Abstract
Alarmins are endogenous mediators capable of enhancing innate and adaptive immune response through induction of concomitant recruitment and activation of antigen-presenting cells. Here we provide a brief overview of various alarmins, highlight their critical roles in innate and adaptive antimicrobial immunity, and speculate on potential usage of alarmins in combating aspergillosis.
Keywords: Alarmins, aspergillosis, dendritic cell, immunity, antimicrobial
What are alarmins?
Alarmins are defined as endogenous mediators that can simultaneously induce the chemotactic migration and activation of antigen-presenting cells (APC) and consequently promote the induction of immune responses [1,2]. At present, alarmins include defensins, cathelicidin, eosinophil-derived neurotoxin (EDN, all abbreviations are listed in Table 1), and high-mobility group box 1 (HMGB1) protein (Table 2). They belong to several structurally distinct superfamilies of proteins that have historically been identified as antimicrobial peptides and proteins (AMP), enzymes, or chromosome-binding proteins [3–6]. Alarmins are present in leukocytes (granulocytes in particular) and various epithelial cells (including keratinocytes) as either granule products or nuclear proteins that are rapidly released upon microbial invasion or tissue injury. In addition, the expression of most alarmins can also be induced in the course of innate host defenses in response to pathogen-associated molecular patterns (PAMP) such as bacterial LPS or proinflammatory cytokines such as IL-1, TNF, and IFN [7].
Table 1.
Abbreviations used in this paper
| AMP | Antimicrobial peptide or protein |
| AP-1 | Activator protein 1 |
| AP-2 | Activator protein 2 |
| APC | Antigen-presenting cell |
| CCR | CC chemokine receptor |
| CD | Cluster of differentiation |
| CRAMP | Cathelin-related antimicrobial peptide |
| CTL | Cytotoxic T lymphocyte |
| CXCR | CXC chemokine receptor |
| DC | Dendritic cell |
| EAR | Eosinophil-associated ribonuclease |
| EDN | Eosinophil-derived neurotoxin |
| ERK | Extracellular signal-regulated kinase |
| FPR2 | Formyl peptide receptor 2 |
| FPRL1 | Formyl peptide receptor-like 1 |
| GαiPCR | Gαi protein-coupled receptor |
| G-CSF | Granulocyte colony-stimulating factor |
| GM- | Granulocyte macrophage colony-stimulating factor |
| CSF | |
| HBD | Human beta (β)-defensin |
| HMG | High-mobility group protein |
| HMGB1 | High-mobility group box 1 protein |
| HNP | Human neutrophil (α-defensin) peptide |
| I-A/I-E | Mouse class II MHC antigen encoded by the A and E lololoci |
| loci of the I region | |
| IFN | Interferon |
| IL | Interleukin |
| LPS | Lipopolysaccharide |
| M-CSF | Macrophage colony-stimulating factor |
| MBD | Mouse beta (β)-defensin |
| MCP | Monocyte chemoattractant protein |
| MHC | Major histocompatibility |
| NF-IL-6 | Nuclear factor-interleukin-6 |
| NF-κB | Nuclear factor kappa (κ) B |
| NK | Natural killer cell |
| NKT | Natural killer T cell |
| PAMP | Pathogen-associated molecular pattern |
| RAGE | Receptor for advanced glycation endproducts |
| TLR | Toll-like receptor |
Table 2.
Immunologic properties of alarmins
| Alarmin | APC recruitment | APC activation | Immune enhancement | ||
|---|---|---|---|---|---|
| Target cell | Receptor | Target cell | Receptor | ||
| Defensin | Dendritic cell | CCR6 | Dendritic cell | TLR2/1 | Th1 or Th2 |
| Macrophage | ?GαiPCR | Macrophage | TLR4 | ||
| Cathelicidin | Dendritic cell | FPRL1 | Dendritic cell | TLR9 | Th1 and Th2 |
| Macrophage | (mFPR2) | Macrophage | P2X7 | ||
| EDN | Dendritic cell | ?GαiPCR | Dendritic cell | TLR2 | Th2 |
| HMGB1 | Dendritic cell | RAGE | Dendritic cell | RAGE | Th1 |
| Macrophage | ?GαiPCR | Macrophage | TLR2;4;9 | ||
?GαiPCR = an unidentified GαiPCR. (Refer to Table 1 for the abbreviations).
Defensins
Defensins were the first mediators to be shown to have alarmin characteristics. Mammalian defensins are classified into α-, β-, and θ-subfamilies that differ in the distribution of the six conserved cysteine residues that form three distinct intramolecular disulfide bonds. Human α-defensin-1~4 are conventionally called human neutrophil peptides (HNP1~4) owing to their presence in the primary granules of neutrophils [8]. Mice have no neutrophil α-defensin, but possess multiple Paneth cell α-defensins called cryptidins, similar to human Paneth cell α-defensin 5 and 6 [9]. More than thirty human β-defensins (HBD) and mouse β-defensins (MBD) have been identified, which are predominantly generated by epithelial cells of various origins, including keratinocytes [10]. θ-defensin is only present in nonhuman primates [11]. Most defensins can be induced by proinflammatory stimuli via the activation of multiple transcriptional factors such as NF-κB, AP-1, AP-2, NF-IL-6, and IFN-activated transcriptional activators [12]. Both α- and β-defensins form a compact globular structure consisting of three anti-parallel β-sheets constrained by three disulfide bridges [3].
Several α- and β-defensins have been shown to have the dual capability of chemoattracting and activating APC (Table 2). HNP1~3 and several β-defensins are chemotactic for various subsets of leukocytes including dendritic cells (DC), monocytes, and macrophages [13–20]. The chemotactic effect of defensins is mediated by Gαi protein-coupled receptors (GαiPCRs) because it can be inhibited by pretreatment of the target cells with a Gαi protein-specific inhibitor pertussis toxin [14 16,19]. Certain β-defensins use the CC chemokine receptor (CCR) 6 to mediate their chemoattraction of DC and T cells [14,19,21]. Since monocytes and macrophages do not express functional CCR6, the GαiPCR(s) responsible for β-defensin chemoattraction of monocytes and macrophages remains unidentified [18,20]. The GαiPCR(s) used by HNP to chemoattract target cells also remains to be characterized [2,7]. In addition to their APC-chemoattracting effects, several defensins have the ability to activate leukocytes and epithelial cells. HBD2 and several α-defensins can activate mast cells and epithelial cells, leading to the release of prostaglandins and histamine and the production of many cytokines and chemokines [22–27]. MBD2 induces the three hallmarks of DC activation, including upregulation of DC surface costimulatory and major histocompatibility complex (MHC) molecules (CD40, CD86, and I-A/I-E), elevation of many cytokines including IL-12, and switch of chemokine receptor from CCR5 to CCR7, in a TLR4-dependent manner [28]. HBD3 has recently been reported to activate APC via a heterodimeric receptor consisting of Toll-like receptor (TLR) 1 and TLR2 [29]. Most of the more than thirty human β-defensins have not been studied at the protein level in the context of APC chemoattraction and activation, and therefore, it remains to be determined whether all β-defensins use TLRs as APC-activating receptors or not.
Cathelicidin
Cathelicidin represents another superfamily of mammalian AMPs that possesses the properties of alarmins (Table 2). About 40 cathelicidin members have been identified [30], however, humans and mice generate only one cathelicidin, called human cationic antimicrobial protein 18 (hCAP18)/LL-37 [31] and cathelin-related antimicrobial peptide (CRAMP) [32], respectively. All cathelicidins contain an N-terminal putative signal peptide, a conserved cathelin-like domain and a C-terminal antimicrobial domain that varies remarkably in size (ranging from 12–97 amino acid residues) [30]. Cathelicidins are predominantly stored constitutively in the secondary granules of neutrophils, however, they are also generated by other leukocytes and epithelial cells in response to proinflammatory stimuli including cytokines, PAMPs, or tissue injury [33–36]. LL-37 and CRAMP are α-helical peptides with a wide spectrum of antimicrobial effects [30]. Many cathelicidins are chemotactic for various leukocytes [37–41]. LL-37 utilizes the GαiPCR formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract neutrophils, monocytes and T cells [39,40]. CRAMP induces chemotaxis of mouse leukocytes using formyl peptide receptor 2 (FPR2), the mouse homolog of human FPRL1, as the receptor [42]. Cathelicidins can also induce the activation of many types of cells, resulting in the mobilization of intracellular calcium in monocytes [39], upregulation of a variety of genes (e.g., IL-8, MCP-1, CXCR2, and CCR2) by macrophages [43], release of proinflammatory mediators (e.g., histamine and prostaglandins, cytokines) by mast cells and keratinocytes [22,23], and proliferation of endothelial and epithelial cells [44,45]. Similar to its chemotactic activity, the angiogenic activity of LL-37 is mediated by FPRL1 expressed on endothelial cells [44]. Although the capacity of LL-37 to promote IL-1β production by monocytes is reported to be mediated by the ionotrophic purinergic receptor P2X7 [46], additional experimental evidence indicates that both FPRL1 and P2X7 are responsible for mediating the leukocyte-activating effect of LL-37[47]. LL-37 has recently been reported to form a complex with otherwise inert mammalian DNA fragments to activate plasmacytoid DC via TLR9 [48].
The direct microbicidal effect of most defensins and cathelicidins can only been seen at micromolar concentrations in vitro in buffers that are hypotonic (e.g., 10 mM phosphate buffer) and free of protein [3,4,8,9,20]. However, defensins or cathelicidins induce the migration and/or activation of leukocytes (including APCs) at nanomolar concentrations, even under isotonic and serum protein-containing conditions [13–19]. Therefore, it is speculated that the alarmin properties of defensins or cathelicidins may play more prominent roles than their direct microbicidal effects in combating invading pathogens in vivo.
EDN. EDN is a member of eosinophil-associated ribonuclease (EAR) superfamily that also includes eosinophil cationic protein in humans as well as multiple orthologous EARs in murine and other species [5]. EDN is a 134-amino acid residue protein with heavy glycosylation [49]. Structurally, EDN shows a V-shaped two-lobe folding typical of members of ribonuclease super-family, each consisting of three anti-parallel β-strands and one α-helix with two additional α-helices positioned between the two lobes [50]. EDN is stored in eosinophil granules and expressed by liver, spleen, neutrophils, and activated monocytes/macrophages [5,51,52]. Aside from its ribonuclease activity, EDN has antiviral effect against respiratory syncytial virus and HIV [53,54] and possesses alarmin properties (Table 2). EDN and its mouse ortholog mEAR2 are selectively chemotactic for human and mouse DC [55]. EDN treatment of DC induces DC activation as evidenced by an increase in the phosphorylation of extracellular signal-regulated kinases (ERK), production of numerous inflammatory cytokines, and expression of surface costimulatory (CD80, CD86) and MHC molecules by DCs [55,56]. Although the GaiPCR responsible for EDN’s chemotactic effect is unidentified, the receptor that mediates EDN’s DC-activating effect has recently been identified as TLR2 [56].
HMGB1. HMGB1 is a member of the high-mobility group (HMG) chromatin-binding protein superfamily. HMGs are divided into three subfamilies based on their distinct N-terminal functional domains: HMGBs contain two box domains, HMGNs possess a nucleosome-binding domain, while HMGAs have several ‘AT-hooks’ (AT-hook is a small DNA-binding protein motif that binds selectively to AT-rich DNA sequences) [6]. All HMGs have a C-terminus rich in acidic amino acids and play important roles in development and control of expression of numerous genes by regulating the structural changes of chromatin fibers. HMGB1 consists of 215 amino acid residues and is released by dying cells as a result of injury or by monocytes, macrophages, and NK cells in response to danger signals such as PAMPs [57–62]. Extracellular HMGB1 acts as an alarmin (Table 2) because it also possesses the dual capability of chemoattracting and activating APCs [63–67]. The chemotactic effect of HMGB1 on mesoangioblasts, monocytes and DC can be inhibited by pretreatment of target cells with pertussis toxin, indicative of the usage of a GαiPCR [63,65,68]. However, neutralizing antibody against receptor for advanced glycation end-products (RAGE) also reduces the chemotactic activity, suggesting both RAGE and an unidentified GαiPCR are somehow involved [63,65]. HMGB1 activation of macrophages and DC has been shown to be mediated by RAGE, TLR2, TLR4, and/or TLR9 [66,69–71]. Since HMGB1 has a great propensity to form complexes with DNA, LPS, and certain lipoproteins, activation of TLR2, 4, or 9 by the resultant complexes may account for the capacity of HMGB1 to induce cell activation.
Alarmins and antimicrobial immunity
Alarmins play important roles in galvanizing antimicrobial innate immunity (Fig. 1). Upon the entry of microorganisms into the host, alarmins (e.g., defensins, cathelicidins, EDN, or HMGB1) are rapidly released by epithelial cells and local resident leukocytes in response to cell death or stimulation by PAMPs. These alarmins, based on their antimicrobial activities may directly kill bacteria, fungi, parasites, and inactivate viruses or toxins, therefore, would greatly reduce the burden of invading pathogens and the pathogenic effects of toxins [3,5,30,72,73]. In addition, alarmins also contribute to the recruitment of phagocytes and APC (e.g., granulocytes, monocytes, and DC) owing to their chemotactic effects [2,13–20,37–41,55,63,65,68]. Furthermore, activation of local epithelial cells and leukocytes by alarmins would lead to enhanced phagocytosis and generation of inflammatory mediators (e.g., cytokines, chemokines, histamine, prostaglandins, etc) that further amplify local inflammatory innate immune responses [22–29,43,66,69–71]. Invading pathogens are contained/eliminated by these orchestrated actions of alarmins and other components of the innate immune response.
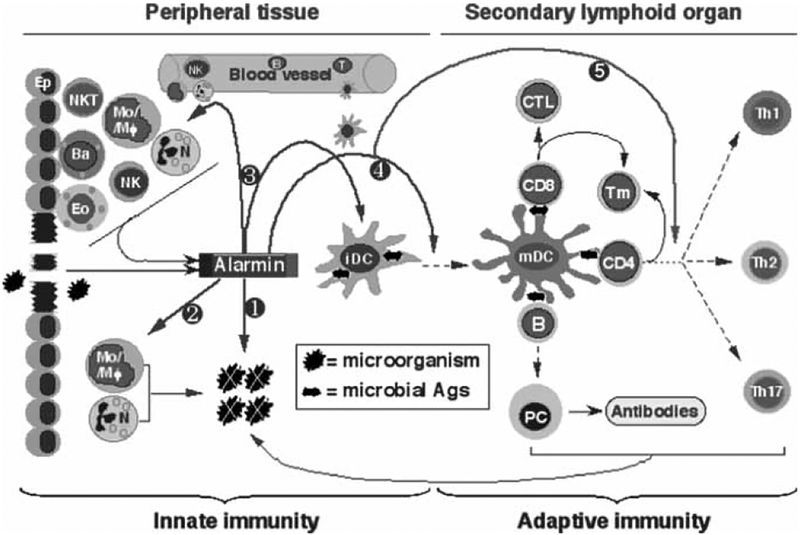
Fig. 1.
Schematic illustration of the roles and mechanisms by which alarmins enhance host antimicrobial immunity. Microorganisms enter the tissue during infection and/or tissue injury. In infected tissue, various alarmins are released by cells of the innate immune system, such as epithelial cells (Ep, including keratinocytes) and infiltrating leukocytes including neutrophils (N), basophils (Ba), eosinophils (Eo), monocytes/macrophages (Mo/Mϕ), NK, and NKT cells. Alarmins contribute to innate antimicrobial defense by directly killing and/or inactivating microorganisms (), activating phagocytes that, in turn, destroy microorganisms (⊄), and/or recruit additional phagocytes into infected tissue (⊂). In addition, alarmins induce the recruitment of immature DCs (iDC) into the infected tissue, which would promote the uptake of microbial antigens (⊂). Alarmins also stimulate the maturation of DCs that have engulfed microbial antigens into fully activated mature DCs (mDC), which not only enhances the antigen-presenting capacity, but also enable the resulting DCs to migrate into the secondary lymphoid organs for inducing the activation of antigen-specific B and T lymphocytes (⊆). Furthermore, alarmins can regulate the types (e.g., Th1 vs Th2) of adaptive antimicrobial immune responses through controlling the characteristics of DC maturation (∈). The products of the adaptive immune responses contribute to the elimination of the invading microorganisms either directly (e.g., CTLs) or indirectly by facilitating innate effector cells (e.g. antibodies and cytokines).
Innate immune responses not only efficiently contain infection, but also set the stage for the induction of antigen-specific adaptive immune response that is required to completely eliminate many types of pathogens and to generate immunologic memory. The process of adaptive antimicrobial immune response is initiated at the sites of pathogen entry where APC (in particular DC) engulf microbial antigens. Alarmins enhance antigen uptake by recruiting DCs into sites of pathogen entry (Fig. 1). After antigen uptake, DCs need to mature while processing antigens in order to acquire the capacities of trafficking to secondary lymphoid organs and presenting antigens to naïve T cells [2,74]. Alarmins rapidly activate DC to mature, which plays a critical role in the induction of adaptive immune response (Fig. 1). This has been established by the capacity of various alarmins to enhance antigen-specific immune responses to a number of antigens when the antigen is administered together with a single alarmin or as an alarmin-antigen fusion product [19,28,42,63,67,75,76]. In addition to enhancing the development of antigen-specific immune response, alarmins also regulate the type of immune responses by controlling the activation of DCs (Fig. 1). For example, MBD2 and HMGB1 polarize T cell responses predominantly in a Th1 direction [19,28,67,76]. Thus, DC activated by MBD2 and HMGB1 generate high amount of IL-12p70, a cytokine critical for Th1 polarization [28,63,64,67]. In contrast, EDN-activated DC generate more IL-10 without much IL-12p70 and polarizes immune responses predominantly into a Th2 type, resulting in the production of large amount of IL-5, IL-13, and IL-10 by antigen-specific T lymphocytes [56].
Thus, alarmins play critical roles in host antimicrobial immunity by initiating and augmenting both innate and adaptive immune responses through multiple mechanisms. The importance of alarmins to mammalian antimicrobial immunity is validated by various animal models. Knockout of matrilysin, an enzyme required for the generation of mature mouse cryptidins, renders mice more susceptible to Salmonella typhimurium infection due to the lack of functional Paneth cell α-defensins [77]. Knockout of MBD1 or mouse cathelicidin results in reduced resistance to several bacterial infections [78–80]. Conversely, overexpression of α-defensin HD5 and cathelicidin by transgenic technique or adenovirus-mediated gene transfer enhances antibacterial defenses of mice [81–83]. Furthermore, the requirement of recruited leukocytes to participate in the in vivo anti-bacterial effect of HNP1 [84], together with the simultaneous induction of Th1-type cytokines (IL-12 and IFNγ), leukocyte infiltration, and resistance to Bordetella pertussis challenge in the piglet lung tissue by intra-pulmonary administration of porcine β-defensin 1, provide additional support for the participation of alarmins in both innate and adaptive antimicrobial immunity.
Potential implication of alarmins in combating Aspergillus infection
Aspergillosis due to infection by Aspergillus fumigatus has become a serious clinical problem in immunocompromised patients [85]. Aspergillus fumigatus infection is initiated by inhalation of A. fumigatus spores (conidia) that germinate to form hyphae, resulting in the destruction of affected tissues. Macrophages can kill conidia whereas hyphal invasion is predominantly controlled by neutrophils [85–87]. Cytokines capable of mobilizing and activating phagocytes and DC (e.g., GCSF and GM-CSF, M-CSF) as well as mounting an A. fumigatus-specific Th1 immune response (e.g., TNF and IFNγ) are also critical to the combat against infection [85,88,89]. The ideal approach for preventing the occurrence of aspergillosis would be vaccination, however, there is no such a vaccine available at present. Given their critical roles in antimicrobial immunity, alarmins may potentially be utilized in various ways for the prevention and/or treatment of Aspergillus fumigatus infection. One simple approach would be to directly use certain alarmins as antibiotics against Aspergillus fumigatus. Although many alarmins, particularly defensins and cathelicidins, have been shown to directly kill various fungi such as Candida albicans and Cryptococcus neoformans, most alarmins have not been tested against Aspergillus fumigatus [3,20,30,90]. The identification of a human defensin with selective killing against Aspergillus spp. offers some optimism in this regard [91]. Alternatively, alarmins may be delivered into infected tissues to promote anti-Aspergillus fumigatus immune defense based on their capabilities to induce the recruitment and activation of phagocytes. Because certain alarmins (e.g., MBD2, HMGB1, etc) can selectively induce Th1-polarized antigen-specific immune response, they may potentially be used as molecular adjuvants for vaccine development or to enhance therapeutic interventions against aspergillosis.
Acknowledgements
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract N01-CO-12400. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This Research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Yang D, Oppenheim JJ. Antimicrobial proteins act as ‘alarmins’ in joint immune defense. Arthritis Rheum 2004; 50: 3401–3403. [DOI] [PubMed] [Google Scholar]
- 2.Oppenheim JJ, Yang D. Alarmins: chemotactic activators of immune responses. Curr Opin Immunol 2005; 17: 359–365. [DOI] [PubMed] [Google Scholar]
- 3.Ganz T Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol 2003; 3: 710–720. [DOI] [PubMed] [Google Scholar]
- 4.Cathelicidins Zanetti M., multifunctional peptides of the innate immunity. J Leukoc Biol 2004; 75: 39–48. [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg HF, Domachowke JB. Eosinophils, eosinophil ribonucleases, and their role in host defense against respiratory virus pathogens. J Leukoc Biol 2001; 70: 691–698. [PubMed] [Google Scholar]
- 6.Hock R, Furusawa T, Ueda T, Bustin M. HMG chromosomal proteins in development and disease. Trends Cell Biol 2007; 17: 72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang D, Biragyn A, Hoover DM, Lubkowski J, Oppenheim JJ. Multiple roles of antimicrobial defensins, cathelicidins, and eosinophil-derived neurotoxin in host defense. Annu Rev Immunol 2004; 22: 181–315. [DOI] [PubMed] [Google Scholar]
- 8.Ganz T, Selsted ME, Szklarek D, et al. Defensins: natural peptide antibiotics of human neutrophils. J Clin Invest 1985; 76: 1427–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ouellette AJ, Selsted ME. Paneth cell defensins: endogenous peptide components of intestinal host defense. FASEB J 1996; 10: 1280–1289. [DOI] [PubMed] [Google Scholar]
- 10.Schutte BC, Mitros JP, Bartlett JA, et al. Discovery of five conserved beta -defensin gene clusters using a computational search strategy. Proc Natl Acad Sci USA 2002; 99: 2129–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang Y-Q, Yuan J, Osapay G, et al. A cyclic antimicrobial peptide produced in primate leukocytes by the ligation of two truncated adefensins. Science 1999; 286: 498–502. [DOI] [PubMed] [Google Scholar]
- 12.Kaiser V, Diamond G. Expression of mammalian defensin genes. J Leukoc Biol 2000; 68: 779–784. [PubMed] [Google Scholar]
- 13.Chertov O, Michiel DF, Xu L, et al. Identification of defensin-1, defensin-2, and CAP37/azurocidin as T-cell chemoattractant proteins released from interleukin-8-stimulated neutrophils. J Biol Chem 1996; 271: 2935–2940. [DOI] [PubMed] [Google Scholar]
- 14.Yang D, Chertov O, Bykovskaia SN, et al. b-Defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science 1999; 286: 525–528. [DOI] [PubMed] [Google Scholar]
- 15.Yang D, Chen Q, Chertov O, Oppenheim JJ. Human neutrophil defensins selectively chemoattract naïve T and immature dendritic cells. J Leukoc Biol 2000; 68: 9–14. [PubMed] [Google Scholar]
- 16.Niyonsaba F, Iwabuchi K, Matsuda H, Ogawa H, Nagaoka I. Epithelial cell-derived human b-defensin-2 acts as a chemotaxin for mast cells through a pertussis toxin-sensitive and phospholipase C-dependent pathway. Int Immunol 2002; 14: 421–426. [DOI] [PubMed] [Google Scholar]
- 17.Niyonsaba F, Ogawa H, Nagaoka I. Human b-defensin-2 functions as a chemotactic agent for tumour necrosis factor-alpha-treated human neutrophils. Immunology 2004; 111: 273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Z, Hoover DM, Yang D, et al. Engineering disulfide bridges to dissect antimicrobial and chemotactic activities of human b-defensin 3. Proc Natl Acad Sci USA 2003; 100: 8880–8885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biragyn A, Surenhu M, Yang D, et al. Mediators of innate immunity that target immature, but not mature, dendritic cells induce antitumor immunity when genetically fused with nonimmunogenic tumor antigens. J Immunol 2001; 167: 6644–6653. [DOI] [PubMed] [Google Scholar]
- 20.Garcia JR, Jaumann F, Schulz S, et al. Identification of a novel, multifunctional b-defensin (human b-defensin 3) with specific antimicrobial activity: its interaction with plasma membranes of Xenopus oocytes and the induction of macrophage chemoattraction. Cell Tissue Res 2001; 306: 257–264. [DOI] [PubMed] [Google Scholar]
- 21.Conejo-Garcia JR, Benencia F, Courreges MC, et al. Tumor-infiltrating dendritic cell precursors recruited by a b-defensin contribute to vasculogenesis under the influence of Vegf-A. Nat Med 2004; 10: 950–958. [DOI] [PubMed] [Google Scholar]
- 22.Niyonsaba F, Someya A, Hirata M, Ogawa H, Nagaoka I. Evaluation of the effects of peptide antibiotics human bdefensin-1/2 and LL-37 on histamine release and prostaglandin D2 production from mast cells. Eur J Immunol 2001; 31: 1066–1075. [DOI] [PubMed] [Google Scholar]
- 23.Niyonsaba F, Ushio H, Nagaoka I, Okumura K, Ogawa H. The human beta-defensins (−1, −2, −3, −4) and cathelicidin LL-37 induce IL-18 secretion through p38 and ERK MAPK activation in primary human keratinocytes. J Immunol 2005; 175: 1776–1784. [DOI] [PubMed] [Google Scholar]
- 24.Yamashita T, Saito K. Purification, primary structure, and biological activity of guinea pig neutrophil cationic peptides. Infect Immun 1989; 57: 2405–2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Befus AD, Mowat C, Gilchrist M, et al. Neutrophil defensins induce histamine secretion from mast cells: mechanisms of action. J Immunol 1999; 163: 947–953. [PubMed] [Google Scholar]
- 26.van Wetering S, Mannesse-Lazeroms SPG, Dijkman JH, Hiemstra PS. Effect of neutrophil serine proteinases and defensins on lung epithelial cells: modulation of cytotoxicity and IL-8 production. J Leukoc Biol 1997; 62: 217–226. [DOI] [PubMed] [Google Scholar]
- 27.Niyonsaba F, Ushio H, Nakano N, et al. Antimicrobial peptides human b-defensins stimulate epidermal keratinocyte migration, proliferation and production of proinflammatory cytokines and chemokines. J Invest Dermatol 2007; 127: 594–604. [DOI] [PubMed] [Google Scholar]
- 28.Biragyn A, Ruffini PA, Leifer CA, et al. Toll-like receptor 4-dependent activation of dendritic cells by b-defensin 2. Science 2002; 298: 1025–1029. [DOI] [PubMed] [Google Scholar]
- 29.Funderburg N, Lederman MM, Feng Z, et al. Human-defensin-3 activates professional antigen-presenting cells via Toll-like receptors 1 and 2. Proc Natl Acad Sci USA 2007; 104: 18631–18635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zanetti M, Gennaro R, Romeo D. Cathelicidins: a novel protein family with a common proregion and a variable C-terminal antimicrobial domain. FEBS Lett 1995; 374: 1–5. [DOI] [PubMed] [Google Scholar]
- 31.Larrick JW, Hirata M, Zheng H, et al. A novel granulocyte-derived peptide with lipopolysaccharide-neutralizing activity. J Immunol 1994; 152: 231–240. [PubMed] [Google Scholar]
- 32.Gallo RL, Kim KJ, Bernfield M, et al. Identification of CRAMP, a cathelin-related antimicrobial peptide expressed in the embryonic and adult mouse. J Biol Chem 1997; 272: 13088–13093. [DOI] [PubMed] [Google Scholar]
- 33.Agerberth B, Gunne H, Odeberg J, et al. FALL-39, a putative human peptide antibiotic, is cysteine-free and expressed in bone marrow and testis. Proc Natl Acad Sci USA 1995; 92: 195–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bals R, Wang X, Zasloff M, Wilson JM. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad anti-microbial activity at the airway surface. Proc Natl Acad Sci USA 1998; 95: 9541–9546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dorschner RA, Pestonjamasp VK, Tamakuwala S, et al. Cutaneous injury induces the release of cathelicidin anti-microbial peptides active against group A Streptococcus. J Invest Dermatol 2001; 117: 91–97. [DOI] [PubMed] [Google Scholar]
- 36.Di Nardo A, Vitiello A, Gallo RL. Cutting Edge: mast cell antimicrobial activity is mediated by expression of cathelicidin antimicrobial peptide. J Immunol 2003; 170: 2274–2278. [DOI] [PubMed] [Google Scholar]
- 37.Verbanac D, Zanetti M, Romeo D. Chemotactic and protease-inhibiting activities of antibiotic peptide precursors. FEBS Lett 1993; 371: 255–258. [DOI] [PubMed] [Google Scholar]
- 38.Huang HJ, Ross CR, Blecha F. Chemoattractant properties of PR-39, a neutrophil antibacterial peptide. J Leukoc Biol 1997; 61: 624–629. [DOI] [PubMed] [Google Scholar]
- 39.Yang D, Chen Q, Schmidt AP, et al. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med 2000; 192: 1069–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Agerberth B, Charo J, Werr J, et al. The human antimicrobial and chemotactic peptides LL-37 and a-defensins are expressed by specific lymphocyte and monocyte populations. Blood 2000; 96: 3086–3093. [PubMed] [Google Scholar]
- 41.Niyonsaba F, Iwabuchi K, Someya A, et al. A cathelicidin family of human antibacterial peptide LL-37 induces mast cell chemo-taxis. Immunology 2002; 106: 20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kurosaka K, Chen Q, Yarovinsky F, Oppenheim JJ, Yang D. Mouse cathelin-related antimicrobial peptide chemoattracts leukocytes using formyl peptide receptor-like 1/mouse formyl peptide receptor-like 2 as the receptor and acts as an immune adjuvant. J Immunol 2005; 174: 6257–6265. [DOI] [PubMed] [Google Scholar]
- 43.Scott MG, Davidson DJ, Gold MR, Bowdish D, Hancock REW. The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune response. J Immunol 2002; 169: 3883–3891. [DOI] [PubMed] [Google Scholar]
- 44.Koczulla R, von Degenfeld G, Kupatt C, et al. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J Clin Invest 2003; 111: 1665–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heilborn JD, Nilsson MF, Kratz G, et al. The cathelicidin antimicrobial peptide LL-37 is involved in re-epithelialization of human skin wounds and is lacking in chronic ulcer epithelium. J Invest Dermatol 2003; 120: 379–389. [DOI] [PubMed] [Google Scholar]
- 46.Elssner A, Duncan M, Gavrilin M, Wewers MD. A novel P2X7 receptor activator, the human cathelicidin-derived peptide LL37, induces IL-1b processing and release. J Immunol 2004; 172: 4987–4994. [DOI] [PubMed] [Google Scholar]
- 47.Nagaoka I, Tamura H, Hirata M. An antimicrobial cathelicidin peptide, human CAP18/LL-37, suppresses neutrophil apoptosis via the activation of formyl-peptide receptor-like 1 and P2X7. J Immunol 2006; 176: 3044–3052. [DOI] [PubMed] [Google Scholar]
- 48.Lande R, Gregorio J, Facchinetti V, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 2007; 449: 564–569. [DOI] [PubMed] [Google Scholar]
- 49.Rosenberg HF, Tenen DG, Ackerman SJ. Molecular cloning of human eosinophil-derived neurotoxin: a member of the ribonuclease gene family. Proc Natl Acad Sci USA 1989; 86: 4460–4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mosimann SC, Newton DL, Youle RJ, James MN. X-ray crystallographic structure of recombinant eosinophil-derived neurotoxin at 1.83 A resolution. J Mol Biol 1996; 26: 540–552. [DOI] [PubMed] [Google Scholar]
- 51.Sur S, Glitz DG, Kita H, et al. Localization of eosinophil-derived neurotoxin and eosinophil cationic protein in neutrophilic leukocytes. J Leukoc Biol 1998; 63: 715–722. [DOI] [PubMed] [Google Scholar]
- 52.Egesten A, Dyer KD, Batten D, Domachowske JB, Rosenberg HF. Ribonucleases and host defense: identification, localization and gene expression in adherent monocytes in vitro. Biochim Biophys Acta 1997; 1358: 255–260. [DOI] [PubMed] [Google Scholar]
- 53.Domachowske JB, Dyer KD, Bonville CA, Rosenberg HF. Recombinant human eosinophil-derived neurotoxin/RNase 2 functions as an effective antiviral agent against respiratory syncytial virus. J Infect Dis 1998; 177: 1458–1464. [DOI] [PubMed] [Google Scholar]
- 54.Rugeles MT, Trubey CM, Bedoya VI, et al. Ribonuclease is partly responsible for the HIV-1 inhibitory effect activated by HLA alloantigen recognition. AIDS 2003; 17: 481–486. [DOI] [PubMed] [Google Scholar]
- 55.Yang D, Rosenberg HF, Chen Q, et al. Eosinophil-derived neurotoxin (EDN), an antimicrobial protein with chemotactic activities for dendritic cells. Blood 2003; 102: 3396–3403. [DOI] [PubMed] [Google Scholar]
- 56.Yang D, Chen Q, Su SB, et al. Eosinophil-derived neurotoxin acts as an alarmin to activate the TLR2-MyD88 signal pathway in dendritic cells and enhances Th2 immune responses. J Exp Med 2008; 205: 79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang H, Bloom O, Zhang M, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science 1999; 285: 248–251. [DOI] [PubMed] [Google Scholar]
- 58.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 2002; 418: 191–195. [DOI] [PubMed] [Google Scholar]
- 59.Bonaldi T, Talamo F, Scaffidi P, et al. Monocytic cells hyper-acetylate chromatin protein HMGB1 to redirect it towards secretion. Embo J 2003; 22: 5551–5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rendon-Mitchell B, Ochani M, Li J, et al. IFN-gamma induces high mobility group box 1 protein release partly through a TNF-dependent mechanism. J Immunol 2003; 170: 3890–3897. [DOI] [PubMed] [Google Scholar]
- 61.Dumitriu IE, Baruah P, Valentinis B, et al. Release of high mobility group box 1 by dendritic cells controls T cell activation via the receptor for advanced glycation end products. J Immunol 2005; 174: 7506–7515. [DOI] [PubMed] [Google Scholar]
- 62.Semino C, Angelini G, Poggi A, Rubartelli A. NK/iDC interaction results in IL-18 secretion by DCs at the synaptic cleft followed by NK cell activation and release of the DC maturation factor HMGB1. Blood 2005; 106: 609–616. [DOI] [PubMed] [Google Scholar]
- 63.Yang D, Chen Q, Yang H, et al. High mobility group box-1 protein induces the migration and activation of human dendritic cells and acts as an alarmin. J Leukoc Biol 2007; 81: 59–66. [DOI] [PubMed] [Google Scholar]
- 64.Messmer D, Yang H, Telusma G, et al. High mobility group box protein 1: an endogenous signal for dendritic cell maturation and Th1 polarization. J Immunol 2004; 173: 307–313. [DOI] [PubMed] [Google Scholar]
- 65.Rouhiainen A, Kuja-Panula J, Wilkman E, et al. Regulation of monocyte migration by amphoterin (HMGB1). Blood 2004; 104: 1174–1182. [DOI] [PubMed] [Google Scholar]
- 66.Kokkola R, Andersson A, Mullins G, et al. RAGE is the major receptor for the proinflammatory activity of HMGB1 in rodent macrophages. Scand J Immunol 2005; 61: 1–9. [DOI] [PubMed] [Google Scholar]
- 67.Rovere-Querini P, Capobianco A, Scaffidi P, et al. HMGB1 is an endogenous immune adjuvant released by necrotic cells. EMBO Rep 2004; 5: 825–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Palumbo R, Sampaolesi M, De Marchis F, et al. Extracellular HMGB1, a signal of tissue damage, induces mesoangioblast migration and proliferation. J Cell Biol 2004; 164: 441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Park JS, Gamboni-Robertson F, He Q, et al. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am J Physiol Cell Physiol 2006; 290: C917–924. [DOI] [PubMed] [Google Scholar]
- 70.Tian J, Avalos AM, Mao SY, et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol 2007; 8: 487–496. [DOI] [PubMed] [Google Scholar]
- 71.Ivanov S, Dragoi AM, Wang X, et al. A novel role for HMGB1 in TLR9-mediated inflammatory responses to CpG-DNA. Blood 2007; 110: 1970–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zetterstrom CK, Bergman T, Rynnel-Dagoo B, et al. High mobility group box chromosomal protein 1 (HMGB1) is an antibacterial factor produced by the human adenoid. Pediatr Res 2002; 52: 148–154. [DOI] [PubMed] [Google Scholar]
- 73.Kim C, Gajendran N, Mittrucker HW, et al. Human alpha-defensins neutralize anthrax lethal toxin and protect against its fatal consequences. Proc Natl Acad Sci USA 2005; 102: 4830–4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Steinman RM, Hemmi H. Dendritic cells: translating innate to adaptive immunity. Curr Top Microbiol Immunol 2006; 311: 17–58. [DOI] [PubMed] [Google Scholar]
- 75.Jr Lillard. JW, Boyaka PN, Chertov O, Oppenheim JJ, McGhee JR. Mechanisms for induction of acquired host immunity by neutrophil peptide defensins. Proc Natl Acad Sci USA 1999; 96: 651–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Biragyn A, Ruffini PA, Coscia M, et al. Chemokine receptor-mediated delivery directs self-tumor antigen efficiently into the class II processing pathway in vitro and induces protective immunity in vivo. Blood 2004; 104: 1961–1969. [DOI] [PubMed] [Google Scholar]
- 77.Wilson CL, Ouellette AJ, Satchell DP, et al. Regulation of intestinal a-defensin activation by the metalloproteinase matrilysin in innate host defense. Science 1999; 286: 113–117. [DOI] [PubMed] [Google Scholar]
- 78.Morrison G, Kilanowski F, Davidson D, Dorin J. Characterization of the mouse b defensin 1, Defb1, mutant mouse model. Infect Immun 2002; 70: 3053–3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moser C, Weiner DJ, Lysenko E, et al. b-Defensin 1 contributes to pulmonary innate immunity in mice. Infect Immun 2002; 70: 3068–3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nizet V, Ohtake T, Lauth X, et al. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature 2001; 414: 454–457. [DOI] [PubMed] [Google Scholar]
- 81.Salzman NH, Ghosh D, Huttner KM, Paterson Y, Bevins CL. Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature 2003; 422: 522–526. [DOI] [PubMed] [Google Scholar]
- 82.Bals R, Weiner DJ, Meegalla RL, Wilson JM. Transfer of a cathelicidin peptide antibiotic gene restores bacterial killing in a cystic fibrosis xenograft model. J Clin Invest 1999; 103: 1113–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bals R, Weiner DJ, Moscioni AD, Meegalla RL, Wilson JM. Augmentation of innate host defense by expression of a cathelicidin antimicrobial peptide. Infect Immun 1999; 67: 6084–6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Welling MM, Hiemstra PS, van-den-Barselaar MT, et al. Antibacterial activity of human neutrophil defensins in experimental infections in mice is accompanied by increased leukocyte accumulation. J Clin Invest 1998; 102: 1583–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Steinbach WJ, Stevens DA, Denning DW, Moss RB. Advances against aspergillosis. Clin Infect Dis 2003; 37: S155–S156. [DOI] [PubMed] [Google Scholar]
- 86.Philippe B, Ibrahim-Granet O, Prevost MC, et al. Killing of Aspergillus fumigatus by alveolar macrophages is mediated by reactive oxidant intermediates. Infect Immun 2003; 71: 3034–3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schaffner A, Douglas H, Braude A. Selective protection against conidia by mononuclear and against mycelia by polymorphonuclear phagocytes in resistance to Aspergillus. Observations on these two lines of defense in vivo and in vitro with human and mouse phagocytes. J Clin Invest 1982; 69: 617–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bozza S, Perruccio K, Montagnoli C, et al. A dendritic cell vaccine against invasive aspergillosis in allogeneic hematopoietic transplantation. Blood 2003; 102: 3807–3814. [DOI] [PubMed] [Google Scholar]
- 89.Cenci E, Mencacci A, Fe d’Ostiani C, et al. Cytokine- and T helper-dependent lung mucosal immunity in mice with invasive pulmonary aspergillosis. J Infect Dis 1998; 178: 1750–1760. [DOI] [PubMed] [Google Scholar]
- 90.Harder J, Bartels J, Christophers E, Schröder JM. Isolation and characterization of human b-defensin-3, a novel human inducible peptide antibiotics. J Biol Chem 2001; 276: 5707–5713. [DOI] [PubMed] [Google Scholar]
- 91.Simon A, Kullberg BJ, Tripet B, et al. Drosomycin-like defensin (DLD): a human homologue of Drosophila melanogaster drosomycin with antifungal activity. Antimicrob Agents Chemother 2008; 52: 1407–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]