Abstract
Obesity and hypertension are important risk factors of arterial stiffness. However, the complex relationship between increased body mass index (BMI), elevated blood pressure (BP) and arterial stiffness is largely unknown. We aim to examine the mediation effect of elevated BP on the association of early life BMI, long-term burden and trend of BMI with arterial stiffness in midlife. The longitudinal study cohort consisted of 1,190 participants (829 whites and 361 blacks, 518 males, mean age=40.0 years at follow-up) who had been examined for BMI and BP 4–15 times from childhood and aortic-femoral pulse wave velocity (afPWV) in adulthood, with a mean follow-up period of 30.3 years. Total area under the curve (AUCt) and incremental AUC (AUCi) were calculated in random-effects models and used as long-term measures of BMI and BP. Total effects of BMI measures on adult afPWV, adjusted for covariates were all significant without adult BMI and systolic BP (SBP) measures included in the models. The mediation effects of adult SBP (20.2%) and SBP AUCi (16.9%) were significant on the childhood BMI-afPWV association. Adult SBP showed significant mediation effects of 36.7% on the BMI AUCi-afPWV association and 36.4% on the BMI AUCt-afPWV association. The mediation effect of SBP AUCi was estimated at 63.3% (p<0.01) on the BMI AUCi-afPWV association. DBP had similar total and mediation effects. These findings suggest that the association of increased childhood BMI and its cumulative burden with adult arterial stiffness measured as afPWV is predominantly mediated through the long-term and increasing trend of BP.
Keywords: Body mass index, Blood pressure, Arterial stiffness, Mediation effect, Longitudinal study
INTRODUCTION
Arterial stiffening is an important independent risk factor of cardiovascular disease and starts many years before clinical manifestations of coronary artery disease, stroke and peripheral arterial disease.1–3 It is well-known that obesity and hypertension play important roles in the development of arterial stiffness.4,5 Extensive studies have shown that obesity plays an initial role in the process of vascular stiffening.5–8 Elevated blood pressure (BP) is one of the accelerating risk factors for arterial stiffness in children and adults.9 In a systematic review of 77 cross-sectional studies in adults, BP was consistently and independently associated with aortic pulse wave velocity (PWV).10 Longitudinal cohort studies have shown that elevated BP in children and young adults is a strong predictor of arterial stiffness measures.11–13 Despite overwhelming evidence for the association of obesity and BP with vascular stiffness, how obesity and elevated BP interact in accelerating the vascular stiffening process is unclear. Many studies found that the effect of overweight/obesity on PWV became nonsignificant when BP was included in the association analyses;10 on the other hand, BP still remained significantly associated with arterial stiffness after adjustment for body mass index (BMI) in young adult life.9,11 To date, the complex relationships between BMI, BP and arterial stiffness have not been elucidated.
BP levels and arterial stiffness measures are highly correlated and influenced by obesity and other cardiovascular risk factors during the development of hypertension.12–14 Although the role of arterial stiffness in isolated systolic hypertension and pulse pressure amplitude has been well documented in the elderly population,9,15,16 the Bogalusa Heart Study has shown that elevation of BP determines arterial stiffening instead of the other way around in a longitudinal cohort of young and middle-aged adults.9 Existing observations on the close correlation between obesity and hypertension and their impact on vascular stiffness have raised a study question about whether obesity affects the arterial stiffening process directly or indirectly through the path of BP elevation. The current study aims to examine quantitatively the mediation effect of BP on the association of long-term burden and trends of increased BMI with adult arterial stiffness by utilizing a longitudinal cohort followed from childhood to adulthood in the Bogalusa Heart Study.
METHODS
All data and materials have been made publicly available at the NHLBI Biologic Specimen and Data Repository and can be accessed at https://biolincc.nhlbi.nih.gov/studies/bhs.
Study cohort
The Bogalusa Heart Study, founded by Dr. Gerald Berenson in 1973, is a series of long-term cardiovascular epidemiologic studies in a semi-rural, biracial (65% white and 35% black) community in Bogalusa, Louisiana. This study focuses on the early natural history of cardiovascular disease from childhood.17 Between 1973 and 2010, nine cross-sectional surveys of children aged 4–19 years and eleven cross-sectional surveys of adults aged 20–51 years, who had been previously examined as children, were conducted. These repeated cross-sectional surveys conducted every 2–3 years have resulted in serial observations from childhood to adulthood. The present longitudinal study cohort consisted of 1,190 adult subjects (829 whites and 361 blacks; 43.5% males; mean age=40.0 years in the last survey). These participants were examined 4–15 times for BMI and BP (at least 2 times in childhood and at least 2 times in adulthood) and aortic-femoral pulse wave velocity (afPWV) in adulthood. The mean follow-up period was 30.3 years (range=14.2–41.9 years) from the first childhood to the last adult survey.
All subjects in this study gave informed consent for each survey, and for those <18 years of age, consent of a parent/guardian was obtained. Study protocols were approved by the Institutional Review Board of the Tulane University Health Sciences Center (New Orleans, Louisiana).
General examinations
Standardized protocols were used by trained examiners across all surveys since 1973.17 Replicate measurements of height and weight were made, and the mean values were used for analysis. BMI (weight in kilograms divided by height in meters squared) was used as a measure of overall adiposity.
Participants’ BP levels were obtained on right arms in a relaxed sitting position by 2 trained observers (3 times each) between 8:00 AM and 10:00 AM. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were recorded using a mercury sphygmomanometer. The fourth Korotkoff phase was used for DBP in children and the fifth Korotkoff phase was used for adults. The mean values of the 6 readings were used for analysis. For hypertensive patients (n=206) who were under anti-hypertensive treatment and had SBP/DBP<140/90 mmHg, forced values (140/90 mmHg) were assigned for measured SBP/DBP.
Aortic-femoral pulse wave velocity
Aortic-femoral pulse wave velocity (afPWV) was measured through a Toshiba digital ultrasound instrument (Xario SSA-660A; Toshiba America Medical Systems, Tustin, California). We placed a nondirectional transcutaneous Doppler flow probe (Toshiba PSK25AT, 2.5 MHz; Toshiba America Medical Systems) at the suprasternal notch, and put another probe (Toshiba PCK703AT, 7.5 MHz; Toshiba America Medical Systems) at the left femoral artery with the subject lying in a supine position. The output from the electrocardiogram and the 2 Doppler probes was displayed and recorded through a computer system. This system also recorded and stored the arterial flow waves from the 2 arterial sites and captured the output for subsequent scoring. After collection of the waveform data, we measured the distance between the aorta and femoral arteries with a caliper instrument to reduce the influence of body contours on the distance measured. The software averages the selected waveforms and determines the time from the R wave of the electrocardiogram to the foot of each waveform. The difference in timing between the 2 waves represents the time component of the velocity equation. We then calculated afPWV by dividing the distance traveled by the time differential between the 2 waveforms.18 To examine the reproducibility of afPWV, 46 randomly selected subjects were re-examined. The correlation between the 2 measurements was 0.91 on the same day and 0.68 on different days. The day-to-day variations were influenced by both measurement errors and physiological fluctuations.
Statistical methods
Long-term burden and trends of BMI and BP were measured as the area under the curve (AUC), which was calculated using statistical models we previously described.19–21 In brief, growth curves of BMI and BP measured multiple times from childhood to adulthood were constructed using a random-effects model by SAS proc MIXED (SAS Institute Inc., Cary, North Carolina). A quadratic curve was fitted for BMI, and cubic curves for SBP and DBP in race-sex groups. The AUCs were calculated as the integral of the curve parameters during the follow-up period for each participant. Because participants had different follow-up years, the AUC values were divided by the number of follow-up years. The AUC measures as shown in Supplemental Figures S1 and S2 have advantages over other conventional longitudinal analysis models in that they measure both long-term burden and trends. Total AUC (AUCt) can be considered a measure of a long-term cumulative burden; incremental AUC (AUCi) determined by within-subject variability represents a combination of linear and nonlinear longitudinal trends.
Multivariable linear regression analyses were performed to examine the total effect (standardized regression coefficients, β) of BMI measures on adult afPWV, adjusted for race, sex, adult age, heart rate (HR), smoking and alcohol drinking. Race differences in the total effects were examined in linear regression interaction models by including race-BMI interaction terms, adjusted for covariates. Causal mediation analysis models were constructed as previously proposed by VanderWeele and Sobel.22,23 In mediation analysis models with multiple mediators (Supplemental Figure S3), predictor variables (X) were childhood BMI, BMI AUCt or BMI AUCi; mediator variables (M) were adult BMI, adult BP, BP AUCt or BP AUCi; the outcome variable (Y) was adult afPWV. Four steps of the mediation analysis were involved in the calculation of the mediating effect:
Step 1: Showing that the predictor variable determines the outcome (Model Y=cX) (c=total effect).
Step 2: Showing that the predictor variable affects the mediators (Model M1=β1X and Model M2=β3X) (β1=indirect effect 1, β3=indirect effect 3).
Step 3: Showing that the mediators determine the outcome controlling for the predictor (Model Y=β2M1 + β4M2 + c’X) (β2=indirect effect 2, β4=indirect effect 4, c’=direct effect).
Step 4: Calculating the proportion of mediation effects by (β1×β2 / c) ×100% and (β3×β4 / c) ×100%.
In mediation analyses, testing the significance of the mediation effect is equivalent to testing the null hypothesis H0: β2=0 and/or β4=0 versus the alternative hypothesis Ha: β2≠0 and/or β4≠0, using the procedures of R package mediation.24 In the mediation models with childhood BMI as the predictor, two mediators (BP measures and adult BMI) were included in the models. In the mediation models with BMI AUCt or BMI AUCi as the predictor, only one mediator (adult BP or BP AUCi) was included in the models. Adult BMI could not be included in these models as a mediator because it was included in the calculation of BMI AUC measures.
RESULTS
Table 1 summarizes mean values (SD) of the study variables in childhood, adulthood and long-term measures of BMI and BP by race and sex. There were no significant differences in childhood BMI, SBP and DBP between race and sex groups; adult BMI, SBP and DBP showed significant race and sex differences except for race difference in BMI for males; adult HR showed significant sex difference in whites and race difference in males. Adult afPWV did not differ significantly between race and sex groups. Race and sex differences in AUC measures of BMI, SBP and DBP were all significant except for race difference in BMI AUCt and BMI AUCi in males.
Table 1.
Characteristics of Study Participants by Race and Sex
| Variable | White |
Black |
P for race difference |
|||
|---|---|---|---|---|---|---|
| Male (n=375) | Female (n=454) | Male (n=143) | Female (n=218) | Male | Female | |
| Childhood | ||||||
| Age (y) | 9.9 (3.3) | 9.9 (3.4) | 9.7 (3.0) | 9.4 (2.9) | 0.367 | 0.067 |
| BMI (kg/m2) | 17.9 (3.6) | 17.8 (3.7) | 17.5 (3.5) | 17.4 (3.6) | 0.293 | 0.234 |
| SBP (mmHg) | 100.8 (10.0) | 99.9 (9.8) | 100.0 (11.8) | 98.4 (10.2) | 0.441 | 0.081 |
| DBP (mmHg) | 61.6 (8.1) | 62.1 (8.5) | 62.8 (8.5) | 61.3 (8.7) | 0.132 | 0.240 |
| Adulthood | ||||||
| Age (y) | 40.4 (5.9) | 40.0 (5.8) | 40.4 (6.0) | 39.3 (6.5) | 0.987 | 0.125 |
| BMI (kg/m2) | 30.2 (6.0)* | 29.2 (7.4) | 30.5 (8.0)* | 32.7 (8.9) | 0.685 | <0.001 |
| SBP (mmHg) | 122.0 (12.8)† | 114.3 (14.0) | 132.2 (18.0)† | 125.6 (19.1) | <0.001 | <0.001 |
| DBP (mmHg) | 83.0 (8.1)† | 77.9 (8.4) | 88.9 (12.2)† | 83.7 (11.7) | <0.001 | <0.001 |
| HR (beat/min) | 68.3 (9.0)† | 70.6 (9.0) | 70.5 (9.8) | 71.3 (9.2) | 0.020 | 0.347 |
| Smoker, n (%) | 100 (26.7) | 135 (29.7) | 56 (39.2)* | 62 (28.4) | 0.006 | 0.729 |
| Drinker, n (%) | 128 (34.1) | 184 (40.5) | 29 (20.3)† | 72 (33.0) | 0.001 | 0.061 |
| afPWV (m/s) | 6.7 (4.3) | 6.5 (4.5) | 6.9 (2.5) | 6.7 (3.0) | 0.553 | 0.437 |
| Long-term Measures | ||||||
| Average age (y) | 23.2 (5.1) | 23.2 (4.9) | 22.6 (4.9) | 22.2 (4.5) | 0.238 | 0.013 |
| BMI AUCt (kg/m2) | 25.4 (4.5)† | 24.3 (5.1) | 25.2 (5.5)* | 26. 6 (5.9) | 0.762 | <0.001 |
| BMI AUCi (kg/m2) | 7.5 (2.9)† | 6.7 (3.7) | 7.7 (3.6)† | 9.0 (4.3) | 0.519 | <0.001 |
| SBP AUCt (mmHg) | 114.1 (7.7)† | 108.4 (7.1) | 118.3 (9.8)† | 112.9 (9.0) | <0.001 | <0.001 |
| SBP AUCi (mmHg) | 13.3 (5.3)† | 8.6 (5.8) | 18.4 (7.6)† | 14.3 (6.8) | <0.001 | <0.001 |
| DBP AUCt (mmHg) | 73.4 (5.6)† | 70.9 (4.9) | 75.4 (7.3)† | 73.1 (6.3) | 0.003 | <0.001 |
| DBP AUCi (mmHg) | 12.3 (3.4)† | 9.2 (3.8) | 13.6 (5.0)† | 11.6 (4.5) | 0.004 | <0.001 |
Values are mean (SD) or n (%).
BMI=body mass index; SBP=systolic blood pressure; DBP=diastolic blood pressure; HR=heart rate; afPWV=aortic-femoral pulse wave velocity; AUCt=total area under the curve; AUCi=incremental area under the curve
Sex difference within racial groups:
P<0.05;
P<0.01
Table 2 presents total effect of BMI measures on adult afPWV in linear regression models, adjusted for race, sex, adult age, HR, smoking and alcohol drinking. Adult afPWV was associated with childhood BMI (standardized regression coefficient, β=0.084, p=0.003), adult BMI (β=0.091, p=0.002), BMI AUCt (β=0.088, p=0.002), and BMI AUCi (β=0.079, p=0.005). When adult BMI, adult SBP, SBP AUCt and SBP AUCi were included in the models, respectively, for additional adjustment, the above associations became nonsignificant except for the BMI AUCt-afPWV association with adjustment for SBP AUCt. The effects of childhood and adult BMI, BMI AUCt and BMI AUCi on adult afPWV, adjusted for adult BMI, adult DBP, DBP AUCt and DBP AUCi were substantially similar to those with adjustment for SBP measures (Supplemental Table S1). Additional analyses were conducted to examine race differences in the total effects of BMI measures on adult afPWV (BMI-race interactions) adjusted for sex and other covariates. Although the total effects were weaker in blacks, the racial differences were not significant as indicated by the interaction P-values (all P>0.05). The results of racial differences in the total effects are presented in Supplemental Table S2.
Table 2.
Linear Regression of Adult afPWV on BMI Measures since Childhood
| Independent variable | β (95% CI) | P |
|---|---|---|
| Childhood BMI | 0.084 (0.029 ~ 0.139) | 0.003 |
| Childhood BMI * | 0.053 (−0.012 ~ 0.118) | 0.107 |
| Adult BMI | 0.091 (0.034 ~ 0.148) | 0.002 |
| Adult BMI † | 0.056 (−0.005 ~ 0.117) | 0.069 |
| BMI AUCt | 0.088 (0.033 ~ 0.143) | 0.002 |
| BMI AUCt ‡ | 0.081 (0.020 ~ 0.142) | 0.010 |
| BMI AUCi | 0.079 (0.024 ~ 0.134) | 0.005 |
| BMI AUCi § | 0.030 (−0.033 ~ 0.093) | 0.350 |
Race, sex, adult age, heart rate, smoking and alcohol drinking were included for adjustment.
Additional adjustment for adult SBP and adult BMI
Additional adjustment for adult SBP
Additional adjustment for SBP AUCt
Additional adjustment for SBP AUCi
afPWV=aortic-femoral pulse wave velocity; BMI=body mass index; AUCt=total area under the curve; AUCi=incremental area under the curve; SBP=systolic blood pressure; β=standardized regression coefficient; CI=confidence interval
Table 3 presents the results of mediation analyses of SBP measures and adult BMI on the childhood BMI-adult afPWV association, adjusted for race, sex, adult age, HR, smoking and alcohol drinking. The total effect of childhood BMI on adult afPWV (c=0.084, p<0.01) was estimated without SBP measures and adult BMI in the model. The mediation effects of adult SBP (20.2%, p<0.01) and SBP AUCi (16.9%, p<0.01) were significant on the association between childhood BMI and adult afPWV. In all three models with SBP measures and adult BMI included, the direct effects of childhood BMI on adult afPWV were not significant. Of note, the associations between adult BMI and afPWV (β4s) became nonsignificant when SBP measures included in the mediation models. In Supplemental Table S3, the mediation effect patterns of DBP were similar to those of SBP.
Table 3.
Mediation effect (standardized regression coefficient) of SBP measures and adult BMI on the childhood BMI-afPWV association
| Effect | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| Total effect (c) | 0.084† | 0.084† | 0.084† |
| Mediator 1: | Childhood SBP | Adult SBP | SBP AUCi |
| Direct effect (c’) | 0.052 | 0.053 | 0.056 |
| β1 | 0.396† | 0.168† | 0.135† |
| β2 | −0.003 | 0.101† | 0.105† |
| βInd | −0.001 | 0.017† | 0.014† |
| Mediation effect (%) | −1.2 | 20.2† | 16.9† |
| Mediator 2: | Adult BMI | Adult BMI | Adult BMI |
| Direct effect (c’) | 0.052 | 0.053 | 0.056 |
| β3 | 0.525† | 0.525† | 0.525† |
| β4 | 0.064 | 0.027 | 0.027 |
| βInd | 0.033 | 0.014 | 0.014 |
| Mediation effect (%) | 39.3 | 16.7 | 16.9 |
Race, sex, adult age, heart rate, smoking and alcohol drinking were included for adjustment.
c=total effect; c’=direct effect; β1=indirect effect 1; β2=indirect effect 2; β3=indirect effect 3; β4=indirect effect 4; βInd=total indirect effect. BMI=body mass index; SBP=systolic blood pressure; AUCi=incremental area under the curve; afPWV=aortic-femoral pulse wave velocity
P<0.05;
P<0.01
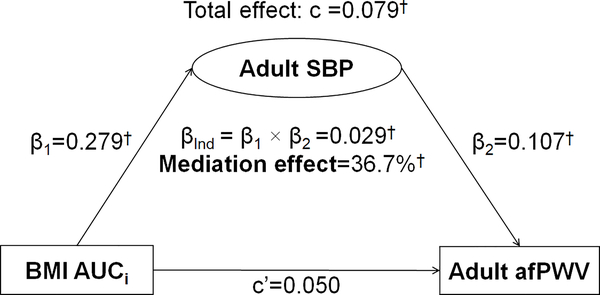
Figure 1 presents the mediation effect of adult SBP on the BMI AUCi-adult afPWV association in a model with one mediator, adjusting for covariates. As mentioned above, adult BMI could not be included in the model as a mediator because it was included in the calculation of BMI AUCi. The total effect of BMI AUCi on adult afPWV (c=0.079, p<0.01) was estimated without adult SBP in the model. The mediation effect of adult SBP on the BMI AUCi-adult afPWV association was estimated at 36.7%, with a significant indirect effect (βInd=0.029, p<0.01). The direct effect (c’=0.050) was not significant.
Figure 1.
Mediation Analysis Model of Adult SBP on the BMI AUCi-afPWV Association
β, c and c’ are standardized regression coefficients; c=total effect; c’=direct effect; β1=indirect effect 1; β2=indirect effect 2; βInd=total indirect effect; BMI=body mass index; SBP=systolic blood pressure; AUCi=incremental area under the curve; afPWV=aortic-femoral pulse wave velocity
* P<0.05; † P<0.01
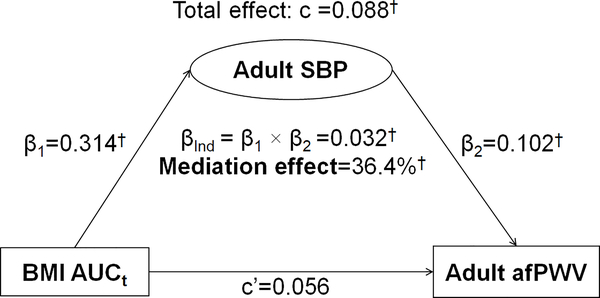
Figure 2 presents the mediation effect of adult SBP on the BMI AUCt-adult afPWV association in a one-mediator model without adult BMI. The total effect of BMI AUCt on adult afPWV (c=0.088, p<0.01) was estimated without adult SBP in the model. The mediation effect of adult SBP on the BMI AUCt-adult afPWV association was estimated at 36.4% (p<0.01). The direct effect (c’=0.056) was not significant.
Figure 2.
Mediation Analysis Model of Adult SBP on the BMI AUCt-afPWV Association
β, c and c’ are standardized regression coefficients; c=total effect; c’=direct effect; β1=indirect effect 1; β2=indirect effect 2; βInd=total indirect effect; BMI=body mass index; SBP=systolic blood pressure; AUCt=total area under the curve; afPWV=aortic-femoral pulse wave velocity
* P<0.05; † P<0.01
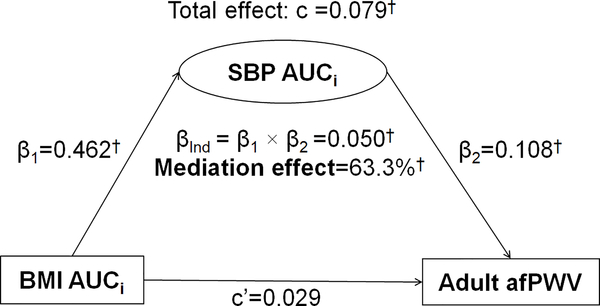
Figure 3 shows the mediation effect of SBP AUCi on the BMI AUCi-adult afPWV association, adjusting for covariates. In this model, incremental AUCs of both BMI and SBP were included as the predictor and mediator, respectively. The mediation effect of SBP AUCi on the BMI AUCi-adult afPWV association increased to 63.3% (p<0.01), with a significant indirect effect (βInd=0.050, p<0.01). The direct effect (c’=0.029) was not significant.
Figure 3.
Mediation Analysis Model of SBP AUCi on the BMI AUCi-afPWV Association
β, c and c’ are standardized regression coefficients; c=total effect; c’=direct effect; β1=indirect effect 1; β2=indirect effect 2; βInd=total indirect effect; BMI=body mass index; SBP=systolic blood pressure; AUCi=incremental area under the curve; afPWV=aortic-femoral pulse wave velocity
* P<0.05; † P<0.01
Supplemental Figures S4-S6 show mediation effects of DBP measures on the BMI AUC-adult afPWV associations substantially similar to those of SBP measures.
DISCUSSION
Hypertension and obesity in children and adults are the most important risk factors related to arterial stiffening.4–8 Extensive studies, including the Bogalusa Heart Study, have demonstrated that increased BMI since early life and elevated BP have an adverse effect on arterial stiffness.6–10,12,25 It is well known that childhood obesity tracks into adulthood and associates with elevated adult BP.26–28 Although it is widely accepted that childhood obesity affects arterial stiffness through adult BMI and BP, the degree of their mediation effects has never been reported, especially by using long-term BMI and BP measured from childhood to adulthood. In the present study, we quantified the mediation effect of adult BMI and BP and their long-term measures and found that the BMI-afPWV association was significantly mediated by adult BP and its long-term values, but not by adult BMI. The association between childhood BMI and adult afPWV was significantly mediated by adult BP (20.2% and 25.0%) and the increasing trends of BP measured by AUCi (16.9%−21.4%). When long-term measures of both BMI and BP were used as the predictor and mediator, respectively, BP AUCi showed considerably greater mediation effects (63.3% and 64.6%) than adult BP measured at one time-point on the association between BMI AUCi and adult afPWV. These findings indicate that long-term burden of increased BMI from early life is associated with adult arterial stiffness predominantly through elevated BP, especially in terms of its long-term burden and trends.
Studies have indicated that childhood obesity is associated with adult cardiovascular morbidity and mortality.29 The International Childhood Cardiovascular Cohort (i3C) Consortium found that obese children who were obese as adults had increased risks of type 2 diabetes, hypertension, dyslipidemia and carotid-artery atherosclerosis, but the risks of these outcomes among overweight/obese children who became nonobese by adulthood were similar to those among persons who were never obese.30 Other longitudinal cohort studies followed since childhood have also shown that obesity in both childhood and adulthood are associated with higher values of PWV.5,7,8 These observations suggest that adult obesity is more important, and early life obesity might increase the future cardiovascular risk through later life obesity. Despite the strong tracking correlation of obesity measures from childhood to adulthood,27,28,31 whether and in what degree the association between childhood obesity and adult arterial stiffness is mediated by adult body weight is largely unknown. We found in this study that although childhood BMI was strongly associated with adult afPWV, adult BMI had a marginally significant mediation effect (39.3%, p=0.061) on the association, and the mediation effect was substantially reduced with adjustment for BP in the models.
Elevated BP has been identified as the most important risk factor of vascular stiffness in enormous studies, including the Bogalusa Heart Study.9–11,25 Based on our previous findings on the one-directional relation from BP to PWV in young and middle-aged adults,9 a mediation analysis model was constructed in the present study with BP as the mediator in the indirect pathway from BMI to afPWV. We noted that adult BP had a significant mediation effect on the childhood BMI-afPWV association, adjusting for adult BMI, and a greater mediation effect on the association between long-term measures of BMI and adult afPWV. Of note, when the long-term increasing trend (AUCi) of BMI was used as the predictor in the mediation analysis models, BP AUCis had the strongest mediation effects (63.3% for SBP and 64.6% for DBP). In a systematic review of 77 cross-sectional studies in adults, BP was found to be consistently and independently associated with aortic PWV in 90% of studies.10 The Bogalusa Heart Study has shown that elevated BP is one of the important risk factors of arterial stiffness measures in children and young adults.9,13,25 Recently, the Cardiovascular Risk in Young Finns Study reported that individuals with elevated adult BP had an increased risk of high adult PWV, irrespective of childhood BP and independent of adult BMI.11 Taken together, the evidence from the current and previous studies support the notion that increased adult BP plays a predominant role in mediating the obesity-arterial stiffness association.
This community-based longitudinal cohort provides an unparalleled opportunity to examine the mediation effects of BP in terms of both adult values and incremental trends in relation to BMI and afPWV. There were, however, a few limitations in this study. First, hypertensive subjects under pharmacological treatment represent a subgroup who would be expected to have the highest BP levels without treatment; the forced values of 140/90 mmHg assigned to the measured SBP/DBP for these hypertensive patients would result in some bias in the association and mediation analyses. Second, PWV can be reversed by long-term antihypertensive treatment; but, such an effect cannot be assessed without the PWV progression data in this study.
In conclusion, we demonstrated that increased BMI in childhood and adulthood and its life-long burden and incremental trends are all significantly associated with adult arterial stiffness measured as afPWV. The relationship between high BMI and increased afPWV is mainly through elevated BP. The long-term burden of elevated BP plays a more important role than adult BMI in mediating the vascular stiffening process in middle-aged adults. In addition, the long-term measures of BP have stronger mediation effects on the association of cumulative burden and increasing trends of BMI with adult arterial stiffness.
PERSPECTIVES
The present study provides new insights into the life-long impact of BMI and BP on the arterial stiffening process and better understanding of the underlying mechanisms. These findings will help promote the development of new prevention and intervention strategies for controlling the modifiable predictors and mediators beginning in childhood to improve vascular health and reduce the risk of cardiovascular disease in later life.
Supplementary Material
Novelty and Significance:
What Is New?
The current study demonstrated that BMI in childhood and adulthood and its life-long burden and incremental trends are all significantly associated with adult arterial stiffness measured as aortic-femoral pulse wave velocity (afPWV).
The association between high BMI and increased afPWV is mainly through long-term burden and trends of elevated blood pressure (BP).
The long-term measures of BP have stronger mediation effects on the association of childhood BMI and its cumulative burden with adult arterial stiffness.
What Is Relevant?
The observations on the life-long impact of BMI and BP on the arterial stiffening process would improve our understanding of the underlying mechanisms. These findings will facilitate selection of new prevention and intervention strategies for controlling the modifiable predictors and mediators beginning in childhood to improve vascular health and reduce the risk of cardiovascular disease in later life.
Summary
Higher childhood and adulthood BMI and its life-long burden and incremental trends were all significantly associated with adult arterial stiffness measured as afPWV. The association between high BMI and increased afPWV was predominantly through elevated BP. The long-term burden of elevated BP played a more important role than adult BMI in mediating the vascular stiffening process in middle-aged adult life.
ACKNOWLEDGMENTS
The Bogalusa Heart Study is a joint effort of many investigators and staff members whose contribution is gratefully acknowledged.
SOURCES OF FUNDING
This study was supported by grants R01HL121230 from the National Heart, Lung and Blood Institute, R03AG060619 from National Institute on Aging, and P20GM109036 from the National Institute of General Medical Sciences of the National Institutes of Health. Yang Liu was supported by a research training grant (D43TW009107) from the Fogarty International Center of the National Institutes of Health, Bethesda, Maryland, USA.
ABBREVIATIONS
- afPWV
aortic-femoral pulse wave velocity
- AUCt
total area under the curve
- AUCi
incremental area under the curve
- BMI
body mass index
- BP
blood pressure
- DBP
diastolic blood pressure
- HR
heart rate
- SBP
systolic blood pressure
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Avolio AP, Deng FQ, Li WQ, Luo YF, Huang ZD, Xing LF, O’rourke MF. Effects of aging on arterial distensibility in populations with high and low prevalence of hypertension: comparison between urban and rural communities in China. Circulation. 1985;71:202–210. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liao J, Farmer J. Arterial Stiffness as a Risk Factor for Coronary Artery Disease. Curr Atheroscler Rep. 2014;16:387. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell GF. Arterial stiffness and hypertension: chicken or egg? Hypertension. 2014;64:210–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herouvi D, Karanasios E, Karayianni C, Karavanaki K. Cardiovascular disease in childhood: the role of obesity. Eur J Pediatr. 2013;172:721–732. [DOI] [PubMed] [Google Scholar]
- 6.Hudson LD, Rapala A, Khan T, Williams B, Viner RM. Evidence for contemporary arterial stiffening in obese children and adolescents using pulse wave velocity: A systematic review and meta-analysis. Atherosclerosis. 2015;241:376–386. [DOI] [PubMed] [Google Scholar]
- 7.Cote AT, Phillips AA, Harris KC, Sandor GG, Panagiotopoulos C, Devlin AM. Obesity and arterial stiffness in children: systematic review and meta-analysis. Arterioscler Thromb Vasc Biol. 2015;35:1038–1044. [DOI] [PubMed] [Google Scholar]
- 8.Zebekakis PE, Nawrot T, Thijs L, Balkestein EJ, van der Heijden-Spek J, Van Bortel LM, Struijker-Boudier HA, Safar ME, Staessen JA. Obesity is associated with increased arterial stiffness from adolescence until old age. J Hypertens. 2005;23:1839–1846. [DOI] [PubMed] [Google Scholar]
- 9.Chen W, Li S, Fernandez C, Sun D, Lai CC, Zhang T, Bazzano L, Urbina EM, Deng HW. Temporal Relationship Between Elevated Blood Pressure and Arterial Stiffening Among Middle-Aged Black and White Adults. Am J Epidemiol. 2016;183:599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cecelja M, Chowienczyk P. Dissociation of Aortic Pulse Wave Velocity With Risk Factors for Cardiovascular Disease Other Than Hypertension A Systematic Review. Hypertension. 2009;54:1328–U1103. [DOI] [PubMed] [Google Scholar]
- 11.Aatola H, Koivistoinen T, Tuominen H, Juonala M, Lehtimäki T, Viikari JS, Raitakari OT, Kähönen M, Hutri-Kähönen N. Influence of Child and Adult Elevated Blood Pressure on Adult Arterial Stiffness: The Cardiovascular Risk in Young Finns Study. Hypertension. 2017;70:531–536. [DOI] [PubMed] [Google Scholar]
- 12.Li S, Chen W, Srinivasan SR, Berenson GS. Influence of metabolic syndrome on arterial stiffness and its age-related change in young adults: the Bogalusa Heart Study. Atherosclerosis. 2005;180:349–354. [DOI] [PubMed] [Google Scholar]
- 13.Urbina EM, Srinivasan SR, Kieltyka RL, Tang R, Bond MG, Chen W, Berenson GS. Correlates of carotid artery stiffness in young adults: The Bogalusa Heart Study. Atherosclerosis. 2004;176:157–164. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell GF, Guo CY, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Cross-sectional correlates of increased aortic stiffness in the community: the Framingham Heart Study. Circulation. 2007;115:2628–2636. [DOI] [PubMed] [Google Scholar]
- 15.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. 2012;308:875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takase H, Dohi Y, Toriyama T, Okado T, Tanaka S, Sonoda H, Sato K, Kimura G. Brachial-ankle pulse wave velocity predicts increase in blood pressure and onset of hypertension. Am J Hypertens. 2011;24:667–673. [DOI] [PubMed] [Google Scholar]
- 17.Berenson GS, McMahan CA, Voors AW, Webber LS, Srinivasan SR, Frank GC, Foster TA, Blonde CV. Cardiovascular risk factors in children: The early natural history of atherosclerosis and essential hypertension. New York, NY: Oxford University Press; 1980:47–123. [Google Scholar]
- 18.Jo CO, Lande MB, Meagher CC, Wang HY, Vermilion RP. A Simple Method of Measuring Thoracic Aortic Pulse Wave Velocity in Children: Methods and Normal Values. J Am Soc Echocardiog. 2010;23:735–740. [DOI] [PubMed] [Google Scholar]
- 19.Chen W, Li S, Cook NR, Rosner BA, Srinivasan SR, Boerwinkle E, Berenson GS. An autosomal genome scan for loci influencing longitudinal burden of body mass index from childhood to young adulthood in white sibships: The Bogalusa Heart Study. Int J Obesity. 2004;28:462–469. [DOI] [PubMed] [Google Scholar]
- 20.Cook NR, Rosner BA, Wei C, Srinivasan SR, Berenson GS. Using the area under the curve to reduce measurement error in predicting young adult blood pressure from childhood measures. Stat Med. 2004;23:3421–3435. [DOI] [PubMed] [Google Scholar]
- 21.Chen W, Li SX, Srinivasan SR, Boerwinkle E, Berenson GS. Autosomal genome scan for loci linked to blood pressure levels and trends since childhood - The Bogalusa Heart Study. Hypertension. 2005;45:954–959. [DOI] [PubMed] [Google Scholar]
- 22.VanderWeele TJ. Mediation Analysis: A Practitioner’s Guide. Annu Rev Publ Health. 2016;37:17–32. [DOI] [PubMed] [Google Scholar]
- 23.Sobel ME. Asymptotic Confidence Intervals for Indirect Effects in Structural Equation Models. Sociological Methodology. 1982;13:290–312. [Google Scholar]
- 24.Tingley D, Yamamoto T, Hirose K, Keele L, Imai K. Mediation: R Package for Causal Mediation Analysis. J Stat Softw. 2014;59:1–38.26917999 [Google Scholar]
- 25.Li SX, Chen W, Srinivasan SR, Berenson GS. Childhood blood pressure as a predictor of arterial stiffness in young adults: The Bogalusa Heart Study. Hypertension. 2004;43:541–546. [DOI] [PubMed] [Google Scholar]
- 26.Dwyer T, Sun C, Magnussen CG, Raitakari OT, Schork NJ, Venn A, Burns TL, Juonala M, Steinberger J, Sinaiko AR, Prineas RJ. Cohort Profile: The International Childhood Cardiovascular Cohort (i3C) Consortium. Int J Epidemiol. 2013;42:86–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh AS, Mulder C, Twisk JWR, van Mechelen W, Chinapaw MJM. Tracking of childhood overweight into adulthood: a systematic review of the literature. Obes Rev. 2008;9:474–488. [DOI] [PubMed] [Google Scholar]
- 28.Johannsson E, Arngrimsson SA, Thorsdottir I, Sveinsson T. Tracking of overweight from early childhood to adolescence in cohorts born 1988 and 1994: overweight in a high birth weight population. Int J Obes (Lond). 2006;30:1265–1271. [DOI] [PubMed] [Google Scholar]
- 29.Twig G, Yaniv G, Levine H, Leiba A, Goldberger N, Derazne E, Ben-Ami Shor D, Tzur D, Afek A, Shamiss A, Haklai Z. Body-Mass Index in 2.3 Million Adolescents and Cardiovascular Death in Adulthood. New England Journal of Medicine. 2016;374:2430–2440. [DOI] [PubMed] [Google Scholar]
- 30.Juonala M, Magnussen CG, Berenson GS, Venn A, Burns TL, Sabin MA, Srinivasan SR, Daniels SR, Davis PH, Chen W, Sun C. Childhood Adiposity, Adult Adiposity, and Cardiovascular Risk Factors. New England Journal of Medicine. 2011;365:1876–1885. [DOI] [PubMed] [Google Scholar]
- 31.Freedman DS, Khan LK, Serdula MK, Dietz WH, Srinivasan SR, Berenson GS. Racial differences in the tracking of childhood BMI to adulthood. Obes Res. 2005;13:928–935. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.