Abstract
Electrical pacemaker activity generates phasic contractions and motility patterns such as segmentation and peristalsis in the gastrointestinal tract. Pacemaker currents are generated in interstitial cells of Cajal (ICC), which release Ca2+ from intracellular stores that stimulates Ca2+-activated Cl− channels (CaCCs) in the plasma membrane. Thus, Ca2+ stores must be maintained to sustain pacemaker activity. Store-operated Ca2+ entry (SOCE) facilitates the refilling of Ca2+ stores by a mechanism dependent upon interactions between STIM and Orai proteins. We investigated the role of SOCE in ICC pacemaker activity. Reintroduction of extracellular Ca2+ in store-depleted ICC resulted in CaCC activation. Blocking CaCCs revealed an inwardly rectifying current with properties of a Ca2+ release–activated current (ICRAC). An inhibitory peptide that interfered with the STIM-Orai interaction blocked ICRAC in HEK 293 cells expressing STIM1 and Orai1 and blocked spontaneous transient inward currents (STICs) and slow wave currents in ICC. STICs, which are fundamental pacemaker events in ICC, were blocked by an Orai antagonist. Imaging of Ca2+ transients linked to pacemaker activity in ICC in intact muscles showed that the Orai antagonist blocked Ca2+ transients in ICC. These data suggest that Ca2+ recovery through STIM-Orai interactions is necessary to maintain ICC pacemaker activity.
INTRODUCTION
In the gastrointestinal (GI) tract, phasic contractions and more integrated, whole-organ motility behaviors, such as gastric peristalsis and intestinal segmentation, depend upon pacemaker activity generated by interstitial cells of Cajal (ICC) (1–4). Pacemaker events generate electrical slow waves that propagate through networks of electrically coupled ICC and conduct to smooth muscle cells (SMCs) to initiate Ca2+ entry through L-type Ca2+ channels and excitation-contraction coupling (3). Slow waves depend on the activation of Ca2+-activated Cl− channels (CaCCs) (Anol) and T-type Ca2+ channels (5–8). From sites of pacemaker initiation, slow waves propagate through networks of ICC by means of voltage-dependent Ca2+ entry and amplification of [Ca2+] by Ca2+-induced Ca2+ release from ryanodine and inositol 1,4,5-trisphosphate (IP3) receptors (9). Ca2+ release events couple to activation of Anol channels. Thus, Ca2+ handling mechanisms in ICC are fundamental to GI motility.
Because of its important role in motility, Ca2+ signaling in ICC must be tightly regulated. As in other cells (10), small excluded volumes (nanodomains) created by close associations (≈20 nm) between the endoplasmic reticulum (ER) and the plasma membrane appear to be crucial structures that enable pacemaker activity. Because of their small volumes, the dynamic range of Ca2+ and other ion concentrations may be far greater in nanodomains than in the cytoplasm at large. The ER has a finite capacity to store Ca2+, so frequent release of Ca2+ from ER stores, as observed in ICC (9, 11), requires a mechanism to replenish the stores. This occurs, in part, by reuptake of Ca2+ through SERCA [sarco/endoplasmic reticulum Ca2+ ATPase (adenosine triphosphatase)] that regulates the Ca2+ concentration in the ER (12), but SERCA pumps compete with extrusion mechanisms, such as Na+/Ca2+ exchange and the plasma membrane Ca2+ATPases, such that, over time, net loss of stored Ca2+ occurs without a refilling mechanism (13). A mechanism linked to store refilling is store-operated Ca2+ entry (SOCE). Passive emptying of ER Ca2+ by treatment with SERCA inhibitors results in Ca2+ influx (14–16). The ER transmembrane Ca2+ sensing molecule known as STIM (stromal interacting molecule) and the Ca2+-selective plasma membrane ion channel Orai provide the central mechanism for ER Ca2+ refilling (17–21). Mouse and human genomes contain two STIM paralogs (STIM1 and STIM2) and three Orai paralogs (ORAI1 to ORAI3). Emptying of stores causes rearrangement and oligomerization of STIMs, which translocate within the ER membrane such that the C-terminal cytoplasmic fragments of STIM bind to Orai. STIM association opens and increases the Ca2+ selectivity of Orai (22, 23).
In most cells, formation of this SOCE junctional assembly is slow (10 to 30 s), but in skeletal muscles, SOCE occurs with much faster kinetics than in nonexcitable cells because of either preassembly of STIM1-Orai1 complexes without store depletion (24) or the expression of a specialized splice variant of STIM1 that can respond to store depletion and form junctional complexes with Orai within 1 s (25). We investigated the role of SOCE in sustaining pacemaker activity of ICC of the murine small intestine. ER stores release Ca2+ during slow waves (9) and have only about 1 s to reset before the next slow wave cycle. We identified multiple STIM and Orai paralogs in ICC. Blocking the STIM-Orai complex with an antagonist or a peptide that disrupts STIM-Orai binding resulted in rundown of pacemaker activity, suggesting a key role for SOCE in the pacemaker activity of the GI tract.
RESULTS
Expression of Orai and Stim paralogs in small intestinal ICC
We have previously used fluorescence-activated cell sorting (FACS) to purify ICC, which increases Kit transcript abundance in sorted cells and reduces or eliminates other cell-specific markers, such as Pdgfra (a biomarker for fibroblast-like cells), Myh11 (a biomarker for SMCs), and Uchl1 (a biomarker for neurons) (26). We compared the expression of Orai1, Orai2, Orai3, Stim1, and Stim2 transcripts in extracts of enzymatically dispersed cells from the tunica muscularis of the small intestine (which consisted of unsorted cells) and in FACS-sorted, purified ICC. All paralogs of Orai and Stim were expressed in ICC, and Orai1, Orai2, Orai3, and Stim2 displayed increased expression in ICC compared to unsorted cells (fig. S1, A and B).
Activation of a Cl− conductance by restoration of Ca2+ in ICC
The effects of SOCE in ICC were first investigated with voltage-clamp experiments performed on isolated and identified ICC from small intestine. ICC were pretreated with the SERCA pump inhibitor cyclopiazonic acid (CPA) in a Ca2+-free solution (solution II, Table 1) to induce passive depletion of ER Ca2+ stores, then dialyzed with Cs+-rich pipette solution (to block K+ currents; solution V, Table 1), and held at −80 mV. Restoring extracellular Ca2+ ([Ca2+]o) to 2 mM (solution I, Table 1) caused development of inward current, which was inhibited by returning [Ca2+]0 to 0 mM (solution II, Table 1) and reactivated by restoring 2 mM [Ca2+]o (Fig. 1A). To identify the inward current, ramp protocols (400-ms ramps from −80 to 80 mV) were applied before and in the presence of 2 mM [Ca2+]o. The inward current (Fig. 1B) that developed in response to 2 mM [Ca2+]o was outwardly rectifying and was due to a Cl− conductance because the current reversed at ECl, or −39 ± 0.7 mV after correction for a 15-mV junction potential. Restoring 2 mM [Ca2+]o increased the inward current by 11.8-fold at 0 mV. The Cl− conductance activated by restoration of 2 mM [Ca2+]o is likely due to activation of Ano1 channels, the dominant functional Cl− conductance in ICC (5, 8). Ano1 channels are CaCC, so activation of this conductance by restoration of 2 mM [Ca2+]o suggests Ca2+ entry into ICC.
Table 1. The composition of pipette solutions and bath solutions for patch clamp.
Solutions I, II, and VII were adjusted to pH 7.4 with tris, and solutions III, IV, V, VI, and VIII were adjusted to pH 7.2 with tris. BAPTA, 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid; ATP, adenosine 5′-triphosphate.
| Solutions (mM) | I | II | III | IV | V | VI | VII | VIII |
|---|---|---|---|---|---|---|---|---|
| NaCl | 140 | 140 | ||||||
| NMDGCl | 150 | 147.7 | ||||||
| KCl | 5 | 5 | 140 | |||||
| CaCl2 | 2 | 2 | 4.1 | |||||
| MgCl2 | 1.2 | 1.2 | 1 | |||||
| Glucose | 10 | 10 | ||||||
| CsCl | 140 | 30 | 140 | |||||
| Cesium aspartate | 110 | |||||||
| MgATP | 3 | 3 | 3 | 3 | ||||
| NaGTP | 0.1 | 0.1 | 0.1 | 0.1 | ||||
| Creatine phosphate disodium | 2.5 | 2.5 | 2.5 | 2.5 | ||||
| EGTA | 0.1 | 0.1 | 0.1 | 10 | ||||
| BAPTA | 5 | |||||||
| Hepes | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
Fig. 1. SOCE activates a Cl-conductance in ICC.
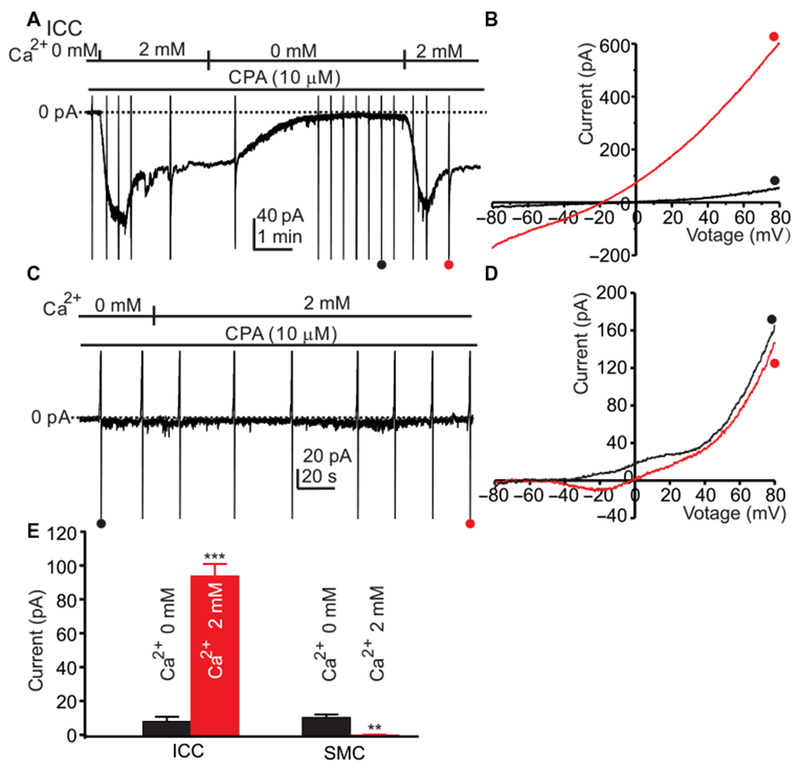
(A) ICC were dialyzed with internal solution V (Table 1; ECl = −40 mV), held at −80 mV, and pretreated with CPA (10 μM) in Ca2+-free external solution (solution II) for 10 min. Inward current was activated when 2 mM Ca2+ (CaPSS, solution I) was restored. Ramp potentials from −80 to +80 mV were run before and after addition of 2 mM Ca2+ (examples noted by • and •). (B) Responses to ramp potentials in (A) [• (black trace) is in 0 mM Ca2+, and • (red trace) is after addition of 2 mM Ca2+]. The current activated by 2 mM Ca2+ was outwardly rectifying and reversed at ECl when corrected for junction potential. (C) SMCs were dialyzed with internal solution V (Table 1; ECl = −40 mV), held at −80 mV, and pretreated with CPA (10 μM) in Ca2+-free external solution (solution II) for 10 min. Ramp potentials from −80 to +80 mV were run before and after addition of 2 mM Ca2+ (examples noted by • and •). (D) Responses to ramp potentials in (C) [• (black trace) is in 0 mM Ca2+, and • (red trace) is after addition of 2 mM Ca2+]. Small inward current after addition of 2 mM Ca2+ is voltage-dependent Ca2+ current common in SMCs. (E) Summary data showing currents induced by reintroducing 2 mM [Ca2+]o at 0 mV in ICC and SMCs. Data are means ± SEM (n = 5 cells for each group; **P < 0.01, ***P < 0.001, Student’s two-tailed t test).
The same experiments were performed on SMCs dispersed from the same tissues from which ICC were obtained. The cells were dialyzed with Cs+-rich pipette solution (solution V) and held at −80 mV. Restoration of 2 mM [Ca2+]o in these cells, after pretreatment with CPA in the presence of 0 mM [Ca2+]o solution, failed to induce inward current at the holding potential (Fig. 1, C and D). These data suggest that, if SOCE is functional in SMCs, the current generated is very small. Increasing [Ca2+]o to 2 mM increased inward current in store-depleted ICC but not in SMCs (Fig. 1E).
Block of SOCE currents in HEK 293 cells and ICC by an inhibitory STIM1 peptide
Selective pharmacological inhibitors of STIM1 are not available. Therefore, we developed novel peptides designed to act as dominant negative inhibitors to interrupt STIM1 activity. These peptides target a cytosolic domain responsible for functional interactions with Orai channels in the plasma membrane. We identified a 27-residue amino acid sequence within the STIM-Orai activation region (SOAR) that forms the second cytoplasmic coiled-coil domain (CC2) peptide of STIM1. Patch clamp experiments were performed on human embryonic kidney (HEK) 293 cells stably expressing Orai1 and Stim1 (26) to determine the effects of this peptide on ICRAC. Cells were treated with thapsigargin and 0 mM [Ca2+]o to passively deplete ER Ca2+ stores and the CaCC antagonist 5-nitro-2-(3-phenylpropylamino)-benzoic acid (NPPB) to block Cl− current. The CC2 peptide or scrambled CC2 peptide was added to pipette solutions and dialyzed into cells held at −50 mV (Fig. 2A). In cells treated with the scrambled CC2 peptide, increasing [Ca2+]o from 0 to 2 mM caused development of inward current, a 10.4-fold increase at −80 mV (Fig. 2B). In cells treated with the CC2 peptide, the inward current resulting from restoration of 2 mM [Ca2+]o was reduced by 2.8-fold (Fig. 2, C and D). In comparison to the scrambled CC2 peptide, the CC2 peptide decreased the inward current induced by 2 mM [Ca2+]o by 6.9-fold at −80 mV (Fig. 2E). These data demonstrate that the CC2 peptide (Fig. 2F) effectively inhibits ICRAC initiated by store depletion in this model system.
Fig. 2. CRAC current in HEK 293 cells expressing Orai1 and Stim1.
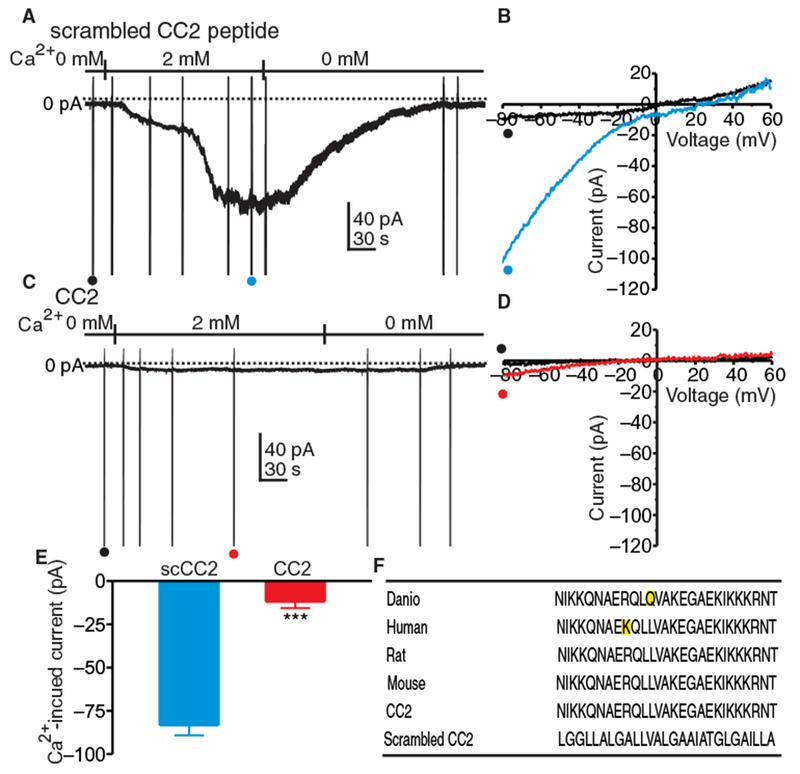
(A) Currents elicited in HEK 293 cells expressing Orai1 and Stim1 in whole-cell voltage-clamp at a holding potential of −50 mV. Cells were treated with Ca2+-free external solution (solution II) containing NPPB (30 μM) and thapsigargin (1 μM) for 10 min to deplete ER Ca2+ and block CaCC. HEK 293 cells were dialyzed with Cs+-rich solution containing BAPTA (solution VI). When cells were dialyzed with scrambled CC2 peptide (10 μM), reintroduction of 2 mM [Ca2+]o caused development of inward current. Ramp potentials from −80 to +60 mV were run before and after addition of 2 mM [Ca2+]o [examples noted by • and • in (A)]. (B) Currents elicited by ramp potentials [• (black trace) is control, and • (blue trace) is after addition of 2 mM [Ca2+]i] in (A). (C) Currents elicited in HEK 293 cells expressing Orai1 and Stim1 in whole-cell voltage-clamp at a holding potential of −50 mV. Cells were treated with Ca2+-free external solution (solution II) containing NPPB (30 μM) and thapsigargin (1 μM) for 10 min to deplete ER Ca2+. HEK 293 cells were dialyzed with Cs+-rich solution containing BAPTA (solution VI). (D) Current responses to ramp potentials [• (black trace) is control, and • (red trace) is after addition of 2 mM Ca2+] in (C). (E) Summary data showing the effects of scrambled CC2 (scCC2) peptide and CC2 peptide on current elicited by reintroducing 2 mM [Ca2+]o at −80 mV. Data are means ± SEM (n = 5 cells for each group; ***P < 0.001, Student’s two-tailed t test). (F) STIM1 sequence in several species and the sequences of the CC2 and scrambled CC2 peptides.
Activation of ICRAC in ICC was tested by treating cells with thapsigargin and 0 mM [Ca2+]o (solution II, Table 1) to passively deplete ER Ca2+ stores and the CaCC antagonist NPPB to block the Cl− current that was activated by restoring [Ca2+]o to 2 mM (see Fig. 1, A and B). A Cs+-rich pipette solution containing BAPTA (solution VI, Table 1) was used in these experiments to further eliminate contamination from CaCC, and cells were held at −50 mV. In cells dialyzed with the scrambled CC2 peptide, restoring [Ca2+]o to 2 mM induced inward current (Fig. 3A). Ramp potentials (from −80 to +80 mV) were applied before and after adding [Ca2+]o to determine the current-voltage characteristics of ICRAC. [Ca2+]o (2 mM) induced ICRAC-like currents (Fig. 3B), increasing inward current by 2.6-fold at −80 mV. The reversal potential of the current responses to ramp potentials shifted from 0.4 ± 1.8 mV to 19.8 ± 3.3 mV, consistent with the conductance activated by 2 mM [Ca2+]o being permeable to Ca2+ (Fig. 3B). In cells dialyzed with the CC2 peptide, increasing [Ca2+]o to 2 mM did not induce appreciable inward current at −80 mV (Fig. 3, C and D). In comparison with the scrambled CC2 peptide, the CC2 peptide reduced the inward current induced by restoration of 2 mM [Ca2+]o by 12-fold (Fig. 3E). Cells dialyzed with CC2 peptide but not pretreated with thapsigargin did not respond to restoration of 2 mM [Ca2+]o (fig. S2, A to C).
Fig. 3. CRAC current (ICRAC) in ICC.
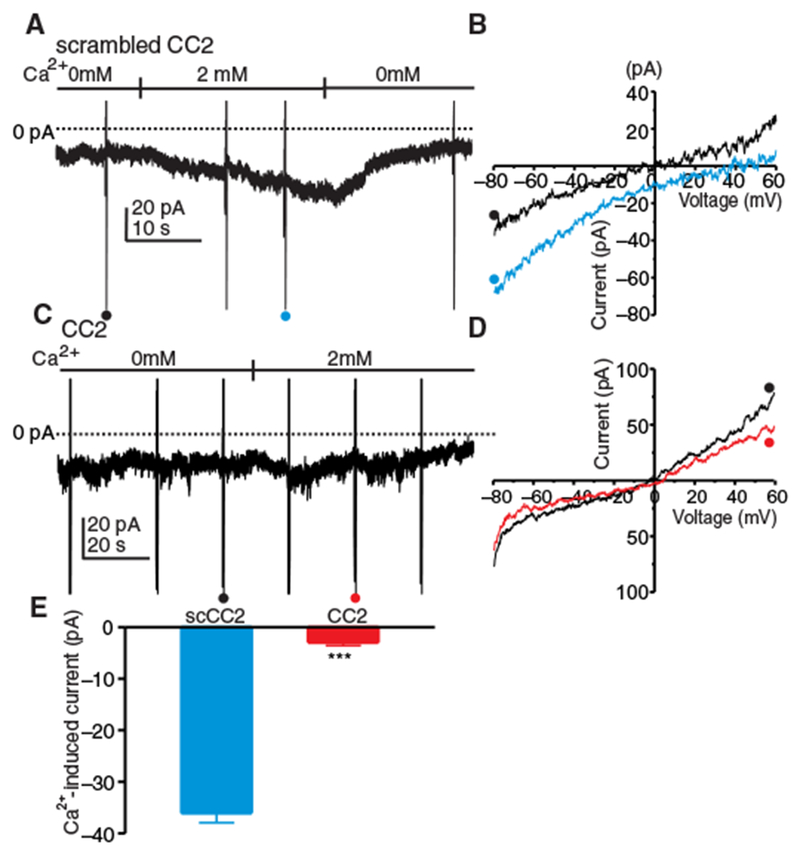
(A) ICC were held at −50 mV in whole-cell voltage-clamp and exposed to Ca2+-free external solution (solution II) with NPPB (30 μM) and thapsigargin (1 μM) for 10 min to deplete ER Ca2+ and block CaCC and dialyzed with Cs+-rich solution containing BAPTA (solution VI). Inward current developed in cells dialyzed with scrambled CC2 peptide (10 μM) upon reintroduction of 2 μM [Ca2+]o. Ramp potentials from −80 to +60 mV were run before and after addition of 2 mM [Ca2+]o (examples noted by • and •). (B) Current responses to ramp potentials in (A) [• (black trace) is control, and • (blue trace) is after addition of 2 mM [Ca2+]o]. (C) ICC were held at −50 mV in whole-cell voltage-clamp, treated with Ca2+-free external solution (solution II) with NPPB (30 μM) and thapsigargin (1 μM) for 10 min to deplete ER Ca2+ and block CaCC, and dialyzed with Cs+-rich solution containing BAPTA (solution VI). (D) Responses to ramp potentials in (C) [• (black trace) is control, and • (red trace) is after addition of 2 mM [Ca2+]o]. (E) Summary data showing the effects of scrambled CC2 peptide and CC2 peptide on current induced at −80 mV by reintroducing 2 mM [Ca2+]o. Data are means ± SEM (n = 5 cells for each group; ***P < 0.001, Student’s two-tailed t test).
Blocking ICRAC in ICC with the Orai antagonist GSK-7975A
The Orai antagonist GSK-7975A abolished ICRAC developed in response to restoring 2 mM [Ca2+]o (Fig. 4, A to C) in cells in which stores were depleted with thapsigargin and 0 mM [Ca2+]o. Contamination from CaCC was reduced by pretreating cells with NPPB and using a Cs+-rich pipette solution containing BAPTA (solution VI; Table 1). The calcium channel Cav3.2 and the chloride channel Ano1 are involved in pacemaker activity in ICC (3). However, GSK-7975A did not inhibit Cav3.2 current over a broad range of test potentials (fig. S3, A to C) and had no resolvable effects on Ano1 currents, except at very positive potentials that do not occur physiologically (fig. S3, D to F).
Fig. 4. Block of ICRAC in ICC with GSK-7975A.
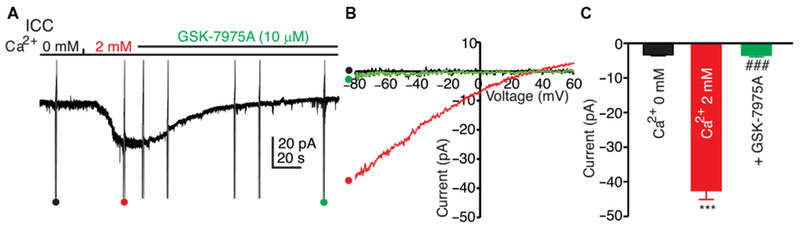
(A) ICC in whole-cell voltage-clamp were held at −50 mV and treated with 0 mM [Ca2+]o solution (solution II) containing NPPB (30 μM) and thapsigargin (1 μM) for 10 min to deplete ER Ca2+ and block CaCC. The cells were dialyzed with Cs+-rich solution containing BAPTA (solution VI). Reintroduction of 2 mM [Ca2+]o (solution I) activated inward current (ICRAC). GSK-7975A (10 μM) completely abolished ICRAC. Ramp potentials from −80 to +60 mV were run during the experiment (examples noted by •, •, and •). (B) Responses to ramp potentials in (A) [• (black trace) is 0 mM [Ca2+]o, • (red trace) is after addition of 2 mM [Ca2+]o, and • (green trace) is after addition of GSK-7975A (10 μM)]. (C) Summary of the effects of GSK-7975A on ICRAC. Data are means ± SEM [n = 5 cells for each group; ***P < 0.001 compared to 0 mM [Ca2+]o, ###P < 0.001 compared to 2 mM [Ca2+]o, one-way analysis of variance (ANOVA)].
Effects of 2-APB on ICRAC in ICC
2-Aminoethoxydiphenyl borate (2-APB) has complicated effects on Orai channels. Low concentrations of this agent increase the unitary conductance of Orai1 (27), but higher concentrations block the conductance (28,29). We used this signature to further verify that the current observed in ICC upon restoring [Ca2+]o to 2 mM after treatment with thapsigargin and 0 mM [Ca2+]o was ICRAC. Restoring [Ca2+]o to 2 mM induced inward current. 2-APB (10 μM) enhanced the inward current, whereas 100 μM 2-APB inhibited the current (Fig. 5A). Ramp potentials (−80 to +80 mV) were applied during the experiments to quantify ICRAC (Fig. 5B). The ICRAC-like current increased by fivefold at −80 mV after restoring [Ca2+]o to 2 mM. 2-APB at 10 μM enhanced ICRAC-like current by 1.5-fold, and 2-APB at 100 μM decreased the current by 5-fold (Fig. 5C).
Fig. 5. Effects of 2-APB on CRAC current in ICC.
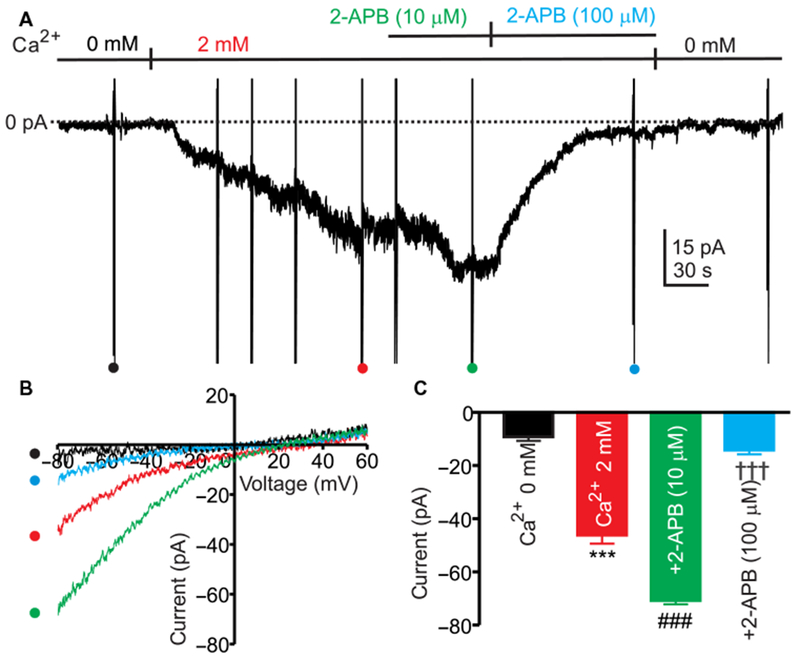
(A) ICC were treated with Ca2+-free external solution (solution II), NPPB (30 μM), and thapsigargin (1 μM) for 10 min to deplete ER Ca2+ and block CaCC. ICC were dialyzed with Cs+-rich solution containing BAPTA (solution VI) and held at −50 mV. Restoration of 2 mM [Ca2+]o (CaPSS, solution I) activated inward current. 2-APB (10 μM) enhanced the inward current, and 2-APB (100 μM) blocked the inward current. Ramp potentials from −80 to +60 mV were run during the experiment (ramps run during different experimental conditions denoted by,•,•,• and •). (B) Responses to ramp potentials in (A) [• (black trace) is control, • (red trace) is after addition of 2 μM [Ca2+]o, • (green trace) is after addition of 10 μM 2-APB, and • (blue trace) is after addition of 100 μM 2-APB]. (C) Summary data showing the effects of 2-APB on current induced by restoring 2 μM [Ca2+]o at −80 mV. Data are means ± SEM [n = 5 cells for each group; ***P < 0.001 compared to 0 mM [Ca2+]o, ###P < 0.001 compared to 2 mM [Ca2+]o, †††P < 0.001 compared to 2-APB (10 μM), one-way ANOVA].
Activation of ICRAC in ICC by store unloading with IP3
SOCE can also be activated by emptying of Ca2+ stores mediated by Ca2+ release through ligand binding to IP3 receptors (14, 15). ICC were dialyzed with Cs+-rich pipette solution containing BAPTA (solution VI, Table 1) and IP3 to cause store emptying. Dialysis of cells with IP3 caused development of an inward current that was abolished by GSK-7975A (Fig. 6A). Ramp potentials applied during whole-cell recording showed that IP3 increased inward currents by 4.6-fold and that GSK-7975A reduced inward current by 4.7-fold at −80 mV (Fig. 6, B and C).
Fig. 6. CRAC current induced by IP3 in ICC.
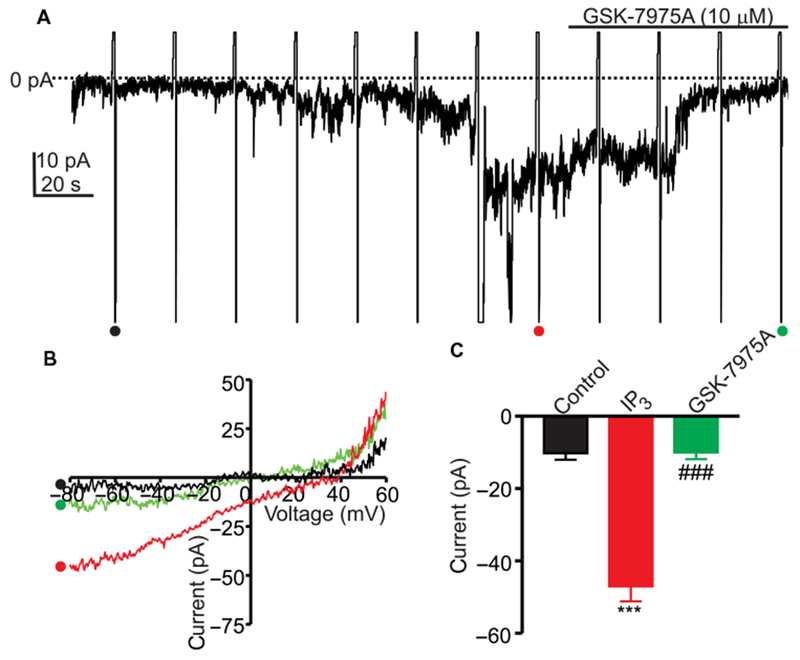
NPPB (30 μM) was added to CaPSS (solution I) to block Ca2+-activated Cl− current. IP3 (30 μM) was included in the Cs+-rich pipette solution also containing BAPTA (solution VI) to cause depletion of ER Ca2+. ICC in the whole-cell configuration were held at −50 mV. (A) Representative trace showing the effect of IP3 in the presence or absence of GSK-7975A on inward current. Ramp potentials from −80 to +60 mV were run during recording. (B) Responses to ramp potentials in (A) [• (black trace) is control, • (red trace) is with IP3 dialysis, and • (green trace) is with IP3 dialysis and GSK-7975A treatment]. (C) Summary data showing the effects of IP3 and GSK-7975A on CRAC currents. Data are means ± SEM (n = 5 cells for each group; ***P < 0.001 compared to control, ###P < 0.01 compared to IP3, one-way ANOVA).
Reduced STICs and slow wave currents in ICC by the STIM1 inhibitory peptide
To investigate the effects of SOCE on spontaneous transient inward currents (STICs) and slow wave currents in ICC (8, 30), voltage-clamp experiments on cells held at −80 mV were performed using a Cs+-rich pipette solution to prevent contamination from K+ conductances. Under these conditions, ongoing STICs were recorded and slow wave currents were initiated by step depolarization from −80 to −35 mV (8). When ICC were dialyzed with the CC2 peptide, the frequency of STICs was reduced by 4-fold, and amplitude decreased by 4.7-fold (Fig. 7A). Peak slow wave current was also reduced by fourfold by CC2 peptide dialysis (Fig. 7B). Dialysis of the scrambled CC2 peptide into a different group of cells did not affect slow wave currents or the frequency or amplitude of STICs (Fig. 7, C to G). CC2 peptide, but not scrambled CC2 peptide, reduced the amplitude of STICs as a function of dialysis time (Fig. 7G). CC2 peptide had no effect on Ano1 current (fig. S4, A to C).
Fig. 7. Inhibition of STICs and slow wave currents by the CC2 peptide.
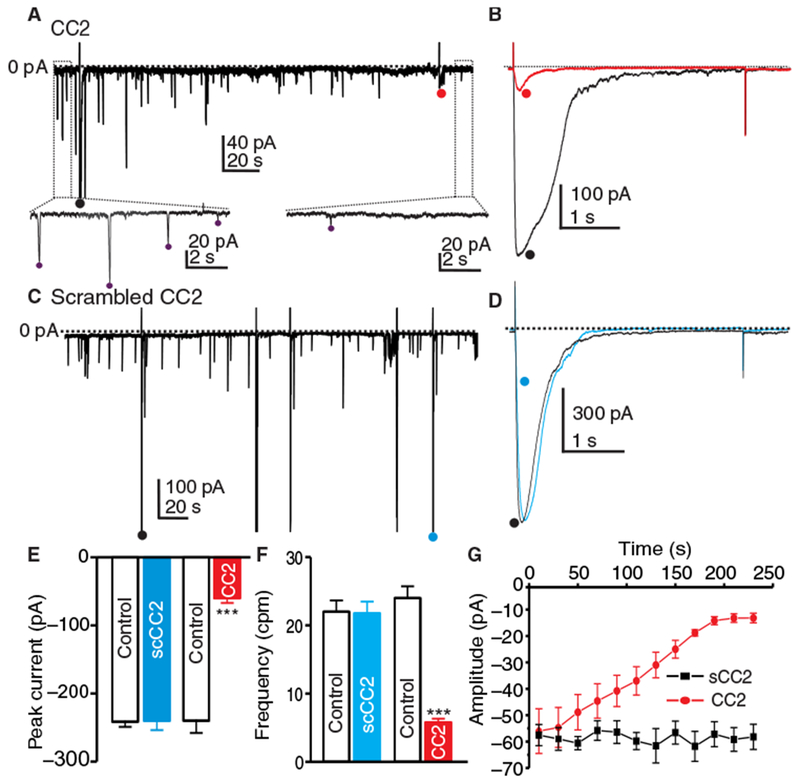
(A) Representative trace showing the effect of CC2 peptide (10 μM) added to Cs+-rich pipette solution (solution IV) on STICs recorded from ICC at −80 mV. Insets show expanded time scale from upper panel (dotted boxes) to display how STIC amplitude and frequency were measured. Purple dots (•) denote detection of STICs using event detection analysis. (B) Representative traces showing the effect of dialysis of the CC2 peptide on slow wave currents induced in ICC by step depolarization from −80 mV to −35 mV (5 s) [• (black trace) is the initial slow wave current, and • (red trace) is after CC2 peptide dialysis]. (C) Representative trace showing the effect of scrambled CC2 peptide (10 μM) on STICs at −80 mV. Stepping from −80 to −35 mV (5 s) induced slow wave currents in (B). (D) Representative traces showing the effect of dialysis of scrambled CC2 peptide on slow wave currents induced in ICC by step depolarization from −80 to −35 mV (5 s) [• (black trace) is the initial current, and • (blue trace) is after dialysis with scrambled CC2 peptide]. (E and F) Summary data showing the effects of CC2 peptide and scrambled CC2 peptide on the peak amplitude of slow wave currents (E) and frequency [cycles per minute (cpm)] of STICs (F). Data are means ± SEM (n = 5 cells for each group; ***P < 0.001, Student’s two-tailed t test). (G) Summary data showing amplitudes of STICs as a function of time during CC2 peptide and scrambled CC2 peptide dialysis. Data are means ± SEM (n = 5 cells for each group).
Reduction in STICs and STDs in ICC by GSK-7975A
Whole-cell patch clamp experiments were performed to determine whether Orai channels are involved in sustaining STICs and spontaneous transient depolarizations (STDs). Cs+-rich solution (solution IV) was used to record STICs and to eliminate contamination from K+ currents. GSK-7975A reduced the frequency and amplitude of STICs by 2.3-fold (Fig. 8, A and B) and 5.7-fold (Fig. 8, A and C), respectively. STDs were recorded in ICC under current-clamp (I = 0) in a K+-rich pipette solution and CaPSS. GSK-7975A did not affect the resting membrane potential (Fig. 8, D and E) but inhibited the amplitude of STDs by 14.8-fold (Fig. 8, D and F) and the frequency by 6.6-fold (Fig. 8, D and G).
Fig. 8. Inhibition of STICs and STDs by GSK-7975A.
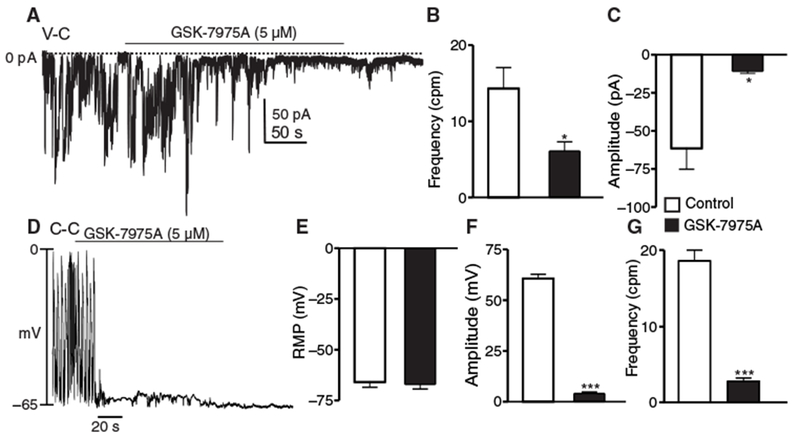
(A) STIC activity under voltage-clamp (V-C) conditions before and after the addition of GSK-7975A (5 μM; Cs+-rich pipette solution, solution IV). (B and C) Summary data showing the effects of GSK-7975A on the frequency and amplitude of STICs, respectively. Data are means ± SEM (n = 5 cells; *P < 0.05, Student’s two-tailed t test). (D) Under current-clamp (C-C) conditions (I = 0), STICs manifest as STDs. STD activity under current-clamp conditions before and after the addition of GSK-7975A. CaPSS (solution I) and K+-rich solution (solution III) were used as the external and pipette solution, respectively. (E to G) Summary data showing the effects of GSK-7975A on the resting membrane potential (RMP) (E), amplitude of STDs (F), and frequency of STDs (G), respectively. Data are means ± SEM (n = 5 cells for each group; no symbol = not significant; ***P < 0.001, Student’s two-tailed t test).
Reduction in Ca2+ transients in small intestinal ICC by GSK-7975A
In ICC, STICs are caused by the activation of CaCC (30), and Ca2+ release events appear to be the source of Ca2+ for activation of CaCC (9, 11). Recordings of pacemaker ICC in the plane of the myenteric plexus (ICC-MY) in situ (Fig. 9A) revealed a pattern of rhythmic Ca2+ transients throughout the ICC-MY network. Firing of Ca2+ transients consisted of temporal clusters of ~1-s duration from multiple firing sites within each field of view (FOV), which are termed Ca2+ transient clusters (CTCs) (9). Analysis of Ca2+ events in ICC-MY was performed with an accumulation map of all Ca2+ transient particles (PTCLs) recorded during a 20-s period from one FOV, displayed as a heat map. When GSK-7975A was added, CTCs were greatly diminished, as shown by the PTCL accumulation map (Fig. 9, B and C). All firing sites in an FOV were also displayed as an occurrence map in which each firing site in the FOV was color-coded and shown as a “lane,” and the PTCL area and count for Ca2+ events were plotted below the occurrence map (Fig. 9D). GSK-7975A reduced the number of firing sites, the PTCL area, and count of Ca2+ events within the same FOV (Fig. 9E). Ca2+ firing sites were reduced by 10.6-fold by GSK-7975A treatment (Fig. 10A), and the regular temporal clustering of Ca2+ transients was reduced. The probability that an individual Ca2+ firing site would generate a Ca2+ transient during a CTC was reduced by 31.9-fold after addition of GSK-7975A (Fig. 10B). The overall frequency of CTCs was also reduced by 2.5-fold (Fig. 10C). Furthermore, the PTCL area was reduced by 117-fold (Fig. 10D), and the PTCL count/frame was reduced by 56-fold after GSK-7975A treatment (Fig. 10E).
Fig. 9. Block of Ca2+ transients in ICC by GSK-7975A.
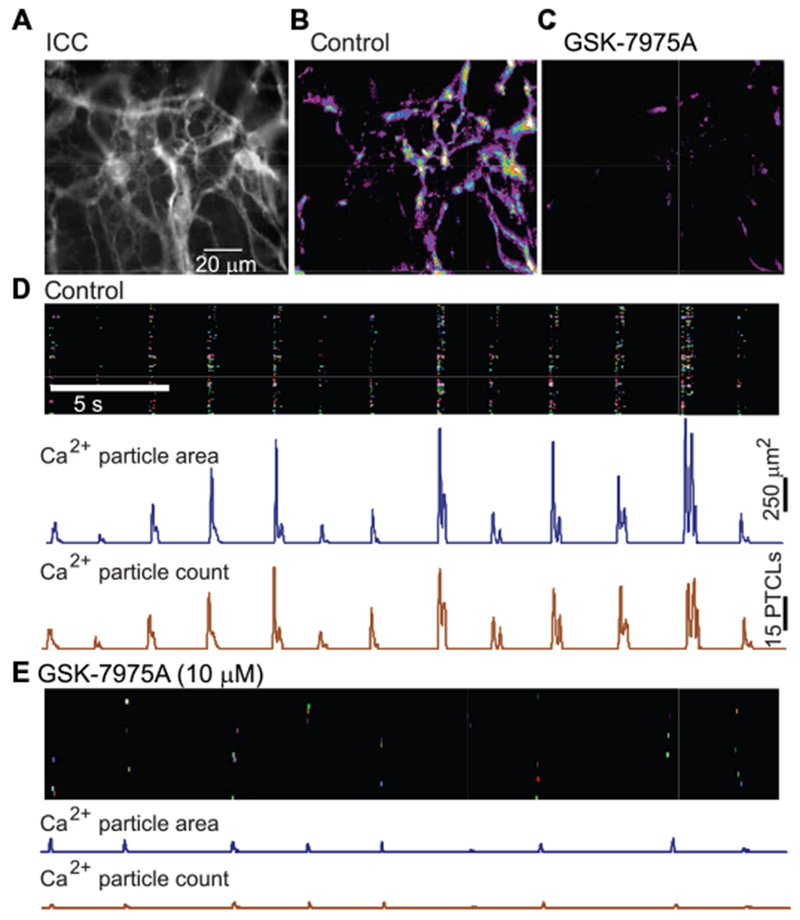
(A) Representative image of an ICC-MY network in situ in the small intestine of a Kit-Cre-GCaMP3 mouse (scale bar, 20 μm). (B) Representative heat map showing summation of Ca2+ transient PTCLs for a 20-s recording period under control conditions [same FOV as in (A)]. (C) Representative heat map showing summation of Ca2+ transient PTCLs for a second recording period after addition of GSK-7975A (10 μM) [same FOV as in (A) and (B)]. (D) Occurrence map of Ca2+ firing sites within the ICC-MY network shown in (A) and (B) during the control period [time scale in (D) also applies to (E)]. Each Ca2+ firing site within the FOV was individually color-coded and positioned in a lane and plotted against time. Summaries of Ca2+ PTCL area (in μm2) and PTCL count are shown in the traces below the occurrence map. (E) Occurrence map of Ca2+ transients from the same FOV in (A) and (C) after addition of GSK-7975A (10 μM). Summaries of Ca2+ PTCL area (in μm2) and PTCL count are shown in the traces below the occurrence map.
Fig. 10. Effects of GSK-7975A on Ca2+ signaling in ICC.
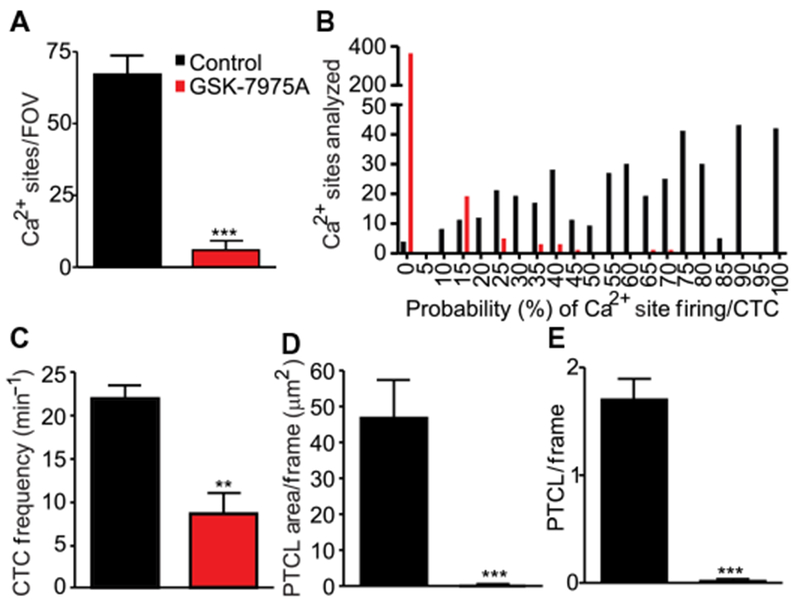
(A) Summary data showing the number of Ca2+ firing sites observed during control recordings (black bar) and after addition of GSK-7975A (10 µM, red bar). Data are means ± SEM (n = 6 animals for each group; ***P < 0.001, Student’s two-tailed t test). (B) Histogram describing the probability (%) of individual Ca2+ firing sites in the ICC-MY network discharging a Ca2+ transient during a CTC in control conditions (black bars) and after addition of GSK-7975A (10 µM, red bars; n = 6 animals for each group). (C to E) Summary data showing the CTC frequency (in min−1) (C), CTC PTCL area (in µm2) (D), and CTC PTCL count (E) observed in control conditions (black bar) and after addition of GSK-7975A (10 µM, red bar). Data are means ± SEM (n = 6 animals for each group; **P<0.01, ***P < 0.001, Student’s two-tailed t test).
DISCUSSION
ICC display dynamic Ca2+ signaling characterized by localized Ca2+ transients that activate CaCC in the plasma membrane (8, 9,11, 31). Ca2+ signaling is fundamental to pacemaker activity of ICC and is also likely to play an important role in neurotransmission in ICC. We investigated how SOCE, mediated by STIM-Orai interactions, is involved in sustaining Ca2+ transients and STICs in ICC of the murine small intestine. ICC expressed the two components of the molecular apparatus required for SOCE (STIM and Orai), and SOCE currents were apparent in isolated and identified ICC. Activation of SOCE enhanced CaCC, but when this conductance was blocked, small amplitude inward currents, consistent with ICRAC, were measured in ICC. A dominant-negative peptide, designed to inhibit STIM-Orai binding, blocked ICRAC in HEK 293 cells expressing Orai1 and STIM1 and in ICC. The same peptide blocked STICs and slow wave currents in ICC. The Orai antagonist GSK-7975A had similar effects on ICRAC and STICs in ICC, and it also blocked Ca2+ transients in ICC networks in intact muscles. Together, these observations demonstrate that SOCE assists in maintenance of the Ca2+ stores required for pacemaker activity in ICC.
STIM and Orai mediate SOCE in nonexcitable cells (32) and in some excitable cells, such as skeletal muscle cells (33, 34). Because of the relatively slow kinetics of STIM1-Orai1 interactions, SOCE is unlikely to contribute to cytoplasmic Ca2+ during twitch responses. It is more likely that this mechanism contributes to longer-term maintenance of store Ca2+ or participates in other cell signaling mechanisms (35). Cardiac and smooth muscle cells also express STIM and Orai genes (35, 36); however, lower expression levels suggest functions for SOCE other than regulation of contraction (37, 38). STIM1 and Oraii are expressed in pacemaker cells isolated from the sinoatrial node (and immunopositive for HCN4) (39), and these cells display SOCE, as determined by increased [Ca2+]i after store depletion by thapsigargin and restoration of [Ca2+]o. A role for SOCE in maintenance of pacemaker activity, however, is unclear because blockers of SOCE only modestly affect pacemaker frequency, and their nonspecific effects preclude clear interpretation of the results. In cultured intestinal ICC, STIM1 overexpression increases pacemaker potentials, and overexpression of the SOCE inhibitor SARAF (40) suppresses pacemaker activity (41). The present study further explored the presence and importance of SOCE in pacemaker activity of ICC. ICRAC was measured directly in freshly dispersed ICC. STICs and STDs, which are fundamental for generation of pacemaker activity in ICC (6, 8, 42), were inhibited by an Orai antagonist and by a dominant-negative peptide. The Orai antagonist also inhibited the Ca2+ transients that underlie pacemaker activity in ICC (9).
The role of SOCE in pacemaker activity might be questioned on the basis of kinetics. The frequency of electrical slow waves in murine small intestinal ICC is about 30 cycles per minute, depending upon experimental conditions (9). The duration of the slow wave event is about 1 s from the onset of depolarization (upstroke phase) to completion of the plateau phase (9). Ca2+ transients occur asynchronously throughout the plateau phase to sustain the activation of CaCC, and depolarization to approximately ECl is maintained during the plateau (9, 43). At the depolarized potential of the plateau (≈−10 mV), the driving force for Ca2+ entry [difference between reversal potential for ICRAC (ECRAC) and membrane potential at the peak of the plateau phase (EPlat)] is relatively small, so the period between slow waves is likely to be the period of greatest SOCE. Thus, under normal circumstances, Ca2+ stores have about 1 s to refill after each slow wave. The rate of activation of SOCE in nonexcitable cells is slow, requiring tens of seconds for full activation (44). These characteristics are too slow to maintain stores that release Ca2+ every few seconds. In skeletal muscles, the kinetics of STIM-Orai due to Ca2+ depletion are faster because of a specialized splice variant or sustained coupling of the complex (25, 45, 46). RNA sequencing analysis of ICC from small intestinal muscles shows that several splice variants of STIM1 are expressed in these cells (47). Although the skeletal muscle isoform was not detected, other splice variants of STIM may convey kinetic properties suitable for the maintenance of pacemaker activity.
When expressed with STIM proteins, the three homologs of Orai can reconstitute SOCE or CRAC currents (ICRAC). CRAC channels can be composed of single-or multiple-type homologs of Orai (48). Transcripts of all three Orai homologs of Orai were more abundant in ICC than in unsorted cells. ICC expressed Stim1 at relatively higher levels than Stim2 (fig. S1B), and Stim1 was expressed at relatively higher levels than Stim2. STIM1 and STIM2 both translocate to the plasma membrane–ER junctions and facilitate development of ICRAC upon store depletion (49). However, STIM2 appears to regulate basal Ca2+ due to its lower affinity for Ca2+ and ability to respond to smaller decreases in ER Ca2+ content (50). STIM2 may be partially activated at normal ER Ca2+ levels. STIM1 has little or no response to minor changes in basal Ca2+ levels but becomes activated when larger Ca2+ release events reduce ER [Ca2+] to below 200 μM (50, 51). Thus, expression of these two isoforms in ICC may have consequences for maintenance of basal ER Ca2+ levels (in preparation for pacemaker events) and store-depleting Ca2+ release events (recovery from post-pacemaker activity).
It is difficult to establish the functional influence of STIM-Orai activity in physiological settings because selective pharmacological inhibitors of STIM are not available, and global knockout animals are not viable (52). Acute knockdown has been used effectively in cultured cells, but this approach would be problematic for studies of ICC because the pacemaker mechanism changes substantially in culture (53). Conditional knockouts are also problematic because of the multiple STIM and Orai isoforms (fig. S1, A and B) and because of incomplete gene deactivation with inducible Cre recombinase (42). To overcome these obstacles, we developed a new strategy using an inhibitory peptide that is homologous to CC2 within the SOAR of the mouse STIM1 gene. Site-directed mutagenesis studies have shown that this region is critical for physical interactions between STIM1 and Orai channels and initiation of SOCE (54). This peptide displays only limited homology with STIM2 (20 of 27 residues) and may selectively inhibit STIM1 activity, but this effect has not been confirmed experimentally. The inhibitory peptide abolished whole-cell CRAC channels in HEK cells expressing STIM1 and Orai1. We believe that by acting in a dominant-negative manner, the peptide prevents STIM1 binding to Orai1, thereby inactivating SOCE. The inhibitory peptide also blocked ICRAC, STICs, and slow wave currents in ICC, providing evidence for involvement of STIM1/Orai binding in pacemaker activity.
Early studies on SOCE (called CRAC channels in many papers) suggested that canonical transient receptor potential channels (TRPCs) are involved in SOCE because of enhanced responses in cells ectopically expressing TRPCs (55–57). Although this topic is controversial, it should be noted that ICC of the small intestine express Trpc1 and other Trpc genes to a lesser extent (47). Our results, which do not exclude interactions of TRPC1 with Orai, are compatible with the idea that STIM-Orai interactions are sufficient to sustain pacemaker currents and underlying Ca2+ transients because the CC2 peptide blocked ICRAC and pacemaker currents.
Limitations of the current study include the expression of multiple paralogs of Orai and Stim in ICC, making it difficult to validate pharmacological findings by gene knockout studies. Interactions between STIM and Orai need to be rapid to accomplish store recovery between slow wave events. This might be accomplished by specialized splice variants of Stim genes, and several splice variants are present in ICC (47). The importance of this diversity has not yet been evaluated. We were not able to directly visualize Ca2+ entry into ICC after passive store depletion and restoration of 2 mM [Ca2+]o because of large muscle movements upon addition of 2 mM [Ca2+]o. There are also several auxiliary proteins that modulate STIM-Orai interactions. Genes encoding many of these auxiliary proteins are expressed in ICC (47), and future studies will need to be performed to understand their role in modulating pacemaker activity. Furthermore, additional mechanisms may contribute to store maintenance in ICC, and these have not yet been evaluated in a manner that allows a complete description of Ca2+ handling during pacemaker activity. For example, slow waves require activation of voltage-dependent Ca2+ channels to sustain propagation (7, 9), and, as in SMCs, store refilling may be accomplished, in part, by Ca2+ entry through this pathway (58). Additional mechanisms, such as Na+/Ca2+ exchange working in reverse mode, may also contribute to store replenishment.
In summary, SOCE appears to be critical for maintenance of pacemaker activity in ICC because we observed rapid loss of Ca2+ release events and STICs, the fundamental steps in initiation and propagation of pacemaker activity in ICC, when SOCE was blocked. How STIM-Orai function at a rate appropriate to the 30 cycle per minute frequency of slow waves in intestinal ICC, whether by continuous association or by the expression of STIM isoforms that facilitate rapid STIM-Orai binding and activation of Ca2+ entry, are questions yet to be answered.
MATERIALS AND METHODS
Animals
C57BL/6 (Charles River Laboratories) and KitcopGFP/+ mice, as described previously (8), were used for electrophysiological experiments. For Ca2+ imaging experiments, we used a genetically encoded Ca2+ sensor expressed selectively in ICC to monitor Ca2+ dynamics in ICC in situ, as described previously (9, 11). GCaMP3-floxed mice [B6.129S-Gt(ROSA)26Sortm38(CAG-GCaMP3)Hze/J] and their wild-type littermates (C57BL/6) were purchased from The Jackson Laboratory. Kit-Cre mice (c-Kit+/Cre-ERT2) were a gift from D. Saur (Technical University of Munich, Munich, Germany). From crosses of these mice, Kit-Cre-GCaMP3 mice were generated, and Cre recombinase activation, along with subsequent GCaMP3 expression, was induced by injection with tamoxifen at 6 to 8 weeks of age (2 mg for three consecutive days), as described previously (9, 11). Animals were used for experiments 50 days after tamoxifen injections. Animals were anesthetized with isoflurane (Aerrane, Baxter) before decapitation, and the small intestines were removed. The institutional Animal Use and Care Committee at the University of Nevada approved all procedures used in the breeding and sacrificing of animals.
Isolation of ICC and preparation of HEK 293 cells
ICC were isolated from Kit+/copGFP mice, as described previously (8). Briefly, jejunal muscles were cut into small pieces, put in Ca2+-free Hanks’ solution for 20 min, and then incubated in a Ca2+-free enzyme solution containing (per milliliter) collagenase (1.3 mg, Worthington type II), bovine serum albumin (2 mg, Sigma-Aldrich), trypsin inhibitor (2 mg, Sigma-Aldrich), and ATP (0.27 mg). ICC were plated onto coverslips coated with murine collagen (2.5 mg ml−1, BD Falcon) in 35-mm culture dishes. The cells were allowed to stick to the coverslips, which required at least 3 hours of incubation at 37°C (atmosphere, 95% O2–5% CO2) in culture medium (Clonetics) supplemented with 1% antibiotic-antimycotic (Gibco) and stem cell factor (5 ng ml−1, Sigma-Aldrich). SMCs were isolated from the jejunum using similar techniques; however, the enzyme solution used to disperse SMCs contained (per milliliter) collagenase (4 mg, Worthington type II), bovine serum albumin (8 mg), trypsin inhibitor (8 mg), papain (1 mg), and l-dithiothreitol (0.15 mg, Sigma-Aldrich) for about 25 min. SMCs were stored in Ca2+-free Hanks’ solution on ice for less than 6 hours before using for experiments.
HEK 293 cells stably expressing Orai1-V102C-CFP (cyan fluorescent protein) and STIM1-YFP (yellow fluorescent protein) were donated by D. Gill (The Pennsylvania State University College of Medicine, Hershey, PA). The generation of cell lines and the electrophysiological properties of the currents were described previously (26). HEK 293 cells with stable expression of Cav3.2 (T-type Ca2+ channels) were provided by E. Perez-Reyes (University of Virginia). Generation of cell lines and cell cultures was described previously (59). HEK 293 cells were generated with expression of the Ano1 AC splice variant tagged with green fluorescent protein (GFP), as described previously (60). HEK 293 cells were maintained in Dulbecco’s modified Eagle’s medium (Mediatech) supplemented with 10% fetal bovine serum (FBS), penicillin, and streptomycin. The Bio-Rad Gene Pulser Xcell System was used to perform all the plasmid transfections via electroporation in Opti-MEM medium. Transfected cells were cultured on coverslips in Opti-MEM with 5% FBS.
Electrophysiological recording
ICC were identified in mixed cell populations by the expression of GFP (copGFP) using an inverted fluorescence microscope. The whole-cell patch clamp configuration was used to record membrane currents (voltage-clamp mode) and membrane potential (current-clamp mode, I = 0). Currents were amplified with an Axopatch 200B patch-clamp amplifier (Axon Instruments) and digitized with a 16-bit analog to digital converter (Digidata 1440A, Axon Instruments) and stored using pCLAMP software (version 10.2, Axon Instruments). Data were sampled at 4 kHz and filtered at 2 kHz using an eight-pole Bessel filter for whole-cell experiments. The amplitude and frequency of STICs were analyzed using a threshold search (0.5 pA) in Event Detection of Clampfit (see Fig. 7A, inset; pCLAMP version 10.2, Axon Instruments) and GraphPad Prism software (version 3.0, GraphPad Software Inc.).
To induce ICRAC in electrophysiological conditions, ICC were pretreated (10 min) with CPA (10 μM) or thapsigargin (1 μM) (SERCA pump inhibitors) in the presence of Ca2+-free external solution (solution II) to induce passive depletion of ER Ca2+ stores. The cells were dialyzed with Cs+-rich pipette solution (solution V; ECl = −40 mV) and held at −80 mV. Restoring extracellular Ca2+ (2 mM; solution I) caused development of ICRAC.
Solutions and chemicals
The external solution for whole-cell recordings was a Ca2+-containing and Ca2+-free physiological salt solution (CaPSS and Ca2+-free external solution; see solutions I and II, Table 1). The pipette solutions (solutions III, IV, V, and VI) for these experiments were described in Table 1. Thapsigargin, NPPB, IP3, and 2-APB were purchased from Sigma-Aldrich. CPA was purchased from Tocris Bioscience. 2,6-Difluoro-N-(1-(4-hydroxy-1-(trifluoromethyl)benzyl)-1H-pyrazol-3-yl)benzamide (GSK-7975A) was purchased from Aobious. Stock solutions were made by dissolving NPPB, CPA, thapsigargin, and GSK-7975A in dimethyl sulfoxide (DMSO), and IP3 was dissolved in water. Then, these chemicals were diluted in the bath solution to the specific concentrations. Final concentrations of DMSO were <0.1%.
The CC2 peptide and scrambled CC2 peptide were synthesized by NEO BioScience. The sequences for CC2 peptide and scrambled CC2 peptide are provided in Fig. 2F. CC2 peptide and scrambled CC2 peptide were dissolved in pipette solutions (solution IV or VI) for cell dialysis, and final concentrations were 10 μM.
Cell purification and qPCR
ICC from KitcopGFP/+ mice were purified by FACS (Becton Dickinson FACSAria) using 488-nm excitation and a 530/30-nm bandpass filter for GFP, as previously described (61). Total RNA was isolated from ICC, and the mixed cell population was obtained after dispersion (pre-unsorted cells) from three mice (n = 3) using illustra RNAspin Mini RNA Isolation kit (GE Healthcare). Concentration and purity of RNA were measured using a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific). Comparative amounts of RNA were used for first-strand complementary DNA (cDNA) and synthesized using SuperScript III (Invitrogen), according to the manufacturer’s instructions. Polymerase chain reaction (PCR) was performed with specific primers using the AmpliTaq Gold PCR Master Mix (Applied Biosystems). The following numbers given in parentheses for the reference nucleotide sequence were used: Gapdh (NM_008084), Orai1 (NM_175423), Orai2 (NM_178751), Orai3 (NM_198424), Stim1 (NM_009287), and Stim2 (NM001081103). PCR products were analyzed on 2% agarose gels and visualized by ethidium bromide. Quantitative PCR (qPCR) was performed with the same primers used for PCR using SYBR Green Chemistry on the 7900HT Real-Time PCR System (Applied Biosystems). cDNA was prepared from three mice (n = 3), and each cDNA was tested independently with triplicate replicates. Negative (no template) controls were included in each qPCR run. Melting curve analysis was performed using the manufacturer’s standard protocol with no evidence for primer dimer or nonspecific products. Regression analysis using the serial 10-fold dilutions of cDNA was used to generate standard curves. Unknown amounts of mRNA were plotted relative to the standard curve for each set of primers and plotted graphically with Microsoft Excel. This gave transcriptional quantification of each gene relative to the endogenous Gapdh standard after log transformation of the corresponding raw data. In pilot studies, Gapdh was tested on ICC cells used in the present study and found to be an appropriate control for qPCR. Normalized values and SDs were calculated for differences of relative gene expression from four dilutions of technical duplicates from each animal.
Calcium imaging
After removal of the small intestines from Kit-Cre-GCaMP3 mice, the intestines were opened along the mesenteric border, and the mucosal layers were removed. Segments of jejunum (2 cm) were pinned flat in a Sylgard coated dish (serosa facing upward). Muscles were perfused continuously with KRB solution at 37°C and allowed to equilibrate for 1 hour. Images were acquired using an Eclipse E600FN microscope (Nikon Inc.) with a 60× 1.0 numerical aperture CFI Fluor lens (Nikon instruments Inc.). GCaMP3 was excited at 488 nm (Polychrome IV, TILL Photonics). After acquisition, movie sequences of Ca2+ imaging data were imported into custom-written software (Volumetry G8d, GWH) for post hoc analysis. Ca2+ imaging experiments were performed in the presence of 100 nM nicardipine to minimize movement artifacts resulting from tissue contractions and associated loss of focus.
Ca2+ transients observed in ICC-MY were quantified using particle analysis, as described previously (9). Briefly, movies were imported into Volumetry G8d and processed to minimize residual motion artifacts. A differential (Δt = ±66 to 70 ms) and Gaussian filter (1.5 μm × 1.5 μm; SD, 1.0) was applied to better distinguish Ca2+ transients from background. A particle analysis routine was applied to movies by use of a flood-fill algorithm that marked the structure of all adjoining pixels that had intensities above threshold. Ca2+ transient particles (PTCLs) were brighter and larger than noise particles. The point at which noise particles emerged and reduced the average particle size was thresholded, and PTCLs above this threshold were then saved as a coordinate-based PTCL movie. Residual noise in the PTCL file was filtered out by only including PTCLs > 6 μm2 (diameter, ~2 pm or smaller) in analysis. Ca2+ transients in ICC-MY manifested as temporal clusters from multiple sites within the FOV, and these temporally clustered events were defined as CTC (9). CTCs were defined as periods of time in which Ca2+ transients were separated by less than 300 ms. In a 30-s recording, 1000 frames were acquired per experiment. The probability (%) that a Ca2+ firing site in the FOV fired during a CTC, along with PTCL area (in square micrometers) and total PTCL count for the entire recording, was calculated. These data were expressed as PTCL area/frame and PTCL count/frame.
Statistical analyses
Molecular data are expressed as means ± SDs, and all other data are expressed as means ± SEM of n cells. All statistical analyses were performed using GraphPad Prism. We used Student’s t test and ANOVA, followed by Bonferroni’s multiple comparisons test to compare two groups and more than two groups, respectively. In all statistical analyses, P < 0.05 was considered statistically significant.
Supplementary Material
Fig. S1. Expression of Orai and Stim paralogs in ICC of the small intestine.
Fig. S2. CC2 peptide has no effect on HEK 293 cells expressing Orai1 and Stim1 without thapsigargin pretreatment.
Fig. S3. Cav3.2 current and Ano1 current are not significantly affected by GSK-7975A.
Fig. S4. Effect of CC2 peptide on Ano1 current.
Acknowledgments:
We are grateful to D. Gill for providing HEK 293 cells with stable expression of STIM1 and Orai1, L. O’Kane for performing analysis of gene transcripts by qPCR, and N. Horowitz for cell cultures.
Funding: H.Z., B.T.D., T.S.S., S.D.K., and K.M.S. were funded by P01 DK41315 (awarded to K.M.S. and S.D.K.), and S.E. was funded by R01 HL091905 from the NIH (awarded to S.E.).
Footnotes
SUPPLEMENTARY MATERIALS
www.sciencesignaling.org/cgi/content/full/11/534/eaaq0918/DC1
Competing interests: The authors declare that they have no competing interests.
REFERENCES AND NOTES
- 1.Huizinga JD, Thuneberg L, Klüppel M, Malysz J, Mikkelsen HB, Bernstein A, W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature 373, 347–349 (1995). [DOI] [PubMed] [Google Scholar]
- 2.Langton P, Ward SM, Carl A, Norell MA, Sanders KM, Spontaneous electrical activity of interstitial cells of Cajal isolated from canine proximal colon. Proc. Natl. Acad. Sci. U.S.A 86, 7280–7284 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanders KM, Ward SM, Koh SD, Interstitial cells: Regulators of smooth muscle function. Physiol. Rev 94, 859–907 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ward SM, Burns AJ, Torihashi S, Sanders KM, Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J. Physiol 480 (Pt. 1), 91–97 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gomez-Pinilla PJ, Gibbons SJ, Bardsley MR, Lorincz A, Pozo MJ, Pasricha PJ, Van de Rijn M, West RB, Sarr MG, Kendrick ML, Cima RR, Dozois EJ, Larson DW, Ordog T, Farrugia G, Ano1 is a selective marker of interstitial cells of Cajal in the human and mouse gastrointestinal tract. Am. J. Physiol. Gastrointest. Liver Physiol 296, G1370–G1381 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hwang SJ, Blair PJA, Britton FC, O’Driscoll KE, Hennig G, Bayguinov YR, Rock JR, Harfe BD, Sanders KM, Ward SM, Expression of anoctamin 1/TMEM16A by interstitial cells of Cajal is fundamental for slow wave activity in gastrointestinal muscles. J. Physiol 587, 4887–4904 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng H, Park KS, Koh SD, Sanders KM, Expression and function of a T-type Ca2+ conductance in interstitial cells of Cajal of the murine small intestine. Am. J. Physiol. Cell Physiol 306, C705–C713 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu MH, Kim TW, Ro S, Yan W, Ward SM, Koh SD, Sanders KM, A Ca2+-activated Cl− conductance in interstitial cells of Cajal linked to slow wave currents and pacemaker activity. J. Physiol 587, 4905–4918 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drumm BT, Hennig GW, Battersby MJ, Cunningham EK, Sung TS, Ward SM, Sanders KM, Baker SA, Clustering of Ca2+ transients in interstitial cells of Cajal defines slow wave duration. J. Gen. Physiol 149, 703–725 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berridge MJ, Calcium microdomains: Organization and function. Cell Calcium 40, 405–412 (2006). [DOI] [PubMed] [Google Scholar]
- 11.Baker SA, Drumm BT, Saur D, Hennig GW, Ward SM, Sanders KM, Spontaneous Ca2+ transients in interstitial cells of Cajal located within the deep muscular plexus of the murine small intestine. J. Physiol 594, 3317–3338 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brini M, Carafoli E, Calcium pumps in health and disease. Physiol. Rev 89, 1341–1378 (2009). [DOI] [PubMed] [Google Scholar]
- 13.Berridge MJ, Bootman MD, Roderick HL, Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol 4, 517–529 (2003). [DOI] [PubMed] [Google Scholar]
- 14.Berridge MJ, Capacitative calcium entry. Biochem. J 312 (Pt. 1), 1–11 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Putney JW Jr., A model for receptor-regulated calcium entry. Cell Calcium 7, 1–12 (1986). [DOI] [PubMed] [Google Scholar]
- 16.Putney JW Jr., Capacitative calcium entry revisited. Cell Calcium 11,611–624 (1990). [DOI] [PubMed] [Google Scholar]
- 17.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel S-H, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A, A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 441,179–185 (2006). [DOI] [PubMed] [Google Scholar]
- 18.Liou J,Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE Jr., T. Meyer, STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr. Biol 15, 1235–1241 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Veliqelebi G, Stauderman KA, STIM1, an essential and conserved component of store-operated Ca2+ channel function. J. Cell. Biol 169, 435–445 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soboloff J, Rothberg BS, Madesh M, Gill DL, STIM proteins: Dynamic calcium signal transducers. Nat. Rev. Mol. Cell Biol 13, 549–565 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang SL, Yeromin AV, Zhang XH-F, Yu Y, Safrina O, Penna A, Roos J, Stauderman KA, Cahalan MD, Genome-wide RNAi screen of Ca2+ influx identifies genes that regulate Ca2+ release-activated Ca2+ channel activity. Proc. Natl. Acad. Sci. U.S.A 103, 9357–9362 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McNally BA, Somasundaram A, Yamashita M, Prakriya M, Gated regulation of CRAC channel ion selectivity by STIM1. Nature 482, 241–245 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet J-P, CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science 312, 1220–1223 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dirksen RT, Checking your SOCCs and feet: The molecular mechanisms of Ca2+ entry in skeletal muscle. J. Physiol 587, 3139–3147 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Darbellay B, Arnaudeau S, Bader CR, Konig S, Bernheim L, STIM1L is a new actin-binding splice variant involved in fast repetitive Ca2+ release. J. Cell Biol 194, 335–346 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X, Wang Y, Zhou Y, Hendron E, Mancarella S, Andrake MD, Rothberg BS, Soboloff J, Gill DL, Distinct Orai-coupling domains in STIM1 and STIM2 define the Orai-activating site. Nat. Commun 5, 3183 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu X, Ali S, Li Y, Yu H, Zhang M, Lu J, Xu T, 2-Aminoethoxydiphenyl borate potentiates CRAC current by directly dilating the pore of open Orai1. Sci. Rep 6, 29304 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prakriya M, Lewis RS, Potentiation and inhibition of Ca2+ release-activated Ca2+ channels by 2-aminoethyldiphenyl borate (2-APB) occurs independently of IP3 receptors. J. Physiol 536, 3–19 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei M, Zhou Y, Sun A, Ma G, He L, Zhou L, Zhang S, Liu J, Zhang SL, Gill DL, Wang Y, Molecular mechanisms underlying inhibition of STIM1-Orai1-mediated Ca2+ entry induced by 2-aminoethoxydiphenyl borate. Pflugers Arch. 468, 2061–2074 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu MH, Sung IK, Zheng H, Sung TS, Britton FC, O’Driscoll K, Koh SD, Sanders KM, Muscarinic activation of Ca2+-activated Cl− current in interstitial cells of Cajal. J. Physiol 589, 4565–4582 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu MH, Sung TS, O’Driscoll K, Koh SD, Sanders KM, Intracellular Ca2+ release from endoplasmic reticulum regulates slow wave currents and pacemaker activity of interstitial cells of Cajal. Am. J. Physiol. Cell Physiol 308, C608–C620 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoth M, Penner R, Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature 355, 353–356 (1992). [DOI] [PubMed] [Google Scholar]
- 33.Kurebayashi N, Ogawa Y, Depletion of Ca2+ in the sarcoplasmic reticulum stimulates Ca2+ entry into mouse skeletal muscle fibres. J. Physiol 533, 185–199 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lyfenko AD, Dirksen RT, Differential dependence of store-operated and excitation-coupled Ca2+ entry in skeletal muscle on STIM1 and Orai1. J. Physiol 586, 4815–4824 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trebak M, Zhang W, Ruhle B, Henkel MM, Gonzalez-Cobos JC, Motiani RK, Stolwijk JA, Newton RL, Zhang X, What role for store-operated Ca2+ entry in muscle? Microcirculation 20, 330–336 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trebak M, STIM/Orai signalling complexes in vascular smooth muscle. J. Physiol 590, 4201–4208 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bisaillon JM, Motiani RK, Gonzalez-Cobos JC, Potier M, Halligan KE, Alzawahra WF, Barroso M, Singer HA, Jourd’heuil D, Trebak M, Essential role for STIM1/Orai1-mediated calcium influx in PDGF-induced smooth muscle migration. Am. J. Physiol. Cell Physiol 298, C993–C1005 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luo X, Hojayev B, Jiang N, Wang ZV, Tandan S, Rakalin A, Rothermel BA, Gillette TG, Hill JA, STIM1-dependent store-operated Ca2+ entry is required for pathological cardiac hypertrophy. J.Mol. Cell. Cardiol 52, 136–147 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu J, Xin L, Benson VL, Allen DG, Ju Y-K, Store-operated calcium entry and the localization of STIM1 and Orai1 proteins in isolated mouse sinoatrial node cells. Front. Physiol 6, 69 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palty R, Raveh A, Kaminsky I, Meller R, Reuveny E, SARAF inactivates the store operated calcium entry machinery to prevent excess calcium refilling. Cell 149, 425–438 (2012). [DOI] [PubMed] [Google Scholar]
- 41.Park CG, Wu MJ, Hong C, Jo JY, Jiao HY, Park H, Jun JY, Choi S, Regulation of intracellular calcium by endoplasmic reticulum proteins in small intestinal interstitial cells of Cajal. J. Neurogastroenterol. Motil 24, 128–137 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malysz J, Gibbons SJ, Saravanaperumal SA, Du P, Eisenman ST, Cao C, Oh U, Saur D, Klein S, Ordog T, Farrugia G, Conditional genetic deletion of Ano1 in interstitial cells of Cajal impairs Ca2+ transients and slow waves in adult mouse small intestine. Am. J. Physiol. Gastrointest. Liver Physiol 312, G228–G245 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu MH, Sung TS, Kurahashi M, O’Kane LE, O’Driscoll K, Koh SD, Sanders KM, Na+-K+-Cl− cotransporter (NKCC) maintains the chloride gradient to sustain pacemaker activity in interstitial cells of Cajal. Am. J. Physiol. Gastrointest. Liver Physiol 311, G1037–G1046 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lis A, Peinelt C, Beck A, Parvez S, Monteilh-Zoller M, Fleig A, Penner R, CRACM1, CRACM2, and CRACM3 are store-operated Ca2+ channels with distinct functional properties. Curr. Biol 17, 794–800 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Edwards JN, Blackmore DG, Gilbert DF, Murphy RM, Launikonis BS, Store-operated calcium entry remains fully functional in aged mouse skeletal muscle despite a decline in STIM1 protein expression. Aging Cell 10, 675–685 (2011). [DOI] [PubMed] [Google Scholar]
- 46.Stiber JA, Rosenberg PB, The role of store-operated calcium influx in skeletal muscle signaling. Cell Calcium 49, 341–349 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee MY, Ha SE, Park C, Park PJ, Fuchs R, Wei L, Jorgensen BG, Redelman D, Ward SM, Sanders KM, Ro S, Transcriptome of interstitial cells of Cajal reveals unique and selective gene signatures. PLOS ONE 12, e0176031 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hou X, Pedi L, Diver MM, Long SB, Crystal structure of the calcium release-activated calcium channel Orai. Science 338, 1308–1313 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brandman O, Ferrell JE Jr., R. Li, T. Meyer, Interlinked fast and slow positive feedback loops drive reliable cell decisions. Science 310, 496–498 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou Y, Mancarella S, Wang Y, Yue C, Ritchie M, Gill DL, Soboloff J, The short N-terminal domains of STIM1 and STIM2 control the activation kinetics of Orai1 channels. J. Biol. Chem 284, 19164–19168 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berna-Erro A, Jardin I, Salido GM, Rosado JA, Role of STIM2 in cell function and physiopathology. J. Physiol 595, 3111–3128 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oh-Hora M, Yamashita M, Hogan PG, Sharma S, Lamperti E, Chung W, Prakriya M, Feske S, Rao A, Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance. Nat. Immunol 9, 432–443 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koh SD, Ward SM, Sanders KM, Ionic conductances regulating the excitability of colonic smooth muscles. Neurogastroenterol. Motil 24, 705–718 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yuan JP, Zeng W, Dorwart MR, Choi Y-J, Worley PF, Muallem S, SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nat. Cell. Biol 11,337–343 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liao Y, Erxleben C, Abramowitz J, Flockerzi V, Zhu MX, Armstrong DL, Birnbaumer L, Functional interactions among Orai1, TRPCs, and STIM1 suggest a STIM-regulated heteromeric Orai/TRPC model for SOCE/Icrac channels. Proc. Natl. Acad. Sci U.S.A 105, 2895–2900 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liao Y, Erxleben C, Yildirim E, Abramowitz J, Armstrong DL, Birnbaumer L, Orai proteins interact with TRPC channels and confer responsiveness to store depletion. Proc. Natl. Acad. Sci. U.S.A 104, 4682–4687 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liao Y, Plummer NW, George MD, Abramowitz J, Zhu MX, Birnbaumer L, A role for Orai in TRPC-mediated Ca2+ entry suggests that a TRPC:Orai complex may mediate store and receptor operated Ca2+ entry. Proc. Natl. Acad. Sci. U.S.A 106, 3202–3206 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McCarron JG, Flynn ERM, Bradley KN, Muir TC, Two Ca2+ entry pathways mediate InsP3-sensitive store refilling in guinea-pig colonic smooth muscle. J. Physiol 525 (Pt. 1), 113–124 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cribbs LL, Lee J-H, Yang J, Satin J, Zhang Y, Daud A, Barclay J, Williamson MP, Fox M, Rees M, Perez-Reyes E, Cloning and characterization of α1H from human heart, a member of the T-type Ca2+ channel gene family. Circ. Res 83, 103–109 (1998). [DOI] [PubMed] [Google Scholar]
- 60.Sung TS, O’Driscoll K, Zheng H, Yapp NJ, Leblanc N, Koh SD, Sanders KM, Influence of intracellular Ca2+ and alternative splicing on the pharmacological profile of ANO1 channels. Am. J. Physiol. Cell Physiol 311, C437–C451 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peri LE, Sanders KM, Mutafova-Yambolieva VN, Differential expression of genes related to purinergic signaling in smooth muscle cells, PDGFRα-positive cells, and interstitial cells of Cajal in the murine colon. Neurogastroenterol.Motil 25, e609–e620 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Expression of Orai and Stim paralogs in ICC of the small intestine.
Fig. S2. CC2 peptide has no effect on HEK 293 cells expressing Orai1 and Stim1 without thapsigargin pretreatment.
Fig. S3. Cav3.2 current and Ano1 current are not significantly affected by GSK-7975A.
Fig. S4. Effect of CC2 peptide on Ano1 current.


