Abstract
Aims:
Right ventricular failure (RVF) varies significantly from the more common left ventricular failure (LVF). This study was undertaken to determine potential molecular pathways that are important in human right ventricular (RV) function and may mediate RVF.
Materials and methods:
We analyzed mRNA of human non-failing LV and RV samples and RVF samples from patients with pulmonary arterial hypertension (PAH), and post-LVAD implantation. We then performed transcript analysis to determine differential expression of genes in the human heart samples. Immunoblot quantification was performed followed by analysis of non-failing and failing phenotypes.
Key findings:
Inflammatory pathways were more commonly dysregulated in RV tissue (both non-failing and failing phenotypes). In non-failing human RV tissue we found important differences in expression of FIGF, TRAPPAC, and CTGF suggesting that regulation of normal RV and LV function are not the same. In failing RV tissue, FBN2, CTGF, SMOC2, and TRAPP6AC were differentially expressed, and are potential targets for further study.
Significance:
This work provides some of the first analyses of the molecular heterogeneity between human RV and LV tissue, as well as key differences in human disease (RVF secondary to pulmonary hypertension and LVAD mediated RVF). Our transcriptional data indicated that inflammatory pathways may be more important in RV tissue, and changes in FIGF and CTGF supported this hypothesis. In PAH RV failure samples, upregulation of FBN2 and CTGF further reinforced the potential significance that altered remodeling and inflammation play in normal RV function and failure.
Keywords: Right ventricular failure, Right heart failure, Pulmonary hypertension, Molecular mechanisms
1. Introduction
Heart failure (HF) currently affects 5.7 million adults in the United States [1], with nearly 1 million new cases diagnosed each year. Further, this number is projected to increase by nearly 50% by the year 2030 [2]. Coronary heart disease accounts for more than half of all cardiovascular events in those under age 75 [3], and is largely characterized by acute coronary syndromes that occur at a rate of 750,000 annually in the United States [1]. Coronary disease predominantly affects the left heart, and given the prevalence, it is not surprising that the study of HF has primarily involved the left ventricle (LV). Subsequently, diseases that affect the right ventricle (RV), such as pulmonary hypertension, congenital heart disease, and right-sided valvular disease, are less studied and understood.
In the past two decades, advances in medicine have highlighted the importance of the RV. The two largest groups of patients that have brought attention to the RV are congenital heart disease (CHD) survivors and those with pulmonary vascular disease, known as pulmonary arterial hypertension (PAH). Each of these conditions underscores the importance of right ventricular function in chronic disease. In fact, given the increasing recognition of this, the National Heart, Lung, and Blood Institute organized a working group to define understanding of RV disease and proposed areas of importance for investigation in an effort to improve knowledge [4]. While it has been a decade since this group organized, due to lack of translational animal models and data on specific molecular pathways altered in tissue from patients with RV failure, we unfortunately still lack knowledge of the molecular pathophysiology of these diseases. Further, we lack even a fundamental baseline of how RV dysfunction differs from LV dysfunction in the human heart. To provide new information on the pathways associated with RV dysfunction, we performed transcriptional profiling on tissue from hearts with LV failure, hearts with RV failure, and non-failing hearts. We report that the molecular pathways associated with RV dysfunction are significantly different from LV failure. Further, we validate key surrogate pathways unique to RV failure by protein analyses. Our findings provide new data on the unique pathways associated with human RV failure as well as identify potential markers for disease assessment/progression and possible future therapeutic avenues.
2. Materials and methods
2.1. Human tissue acquisition
Non-failing non-transplantable donor hearts (control) were acquired through Lifeline of Ohio Organ Procurement, and end-stage diseased hearts were obtained at the time of cardiac transplantation, as described by our group [5]. Samples were obtained from the free wall of the LV and RV so that septal tissue, which contains myocytes from both the LV and RV could be avoided, thereby eliminating the possibility of “cross-contamination” between left and right ventricular tissue. Informed consent was obtained from transplant and left ventricular assist device (LVAD) patients and the institutional review board approved this study. Based upon available phenotypes within the repository, we included normal RV (n = 5) and LV samples (n = 5), RV samples from patients with PAH (n = 2), and both normal and RV failure samples from patients post-LVAD implantation (non-failing RV n = 2, mild RV failure n = 2, severe RV failure n = 2). We conducted 3 experiments evaluating the relationship between: 1) normal RV and LV tissue, 2) normal RV tissue and failing RV-PAH tissue, and 3) RV tissue from LVAD patients with normal systolic function, mild-RV failure and severe-RV failure.
2.2. Phenotype definitions
Within our institutional biorepository we searched for samples with phenotypes representative of 2 right heart failure disease states: PAH with RV failure (RVF) and post-LVAD with RVF, in addition to non-failing control RV and LV samples (Table 1). Using a de-identified dataset, patients with right heart dysfunction due to pulmonary hypertension out of proportion to left heart failure were identified by evaluating standard invasive hemodynamic criteria (mean pulmonary artery pressure (mPAP) ≥25 mmHg, pulmonary capillary wedge pressure (PCWP) ≥15 mmHg, pulmonary vascular resistance (PVR) ≥3 Wood units, and transpulmonary gradient ≥12 mmHg) [6]. Post-LVAD patients with right heart failure were identified by applying the Inter-agency Registry for Mechanically Assisted Circulatory Support (INTERMACS) diagnostic criteria for RVF and severity score [7,8]. The LVAD-RVF group was further subdivided into those with mild (LVAD-mild-RVF) and severe (LVAD-sev-RVF) right heart failure (Tables 1, 2).
Table 1.
Experimental samples.
| Control | Non-failing tissue (RV and LV) | Lifeline of Ohio, no known cardiovascular disease |
|---|---|---|
| RVF groups | PAH with RVF | • Tissue from, patients with known PAH (PH out of proportion to LV dysfunction), explanted at time of OHT6 |
| LVAD control (normal RV function) | • Post-LVAD with normal right heart function, explanted at time of OHT | |
| LVAD-mild-RVF | • Post-LVAD with INTERMACS criteria for mild RVF, explanted at time of OHT8 | |
| LVAD-sev-RVF | • Post-LVAD with INTERMACS criteria for severe RVF, explanted at time of OHT8 |
LV: left ventricle, LVAD: left ventricular assist device, OHT: orthotopic heart transplant, RV: right ventricle, RVF: right ventricular failure.
Table 2.
Diagnostic criteria for RVF and severity score of RVF.
| Diagnostic criteria for RV failure |
|---|
| Symptoms and. signs of persistent right ventricular dysfunction, CVP > 18 mmHg with a CI < 2.0L/min/m2 |
| In the absence of elevated left atrial/pulmonary capillary wedge pressure > 18 mmHg, tamponade, ventricular arrhythmias or pneumothorax |
| Requiring RVAD implantation; or requiring inhaled nitric oxide or inotropic therapy for duration of more than one week at any time after LVAD implantation |
| Severity scale |
| Severe: RVAD implantation |
| Moderate: inotropes or use of IV or inhaled pulmonary vasodilator (iNO or prostaglandin E) |
| Mild: 2 of the 4 following criteria |
| CVP > 18 mmHg or mean RA pressure > 18 mmHg |
| CI < 2.3L/min/m2 (using a pulmonary artery catheter) |
| Ascites or evidence of moderate to worse peripheral edema |
| Evidence of elevated CVP by echo cardiogram (dilated inferior vena cava without collapse), and in physical exam (signs of increased jugular venous pressure). |
CI: cardiac index; CVP: central venous pressure; LVAD, left ventricular assist device; RV: right ventricle; RVAD: right ventricular assist device; RVF: right ventricular failure. Modified from Argiriou M, et.al.8
2.3. mRNA and immunoblot experiments
mRNA isolation and analysis was performed according to methods previously described [9]. Briefly, tissue is flash frozen at the time of acquisition. RNA was extracted using the Qiagen RNeasy Mini Kit and RNA yield was measured using a Nanodrop 1000 Spectrophotometer. RNA was then converted to cDNA using a reverse transcription kit. Custom-designed gene arrays were used to probe for a number of targets using the Affymetrix system as previously described [9]. We then used analysis of variance (ANOVA) to measure differences (> 2 fold differences, both positive and negative) in expression between the listed groups (Fig. 1). Immunoblots were performed as described [9–11]. All proteins were normalized to Glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Antibodies utilized include (in albumin, or blotting-grade blocker): Anti-CTGF (Abcam, ab6990, 1:1000), Anti-Fibrillin 2 (Abcam, ab128026, 1:1000), Anti VEGFD (Bosterbio, PA1332, 1:4000), Anti-MYBPC2 monoclonal antibody (Invitrogen,MA1–26180, 1:4000), Anti-AACT antibody (Origene, TA323307, 1:1000), Anti-SMOC2 antibody (Novus Biologicals, NBP2–20425, 1:1000), Anti-S1PR3 antibody (Sigma-Aldrich, HPA059513, 1:1000) and Anti-TRAPP6AC antibody (Novus Biologicals, NBP1–83167, 1:1000). Secondary antibodies (Peroxidase AffiniPure Donkey Anti-Mouse IgG and Peroxidase AffiniPure Donkey Anti-Rabbit IgG both from Jackson Immunoreserach Laboratories, 715–035-150 and 711-035-152 respectively) were all prepared at concentrations of 1:10,000.
Fig. 1.
Differential gene expression in non-failing LV and RV tissue.
Representative heat map of genes of interest, evaluating for differences between expression in LV and RV tissue (A). Sample of upregulated (positive fold-change, B) and downregulated (negative fold-change, C) genes in RV myocardial tissue.
2.4. Statistical analysis
Clinical data were reviewed and basic demographic information and descriptive statistics were generated. Data is expressed as mean ± SEM with significance occurring at an α level < 0.05. To better understand the basic differences between human left and right ventricular tissue, we identified transcripts up or down-regulated in non-failing LV and RV samples (Fig. 2) [12]. mRNA was analyzed for ≥2 fold change between groups by ANOVA. From this list, key transcripts of interest were identified (Table 3).
Fig. 2.
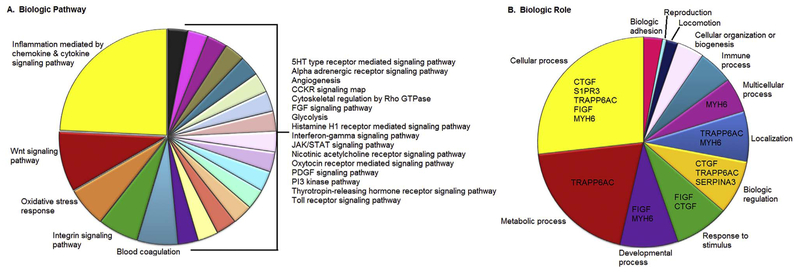
Distribution of differentially expressed genes by biologic pathway and biologic role.
Ontologic pathway of genes differentially expressed in RV tissue, highlighting inflammatory signaling pathways (A) and biologic role of genes of interest (B).
Table 3.
Description of key transcripts.
| Symbol | Protein nomenclature | Implication in cardiovascular disease |
|---|---|---|
| CTGF | Connective tissue growth factor | • Role in cardiac fibrosis and susceptibility to systemic sclerosis |
| FBN2 | Fibrillin 2 | • Role in non-cardiac disease, but no role yet established in cardiac disease; functions as a part of the extracellular matrix in connective tissue disease |
| FIGF | c-fos induced growth factor (VEGFD) | • Modulates endothelial cell growth and function |
| MYH6 | Myosin heavy chain 6, cardiac muscle, alpha | • Associated with several congenital heart defects, including hypoplastic left heart syndrome (recessive) |
| SERPINA3 | Serpin peptidase inhibitor (alpha 1 antiproteinase) | • Clear relationship to occlusive cerebrovascular disease (alpha 2 antichymotrypsin deficiency), also serves as an acute phase reactant |
| SMOC2 | SPARC-related modular calcium binding 2 | • Acts in proliferation and migration of vascular smooth muscles to the intima in a model of carotid artery injury |
| S1PR3 | Sphingosine-1-phosphate receptor 3 | • Regulates cell proliferation, apoptosis, motility, has been shown to be increased in an ischemia-reperfusion myocardial model |
| TRAPP6AC | TRAnsport protein particle | • Functions in protein transport |
Protein quantification from immunoblot in the genes of interest were normalized to GAPDH and differences between the following groups were compared using an independent t-test so long as there were at least n = 5 in each group (Control RV vs. Control LV, Control RV vs. PAH, Control RV vs. LVAD RV, LVAD RV vs. LVAD-mild-RVF, LVAD RV vs. LVAD-sev-RVF). For groups with n < 5 samples, standard statistical analysis was performed, but due to the increased likelihood of a statistical type II error we also examined and highlight descriptively any findings with > 2-fold change between groups.
3. Results
3.1. Defining transcriptional pathways unique to human right ventricle (RV)
To date, there is little information on the molecular pathways associated with RV failure. As an initial step to define inherent differences between left and right ventricle, we performed transcript analysis of non-failing human right and left ventricle samples. Notably, despite analysis of over 25,000 transcripts, we observed only 215 coding transcripts that displayed statistically significant 2-fold increase or decrease in expression (p < 0.05). Of these, 129 transcripts were upregulated in non-failing RV tissue versus non-failing LV tissue. Eighty-six of the transcripts were decreased > 2 fold in non-failing human RV versus LV tissue. Gene ontologic analysis revealed that 25% of these genes were associated with chemokine/cytokine-mediated inflammatory pathways, with another 21% functioning in important signaling pathways (Wnt, oxidative stress response, and integrin signaling) (Fig. 2A). Further grouping of altered transcripts by biologic role revealed a wide distribution of cellular roles including metabolic, developmental, regulation, and localization, among others (Fig. 2B). As altered transcriptional regulation may not necessarily translate to altered protein expression, we performed immunoblot analysis on eight genes, representing multiple protein classes that were significantly altered in non-failing human RV versus non-failing LV samples.
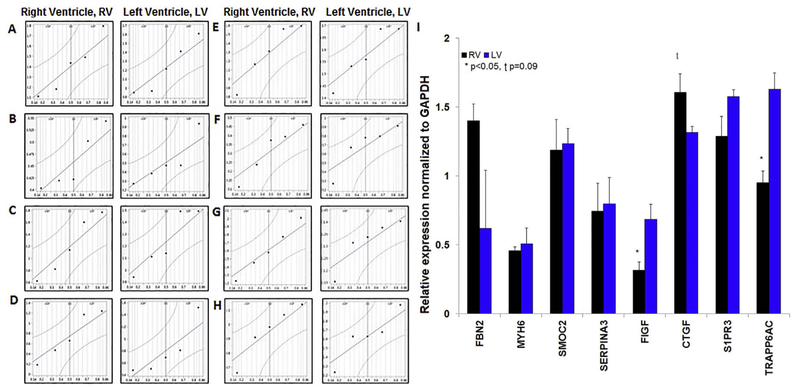
Quantile plots of the eight proteins (Fig. 3) in RV and LV tissue demonstrated normal distribution. In RV tissue there was a statistically significant reduction in expression of c-fos induced growth factor (FIGF, also known as VEGFD) (0.32 ± 0.06 vs. 0.69 ± 0.11, p < 0.05) and transport protein particle 6AC/TRAPP6AC (0.95 ± 0.08 vs. 1.63 ± 0.11, p < 0.05). Further, we observed an insignificant trend for increased expression of connective tissue growth factor (CTGF) in non-failing human RV samples versus non-failing human LV samples (1.61 ± 0.13 vs. 1.32 ± 0.04, p = 0.09). We observed no significant difference in the expression of FBN2 (p = 0.36), MYH6 (p = 0.69), SMOC2 (p = 0.86), Serpina3 (p = 0.85), or S1PR3 (p = 0.12) (Fig. 3).
Fig. 3.
Expression of key proteins in non-failing LV and RV tissue.
Quantile plots illustrating distribution of RV and LV non-failing samples (n = 5 each group; logarithmic scale): FBN2 (A), MYH6 (B), SMOC2 (C), Serpina3 (D), FIGF (E), CTGF (F), S1PR3 (G), TRAPP6AC (H). Relative protein expression (normalized to GAPDH) in non-failing RV and LV tissue with significant differences (*) in FIGF, TRAPP6AC, and CTGF (I). Representative (single sample) immunoblots of each protein in RV and LV tissue in Supplementary Fig. 1.
3.2. Analysis of altered protein expression in human pulmonary arterial hypertension (PAH)
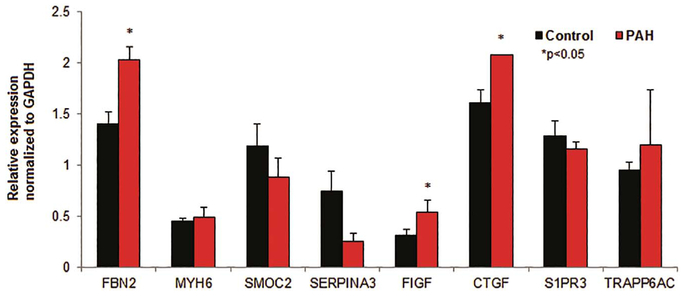
In PAH there is increased vascular resistance in the pulmonary bed, which drives pulmonary artery pressures to be severely elevated. In this condition, the RV is required to pump against this higher pressure, and it frequently results in dilatation and failure of the ventricle. PAH is an important clinical model of RV failure. Therefore we next evaluated the expression of key surrogate proteins identified in Table 3 in non-failing human RV samples (n = 5) versus human PAH (n = 2) RV samples. It is worth noting that only two samples were utilized in the PAH group as these were the only samples available in the institutional biorepository that met PAH hemodynamic criteria (in this case, pulmonary hypertension out of proportion to left heart disease defined as transpulmonary gradient > 12 mmHg). Both patients had biventricular failure and received orthotopic heart transplant for a primary indication of LV failure secondary to ischemic cardiomyopathy. Both died within one year from persistent post-transplant RVF (Table 4) despite RVAD (right ventricular assist device) use in one patient. Tissue analysis of these specimens showed increased expression of Fibrillin 2 in PAH RV tissue (2.03 ± 0.10 vs. 1.40 ± 0.12, p < 0.05) and Connective tissue growth factor (2.10 ± 0.002 vs. 1.61 ± 0.134, p < 0.05). There was reduced RV expression of Serpin peptidase inhibitor (0.26 ± 0.09 vs. 0.74 ± 0.20, p < 0.05). (Fig. 4) We observed no difference in the expression of MYH6 (p = 0.76), SMOC2 (p = 0.36), FIGF (p = 0.28), S1PR3 (p = 0.45) or TRAPP6AC (p = 0.73).
Table 4.
Clinical characteristics of PH patients.
| Clinical characteristic, ID | PH patient 1, 961536 | PH patient 2, |
|---|---|---|
| 537263 | ||
| Age | 61 | 62 |
| Sex | Female | Male |
| Ethnicity | Caucasian | African American |
| Measures of RV function, echo | ||
| S’ (mm) | 7.9 | 4.6 |
| RV systolic pressure (mmHg) | 35 | 51 |
| TAPSE (mm) | 9 | 6 |
| Invasive hemodynamics | ||
| RA pressure (mmHg) | 14 | 21 |
| Mean PA pressure (mmHg) | 35 | 42 |
| PCWP (mmHg) | 22 | 28 |
| TPG (mmHg) | 13 | 14 |
| PVR (Woods units) | 6.5 | 7.8 |
| CO (Liters/minute) | 2.0 | 1.8 |
| CI (Liters/min/m2) | 1.3 | 0.8 |
CI: cardiac index; CO: cardiac output; ID: identification; PA: pulmonary artery, PAH: pulmonary arterial hypertension; PCWP: pulmonary capillary wedge pressure; PVR: pulmonary vascular resistance; RA: right atrium; RV: right ventricle; S’: A measure of tricuspid annular systolic motion, TAPSE: tricuspid annular plane systolic excursion; TPG: trans-pulmonary gradient (mean PA – PCWP).
Fig. 4.
Expression of key proteins in non-failing RV and PAH-mediated RV failure.
Relative protein expression (normalized to GAPDH) in non-failing (control) RV samples and PAH-mediated RV failure samples; note significant increase (*) in expression of FBN2, FIGF, and CTGF in PAH-mediated RV failure samples. Representative (single sample) immunoblots of each protein in control non-failing RV and PAH-mediated RV failure tissue in Supplementary Fig. 1.
3.3. Altered expression of key proteins in human left ventricular assist device (LVAD) and right heart failure (RHF)
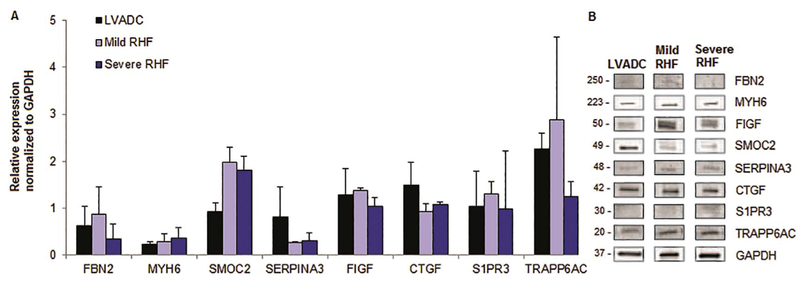
Right heart failure is seen frequently after implantation of an LVAD in the setting of biventricular failure, and so as a final step to define differences in human heart samples, we evaluated samples taken from three groups: post-LVAD with normal right heart function (LVAD Control), mild RHF, and severe RHF. Basic demographic characteristics and detailed analysis of INTERMACS criteria for RVF are outlined in Table 5. Surprisingly, we did not observe striking differences in expression between the groups. However, we did observe a non-significant ~ 2-fold increase in SPARC-related modular calcium binding 2 protein that occurred in both the mild (1.97 ± 0.32 vs. 0.93 ± 0.19, p = 0.14) and severe RVF groups (1.82 ± 0.29 vs. 0.93 ± 0.19, p = 0.14). There was a 2.4 fold increase in Transport protein particle 6AC expression in LVAD patients with normal RV function as compared to normal RV samples without LVAD (2.27 ± 0.33 vs. 0.95 ± 0.08, p = 0.14) (Fig. 5).
Table 5.
Clinical characteristics of LVAD patients.
| Clinical characteristic, ID | LVAD normal RV function 1, 733252 | LVAD normal RV function 2, 233587 | LVAD milds RHF 1, 537114 | LVAD mild RHF 2, 203056 | LVAD severe RHF 1, 371558 | LVAD severe RHF 2, 956256 |
|---|---|---|---|---|---|---|
| Age | 61 | 65 | 48 | 51 | 36 | 64 |
| Sex | Male | Male | Male | Male | Male | Male |
| Ethnicity | African American | Caucasian | East Indian | African American | African American | Caucasian |
| Cause of HF | ICM | ICM | NICM, Viral | NICM | NICM, Viral | ICM |
| Received transplant | Yes | Yes | Yes | Yes | Yes | Yes |
| Diagnostic criteria RVF (INTERMACS) CVP > 18 mmHg with CI < 2.0 L/min | No | No | No | No | Yes | No |
| PCWP < 18 mmHg with Tamponade, VT, PnTx | No | No | No | No | No | No |
| RVAD, iNO, Inotrope > 1 week post LVAD | No | No | Yes, inotrope < 1 month | Yes, inotrope < 1 month | Yes, inotrope > 1 month | Yes, inotrope > 1 month |
| Severity criteria RVF (INTERMACS) | ||||||
| Severe: RVAD implanted | No | No | No | No | No | No |
| Moderate: Inotrope or iNO | No | No | No | No | Yes | Yes |
| Mild: (> 2 of the following) | No | No | Yes | Yes | NA | NA |
| CVP or RAP > 18 mmHg | No | No | No | No | No | No |
| CI < 2.3 L/min/m2 | Yes | No | No | No | Yes | No |
| Ascites or evidence significant peripheral edema | No | No | Yes | Yes | No | Yes |
| Increased CVP by echo (dilated IVC) or exam (increased JVP) | No | No | Yes | Yes | Yes | Yes |
CI: cardiac index; CVP: central venous pressure, HF: heart failure; ICM: ischemic cardiomyopathy, ID: identification number, iNO: inhaled nitric oxide; INTERMACS: Interagency Registry for Mechanically Assisted Circulatory Support; IVC: inferior vena cava; JVP: jugular venous pressure; LVAD: left ventricular assist device; NICM: non-ischemic cardiomyopathy; PCWP: pulmonary capillary wedge pressure, PnTx: Pneumothorax, RAP: right atrial pressure, RVAD: right ventricular assist device, VT: ventricular tachycardia.
Fig. 5.
Expression of key proteins in an LVAD model of RV failure.
Relative protein expression (normalized to GAPDH) in LVAD-RV samples with no RV failure (LVAD control), LVAD-mild RVF, LVAD-severe RVF (A). Representative (single sample) immunoblots of each protein in all 4 sample subtypes (B).
4. Discussion
4.1. Heart failure: LV versus RV
Over the past several decades, there has been progressive understanding of the molecular mechanisms that contribute to LV failure. Hemodynamic abnormalities were thought to explain the syndrome of heart failure (both cardiorenal and cardiocirculatory models) [13]. It later became clear that neurohormonal mechanisms at the very least, contributed to the progressive nature of HF, and that when antagonized, improved clinical status [14–21]. The field was further advanced as progressive maladaptive remodeling became better characterized and understood [22,23].Standard heart failure medications have substantially changed morbidity and mortality in left heart failure; however, have not had the same effect on patients with RVF, suggesting that there is a difference in the molecular mechanisms underlying RVF. Our data provides new information not only detailing differences in molecular pathways between human LV and RV in non-failing hearts, but further defines key proteins that may be altered in a host of unique diseases. For example, transcript analysis identified inflammation- mediated pathways and cellular and metabolic processes as important in normal RV function, disparate from mechanisms in LV function. Previous studies have evaluated ischemic explanted hearts but our data is unique in that we compare non-failing LV and RV samples as well as diseased RV samples with PAH out of proportion to LV disease [24,25].
C-fos induced growth factor (FIGF)/VEGFD is a member of the platelet-derived growth factor family with mitogenic activity for endothelial cells and hypoxia-induced stimulation of angiogenesis. FIGF itself plays an important role in and in heart failure [26,27]. FIGF in infarct models acts as a profibrogenic mediator stimulating myofibroblast growth, migration, and type I collagen synthesis [28]. Extrapolating from this data, it seems reasonable that in RV tissue if there is reduced expression of FIGF, physiologic remodeling may be impaired. And so it is possible that failure of the RV may not be due to a primary insult perse, but rather may stem from a compromise in ability to undergo necessary physiologic remodeling in the face of an insult, such as increased pressure, infarction or volume overload.
Connective tissue growth factor (CTGF) (also known as CCN2) is a members of the CCN family are cysteine-rich matricellular proteins containing an N-terminal secretory peptide and 4 multi-functional domains that bind to multiple proteins: integrins, low-density lipoprotein receptor-related proteins, growth factors, and extracellular matrix components [29]. This protein plays a role normal embryonic and postnatal development, however in adults, has been shown to play a role in pathologic conditions such as: fibrosis, atherosclerosis, osteoarthritis, and malignancy [30]. We observed a trend toward increased expression of connective tissue growth factor CTGF in RV tissue. It is unknown whether CTGF causes fibrosis, or is a marker of the extent of fibrosis in different tissue subtypes. In cardiovascular disease models it has been shown to be both upregulated [31,32] and down-regulated [33,34] We have found only one study evaluating the role of CTGF in RV function and failure, where in patients with carcinoid disease there was an inverse correlation between RV function and CTGF levels, indicating that CTGF may play a role in mediating RV systolic dysfunction, and could potentially be used as a marker of this dysfunction [35]. Our findings, that CTGF may have higher expression in normal functioning RV (versus LV) tissue, suggests that the role of CTGF in fibrosis and cardiomyopathy, may be magnified in right heart disease and RV failure.
Changes in FIGF or CTGF don’t entirely explain why RV function and failure are different than LV function/failure. However, we postulate that these changes together (downregulation of FIGF and upregulation of CTGF) may indicate that there are distinctive differences in RV response to stress and/or injury. Specifically, RV dysfunction may be a result of impaired remodeling and inflammation. This hypothesis would support the transcriptional data we generated, which showed the importance of inflammatory pathways in RV tissue.
4.2. RV failure in PAH
Advances in medical therapy for pulmonary vascular disease have led to improved survival in patients with pulmonary arterial hypertension; despite this, morbidity and mortality remain high [36]. Presently available medical therapy has targeted vasoactive and proliferative compounds found to contribute to the pathobiological abnormalities within the pulmonary vasculature. It has become clear that although changes in the pulmonary vasculature are important, the RV plays a central role in survival and prognosis [37–43].In fact, RV function according to many, is the most important determinant of survival in PAH [44,45]. In the presence of PAH, the RV is forced to deal with increased afterload, frequently resulting in hypertrophy and dilatation of the right ventricle. It remains unclear why some individuals have a more favorable and compensatory RV, and others have progressive dilatation and dysfunction leading to decline in functional capacity and ultimately worse prognosis. At present we lack knowledge about the differential response of the RV in pulmonary vascular disease, and as such there are no medical therapies targeted specifically to the right heart.
In this study, we identified two patients within our institutional biorepository who met hemodynamic criteria for PH out of proportion to left heart disease (PAH) and underwent transplant, thereby making the explanted RV available for study. From a hemodynamic standpoint, the PAH in these individuals was severe. In fact, in both cases despite undergoing orthotopic heart transplant, survival was poor (~1 year) due to recalcitrant RHF in the setting of pulmonary hypertension. We showed that CTGF may be higher in the normal functioning RV and was higher yet in the failing right hearts of PAH patients. Our study isn’t the only one to show upregulation of CTGF in pulmonary disease and in patients with PAH [46,47]. This finding further supports the theory that RV dysfunction may be largely mediated by impaired remodeling and subsequent inflammation.
The upregulation of Fibrillin-2 (FBN2) may also support this hypothesis. FBN2 is a component of the extracellular matrix functioning in elastic fiber formation/anchoring; in humans FBN2 gene variants are linked to the microfibrillopathy condition termed congenital contractural arachnodactyly; this is clinically similar to the more well-known Marfan syndrome – an important example of a vascular connective tissue disease [48]. Likewise, Serpin peptidase inhibitor (SER-PINA3), also known as alpha-1-antiproteinase, has played a role in diseases characterized by abnormal remodeling and regeneration: alpha 2 antichymotrypsin deficiency, [49] Alzheimer’s disease, Parkinson disease, and multiple system atrophy [50–53]. And so it is not surprising that downregulation of SERPINA3 occurred in PAH-mediated right heart failure, and again supports the hypothesis that the development of RV failure may rely more heavily on incomplete or flawed tissue remodeling and inflammation.
4.3. RV failure post-LVAD
RVF is one of the leading complications post-LVAD implantation occurring in 10–40% of patients [54–57], and is associated with worse survival [57]. RVF post-LVAD implantation is due to multiple factors including: increased LV cardiac output leading to increased venous return (preload) to the RV [58], exaggerated left shift of the inter-ventricular septum leading to decreased contribution to RV contraction [59,60], tachyarrhythmias [61], and in the case of underlying tricuspid valve disease, worsening regurgitation may ensue leading to progressive RVF. There are also several patient-related factors that increase the risk of developing RVF, including elevated PVR, which increases risk for RVF linearly [62]. Although RVF risk prediction models exist, there is no generally accepted work-up to accurately predict risk of RVF post-LVAD implantation.
There is not significant data on molecular mechanisms of RVF in the post-LVAD population. Due to a small number of tissue samples available in all LVAD categories we were only able to report fold-changes and no statistical analysis was done. In this very preliminary analysis we did not find any changes that were > 2-fold different between control, mild RHF, and severe RHF samples. There may have been some trends in differences between SPARC-related modular calcium-binding 2 protein (SMOC2) and TRAPP6AC, however with small sample size it is difficult to determine what role these proteins may play in RVF post LVAD implantation. However, with an increasing number of mechanical circulatory devices placed, this is an ideal target for further research.
4.4. Limitations
This study is limited by the assumptions inherent in analysis of molecular signatures in human control and diseased tissue. In particular, this relies on the supposition that protein levels (as identified by mRNA expression) have a linear relationship with function. Alternative splicing and posttranslational modification along with cellular differentiation commonly play a role in protein variation and function. These are less well examined with this methodology in order to narrow the scope of this project so that results can be reasonably achieved in the study timeframe. The tissue samples analyzed are categorized by underlying cause of RV function (or failure). We recognize that phenotypic heterogeneity between the subgroups analyzed exists, and while the groupings may be overly simplistic, it is a necessary categorization to allow comparison between reasonably different clinical phenotypes. It is important to acknowledge that we did not find linear changes in protein expression related to the degree or RV failure. We postulate that this is because there is some level of tissue degradation during the acquisition and storage process. Our tissue repository dates back almost 10 years, and it is also likely that storage time also affects tissue integrity. Therefore, we can detect changes in expression, but not necessarily infer that degree of change in expression is correlated to the amount of RV dysfunction. Finally, small numbers of patients with RVF go on to heart transplant, and this limited our ability to look at large sample numbers. Transplant medicine has evolved rapidly over the last several years, and in particular with human heart transplant, we have learned that patients with RVF do poorly. Therefore, right heart function is scrutinized during the heart transplant work-up and listing process. As a result, tissue from patients similar to those included in this study, are no longer available as they do not go on to be listed or receive heart transplant if significant right heart dysfunction is present. We fully acknowledge that a larger sample size would increase the power and potential impact of the results. However, we believe that this work in human samples will serve as a platform for new hypothesis generation in the study of right ventricular failure. Despite these limitations, this model is a practical way to take a “first look” at the genomics underlying RV function and failure.
5. Conclusions
This work provides some of the first analyses of the molecular heterogeneity between human RV and LV tissue, as well as key differences in human disease (RVF secondary to pulmonary hypertension and LVAD mediated RVF). Our transcriptional data indicated that inflammatory pathways may be more important in RV tissue, and changes in FIGF and CTGF supported this hypothesis. In PAH RV failure samples, upregulation of FBN2 and CTGF further reinforced the potential significance that altered remodeling and inflammation play in normal RV function and failure.
Supplementary Material
Acknowledgments
Erin Bumgardner.
Emily Jarvis.
Kelly MacBrair.
Susan Montgomery.
We would also like to thank Lifeline of Ohio Organ Procurement program and their collaboration on the procurement of non-failing hearts.
Funding
This work was supported by The Courtney Williams Heart Failure Research Fund.
Disclosures: (support or conflict of interest)
Jordan L. Williams, BS: None.
Emefah C. Loccoh, BS: None.
Omer Cavus, MD: None.
Sara Adelman, BS: None.
John C. Daugherty, BA: None.
Sakima A. Smith, MD, MPH: None.
Benjamin Canan, BS, MPH: None.
Paul ML Janssen, PhD: None.
Sara Koenig, PhD: None.
C. Faith Kline, PhD: None.
Peter J. Mohler, PhD: None.
Elisa A. Bradley, MD: None
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.lfs.2018.01.021.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
References
- [1].Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB, Heart disease and stroke statistics-2016 update: a report from the American Heart Association, Circulation 133 (2016) e38–360. [DOI] [PubMed] [Google Scholar]
- [2].Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, Nichol G, Pham M, Pina IL, Trogdon JG, Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association, Circ. Heart Fail. 6 (2013) 606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Thom TJKW, Silbershatz H, d’Agostino RBSr, Cardiovascular Diseases in the United States and Prevention Approaches, 10 ed., McGraw-Hill, New York, NY, 2001. [Google Scholar]
- [4].Voelkel NF, Quaife RA, Leinwand LA, Barst RJ, McGoon MD, Meldrum DR, Dupuis J, Long CS, Rubin LJ, Smart FW, Suzuki YJ, Gladwin M, Denholm EM, Gail DB, Right ventricular function and failure: report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure, Circulation 114 (2006) 1883–1891. [DOI] [PubMed] [Google Scholar]
- [5].Milani-Nejad N, Canan BD, Elnakish MT, Davis JP, Chung JH, Fedorov VV, Binkley PF, Higgins RS, Kilic A, Mohler PJ, Janssen PM, The frank-Starling mechanism involves deceleration of cross-bridge kinetics and is preserved in failing human right ventricular myocardium, Am. J. Physiol. Heart Circ. Physiol 309 (2015) H2077–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, McGoon MD, Park MH, Rosenson RS, Rubin LJ, Tapson VF, Varga J, Harrington RA, Anderson JL, Bates ER, Bridges CR, Eisenberg MJ, Ferrari VA, Grines CL, Hlatky MA, Jacobs AK, Kaul S, Lichtenberg RC, Lindner JR, Moliterno DJ, Mukherjee D, Pohost GM, Rosenson RS, Schofield RS, Shubrooks SJ, Stein JH, Tracy CM, Weitz HH, Wesley DJ, ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation task force on expert consensus documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association, Circulation 119 (2009) 2250–2294. [DOI] [PubMed] [Google Scholar]
- [7].Holman WL, Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS): what have we learned and what will we learn? Circulation 126 (2012) 1401–1406. [DOI] [PubMed] [Google Scholar]
- [8].Argiriou M, Kolokotron SM, Sakellaridis T, Argiriou O, Charitos C, Zarogoulidis P, Katsikogiannis N, Kougioumtzi I, Machairiotis N, Tsiouda T, Tsakiridis K, Zarogoulidis K, Right heart failure post left ventricular assist device implantation, J. Thorac. Dis 6 (Suppl. 1) (2014) S52–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Smith SA, Sturm AC, Curran J, Kline CF, Little SC, Bonilla IM, Long VP, Makara M, Polina I, Hughes LD, Webb TR, Wei Z, Wright P, Voigt N, Bhakta D, Spoonamore KG, Zhang C, Weiss R, Binkley PF, Janssen PM, Kilic A, Higgins RS, Sun M, Ma J, Dobrev D, Zhang M, Carnes CA, Vatta M, Rasband MN, Hund TJ, Mohler PJ, Dysfunction in the betaII spectrin-dependent cytoskeleton underlies human arrhythmia, Circulation 131 (2015) 695–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gygi SP, Rochon Y, Franza BR, Aebersold R, Correlation between protein and mRNA abundance in yeast, Mol. Cell. Biol 19 (1999) 1720–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Liu ZQ, Mahmood T, Yang PC, Western blot: technique, theory and trouble shooting, N. Am. J. Med. Sci 6 (2014) 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mi H, Huang X, Muruganujan A, Tang H, Mills C, Kang D, Thomas PD, PANTHER version 11: expanded annotation data from gene ontology and reactome pathways, and data analysis tool enhancements, Nucleic Acids Res. 45 (2017) D183–d189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Packer M, The neurohormonal hypothesis: a theory to explain the mechanism of disease progression in heart failure, J. Am. Coll. Cardiol 20 (1992) 248–254. [DOI] [PubMed] [Google Scholar]
- [14].Cohn JN, Johnson G, Ziesche S, Cobb F, Francis G, Tristani F, Smith R, Dunkman WB, Loeb H, Wong M, et al. , A comparison of enalapril with hydralazine-isosorbide dinitrate in the treatment of chronic congestive heart failure, N. Engl. J. Med 325 (1991) 303–310. [DOI] [PubMed] [Google Scholar]
- [15].Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, Shusterman NH, The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol heart failure study group, N. Engl. J. Med 334 (1996) 1349–1355. [DOI] [PubMed] [Google Scholar]
- [16].Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN, Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure, N. Engl. J. Med 325 (1991) 293–302. [DOI] [PubMed] [Google Scholar]
- [17].Yusuf S, Pitt B, Davis CE, Hood WB Jr., J.N. Cohn, Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions, N. Engl. J. Med 327 (1992) 685–691. [DOI] [PubMed] [Google Scholar]
- [18].Bristow MR, Gilbert EM, Abraham WT, Adams KF, Fowler MB, Hershberger RE, Kubo SH, Narahara KA, Ingersoll H, Krueger S, Young S, Shusterman N, Carvedilol produces dose-related improvements in left ventricular function and survival in subjects with chronic heart failure. MOCHA investigators, Circulation 94 (1996) 2807–2816. [DOI] [PubMed] [Google Scholar]
- [19].Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF), Lancet 353 (1999) 2001–2007 (London, England). [PubMed] [Google Scholar]
- [20].Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J, The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone evaluation study investigators, N. Engl. J. Med 341 (1999) 709–717. [DOI] [PubMed] [Google Scholar]
- [21].Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J, Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-preserved trial, Lancet 362 (2003) 777–781 (London, England). [DOI] [PubMed] [Google Scholar]
- [22].Cohn JN, Structural basis for heart failure. Ventricular remodeling and its pharmacological inhibition, Circulation 91 (1995) 2504–2507. [DOI] [PubMed] [Google Scholar]
- [23].Mann DL, Mechanisms and models in heart failure: a combinatorial approach, Circulation 100 (1999) 999–1008. [DOI] [PubMed] [Google Scholar]
- [24].Su YR, Chiusa M, Brittain E, Hemnes AR, Absi TS, Lim CC, Di Salvo TG, Right ventricular protein expression profile in end-stage heart failure, Pulm. Circ 5 (2015) 481–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].di Salvo TG, Yang KC, Brittain E, Absi T, Maltais S, Hemnes A, Right ventricular myocardial biomarkers in human heart failure, J. Card. Fail 21 (2015) 398–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Huusko J, Lottonen L, Merentie M, Gurzeler E, Anisimov A, Miyanohara A, Alitalo K, Tavi P, Yla-Herttuala S, AAV9-mediated VEGF-B gene transfer improves systolic function in progressive left ventricular hypertrophy, J. Am. Soc. Gene Ther. 20 (2012) 2212–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Abraham D, Hofbauer R, Schafer R, Blumer R, Paulus P, Miksovsky A, Traxler H, Kocher A, Aharinejad S, Selective downregulation of VEGF-A(165), VEGF-R(1), and decreased capillary density in patients with dilative but not ischemic cardiomyopathy, Circ. Res 87 (2000) 644–647. [DOI] [PubMed] [Google Scholar]
- [28].Zhao T, Zhao W, Chen Y, Liu L, Ahokas RA, Sun Y, Differential expression of vascular endothelial growth factor isoforms and receptor subtypes in the infarcted heart, Int. J. Cardiol 167 (2013) 2638–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hall-Glenn F, Lyons KM, Roles for CCN2 in normal physiological processes, Cell. Mol. Life Sci. 68 (2011) 3209–3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Chen CC, Lau LF, Functions and mechanisms of action of CCN matricellular proteins, Int. J. Biochem. Cell Biol. 41 (2009) 771–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chen MM, Lam A, Abraham JA, Schreiner GF, Joly AH, CTGF expression is induced by TGF- beta in cardiac fibroblasts and cardiac myocytes: a potential role in heart fibrosis, J. Mol. Cell. Cardiol 32 (2000) 1805–1819. [DOI] [PubMed] [Google Scholar]
- [32].Ahmed MS, Oie E, Vinge LE, Yndestad A, Oystein Andersen G, Andersson Y, Attramadal T, Attramadal H, Connective tissue growth factor—a novel mediator of angiotensin II-stimulated cardiac fibroblast activation in heart failure in rats, J. Mol. Cell. Cardiol 36 (2004) 393–404. [DOI] [PubMed] [Google Scholar]
- [33].Koshman YE, Sternlicht MD, Kim T, O’Hara CP, Koczor CA, Lewis W, Seeley TW, Lipson KE, Samarel AM, Connective tissue growth factor regulates cardiac function and tissue remodeling in a mouse model of dilated cardiomyopathy, J. Mol. Cell. Cardiol 89 (2015) 214–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gravning J, Orn S, Kaasboll OJ, Martinov VN, Manhenke C, Dickstein K, Edvardsen T, Attramadal H, Ahmed MS, Myocardial connective tissue growth factor (CCN2/CTGF) attenuates left ventricular remodeling after myocardial infarction, PLoS One 7 (2012) e52120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bergestuen DS, Gravning J, Haugaa KH, Sahakyan LG, Aakhus S, Thiis-Evensen E, Oie E, Aukrust P, Attramadal H, Edvardsen T, Plasma CCN2/connective tissue growth factor is associated with right ventricular dysfunction in patients with neuroendocrine tumors, BMC Cancer 10 (2010) 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Badesch DB, Raskob GE, Elliott CG, Krichman AM, Farber HW, Frost AE, Barst RJ, Benza RL, Liou TG, Turner M, Giles S, Feldkircher K, Miller DP, McGoon MD, Pulmonary arterial hypertension: baseline characteristics from the REVEAL registry, Chest 137 (2010) 376–387. [DOI] [PubMed] [Google Scholar]
- [37].Benza RL, Miller DP, Gomberg-Maitland M, Frantz RP, Foreman AJ, Coffey CS, Frost A, Barst RJ, Badesch DB, Elliott CG, Liou TG, McGoon MD, Predicting survival in pulmonary arterial hypertension: insights from the registry to evaluate early and long-term pulmonary arterial hypertension disease management (REVEAL), Circulation 122 (2010) 164–172. [DOI] [PubMed] [Google Scholar]
- [38].Campo A, Mathai SC, Le Pavec J, Zaiman AL, Hummers LK, Boyce D, Housten T, Champion HC, Lechtzin N, Wigley FM, Girgis RE, Hassoun PM, Hemodynamic predictors of survival in scleroderma-related pulmonary arterial hypertension, Am. J. Respir. Crit. Care Med. 182 (2010) 252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Forfia PR, Fisher MR, Mathai SC, Housten-Harris T, Hemnes AR, Borlaug BA, Chamera E, Corretti MC, Champion HC, Abraham TP, Girgis RE, Hassoun PM, Tricuspid annular displacement predicts survival in pulmonary hypertension, Am. J. Respir. Crit. Care Med. 174 (2006) 1034–1041. [DOI] [PubMed] [Google Scholar]
- [40].Ghio S, Pazzano AS, Klersy C, Scelsi L, Raineri C, Camporotondo R, D’Armini A, Visconti LO, Clinical and prognostic relevance of echocardiographic evaluation of right ventricular geometry in patients with idiopathic pulmonary arterial hypertension, Am. J. Cardiol 107 (2011) 628–632. [DOI] [PubMed] [Google Scholar]
- [41].Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaici A, Weitzenblum E, Cordier JF, Chabot F, Dromer C, Pison C, Reynaud-Gaubert M, Haloun A, Laurent M, Hachulla E, Cottin V, Degano B, Jais X, Montani D, Souza R, Simonneau G, Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era, Circulation 122 (2010) 156–163. [DOI] [PubMed] [Google Scholar]
- [42].Sachdev A, Villarraga HR, Frantz RP, McGoon MD, Hsiao JF, Maalouf JF, Ammash NM, McCully RB, Miller FA, Pellikka PA, Oh JK, Kane GC, Right ventricular strain for prediction of survival in patients with pulmonary arterial hypertension, Chest 139 (2011) 1299–1309. [DOI] [PubMed] [Google Scholar]
- [43].van Wolferen SA, Marcus JT, Boonstra A, Marques KM, Bronzwaer JG, Spreeuwenberg MD, Postmus PE, Vonk-Noordegraaf A, Prognostic value of right ventricular mass, volume, and function in idiopathic pulmonary arterial hypertension, Eur. Heart J. 28 (2007) 1250–1257. [DOI] [PubMed] [Google Scholar]
- [44].d’Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Kernis JT, et al. , Survival in patients with primary pulmonary hypertension. Results from a national prospective registry, Ann. Intern. Med 115 (1991) 343–349. [DOI] [PubMed] [Google Scholar]
- [45].Sandoval J, Bauerle O, Palomar A, Gomez A, Martinez-Guerra ML, Beltran M, Guerrero ML, Survival in primary pulmonary hypertension. Validation of a prognostic equation, Circulation 89 (1994) 1733–1744. [DOI] [PubMed] [Google Scholar]
- [46].Lee YS, Byun J, Kim JA, Lee JS, Kim KL, Suh YL, Kim JM, Jang HS, Lee JY, Shin IS, Suh W, Jeon ES, Kim DK, Monocrotaline-induced pulmonary hypertension correlates with upregulation of connective tissue growth factor expression in the lung, Exp. Mol. Med 37 (2005) 27–35. [DOI] [PubMed] [Google Scholar]
- [47].Li G, Tang L, Jia P, Zhao J, Liu D, Liu B, Elevated plasma connective tissue growth factor levels in children with pulmonary arterial hypertension associated with congenital heart disease, Pediatr. Cardiol 37 (2016) 714–721. [DOI] [PubMed] [Google Scholar]
- [48].Takeda N, Morita H, Fujita D, Inuzuka R, Taniguchi Y, Imai Y, Hirata Y, Komuro I, Congenital contractural arachnodactyly complicated with aortic dilatation and dissection: case report and review of literature, Am. J. Med. Genet. A 167a (2015) 2382–2387. [DOI] [PubMed] [Google Scholar]
- [49].Hu X, Li Y, Li H, Fang Y, Liu M, You C, Is Alpha-1 antichymotrypsin gene polymorphism a risk factor for primary intracerebral hemorrhage? A case-control study and meta-analysis, Int. Med. J. Exp. Clin. Res 21 (2015) 2149–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Rowan AJ, Ramsay RE, Collins JF, Pryor F, Boardman KD, Uthman BM, Spitz M, Frederick T, Towne A, Carter GS, Marks W, Felicetta J, Tomyanovich ML, New onset geriatric epilepsy: a randomized study of gabapentin, lamotrigine, and carbamazepine, Neurology 64 (2005) 1868–1873. [DOI] [PubMed] [Google Scholar]
- [51].Kamboh MI, Sanghera DK, Ferrell RE, DeKosky ST, APOE*4-associated Alzheimer’s disease risk is modified by alpha 1-antichymotrypsin polymorphism, Nat. Genet 10 (1995) 486–488. [DOI] [PubMed] [Google Scholar]
- [52].Yamamoto M, Kondo I, Ogawa N, Asanuma M, Yamashita Y, Mizuno Y, Genetic association between susceptibility to Parkinson’s disease and alpha1-antic-hymotrypsin polymorphism, Brain Res. 759 (1997) 153–155. [DOI] [PubMed] [Google Scholar]
- [53].Furiya Y, Hirano M, Kurumatani N, Nakamuro T, Matsumura R, Futamura N, Ueno S, Alpha-1-antichymotrypsin gene polymorphism and susceptibility to multiple system atrophy (MSA), Brain Res. Mol. Brain Res. 138 (2005) 178–181. [DOI] [PubMed] [Google Scholar]
- [54].Dang NC, Topkara VK, Mercando M, Kay J, Kruger KH, Aboodi MS, Oz MC, Naka Y, Right heart failure after left ventricular assist device implantation in patients with chronic congestive heart failure, J Heart Lung Transplant 25 (2006) 1–6. [DOI] [PubMed] [Google Scholar]
- [55].Patel ND, Weiss ES, Schaffer J, Ullrich SL, Rivard DC, Shah AS, Russell SD, Conte JV, Right heart dysfunction after left ventricular assist device implantation: a comparison of the pulsatile HeartMate I and axial-flow HeartMate II devices, Ann. Thorac. Surg 86 (2008) 832–840 (discussion 832–40). [DOI] [PubMed] [Google Scholar]
- [56].Matthews JC, Koelling TM, Pagani FD, Aaronson KD, The right ventricular failure risk score a pre-operative tool for assessing the risk of right ventricular failure in left ventricular assist device candidates, J. Am. Coll. Cardiol 51 (2008) 2163–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Kormos RL, Teuteberg JJ, Pagani FD, Russell SD, John R, Miller LW, Massey T, Milano CA, Moazami N, Sundareswaran KS, Farrar DJ, Right ventricular failure in patients with the HeartMate II continuous-flow left ventricular assist device: incidence, risk factors, and effect on outcomes, J. Thorac. Cardiovasc. Surg 139 (2010) 1316–1324. [DOI] [PubMed] [Google Scholar]
- [58].Farrar DJ, Compton PG, Hershon JJ, Fonger JD, Hill JD, Right heart interaction with the mechanically assisted left heart, World J. Surg 9 (1985) 89–102. [DOI] [PubMed] [Google Scholar]
- [59].Farrar DJ, Ventricular interactions during mechanical circulatory support, Semin. Thorac. Cardiovasc. Surg 6 (1994) 163–168. [PubMed] [Google Scholar]
- [60].Moon MR, Bolger AF, DeAnda A, Komeda M, Daughters GT 2nd, S.D. Nikolic, D.C. Miller, N.B. Ingels Jr., Septal function during left ventricular unloading, Circulation 95 (1997) 1320–1327. [DOI] [PubMed] [Google Scholar]
- [61].Krishan K, Nair A, Pinney S, Adams DH, Anyanwu AC, Liberal use of tricuspid-valve annuloplasty during left-ventricular assist device implantation, Eur. J. Cardiothorac. Surg 41 (2012) 213–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Drakos SG, Janicki L, Horne BD, Kfoury AG, Reid BB, Clayson S, Horton K, Haddad F, Li DY, Renlund DG, Fisher PW, Risk factors predictive of right ventricular failure after left ventricular assist device implantation, Am. J. Cardiol 105 (2010) 1030–1035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.