Abstract
Rationale:
Myofibroblasts are believed to evolve from precursor cells; however, whether non-cardiomyocyte cardiac cells (NMCCs; i.e., endothelial cells [ECs], smooth muscle cells [SMCs], pericytes, and fibroblasts) that have been derived from human induced-pluripotent stem cells (hiPSCs) can transdifferentiate into myofibroblast-like cells, and if so, whether this process reduces the efficacy of hiPSC-NMCC therapy, is unknown.
Objective:
To determine whether hiPSC-NMCCs can differentiate to myofibroblast-like cells, and whether limiting the transdifferentiation of hiPSC-NMCCs can improve their effectiveness for myocardial repair.
Methods and Results:
When ECs, SMCs, pericytes, and fibroblasts that had been generated from hiPSCs were cultured with transforming growth factor β (TGFβ), the expression of myofibroblast markers increased, while EC-, SMC-, pericyte-, and fibroblast-marker expression declined. TGFβ-associated myofibroblast differentiation was accompanied by increases in the signaling activity of Smad, Snail, and mammalian target of rapamycin. However, measures of pathway activation, proliferation, apoptosis, migration, and protein expression in hiPSC-EC–, -SMC–, -pericyte–, and -fibroblast–derived myofibroblast-like cells differed. Furthermore, when hiPSC-NMCCs were transplanted into the hearts of mice after myocardial infarction, ~21–35% of the transplanted hiPSC-NMCCs expressed myofibroblast markers one week later, compared to <7% of transplanted cells (p<0.01, each cell type) in animals that were treated with both hiPSC-NMCCs and the TGFβ-inhibitor galunisertib. Galunisertib co-administration was also associated with significant improvements in fibrotic area, left-ventricular dilatation, vascular density, and cardiac function.
Conclusions:
hiPSC-NMCCs differentiate into myofibroblast-like cells when cultured with TGFβ or when transplanted into infarcted mouse hearts, and the phenotypes of the myofibroblast-like cells can differ depending on the lineage of origin. TGFβ inhibition significantly improved the efficacy of transplanted hiPSC-NMCCs for cardiac repair, perhaps by limiting the differentiation of hiPSC-NMCCs into myofibroblast-like cells.
Keywords: Heart failure, stem cells, myofibroblasts, fibrosis, transforming growth factor β, Cell Therapy, Fibrosis, Stem Cells
INTRODUCTION
Although myofibroblasts are believed to evolve from precursor cells,1–4 recent studies using fate mapping technologies and engineered mouse models,5, 6 have demonstrated that endothelial cells do not transdifferentiate into fibroblasts or myofibroblasts. Furthermore, whether the phenotypes of specific myofibroblast populations differ depending on the lineage of the cells from which they originated7 and, by extension, whether these potential differences can impact the cells’ role during myocardial recovery, remains unclear.
Human induced-pluripotent stem cells (hiPSCs) are exceptionally useful tools for studies in the life sciences;8 however, hiPSC-derived cells are less mature than their corresponding populations of adult somatic cells and, consequently, may be more likely to transdifferentiate into cells of unwanted lineages after transplantation. If so, this transdifferentiation could contribute to the limited benefits observed in studies of hiPSC-derived cardiac cell therapy. Here, we begin to address this question by characterizing the differentiation of myofibroblast-like cells from non-cardiomyocyte cardiac cells (NMCCs; eg. endothelial cells [ECs], smooth muscle cells [SMCs], pericytes, and fibroblasts) that had been generated from hiPSCs in vitro, and then evaluating the cellular activity and protein-expression profiles of each hiPSC-NMCC–derived myofibroblast-like cell subpopulation. We also tested our hypothesis that the efficacy of cardiac cell therapy in infarcted mouse hearts could be improved by inhibiting the undesirable differentiation of hiPSC-NMCCs into myofibroblast-like cells and preserving the identity of the transplanted hiPSC-ECs, -SMCs, -pericytes, and -fibroblasts.
METHODS
The data that support the findings of this study are available from the first author upon request.
A detailed description of the experimental procedures used in this investigation is provided in the online Data Supplement.
RESULTS
Transforming growth-factor β (TGFβ) induces the differentiation of hiPSC-NMCCs into myofibroblast-like cells.
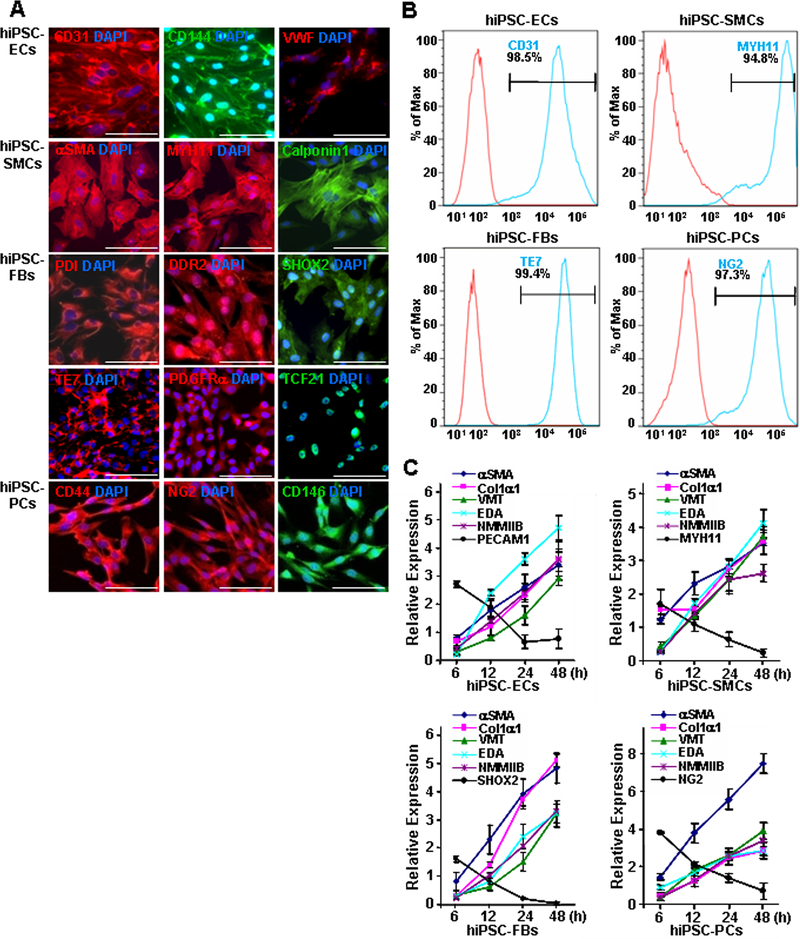
hiPSCs were reprogrammed from mature human cardiac fibroblasts, engineered to express green fluorescent protein (GFP),9 and then differentiated into endothelial cells (ECs),10 smooth muscle cells (SMCs),11 fibroblasts,12, 13 and pericytes14 via established protocols. The lineages of the differentiated cells were confirmed via immunofluorescence analysis of the expression of lineage-marker proteins (ECs: CD31, CD144, and von Willebrand factor [VWF]; SMCs: α smooth muscle actin [αSMA], smooth muscle myosin heavy chain 11 [MYH11], and calponin 1; fibroblasts: protein disulfide isomerase [PDI], discoidin domain receptor 2 [DDR2], short stature homeobox 2 [SHOX2], clone TE7, platelet-derived growth factor receptor alpha [PDGFRα], and transcription factor 21 [TCF21]; pericytes: CD44, neural/glial antigen 2 [NG2], and CD146) (Figure 1A and Online Figure I) and by comparing the results to similar assessments in human umbilical vein endothelial cells (hUVECs), human aortic smooth muscle cells (hASMCs), human dermal fibroblasts (hDFs), and human brain vascular pericytes (hBVPs) (Online Figure II). Flow cytometry analyses of marker expression (ECs: CD31, SMCs: MYH11, fibroblasts: TE7; pericytes: NG2) indicated that each of the differentiated cell populations was >94% pure (Figure 1B), while functional assessments (ECs: tube formation and Dil-conjugated acetylated low-density lipoprotein uptake, SMCs: contractile response to carbachol and gel contraction assay, fibroblasts: wound healing and adhesion, pericytes: osteogenic differentiation and migration) in the hiPSC-derived cells and their corresponding somatic cell lines were comparable (Online Figure III).
Figure 1. hiPSC-derived non-cardiomyocyte cardiac cells (hiPSC-NMCCs) were differentiated into myofibroblast-like cells (MFLCs).
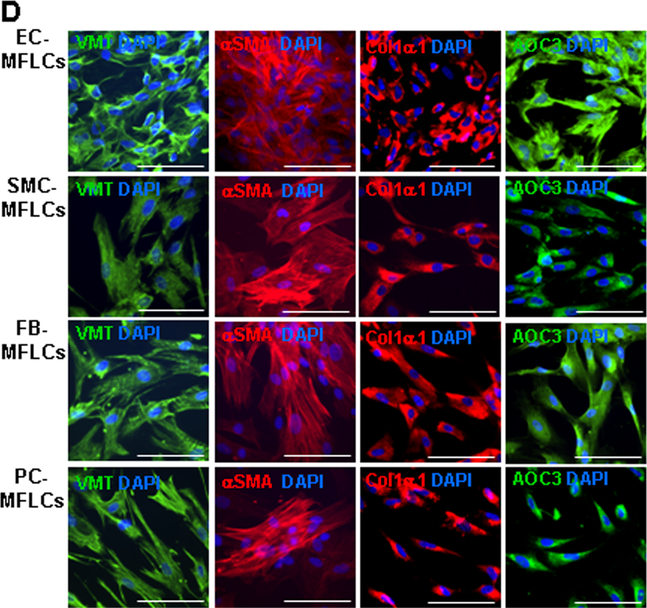
(A) hiPSCs were differentiated into endothelial cells (ECs), smooth-muscle cells (SMCs), fibroblasts (FBs), and pericytes (PCs); then, the lineages of the differentiated cells were confirmed via immunofluorescence analysis of CD31, CD144, and VWF in hiPSC-ECs; αSMA, MYH11, and calponin1 in hiPSC-SMCs; PDI, DDR2, SHOX2, TE7, PDGFRα, and TCF21 in hiPSC-fibroblasts; and CD44, NG2, and CD146 in hiPSC-pericytes. Nuclei were counterstained with DAPI (Bar=100 μm). (B) The purity of the hiPSC-derived NMCCs was determined via flow-cytometry analysis of CD31 (blue) expression for hiPSC-ECs, MYH11 expression (blue) for hiPSC-SMCs, TE7 expression (blue) for hiPSC-fibroblasts, and NG2 expression (blue) for hiPSC-pericytes. Control assessments (red) were performed with non-specific antibodies of the same class and type. (C) hiPSC-NMCCs were differentiated into MFLCs over a 48-hour period, and mRNA levels of the myofibroblast markers αSMA, Col1α1, VMT, NMMIIB, and EDA and of markers for the cells’ lineages of origin (ECs: PECAM1; SMCs MYH11; fibroblasts: SHOX2; pericytes: NG2) were measured at the indicated time points via quantitative RT-PCR. (D) MFLCs differentiated from hiPSC-ECs (EC-MFLCs), -SMCs (SMC-MFLCs), -fibroblasts (FB-MFLCs), and pericytes (PC-MFLCs) were immunofluorescently stained for expression of the myofibroblast markers VMT, αSMA, Col1α1, and AOC3; nuclei were counterstained with DAPI (bar=100 μm). VWF: von Willebrand factor, αSMA: α smooth-muscle actin, MYH11: smooth muscle myosin heavy chain 11, PDI: protein disulfide isomerase, DDR2: discoidin domain receptor 2, SHOX2: short-stature homeobox 2, TE7: fibroblast marker clone TE7, PDGFRα: platelet-derived growth factor receptor alpha, TCF21: transcription factor 21, NG2: neural/glial antigen 2, Col1α1: collagen 1α1, VMT: vimentin, NMMIIB: non-muscle myosin IIB, EDA: fibronectin extra domain A, PECAM1 (CD31): platelet and endothelial cell adhesion molecule 1, AOC3: amine oxidase copper-containing 3.
The non-cardiomyocyte cell populations were differentiated into myofibroblast-like cells by culturing them on Matrigel-coated plates with transforming growth-factor β1 (TGFβ1).15–18 Quantitative RT-PCR (qRT-PCR) analyses indicated that the mRNA levels of EC-, SMC-, fibroblast-, and pericyte-specific markers (platelet and endothelial cell adhesion molecule 1 [PECAM1, also known as CD31], MYH11, SHOX2, and NG2 respectively) declined during the 48-hour culture period, while mRNA levels of myofibroblast markers (αSMA, type I collagen α1 [Col1α1], vimentin [VMT], non-muscle myosin IIB [NMMIIB], and fibronectin extra domain A [EDA]) increased (Figure 1C). Upon completion of the myofibroblast differentiation protocol, immunofluorescence assessments confirmed that the cells were morphologically similar to myofibroblasts, as defined previously,19 and expressed the myofibroblast marker proteins VMT, αSMA, Col1α1, and amine oxidase copper-containing 3 (AOC3) (Figure 1D).
Activity and protein expression of hiPSC-EC–, -SMC–, -pericyte–, and -fibroblast–myofibroblast-like cells can vary depending on the hiPSC-NMCC lineage.
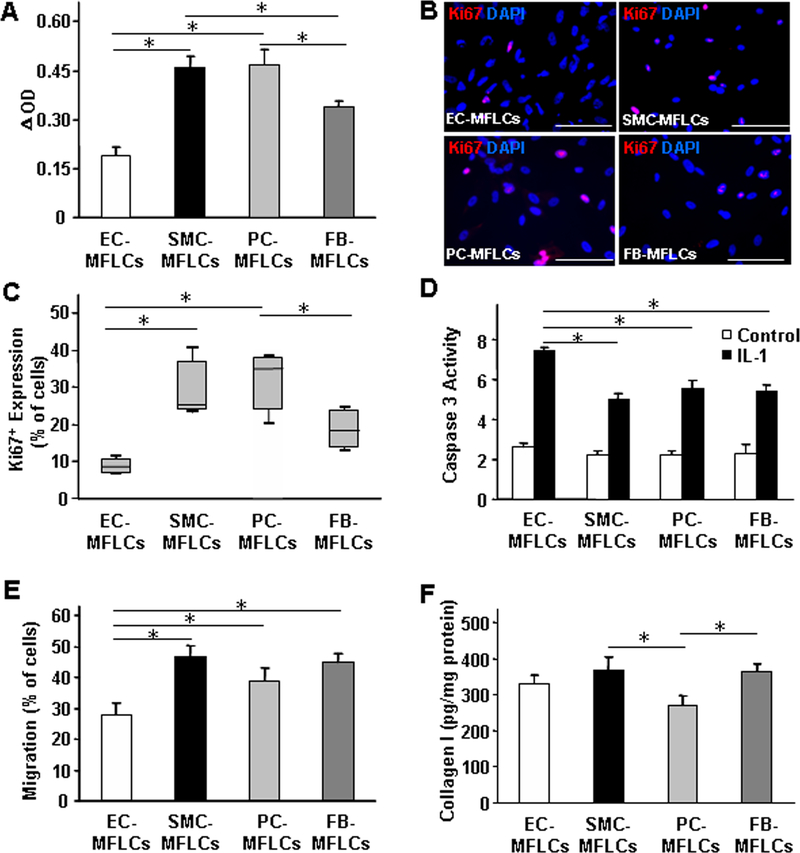
Assessments in cultured hiPSC-NMCC–myofibroblast-like cells indicated that some cellular functions varied depending on the NMCC lineage. When evaluated via measurements of optical density, proliferation was significantly greater in hiPSC-SMC–myofibroblast-like cells and -pericyte–myofibroblast-like cells than in hiPSC-EC–myofibroblast-like cells and -fibroblast–myofibroblast-like cells (Figure 2A), while expression of the proliferation marker Ki67 was significantly greater in both hiPSC-SMC–myofibroblast-like cells and -pericyte–myofibroblast-like cells than in hiPSC-EC–myofibroblast-like cells, and in hiPSC-pericyte–myofibroblast-like cells than in hiPSC-fibroblast–myofibroblast-like cells (Figure 2B–2C). Measurements of apoptosis (interleukin-1 [IL-1] induced caspase 3 activity) (Figure 2D) were significantly higher, and of cell migration (Figure 2E) were significantly lower, in hiPSC-EC–myofibroblast-like cells than in hiPSC-SMC–, -pericyte–, and -fibroblast–myofibroblast-like cells while collagen I production was lower in myofibroblast-like cells differentiated from hiPSC-pericytes than in myofibroblast-like cells from the other three hiPSC-NMCC lineages (Figure 2F).
Figure 2. Properties of hiPSC-NMCC–myofibroblast-like cells (MFLCs) can vary depending on NMCC lineage.
(A) hiPSC-NMCC–MFLCs (i.e., EC-MFLCs, SMC-MFLCs, pericyte [PC]-MFLCs, and fibroblast [FB]-MFLCs) were cultured for 16 hours, and proliferation was calculated as the difference between optical density measurements (ΔOD) taken before and after the culture period. (B) hiPSC-NMCC–MFLCs were cultured for 12 hours and stained for expression of Ki67 (bar=100 μm); (C) then, the percentage of Ki67-positive cells was quantified. (D) hiPSC-NMCC–MFLCs were cultured with 6 ng/mL IL-1 for 16 hours, and then apoptosis was evaluated by measuring caspase 3 activity with a cell apoptosis kit. (E) hiPSC-NMCC–MFLCs (2104) were seeded onto one surface of a gelatin-coated plate; 24 hours later, the number of cells that had migrated to the other surface of the gelatin was determined and presented as a percentage of the total number of seeded cells. (F) hiPSC-NMCC–MFLCs (5105) were cultured for 24 hours in 12 well plates and lysed; then, the concentration of collagen I in the lysate was measured. *P<0.05, **P<0.01; One-way ANOVA followed by Tukey post-hoc test for A, D, E, F; Kruskal-Wallis followed by Dunn post-hoc test for C. n=3–4 independent experiments.
TGFβ binds to serine-threonine kinase receptors at the cell surface, which activates the intracellular Smad/Snail signaling cascade, and TGF-β/Smad3 signaling has been shown to promote collagen production20 during fibrogenesis by activating mammalian target of rapamycin (mTOR).21, 22 Thus, we investigated the mechanisms responsible for the TGFβ1-induced differentiation of hiPSC-NMCCs into myofibroblast-like cells by comparing Smad, Snail, and mTOR levels in differentiating hiPSC-EC–, -SMC–, -pericyte–, and -fibroblast–myofibroblast-like cells. Western blot and qRT-PCR analyses indicated that TGFβ1 significantly increased Snail1 levels in differentiating hiPSC-EC–, -pericyte–, and -fibroblast–myofibroblast-like cells (Online Figure IVA), mTOR levels during hiPSC-EC–, -SMC–, and -pericyte–myofibroblast-like cell differentiation (Online Figure IVB), Smad3 levels during hiPSC-EC– and hiPSC-SMC–myofibroblast-like cell differentiation (Online Figure IVC), and phosphorylated Smad3 levels during hiPSC-EC–, -SMC–, -pericyte–, and -fibroblast–myofibroblast-like cell differentiation (Online Figure IVD), but not in the presence of an anti-TGFβ antibody or galunisertib, which inhibits the TGFβ receptor (Online Figure IV).
Protein expression in hiPSC-EC–, -SMC–, -pericyte–, and -fibroblast–myofibroblast-like cells was evaluated via isobaric tagging for relative and absolute quantification (iTRAQ) liquid chromatography tandem-mass spectrometry (LC-MS/MS). Quantifiable data suitable for statistical analysis (n=2 or more) was available for 2296 proteins, and the expression of a number of proteins involved in fibrosis and cellular remodeling (Online Figure VA), proliferation (Online Figure VB), and apoptosis (Online Figure VC) varied significantly across the four hiPSC-NMCC–lineage myofibroblast-like cell populations. Thus, the activity and protein expression of TGFβ-induced hiPSC-EC–, -SMC–, -pericyte–, and -fibroblast–myofibroblast-like cells may vary somewhat depending on the lineage of the hiPSC-NMCCs.
Pathways involved in integrin signaling, endocytosis, and remodeling are activated in hiPSC-EC–, -SMC–, -pericyte–, and -fibroblast–myofibroblast-like cells.
A total of 701 proteins were expressed at significantly higher or lower levels in hiPSC-NMCC–myofibroblast-like cells than in the hiPSC-NMCCs from which they were differentiated (Online Table I). When evaluated with Ingenuity Pathway Analysis software (Online Figure VI), the most significant differences were observed for pathways involved in integrin-, actin-cytoskeletal–, and agrin-signaling (remodeling), which is consistent with the roles of myofibroblasts in fibrosis and injury repair. The most significantly different cellular functions were growth and proliferation, death and survival, and movement, which are consistent with the results from our in vitro functional analyses, and the top disease/disorder categories were organismal injury and abnormalities, infectious response, and cancer (Online Table II). Notably, actin-cytoskeletal–signaling and agrin-signaling appeared to be more and less strongly associated, respectively, with hiPSC-EC–myofibroblast-like cells than with the other hiPSC-NMCC–myofibroblast-like cell lineages.
Protein secretion by hiPSC-EC–, -SMC–, -pericyte–, and -fibroblast–myofibroblast-like cells is partially dependent on the hiPSC-NMCC lineage.
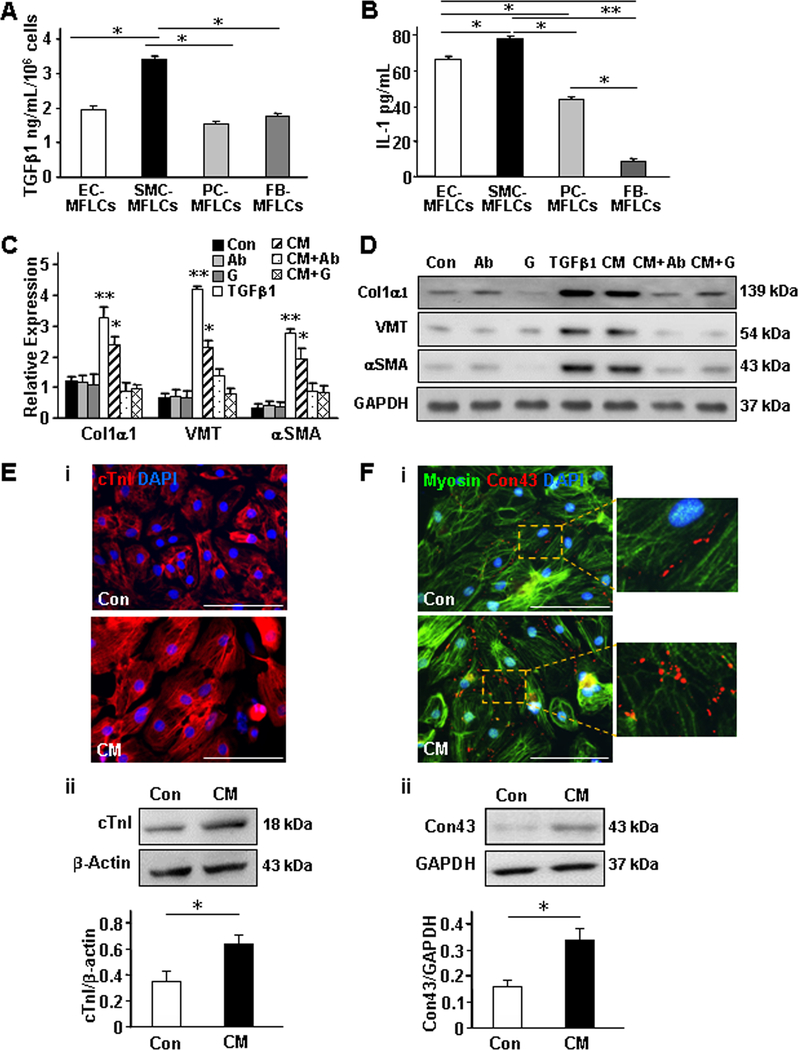
Proteins secreted into the medium of cultured hiPSC-NMCC–myofibroblast-like cells were collected and evaluated via orbitrap mass spectrometry (Online Table III) and enzyme-linked immunosorbent assay (ELISA). Both TGFβ1 (Figure 3A) and IL-1 (Figure 3B) levels were significantly greater in hiPSC-SMC–myofibroblast-like cell medium than in medium from the other three hiPSC-NMCC–myofibroblast-like cells, while IL-1 levels were significantly higher in hiPSC-pericyte–myofibroblast-like cell medium than in hiPSC-fibroblast–myofibroblast-like cell medium and in hiPSC-EC–myofibroblast-like cell medium than in either hiPSC-pericyte– or -fibroblast–myofibroblast-like cell medium. Furthermore, the medium from hiPSC-SMC–myofibroblast-like cells induced hiPSC-ECs, -SMCs, -pericytes, and -fibroblasts to express Col1α1, VMT, and αSMA, but not in the presence of the anti-TGFβ antibody or galunisertib, and even higher expression levels were achieved when the cells were treated with TGFβ1 (Figures 3C and 3D). Conditioned medium from one or more lineages of hiPSC-NMCC–myofibroblast-like cells also promoted the expression of cardiac troponin I (cTnI) (Figure 3E) and connexin 43 (Con43) (Figure 3F) in cultured hiPSC-derived cardiomyocytes, enhanced both migration and cytokine expression (e.g., IL-1 and vascular endothelial growth factor [VEGF]) in macrophages (Online Figure VII); and stimulated the production of Col1α1, VMT, and αSMA in hiPSC-NMCCs (Online Figure VIII). Collectively, these results suggest that factors secreted by the hiPSC-NMCC–myofibroblast-like cells promoted the growth and maturation of cardiomyocytes, the inflammatory response in macrophages, and other reparative functions in hiPSC-NMCCs.
Figure 3. Conditioned medium from cultured hiPSC-SMC–myofibroblast-like cells (MFLCs) alters protein expression in cultured hiPSC-NMCCs and cardiomyocytes.
(A-B) The media of cultured hiPSC-EC–, -SMC–, -pericyte (PC)–, and -fibroblast (FB)–MFLCs was collected, and (A) TGFβ1 and (B) IL-1 levels were evaluated via ELISA. (C-D) A combined population of hiPSC-ECs, -SMCs, -pericytes, and -fibroblasts was cultured with TGFβ1, with an anti-TGFβ antibody (Ab), with the TGFβ-receptor 1-blocker galunisertib (G), with conditioned medium from hiPSC-SMC–MFLCs (CM), with CM and an anti-TGFβ antibody (CM+Ab), with CM and galunisertib (CM+G), or under standard conditions (Con); then, Col1α1, VMT, and αSMA (C) mRNA and (D) protein levels in the medium from the cultured cells were evaluated via quantitative RT-PCR and Western blot, respectively. (E-F) hiPSC-derived cardiomyocytes were cultured with CM from hiPSC-SMC–MFLCs or under standard conditions; then, (E) Cardiac troponin I (cTnI) and (F) connexin 43 (Con43) expression were evaluated via (i) immunofluorescence and (ii) Western blot. Nuclei were counterstained with DAPI, and Western blots of β-actin or GAPDH levels were evaluated to confirm equal loading. Bar=100 μm; *P<0.05, **P<0.01 versus Control, CM+AB, and CM+G; One-way ANOVA followed by Tukey post-hoc test for A, B, C; Two-tailed Student’s t-test for E, F. n=3–4 independent experiments.
hiPSC-NMCCs can differentiate into myofibroblast-like cells after transplantation into infarcted mouse hearts.
Because the hiPSC-derived ECs, SMCs, pericytes, and fibroblasts readily differentiated into myofibroblast-like cells after treatment with TGFβ1 in vitro, we investigated whether they also differentiate into myofibroblast-like cells after transplantation into infarcted mouse hearts. Myocardial infarction (MI) was surgically induced by ligating the left anterior descending coronary artery; then, animals in the MI+EC group, the MI+SMC group, the MI+PC group, and the MI+FB group were treated with hiPSC-ECs, -SMCs, -pericytes, and -fibroblasts, respectively, and animals in the MI+EC+G, MI+SMC+G, MI+PC+G, and MI+FB+G groups were treated with the indicated hiPSC-NMCCs and the TGFβ receptor 1 inhibitor galunisertib. The cells (3105 per mouse) were injected 15 minutes after MI induction into three sites of the heart (1×105 cells per injection site); one site was located in the infarcted region, and two were located in the region surrounding the infarct. Galunisertib (75 mg/kg per day) was administered orally from the day of MI induction through day 7 after MI, when the animals were sacrificed.
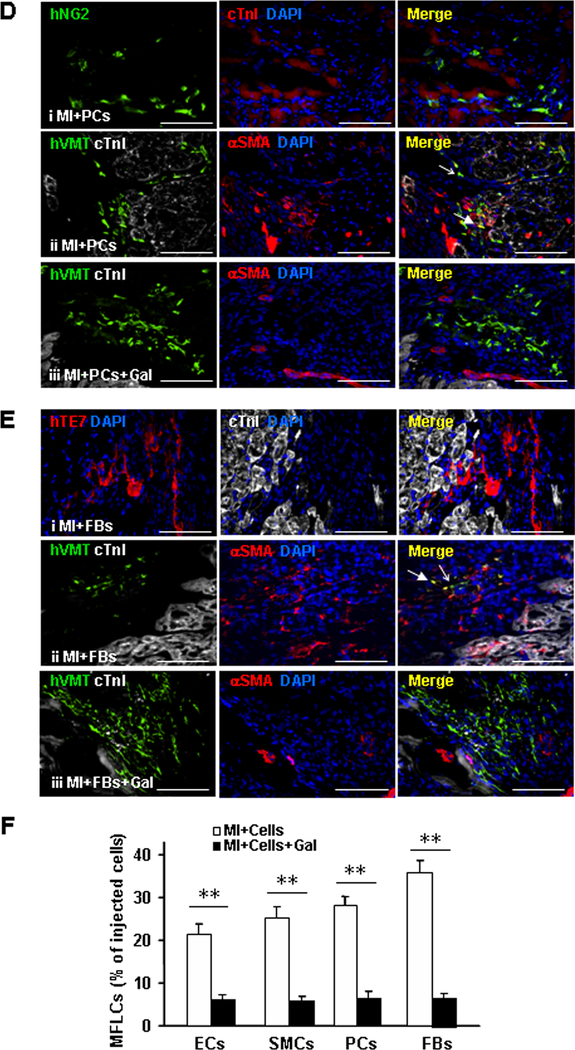
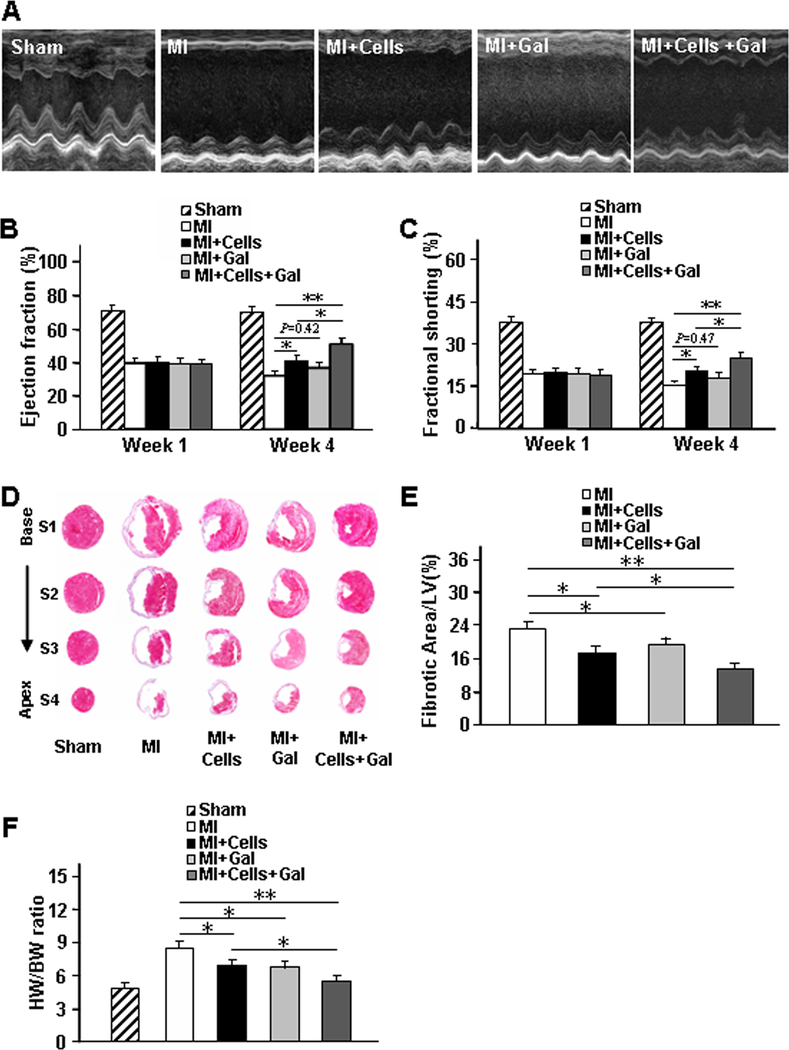
Sections from animals in the MI+EC and MI+EC+G groups were stained for expression of the human CD31 variant (hCD31) to identify hiPSC-ECs that had retained their EC lineage and for the co-expression of human vimentin (hVMT) and αSMA to identify hiPSC-ECs that had differentiated into myofibroblast-like cells (Figures 4A and 4B). Similarly, hiPSC-SMCs that had retained their SMC lineage after transplantation were identified by the co-expression of human calponin 1 (hCalp1) and MYH11 or by hCalp1 expression in the absence of hVMT expression (Figures 4A and 4C), while hiPSC-pericytes (Figures 4A and 4D) and hiPSC-fibroblasts (Figures 4A and 4E) that had retained their lineages were identified by the expression of human NG2 (hNG2) and human TE7 (hTE7), respectively; cells that had differentiated into myofibroblast-like cells were identified via the co-expression of hVMT and αSMA. The proportion of hiPSC-NMCCs that differentiated into myofibroblast-like cells ranged from ~21% to ~35% in the MI+EC, MI+SMC, MI+PC, and MI+FB groups (Figure 4F); however, less than 7% of the transplanted cells differentiated into myofibroblast-like cells in the groups that were administered galunisertib (Figure 4B–4F). Evidence of the differentiation of hiPSC-NMCCs into myofibroblast-like cells was also observed in swine hearts: the animals were injected with GFP-labelled hiPSC-SMCs and -ECs after experimentally induced MI, and a number of the cells in sections collected four weeks after treatment co-expressed hVMT and αSMA or hCalp1 and VMT (Online Figure IX). Thus, a substantial proportion of hiPSC-NMCCs differentiated into myofibroblast-like cells after transplantation into infarcted hearts, but this conversion appeared to be attenuated via the blockade of TGFβ activity.
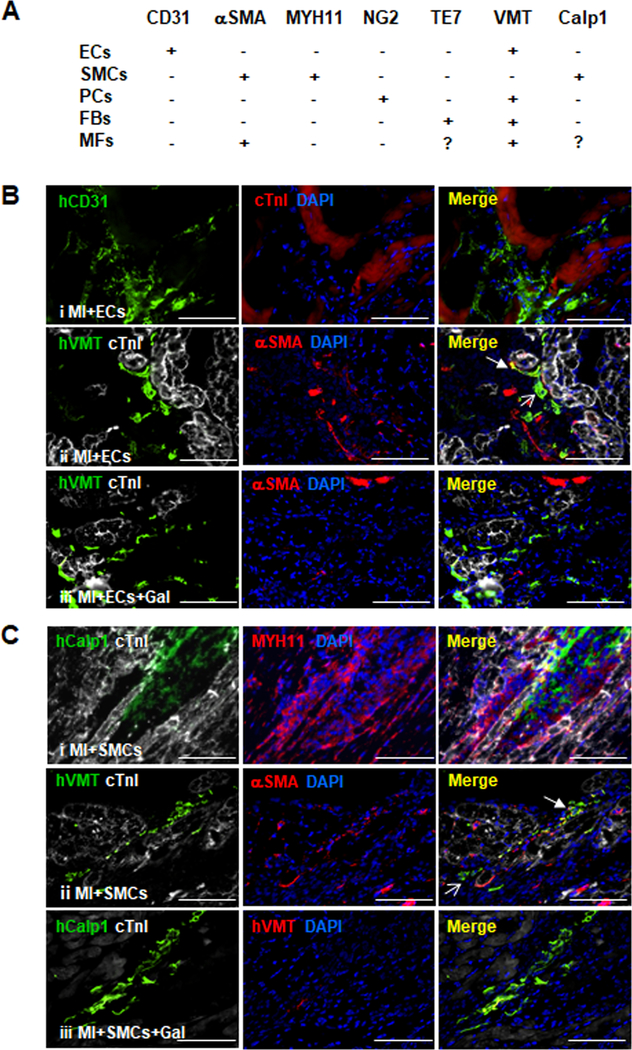
Figure 4. hiPSC-NMCCs can differentiate into myofibroblast-like cells after transplantation into infarcted.
mouse hearts. (A) Marker expression is summarized for ECs, SMCs, pericytes (PCs), fibroblasts (FBs), and myofibroblasts (MFs). (B-F) MI was surgically induced in mice; then, animals were treated with hiPSC-ECs, -SMCs, -pericytes, or -fibroblasts (i.e., the MI+ECs, MI+SMCs, MI+PCs, or MI+FBs groups, respectively) or with hiPSC-ECs, -SMCs, -pericytes, or -fibroblasts and the TGFβ1 inhibitor galunisertib. 7 days later, the mice were sacrificed, and immunofluorescence analyses of marker expression were performed in sections from the site of cell administration. (B) Sections from the MI+ECs group were stained for expression of the human CD31 isoform (hCD31), cTnI, the human isoform of vimentin (hVMT), and αSMA; (C) sections from the MI+SMCs group were stained for expression of the human isoform of calponin 1 (hCalp1), MYH11, hVMT, cTnI, and αSMA; (D) sections from the MI+PCs group were stained for expression of the human isoform of neural/glial antigen 2 (hNG2), hVMT, cTnI, and αSMA; and (E) sections from the MI+FBs group were stained for expression of the human isoform TE7 (hTE7), hVMT, cTnI, and αSMA. Nuclei were counter-stained with DAPI. Examples of cells that continued to express markers of the injected cell lineages or that differentiated to myofibroblasts are identified with open- and closed-headed arrows, respectively. (F) The proportion of hiPSC-derived cells that expressed myofibroblast markers was determined for animals treated with hiPSC-ECs, -SMCs, -pericytes, and -fibroblasts alone (MI+Cells) or with both the hiPSC-derived cells and galunisertib (MI+Cells+Gal) and presented as a percentage (n=6 sections per mouse, 5 mice per group, a minimum of 800 cells from each group were counted). Bar=100 μm; **P<0.01. Two-tailed Student’s t-test for F.
TGFβ inhibition limits the differentiation of hiPSC-NMCCs into myofibroblast-like cells in vivo and improves the potency of transplanted hiPSC-NMCCs for myocardial recovery.
One of the primary goals of hiPSC-NMCC transplantation after MI is to limit the size of the infarct by providing cellular components that can contribute directly to angiogenesis and other processes involved in myocardial repair. Thus, the differentiation of transplanted hiPSC-NMCCs into myofibroblast-like cells likely limits the cells’ therapeutic potency. Furthermore, although TGFβ inhibition during the early stage of recovery from MI has been associated with increases in mortality and left ventricular remodeling,23, 24 inhibition during the later stages appears to reduce fibrosis,25 so we investigated whether combining hiPSC-NMCC transplantation with galunisertib administration at Week 1 after MI would improve measures of cardiac function, fibrosis, hypertrophy, and vessel density. Experiments were conducted in mice that had been treated with hiPSC-NMCCs (0.75105 of each of the four cell types) and galunisertib both alone (i.e., the MI+Cells and MI+Gal groups, respectively) and in combination (the MI+Cells+Gal group), or neither experimental treatment (i.e., the MI group); the dose of galunisertib was chosen to be sufficient for inhibiting hiPSC-NMCCs–myofibroblast-like cell differentiation. A fifth group of animals underwent all surgical procedures for MI induction except coronary artery ligation and recovered without either experimental treatment (the Sham group).
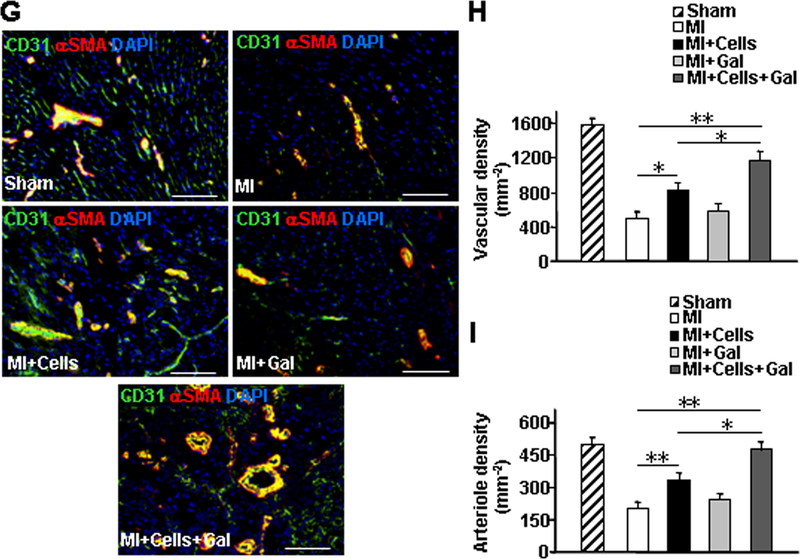
Echocardiographic assessments (Figure 5A) of left-ventricular ejection fraction (EF) (Figure 5B) and fractional shortening (FS) (Figure 5C) were significantly greater in the MI+Cells group than in MI animals, and in the MI+Cells+Gal group than in either MI+Cells or MI animals, at week 4. Furthermore, measurements of fibrotic area (Figure 5D and 5E), heart-weight to bodyweight ratio (HW/BW) (Figure 5F), total vascular density (Figure 5G and 5H), and arteriole density (Figure 5G and 5I) were significantly better after treatment with both hiPSC-NMCCs and galunisertib than after treatment with hiPSC-NMCCs alone, and in the MI+Cells group than in MI animals, while fibrotic areas and HW/BW ratios, but no other measured parameter, were significantly better in the MI+Gal group than in the MI group. Notably, the engraftment rate in MI+Cells and MI+Cells+Gal animals were similar (Online Figure X), indicating that the difference in recovery cannot be attributed to a TGFβ-induced improvement in engraftment. Thus, the effectiveness of transplanted hiPSC-ECs, -SMCs, -pericytes, and -fibroblasts for treatment of MI in mice appeared to improve when a TGFβ receptor inhibitor was used to prevent the transplanted cells from differentiating into myofibroblast-like cells.
Figure 5. TGFβ inhibition limits the differentiation of transplanted hiPSC-NMCCs into myofibroblast-like cells and improves cardiac functional recovery and vascularity in hiPSC-NMCC–treated animals.
MI was surgically induced in mice; one week later, the mice were randomly divided into four experimental groups: the MI, MI+Cells, MI+Gal, and MI+Cells+Gal groups. Animals in the MI+Cells group were treated with intramyocardial injections of hiPSC-ECs, -SMCs, -pericytes, and -fibroblasts (0.75×105 of each of the four cell types), animals in the MI+Gal group were treated with the TGFβ receptor 1 blocker galunisertib, animals in the MI+Cells+Gal group were treated with both the hiPSC-derived cells and galunisertib, and animals in the MI group recovered without either of the experimental treatments. The Sham group underwent all surgical procedures for MI induction except the ligation step and recovered without either experimental treatment. (A-C) Cardiac function was evaluated 1 and 4 weeks after MI via (A) echocardiographic assessments of (B) left ventricular ejection fraction and (C) fractional shortening. (D) Sections of hearts harvested from each group of animals were Masson-trichrome-stained for histological assessments; (E) then, fibrotic area was assessed and reported as a percentage of the total area of the left ventricle. (F) The hypertrophic response to MI injury was evaluated by determining the heart weight/bodyweight (HW/BW) ratio. (G-I) Sections from the border-zone of ischemia in the hearts were (G) immunofluorescently stained for the presence of CD31 and αSMA and nuclei were counterstained with DAPI (bar=100 μm); then, (H) vascular density was determined by quantifying the expression of CD31, and (I) arteriole density was determined by quantifying the co-expression of CD31 and αSMA. Data displayed in panels D-I were obtained from animals sacrificed four weeks after MI. *P<0.05, **P<0.01; One-way ANOVA followed by Tukey post-hoc test for B, C, E, F, H, I. n=8–10 mice per group, 6 sections per mouse.
DISCUSSION
Over the last two decades, numerous preclinical studies have reported that the functional benefits of cardiac cell therapy are mediocre and accompanied by an extremely low rate of cell engraftment. Here, we investigated whether the limited potency of hiPSC-derived cells for cardiac repair may be at least partially attributable to their transdifferentiation into myofibroblast-like cells after transplantation. The primary findings of our current investigation were that 1) hiPSC-derived NMCCs (hiPSC-ECs, -SMCs, -pericytes, and -fibroblasts) transdifferentiate into myofibroblast-like cells when exposed to TGFβ in culture and when transplanted into infarcted mouse hearts, and 2) inhibiting hiPSC-NMCC–myofibroblast-like cell transdifferentiation via oral administration of the TGFβ receptor inhibitor galunisertib improves the therapeutic potency of transplanted hiPSC-NMCCs for myocardial repair by attenuating the unwanted transdifferentiation of hiPSC-derived cells.
In response to myocardial injury, myofibroblasts regulate and contribute to the synthesis and secretion of ECM components (collagens, fibronectin and laminin), as well as the production of proteins and metalloproteinases (MMPs) that are involved in fibrosis26, 27 which, some evidence suggests,28 may be a direct response to the ischemic event itself, rather than a secondary response to the loss of functioning cardiomyocytes. Although the origins of the myofibroblasts that participate in myocardial repair remain somewhat unclear,2, 3, 29, 30 a number of recent studies2, 5, 6 have conclusively demonstrated (via lineage tracing and other techniques) that few, if any, endogenous non-myocyte cardiac cells transdifferentiate into fibroblasts or myofibroblasts. Thus, our observation that 21%−35% of hiPSC-NMCCs transdifferentiate into myofibroblast-like cells after transplantation does not imply that endogenous cardiac cells also transdifferentiate in response to myocardial injury; hiPSC-derived cells tend to be less mature than the cells in adult tissues and, consequently, may be more susceptible than endogenous cells to changes in cell identity.
TGFβ contributes to tumor growth, inflammatory fibrosis, and tissue repair by promoting cell growth and differentiation, inducing the epithelial-to-mesenchymal transition, modulating angiogenesis and stromal ECM production, and regulating immune surveillance.31 Thus, galunisertib, which inhibits TGFβ receptor 1, has been evaluated as a treatment for cancer, as well as fibrosis of the liver, lung, and other organs,32, 33 and has been shown to reduce fibroblast proliferation, the production of fibrotic ECM proteins34 and immune-cell infiltration.35 TGFβ also has a role in myocardial infarction, cardiac remodeling, and fibrosis,23, 36 but the effect of TGFβ inhibition on myocardial function after MI is debatable and appears to be time dependent. During the early stage of recovery, TGFβ inhibition has been associated with declines in cardiac function, increases in mortality, and the exacerbation of left ventricular remodeling,24 whereas inhibition during later stages appears to reduce fibrosis.25 In the present study, TGFβ inhibition with galunisertib effectively improved the efficacy of cell therapy, likely by decreasing the unwanted differentiation of the transplanted hiPSC-NMCCs.
The results from these earlier studies are consistent with those in our current report, because the improvements in measures of cardiac function, remodeling, fibrotic area, and vascularity achieved by adding galunisertib to hiPSC-NMCC therapy was observed in animals treated one week (rather than a few minutes) after MI induction. Furthermore, although the low dose of galunisertib used here was sufficient to prevent the unwanted transdifferentiation of transplanted hiPSC-NMCCs, it was not associated with any obvious side effects over the 3 weeks of this study. However, the broad and complex role of TGFβ signaling in the infarcted heart is difficult to evaluate in experiments with hiPSCs, because TGFβ is required for maintaining the pluripotency of human stem cells37, 38 and, consequently, cannot be knocked out of hiPSCs before the cells are differentiated, while pharmacological agents used to inhibit TGFβ signaling can have off-target effects. Thus, a more thorough investigation of the involvement of TGFβ signaling in differentiating hiPSC-NMCCs and in the regenerative potency of hiPSC-derived cells is warranted with hiPSCs carrying a conditional TGFβ-knockout mutation.
Although the results from our study convincingly demonstrate that hiPSC-NMCCs can transdifferentiate into myofibroblast-like cells when treated with TGFβ in vitro or after transplantation into infarcted mouse hearts, we have not shown that endogenous cardiac cells undergo changes in cell identity after MI, and our experiments do not address whether the differentiation of hiPSC-NMCCs into myofibroblast-like cells contributes to pathological fibrosis.
Conclusion.
The results presented here demonstrate that hiPSC-derived ECs, SMCs, pericytes, and fibroblasts transdifferentiated into myofibroblast-like cells when cultured with TGFβ or when transplanted into infarcted mouse hearts. Furthermore, the differentiation of transplanted hiPSC-NMCCs into myofibroblast-like cells was attenuated by inhibiting TGFβ signaling, and measurements of infarct size and cardiac function in a murine MI model were significantly better in animals treated with both hiPSC-NMCCs and a TGFβ inhibitor than with hiPSC-NMCCs alone.
Supplementary Material
NOVELTY AND SIGNIFICANCE
What Is Known?
Myofibroblasts are believed to evolve from precursor cells; however, their origins remain somewhat unclear, and recent studies using fate mapping technologies and engineered mouse models have demonstrated that endothelial cells do not transdifferentiate into fibroblasts or myofibroblasts.
Non-cardiomyocyte cardiac cells (NMCCs; e.g., endothelial cells, smooth muscle cells, pericytes, and fibroblasts) derived from human induced-pluripotent stem cells (hiPSCs) are less mature than their corresponding populations of adult somatic cells and, consequently, may transdifferentiate into cells of unwanted lineages after transplantation.
What New Information Does This Article Contribute?
hiPSC-NMCCs can differentiate into myofibroblast-like cells.
Limiting the differentiation of transplanted hiPSC-NMCCs can improve their effectiveness for myocardial repair.
Here, we show that hiPSC-NMCCs differentiate into myofibroblast-like cells when cultured with transforming growth factor β (TGFβ) or when transplanted into infarcted mouse hearts, and that the phenotypes of the myofibroblast-like cells can differ depending on the lineage of origin. Our results also demonstrate that TGFβ inhibition significantly improved the efficacy of transplanted hiPSC-NMCCs for cardiac repair, perhaps by limiting the unwanted differentiation of hiPSC-NMCCs into myofibroblast-like cells.
ACKNOWLEDGMENTS
The authors would like to thank W. Kevin Meisner, PhD, ELS, for editorial assistance, and Yanwen Liu, PhD, for excellent technical assistance.
SOURCES OF FUNDING
This work was supported by the following funding sources: NIH RO1 HL 95077, HL114120, HL 131017, HL 138023, UO1 HL134764.
Nonstandard Abbreviations and Acronyms:
- NMCCs
non-cardiomyocyte cardiac cells
- ECs
endothelial cells
- SMCs
smooth muscle cells
- ECM
extracellular matrix
- MMPs
metalloproteinases
- TGFβ
transforming growth-factor β
- hiPSC
human induced-pluripotent stem cells
- αSMA
α smooth muscle actin
- Col1α1
type I collagen α1
- IL-1
interleukin-1
- mTOR
mammalian target of rapamycin
- VEGF
vascular endothelial growth factor
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Crawford JR, Haudek SB, Cieslik KA, Trial J and Entman ML. Origin of developmental precursors dictates the pathophysiologic role of cardiac fibroblasts. J Cardiovasc Transl Res. 2012;5:749–59. doi: 10.1007/s12265–012-9402–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alex L and Frangogiannis NG. The Cellular Origin of Activated Fibroblasts in the Infarcted and Remodeling Myocardium. Circulation research. 2018;122:540–542. doi: 10.1161/CIRCRESAHA.118.312654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kramann R, Schneider RK, DiRocco DP, Machado F, Fleig S, Bondzie PA, Henderson JM, Ebert BL and Humphreys BD. Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell stem cell. 2015;16:51–66. doi: 10.1016/j.stem.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.LeBleu VS, Taduri G, O’Connell J, Teng Y, Cooke VG, Woda C, Sugimoto H and Kalluri R. Origin and function of myofibroblasts in kidney fibrosis. Nat Med. 2013;19:1047–53. doi: 10.1038/nm.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y, He L, Huang X, Issa Bhaloo S, Zhao H, Zhang S, Pu W, Tian X, Li Y, Liu Q, Yu W, Zhang L, Liu X, Liu K, Tang J, Zhang H, Cai D, Adams RH, Xu Q, Lui KO and Zhou B. Genetic Lineage Tracing of Non-Myocyte Population by Dual Recombinases. Circulation. 2018. doi: 10.1161/CIRCULATIONAHA.118.034250. [DOI] [PubMed] [Google Scholar]
- 6.Moore-Morris T, Cattaneo P, Guimaraes-Camboa N, Bogomolovas J, Cedenilla M, Banerjee I, Ricote M, Kisseleva T, Zhang L, Gu Y, Dalton ND, Peterson KL, Chen J, Puceat M and Evans SM. Infarct Fibroblasts Do Not Derive From Bone Marrow Lineages. Circulation research. 2018;122:583–590. doi: 10.1161/CIRCRESAHA.117.311490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Micallef L, Vedrenne N, Billet F, Coulomb B, Darby IA and Desmouliere A. The myofibroblast, multiple origins for major roles in normal and pathological tissue repair. Fibrogenesis Tissue Repair. 2012;5:S5. doi: 10.1186/1755–1536-5-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sayed N, Liu C and Wu JC. Translation of Human-Induced Pluripotent Stem Cells: From Clinical Trial in a Dish to Precision Medicine. J Am Coll Cardiol. 2016;67:2161–2176. doi: 10.1016/j.jacc.2016.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang L, Guo J, Zhang P, Xiong Q, Wu SC, Xia L, Roy SS, Tolar J, O’Connell TD, Kyba M, Liao K and Zhang J. Derivation and high engraftment of patient-specific cardiomyocyte sheet using induced pluripotent stem cells generated from adult cardiac fibroblast. Circ Heart Fail. 2015;8:156–66. doi: 10.1161/CIRCHEARTFAILURE.114.001317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao L, Gregorich ZR, Zhu W, Mattapally S, Oduk Y, Lou X, Kannappan R, Borovjagin AV, Walcott GP, Pollard AE, Fast VG, Hu X, Lloyd SG, Ge Y and Zhang J. Large cardiac muscle patches engineered from human induced-pluripotent stem cell-derived cardiac cells improve recovery from myocardial infarction in swine. Circulation. 2018;137:1712–1730. doi: 10.1161/CIRCULATIONAHA.117.030785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang L, Gao L, Nickel T, Yang J, Zhou J, Gilbertsen A, Geng Z, Johnson C, Young B, Henke C, Gourley GR and Zhang J. Lactate Promotes Synthetic Phenotype in Vascular Smooth Muscle Cells. Circulation research. 2017;121:1251–1262. doi: 10.1161/CIRCRESAHA.117.311819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shu B, Xie JL, Xu YB, Yu JX, Shi Y, Liu J, Wang P, Liu XS and Qi SH. Directed differentiation of skin-derived precursors into fibroblast-like cells. Int J Clin Exp Pathol. 2014;7:1478–86. doi: [PMC free article] [PubMed] [Google Scholar]
- 13.Xu R, Taskin MB, Rubert M, Seliktar D, Besenbacher F and Chen M. hiPS-MSCs differentiation towards fibroblasts on a 3D ECM mimicking scaffold. Sci Rep. 2015;5:8480. doi: 10.1038/srep08480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orlova VV, van den Hil FE, Petrus-Reurer S, Drabsch Y, Ten Dijke P and Mummery CL. Generation, expansion and functional analysis of endothelial cells and pericytes derived from human pluripotent stem cells. Nat Protoc. 2014;9:1514–31. doi: 10.1038/nprot.2014.102. [DOI] [PubMed] [Google Scholar]
- 15.Santiago JJ, Dangerfield AL, Rattan SG, Bathe KL, Cunnington RH, Raizman JE, Bedosky KM, Freed DH, Kardami E and Dixon IM. Cardiac fibroblast to myofibroblast differentiation in vivo and in vitro: expression of focal adhesion components in neonatal and adult rat ventricular myofibroblasts. Dev Dyn. 2010;239:1573–84. doi: 10.1002/dvdy.22280. [DOI] [PubMed] [Google Scholar]
- 16.Wu CF, Chiang WC, Lai CF, Chang FC, Chen YT, Chou YH, Wu TH, Linn GR, Ling H, Wu KD, Tsai TJ, Chen YM, Duffield JS and Lin SL. Transforming growth factor beta-1 stimulates profibrotic epithelial signaling to activate pericyte-myofibroblast transition in obstructive kidney fibrosis. Am J Pathol. 2013;182:118–31. doi: 10.1016/j.ajpath.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hegner B, Schaub T, Catar R, Kusch A, Wagner P, Essin K, Lange C, Riemekasten G and Dragun D. Intrinsic deregulation of vascular smooth muscle and myofibroblast differentiation in mesenchymal stromal cells from patients with systemic sclerosis. PLoS One. 2016;11:e0153101. doi: 10.1371/journal.pone.0153101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piera-Velazquez S, Mendoza FA and Jimenez SA. Endothelial to mesenchymal transition (EndoMT) in the pathogenesis of human fibrotic diseases. J Clin Med. 2016;5. doi: 10.3390/jcm5040045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsia LT, Ashley N, Ouaret D, Wang LM, Wilding J and Bodmer WF. Myofibroblasts are distinguished from activated skin fibroblasts by the expression of AOC3 and other associated markers. Proc Natl Acad Sci U S A. 2016;113:E2162–71. doi: 10.1073/pnas.1603534113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dobaczewski M, Bujak M, Li N, Gonzalez-Quesada C, Mendoza LH, Wang XF and Frangogiannis NG. Smad3 signaling critically regulates fibroblast phenotype and function in healing myocardial infarction. Circ Res. 2010;107:418–28. doi: 10.1161/CIRCRESAHA.109.216101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rozen-Zvi B, Hayashida T, Hubchak SC, Hanna C, Platanias LC and Schnaper HW. TGF-beta/Smad3 activates mammalian target of rapamycin complex-1 to promote collagen production by increasing HIF-1alpha expression. Am J Physiol Renal Physiol. 2013;305:F485–94. doi: 10.1152/ajprenal.00215.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morikawa M, Derynck R and Miyazono K. TGF-beta and the TGF-beta family: Context-dependent roles in cell and tissue physiology. Cold Spring Harb Perspect Biol. 2016;8. doi: 10.1101/cshperspect.a021873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Good Euler G. and bad sides of TGFbeta-signaling in myocardial infarction. Frontiers in physiology. 2015;6:66. doi: 10.3389/fphys.2015.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frantz S, Hu K, Adamek A, Wolf J, Sallam A, Maier SK, Lonning S, Ling H, Ertl G and Bauersachs J. Transforming growth factor beta inhibition increases mortality and left ventricular dilatation after myocardial infarction. Basic Res Cardiol. 2008;103:485–92. doi: 10.1007/s00395–008-0739–7. [DOI] [PubMed] [Google Scholar]
- 25.Okada H, Takemura G, Kosai K, Li Y, Takahashi T, Esaki M, Yuge K, Miyata S, Maruyama R, Mikami A, Minatoguchi S, Fujiwara T and Fujiwara H. Postinfarction gene therapy against transforming growth factor-beta signal modulates infarct tissue dynamics and attenuates left ventricular remodeling and heart failure. Circulation. 2005;111:2430–7. doi: 10.1161/01.CIR.0000165066.71481.8E. [DOI] [PubMed] [Google Scholar]
- 26.Snider P, Hinton RB, Moreno-Rodriguez RA, Wang J, Rogers R, Lindsley A, Li F, Ingram DA, Menick D, Field L, Firulli AB, Molkentin JD, Markwald R and Conway SJ. Periostin is required for maturation and extracellular matrix stabilization of noncardiomyocyte lineages of the heart. Circ Res. 2008;102:752–60. doi: 10.1161/CIRCRESAHA.107.159517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weber KT, Sun Y, Bhattacharya SK, Ahokas RA and Gerling IC. Myofibroblast-mediated mechanisms of pathological remodelling of the heart. Nat Rev Cardiol. 2013;10:15–26. doi: 10.1038/nrcardio.2012.158. [DOI] [PubMed] [Google Scholar]
- 28.Nural-Guvener HF, Zakharova L, Nimlos J, Popovic S, Mastroeni D and Gaballa MA. HDAC class I inhibitor, Mocetinostat, reverses cardiac fibrosis in heart failure and diminishes CD90+ cardiac myofibroblast activation. Fibrogenesis Tissue Repair. 2014;7:10. doi: 10.1186/1755–1536-7–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tallquist MD and Molkentin JD. Redefining the identity of cardiac fibroblasts. Nature reviews Cardiology. 2017;14:484–491. doi: 10.1038/nrcardio.2017.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frangogiannis NG. Cardiac fibrosis: Cell biological mechanisms, molecular pathways and therapeutic opportunities. Molecular aspects of medicine. 2018. doi: 10.1016/j.mam.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Neuzillet C, Tijeras-Raballand A, Cohen R, Cros J, Faivre S, Raymond E and de Gramont A. Targeting the TGFbeta pathway for cancer therapy. Pharmacology & therapeutics. 2015;147:22–31. doi: 10.1016/j.pharmthera.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 32.Herbertz S, Sawyer JS, Stauber AJ, Gueorguieva I, Driscoll KE, Estrem ST, Cleverly AL, Desaiah D, Guba SC, Benhadji KA, Slapak CA and Lahn MM. Clinical development of galunisertib (LY2157299 monohydrate), a small molecule inhibitor of transforming growth factor-beta signaling pathway. Drug design, development and therapy. 2015;9:4479–99. doi: 10.2147/DDDT.S86621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hammad S, Cavalcanti E, Werle J, Caruso ML, Dropmann A, Ignazzi A, Ebert MP, Dooley S and Giannelli G. Galunisertib modifies the liver fibrotic composition in the Abcb4Ko mouse model. Archives of toxicology. 2018;92:2297–2309. doi: 10.1007/s00204–018-2231-y. [DOI] [PubMed] [Google Scholar]
- 34.Mazzocca A, Fransvea E, Dituri F, Lupo L, Antonaci S and Giannelli G. Down-regulation of connective tissue growth factor by inhibition of transforming growth factor beta blocks the tumor-stroma cross-talk and tumor progression in hepatocellular carcinoma. Hepatology. 2010;51:523–34. doi: 10.1002/hep.23285. [DOI] [PubMed] [Google Scholar]
- 35.Yang XJ, Chen GL, Yu SC, Xu C, Xin YH, Li TT, Shi Y, Gu A, Duan JJ, Qian C, Cui YH, Zhang X and Bian XW. TGF-beta1 enhances tumor-induced angiogenesis via JNK pathway and macrophage infiltration in an improved zebrafish embryo/xenograft glioma model. International immunopharmacology. 2013;15:191–8. doi: 10.1016/j.intimp.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 36.Yue Y, Meng K, Pu Y and Zhang X. Transforming growth factor beta (TGF-beta) mediates cardiac fibrosis and induces diabetic cardiomyopathy. Diabetes research and clinical practice. 2017;133:124–130. doi: 10.1016/j.diabres.2017.08.018. [DOI] [PubMed] [Google Scholar]
- 37.Puceat M TGFbeta in the differentiation of embryonic stem cells. Cardiovasc Res. 2007;74:256–61. doi: 10.1016/j.cardiores.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 38.James D, Levine AJ, Besser D and Hemmati-Brivanlou A. TGFbeta/activin/nodal signaling is necessary for the maintenance of pluripotency in human embryonic stem cells. Development. 2005;132:1273–82. doi: 10.1242/dev.01706. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.