Until recently, many viewed atherosclerosis as a lipid storage disease and/or a bland accumulation of proliferated smooth muscle cells. How the landscape has changed! Abundant evidence now implicates intertwining pathways of inflammation as crucial to atherogenesis and its clinical consequences.1 Inflammatory mediators modulate the functions of vascular wall cells that provoke the disease. Multiple cell types beckoned to enter the arterial intima during atherogenesis can produce these inflammatory mediators. Actions of monocyte/macrophages and T lymphocytes implicated both adaptive and innate immunity in atherosclerosis. Indeed, many leukocyte lineages participate in chronic inflammation in the plaque. Recent evidence points to participation of the acute inflammatory cell, the polymorphonuclear leukocytes (PMN), in plaque complication. An ongoing struggle between pro- and anti-inflammatory and pro-resolving stimuli determine the evolution of the plaque, from its initiation through its thrombotic complications.
In addition to arterial disease, leukocytes and inflammation also modulate venous thromboembolism, heart failure, and the healing of myocardial infarction. Indeed, the brain, autonomic nervous system, and hematopoietic organs (the bone marrow and the spleen) engage in complex crosstalk with the heart and blood vessels.2 We now recognize yet another a link between leukocytes and atherothrombosis: clonal hematopoiesis of indeterminate potential (CHIP).3
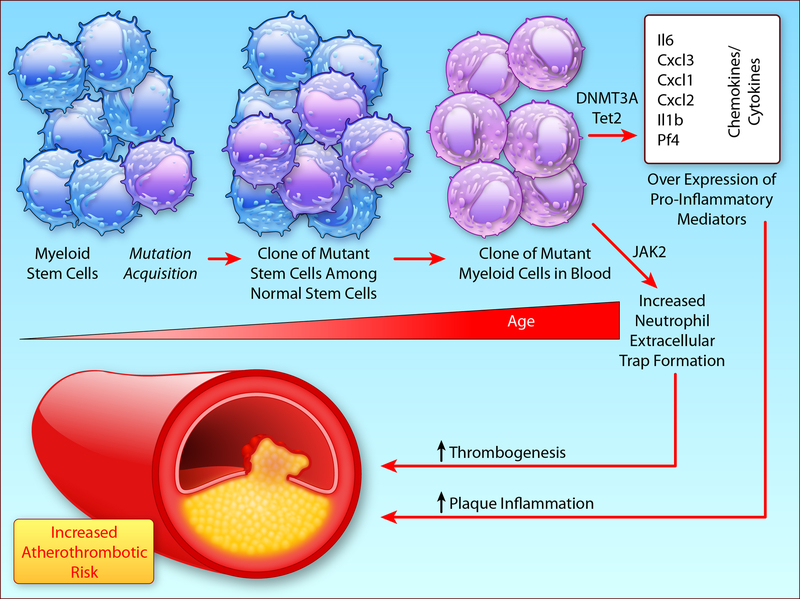
With age, we commonly accumulate clones of leukocytes that circulate in peripheral blood derived from bone marrow stem cells that have acquired somatic mutations that confer a proliferative advantage (Figure 1A). Over 10% of septuagenarians harbor such clones. Unsurprisingly, individuals with these leukocyte clones have a markedly elevated risk of developing hematologic malignancies. But most bearers of these mutant clones of blood leukocytes will never develop hematologic malignancies (hence the “indeterminate” in CHIP). Yet, total mortality in individuals with these clones by far exceeds the deaths attributable to hematologic malignancies. Unexpectedly, cardiovascular events account for this excess mortality. This cardiovascular risk appears independent of and at least as powerful as traditional risk factors. Thus, CHIP constitutes a previously unrecognized and potent cardiovascular risk factor.4
Figure 1A:
Clonal Hematopoiesis, as explained in the text, arises from somatic mutations in bone marrow stem cells, associated with ageing, that confer a proliferative advantage on the mutant clone. Mutations in Tet2 and in DNMT3A likely augment expression of pro-inflammatory genes by epigenetic regulation. Jak2 mutation can enhance NET formation, implicated in thrombosis, among other effects shown in B. Modified from reference 4.(Illustration Credit: Ben Smith).
Remarkably, mutations in just four genes account for the majority of these clones. Three of these four mutations involve potential modulators of DNA methylation, and most likely act through epigenetic regulation of gene expression. Mouse experiments support this conjecture, as loss of function of one of the genes commonly mutated in CHIP (Tet2) augments expression of pro-inflammatory cytokines and chemokines, as well as accelerates atherogenesis in response to an atherogenic diet.3,5 These experiments also indicate a causal relationship between CHIP and atherothrombosis, rather than a mere association of two conditions that accompany aging.
A fourth common mutation in CHIP, a V617F mutation in Janus kinase 2 (Jak2V617F), probably elevates atherothrombotic risk through distinct mechanisms. Jak2V617F is the most common genetic lesion in myeloproliferative neoplasms including polycythemia vera. These patients have a thrombotic diathesis, constituting a leading cause of their morbidity and mortality.
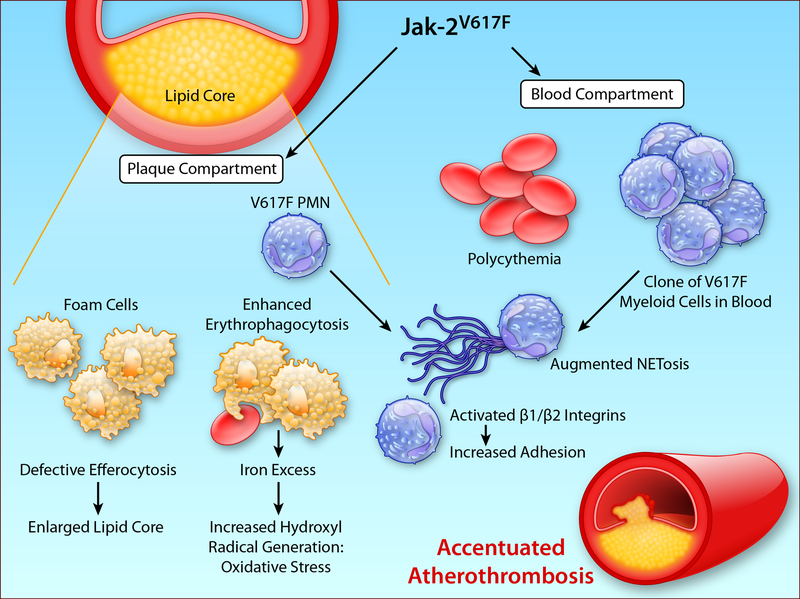
A new study by Wang et al. provides pioneering insight into how Jak2V617F influences atherothrombosis.6 Jak2V617F mice developed atheromata that contain more neutrophils, have larger lipid cores, and demonstrate increased iron deposition (Figure 1B). Mechanistic studies identified ineffective efferocytosis, likely due to reduced MerTK expression, as a contributor to the increased lipid core. Plaques with larger lipid cores associate with rupture and thrombosis, probably predisposing to cardiovascular events in bearers of Jak2V617F. This study also implicates products of the inflammasome in lesion complication in Jak2V617F mice.
Figure 1B:
The JakV617F can influence atherothrombosis both by actions within the plaque and by effects on circulating leukocytes. The left side of this diagram depicts some of the alterations in aspects of plaques demonstrated in mice bearing the JakV617F mutation. Impaired efferocytosis contributes to an enlarged lipid core. Increased erythrocytosis favors deposition of iron derived from heme that can generate the highly oxidant hydroxyl radical augmenting local oxidative stress. Mutant granulocytes in the blood compartment (right side) have a heightened tendency to form NETs, which may favor thrombosis and propagate vascular injury. They also have activation of β1 and β2 integrins, that can enhance attachment to the endothelium, where they may contribute to endothelial damage and thus promote thrombosis. (Illustration Credit: Ben Smith).
The iron excess seems to result from increased erythrophagocytosis. In mouse cells, this process associated with less CD47 on the surface of red blood cells (RBC), a signal that would normally deter phagocytosis (a “don’t eat me” signal). In contrast, in human cells, the authors found increased erythrophagocytosis associated with increased levels of RBCs of the pro-phagocytic signal calreticulin. The rich and often leaky microvasculature of human plaques provides a potential portal for erythrocyte entry. Plexi of plaque microvessels colocalize with regions of iron deposition, which can promote local generation of OH via the Fenton reaction (Figure).7 Recent work identified biliverdin reductase as one of the most dysregulated genes in human atheromata.8 Elevation in this enzyme, involved in heme catabolism, may reflect a response erythrocyte products within the plaque. These human observations bolster the findings regarding abnormal erythrocyte trafficking and iron deposition described by Wang et al.6
Jak2V617F neutrophils have a heightened tendency to form neutrophil extracellular traps (NETs), another contributor to thrombosis.9 In addition, Jak2V617 PMN translocate Rap1 to the surface membrane, activating β1/β2 integrins and enhancing leukocyte binding to endothelial adhesion molecules, and augmenting experimental thrombosis.10 Thus, the Jak2V617F mutation can alter atherosclerosis within the lesion itself, and also in the blood compartment, sensitizing granulocytes to NET formation (Figure 1B).
The study of Wang et al. has some limitations. The promoter used directed not only expansion of myeloid cells, but also yielded polycythemia and thrombocytosis, and the mutant clone accounts for the vast majority of bone marrow-derived cells. Thus, while this pattern mimics myeoproliferative neoplasms, individuals with CHIP generally have smaller clones (a median variant allele fraction under 20%), and by definition do not have abnormalities in blood cell numbers.11 These complexities render it difficult to dissect out the contributions of the quantitative elevations in the different lineages, as opposed to qualitative changes in cells, and the alterations in plaque character described. This circumstance, however, may have serendipitously permitted discovery of the altered RBC fates they found.
The involvement of granulocytes in human atherosclerosis has engendered controversy. While mouse lesions incorporate PMN in all stages of atherogenesis, granulocytes in human atherosclerosis appear predominantly in lesions previously disrupted either from leakage from friable neovessels, or by fibrous cap fissure followed by healing. Granulocyte involvement in the earlier phases of human atherosclerosis requires further study.
The extreme hypercholesterolemia generally produced in mouse atherosclerosis experiments presents another gap between the experimental conditions and the human disease. The increased use of ever more effective LDL-lowering therapies has led to an almost twentyfold difference in the cholesterol concentrations in typical mouse experiments and what is currently encountered in many patients. As cholesterol crystals can co-activate the inflammasome, extreme hypercholesterolemia could exaggerate the contribution the inflammasome and its products mature interleukin (IL)-1 beta and IL-18 in such mouse experiments.
Effective LDL lowering in the clinic may also lessen the prevalence of the so-called “vulnerable plaque.” Recent observations in mouse atherosclerosis that loss of function of IL-1 can augment characteristics of atheromata associated with propensity to rupture12 contrast starkly with studies of interruption of IL-1 beta signaling in humans that show no structural changes in arteries and reduced risk of cardiovascular events.13,14 These directionally opposite findings in experimental atherosclerosis in mice and human studies inject a sobering note of caution in the extrapolation of results obtained in mice to humans.15 We should use mice as an invaluable tool to probe mechanisms, as done in the elegant study by Wang and colleagues, rather than as a reliable predictor of translation to humans.
The recent recognition of the link between CHIP and worsened cardiovascular outcomes offers the community a cornucopia of opportunity to expand understanding of atherogenesis beyond traditional risk factors. Individuals in the CHIP demographic have often experienced decades of exposure to unnecessarily high concentrations of LDL, a indubitably causal risk factor. For such individuals, targeting pathways uncovered by the study of CHIP suggests treatments beyond the current standard of care (e.g. JAK inhibitors, or blockers of IL-1β, IL-18, or IL-6), directed by CHIP status, that might address the remaining unacceptable burden of risk in a more personalized and precision manner than heretofore possible.
Acknowledgments
Funding
PL receives support from the Heart, Lung and Blood Institute (R01HL080472) and the RRM Charitable Fund, and the Collaborative Sciences Award (18CSA34080399). RSS receives support from The Kay Kendall Leukaemia Fund.
Footnotes
Disclosures
RSS and BLE have filed a patent application covering the use of JAK-STAT inhibition with Ruxolitinib to inhibit NETosis. BLE has consulted for Celgene.
References
- 1.Libby P and Hansson GK. Inflammation and Immunity in Diseases of the Arterial Tree: Players and Layers. Circ Res. 2015;116:307–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Libby P, Nahrendorf M and Swirski FK. Leukocytes Link Local and Systemic Inflammation in Ischemic Cardiovascular Disease. J Am Coll Cardiol. 2016;67:1091–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, McConkey M, Gupta N, Gabriel S, Ardissino D, Baber U, Mehran R, Fuster V, Danesh J, Frossard P, Saleheen D, Melander O, Sukhova GK, Neuberg D, Libby P, Kathiresan S and Ebert BL. Clonal Hematopoiesis and Risk of Atherosclerotic Cardiovascular Disease. N Engl J Med. 2017;377:111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Libby P and Ebert B. CHIP (Clonal Hematopoiesis of Indeterminate Potential): Potent and Newly Recognized Contributor to Cardiovascular Risk. Circulation. 2018;138:666–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuster JJ, MacLauchlan S, Zuriaga MA, Polackal MN, Ostriker AC, Chakraborty R, Wu C-L, Sano S, Muralidharan S, Rius C, Vuong J, Jacob S, Muralidhar V, Robertson AAB, Cooper MA, Andrés V, Hirschi KK, Martin KA and Walsh K. Clonal hematopoiesis associated with Tet2 deficiency accelerates atherosclerosis development in mice. Science. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang W, Liu W, Fidler T, Wang Y, Tang Y, Woods B, Welch C, Cai B, Silvestre-Roig C, Ai D, Yang YG, Hidalgo A, Soehnlein O, Tabas I, Levine RL, Tall AR and Wang N. Macrophage Inflammation, Erythrophagocytosis and Accelerated Atherosclerosis in Jak2V617F Mice. Circ Res. 2018;in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michel JB, Libby P and Franck G. Intraplaque Hemorrhage: Decoration or Driver?. JACC: Basic to Translational Science. 2018;3:481–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matic LP, Jesus Iglesias M, Vesterlund M, Lengquist M, Hong MG, Saieed S, Sanchez-Rivera L, Berg M, Razuvaev A, Kronqvist M, Lund K, Caidahl K, Gillgren P, Ponten F, Uhlen M, Schwenk JM, Hansson GK, Paulsson-Berne G, Fagman E, Roy J, Hultgren R, Bergstrom G, Lehtio J, Odeberg J and Hedin U. Novel Multiomics Profiling of Human Carotid Atherosclerotic Plaques and Plasma Reveals Biliverdin Reductase B as a Marker of Intraplaque Hemorrhage. JACC Basic Transl Sci. 2018;3:464–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolach O, Sellar RS, Martinod K, Cherpokova D, McConkey M, Chappell RJ, Silver AJ, Adams D, Castellano CA, Schneider RK, Padera RF, DeAngelo DJ, Wadleigh M, Steensma DP, Galinsky I, Stone RM, Genovese G, McCarroll SA, Iliadou B, Hultman C, Neuberg D, Mullally A, Wagner DD and Ebert BL. Increased neutrophil extracellular trap formation promotes thrombosis in myeloproliferative neoplasms. Sci Transl Med. 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edelmann B, Gupta N, Schnoeder TM, Oelschlegel AM, Shahzad K, Goldschmidt J, Philipsen L, Weinert S, Ghosh A, Saalfeld FC, Nimmagadda SC, Müller P, Braun-Dullaeus R, Mohr J, Wolleschak D, Kliche S, Amthauer H, Heidel FH, Schraven B, Isermann B, Müller AJ and Fischer T. JAK2-V617F promotes venous thrombosis through β1/β2 integrin activation. J Clin Invest. 2018;128:4359–4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steensma DP, Bejar R, Jaiswal S, Lindsley RC, Sekeres MA, Hasserjian RP and Ebert BL. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood. 2015;126:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomez D, Baylis RA, Durgin BG, Newman AAC, Alencar GF, Mahan S, St Hilaire C, Muller W, Waisman A, Francis SE, Pinteaux E, Randolph GJ, Gram H and Owens GK. Interleukin-1beta has atheroprotective effects in advanced atherosclerotic lesions of mice. Nat Med. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P and Glynn RJ. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 14.Choudhury RP, Birks JS, Mani V, Biasiolli L, Robson MD, L’Allier PL, Gingras M-A, Alie N, McLaughlin MA, Basson CT, Schecter AD, Svensson EC, Zhang Y, Yates D, Tardif J-C and Fayad ZA. Arterial Effects of Canakinumab in Patients With Atherosclerosis and Type 2 Diabetes or Glucose Intolerance. J Am Coll Cardiol. 2016;68:1769–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Libby P. Murine “Model” Monotheism: An Iconoclast at the Altar of Mouse. Circ Res. 2015;117:921–925. [DOI] [PubMed] [Google Scholar]