Abstract
Human NK cells are innate immune effectors that play a critical roles in the control of viral infection and malignancy. The importance of their homeostasis and function can be demonstrated by the study of patients with primary immunodeficiencies (PID), which are part of the family of diseases known as inborn defects of immunity. While NK cells are affected in many PIDs in ways that may contribute to a patient’s clinical phenotype, a small number of PIDs have an NK cell abnormality as their major immunological defect. These PIDs can be collectively referred to as NK cell deficiency disorders (NKD), and include effects upon NK cell numbers, subsets and/or functions. The clinical impact of NKD can be severe including fatal viral infection, with particular susceptibility to herpesviral infections, such as cytomegalovirus, varicella zoster virus and Epstein-Barr virus. While NKD is rare, studies of these diseases are important for defining specific requirements for human NK cell development and homeostasis. New themes in NK cell biology are emerging through the study of both known and novel NKD, particularly those affecting cell cycle and DNA damage repair, as well as broader PIDs having substantive impact upon NK cells. In addition, the discovery of NKD that affect other innate lymphoid cell (ILC) subsets opens new doors for better understanding the relationship between conventional NK cells and other ILC subsets. Here we describe the biology underlying human NKD, particularly in the context of new insights into innate immune cell function, including a discussion of recently described NKD with accompanying effects on ILC subsets. Given the impact of these disorders upon human immunity with a common focus upon NK cells, the unifying message of a critical role for NK cells in human host defense singularly emerges.
Introduction
Natural killer (NK) cells are innate lymphocytes that critically function in defense against viral infection and malignancy. They serve these roles through multiple mechanisms that collectively exert both direct anti-viral and anti-tumor responses, while also helping to shape the adaptive and innate immune responses. According to current paradigms, NK cells are considered within the innate lymphoid cell (ILC) family, which includes both conventional NK cells and ILCs that primarily reside within tissue and have specialized functions that parallel T cell helper subsets1,2. Based upon this nomenclature, conventional NK cells are functionally and phenotypically similar to the ILC1 group, as they are potent producers of interferon gamma and express Tbx211,3. However, conventional NK cells are distinct from the other ‘helper’ ILCs as NK cells are the cytotoxic effectors of the ILC family, which functionally also aligns them with CD8+ T cells. They perform targeted lysis through the directed secretion of perforin and granzymes, contained within specialized organelles termed lytic granules, in response to activating signals transmitted through germline-encoded receptors. It is this latter characteristic, in concert with the general preparedness of human NK cells attributed to their baseline high lytic granule content4, that allows them to fill a unique and non-redundant role in the innate immune response.
Primary immunodeficiency (PID) occurs when genetic abnormalities impacting immunity lead to immune dysregulation, immune impairment or both. Recent advances in genetic and genomic technology have enabled the acceleration of this field, and there are currently more than 350 described monogenic disorders of immunity that can cause PID5. These may affect single cell subsets, or may stretch across the immune system and affect multiple lineages, reflecting a conserved requirement for the gene or protein in immune function. While they are rare, PID that primarily affect a single immune cell lineage, or that have a unique phenotype in a single lineage, can be remarkably informative about the function and natural history of these cells. This approach has led to important discoveries about the uniquely human aspects of immune function in cases where human immunodeficiency phenotypes differ significantly from those seen in a mouse model. These include human CD19 deficiency as a cause of hypogammaglobulinemia6, and Zap70 deficiency, which leads to significant differences in its effect on circulating T cell subsets between mice and humans7–10. Such differences continue to be uncovered, including the recent discovery of human RIPK1 deficiency, which leads to immunodeficiency, gut inflammation and polyarthritis, a surprisingly different phenotype from the systemic inflammation and early morbidity seen in Ripk1-knockout mice11–14. Finally, the study of patients with primary immunodeficiency has the potential to provide important therapeutic insights and help in the design and guidance of new treatments for others.
With this in mind, the study of patients with PID that affect the NK cell subset has shaped our understanding of uniquely human aspects of immunology. Innate immune cells are finely tuned to respond to environmental cues for their function and homeostasis. While model organisms are an irreplaceable tool in our understanding of the requirements for immunity, we must turn to the human model to fully understand the human system. The differing environs between humans and experimental mice, who are frequently housed in the absence of specific pathogens, are likely at least partially responsible for the functional distinctions between innate immune cell subsets between the species15. There is also notable divergent evolution with a number of important of functional orthologues being represented by distinct gene families in humans and mice, such as Ly49 in mice and KIR in humans16. As technologies improve for studying specifically human immunology, we can also take advantage of these to drive new investigations into lesser-understood immune subsets, including NK cell subsets, adaptive and memory-like NK cells, and ILCs.
Here, we will summarize the findings from the study of patients with PID that can primarily or solely affect NK cells, and place these in the context of our current understanding of human NK cell development and homeostasis. These NKD have traditionally been divided into those that affect maturation, survival and development, as opposed to those that singularly affect NK cell function. We are increasingly becoming aware of the complexity of drawing such distinctions, however, and will discuss current thinking about the relationship between phenotype and function in NKD. Similarly, we will also include a brief discussion of select combined immunodeficiencies in which an NK cell abnormality may be a minor component of the immunological phenotype, but provides significant insight into human NK cell biology. While these affect other immune subsets, they additionally provide valuable information about the mechanistic requirements for human NK cells. Finally, we will consider the implication of these findings given the emerging understanding of the relationship between NK cells and ILCs. The careful study of patients with NKD gives us the opportunity to better understand how inherent defects in NK cell development, differentiation and function can inform our current understanding of the requirement for this particular subset of human immune cells.
Human NK cell development, differentiation and function
Human NK cells arise from bone marrow hematopoietic precursors, and in both mice and humans, ablation of the bone marrow abrogates NK cell development17–19. While the generation of mature NK cells in bone marrow has been described, it is thought that bone marrow precursors traffic to peripheral tissues, the best-described of which is secondary lymphoid tissue, to undergo terminal maturation and subsequently exit to circulation20–22. Despite the first description of this natural history of human NK cells in 2005, it is still poorly understood how these steps of maturation are regulated, and the precise localization and regulation of NK cell generation has not been well defined.
Within peripheral blood, the primary NK cell subset is the CD56dim subset, which has cytolytic capacity at baseline and is thought to circulate to enable recruitment to sites of infection and inflammation. The minor subset in peripheral blood, CD56bright, are potent cytokine producers, particularly of IFNγ and TNFα. While CD56dim NK cells predominate in peripheral blood, within tissue most NK cells fall within the CD56bright subset, although tissue specialization ultimately shapes their receptor repertoire23. CD56bright NK cells are thought to give rise to CD56dim NK cells based upon in vitro, in vivo, transplantation and humanized mouse models24–29. Despite these findings, which include longer telomeres in CD56bright NK cells27 and the early appearance of CD56bright NK cells following transplantation28,29, the mechanism by which this transition is regulated is not defined, and the precise physiological relationship between the two subsets is called into question by recent studies in non-human primates that suggests the two subsets have independent origins30,31.
The difficulty in understanding human NK cell development has arisen from several factors. NK cell development and terminal maturation is a process with much greater plasticity than T or B cell development, including much less rigorous tissue restriction. While T and B cell developmental subsets can be spatially and temporally mapped within thymic and bone marrow environments respectively, NK cell precursors and developmental intermediates in adults can be isolated from a number of sites, including bone marrow32, spleen32, thymus33–36, intestine37, secondary lymphoid tissue20,32,38, uterus39, and liver40. With the exception of bone marrow, which generates hematopoietic precursors, none of these sites are critical for the generation of mature circulating NK cells. Instead, it is becoming increasingly clear that NK cell development is shaped by the microenvironment, leading to generation of diverse, tissue specific subsets with unique functions41. This model highlights the plasticity of NK cell precursors, which includes common NK-ILC precursors, and underscores the role that the cytokine and tissue microenvironment play in shaping functionally mature NK cells.
A second factor that has confounded the study of human NK cell development lies in the difficulty in drawing comparisons between mouse and human models. While analogous functional subsets are commonly identified, key differences in receptor expression make this difficult. This includes the lack of CD56 or a known CD56 homologue on murine NK cells. Perhaps more important is the role that environmental exposure plays in human NK cell maturation. The phenotype of circulating NK cells is frequently described in terms of CD56bright or CD56dim subsets, however careful dissection of peripheral blood NK cells reveals receptor expression that changes through life and in response to immune experiences. These changes include distinct patterns of receptor diversity that are induced in response to genetic and environmental factors42, and the formation of adaptive or memory NK cells in response to common viruses. The best characterized of these is CMV infection, which leads to the generation of an adaptive pool of NK cells that were originally identified as expressing NKG2C43–45, but have also been identified in NKG2C-negative individuals46. Further studies have identified other characteristics of these cells, including their down-regulation of the intracellular adaptors Syk and FcεRγ and the transcription factors EAT2 and PLZF147,48. Adaptive NK cells are also present following diverse viral infections including hantavirus49, chikungunya virus50 and HIV51–53, and memory-like NK cells are generated in response to cytokine stimulation54. It is likely that there are additional environmental factors that similarly shape NK cell phenotype and function that are even less well understood and are also not well replicated in mice housed under specific pathogen-free conditions. These could include the role of the microbiome in shaping innate immune cell function, and exposure to fungi and other non-viral pathogens55–57.
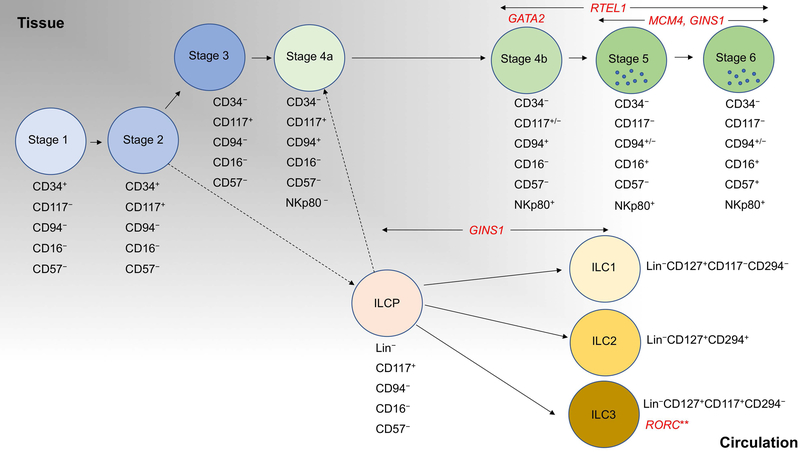
The commonly used model of NK cell development now assigns this process to 7 stages (Stages 1–3, 4a, 4b, 5 and 6) but can be minimally defined by expression of CD34, CD117 (c-Kit), CD94 and CD16 or KIRs58,59 (Figure 1). These stages can be isolated from tissue, including secondary lymphoid tissue, but can also be recapitulated using in vitro differentiation60,61. CD34+ cells from cord blood, circulating blood or mobilized bone marrow are a common source of NK cell progenitors for experimental modeling of human NK cell development, although the more restricted precursor, and technically the Stage 1 cell, is defined as CD34+CD45RA+CD10+. Progression to Stage 2 minimally includes the up-regulation of CD117 with CD34 retained on the cell surface, and Stage 3 is defined by loss of CD34 with retained CD117 expression. Acquisition of CD94 defines progression to Stage 4, which is followed by terminal maturation marked by acquisition of CD16 and/or KIRs (Stages 5 and 6). The recent delineation of Stage 4 NK cells to ‘4a’ and ‘4b’ subsets, and the important phenotypic and functional differences between the two, suggests that the current model retains opportunity for further dissection58.
Figure 1. Human NK cell and ILC developmental subsets and NK cell/ILC deficiencies that affect them.
Key cell surface receptors that identify NK cell and ILC developmental subsets are shown. Monogenic causes of NKD or ILC deficiency are shown in red. This model of human NK cell development is adapted from59,237, with circulating ILC subsets and precursors68 shown as described in Cottineau et al91 to be affected in some patients with GINS1 mutations. Lineage markers are CD3, CD4, CD5, CD14, CD16, CD94, CD123, CD34, CD303, CD19, FcεR1α. **IL-17 producing subset only. ILCP, systemic innate lymphoid cell precursor68. Dashed line indicates inferred relationship, solid line indicates experimentally defined relationships.
The recent discovery that NK cells are members of a larger group of innate lymphoid cells has prompted a re-examination of what features can be used to identify a conventional NK cell relative to other lineage-negative lymphocytes, namely ILC1, ILC2 and ILC3 subsets. Conventional NK cells are akin to the ILC1 subset due to their capacity for IFNγ production3; however, traditionally it is the capacity for cytotoxic function by a non-B, non-T lymphocyte defines a conventional NK cell62. Therefore, while the expression of lytic effector molecules such as FcγRIIIA (CD16), perforin, and granzymes and the transcription factor EOMES may not be unique to NK cells amongst lymphocytes, using these markers in combination with lineage exclusion is effective for identifying conventional NK cells amongst other ILCs3.
NK cells in peripheral blood are approximately 1–17% of the lymphocyte population in healthy donors63,64. In contrast, circulating ILC subsets are found at even lower frequency (<0.2%) as they are predominantly found within tissue65. However, while at low frequency, both mature ILC subsets and ILC precursors can be isolated from peripheral blood66–68. These are negative for multiple lymphocyte lineages but identified by expression of CD117 (c-Kit), and in some cases CD294 (CRTH2), CD161 and NKp44. The circulating lineage negative CD117+ ILCP can give rise to all ILC subsets, including conventional NK cells, distinguishing it from gut CD117+ ILCs that express NKp44 and RORγT68. As Stage 3 NK cells are minimally defined as being lineage negative CD117+ cells, it is important to be aware of these subsets, particularly when using tissue or in vitro samples, as it is likely that phenotypic heterogeneity ascribed to Stage 3 NK cells arises from the inclusion of ILC3 or ILCP in analyses.
NK cell developmental subsets have unique and defining phenotypes, however they also have unique functions. The CD56dim (Stage 5) subset is considered the canonical cytolytic subset, due to its lytic granule content, expression of perforin and granzymes, as well as the KIR molecules and low affinity Fc receptor FcγRIIIA (CD16). In contrast, the CD56bright subset (Stage 4) is considered the most potent for cytokine production. However, recent studies of cytokine primed peripheral blood NK cells demonstrate that the CD56bright subset can be highly functional for contact dependent killing, and CD56dim NK cells can be potent producers of cytokines, including IFNγ69,70. These studies highlight the plasticity of all NK cell subsets and further underscore the context-dependent regulation of their function.
NK cell lytic function is exerted through the formation of an immunological synapse, a highly organized signaling platform that directs secretion towards a susceptible target cell (reviewed in71). This function is controlled by the expression of activating and inhibitory receptors that tune NK cell responsiveness to MHC class I and virally infected or stress-induced ligands on target cells. In an interaction with a potential target cell, surpassing the threshold of NK cell restraint leads to the cytoskeletal polarization of the NK cell toward to target. This is accompanied by the re-orientation of lytic granules towards the immune synapse following the rapid recruitment of lytic granules to the microtubule organizing center (MTOC) also known as convergence72,73. Following delivery to the immune synapse, granules pass through the cortical actin network to the plasma membrane, where they fuse with the NK cell membrane and release their contents. NK cell function can be modulated for efficiency within the microenvironment, both by the positioning of lytic granules within the cell, and by the use of serial killing to rapidly eliminate multiple targets73–76.
NK cell subsets are frequently enumerated as CD56bright and CD56dim, or simply as CD56+CD16+. However, the comprehensive interrogation of NK cell receptors, including activating and inhibitory receptors and those associated with developmental subsets, can provide important insight to functional questions. The application of mass cytometry has provided the capacity to evaluate these parameters together on a single cell level and derive new and important information about receptor diversity42. In addition, it has allowed for the targeted analyses of specific signaling pathways or signaling axes54,69. In addition to characterization by cell surface phenotype, NK cells can be functionally interrogated using standard cytotoxicity assays and microscopy-based single cell killing assays against susceptible MHC class I-negative target cells. This approach can also be used to measure ADCC, which is cytotoxicity mediated by FcγRIIIA binding to IgG on opsonized target cells. Cytokine production may be measured by ELISA, or increasingly commonly by intracellular staining or cytokine bead array, both of which are flow cytometric-based approaches. Ideally, both phenotype and function are thoughtfully and carefully measured when undertaking the study of human NK cells in health and disease. The comprehensive measurement of both functional and phenotypic parameters enables the high-resolution characterization of both known and novel NK cell subsets42,63.
Clinical hallmarks of NK cell deficiencies
The term NK cell deficiency, or NKD, defines a PID in which the major immunological defect is that of the NK cell subset77. At present this includes 6 monogenetic disorders (Table 1). As we will discuss in detail below, there are some cases where clinical phenotypes may vary between patients having an abnormality within a given gene, but progress has been made to identify, as best possible, “pure” cases of NKD in which NK cells are solely or primarily affected. NKD may be manifest by an absence or decrease of NK cells or specific NK cell subsets, or it may be defined by impaired NK cell function in the context of seemingly normal phenotype. However, it is expected that when NK cell phenotype is affected, NK cell function will be measurably diminished, either as a feature of reduced number or aberrant functional maturation. NKD may be defined as ‘classical’ (cNKD) or ‘functional’ (fNKD), with the former referring to cases in which NK cell development or maturation is affected, and the latter referring to aberrant function in the presence of seemingly normal numbers, including distribution of subsets, in peripheral blood78. The specific reason for the term ‘classical’ as opposed to ‘developmental’ or ‘maturational’ is that the first reported cases of NKD (which were ultimately genetically solved) were ones in which NK cells were initially reported or subsequently defined as absent or greatly reduced in the peripheral circulation79–88.
Table 1:
Monogenic causes of human NK cell deficiency
| Gene | Infectious Susceptibility | NK cell Features | Key Reference |
|---|---|---|---|
| cNKD | |||
| GATA2 | VZV, HSV, CMV, HPV, mycobacteria | Low NK cell number, decreased CD56bright subset, cytotoxicity | 87 |
| MCM4 | EBV, recurrent respiratory infections | Low NK cell number, decreased CD56dim subset | 89 |
| RTEL1 | VZV | Absent NK cells | 90 |
| GINS1 | CMV | Low NK cell number, decreased CD56dim subset | 91 |
| IRF8 | EBV | Low NK cell number, decreased CD56dim subset, cytotoxicity | 92 |
| fNKD | |||
| FCGR3A | EBV, HSV, HPV | Normal NK cell numbers, cytotoxicity | 93 |
cNKD, classical NKD; fNKD, functional NKD; VZV, varicella zoster virus; HSV, herpes simplex virus; CMV, cytomegalovirus; HPV, human papillomavirus; EBV, Epstein-Barr virus
Clinically, the presentation of NKD is variable, however the hallmark of NKD is generally considered to be herpesviral infections including varicella zoster virus (VZV), herpes simplex virus (HSV), Epstein-Barr Virus (EBV) and cytomegalovirus (CMV), with these viruses present in almost 60% of reported NKD cases78. While some NKD may affect other immune compartments or lead to other clinical phenotypes, the unusual susceptibility to these viruses is a conserved feature of each of the monogenic causes of NKD reported to date (Figure 2). This is especially important as while all of these monogenic defects impact the individual in some way outside of the NK cell subset, the common infectious susceptibility underlies the particular clinical nature of having a more isolated NK cell abnormality. Conversely it points to an importance of NK cells in isolation in defense against these infections. This was first suggested in humans over 3 decades ago in post-transplant patients who had defective NK cell killing despite intact cytotoxic T cell function in the context of CMV infection94. This unusual susceptibility to these viruses can be defined by multiple severe infections, recurrences of infection for viruses that are typically not recurrent, clinically relevant infection from multiple herpesviruses, infections that are refractory to common treatments, and infection at multiple or unusual sites of the body. In addition to herpesviral infections, human papillomavirus infections are reported, as well as increased frequencies of some generally experienced viral infections. Fungal and bacterial infections have also been described in some patients and although there are experimental evidence for roles of NK cells in defense against these types of organisms it is less well understood. Of those patients that have been reported with NKD, almost half had died prematurely, underscoring a likely substantive reporting bias but also the severity their disease78. Correlation between NK cell function and the control of viral infections can be additionally defined by the study of GATA2 deficient patients, which demonstrates that decreasing frequency of NK cell counts were associated with increasing complications of disease95.
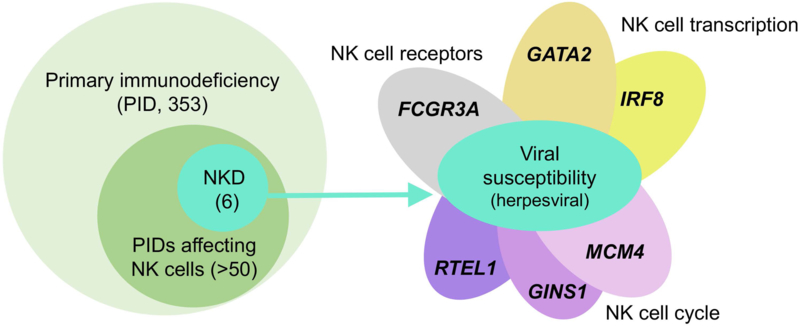
Figure 2. Herpesviral susceptibility is a unifying feature of human NK cell deficiencies.
Within the >350 primary immunodeficiencies registered by the International Union of Immunological Societies, an effect on NK cell function or phenotype has been noted in >50 of these (left). There are currently 6 published monogenic causes of human NK cell deficiency, in which disrupted NK cell development is the sole or predominant feature. While these may have other clinical features, the underlying clinical theme is that of viral susceptibility, particularly severe or refractory herpesviral infections (right). Despite the diverse functions of these genes, this shared susceptibility to viral infection, particularly by herpesviruses, underscores the common theme of NK cell deficiency amongst each of these defects and therefore the critical role that human NK cells play in antiviral immunity.
While NK cells have an inherent ability to provide surveillance for cancerous cells and powerful anti-tumor functions, the susceptibility to malignancy in NKD patients has been less well-defined. This is perhaps in part because of the high morbidity of NKD decreasing the mean lifespan of many of the reported patients. There are, however, susceptibilities to certain malignancies associated with some NKD, specifically those that are driven by oncogenic viruses. EBV-driven cancers have been described in NKD patients, including lymphomas in GATA2 deficiency96, an EBV-smooth muscle tumor in a patient with cNKD79, and EBV-driven Castleman’s disease in two patients with fNKD93,97. HPV-driven dysplasia was detected in 35% of patients with GATA2 deficiency, and HPV-related head and neck cancers have also been observed95. The importance of NK cells in the control of malignancy has also been illustrated by the study of their role in hematopoietic stem cell transplant. These studies show that the presence and functionality of NK cells, specifically donor-derived cells with alloreactivity to the leukemia, is a determinant of patient survival98. This has led to a number of efforts to harness this functionality to improve transplant rates, and there has been an explosion of exciting new NK cell-based therapies that are showing promise in the clinic99. Taken together, the severe clinical phenotype of patients with NKD, combined with clinical outcomes of transplant, reinforces the conclusion that NK cells play at least some non-redundant role in human health.
Functional NKD (fNKD)
To date, the only example of an NKD that strictly affects function (fNKD) but not maturation, development or more broadly phenotype, is a result of homozygous mutations in FCGRIIIA leading to the protein having an L66H substitution, which has been reported in 3 unrelated patients93,97,100. No changes in NK cell maturation or other NK cell phenotypic markers were noted, however all had impaired NK cell cytotoxicity. These patients experienced recurrent HSV and HPV, as well as one with EBV-driven Castleman’s disease. Despite CD16’s role as the IgG Fc receptor, these patients have only impaired natural cytotoxicity, while their ADCC function is intact93,100. This seemingly paradoxical finding is explained by the fact that the variant amino acid is outside of the CD16 IgG Fc-binding domain and is not predicted to change the structure of that region. It does, however, cause variation in the distal Ig domain of CD16 and interrupts the role of CD16 in stabilizing the activating receptor CD2 at the immunological synapse93. Thus, while the ability of this aberrant CD16 to recognize an opsonized target cell is normal, it is unable to be utilized by CD2 when CD2 is engaged at the immunological synapse. This prevents ligated CD2 from accessing CD16 costimulation and inducing CD3ζ signaling downstream of CD16. As a result the signaling for natural cytotoxicity induced by susceptible target cells is reduced, leading to decreased NK cell killing. While CD16 can be expressed by non-NK cells, neutrophils express a form lacking the intracellular signaling domain as it is replaced with a GPI-linkage101. Thus this defect owing to the variation in the CD16 extracellular domain is likely NK cell specific.
Importantly, while these particular FCGRIIIA mutations do not affect NK cell phenotype, the single amino acid change in these patients disrupts the epitope of CD16 recognized by monoclonal antibody B73.1. Thus NK cells from the patients harboring the homozygous CD16 mutation can be detected by flow cytometry through loss of detection of CD16 by the B73.1 clone, provided it can still be recognized by a monoclonal antibody outside of the variant domain such as 3G893. This abnormal ‘dual epitope assay’ can help in screening patients for this abnormality, but does not serve as a replacement for direct gene sequencing.
While it is somewhat surprising that to date there is only one fNKD described, the difficulty in defining fNKD lies not necessarily in their rarity, but in identifying the patients themselves as well as those function-imparing genes that are unique to NK cells. The first challenge arises as from a clinical standpoint, as the main assays used are generally a simple functional test of cytotoxicity and singular NK cell count. In fNKD cytotoxic function will be reduced and the count will be normal. Access to more detailed diagnostics to screen for specific NK cell receptors or other functional proteins are not yet clinically available. As for specificity, while there are a number of PID and other conditions that include impaired effector cell function, the commonality of signaling for secretion between NK cells, T cells and macrophages and dendritic cells generally makes these more broadly affected. These are valuable for defining how NK cells work, and patients may suffer from having aberrations of NK cell function, but they leave questions as to whether there are NK cell-specific components of common functional pathways. Better understanding of the molecular signaling for cytotoxicity and rigorous comparison of this process across multiple immune effector subtypes in concert with ongoing studies of patients with suggestive clinical histories will no doubt lead to the discovery of other uniquely NK cell functional deficiencies.
Classical NKD (cNKD)
The majority of genetic explanations for NKD that have been published in the peer-reviewed literature (5 to date) are classical NKD, which can also be referred to as developmental NKD. These are defined by impaired NK cell development, which can range from a complete absence of NK cells in peripheral blood to a stable, dysregulated subset distribution most frequently reflected by a low overall number of peripheral blood NK cells (Figure 1). As the primary subsets in peripheral blood, CD56bright and CD56dim, also represent stages of NK cell development, the loss of these specific subsets as a result of NKD are informative about the requirements for NK cell development and homeostasis.
GATA2
Perhaps the best-known case of NKD was that of a teenage girl, first described in 1989, who had extreme susceptibility to herpesvirus infections87. Beginning at the age of 13, she had recurrent and severe infections with CMV, VZV (including varicella pneumonia) and HSV. Given the described link between NK cells and control of herpesviral infection102–104, NK cell functional testing was performed and revealed that the patient had essentially absent NK cells, accompanied by severely impaired NK cell function87. T cell numbers and function were mostly normal, underscoring the observation that human NK cells seemed particularly important for the control of herpesviral infections; an observation in part based upon earlier experience with CMV disease post-transplantation where CTL functions were intact but NK cell cytotoxicity was impaired94. The genetic origin of this patient’s disease was subsequently found to be a particular heterozygous GATA2 mutation, and this finding was reported with a cohort of other GATA2-deficient patients who had a range of frequencies of NK cells within peripheral blood105. This included many patients with <1% NK cells within the lymphocyte gate, as well as those who had higher frequencies of NK cells; however, it should be noted that even patients with NK cell frequencies that fell within normal range had reduced NK cell function as well as aberrant NK cell subsets105.
A consistent feature of GATA2-deficient NK cell populations is the decreased relative frequency of the CD56bright subset105–108. The aberration in subset distribution has been identified both in patient peripheral blood and in NK cell subsets that have been differentiated from patient CD34+ hematopoietic stem cells in vitro. This is surprising, given that the CD56bright cells are thought to be precursors of the CD56dim subset. The mechanism of this relationship is still unknown however, and it is unclear how a CD56bright NK cell may undergo differentiation to a CD56dim cell, both in terms of regulation of CD56 itself and also the host of receptors and functions associated with this transition. The role that GATA2 plays in NK cell homeostasis can be explained by the finding that the remaining CD56dim NK cells in GATA2-deficient patients have an adaptive phenotype identified by down-regulation of SYK, EAT2, PLZF1 and/or FcεGRγ47,48, suggesting that these are longer-lived and that conventional NK cells have been lost from circulation due to attrition of stem cell precursors from the bone marrow108. In addition, there may be effects on NK cell trafficking, given the loss of CXCR4 expression on NK cells from GATA2 deficient patients107. These findings highlight compelling biology that remains to be defined about the relationship between stem cell precursors, viral infection and NK cell differentiation and homeostasis. Another puzzling feature of GATA2 deficiency is the clinical heterogeneity found even between individuals with disease, even amongst those having the same genetic lesion95. While some patients have seemingly isolated NKD, others have monocytopenia, B cell deficiency, dendritic cell deficiency, or Emberger’s disease. This suggests that there are either environmental events that shape the nature of disease progression, or other background genetics that contribute. Regardless, 82% of patients experienced major infections, and of these, 70% are herpesviral infections that are commonly one of the earliest clinical features of disease95. There additionally is a correlation between decreasing NK cell numbers in the periphery and increasing complications of disease, and asymptomatic carriers of GATA2 mutations are often CMV seronegative and have higher frequencies of NK cells in peripheral blood95,108. Taken together, these observations suggest that viral infection in the face of impaired NK cell homeostasis and function contributes to NK cell attrition and progression of disease. Better delineating the role that viral infection may play in shaping both the NK and stem cell populations will be important for improved therapeutic interventions, given the impaired survival time of patients following the onset of symptoms95. Further studies are necessary to better understand the natural history of this complex disease and where NKD fits into the spectrum of clinical possibilities that derive from aberrations of this singular transcription factor.
CMG helicase mutations: MCM4 and GINS1
Discovery of the Cell Division Cycle (CDC) 45-Mini Chromosome Maintenance (MCM)-Go-Ichi-Ni-San (GINS) complex (CMG) helicase mutations as a cause of NKD arose out of the study of cohorts of patients with isolated NK cell deficiency83,84,109,110. The first of these was a population of endogamous Irish travelers with unusual susceptibility to EBV, CMV, HSV and VZV infections83,110. In addition, these patients had extra-immune features, including short stature, adrenal insufficiency and microcephaly. While their T cell phenotype and function were relatively unaffected, these patients had stable and significant decreases in NK cell numbers in peripheral blood. In addition, they had stable relative over-representation of the CD56bright subset within peripheral blood as a result of reduced numbers of CD56dim NK cells89,111. Whole exome sequencing identified homozygous mutations in MCM4, a component of the CMG complex that comprises the eukaryotic DNA helicase89,111.
The CMG complex is comprised of CDC45, MCM2–7, MCM10 and GINS1–4, which interact with double- and single- stranded DNA to promote DNA replication. During licensing of origins of replication, MCM2–7 hexamers are loaded onto double-stranded DNA. Initiation of DNA synthesis (‘firing’) requires helicase co-factors CDC45 and GINS, and firing is followed by DNA synthesis and replication elongation throughout the S phase of cell cycle. MCM10 is recruited following formation of the pre-recognition complex, and is thought to play a critical role in assembly and activation of the helicase112. Stalling of replication forks or activation of the checkpoint response leads to induction of DNA damage response (DDR) pathways if the replication fork cannot be restarted. Induction of the DDR as a result of stalled replication forks or single- or double-stranded breaks is associated with increased expression and phosphorylation of ATM, CHK1/2, and p53, and phosphorylation of the histone γH2AX. Given the ubiquitous role that these proteins play in replication and genomic integrity, complete deletion of CMG helicase complex members is not supportive of life; however, partial deletion or loss of function leads to DNA damage marked by the presence of γH2AX foci, and impaired cell cycle progression113,114.
Patient mutations lead to MCM4 deficiency through the introduction of a premature termination codon and 2 new translation initiation codons downstream89,111. While expression of other MCM2–7 complex members is unaffected, progression through cell cycle is impaired as demonstrated by the decreased frequency of patient-derived SV40 transformed fibroblasts in G2/M phase of cell cycle relative to control cells89. This is accompanied by genomic instability and increased chromosome breakage in patient SV40 fibroblasts. In addition to the previously noted decreased frequency of NK cells in peripheral blood and over-representation of the CD56bright subset, NK cell proliferation in response to cytokine stimulation was impaired, and increased apoptosis specifically of the CD56bright subset was observed89. This careful analysis of selective NK cell deficiency in these patients suggested that there was a unique role for helicase function in the generation and/or homeostasis of the CD56dim NK cell subset in particular. Despite the fact that this discovery was made by two groups simultaneously, it was hard to conceive a role for the CMG complex that would be specific to NK cells.
The further study of additional patients with NKD put further emphasis upon the CMG complex mandating further understanding of how NK cells may be specifically intolerant to mutational pressure on the complex. In particular, there was an additional cohort of individuals with susceptibility to CMV infections, accompanied by a similar NK cell phenotype84,109. One of the families described represented one of the earliest reports of inherited NKD84. These patients also had neutropenia, and mild T cell lymphopenia, but otherwise had a phenotype seemingly restricted to NK cells. Like the MCM4 deficient patients, they also had some extra-immune manifestations including short stature and dysmorphic features. Whole exome sequencing identified mutations in GINS1, leading to decreased protein expression of GINS1 and other GINS complex members91. As with MCM4 mutations, biochemical analysis demonstrated impaired initiation of DNA replication in patient fibroblasts, and cells containing damaging GINS1 variants had cell cycle arrest and induction of DNA damage repair pathways measured by γH2AX staining.
In addition to NK cell deficiency, individuals with GINS1 mutations had stable neutropenia. This observation is noteworthy given the previously described link between neutrophils and NK cell also derived from the study of patients with PID115. Patients with severe congenital neutropenia due to mutations in ELANE, or unidentified variants, were shown to have decreased NK cell number and function. Notably, neutropenic patients had reduced numbers of CD56dim NK cells, a phenotype very similar to that of MCM4- and GINS1-deficient patients. Through the study of patients with autoimmune neutropenia, who lack only peripheral neutrophils, this phenotype was shown to be at least partially dependent on events in the periphery115. While the NK cell defect in GINS1 patients is intrinsic, there remains an interesting and poorly understood link between neutropenia and NK cell subsets. In addition, some individuals with GINS1 mutations had decreased frequency of circulating ILC subsets, demonstrating another poorly understood but exciting insight that can be derived from these patients. In aggregate these two CMG-related NKDs underscore a critical role for the complex in aspects of NK cell development, terminal maturation, and/or subset survival. Additional biological investigation as well as hopeful discoveries of new etiologies through ongoing NKD patient studies will give further clarity to the seemingly special relationship between the CMG complex and NK cells.
IRF8
An additional classic case of NK cell deficiency was first published in the Journal of Pediatrics in 1982 and described a family whose children were highly susceptible to EBV infection88. In a family of 4 children, 1 died as a result of severe EBV, a second had life-threatening prolonged illness and died several years later, and a third was hospitalized extensively and has continued ongoing sequelae. The fourth child was unaffected. Whole exome sequencing and analysis identified biallelic variants in the IRF association domain (IAD) of IRF8 that segregated appropriately within the family and were novel and damaging92. Additional families were identified, including a child with homozygous variants in IRF8 and disseminated BCG disease, and a second child with compound heterozygous mutations and recurrent respiratory infections116,117.
Similarly to MCM4 and GINS1 deficiency, the distinguishing feature of NK cell subsets from patients with biallelic IRF8 deficiency is over-representation of the CD56bright subset, with concomitant decreased frequency of the CD56dim subset92,116,117. This particular phenotype is suggestive of an arrest in terminal NK cell maturation, particularly given that the NK cells found in peripheral blood in these patients do not merely have increased surface density of CD56, but are seemingly prototypical CD56bright NK cells when all receptors associated with the subset are taken into consideration92. NK cell cytolytic function in these patients is reduced, however IFNγ production is retained, further supporting the observation that the CD56bright NK cells found in these patients are bona fide and functional. Importantly, family members and unrelated individuals carrying a single IRF8 variant did not have any NK cell abnormalities. This is relevant as a mild dendritic cell abnormality associated with a decrease in CD1c+ subsets has been identified in some patients harboring single IRF8 alleles116. These patients have been reported as having susceptibility to mycobacterial infections116. Interestingly, the patient with biallelic IRF8 deficiency from the original NKD family, or his heterozygous children, did not have overt deficiency of these dendritic cell subsets. This might be attributed to the fact that both of the IRF8 mutations in this family were missense mutations that affect the IRF association domain of the protein, which might be preferentially impactful in NK cell development.
IRF transcription factors are master regulators of the immune response, and IRF8 can mediate co-activation of other transcription factors, including PU.1 and Spi118–122. Despite the previously demonstrated role for PU.1 in NK cell lineage commitment, however, the NK cell phenotype in IRF8-deficient patients is not as a result of impaired PU.1 transactivation92. This suggests that IRF8 has novel, NK cell-specific regulatory functions.
Given the phenotype of impaired terminal maturation identified in biallelic IRF8 deficiency, it seems that IRF8 plays a crucial role specifically in the maturation of CD56dim NK cells from precursors. This had been recapitulated in CD34+ hematopoietic cells from patients with biallelic IRF8 deficiency that had been differentiated in vitro to NK cells as it was difficult to expand terminally matured cells92. Gene expression analyses of NK cells from IRF8-deficient patients gave clues as to mechanism and identified deregulation of transcription factors previously shown to be important for NK cell development, including PRDM1 and E4BP492. Therefore, it seems that the role of IRF8 could be to control a transcriptional program that regulates NK cell terminal maturation. This hypothesis was strengthened by the study of Irf8−/− mice, who were found to have decreased frequency of terminally mature NK cells in peripheral blood and spleen92. Further study of these mice showed that they are particularly susceptible to LCMV infection and malignancy, and that generation of adaptive NK cells in these mice following infection is impaired123. In both murine123 and human92 NK cells, IRF8 is up-regulated in response to cytokine signaling, and in mice this leads to Stat4-mediated binding of Irf8 to the cell cycle regulator Zbtb32. In this way, viral infection or expansion in response to tumor challenge leads to Irf8-mediated NK cell expansion and generation of the NK cell memory pool123.
Taken together, these data suggest that at least part of the mechanism of NK cell deficiency in patients with IRF8 deficiency may be impaired NK cell expansion in response to viral challenge. This would link the phenotype found in these individuals with the very similar NK cell phenotype in patients with CMG complex mutations, which have defined impairment in NK cell proliferation and cell cycle progression. Further study of the relationship between NK cell proliferation, antiviral response and phenotype as informed by patients with NKD will be highly informative in understanding the relationship between these NK cell phenotypes and their manifestations in human health and disease.
RTEL1
Biallelic mutations in regulator of telomerase elongation 1 (RTEL1) lead to Hoyeraal-Hreidersson syndrome, which includes dyskeratosis congenita, bone marrow failure, cerebellar hypoplasia, intrauterine growth retardation, developmental delay and short telomeres124–126. Immunodeficiency is also frequently present, including progressive lymphopenia affecting T, B and NK cell subsets124. However, in at least one case, homozygous RTEL1 mutations led to isolated NKD in a young girl with fatal varicella infection82,90. While NK cell subsets were not measured, total NK cell numbers were significantly decreased and NK cell cytotoxic function was impaired82. Other lymphocyte subsets were unaffected, and no extra-immune features were noted. It is unclear why NK cells were the sole affected population in this case, particularly as the mutation that was identified in the index case is a founder mutation within the Ashkenazi Jewish population90. This may be an additional manifestation of the sensitivity of NK cells to genetic lesions that affect proliferation and DNA replication, as seen in helicase mutations. It may also reflect a tendency, as with GATA2 deficiency, for disorders of bone marrow failure to selectively progress to NKD in advance of broader immune abnormalities and cellular deficiencies.
Primary immune deficiencies with NK cell features
In addition to NKD, in which NK cell aberration is the primary, majority or only clinically relevant immune manifestation, there are other monogenic causes of PID that have an effect on NK cell phenotype or function (Table 2). While less easily defined, there are aspects of these diseases that can be attributed to impaired NK cell function, given what
Table 2:
Primary Immunodeficiencies with an NK cell component
| Disease | Gene | Infectious susceptibility | NK cell Features | Key Reference |
|---|---|---|---|---|
| Cytokine signaling and pathways | ||||
| X-linked SCID | IL2RG | Multiple infections | Absent NK cells | 129 |
| Autosomal recessive SCID | JAK3 | Multiple infections | Absent or low NK cells | 130 |
| Autosomal dominant chronic mucocutaneous candidiasis | STAT1 (GOF) | Multiple infections | Low NK cell number, aberrant subsets, cytotoxicity | 131 |
| Hyper IgE syndrome | STAT3 | Multiple infections | Decreased NKG2D expression, cytotoxicity | 132 |
| STAT5B deficiency | STAT5B | Multiple infections | Low NK cell number, aberrant subsets, cytotoxicity | 133 |
| IL-12/IL-12 receptor deficiency | IL12RB1, IL12B | Mycobacteria, salmonella | Cytokine production, cytotoxicity | 134 |
| IL-21 receptor deficiency | IL21R | Multiple infections | Cytotoxicity | 135 |
| CD25 deficiency | IL2RA | CMV | Low NK cell number | 136 |
| Lytic machinery | ||||
| Familial hemophagocytic lymphohistiocytosis 2 | PRF1 | Herpesviruses | Cytotoxicity | 137 |
| Familial hemophagocytic lymphohistiocytosis 3 | UNC13D | Herpesviruses | Cytotoxicity | 138 |
| Familial hemophagocytic lymphohistiocytosis 4 | STX11 | Herpesviruses | Cytotoxicity | 139 |
| Familial hemophagocytic lymphohistiocytosis 5 | STXBP2 | Herpesviruses | Cytotoxicity | 140 |
| Hermansky-Pudliak Syndrome type 2 | AP3BP1 | Herpesviruses, bacteria | Cytotoxicity | 141 |
| Hermansky-Pudliak Syndrome type 9 | BLOC1S6 | Herpesviruses, bacteria | Cytotoxicity | 142 |
| Papillon-Lefevre Syndrome | CTSC | Herpesviruses, bacteria | Cytotoxicity | 143 |
| Chediak-Higashi syndrome | LYST | EBV, fungi | Cytotoxicity | 144 |
| Griscelli syndrome type 2 | RAB27A | Herpesviruses, bacteria | Cytotoxicity | 145 |
| DNA damage response or transcription/translation | ||||
| POLE2 deficiency | POLE2 | BCG, respiratory infections | Low NK cell number, decreased CD56dim subset | 146 |
| Immunodeficiency-centromeric instability-facial anomalies syndrome 2 | ZBTB24 | Upper respiratory infections, bacteria | Progressive decrease in NK cell numbers, cytotoxicity | 147 |
| Transcriptional control | ||||
| Autosomal recessive SCID | IKZF1 (biallelic) | Multiple infections | Absent NK cells | 148 |
| Combined variable immune deficiency | IKZF1 (heterozygous) | Low NK cell number | 149 | |
| Rett Syndrome-like MCP2 duplication | MECP2 duplication (T-bet function) | Fungi, pneumonia | Low NK cell number | 150 |
| NFAT5 deficiency | NFAT5 | Multiple infections | Low NK cell number | 151 |
| Metabolism or bone marrow environment | ||||
| Autosomal recessive SCID | ADA | Multiple infections | Absent or low NK cells | 129 |
| Fanconi’s anemia | FANCA-G | Multiple infections | Low NK cells | 152 |
| Dyskeratosis congenita | DKC1 | Multiple infections | Low NK cells | 153 |
| AK2 deficiency | AK2 | Multiple infections | Absent NK cells | 154 |
| Autosomal recessive SCID | MTHFD1 | Multiple infections | Low NK cells | 155 |
| Bloom Syndrome | BLM | Fungi, bacteria | Cytotoxicity | 156 |
| Other (including non-NK intrinsic) | ||||
| Comel-Netherton Syndrome | SPINK5 | Cutaneous infections | Cytotoxicity | 157 |
| Bare lymphocyte syndrome | TAP1 | Multiple infections | Unlicensed NK cells, cytotoxicity | 158 |
| Bare lymphocyte syndrome | TAP2 | Multiple infections | Unlicensed NK cells, cytotoxicity | 159 |
| β2 microglobulin deficiency | B2M | Respiratory tract infections | Unlicensed NK cells, cytotoxicity | 160 |
| Severe congenital neutropenia | ELANE | Bacteria | Impaired terminal maturation, cytotoxicity | 115 |
| X-linked hyper IgM | CD40LG | Enteroviruses, bacteria, pneumocystis | Cytotoxicity | 161 |
| Lipid signaling pathways | ||||
| Activated PI3Kδ syndrome type 1 | PIK3CD (GOF) | EBV, CMV | Cytotoxicity, aberrant NK cell subsets | 162 |
| Activated PI3Kδ syndrome type 2 | PIK3R1 (GOF) | Multiple infections | Low NK cell numbers, cytotoxicity, cytokine secretion | 163 |
| PIK3R1 deficiency | PIK3R1 | Multiple infections | Low NK cell numbers | 164 |
| PIK3CD deficiency |
PIK3CD (LOF) |
Sinopulmonary infections | Aberrant subsets | 165 |
| Cowden’s syndrome | PTEN | Multiple infections | Low NK cell number | 166 |
| PLCG2 associated antibody deficiency and immune dysregulation | PLCG2 | Respiratory infections | Cytotoxicity | 167 |
| PRKCD deficiency | PRKCD | Recurrent infections, lymphoproliferation | Low NK cell numbers, cytotoxicity | 168 |
| Cytoskeleton and actin regulatory proteins | ||||
| Wiskott-Aldrich Syndrome | WAS | Herpesviruses, multiple infections | Cytotoxicity | 169 |
| Coronin 1a deficiency | CORO1A | Multiple infections | Cytotoxicity | 170 |
| DOCK8 immunodeficiency syndrome | DOCK8 | HPV, multiple infections | Cytotoxicity | 171 |
| MYH9 related disorder, May-Heggelin Anomaly | MYH9 | Intracellular bacteria | Cytotoxicity | 172 |
| WIPF1 deficiency | WIPF1 | Herpesviruses, multiple infections | Cytotoxicity | 173 |
| DOCK2 deficiency | DOCK2 | Multiple infections | Cytotoxicity | 174 |
| RASGRP1 deficiency | RASGRP1 | Herpesvirus, multiple infections | Cytotoxicity | 175 |
| Ion channel signaling | ||||
| X-linked immunodeficiency with magnesium defect, EBV infection, and neoplasia | MAGT1 | EBV, multiple infections | Aberrant subsets, cytotoxicity | 176 |
| ORAI1 deficiency | ORAI1 | Multiple infections | Cytotoxicity | 177 |
| STIM1 deficiency | STIM1 | Multiple infections | Cytotoxicity | 178 |
| Integrins and integrin signaling | ||||
| Leukocyte adhesion deficiency-I | ITGB2 | Multiple infections | Cytotoxicity | 72 |
| Leukocyte adhesion deficiency-III | FERMT3 | Multiple infections | Cytotoxicity | 179 |
| Other signaling for function | ||||
| X-linked lymphoproliferative syndrome type 1 | SH2D1A | EBV | Cytotoxicity | 180 |
| X-linked lymphoproliferative syndrome type 2 | XIAP | EBV | Low NK cell numbers, cytotoxicity | 181 |
| Non-X linked lymphoproliferative syndrome | ITK | EBV | Low NK cell numbers, cytotoxicity | 182 |
| NEMO deficiency | IKBKG | Mycobacteria, CMV, bacteria | Cytotoxicity | 183 |
| Autoimmune lymphoproliferative syndrome | CASP8 | Herpesviruses, bacteria | Cytotoxicity | 184 |
| NFKB2 deficiency | NFKB2 | Upper respiratory infections | Cytotoxicity | 185 |
| NIK deficiency | MAP3K14 | Multiple infections | Low NK cell numbers, cytotoxicity | 186 |
| Partial DiGeorge Syndrome | CRKL | Multiple infections | Cytotoxicity | 187 |
| IKK2 deficiency | IKBKB | Candida, CMV | Low NK cell numbers, cytotoxicity | 188 |
| CTLA4 deficiency | CTLA4 | EBV, CMV | Cytotoxicity, cytokine production | 189 |
| Syndromic diarrhea/tricho-hepatoenteric syndrome | TTC37, SKIV2L | EBV | Low NK cell number, aberrant subsets, cytotoxicity, cytokine production | 190 |
| NFKB1 deficiency | NFKB1 | Multiple infections | Aberrant subsets, cytotoxicity, cytokine production | 191 |
| RLTPR deficiency | CARMIL2/RLTPR | HPV | Cytokine production | 192 |
VZV, varicella zoster virus; HSV, herpes simplex virus; CMV, cytomegalovirus; HPV, human papillomavirus; EBV, Epstein-Barr virus; BCG, bacille Calmette-Guerin
we know about the function of NK cells in host defense based upon NKD and experimental studies. There are more than 350 PIDs now recognized, and of these more than 50 include an effect on NK cell phenotype and function. Some of these, such as hemophagocytic lymphohistiocytosis (HLH), are quite well defined and understood, while others are still emerging and yielding important insight into the regulation of human NK function. These include mutations in the STAT and PI3K signaling pathways, as well as many cytoskeletal regulators. While full consideration of all of these is beyond the scope of this review, it is worthwhile to consider a few more recent advances beyond what has been already reviewed elsewhere78,127,128 to help understand what can be learned about NK cells from PID with NK cell features.
Familial hemophagocytic lymphohistiocytosis
One of the most easily understood causes of impaired NK cell function can be found in cases of HLH due to mutations in lytic granule components or the secretory machinery. These affect other lytic and secretory cells as well, namely CD8+ T cells and macrophage/DCs as well as certain biologically analogous non-immune cells, such as melanocytes which are responsible for pigmentation. As a result, while NK cell killing is impaired, this is generally overshadowed clinically by massive cytokine over-production due to impaired control of the immune response resulting from an inability of cytotoxic cells to eliminate sources of infection, as well as albinism for certain types of mutations. These particular lesions still drive a greater understanding of NK cell function through the mechanistic study of the requirements for lytic granule secretion and function, and despite decades of study, new insights are still being derived. In particular, several new publications have been focused on the biology of LYST, which when aberrant causes Chediak-Higashi syndrome. These have demonstrated differences in the severity of disease associated with mutations that affect different domains of LYST144. While lytic granules from patients with Chediak-Higashi syndrome have previously been shown to be unusually large193,194, this was recently advanced further for granules in NK cells from patients with LYST mutations144,195. New studies have demonstrated that the size of lytic granules can be a physical barrier to their directed secretion, as they are unable to transverse the cortical actin network located below the NK cell synaptic embrane195. This network has been shown to regulate granule exocytosis196 and the fact that large granules cannot find egress is further evidence to support the actin meshwork as a checkpoint that lytic granules must navigate and pass. These studies complement those that show that mutations in the actin regulating protein Coronin 1A that cause primary immune deficiency lead to impaired NK cell function due to increased density of actin at the NK cell lytic synapse (discussed further below)170.
A second emerging theme in the cell biology of HLH is the recent description of patients with disease-causing RAB27A mutations who have normal skin and hair pigmentation197–199. To date, there have been three cohorts of patients described in which this is the case. In the first two, novel mutations lead to selective disruption of the interaction between RAB27A and Munc13–4, but not melanophilin. This leads to impaired immune cell secretion, but normal melanocyte function197,198. In the third cohort of 5 patients from 5 families, whole genome sequencing identified distinct transcriptional start sites differentially utilized by lymphocytes and melanocytes199. Mutations in the start site used primarily by lymphocytes leads to HLH, however melanocyte function is retained through the utilization of the alternate transcriptional start site. Both of these led to complex and difficult to diagnose cases, particularly the second in which whole exome sequencing failed to uncover the cause of disease and the tell-tale hypopigmentation associated with Griscelli’s Syndrome Type 2 was not present.
Actin regulators
Wiskott-Aldrich syndrome (WAS) was amongst the earliest primary immune deficiencies to be described, and is classically described as a triad of eczema, thrombocytopenia and infections200,201. Early cell biological studies identified the role for WASp in cytoskeletal regulation however, and these led the way for the description of the requirement for actin remodeling in NK cell immune synapse formation and function169. Impaired NK cell function in WAS patients leads to unusual susceptibility to herpesviral infections, with clinically relevant infections in approximately one third of WAS patients202. The functional defect in actin remodeling at the NK cell synapse can be corrected by addition of IL-2, which activates alternative actin remodeling pathways through WAVE2203,204. Further mechanistic studies have identified the importance of actin nucleation and remodeling throughout immune synapse formation and function, including mediating adhesion to target cells, sustaining signaling through receptor dynamics, and positioning and extruding lytic granules.
Given this central importance of actin remodeling, it is not surprising that as new PID are identified that affect actin regulatory proteins, many of these will likely have a component of NK cell dysfunction as well. WIPF1 and DOCK8 mutations lead to PID having also associated with an NK cell abnormality171,173. As with WASp, each of these are required for the generation of branched actin networks, and mutations in these proteins impair the remodeling of actin at the immunological synapse. This prevents multiple critical steps of cytotoxic function, including receptor clustering leading to the amplification of signal that is required for MTOC and granule polarization. In addition, given the requirement for actin remodeling in cell migration, many of the accompanying symptoms in these patients can be attributed to defects in lymphocyte migration and trafficking. Interestingly, while defects in WASp lead to an overall reduction in F-actin content in NK cells169,204, DOCK8 aberrations do not and instead only prevent actin accumulation and synaptic reorganization171. This is likely due to DOCK8 serving not as an actin nucleator but in a regulator of WASp activation and the induction of its function at the lytic synapse. DOCK2 mutations similarly lead to impaired NK cell degranulation, IFNγ production and activation-induced actin polymerization, with impaired activation of key signaling molecules likely reflecting a requirement for actin polymerization in receptor clustering and signal transduction174.
Additional novel insight into signaling for NK cell cytotoxicity was revealed by the study of a single patient with biallelic RASGRP1 mutations175. RASGRP1 is a key regulator of RAS signaling, and the patient had profound T and B cell abnormalities that reflect a requirement for RAS signaling in T cell development, survival and proliferation. In addition, NK cell lytic function in patient cells was impaired despite normal NK cell numbers and expression of perforin and granzymes. Formation of the NK cell lytic synapse was marked by impaired actin accumulation, consistent with the finding that activation signaling, including Erk1/2 phosphorylation, was decreased in RASGRP1-deficient T cells. An unexpected finding was the observation of impaired lytic granule convergence to the MTOC, which was subsequently explained by the identification of a previously unknown interaction between RASGRP1 and the minus-ended motor dynein, which mediates trafficking of lytic granules along microtubules to the MTOC73. Together, these findings demonstrate a previously unknown role for RASGRP1 in orchestrating both lytic granule and cytoskeletal dynamics at the NK cell lytic synapse.
Coronin 1A (also known as p57, clabp and TACO), is a WD-repeat domain containing protein that binds to both Arp2 and cofilin, as well as actin directly, and promotes actin turnover. Patients with biallelic mutations in Coronin 1A have a combined immune deficiency, with marked T cell lymphopenia despite the presence of a thymus, variable B cell lymphopenia, and an impaired T cell proliferative response to mitogens205–207. NK cell numbers are sometimes reduced, however NK cells are present with a seemingly normal phenotype. Patients frequently have unusually severe EBV infections and EBV-driven lymphomas, which can likely be attributed to the demonstrated functional impairment of NK cells in peripheral blood170. In contrast with WASp, Arp2 and other actin nucleation promoting factors, however, immunological synapse formation occurs in NK cells from patients with Coronin 1A mutations, and MTOC and granule polarization to the synapse are unaffected170. Instead, decreased NK cell function can be attributed to impaired translocation of lytic granules across the cortical actin network to enable membrane fusion and exocytosis. Reduced actin remodeling in the absence of Coronin 1A function leads to an actin barrier that physically impedes the transit of granules through minimally sized clearances in the actin network. As opposed to mutations that lead to gross actin defects, these changes are only detectable on the nanoscale. The increased optical resolution afforded by super-resolution microscopy will likely lead to the identification of similar defects that result in fine changes in molecular tuning that translate to significant biological effects208.
Recent developments in combined immune deficiencies having phenotypic and functional NK cell impairments
While the majority of PID that are associated with an NK cell abnormality include both functional and phenotypic aberrations a number of relevant developments have been reported since recent reviews on NK cells in PID have been published78,127,128. Several of these are reviewed here in order to be able to contextualize the derivative biology in light of advances in the NK cell field. Both STAT deficiencies and mutations in the PI3K signaling pathway lead to combined immune deficiencies, with multi-lineage impairments reflective of the conserved requirements for these pathways in immune cell function. In each case there is also an NK cell component that contributes to clinical phenotype in many patients. While an in-depth review of these diseases is beyond our current scope, it is again worth considering how these contribute to an NK cell phenotype that is abnormal in both phenotype and function.
Gain-of-function mutations in the PI3K signaling pathway
The PI3K signaling pathway is a ubiquitous and important pathway in lymphocyte function, and the recent discovery of patients with gain-of-function, and far less frequently, loss-of-function mutations in this pathway has led to increased understanding of the respective contribution of PI3K signaling to NK cell development, homeostasis and function (reviewed in128). Gain-of-function mutations in PIK3CD lead to p110 delta activating mutation causing senescent T cells, lymphadenopathy, and immunodeficiency (PASLI) disease, also referred to as activated PI3K delta syndrome type 1 (APDS1), whereas those in PIK3R1 lead to PASLI-R1, or APDS2209–212. In each case, mutations lead to hyperactivation through the S6, mTOR and AKT pathways through constitutive activation of the PI3K110δ signaling complex at the cell membrane.
Patients with PASLI disease have increased frequencies of senescent T cells in the periphery, as well as increased transitional B cells, and unusual susceptibility to EBV and CMV infections are frequently reported209–211. NK cell numbers are low, with a relative increase in the frequency of the CD56bright subset; accordingly, overall expression of CD16 is low in the total NK cell population, as is expression of perforin162. In addition to these phenotypic features, NK cell function is impaired due to defective immune synapse formation and polarization of the NK cell lytic machinery towards target cells.
Treatment of patients with rapamycin or new small molecule inhibitors specifically targeting PI3K110δ leads to clinical improvement, and in the case of rapamycin this is accompanied by partial restoration of NK cell function but no detectable change in NK cell phenotype162,213. It is interesting to speculate as to the source of cytolytic dysfunction in the NK cells in these patients, particularly in the context of their phenotype. While some downstream effectors of the PI3K pathway are hyperactivated in patient cells, such as AKT, the phosphorylation of ERK in NK cells is significantly decreased when compared to healthy donors162. This suggests hyporesponsiveness of NK cells, which is also reflected by their impaired function. The modulation of this responsiveness by the administration of rapamycin suggests that it originates with the hyperactive mTOR signal. Given the known tunability of NK cell function in response to signaling (for example in the case of licensing), and the potential for damage if NK cell function itself were to become hyperactivated, it is not surprising that the responsive to excess receptor-mediated activation is to shut down the cytotoxic response.
The NK cell phenotype in these patients is less easily explained. While in some senses the phenotype seems to indicate that the cells are immature, the increase in CD56bright cells is distinct from that seen in patients with MCM4, GINS1 or IRF8 mutations. The selective loss of some receptors associated with the CD56dim subset, even when combined with high expression of CD56, is less indicative of a block in terminal maturation and more suggestive of deregulation of specific receptors in response to dysregulated activation signaling. This is an important point to remember when considering primary immunodeficiencies that appear to interfere with NK cell development, as it is relevant to determine whether the defect truly impacts development or whether there is instead a deregulation of the expression of receptors associated with maturation.
JAK/STAT signaling pathway
STAT molecules have a long-appreciated central role in NK cell development and function, which includes the role of STAT5 as the critical signaling intermediate downstream of IL-15 receptor mediated signaling214. The requirement for IL-15 signaling in human NK cell development is defined by patients with mutations in the common gamma chain (IL2Rγ) that interrupt signaling downstream of the IL-15 receptor and lead to T−B−NK− SCID129,215. Underscoring the importance of IL-15 signaling specifically, are patients with particular mutations in IL2Rγ that predominantly affect IL-15 signaling as opposed to those that affect both IL-2- and IL-15-mediated signaling. In the first case, NK cells are absent, however if IL-15-mediated signaling is retained, NK cells are present despite impaired IL-2-mediated signaling216.
Perhaps unsurprisingly, patients with STAT5B mutations have profoundly impaired NK cell development, with few NK cells in the periphery and accompanying poor NK cell function133. NK cell phenotype and function are also affected in patients with gain-of-function mutations in STAT1, or loss-of-function mutations in STAT3131,132. In the case of STAT3 mutations, which lead to hyper-IgE syndrome, poor NK cell function can be attributed to decreased expression of the activating receptor NKG2D on peripheral blood NK cells132. In addition, deregulated STAT signaling may impair NK cell function or homeostasis through downstream signaling, as is the case in NK cells from patients with STAT1-GOF mutations131. NK cells from these patients are poorly functional, and additionally have altered phenotype and homeostasis. Similarly to those seen in PASLI disease patients, these cells seem to be immature, with an increase in the CD56bright subset and decreased expression of CD16, perforin and KIR131. However, again it is unclear whether this phenotype represents a true halt in terminal maturation, or whether this is more reflective of impaired homeostasis or regulation of specific receptors. The complexity of STAT signaling makes it difficult to isolate non-redundant roles for individual STATs in NK cell development, however the identification of rare patients with STAT mutations provides an invaluable opportunity to better understand this critical signaling axis and its role specifically in NK cell maturation and function.
Finally, JAK1 and JAK3 are important adaptors that mediate STAT signaling, and treatment of STAT1-GOF patients with the JAK inhibitor ruxolitinib improves NK cell function and phenotype131. Patients with JAK3 mutations generally present with T−B+NK− SCID, demonstrating that JAK3 function is non-redundant for IL-15 mediated signaling supporting NK cell development130. A single patient identified with biallelic JAK1 mutations did not have significant alterations in NK cell numbers, however NK cell subsets and function were not specifically tested217.
Other informative PIDs affecting NK cells
There are a number of other combined immune deficiencies with more recently reported effects on NK cell phenotype and function. These include molecules that promote lymphocyte effector function in both T cells and NK cells, such as SAP, mutations in which cause XLP and HLH. In patients with XMEN syndrome, caused by mutations in MAGT1, NK cells are poorly functional in part due to decreased expression of NKG2D176,218. There are others that are less well understood that are not NK cell intrinsic but affect the cellular microenvironment. This includes the molecular basis for NK cell deficiency in Comel-Netherton syndrome, in which SPINK5 mutations lead to loss of LEKTI expression in epithelial cells and accompanying NK cell dysfunction157. There are also genes that control cellular metabolism that affect NK cell development at early stages, such as MTHFD1, ADA and AK2 mutations129,154,155. These likely affect lineage commitment in bone marrow or peripheral tissue, however the mechanism by which they exert function is poorly understood. Similarly, homozygous mutations in IKZF1, which encodes the master transcriptional regulator Ikaros, leads to profound multi-lineage cytopenias that in some patients includes reduced NK cell numbers148; heterozygous IKZF1 mutations also lead to reduced frequency of circulating NK cells in some patients149. Even though an abnormality of NK cells in these conditions represents a minor immunological aberration, the study of NK cells in these conditions can yield often unexpected biological insights into NK cell biology.
Emerging themes in NK cell deficiency
Helicase mutations, proliferation and cell cycle
Given the rarity of NKD, there is an increasingly strong signal around the theme of genes associated with cell cycle and proliferation in NKD. Mutations in MCM4 and GINS1 all lead to a strikingly similar NK cell phenotype with seemingly little involvement of other lymphocyte lineages89,91,111. While less well described, mutations in other DNA replication and repair enzymes also lead to NK cell aberrations, including RTEL1, ZBTB24, and POLE282,90,146,219.
The obvious question, given the clear signal around this pathway in human NK cells and the ubiquitous requirement for these proteins, is why would these mutations in the helicase complex selectively affect NK cell maturation? While this is still not well understood, there are several potential explanations. The first consideration is that each mutation leads to a fairly narrow window of insufficiency, as mouse models demonstrate that complete deletion of CMG complex members are embryonic lethal113,114. Therefore, the mutations in question have the effect of impairing function without completely abrogating it. It is possible that other, more damaging mutations may have more severe effects but have not been identified because they lead to more systemic phenotypes. Similarly, if T cell proliferation was seriously affected, one could predict a SCID-like phenotype, which may also be less supportive of life. The rare incidence of these patients, combined with an insufficient phenotype that is permissive of early life yet leads to manifestation of disease in later childhood or adolescence, may enable their detection.
The question still remains of why, even in this case, NK cells are primarily or solely affected. One hypothesis is that there is an NK-specific transcriptional program that is promoted by the CMG complex, DNA damage repair, or cell cycle. MCM5 binds directly to the STAT1b promoter to promote transcription of interferon response genes220, and it is conceivable that a similar mechanism may be required for NK cell maturation. In both T and B cells, there is a link between cell cycle and gene transcription, suggesting that impaired cell cycle may directly impact transcriptional programs required for NK cell terminal maturation221,222. A more straightforward explanation, however, is that other cell types have better protection from CMG complex insufficiency. Accessibility to origins of replication is a critical requirement for CMG complex function, and as such complex members are present far in excess of what is required to minimally enable replication223. Given the clonal expansion that is a key feature of T and B cell lymphocyte function, it is possible that these cells have a baseline higher expression of CMG complex components to accommodate these needs, or have developed better failsafe mechanisms to protect this critical aspect of their activation and homeostasis.
In addition to CMG complex mutations, there are other signs that pressure on this signaling pathway may lead to impaired NK cell maturation and function in the context of other combined immune deficiencies. This includes Hoyeraal-Hreidarsson syndrome caused by mutations in RTEL1, which in at least in one patient has led to cNKD82,90. In addition, there are reports of other proteins associated with cell cycle, DNA damage repair or homologous recombination leading to NK cell phenotypes. Mutations in ZBTB24, which cause immunodeficiency-centromeric instability-facial anomalies syndrome, also can cause progressive loss of NK cell subsets and function in peripheral blood219. More acutely, mutations in FANCA-G and DKC1 lead to immune deficiency that can include low frequencies of NK cells, although this is likely due to impaired generation of precursors in bone marrow152,153. Finally, as discussed above, there may be a component of the NKD found in IRF8-deficient patients that is a feature of impaired NK cell expansion, a phenotype that could help explain the striking phenotypic similarities between the NK cells in these patients and those with CMG helicase mutations.
This leads to the question of the significance of over-representation of CD56bright NK cells in peripheral blood. The simplest explanation may be that there is a block in terminal maturation, suggesting that the transition from CD56bright to CD56dim is not occurring, and that instead the CD56bright NK cells are exiting, presumably from tissue to peripheral blood, without undergoing terminal maturation. An alternative explanation is that there is selective survival of the CD56bright subset, and that CD56dim NK cells are undergoing increased rates of apoptosis. This could reflect a requirement for a ‘proliferative burst’ that accompanies the terminal maturation step, and modeling of turnover rates in peripheral blood supports this hypothesis224.
A similar NK cell phenotype in peripheral blood, in the absence of impaired function, is seen in patients with SCID due to hypomorphic RAG mutations, or mutations in DCLRE1C (Artemis)225. Specifically, in these patients approximately 40% of their circulating NK cells were the CD56bright phenotype225. While lytic function against tumor targets has not directly been tested, the NK cells from these patients have increased degranulation in response to co-culture with K562 tumor targets225. This phenotype is supported by studies in mice that demonstrate that up to 30% of mature NK cells in the periphery have expressed RAG at some point, and that RAG expression is associated with longer-lived NK cells with a mature phenotype226. Murine RAG-deficient NK cells, while highly functional, have increased rates of apoptosis and fail to expand or persist following viral infection. This is accompanied by increased rates of DNA double-stranded breaks and increased phosphorylation of γH2AX, thereby linking RAG-mediated DNA damage repair directly to this phenotype. Together, these data suggest that expression of RAG is associated with increased cellular fitness that is required to sustain mature or developing NK cells through periods of proliferation or cellular stress. Additional evidence can be gleaned from linkage analysis studies that identified p53 as a master regulator of NK cell terminal maturation in mice227. This study sheds light on the puzzling observation that NK cells from non-obese diabetic (NOD) mice were poorly functional and demonstrated that this decreased functionality was associated with decreased frequencies of terminally mature NK cells. While the mechanism of this impairment was not fully defined, this provides another compelling link between cell cycle, proliferation and NK cell terminal maturation.
Together, these data suggest that, in both mice and humans, there is a critical link between NK cell development, proliferation, and functional maturation. Given the requirement for IRF8-driven NK cell expansion, mediated by the cell cycle regulator Zbtb32, in the generation of adaptive NK cells in mice123 it is plausible to think that this expansion is the feature that is ‘broken’ in NK cells from patients with CMG complex or biallelic IRF8 mutations. It remains to be seen how this so dramatically affects the function of remaining NK cells in these patients, however, particularly given the presumed retained function of the NK cells in patients with hypomorphic RAG mutations225.
Finally, there is evidence to suggest that the loss of NK cells in these patients may be a result of reduced stem cell fitness and functionality. While T and B cell precursor populations are largely set early in life, NK cells and other innate cells require continuous regeneration from bone marrow precursors. Repeated infection and chronic interferon signaling can drive premature terminal differentiation and subsequent depletion of the hematopoietic stem cell pool228,229. In addition, aging has a measurable effect on the generation of mature NK cells and their subsets, an observation that is due at least in part to changes in environmental and infectious exposures, but also likely reflects changes in the stem cell population230. A reduction in NK cell production and proliferation in elderly adults is interesting in the context of reduced MCM complex expression in aging stem cells and accompanying replication stress231–233. In healthy adults, these changes are likely balanced by an increase in the population of longer-lived adaptive cells and increased diversity of NK cell receptors, in part due to exposure to viral antigens. However, it is likely that progressive changes in the HSC pool due to infection and inflammation may drive increasing NK cell deficiency in the periphery in patients with NKD. This could account for the delayed onset, in some cases well into adulthood, of NKD that could affect the stem cell subset, particularly GATA2 deficiency. It is also important to consider the natural history of exposure to herpesviral infections as a driver of NK cell subsets and activation/maturation234, particularly as many of these happen in adolescence and likely account for the timing of appearance of symptoms, particularly in patients with IRF8 and CMG helicase mutations83,84,88,89,91,92,109,111.
Given the emerging importance of NK cells as tools for immune therapy99, and the demonstrated role that they play in the setting of transplant235, it will be critical to better understand the relationship between proliferation, transcription, cellular fitness, and NK cell maturation and function. The advantage of doing so will hopefully be the ability to make improvements to these cells as therapeutic vehicles.
Adaptive NK cells and NKD
The identification of a persistent adaptive NK cell population in GATA2-deficient patients108 highlights the distinct longevity of these cells and suggests that there may be other cases whether the generation of adaptive cells is either impaired, or where the progressive loss of non-adaptive cells leads to their selective survival. Identifying an NKD with a specific deficit in adaptive or memory-like NK cell generation would be informative in understanding their role in control of viral infection and malignancy. In particular, other immune deficiencies with bone marrow failure and progressive loss of NK cell subsets, such as RTEL1 and Zbtb24 deficiencies, would be of interest. Given the recently described link between STAT4-mediated induction of IRF8 leading to Zbtb32-mediated cell proliferation and the generation of adaptive NK cells in mice123, it would also be of interest to more closely examine the presence of adaptive NK cells in patients with helicase complex mutations. As clonal expansion is a prerequisite for generation of an adaptive immune response, it will be important to determine whether impaired proliferation has an impact on the generation of long-lived memory-like NK cells in these patients. It would also be invaluable to be able to probe the function and inherent value of these populations in the human system via expanded specific study of these disorders.
What can NKD and ILCD tell us about the requirements for NK and ILC development?
The decreased frequency of circulating ILC subsets in some patients with GINS1 deficiency was exciting as it was the first report of a common cause of NK and ILC deficiency91. Given that non-NK cell ILC subsets are predominantly found in tissue, the question of the relevance of circulating ILCs in peripheral blood had dogged the field for some time. However, the discovery of ILC precursors and mature subsets in circulation has made the measurement of ILCs in peripheral blood more common and informative68. It will be important, as tools for ILC detection and classification continue to be developed, that we define normal ranges of ILC subsets and better understand how these are affected by age, infection, transplant and immunodeficiency. This will include, when possible, the cataloguing of these cells in tissues and the comprehensive study of their phenotype and ontology, an undertaking that has already been significantly advanced by single cell analyses236.
The relationship between NK cells and ILCs is plastic and the study of this relationship is a rapidly evolving field. The identity of common precursors, particularly in humans, has been difficult to definitively define and study. An NK- and ILC- restricted precursor, the common innate lymphoid precursor (CILP), has been identified in tissue and can be identified by expression of RORγT237. In addition, it is widely cited that ILC3 subset generation arises from a CD34+ RORγT+ precursor, and ILC3 are defined by RORγT expression3,238. However, the presence of conventional NK cells in patients with immune deficiency as a result of mutations in the RORC gene encoding RORγ and RORγT, suggests that RORC is not required for conventional human NK cell development68,239. In addition, in two patients examined with biallelic mutations in RORC, ILC precursors were present in peripheral blood and gave rise to functional conventional NK cells, ILC1, and ILC2 subsets, as well as IL-13-, but not IL-17-producing, ILC3s68. Given that RORC deficiency affects all IL-17 producing subsets, including Th17 cells, it is not an isolated ILCD. The pan-cytopenic impairment in IL-17 production accounts for the overwhelming susceptibility to candidiasis in these patients; in addition, decreased IFNγ production by αβ and γδ T cell subsets leads to increased susceptibility to Mycobacterium infection239.
While transcription factors such as GATA3, EOMES and RORγT are used to define ILC subsets, ILCs are highly reliant on cytokine signaling for their development and homeostasis, and their primary function is cytokine production. As such, their phenotype and function can be changed in response to inflammatory conditions, and they are thought to play a role in the pathology of IBD, psoriasis and rheumatoid diseases. Within the context of primary immunodeficiency, the emerging understanding of diseases with interferon gene signatures includes an awareness of the effect that this has on ILC subsets. In patients with CVID, the expansion of an IFNγ+ circulating ILC subset is found in patients with non-infectious complications, which frequently include autoimmune cytopenias, lymphoid hyperplasia and granulomatous disease240. Therefore, particularly as new diseases emerge with interferon signatures and existing conditions are better delineated, it will be important to place the ILC subsets in context of both immunity and pathology in these diseases.
In contrast to RORC-deficient patients, the reduced frequency of both conventional NK cells and ILCs, as well as MAIT and NKT cells, in some, but not all, GINS1-deficient patients suggests a common requirement for GINS1 in their development91. Should the requirement for CMG helicase components reflect a need for a proliferative burst of development by NK precursors, this suggests that generation or maturation of ILCs may require a similar stage. Alternatively, this common loss of subsets may indicate depletion or restriction of the stem cell pool. It is important to note that these patients developed lymph nodes, suggesting function of the lymphoid tissue inducer (LTi) ILC subset is intact. The neutrophil defect in GINS1 patients was found to be primarily manifested in the periphery, as opposed to at the bone marrow precursor level, suggesting that the overall defect may be a result of dysregulated balance between apoptosis and proliferation in the periphery91. Mouse models of the effect of reduced helicase or DNA damage pathway function, however, reveal skewing of multipotent progenitors at the earliest stages, suggesting there may be effects on lineage potential at the earliest stages of hematopoiesis241. In addition, it is likely that these may become more damaging as the effects of aging and, potentially, inflammation change the function and kinetics of the stem cell population232,233. It will be of value to determine the changes in gene expression that accompany NK and ILC precursors in patients with helicase mutations throughout development in comparison to healthy donors. Ultimately, a better understanding of the relationship between the requirements for stem cell fitness and function and NK cell generation, homeostasis and function will be important in defining the mechanism of disease in these patients and the role that all innate lymphoid cells play in maintaining human health. This includes a better understanding of tissue resident NK cell and ILC subsets, and their origins and kinetics.
Conclusion
Our understanding of NK cell biology has undergone rapid expansion in recent years. The discovery of other ILC subsets, advances in our understanding of NK cell memory and the generation of adaptive NK cells, and advanced technologies such as single cell sequencing have provided new insights into the origins and functions of human NK cells. In addition, the extraordinary promise of NK cell-based immunotherapy and advances in understanding the role of NK cells in transplantation has helped to underscore their therapeutic relevance and potential. In parallel, the field of NKD has continued to evolve and support the findings made in these respective biological displines. The critical role that NK cells play in human health has been further defined by the discovery of new NKDs that continue to define and strengthen the clinical hallmarks associated with the disease. In other words, the common features between the common clinical presentations is increasingly clear to be the aberration in NK cells (Figure 2). In addition, we see new themes emerging, particularly around replication, cell cycle and replication stress. Finally, the effect of NKD on more recently described subsets, including adaptive NK cells and ILC, provides further nuanced insight into the biology of human innate immune cells. These are merging with exciting new themes within immunology, including immune cell metabolism and tissue-dependent immune cell regulation.
The field of primary immunodeficiency as a whole has also undergone tremendous changes in recent years, particularly powered by whole exome-based gene discovery. The explosion of novel genes has been followed by increased awareness that we are only beginning to scratch the surface of understanding the genetic and biological factors that contribute to infectious susceptibility. The days of monogenic discoveries are giving way to more complex analyses of the contribution of multiple genetic elements, non-coding genetic regulators and epigenetic modifiers. With evolving technology will come exciting new insights to drive deeper understanding and, ultimately, improved patient care.
Acknowledgments:
This work was supported by NIH-NIAID Grants R01AI067946 and R01 AI120989 to JSO, the Jeffrey Modell Foundation, the American Society of Hematology Junior Scholar Award to EMM, and NIH-NIAID R01 AI137073 to EMM.
Footnotes
Conflict of interest: The authors have no conflict of interest to declare.
References
- 1.Vivier E, Artis D, Colonna M, et al. Innate Lymphoid Cells: 10 Years On. Cell. 2018;174(5):1054–1066. [DOI] [PubMed] [Google Scholar]
- 2.Cherrier DE, Serafini N, Di Santo JP. Innate Lymphoid Cell Development: A T Cell Perspective. Immunity. 2018;48(6):1091–1103. [DOI] [PubMed] [Google Scholar]
- 3.Spits H, Artis D, Colonna M, et al. Innate lymphoid cells--a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13(2):145–149. [DOI] [PubMed] [Google Scholar]
- 4.Gwalani LA, Orange JS. Single Degranulations in NK Cells Can Mediate Target Cell Killing. J Immunol. 2018;200(9):3231–3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Picard C, Bobby Gaspar H, Al-Herz W, et al. International Union of Immunological Societies: 2017 Primary Immunodeficiency Diseases Committee Report on Inborn Errors of Immunity. J Clin Immunol. 2018;38(1):96–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Zelm MC, Reisli I, van der Burg M, et al. An antibody-deficiency syndrome due to mutations in the CD19 gene. N Engl J Med. 2006;354(18):1901–1912. [DOI] [PubMed] [Google Scholar]
- 7.Chan AC, Kadlecek TA, Elder ME, et al. ZAP-70 deficiency in an autosomal recessive form of severe combined immunodeficiency. Science. 1994;264(5165):1599–1601. [DOI] [PubMed] [Google Scholar]
- 8.Elder ME, Lin D, Clever J, et al. Human severe combined immunodeficiency due to a defect in ZAP-70, a T cell tyrosine kinase. Science. 1994;264(5165):1596–1599. [DOI] [PubMed] [Google Scholar]
- 9.Arpaia E, Shahar M, Dadi H, Cohen A, Roifman CM. Defective T cell receptor signaling and CD8+ thymic selection in humans lacking zap-70 kinase. Cell. 1994;76(5):947–958. [DOI] [PubMed] [Google Scholar]
- 10.Negishi I, Motoyama N, Nakayama K, et al. Essential role for ZAP-70 in both positive and negative selection of thymocytes. Nature. 1995;376(6539):435–438. [DOI] [PubMed] [Google Scholar]
- 11.Kelliher MA, Grimm S, Ishida Y, Kuo F, Stanger BZ, Leder P. The death domain kinase RIP mediates the TNF-induced NF-kappaB signal. Immunity. 1998;8(3):297–303. [DOI] [PubMed] [Google Scholar]
- 12.Rickard JA, O’Donnell JA, Evans JM, et al. RIPK1 regulates RIPK3-MLKL-driven systemic inflammation and emergency hematopoiesis. Cell. 2014;157(5):1175–1188. [DOI] [PubMed] [Google Scholar]
- 13.Dannappel M, Vlantis K, Kumari S, et al. RIPK1 maintains epithelial homeostasis by inhibiting apoptosis and necroptosis. Nature. 2014;513(7516):90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cuchet-Lourenco D, Eletto D, Wu C, et al. Biallelic RIPK1 mutations in humans cause severe immunodeficiency, arthritis, and intestinal inflammation. Science. 2018;361(6404):810–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masopust D, Sivula CP, Jameson SC. Of Mice, Dirty Mice, and Men: Using Mice To Understand Human Immunology. J Immunol. 2017;199(2):383–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rahim MM, Makrigiannis AP. Ly49 receptors: evolution, genetic diversity, and impact on immunity. Immunol Rev. 2015;267(1):137–147. [DOI] [PubMed] [Google Scholar]
- 17.Haller O, Wigzell H. Suppression of natural killer cell activity with radioactive strontium: effector cells are marrow dependent. J Immunol. 1977;118(4):1503–1506. [PubMed] [Google Scholar]
- 18.Kumar V, Bennett M, Eckner RJ. Mechanisms of genetic resistance to friend virus leukemia in mice. J Exp Med. 1974;139(5):1093–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dokhelar MC, Wiels J, Lipinski M, et al. Natural killer cell activity in human bone marrow recipients: early reappearance of peripheral natural killer activity in graft-versus-host disease. Transplantation. 1981;31(1):61–65. [DOI] [PubMed] [Google Scholar]
- 20.Freud AG, Becknell B, Roychowdhury S, et al. A human CD34(+) subset resides in lymph nodes and differentiates into CD56bright natural killer cells. Immunity. 2005;22(3):295–304. [DOI] [PubMed] [Google Scholar]
- 21.Freud AG, Caligiuri MA. Human natural killer cell development. Immunol Rev. 2006;214:56–72. [DOI] [PubMed] [Google Scholar]
- 22.Freud AG, Yokohama A, Becknell B, et al. Evidence for discrete stages of human natural killer cell differentiation in vivo. J Exp Med. 2006;203(4):1033–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melsen JE, Lugthart G, Lankester AC, Schilham MW. Human Circulating and Tissue-Resident CD56(bright) Natural Killer Cell Populations. Front Immunol. 2016;7:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan A, Hong DL, Atzberger A, et al. CD56bright human NK cells differentiate into CD56dim cells: role of contact with peripheral fibroblasts. J Immunol. 2007;179(1):89–94. [DOI] [PubMed] [Google Scholar]
- 25.Cooley S, Xiao F, Pitt M, et al. A subpopulation of human peripheral blood NK cells that lacks inhibitory receptors for self-MHC is developmentally immature. Blood. 2007;110(2):578–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huntington ND, Legrand N, Alves NL, et al. IL-15 trans-presentation promotes human NK cell development and differentiation in vivo. J Exp Med. 2009;206(1):25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Romagnani C, Juelke K, Falco M, et al. CD56brightCD16- killer Ig-like receptor- NK cells display longer telomeres and acquire features of CD56dim NK cells upon activation. J Immunol. 2007;178(8):4947–4955. [DOI] [PubMed] [Google Scholar]
- 28.Dulphy N, Haas P, Busson M, et al. An unusual CD56(bright) CD16(low) NK cell subset dominates the early posttransplant period following HLA-matched hematopoietic stem cell transplantation. J Immunol. 2008;181(3):2227–2237. [DOI] [PubMed] [Google Scholar]
- 29.Shilling HG, McQueen KL, Cheng NW, Shizuru JA, Negrin RS, Parham P. Reconstitution of NK cell receptor repertoire following HLA-matched hematopoietic cell transplantation. Blood. 2003;101(9):3730–3740. [DOI] [PubMed] [Google Scholar]
- 30.Wu C, Li B, Lu R, et al. Clonal tracking of rhesus macaque hematopoiesis highlights a distinct lineage origin for natural killer cells. Cell Stem Cell. 2014;14(4):486–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu C, Espinoza DA, Koelle SJ, et al. Geographic clonal tracking in macaques provides insights into HSPC migration and differentiation. J Exp Med. 2018;215(1):217–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eissens DN, Spanholtz J, van der Meer A, et al. Defining early human NK cell developmental stages in primary and secondary lymphoid tissues. PloS one. 2012;7(2):e30930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hao QL, George AA, Zhu J, et al. Human intrathymic lineage commitment is marked by differential CD7 expression: identification of CD7- lympho-myeloid thymic progenitors. Blood. 2008;111(3):1318–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McClory S, Hughes T, Freud AG, et al. Evidence for a stepwise program of extrathymic T cell development within the human tonsil. J Clin Invest. 2012;122(4):1403–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mingari MC, Vitale C, Cantoni C, et al. Interleukin-15-induced maturation of human natural killer cells from early thymic precursors: selective expression of CD94/NKG2-A as the only HLA class I-specific inhibitory receptor. Eur J Immunol. 1997;27(6):1374–1380. [DOI] [PubMed] [Google Scholar]
- 36.Res P, Martinez-Caceres E, Cristina Jaleco A, et al. CD34+CD38dim cells in the human thymus can differentiate into T, natural killer, and dendritic cells but are distinct from pluripotent stem cells. Blood. 1996;87(12):5196–5206. [PubMed] [Google Scholar]
- 37.Chinen H, Matsuoka K, Sato T, et al. Lamina propria c-kit+ immune precursors reside in human adult intestine and differentiate into natural killer cells. Gastroenterology. 2007;133(2):559–573. [DOI] [PubMed] [Google Scholar]
- 38.Freud AG, Yu J, Caligiuri MA. Human natural killer cell development in secondary lymphoid tissues. Semin Immunol. 2014;26(2):132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vacca P, Vitale C, Montaldo E, et al. CD34+ hematopoietic precursors are present in human decidua and differentiate into natural killer cells upon interaction with stromal cells. Proc Natl Acad Sci U S A. 2011;108(6):2402–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moroso V, Famili F, Papazian N, et al. NK cells can generate from precursors in the adult human liver. Eur J Immunol. 2011;41(11):3340–3350. [DOI] [PubMed] [Google Scholar]
- 41.Freud AG, Mundy-Bosse BL, Yu J, Caligiuri MA. The Broad Spectrum of Human Natural Killer Cell Diversity. Immunity. 2017;47(5):820–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horowitz A, Strauss-Albee DM, Leipold M, et al. Genetic and environmental determinants of human NK cell diversity revealed by mass cytometry. Sci Transl Med. 2013;5(208):208ra145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guma M, Angulo A, Vilches C, Gomez-Lozano N, Malats N, Lopez-Botet M. Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood. 2004;104(12):3664–3671. [DOI] [PubMed] [Google Scholar]
- 44.Guma M, Budt M, Saez A, et al. Expansion of CD94/NKG2C+ NK cells in response to human cytomegalovirus-infected fibroblasts. Blood. 2006;107(9):3624–3631. [DOI] [PubMed] [Google Scholar]
- 45.Lopez-Verges S, Milush JM, Schwartz BS, et al. Expansion of a unique CD57(+)NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc Natl Acad Sci U S A. 2011;108(36):14725–14732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu LL, Landskron J, Ask EH, et al. Critical Role of CD2 Co-stimulation in Adaptive Natural Killer Cell Responses Revealed in NKG2C-Deficient Humans. Cell Rep. 2016;15(5):1088–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee J, Zhang T, Hwang I, et al. Epigenetic Modification and Antibody-Dependent Expansion of Memory-like NK Cells in Human Cytomegalovirus-Infected Individuals. Immunity. 2015;42(3):431–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schlums H, Cichocki F, Tesi B, et al. Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity. 2015;42(3):443–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bjorkstrom NK, Lindgren T, Stoltz M, et al. Rapid expansion and long-term persistence of elevated NK cell numbers in humans infected with hantavirus. J Exp Med. 2011;208(1):13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petitdemange C, Becquart P, Wauquier N, et al. Unconventional repertoire profile is imprinted during acute chikungunya infection for natural killer cells polarization toward cytotoxicity. PLoS Pathog. 2011;7(9):e1002268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gregson JN, Kuri-Cervantes L, Mela CM, Gazzard BG, Bower M, Goodier MR. Short communication: NKG2C+ NK cells contribute to increases in CD16+CD56- cells in HIV type 1+ individuals with high plasma viral load. AIDS Res Hum Retroviruses. 2013;29(1):84–88. [DOI] [PubMed] [Google Scholar]
- 52.Brunetta E, Fogli M, Varchetta S, et al. Chronic HIV-1 viremia reverses NKG2A/NKG2C ratio on natural killer cells in patients with human cytomegalovirus co-infection. AIDS. 2010;24(1):27–34. [DOI] [PubMed] [Google Scholar]
- 53.Guma M, Cabrera C, Erkizia I, et al. Human cytomegalovirus infection is associated with increased proportions of NK cells that express the CD94/NKG2C receptor in aviremic HIV-1-positive patients. J Infect Dis. 2006;194(1):38–41. [DOI] [PubMed] [Google Scholar]
- 54.Romee R, Schneider SE, Leong JW, et al. Cytokine activation induces human memory-like NK cells. Blood. 2012;120(24):4751–4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ganal SC, Sanos SL, Kallfass C, et al. Priming of natural killer cells by nonmucosal mononuclear phagocytes requires instructive signals from commensal microbiota. Immunity. 2012;37(1):171–186. [DOI] [PubMed] [Google Scholar]
- 56.Sonnenberg GF, Artis D. Innate lymphoid cell interactions with microbiota: implications for intestinal health and disease. Immunity. 2012;37(4):601–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schmidt S, Zimmermann SY, Tramsen L, Koehl U, Lehrnbecher T. Natural killer cells and antifungal host response. Clin Vaccine Immunol. 2013;20(4):452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Freud AG, Keller KA, Scoville SD, et al. NKp80 Defines a Critical Step during Human Natural Killer Cell Development. Cell Rep. 2016;16(2):379–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu J, Freud AG, Caligiuri MA. Location and cellular stages of natural killer cell development. Trends Immunol. 2013;34(12):573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mace EM, Gunesch JT, Dixon A, Orange JS. Human NK cell development requires CD56-mediated motility and formation of the developmental synapse. Nature communications. 2016;7:12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McCullar V, Oostendorp R, Panoskaltsis-Mortari A, et al. Mouse fetal and embryonic liver cells differentiate human umbilical cord blood progenitors into CD56-negative natural killer cell precursors in the absence of interleukin-15. Exp Hematol. 2008;36(5):598–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Caligiuri MA. Human natural killer cells. Blood. 2008;112(3):461–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mahapatra S, Mace EM, Minard CG, et al. High-resolution phenotyping identifies NK cell subsets that distinguish healthy children from adults. PloS one. 2017;12(8):e0181134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Angelo LS, Banerjee PP, Monaco-Shawver L, et al. Practical NK cell phenotyping and variability in healthy adults. Immunol Res. 2015;62(3):341–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mjosberg J, Spits H. Human innate lymphoid cells. J Allergy Clin Immunol. 2016;138(5):1265–1276. [DOI] [PubMed] [Google Scholar]
- 66.Hazenberg MD, Spits H. Human innate lymphoid cells. Blood. 2014;124(5):700–709. [DOI] [PubMed] [Google Scholar]
- 67.Munneke JM, Bjorklund AT, Mjosberg JM, et al. Activated innate lymphoid cells are associated with a reduced susceptibility to graft-versus-host disease. Blood. 2014;124(5):812–821. [DOI] [PubMed] [Google Scholar]
- 68.Lim AI, Li Y, Lopez-Lastra S, et al. Systemic Human ILC Precursors Provide a Substrate for Tissue ILC Differentiation. Cell. 2017;168(6):1086–1100 e1010. [DOI] [PubMed] [Google Scholar]
- 69.Wagner JA, Rosario M, Romee R, et al. CD56bright NK cells exhibit potent antitumor responses following IL-15 priming. J Clin Invest. 2017;127(11):4042–4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.De Maria A, Bozzano F, Cantoni C, Moretta L. Revisiting human natural killer cell subset function revealed cytolytic CD56(dim)CD16+ NK cells as rapid producers of abundant IFN-gamma on activation. Proc Natl Acad Sci U S A. 2011;108(2):728–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mace EM, Dongre P, Hsu HT, et al. Cell biological steps and checkpoints in accessing NK cell cytotoxicity. Immunol Cell Biol. 2014;92(3):245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.James AM, Hsu HT, Dongre P, et al. Rapid activation receptor- or IL-2-induced lytic granule convergence in human natural killer cells requires Src, but not downstream signaling. Blood. 2013;121(14):2627–2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mentlik AN, Sanborn KB, Holzbaur EL, Orange JS. Rapid lytic granule convergence to the MTOC in natural killer cells is dependent on dynein but not cytolytic commitment. Mol Biol Cell. 2010;21(13):2241–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hsu HT, Mace EM, Carisey AF, et al. NK cells converge lytic granules to promote cytotoxicity and prevent bystander killing. J Cell Biol. 2016;215(6):875–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bhat R, Watzl C. Serial killing of tumor cells by human natural killer cells--enhancement by therapeutic antibodies. PloS one. 2007;2(3):e326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Olofsson PE, Forslund E, Vanherberghen B, et al. Distinct Migration and Contact Dynamics of Resting and IL-2-Activated Human Natural Killer Cells. Front Immunol. 2014;5:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Orange JS. Human natural killer cell deficiencies and susceptibility to infection. Microbes Infect. 2002;4(15):1545–1558. [DOI] [PubMed] [Google Scholar]
- 78.Orange JS. Natural killer cell deficiency. J Allergy Clin Immunol. 2013;132(3):515–525; quiz 526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shaw RK, Issekutz AC, Fraser R, et al. Bilateral adrenal EBV-associated smooth muscle tumors in a child with a natural killer cell deficiency. Blood. 2012;119(17):4009–4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wendland T, Herren S, Yawalkar N, Cerny A, Pichler WJ. Strong alpha beta and gamma delta TCR response in a patient with disseminated Mycobacterium avium infection and lack of NK cells and monocytopenia. Immunol Lett. 2000;72(2):75–82. [DOI] [PubMed] [Google Scholar]
- 81.Notarangelo LD, Mazzolari E. Natural killer cell deficiencies and severe varicella infection. J Pediatr. 2006;148(4):563–564; author reply 564. [DOI] [PubMed] [Google Scholar]
- 82.Etzioni A, Eidenschenk C, Katz R, Beck R, Casanova JL, Pollack S. Fatal varicella associated with selective natural killer cell deficiency. J Pediatr. 2005;146(3):423–425. [DOI] [PubMed] [Google Scholar]
- 83.Eidenschenk C, Dunne J, Jouanguy E, et al. A novel primary immunodeficiency with specific natural-killer cell deficiency maps to the centromeric region of chromosome 8. American journal of human genetics. 2006;78(4):721–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bernard F, Picard C, Cormier-Daire V, et al. A novel developmental and immunodeficiency syndrome associated with intrauterine growth retardation and a lack of natural killer cells. Pediatrics. 2004;113(1 Pt 1):136–141. [DOI] [PubMed] [Google Scholar]
- 85.Ballas ZK, Turner JM, Turner DA, Goetzman EA, Kemp JD. A patient with simultaneous absence of “classical” natural killer cells (CD3-, CD16+, and NKH1+) and expansion of CD3+, CD4-, CD8-, NKH1+ subset. J Allergy Clin Immunol. 1990;85(2):453–459. [DOI] [PubMed] [Google Scholar]
- 86.Akiba H, Motoki Y, Satoh M, Iwatsuki K, Kaneko F. Recalcitrant trichophytic granuloma associated with NK-cell deficiency in a SLE patient treated with corticosteroid. Eur J Dermatol. 2001;11(1):58–62. [PubMed] [Google Scholar]
- 87.Biron CA, Byron KS, Sullivan JL. Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med. 1989;320(26):1731–1735. [DOI] [PubMed] [Google Scholar]
- 88.Fleisher G, Starr S, Koven N, Kamiya H, Douglas SD, Henle W. A non-x-linked syndrome with susceptibility to severe Epstein-Barr virus infections. J Pediatr. 1982;100(5):727–730. [DOI] [PubMed] [Google Scholar]
- 89.Gineau L, Cognet C, Kara N, et al. Partial MCM4 deficiency in patients with growth retardation, adrenal insufficiency, and natural killer cell deficiency. J Clin Invest. 2012;122(3):821–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hanna S, Beziat V, Jouanguy E, Casanova JL, Etzioni A. A homozygous mutation of RTEL1 in a child presenting with an apparently isolated natural killer cell deficiency. J Allergy Clin Immunol. 2015;136(4):1113–1114. [DOI] [PubMed] [Google Scholar]
- 91.Cottineau J, Kottemann MC, Lach FP, et al. Inherited GINS1 deficiency underlies growth retardation along with neutropenia and NK cell deficiency. J Clin Invest. 2017;127(5):1991–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mace EM, Bigley V, Gunesch JT, et al. Biallelic mutations in IRF8 impair human NK cell maturation and function. J Clin Invest. 2017;127(1):306–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Grier JT, Forbes LR, Monaco-Shawver L, et al. Human immunodeficiency-causing mutation defines CD16 in spontaneous NK cell cytotoxicity. J Clin Invest. 2012;122(10):3769–3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Quinnan GV Jr., Kirmani N, Rook AH, et al. Cytotoxic t cells in cytomegalovirus infection: HLA-restricted T-lymphocyte and non-T-lymphocyte cytotoxic responses correlate with recovery from cytomegalovirus infection in bone-marrow-transplant recipients. N Engl J Med. 1982;307(1):7–13. [DOI] [PubMed] [Google Scholar]
- 95.Spinner MA, Sanchez LA, Hsu AP, et al. GATA2 deficiency: a protean disorder of hematopoiesis, lymphatics, and immunity. Blood. 2014;123(6):809–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cohen JI, Dropulic L, Hsu AP, et al. Association of GATA2 Deficiency With Severe Primary Epstein-Barr Virus (EBV) Infection and EBV-associated Cancers. Clin Infect Dis. 2016;63(1):41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.de Vries E, Koene HR, Vossen JM, et al. Identification of an unusual Fc gamma receptor IIIa (CD16) on natural killer cells in a patient with recurrent infections. Blood. 1996;88(8):3022–3027. [PubMed] [Google Scholar]
- 98.Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295(5562):2097–2100. [DOI] [PubMed] [Google Scholar]
- 99.Guillerey C, Huntington ND, Smyth MJ. Targeting natural killer cells in cancer immunotherapy. Nat Immunol. 2016;17(9):1025–1036. [DOI] [PubMed] [Google Scholar]
- 100.Jawahar S, Moody C, Chan M, Finberg R, Geha R, Chatila T. Natural Killer (NK) cell deficiency associated with an epitope-deficient Fc receptor type IIIA (CD16-II). Clin Exp Immunol. 1996;103(3):408–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ravetch JV, Perussia B. Alternative membrane forms of Fc gamma RIII(CD16) on human natural killer cells and neutrophils. Cell type-specific expression of two genes that differ in single nucleotide substitutions. J Exp Med. 1989;170(2):481–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Quinnan GV Jr., Manischewitz JF, Kirmani N. Involvement of natural killer cells in the pathogenesis of murine cytomegalovirus interstitial pneumonitis and the immune response to infection. J Gen Virol. 1982;58 Pt 1:173–180. [DOI] [PubMed] [Google Scholar]
- 103.Padgett GA, Reiquam CW, Henson JB, Gorham JR. Comparative studies of susceptibility to infection in the Chediak-Higashi syndrome. J Pathol Bacteriol. 1968;95(2):509–522. [DOI] [PubMed] [Google Scholar]
- 104.Anderson DC, Schmalsteig FC, Finegold MJ, et al. The severe and moderate phenotypes of heritable Mac-1, LFA-1 deficiency: their quantitative definition and relation to leukocyte dysfunction and clinical features. J Infect Dis. 1985;152(4):668–689. [DOI] [PubMed] [Google Scholar]
- 105.Mace EM, Hsu AP, Monaco-Shawver L, et al. Mutations in GATA2 cause human NK cell deficiency with specific loss of the CD56(bright) subset. Blood. 2013;121(14):2669–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dickinson RE, Milne P, Jardine L, et al. The evolution of cellular deficiency in GATA2 mutation. Blood. 2014;123(6):863–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Maciejewski-Duval A, Meuris F, Bignon A, et al. Altered chemotactic response to CXCL12 in patients carrying GATA2 mutations. J Leukoc Biol. 2016;99(6):1065–1076. [DOI] [PubMed] [Google Scholar]
- 108.Schlums H, Jung M, Han H, et al. Adaptive NK cells can persist in patients with GATA2 mutation depleted of stem and progenitor cells. Blood. 2017;129(14):1927–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Eidenschenk C, Jouanguy E, Alcais A, et al. Familial NK cell deficiency associated with impaired IL-2- and IL-15-dependent survival of lymphocytes. J Immunol. 2006;177(12):8835–8843. [DOI] [PubMed] [Google Scholar]
- 110.O’Riordan SM, Lynch SA, Hindmarsh PC, Chan LF, Clark AJ, Costigan C. A novel variant of familial glucocorticoid deficiency prevalent among the Irish Traveler population. J Clin Endocrinol Metab. 2008;93(7):2896–2899. [DOI] [PubMed] [Google Scholar]
- 111.Hughes CR, Guasti L, Meimaridou E, et al. MCM4 mutation causes adrenal failure, short stature, and natural killer cell deficiency in humans. J Clin Invest. 2012;122(3):814–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Baxley RM, Bielinsky AK. Mcm10: A Dynamic Scaffold at Eukaryotic Replication Forks. Genes (Basel). 2017;8(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lim HJ, Jeon Y, Jeon CH, Kim JH, Lee H. Targeted disruption of Mcm10 causes defective embryonic cell proliferation and early embryo lethality. Biochim Biophys Acta. 2011;1813(10):1777–1783. [DOI] [PubMed] [Google Scholar]
- 114.Shima N, Alcaraz A, Liachko I, et al. A viable allele of Mcm4 causes chromosome instability and mammary adenocarcinomas in mice. Nat Genet. 2007;39(1):93–98. [DOI] [PubMed] [Google Scholar]
- 115.Jaeger BN, Donadieu J, Cognet C, et al. Neutrophil depletion impairs natural killer cell maturation, function, and homeostasis. J Exp Med. 2012;209(3):565–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hambleton S, Salem S, Bustamante J, et al. IRF8 mutations and human dendritic-cell immunodeficiency. N Engl J Med. 2011;365(2):127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bigley V, Maisuria S, Cytlak U, et al. Biallelic interferon regulatory factor 8 mutation: A complex immunodeficiency syndrome with dendritic cell deficiency, monocytopenia, and immune dysregulation. J Allergy Clin Immunol. 2018;141(6):2234–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Aliberti J, Schulz O, Pennington DJ, et al. Essential role for ICSBP in the in vivo development of murine CD8alpha + dendritic cells. Blood. 2003;101(1):305–310. [DOI] [PubMed] [Google Scholar]
- 119.Grajales-Reyes GE, Iwata A, Albring J, et al. Batf3 maintains autoactivation of Irf8 for commitment of a CD8alpha(+) conventional DC clonogenic progenitor. Nat Immunol. 2015;16(7):708–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lu R, Medina KL, Lancki DW, Singh H. IRF-4,8 orchestrate the pre-B-to-B transition in lymphocyte development. Genes Dev. 2003;17(14):1703–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tamura T, Tailor P, Yamaoka K, et al. IFN regulatory factor-4 and −8 govern dendritic cell subset development and their functional diversity. J Immunol. 2005;174(5):2573–2581. [DOI] [PubMed] [Google Scholar]
- 122.Tamura T, Yanai H, Savitsky D, Taniguchi T. The IRF family transcription factors in immunity and oncogenesis. Annu Rev Immunol. 2008;26:535–584. [DOI] [PubMed] [Google Scholar]
- 123.Adams NM, Lau CM, Fan X, et al. Transcription Factor IRF8 Orchestrates the Adaptive Natural Killer Cell Response. Immunity. 2018;48(6):1172–1182 e1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ballew BJ, Yeager M, Jacobs K, et al. Germline mutations of regulator of telomere elongation helicase 1, RTEL1, in Dyskeratosis congenita. Hum Genet. 2013;132(4):473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Deng Z, Glousker G, Molczan A, et al. Inherited mutations in the helicase RTEL1 cause telomere dysfunction and Hoyeraal-Hreidarsson syndrome. Proc Natl Acad Sci U S A. 2013;110(36):E3408–3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Le Guen T, Jullien L, Touzot F, et al. Human RTEL1 deficiency causes Hoyeraal-Hreidarsson syndrome with short telomeres and genome instability. Hum Mol Genet. 2013;22(16):3239–3249. [DOI] [PubMed] [Google Scholar]
- 127.Mace EM, Orange JS. Genetic Causes of Human NK Cell Deficiency and Their Effect on NK Cell Subsets. Front Immunol. 2016;7:545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mace EM. Phosphoinositide-3-Kinase Signaling in Human Natural Killer Cells: New Insights from Primary Immunodeficiency. Front Immunol. 2018;9:445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Buckley RH, Schiff RI, Schiff SE, et al. Human severe combined immunodeficiency: genetic, phenotypic, and functional diversity in one hundred eight infants. J Pediatr. 1997;130(3):378–387. [DOI] [PubMed] [Google Scholar]
- 130.Roberts JL, Lengi A, Brown SM, et al. Janus kinase 3 (JAK3) deficiency: clinical, immunologic, and molecular analyses of 10 patients and outcomes of stem cell transplantation. Blood. 2004;103(6):2009–2018. [DOI] [PubMed] [Google Scholar]
- 131.Vargas-Hernandez A, Mace EM, Zimmerman O, et al. Ruxolitinib partially reverses functional natural killer cell deficiency in patients with signal transducer and activator of transcription 1 (STAT1) gain-of-function mutations. J Allergy Clin Immunol. 2018;141(6):2142–2155 e2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhu S, Phatarpekar PV, Denman CJ, et al. Transcription of the activating receptor NKG2D in natural killer cells is regulated by STAT3 tyrosine phosphorylation. Blood. 2014;124(3):403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bezrodnik L, Di Giovanni D, Caldirola MS, Azcoiti ME, Torgerson T, Gaillard MI. Long-term follow-up of STAT5B deficiency in three argentinian patients: clinical and immunological features. J Clin Immunol. 2015;35(3):264–272. [DOI] [PubMed] [Google Scholar]
- 134.Guia S, Cognet C, de Beaucoudrey L, et al. A role for interleukin-12/23 in the maturation of human natural killer and CD56+ T cells in vivo. Blood. 2008;111(10):5008–5016. [DOI] [PubMed] [Google Scholar]
- 135.Kotlarz D, Zietara N, Uzel G, et al. Loss-of-function mutations in the IL-21 receptor gene cause a primary immunodeficiency syndrome. J Exp Med. 2013;210(3):433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Goudy K, Aydin D, Barzaghi F, et al. Human IL2RA null mutation mediates immunodeficiency with lymphoproliferation and autoimmunity. Clin Immunol. 2013;146(3):248–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Risma KA, Frayer RW, Filipovich AH, Sumegi J. Aberrant maturation of mutant perforin underlies the clinical diversity of hemophagocytic lymphohistiocytosis. J Clin Invest. 2006;116(1):182–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Feldmann J, Callebaut I, Raposo G, et al. Munc13–4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lymphohistiocytosis (FHL3). Cell. 2003;115(4):461–473. [DOI] [PubMed] [Google Scholar]
- 139.Bryceson YT, Rudd E, Zheng C, et al. Defective cytotoxic lymphocyte degranulation in syntaxin-11 deficient familial hemophagocytic lymphohistiocytosis 4 (FHL4) patients. Blood. 2007;110(6):1906–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Introne W, Boissy RE, Gahl WA. Clinical, molecular, and cell biological aspects of Chediak-Higashi syndrome. Mol Genet Metab. 1999;68(2):283–303. [DOI] [PubMed] [Google Scholar]
- 141.Badolato R, Parolini S. Novel insights from adaptor protein 3 complex deficiency. J Allergy Clin Immunol. 2007;120(4):735–741; quiz 742–733. [DOI] [PubMed] [Google Scholar]
- 142.Badolato R, Prandini A, Caracciolo S, et al. Exome sequencing reveals a pallidin mutation in a Hermansky-Pudlak-like primary immunodeficiency syndrome. Blood. 2012;119(13):3185–3187. [DOI] [PubMed] [Google Scholar]
- 143.Meade JL, de Wynter EA, Brett P, et al. A family with Papillon-Lefevre syndrome reveals a requirement for cathepsin C in granzyme B activation and NK cell cytolytic activity. Blood. 2006;107(9):3665–3668. [DOI] [PubMed] [Google Scholar]
- 144.Gil-Krzewska A, Wood SM, Murakami Y, et al. Chediak-Higashi syndrome: Lysosomal trafficking regulator domains regulate exocytosis of lytic granules but not cytokine secretion by natural killer cells. J Allergy Clin Immunol. 2016;137(4):1165–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wood SM, Meeths M, Chiang SC, et al. Different NK cell-activating receptors preferentially recruit Rab27a or Munc13–4 to perforin-containing granules for cytotoxicity. Blood. 2009;114(19):4117–4127. [DOI] [PubMed] [Google Scholar]
- 146.Frugoni F, Dobbs K, Felgentreff K, et al. A novel mutation in the POLE2 gene causing combined immunodeficiency. J Allergy Clin Immunol. 2016;137(2):635–638 e631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.von Bernuth H, Ravindran E, Du H, et al. Combined immunodeficiency develops with age in Immunodeficiency-centromeric instability-facial anomalies syndrome 2 (ICF2). Orphanet J Rare Dis. 2014;9:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Goldman FD, Gurel Z, Al-Zubeidi D, et al. Congenital pancytopenia and absence of B lymphocytes in a neonate with a mutation in the Ikaros gene. Pediatr Blood Cancer. 2012;58(4):591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kuehn HS, Boisson B, Cunningham-Rundles C, et al. Loss of B Cells in Patients with Heterozygous Mutations in IKAROS. N Engl J Med. 2016;374(11):1032–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yang T, Ramocki MB, Neul JL, et al. Overexpression of methyl-CpG binding protein 2 impairs T(H)1 responses. Sci Transl Med. 2012;4(163):163ra158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Boland BS, Widjaja CE, Banno A, et al. Immunodeficiency and autoimmune enterocolopathy linked to NFAT5 haploinsufficiency. J Immunol. 2015;194(6):2551–2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Korthof ET, Svahn J, Peffault de Latour R, et al. Immunological profile of Fanconi anemia: a multicentric retrospective analysis of 61 patients. Am J Hematol. 2013;88(6):472–476. [DOI] [PubMed] [Google Scholar]
- 153.Cossu F, Vulliamy TJ, Marrone A, Badiali M, Cao A, Dokal I. A novel DKC1 mutation, severe combined immunodeficiency (T+B-NK- SCID) and bone marrow transplantation in an infant with Hoyeraal-Hreidarsson syndrome. Br J Haematol. 2002;119(3):765–768. [DOI] [PubMed] [Google Scholar]
- 154.Lagresle-Peyrou C, Six EM, Picard C, et al. Human adenylate kinase 2 deficiency causes a profound hematopoietic defect associated with sensorineural deafness. Nat Genet. 2009;41(1):106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Keller MD, Ganesh J, Heltzer M, et al. Severe combined immunodeficiency resulting from mutations in MTHFD1. Pediatrics. 2013;131(2):e629–634. [DOI] [PubMed] [Google Scholar]
- 156.Etzioni A, Lahat N, Benderly A, Katz R, Pollack S. Humoral and cellular immune dysfunction in a patient with Bloom’s syndrome and recurrent infections. J Clin Lab Immunol. 1989;28(3):151–154. [PubMed] [Google Scholar]
- 157.Renner ED, Hartl D, Rylaarsdam S, et al. Comel-Netherton syndrome defined as primary immunodeficiency. J Allergy Clin Immunol. 2009;124(3):536–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Furukawa H, Yabe T, Watanabe K, et al. Tolerance of NK and LAK activity for HLA class I-deficient targets in a TAP1-deficient patient (bare lymphocyte syndrome type I). Hum Immunol. 1999;60(1):32–40. [DOI] [PubMed] [Google Scholar]
- 159.Markel G, Mussaffi H, Ling KL, et al. The mechanisms controlling NK cell autoreactivity in TAP2-deficient patients. Blood. 2004;103(5):1770–1778. [DOI] [PubMed] [Google Scholar]
- 160.Ardeniz O, Unger S, Onay H, et al. beta2-Microglobulin deficiency causes a complex immunodeficiency of the innate and adaptive immune system. J Allergy Clin Immunol. 2015;136(2):392–401. [DOI] [PubMed] [Google Scholar]
- 161.Ostenstad B, Giliani S, Mellbye OJ, Nilsen BR, Abrahamsen T. A boy with X-linked hyper-IgM syndrome and natural killer cell deficiency. Clin Exp Immunol. 1997;107(2):230–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ruiz-Garcia R, Vargas-Hernandez A, Chinn IK, et al. Mutations in PI3K110delta cause impaired natural killer cell function partially rescued by rapamycin treatment. J Allergy Clin Immunol. 2018;142(2):605–617 e607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lougaris V, Patrizi O, Baronio M, et al. p85alpha is an intrinsic regulator of human natural killer cell effector functions. J Allergy Clin Immunol. 2016;138(2):605–608 e603. [DOI] [PubMed] [Google Scholar]
- 164.Conley ME, Dobbs AK, Quintana AM, et al. Agammaglobulinemia and absent B lineage cells in a patient lacking the p85alpha subunit of PI3K. J Exp Med. 2012;209(3):463–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhang KJ, Husami A, Marsh R & Jordan MB Identification of a phosphoinositide 3-kinase (PI-3K) p110δ (PIK3CD) deficient individual. Journal of Clinical Immunology. 2013;33:673–674. [Google Scholar]
- 166.Tsujita Y, Mitsui-Sekinaka K, Imai K, et al. Phosphatase and tensin homolog (PTEN) mutation can cause activated phosphatidylinositol 3-kinase delta syndrome-like immunodeficiency. J Allergy Clin Immunol. 2016;138(6):1672–1680 e1610. [DOI] [PubMed] [Google Scholar]
- 167.Ombrello MJ, Remmers EF, Sun G, et al. Cold urticaria, immunodeficiency, and autoimmunity related to PLCG2 deletions. N Engl J Med. 2012;366(4):330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kuehn HS, Niemela JE, Rangel-Santos A, et al. Loss-of-function of the protein kinase C delta (PKCdelta) causes a B-cell lymphoproliferative syndrome in humans. Blood. 2013;121(16):3117–3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Orange JS, Ramesh N, Remold-O’Donnell E, et al. Wiskott-Aldrich syndrome protein is required for NK cell cytotoxicity and colocalizes with actin to NK cell-activating immunologic synapses. Proc Natl Acad Sci U S A. 2002;99(17):11351–11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mace EM, Orange JS. Lytic immune synapse function requires filamentous actin deconstruction by Coronin 1A. Proc Natl Acad Sci U S A. 2014;111(18):6708–6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mizesko MC, Banerjee PP, Monaco-Shawver L, et al. Defective actin accumulation impairs human natural killer cell function in patients with dedicator of cytokinesis 8 deficiency. J Allergy Clin Immunol. 2013;131(3):840–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sanborn KB, Mace EM, Rak GD, et al. Phosphorylation of the myosin IIA tailpiece regulates single myosin IIA molecule association with lytic granules to promote NK-cell cytotoxicity. Blood. 2011;118(22):5862–5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lanzi G, Moratto D, Vairo D, et al. A novel primary human immunodeficiency due to deficiency in the WASP-interacting protein WIP. J Exp Med. 2012;209(1):29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Dobbs K, Dominguez Conde C, Zhang SY, et al. Inherited DOCK2 Deficiency in Patients with Early-Onset Invasive Infections. N Engl J Med. 2015;372(25):2409–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Salzer E, Cagdas D, Hons M, et al. RASGRP1 deficiency causes immunodeficiency with impaired cytoskeletal dynamics. Nat Immunol. 2016;17(12):1352–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chaigne-Delalande B, Li FY, O’Connor GM, et al. Mg2+ regulates cytotoxic functions of NK and CD8 T cells in chronic EBV infection through NKG2D. Science. 2013;341(6142):186–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Maul-Pavicic A, Chiang SC, Rensing-Ehl A, et al. ORAI1-mediated calcium influx is required for human cytotoxic lymphocyte degranulation and target cell lysis. Proc Natl Acad Sci U S A. 2011;108(8):3324–3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Fuchs S, Rensing-Ehl A, Speckmann C, et al. Antiviral and regulatory T cell immunity in a patient with stromal interaction molecule 1 deficiency. J Immunol. 2012;188(3):1523–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gruda R, Brown AC, Grabovsky V, et al. Loss of kindlin-3 alters the threshold for NK cell activation in human leukocyte adhesion deficiency-III. Blood. 2012;120(19):3915–3924. [DOI] [PubMed] [Google Scholar]
- 180.Tangye SG, Phillips JH, Lanier LL, Nichols KE. Functional requirement for SAP in 2B4-mediated activation of human natural killer cells as revealed by the X-linked lymphoproliferative syndrome. J Immunol. 2000;165(6):2932–2936. [DOI] [PubMed] [Google Scholar]
- 181.Marsh RA, Madden L, Kitchen BJ, et al. XIAP deficiency: a unique primary immunodeficiency best classified as X-linked familial hemophagocytic lymphohistiocytosis and not as X-linked lymphoproliferative disease. Blood. 2010;116(7):1079–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Huck K, Feyen O, Niehues T, et al. Girls homozygous for an IL-2-inducible T cell kinase mutation that leads to protein deficiency develop fatal EBV-associated lymphoproliferation. J Clin Invest. 2009;119(5):1350–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Orange JS, Brodeur SR, Jain A, et al. Deficient natural killer cell cytotoxicity in patients with IKK-gamma/NEMO mutations. J Clin Invest. 2002;109(11):1501–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Su H, Bidere N, Zheng L, et al. Requirement for caspase-8 in NF-kappaB activation by antigen receptor. Science. 2005;307(5714):1465–1468. [DOI] [PubMed] [Google Scholar]
- 185.Lougaris V, Tabellini G, Vitali M, et al. Defective natural killer-cell cytotoxic activity in NFKB2-mutated CVID-like disease. J Allergy Clin Immunol. 2015;135(6):1641–1643. [DOI] [PubMed] [Google Scholar]
- 186.Willmann KL, Klaver S, Dogu F, et al. Biallelic loss-of-function mutation in NIK causes a primary immunodeficiency with multifaceted aberrant lymphoid immunity. Nature communications. 2014;5:5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zheng P, Noroski LM, Hanson IC, et al. Molecular mechanisms of functional natural killer deficiency in patients with partial DiGeorge syndrome. J Allergy Clin Immunol. 2015;135(5):1293–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Mousallem T, Yang J, Urban TJ, et al. A nonsense mutation in IKBKB causes combined immunodeficiency. Blood. 2014;124(13):2046–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lougaris V, Tabellini G, Baronio M, et al. CTLA-4 regulates human Natural Killer cell effector functions. Clin Immunol. 2018;194:43–45. [DOI] [PubMed] [Google Scholar]
- 190.Vely F, Barlogis V, Marinier E, et al. Combined Immunodeficiency in Patients With Trichohepatoenteric Syndrome. Front Immunol. 2018;9:1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lougaris V, Patrizi O, Baronio M, et al. NFKB1 regulates human NK cell maturation and effector functions. Clin Immunol. 2017;175:99–108. [DOI] [PubMed] [Google Scholar]
- 192.Sorte HS, Osnes LT, Fevang B, et al. A potential founder variant in CARMIL2/RLTPR in three Norwegian families with warts, molluscum contagiosum, and T-cell dysfunction. Mol Genet Genomic Med. 2016;4(6):604–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Windhorst DB, Zelickson AS, Good RA. Chediak-Higashi syndrome: hereditary gigantism of cytoplasmic organelles. Science. 1966;151(3706):81–83. [DOI] [PubMed] [Google Scholar]
- 194.Burkhardt JK, Wiebel FA, Hester S, Argon Y. The giant organelles in beige and Chediak-Higashi fibroblasts are derived from late endosomes and mature lysosomes. J Exp Med. 1993;178(6):1845–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Gil-Krzewska A, Saeed MB, Oszmiana A, et al. An actin cytoskeletal barrier inhibits lytic granule release from natural killer cells in patients with Chediak-Higashi syndrome. J Allergy Clin Immunol. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Carisey AF, Mace EM, Saeed MB, Davis DM, Orange JS. Nanoscale Dynamism of Actin Enables Secretory Function in Cytolytic Cells. Curr Biol. 2018;28(4):489–502 e489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Cetica V, Hackmann Y, Grieve S, et al. Patients with Griscelli syndrome and normal pigmentation identify RAB27A mutations that selectively disrupt MUNC13–4 binding. J Allergy Clin Immunol. 2015;135(5):1310–1318 e1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Netter P, Chan SK, Banerjee PP, et al. A novel Rab27a mutation binds melanophilin, but not Munc13–4, causing immunodeficiency without albinism. J Allergy Clin Immunol. 2016;138(2):599–601 e593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Tesi B, Rascon J, Chiang SCC, et al. A RAB27A 5’ untranslated region structural variant associated with late-onset hemophagocytic lymphohistiocytosis and normal pigmentation. J Allergy Clin Immunol. 2018;142(1):317–321 e318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Aldrich RA, Steinberg AG, Campbell DC. Pedigree demonstrating a sex-linked recessive condition characterized by draining ears, eczematoid dermatitis and bloody diarrhea. Pediatrics. 1954;13(2):133–139. [PubMed] [Google Scholar]
- 201.Wiskott A Familiärer, angeborener Morbus Werlhofii?. Monatsschr Kinderheilkd. 1937;68:212–216. [Google Scholar]
- 202.Sullivan KE, Mullen CA, Blaese RM, Winkelstein JA. A multiinstitutional survey of the Wiskott-Aldrich syndrome. J Pediatr. 1994;125(6 Pt 1):876–885. [DOI] [PubMed] [Google Scholar]
- 203.Orange JS, Roy-Ghanta S, Mace EM, et al. IL-2 induces a WAVE2-dependent pathway for actin reorganization that enables WASp-independent human NK cell function. J Clin Invest. 2011;121(4):1535–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Gismondi A, Cifaldi L, Mazza C, et al. Impaired natural and CD16-mediated NK cell cytotoxicity in patients with WAS and XLT: ability of IL-2 to correct NK cell functional defect. Blood. 2004;104(2):436–443. [DOI] [PubMed] [Google Scholar]
- 205.Moshous D, Martin E, Carpentier W, et al. Whole-exome sequencing identifies Coronin-1A deficiency in 3 siblings with immunodeficiency and EBV-associated B-cell lymphoproliferation. J Allergy Clin Immunol. 2013;131(6):1594–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Shiow LR, Roadcap DW, Paris K, et al. The actin regulator coronin 1A is mutant in a thymic egress-deficient mouse strain and in a patient with severe combined immunodeficiency. Nat Immunol. 2008;9(11):1307–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Stray-Pedersen A, Jouanguy E, Crequer A, et al. Compound heterozygous CORO1A mutations in siblings with a mucocutaneous-immunodeficiency syndrome of epidermodysplasia verruciformis-HPV, molluscum contagiosum and granulomatous tuberculoid leprosy. J Clin Immunol. 2014;34(7):871–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Mace EM, Orange JS. Insights into primary immune deficiency from quantitative microscopy. J Allergy Clin Immunol. 2015;136(5):1150–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Angulo I, Vadas O, Garcon F, et al. Phosphoinositide 3-kinase delta gene mutation predisposes to respiratory infection and airway damage. Science. 2013;342(6160):866–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Lucas CL, Chandra A, Nejentsev S, Condliffe AM, Okkenhaug K. PI3Kdelta and primary immunodeficiencies. Nat Rev Immunol. 2016;16(11):702–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Lucas CL, Kuehn HS, Zhao F, et al. Dominant-activating germline mutations in the gene encoding the PI(3)K catalytic subunit p110delta result in T cell senescence and human immunodeficiency. Nat Immunol. 2014;15(1):88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Lucas CL, Zhang Y, Venida A, et al. Heterozygous splice mutation in PIK3R1 causes human immunodeficiency with lymphoproliferation due to dominant activation of PI3K. J Exp Med. 2014;211(13):2537–2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Rao VK, Webster S, Dalm V, et al. Effective “activated PI3Kdelta syndrome”-targeted therapy with the PI3Kdelta inhibitor leniolisib. Blood. 2017;130(21):2307–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Eckelhart E, Warsch W, Zebedin E, et al. A novel Ncr1-Cre mouse reveals the essential role of STAT5 for NK-cell survival and development. Blood. 2011;117(5):1565–1573. [DOI] [PubMed] [Google Scholar]
- 215.Noguchi M, Yi H, Rosenblatt HM, et al. Interleukin-2 receptor gamma chain mutation results in X-linked severe combined immunodeficiency in humans. Cell. 1993;73(1):147–157. [DOI] [PubMed] [Google Scholar]
- 216.Ginn SL, Smyth C, Wong M, Bennetts B, Rowe PB, Alexander IE. A novel splice-site mutation in the common gamma chain (gammac) gene IL2RG results in X-linked severe combined immunodeficiency with an atypical NK+ phenotype. Hum Mutat. 2004;23(5):522–523. [DOI] [PubMed] [Google Scholar]
- 217.Eletto D, Burns SO, Angulo I, et al. Biallelic JAK1 mutations in immunodeficient patient with mycobacterial infection. Nature communications. 2016;7:13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Li FY, Chaigne-Delalande B, Kanellopoulou C, et al. Second messenger role for Mg2+ revealed by human T-cell immunodeficiency. Nature. 2011;475(7357):471–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.de Greef JC, Wang J, Balog J, et al. Mutations in ZBTB24 are associated with immunodeficiency, centromeric instability, and facial anomalies syndrome type 2. Am J Hum Genet. 2011;88(6):796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Zhang JJ, Zhao Y, Chait BT, et al. Ser727-dependent recruitment of MCM5 by Stat1alpha in IFN-gamma-induced transcriptional activation. EMBO J. 1998;17(23):6963–6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Wells AD, Morawski PA. New roles for cyclin-dependent kinases in T cell biology: linking cell division and differentiation. Nat Rev Immunol. 2014;14(4):261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Tangye SG, Hodgkin PD. Divide and conquer: the importance of cell division in regulating B-cell responses. Immunology. 2004;112(4):509–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Ibarra A, Schwob E, Mendez J. Excess MCM proteins protect human cells from replicative stress by licensing backup origins of replication. Proc Natl Acad Sci U S A. 2008;105(26):8956–8961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Lutz CT, Karapetyan A, Al-Attar A, et al. Human NK cells proliferate and die in vivo more rapidly than T cells in healthy young and elderly adults. J Immunol. 2011;186(8):4590–4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Dobbs K, Tabellini G, Calzoni E, et al. Natural Killer Cells from Patients with Recombinase-Activating Gene and Non-Homologous End Joining Gene Defects Comprise a Higher Frequency of CD56(bright) NKG2A(+++) Cells, and Yet Display Increased Degranulation and Higher Perforin Content. Front Immunol. 2017;8:798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Karo JM, Schatz DG, Sun JC. The RAG recombinase dictates functional heterogeneity and cellular fitness in natural killer cells. Cell. 2014;159(1):94–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Collin R, St-Pierre C, Guilbault L, et al. An Unbiased Linkage Approach Reveals That the p53 Pathway Is Coupled to NK Cell Maturation. J Immunol. 2017;199(4):1490–1504. [DOI] [PubMed] [Google Scholar]
- 228.Matatall KA, Jeong M, Chen S, et al. Chronic Infection Depletes Hematopoietic Stem Cells through Stress-Induced Terminal Differentiation. Cell Rep. 2016;17(10):2584–2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Baldridge MT, King KY, Boles NC, Weksberg DC, Goodell MA. Quiescent haematopoietic stem cells are activated by IFN-gamma in response to chronic infection. Nature. 2010;465(7299):793–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Hazeldine J, Lord JM. The impact of ageing on natural killer cell function and potential consequences for health in older adults. Ageing Res Rev. 2013;12(4):1069–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Zhang Y, Wallace DL, de Lara CM, et al. In vivo kinetics of human natural killer cells: the effects of ageing and acute and chronic viral infection. Immunology. 2007;121(2):258–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Alvarez S, Diaz M, Flach J, et al. Replication stress caused by low MCM expression limits fetal erythropoiesis and hematopoietic stem cell functionality. Nature communications. 2015;6:8548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Flach J, Bakker ST, Mohrin M, et al. Replication stress is a potent driver of functional decline in ageing haematopoietic stem cells. Nature. 2014;512(7513):198–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.White DW, Keppel CR, Schneider SE, et al. Latent herpesvirus infection arms NK cells. Blood. 2010;115(22):4377–4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Cooley S, Parham P, Miller JS. Strategies to activate NK cells to prevent relapse and induce remission following hematopoietic stem cell transplantation. Blood. 2018;131(10):1053–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Bjorklund AK, Forkel M, Picelli S, et al. The heterogeneity of human CD127(+) innate lymphoid cells revealed by single-cell RNA sequencing. Nat Immunol. 2016;17(4):451–460. [DOI] [PubMed] [Google Scholar]
- 237.Scoville SD, Mundy-Bosse BL, Zhang MH, et al. A Progenitor Cell Expressing Transcription Factor RORgammat Generates All Human Innate Lymphoid Cell Subsets. Immunity. 2016;44(5):1140–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Montaldo E, Teixeira-Alves LG, Glatzer T, et al. Human RORgammat(+)CD34(+) cells are lineage-specified progenitors of group 3 RORgammat(+) innate lymphoid cells. Immunity. 2014;41(6):988–1000. [DOI] [PubMed] [Google Scholar]
- 239.Okada S, Markle JG, Deenick EK, et al. IMMUNODEFICIENCIES. Impairment of immunity to Candida and Mycobacterium in humans with bi-allelic RORC mutations. Science. 2015;349(6248):606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Cols M, Rahman A, Maglione PJ, et al. Expansion of inflammatory innate lymphoid cells in patients with common variable immune deficiency. J Allergy Clin Immunol. 2016;137(4):1206–1215 e1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Pilzecker B, Buoninfante OA, van den Berk P, et al. DNA damage tolerance in hematopoietic stem and progenitor cells in mice. Proc Natl Acad Sci U S A. 2017;114(33):E6875–E6883. [DOI] [PMC free article] [PubMed] [Google Scholar]