Abstract
Nipah virus is a paramyxovirus in the genus Henipavirus, which has caused outbreaks in humans in Malaysia, India, Singapore and Bangladesh. Whereas the human cases in Malaysia were characterized mainly by neurological symptoms and a case fatality rate of ~40%, cases in Bangladesh additionally exhibited respiratory disease and had a case fatality rate of ~70%. Here, we compared the histopathological changes in the respiratory tract of Syrian hamsters, a well-established small animal disease model for Nipah virus, inoculated oronasally with Nipah virus isolates from human cases in Malaysia and Bangladesh. The Nipah virus isolate from Bangladesh caused slightly more severe rhinitis and bronchointerstitial pneumonia 2 days after inoculation in Syrian hamsters. By day 4, differences in lesion severity could no longer be detected. Immunohistochemistry demonstrated Nipah virus antigen in nasal cavity and pulmonary lesions; the amount of Nipah virus antigen present correlated with lesion severity. Immunohistochemistry indicated that both Nipah virus isolates exhibited endotheliotropism in small and medium caliber arteries and arterioles, but not in veins, in the lung. This correlated with the location of ephrin B2, the main receptor for Nipah virus, in the vasculature. In conclusion, Nipah virus isolates from outbreaks in Malaysia and Bangladesh caused a similar type and severity of respiratory tract lesions in Syrian hamsters, suggesting that the differences in human disease reported in the outbreaks in Malaysia and Bangladesh are unlikely to have been caused by intrinsic differences in these two virus isolates.
Keywords: histopathology, Nipah virus, respiratory system, Syrian hamster
Nipah virus is a paramyxovirus in the genus Henipavirus. Nipah virus was first identified in an outbreak in 1998–1999 in Malaysia where it caused encephalitis and respiratory signs in pigs and encephalitis in humans that had contact with infected pigs.2,4 Since 2001, Nipah virus has caused outbreaks in humans in Bangladesh almost every year. Whereas the initial outbreak in humans in Malaysia and Singapore was characterized mainly by neurological signs with occasional respiratory signs and a case fatality rate of approximately 40%,2 human cases in Bangladesh exhibited respiratory as well as neurological disease with a case fatality rate up to 92% in individual outbreaks.14 The main histologic changes in humans infected with Nipah virus in Malaysia were multi-organ vasculitis, fibrin thrombi, fibrinoid necrosis and occasional endothelial syncytia; vascular lesions were often associated with parenchymal necrosis and hemorrhage, especially in the brain with lesser involvement of the lung.19 In the Malaysia outbreak, Nipah virus was transmitted from its natural reservoir, Pteropus spp. fruit bats, to pigs. It is suspected that pigs were infected by ingesting fruit contaminated with urine, feces or saliva from Nipah virus-infected fruit bats.13 Pigs acted as an intermediate, amplifying host for Nipah virus and transmitted this virus to humans through direct contact.3,15 Histologic lesions in Nipah virus-infected individuals from Bangladesh have not been described; however, the Bangladesh isolate has been reported to cause clinical signs similar to those in the Malaysia outbreak with the addition of acute respiratory distress syndrome.12 In the outbreaks in Bangladesh, epidemiological studies suggest that Nipah virus was transmitted from fruit bats to humans through ingestion of raw date palm sap contaminated with Nipah virus through bat saliva, urine or feces.14 Infected humans then transmitted the virus to other humans, likely through close contact with respiratory tract secretions.10 Nipah virus isolates from Malaysia and Bangladesh have been shown to share a sequence identity of 91.8% on the nucleotide level and >92% on the amino acid level.11
Comparisons of a Nipah virus isolate from Malaysia to a Nipah virus isolate from Bangladesh in oronasally inoculated ferrets and intraperitoneally inoculated hamsters has been reported. Clayton et al. showed that ferrets inoculated oronasally with either Nipah virus isolate developed similar clinical signs and multisystemic inflammation with vasculitis, yet ferrets inoculated with the Nipah virus isolate from Malaysia occasionally developed meningitis, while those inoculated with the Bangladesh isolate did not.5 Intraperitoneal inoculation of Syrian hamsters with either the Nipah virus isolate from Malaysia or Bangladesh resulted in similar histologic lesions, although the lesions developed more rapidly in hamsters inoculated with the Nipah virus isolate from Malaysia.8
Here, we compare histologic lesions in the respiratory tract of Syrian hamsters oronasally inoculated with 107 TCID50 (50% tissue culture infectious dose) of a Nipah virus isolate from Malaysia (NiV-M) or Bangladesh (NiV-B). Although a previous study compared lesions caused by intraperitoneal inoculation of NiV-M or NiV-B in hamsters, this inoculation route may not accurately represent natural disease progression in humans. In our study comparing oronasally inoculated hamsters, differences in the severity of respiratory tract lesions in hamsters caused by NiV-B and NiV-M were minimal and only noted at 2 dpi, with NiV-B causing slightly more severe lesions.
Materials and Methods
Ethics statement
All animal experiments were approved by the Institutional Animal Care and Use Committee of the Rocky Mountain Laboratories and performed following the guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC) by certified staff in an AAALAC-approved facility.
Case Selection and Histology
Archived microscope slides from 6–8 week old female Syrian hamsters (HsdHan®:AURA, Harlan Laboratories), from previously published studies,6,7 euthanized 2 or 4 days after oronasal inoculation with 107 TCID50 NiV-M or NiV-B in a total volume of 100 μl or 80 μl respectively (50 or 40 μl/nostril) were analyzed histologically. Slides were originally prepared as 5 μm-thick sections of formalin-fixed paraffin-embedded tissues adhered to microscope slides and then stained with hematoxylin and eosin. The Nipah virus isolates from Malaysia and Bangladesh were kindly provided by the Special Pathogens Branch of the Center for Disease Control and Prevention, Atlanta, GA, USA. NiV-M was isolated from the cerebrum of an infected fatal human case in 1999. NiV-B was isolated from a throat swab collected from a fatal human case in 2004.
In the archived Nipah virus microscope slides, there were five hamsters inoculated with NiV-M; three of these hamsters were euthanized at 2 dpi (case Nos. 1M-3M) and two hamsters (case Nos. 4M, 5M) were euthanized at 4 dpi. Eight hamsters were inoculated with NiV-B; four hamsters each were euthanized at 2 dpi (case Nos. 6B-9B) and 4 dpi (case Nos. 10B-13B). Examined tissues included the lung, nasal cavity (transverse or mid-sagittal sections through the skull) and brain. All tissues were analyzed for the presence of lesions and scored in a non-masked manner by two pathologists (LB, DPS). Any discrepancies in scoring values were discussed and a final score was mutually agreed upon by the pathologists. Lesion severity was scored as follows: 0 = the lesion was absent; 1 = up to 25% of the described tissue or cell type was affected; 2 = up to 50% of the described tissue or cell type was affected; 3 = up to 75% of the described tissue or cell type was affected; 4 = 75–100% of the described tissue or cell type was affected.
Immunohistochemistry
Immunohistochemistry was performed on all tissues examined histologically using a rabbit polyclonal antiserum against the Nipah virus nucleoprotein1 (1:5000; kindly provided by L. Wang, CSIRO Livestock Industries, Australian Animal Health Laboratory, Australia) as a primary antibody for detection of Nipah virus antigen. The tissues were then processed for immunohistochemistry using the Discovery XT automated processor (Ventana Medical Systems) with a DABMap kit (Ventana Medical Systems). All tissues were scored based on the percentage of the tissue that was immunopositive for Nipah virus nucleoprotein: 0 = negative, 1 = up to 25% of the tissue was immunopositive; 2 = up to 50% of the tissue was immunopositive; 3 = up to 75% of the tissue was immunopositive; 4 = 75–100% of the tissue was immunopositive. Scoring was performed in a non-masked manner by two pathologists (LB, DPS); any discrepancies in scoring values were discussed and a final score was mutually agreed upon by the pathologists.
Verhoeff-Van Gieson Histochemistry
5 μm-thick sections of formalin-fixed paraffin-embedded lung adhered to charged microscope slides were deparaffinized, hydrated with distilled water and then placed in Verhoeff elastic tissue stain for one hour. Tissue sections were washed twice with distilled water and then placed in 2% ferric chloride until elastic fibers were black and the background was colorless after which the sections were rinsed with distilled water. Tissue sections were placed in sodium thiosulfate for one minute and then washed in running tap water for five minutes. Sections were counterstained with Van Gieson stain for 1 minute, differentiated in 95% alcohol and then dehydrated in absolute alcohol, cleared in xylene and mounted in Cytoseal XYL mounting media.
Statistical Analysis
A Fisher’s exact test was performed using GraphPad (Prism version 6.02 for Windows, GraphPad Software, La Jolla, CA) to determine statistical significance between the scoring values of animals inoculated with NiV-B or NiV-M at each time point and for the presence or absence of Nipah virus nucleoprotein in arteries or veins in the lung. P < 0.05 was considered statistically significant.
Results
Nasal Cavity
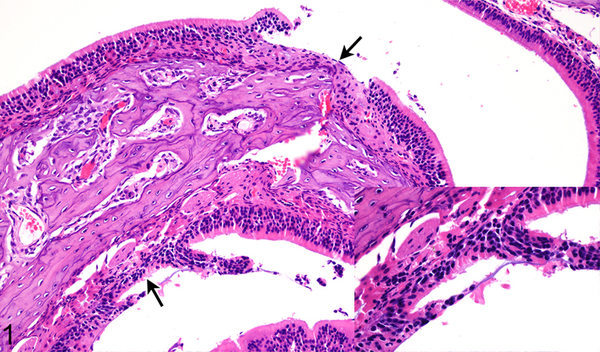
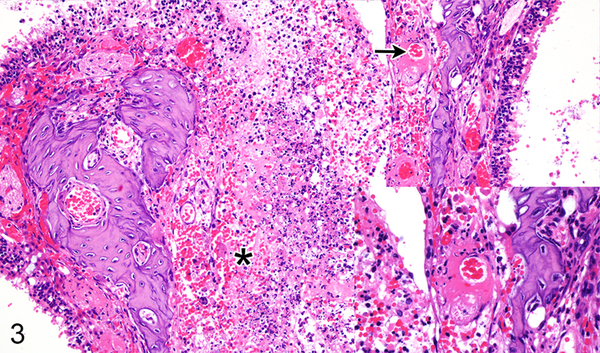
In the nasal cavity, the histologic differences between hamsters inoculated with NiV-B and NiV-M were minimal and observed on day 2 only (Table 1). Differences in lesion severity were seen in the submucosal glands and respiratory and olfactory epithelium. Submucosal gland epithelial degeneration on 2 dpi was only present in hamsters inoculated with NiV-B. NiV-B caused slightly more severe olfactory epithelial lesions than NiV-M 2 dpi; whereas NiV-M caused slightly more severe respiratory epithelial lesions. Both Nipah virus isolates caused mild neutrophilic rhinitis with respiratory and olfactory epithelial degeneration, necrosis and syncytia formation (Figs. 1, 2). Rhinitis caused by NiV-B was multifocal to coalescing and affected up to 10% of the nasal cavity; whereas rhinitis produced by NiV-M was often focal and affected 5% or less of the nasal cavity. Nasal cavity lesions were more severe at 4 dpi than at 2 dpi for both NiV-B and NiV-M. Lesion severity and distribution was similar at 4 dpi for both virus isolates (Table 2), with up to 50% of the nasal cavity being filled with inflammatory infiltrate (Figs. 3, 4). Multifocal to coalescing ulcers and submucosal gland epithelial degeneration, necrosis and syncytia were present. Prominent vascular lesions were observed at 4 dpi, including fibrinoid degeneration and fibrin thrombi in multiple nasal submucosal small and medium caliber vessels. Nipah virus antigen was detected in the same cell types in hamsters inoculated with either NiV-M or NiV-B and antigen was more abundant at 4 dpi compared to 2 dpi (Table 3). Multifocally, Nipah virus antigen was present in the cytoplasm of nasal cavity olfactory, respiratory and submucosal gland acinar epithelium, mononuclear leukocytes and neutrophils (Supplementary Figs. S1-4). Nipah virus antigen was also present in the cytoplasm of endothelial cells in small to medium caliber vessels in the nasal submucosa in all hamsters inoculated with NiV-M at 4 dpi, but was not present at 2 dpi. In hamsters inoculated with NiV-B, 1 out of 4 hamsters (25%) at 2 dpi and 3 out of 4 hamsters (75%) at 4 dpi expressed Nipah virus antigen in endothelial cells. The only statistically significant difference (P < 0.03) between NiV-M and NiV-B in the nasal cavity was the presence of submucosal gland epithelial degeneration in hamsters inoculated with NiV-B, but not NiV-M, at 2 dpi, as determined by a Fisher’s exact test.
Table 1.
Histologic severity of respiratory tract lesions in Syrian hamsters inoculated with a Nipah virus isolate from Bangladesh or Malaysia at 2 days post inoculation
| Hamster No. | Isolate | Rhinitis | Respiratory epithelial degeneration/necrosis | Olfactory epithelial degeneration/necrosis | Submucosal gland acinar degeneration/necrosis | Bronchointerstitial pneumonia |
|---|---|---|---|---|---|---|
| 1M | Mal | 1 | 1/1 | 1/1 | 0/0 | 1 |
| 2M | Mal | 1 | 2/0 | 1/1 | 0/0 | 1 |
| 3M | Mal | 1 | 2/2 | 0/0 | 0/0 | 1 |
| 6B | Bang | 1 | 1/0 | 1/1 | 1/0 | 2 |
| 7B | Bang | 1 | 3/1 | 1/1 | 1/0 | 2 |
| 8B | Bang | 1 | 1/0 | 1/1 | 1/0 | 1 |
| 9B | Bang | 1 | 1/0 | 2/1 | 1/0 | 2 |
Mal, Nipah virus isolate from Malaysia; Bang, Nipah virus isolate from Bangladesh.
Tissues from the nasal cavity (respiratory epithelium, olfactory epithelium and submucosal glands) and lung (bronchointerstitial pneumonia) were scored. Scoring protocol: 0 = no lesions; 1 = up to 25% of the described tissue or cell type affected; 2 = up to 50% of the described tissue or cell type affected; 3 = up to 75% of the described tissue or cell type affected; 4 = 75–100% of the described tissue or cell type affected.
Figure 1.
Case No. 1M. Rhinitis with olfactory epithelial degeneration and necrosis and multifocal erosions (arrows; inset) 2 days post inoculation (dpi) with NiV-M. HE.
Figure 2.
Case No. 6B. Rhinitis with olfactory epithelial degeneration and necrosis (arrows; inset) 2 dpi with NiV-B. HE.
Table 2.
Histologic severity of respiratory tract lesions in Syrian hamsters inoculated with a Nipah virus isolate from Bangladesh or Malaysia at 4 days post inoculation
| Hamster No. | Isolate | Rhinitis | Respiratory epithelial degeneration/necrosis | Olfactory epithelial degeneration/necrosis | Submucosal gland acinar degeneration/necrosis | Bronchointerstitial pneumonia |
|---|---|---|---|---|---|---|
| 4M | Mal | 2 | 2/1 | 2/1 | 1/1 | 3 |
| 5M | Mal | 2 | 3/1 | 1/2 | 1/2 | 1 |
| 10B | Bang | 1 | 1/1 | 1/0 | 1/0 | 3 |
| 11B | Bang | 2 | 0/0 | 2/2 | 2/1 | 2 |
| 12B | Bang | 1 | 1/0 | 1/2 | 1/1 | 3 |
| 13B | Bang | 2 | 1/1 | 2/2 | 2/2 | 2 |
Mal, Nipah virus isolate from Malaysia; Bang, Nipah virus isolate from Bangladesh.
Tissues from the nasal cavity (respiratory epithelium, olfactory epithelium and submucosal glands) and lung (bronchointerstitial pneumonia) were scored. Scoring protocol: 0 = no lesions; 1 = up to 25% of the described tissue or cell type affected; 2 = up to 50% of the described tissue or cell type affected; 3 = up to 75% of the described tissue or cell type affected; 4 = 75–100% of the described tissue or cell type affected.
Figure 3.
Case No. 5M. Rhinitis with multifocal ulcers (asterisk) and submucosal vascular fibrinoid degeneration (arrow; inset) 4 dpi with NiV-M. HE.
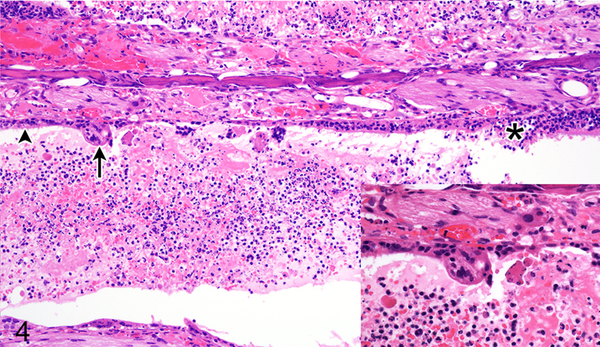
Figure 4.
Case No. 13B. Rhinitis affecting olfactory epithelium (asterisk) and respiratory epithelium (arrowhead) with formation of epithelial syncytium (arrow; inset) 4 dpi with NiV-B. HE.
Table 3.
Immunohistochemistry scoring for Nipah virus nucleoprotein in the respiratory tract of Syrian hamsters inoculated with either a Nipah virus isolate from Bangladesh or Malaysia
| Hamster No. | Isolate | Necropsy, dpi | Nasal cavity | Lung |
|---|---|---|---|---|
| 1M | Mal | 2 | 1 | 2 |
| 2M | Mal | 2 | 1 | 2 |
| 3M | Mal | 2 | 1 | 2 |
| 4M | Mal | 4 | 2 | 3 |
| 5M | Mal | 4 | 3 | 1 |
| 6B | Bang | 2 | 2 | 2 |
| 7B | Bang | 2 | 2 | 3 |
| 8B | Bang | 2 | 2 | 2 |
| 9B | Bang | 2 | 2 | 2 |
| 10B | Bang | 4 | 1 | 3 |
| 11B | Bang | 4 | 3 | 3 |
| 12B | Bang | 4 | 2 | 2 |
| 13B | Bang | 4 | 2 | 2 |
Dpi, days post inoculation; Mal, Nipah virus isolate from Malaysia; Bang, Nipah virus isolate from Bangladesh.
Scoring protocol: 0 = negative, 1 = up to 25% of the tissue was immunopositive; 2 = up to 50% of the tissue was immunopositive; 3 = up to 75% of the tissue was immunopositive; 4 = 75–100% of the tissue was immunopositive.
Lung
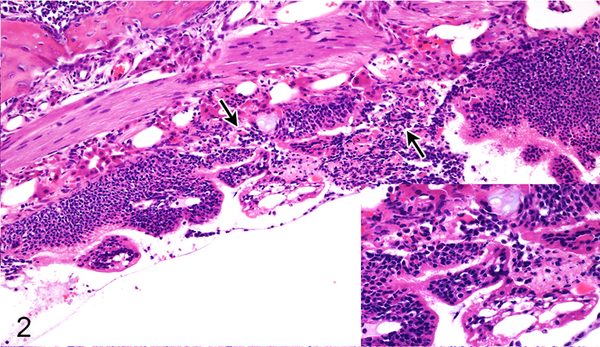
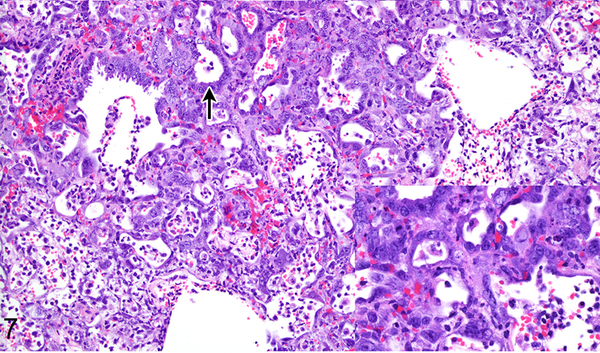
Differences in lesion severity caused by NiV-B and NiV-M were minor, only noted at 2 dpi and involved the extent of the bronchointerstitial pneumonia (Table 1) and formation of bronchiolar epithelial syncytia. Both Nipah virus isolates caused a multifocally distributed bronchointerstitial pneumonia centered on terminal bronchioles with scattered fibrin thrombi in alveolar septal capillaries in all hamsters at 2 dpi. NiV-B caused more extensive bronchointerstitial pneumonia at 2 dpi than NiV-M, affecting up to 50% of the lung as compared to less than 25% of the lung, respectively (Figs. 5, 6). Multiple small and medium caliber blood vessels in the lung exhibited vasculitis with disruption of the vascular wall and rare endothelial syncytia in hamsters inoculated with either virus isolate. Bronchiolar epithelial syncytia were seen at 2 dpi in all hamsters inoculated with NiV-M, but were only present in 25% of hamsters (1 of 4 cases) inoculated with NiV-B. At 4 dpi, bronchiolar epithelial syncytia were present in 50% of the hamsters (1 of 2 cases) inoculated with NiV-M and 25% of hamsters (1 of 4 cases) inoculated with NiV-B. At 4 dpi the bronchointerstitial pneumonia caused by NiV-B and NiV-M had a similar distribution and severity (Table 2), was more extensive than at 2 dpi, affected up to 75% of the lung, and exhibited multifocal type II pneumocyte hyperplasia (Figs. 7, 8). In the lung, the location of Nipah virus antigen was similar for both NiV-B and NiV-M (Supplementary Figs. S5-8) and the amount of antigen present for both virus isolates increased from 2 dpi to 4 dpi (Table 3). Nipah virus antigen was detected in the cytoplasm of multiple alveolar septal capillary endothelial cells, type I pneumocytes and alveolar mononuclear leukocytes at 2 dpi in all hamsters. Nipah virus antigen was present in endothelium of rare small caliber blood vessels at 2 dpi in one hamster inoculated with NiV-M (case No. 2M) and one hamster inoculated with NiV-B (case No. 9B). At 4 dpi, Nipah virus antigen was also present in scattered type II pneumocytes, endothelial cells and vascular smooth muscle cells in hamsters inoculated with either NiV-B or NiV-M.
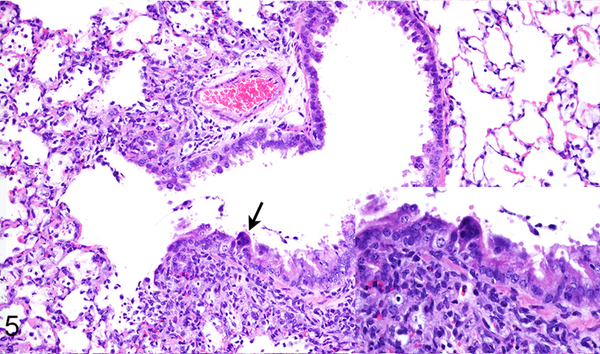
Figure 5.
Case No. 1M. Bronchointerstitial pneumonia with bronchiolar epithelial syncytium (arrow; inset) 2 dpi with NiV-M. HE.
Figure 6.
Case No. 7B. Bronchointerstitial pneumonia 2 dpi with NiV-B. Inset: higher magnification of pneumonia. HE.
Figure 7.
Case No. 4M. Bronchointerstitial pneumonia with type II pneumocyte hyperplasia (arrow; inset) 4 dpi with NiV-M. HE.
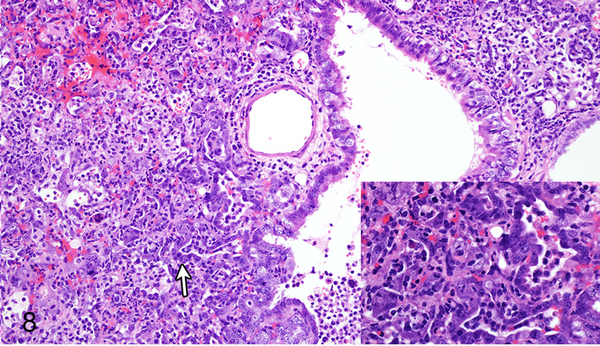
Figure 8.
Case No. 10B. Bronchointerstitial pneumonia with type II pneumocyte hyperplasia (arrow; inset) 4 dpi with NiV-B. HE.
Pulmonary Vasculature
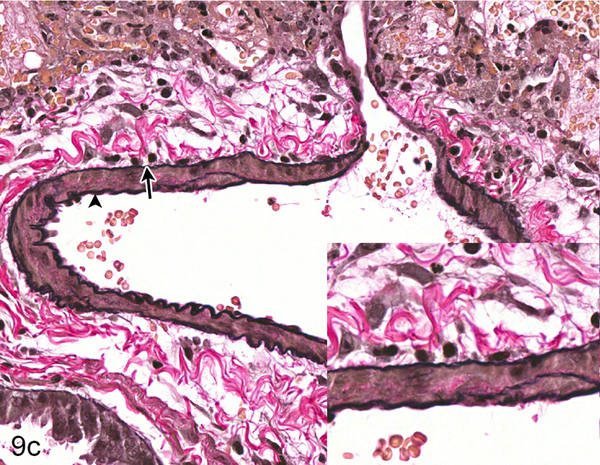
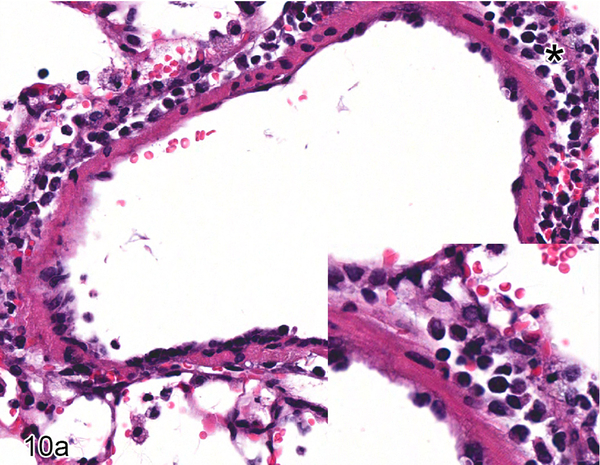
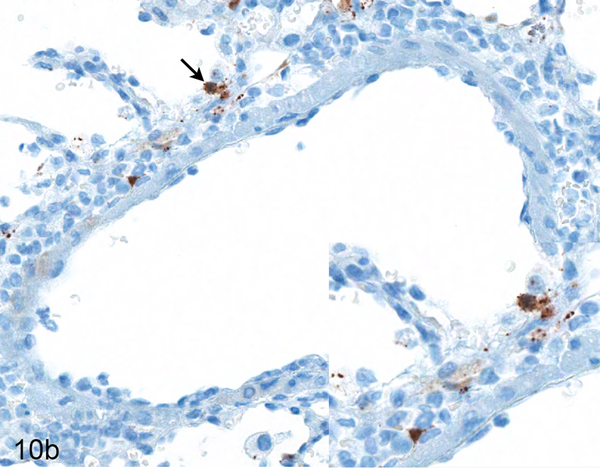
Nipah virus antigen was present in endothelial and smooth muscle cells in multiple small and medium caliber blood vessels at 4 dpi in the lung of hamsters inoculated with either NiV-B or NiV-M. Through the comparison of hematoxylin and eosin (HE) and Nipah virus antigen immunohistochemistry on sequential sections of lung, it appeared that Nipah virus antigen had a tropism for arteries rather than veins. Since it can be difficult to differentiate small and medium caliber arteries and veins in the lung on HE, a Verhoeff-Van Gieson stain was performed on sections of lung to identify the presence or absence of internal and external elastic laminae in blood vessels. A comparison of HE, Verhoeff-Van Gieson and immunohistochemistry using anti-Nipah virus nucleoprotein antibody on serial sections of the lung was performed on all hamsters inoculated with either NiV-B or NiV-M that were euthanized at 4 dpi to elucidate if there was an arterial tropism. Ten arteries and ten veins were analyzed in each hamster. Blood vessels that could not definitively be characterized as arteries or veins were not included. In each hamster, the endothelium of at least five out of ten arteries examined contained Nipah virus nucleoprotein (Fig. 9), whereas none of the veins did (Fig. 10). This arterial tropism was observed in hamsters inoculated with either virus isolate and was statistically significant (P < 0.003) when compared to the absence of Nipah virus nucleoprotein in venous endothelium using a Fisher’s exact test.
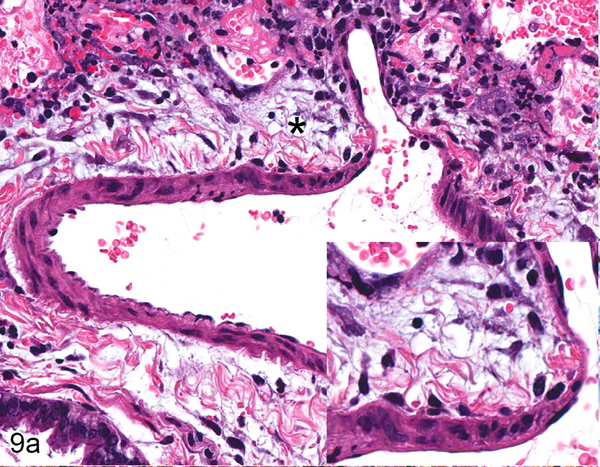
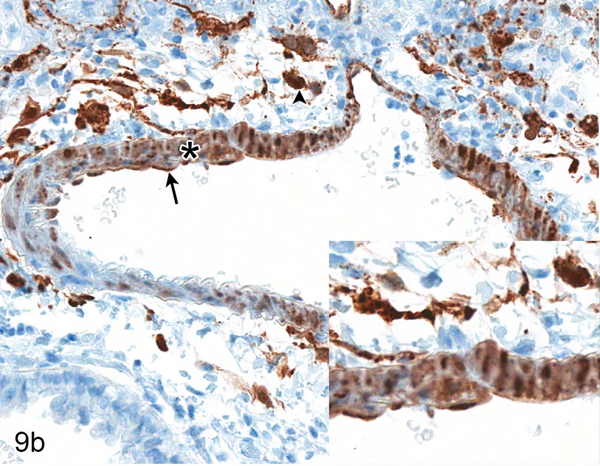
Figure 9.
Serial sections of an artery in the lung of Case No. 4M. (a) Artery in the lung. The tunica adventitia is expanded by edema and a mild infiltrate of neutrophils, lymphocytes and macrophages (asterisk; inset). HE. (b) Cytoplasmic expression of Nipah virus nucleoprotein in arterial endothelial cells (arrow), tunica media smooth muscle cells (asterisk) and perivascular leukocytes (arrowhead). Inset: higher magnification of viral antigen expression. Nipah virus nucleoprotein IHC. (c) Internal (arrowhead) and external (arrow) elastic laminae are prominent in this vessel, indicating the vessel is an artery. Inset: higher magnification of vascular elastic laminae. Verhoeff-Van Gieson stain.
Figure 10.
Serial sections of a vein in the lung of Case No. 4M. (a) Vein in the lung. The tunica adventitia is moderately expanded by an infiltrate of neutrophils and macrophages with fewer lymphocytes and plasma cells (asterisk; inset). HE. (b) Cytoplasmic expression of Nipah virus nucleoprotein in perivascular leukocytes (arrow; inset). Nipah virus antigen was not present in endothelial cells. Nipah virus nucleoprotein IHC. (c) The vessel exhibits an incomplete internal elastic lamina (arrow; inset) and lacks an external elastic lamina, indicating this vessel is a vein. Verhoeff-Van Gieson stain.
Brain
Lesions were not present in the brain in any hamster inoculated with either Nipah virus isolate. Nipah virus antigen was not observed in the brain parenchyma in any hamster at either 2 dpi or 4 dpi.
Discussion
Syrian hamsters were used as an animal model to investigate histopathologic differences between NiV-M and NiV-B since they recapitulate the disease caused by Nipah virus in humans.6,8,16,18 In Nipah virus-infected hamsters, clinical signs and histologic lesions depend on the infectious dose and route of inoculation. Hamsters oronasally inoculated with a high dose of Nipah virus develop acute severe respiratory disease, while low doses initially cause mild respiratory disease and then subsequent neurological signs.16 High doses of NiV-B or NiV-M have both been shown to be uniformly lethal in oronasally inoculated hamsters.6,7 In this study, all hamsters were inoculated with a high dose of either NiV-B or NiV-M to evaluate the respiratory component of these diseases. At the time points that were examined, Nipah virus antigen had not yet disseminated to the brain, as determined by immunohistochemistry, and lesions were not present in this tissue. Oronasal inoculation of hamsters with NiV-B or NiV-M resulted in subtle differences in lesion severity in the respiratory tract at 2 dpi, with NiV-B causing slightly more severe lesions at that time point. Immunohistochemistry for Nipah virus antigen in the nasal cavity 2 days after oronasal inoculation with NiV-M or NiV-B in Syrian hamsters showed that a higher percentage of epithelial cells in the nasal cavity contained Nipah virus antigen in hamsters inoculated with NiV-B. Although this may have been due to the different inoculum volumes used, it could also indicate that NiV-B entry into epithelial cells lining the nasal cavity is more rapid than NiV-M entry into cells or that NiV-B replicates faster within epithelial cells than NiV-M. This is one scenario that may also explain the observed lesions in the submucosal gland epithelium in NiV-B infected hamsters on 2 dpi, as a faster replication rate may have resulted in a faster progression to infection of this deeper-laying tissue.
On 2 dpi, NiV-B showed a slight predilection for damaging olfactory epithelium and NiV-M exhibited a slight predilection for damaging respiratory epithelium in oronasally infected hamsters. The exact mechanism behind these predilections is unknown, but could possibly be due to genetic differences resulting in phenotypic variation in the glycoproteins G and F between these two isolates11 which may affect cell entry of these viruses through host receptor binding on olfactory or respiratory epithelium. Additionally, a combination of viral genetic differences and variations in cellular structure and function between respiratory and olfactory epithelium may have caused differences in the viral replication rates in these two epithelial cell types.
By 4 dpi, the type and severity of lesions in the lung and nasal cavity was similar for both Nipah virus isolates. Although disease progression was initially more rapid for NiV-B, by 4 dpi both Nipah virus isolates caused essentially the same severity of pulmonary and nasal cavity lesions in Syrian hamsters. Similarly, in ferrets oronasally inoculated with NiV-B or NiV-M, the clinical respiratory signs and respiratory tract histologic lesions were comparable.5 Intraperitoneal inoculation of Syrian hamsters with NiV-B or NiV-M also did not result in differences in lesion severity between the two isolates, although disease onset and lesion development were faster with NiV-M than NiV-B.8 However, all studies to date comparing NiV-M and NiV-B have used the same two isolates and the results would be strengthened if these studies could be repeated using virus isolates from different outbreaks in Bangladesh.
To our knowledge, this is the first study that describes the tropism of Nipah virus for endothelium within arteries and arterioles, but not veins. Other studies have examined the presence of Nipah virus antigen within blood vessels, but have not exclusively localized antigen to endothelium in arteries and arterioles rather than veins.9,19 This tropism correlates with the expression of ephrin B2, the main receptor for Nipah virus, in arterial but not venous endothelium; venous endothelium typically expresses Eph-B417 which is not a receptor for Nipah virus.
Taken together, the results presented here do not explain the increased case fatality rate seen in humans infected with NiV-B; rather, the results suggest that differences between the outbreaks in Malaysia and Bangladesh were not caused by intrinsic differences between NiV-B and NiV-M. Other factors, such as the route of infection or dose of the virus received, which were identical in hamsters inoculated with either NiV-B or NiV-M in this study, may have played a role in the differences in disease outcome in infected humans in Malaysia and Bangladesh. For instance, in the Malaysia outbreak, humans were exposed to the virus through close contact with pigs15 whereas in the Bangladesh outbreaks, humans are thought to be infected by drinking Nipah virus-contaminated date palm sap or through direct contact with respiratory secretions from infected humans.10,14 Additionally, differences in the availability of health care facilities, willingness to seek medical care early in the infection, the role that family members play in caring for the ill or other cultural differences may play a role in the differences noted between the outbreaks in Malaysia and Bangladesh in humans.
Supplementary Material
Figure S1. Case No. 1M. Cytoplasmic expression of viral antigen in olfactory epithelium (arrow) 2 days post inoculation (dpi) with NiV-M. IHC for Nipah virus nucleoprotein.
Figure S2. Case No. 6B. Cytoplasmic expression of viral antigen in olfactory (arrow) and submucosal gland acinar epithelium (arrowhead) 2 dpi with NiV-B. IHC for Nipah virus nucleoprotein.
Figure S3. Case No. 5M. Cytoplasmic expression of viral antigen in olfactory (arrow) and submucosal gland acinar epithelium (arrowhead) 4 dpi with NiV-M. IHC for Nipah virus nucleoprotein.
Figure S4. Case No. 13B. Cytoplasmic expression of viral antigen in olfactory (arrow) and submucosal gland acinar epithelium (arrowhead) 4 dpi with NiV-B. IHC for Nipah virus nucleoprotein.
Figure S5. Case No. 1M. Cytoplasmic expression of viral antigen in type I pneumocytes (arrow) and bronchiolar epithelium (arrowhead) 2 dpi with NiV-M. IHC for Nipah virus nucleoprotein.
Figure S6. Case No. 7B. Cytoplasmic expression of viral antigen in type I pneumocytes (arrow) 2 dpi with NiV-B. IHC for Nipah virus nucleoprotein.
Figure S7. Case No. 4M. Cytoplasmic expression of viral antigen in type I (black arrow) and type II pneumocytes (arrowhead) and mononuclear leukocytes (white arrow) 4 dpi with NiV-M. IHC for Nipah virus nucleoprotein.
Figure S8. Case No. 10B. Cytoplasmic expression of viral antigen in type I (black arrow) and type II pneumocytes (arrowhead) and mononuclear leukocytes (white arrow) 4 dpi with NiV-B. IHC for Nipah virus nucleoprotein.
Acknowledgements
The authors would like to thank Trenton Bushmaker, Tina Thomas, Rebecca Rosenke and Dan Long for their excellent technical assistance and Anita Mora and Austin Athman for help with graphics.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases. Dr. Baseler is supported in part by the National Institutes of Health Comparative Biomedical Scientist Training Program.
References
- 1.Bossart KN, Zhu Z, Middleton D, et al. A neutralizing human monoclonal antibody protects against lethal disease in a new ferret model of acute nipah virus infection. PLoS Pathog. 2009;5:e1000642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Outbreak of Hendra-like virus - Malaysia and Singapore, 1998–1999. MMWR Morb Mortal Wkly Rep. 1999;48:265–269. [PubMed] [Google Scholar]
- 3.Chew MH, Arguin PM, Shay DK, et al. Risk factors for Nipah virus infection among abattoir workers in Singapore. J Infect Dis. 2000;181:1760–1763. [DOI] [PubMed] [Google Scholar]
- 4.Chua KB, Bellini WJ, Rota PA, et al. Nipah virus: a recently emergent deadly paramyxovirus. Science. 2000;288:1432–1435. [DOI] [PubMed] [Google Scholar]
- 5.Clayton BA, Middleton D, Bergfeld J, et al. Transmission routes for Nipah virus from Malaysia and Bangladesh. Emerg Infect Dis. 2012;18:1983–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Wit E, Bushmaker T, Scott D, et al. Nipah virus transmission in a hamster model. PLoS Negl Trop Dis. 2011;5:e1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Wit E, Prescott J, Falzarano D, et al. Foodborne transmission of nipah virus in Syrian hamsters. PLoS Pathog. 2014;10:e1004001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeBuysscher BL, de Wit E, Munster VJ, et al. Comparison of the pathogenicity of Nipah virus isolates from Bangladesh and Malaysia in the Syrian hamster. PLoS Negl Trop Dis. 2013;7:e2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geisbert TW, Daddario-DiCaprio KM, Hickey AC, et al. Development of an acute and highly pathogenic nonhuman primate model of Nipah virus infection. PLoS One. 2010;5:e10690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gurley ES, Montgomery JM, Hossain MJ, et al. Person-to-person transmission of Nipah virus in a Bangladeshi community. Emerg Infect Dis. 2007;13:1031–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harcourt BH, Lowe L, Tamin A, et al. Genetic characterization of Nipah virus, Bangladesh, 2004. Emerg Infect Dis. 2005;11:1594–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hossain MJ, Gurley ES, Montgomery JM, et al. Clinical presentation of Nipah virus infection in Bangladesh. Clin Infect Dis. 2008;46:977–984. [DOI] [PubMed] [Google Scholar]
- 13.Luby SP, Gurley ES, Hossain MJ. Transmission of human infection with Nipah virus. Clin Infect Dis. 2009;49:1743–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luby SP, Rahman M, Hossain MJ, et al. Foodborne transmission of Nipah virus, Bangladesh. Emerg Infect Dis. 2006;12:1888–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parashar UD, Sunn LM, Ong F, et al. Case-control study of risk factors for human infection with a new zoonotic paramyxovirus, Nipah virus, during a 1998–1999 outbreak of severe encephalitis in Malaysia. J Infect Dis. 2000;181:1755–1759. [DOI] [PubMed] [Google Scholar]
- 16.Rockx B, Brining D, Kramer J, et al. Clinical outcome of henipavirus infection in hamsters is determined by the route and dose of infection. J Virol. 2011;85:7658–7671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93:741–753. [DOI] [PubMed] [Google Scholar]
- 18.Wong KT, Grosjean I, Brisson C, et al. A golden hamster model for human acute Nipah virus infection. Am J Pathol. 2003;163:2127–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong KT, Shieh WJ, Kumar S, et al. Nipah virus infection: pathology and pathogenesis of an emerging paramyxoviral zoonosis. Am J Pathol. 2002;161:2153–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Case No. 1M. Cytoplasmic expression of viral antigen in olfactory epithelium (arrow) 2 days post inoculation (dpi) with NiV-M. IHC for Nipah virus nucleoprotein.
Figure S2. Case No. 6B. Cytoplasmic expression of viral antigen in olfactory (arrow) and submucosal gland acinar epithelium (arrowhead) 2 dpi with NiV-B. IHC for Nipah virus nucleoprotein.
Figure S3. Case No. 5M. Cytoplasmic expression of viral antigen in olfactory (arrow) and submucosal gland acinar epithelium (arrowhead) 4 dpi with NiV-M. IHC for Nipah virus nucleoprotein.
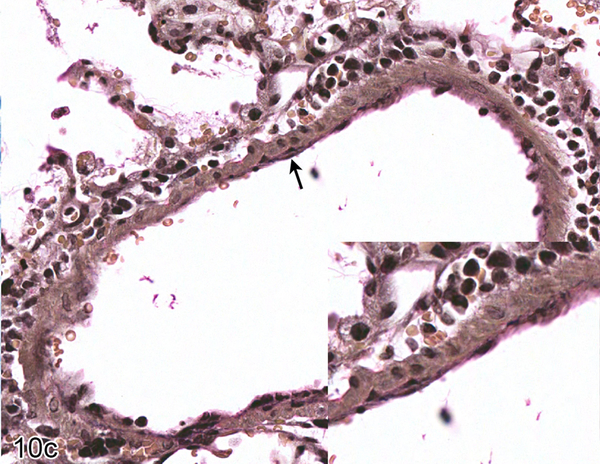
Figure S4. Case No. 13B. Cytoplasmic expression of viral antigen in olfactory (arrow) and submucosal gland acinar epithelium (arrowhead) 4 dpi with NiV-B. IHC for Nipah virus nucleoprotein.
Figure S5. Case No. 1M. Cytoplasmic expression of viral antigen in type I pneumocytes (arrow) and bronchiolar epithelium (arrowhead) 2 dpi with NiV-M. IHC for Nipah virus nucleoprotein.
Figure S6. Case No. 7B. Cytoplasmic expression of viral antigen in type I pneumocytes (arrow) 2 dpi with NiV-B. IHC for Nipah virus nucleoprotein.
Figure S7. Case No. 4M. Cytoplasmic expression of viral antigen in type I (black arrow) and type II pneumocytes (arrowhead) and mononuclear leukocytes (white arrow) 4 dpi with NiV-M. IHC for Nipah virus nucleoprotein.
Figure S8. Case No. 10B. Cytoplasmic expression of viral antigen in type I (black arrow) and type II pneumocytes (arrowhead) and mononuclear leukocytes (white arrow) 4 dpi with NiV-B. IHC for Nipah virus nucleoprotein.