: Single nucleotide polymorphisms associated with height in GWA studies
Candidate gene: gene within locus that was implicated as having a growth plate function
All genes on this list are expressed specifically in the growth plate: expression in 1-wk mouse whole growth plate exceeded expression in lung, kidney, heart (≥ 2.0-fold, FDR < 1%, microarray analysis)
GP spatially regulated: Expression between adjacent zones in 1-wk rat growth plate differ significantly (resting vs proliferative or proliferative vs hypertrophic, ≥ 2.0-fold, FDR < 5%, microarray analysis)
GP temporally regulated: Expression in the proliferative zone changed significantly from 3-wk-old rat to 12-wk-old rat (≥ 2.0-fold, FDR < 5%, microarray analysis)
↑R-P-H : upregulated from RZ (resting zone) to PZ (proliferative zone) to HZ (hypertrophic zone)
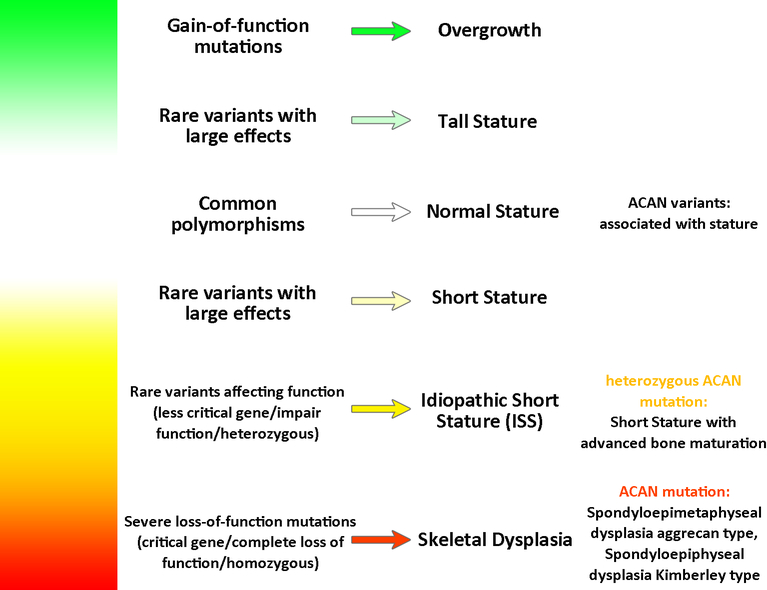
These height association studies also point to another important concept about childhood growth, short stature, and skeletal disorders; different sequence variants in a single gene required for normal growth plate function can produce a broad phenotypic spectrum (Fig.2). Severe genetic abnormalities in a gene required for growth plate function often produce a skeletal dysplasia, in which the bones are very short and malformed. Such mutations often disrupt protein function or expression severely and often are present in the homozygous state. Less severe genetic abnormalities in the same gene may present as “idiopathic” short stature, without clinical or radiological evidence of a skeletal dysplasia. These mutations may only partially impair protein function or expression or may occur in the heterozygous state. Even milder common variants in the same gene can lead to slight variation in stature. These small effects can be detected by GWAS. This concept is nicely demonstrated by recent findings related to the ACAN gene, which encodes a cartilage extracellular matrix proteoglycan, aggrecan. Homozygous mutations present as a severe skeletal dysplasia, spondyloepimetahyseal dysplasia, Aggrecan type (81). Heterozygous mutations in ACAN can present as a milder skeletal dysplasia, spondyloepiphyseal dysplasia, Kimberley type (82) whereas other heterozygous mutation can present as short stature with advanced skeletal maturation (83). Finally, ACAN lies within one of the height-associated loci found by GWA studies (78), suggesting that common polymorphism modulate height within the normal range.
Fig 2. Sequence variants in genes that affect growth plate function can produce a broad phenotypic spectrum.
For genes that positive regulate growth plate function, severe loss-of-function mutations often present as a skeletal dyplasias whereas milder mutations may present as short stature without an overt dysplasia. Common polymorphisms can modulate height, and gain-of-function mutations can cause tall stature.
Overgrowth Syndromes and Epigenetics
Recently, the genetic causes of several overgrowth syndromes have been found to involve mutations in genes responsible for epigenetic regulation. Weaver syndrome and Sotos syndrome were found to be caused by mutations in genes that encode histone methyltransferases. Weaver syndrome is caused by mutations in EZH2, which is part of the polycomb repressor complex 2 (PRC2) and catalyzes the trimethylation of lysine residue 27 in histone H3 (H3K27me3) which is associated with transcriptional repression and chromatin condensation (84;85). Similarly, Sotos syndrome is caused by mutations in NSD1 (86), a histone methyltransferase responsible for H3K36 methylation which is associated with transcriptional activation. Although the initial report on NSD1 mutations in Sotos syndrome was published in 2002, a more recent study in two children with ‘Sotos-like’ syndrome identified heterozygous mutations in SETD2 gene, which is a different histone methyltransferase for H3K36 trimethylation (87). In addition to histone methylation, DNA methylation has been implicated in an overgrowth syndrome; a recent study identified de novo mutations of DNMT3A in 13 overgrowth patients by exome sequencing (88). The DNMT3A gene encodes a DNA methyltransferase, which converts cytosine to 5-methylcytosine to establish new methylation marks after the removal of parental methylation during development. These recent discoveries on the genetics of overgrowth syndromes suggest a role for epigenetic mechanisms in body size regulation.
Conclusion
Mammalian body growth is governed by a complex interaction among environmental factors, endocrine, and local mechanisms in growing tissues. Some of these mechanisms specifically regulate the local function of the growth plate, which is responsible for longitudinal bone growth and thus determines adult height, whereas other signals regulate growth more generally in multiple organs. Our improved understanding about human childhood growth provides insights into some longstanding mysteries in biology, such as determination of body proportionality, body size and organ size. New human genetic approaches have helped identify the molecular causes of childhood growth disorders and the mechanisms governing normal variation in adult stature.
Key points:
Body growth is regulated by genetic, nutritional, environmental, and hormonal factors, and usually follows the pattern of being rapid in early life but gradually decelerating with age until a final body size is attained.
Recent advances in genetic technology has allowed rapid discovery of the molecular causes of growth disorders, such as a microduplication of Xq26.3 responsible for X-linked acrogigantism (X-LAG), EZH2 mutations leading to Weaver syndrome, and DNMT3A mutations causing a new overgrowth syndrome.
We are only beginning to understand the local mechanisms responsible for growth regulation, for example, the Hippo signaling pathway that regulates organ size; and a growth-limiting genetic program that helps explain the slowing of growth with age.
Recent GWAS and findings from whole exome sequencing suggest that sequence variants in growth plate genes may produce a broad phenotypic spectrum, including skeletal dysplasias, “idiopathic” short stature, or stature variation within the normal range.
Acknowledgements
Financial support and sponsorship
This work was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH.
Footnotes
Conflicts of interest
All listed authors have no conflicts of interest.
Reference List
- (1).Gomer RH. Not being the wrong size. Nat Rev Mol Cell Biol 2001. January;2(1):48–54. [DOI] [PubMed] [Google Scholar]
- (2).Reiter EO, Rosenfeld RG. Normal and Aberrant Growth In: Larsen PR, Kronenberg HM, Melmed S, Polonsky KS, editors. Williams Textbook of Endocrinology. 10 ed Philadelphia: Saunders; 2003. p. 1003–114. [Google Scholar]
- (3).Bogin B Evolutionary perspective on human growth. Annu Rev Anthropol 1999;28:109–53. [DOI] [PubMed] [Google Scholar]
- (4).Abad V, Meyers JL, Weise M, Gafni RI, Barnes KM, Nilsson O, et al. The role of the resting zone in growth plate chondrogenesis. Endocrinology 2002. May;143(5):1851–7. [DOI] [PubMed] [Google Scholar]
- (5).Rosenfeld RG, Belgorosky A, Camacho-Hubner C, Savage MO, Wit JM, Hwa V. Defects in growth hormone receptor signaling. Trends Endocrinol Metab 2007. May;18(4):134–41. [DOI] [PubMed] [Google Scholar]
- (6).Lecoq AL, Kamenicky P, Guiochon-Mantel A, Chanson P. Genetic mutations in sporadic pituitary adenomas--what to screen for? Nat Rev Endocrinol 2015. Jan;11(1):43–54. [DOI] [PubMed] [Google Scholar]
- (7).Trivellin G, Daly AF, Faucz FR, Yuan B, Rostomyan L, Larco DO, et al. Gigantism and acromegaly due to Xq26 microduplications and GPR101 mutation. N Engl J Med 2014. December 18;371(25):2363–74. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This study described a microduplication of Xq26.3 as new genetic cause of early-onset gigantism and GH hypersecretion
- (8).Beckers A, Lodish M, Giampaolo T, Rostomyan L, Lee M, Faucz FR, et al. X-linked acrogigantism (X-LAG) syndrome: clinical profile and therapeutic responses. Endocr Relat Cancer 2015. February 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Garcia-Aragon J, Lobie PE, Muscat GE, Gobius KS, Norstedt G, Waters MJ. Prenatal expression of the growth hormone (GH) receptor/binding protein in the rat: a role for GH in embryonic and fetal development? Development 1992. April;114(4):869–76. [DOI] [PubMed] [Google Scholar]
- (10).Abuzzahab MJ, Schneider A, Goddard A, Grigorescu F, Lautier C, Keller E, et al. IGF-I receptor mutations resulting in intrauterine and postnatal growth retardation. N Engl J Med 2003. December 4;349(23):2211–22. [DOI] [PubMed] [Google Scholar]
- (11).Fang P, Hi CY, Derr MA, Rosenfeld RG, Hwa V, Cowell CT. Severe Short Stature Caused by Novel Compound Heterozygous Mutations of the Insulin-Like Growth Factor 1 Receptor (IGF1R). J Clin Endocrinol Metab 2012. February;97(2):E243–E247. [DOI] [PubMed] [Google Scholar]
- (12).Giudice LC, de ZF, Gargosky SE, Dsupin BA, de las FL, Crystal RA, et al. Insulin-like growth factors and their binding proteins in the term and preterm human fetus and neonate with normal and extremes of intrauterine growth. J Clin Endocrinol Metab 1995. May;80(5):1548–55. [DOI] [PubMed] [Google Scholar]
- (13).Czeszynska MB, Janus D, Pankiewicz E, Konefal H, Hnatyszyn G, Jaskot B. 74 Gestational Diabetes: Cord Blood Insulin, IGF-I and IGF-II Levels in Relation to Anthropometric Parameters of Neonates Born as Aga or LGA. Pediatr Res 2005. August;58(2):367. [Google Scholar]
- (14).Baker J, Liu JP, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell 1993. October 8;75(1):73–82. [PubMed] [Google Scholar]
- (15).Zachmann M, Prader A, Sobel EH, Crigler JF Jr., Ritzen EM, Atares M, et al. Pubertal growth in patients with androgen insensitivity: indirect evidence for the importance of estrogens in pubertal growth of girls. J Pediatr 1986. May;108(5 Pt 1):694–7. [DOI] [PubMed] [Google Scholar]
- (16).Grumbach MM. Estrogen, bone, growth and sex: a sea change in conventional wisdom. J Pediatr Endocrinol Metab 2000;13 Suppl 6:1439–55. [DOI] [PubMed] [Google Scholar]
- (17).Leung KC, Johannsson G, Leong GM, Ho KK. Estrogen regulation of growth hormone action. Endocr Rev 2004. October;25(5):693–721. [DOI] [PubMed] [Google Scholar]
- (18).Nilsson O, Chrysis D, Pajulo O, Boman A, Holst M, Rubinstein J, et al. Localization of estrogen receptors-alpha and -beta and androgen receptor in the human growth plate at different pubertal stages. J Endocrinol 2003. May;177(2):319–26. [DOI] [PubMed] [Google Scholar]
- (19).Blanchard O, Tsagris L, Rappaport R, Duval-Beaupere G, Corvol M. Age-dependent responsiveness of rabbit and human cartilage cells to sex steroids in vitro. J Steroid Biochem Mol Biol 1991;40(4–6):711–6. [DOI] [PubMed] [Google Scholar]
- (20).Weise M, De-Levi S, Barnes KM, Gafni RI, Abad V, Baron J. Effects of estrogen on growth plate senescence and epiphyseal fusion. Proc Natl Acad Sci U S A 2001. June 5;98(12):6871–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Sigurjonsdottir TJ, Hayles AB. Precocious puberty. A report of 96 cases. Am J Dis Child 1968. March;115(3):309–21. [DOI] [PubMed] [Google Scholar]
- (22).Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, et al. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med 1994. October 20;331(16):1056–61. [DOI] [PubMed] [Google Scholar]
- (23).Smith EP, Specker B, Bachrach BE, Kimbro KS, Li XJ, Young MF, et al. Impact on bone of an estrogen receptor-alpha gene loss of function mutation. J Clin Endocrinol Metab 2008. August;93(8):3088–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Quaynor SD, Stradtman EW Jr., Kim HG, Shen Y, Chorich LP, Schreihofer DA, et al. Delayed puberty and estrogen resistance in a woman with estrogen receptor alpha variant. N Engl J Med 2013. July 11;369(2):164–71. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This is the second ever clinical report of estrogen receptor α mutation in human, describing a women with delayed puberty and estrogen resistance due to homozygous loss-of-function ESR1 mutation.
- (25).Hero M, Wickman S, Dunkel L. Treatment with the aromatase inhibitor letrozole during adolescence increases near-final height in boys with constitutional delay of puberty. Clin Endocrinol (Oxf) 2006. May;64(5):510–3. [DOI] [PubMed] [Google Scholar]
- (26).Hero M, Norjavaara E, Dunkel L. Inhibition of estrogen biosynthesis with a potent aromatase inhibitor increases predicted adult height in boys with idiopathic short stature: a randomized controlled trial. J Clin Endocrinol Metab 2005. December;90(12):6396–402. [DOI] [PubMed] [Google Scholar]
- (27).Weise M, Flor A, Barnes KM, Cutler GB Jr., Baron J. Determinants of growth during gonadotropin-releasing hormone analog therapy for precocious puberty. J Clin Endocrinol Metab 2004. January;89(1):103–7. [DOI] [PubMed] [Google Scholar]
- (28).Nilsson O, Weise M, Landman EB, Meyers JL, Barnes KM, Baron J. Evidence that estrogen hastens epiphyseal fusion and cessation of longitudinal bone growth by irreversibly depleting the number of resting zone progenitor cells in female rabbits. Endocrinology 2014. August;155(8):2892–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Victora CG, Adair L, Fall C, Hallal PC, Martorell R, Richter L, et al. Maternal and child undernutrition: consequences for adult health and human capital. Lancet 2008. January 26;371(9609):340–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Black RE, Allen LH, Bhutta ZA, Caulfield LE, de OM, Ezzati M, et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 2008. January 19;371(9608):243–60. [DOI] [PubMed] [Google Scholar]
- (31).Jain S, Golde DW, Bailey R, Geffner ME. Insulin-like growth factor-I resistance. Endocr Rev 1998. October;19(5):625–46. [DOI] [PubMed] [Google Scholar]
- (32).Straus DS, Takemoto CD. Effect of fasting on insulin-like growth factor-I (IGF-I) and growth hormone receptor mRNA levels and IGF-I gene transcription in rat liver. Mol Endocrinol 1990. January;4(1):91–100. [DOI] [PubMed] [Google Scholar]
- (33).Bornfeldt KE, Arnqvist HJ, Enberg B, Mathews LS, Norstedt G. Regulation of insulin-like growth factor-I and growth hormone receptor gene expression by diabetes and nutritional state in rat tissues. J Endocrinol 1989. September;122(3):651–6. [DOI] [PubMed] [Google Scholar]
- (34).Thissen JP, Underwood LE, Maiter D, Maes M, Clemmons DR, Ketelslegers JM. Failure of insulin-like growth factor-I (IGF-I) infusion to promote growth in protein-restricted rats despite normalization of serum IGF-I concentrations. Endocrinology 1991. February;128(2):885–90. [DOI] [PubMed] [Google Scholar]
- (35).Lawson EA, Donoho D, Miller KK, Misra M, Meenaghan E, Lydecker J, et al. Hypercortisolemia is associated with severity of bone loss and depression in hypothalamic amenorrhea and anorexia nervosa. J Clin Endocrinol Metab 2009. December;94(12):4710–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Warner MH, Beckett GJ. Mechanisms behind the non-thyroidal illness syndrome: an update. J Endocrinol 2010. April;205(1):1–13. [DOI] [PubMed] [Google Scholar]
- (37).Chou SH, Chamberland JP, Liu X, Matarese G, Gao C, Stefanakis R, et al. Leptin is an effective treatment for hypothalamic amenorrhea. Proc Natl Acad Sci U S A 2011. April 19;108(16):6585–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Potthoff MJ, Inagaki T, Satapati S, Ding X, He T, Goetz R, et al. FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc Natl Acad Sci U S A 2009. June 30;106(26):10853–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Inagaki T, Lin VY, Goetz R, Mohammadi M, Mangelsdorf DJ, Kliewer SA. Inhibition of growth hormone signaling by the fasting-induced hormone FGF21. Cell Metab 2008. July;8(1):77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Yamamoto M, Iguchi G, Fukuoka H, Suda K, Bando H, Takahashi M, et al. SIRT1 regulates adaptive response of the growth hormone--insulin-like growth factor-I axis under fasting conditions in liver. Proc Natl Acad Sci U S A 2013. September 10;110(37):14948–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Fan Y, Menon RK, Cohen P, Hwang D, Clemens T, DiGirolamo DJ, et al. Liver-specific deletion of the growth hormone receptor reveals essential role of growth hormone signaling in hepatic lipid metabolism. J Biol Chem 2009. July 24;284(30):19937–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Wu S, Grunwald T, Kharitonenkov A, Dam J, Jockers R, De LF. Increased expression of fibroblast growth factor 21 (FGF21) during chronic undernutrition causes growth hormone insensitivity in chondrocytes by inducing leptin receptor overlapping transcript (LEPROT) and leptin receptor overlapping transcript-like 1 (LEPROTL1) expression. J Biol Chem 2013. September 20;288(38):27375–83. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- (43).Guasti L, Silvennoinen S, Bulstrode NW, Ferretti P, Sankilampi U, Dunkel L. Elevated FGF21 leads to attenuated postnatal linear growth in preterm infants through GH resistance in chondrocytes. J Clin Endocrinol Metab 2014. November;99(11):E2198–E2206. [DOI] [PubMed] [Google Scholar]; * This study discovered elevated FGF21 in preterm infants and investigated the potential role of FGF21 in GH resistance of these infants.
- (44).Kubicky RA, Wu S, Kharitonenkov A, De LF. Role of fibroblast growth factor 21 (FGF21) in undernutrition-related attenuation of growth in mice. Endocrinology 2012. May;153(5):2287–95. [DOI] [PubMed] [Google Scholar]
- (45).Tapon N, Harvey KF, Bell DW, Wahrer DC, Schiripo TA, Haber DA, et al. salvador Promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell 2002. August 23;110(4):467–78. [DOI] [PubMed] [Google Scholar]
- (46).Wu S, Huang J, Dong J, Pan D. hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell 2003. August 22;114(4):445–56. [DOI] [PubMed] [Google Scholar]
- (47).Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell 2005. August 12;122(3):421–34. [DOI] [PubMed] [Google Scholar]
- (48).Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 2007. September 21;130(6):1120–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Plouffe SW, Hong AW, Guan KL. Disease implications of the Hippo/YAP pathway. Trends Mol Med 2015. February 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Jia J, Zhang W, Wang B, Trinko R, Jiang J. The Drosophila Ste20 family kinase dMST functions as a tumor suppressor by restricting cell proliferation and promoting apoptosis. Genes Dev 2003. October 15;17(20):2514–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Tao W, Zhang S, Turenchalk GS, Stewart RA, St John MA, Chen W, et al. Human homologue of the Drosophila melanogaster lats tumour suppressor modulates CDC2 activity. Nat Genet 1999. February;21(2):177–81. [DOI] [PubMed] [Google Scholar]
- (52).Trofatter JA, MacCollin MM, Rutter JL, Murrell JR, Duyao MP, Parry DM, et al. A novel moesin-, ezrin-, radixin-like gene is a candidate for the neurofibromatosis 2 tumor suppressor. Cell 1993. November 19;75(4):826. [DOI] [PubMed] [Google Scholar]
- (53).Steinhardt AA, Gayyed MF, Klein AP, Dong J, Maitra A, Pan D, et al. Expression of Yes-associated protein in common solid tumors. Hum Pathol 2008. Nov;39(11):1582–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Wang X, Su L, Ou Q. Yes-associated protein promotes tumour development in luminal epithelial derived breast cancer. Eur J Cancer 2012. May;48(8):1227–34. [DOI] [PubMed] [Google Scholar]
- (55).Wang L, Shi S, Guo Z, Zhang X, Han S, Yang A, et al. Overexpression of YAP and TAZ is an independent predictor of prognosis in colorectal cancer and related to the proliferation and metastasis of colon cancer cells. PLoS One 2013;8(6):e65539. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- (56).Wang J, Ma L, Weng W, Qiao Y, Zhang Y, He J, et al. Mutual interaction between YAP and CREB promotes tumorigenesis in liver cancer. Hepatology 2013. September;58(3):1011–20. [DOI] [PubMed] [Google Scholar]
- (57).Song H, Mak KK, Topol L, Yun K, Hu J, Garrett L, et al. Mammalian Mst1 and Mst2 kinases play essential roles in organ size control and tumor suppression. Proc Natl Acad Sci U S A 2010. January 26;107(4):1431–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Heallen T, Zhang M, Wang J, Bonilla-Claudio M, Klysik E, Johnson RL, et al. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science 2011. April 22;332(6028):458–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Zhao R, Fallon TR, Saladi SV, Pardo-Saganta A, Villoria J, Mou H, et al. Yap tunes airway epithelial size and architecture by regulating the identity, maintenance, and self-renewal of stem cells. Dev Cell 2014. July 28;30(2):151–65. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This study, along with ref (58), independently described the novel roles for the Hippo pathway in regulation of lung progenitor cell differentiation during lung development
- (60).Mahoney JE, Mori M, Szymaniak AD, Varelas X, Cardoso WV. The hippo pathway effector Yap controls patterning and differentiation of airway epithelial progenitors. Dev Cell 2014. July 28;30(2):137–50. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This study, along with ref (59), independently described the novel roles for the Hippo pathway in regulation of lung progenitor cell differentiation during lung development
- (61).Lui JC, Finkielstain GP, Barnes KM, Baron J. An imprinted gene network that controls mammalian somatic growth is down-regulated during postnatal growth deceleration in multiple organs. Am J Physiol Regul Integr Comp Physiol 2008. July;295(1):R189–R196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Finkielstain GP, Forcinito P, Lui JC, Barnes KM, Marino R, Makaroun S, et al. An extensive genetic program occurring during postnatal growth in multiple tissues. Endocrinology 2009. April;150(4):1791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Delaney A, Padmanabhan V, Rezvani G, Chen W, Forcinito P, Cheung CS, et al. Evolutionary conservation and modulation of a juvenile growth-regulating genetic program. J Mol Endocrinol 2014. June;52(3):269–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Lui JC, Forcinito P, Chang M, Chen W, Barnes KM, Baron J. Coordinated postnatal down-regulation of multiple growth-promoting genes: evidence for a genetic program limiting organ growth. FASEB J 2010. August;24(8):3083–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Rezvani G, Lui JC, Barnes KM, Baron J. A set of imprinted genes required for normal body growth also promotes growth of rhabdomyosarcoma cells. Pediatr Res 2012. January;71(1):32–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Chase A, Cross NC. Aberrations of EZH2 in cancer. Clin Cancer Res 2011. May 1;17(9):2613–8. [DOI] [PubMed] [Google Scholar]
- (67).Kishida S, Mu P, Miyakawa S, Fujiwara M, Abe T, Sakamoto K, et al. Midkine promotes neuroblastoma through Notch2 signaling. Cancer Res 2013. February 15;73(4):1318–27. [DOI] [PubMed] [Google Scholar]
- (68).Lui JC, Baron J. Evidence that Igf2 down-regulation in postnatal tissues and up-regulation in malignancies is driven by transcription factor E2f3. Proc Natl Acad Sci U S A 2013. April 9;110(15):6181–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Lui JC, Baron J. Mechanisms limiting body growth in mammals. Endocr Rev 2011. June;32(3):422–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Nilsson O, Marino R, De LF, Phillip M, Baron J. Endocrine regulation of the growth plate. Horm Res 2005;64(4):157–65. [DOI] [PubMed] [Google Scholar]
- (71).Hao Y, Liu X, Lu X, Yang X, Wang L, Chen S, et al. Genome-wide association study in Han Chinese identifies three novel loci for human height. Hum Genet 2013. June;132(6):681–9. [DOI] [PubMed] [Google Scholar]
- (72).Okada Y, Kamatani Y, Takahashi A, Matsuda K, Hosono N, Ohmiya H, et al. A genome-wide association study in 19 633 Japanese subjects identified LHX3-QSOX2 and IGF1 as adult height loci. Hum Mol Genet 2010. June 1;19(11):2303–12. [DOI] [PubMed] [Google Scholar]
- (73).Lei SF, Tan LJ, Liu XG, Wang L, Yan H, Guo YF, et al. Genome-wide association study identifies two novel loci containing FLNB and SBF2 genes underlying stature variation. Hum Mol Genet 2009. May 1;18(9):1661–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Lettre G, Jackson AU, Gieger C, Schumacher FR, Berndt SI, Sanna S, et al. Identification of ten loci associated with height highlights new biological pathways in human growth. Nat Genet 2008. May;40(5):584–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Sanna S, Jackson AU, Nagaraja R, Willer CJ, Chen WM, Bonnycastle LL, et al. Common variants in the GDF5-UQCC region are associated with variation in human height. Nat Genet 2008. February;40(2):198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Weedon MN, Lettre G, Freathy RM, Lindgren CM, Voight BF, Perry JR, et al. A common variant of HMGA2 is associated with adult and childhood height in the general population. Nat Genet 2007. October;39(10):1245–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Gudbjartsson DF, Walters GB, Thorleifsson G, Stefansson H, Halldorsson BV, Zusmanovich P, et al. Many sequence variants affecting diversity of adult human height. Nat Genet 2008. May;40(5):609–15. [DOI] [PubMed] [Google Scholar]
- (78).Lango AH, Estrada K, Lettre G, Berndt SI, Weedon MN, Rivadeneira F, et al. Hundreds of variants clustered in genomic loci and biological pathways affect human height. Nature 2010. October 14;467(7317):832–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Lui JC, Nilsson O, Chan Y, Palmer CD, Andrade AC, Hirschhorn JN, et al. Synthesizing genome-wide association studies and expression microarray reveals novel genes that act in the human growth plate to modulate height. Hum Mol Genet 2012. December 1;21(23):5193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Wood AR, Esko T, Yang J, Vedantam S, Pers TH, Gustafsson S, et al. Defining the role of common variation in the genomic and biological architecture of adult human height. Nat Genet 2014. November;46(11):1173–86. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This is the latest genome-wide association studies reporting over 400 genomic loci associated with human height variations.
- (81).Tompson SW, Merriman B, Funari VA, Fresquet M, Lachman RS, Rimoin DL, et al. A recessive skeletal dysplasia, SEMD aggrecan type, results from a missense mutation affecting the C-type lectin domain of aggrecan. Am J Hum Genet 2009. January;84(1):72–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Gleghorn L, Ramesar R, Beighton P, Wallis G. A mutation in the variable repeat region of the aggrecan gene (AGC1) causes a form of spondyloepiphyseal dysplasia associated with severe, premature osteoarthritis. Am J Hum Genet 2005. September;77(3):484–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Nilsson O, Guo MH, Dunbar N, Popovic J, Flynn D, Jacobsen C, et al. Short stature, accelerated bone maturation, and early growth cessation due to heterozygous aggrecan mutations. J Clin Endocrinol Metab 2014. August;99(8):E1510–E1518. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This study described a heterozygous mutation of the ACAN gene associated with idiopathic short stature in three families.
- (84).Tatton-Brown K, Hanks S, Ruark E, Zachariou A, Duarte S,V, Ramsay E, et al. Germline mutations in the oncogene EZH2 cause Weaver syndrome and increased human height. Oncotarget 2011. December;2(12):1127–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Gibson WT, Hood RL, Zhan SH, Bulman DE, Fejes AP, Moore R, et al. Mutations in EZH2 cause Weaver syndrome. Am J Hum Genet 2012. January 13;90(1):110–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Kurotaki N, Imaizumi K, Harada N, Masuno M, Kondoh T, Nagai T, et al. Haploinsufficiency of NSD1 causes Sotos syndrome. Nat Genet 2002. April;30(4):365–6. [DOI] [PubMed] [Google Scholar]
- (87).Luscan A, Laurendeau I, Malan V, Francannet C, Odent S, Giuliano F, et al. Mutations in SETD2 cause a novel overgrowth condition. J Med Genet 2014. August;51(8):512–7. [DOI] [PubMed] [Google Scholar]
- (88).Tatton-Brown K, Seal S, Ruark E, Harmer J, Ramsay E, Del Vecchio DS, et al. Mutations in the DNA methyltransferase gene DNMT3A cause an overgrowth syndrome with intellectual disability. Nat Genet 2014. April;46(4):385–8. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This study discovered mutation of the DNMT3A gene as the genetic cause of a new overgrowth syndrome.