Abstract
Diffusion tensor imaging studies report childhood adversity (CA) is associated with reduced fractional anisotropy (FA) in multiple white matter tracts in adults. Reduced FA may result from changes in tissue, suggesting myelin/axonal damage, and/or from increased levels of extracellular free-water, suggesting atrophy or neuroinflammation. Free-water imaging can separately identify FA in tissue (FAT) and the fractional volume of free-water (FW). We tested whether CA was associated with altered FA, FAT, and FW in seven white matter regions of interest (ROI), in which FA changes had been previously linked to CA (corona radiata, corpus callosum, fornix, cingulum bundle: hippocampal projection, inferior fronto-occipital fasciculus, superior longitudinal fasciculus, uncinate fasciculus). Tract-based spatial statistics were performed in 147 psychiatrically healthy adults who had completed a self-report questionnaire on CA primarily stemming from parental maltreatment. ROI were extracted according to the protocol provided by the ENIGMA-DTI working group. Analyses were performed both treating CA as a continuous and a categorical variable. CA was associated with reduced FA in all ROI (although categorical analyses failed to find an association in the fornix). In contrast, CA was only associated with reduced FAT in the corona radiata, corpus callosum, and uncinate fasciculus (with the continuous measure of CA finding evidence of a negative relation between CA and FAT in the fornix). There was no association between CA on FW in any ROI. These results provide preliminary evidence that childhood adversity is associated with changes to the microstructure of white matter itself in adulthood. However, these results should be treated with caution until they can be replicated by future studies which address the limitations of the present study.
Keywords: Abuse, diffusion tensor imaging, free-water, trauma
Childhood adversity is associated with an increased risk of physical and mental health problems in adulthood (Anda et al., 2006; Felitti et al., 1998; Kessler et al., 2010; McCarthy-Jones & McCarthy-Jones, 2015; Wegman & Stetler, 2009). There has recently been increased interest in how neural changes may mediate these relations. A number of studies have documented white matter changes in people who experienced childhood adversity, with the strongest evidence of change being a reduction in the volume of the corpus callosum and changes to its microstructure (e.g., de Bellis et al., 2002; Huang et al., 2012; Lu et al., 2013; Paul et al., 2008; Teicher et al., 2008, 2010).
The corpus callosum (CC) is the largest fiber tract in the brain, comprising over 190 million axons and connecting the two cerebral hemispheres, linking distant regions of the cerebral cortex (Edwards et al., 2014). Early volumetric studies reported that adverse childhood experiences were associated with reductions in the volume of the corpus callosum in children and adolescents (Teicher et al., 1997; de Bellis et al., 1999; de Bellis et al., 2002; Teicher et al., 2004). The development of Diffusion Tensor Imaging (DTI) has now allowed the field to clarify further the microstructural underpinnings of these callosal abnormalities. The most commonly used index in DTI studies is fractional anisotropy (FA), a measure of the directionality of water diffusion. Relative decreases in FA in white matter are suggestive of reduced tract integrity, potentially due to factors such as demyelination or axonal injury (Mori & Zhang, 2006). Maltreated children with PTSD have been found to have lower FA in their CC than healthy controls (Jackowski et al., 2008; Rinne-Albers et al., 2015). These changes appear to persist into adulthood, as healthy adolescents and adults exposed to childhood adversity also have reduced FA in their CC (Huang et al., 2012; Lu et al., 2013; Paul et al., 2008; Teicher et al., 2010; although see Seckfort et al., 2008 for a partial failure to replicate).
In addition to the CC, reductions in the FA of other white matter tracts in adulthood have also been linked to various forms of childhood adversity. We focus first on findings that have been replicated by at least one other study. Childhood adversity has been reported to be associated with FA reductions in the hippocampal projection of the cingulum bundle in children (Hanson et al., 2013), adolescents (Huang et al., 2012), and young adults (Choi et al., 2009), although Rinne-Albers et al. (2015) failed to replicate this finding in adolescent survivors of child sexual abuse with PTSD. The superior longitudinal fasciculus has also been reported to have lower FA in both adolescent (Huang et al., 2012) and young adult (Choi et al., 2009) survivors of childhood adversity. Finally, both child (Hanson et al., 2013) and adolescent (Huang et al., 2012) survivors of adversity have been found to have reductions in FA in the inferior fronto-occipital fasciculus. Individual studies have also found childhood adversity-related FA reductions in other white matter tracts, namely the fornix (Choi et al., 2009), the uncinate fasciculus (Eluvathingal et al., 2006), inferior longitudinal fasciculus (Hanson et al., 2013) and corona radiata (Hanson et al., 2013). Comparability between these studies is limited due to some focusing on specific types of child abuse (e.g., parental verbal abuse, Choi et al., 2009; neglect, Hanson et al., 2013; socioeconomic deprivation, Eluvathingal et al., 2006) and others using a variety of broader measures of childhood adversity (e.g., Childhood Adversity Interview, Huang et al., 2012; Childhood Trauma Questionnaire, Lu et al., 2013; Early Life Stress Questionnaire, Paul et al., 2008). Nevertheless, these studies can all inform the identification of ROI for future investigations.
A limitation common to all previous studies is their interpretation of FA as being a measure of the structural integrity of a tract itself. In reality, changes to FA may result from changes to the diffusion of water in tissue and/or changes to levels of freely diffusing water molecules in the extracellular space (termed free-water; Pasternak et al., 2009). Free-water is predominantly present in the cerebrospinal fluid (CSF), making CSF contamination a major problem for estimating DTI metrics in fiber tracts that are in close proximity to the ventricles (Chou et al., 2005), of which the CC is one. Whilst reductions in directional water diffusion in tissue may point to myelin degeneration and/or axonal damage, increases in free-water point to factors such as atrophy and/or neuroinflammation (Pasternak et al., 2012). The recent development of free-water imaging (Pasternak et al., 2009) offers a method to measure the fractional volume of freely diffusing water molecules (or, in short, FW) in the extracellular space and to then calculate the diffusion properties of water in the tissue itself after eliminating free-water, thereby creating a map of tissue-FA (FAT), which is a free-water corrected (non-contaminated) FA map.
The first aim of the current study was to use DTI to attempt to replicate previous studies and reassess whether childhood adversity was associated with reduced FA, in a large dataset of adults, in the seven regions of interest (ROI) suggested by previous research (corona radiata, corpus callosum, fornix, hippocampal projection of the cingulum bundle, inferior fronto-occipital fasciculus, superior longitudinal fasciculus, and the uncinate fasciculus). The second aim was to use free-water imaging to assess if childhood adversity was specifically associated with changes to either FAT or FW in these ROI, and hence to better understand the nature of biological changes associated with childhood adversity.
Method
Participants
The data for this study was provided by the Australian Schizophrenia Research Bank. The original data collection process is outlined in detail elsewhere (Loughland et al., 2010). Of 188 healthy controls with DTI data available, 150 had completed all twenty items of the Childhood Adversity Questionnaire (Rosenman & Rodgers, 2004) and were included in the study. These individuals had been screened to ensure they neither had a family history of, nor had themselves been treated for, psychiatric illness. Exclusionary criteria also included a history of epilepsy, seizures, dementia, neurological illnesses, movement disorders, organic brain disorders, brain injury or head injury with posttraumatic amnesia > 24 hours.
Measures
Childhood adversity.
Childhood adversity (CA) was assessed using the twenty-item Childhood Adversity Questionnaire (CAQ; Rosenman & Rodgers, 2004). The CAQ is comprised of items adapted from the Parental Bonding Instrument (Parker, 1979), the British National Survey of Health and Development (Rodgers, 1996), the US National Comorbidity Survey (NCS; Riso et al., 2002), with additional items developed from an Australian study (Henderson et al., 1998). It focusses on primarily childhood adversity stemming from parental maltreatment. CAQ questions assess lack of parental affection, parental emotional/depressive disorders, parental substance abuse, parental conflict, parental divorce/separation, abuse (e.g., sexual abuse by a parent; verbal abuse by a parent), neglect, authoritarian upbringing, parental indifference, as well as growing up in poverty/financial hardship. Items are scored in a dichotomous manner (present = 1, absent = 0), with the exception of parental mental health problems and parental substance use, which are scored on a 0–2 scale. Following McCabe et al., (2012) we recoded these latter items into a dichotomous form (0 scored 0, 1 and 2 scored as 1). The upper age limit for the experience of adversity in childhood was 18 years (McCabe et al., 2012).
Concerns can be raised as to whether a multi-item measure of childhood adversity can be validly treated as a continuous variable, given that intervals between the scale values are not equal. Previous studies employing such measures have treated the ensuing data as a categorical variable (e.g., Filippon et al., 2013), a continuous variable (e.g., Majer et al., 2010), or have reported analyses using both methods for completeness (e.g., Heim et al., 2009; Thompson et al., 2013). We followed this latter approach, performing analyses using both categorical and continuous scores derived from the CAQ, although our primary analyses were based on treating CAQ scores as a categorical variable. When the CAQ was treated as a categorical variable, participants were to be split into three groups; no recorded-CA (score of zero on the CAQ), low-CA, and high-CA. A cut-off point for the low- and high-CA groups was to be chosen based on the distribution of scores, and aimed to create groups of approximately equal size, to allow analyses comparing each of these CA groups to the no recorded-CA group to have equivalent statistical power.
Handedness.
Handedness was assessed using the Edinburgh Handedness Inventory (Oldfield, 1971) that produces a laterality quotient (LQ). This is a continuous variable that runs from −100 (fully left-handed) to 100 (fully right-handed).
Data Acquisition
Diffusion MRI scans were acquired on 1.5T Siemens Avanto scanners from five Australian locations. Identical imaging parameters were used across all scanners. Sixty-five axial slices were acquired that enabled whole-brain coverage in 64 gradient directions with a b-value of 1000s/mm2 and one non-diffusion-weighted (b0). Diffusion acquisition had a repetition time (TR) of 8.4s, an echo time (TE) of 88ms, a field of view (FOV) of 25×25cm, a matrix size of 104×104 and 2.5mm slice thickness without gap, producing 2.4mm voxels.
Processing of DTI
All scans were individually inspected for movement artifacts. In order to remove intra-scan misalignments due to head movements and eddy current-induced distortions, affine registration of the diffusion weighted images to the baseline image (b0) was performed for each individual participant (FSL, Functional MRI of the Brain [FMRIB] Software Library [FSL]). Slices with signal loss due to head movements were replaced by non-parametric predictions of the Gaussian Process, which is part of the ‘eddy’ tool implemented in FSL. Manual editing of a label map, initialized using the OTSU method in the software 3D Slicer (www.slicer.org) was used to remove non-brain areas and background noise after masking. The FW map and FAT maps were generated from the realigned volumes (Pasternak et al., 2009; 2012).
Tract-based spatial statistics
White matter was investigated using whole-brain tract-based spatial statistics (TBSS; Smith et al., 2006), according to the protocol provided by the ENIGMA-DTI working group (http://enigma.ini.usc.edu/ongoing/dti-working-group/). In brief, FA images from all participants were co-registered into the ENIGMA-DTI template. Each participant’s aligned FA image was then projected onto the ENIGMA-DTI skeleton, which represents a structural core of the white-matter (Jahanshad et al., 2013). In this way a skeletonized FA map was created, such that the central core of each participant’s white matter fiber tracts was represented on the skeleton. FAT and FW maps were projected onto this skeleton. Seven ROI (corona radiata, corpus callosum, fornix, hippocampal projection of the cingulum bundle, inferior fronto-occipital fasciculus, superior longitudinal fasciculus, and the uncinate fasciculus) were parcellated from the ENGIMA-DTI target according to the Johns Hopkins University atlas (Mori et al., 2008; Oishi et al., 2008). An average of the FA, FAT and FW measures over all skeleton voxels (i.e., averaging over both hemispheres for bilateral tracts) included in each of the ROI was calculated, and subjected to statistical analysis.
Statistical Analysis
When treating CAQ scores as a categorical variable, analysis of covariance (ANCOVA) was performed using SPSS (version 22, www.spss.com), testing for an effect of group (no recorded-CA, low-CA, high CA) on each diffusivity metric, whilst controlling for age, gender, handedness (laterality co-efficient), and scanner location (dummy coded). When testing for the significance of an effect of group on a specific measure (FA, FAT or FW) over the seven ROIs, a correction was made to alpha for multiple testing using the False Discovery Rate (FDR) method (Benjamini & Hochberg, 1995). Any significant group effects were to be followed up by post-hoc tests (with 1000 boot-strapped samples), correcting alpha for the multiple tests performed (three; no recorded-CA vs. low-CA, no recorded-CA vs. high-CA, low-CA vs. high-CA) using a Bonferroni-correction, resulting in p’=.017. When treating CAQ scores as a continuous variable, multiple linear regression was performed, with FA/FAT/FW as dependent variables. Age, gender, laterality quotient, and scanner location (dummy coded) were entered in a first step, with CAQ scores entered in a second step. The relation between CAQ scores and FA/FAT/FW was assessed by the significance of this second step.
Results
DTI data were screened for outliers, defined as values more than three standard deviations from the group mean. Three participants had outlying values of FW for multiple ROIs, and were hence removed from the analysis. In the remaining 147 participants, there were no outlying values of FA, 4 outlying values of FAT (all in the fornix; being one in what would come to be defined as the no-reported CA group, two in the low-CA group, and one in the high-CA group; see below for how these groups were identified and defined), and 12 outlying values of FW, being 1 in the corona radiate (high-CA group), 4 in the fornix (one in either of the no-reported and low CA group, two in the high-CA group), 2 in the inferior frontal-occipital fasciculus (both in the high-CA group), 1 in the superior longitudinal fasciculus (low-CA group), and 4 in the uncinate fasciculus (one in the low-CA group, three in the high-CA group). These outlying data points were removed from the analyses, with further tests establishing that this left no further outliers present.
The distribution of CAQ scores is shown in Figure 1. On the basis of this, participants were split into three groups; no recorded-CA (CAQ score = 0), low-CA (CAQ score of 1 or 2), high-CA (CAQ score of 3 or more). In order to characterize the low- and high-CA groups, the percentage of each group who endorsed each CAQ item are detailed in Table 1. As can be seen, the forms of childhood adversity reported by the low-CA group were typically either household conflict (reported by a slight majority of this group) or strict parenting. In contrast, nearly all the high-CA group had experienced household conflict, and a significant proportion reported that they had experienced multiple difficulties with their father, had a mother with emotional problems, had divorced/separated parents, had grown up in poverty, had strict parents, and had experienced verbal abuse, physical abuse, excessive punishment, and humiliation from a parent.
Figure 1.
Distributions of Childhood Adversity Questionnaire scores
Table 1.
Characterisation of low- and high-childhood adversity groups
| Childhood Adversity Questionnaire item | Low-CA (n=58) | High-CA (n=55) |
|---|---|---|
| Lack of father or lack of father’s affection | 8 (14%) | 17 (31%) |
| Father emotional problems | 2 (3%) | 15 (27%) |
| Father drinking or drug problem | 6 (10%) | 20 (36%) |
| Lack of mother or lack of mother’s affection | 2 (3%) | 6 (11%) |
| Mother emotional problems | 0 | 20 (36%) |
| Mother drinking or drug problem | 2 (5%) | 7 (13%) |
| Conflict in household | 35 (59%) | 50 (91%) |
| Parents divorced/separated | 3 (5%) | 17 (31%) |
| Unhappy childhood | 1 (2%) | 12 (22%) |
| Parents didn’t do their best | 0 | 5 (9%) |
| Neglected | 1 (2%) | 8 (15%) |
| Strict parents | 17 (29%) | 25 (46%) |
| Grew up in poverty | 1 (2%) | 23 (42%) |
| Verbal abuse by parent | 2 (3%) | 23 (42%) |
| Humiliated by parent | 0 | 14 (26%) |
| Witnessed physical/sexual abuse in family | 0 | 15 (27%) |
| Physically abused by parent | 3 (5%) | 16 (29%) |
| Too much physical punishment | 1 (2%) | 13 (24%) |
| Sexually abused | 0 | 2 (4%) |
| Abnormal childhood | 0 | 12 (22%) |
Note. CA = Childhood adversity
Descriptive statistics for each group are presented in Table 2. There were significantly more women in the low- and high-CA groups than in the no recorded-CA group but the groups did not differ in age, laterality quotients, or scan location.
Table 2.
Demographic and clinical characteristics of sample
| Variable | No recorded-CA (n=34) | Low-CA (n=58) | High-CA (n=55) | Difference |
|---|---|---|---|---|
| Age | 37.15 (14.72) | 40.64 (14.26) | 42.05 (13.05) | F(2,144)=1.32, p=.27 |
| Gender (% female) | 29% | 59% | 56% | χ2(2)=8.32, p=.02 |
| Laterality quotient | 61.52 (69.20) | 47.10 (76.88) | 59.63 (68.05) | F(2,144)=.60, p=.55 |
| Childhood Adversity Questionnaire | 0 (0) | 1.45 (.50) | 5.82 (3.18) | F(2,144)=112.16, p<.001 |
| Scan location | ||||
| Sydney | 9 | 13 | 11 | |
| Melbourne | 12 | 25 | 31 | χ2(6)=5.34, p=.50 |
| Brisbane | 8 | 15 | 8 | |
| Newcastle | 5 | 5 | 5 | |
Note. CA = Childhood adversity
ANCOVA, controlling for age, gender, laterality quotient, and scanner location (dummy coded), found an effect of CA-group on FA in six of the seven ROI, with the exception being the fornix (Table 3). In all six of these ROI, post-hoc tests indicated that the high-CA group had lower FA than the no recorded-CA group, with no other group comparisons being significant. Multiple linear regression was also performed, treating CAQ scores as a continuous variable. These analyses entered age, gender, laterality quotient, and scanner location (dummy coded) in a first step, and CAQ score in the second step. These found an effect of CAQ on FA in all seven regions of interest, namely the cingulate (hippocampus), ΔF(1,139)=7.25, p=.008, ΔR2=.04, corpus callosum, ΔF(1,139)=4.96, p=.028, ΔR2=.03, corona radiate, ΔF(1,139)=5.12, p=.025, ΔR2=.03, fornix, ΔF(1,139)=4.04, p=.047, ΔR2=.02, inferior fronto-occipital fasciculus, ΔF(1,139)=5.23, p=.024, ΔR2=.03, superior longitudinal fasciculus, ΔF(1,139)=4.19, p=.043, ΔR2=.03, and uncinate fasciculus, ΔF(1,139)=4.73, p=.031, ΔR2=.03.
Table 3.
Associations between childhood adversity and fractional anisotropy (FA)
| Tract | Adjusted meana (standard error) |
Group effect F(2,138) | Significant post-hoc testsb,c | ||
|---|---|---|---|---|---|
| No-recorded CA | Low-CA | High-CA | |||
| Cingulum (hippocampus) | .713 (.021) | .671 (.016) | .621 (.016) | 6.05, p=.003*, η2=.09 | High-CA<No-CA p=.001, d=0.77 (0.33–1.22) |
| Corona radiata | .656 (.024) | .621 (.018) | .567 (.018) | 4.77, p=.010*, η2=.07 | High-CA<No-CA p=.010, d=0.66 (0.22–1.10) |
| Corpus callosum | .821 (.013) | .804 (.009) | .773 (.010) | 4.97, p=.008*, η2=.07 | High-CA<No-CA p=.005, d=0.65 (0.21–1.09) |
| Fornix | .614 (.021) | .584 (.016) | .555 (.016) | 2.39, p=.095, η2=.03 | - |
| Inferior frontal-occipital fasciculus | .715 (.023) | .673 (.017) | .625 (.018) | 4.74, p=.010*, η2=.06 | High-CA<No-CA p=.006, d=0.68 (0.24–1.12) |
| Superior longitudinal fasciculus | .666 (.021) | .631 (.015) | .589 (.016) | 4.49, p=.013*, η2=.06 | High-CA<No-CA p=.010, d=0.64 (0.21–1.09) |
| Uncinate fasciculus | .735 (.022) | .703 (.017) | .653 (.017) | 4.50, p=.013*, η2=.06 | High-CA<No-CA p=.006, d=0.65 (0.22–1.09) |
= Significant after False Discovery Rate correction.
= Marginal means after adjustment for age, gender, laterality quotient, and scanner location.
= Significant differences determined using a Bonferroni corrected alpha of p’=.017.
= Cohen’s d, calculated based on the marginal means, is reported with 95% confidence intervals. CA = Childhood adversity.
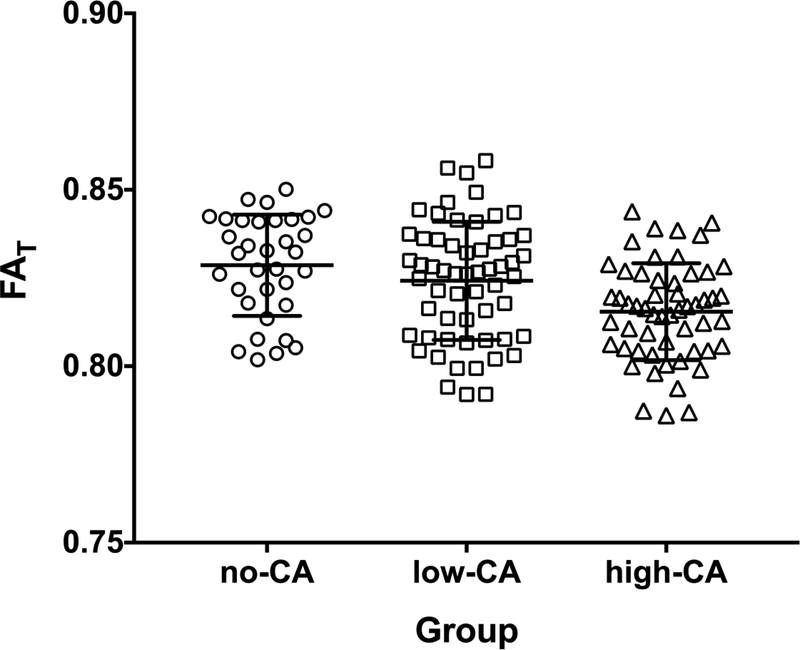
ANCOVA only found an effect of CA-group on FAT in the corona radiata, corpus callosum, and uncinate fasciculus (Table 4). Post-hoc tests revealed that for all three of these ROIs, the high-CA group had lower FAT than both the no recorded-CA group and the low-CA group (Table 4). There were no differences between the low-CA and no recorded-CA groups. Despite these significant differences, there were still notable overlap between the groups, with the distribution of FAT values in the corpus callosum by CA-group being shown in Figure 2 to illustrate this. Multiple linear regression found an effect of CAQ score on FAT in the same three regions of interest [corpus callosum, ΔF(1,139)=4.55, p=.035, ΔR2=.03, corona radiate, ΔF(1,139)=5.60, p=.019 ΔR2=.03, and uncinate fasciculus, ΔF(1,139)=5.47, p=.021, ΔR2=.04] but also in the fornix, ΔF(1,135)=5.24, p=.024, ΔR2=.04. There was no evidence of an association between CAQ scores and FAT in the cingulate (hippocampus), ΔF(1,139)=0.05, p=.821, ΔR2=.00, inferior fronto-occipital fasciculus, ΔF(1,139)=3.16, p=.078, ΔR2=.02, or superior longitudinal fasciculus, ΔF(1,139)=0.13, p=.718, ΔR2=.00.
Table 4.
Associations by tract between childhood adversity and fractional anisotropy in tissue (FAT)
| Tract | Adjusted meana (standard error) |
Group effect F(2,138) | Significant post-hoc testsb,c | ||
|---|---|---|---|---|---|
| No recorded-CA | Low-CA | High-CA | |||
| Cingulum (hippocampus) | .669 (.006) | .661 (.004) | .657 (.004) | 1.41, p=.249, η2=.02 | - |
| Corona radiate | .616 (.004) | .608 (.003) | .598 (.003) | 7.42, p=.001*, η2=.10 | High-CA<No-CA p=.001, d=0.80 (0.36–1.25)High-CA<Low-CA p=.006, d=0.45 (0.07–0.82) |
| Corpus callosum | .826 (.003) | .825 (.002) | .817 (.002) | 6.07, p=.003*, η2=.08 | High-CA<No-CA p=.005, d=0.57 (0.14–1.01)High-CA<Low-CA p=.004, d=0.54 (0.16–0.91) |
| Fornix | .754 (.010) | .761 (.007) | .771 (.007) | 1.13d, p=.325, η2=.02 | |
| Inferior frontal-occipital fasciculus | .660 (.005) | .656 (.004) | .656 (.004) | 0.23, p=.794, η2<.01 | - |
| Superior longitudinal fasciculus | .627 (.003) | .621 (.002) | .618 (.003) | 2.14, p=.122, η2=.03 | - |
| Uncinate fasciculus | .771 (.007) | .771 (.005) | .750 (.005) | 5.23, p=.006*, η2=.07 | High-CA<No-CA p=.015, d=0.55 (0.12–0.99)High-CA<Low-CA p=.005, d=0.56 (0.19–0.94) |
= Significant after False Discovery Rate correction.
= Marginal means after adjustment for age, gender, laterality quotient, and scanner location.
= Significant differences determined using a Bonferroni corrected alpha of p’=.017.
= Cohen’s d, calculated based on the marginal means, is reported with 95% confidence intervals.
= Due to missing data, the degrees of freedom of the F-statistic were 2,134. CA = Childhood adversity.
Figure 2.
Scatterplot of (unadjusted) mean fractional anisotropy in tissue (FAT ) in the corpus callosum by childhood adversity group Note. FAT = fractional anisotropy in tissue. CA = Childhood adversity. Error bars represent +/− one standard deviation.
There was no effect of CA-group on FW in any ROI (Table 5). Similarly, multiple linear regression found no association between CAQ scores and FW in any ROI; cingulate (hippocampus), ΔF(1,139)=0.39, p=.535, ΔR2=.00, corona radiate, ΔF(1,138)=3.73, p=.055, ΔR2=.02, corpus callosum, ΔF(1,139)=2.13, p=.147, ΔR2=.01, fornix, ΔF(1,135)=0.67, p=.416, ΔR2=.00, inferior fronto-occipital fasciculus, ΔF(1,137)=0.20, p=.655, ΔR2=.00, superior longitudinal fasciculus, ΔF(1,138)=0.03, p=.867, ΔR2=.00, and uncinate fasciculus, ΔF(1,135)=0.16, p=.694, ΔR2=.00.
Table 5.
Associations by tract between childhood adversity and fractional volume of free-water (FW)
| Tract | Adjusted meana (standard error) |
Group effect | ||
|---|---|---|---|---|
| No recorded-CA | Low-CA | High-CA | ||
| Cingulum (hippocampus) | .758 (.015) | .773 (.011) | .761 (.012) | F(2,138)=0.40, p=.674, η2<.01 |
| Corona radiata | .409 (.011) | .390 (.008) | .381 (.008) | F(2,137)=1.97, p=.143, η2=.03 |
| Corpus callosum | .617 (.011) | .599 (.008) | .582 (.008) | F(2,138)=3.25, p=.042, η2=.05 |
| Fornix | .885 (.013) | .881 (.010) | .870 (.010) | F(2,134)=0.48, p=.619, η2<.01 |
| Inferior frontal-occipital fasciculus | .487 (.015) | .463 (.011) | .480 (.012) | F(2,136)=0.96, p=.387, η2=.01 |
| Superior longitudinal fasciculus | .420 (.012) | .440 (.009) | .429 (.009) | F(2,137)=0.97, p=.383, η2=.01 |
| Uncinate fasciculus | .398 (.025) | .427 (.019) | .386 (.020) | F(2,134)=1.22, p=.299, η2=.02 |
= Marginal means after adjustment for age, gender, laterality quotient, and scanner location.
CA = Childhood Adversity.
Discussion
The first aim of this study was to reassess, in a large dataset, the association of childhood adversity in psychiatrically healthy adults with changes in conventionally measured fractional anisotropy (FA) in seven ROI selected on the basis of previous research. Our categorical analyses found that adults who had experienced high levels of childhood adversity had reductions in FA in six of the seven ROIs studied, namely the hippocampal projection of the cingulum bundle, the corona radiata, the corpus callosum, the superior longitudinal fasciculus, the inferior frontal-occipital fasciculus, and the uncinate fasciculus, compared to adults with no reported childhood adversity. Our continuous analyses also found greater levels of childhood adversity were associated with reductions in FA in these tracts, as well as in the fornix. These results are broadly in line with the research literature in this area (Choi et al., 2009; Eluvathingal et al., 2006; Hanson et al., 2013; Huang et al., 2012; Lu et al., 2013; Paul et al., 2008; Teicher et al., 2010). Yet, whilst these previous studies each typically reported reduced FA in only one or two tracts to be associated with childhood adversity, we found evidence of FA reductions across this wide range of spatially distinct tracts. This may be explained by our larger sample, and hence the greater power of our study. Another possible reason for this could be that our measure of childhood adversity was primarily focussed on adversity resulting from parental maltreatment, which may be associated with a greater chronicity of exposure (e.g., Dube & Hébert, 1988), and a greater negative impact on security of attachment (Lamb et al., 1985), than a more general measure of childhood adversity.
The second aim of our study was to test whether or not childhood adversity was associated with the measures of free-water corrected fractional anisotropy (pertaining to diffusion in white matter tissue itself; FAT), and free-water (the fractional volume of freely diffusing water molecules in the extracellular space; FW), which are arguably more sensitive and biologically specific than FA. Our categorical analyses found that the high-childhood adversity group had reduced FAT in the corona radiata, corpus callosum, and uncinate fasciculus, compared to both the no-childhood and low-childhood adversity groups. Our continuous analyses similarly found greater levels of childhood adversity to be associated with reductions in FAT in these three ROIs, and also in the fornix. This suggests that childhood adversity is associated with alterations to the white matter itself in these tracts. As the significant associations between childhood adversity and FA in the hippocampal projection of the cingulum bundle, superior longitudinal fasciculus, the inferior frontal-occipital fasciculus, were not found for FAT, this suggests that these findings may have reflected alterations in extracellular volume as opposed to tissue microstructure per se. However, since no significant changes in FW were observed, these extracellular changes are likely subtle and may not be specific to childhood adversity. Due to the removal of outlying data points, the participants in the three different FA metric groups were subtly different for some ROIs, which maximised the power of our within-group analyses (i.e., associations between CA and FA metrics) but necessitated some caution in concluding on the nature of between-group differences (i.e., associations between CA and FAT but not CA and FA).
Our study adds to our current understandings in two ways. First, it shows that associations between white matter microstructure and retrospective reports of childhood adversity, previously reported predominantly in child or young adult populations, can also be documented in a psychiatrically healthy middle-aged population. Second, it suggests that the white matter changes associated with childhood adversity are likely to involve the tissue of tracts specifically, rather than increased extracellular water. Although these findings are consistent with a model in which childhood adversity affects neurodevelopment, resulting in microstructural alterations to white matter tracts, we are unable to conclude that this causal relation exists due to the cross-sectional nature of our study and its specific limitations.
A first set of limitations stemmed from our measure of childhood adversity. Our measure was open to the criticism of any self-report measure of childhood adversity, namely that it is subject to bias (Susser & Widom, 2012). Responses to self-report childhood adversity questionnaires can be inconsistent over time (Widom & Czaja, 2012), with there being some evidence that responses are influenced by the respondent’s current emotional state (Prescott et al., 2000). Consistent with this, prospective and retrospective studies of the effects of childhood adversity can find differential associations with psychopathology (e.g., Widom et al., 1999). Despite this, a review of studies of retrospective recall of childhood adversity suggested that valid conclusions might be drawn when asking about “major adversities of an easily defined kind”, such as those inquired about by our measure of childhood adversity (Hardt & Rutter, 2004). Nevertheless, future studies would benefit from determination of the presence of childhood adversity through other means, such as court records. Our measure of childhood adversity also amalgamated different types of abuse and hence did not allow us to assess the association between specific white matter changes and specific types of child abuse. Further research may wish to explore whether or not specific types of abuse (e.g., neglect, sexual abuse) have differential effects on specific white tracts (e.g., Choi et al., 2009). Our childhood adversity measure also did not assess the severity of the adversities reported, and hence may have lacked sensitivity. It was also focussed primarily on parental maltreatment, and did not account for adversity of extra-familial origins.
Other limitations were also present in our study. The data employed in this study were acquired from five separate scanners. While all scanners were the same model and built according to the same specifications, disparities between the scanners could have potentially impacted the DTI measures. Furthermore, diffusion MRI data from this study was acquired with a single b-value shell, which reduces the specificity and sensitivity of the free-water, bi-tensor model (Pasternak et al. 2012). However, the difference between single- and multi-shell acquisitions for extracellular volume estimations is subtle, particularly when the algorithm to fit the free-water model involves spatial regularization as in the present study (Pasternak et al., 2012). A further limitation pertained to our sample. We had no data on the presence of events that may have mitigated or enhanced the impact of childhood adversity, such as levels of social support or adult re-victimisation, for example. Finally, our approach was a hypothesis driven approach. However, it would also have been useful to undertake an exploratory whole-brain voxel-wise TBSS analysis. This could have examined if white matter changes in other parts of the brain, not predicted by previous work, are also associated with childhood adversity. Such an approach should be undertaken by future studies.
In summary, this study found evidence consistent with high levels of childhood adversity being associated with alterations to the tissue of the corona radiata, corpus callosum, uncinate fasciculus, and perhaps the fornix, in psychiatrically healthy adults. In contrast, there was no evidence that low levels of parental-related childhood adversity (i.e., parental conflict and strict parenting) were associated with changes to the tissue of white matter, and no evidence that parental-related childhood adversity was associated with altered levels of extracellular free-water. There is now the need for alternative research designs to be employed to test if our findings can be replicated when the limitations of our study are remedied. Specifically, there is the need for a large, single-site study that does not rely on self-report measures of childhood adversity, and which takes into account the potential presence of factors that may have mitigated or enhanced the impact of childhood adversity. As noted above, such studies may also wish to consider exploratory whole-brain voxel-wise TBSS analyses. Future research may also wish to examine whether white matter changes, such as we have reported in the present study, are associated with detectable cognitive changes, even in psychiatrically healthy adults. The uncinate fasciculus is involved in emotional regulation (Versace et al., 2015) and, through memory, the valence-based biasing of decisions (Von Der Heide et al., 2013). The corona radiata plays a role in attentional control (Niogi et al., 2008), and alterations of the corpus callosum have been linked to a wide range of neuropsychological impairments (Fryer et al., 2008). Alterations to such abilities are broadly consistent with the cognitive and affective changes associated with early life stress (Pechtel & Pizzagalli, 2011). Finally, if childhood adversity-related neurological changes are found to exist, it will be important to assess the meaning of these to survivors (although it should be emphasized that the changes found here were at the level of the group, rather than the individual). That is, what does it mean to an individual to be psychiatrically healthy yet still carrying the signature of adversity in one’s white matter? More work is needed that considers the hermeneutics of biological research findings.
Acknowledgments
Funding. This study was supported by the Schizophrenia Research Institute using data from the Australian Schizophrenia Research Bank, funded by NHMRC Enabling Grant (No.386500) held by V Carr, U Schall, R Scott, A Jablensky, B Mowry, P Michie, S Catts, F Henskens and C Pantelis (Chief Investigators), and the Pratt Foundation, Ramsay Health Care, the Viertel Charitable Foundation, as well the Schizophrenia Research Institute, using an infrastructure grant from the NSW Ministry of Health. Simon McCarthy-Jones’s work was in part supported by Australian Research Council Discovery Early Career Researcher Award (DE140101077). Thomas Whitford is supported by a Discovery Project from the Australian Research Council (DP140104394), a Career Development Fellowship from the National Health and Medical Research Council of Australia (APP1090507). Ofer Pasternak, Marek Kubicki, Martha E Shenton and Amanda Lyall are supported by the following National Institutes of Health (NIH) grants: RO1MH108574 (OP, MK and MES), R01AG04252 (MK and OP), R01MH102377 (MK, MES and OP), R01MH074794 (OP), P41EB015902 (OP, MES), T32MH016259 (AL). Peter Savadjiev is supported by a Young Investigator Award from the NARSAD Brain and Behavior Research Foundation (22591).
Footnotes
Conflict of Interest. The authors declare they have no conflict of interest.
Compliance with Ethical Standards
Ethical approval. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent. Informed consent was obtained from all individual participants included in the study by the Australian Schizophrenia Research Bank at the time of data collection.
References
- Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield CH, Perry BD,…& Giles WH (2006). The enduring effects of abuse and related adverse experiences in childhood. European Archives of Psychiatry and Clinical Neuroscience, 256(3), 174–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnone D, Barrick TR, Chengappa S, Mackay CE, Clark CA, & Abou-Saleh MT (2008). Corpus callosum damage in heavy marijuana use: preliminary evidence from diffusion tensor tractography and tract-based spatial statistics. Neuroimage, 41(3), 1067–1074. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological), 289–300. [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T,…& Zule W (2003). Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse & Neglect, 27(2), 169–190. [DOI] [PubMed] [Google Scholar]
- Choi J, Jeong B, Rohan ML, Polcari AM, & Teicher MH (2009). Preliminary evidence for white matter tract abnormalities in young adults exposed to parental verbal abuse. Biological Psychiatry, 65(3), 227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Clark DB, Casey BJ, Giedd JN, Boring AM,…& Ryan ND (1999). Developmental traumatology part II: brain development. Biological Psychiatry, 45(10), 1271–1284. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Shifflett H, Iyengar S, Beers SR, Hall J, & Moritz G (2002). Brain structures in pediatric maltreatment-related posttraumatic stress disorder: a sociodemographically matched study. Biological Psychiatry, 52(11), 1066–1078. [DOI] [PubMed] [Google Scholar]
- Dragovic M (2004). Categorization and validation of handedness using latent class analysis. Acta Neuropsychiatrica, 16, 212–8. [DOI] [PubMed] [Google Scholar]
- Edwards TJ, Sherr EH, Barkovich AJ, & Richards LJ (2014). Clinical, genetic and imaging findings identify new causes for corpus callosum development syndromes. Brain, awt358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eluvathingal TJ, Chugani HT, Behen ME, Juhász C, Muzik O, Maqbool M,…& Makki M (2006). Abnormal brain connectivity in children after early severe socioemotional deprivation: a diffusion tensor imaging study. Pediatrics, 117(6), 2093–2100. [DOI] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V,…& Marks JS (1998). Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: the Adverse Childhood Experiences (ACE) study. American Journal of Preventive Medicine, 14(4), 245–258. [DOI] [PubMed] [Google Scholar]
- Filippon APM, Bassani DG, Aguiar RWD, & Ceitlin LHF (2013). Association between childhood trauma and loss of functionality in adult women with fibromyalgia. Trends in Psychiatry and Psychotherapy, 35(1), 46–54. [DOI] [PubMed] [Google Scholar]
- Fryer SL, Frank LR, Spadoni AD, Theilmann RJ, Nagel BJ, Schweinsburg AD, & Tapert SF (2008). Microstructural integrity of the corpus callosum linked with neuropsychological performance in adolescents. Brain and Cognition, 67(2), 225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Adluru N, Chung MK, Alexander AL, Davidson RJ, & Pollak SD (2013). Early neglect is associated with alterations in white matter integrity and cognitive functioning. Child Development, 84(5), 1566–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardt J, & Rutter M (2004). Validity of adult retrospective reports of adverse childhood experiences: review of the evidence Journal of Child Psychology and Psychiatry, 45, 260–273. [DOI] [PubMed] [Google Scholar]
- Heim C, Nater UM, Maloney E, Boneva R, Jones JF, & Reeves WC (2009). Childhood trauma and risk for chronic fatigue syndrome: association with neuroendocrine dysfunction. Archives of General Psychiatry, 66(1), 72–80. [DOI] [PubMed] [Google Scholar]
- Henderson AS, Jorm AF, Korten AE, Jacomb P, Christensen H, & Rodgers B (1998). Symptoms of depression and anxiety during adult life: evidence for a decline in prevalence with age. Psychological Medicine, 28(6), 1321–8. [DOI] [PubMed] [Google Scholar]
- Huang H, Gundapuneedi T, & Rao U (2012). White matter disruptions in adolescents exposed to childhood maltreatment and vulnerability to psychopathology. Neuropsychopharmacology, 37(12), 2693–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackowski MP, Douglas-Palumberi H, Jackowskie M, Win L, Schultz RT, Staib LW, … Kaufman J (2008). Corpus callosum in maltreated children with PTSD: A diffusion tensor imaging study. Psychiatry Research, 162(3), 256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanshad N, Kochunov PV, Sprooten E, Mandl RC, Nichols TE, Almasy L,…,Glahn DC (2013). Multi-site genetic analysis of diffusion images and voxelwise heritability analysis: a pilot project of the ENIGMA-DTI working group. NeuroImage, 81, 455–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM,…& Benjet C (2010). Childhood adversities and adult psychopathology in the WHO World Mental Health Surveys. The British Journal of Psychiatry, 197(5), 378–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, Williamson DE, Lancaster J, Fox P, Cornell J, Blangero J, & Glahn DC (2012). Fractional anisotropy of water diffusion in cerebral white matter across the lifespan. Neurobiology of Aging, 33(1), 9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb ME, Gaensbauer TJ, Malkin CM, & Schultz LA (1985). The effects of child maltreatment on security of infant-adult attachment. Infant Behavior and Development, 8(1), 35–45. [Google Scholar]
- Loughland C, Draganic D, McCabe K, Richards J, Nasir A, Allen J, … Carr V (2010). Australian Schizophrenia Research Bank: a database of comprehensive clinical, endophenotypic and genetic data for aetiological studies of schizophrenia. Australian and New Zealand Journal of Psychiatry, 44(11), 1029–1035. [DOI] [PubMed] [Google Scholar]
- Lu S, Wei Z, Gao W, Wu W, Liao M, Zhang Y,…& Li L (2013). White matter integrity alterations in young healthy adults reporting childhood trauma: A diffusion tensor imaging study. Australian and New Zealand Journal of Psychiatry, 47(12), 1183–1190. [DOI] [PubMed] [Google Scholar]
- Majer M, Nater UM, Lin JMS, Capuron L, & Reeves WC (2010). Association of childhood trauma with cognitive function in healthy adults: a pilot study. BMC Neurology, 10, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe KL, Maloney EA, Stain HJ, Loughland CM, & Carr VJ (2012). Relationship between childhood adversity and clinical and cognitive features in schizophrenia. Journal of Psychiatric Research, 46(5), 600–607. [DOI] [PubMed] [Google Scholar]
- McCarthy-Jones S, & McCarthy-Jones R (2014). Body mass index and anxiety/depression as mediators of the effects of child sexual and physical abuse on physical health disorders in women. Child Abuse & Neglect, 38(12), 2007–2020. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Hasan KM, Steinberg JL, Kramer LA, Dougherty DM, Santos RM,…& Narayana PA (2005). Reduced anterior corpus callosum white matter integrity is related to increased impulsivity and reduced discriminability in cocaine-dependent subjects: diffusion tensor imaging. Neuropsychopharmacology, 30(3), 610–617. [DOI] [PubMed] [Google Scholar]
- Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K,…, Mazziotta J (2008). Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. NeuroImage, 40(2), 570–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, & Zhang J (2006). Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron, 51(5), 527–539. [DOI] [PubMed] [Google Scholar]
- Narayana PA, Herrera JJ, Bockhorst KH, Esparza-Coss E, Xia Y, Steinberg JL, & Moeller FG (2014). Chronic cocaine administration causes extensive white matter damage in brain: Diffusion tensor imaging and immunohistochemistry studies. Psychiatry Research: Neuroimaging, 221(3), 220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niogi S, Mukherjee P, Ghajar J, & McCandliss BD (2010). Individual differences in distinct components of attention are linked to anatomical variations in distinct white matter tracts. Frontiers in Neuroanatomy, 4(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC (1971). The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia, 9, 97–113. [DOI] [PubMed] [Google Scholar]
- Oishi K, Zilles K, Amunts K, Faria A, Jiang H, Li X,…, Mori S (2008). Human brain white matter atlas: identification and assignment of common anatomical structures in superficial white matter. Neuroimage, 43(3), 447–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker G, Tupling H, & Brown LB (1979). A parental bonding instrument. British Journal of Medical Psychology, 52(1), 1–10. [Google Scholar]
- Pasternak O, Sochen N, Gur Y, Intrator N, & Assaf Y (2009). Free water elimination and mapping from diffusion MRI. Magnetic Resonance in Medicine, 62(3), 717–730. [DOI] [PubMed] [Google Scholar]
- Pasternak O, Shenton ME, & Westin CF (2012). Estimation of extracellular volume from regularized multi-shell diffusion MRI. Medical Image Computing and Computer Assisted Interventions, 15(2), 305–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak O, Westin CF, Bouix S, Seidman LJ, Goldstein JM, Woo TUW,…& Shenton ME (2012). Excessive extracellular volume reveals a neurodegenerative pattern in schizophrenia onset. The Journal of Neuroscience, 32(48), 17365–17372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R, Henry L, Grieve SM, Guilmette TJ, Niaura R, Bryant R,…& Gordon E (2008). The relationship between early life stress and microstructural integrity of the corpus callosum in a non-clinical population. Neuropsychiatric Disease and Treatment, 4(1B), 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechtel P, & Pizzagalli DA (2011). Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology, 214(1), 55–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Hedehus M, Adalsteinsson E, Lim KO, & Moseley M (2000). In vivo detection and functional correlates of white matter microstructural disruption in chronic alcoholism. Alcoholism: Clinical and Experimental Research, 24(8), 1214–1221. [PubMed] [Google Scholar]
- Prescott A, Bank L, Reid JB, Knutson JF, Burraston BO, & Eddy JM (2000). The veridicality of punitive childhood experiences reported by adolescents and young adults. Child Abuse & Neglect, 24, 411–423. [DOI] [PubMed] [Google Scholar]
- Rinne-Albers MA, van der Werff SJ, van Hoof MJ, van Lang ND, Lamers-Winkelman F, Rombouts SA,…& van der Wee NJ (2015). Abnormalities of white matter integrity in the corpus callosum of adolescents with PTSD after childhood sexual abuse: a DTI study. European Child & Adolescent Psychiatry, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riso LP, Miyatake RK, & Thase ME (2002). The search for determinants of chronic depression: a review of six factors. Journal of Affective Disorders, 70(2), 103–15. [DOI] [PubMed] [Google Scholar]
- Rodgers B (1996). Reported parental behaviour and adult affective symptoms. 1. Associations and moderating factors. Psychological Medicine, 26(1), 51–61. [DOI] [PubMed] [Google Scholar]
- Rosenman S, & Rodgers B (2004). Childhood adversity in an Australian population. Social Psychiatry and Psychiatric Epidemiology, 39, 695–702. [DOI] [PubMed] [Google Scholar]
- Seckfort DL, Paul R, Grieve SM, Vandenberg B, Bryant RA, Williams LMCCR, … Gordon E (2008) Early life stress on brain structure and function across the lifespan: a preliminary study. Brain Imaging & Behaviour, 2, 49–58. [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE,…, Behrens TE (2006). Tract-based spatial statistics: voxelwise analysis of multi subject diffusion data. NeuroImage, 31(4), 1487–1505. [DOI] [PubMed] [Google Scholar]
- Susser E, & Widom CS (2012). Still searching for lost truths about the bitter sorrows of childhood. Schizophrenia Bulletin, 38(4), 672–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Samson JA, Sheu YS, Polcari A, & McGreenery CE (2010). Hurtful words: association of exposure to peer verbal abuse with elevated psychiatric symptom scores and corpus callosum abnormalities. American Journal of Psychiatry, 167, 1464–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Dumont NL, Ito Y, Vaituzis C, Giedd JN, & Andersen SL (2004). Childhood neglect is associated with reduced corpus callosum area. Biological Psychiatry, 56(2), 80–85. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Ito Y, Glod CA, Andersen SL, Dumont N, & Ackerman E (1997). Preliminary evidence for abnormal cortical development in physically and sexually abused children using EEG coherence and MRI. Annals of the New York Academy of Sciences, 821(1), 160–175. [DOI] [PubMed] [Google Scholar]
- Thompson AD, Nelson B, Yuen HP, Lin A, Amminger GP, McGorry PD,…& Yung AR (2013). Sexual trauma increases the risk of developing psychosis in an ultra high-risk “prodromal” population. Schizophrenia Bulletin, 40 (3), 697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari-Woodruff S, Morales LBJ, Lee R, & Voskuhl RR (2007). Differential neuroprotective and antiinflammatory effects of estrogen receptor (ER) α and ERβ ligand treatment. Proceedings of the National Academy of Sciences, 104(37), 14813–14818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versace A, Acuff H, Bertocci MA, Bebko G, Almeida JR, Perlman SB,…& Bonar L (2015). White matter structure in youth with behavioral and emotional dysregulation disorders: a probabilistic tractographic study. JAMA Psychiatry, 72(4), 367–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Der Heide RJ, Skipper LM, Klobusicky E, & Olson IR (2013). Dissecting the uncinate fasciculus: disorders, controversies and a hypothesis. Brain, 136(6), 1692–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegman HL, & Stetler C (2009). A meta-analytic review of the effects of childhood abuse on medical outcomes in adulthood. Psychosomatic Medicine, 71, 805–812. [DOI] [PubMed] [Google Scholar]
- Widom CS, & Czaja SJ (2012). Childhood trauma, psychopathology and violence: disentangling causes, consequences, and correlates, in Widom CS (ed.) Trauma, Psychopathology, and Violence: Causes, Consequences, or Correlates? New York, NY: Oxford University Press. [Google Scholar]
- Widom CS, Weiler BL, & Cottler LB (1999). Childhood victimization and drug abuse: A comparison of prospective and retrospective findings. Journal of Consulting and Clinical Psychology, 67, 867–880 [DOI] [PubMed] [Google Scholar]