Abstract
Background:
To date, studies evaluating outcome improvements associated with participation in physician-led collaboratives have been limited by the absence of a contemporaneous control group. We examined post cardiac surgery pneumonia rates associated with participation in a statewide, quality improvement collaborative relative to a national physician reporting program.
Methods and Results:
We evaluated 911,754 coronary artery bypass operations (July 1, 2011 to June 30, 2017) performed across 1,198 hospitals participating in a voluntary national physician reporting program (Society of Thoracic Surgeons, “STS”), including 33 that participated in a Michigan-based collaborative (MI-Collaborative). Unlike STS hospitals not participating in the MI-Collaborative (STSnonMI) that solely received blinded reports, MI-Collaborative hospitals received a multi-faceted intervention starting November 2012 (quarterly in-person meetings showcasing unblinded data, webinars, site visits). Eighteen of the MI-Collaborative hospitals received additional support to implement recommended pneumonia prevention practices (MI-CollaborativePlus), while 15 did not (MI-CollaborativeOnly). We evaluated rates of postoperative pneumonia, adjusting for patient mix and hospital effects. Baseline patient characteristics were qualitatively similar between groups and time. During the pre-intervention period, there was a 2.53% per quarter reduction in the adjusted pneumonia odds ratio for STSnonMI (p<0.001), which was equivalent to the MI-Collaborative (p>0.05). During the intervention period, there was a significant 2% reduction in the adjusted odds ratio for pneumonia for MI-Collaborative hospitals relative to the STSnonMI, although was 3% significantly lower among the MI-CollaborativeOnly hospitals. The STSnonMI had a 1.96% reduction in risk adjusted pneumonia, which was less than the MI-Collaborative (3.23%, p=0.011). The MI-CollaborativePlus reduced adjusted pneumonia rates by 10.29%, p=0.001.
Conclusions:
Participation in a physician-led collaborative was associated with significant reductions in pneumonia relative to a national quality reporting program. Interventions including collaborative learning may yield superior outcomes relative to solely using physician feedback reporting.
Keywords: infection, surgery, cardiopulmonary bypass, Intervention, Surgery, Transplantation, Cardiovascular Surgery, Quality and Outcomes, Quality and Outcomes
Introduction
The federal government began using administrative data to publicly report hospital mortality rates following coronary artery bypass grafting (CABG) surgery in the 1980s. Shortly thereafter, large, clinically-based registries (e.g., The Society of Thoracic Surgeons Adult Cardiac Surgery Database and STS) emerged in part to address perceived concerns over inadequate risk adjustment inherent within administrative claims. Currently, nearly 100% of all U.S. cardiac surgical programs voluntarily submit data to the STS. While STS participants in turn receive quarterly benchmarking reports, some have argued gains in surgical outcomes are largely unrelated to voluntary reporting programs1.
Physician-led quality collaboratives have developed as a mechanism for using data (e.g., STS for cardiac surgery) to improve the quality and safety of care. As an example, all 33-non-federal cardiac surgical programs in Michigan participate voluntarily both in the STS and the Michigan Society of Thoracic and Cardiovascular Surgeons Quality Collaborative, the latter which is funded in part by the largest statewide private health care payer. This collaborative supplements STS reporting with quarterly face-to-face meetings showcasing unblinded center-level data to discuss barriers and obstacles to driving local quality improvement2. While a number of reports have evaluated the effectiveness of such a collaborative learning model3, 4, most have been limited by their lack of a contemporaneous control population.
To understand the role of participation in a physician-led quality improvement collaborative relative to a national physician reporting program, we evaluated reductions in post cardiac surgery pneumonia rates associated with a statewide pneumonia prevention initiative. Pneumonia, the most common healthcare-associated infection in this setting, occurs among 3% of patients, is associated with a 4-fold increased odds of mortality and 3-fold increased length of stay5. Specifically, we compared pneumonia rates between hospitals participating in a statewide collaborative initiative and hospitals that solely participated in a national physician reporting program. We secondarily assessed whether there were further reductions in pneumonia rates associated with receipt of additional feedback on recommended pneumonia practices among a subset of collaborative hospitals.
Methods
The data, analytic methods and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure. This study was approved by the Institutional Review Boards at Duke University (Pro00056095), the University of Michigan (HUM00084088) and registered at Clinicaltrials.gov (NCT02068716). No informed consent was required.
Site Selection
During the period of 07/01/2011 through 06/30/2017; 1,198 hospitals participated in the Society of Thoracic Surgeons Adult Cardiac Surgery Database (STS, n = 1,198). All 33 Michigan nonfederal hospitals performing cardiac surgery voluntarily participated in both the STS and in a physician-led, quality improvement collaborative.
Data Source and Collaborative Intervention
Hospitals participating in the STS submit data harvest files quarterly to the STS data warehouse and in turn receive standardized, blinded, comprehensive benchmarking reports for quality assurance and improvement. These reports provide each hospital with its observed performance relative to both a comparable group (based on case volume and presence of a surgical residency program) and national outcomes.
In addition to their participation in the STS, all 33 Michigan hospitals also participate in the Michigan Society of Thoracic and Cardiovascular Surgeons Quality Collaborative (MI-Collaborative). The MI-Collaborative’s quarterly face-to-face meetings (attended by surgeons, database managers and perfusion representatives from each hospital) include unblinded center results along with STS benchmarks and discussions concerning perceived barriers and facilitators for local quality improvement projects. The MI-Collaborative also shares quarterly electronic benchmarking reports, which are designed to supplement the existing STS reports, that graphically depict each center’s performance against the other 32 hospitals along with a STS benchmark (when available).
At the MI-Collaborative November 2012 meeting, the attendees agreed to undertake a statewide, pneumonia prevention intervention to address observed variation in post-cardiac surgery infection rates6. During this meeting, attendees were presented with data concerning the variability and impact of healthcare-associated infections following coronary artery bypass grafting (CABG) surgery across the MI-Collaborative hospitals. The MI-Collaborative embarked on a multifaceted initiative including benchmarking site visits to high and low performing centers, face-to-face meetings and educational webinars.
Project materials are described in detail in the Supplement (Supplementary Methods). As previously described, the MI-Collaborative developed, endorsed and distributed to all its hospitals a set of Pneumonia Prevention practice recommendations (Supplementary Table 1), which were based on benchmarking site visits and structured literature review (unpublished data)7. Providers at each MI-Collaborative hospital attended each quarterly meeting and webinar materials were distributed to each surgical practice.
Additionally, between Q1/2016 through Q4/2106, 18 of the 33 centers (MI-CollaborativePlus) agreed to voluntarily receive (in addition to their ongoing STS- and collaborative quarterly reports) monthly data feedback detailing CABG-specific pneumonia prevention practices. None of the interventions were mandated. While encouraged to be multi-disciplinary, the cardiac surgeon lead at each of the 18 hospitals independently chose which specialties and disciplines (e.g., respiratory therapy, nurse practitioner, anesthesiologist) to include in its quality improvement team. The remaining 15 hospitals (MI-CollaborativeOnly) solely participated in ongoing collaborative and STS activities. Quarterly collaborative meetings were used to examine and evaluate the effectiveness of our intervention in reducing pneumonia rates.
Primary Outcomes
The primary outcome was postoperative pneumonia. All STS participating centers adhere to a common STS pneumonia definition, which is based on laboratory findings (e.g. positive sputum culture results from transtracheal fluid and/or bronchial washings) and/or radiological evidence (e.g. chest roentgenogram diagnostic of pulmonary infiltrates)8. Fever and/or white blood cell count are considered as evidence of pneumonia.
Statistical Analysis
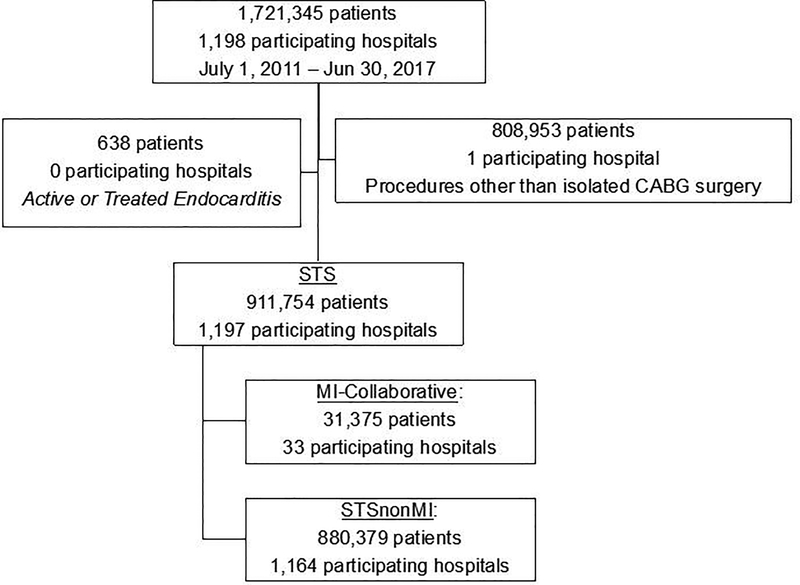
We identified 1,721,345 patients undergoing cardiac surgery between 07/01/2011 and 06/30/2017 at 1,198 hospitals participating in the STS. After excluding patients receiving procedures other than isolated CABG surgery or those presenting with active or treated endocarditis, equaled 911,754 patients (n=196,954 pre-intervention, 714,800 intervention), representing 1,197 participating hospitals, were available for subsequent analysis (Figure 1).
Figure 1:
Selection criteria schema for CABG patients from STS. STS (Society of Thoracic Surgeons Adult Cardiac Surgery Database); CABG (coronary artery bypass grafting); STS-nonMI: 1,164 Hospitals (other than those located in Michigan) that participated in the Society of Thoracic Surgeons Adult Cardiac Surgical Database; MI-Collaborative: 33 Michigan hospitals that participated a physician-led collaborative
Descriptive statistics were compared using Chi-square analysis for binary or nominal categorical variables and the Wilcoxon test or Kruskal-Wallis test for continuous or ordinal categorical variables. Our analysis captured the effect of the intervention using two linear splines to account for time from the start of the study (July 2011) as well as start of the intervention (November 2012). A Multivariable logistic regression model using a unique random intercept for each hospital was used to model the risk of pneumonia. Model fit was analyzed using C-statistic, as well as looking at the calibration between observed and expected. Additional sensitivity analyses examined the effect of each hospital in the MI-Collaborative to determine if hospitals that had the highest baseline risk for pneumonia exhibited the strongest decline during the study period.
Furthermore, we calculated O/E ratios for stratum specific results with the goal to assess the rates of change over time. These were presented for both pre-intervention and intervention time periods for MI-Collaborative (and separately for the MI-CollaborativeOnly and MI-CollaborativePlus groups), STSnonMI and all STS hospitals. Where the O/E is the ratio of a specific stratum’s (i.e., pre-intervention period for MI-CollaborativePlus) mean number of observed post-operative pneumonia events relative to the mean number of pneumonia events expected by the model we generated, while accounting for patient mix and hospital effects. Risk-adjusted rates were calculated by multiplying the O/E ratio by the overall unadjusted rate of pneumonia during our study period. P-values comparing O/E ratios between groups and time periods were obtained using a Wald test statistic for testing the null hypothesis that the two groups had equal O/E ratios.
Statistical analyses were performed using SAS version 9.4 (SAS Institute, Inc. Cary, NC) and R version 3.4.2 (R Foundation for Statistical Computing, Vienna, Austria). The two-tailed tests were considered significant at p<0.05.
Results
There were 196,954 isolated CABG patients in the pre-intervention period [189,774: STSnonMI; 7,180: MI-Collaborative (3,410: MI-CollaborativeOnly; 3,770: MI-CollaborativePlus)] and 714,800 in the intervention period [690,605: STSnonMI; 24,195: MI-Collaborative (10,829: MI-CollaborativeOnly; 13,366: MI-CollaborativePlus)], Table 1.
Table 1:
Pre-operative Characteristics by Cohort
| Characteristics | Pre-Intervention | Intervention | ||||||
|---|---|---|---|---|---|---|---|---|
| Overall | STSnonMI | MI-Collaborative | p-value | Overall | STSnonMI | MI-Collaborative | p-value | |
| N | 196,954 | 189,774 | 7,180 | 714,800 | 690,605 | 24,195 | ||
| Demographics | ||||||||
| Age, mean (SD), y | 64.9 (10.5) | 64.9 (10.5) | 65.2 (10.5) | 0.109 | 65.2 (10.3) | 65.2 (10.3) | 65.6 (10.2) | <0.001 |
| Caucasian | 85.5 | 85.4 | 89.0 | <0.001 | 84.8 | 84.6 | 89.7 | <0.001 |
| Female | 26.1 | 26.1 | 26.5 | 0.38 | 24.8 | 24.8 | 25.2 | 0.190 |
| Laboratory Values | ||||||||
| Last Preoperative Hematocrit, mean (SD) | 38.5 (5.4) | 38.6 (5.4) | 38.3 (5.2) | 0.0007 | 39.2 (5.5) | 39.2 (5.5) | 39.1 (5.4) | 0.057 |
| Last White Blood Cell Count, mean (SD) | 8.2 (3.6) | 8.2 (3.6) | 8.0 (3.7) | <0.001 | 8.2 (3.3) | 8.2 (3.3) | 8.0 (3.1) | <0.001 |
| Comorbid Disease | ||||||||
| Dyslipidemia | 86.2 | 86.1 | 90.6 | <0.001 | 88.0 | 87.8 | 91.2 | <0.001 |
| Peripheral Arterial Disease | 14.3 | 14.2 | 17.1 | <0.001 | 14.2 | 14.2 | 16.0 | <0.001 |
| Cerebrovascular Disease | 14.1 | 14.0 | 15.9 | <0.001 | 18.6 | 18.5 | 21.8 | <0.001 |
| Diabetes Mellitus | 44.5 | 44.5 | 45.0 | 0.39 | 47.9 | 47.9 | 46.6 | <0.001 |
| Liver Disease | 2.2 | 2.2 | 2.2 | 0.96 | 3.0 | 3.0 | 3.3 | 0.0067 |
| Pulmonary Function | ||||||||
| Home Oxygen Therapy | 1.7 | 1.7 | 1.8 | 0.59 | 1.8 | 1.8 | 1.7 | 0.54 |
| History of Pneumonia | <0.001 | <0.001 | ||||||
| Recent | 2.9 | 2.9 | 2.9 | 2.9 | 3.0 | 2.4 | ||
| Remote | 3.4 | 3.3 | 5.0 | 4.1 | 4.0 | 5.7 | ||
| Current Cigarette User | 22.8 | 22.8 | 23.6 | 0.087 | 22.7 | 22.7 | 23.2 | 0.071 |
| History of Chronic Lung Disease | <0.001 | <0.001 | ||||||
| Unknown Severity | Not collected | Not collected | Not collected | 3.9 | 3.9 | 3.5 | ||
| Severe | 4.3 | 4.2 | 6.5 | 4.3 | 4.2 | 5.3 | ||
| Moderate | 6.2 | 6.1 | 6.4 | 5.3 | 5.3 | 5.6 | ||
| Mild | 13.5 | 13.4 | 17.3 | 11.5 | 11.3 | 16.9 | ||
| Cardiac Function | ||||||||
| Pre-operative Intra-aortic Balloon Pump | 10.2 | 10.3 | 8.3 | <0.001 | 9.6 | 9.6 | 7.9 | <0.001 |
| History of Arrhythmia | 12.7 | 12.7 | 13.6 | 0.021 | 13.5 | 13.4 | 14.9 | <0.001 |
| Ejection Fraction | 0.040 | 0.0002 | ||||||
| >=35 and <50 | 21.5 | 21.5 | 21.6 | 21.0 | 21.0 | 20.6 | ||
| <35 | 10.4 | 10.5 | 9.5 | 10.8 | 10.8 | 10.2 | ||
| Operative Status | <0.001 | 0.014 | ||||||
| Urgent | 56.0 | 55.9 | 61.2 | 57.6 | 57.5 | 59.8 | ||
| Emergent/Salvage | 4.9 | 4.9 | 3.5 | 4.6 | 4.6 | 2.8 |
Data are expressed as No. (%) unless otherwise indicated.
P-values are based on Pearson chi-square tests for all categorical row variables.
Unknown severity of history of chronic lung disease was not a category option in the Society of Thoracic Surgeons version 2.73, which was used in the pre-intervention period.
Overall, in the pre-intervention period, nearly 60% of patients were urgent, mean age was 64.9 years, 26.1% were female, average white blood cell count before surgery was 8.2, 14.3% had peripheral arterial disease, 44.5% had diabetes, 2.9% had a recent history of pneumonia, 22.8% were current cigarette users, 10.5% had moderate to severe chronic lung disease and 10.4% had ejection fractions less than 35% (Table 1, Supplementary Table 2). Relatively small absolute differences existed across groups and over time with some exceptions: MI-Collaborative patients presented with more cerebrovascular disease (in the intervention period), more recent history of pneumonia (in both time periods), more mild and severe chronic lung disease (both time periods), all p<0.001.
In aggregate, more than 80% of procedures utilized cardiopulmonary bypass in the pre-intervention period (Table 2). The mean bypass duration was 95.7 minutes, while cross clamp duration was 58.3 minutes. Almost all patients received appropriate selection, timing and discontinuation of antibiotics. Nearly 35% of patients received blood products intraoperatively (average of 1.8 units transfused) and 38.9% were transfused postoperatively (average of 2.5 units transfused). More than 10% of patients were intubated more than 24 hours, 13.7% developed major morbidity/mortality and 2.4% died within 30 days. Relatively small absolute differences existed across groups and over time, with some exceptions: MI-Collaborative patients were more likely to receive surgery utilizing cardiopulmonary bypass (both time periods), had longer bypass (both time periods) and cross clamp durations (both time periods), although they were less likely to receive blood (pre-intervention) products intra or postoperatively. Blood product utilization diminished over time for both groups, as did rates of prolonged intubation and major morbidity. Operative mortality did not appreciably change over time. In addition, cardiopulmonary bypass and cross clamp durations were longer for patients operated at MI-CollaborativePlus hospitals relative to other groups (Supplementary Table 3).
Table 2:
Intra- and Postoperative Characteristics by Cohort
| Characteristics | Pre-Intervention | Intervention | ||||||
|---|---|---|---|---|---|---|---|---|
| Overall | STSnonMI | MI-Collaborative | p-value | Overall | STSnonMI | MI-Collaborative | p-value | |
| N | 196,954 | 189,774 | 7,180 | 714,800 | 690,605 | 24,195 | ||
| Intraoperative | ||||||||
| Cardiopulmonary Bypass Utilization | <0.001 | <0.001 | ||||||
| Full | 80.8 | 80.4 | 91.7 | 84.6 | 84.3 | 92.4 | ||
| None | 18.1 | 18.5 | 8.0 | 14.4 | 14.6 | 7.4 | ||
| Cardiopulmonary Bypass Duration, mean (SD), minutes | 95.7 (37.8) | 95.3 (37.6) | 104.1 (42.7) | <0.001 | 94.8 (37.4) | 94.6 (37.3) | 100.5 (39.5) | <0.001 |
| Cross Clamp Duration, mean (SD), minutes | 68.3 (29.4) | 67.9 (29.1) | 77.1 (36.1) | <0.001 | 68.5 (29.3) | 68.2 (29.2) | 75.5 (32.4) | <0.001 |
| Nadir Hematocrit, mean (SD) | 24.7 (4.8) | 24.7 (4.8) | 25.6 (4.8) | <0.001 | 25.2 (4.9) | 25.2 (4.9) | 25.7 (5.0) | <0.001 |
| Blood Products Used | 33.9 | 34.3 | 24.6 | <0.001 | 28.5 | 28.8 | 21.1 | <0.001 |
| Red Blood Cells, mean (SD), number | 1.81 (1.72) | 1.81 (1.72) | 1.7 (1.5) | 0.78 | 1.7 (1.7) | 1.7 (1.7) | 1.5 (1.4) | 0.0002 |
| Antibiotic Delivery | ||||||||
| Appropriate Selection | 97.7 | 97.7 | 97.5 | 0.47 | 98.6 | 98.6 | 99.0 | <0.001 |
| Appropriate Timing | 98.9 | 98.9 | 99.0 | 0.55 | 98.9 | 98.9 | 98.7 | 0.0023 |
| Postoperative | ||||||||
| Blood Products Used | 38.9 | 39.1 | 34.2 | <0.001 | 31.0 | 31.1 | 27.8 | <0.001 |
| Red Blood Cells, mean (SD), number | 2.5 (2.7) | 2.5 (2.7) | 2.6 (2.7) | 0.0043 | 2.4 (2.9) | 2.4 (2.9) | 2.3 (2.6) | 0.46 |
| Prolonged Intubation | 10.1 | 10.1 | 10.9 | 0.030 | 8.5 | 8.6 | 8.2 | 0.071 |
| Major Morbidity/Mortality | 13.7 | 13.7 | 14.2 | 0.27 | 12.2 | 12.2 | 11.8 | 0.030 |
| Operative Mortality | 2.4 | 2.4 | 1.9 | 0.0071 | 2.3 | 2.3 | 1.9 | <0.001 |
| Postoperative Length of Stay, mean (SD), days | 6.9 (5.1) | 6.8 (5.2) | 7.0 (5.0) | <0.001 | 6.8 (5.0) | 6.8 (5.0) | 7.1 (4.7) | <0.001 |
| Antibiotic | ||||||||
| Appropriate Discontinuation | 97.7 | 97.7 | 98.3 | 0.0003 | 97.8 | 97.8 | 98.4 | <0.001 |
Data are expressed as No. (%) unless otherwise indicated.
P-values are based on Pearson chi-square tests for all categorical row variables.
Overall, the observed rate of pneumonia was 3.09% (CI95%: 3.01%, 3.17%) in the pre-intervention period [STSnonMI: 3.07% versus MI-Collaborative: 3.62% (MI-CollaborativeOnly 4.11%, MI-CollaborativePlus: 3.18%)] and 2.60% (CI95%: 2.56%, 2.64%) in the intervention period (STSnonMI: 2.61% versus MI-Collaborative: 2.47% (MI-CollaborativeOnly 2.96%, MI-CollaborativePlus: 2.07%)]. While risk adjusted pneumonia rates declined by 1.96% (pre-intervention: 3.06% versus intervention: 3.00%) for STSnonMI, they declined by 3.23% (2.79% versus 2.70%) among MI-Collaborative (Supplementary Figure 1). While risk adjusted pneumonia rates for MI-CollaborativeOnly hospitals increased non-significantly by 4.93% (2.84% versus 2.98%), those in MI-CollaborativePlus decreased by 10.29% (2.72% versus 2.44%).
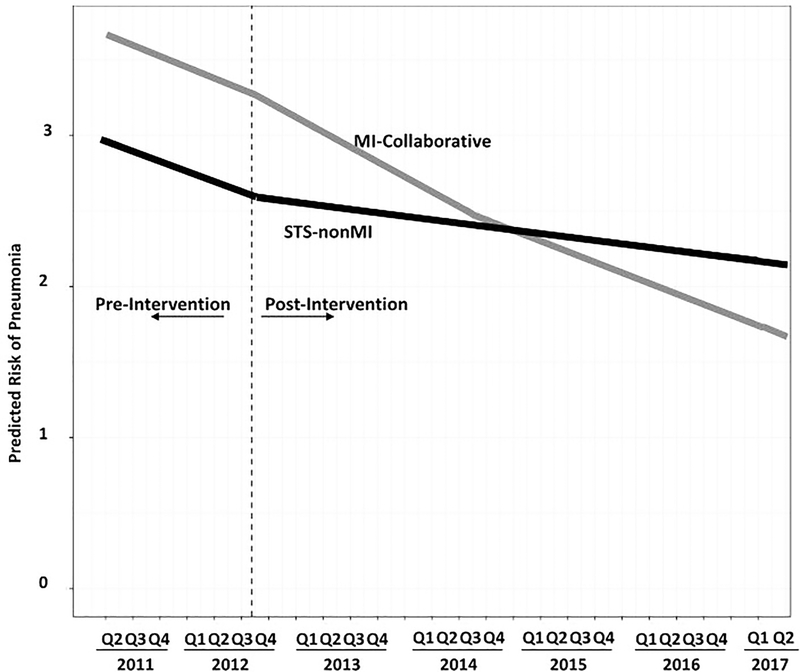
During the pre-intervention period, there was a 2.53% per quarter reduction in the adjusted odds of pneumonia for STSnonMI (p<0.001). For each quarter prior to the intervention, there was a 0.97 adjusted odds of pneumonia (CI95%: 0.96, 0.99), Figure 2. There was no statistical interaction between time and the MI-Collaborative as a whole (p=0.92) for the odds of pneumonia – this finding was consistent for the MI-CollaborativePlus (p=0.55) and MI-CollaborativeOnly (p=0.49) groups. For each quarter after the intervention, there was a 0.99 adjusted odds of pneumonia (CI95%: 0.99, 0.99). During this period, there was a 0.98 (CI95%: 0.96, 0.99) adjusted odds of pneumonia for MI-Collaborative relative to STSnonMI, 0.98 (CI95%: 0.96, 1.00) for MI-CollaborativePlus and 0.97 (CI95%: 0.95, 0.99) for the MI-CollaborativeOnly (Supplementary Figure 2).
Figure 2:
Adjusted Postoperative Pneumonia Rates by Group and Calendar Quarter. STS: Society of Thoracic Surgeons; MI: Michigan. STS-nonMI: 1,164 Hospitals (other than those located in Michigan) that participated in the Society of Thoracic Surgeons Adult Cardiac Surgical Database; MI-Collaborative: 33 Michigan hospitals that participated a physician-led collaborative.
There were decreases over time in observed pneumonia rates in each of the patient subgroups (Table 3, Supplementary Table 4). Pneumonia rates were either equivalent or lower in Michigan (MI-Collaborative or by MI-CollaborativePlus and CollaborativeOnly) relative to STSnonMI.
Table 3.
Unadjusted Pneumonia Rates Across Important Clinical Subgroup Analyses
| Characteristics | Pre-Intervention | Intervention | ||||||
|---|---|---|---|---|---|---|---|---|
| Overall | STSnonMI | MI-Collaborative | p-value | Overall | STSnonMI | MI-Collaborative | p-value | |
| N | 196,954 | 189,774 | 7,180 | 714,800 | 690,605 | 24,195 | ||
| Demographics | ||||||||
| Age Groups | ||||||||
| 65–74 | 3.0 | 3.0 | 3.3 | 0.38 | 2.5 | 2.6 | 2.2 | 0.048 |
| 75–84 | 4.1 | 4.1 | 4.2 | 0.85 | 3.5 | 3.5 | 3.1 | 0.15 |
| >=85 | 5.2 | 5.3 | 3.5 | 0.34 | 4.5 | 4.5 | 4.4 | 0.90 |
| Race | ||||||||
| White | 3.0 | 3.0 | 3.3 | 0.12 | 2.5 | 2.6 | 2.4 | 0.10 |
| Non-White | 3.5 | 3.5 | 5.9 | 0.0002 | 3.0 | 3.0 | 3.5 | 0.14 |
| Sex | ||||||||
| Female | 3.2 | 3.2 | 4.3 | 0.0094 | 2.8 | 2.8 | 2.8 | 0.90 |
| Male | 3.0 | 3.0 | 3.4 | 0.13 | 2.6 | 2.6 | 2.4 | 0.10 |
| Body Mass Index (kg/m2) | ||||||||
| <18.5 | 5.8 | 5.8 | 5.9 | 0.99 | 5.4 | 5.4 | 3.7 | 0.38 |
| 15.8–24.9 | 3.7 | 3.7 | 4.1 | 0.48 | 3.1 | 3.1 | 3.1 | 0.96 |
| 25.0–29.9 | 2.7 | 2.7 | 2.6 | 0.78 | 2.3 | 2.3 | 2.1 | 0.23 |
| 30.0–34.9 | 2.8 | 2.8 | 3.4 | 0.081 | 2.4 | 2.4 | 2.2 | 0.17 |
| 35.0–39.9 | 3.5 | 3.4 | 5.5 | 0.0010 | 2.7 | 2.7 | 2.7 | 0.99 |
| >=40 | 3.9 | 3.8 | 5.1 | 0.12 | 3.2 | 3.2 | 3.5 | 0.38 |
| Operative Status | ||||||||
| Elective | 2.3 | 2.2 | 2.8 | 0.081 | 1.9 | 1.9 | 1.8 | 0.97 |
| Urgent | 3.3 | 3.3 | 3.7 | 0.099 | 2.8 | 2.8 | 2.6 | 0.30 |
| Emergent/Salvage | 7.7 | 7.6 | 10.8 | 0.062 | 6.9 | 6.9 | 7.8 | 0.38 |
| Cigarette Smoking, Current | ||||||||
| No | 2.7 | 2.7 | 2.8 | 0.68 | 2.2 | 2.2 | 2.0 | 0.45 |
| Yes | 4.5 | 4.4 | 6.4 | 0.0001 | 3.9 | 3.9 | 4.0 | 0.63 |
| Chronic Lung Disease | ||||||||
| No | 2.5 | 2.5 | 2.7 | 0.53 | 2.0 | 2.0 | 1.8 | 0.047 |
| Yes | 4.9 | 4.9 | 5.9 | 0.039 | 4.2 | 4.2 | 3.8 | .079 |
| Off-Pump Surgery | ||||||||
| No | 2.7 | 2.7 | 3.0 | 0.72 | 2.3 | 2.3 | 2.1 | 0.59 |
| Yes | 3.2 | 3.2 | 3.7 | 0.017 | 2.7 | 2.7 | 2.5 | 0.13 |
| Cardiopulmonary Bypass Duration (min) | ||||||||
| <60 | 2.3 | 2.3 | 2.1 | 0.73 | 1.9 | 1.9 | 1.4 | 0.079 |
| 60–89 | 2.7 | 2.7 | 3.5 | 0.034 | 2.3 | 2.3 | 2.1 | 0.24 |
| 90–119 | 3.2 | 3.2 | 3.3 | 0.79 | 2.7 | 2.7 | 2.4 | 0.16 |
| >=120 | 4.4 | 4.4 | 4.8 | 0.42 | 3.8 | 3.8 | 3.6 | 0.43 |
| Intraoperative Red Blood Cell Transfusions Used | ||||||||
| No | 2.3 | 2.3 | 2.8 | 0.019 | 2.0 | 2.0 | 2.0 | 0.91 |
| Yes | 4.6 | 4.6 | 6.2 | 0.0015 | 4.2 | 4.2 | 4.3 | 0.71 |
Data are expressed as No. (%) unless otherwise indicated.
P-values are based on Pearson chi-square tests for all categorical row variables.
Discussion
In this large, statewide quality improvement study utilizing clinical registry data, we found that hospitals participating in a physician-led quality collaborative had a significant, greater decline in adjusted pneumonia rates relative to the rest of the country. Declines in adjusted pneumonia rates were greatest among the MI-Collaborative hospitals with enhanced feedback about pneumonia prevention practices (10% versus 2% reduction). We estimate that broader adoption of collaborative learning would result in 2,017 fewer pneumonia cases across the U.S. over the course of the intervention period. To our knowledge, these findings are the first to quantitatively assess the effectiveness of participating in a physician-led quality improvement collaborative relative to a national reporting program.
Approximately 1 out of every 25 hospitalized patients develops a HAI9. Cardiac surgical patients are more likely to develop major morbidity and mortality at high (17.9%) versus low (8.6%) HAI rate hospitals (p<0.001)10. Rates of HAIs following cardiac surgery, which are predominantly accounted for by pneumonia, vary considerably across hospitals6, 10. Perhaps surprisingly, only 2% of the overall variability in pneumonia rates is accounted for by differences in patient-level risk factors11, suggesting that pneumonia rates are likely driven by factors other than patient comorbidities and the duration of the operation.
The STS is the largest clinical database for cardiac surgery, covering nearly 100% of all U.S.-based hospitals. Data from the STS is used for research, public reporting and quality improvement. We found that hospitals participating in the STS had reductions in pneumonia. We hypothesize that the underlying national reduction in HAIs, including pneumonia, may be attributed in part to the general increased awareness of HAIs, in part due to the efforts of the Agency for Healthcare Research and Quality12. During the time period of our study, hospitals participating in the STS received quarterly feedback reports documenting each center’s performance over time relative to a comparable hospital and the national average. These detailed reports contain risk-adjusted outcomes and are designed to be comprehensive, spanning over 200-pages. Nonetheless, evidence suggests that by itself, feedback reporting does not result in large-scale reductions in morbidity or mortality1.
Prior reports have documented targeted quality improvement associated with physician-led quality improvement collaboratives. One of the earliest efforts was conducted by the Northern New England Cardiovascular Disease Study Group13. Use of validated, clinically-derived data feedback to frontline care providers, along with quality improvement training was associated with a 24% reduction in in-hospital mortality following CABG surgery, p=0.001. Subgroup analyses documented robust findings across important patient subgroups. This model has been replicated both within3, 14 and outside of cardiac surgery15, 16. Indeed, the largest commercial insurer in Michigan (Blue Cross and Blue Shield of Michigan/Blue Care Network) financially supports 17 of these collaboratives, including those focused on cardiothoracic surgery, interventional cardiology, orthopedic surgery, bariatric surgery and anticogulation17. Prior reports have documented the improvements in quality and expenditure associated with this partnership16. The MI-Collaborative, is the only participating value collaborative that is organized through a statewide professional society.
Current evaluations of physician-led quality improvement initiatives have predominantly focused on local, state and regional experiences. Paone and colleagues reported findings from a statewide blood conservation initiative involving the MI-Collaborative occurring between 2008 through 20124. The investigators noted a 32% reduction in red blood cell transfusions, a 39% drop in fresh frozen plasma and 35% drop in use of platelets for patients undergoing isolated CABG surgery. LaPar and colleagues additionally reported reductions in transfusions from 2006 to 2010 (intraoperative: 24% to 18%, postoperative 39% to 33%) through the Virginia Cardiac Surgery Quality Initiative18. While informative, these studies have been unable to evaluate whether their improvement efforts are attributed to their targeted intervention, or alternatively a function of secular declines in blood product transfusions.
In this present study, participation in a physician-led quality improvement collaborative was associated with greater reductions in postoperative pneumonia relative to national norms. Several reasons might help explain these findings. First, the MI-Collaborative identified discrete, pneumonia prevention measures that clinical teams could focus on to improve their performance. Although the Michigan intervention hospitals received monthly feedback reports, all Michigan hospitals participated in our quarterly MI-Collaborative conferences. These conferences routinely were preceded with a one-hour infection prevention workgroup meeting that was open to all MI-Collaborative conference attendees. Second, the MI-Collaborative is one of the few surgical collaboratives to unblind center data at their quarterly collaborative conferences. Based on trust and mutual respect, unblinding has facilitated sharing of best practices as well as fostered peer-to-peer accountability of each center’s results. Third, several resources were made available to Michigan hospitals, including education materials (e.g., webinars, summary of published literature) focusing on pneumonia prevention practices to support local adoption of best practices. Fourth, while other clinical teams beyond Michigan have been exposed to the same evidence-based literature detailing pneumonia-preventive practices, we are unaware of any systematic, pneumonia prevention initiatives underway outside of our collaborative during this time period.
A number of other regional quality improvement collaboratives exist, with many participating in a voluntary network -- The IMPROVE Network19. The IMPROVE Network is comprised of 7 physician-led quality collaboratives that collectively represent nearly 10% of all U.S.-based cardiac surgery hospitals. Hospitals in each collaborative contribute surgical data to a robust data coordinating center. Adoption of the pneumonia prevention practices across hospitals participating in this voluntary network would serve to externally validate our current findings.
We recognize some limitations to the present study. First, we cannot rule out the impact of selection bias (MSTCVS-QC centers voluntarily chose to participate in the MI-CollaborativePlus relative to MI-CollaborativeOnly), which may help explain the significant reduction in pneumonia rates relative to national norms achieved by the MI-CollaborativeOnly hospitals (Supplementary Figure 2). Second, we cannot rule out systematic differences in reporting pneumonia rates across the three groups. Nonetheless, all hospitals participating in this study are subjects to the STS’ random external data auditing process20. Each MI-Collaborative center additionally undergoes data quality audits every three years. Third, we cannot rule out other factors that may have contributed to the observed findings, including other quality improvement initiatives undertaken by the hospitals in Michigan. Our findings appear to be robust, as evidenced through subgroup analyses. Finally, our findings are subject to unmeasured confounding given the absence of randomization in our study design. We addressed this concern through risk adjustment, accounting for patient and center-level effects.
Post-operative pneumonia rates have significantly declined over time for hospitals participating in a national cardiac surgery reporting program. Nonetheless, the rates of decline were significantly accelerated for hospitals participating in a physician-led statewide quality improvement study. Beyond the need to evaluate broader dissemination of these pneumonia prevention practices, our findings provide further evidence supporting the benefit of physician-led collaboratives.
Supplementary Material
“What is Known”.
Nearly 100% of all hospitals performing adult cardiac surgery in the United States participate in The Society of Thoracic Surgeons Adult Cardiac Surgery Database (“STS”), which is often used to benchmark quality in cardiac surgery.
It is unknown whether participation in a physician-led collaborative in Michigan (“MI-Collaborative”) leveraging collaborative learning and quality benchmarking is independently associated with improved outcomes relative to quality benchmarking alone.
“What the Study Adds”.
We found that a collaborative learning intervention focusing on postoperative pneumonia following cardiac surgery yielded significant reductions in pneumonia relative to quality benchmarking alone.
Risk adjusted pneumonia rates declined by 1.96% for STS hospitals outside of Michigan and declined by 3.23% among MI-Collaborative hospitals, p=0.011.
A subgroup of 18 Michigan Collaborative hospitals that chose to receive pneumonia-specific implementation support reduced adjusted pneumonia rates by 10.29%, p=0.001.
Acknowledgments
Sources of Funding:
This project was supported by grant number R01HS022535 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality. Support for the MSTCVS Quality Collaborative is provided by the Blue Cross and Blue Shield of Michigan and Blue Care Network as part of the BCBSM Value Partnerships program.
Footnotes
Disclosures: The authors have no disclosures.
References
- 1.Osborne NH, Nicholas LH, Ryan AM, Thumma JR and Dimick JB. Association of hospital participation in a quality reporting program with surgical outcomes and expenditures for Medicare beneficiaries. JAMA. 2015;313:496–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prager RL, Armenti FR, Bassett JS, Bell GF, Drake D, Hanson EC, Heiser JC, Johnson SH, Plasman FB, Shannon FL, Share D, Theurer P, Williams J and Surgeons MSoTaC. Cardiac surgeons and the quality movement: the Michigan experience. Semin Thorac Cardiovasc Surg. 2009;21:20–7. [DOI] [PubMed] [Google Scholar]
- 3.Johnson SH, Theurer PF, Bell GF, Maresca L, Leyden T, Prager RL and Surgeons MSoTaC. A statewide quality collaborative for process improvement: internal mammary artery utilization. Ann Thorac Surg. 2010;90:1158–64; discussion 1164. [DOI] [PubMed] [Google Scholar]
- 4.Paone G, Brewer R, Likosky DS, Theurer PF, Bell GF, Cogan CM, Prager RL and Surgeons MotMSoTaC. Transfusion rate as a quality metric: is blood conservation a learnable skill? Ann Thorac Surg. 2013;96:1279–86. [DOI] [PubMed] [Google Scholar]
- 5.Kinlin LM, Kirchner C, Zhang H, Daley J and Fisman DN. Derivation and validation of a clinical prediction rule for nosocomial pneumonia after coronary artery bypass graft surgery. Clin Infect Dis. 2010;50:493–501. [DOI] [PubMed] [Google Scholar]
- 6.Shih T, Zhang M, Kommareddi M, Boeve TJ, Harrington SD, Holmes RJ, Roth G, Theurer PF, Prager RL, Likosky DS and Collaborative MSoTaCSQ. Center-level variation in infection rates after coronary artery bypass grafting. Circ Cardiovasc Qual Outcomes. 2014;7:567–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Society of Thoracic Surgeons. Society of Thoracic Surgeons Adult Cardiac Surgery Database. 2018. https://www.sts.org/registries-research-center/sts-national-database. Accessed October 02, 2018.
- 8.Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, Lynfield R, Maloney M, McAllister-Hollod L, Nadle J, Ray SM, Thompson DL, Wilson LE, Fridkin SK and Team EIPH-AIaAUPS. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370:1198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Likosky DS, Wallace AS, Prager RL, Jacobs JP, Zhang M, Harrington SD, Saha-Chaudhuri P, Theurer PF, Fishstrom A, Dokholyan RS, Shahian DM, Rankin JS and Collaborative MSoTaCSQ. Sources of Variation in Hospital-Level Infection Rates After Coronary Artery Bypass Grafting: An Analysis of The Society of Thoracic Surgeons Adult Heart Surgery Database. Ann Thorac Surg. 2015;100:1570–5; discussion 1575–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brescia AA, Rankin JS, Cyr DD, Jacobs JP, Prager RL, Zhang M, Matsouaka RA, Harrington SD, Dokholyan RS, Bolling SF, Fishstrom A, Pasquali SK, Shahian DM, Likosky DS and Collaborative MSoTaCSQ. Determinants of Variation in Pneumonia Rates After Coronary Artery Bypass Grafting. Ann Thorac Surg. 2017. 105(2):513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agency for Healthcare Research and Quality’s Healthcare-Associated Infections Program. 2018. https://www.ahrq.gov/professionals/quality-patient-safety/hais/index.html. Accessed October 02, 2018.
- 12.O’Connor GT, Plume SK, Olmstead EM, Morton JR, Maloney CT, Nugent WC, Hernandez F, Clough R, Leavitt BJ, Coffin LH, Marrin CA, Wennberg D, Birkmeyer JD, Charlesworth DC, Malenka DJ, Quinton HB and Kasper JF. A regional intervention to improve the hospital mortality associated with coronary artery bypass graft surgery. The Northern New England Cardiovascular Disease Study Group. JAMA. 1996;275:841–6. [PubMed] [Google Scholar]
- 13.Groom RC, Quinn RD, Lennon P, Donegan DJ, Braxton JH, Kramer RS, Weldner PW, Russo L, Blank SD, Christie AA, Taenzer AH, Forest RJ, Clark C, Welch J, Ross CS, O’Connor GT, Likosky DS and Group NNECDS. Detection and elimination of microemboli related to cardiopulmonary bypass. Circ Cardiovasc Qual Outcomes. 2009;2:191–8. [DOI] [PubMed] [Google Scholar]
- 14.Hemmila MR, Cain-Nielsen AH, Wahl WL, Vander Kolk WE, Jakubus JL, Mikhail JN and Birkmeyer NJ. Regional collaborative quality improvement for trauma reduces complications and costs. J Trauma Acute Care Surg. 2015;78:78–85; discussion 85–7. [DOI] [PubMed] [Google Scholar]
- 15.Share DA, Campbell DA, Birkmeyer N, Prager RL, Gurm HS, Moscucci M, Udow-Phillips M and Birkmeyer JD. How a regional collaborative of hospitals and physicians in Michigan cut costs and improved the quality of care. Health Aff (Millwood). 2011;30:636–45. [DOI] [PubMed] [Google Scholar]
- 16.Blue Cross/Blue Shield Blue Care Network. Collaborative Quality Initiatives. 2018.
- 17.LaPar DJ, Crosby IK, Ailawadi G, Ad N, Choi E, Spiess BD, Rich JB, Kasirajan V, Fonner E, Kron IL, Speir AM and Initiative IftVCSQ. Blood product conservation is associated with improved outcomes and reduced costs after cardiac surgery. J Thorac Cardiovasc Surg. 2013;145:796–803; discussion 803–4. [DOI] [PubMed] [Google Scholar]
- 18.IMRPOVE Network. http://www.improvenetwork.org. Accessed August 31, 2018.
- 19.Winkley Shroyer AL, Bakaeen F, Shahian DM, Carr BM, Prager RL, Jacobs JP, Ferraris V, Edwards F and Grover FL. The Society of Thoracic Surgeons Adult Cardiac Surgery Database: The Driving Force for Improvement in Cardiac Surgery. Semin Thorac Cardiovasc Surg. 2015;27:144–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.