Abstract
In vitro models of the human intestinal epithelium derived from primary stem cells are much needed for the study of intestinal immunology in health and disease. Here we describe an intestinal monolayer cultured on a porous membrane with accessible basal and apical surfaces for assay of intestinal cytokine production in response to stimuli. The system was composed of a differentiated, confluent epithelial monolayer derived from human primary stem cells obtained from small or large intestine. Interleukin 8 (IL-8) and monocyte chemoattractant protein 1 (MCP-1) were the most abundant inflammatory cytokines produced by the intestinal epithelium. The epithelium from all five tested regions of the intestine preferentially secreted into the apical reservoir of the monolayer with a twenty-six-fold greater concentration of IL-8 present in the apical reservoir of the colonic monolayer relative to that in the basal reservoir. Upon application of tumor necrosis factor alpha (TNF-α) to the basal surface of the colonic monolayer, the IL-8 concentration significantly increased in the basal, but not the apical reservoir. A dose-dependent elevation of IL-8 in the basal reservoir was observed for TNF-α-stimulation of the monolayer, but not for an organoid-based platform. To demonstrate the utility of the monolayer system, 88 types of dietary metabolites or compounds were screened for their ability to modulate IL-8 production in the basal reservoir of the intestinal monolayer in the absence or presence of TNF-α. No dietary metabolite or compound caused an increase in IL-8 in the basal reservoir in the absence of TNF-α. After addition of TNF-α to the monolayer, two compounds (butyrate, gallic acid) suppressed IL-8 production, suggesting their potential anti-inflammatory effect while the dietary factor forskolin significantly increased production. These results demonstrate that the described human intestinal monolayer platform has the potential for assay and screening of metabolites and compounds that alter the inflammatory response of the intestine.
Graphical Abstract
INTRODUCTION
The human intestinal epithelium and its surrounding immune cells constitute the largest immune organ and the epithelium continuously interacts with a vast array of microbes and dietary factors. Normal functioning of the intestinal immune system and integrity of the epithelial barrier are essential for protecting the host against leakage of bacterial and waste products from the gut lumen into the underlying tissue.1 The epithelial cells dynamically sense their environment acting as first responders in the event of a barrier breach by inflammatory responses, secreting different immune factors including interleukin 8 (IL-8), which then act as chemoattractants for rapid recruitment of immune cells. In vitro intestinal epithelial models hold immense potential in deciphering how the intestine surveys its immediate microenvironment monitoring for barrier defects and then responds interactively with cells of the immune system, such as lymphocytes, dendritic cells, macrophages, etc.
Adenocarcinoma cell lines including Caco-2 and HT-29 have been widely used as in vitro models to simulate the immunological response of the intestinal epithelium.2–8 The tumor cells, cultured on a variety of platforms (multiwell plates,2–5 Transwell inserts,6,7 and microfluidic devices8), have been shown to respond to stimuli (e.g. bacteria, bacterial antigens, inflammation mediators, toxins, etc.) by producing inflammatory cytokines. And yet these tumor cell lines suffer from a number of limitations due in part to genetic mutations, genetic drift and lab-to-lab variability.9 In recent years, breakthroughs in intestinal stem-cell biology enabled the construction of in vitro models based on primary intestinal epithelial cells in lieu of tumor cell lines. The intestinal organoid culture system for the first time realized the in vitro expansion and lineage manipulation of adult intestinal epithelial stem cells to produce “mini-gut” organoids comprised of the cell lineages found within the in vivo epithelium.10–13 Cells of the organoid are polarized so that the apical surface faces the enclosed lumen with the basal face in contact with the surrounding Matrigel.14 The organoid model has been used to study IL-6 secretion in response to infection with Salmonella.15 However, due to the hydrogel-embedded state and enclosed lumen, it is not straightforward to independently stimulate and analyze the immunological response at the apical and/or basal sides in the organoid system.
Primary intestinal cells grown as a monolayer on a porous substrate under defined conditions has enabled access to both the apical and basal cell surfaces.16–21 The intestinal stem cells cultured on the surfaces differentiate to form confluent monolayers utilized for transport and secretion assays. Such models have been used to study IgA transcytosis,16 co-culture with bacteria,17,18 ion transport,19 hormone secretion,19 macrophage,20 and myofibroblast and enteric neuron co-cultures.21 Recently, a small intestinal monolayer was shown to secrete cytokines such as transforming growth factor-beta 1 (TGF-β1), interferon gamma (IFN-γ) and IL-8; however, monolayers derived from the large intestine have not been demonstrated to secrete similar cytokines.20,21
The monolayer culture format using a porous membrane as a substrate offers well segregated compartments above (apical) and below (basal) the intestinal tissue as well as independent access to these fluid reservoirs. Thus, this system provides a valuable in vitro tool to study the immunological properties of primary intestinal epithelium, particularly the induction and production of inflammatory cytokines in response to external perturbants such as drugs or dietary metabolites. In the current study, intestinal epithelial stem cells were expanded using a recently developed monolayer system based on a collagen hydrogel scaffold,22 and plated on porous membranes to form a confluent, fully differentiated epithelium. We compare intestinal monolayers derived from different regions of the small and large intestine and systematically characterize this stem cell-derived model and its ability to act as an analytical tool to assess the impact of chemicals on the gut epithelium. The production of IL-8, the most abundant inflammatory cytokine produced by the intestinal epithelium, was extensively characterized for use of this system as a screening tool to detect induction of an inflamed epithelial state. The utility of this model for high-throughput screening was demonstrated by studying the effect of 88 dietary factors on IL-8 production followed by verification of the impact of these compounds on the intestinal epithelium.
EXPERIMENTAL SECTION
In vitro expansion of human intestinal epithelial stem cells on collagen hydrogel.
To expand human-derived intestinal epithelial stem cells, a monolayer culture technique was used according to a previously published protocol.22,23 Briefly, intestinal crypts were isolated from biopsy specimens of a patient (male, 52 years old), and plated directly on collagen hydrogel at a density of 1,000 crypts/well of a standard 6-well culture plate and overlaid with 4 mL stem medium (SM- Table S1). The medium was changed every 48 hours. When the cell confluency reached ≥ 80% (typically 5–8 days), the monolayers were passaged and sub-cultured on fresh collagen hydrogel at a passage ratio of 1:3.22,23 To ensure the cells possessed normal chromosomes, the cells were karyotyped (KaryoLogic, Inc, Research Triangle Park, NC) at passages P7 and P15. All experiments herein used cells between P5 and P15 unless otherwise specified. To study IL-8 production in five different regions of the intestine, intestinal epithelial stem cells were also expanded from tissue specimens of a cadaveric donor (male, 23 years old) following this same procedure.
Production of a monolayer of human intestinal epithelium on a porous substrate.
Transwell inserts possessing a porous membrane (0.4 μm pore size, either 12-well [Corning, #3460] or 96-well [Millipore, #PSHT004S5]) were coated with 1 vol% Matrigel in phosphate-buffered saline (PBS) at 37 °C overnight. The inserts were rinsed with PBS ×1 prior to cell plating. Intestinal epithelial cells were passaged according to the procedure described above except that the cells were suspended in expansion medium (EM- Table S1) and plated directly onto the top compartment of Transwell inserts. Cells from 1 well of the 6-well plate were dispersed into 6 separate 12-well Transwell inserts (1 mL in the upper [apical] reservoir, and 2 mL in the lower [basal] reservoir), or 48 separate 96-well inserts (0.1 mL in the apical reservoir, and 0.2 mL in the basal reservoir). The medium was exchanged every 48 h. To induce cell differentiation, the medium was switched to differentiation medium (DM- Table S1) after 6 days, and the medium was exchanged every 48 h thereafter. By day 12, the monolayer was fully differentiated and suitable for characterization and cytokine assay. Several compounds were used to stimulate IL-8 production including tumor necrosis factor alpha (TNF-α, Peprotech, #300–01A), phorbol 12-myristate 13-acetate (PMA, Sigma, #P8139), lipopolysaccharides (LPS, Sigma, #L3012) and lipoteichoic acid (LTA, Invivogen, #tlrl-slta).
Characterization of human intestinal epithelium.
To reveal phenotypes, monolayers were simultaneously stained with 5-ethynyl-2-deoxyuridine (EdU, for proliferative cells), alkaline phosphatase (ALP, for colonocytes), mucin 2 (Muc2, for goblet cells) and Hoechst 33342 (for nuclei of all cells) according to a previously published protocol.22,23 The cells were then imaged using a Nikon Eclipse TE300 inverted epifluorescence microscope equipped with DAPI/FITC/Texas Red/CY5 filter sets. Images were acquired at randomly selected locations within a specimen using a 40× (N.A. = 0.6) objective. S-phase cells stained with EdU were imaged using a CY5 filter (excitation filter 604–644 nm, emission 672–712 nm), ALP was imaged with a Texas Red filter (excitation filter 542–582 nm, emission 604–644 nm), Muc2 immunofluorescence was visualized with a fluorescein filter (excitation filter 450–490 nm, emission 520 nm long pass), and nuclei stained by Hoechst 33342 was identified using a DAPI filter (excitation filter 352–402 nm, emission 417–477 nm). To reveal the apical features of the cells, the monolayer was fixed with 4% paraformaldehyde for 15 minutes, dried with a critical point dryer (Tousimis Semidri PVT-3), coated with 10-nm metal by a sputter coater (Cressington 108), and inspected by a SEM (FEI Quanta 200 ESEM, FEI Company).
Culture of Matrigel-embedded organoids and monolayers on a thick scaffold for IL-8 measurements.
In the organoid model, the cells were embedded in Matrigel in a 96-well plate following a previously reported protocol.24 The cells were cultured in EM for 4 days followed by differentiation in DM for 4 days. In the monolayer model on a thick scaffold, the cells were plated on the top of a collagen hydrogel (thickness of ~2.5 mm) in a 96-well plate and cultured in EM for 4 days followed by in DM for 4 days.22 To induce IL-8 production, the organoids and monolayers were exposed to 0, 0.4, 2 and 10 ng/mL TNF-α for 4 h, and the medium was collected for IL-8 measurement. Due to the technical challenge in accurately counting cell numbers within hydrogel-embedded organoids, the IL-8 production was not normalized to cell number. Instead, IL-8 induction was obtained by normalization to the unstimulated controls in each model.
Screening 88 dietary factors for induction or inhibition of IL-8 secretion.
Table S2 lists 88 dietary metabolites and compounds and their working concentrations used in the screen. The working concentration for each compound was based on that found in the literature (Table S2). On the 96-well Transwell plate, the cells were rinsed with DM (100 μL in the apical and 200 μL in the basal reservoir) ×1. To study the induction of IL-8, 80 μL DM with a dietary factor was added to the apical reservoirs, and 200 μL DM without the factor was added to the basal reservoirs. At 4 h, the supernatants from the basal reservoirs were collected. To study the inhibition of TNF-α induced stimulation of IL-8 production, 200 μL DM containing 10 ng/mL TNF-α was added to the basal reservoirs and incubated for 4 h. The supernatants from the basal reservoirs were then collected for measurement. One sample was used for each compound for the screen, and three samples were used for verifying each hit compound.
Quantification of IL-8 and other cytokines.
The IL-8 concentration was determined using an ELISA kit (ThermoFisher, #88–8086-88) according to the manufacturer’s instructions. The samples were diluted 10× (for unstimulated samples) or 40× (for TNF-α-stimulated samples) so that all concentrations measured fell well within the linear range (10–250 pg/mL) of the IL-8 ELISA assay. Human Inflammation Antibody Array (RayBiotech, #QAH-INF-1) was used to quantitatively measure 10 human inflammatory cytokines including IL-8. Unless otherwise specified, three samples were used for each condition. The change of IL-8 concentration compared with untreated control was analyzed statistically by two-tailed unpaired t-test. In all figures, ‘*’ denotes p <0.05, ‘**’ p <0.005 and ‘#’ not statistically significant
RESULTS AND DISCUSSION
Cytokine Secretion from Human Colonic Epithelium Monolayer
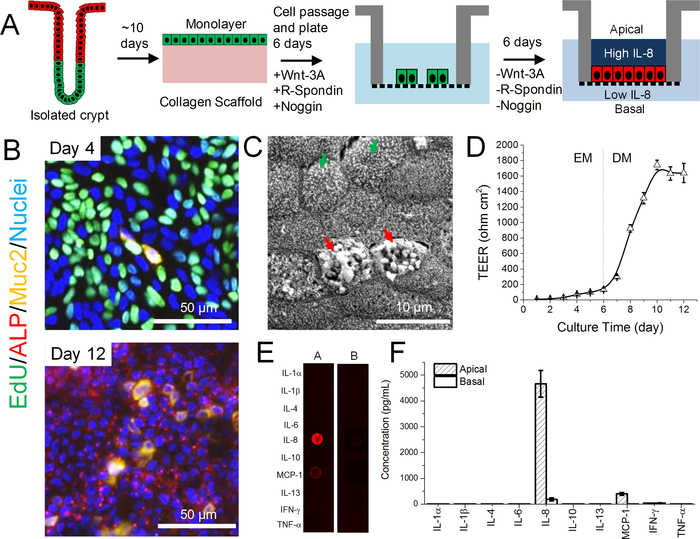
To create stem-cell-derived, human colonic epithelium on a porous substrate for the assay of cytokine production in the apical and basal reservoirs, stem cells were first expanded from isolated colon crypts using a culture technique on a neutralized collagen hydrogel scaffold in medium rich in growth factors such as Wnt-3A, R-spondin 3, and Noggin (Figure 1A).22 This technique has a unique advantage over the widely used organoid culture model in its ability to scale up rapidly with respect to the numbers of stem cells present in the culture.25 The intestinal cells were then plated on Matrigel-coated porous membranes and cultured for 6 days. The cells were highly proliferative (EdU+) due to the abundance of growth factors in the medium (Figure 1B). At day 6 of culture when the cells covered the entire surface of the porous membrane, the Wnt-3A, R-spondin 3, and Noggin were removed from the medium (differentiation medium, DM). Due to the absence of growth factors, cells ceased proliferating (EdU-) and spontaneously differentiated to mature cell lineages composed of a mixture of colonocytes (ALP+) and goblet cells (Muc2+) (Figure 1B). Imaging by electron microscopy confirmed the presence of colonocytes (microvilli on the apical surface, green arrows) and goblet cells (secretory granules, red arrows) (Figure 1C). The transepithelial electrical resistance (TEER) was low at day 6 (130±20 Ω cm2, n=6) when the cells were cultured in EM but increased rapidly and reached 1740±60 Ω cm2 after 4 days without growth factors (Figure 1D). After this time, the TEER remained stable up to day 12 of culture, and cytokine assays were performed at day 12 when the TEER was 1600±100 Ω cm2. The high TEER indicated that the reservoirs above the apical and below the basal cell membrane were compartmentalized without passive fluid and ion movement across the epithelium. The segregation suggested that the cells could be stimulated, and cytokines assayed independently on both the apical and basal sides of the cells.
Figure 1.
Human stem-cell-derived intestinal epithelial monolayer for studying cytokine production. (A) Schematic showing the generation of fully differentiated, confluent monolayer derived from primary intestinal crypts. (B) Fluorescence microscopy of monolayers. Day 4 (upper panel) shows monolayers grown with Wnt-3A, R-spondin 3, and Noggin (day 4: 4 days in EM) and then fully differentiated (day 12: 6 days in EM, 6 days in DM). Colors as follows: EdU, green; ALP, red; Muc2, yellow; Hoechst 33342-stained nuclei, blue. (C) Electron microscopy of the apical surface of the differentiated monolayer at day 12. (D) Change in TEER over time. Each point represents the mean ± standard deviation, n=6. (E) Fluorescence image of the cytokine antibody array for assay of the 7-h supernatants. A: apical; B: basal. (F) Quantification of the concentration of inflammatory cytokines in the 7-h supernatant at both apical and basal sides (n=3).
To evaluate the cytokines produced, the cells were incubated in serum-free medium for 7 h and then the medium from the apical and basal reservoirs was assayed for 10 common inflammatory cytokines (Figure 1E and F). IL-8, a potent chemoattractant that activates neutrophils, was the most abundant cytokine with a concentration of 4700±500 pg/mL (n=3) in the apical reservoir, and 180±60 pg/mL in the basal reservoir. Monocyte chemoattractant protein 1 (MCP-1) was the second most abundant: 400±50 pg/mL (apical) and 6±1 pg/mL (basal). Other cytokines were not detected. Since the apical concentrations of IL-8 and MCP-1 were much higher than in the basal reservoir (IL-8 [26×] and MCP-1 [67×]), a steep gradient of cytokines was established across the intestinal epithelium. This directional production of chemotactic cytokines is thought to be utilized in vivo as an early signal system for intestinal immune response, where in the event of intestinal epithelial barrier breakdown, IL-8 and MCP-1 rush into the basal epithelial side to attract immune cells (neutrophils, monocytes) from the underlying lamina propria towards the apical surface. Thus IL-8 production and apical secretion by the epithelial cells is used as a sentinel signal for epithelial damage.20 As IL-8 is the most abundant cytokine produced by the colonic epithelium, it became the focus of the following studies to validate the platform.
IL-8 Induction
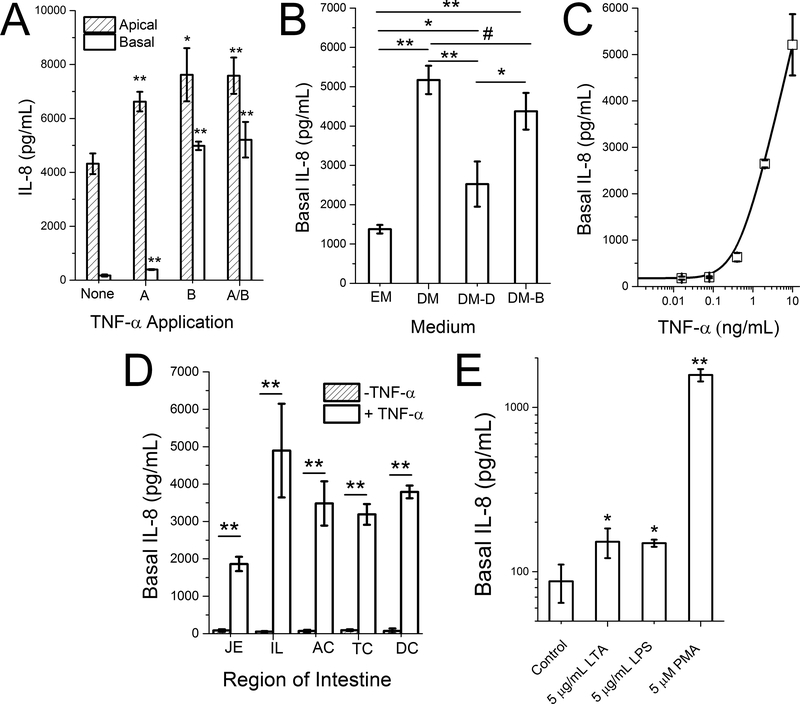
Inflammatory mediators, such as TNF-α, have been widely used to induce IL-8 production in Caco-2 and HT-29 tumor intestinal cell models.2,3 To study IL-8 production from the primary colonic epithelial cells, the monolayers were stimulated with 10 ng/mL TNF-α for 4 h in either reservoir (apical or basal) or both reservoirs (Figure 2A). Apical application of TNF-α minimally increased the IL-8 production in both apical [1.5×] and basal [2.3×] reservoirs. Basal application of TNF-α also slightly increased the IL-8 concentration apically [1.8×], but significantly increased that in the basal reservoir [28.5×]. A similar IL-8 result (apical [1.8×], basal [29.7×]) was observed when TNF-α was added to both reservoirs. White blood cell production of TNF-α creates an “inflamed” epithelium which then results in increased IL-8 secretion by the colonic epithelium potentiating the impact of the TNF-α and attracting more immune cells into the epithelium.26 Thus, this colonic monolayer culture system recapitulated key features of the intestinal inflammatory response.
Figure 2.
IL-8 production. (A) IL-8 concentration in the apical and basal reservoirs when the cells were stimulated with 0 (None) or 10 ng/mL TNF-α either apically (A), basally (B), or both (A/B). (B) IL-8 production in the basal reservoir after the monolayer was cultured in EM for 6 days, followed by various media for an additional 6 days. EM: expansion medium; DM: differentiation medium; DM-D: differentiation medium with 10 μM DAPT. DM-B: differentiation medium with 2 mM butyrate. (C) Dose dependent basal production of IL-8 in response to basal TNF-α stimulation. (D) Basal IL-8 production in monolayers derived from five different regions of the human intestine: jejunum (JE), ileum (IL), ascending colon (AC), transverse colon (TC), and descending colon (DC). (E) Basal IL-8 production when the monolayer was apically stimulated with lipoteichoic acid (LTA), lipopolysaccharide (LPS) or PMA. IL-8 production was quantified in the basal reservoir after stimulation with 10 ng/mL TNF-α on the basal side for 4 h. Unpaired t test: * P < 0.05; ** P < 0.005.
To understand which of the epithelial cell types might be responsible for IL-8 production, the degree of Wnt and Notch pathway stimulation applied to the monolayers was titrated using defined media to force the cells into one or more of the differentiated cell lineages.13,23 The monolayers were then assayed for IL-8 production. Prior to forced differentiation, the colonic monolayers were cultured in a growth medium rich in growth factors for 6 days to enable the cells to expand and cover the porous membrane. The monolayers were then placed into one of four types of media for an additional 6 days: EM (+Wnt-3A, R-spondin 3, and Noggin), DM, DM plus 10 μM DAPT (DM-D), or DM plus 2 mM butyrate (DM-B). Cells remained proliferative under EM, and underwent spontaneous differentiation in DM into a mixture of colonocytes and goblet cells.23 Goblet cells were enriched by application of DM-D (DAPT is a Notch inhibitor), while colonocytes are enhanced by using DM-B (butyrate may function as Notch activator).23 The basal IL-8 production (after 4-h basal stimulation with 10 ng/mL TNF-α) was highest in DM, followed by DM-B, DM-D, and EM (Figure 2B). Colonocyte-enriched monolayers produced greater amounts of IL-8 (in DM and DM-B) than that for monolayers enriched with other cell types. The slightly reduced IL-8 production in DM-B vs. DM is likely due to the growth inhibitory effect of butyrate.23,27 For cells placed into DM on day 6, basal IL-8 production was dependent on the concentration of TNF-α applied to the basal tissue surface (Figure 2C).
IL-8 Production in Different Regions of the Small Intestine and Colon
All the above IL-8 studies are based on the cells derived from a colonic biopsy from a single patient. To investigate if IL-8 production is region-specific and would be exhibited by an additional patient, cells from five regions (jejunum: JE; ileum: IL; ascending colon: AC; transverse colon: TC; descending colon: DC) of the intestine were isolated from a second donor, and their response to the basal addition of 0 and 10 ng/mL TNF-α was measured (Figure 2D). Cells from all regions demonstrated high IL-8 production in the basal compartment in response to TNF-α stimulation: JE [22×], IL [92×], AC [50×], TC [35×] and DC [53×]. These data demonstrate that all regions of intestine have the capacity to use IL-8 as a sentinel molecule for damage to the intestinal epithelium.
Apical Stimulation of IL-8 Production
While TNF-α is presented to the epithelium in vivo through basal exposure from cells in the bloodstream and lamina propria, dietary and microbial factors will arrive at the cells on the apical face of the intestinal epithelium. To determine whether apical simulation by bacterial and food components could induce IL-8 production and secretion into the lower or basal reservoir of the epithelial monolayer, the monolayer was exposed to LTA (5 μg/mL), LPS (5 μg/mL) or PMA (5 μM) by addition of the molecules to the apical or upper reservoir. After 4 h, IL-8 was measured in the basal reservoir (Figure 2E). Slight increases in IL-8 was observed for LTA [1.7×] and LPS [1.7×], while greater production was observed for PMA [18.0×]. These data are consistent with that obtained using the Caco-2 tumor cell model where apical stimulation with lipopolysaccharide (LPS), lipoteichoic acid (LTA), PMA, endotoxin, or bacteria is known to lead to increased IL-8 production.2,6,28–30
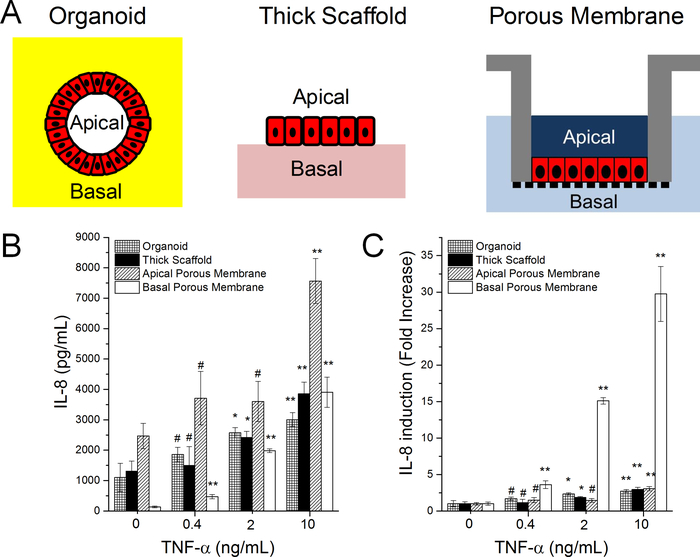
IL-8 Induction in Cells within an Organoid, on a Thick Collagen Scaffold, or Porous Membrane
Two primary cell models, the Matrigel embedded organoid system31 and the collagen hydrogel based monolayer system,22 have been reported for self-renewing culture of intestinal epithelial stem cells. For the organoid system, the basal surface of the cells faces outward, but is buried within a thick layer of Matrigel, while the apical surface is enclosed within the lumen of the organoid (Figure 3A). For the monolayer on a collagen hydrogel, the apical face is in direct contact with the overlying media and is easily accessible, but the basal face abuts a hydrogel on a non-porous polystyrene surface (Figure 3A). Consequently, the basal surface can only be accessed by application of compounds to the apical surface with leakage through the cell layer to the basal cell side. With the monolayer on the porous surface, both the basal and apical surfaces of the cells are accessible via the two reservoirs, although diffusion through the thin Matrigel coating and porous membrane is required to access the basal surface (Figure 3A). To compare the IL-8 production of these three platforms, the organoids and monolayers were stimulated by adding TNF-α (0, 0.4, 2 and 10 ng/mL) to the media overlying the Matrigel paddy (organoid), to the media above the monolayer (collagen hydrogel platform), or to the basal reservoir (monolayer on a porous membrane) for 4 hours followed by measurement of the IL-8 concentration (Figure 3B and 3C). Without TNF-α stimulation (i.e. 0 ng/mL), the baseline IL-8 production was high for the organoid platform (1100±500 pg/mL), the collagen hydrogel monolayer (1300±300 pg/mL) and at the apical side of monolayer on the porous membrane (2500±400 pg/mL). However, a low baseline concentration of IL-8 (130±30 pg/mL) was observed when measuring from the basal reservoir of the latter platform. After exposure to 10 ng/mL TNF-α, stimulated IL-8 production (or the fold increase of the reservoir IL-8 concentration) was small for the organoids (2.7×), monolayers on a collagen hydrogel (2.9×), and the apical reservoir of the monolayer on a porous membrane (3.1×). However stimulated IL-8 production was very prominent in the basal reservoir of the cells grown on the porous membrane (29.7×). The low induction of IL-8 by TNF-α in the organoid and thick collagen scaffold model is most likely due to leakage from the apical side across to the basal side of the epithelium or to the impact of the scaffold (Matrigel or collagen) on IL-8 production by the cells. In either case, the monolayer on a porous substrate offers a unique advantage over the other two models in that it is uniquely suited to assay immune responses by the epithelium due to its segregated and independently accessible apical and basal sides.
Figure 3.
IL-8 production by Matrigel-embedded organoids, monolayer cells on a thick collagen substrate, and monolayer cells on a porous membrane. (A) Schematic of the three model systems. In the organoid model the Matrigel patty is marked by the yellow while in the thick scaffold model the collagen hydrogel (2.5 mm thick) is marked in light pink. In the porous membrane model, the membrane is marked by the black vertical pattern below the cells which are marked red in each image. (B,C) IL-8 production in response to TNF-α stimulation. Panel C shows the absolute IL-8 concentration while panel D shows the fold change relative to the control without TNF-α. Unpaired t test: * P < 0.05; ** P < 0.005; #: not statistically significant.
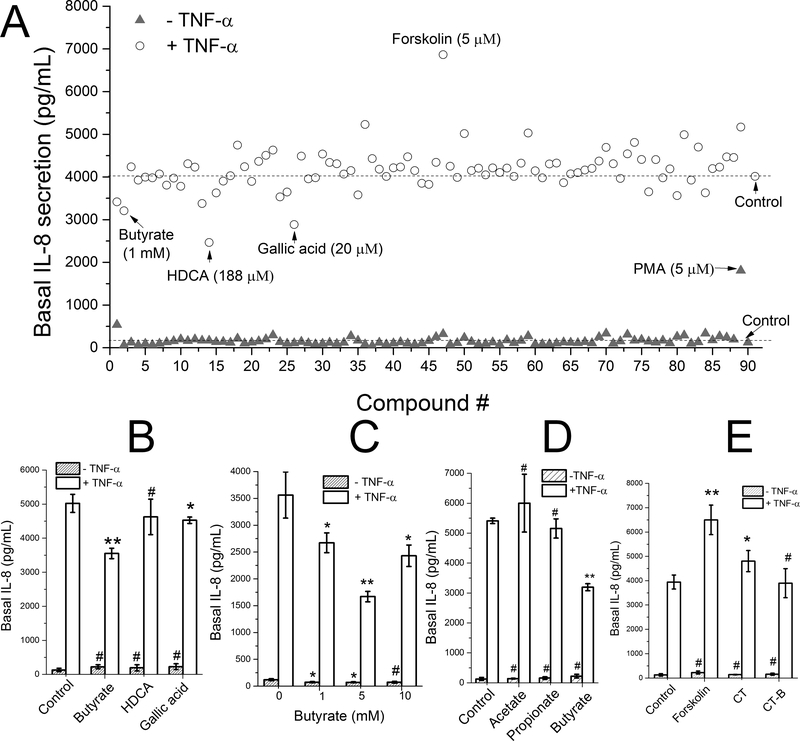
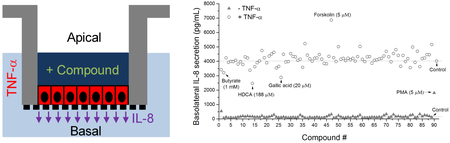
Compound Screen for Increased or Decreased Secretion of IL-8
To demonstrate the screening capability of the platform, 88 dietary metabolites and compounds were assayed for their ability to alter IL-8 production in the absence or presence of 10 ng/mL TNF-α stimulation (Figure 4A). The compounds were added to the apical surface of the monolayer followed by measurement of the IL-8 concentration on the basal side of the epithelium. In the absence of TNF-α, only PMA, a compound found in croton shrub leaves, substantially increased IL-8 production. PMA is a potent activator of the intracellular protein kinase, protein kinase C, which is known to participate in a wide range of intracellular signaling cascades particularly those regulating immune responses.32 The other tested compounds did not stimulate a direct inflammatory response in the colonic epithelium as assessed by this assay.
Figure 4.
Compound screen for modulation of IL-8 secretion. (A) Screening of 88 dietary metabolites and compounds in the absence or presence of TNF-α (#89: PMA as positive control sample, #90: negative control sample). The cells were exposed to the compounds at their apical surface for 4 h, then were stimulated with 0 (closed triangles) or 10 ng/mL (open circles) TNF-α on the basal side for 4 h. One sample was used for each compound. (B-E) Measurement of IL-8 production of selected compounds performed in triplicate. (B) IL-8 production in response to butyrate (1 mM), HDCA (188 μM) and gallic acid (20 μM). (C) IL-8 production in response to different concentrations of butyrate. (D) Effect of short chain fatty acids on IL-8 production: acetate (15 mM), propionate (5 mM), and butyrate (5 mM). (E) Effect of cAMP activators on IL-8 production: forskolin (5 μM), cholera toxin (CT, 10 μg/mL), cholera toxin subunit B (CT-B, 10 μg/mL). Unpaired t test: * P < 0.05; ** P < 0.005; #: not statistically significant.
To investigate whether the compounds possessed an anti-inflammatory effect on the colonic epithelium, the compounds were assayed for their ability to block TNF-α-mediated IL-8 production. The compounds were added to the apical side of the monolayers for 4 h after which time TNF-α was added to the basal side for 4 h. With basal TNF-α stimulation, a significant basal increase in IL-8 production was observed for all compounds (Figure 4A). Three compounds (butyrate, hyodeoxycholic acid [HDCA] and gallic acid) were identified that suppressed IL-8 production. To verify this result, the assay was repeated in triplicate using these compounds (Figure 4B). Compared with to the control with only TNF-α (100% induction), butyrate significantly reduced the level of TNF-α-induced IL-8 production to 71%, and gallic acid slightly reduced production to 92% of the control. The effect of HDCA was not statistically significant. As butyrate showed the greatest IL-8 suppressive effect among the screened compounds, the butyrate dose dependence of the suppression was measured (Figure 4C). The optimal butyrate concentration (5 mM) reduced the level of TNF-α-induced IL-8 production to 47% of the control, while 1 and 10 mM reduced production to 75% and 68%, respectively. Butyrate, a short-chain fatty acid that is the microbial fermentation product of dietary fiber, is reported to suppress intestinal inflammation and one mechanism may be through the inhibition of basal IL-8 secretion.33 Two other short chain fatty acids (SCFAs), propionate and acetate also end products of fermentation by anaerobic colonic microbiota were assessed for their impact on TNF-α-induced IL-8 production (Figure 3D). In contrast to butyrate, acetate (15 mM) and propionate (5 mM) failed to suppress TNF-α-induced IL-8 production. This is consistent with the known potency of SCFAs to modulate gut inflammation in which butyrate is the most potent.34
One compound (forskolin) enhanced TNF-α-induced IL-8 production. To verify this result, we repeated this assay in triplicate demonstrating that forskolin significantly and reproducibly enhanced TNF-α-mediated IL-8 secretion (Figure 3E). Forskolin, derived from the Coleus plant, is a potent activator of adenylate cyclase which acts to produce cyclic adenosine monophosphate (cAMP), an intracellular second messenger and modulator of inflammation.35 Cholera toxin is known to produce its toxic effect by activating adenylate cyclase to increase cAMP and ion/water movement into the intestinal lumen.36 For this reason the impact of cholera toxin (CT), and its inactive subunit B (CT-B) on TNF-α-induced IL-8 production was also measured. Cholera toxin but not CT-B enhanced IL-8 production. The above results demonstrated that the human colonic monolayer functioned in a physiologically relevant manner in the study of IL-8 production in response to multiple stimuli. A variety of pathways such as nuclear factor NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells), mitogen-activated protein kinase (MAPK) and peroxisome proliferator-activated receptor gamma (PPAR-γ) modulate inflammatory responses by regulating IL-8 secretion via TNF-α expression and drugs modulating these pathways are future targets for screens using this platform.37 The method also has significant potential for study of the effect of TNF-α modulators on TNF-α-induced IL-8 production given that the global TNF inhibitors market was valued at US $43 billion in 2017 and expected to reach US $181 billion by 2026.38
CONCLUSIONS
Adult intestinal epithelial stem cells on a porous substrate were utilized to create a functional monolayer of primary fully differentiated epithelium for cytokine assays. High TEER ensured the strict segregation between the apical and basal aspects of the epithelium to allow cells to be stimulated and assayed from either side independently. IL-8 was the most abundant inflammatory cytokine produced by the in vitro colonic epithelium. Without stimulation, directional production of IL-8 was observed apically. The basal IL-8 production was highly inducible to stimulation applied basally (e.g. TNF-α) or apically (e.g. PMA). This highly responsive induction was not observed in other primary cell models including the organoid system. A 96-well platform of this monolayer model system enabled high-throughput screening of 88 dietary compounds to study their effect on IL-8 secretion. Two compounds (butyrate and gallic acid) were identified that suppressed TNF-α-induced IL-8 production, while one compound (forskolin) significantly enhanced IL-8 production. This simple model made up of primary cells is anticipated to be useful to study a number of aspects of intestinal immunology.
Supplementary Material
ACKNOWLEDGEMENTS
Research reported in this publication was supported by the National Institutes of Health under Award R01DK109559 to N.L.A. We thank Scott Magness’ Laboratory for kindly providing human colonic biopsies and intestinal tissues. This work was performed in part at the Chapel Hill Analytical and Nanofabrication Laboratory, CHANL, a member of the North Carolina Research Triangle Nanotechnology Network, RTNN, which is supported by the National Science Foundation, Grant ECCS-1542015, as part of the National Nanotechnology Coordinated Infrastructure, NNCI.
Footnotes
These authors disclose the following: Yuli Wang, Christopher E. Sims, and Nancy L. Allbritton have a financial interest in Altis Biosystems. The remaining authors disclose no conflicts.
ASSOCIATED CONTENT
Supporting Information
Formulation of culture medium and list of dietary metabolites for screening.
REFERENCES
- (1).Round JL; Mazmanian SK Nat. Rev. Immunol 2009, 9, 313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Schuerermaly CC; Eckmann L; Kagnoff MF; Falco MT; Maly FE Immunology 1994, 81, 85–91. [PMC free article] [PubMed] [Google Scholar]
- (3).Abreumartin MT; Vidrich A; Lynch DH; Targan SR J. Immunol 1995, 155, 4147–4154. [PubMed] [Google Scholar]
- (4).Liboni KC; Li N; Scumpia PO; Neu J Journal of Nutrition 2005, 135, 245–251. [DOI] [PubMed] [Google Scholar]
- (5).Asarat M; Vasiljevic T; Apostolopoulos V; Donkor O Immunological Investigations 2015, 44, 678–693. [DOI] [PubMed] [Google Scholar]
- (6).Yang JS; Jeon JH; Jang MS; Kang SS; Ahn KB; Song M; Yun CH; Han SH Mol. Immunol 2018, 93, 47–54. [DOI] [PubMed] [Google Scholar]
- (7).Nishitani Y; Zhang L; Yoshida M; Azuma T; Kanazawa K; Hashimoto T; Mizuno M Plos One 2013, 8, e62441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Kim HJ; Li H; Collins JJ; Ingber DE Proc. Natl. Acad. Sci. U. S. A 2016, 113, E7–E15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Larregieu CA; Benet LZ AAPS Journal 2013, 15, 483–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Sato T; Vries RG; Snippert HJ; van de Wetering M; Barker N; Stange DE; van Es JH; Abo A; Kujala P; Peters PJ; Clevers H Nature 2009, 459, 262–265. [DOI] [PubMed] [Google Scholar]
- (11).Date S; Sato T In Annual Review of Cell and Developmental Biology, Vol 31, Schekman R, Ed., 2015, pp 269–289. [DOI] [PubMed] [Google Scholar]
- (12).Sato T; Stange DE; Ferrante M; Vries RGJ; van Es JH; van den Brink S; van Houdt WJ; Pronk A; van Gorp J; Siersema PD; Clevers H Gastroenterology 2011, 141, 1762–1772. [DOI] [PubMed] [Google Scholar]
- (13).Yin XL; Farin HF; van Es JH; Clevers H; Langer R; Karp JM Nature Methods 2014, 11, 106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Fatehullah A; Appleton PL; Nathke IS Philos. Trans. R. Soc. Lond. B Biol. Sci 2013, 368, 20130014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Zhang Y-G; Wu S; Xia Y; Sun J Physiol. Rep 2014, 2, e12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Moon C; VanDussen KL; Miyoshi H; Stappenbeck TS Mucosal Immunology 2014, 7, 818–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).VanDussen KL; Marinshaw JM; Shaikh N; Miyoshi H; Moon C; Tarr PI; Ciorba MA; Stappenbeck TS Gut 2015, 64, 911–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).In J; Foulke-Abel J; Zachos NC; Hansen A-M; Kaper JB; Bernstein HD; Halushka M; Blutt S; Estes MK; Donowitz M; Kovbasnjuk O Cell. Mol. Gastroenterol. Hepatol 2016, 2, 48–62.e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Kozuka K; He Y; Koo-Mccoy S; Kumaraswamy P; Nie B; Shaw K; Chan P; Leadbetter M; He L; Lewis JG; Zhong Z; Charmot D; Balaa M; King AJ; Caldwell JS; Siegel M Stem Cell Reports 2017, 9, 1976–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Noel G; Baetz NW; Staab JF; Donowitz M; Kovbasnjuk O; Pasetti MF; Zachos NC Sci. Rep 2017, 7, 45270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Puzan M; Hosic S; Ghio C; Koppes A Sci. Rep 2018, 8, 6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Wang YL; DiSalvo M; Gunasekara DB; Dutton J; Proctor A; Lebhar MS; Williamson IA; Speer J; Howard RL; Smiddy NM; Bultman SJ; Sims CE; Magness ST; Allbritton NL Cell. Mol. Gastroenterol. Hepatol 2017, 4, 165–182.e167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Wang YL; Kim R; Gunasekara DB; Reed MI; DiSalvo M; Nguyen DL; Bultman SJ; Sims CE; Magness ST; Allbritton NL Cell. Mol. Gastroenterol. Hepatol 2018, 5, 113–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Miyoshi H; Stappenbeck TS Nature Protocols 2013, 8, 2471–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Spence JR Cell. Mol. Gastroenterol. Hepatol 2017, 4, 203–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Scapini P; Lapinet-Vera JA; Gasperini S; Calzetti F; Bazzoni F; Cassatella MA Immunol. Rev 2000, 177, 195–203. [DOI] [PubMed] [Google Scholar]
- (27).Kaiko GE; Ryu SH; Koues OI; Collins PL; Solnica-Krezel L; Pearce EJ; Pearce EL; Oltz EM; Stappenbeck TS Cell 2016, 165, 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Kim KW; Kang SS; Woo SJ; Park OJ; Ahn KB; Song KD; Lee HK; Yun CH; Han SH Front. Microbiol 2017, 8, 1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Eckmann L; Kagnoff MF; Fierer J Infection and Immunity 1993, 61, 4569–4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Ma’ayeh SY; Knorr L; Skold K; Granham A; Ansell BRE; Jex AR; Svard SG Front. Cell. Infect. Microbiol 2018, 8, 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Clevers H Cell 2016, 165, 1586–1597. [DOI] [PubMed] [Google Scholar]
- (32).Ballester R; Rosen OM J. Biol. Chem 1985, 260, 5194–5199. [PubMed] [Google Scholar]
- (33.) Canani RB; Costanzo MD; Leone L; Pedata M; Meli R; Calignano A World Journal of Gastroenterology : WJG 2011, 17, 1519–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Vinolo MAR; Rodrigues HG; Nachbar RT; Curi R Nutrients 2011, 3, 858–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Alasbahi RH; Melzig MF Pharmazie 2012, 67, 5–13. [PubMed] [Google Scholar]
- (36).Insel PA; Ostrom RS Cell. Mol. Neurobiol 2003, 23, 305–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Hoffmann E; Dittrich-Breiholz O; Holtmann H; Kracht MJ Leukoc. Biol 2002, 72, 847–855. [PubMed] [Google Scholar]
- (38).Tumor Necrosis Factor (TNF) Inhibitor Market Projected to Grow With Double Digit CAGR during the Forecast Period; Credence Research 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.