Abstract
Scope:
Several lines of evidence suggest that the consumption of cruciferous vegetables is beneficial to human health. Yet, underlying mechanisms and key molecular targets that are involved with achieving these benefits in humans are still not fully understood. To accelerate this research, we conducted a human study to identify potential molecular targets of crucifers for further study. This study aimed to characterize plasma metabolite profiles in humans before and after consuming fresh broccoli sprouts (a rich dietary source of bioactive sulforaphane).
Methods and Results:
Ten healthy adults consumed fresh broccoli sprouts (containing 200 μmol sulforaphane equivalents) at time 0 and provided blood samples at 0, 3, 6, 12, 24 and 48 hours. An untargeted metabolomics screen revealed that levels of several plasma metabolites were significantly different before and after sprout intake, including fatty acids (14:0, 14:1, 16:0, 16:1, 18:0, 18:1), glutathione, glutamine, cysteine, dehydroepiandrosterone, and deoxyuridine monophosphate. Evaluation of all time points was conducted using paired t test (R software) and repeated measures ANOVA for a within-subject design (Progenesis QI).
Conclusion:
This investigation identified several potential molecular targets of crucifers that may aid in studying established and emerging health benefits of consuming cruciferous vegetables and related bioactive compounds.
Keywords: broccoli sprouts, human, metabolomics, plasma, sulforaphane
INTRODUCTION
Consuming cruciferous vegetables is associated with many health benefits including cancer prevention [1–3]. Many of these health effects have been attributed to the isothiocyanate (ITC), sulforaphane (SFN), which is derived from crucifers such as broccoli. Cruciferous vegetables contain the glucosinolate precursor of SFN, glucoraphanin (GFN), which is hydrolyzed to yield SFN by plant myrosinase enzymes upon tissue damage (e.g., chopping or chewing the vegetable) or from myrosinase-like activity produced by certain gut microbes. After SFN is formed and ingested, it is rapidly absorbed and metabolized via the mercapturic acid pathway, yielding several additional metabolites that are thought to be important for some of the health effects of consuming crucifers [4]. Cruciferous vegetables are known to deliver high amounts of SFN in particular when consumed raw or lightly cooked. Fresh broccoli sprouts are an extremely rich source of SFN, because they contain high levels of GFN (up to 50x higher than mature broccoli) and intact myrosinase [5].
SFN has largely been studied for its roles in cancer chemoprevention, and emerging evidence suggests that consuming dietary sources of SFN may be beneficial in the prevention or management of other morbidities as well, such as cardiovascular disease and diabetes [2, 6–8]. In patients with type 2 diabetes, lipid profiles were improved after 4 weeks consumption of a broccoli sprout powder [9]. Several molecular mechanisms of SFN have been characterized in cultured cells and animal models, including the identification of both genetic and epigenetic targets. Targets include those under the transcriptional control of nuclear factor (erythroid-derived 2)-like 2 (Nrf2), such as quinone reductase 1 [5]. There is also evidence to suggest that SFN influences epigenetic landscapes [10, 11]. In humans there appear to be differences in the responses to the consumption of dietary sources of SFN compared to studies in cultured cells and animals exposed to high doses of SFN and a purified chemical source [12, 13]. Establishing the targets and events that are associated with dietary exposures to SFN is critical to understanding and capitalizing on the health benefits of cruciferous vegetable consumption. To advance the study of these dietary components in human health, we performed an unbiased metabolomics screen on plasma isolated from humans before and after they consumed fresh broccoli sprouts to identify potential molecular targets of crucifers and their bioactive components.
Metabolomics is a powerful approach to screen for physiological responses to diet, drugs and disease [14]. This analysis detects small molecule metabolites in blood or tissues. Monitoring changes in metabolites provides information on the functioning of biochemical pathways upstream. The results from the present study provide direction for future studies to determine the role of crucifers and their bioactive components in preventing diseases such as cardiovascular disease.
MATERIALS AND METHODS
Participants
This human feeding study included ten healthy adults, 19–50 years old, recruited in Corvallis, Oregon and was conducted in the Moore Family Center metabolic kitchen and laboratory at Oregon State University (OSU). Subjects were excluded for smoking, having a BMI < 18.5 or > 30 kg/m2, consuming a vegetarian diet, or using drugs known to alter lipid metabolism. All participants provided informed, written consent. The Institutional Review Board (IRB) at OSU approved all study protocols (OSU IRB #4995). This study was part of a separate investigation of SFN bioavailability in humans consuming fresh broccoli sprouts and a SFN-rich broccoli spout preparation [12]. The original study design did not include a crucifer-free intervention. Thus, the present analysis was intended to identify potential molecular targets of crucifers to direct future investigations.
Study Design
Following an overnight fast, subjects consumed a single dose of fresh broccoli sprouts (providing 200 μmol SFN equivalents) at 8 AM on study day 1. Along with sprouts, subjects consumed a standardized breakfast meal containing no additional cruciferous vegetable components or related bioactives. The same breakfast was provided on days 2 and 3 to improve consistency among the 0-, 24- and 48-hour measurements. Other meals were not standardized due to the free-living conditions of the study design. Three-day diet records were maintained by participants during the study and analyzed using Food Processer® SQL (ESHA, Salem, OR). Sprouter’s Northwest (Kent, WA) provided fresh broccoli sprouts. Subjects consumed no crucifers or ITCs 1 week before or during the study.
Sample Collection and Processing
Whole blood (20 ml) was collected into EDTA vacutainers (VWR, Radnor, PA) before sprout consumption and following consumption at 3, 6, 12, 24 and 48 hours. Plasma was isolated as described by Clarke, et al. [10], snap frozen in liquid nitrogen, and stored at −80°C. For metabolomics analysis, 20 μl plasma was mixed with 80 μl of ice-cold 50:50 (v/v) methanol:ethanol, vortexed, and spun 15 min at 13,000 x g at 4°C. The supernatant was collected and analyzed by HPLC-MS/MS. Quality control samples were prepared in a similar fashion and consisted of aliquots from all study samples. Extraction and HPLC-MS/MS methods were developed to capture and detect plasma metabolites associated with a variety of biological pathways as described by Kirkwood et al. except where noted [15].
Sulforaphane Content Validation
To determine maximal potential for SFN yield from sprouts and to standardize doses among sprout batches, sulforaphane content derived from ~215 mg fresh broccoli sprouts from each batch (n=3) was measured using HPLC-MS/MS as described previously [12].
LC-MS/MS-based Metabolomics
Plasma supernatants were analyzed by HPLC-MS/MS using methods described previously [16]. Briefly, HPLC was performed using a Shimadzu Nexera system (Shimadzu, Columbia, MD) coupled to a TripleTOF™ 5600 mass spectrometer (AB SCIEX, Framingham, MA). Compounds were separated using an Inertsil phenyl-3 column (150 × 4.6 mm, 5 μm, MetaChem Technologies, Torrence, CA) held constant at 50°C while utilizing a flow rate of 0.4 ml/min with mobile phases of water and methanol, both containing 0.1% formic acid. All injections had a volume of 10 μl. All metabolite analyses were conducted using both negative and positive electrospray ionization modes. For MS/MS, the TripleTOF™ 5600 was operated in the information-dependent MS/MS (IDA-MS) acquisition mode using setting described in detail by Kirkwood, et al [16]. To evaluate system and biological variance, quality control samples were injected at random intervals.
Data Processing and Statistical Analysis
Raw HPLC-MS/MS data were imported into MarkerView™ software (AB SCIEX) to perform data normalization (e.g., feature detection, peak alignment, peak integration) using software-designed algorithms. Principal component analysis (PCA) was performed in MetaboAnalyst 3.0 using log transformation. At this step, a feature was defined as a detected analyte with a unique m/z value and retention time combination and the log transformation was applied on the data for normality. PCA determined degree of separation based on features detected in the positive and negative ion modes merged. This analysis shows that there are changes in the pattern of metabolites over time. Since levels of SFN ITCs are known to change over time, we identified and excluded the corresponding features in the data set prior to performing PCA to avoid falsely observing differences in metabolite patterns over time [12, 17].
To identify significantly altered features, paired t tests were performed in R comparing peak areas at each time point to time 0, separately for features detected in the positive and negative ion modes after log transformation. To adjust for multiple comparisons given the large number of features, we computed the q-value based on p-values and consider those features with a q-value < 0.2. This implies that the false discovery rate of this analysis is controlled at 20%. Additionally, fold changes were determined and reported after normalizing values to the group mean at time 0.
The paired t tests compared a single time point to time 0. To include all time points in one analysis, a repeated measures ANOVA was performed using Progenesis QI software v2.0 (Waters Corp.). This analysis was a complement to the paired t-tests described as above. Similarly, we computed the q-values based on p-values to adjust for multiple comparison given the large number of features in this analysis.
Metabolite Identification
Complete raw metabolomics data were imported into PeakView® software (AB SCIEX) to identify metabolomic features using an established in-house MS database [15, 16]. A major strength of comparing our dataset to this database is that the database was created by analyzing purchased metabolite standards (IROA Technologies, Bolton, MA, USA) using identical analytical protocols and equipment as used to analyze plasma samples in the present study, unlike using composite online databases. Thus, we have high confidence in the identification of the compounds in this report. Using this database, metabolomic features in plasma samples were matched to compound standards based on accurate mass and retention time [15, 16]. For the repeated measures ANOVA, metabolite identifications were performed using Progenesis QI which integrates the established in-house IROA MS database and public databases. To reduce unintentional bias in metabolite identification from over- or underrepresentation of metabolites in the database, features were also compared to online metabolite databases, including METLIN and the Human Metabolome Database. Here, we report the endogenous metabolites (not those arising solely from the diet) that were identified using these approaches and that were determined to be significantly different at time 0 compared to at least one other time point.
RESULTS & DISCUSSION
Subject Characteristics
The study population included 4 adult males (mean age: 36 ± 5.3 years; mean BMI: 24 ± 1.7 kg/m2) and 6 adult females (mean age: 27 ± 2.6 years; mean BMI: 22 ± 0.9 kg/m2). We experimentally confirmed that subjects avoided confounding food items the week before and during the study using dietary records and plasma and urinary SFN ITC measurements (data published previously [12]). One subject’s data was missing at 48 hours due to issues with sample collection.
Discovery of Metabolites and Identification
PCA revealed significant differences in metabolite profiles over time following sprout consumption (Fig. 1). Importantly, metabolite profiles at 0 and 24 hours were clearly separated on PCA plots suggesting changes induced by broccoli sprout consumption beyond a basic time-of-day effect. Analysis of quality control samples confirmed low system variance and absence of time drifts, with coefficient of variation below 4.1% (data not shown).
Figure 1. Plasma metabolomic profiles differ over time following broccoli sprout consumption.
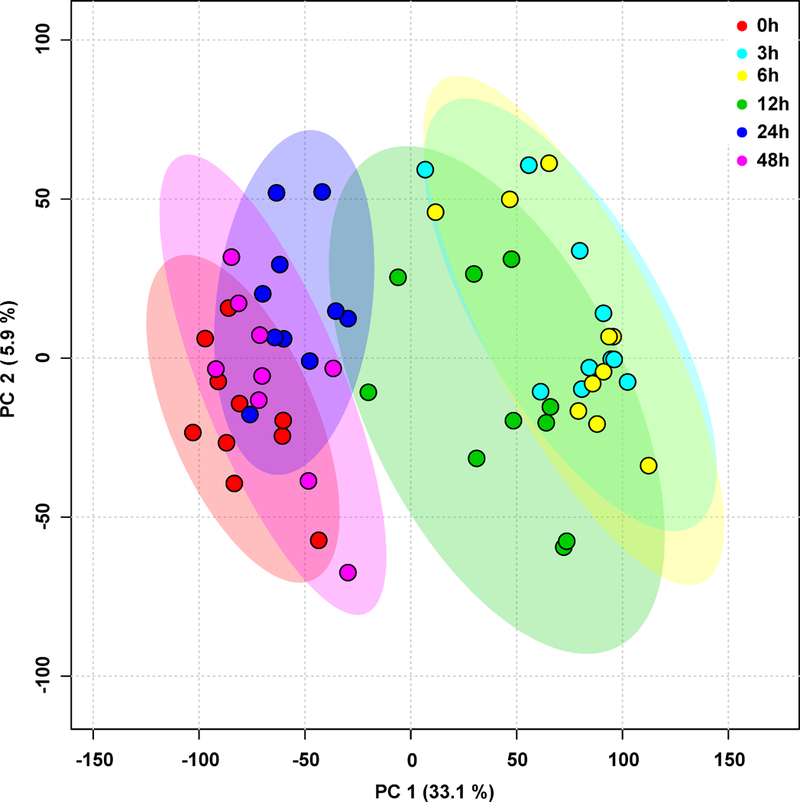
Principal components analysis (PCA) was conducted after log transformation on all features detected in plasma of subjects (positive and negative ion modes merged, SFN and SFN isothiocyanates excluded). Each symbol represents a subject at a specific time point. n = 10 for all time points (except 48h, n = 9).
Initially, 9,367 and 10,510 features were detected in plasma samples in positive and negative ion modes, respectively. For the repeated measures ANOVA, 470 and 494 features were significantly altered across the time course experiment (q ≤0.2) in positive and negative ion modes, respectively. For more information, a full list of identified metabolites is in Supporting Information Table S1. Among them, 60 were significant (Table S2). This implies that 60 identified metabolites altered their levels during some time interval within the 48 hours.
To be more specific about timing, paired t tests were used to compare all time points after sprout consumption to time 0. Of the features we identified using metabolite databases and classified as endogenous, eleven were significantly altered by comparing before and sometime after sprout consumption from paired t tests (q-value < 0.2) (Table 1 and Fig. 2).
Table 1.
Endogenous metabolites detected in human plasma following broccoli sprout consumption.
| Metabolite | Formula | Adduct | Theoretical m/za |
Experimental m/z |
Mass Error (ppm) |
|---|---|---|---|---|---|
| Glutathione | C10H17N3O6S | + H | 308.0911 | 308.0914 | 1 |
| Cysteine | C3H7NO2S | + H | 122.0270 | 122.0266 | −3.4 |
| Glutamine | C5H10N2O3 | + H | 147.0764 | 147.0765 | 0.4 |
| DHEA | C19H28O2 | + H | 289.2162 | 289.2154 | −2.7 |
| dUMP | C9H13N2O8P | + H | 309.0482 | 309.0485 | 0.9 |
| FA 14:0 | C14H28O2 | − H | 227.2017 | 227.2019 | 1.2 |
| FA 14:1 | C14H26O2 | − H | 225.1860 | 225.1856 | −1.6 |
| FA 16:0 | C16H32O2 | − H | 255.2330 | 255.2328 | −0.7 |
| FA 16:1 | C16H30O2 | − H | 253.2173 | 253.2172 | −0.6 |
| FA 18:0 | C18H36O2 | − H | 283.2643 | 283.2643 | 0.1 |
| FA 18:1 | C18H34O2 | − H | 281.2486 | 281.2486 | −0.2 |
Values acquired from database entries. m/z, mass-to-charge ratio.
Figure 2. Plasma metabolites altered following broccoli sprout consumption are associated with multiple biochemical pathways.
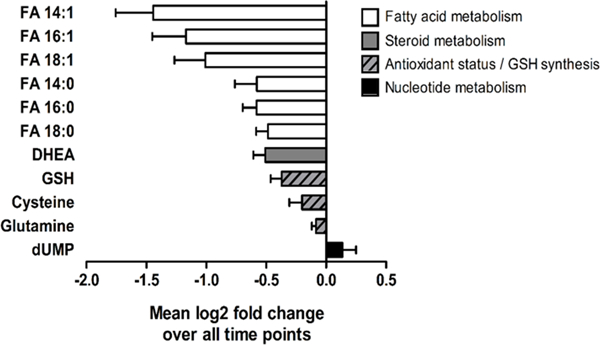
Values represent mean log2 fold change across all time points ± SEM. n = 10 for all time points (except 48h, n = 9).
Glutathione (GSH) was significantly decreased in plasma at 6, 12 and 24 hours following sprout intake (Fig. 3). GSH precursors, glutamine and cysteine, were also decreased at 3 and 24 hours, and 12 and 24 hours, respectively (Fig. 3). GSH is a major intracellular antioxidant that conjugates with SFN during metabolism. Transient decreases of intracellular GSH have previously been reported following SFN exposure as part of the antioxidant response [18]. Decreases in plasma GSH suggest this antioxidant response mechanism also occurs in humans with SFN consumption. Additionally, in human HepG2 cells exposed to SFN, an initial decrease in intracellular GSH was observed, followed by a rebound in GSH levels at 24 hours, which corresponds with our observations [18–20].
Figure 3. Profiles of plasma metabolites following broccoli sprout consumption show significant changes over time.
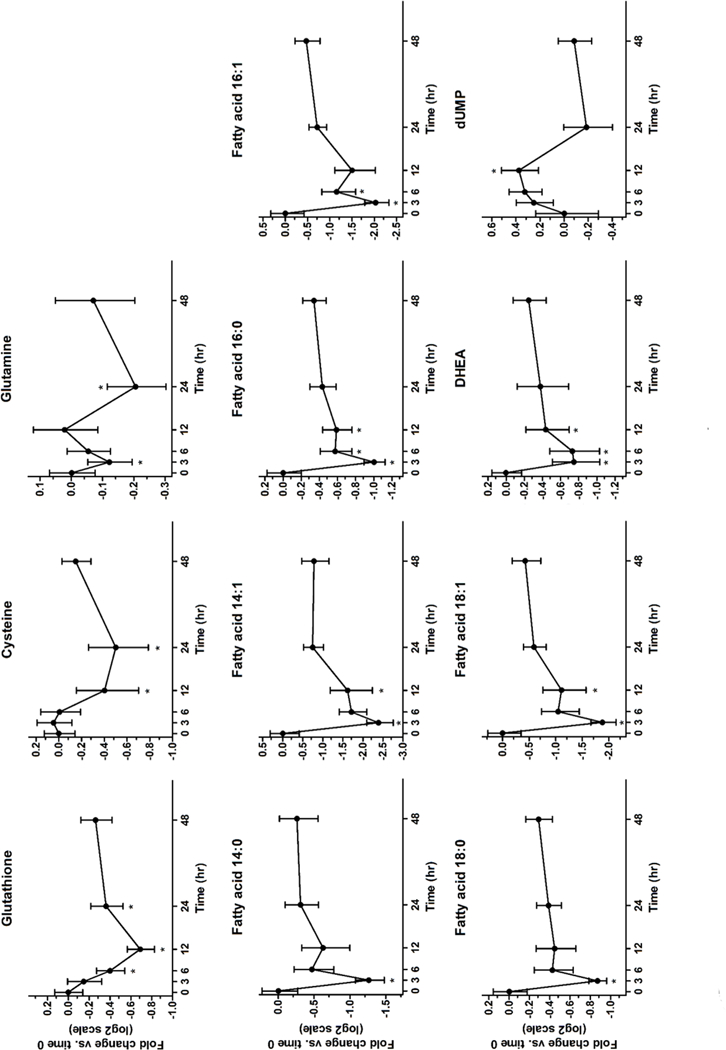
Log2 fold changes are normalized to the group mean value at time 0. Values represent mean fold change ± SEM. n = 10 for all time points (except 48h, n = 9).
This investigation also revealed significant decreases in several fatty acids (FA), including 14:0, 14:1, 16:0, 16:1, 18:0 and 18:1, following sprout intake (Fig. 3). Recent studies suggest that consumption of crucifers could benefit diseases characterized by dyslipidemia [21]. In patients with diabetes, daily consumption of a broccoli sprout powder for 4 weeks reduced serum triglycerides and improved cholesterol ratios [9]. Similar effects were observed in healthy adults that consumed fresh broccoli sprouts daily for 1 week [20]. Several sirtuins (SIRT), a specific class of histone deacetylases (HDACs), including SIRT1, SIRT3, SIRT4 and SIRT6, regulate lipid metabolism at various steps [22]. While SFN is better known as an inhibitor of class I and II HDACs, recent work suggests that SFN may influence SIRTs (class III HDACs) as well, specifically SIRT6 [23]. The decreases in fatty acids reported here suggest that even a single dose of broccoli sprouts may alter plasma lipids in healthy adult populations. Future work is needed to investigate this potential health benefit of broccoli sprouts and elucidate the mechanisms by which broccoli sprout consumption may alter lipid metabolism and related health outcomes.
We observed significant decreases in dehydroepiandrosterone (DHEA), specifically at 3, 6 and 12 hours. DHEA is a steroid that is converted to androgens and a portion can be converted to estrogen by aromatase [24]. The steroid 17A,21-Dihydroxy-4-pregnene-3,20-dione (11-deoxycortisol) that often follows DHEA during development showed similar decreases (data not shown, TableS2). A study in human breast cancer cell lines revealed evidence that metabolic derivatives of DHEA (e.g., 7β-hydroxy-epiandrosterone) may have anti-cancer effects by serving as an anti-estrogenic agent and preventing cell proliferation [25]. In the central nervous system, DHEA has also been shown to bind neuroreceptors that activate apoptotic signaling pathways [26]. Studies have reported altered levels of sex steroid receptors following SFN exposure, but direct evidence of an effect of SFN on DHEA levels is limited [27]. Due to the major roles of DHEA in human health and possible links to cancer development, studying the impact of crucifer consumption on steroid metabolism and DHEA levels will be an interesting area of future research.
The only metabolite (out of the 11 discriminant metabolites) that increased significantly following sprout consumption was deoxyuridine monophosphate (dUMP), which was increased at 12 hours (Fig. 3). dUMP is a precursor of the dinucleotide, deoxythymidine triphosphate (dTTP), which is one of the building blocks of DNA. SFN is well-known to alter proteins such as cyclins that govern cell cycle and cell proliferation - events that impact DNA synthesis and nucleotide metabolism [1, 28]. Our observations support that SFN may have similar effects in humans. Additional studies are needed to clarify mechanisms in human tissues.
While this study focuses largely on the potential effects of SFN, broccoli sprouts contain many other bioactive components (e.g., indoles) that could be responsible for our observations as well as additional health benefits. Future research is needed to determine the role of these bioactive components and any combinatory effects.
CONCLUDING REMARKS
Limitations of this study include small sample size and lack of a crucifer-free intervention arm. To improve confidence in our results, we compared metabolite changes to those observed in a separate intervention arm, where 10 additional subjects completed the study but consumed a SFN-rich broccoli sprout extract supplement instead of fresh broccoli sprouts. This comparison helped rule out the possibility that the changes observed were due to other food items consumed, or circadian rhythms [29] (Supporting Information Fig. S1). Ten of the 11 metabolites (from Fig. 3) were differentially altered between the two groups, where broccoli sprouts generally had a larger effect. In the original bioavailability study, the sprouts delivered 3x more SFN to plasma than the SFN supplement [12]. This suggests that SFN may be contributing in part to the metabolite changes observed. Glutamine was the only exception, where changes over time were similar between the two treatment groups. Thus, we cannot rule out an influence of the other food items consumed on this metabolite. However, since glutamine levels were significantly different at 0 and 24 hours in the sprout group (Fig. 3), we can conclude that circadian rhythm was not the only contributing factor to the changes observed.
We used untargeted metabolomics to screen for metabolomic phenotypes associated with broccoli sprout consumption. A major strength of this approach is the ability to discover novel targets with health implications from dietary exposures. This screen identified changes in several endogenous metabolites in human plasma before and after broccoli sprouts consumption, including specific fatty acids and metabolites associated with GSH biosynthesis, steroid metabolism, and nucleotide metabolism (Figs. 2 and 3). These data provide critical information for future human studies that evaluate both known and emerging health benefits of cruciferous vegetable consumption. Future studies that include larger study populations, additional control groups, and targeted approaches are warranted to confirm the effects of crucifer consumption on the pathways identified from this screen.
Supplementary Material
ACKNOWLEDGMENTS
We thank the Center for Genome Research and Biocomputing, the OSU Mass Spectrometry Center, Karin Hardin, Jeannie Allen, Jeff Morré, and Traci Beckman for technical assistance. We thank Dr. Jay Kirkwood for database development/validation and all study participants.
FUNDING SOURCES
This study was supported by the Oregon Agricultural Experimental Station, National Institute of Health (P01 CA090890 and S10RR02787801). Funders had no role in study design, data collection or analysis, decision to publish, or manuscript preparation.
Abbreviations:
- DHEA
dehydroepiandrosterone
- dUMP
deoxyuridine monophosphate
- FA
fatty acid
- GFN
glucoraphanin
- GSH
glutathione
- HDAC
histone deacetylase
- HMDB
human metabolome database
- ITC
isothiocyanate
- Nrf2
nuclear factor (erythroid-derived 2)-like 2
- OSU
Oregon State University
- PCA
principal components analysis
- SFN
sulforaphane
- SIRT
sirtuin
Footnotes
CONFLICTS OF INTEREST
No conflicts of interest to disclose.
REFERENCES
- [1].Bever LM, Williams DE, Dashwood RH, Ho E, in: Shankar S, Srivastava RK (Eds.), Nutrition, Diet and Cancer, Springer; Netherlands: 2012, pp. 49–81. [Google Scholar]
- [2].Evans PC, The influence of sulforaphane on vascular health and its relevance to nutritional approaches to prevent cardiovascular disease. EPMA J 2011, 2, 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kim MK, Park JH, Conference on “Multidisciplinary approaches to nutritional problems”. Symposium on “Nutrition and health”. Cruciferous vegetable intake and the risk of human cancer: epidemiological evidence. Proc Nutr Soc 2009, 68, 103–110. [DOI] [PubMed] [Google Scholar]
- [4].Dashwood RH, Myzak MC, Ho E, Dietary HDAC inhibitors: time to rethink weak ligands in cancer chemoprevention? Carcinogenesis 2006, 27, 344–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Fahey JW, Zhang Y, Talalay P, Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc Natl Acad Sci U S A 1997, 94, 10367–10372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bahadoran Z, Tohidi M, Nazeri P, Mehran M, Azizi F, Mirmiran P, Effect of broccoli sprouts on insulin resistance in type 2 diabetic patients: a randomized double-blind clinical trial. Int J Food Sci Nutr 2012, 63, 767–771. [DOI] [PubMed] [Google Scholar]
- [7].Cui W, Bai Y, Miao X, Luo P, Chen Q, Tan Y, Rane MJ, Miao L, Cai L, Prevention of diabetic nephropathy by sulforaphane: possible role of Nrf2 upregulation and activation. Oxid Med Cell Longev 2012, 2012, 821936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Dinkova-Kostova AT, Fahey JW, Kostov RV, Kensler TW, KEAP1 and done? Targeting the NRF2 pathway with sulforaphane. Trends Food Sci Technol 2017, 69, 257–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bahadoran Z, Mirmiran P, Hosseinpanah F, Rajab A, Asghari G, Azizi F, Broccoli sprouts powder could improve serum triglyceride and oxidized LDL/LDL-cholesterol ratio in type 2 diabetic patients: a randomized double-blind placebo-controlled clinical trial. Diabetes Res Clin Pract 2012, 96, 348–354. [DOI] [PubMed] [Google Scholar]
- [10].Clarke JD, Riedl K, Bella D, Schwartz SJ, Stevens JF, Ho E, Comparison of isothiocyanate metabolite levels and histone deacetylase activity in human subjects consuming broccoli sprouts or broccoli supplement. J Agric Food Chem 2011, 59, 10955–10963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hsu A, Wong CP, Yu Z, Williams DE, Dashwood RH, Ho E, Promoter de-methylation of cyclin D2 by sulforaphane in prostate cancer cells. Clin Epigenetics 2011, 3, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Atwell LL, Hsu A, Wong CP, Stevens JF, Bella D, Yu TW, Pereira CB, Lohr CV, Christensen JM, Dashwood RH, Williams DE, Shannon J, Ho E, Absorption and chemopreventive targets of sulforaphane in humans following consumption of broccoli sprouts or a myrosinase-treated broccoli sprout extract. Mol Nutr Food Res 2015, 59, 424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Shen G, Xu C, Chen C, Hebbar V, Kong AN, p53-independent G1 cell cycle arrest of human colon carcinoma cells HT-29 by sulforaphane is associated with induction of p21CIP1 and inhibition of expression of cyclin D1. Cancer Chemother Pharmacol 2006, 57, 317–327. [DOI] [PubMed] [Google Scholar]
- [14].Goodacre R, Metabolomics shows the way to new discoveries. Genome Biol 2005, 6, 354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kirkwood JS, Legette LL, Miranda CL, Jiang Y, Stevens JF, A metabolomics-driven elucidation of the anti-obesity mechanisms of xanthohumol. J Biol Chem 2013, 288, 19000–19013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kirkwood JS, Lebold KM, Miranda CL, Wright CL, Miller GW, Tanguay RL, Barton CL, Traber MG, Stevens JF, Vitamin C deficiency activates the purine nucleotide cycle in zebrafish. J Biol Chem 2012, 287, 3833–3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Clarke JD, Hsu A, Riedl K, Bella D, Schwartz SJ, Stevens JF, Ho E, Bioavailability and inter-conversion of sulforaphane and erucin in human subjects consuming broccoli sprouts or broccoli supplement in a cross-over study design. Pharmacol Res 2011, 64, 456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kim BR, Hu R, Keum YS, Hebbar V, Shen G, Nair SS, Kong AN, Effects of glutathione on antioxidant response element-mediated gene expression and apoptosis elicited by sulforaphane. Cancer Res 2003, 63, 7520–7525. [PubMed] [Google Scholar]
- [19].Mizuno K, Kume T, Muto C, Takada-Takatori Y, Izumi Y, Sugimoto H, Akaike A, Glutathione biosynthesis via activation of the nuclear factor E2-related factor 2 (Nrf2)--antioxidant-response element (ARE) pathway is essential for neuroprotective effects of sulforaphane and 6-(methylsulfinyl) hexyl isothiocyanate. J Pharmacol Sci 2011, 115, 320–328. [DOI] [PubMed] [Google Scholar]
- [20].Murashima M, Watanabe S, Zhuo XG, Uehara M, Kurashige A, Phase 1 study of multiple biomarkers for metabolism and oxidative stress after one-week intake of broccoli sprouts. Biofactors 2004, 22, 271–275. [DOI] [PubMed] [Google Scholar]
- [21].Rodriguez-Cantu LN, Gutierrez-Uribe JA, Arriola-Vucovich J, Diaz-De La Garza RI, Fahey JW, Serna-Saldivar SO, Broccoli ( Brassica oleracea var. italica) sprouts and extracts rich in glucosinolates and isothiocyanates affect cholesterol metabolism and genes involved in lipid homeostasis in hamsters. J Agric Food Chem 2011, 59, 1095–1103. [DOI] [PubMed] [Google Scholar]
- [22].Houtkooper RH, Pirinen E, Auwerx J, Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol 2012, 13, 225–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Rajendran P, Kidane AI, Yu TW, Dashwood WM, Bisson WH, Lohr CV, Ho E, Williams DE, Dashwood RH, HDAC turnover, CtIP acetylation and dysregulated DNA damage signaling in colon cancer cells treated with sulforaphane and related dietary isothiocyanates. Epigenetics 2013, 8, 612–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Maninger N, Wolkowitz OM, Reus VI, Epel ES, Mellon SH, Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS). Front Neuroendocrinol 2009, 30, 65–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sandra N, Ester P, Marie-Agnes P, Robert M, Olivier H, The DHEA metabolite 7beta-hydroxy-epiandrosterone exerts anti-estrogenic effects on breast cancer cell lines. Steroids 2012, 77, 542–551. [DOI] [PubMed] [Google Scholar]
- [26].Lazaridis I, Charalampopoulos I, Alexaki VI, Avlonitis N, Pediaditakis I, Efstathopoulos P, Calogeropoulou T, Castanas E, Gravanis A, Neurosteroid dehydroepiandrosterone interacts with nerve growth factor (NGF) receptors, preventing neuronal apoptosis. PLoS Biol 2011, 9, e1001051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Navarro SL, Li F, Lampe JW, Mechanisms of action of isothiocyanates in cancer chemoprevention: an update. Food Funct 2011, 2, 579–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Juge N, Mithen RF, Traka M, Molecular basis for chemoprevention by sulforaphane: a comprehensive review. Cell Mol Life Sci 2007, 64, 1105–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Fahey JW, Wehage SL, Holtzclaw WD, Kensler TW, Egner PA, Shapiro TA, Talalay P, Protection of humans by plant glucosinolates: efficiency of conversion of glucosinolates to isothiocyanates by the gastrointestinal microflora. Cancer Prev Res (Phila) 2012, 5, 603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


