Abstract
Chemoradiation has remained the standard of care treatment for many of the most aggressive cancers. However, despite effective toxicity to cancer cells, current chemoradiation regimens are limited in efficacy due to significant normal cell toxicity. Thus, efforts have been made to identify agents demonstrating selective toxicity, whereby treatments simultaneously sensitize cancer cells to and protect normal cells from chemoradiation. Pharmacological ascorbate (intravenous infusions of vitamin C resulting in plasma ascorbate concentrations >20 mM; P-AscH−) has demonstrated selective toxicity in a variety of pre-clinical tumor models and is currently being assessed as an adjuvant to standard-of-care therapies in several early phase clinical trials. This review summarizes the most current pre-clinical and clinical data available demonstrating the multidimensional role of P-AscH− in cancer therapy including: selective toxicity to cancer cells via a hydrogen peroxide (H2O2)-mediated mechanism; action as a sensitizing agent of cancer cells to chemoradiation; a protectant of normal tissues exposed to chemoradiation; and it’s safety and tolerability in clinical trials.
Introduction
Emergence of P-AscH− in Cancer Therapy –
Vitamin C (ascorbic acid) has traditionally been regarded as a donor antioxidant and enzyme cofactor required for a number of biochemical processes that are essential to human health, including, but not limited to, the maintenance of 2-oxogluturate-dependent dioxygenases involved in the synthesis and maintenance of collagen, epigenetics regulation, and cellular responses to hypoxia.1, 2 In 1976, upon the observation that many cancer patients were nutritionally depleted of vitamin C, a clinical trial by Cameron and Pauling used a combination of supplemental oral and intravenous (IV) administration of ascorbate for patients with a variety of terminal cancers.3 This study found that patients treated with ascorbate experienced a 4.2-fold increase in overall survival as compared to historical controls.3 This study prompted subsequent randomized-controlled clinical trials using orally administered ascorbic acid for cancer treatment. The results of these trials appeared to not support Cameron and Pauling’s findings,4, 5 leading to the abandonment of the use of ascorbate in cancer therapy.
However, the pharmacokinetics of ascorbate were not understood until 1996.6 Using this information, a 2004 analysis of oral and IV ascorbate pharmacokinetics seemed to resolve the different results.7, 8 These informative pharmacokinetic studies revealed that the bioavailability of orally administered ascorbate is tightly controlled while the intravenous route is capable of much higher concentrations, that may provide antitumor activity. The reason for this difference is that high doses of intravenously administered ascorbate (pharmacological ascorbate, P-AscH−) bypass the intestinal transporters responsible for this limitation. Thus, peak plasma levels can be on the order of 200-fold higher than oral supplementation (approximately 20 mM vs. 0.1 mM).9, 10 These findings sparked renewed interest in P-AscH− as a potential antitumor agent. A cascade of studies followed revealing new potential mechanism(s) that could contribute to the possible efficacy of ascorbate as an anti-cancer agent.
A variety of important aspects of P-AscH− have since been characterized. P-AscH− is now known to induce a selective, cytotoxic effect on a variety of cancer cells via a H2O2-mediated mechanism involving redox active metals.10–14 P-AscH− has also been shown to enhance the cytotoxic effects of current standard of care treatment regimens utilizing radiation therapy and/or chemotherapy in pancreatic cancer, non-small cell lung cancer, ovarian cancer, glioblastoma multiforme, gastric cancer, colon cancer, and sarcoma in pre-clinical models.10, 11, 14–17 Furthermore, the systemic antioxidant properties of P-AscH− may reduce normal tissue toxicity associated with current standard of care radiation and/or chemotherapy.10, 11, 15, 18 Finally, numerous clinical trials in a variety of advanced and aggressive cancers (i.e. metastatic pancreatic cancer, metastatic ovarian cancer, metastatic non-small cell lung cancer, and glioblastoma multiforme) have demonstrated the safety and tolerability of P-AscH− treatment and have also demonstrated its potential as an anti-cancer agent in combination with certain standard of care therapies.10, 11, 19, 20 The aims of this review are to present the current evidence supporting the use of P-AscH− as an adjuvant in cancer therapy and to summarize evidence that P-AscH− may mitigate radiation- and chemotherapy-associated normal tissue toxicity.
P-AscH− Selective Toxicity in Cancer vs. Normal Cells —
Currently, the prevailing mechanism underlying the anti-cancer effects of P-AscH− involves the ability of ascorbate to act as a prodrug to deliver extracellular H2O2 to tissue.21, 22 At physiological pH, ascorbate can undergo autoxidation to generate H2O2, likely through a superoxide radical intermediate.23 However, this reaction occurs very slowly (estimate kobs ≈ 1 × 10−2 M−1 s−1, vida infra), possibly underlying the requirement for intravenous administration. Indeed, tissue H2O2 was detectible only after pharmacological administration, and not oral administration, in rats (Figure 1).22
Figure 1. Ascorbate (vitamin C) acid-base and redox chemistry.
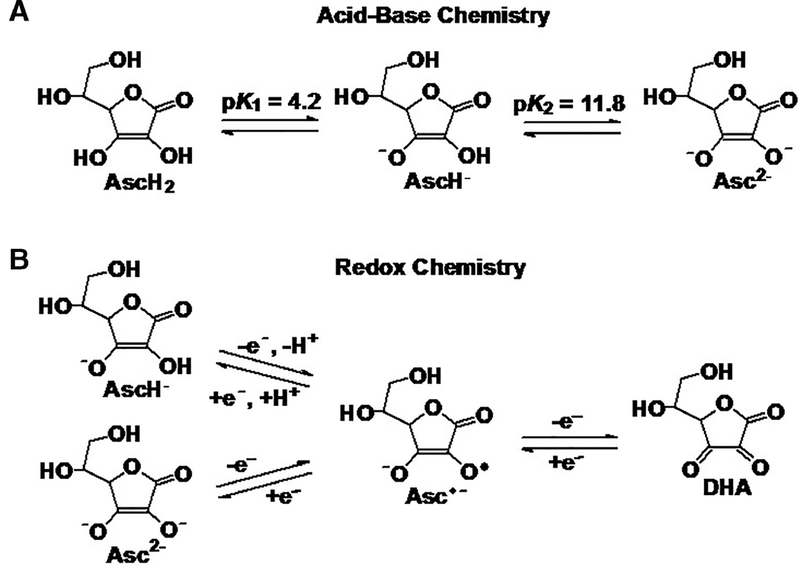
(A) xAscorbate exists primarily as the ascorbate anion (AscH−) at physiologic pH (99.4% as AscH−; 0.06% as AscH2; and 0.004% as Asc2−). As the pH increases, however, the dianion form (Asc2−) increases logarithmically. (B) Autoxidation of the ascorbate monoanion and dianion produces the ascorbate radical (Asc•−) that can undergo further oxidation to form DHA. The rate constant for the autoxidation of the monoanion is approximately one million times smaller than that of the dianion; k = 3 × 102 M−1 s−1 vs. 3 × 10−4 M−1 s−1 for Asc2− and AscH−, respectfully. This will result in a very low value for k3-obs ≈ 1 × 10−2 M−1 s−1 (pH 7.4) for Rxn 3. Thus, metal catalyzed oxidation will be the dominant mechanism for the oxidation of ascorbate at near-neutral pH, reactions 4 – 7.
H2O2 at high steady-state levels is toxic to cells because it can readily cross cell membranes via peroxiporins,24–26 and react with redox-active metals (e.g. the Fenton reaction) to produce the highly oxidizing hydroxyl radical (HO•) causing oxidative damage to cellular lipids, proteins, and most importantly to DNA. The generation of H2O2 has been demonstrated to be absolutely required for ascorbate toxicity, as co-exposure of ascorbate with enzymes that metabolize H2O2 completely inhibits ascorbate toxicity in vitro.10, 21, 27, 28 Several groups have demonstrated that high concentrations of P-AscH− are toxic to a variety of different cancer cells while being relatively innocuous to normal cells.10, 13, 17, 18, 29, 30 The following sections will discuss the current understanding of this selective toxicity, including differential H2O2 metabolic activities and alterations in redox-active iron metabolism.
Differential Capacity for H2O2 Clearance –
Given the requirement for generation of H2O2 in ascorbate toxicity, it follows that the selective toxicity of ascorbate may be dependent on a differential ability to metabolize H2O2. In general, cancer cells have been shown to exist in an increased state of oxidative stress, and therefore may exhibit an inability to effectively metabolize an exogenous bolus of H2O2. Doskey et al. demonstrated that rate constants (kcell) for overall removal of extracellular H2O2 by normal cells are, on average, twice the value of these rate constants for tumor cells.14, 28 The values for kcell are functionally related to the number of active catalase monomers in cells. The ED50’s of P-AscH− for clonogenic survival directly correlate with these rate constants for the cellular removal of H2O2; these in vitro observations translate to in vivo models of pancreatic cancer.13
The differential ability to remove the P-AscH−-mediated flux of H2O2 appears to be related to the differential expression and activity of the antioxidant enzymes that remove H2O2 including catalase, glutathione peroxidase (GPx), and peroxiredoxins (Prx).30–33 Catalase, localized in the peroxisomes of nucleated cells, is regarded as the primary enzyme responsible for eliminating higher fluxes of H2O2 while GPx and Prx are responsible for lower fluxes of H2O2.32, 34–42 Catalase is differentially expressed across tissue types, reflecting the differential metabolic needs across organ systems.43 However, the a majority of malignantly transformed cells tested to date, have low levels of catalase expression and functional capacity to remove extracellular H2O2 compared to their corresponding normal counterparts.13, 43–46 Given these observations, it is likely that the selective toxicity of ascorbate is, at least partially, explained by the differential metabolism of H2O2.
Disruption of Redox Active Fe Metabolism –
The structures of the various acid/base and redox species of ascorbate are shown in Figure 1. The true auto-oxidation of ascorbate, i.e. without the aid of catalytic metals (Rxn 1),47 to generate H2O2 is pH-dependent and occurs relatively slowly in physiologic conditions, likely contributing to the requirement for IV administration to reach supra-physiological plasma ascorbate concentration.23, 48, 49 Ascorbate in the relatively rare dianion form autoxidizes approximately one million times faster than the much more abundant monoanion form at physiologic pH, Rxn 2. The rate constant for the autroxidation of the diacid, would be orders of magnitude lower. Thus
| (1) |
| (2) |
| (3) |
where AscH−/Asc2− represents the equilibrium mixture of these species at pH 7.4 (99.4% as AscH−; 0.06% as AscH2; and 0.004% as Asc2−). The very low value of k3-obs ≈ 1 × 10−2 M−1 s−1 (pH 7.4) of Rxn 3 leads to a very, very slow rate for the autoxidation of ascorbate in the absence of catalytic metals.23 Thus, metal catalyzed oxidation will be the dominant mechanism for the oxidation of ascorbate at near-neutral pH.
However, as little as 40 nM Cu2+ or 50 nM Fe3+, as Fe(III)EDTA, in phosphate buffer pH 7.4 will double the rate of oxidation of ascorbate.48, 50 To observe these effects, the adventious metals in the buffers had to be removed. Typical levels of adventious copper and iron in 100 mM phosphate buffer will be on th order of ≈300 nM and ≈1000 nM, respectively.23 Thus, the rate of ascorbate oxidation and associated production of H2O2 can be dramatically increased in the presence of redox-active metals that are in an appropriate chelating environment, such as ferric iron (Fe3+) as Fe(III)EDTA, but not Fe(III)Desferal.48 The labile iron pool in cells is on the order of 1 – 10 µM,51, 52 indicating that metal catalyzed oxidation of ascorbate will be the dominant mechanism for the production of H2O2 and downstream oxidants.
For example, Fe3+ can be reduced to ferrous iron (Fe2+) by ascorbate and then subsequently be cycled back to ferric iron by O2, generating superoxide radical in the process, Rxns 4 – 6. Superoxide is dismuted by superoxide dismutases forming hydrogen peroxide, Rxn 7.
| (4) |
| (5) |
| (6) |
| (7) |
Labile iron serves two esential roles to bring about the selective toxicity of ascorbate: (1) catalyzing the oxidation of ascorbate to generate H2O2, Rxns 4 – 7; and (2) generation of the hydroxyl free radical from H2O2 via the Fenton reaction, Rxn 8. This is supported by the observation that chelation of labile iron to render it inactive as a catalyst in vitro inhibited ascorbate toxicity and protected from downstream DNA damage without altering H2O2 accumulation in sarcoma cells.17 Ferrous iron reacts with H2O2 to produce the hydroxyl radical, which has direct toxic effects on biomolecules essential for cell survival. In the presence of high levels of ascorbate, labile iron is able to redox cycle and continuously generate H2O2, which in turn leads to HO•.
| (8) |
| (9) |
Interestingly, a growing body of literature has demonstrated increased levels of redox active iron in cancer cells, as compared to normal cells.53–55 Given this metabolic frailty, it follows that the selective toxicity of ascorbate to cancer cells may not only be the generation of H2O2, but also the differential availability of redox-active labile iron.26 Our group has demonstrated that cancer cell-specific disruptions in iron metabolism resulting in increased levels of labile iron in cancer cells is likely secondary to cancer cell-specific alterations in oxidative metabolism leading to increased steady-state levels of reactive oxygen species such as superoxide (O2•−) and H2O2, which are known to be able to disrupt cellular iron metabolism leading to increased labile pools.10, 12, 56–60 Increasing production of mitochondrial-derived O2•− either pharmacologically, with antimycin A, in normal cells or genetically, using CRISPR-Cas9-mediated deletion of SOD2, in cancer cells, increased cellular labile iron and sensitivity to P-AscH−.6 We, and others, have also demonstrated that ascorbate-generated H2O2 itself is capable of disrupting the regulation of labile iron, likely through disruption of iron-containing proteins (such as Fe-S clusters), thereby selectively increasing labile iron in cancer cells.16 This likely reflects the intersection of both mechanisms of selective toxicity, intimately linking steady-state levels of H2O2 with the availability of redox active iron, thereby mediating the toxicity of ascorbate.
P-AscH− Sensitizes Cancer Cells to Radiation Therapy and Chemotherapy
Preclinical studies in xenograft models have provided mixed results on the efficacy of pharmacological ascorbate as a single agent therapy. Importantly, however, results have consistently demonstrated increased therapeutic efficacy of ascorbate in combination with standard-of-care radiation and/or chemotherapy as compared to standard-of-care treatments alone.10, 11, 17, 19, 28, 61 As discussed above, the majority of the toxicity of P-AscH− is thought to be mediated by oxidative damage to DNA. In order to exploit this mechanism of toxicity, the majority of preclinical and clinical studies have combined ascorbate with genotoxic chemotherapies and/or radiation, known to induce DNA damage. Indeed, preclinical studies in a variety of cancer types, including pancreatic, non-small cell lung, ovarian, glioblastoma multiforme, gastric, colon, and sarcoma10, 11, 14–17, have clearly demonstrated increased cell death or tumor growth inhibition with the inclusion of P-AscH− with radiation and/or chemotherapy.
However, only a couple of studies have closely evaluated the relationship of pharmacological ascorbate therapy to radio-chemo-sensitization. Ma and colleagues11 and Espey et al.18 have statistically demonstrated a synergistic relationship with the combination of pharmacological ascorbate and carboplatin (in vitro) and gemcitabine chemotherapy (in vitro and in vivo) using models of ovarian and pancreatic cancer, respectively. Our group has demonstrated the dose-dependent, selective radiosensitization of pancreatic ductal adenocarcinoma cell lines exposed to pharmacological ascorbate.16 We also demonstrated that this relationship was dependent on increased DNA damage that was partially inhibited by catalase. Taken together, the current state of the literature demonstrates the preclinical anti-cancer efficacy of adjuvant ascorbate in a variety of cancers when combined with radiation and a variety of different genotoxic chemotherapeutic agents.
P-AscH− Confers Normal Tissue Protection from Cancer Therapy
Beyond the selectively toxic effects of P-AscH−, there is mounting evidence that P-AscH− may provide benefits beyond tumor cytotoxicity by also ameliorating normal tissue injury that occurs secondarily to standard-of-care chemoradiation. Preliminary data suggest that P-AscH− acts as a pro-oxidant locally on tumor cells, but may behave as an antioxidant systemically in normal tissues. As discussed above, P-AscH− in normal tissues generates undetectable levels of H2O2 because of low levels of redox active labile metal ions and the presence of high levels of metabolic pathways that rapidly remove H2O2.19, 29 Unlike in cancer tissue, where the oxidative damage secondary to ascorbate-mediated H2O2 generation may dictate the redox milieu, in normal tissue, in the absence of the oxidative effects of ascorbate, the reductive capacity of P-AscH− may predominate and ameliorate the oxidative distress induced by radiation and chemotherapy (Figure 2).62
Figure 2. Differences in H2O2 metabolism and redox-active iron metabolism underlie the selective toxicity of pharmacological ascorbate.
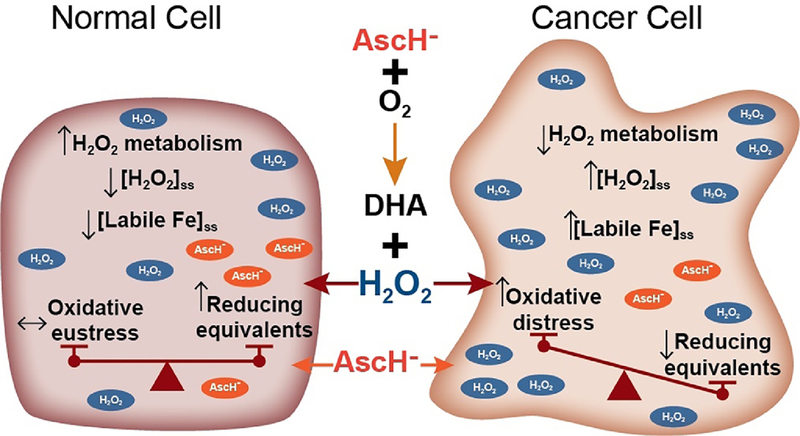
The proposed mechanisms underlying the selective toxicity of pharmacological ascorbate has as its center the production of H2O2 upon its oxidation. This is generally non-toxic to normal cells due to a high capacity to metabolize H2O2 in conjunction with well-regulated iron-metabolism. These properties limit the levels of redox-active, labile iron and the associated production of oxidizing free radicals. In normal cells the absence of ascorbate-mediated oxidative distress allows the reducing capabilities of ascorbate as an antioxidant to surface. Thus, inhibition of chemoradiation-mediated oxidative distress can manifest itself, thereby protecting normal tissue; i.e. a state of oxidative eustress.70 In contrast, decreased capacity of cancer cells to remove H2O2 as well as cancer-cell specific disruptions in iron metabolism result in increased levels of labile iron leading to significant oxidative distress and the selective sensitization of cancer cells to chemoradiation.
A 2011 multi-institutional study performed in Germany evaluated quality of life (QoL) outcomes in post-surgical breast cancer patients receiving adjuvant chemoradiation. Women receiving ascorbate (7.5 g IV weekly) during adjuvant chemoradiation reported significantly fewer side effects (i.e. nausea, loss of appetite, fatigue, depression, sleep disorders, and bleeding diathesis) than women receiving adjuvant chemoradiation alone.63 Furthermore, this study demonstrated a significantly better performance status in patients receiving ascorbate in addition to chemoradiation. However, whether this was due to reduced normal tissue toxicity or increased treatment efficacy was unclear as this study did not report survival data.
Welsh et al. (2013) demonstrated decreased plasma F2-isoprostane levels, a marker of systemic oxidative damage caused by lipid peroxidation, in five patients receiving P-AscH− in combination with gemcitabine chemotherapy for stage IV pancreatic cancer as compared to baseline plasma F2-isoprostane levels.19 Additional evidence suggesting normal tissue protection came from results of a phase I/IIa pilot trial of pharmacological ascorbate in combination with carboplatin/paclitaxel chemotherapy in patients with advanced stage ovarian cancer.11 This study demonstrated decreased grade III and grade IV therapy-related toxicities in patients that were administered adjuvant ascorbate as compared to those in the chemotherapy alone group. However, the results are limited given the small sample size (n = 12 – 13 per group).
Several groups have utilized mouse models to more directly investigate the ability of ascorbate to protect normal tissue from chemoradiation toxicity. Du et al. (2016), demonstrated histologically that P-AscH− administration partially inhibited mouse jejunal crypt loss 48 h following 10 Gy total abdominal radiation.15 Kanter and Akopat (2008) demonstrated that 100 mg kg−1 ascorbate IP significantly inhibited ileal goblet cell toxicity in Wistar rats.64 Another study, utilizing oral administration of ascorbate (in drinking water and/or oral gavage), demonstrated protection of jejunal villi as well as increased mouse survival following 13 Gy total abdominal radiation.65 Administration of ascorbate significantly reduced levels of reactive oxygen species (ROS) in the small intestine as well as tissue necrosis factor (TNF) one week following irradiation. Interestingly, only mice that were administered ascorbate both before and following irradiation demonstrated increased survival. Furthermore, mice that were administered oral ascorbate via gavage 8 h prior to irradiation in addition to ascorbate in the drinking water demonstrated significantly increased survival over mice administered ascorbate in the drinking water only.65 These data tend to support the protective antioxidant effects of ascorbate in the gut.
The protective effects of ascorbate do not appear to be limited to the bowel. Alopecia and achromotrichia are common side effects of radiation therapy. In a pilot study, treatment with daily P-AscH− for two days prior and two weeks following 15 Gy radiation significantly delayed the onset of achromotrichia in C57Bl/6NHsd mice (Figure 3A, B). Furthermore, P-AscH− administration delayed the onset and reduced the incidence of alopecia (Figure 3C). Taken together, these preliminary data suggest that P-AscH− may reduce radiation-mediated achromotrichia and alopecia in patients, a cause of significant patient distress.66 There is also preliminary ex vivo evidence that P-AscH− may protect red blood cells (RBCs) from osmotic hemolysis caused by oxidative distress induced by the taxol class of chemotherapeutic agents.67 Taken together, these preliminary studies offer compelling evidence that P-AscH− may protect normal tissue from chemoradiation while simultaneously sensitizing tumors to the same therapy. However, additional preclinical studies are needed to explore the mechanism of how P-AscH− can mitigate normal tissue toxicity associated with certain cancer therapies. Additional clinical studies are also needed to determine the clinical relevance in therapeutic adverse events and in improving the ability for cancer patients to receive multimodal treatments (i.e. surgery, radiation, and chemotherapy) in a timely and effective manner.
Figure 3. Pharmacological ascorbate protects C57Bl/6NHsd mice from radiation-mediated skin toxicity.
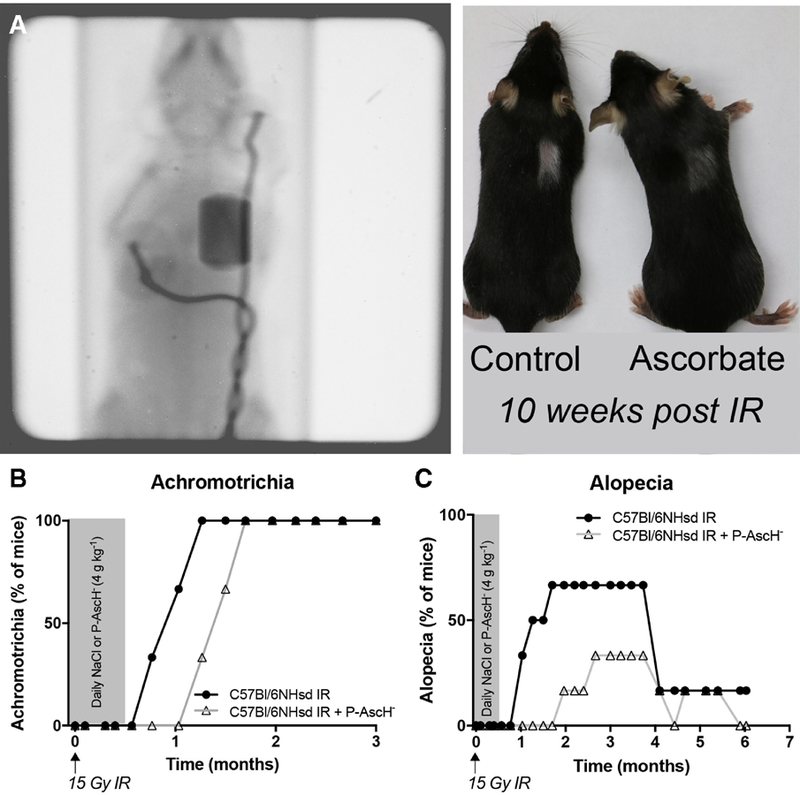
(A) Utilizing a target irradiator developed in-house (See methods.), an average of 13.25 ± 0.005 Gy was delivered to the right lung field. Irradiation was unrestricted in the dorsal-ventral axis, allowing dorsal skin overlying the radiation field to be used to monitor skin toxicity. Ascorbate, or equivalent dose of NaCl, was administered IP for two days prior and for two weeks following radiation treatment. Mice were monitored on a binary scale (present/not present) for (B) achromotrichia and/or (C) alopecia. Each datum is representative of the percent of mice within that group that displayed the phenotype at that time-point (n = 6 mice per group).
Conclusion
Since the re-emergence of P-AscH− as a potential anti-cancer agent, preclinical studies and clinical trials have consistently demonstrated the safety and tolerability of P-AscH− in combination with a variety of chemoradiation therapy regimens in a variety of disease sites10, 11, 19, 61, 68, 69 These same studies have also demonstrated increased efficacy of chemoradiation in combination with P-AscH− in mouse xenograft models. Preliminary clinical data are suggestive of superior clinical outcomes, underscoring the strength of P-AscH− as an adjuvant anti-cancer agent with differential actions on tumor and normal tissue. Future clinical trials, with larger patient cohorts, several of which are currently underway at our institution and elsewhere, are required to more definitively investigate the effects on P-AscH− on chemoradiation-mediated normal tissue damage and therapeutic response. Additionally, there remains much to learn about tumor cell oxidative metabolism that could provide further insight regarding which patients will have the greatest benefit from P-AscH− and how future iterations of ascorbate therapy can be utilized to enhance the selective sensitization of cancer cells to current standard-of-care therapy regimens.
Methods
Ascorbate and Ascorbate Exposure
L-Ascorbate stock solutions (approx. 1 M) were made using L-ascorbic acid (Macron Chemicals, Center Valley, PA) in Nanopure® Type 1 water (18 MΩ) with the pH adjusted to 7.0 with 1 M NaOH, stored in sealed glass tubes with minimal head space. The exact concentration was confirmed spectrophotometrically using ε265 = 14.5 mM−1 cm−1.23 Ascorbate, or equivalent dose of NaCl, was administered to C57Bl/6NHsd mice intraperitoneally (IP) daily at 4 g kg−1.
Mouse Model
Female 4 – 6-week-old c57Bl6/NHsd mice were purchased from Envigo and housed in the Animal Care Facility at The University of Iowa (Iowa City, IA), and all procedures were approved by The University of Iowa Institutional Animal Care and Use Committee and conformed to NIH guidelines. Treatment was initiated with daily ascorbate (4 g kg−1 or equivalent dose of NaCl, IP) two days prior to and two weeks following radiation exposure (See below.) to the right lung field. Following radiation, mice were monitored weekly for dorsal skin/hair changes within the radiation field.
Ionizing Radiation
Ionizing radiation (IR) was delivered in the Iowa Radiation and Free Radical Research Core facility using a Pantak Therapx DXT 300 X-ray machine operated at 200 kVp with added filtration of 0.35 mm Cu + 1.5 mm Al, resulting in a beam quality of 0.95 mm Cu.
For targeted irradiation of mouse lungs, an in-house device, the x-TRAP, was designed and constructed specifically for compatibility with murine irradiation on a Pantak Therapx DXT 300 X-ray machine. A mouse holding tube is capable of being driven in three orthogonal planes within a limited range. Additionally, the source to collimator distance (SCD) may be manually changed. For all experiments, the source to tube axis distance (SAD) used was 57.4 cm. A 3 mm × 6 mm copper collimator was utilized at source to collimator distance (SCD) of 43.1 cm to produce a target field size of 4.1 mm × 8.2 mm. An in-house imaging system was also utilized, similar to that described by Cho et al., 2010.31 Images were acquired using 80 kVp, 30 mA with no 0.20 mm Al. Camera settings were shutter speed of 0.25 s, f/4.5, 3200 ISO. All images were acquired in Canon Digital Photo Professional and image manipulation and overlays were constructed in ImageJ. For treatment, the x-ray machine was operated at 200 kVp, 15 mA with added filtration of 0.35 mm Cu + 1.5 mm Al, resulting in a beam quality of 0.95 mm Cu. Dose calculations were corrected for collimator factor (CF = 0.90 for the 3 mm × 6 mm copper collimator), temperature, pressure, and backscatter (Bw = 1.027). Importantly, Bw = 1.027 is an estimation based upon the assumption that extrapolation of the published Bw data are reasonable below field sizes of 1 cm. Doses at depth were approximated using published tables for these beams (British Institute of Radiology and Institute of Physics and Engineering in Medicine and Biology, 1996). Dosimetric effects of the 2.5 mm thick acrylic tube wall were not specifically included in dosimetry.
To position the mouse within the radiation field, first, a field image was acquired with the copper collimator in place in the absence of a mouse tube and mouse. Next, a mouse, anesthetized as described above, was inserted prone into the mouse holding tube with the aid of a plastic ‘sled,’ and an initial positioning image was acquired without the collimator in place. These images were overlayed, adjustments were made to the position of the mouse, and this process was repeated until the right lung occupied most the radiation field. Effort was expended to spare the left lung as well as to irradiate most the base of the lung; as such, the apex of the right lung was often spared due to the field size. Each mouse was exposed to approximately 4.6 positioning images on average resulting in a total imaging dose of approximately 0.55 ± 0.15 Gy of whole body radiation. Once the mouse was appropriately positioned, the right lung field was treated with 13.25 ± 0.005 Gy for a total dose of 14.63 ± 0.27 Gy.
Acknowledgments
Radiation experiments were assisted by the Ionizing Radiation Services of the Radiation and Free Radical Research Core in the Holden Comprehensive Cancer Center. We also acknowledge the invaluable support of the ESR facility. Graphic support was provided by Gareth Smith
This work was supported by CCSG P30-CA086862 R01CA182804, R01CA184051, R01CA169046, Gateway for Cancer Research award G-17–1500T32-GM007337 and T32CA078586 and T32CA148062
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Buettner GR: The pecking order of free radicals and antioxidants: lipid peroxidation, alpha-tocopherol, and ascorbate. Archives of biochemistry and biophysics, 300:535–543,1993 [DOI] [PubMed] [Google Scholar]
- 2.Kuiper C, Vissers MC: Ascorbate as a co-factor for fe- and 2-oxoglutarate dependent dioxygenases: physiological activity in tumor growth and progression. Frontiers in oncology, 4:359,2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cameron E, Pauling L: Supplemental ascorbate in the supportive treatment of cancer: Prolongation of survival times in terminal human cancer. Proc Natl Acad Sci U S A, 73:3685–3689,1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Creagan ET, Moertel CG, O’Fallon JR et al. : Failure of high-dose vitamin C (ascorbic acid) therapy to benefit patients with advanced cancer. A controlled trial. The New England journal of medicine, 301:687–690,1979 [DOI] [PubMed] [Google Scholar]
- 5.Moertel CG, Frytak S, Hahn RG et al. : Therapy of locally unresectable pancreatic carcinoma: a randomized comparison of high dose (6000 rads) radiation alone, moderate dose radiation (4000 rads + 5-fluorouracil), and high dose radiation + 5-fluorouracil: The Gastrointestinal Tumor Study Group. Cancer, 48:1705–1710,1981 [DOI] [PubMed] [Google Scholar]
- 6.Levine M, Conry-Cantilena C, Wang Y et al. : Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc Natl Acad Sci U S A, 93:3704–3709,1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Padayatty SJ, Sun H, Wang Y et al. : Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann Intern Med, 140:533–537,2004 [DOI] [PubMed] [Google Scholar]
- 8.Padayatty SJ, Katz A, Wang Y et al. : Vitamin C as an antioxidant: evaluation of its role in disease prevention. Journal of the American College of Nutrition, 22:18–35,2003 [DOI] [PubMed] [Google Scholar]
- 9.Levine M, Padayatty SJ, Espey MG: Vitamin C: a concentration-function approach yields pharmacology and therapeutic discoveries. Advances in nutrition (Bethesda, Md), 2:78–88,2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schoenfeld JD, Sibenaller ZA, Mapuskar KA et al. : O2•− and H2O2-Mediated Disruption of Fe Metabolism Causes the Differential Susceptibility of NSCLC and GBM Cancer Cells to Pharmacological Ascorbate. Cancer Cell, 31:487–500 e488,2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma Y, Chapman J, Levine M et al. : High-dose parenteral ascorbate enhanced chemosensitivity of ovarian cancer and reduced toxicity of chemotherapy. Sci Transl Med, 6:222ra218,2014 [DOI] [PubMed] [Google Scholar]
- 12.McCarty MF, Contreras F: Increasing Superoxide Production and the Labile Iron Pool in Tumor Cells may Sensitize Them to Extracellular Ascorbate. Frontiers in oncology, 4:249,2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doskey CM, Buranasudja V, Wagner BA et al. : Tumor cells have decreased ability to metabolize H2O2: Implications for pharmacological ascorbate in cancer therapy. Redox Biol, 10:274–284,2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Leary B, K. Houwen F, L. Johnson C et al. : Pharmacological Ascorbate as an Adjuvant for Enhancing Radiation-Chemotherapy Responses in Gastric Adenocarcinoma; 2018. [DOI] [PMC free article] [PubMed]
- 15.Du J, Cieslak JA 3rd, Welsh JL et al. : Pharmacological Ascorbate Radiosensitizes Pancreatic Cancer. Cancer research, 75:3314–3326,2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moser JC, Rawal M, Wagner BA et al. : Pharmacological ascorbate and ionizing radiation (IR) increase labile iron in pancreatic cancer. Redox Biol, 2:22–27,2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schoenfeld JD, Sibenaller ZA, Mapuskar KA et al. : Redox active metals and H2O2 mediate the increased efficacy of pharmacological ascorbate in combination with gemcitabine or radiation in pre-clinical sarcoma models. Redox Biol, 14:417–422,2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Espey MG, Chen P, Chalmers B et al. : Pharmacologic ascorbate synergizes with gemcitabine in preclinical models of pancreatic cancer. Free radical biology & medicine, 50:1610–1619,2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Welsh JL, Wagner BA, van’t Erve TJ et al. : Pharmacological ascorbate with gemcitabine for the control of metastatic and node-positive pancreatic cancer (PACMAN): results from a phase I clinical trial. Cancer Chemother Pharmacol, 71:765–775,2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polireddy K, Dong R, Reed G et al. : High Dose Parenteral Ascorbate Inhibited Pancreatic Cancer Growth and Metastasis: Mechanisms and a Phase I/IIa study. Scientific Reports, 7:17188,2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Q, Espey MG, Krishna MC, Mitchel l JB, Corpe CP, Buettner GR, Shacter E, Levine M : Pharmacologic ascorbic acid concentrations selectively kill cancer cells: Action as a pro-drug to deliver hydrogen peroxide to tissues. Proc Natl Acad Sci USA, 102:13604–13609,2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Q, Espey MG, Sun AY et al. : Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc Natl Acad Sci U S A, 104:8749–8754,2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buettner GR: In the absence of catalytic metals ascorbate does not autoxidize at pH 7: ascorbate as a test for catalytic metals. Journal of biochemical and biophysical methods, 16:27–40,1988 [DOI] [PubMed] [Google Scholar]
- 24.Bienert GP, Schjoerring JK, Jahn TP: Membrane transport of hydrogen peroxide. Biochimica et biophysica acta, 1758:994–1003,2006 [DOI] [PubMed] [Google Scholar]
- 25.Bienert GP, Moller AL, Kristiansen KA et al. : Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. The Journal of biological chemistry, 282:1183–1192,2007 [DOI] [PubMed] [Google Scholar]
- 26.Erudaitius D, Huang A, Kazmi S et al. : Peroxiporin Expression Is an Important Factor for Cancer Cell Susceptibility to Therapeutic H2O2: Implications for Pharmacological Ascorbate Therapy. PloS one, 12:e0170442,2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olney KE, Du J, van ‘t Erve TJ et al. : Inhibitors of hydroperoxide metabolism enhance ascorbate-induced cytotoxicity. Free radical research, 47:154–163,2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Du J, Martin SM, Levine M et al. : Mechanisms of ascorbate-induced cytotoxicity in pancreatic cancer. Clinical cancer research : an official journal of the American Association for Cancer Research, 16:509–520,2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Q, Espey MG, Sun AY et al. : Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proc Natl Acad Sci U S A, 105:11105–11109,2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Du J, Cullen JJ, Buettner GR: Ascorbic acid: chemistry, biology and the treatment of cancer. Biochimica et biophysica acta, 1826:443–457,2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho CS, Lee S, Lee GT et al. : Irreversible inactivation of glutathione peroxidase 1 and reversible inactivation of peroxiredoxin II by H2O2 in red blood cells. Antioxidants & redox signaling, 12:1235–1246,2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson RM, Ho YS, Yu DY et al. : The effects of disruption of genes for peroxiredoxin-2, glutathione peroxidase-1, and catalase on erythrocyte oxidative metabolism. Free radical biology & medicine, 48:519–525,2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benfeitas R, Selvaggio G, Antunes F et al. : Hydrogen peroxide metabolism and sensing in human erythrocytes: a validated kinetic model and reappraisal of the role of peroxiredoxin II. Free radical biology & medicine, 74:35–49,2014 [DOI] [PubMed] [Google Scholar]
- 34.Nicholls P: ACTIVITY OF CATALASE IN THE RED CELL. Biochimica et biophysica acta, 99:286–297,1965 [DOI] [PubMed] [Google Scholar]
- 35.Jones DP, Eklow L, Thor H et al. : Metabolism of hydrogen peroxide in isolated hepatocytes: relative contributions of catalase and glutathione peroxidase in decomposition of endogenously generated H2O2. Archives of biochemistry and biophysics, 210:505–516,1981 [DOI] [PubMed] [Google Scholar]
- 36.Makino N, Mochizuki Y, Bannai S et al. : Kinetic studies on the removal of extracellular hydrogen peroxide by cultured fibroblasts. The Journal of biological chemistry, 269:1020–1025,1994 [PubMed] [Google Scholar]
- 37.Sasaki K, Bannai S, Makino N: Kinetics of hydrogen peroxide elimination by human umbilical vein endothelial cells in culture. Biochimica et biophysica acta, 1380:275–288,1998 [DOI] [PubMed] [Google Scholar]
- 38.Winterbourn CC: Reconciling the chemistry and biology of reactive oxygen species. Nature chemical biology, 4:278–286,2008 [DOI] [PubMed] [Google Scholar]
- 39.Makino N, Sasaki K, Hashida K et al. : A metabolic model describing the H2O2 elimination by mammalian cells including H2O2 permeation through cytoplasmic and peroxisomal membranes: comparison with experimental data. Biochimica et biophysica acta, 1673:149–159,2004 [DOI] [PubMed] [Google Scholar]
- 40.Mitozo PA, de Souza LF, Loch-Neckel G et al. : A study of the relative importance of the peroxiredoxin-, catalase-, and glutathione-dependent systems in neural peroxide metabolism. Free radical biology & medicine, 51:69–77,2011 [DOI] [PubMed] [Google Scholar]
- 41.Johnson RM, Goyette G Jr., Ravindranath Y et al. : Hemoglobin autoxidation and regulation of endogenous H2O2 levels in erythrocytes. Free radical biology & medicine, 39:1407–1417,2005 [DOI] [PubMed] [Google Scholar]
- 42.De Duve C, Baudhuin P: Peroxisomes (microbodies and related particles). Physiological reviews, 46:323–357,1966 [DOI] [PubMed] [Google Scholar]
- 43.Oberley TD, Oberley LW: Antioxidant enzyme levels in cancer. Histology and histopathology, 12:525–535,1997 [PubMed] [Google Scholar]
- 44.Marklund SL, Westman NG, Lundgren E et al. : Copper- and zinc-containing superoxide dismutase, manganese-containing superoxide dismutase, catalase, and glutathione peroxidase in normal and neoplastic human cell lines and normal human tissues. Cancer research, 42:1955–1961,1982 [PubMed] [Google Scholar]
- 45.Oberley LW, Buettner GR: Role of superoxide dismutase in cancer: a review. Cancer Res, 39:1141–1149,1979 [PubMed] [Google Scholar]
- 46.Huang X, Motea EA, Moore ZR et al. : Leveraging an NQO1 Bioactivatable Drug for Tumor-Selective Use of Poly(ADP-ribose) Polymerase Inhibitors. Cancer cell, 30:940–952,2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miller DM, Buettner GR, Aust SD: Transition metals as catalysts of “autoxidation” reactions. Free Radical Biology and Medicine, 8:95–108,1990 [DOI] [PubMed] [Google Scholar]
- 48.Buettner GR, Jurkiewicz BA: Catalytic metals, ascorbate and free radicals: combinations to avoid. Radiation research, 145:532–541,1996 [PubMed] [Google Scholar]
- 49.Buettner GR: Ascorbate autoxidation in the presence of iron and copper chelates. Free radical research communications, 1:349–353,1986 [DOI] [PubMed] [Google Scholar]
- 50.Buettner GR: Ascorbate oxidation: UV absorbance of ascorbate and ESR spectroscopy of the ascorbyl radical as assays for iron. Free radical research communications, 10:5–9,1990 [DOI] [PubMed] [Google Scholar]
- 51.Espósito BP, Epsztejn S, Breuer W et al. : A Review of Fluorescence Methods for Assessing Labile Iron in Cells and Biological Fluids. Analytical biochemistry, 304:1–18,2002 [DOI] [PubMed] [Google Scholar]
- 52.Petrat F, de Groot H, Sustmann R et al. : The chelatable iron pool in living cells: a methodically defined quantity. Biological chemistry, 383:489–502,2002 [DOI] [PubMed] [Google Scholar]
- 53.Richardson DR, Ponka P: The molecular mechanisms of the metabolism and transport of iron in normal and neoplastic cells. Biochimica et biophysica acta, 1331:1–40,1997 [DOI] [PubMed] [Google Scholar]
- 54.Richardson DR, Kalinowski DS, Lau S et al. : Cancer cell iron metabolism and the development of potent iron chelators as anti-tumour agents. Biochimica et biophysica acta, 1790:702–717,2009 [DOI] [PubMed] [Google Scholar]
- 55.Torti SV, Torti FM: Iron and cancer: more ore to be mined. Nat Rev Cancer, 13:342–355,2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aykin-Burns N, Ahmad IM, Zhu Y et al. : Increased levels of superoxide and H2O2 mediate the differential susceptibility of cancer cells versus normal cells to glucose deprivation. The Biochemical journal, 418:29–37,2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spitz DR, Sim JE, Ridnour LA et al. : Glucose deprivation-induced oxidative stress in human tumor cells. A fundamental defect in metabolism? Annals of the New York Academy of Sciences, 899:349–362,2000 [DOI] [PubMed] [Google Scholar]
- 58.Caltagirone A, Weiss G, Pantopoulos K: Modulation of cellular iron metabolism by hydrogen peroxide. Effects of H2O2 on the expression and function of iron-responsive element-containing mRNAs in B6 fibroblasts. The Journal of biological chemistry, 276:19738–19745,2001 [DOI] [PubMed] [Google Scholar]
- 59.Ibrahim WH, Habib HM, Kamal H et al. : Mitochondrial superoxide mediates labile iron level: evidence from Mn-SOD-transgenic mice and heterozygous knockout mice and isolated rat liver mitochondria. Free radical biology & medicine, 65:143–149,2013 [DOI] [PubMed] [Google Scholar]
- 60.Pantopoulos K, Mueller S, Atzberger A et al. : Differences in the regulation of iron regulatory protein-1 (IRP-1) by extra- and intracellular oxidative stress. The Journal of biological chemistry, 272:9802–9808,1997 [DOI] [PubMed] [Google Scholar]
- 61.Monti DA, Mitchell E, Bazzan AJ et al. : Phase I evaluation of intravenous ascorbic acid in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. PloS one, 7:e29794,2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Conklin KA: Chemotherapy-associated oxidative stress: impact on chemotherapeutic effectiveness. Integrative cancer therapies, 3:294–300,2004 [DOI] [PubMed] [Google Scholar]
- 63.Vollbracht C, Schneider B, Leendert V et al. : Intravenous vitamin C administration improves quality of life in breast cancer patients during chemo-/radiotherapy and aftercare: results of a retrospective, multicentre, epidemiological cohort study in Germany. In vivo (Athens, Greece), 25:983–990,2011 [PubMed] [Google Scholar]
- 64.Kanter M, Akpolat M: Vitamin C protects against ionizing radiation damage to goblet cells of the ileum in rats. Acta histochemica, 110:481–490,2008 [DOI] [PubMed] [Google Scholar]
- 65.Ito Y, Kinoshita M, Yamamoto T et al. : A combination of pre- and post-exposure ascorbic acid rescues mice from radiation-induced lethal gastrointestinal damage. International journal of molecular sciences, 14:19618–19635,2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Freites-Martinez A, Shapiro J, Goldfarb S et al. : CME Part 1: Hair disorders in cancer patients. Journal of the American Academy of Dermatology 2018
- 67.Uzma F, Fatema A, Magda M: Protective role of tocopherol and ascorbic acid in taxol-treated human erythrocytes in vitro. Toxicology Research and Application, 1:2397847317705813,2017 [Google Scholar]
- 68.Hoffer LJ, Levine M, Assouline S et al. : Phase I clinical trial of i.v. ascorbic acid in advanced malignancy. Ann Oncol, 19:1969–1974,2008 [DOI] [PubMed] [Google Scholar]
- 69.Polireddy K, Dong R, Reed G et al. : High Dose Parenteral Ascorbate Inhibited Pancreatic Cancer Growth and Metastasis: Mechanisms and a Phase I/IIa study. Scientific Reports, 7:17188,2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sarsour EH, Kalen AL, Xiao Z et al. : Manganese superoxide dismutase regulates a metabolic switch during the mammalian cell cycle. Cancer research, 72:3807–3816,2012 [DOI] [PMC free article] [PubMed] [Google Scholar]


