Abstract
Background:
Coronary artery disease (CAD) is influenced by genetic variation, and traditional risk factors. Polygenic risk scores (PRS), which can be ascertained prior to the development of traditional risk factors, have been shown to identify individuals at elevated risk of CAD. Here, we demonstrate that a genome-wide PRS for CAD predicts all-cause mortality after accounting for not only traditional cardiovascular risk factors but also angiographic CAD itself.
Methods:
Individuals who underwent coronary angiography and were enrolled in an institutional biobank were included; those with prior myocardial infarction or heart transplant were excluded. Using a pruning-and-thresholding approach, a genome-wide PRS comprised of 139,239 variants was calculated for 1503 participants who underwent coronary angiography and genotyping. Individuals were categorized into high-PRS (hiPRS) and low-PRS control groups using the maximally selected rank statistic. Stratified analysis based on angiographic findings was also performed. The primary outcome was all-cause mortality following the index coronary angiogram.
Results:
Individuals with hiPRS were younger than controls (66 years vs. 69 years; p = 2.1×10−5), but did not differ by sex, BMI, or traditional risk factor profiles. Individuals with hiPRS were at significantly increased risk of all-cause mortality following cardiac catheterization, adjusting for traditional risk factors and angiographic extent of CAD (HR 1.6; 95% CI 1.2-2.2; p = 0.004). The strongest increase in risk of all-cause mortality conferred by hiPRS was seen among individuals without angiographic CAD (HR = 2.4; 95% CI 1.1-5.5; p = 0.04). In the overall cohort, adding hiPRS to traditional risk assessment improved prediction of 5-year all-cause mortality (AUC 0.70 [95% CI 0.66 - 0.75] vs. 0.66 [95% CI 0.61 - 0.70]; p = 0.001).
Conclusions:
A genome-wide PRS improves risk stratification when added to traditional risk factors and coronary angiography. Individuals without angiographic CAD but with hiPRS remain at significantly elevated risk of mortality.
Keywords: genetics, diagnostics, genetic testing, genetic variation, coronary artery disease
INTRODUCTION
Coronary artery disease (CAD) is a leading cause of death worldwide. A number of comorbid conditions, including hypertension, hyperlipidemia, diabetes, and smoking contribute to the risk of developing CAD. Guidelines from the American College of Cardiology and American Heart Association (ACC/AHA) for management of hyperlipidemia and hypertension recommend cardiovascular risk assessment based upon these traditional risk factors to help clinicians identify individuals who may benefit from aggressive primary prevention strategies, and tools, including the Pooled Cohort Equation and scores derived from the Framingham Heart Study, have been developed to facilitate traditional risk factor-based assessment and stratification.1-5
Coronary artery disease is highly heritable, and recent large meta-analyses of genome-wide association studies (GWAS) have identified more than 150 genetic loci associated with coronary artery disease.6,7 Although the causal mechanisms underlying many of these genetic risk factors remain unclear, in total they explain ~30% of CAD heritability.7-9 Polygenic risk scores (PRS), which sum the burden of genetic risk across the genome, have been proposed as a means of identifying individuals at elevated risk of coronary artery disease. For example, a PRS composed of 182 independent risk variants was found to be associated with elevated risk of early-onset CAD, and previous scores have been associated with angiographic extent of coronary obstruction, and incident CAD.10,11 More recently, genome-wide polygenic risk scores, which leverage millions of individual genetic variants to improve predictive power, have been associated with CAD in the UK BioBank population.12-14 Because genetic risk is established prior to the clinical manifestations of traditional cardiovascular risk factors, polygenic risk scores may provide early information for identifying individuals at elevated risk of CAD, as well as provide information that is complementary to traditional risk factors.
In addition to non-invasive assessment of traditional risk factors, coronary artery angiography is also an important tool for risk stratification - the angiographic burden of atherosclerosis is strongly associated with risk of subsequent cardiovascular events including myocardial infarction and death.15 The presence of CAD by coronary angiography weighs heavily into risk management strategies. Individuals with known CAD receive aggressive preventive intervention, whereas those with no angiographic CAD are treated less aggressively. We hypothesized that stratifying individuals by polygenic risk score after coronary artery angiography would identify individuals at increased risk of mortality, even after accounting for traditional risk factors and extent of angiographic CAD.
METHODS
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure, without approval from the University of Pennsylvania Institutional Review Board. The study was approved by the Institutional Review Board of the University of Pennsylvania, and all participants provided written informed consent. Full Methods are available in the Supplemental Materials of the article.
RESULTS
CAD polygenic risk score and obstructive CAD
We focused on cohort of European ancestry individuals with both genotype and cardiac catheterization results (N = 1503). There was a significant, graded increase in traditional risk factor profiles (increased hypertension, diabetes, smoking, statin use, and BMI) associated with increasing burden of angiographic CAD (Supplemental Table 1). We investigated whether our genome-wide PRS was associated with extent of angiographic coronary disease (no angiographic CAD vs. non-obstructive [<50% stenosis or luminal irregularities] vs. obstructive [≥50% stenosis, stent or graft]). There was a significant (Fisher’s Exact Test p = 0.02) association between PRS quartile and the burden of angiographic disease on coronary angiogram (Supplemental Table 2).
To adjust for clinical covariates, we used multinomial logistic regression to examine the effect of a 1 standard deviation change in PRS with the risk of having non-obstructive or obstructive CAD. A 1 standard-deviation increase in polygenic risk was associated with increased odds of obstructive angiographic CAD (OR 1.8; 95% CI 1.5-2.1; p = 2.3×10−9) and non-obstructive CAD (OR 1.3; 95% CI 1.05-1.6; p = 0.02), adjusting for age at catheterization and sex. These associations were attenuated after additional adjustment for traditional cardiovascular risk factors (hypertension, diabetes, smoking, BMI, statin) (OR for obstructive CAD 1.5; 95% CI 1.2-2.0; p = 0.0002; OR for non-obstructive CAD 1.1; 95% CI 0.9-1.4; p = 0.4).
CAD polygenic risk score and mortality following coronary angiography
Individuals who underwent both cardiac catheterization and genotyping were divided into two groups based on the CAD PRS. Individuals in the high polygenic risk score (hiPRS) group were younger than low PRS control subjects (66 years vs. 69 years; p = 2.1×10−5) (Table 1). Individuals with hiPRS had similar rates of diabetes, hypertension, smoking, statin use, lipid profiles (Total cholesterol, LDL-c, HDL-c, Triglycerides), Hemoglobin A1c, and extent of angiographic CAD at time of index cardiac catheterization.
Table 1.
Demographics of PMBB participants of European ancestry with available coronary catheterization data
| Control (N = 654) |
hiPRS (N = 849) |
P-value | |
|---|---|---|---|
| Age | |||
| Median (IQR) | 69 (61, 79) | 66 (58, 76) | P< 0.001 |
| Male | |||
| n (%) | 423 (65) | 517 (61) | P= 0.1 |
| Angiographic CAD | |||
| Obstructive CAD, n (%) | 339 (52) | 472 (56) | P = 0.3 |
| Non-Obstructive CAD, n (%) | 157 (24) | 192 (23) | |
| No Angiographic CAD, n (%) | 158 (24) | 185 (22) | |
| Hypertension | |||
| n (%) | 375 (57) | 502 (59) | P= 0.5 |
| Diabetes | |||
| n (%) | 161 (25) | 211 (25) | P= 1 |
| Smoking | |||
| n (%) | 313/499 (63) | 361/617 (59) | P= 0.2 |
| Statin | |||
| n (%) | 386 (59) | 492 (58) | P= 0.7 |
| BMI | |||
| Median (IQR) | 28 (25, 31) | 28 (25, 32) | P= 0.9 |
| n | 564 | 721 | |
| Hemoglobin A1c | |||
| Median (IQR) | 5.7 (5.4, 6.2) | 5.8 (5.5, 6.3) | P= 0.5 |
| n | 173 | 204 | |
| Total Cholesterol | |||
| Median (IQR) | 188 (154, 218) | 189 (155, 227) | P= 1 |
| n | 356 | 437 | |
| LDL Cholesterol | |||
| Median (IQR) | 110 (83, 137) | 111.0 (83, 142) | P= 0.4 |
| n | 354 | 435 | |
| HDL Cholesterol | |||
| Median (IQR) | 40 (32, 51) | 39 (31, 50) | P= 0.1 |
| n | 354 | 436 | |
| Triglycerides | |||
| Median (IQR) | 135 (98, 186) | 146 (103, 220) | P= 0.3 |
| n | 372 | 457 | |
To determine whether CAD PRS predicts overall survival post angiography independent of angiographic CAD, we used Cox regression modeling. This analysis included a median follow-up of 4.5 years after index cardiac catheterization. After adjustment for traditional cardiovascular risk factors (smoking, diabetes, hypertension, statin use, BMI), extent of angiographic coronary artery disease at index catheterization, and genetic principal components, individuals with hiPRS were at significantly elevated risk of all-cause mortality compared to controls (HR = 1.6; 95% CI 1.2-2.2; p = 0.004 [Figure 1, Supplemental Figure 1]). Similar results were obtained when the PRS was thresholded at the median value (Supplemental Figure 2).
Figure 1.
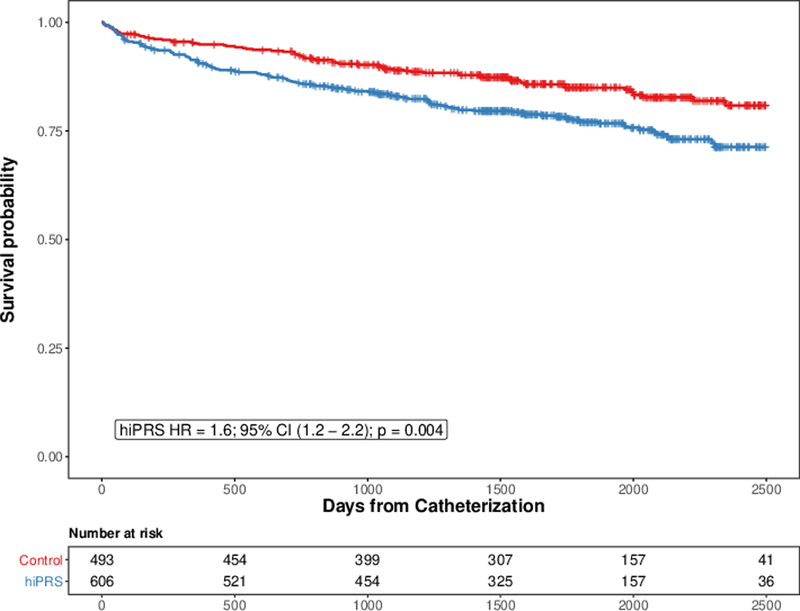
hiPRS is Associated with Increased Risk of All-Cause Mortality following Cardiac Catheterization. Cox regression was performed to assess whether high polygenic risk of CAD (hiPRS) was associated with increased risk of all-cause mortality following cardiac catheterization. Risk of mortality was adjusted for age, sex, hypertension, diabetes, smoking status, statin use, BMI, and genetic principal components (n = 10). Tick-marks represent censoring.
Because angiographic findings may alter prevention strategies, we assessed changes in statin use following index angiogram. The proportion of statin users vs. non-users increased at 30 days in all groups (43%, 59%, 84% in the no angiographic, non-obstructive, and obstructive CAD groups, respectively; p < 0.01). There was no difference in the proportion of statin use in the hiPRS vs. control groups at 30 days (68% vs. 69%; p = 0.5). Adjusting for statin use at 30 days after angiography rather than statin use at time of index angiogram had no effect on the hazard for mortality.
To further examine the difference in survival between hiPRS and control groups, we stratified individuals based on the extent of angiographic CAD, which has been previously been associated with risk of death following cardiac catheterization.15 After stratifying by extent of angiographic CAD, individuals with no angiographic CAD were at significantly increased risk of all-cause mortality (HR = 2.4; 95% CI 1.0-5.5; p = 0.04 [Figure 2, Supplemental Figure 3]). In individuals with non-obstructive or obstructive CAD by angiography, the associated risk of mortality was of lesser magnitude, and did not reach statistical significance (Non-Obstructive CAD HR = 1.6; 95% CI 0.8-3.3; p = 0.16; Obstructive CAD HR = 1.4; 95% CI 0.9-2.2; p = 0.10). There was no significant interaction between hiPRS and angiographic extent of disease.
Figure 2.
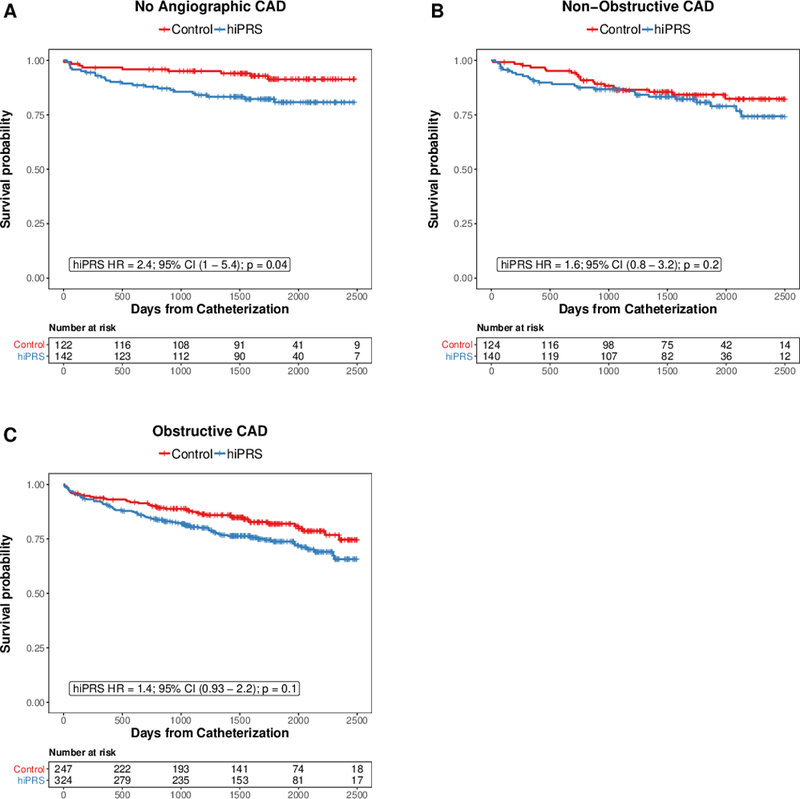
All-Cause Mortality following Cardiac Catheterization, Stratified by Anigographic Burden of Coronary Artery Disease. Cox regression was performed to identify the association between hiPRS and all-cause mortality, stratified by extent of angiographic coronary artery disease: A) No angiographic CAD; B) Non-Obstructive CAD; C) Obstructive CAD. Risk of mortality was adjusted for age, sex, hypertension, diabetes, smoking status, statin use, BMI, and genetic principal components (n = 10). Tick-marks represent censoring.
Risk Assessment
To determine the contribution of our PRS on all-cause mortality we constructed Cox regression models for hiPRS, each traditional risk factor, and angiographic extent of CAD. We compared these models’ predictions of 5-year survival using time-dependent area under the receiver-operating curve (AUC) (Figure 3). The model incorporating conventional risk factors, angiographic CAD, and hiPRS best predicted 5-year overall survival, performing significantly better than the current standard-of-care model that incorporates conventional risk factors and angiographic extent of CAD (AUC 0.70 [95% CI 0.66 - 0.75] vs. 0.65 [95% CI 0.61 - 0.70]; p = 0.001). Similarly, the model incorporating hiPRS had a nominally higher AUC (0.69; 95% CI 0.64 - 0.73) than models incorporating each conventional risk factor, angiographic extent of CAD, or a model combining conventional risk factors with angiographic CAD.
Figure 3.
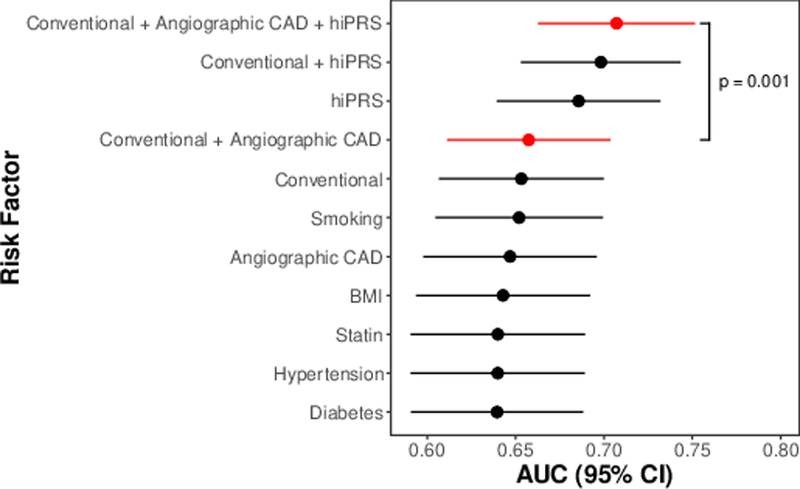
hiPRS Improves Risk Stratification. Time-dependent area under the receiver-operating curve (AUC) for 5-year all-cause mortality was calculated for models built from individual Conventional risk factors (Diabetes, Hypertension, Smoking, Statin-use, BMI), Angiographic CAD, hiPRS, as well as combinations of these risk factors. Whiskers represent 95% confidence intervals. Models in red highlight the predictive capabilities of the current standard of CAD prognostication (conventional risk factors + angiography), with a model adding hiPRS.
Finally, we modeled the absolute risk of the primary outcome in individuals with and without hiPRS. Individuals with hiPRS had 22% median 5 year risk of all-cause mortality, compared to 13% for individuals in the control group (p < 2.2×10−16 [Supplemental Figure 4]). This resulted in hiPRS being associated with an 8.1% (95% CI 6.6 - 9.5%) increase in absolute risk of 5-year all-cause mortality, after controlling for traditional risk factors and the results of cardiac catheterization.
DISCUSSION
In this study we leveraged a large bio-bank population with genetic information linked to electronic health records to evaluate the use of a polygenic risk score to predict survival following coronary angiography. We found that a genome-wide polygenic risk score was significantly associated with both lifetime diagnosis of coronary artery disease, as well as angiographically prevalent disease, consistent with prior studies.6,13,14,16 We extended these findings to show that an elevated polygenic risk score is strongly associated with all-cause mortality following cardiac catheterization. The addition of a polygenic risk score also significantly improved the predictive capabilities of the current best-available risk stratification modalities.
This study adds to the growing evidence that genetic risk is a strong contributor to both prevalent and incident coronary artery disease, independent from traditional cardiovascular risk factors.10,13,14,17,18 More recently, genome-wide polygenic risk scores, which incorporate up to millions of independent variants to improve predictive power, appear to strongly associate with cardiovascular disease risk.12-14 Consistent with those results, our score comprised of 139,239 variants demonstrated clear associations with lifetime diagnosis of coronary artery disease as well as angiographic extent of coronary artery disease.
Interestingly, high polygenic risk was associated with similar traditional risk factor profiles and angiographic burden of disease among individuals with available catheterization data. Notably, though, individuals at high polygenic risk were younger at time of cardiac catheterization. This finding may suggest that lifetime exposure to polygenic risk factors is an important contributor to coronary artery disease, as is the case for many monogenic variants which confer either increased risk or protection from coronary disease.19-24 Adverse genetics may lower the threshold for developing coronary artery disease prior to the clinical manifestations of traditional risk factors.
We found that after adjustment for traditional risk factors and angiographic extent of CAD, high polygenic risk is associated with a considerable increase in all-cause mortality, with a substantial and clinically-significant 8.1% (95% CI 6.6 - 9.5%) absolute risk increase in 5-year mortality after cardiac catheterization. The mechanism behind this association is likely multifactorial. Many of the monogenic variants associated with coronary artery disease have known mechanisms that span the range of traditional risk factors like hypertension, hyperlipidemia, obesity, diabetes, inflammation, and smoking, as well as other cardio-metabolic diseases and gene-environment interactions.25-27 Given the similar traditional risk factor profile in our cohort between individuals with high polygenic risk and controls, non-traditional risk factors may explain the large difference in mortality. Our finding that hiPRS was a better predictor of 5-year mortality when compared to conventional CAD risk factors or angiography may reflect the power of our polygenic risk score to capture both traditional and non-traditional risk. Likewise, despite comparable cross-sectional risk profiles, lifetime exposure to unfavorable genetics may confer additional risk.28
Current guidelines recommend aggressive secondary prevention strategies for individuals with known coronary disease, and aggressive primary prevention strategies for individuals with conventional risk factors like hyperlipidemia, diabetes, smoking and hypertension.1,5 Notably, in a stratified analysis based on angiographic extent of disease, individuals with no angiographic coronary artery disease but with high polygenic risk scores remained at significantly elevated risk of all-cause mortality. This result suggests that absence of angiographic CAD is not necessarily a reassuring finding. Further, this result raises the possibility that genetic risk scores may allow for the identification of individuals who may benefit from more aggressive primary prevention in the absence of other identifiable risk factors or evidence of disease. Similarly, although no significant association between hiPRS and mortality was observed in stratified analysis of individuals with non-obstructive or obstructive CAD, these analyses may be underpowered, and a smaller but significant effect between hiPRS and mortality in these individuals should not be excluded based on our findings.
Traditional cardiovascular risk factors are highly influenced by healthy lifestyle and medications. Similarly, adherence to a healthy lifestyle, cardiorespiratory fitness, physical activity, and grip strength have all been associated with reductions in risk of incident coronary artery disease and cardiovascular death, even in those individuals at highest genetic risk.10,17,18 Our results suggest that incorporating a genome-wide polygenic risk score into traditional risk-factor assessment may improve our ability to identify individuals most likely to benefit from aggressive risk-reductions strategies. These results underscore the need for careful application of genetic risk scores. Further validation in large populations from clinically derived biobanks, as well as prospective studies of genetic risk stratification as an adjunct to traditional risk assessment are warranted.
Our study has several strengths and limitations. First, this study represents a retrospective analysis at a large academic referral center, which may limit its generalizability. However, this focused population allowed us to leverage detailed cardiovascular risk assessment, including traditional risk factor profiles and coronary angiography, which may not be available in larger cohorts. Although information related to reason for cardiac catheterization, as well as intervention at time of catheterization was not available, leaving the potential for confounding by indication, angiography is typically performed in individuals with high clinical probability of CAD. Information related to death was obtained from a large national database, however cause of death was not adjudicated. It is thus possible that our polygenic risk score predicts non-cardiovascular mortality, however this seems unlikely given the strong associations with overall coronary artery disease. Finally, our results were limited to individuals of European ancestry, as summary-level data for genetic risk of CAD in other ancestral populations is lacking. Further study in these populations is strongly warranted.
Overall, after adjusting for traditional cardiovascular risk factors, we found that high genetic risk was associated with substantially elevated risk of incident coronary artery disease.
Supplementary Material
Acknowledgments:
We acknowledge and thank the participants of the Penn Medicine BioBank.
Sources of Funding: ZA was supported by the National Institutes of Health (R01-HL126797). JC was partially supported by the National Institutes of Health (R01-HL138306) This work was supported by the Regeneron Genetics Center. SMD is supported by the U.S. Department of Veterans Affairs (IK2-CX001780). This publication does not represent the views of the Department of Veterans Affairs or the United States government.
Footnotes
Disclosures: Dr. Giri reports serving on the advisory board for AstraZeneca, and receiving research funds to the institution from Recor Medical and St. Jude Medical. The other authors report no conflicts.
References:
- 1.Stone NJ, et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults. J Am Coll Cardiol. 2014;63:2889–2934. [DOI] [PubMed] [Google Scholar]
- 2.Wilson PW, et al. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. [DOI] [PubMed] [Google Scholar]
- 3.D’Agostino RB, et al. General cardiovascular risk profile for use in primary care: The Framingham heart study. Circulation. 2008;117:743–753. [DOI] [PubMed] [Google Scholar]
- 4.D’Agostino RB, et al. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286:180–187. [DOI] [PubMed] [Google Scholar]
- 5.Whelton PK, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults. J Am Coll Cardiol. 2018;71:2199–2269.29146533 [Google Scholar]
- 6.van der Harst P, et al. The Identification of 64 Novel Genetic Loci Provides an Expanded View on the Genetic Architecture of Coronary Artery Disease. Circ Res. 2017:CIRCRESAHA.117.312086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nikpay M, et al. A comprehensive 1000 Genomes-based genome-wide association meta-analysis of coronary artery disease. Nat Genet. 2015;47:1121–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vinkhuyzen AAE, et al. Estimation and Partition of Heritability in Human Populations Using Whole-Genome Analysis Methods. Annu Rev Genet. 2013;47:75–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zdravkovic S, et al. Heritability of death from coronary heart disease: A 36-year follow-up of 20 966 Swedish twins. J Intern Med. 2002;252:247–254. [DOI] [PubMed] [Google Scholar]
- 10.Khera AV, et al. Genetic Risk, Adherence to a Healthy Lifestyle, and Coronary Disease. N Engl J Med. 2016;375:2349–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bjornsson E, et al. Common Sequence Variants Associated With Coronary Artery Disease Correlate With the Extent of Coronary Atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35:1526–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chatterjee N, et al. Projecting the performance of risk prediction based on polygenic analyses of genome-wide association studies. Nat Genet. 2013;45:400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khera AV, et al. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat Genet. 2018;50:1219–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inouye M, et al. Genomic Risk Prediction of Coronary Artery Disease in 480,000 Adults. J Am Coll Cardiol. 2018;72:1883–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maddox TM, et al. Nonobstructive Coronary Artery Disease and Risk of Myocardial Infarction. JAMA. 2014;312:1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bjornsson E et al. Common Sequence Variants Associated With Coronary Artery Disease Correlate With the Extent of Coronary AtherosclerosisSignificance. Arterioscler Thromb Vasc Biol. 2015;35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Said M, et al. Associations of combined genetic and lifestyle risks with incident cardiovascular disease and diabetes in the uk biobank study. JAMA Cardiol. 2018;3:693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tikkanen E, et al. Associations of Fitness, Physical Activity, Strength, and Genetic Risk With Cardiovascular Disease: Longitudinal Analyses in the UK Biobank Study. Circulation. 2018:CIRCULATIONAHA.117.032432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dewey FE, et al. Inactivating Variants in ANGPTL4 and Risk of Coronary Artery Disease. N Engl J Med. 2016;374:1123–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stitziel NO, et al. ANGPTL3 Deficiency and Protection Against Coronary Artery Disease. J Am Coll Cardiol. 2017;69:2054–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dewey FE, et al. Genetic and Pharmacologic Inactivation of ANGPTL3 and Cardiovascular Disease. N Engl J Med. 2017;377:211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen JC, et al. Sequence Variations in PCSK9, Low LDL, and Protection against Coronary Heart Disease. N Engl J Med. 2006;354:1264–1272. [DOI] [PubMed] [Google Scholar]
- 23.Goldstein JL, et al. Familial Hypercholesterolemia. In: The Online Metabolic and Molecular Bases of Inherited Disease. 2014. [Google Scholar]
- 24.Benn M, et al. Mutations causative of familial hypercholesterolaemia: Screening of 98 098 individuals from the Copenhagen General Population Study estimated a prevalence of 1 in 217. Eur Heart J. 2016;37:1384–1394. [DOI] [PubMed] [Google Scholar]
- 25.Leblanc M, et al. Identifying Novel Gene Variants in Coronary Artery Disease and Shared Genes with Several Cardiovascular Risk Factors. Circ Res. 2016;118:83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McPherson R, et al. Genetics of Coronary Artery Disease. Circ Res. 2016;118:564–578. [DOI] [PubMed] [Google Scholar]
- 27.Saleheen D, et al. Loss of Cardioprotective Effects at the ADAMTS7 Locus as a Result of Gene-Smoking Interactions. Circulation. 2017;135:2336–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khera AV, et al. Diagnostic Yield and Clinical Utility of Sequencing Familial Hypercholesterolemia Genes in Patients With Severe Hypercholesterolemia. J Am Coll Cardiol. 2016;67:2578–2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


