Abstract
Alarmins are endogenous mediators that are elicited rapidly in response to danger signals, enhancing innate and adaptive immune responses by promoting the recruitment and maturation of antigen-presenting cells (APCs). The nucleosome-binding protein HMGN1 is a potent alarmin that binds TLR4 and induces antigen-specific Th1 immune responses, but its contributions to antitumor immunity have not been explored. We found that ovalbumin (OVA)-expressing EG7 mouse thymoma cells grew much faster in Hmgn1-deficient mice than littermate-matched controls. Tumor-bearing Hmgn1−/− mice generated fewer OVA-specific CD8 cells in the spleen than EG7-bearing Hmgn1+/+ mice, suggesting that HMGN1 supported T cell-mediated antitumor immunity. Additionally, EG7 tumors expressing HMGN1grew more slowly than control EG7 tumors, suggesting greater resistance to HMGN1-expressing tumors. This resistance relied on T cell mediated immunity because it was abolished by in vivo depletion of CD4+ and CD8+ T cells. Moreover, Mice vaccinated with a DNA vector expressing an HMGN1-gp100 fusion protein manifested gp100-specific, Th1-polarized immune responses, acquiring resistance to challenge with mouse B16F1 melanoma. Overall, our findings show that HMGN1 contributes to antitumor immunity and it may offer an effective adjuvant to heighten responses to cancer vaccines.
Keywords: Alarmin, HMGN1, Melanoma, DNA Vaccine
Introduction
In the past two decades, several anti-tumor immunotherapies including adoptive cell therapy, tumor-specific antibodies and checkpoint inhibition have yielded considerable clinical success (1–3). Although human papilloma virus (HPV) based vaccines have been successful in cancer prevention, other vaccine approaches have so far failed to mediate regression of solid tumors (3, 4). There are many hurdles in therapeutic tumor vaccine approaches, predominantly difficulties in inducing sufficient number of antitumor CD8 effector cells and the immunosuppressive tumor microenvironment that inhibits effector mechanisms (5). Although the immunosuppressive components can be overcome by countering IL-10, TGFβ, VEGF, regulatory T cells, and/or myeloid-derived suppressor cells (3, 5), identifying potent adjuvant(s) capable of generating potent protective antitumor immunity is key to the development of therapeutic antitumor vaccines.
Alarmins are structurally distinct endogenous mediators that can activate the immune system by inducing recruitment and activation of immune cells, particularly antigen-presenting dendritic cells (DCs) (6–8). Alarmins identified so far include defensins, cathelicidins, eosinophil-associated ribonucleases, high-mobility group (HMG) proteins, heat shock proteins (HSPs), saposin-like granulysin, ion-binding proteins (e.g. S100 proteins and lactoferrin), and nucleotides/metabolites (e.g. uric acid) (6–9). Alarmins promotes host defenses by inducing inflammation, immune response, and wound healing (10–23).
Various alarmins appear to have distinct effects on the types of antigen-specific immune responses. Earlier studies show that neutrophil-derived α-defensins can induce both Th1 and Th2 immune responses upon administration together with an antigen via a mucosal route (9, 10), whereas β-defensins stimulate predominantly Th1 responses upon gene gun delivery to mouse epidermis as a defensin-antigen fusion product (9, 12). Cathelicidin also promotes the generation of both Th1 and Th2 immune responses when intraperitoneally administered (13). HMGB1 and HSPs preferentially promote Th1 immune responses involved in protective anti-tumor immunity (9, 15, 24). In contrast, eosinophil-derived neurotoxin, a member of the eosinophil-associated ribonuclease, and uric acid selectively enhance the development of Th2 immune responses (14, 21). Therefore, it has gradually become apparent that various alarmins differentially promote distinct types of antigen-specific immune responses.
We have recently identified HMG nucleosome-binding protein 1 (HMGN1) as a potent Th1 polarizing alarmin (25). Recombinant HMGN1 induced the accumulation and activation of DCs at the site of injection as indicated by upregulation of costimulatory and MHC molecules as well as extracellular secretion of proinflammatory cytokines such as TNFα, IL-12 p70, and IL-1β (25). In addition, immunization of mice with an antigen mixed with HMGN1 augmented antigen-specific immune responses with greatly elevated production of IFNγ, but not IL-4 (25). The critical role of HMGN1 in the induction of Th1-polarized immune response was clearly demonstrated by the lack of such responses in HMGN1 knockout mice (25). Since generation of tumor-associated antigen (TAA)-specific Th1 immune response is vital for antitumor immunity, we investigated 1) whether HMGN1 would contribute to the induction of antitumor immune responses, and 2) whether HMGN1 was an effective anti-cancer adjuvant.
Materials and methods
Cell lines and mice
All cell lines used in the present study were originally obtained from the American Type Culture Collection (Manassas, VA). Human embryonic kidney 293 (HEK293) cells were maintained in DMEM medium [DMEM (Meditech, Manassas, VA) supplemented with 10% FBS (Hyclone, Logan, UT), 2 mM L-glutamine, 25 mM HEPES, 100 U/ml penicillin, 100 µg/ml streptomycin, and 50 µM 2-mercaptoethanol]. Mouse melanoma cell line B16F1 was cultured in DMEM medium additionally supplemented with 1× vitamin solution (Life Technologies, Frederick, MD), and 1× nonessential amino acids solution (Life Technologies). EG7, a cell line derived from EL4 thymoma and constitutively expressing OVA as a surrogate TAA, was maintained in RPMI 1640 medium [RPMI 1640 (Meditech) supplemented with 10% FBS, 2 mM glutamine, 25 mM HEPES, 1.5 g/L sodium bicarbonate, 4.5 g/L glucose, 1 mM sodium pyruvate, 100 U/ml penicillin, 100 µg/ml streptomycin, and 50 µM 2-mercaptoethanol] containing 0.4 mg/ml G418 (Life Technologies). The insect cell lines Sf9 and High-Five™ were purchased from Invitrogen (Carlsbad, CA) and cultured at 27°C in Sf-900 III serum free medium (SFM, Invitrogen) or Express Five SFM (Invitrogen), respectively.
C57BL/6 mice were obtained from Charles River (Frederick, MD). Hmgn1−/− and littermate-matched Hmgn1+/+ mice were generated as reported (25). All mice were kept under specific pathogen–free conditions with water and food given ad libitum. All experiments with mice were performed in compliance with the principles and procedures outlined in the National Institutes of Health Guide for the Care and Use of Animals and were approved by the National Cancer Institute at Frederick Animal Care and Use Committee.
Generation and treatment of mouse dendritic cells
Mouse dendritic cells (DCs) were generated from C57BL/6 bone marrow progenitors as previously reported (14). DCs were incubated with recombinant human HMGN1 at 1~5 µg/ml for 24 ~ 48 h before analysis of DCs. Cytokines produced by DCs in the culture supernatants were quantitated by SearchLight (Aushon, Billerica, MA). Recombinant human HMGN1 used in this study was expressed in insect cells using a baculovirus expressing system and purified as previously reported (25).
Plasmid construction, purification, transfection, and cell line establishment
The recombinant plasmids encoding HMGN1 (Clone Id LIFESEQ1228711) and gp100 (OriGene SC122763) were purchased from Open Biosystems and OriGene Technologies, Inc., respectively. The cDNAs encoding the mature form of the target gene (HMGN1 or gp100) were amplified by PCR, with the introduction of the insulin SP at the N-terminus to facilitate secretion of the targets. The fusion gene HMGN1-gp100 was constructed by overlapping PCR, with the insertion of a flexible linker (Gly4Ser)3 between HMGN1 and gp100. After sequence confirmation, the three target genes were subcloned to eukaryotic expressing plasmid pcDNA3.1-hygromycin (Invitrogen) or pcDNA3.1/myc-His B (Invitrogen) to generate pcDNA3.1-HMGN1, pcDNA3.1-gp100, or pcDNA3.1-HMGN1-gp100, respectively. The plasmid pcDNA3.1/myc-His B also provided a c-myc and a poly-His motifs in-frame after the inserted genes so that the target proteins could be detected using anti-myc or anti-polyHis antibodies. Plasmids were purified using EndoFree Plasmid Maxi Kit (Qiagen, Germantown, MD).
Transfection of HEK293, EG7, or EL4 cells was performed using Lipofectamine™ 2000 (Invitrogen) following the manufacturer’s recommendations. For determining the production of targets, proteins in supernatants collected 24 h after transfection were precipitated by trichloroacetic acid (TCA) and subsequently analyzed by Western blot. Some transfected cells were selected with hygromycin B (Invitrogen) at 700 µg/ml and cloned by limiting dilution.
Western blot
Samples or SeeBlue® Plus2 Pre-Stained Standard (Invitrogen) were loaded onto a NuPAGE® Novex 4–12% Bis-Tris gel (Invitrogen) and separated using either MOPS or MES buffer. After transfer of separated proteins onto PVDF membranes (Immobilon, Millipore), the membranes were rinsed with tris-buffered saline containing 0.05% Tween 20 (TBST), blocked with 5% nonfat dry milk at room temperature for 1h, and incubated overnight at 4°C with rabbit anti-HMGN1 (ProteinTech, Chicago, IL, 11695-1-AP), rabbit anti-polyHis (Cell Signaling, Danvers, MA, #2365), or goat anti-gp100 (Santa Cruz Biotech, Dallas, TX, sc-15010). After washing with TBST, the membranes were reacted with HRP-conjugated goat anti-rabbit IgG (Cell Signaling, #70741) or HRP-conjugated rabbit anti-goat IgG (Calbiochem, Billerica, MA, 401515), washed, and developed in SuperSignal® West Dura Extended Duration Substrate (Thermo-Fisher, Hanover Park, IL). The images were collected using the G BOX Chemi systems (Syngene, Frederick, MD).
Preparation of gene gun bullets and DNA vaccination
Gene gun bullets were prepared as previously described with optimization (26). Briefly, 100 µl of 0.05 M spermidine was added into 45 mg of 1-µm gold beads (Bio-Rad, Hercules, CA) and mixed by brief sonication. Forty-five µl of plasmid (2 mg/ml) was immediately added into the gold/spermidine mixture and the plasmids were coprecipitated onto the gold beads by adding 100 µl of 1 M CaCl2 drop-wise while vortexing. After washing with 100% ethanol, the plasmid-coated gold beads were resuspended in 6 ml of 100% ethanol in a 15-ml tube and loaded onto pre-dried Teflon tubing (Bio-Rad). The Teflon tubing was rotated at 20 rpm in a Tube turner (Bio-Rad) for 1 minute to allow the gold beads smear evenly on the inner surface of the tubing, and finally was dried by flowing dry nitrogen gas through the rotating tubing at a rate of 0.35 liters/minute for about 4 minutes. The dried tubing was subsequently cut into 0.5-inch sections (bullets) and stored at 4°C until use.
For DNA vaccination, C57BL/6 mice were anesthetized and their abdominal skin was shaved. Subsequently, plasmid DNA was delivered into the epidermal layer of the shaved abdominal skin via a Helios Genegun (Bio-Rad, Hercules, CA) with a discharge pressure of 400 psi (4 bullets/mouse/immunization). Mice were vaccinated either once a week for 3 weeks, twice a week for 3 weeks, or only twice in 3 weeks as specified. Each mouse received 4 µg of plasmid DNA at each vaccination.
Mouse tumor models
Mice (C57BL/6, Hmgn1−/−, or Hmgn1+/+, female, 7~10 week-old, n = 5~10), untreated or DNA-vaccinated, were implanted subcutaneously with 0.2 ml sterile PBS containing indicated number (2×104~5×105/injection) of tumor cells (EG7, control/HMGN1-expressing EG7, or B16F1) into the right flank. The appearance and growth of tumors were monitored twice every week. The greatest longitudinal diameter (length) and the greatest transverse diameter (width) of a palpable tumor were measured to the nearest 0.1 mm using a caliper. Tumor volume (mm3) was calculated by the ellipsoidal formula Tumor volume = (length × width2) × π/6. For depleting CD4 or CD8 cells, mice were injected intraperitoneally with 0.2 ml PBS containing 150 µg of either control rat IgG (clone 2A3, BioXcell, West Lebanon, NH), anti-mouse CD4 (clone GK1.5, BioXcell), or anti-mouse CD8α (clone 53-6.72) for three consecutive days prior to tumor inoculation and once a week after tumor inoculation for four weeks.
Immunostaining and flow cytometry
Mouse DCs or splenocytes suspended in FACS buffer (PBS containing 0.5% BSA and 0.05% NaN3) were blocked with 2% normal mouse serum on ice for 20 min and stained with various combinations of fluorophore-conjugated anti-mouse antibodies on ice for 30 min. The antibodies used to stain mouse DCs were FITC-anti-mouse CD80 (clone 16-10A1, BD/Pharmingen™, San Jose, CA), PE-anti-mouse CD86 (clone GL1, BD), FITC-anti-mouse I-A/E (clone 2G9, BD), and PE-anti-mouse CD11c (clone HL3, BD). Stained DCs were analyzed on a flow FACScan cytometer (BD). For determining the frequency of OVA-specific CD8 cells, splenocytes of EG7-bearing Hmgn1−/− and Hmgn1+/+ mice were stained with FITC-anti-mouse CD3 (clone 145-2C11, BD), PE-OVA-specific tetramer (Class I iTAg™ MHC Tetramer, Beckman Coulter, Fullerton, CA), PerCP-Cy5.5-anti-mouse CD8 (clone 53-6.7, eBioscience), and APC-conjugated anti-mouse CD4 (clone RM4-5, BD), and analyzed on a FACSCalibur flow cytometer (BD).
The CD8 epitope of gp100, gp100(25–33) (KVPRNQDWL), was synthesized and purified as reported (27). For intracellular cytokine staining, the splenocytes and lymph node cells of mice vaccinated with various plasmids were stimulated at 5 × 106/ml in RPMI 1640 medium in vitro with 1 µM gp100(25–33) peptide for 6 h at 37°C in humidified air containing 5% CO2, with the addition of GolgiPlug™ (eBioscience) for the last 4 h of culture. Subsequently, the cells were stained with PE-anti-mouse CD3 (clone 17A2; BD), APC-anti-mouse CD4 (clone RM4-5; BD), and PE-Cy7-anti-mouse CD8 (clone 53-6.7; eBioscience) on ice for 30 min, fixed with IC Fixation Buffer (eBioscience Cat. No. 00-8222) at room temperature for 20 min, permeabilized with Permealilization Buffer (eBioscience Cat. No. 00-8333), and incubated with FITC-anti-mouse IFN-γ (clone XMG1.2; BioLegend) and eFluor450-anti-mouse IL-13 (clone eBio13A; eBioscience) in Permeabilization Buffer for 20 min at room temperature. After three washes with Permeabilization Buffer, the samples were resuspended in FACS buffer and data were acquired on a LSR II flow cytometer (BD). All flow cytometry data were analyzed using FlowJo.
Statistical analysis
Unless otherwise specified, all experiments were performed at least three times, and the results of one representative experiment or the mean of multiple experiments are shown. Differences in the in vivo tumor growth were determined by Repeated Measures ANOVA, whereas differences between other control groups and experimentally treated groups were evaluated by one way ANOVA after arcsine square-root transformation.
Results
Endogenous HMGN1 contributed to the development of antitumor immunity
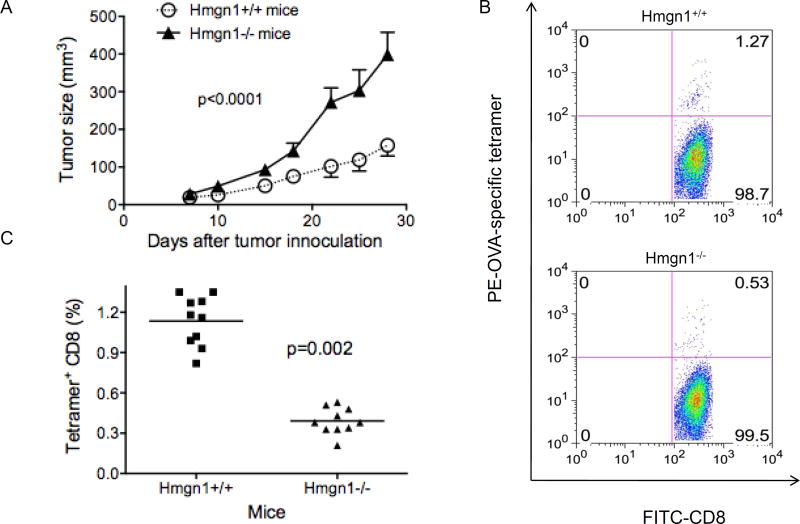
We compared the development and growth of EG7 tumors in Hmgn1−/− and littermate-matched Hmgn1+/+ mice. The use of EG7 mouse thymoma allowed convenient measurement of tumor-specific immune response because EG7 cells express OVA as a surrogate TAA (28). Following subcutaneous implantation, EG7 tumors grew much more rapidly in Hmgn1−/− mice than in Hmgn1+/+ mice (Fig. 1A). The increased rate of EG7 tumor growth in Hmgn1−/− mice was unlikely due to the lack of intranuclear HMGN1, because EG7 tumor cells were not Hmgn1−/−. Given the critical role of HMGN1 in the induction of antigen-specific immune responses (25), we speculated that knockout of HMGN1 might reduce the anti-tumor immune defenses of the host. Analysis of splenic lymphocytes from EG7-bearing mice by tetramer staining and flow cytometry showed that Hmgn1+/+ mice generated considerably higher frequency of splenic OVA-specific CD8 cells than Hmgn1−/− mice (Figs. 1B and 1C). Tumor-draining lymph node also contained higher level of OVA-specific CD8 cells (data not shown). These data showed that Hmgn1−/− mice developed lower antigen-specific anti-tumor immune responses than littermate-matched Hmgn1+/+ mice in response to EG7 tumors, demonstrating the contribution of HMGN1 to the development of antigen-specific anti-tumor immune defense responses.
Figure 1. HMGN1 knockout decreased anti-tumor (EG7) immune response.
HMGN1 knockout (Hmgn1−/−) and littermate-matched wild-type (Hmgn1+/+) mice (7~8 week-old, female, 10/group) were subcutaneously injected with 2×105 EG7 tumor cells into the right flank and the growth of tumors was monitored. On day 28, mouse splenocytes were analyzed by flow cytometry after staining with anti-CD3-FITC, OVA-Tetramer-PE, anti-CD4-APC, and anti-CD8- PerCP-Cy5.5. A, Tumor growth, *p < 0.001 by ANOVA. B, Dot-plot showing OVA-specific CD8 cells of one mouse spleen. C, Percentage of OVA-specific CD8 cells of Hmgn1+/+ and Hmgn1−/− groups. Shown are the results of one experiment representative of two.
Exogenous HMGN1 promoted DC activation and host defense against tumor
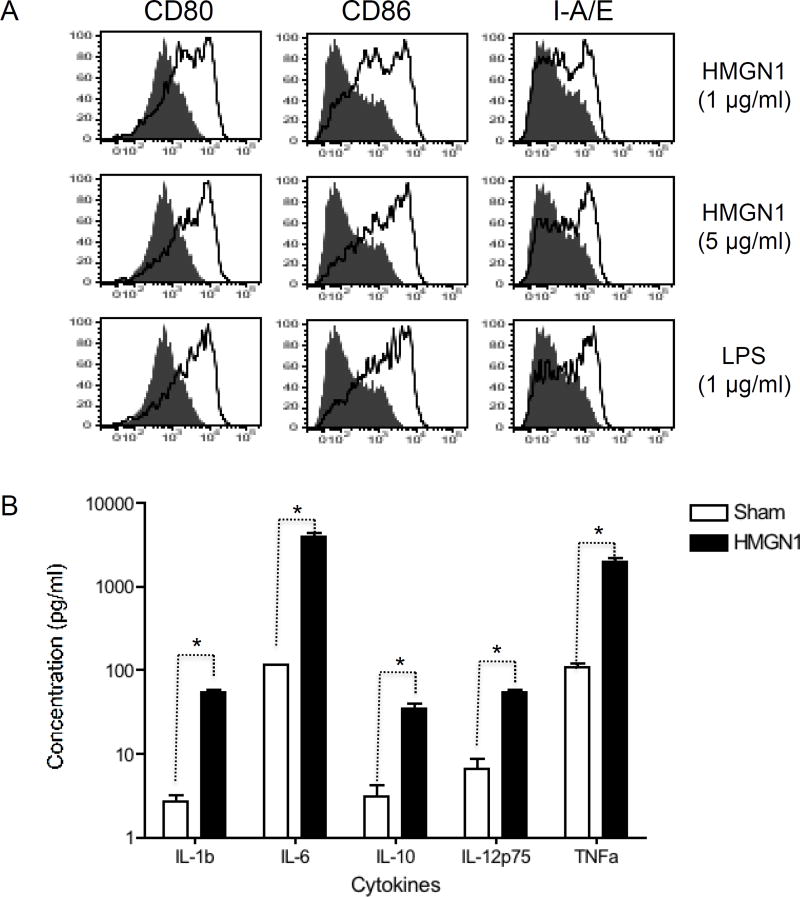
We next determined whether recombinant human HMGN1 could activate mouse DCs. Mouse DCs were therefore incubated without (sham) or with human HMGN1 and subsequently analyzed for the expression of surface markers and production of cytokines, two hallmarks of DC activation. DCs treated with human HMGN1 at 1 or 5 µg/ml for 48 h upregulated their expression of CD80, CD86, and I-A/E in a dose-dependent manner as shown by flow cytometry analysis (Fig. 2A, sFig. 1). Incubation of mouse DCs with human HMGN1 at 5 µg/ml for 48 h induced the production of multiple proinflammatory cytokines including IL-1β, IL-6, IL-10, IL-12p70, and TNFα (Fig. 2B). Thus, human HMGN1 could act as an alarmin in mice.
Figure 2. Activation of mouse bone marrow-derived DCs by recombinant human HMGN1.
A, Mouse BM-derived DCs (5×105/ml) treated without or with recombinant HMGN1 (1 or 5 µg/ml) at 37°C for 48 h in humidified air containing 5% CO2 were immunostained for analysis of surface expression of CD80, CD86, and I-A/E. Shown are the overlay histograms (shaded area = sham treated DCs; solid line = HMGN1-treated DCs) of one experiment representative of three. B, Supernatants of mouse BM-derived DCs (5×105/ml) incubated in the absence (sham) or presence of recombinant human HMGN1 (5 µg/ml) at 37°C for 48 h in humidified air containing 5% CO2 were assayed for the production of indicated cytokines. Shown is the average (mean±SD) of three experiments. *p < 0.05 by Student’s t test.
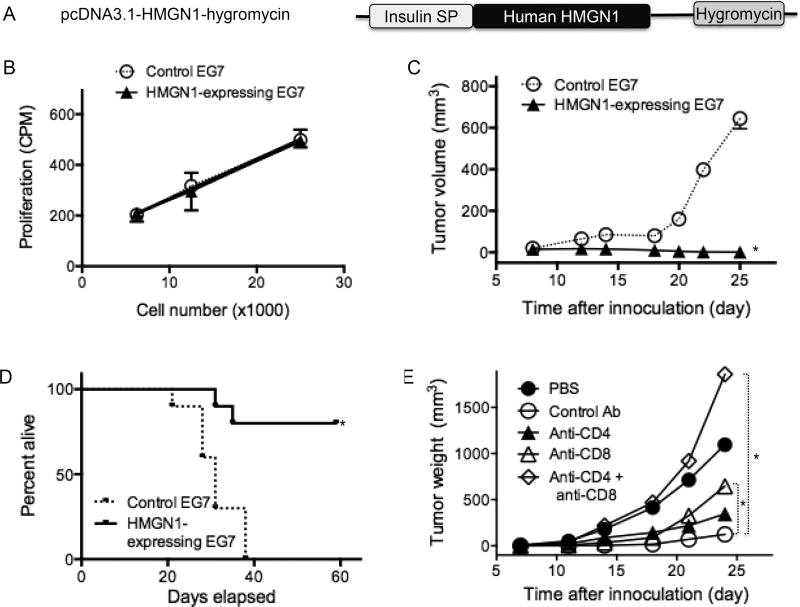
Human HMGN1 gene was cloned into a pcDNA3.1-Hygromycin vector with insulin signal peptide directing extracellular expression of HMGN1 in mammalian cells (Fig. 3A). EG7 cells were transfected with pcDNA3.1 or pcDNA3.1-HMGN1 and subsequently selected with hygromycin to establish control and HMGN1-expressing EG7 cell lines, respectively. Western blot analysis confirmed that HMGN1 was indeed secreted by HMGN1-expressing EG7 cells (Data not shown). The effect of HMGN1 expression on in vitro EG7 growth was determined by comparing 3H-TdR incorporations of control and HMGN1-expressing EG7 cells (Fig. 3B). Both control and HMGN1-expressing EG7 cell lines grew similarly, indicating that expression of human HMGN1 in EG7 did not influence the proliferative capacity of the cells (Fig. 3B).
Figure 3. Human HMGN1-expressing EG7 tumors grew slower than wild type EG7 tumors in vivo.
A, Schematic illustration of pcDNA3.1-hHMGN1-hygromycin. EG7 cells were transfected with pcDNA3.1-hygromycin or pcDNA3.1-hHMGN1-hygromycin and selected in the presence of hygromycin for generating control and human HMGN1-expressing EG7 clones. B, in vitro proliferation of control and HMGN1-expressing EG7 tumor cells. Control and HMGN1-expressing EG7 cells were plated in triplicate in a 96-well plate at the indicated number and incubated overnight. Subsequently, the culture was pulsed with 0.5 µCi/well of 3H-TdR for 4 h, harvested, and measured for the incorporation of 3H-TdR. Shown is the average incorporation of 3H-TdR (mean ± SD) of triplicate wells. C, C57BL/6 mice (8-week old, female) were divided into 2 groups (n = 10) and were subcutaneously injected with 5×105 control or HMGN1-expressing EG7 into the right flank. Tumor growth was monitored twice a week for up to 4 weeks. Shown are the results of one experiment representative of three. *p < 0.05 by Repeated Measures ANOVA. D, Two groups of C57BL/6 mice (8-week old, female, n = 10) were subcutaneously injected with 5×105 control or HMGN1-expressing EG7 into the right flank. Mouse morbidity was monitored for 60 days and compared after graphing with Prism software. *p < 0.05 by Mantel-Cox test. E, Five groups of C57BL/6 mice (8-week old, female, n = 10) were subcutaneously injected with 5×105 control (PBS group) or HMGN1-expressing EG7 (groups with Ab treatment) into the right flank on day 1. For depleting CD4 or CD8 T cells, mice were intraperitoneally injected with anti-CD4, anti-CD8, or a combination of anti-CD4 and anti-CD8α antibodies on day-4, -3, -2, 3, 10, 17, and 24 at 150 µg per mouse. Tumor volume was monitored twice a week. Shown are the results of one experiment representative of two. *p < 0.05 by Repeated Measures ANOVA.
The control and HMGN1-expressing EG7 cells were then implanted subcutaneously into C57BL/6 mice and tumor growth was monitored. Control EG7 formed palpable tumors at about two weeks, and progressed during the third weeks after implantation (Fig. 3C). In contrast, implantation with HMGN1-expressing EG7 cells did not develop palpable tumors within 4 weeks (Fig. 3C). When mice were followed for longer than four weeks, all mice inoculated with control EG7 died or became morbid within 5 weeks, whereas 80% of the mice inoculated with HMGN1-expressing EG7 had no or small tumors and remained alive for at least 60 days (Fig. 3D). Since expression of HMGN1 did not alter the proliferation of HMGN1-expressing EG7 cells in vitro (Fig. 3B), the failure of implanted HMGN1-expressing EG7 to form tumors was unlikely due to any change in tumorigenecity of the implanted tumor cells. Presumably, human HMGN1 secreted by implanted HMGN1-expressing EG7 tumor cells promoted anti-tumor immune defense.
We also studied whether HMGN1 could promote antitumor defense in the absence of surrogate TAA using HMGN1-expressing EL4 cell lines. Both control and HMGN1-expressing EL4 cell lines grew identically in vitro (sFig. 2A). When implanted into C57BL/6 mice, control EL4 formed rapidly growing tumors, whereas HMGN1-expressing EL4 formed smaller, slowly growing tumors (sFig. 2B). Therefore, HMGN1 induced antitumor defense even in the absence of the potent surrogate TAA OVA.
To investigate whether antitumor immunity was responsible for the observed anti-EG7 resistance (Fig. 3C), mice inoculated with HMGN1-expressing EG7 cells were depleted of CD4 and/or CD8 T cells. Mice depleted of CD4 or CD8 T cells and inoculated with HMGN1-expressing EG7 cells showed faster tumor growth (closed and open triangles, respectively) than mice treated with control antibody (open circles, Fig. 3E). Depletion of both CD4 and CD8 cells (open diamonds) resulted in even faster tumor growth than in mice inoculated with control EG7 cells (closed circles, Fig. 3E), indicating that CD4 and CD8 T cells together yielded optimal anti-EG7 immune defense. Therefore, extracellular HMGN1 at the site of tumor elevated host anti-tumor immune defense.
HMGN1 acted as an effective adjuvant for induction of anti-melanoma immunity
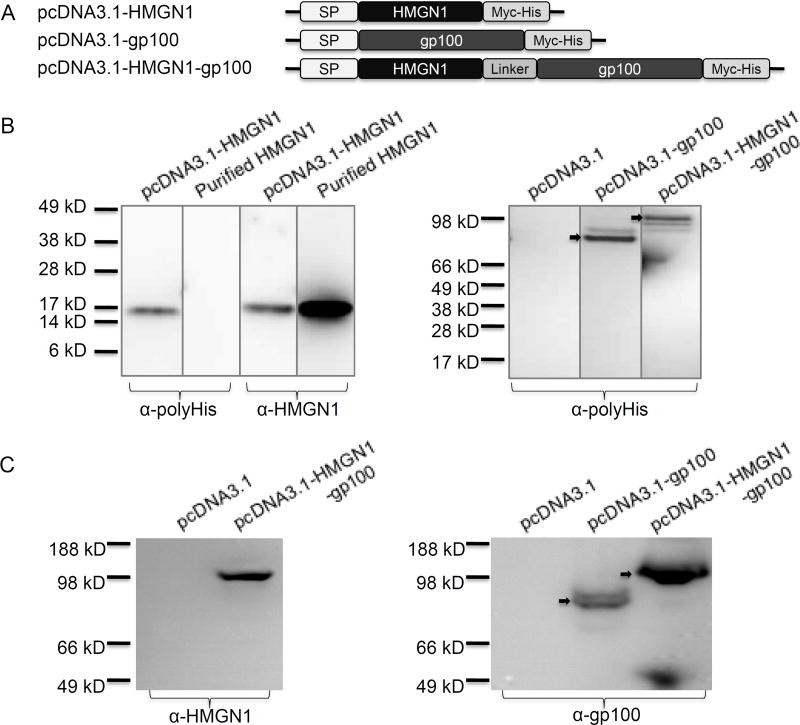
We evaluated the immunoadjuvant activity of HMGN1 using the rapidly growing mouse B16 melanoma with gp100 as the target TAA. It has previously been shown that covalent linkage of an alarmin to a target TAA can better promote TAA-specific immune responses and immunoprotection (12, 29). Therefore, a series of mammalian expression plasmids encoding HMGN1, gp100, or fusion of HMGN1-gp100 were constructed with the insulin signal peptide (SP) fused in-frame with our target proteins (Fig. 4A). A flexible linker consisting of three tandems of Gly4Ser (12, 29) was introduced between HMGN1 and gp100 in the pcDNA3.1-HMGN1-gp100 plasmid (Fig. 4A). To confirm that the target gene products could be expressed and secreted by mammalian cells, the supernatants of HEK293 cells transfected with a particular plasmid, were analyzed by Western blot (Fig. 4B). The supernatant of HEK293 cells transfected with pcDNA3.1-HMGN1 contained a protein band of approximately 16-kDa as probed with either anti-polyHis or anti-HMGN1 antibody (Fig. 4B, left panel). This band consisted of HMGN1 since it showed identical electrophoretic mobility with purified HMGN1 in the same SDS-PAGE gel (Fig. 4B, left panel). When probed with anti-polyHis antibody, the supernatants of HEK293 cells transfected with pcDNA3.1-gp100 and pcDNA3.1-HMGN1-gp100 yielded a detectable protein band of approximately 86- and 102-kDa, respectively, corresponding to the expected size of gp100 and HMGN1-gp100, respectively (Fig. 4B, right panel). The supernatant of HEK293 cells transfected with pcDNA3.1 plasmid had no positive band as expected (Fig. 4B, right panel). Further, probing the supernatants of HEK293 cells transiently transfected with pcDNA3.1, pcDNA3.1-gp100, or pcDNA3.1-HMGN1-gp100 with anti-HMGN1 (Fig. 4C, left panel) or anti-gp100 (Fig. 4C, right panel) revealed a band of approximately 102-kDa for HMGN1-gp100 fusion protein and a band of approximately 86-kDa for gp100 protein (Fig. 4C). Therefore, the plasmids constructed were indeed capable of directing secreted expression of the target proteins in mammalian cells.
Figure 4. Construction of plasmids that direct the expression of human HMGN1, gp100, or human HMGN1-gp100 fusion proteins.
A, Schematic illustration of a series of eukaryotic expressing plasmids (DNA vaccines) harboring the genes of HMGN1, gp100, or HMGN1-gp100 fusion. The eukaryotic expressing vector used in the DNA vaccines is pcDNA3.1/myc-His B (Invitrogen), which provides a myc epitope and a polyhistidine-tag at the C-terminus. The signal peptide of insulin was introduced in front of target genes to ensure secretion. The Kozak sequence was optimized. In the fusion gene HMGN1-gp100, a flexible linker (Gly4Ser)3 was inserted between HMGN1 and gp100. B, HEK293 cells (106/flask) were transfected with 10 µg of pcDNA3.1, pcDNA3.1-HMGN1, pcDNA3.1-gp100, or pcDNA3.1-HMGN1-gp100 plasmid using Lipofectamine™ 2000. Six hours after the transfection, the culture medium was replaced with DMEM containing 1% FBS and after an additional 24 hour-incubation, supernatants were analyzed by Western Blot. Purified human HMGN1 (1 µg) was used as a positive control. Left panel: lanes loaded with the supernatant of HEK293 cells transfected with pcDNA3.1-HMGN1 or purified HMGN1 were probed with α-polyHis (left two lanes) or α-HMGN1 (right two lanes). Right panel: lanes loaded with the supernatants of HEK293 cells transfected with indicated plasmids were probed with α-HMGN1. C, Transfection and subsequent analysis were similar as in B. Supernatants of HEK293 cells transfected with indicated plasmids were analyzed Western blot using anti-HMGN1 (left panel) or anti-gp100 (right panel) antibodies.
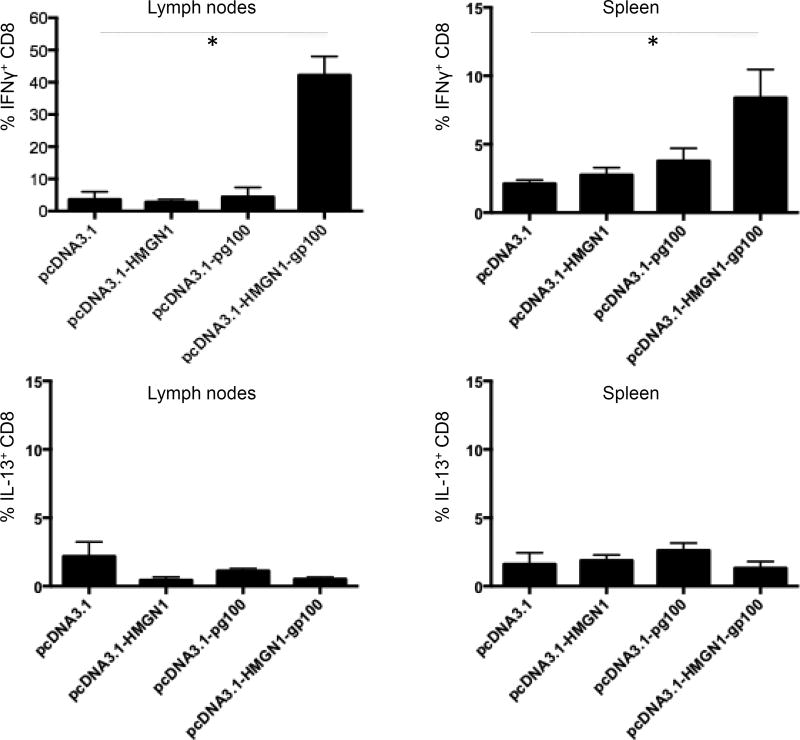
To investigate if HMGN1 could promote an antigen-specific immune response, C57BL/6 mice vaccinated by with various plasmids were analyzed for cytokine-producing T cells in the spleens and draining lymph nodes. Intracellular cytokine staining and flow cytometry analysis (analysis gating illustrated in sFig. 3) showed that in both inguinal lymph nodes and spleen, mice vaccinated with pcDNA3.1-HMGN1-gp100 generated significantly more IFNγ+ CD8 cells than control (vaccinated with pcDNA3.1) mice (Fig. 5). There was no significant increase in IL-13+ CD8 T cells in the inguinal lymph nodes or spleens of mice vaccinated with pcDNA3.1-HMGN1-gp100 in comparison with the control group (Fig. 5). These data demonstrated that the fusion product of HMGN1 and gp100 induced a potent Th1-polarized T cell response against gp100. Mice vaccinated with either pcDNA3.1-HMGN1 or pcDNA3.1-gp100 did not show any increase in the percentage of either IFNγ+ or IL-13+ CD8 T cells, indicating that HMGN1 or gp100 alone had no effect (Fig. 5). Therefore, HMGN1 acted as an adjuvant to stimulate Th1-polarized gp100-specific immune responses.
Figure 5. HMGN1-gp100 DNA vaccination promoted a Th1-polarized anti-gp100 immune response in vivo.
C57BL/6 mice (7-week old, female, 5 mice/group) were DNA vaccinated with indicated plasmids via gene gun once a week for 3 consecutive weeks. Five days after the last vaccination, single cell suspensions of spleens and draining (inguinal) lymph nodes of vaccinated mouse were stimulated in vitro with 1 µM gp100(25–33) peptide for 6 h at 37°C in humidified air containing 5% CO2, with the addition of GolgiPlug™ for the last 4 h of culture. The cells were immunostained with fluorophore-conjugated antibodies against mouse CD3 and CD8, permeabilized, and followed by staining with fluorophore-conjugated anti-mouse IFNγ and anti-IL-13 antibodies as detailed in the Materials and Methods section. The data were analyzed for the presence of IFNγ+ or IL-13+ CD8 T cells. Shown is the average (mean ± SD) of each group (n=5), representative of one of two separate experiments.
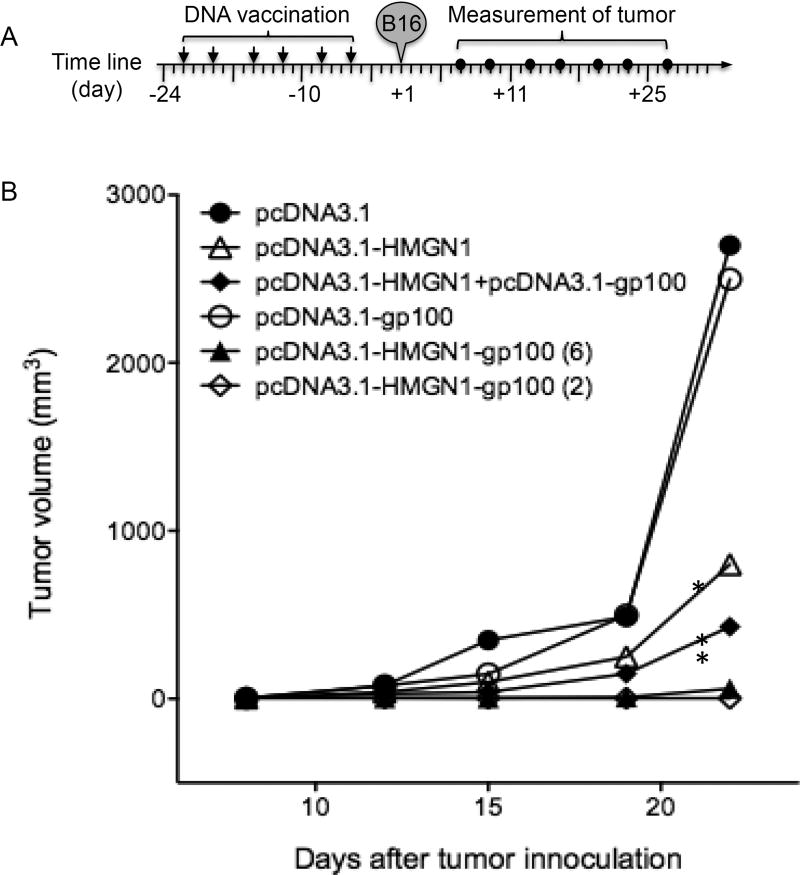
We next determined whether DNA vaccination with pcDNA3.1-HMGN1-gp100 could induce sufficiently potent immune responses capable of protecting against a melanoma challenge. C57BL/6 mice were vaccinated with various plasmids, subcutaneously implanted with B16F1 melanoma, and the appearance and growth of tumors were monitored. Tumors grew at a similar rate in mice vaccinated with pcDNA3.1 (control group) or pcDNA3.1-gp100, suggesting that vaccination with TAA (gp100) alone had no effect (Fig. 6B). Although mice vaccinated with pcDNA3.1-HMGN1 showed a slower tumor growth than controls, the difference was not statistically significant (Fig. 6B). Tumors in mice vaccinated with a mixture of pcDNA3.1-HMGN1 and pcDNA3.1-gp100 grew significantly slower than in the controls, indicative of some immunoprotection (Fig. 6B). In contrast, none of the mice vaccinated with pcDNA3.1-HMGN1-gp100 either six times or just twice, formed any palpable tumors, indicative of the most potent immunoprotection (Fig. 6B). Interestingly, mice given the HMGN1-gp100 fusion construct also developed much greater immunoprotection to subsequent B16F1 challenge than mice given a mixture of HMGN1- and gp100-expressing plasmids (Fig. 6B). Consequently, HMGN1 augments protective anti-tumor immunity and may provide a potent adjuvant for the development of effective anti-tumor vaccines.
Figure 6. DNA vaccination with pcDNA3.1-HMGN1-gp100 plasmid protected mice from B16F1 inoculation.
C57BL/6 mice (7-week old, female, 5 mice/group) were DNA vaccinated with indicated plasmids via gene gun twice a week for 3 consecutive weeks with the exception of group 6, in which mice received only two (the first and the last) vaccinations. Six days after the last vaccination, mice were subcutaneously implanted with B16F1 melanoma (2×104/0.2 ml/mouse). Tumor growth was monitored twice a week for up to 4 weeks, starting on day 6 after B16 implantation. A, Schematic illustration of the time line of various treatments. B, Tumor growth curve. Shown are the results of one experiment representative of two. *p < 0.05 by Repeated Measures ANOVA in comparison with pcDNA3.1 control group.
Discussion
HMGN1 is a nuclear protein that binds specifically to nucleosomes and affects chromatin structure and function (30). We have recently reported that extracellular HMGN1 acts as an alarmin and plays a critical role in the induction of antigen-specific immune responses (25). In this study, we demonstrated the importance of HMGN1 im promoting antitumor immunity in two ways. EG7 tumors grew more rapidly in Hmgn1−/− mice than in littermate-matched Hmgn1+/+ mice, which was accompanied by the generation of lower levels of splenic EG7-specific (OVA-specific) CD8+ cells (Fig. 1). Furthermore, when implanted into C57BL/6 mice, HMGN1-expressing EG7 tumor cells failed to form palpable tumors, whereas control EG7 tumor cells grew progressively into large solid tumors (Fig. 3C). Depletion of CD4 and CD8 T cells in mice inoculated with HMGN1-expressing EG7 cells nullified the antitumor defense and allowed the mice to grow even larger tumors than mice inoculated with control EG7 cells (Fig. 3E). Thus, both loss-of-function and gain-of-function approaches demonstrate that HMGN1 is important for the development of antitumor immune defenses.
Knockout of HMGN1 has been shown to affect DNA repair (31) and to cause increased susceptibility to radiation-induced DNA damage as well as tumorigenicity (30, 32). Therefore, the failure of HMGN1-expressing EG7 to grow into solid tumors might have been due to the adverse growth potential resulting from expression of human HMGN1 in EG7 cells (Fig. 3C). However, this was considered unlikely for two reasons. One is that pcDNA3.1-HMGN1-hygromycin used for transfecting EG7 cells was constructed to achieve secreted expression of HMGN1 (Fig. 3A). Consequently, human HMGN1 expressed by transfected EG7 cells would not reach the nucleus. Secondly, since control and HMGN1-expressing EG7 cell lines proliferated equally in vitro (Fig. 3B), the expression of human HMGN1 in EG7 cells did not adversely influence their growth potential. Therefore, implantation of HMGN1-expressing EG7 cells probably enabled the host immune system to develop greater resistance to the tumor cells. This conclusion is supported by data showing that human HMGN1 activated mouse DCs (Fig. 2), and the induced antitumor defense was dependent on cell-mediated immunity (Fig. 3E).
In this study, human instead of mouse HMGN1 cDNA was used in making various expression constructs. This raises the possibility that adaptive immune responses against xenogenic human HMGN1 instead of antitumor immunity was responsible for the inhibition of HMGN1-expressing EG7 tumors (Fig. 3C, 3D, and 3E). This was considered unlikely since: 1) Human and mouse HMGN1 are highly homologous; 2) Anti-human HMGN1 antibody was not detected in the serum of mice inoculated with HMGN1-expressing EG7 cells (data not shown); and 3) Comparison of the predicted H-2-Kb epitopes of human and mouse HMGN1 shows that the nine likely H-2-Kb epitopes of human HMGN1 are all covered by H-2-Kb epitopes of mouse HMGN1 (Supplemental Table 1), suggesting that it is unlikely for C57BL/6 mice to mount a CD8 response against human HMGN1.
Since most TAAs are weakly immunogenic, it was relevant to determine if HMGN1 could be used to protect against tumors with weaker TAAs. We therefore investigated whether cutaneous vaccination with a plasmid encoding the expression of a fusion protein consisting of HMGN1 and gp100, a melanoma TAA, could induce gp100-specific CD8 response as well as anti-melanoma protection. Vaccination of C57BL/6 mice with pcDNA3.1-HMGN1-gp100 increased gp100-specific IFNγ-producing CD8 cells (Fig. 5). Furthermore, vaccination with pcDNA3.1-HMGN1-gp100 induced full prophylactic protection to a subsequent challenge with mouse B16F1 melanoma (Fig. 6). It is noteworthy that vaccination with pcDNA3.1-HMGN1-gp100 provided more effective protection than vaccination with a mixture of pcDNA3.1-HMGN1 and pcDNA3.1-gp100 (Fig. 6). This was presumably based on observations that antigens linked to mediators capable of interacting with receptors on APCs are more efficiently taken up and processed (9, 33).
Data obtained in this study demonstrate the importance of HMGN1 in the generation of antitumor immune responses. However, EG7 tumor cells do not release HMGN1 at least in vitro (data not shown). Bone marrow chimera experiments reveal that HMGN1 responsible for promotion of antigen-specific immune response does not seem to come from cells of hematopoietic origin (25). Therefore, it remains to be investigated which type of cells is the major source of extracellular HMGN1 that promotes antitumor immunity.
Several alarmins have been reported to have the capacity to enhance anti-tumor immunity. Mouse β-defensin 2 can promote the generation of anti-tumor immunity against leukemia and melanoma through activation of DCs, T cell, and NK cells (12, 29, 34). Heat shock proteins are also reported to be capable of promoting anti-tumor CD8 responses (35, 36). HMGB1 is shown to be an important alarmin for the generation of anti-tumor immunity in both mouse and human species (15, 37). Our data showing the enhancement of antitumor immunity against mouse thymoma and melanoma by HMGN1 suggest that HMGN1 can be an adjuvant of choice for the design of antitumor vaccines. HMGN1 offers some advantages over other adjuvants. First of all, endogenous HMGN1 may exhibit fewer adverse side effects than exogenous pathogen-associated molecular patterns such as bacterial lipopolysaccharides. Secondly, HMGN1 consistently promotes Th1-type immune responses when used intraperitoneally (25) or delivered intradermally as a DNA vaccine (Fig. 5). This preferential Th1-polarizing capability of HMGN1 makes it highly favorable for inclusion in tumor vaccines since only TAA-specific Th1 type immune responses are protective against tumors. Thirdly, HMGN1 is more potent and stable compared to other alarmins capable of promoting anti-tumor immune responses. HMGN1 stimulates DC activation at 0.2~1.0 µg/ml (approximately 10~60 nM), whereas mouse β-defensin 2, heat shock proteins, and HMGB1 need 5- to 10-fold higher concentrations to activate DCs (12, 25, 38–41). Additionally, the presence of intramolecular disulfide bonds in defensins and HMGB1 enables their activities to be affected by their redox status (42, 43), whereas HMGN1 is consistently active due to the lack of disulfide bond. Although it remains to be determined if HMGN1 can be used to treat established solid tumors, our findings suggest that HMGN1 may potentially be a promising tumor vaccine adjuvant.
Supplementary Material
Acknowledgments
Acknowledgements and disclaimers
This project has been funded in part with Federal funds from the Frederick National Lab, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This research was supported in part by the Intramural Research Program of NIH, Frederick National Lab, Center for Cancer Research. The publisher or recipient acknowledges right of the U.S. Government to retain a nonexclusive, royalty-free license in and to any copyright covering the article.
This project has also been funded in part by grants from the National Natural Science Foundation of China (Grant No. 30901376) and the National Key Basic Research Program of China (973 grant 2012CB932503).
Footnotes
Authors’ contributions: F. Wei, D. Yang, and J.J. Oppenheim for Conception and design; F. Wei, D. Yang, and P. Tewary for Development of methodology; F. Wei and D. Yang for Acquisition of data; F. Wei, D. Yang, P. Tewary, Yana Li, Sandra Li, X. Chen, O.M.Z. Howard, Michael Bustin, J.J. Oppenheim for Analysis and interpretation of data; F. Wei, D. Yang, and J. J. Oppenheim for Writing, review and/or revision of the manuscript; Yana Li, Sandra Li, X. Chen, O.M.Z. Howard, and Michael Bustin for Administrative, technical, or material support; D. Yang and J.J. Oppenheim for Study supervision.
References
- 1.Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17:2639–48. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 2.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–9. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–15. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gajewski TF, Meng Y, Blank C, Brown I, Kacha A, Kline J, et al. Immune resistance orchestrated by the tumor microenvironment. Immunol Rev. 2006;213:131–45. doi: 10.1111/j.1600-065X.2006.00442.x. [DOI] [PubMed] [Google Scholar]
- 6.Oppenheim JJ, Yang D. Alarmins: chemotactic activators of immune responses. Curr Opin Immunol. 2005;17:359–65. doi: 10.1016/j.coi.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 8.Yang D, Tewary P, de la Rosa G, Wei F, Oppenheim JJ. The alarmin functions of high-mobility group proteins. Biochim Biophys Acta. 2010;1799:157–63. doi: 10.1016/j.bbagrm.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang D, Biragyn A, Hoover DM, Lubkowski J, Oppenheim JJ. Multiple roles of antimicrobial defensins, cathelicidins, and eosinophil-derived neurotoxin in host defense. Annu Rev Immunol. 2004;22:181–315. doi: 10.1146/annurev.immunol.22.012703.104603. [DOI] [PubMed] [Google Scholar]
- 10.Lillard JW, Jr, Boyaka PN, Chertov O, Oppenheim JJ, McGhee JR. Mechanisms for induction of acquired host immunity by neutrophil peptide defensins. Proc Natl Acad Sci USA. 1999;96:651–6. doi: 10.1073/pnas.96.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang D, Chertov O, Bykovskaia SN, Chen Q, Buffo MJ, Shogan J, et al. β-Defensins: Linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525–8. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- 12.Biragyn A, Ruffini PA, Leifer CA, Klyushnenkova E, Shakhov A, Chertov O, et al. Toll-like receptor 4-dependent activation of dendritic cells by β-defensin 2. Science. 2002;298:1025–9. doi: 10.1126/science.1075565. [DOI] [PubMed] [Google Scholar]
- 13.Kurosaka K, Chen Q, Yarovinsky F, Oppenheim JJ, Yang D. Mouse cathelin-related antimicrobial peptide chemoattracts leukocytes using formyl peptide receptor-like 1/mouse formyl peptide receptor-like 2 as the receptor and acts as an immune adjuvant. J Immunol. 2005;174:6257–65. doi: 10.4049/jimmunol.174.10.6257. [DOI] [PubMed] [Google Scholar]
- 14.Yang D, Chen Q, Su SB, Zhang P, Kurosaka K, Caspi RR, et al. Eosinophil-derived neurotoxin acts as an alarmin to activate the TLR2-MyD88 signal pathway in dendritic cells and enhances Th2 immune responses. J Exp Med. 2008;205:79–90. doi: 10.1084/jem.20062027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rovere-Querini P, Capobianco A, Scaffidi P, Valentinis B, Catalanotti F, Giazzon M, et al. HMGB1 is an endogenous immune adjuvant released by necrotic cells. EMBO Rep. 2004;5:825–30. doi: 10.1038/sj.embor.7400205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Urbonaviciute V, Furnrohr BG, Meister S, Munoz L, Heyder P, De Marchis F, et al. Induction of inflammatory and immune responses by HMGB1-nucleosome complexes: implications for the pathogenesis of SLE. J Exp Med. 2008;205:3007–18. doi: 10.1084/jem.20081165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quintana FJ, Cohen IR. Heat shock proteins as endogenous adjuvants in sterile and septic inflammation. J Immunol. 2005;175:2777–82. doi: 10.4049/jimmunol.175.5.2777. [DOI] [PubMed] [Google Scholar]
- 18.Tewary P, Yang D, de la Rosa G, Li Y, Finn MW, Krensky AM, et al. Granulysin activates antigen-presenting cells through TLR4 and acts as an immune alarmin. Blood. 2010;116:3465–74. doi: 10.1182/blood-2010-03-273953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vogl T, Tenbrock K, Ludwig S, Leukert N, Ehrhardt C, van Zoelen MA, et al. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med. 2007;13:1042–9. doi: 10.1038/nm1638. [DOI] [PubMed] [Google Scholar]
- 20.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–21. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 21.Kool M, Willart MA, van Nimwegen M, Bergen I, Pouliot P, Virchow JC, et al. An unexpected role for uric acid as an inducer of T helper 2 cell immunity to inhaled antigens and inflammatory mediator of allergic asthma. Immunity. 2011;34:527–40. doi: 10.1016/j.immuni.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 22.Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang YH, Homey B, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–9. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 23.Gregorio J, Meller S, Conrad C, Di Nardo A, Homey B, Lauerma A, et al. Plasmacytoid dendritic cells sense skin injury and promote wound healing through type I interferons. J Exp Med. 2010;207:2921–30. doi: 10.1084/jem.20101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flohe SB, Bruggemann J, Lendemans S, Nikulina M, Meierhoff G, Flohe S, et al. Human heat shock protein 60 induces maturation of dendritic cells versus a Th1-promoting phenotype. J Immunol. 2003;170:2340–8. doi: 10.4049/jimmunol.170.5.2340. [DOI] [PubMed] [Google Scholar]
- 25.Yang D, Postnikov YV, Li Y, Tewary P, de la Rosa G, Wei F, et al. High-mobility group nucleosome-binding protein 1 acts as an alarmin and is critical for lipopolysaccharide-induced immune responses. J Exp Med. 2012;209:157–71. doi: 10.1084/jem.20101354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robinson HL, Pertmer TM. Nucleic Acid Immunizations. Curr Protoc Immunol. 2001;27:2.14.1–2..9. doi: 10.1002/0471142735.im0214s27. [DOI] [PubMed] [Google Scholar]
- 27.Palmer DC, Balasubramaniam S, Hanada K, Wrzesinski C, Yu Z, Farid S, et al. Vaccine-stimulated, adoptively transferred CD8+ T cells traffic indiscriminately and ubiquitously while mediating specific tumor destruction. J Immunol. 2004;173:7209–16. doi: 10.4049/jimmunol.173.12.7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore MW, Carbone FR, Bevan MJ. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell. 1988;54:777–85. doi: 10.1016/s0092-8674(88)91043-4. [DOI] [PubMed] [Google Scholar]
- 29.Biragyn A, Surenhu M, Yang D, Ruffini PA, Haines BA, Klyushnenkova E, et al. Mediators of innate immunity that target immature, but not mature, dendritic cells induce antitumor immunity when genetically fused with nonimmunogenic tumor antigens. J Immunol. 2001;167:6644–53. doi: 10.4049/jimmunol.167.11.6644. [DOI] [PubMed] [Google Scholar]
- 30.Hock R, Furusawa T, Ueda T, Bustin M. HMG chromosomal proteins in development and disease. Trends Cell Biol. 2007;17:72–9. doi: 10.1016/j.tcb.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Birger Y, West KL, Postnikov YV, Lim JH, Furusawa T, Wagner JP, et al. Chromosomal protein HMGN1 enhances the rate of DNA repair in chromatin. EMBO J. 2003;22:1665–75. doi: 10.1093/emboj/cdg142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Birger Y, Catez F, Furusawa T, Lim JH, Prymakowska-Bosak M, West KL, et al. Increased tumorigenicity and sensitivity to ionizing radiation upon loss of chromosomal protein HMGN1. Cancer Res. 2005;65:6711–8. doi: 10.1158/0008-5472.CAN-05-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196:1627–38. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma XT, Xu B, An LL, Dong CY, Lin YM, Shi Y, et al. Vaccine with beta-defensin 2-transduced leukemic cells activates innate and adaptive immunity to elicit potent antileukemia responses. Cancer Res. 2006;66:1169–76. doi: 10.1158/0008-5472.CAN-05-2891. [DOI] [PubMed] [Google Scholar]
- 35.Wang L, Rollins L, Gu Q, Chen SY, Huang XF. A Mage3/Heat Shock Protein70 DNA vaccine induces both innate and adaptive immune responses for the antitumor activity. Vaccine. 2009;28:561–70. doi: 10.1016/j.vaccine.2009.09.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang CL, Tsai YC, He L, Wu TC, Hung CF. Cancer immunotherapy using irradiated tumor cells secreting heat shock protein 70. Cancer Res. 2007;67:10047–57. doi: 10.1158/0008-5472.CAN-07-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–9. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 38.Biragyn A, Ruffini PA, Coscia M, Harvey LK, Neelapu SS, Baskar S, et al. Chemokine receptor-mediated delivery directs self-tumor antigen efficiently into the class II processing pathway in vitro and induces protective immunity in vivo. Blood. 2004;104:1961–9. doi: 10.1182/blood-2004-02-0637. [DOI] [PubMed] [Google Scholar]
- 39.Messmer D, Yang H, Telusma G, Knoll F, Li J, Messmer B, et al. High mobility group box protein 1: an endogenous signal for dendritic cell maturation and Th1 polarization. J Immunol. 2004;173:307–13. doi: 10.4049/jimmunol.173.1.307. [DOI] [PubMed] [Google Scholar]
- 40.Kuppner MC, Gastpar R, Gelwer S, Nossner E, Ochmann O, Scharner A, et al. The role of heat shock protein (hsp70) in dendritic cell maturation: hsp70 induces the maturation of immature dendritic cells but reduces DC differentiation from monocyte precursors. Eur J Immunol. 2001;31:1602–9. doi: 10.1002/1521-4141(200105)31:5<1602::AID-IMMU1602>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 41.Yang D, Chen Q, Yang H, Tracey KJ, Bustin M, Oppenheim JJ. High mobility group box-1 protein induces the migration and activation of human dendritic cells and acts as an alarmin. J Leukoc Biol. 2007;81:59–66. doi: 10.1189/jlb.0306180. [DOI] [PubMed] [Google Scholar]
- 42.Wu Z, Hoover DM, Yang D, Boulegue C, Santamaria F, Oppenheim JJ, et al. Engineering disulfide bridges to dissect antimicrobial and chemotactic activities of human b-defensin 3. Proc Natl Acad Sci USA. 2003;100:8880–5. doi: 10.1073/pnas.1533186100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Venereau E, Casalgrandi M, Schiraldi M, Antoine DJ, Cattaneo A, De Marchis F, et al. Mutually exclusive redox forms of HMGB1 promote cell recruitment or proinflammatory cytokine release. J Exp Med. 2012;209:1519–28. doi: 10.1084/jem.20120189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.