Abstract
Radiopharmaceutical therapy (RPT) involves the use of radionuclides that are either conjugated to tumor-targeting agents (e.g., nanoscale constructs, antibodies, peptides, and small molecules) or that concentrate in tumors through natural physiological mechanisms that occur predominantly in neoplastic cells. In the latter category, radioiodine therapy of thyroid cancer is the prototypical and most widely implemented RPT. In the category of radionuclide-ligand conjugates, antibody and peptide conjugates have been studied extensively. The efficacy of RPT relies on the ability to deliver cytotoxic radiation to tumor cells without causing prohibitive normal tissue toxicity. After some 30 y of preclinical and clinical research, a number of recent developments suggest that RPT is poised to emerge as an important and widely-recognized therapeutic modality. These developments include the substantial investment in antibodies by the pharmaceutical industry and the compelling rationale to build upon this already existing and widely-tested platform. In addition, the growing recognition that the signaling pathways responsible for tumor cell survival and proliferation are less easily and durably inhibited than originally envisioned has also provided a rationale for identifying agents that are cytotoxic rather than inhibitory. A number of radiopharmaceutical agents are currently undergoing clinical trial investigation; these include beta-particle emitters, such as 177Lu, that are being used to label anti-somatostatin receptor peptides for neuroendocrine cancers and also prostate-specific membrane antigen targeting small molecules for prostate cancer. Alpha-particle emitting radionuclides have also been studied for RPT; these include 211At for glioblastoma, 225Ac for leukemias and prostate cancer, 212Pb for breast cancer, and 223Ra for prostate cancer. The alpha emitters have tended to show particular promise and there is substantial interest in further developing these agents for therapy of cancers that are particularly difficult to treat.
Keywords: National Council on Radiation Protection and Measurements, radiopharmaceutical therapy, beta-particle emitters, alpha-particle emitters
Radiopharmaceutical therapy (RPT) involves the targeted delivery of radiation to tumor cells or to the tumor microenvironment. This treatment approach is distinguished from external beam radiotherapy and brachytherapy in that the radiation is delivered by unencapsulated radionuclides. RPT agents are systemically (in some cases, also locally) administered and localize to the tumor or its microenvironment. Tumor localization may occur because the radioactive element is involved in relevant tumor-associated biological processes or because the radionuclide is conjugated to a delivery vehicle that confers tumor targeting. Alternatively, passive accumulation due to physiologic mechanisms (e.g., enhanced permeability and retention) may provide targeting. Delivery vehicles that have been investigated include: microspheres, nanoparticles, antibodies, peptides, small molecules, and various constructs of each of these. RPT provides the advantage of beta and Auger electron as well as alpha-particle delivery directly to the targeted cell population. The principal drawback to RPT is that delivery cannot be externally controlled in the way that external beam radiotherapy and brachytherapy may be controlled.
The prototypical RPT, and perhaps the most effective treatment for metastatic cancer, is radioiodine therapy of thyroid cancer. Differentiated thyroid cancer metastases retain the ability to concentrate iodine for thyroglobulin hormone production. High activities of radioiodine (131I) may be delivered via this mechanism leading to tumor cell death by beta particle irradiation. A more recent Food and Drug Administration (FDA) approved RPT, that also concentrates by an endogenous mechanism, is Xofigo™ (223Ra), an alpha-particle emitter that acts as a calcium mimetic and accumulates in blastic bone matrix (i.e., bone matrix that is rapidly being deposited) associated with prostate cancer metastases to bone (Parker et al. 2013).
Within the cancer therapy armamentarium, RPT is a treatment modality that shares features of both chemotherapy and targeted biologic therapy (Table 1). Like chemotherapy and in contrast to targeted biologic therapy which primarily inhibits signaling pathways associated with malignancy, RPT kills cells. Unlike chemotherapy, cell killing is based on the expression of tumor-specific markers rather than proliferation.
Table 1.
Features of RPT relative to chemotherapy and biologic therapy.
| Current cancer therapies |
| After the cancer has spread/metastasized |
| • Chemotherapy -Kill rapidly proliferating cells |
| – Kill rapidly proliferating cells |
| • Radiopharmaceutical Therapy-Kill targeted cells by localized radiation delivery |
| – Kill targeted cells by localized radiation delivery |
| • Targeted Biologic Therapy (hormonal Tx) |
| – Inhibit signaling pathways that tumor cells are addicted to (i.e., rely on to maintain cancer phenotype) |
| • Immunotherapy |
| – Overcome immune tolerance to cancer |
The number of RPTs available or under investigation has been increasing for the past several years (Table 2). The FDA approval and market success of Xofigo™ demonstrated that alpha-emitter based RPTs (αRPT) can be successful. RPT using beta particle emitters (e.g., 131I,177Lu, 90Y) have accumulated a substantial clinical experience but much of this has been outside of rigorous clinical trials. This has made their efficacy relative to other treatment modalities difficult to judge. However, the preponderance of positive clinical outcomes when other treatment options have been exhausted has prompted pharmaceutical companies to initiate FDA registration trials towards commercialization of these agents. The recent FDA approval of 177Lu-labeled anti-somatostatin peptide RPT, Lutathera™, for the treatment of somatostatin receptor positive gastroenteropancreatic neuroendocrine tumors is one such example (Strosberg et al. 2017).
Table 2.
RPT agents that are available or under investigation
| RPT agent | Company | Indication |
|---|---|---|
| 131I-radioiodine | Malincrodt/Jubilant Draximage | Thyroid cancer |
| 131I -MIBG | Progenics | Adrenergic+ tumors |
| 212Pb-trastuzumab | ArevaMed | HER2+ tumors |
| 212Pb -aSSR | ArevaMed/Radiomedix | Somatostatin+ tumors |
| 212Pb -aTEM1 | ArevaMed/Morphotek | TEM1+ tumors |
| 212Pb -aCD37 | ArevaMed/NordicNanovector | Leukemia |
| 131I -aCD45 | Actinium Pharmaceuticals | BM xplant prep |
| 225Ac-aCD33 | Actinium Pharmaceuticals | Leukemia |
| 90Y-microspheres | Varian/Sirtex | Hepatic malignancies |
| 90Y -microspheres | BTG | Hepatic malignancies |
| Lutathera (177Lu) | Novartis/AAA | Somatostatin+ tumors |
| 177Lu -aPSMA-R2 | Novartis/AAA | Prostate, tumor neovasc. |
| 177Lu -NeoBOMB1 | Novartis/AAA | Bombesin+ tumors |
| Xofigo (223Ra) | Bayer | Bone mets |
| HER2-TTC (227Th) | Bayer | HER2+ tumors |
| PSMA-TTC (227Th) | Bayer | Prostate, tumor neovasc. |
| MSLN-TTC (227Th) | Bayer | Mesothelin+ tumors |
| CD22 TTC (227Th) | Bayer | Lymphoma |
| FPX-01 (225Ac) | J&J/Fusion Pharma | NSCLC, pan-cancer target |
All of the RPTs listed on Table 2 may be imaged. In some cases, a surrogate of the therapeutic agent may be used for imaging. Imaging may be used to evaluate tumor accumulation of the RPT, to assess early response to therapy by evaluating the reduction in RPT tumor accumulation, and to perform dosimetry calculations for patient RPT treatment planning. Dosimetry for RPT requires quantitative evaluation of the agents’ biodistribution over time. A three-dimensional (3D) imaging method such as single-photon computed tomography, combined with computed tomography (SPECT/CT) (Dewaraja et al. 2012, 2013; Ljungberg et al. 2016) and a dosimetry calculation that accounts for differences in tissue density and RPT nonuniformity for the treated patient (Kolbert et al. 2007; Prideauz et al. 2007; Hobbs et al. 2009; Dewaraja et al. 2010; Dieudonne et al. 2010; Senthamizhchelvan et al. 2012) provides the highest level of quantitative accuracy needed for RPT dosimetry and therefore the greatest likelihood that the dosimetry calculation will relate to biological outcome (organ toxicity or tumor control).
A 3D imaging-based dosimetry approach may also be used to plan combination RPT and external beam radiotherapy (Bodey et al. 2003, 2004; Hobbs et al. 2011a). The possibility of planning a treatment that combines complementary RPTs such as short-range αRPT with beta-particle RPT or with RPTs that have different half-lives and pharmacokinetics would also require patient-specific imaging-based treatment planning dosimetry (Hobbs et al. 2013). Although one might expect that imaging-based dosimetry would be limited by the resolution of the imaging instrumentation, it is possible to overcome this limitation. By combining imaging-based patient measurements with idealized model representations of the anatomy derived from a priori knowledge, the scale of the absorbed dose calculation need not depend upon the resolution of the anatomical imaging modality. An example of this is provided by imaging-based dosimetry of the arterial wall absorbed dose in lymphoma patients (Hobbs et al. 2010). By pairing human imaging-based measurements with preclinical microscale measurements of the activity distribution, the same conceptual approach leads to a macro-to-micro method that can provide absorbed dose estimates to microscale structures (Hobbs et al. 2012).
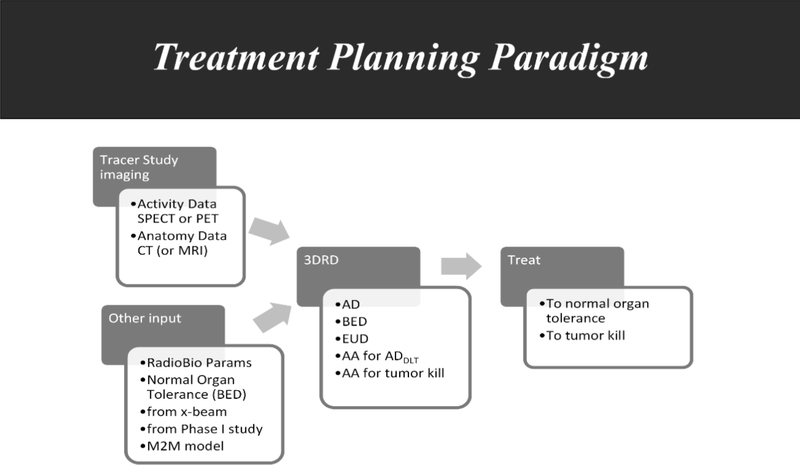
Fig. 1 summarizes how radiobiology may be coupled with patient imaging and with the dosimetric techniques referenced above to implement RPT therapy on a dosimetry-driven treatment planning basis rather than the empirically driven approach that must necessarily be adopted in chemotherapy and biologic therapy.
Fig. 1.
In a treatment planning approach to RPT delivery, data from imaging (e.g., using a pre-therapy tracer study) may be combined with a priori information regarding target and normal organ tissues (“other input”) to obtain a number of dosimetry parameters [e.g., the tolerance dose expressed in biologically effective dose (BED)] for a 3D dosimetry [e.g., using 3D-RD (Prideaux et al. 2007; Hobbs et al. 2008; Sgouros et al. 2008)] calculation that may provide a number of dosimetric parameters, including absorbed dose (AD), BED, and equivalent uniform dose (EUD) (Niemierko 1997; Hobbs et al. 2011b) so that the administered activity (AA) for tumor kill or for the dose-limiting tissue (DLT) may be calculated and then used to treat to normal organ tolerance or tumor kill.
Acknowledgments and Conflicts of Interest
Work supported by NIH/NCI grants R01 CA116477. Dr. Sgouros is founder of Rapid, LLC, a dosimetry services startup. His involvement with this company is managed according to Johns Hopkins conflict of interest policies.
REFERENCES
- Bodey RK, Flux GD, Evans PM. Combining dosimetry for targeted radionuclide and external beam therapies using the biologically effective dose. Cancer Biother Radiopharm 18(1):89–97; 2003. [DOI] [PubMed] [Google Scholar]
- Bodey RK, Evans PM, Flux GD. Application of the linear-quadratic model to combined modality radiotherapy. Int J Radiat Oncol Biol Phys 59(1):228–241; 2004. [DOI] [PubMed] [Google Scholar]
- Dewaraja YK, Schipper MJ, Roberson PL, Wilderman SJ, Amro H, Regan DD, Koral KF, Kaminski MS, Avram AM. 131I-tositumomab radioimmunotherapy: initial tumor dose–response results using 3-dimensional dosimetry including radiobiologic modeling. J Nucl Med 51(7):1155–1162; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewaraja YK, Frey EC, Sgouros G, Brill AB, Roberson P, Zanzonico PB, Ljungberg M. MIRD pamphlet No. 23: quantitative SPECT for patient-specific 3-dimensional dosimetry in internal radionuclide therapy. J Nucl Med 53(8):1310–1325; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewaraja YK, Ljungberg M, Green AJ, Zanzonico PB, Frey EC; SNMMI MIRD Committee, Bolch WE, Brill AB, Dunphy M, Fisher DR, Howell RW, Meredith RF, Sgouros G, Wessels BW. MIRD Pamphlet No. 24: guidelines for quantitative 131I SPECT in dosimetry applications. J Nucl Med 54(12):2182–2188; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieudonne A, Hobbs RF, Bolch WE, Sgouros G, Gardin I. Fine-resolution voxel S values for constructing absorbed dose distributions at variable voxel size. J Nucl Med 51(10):1600–1607; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs R et al. Salivary gland Monte Carlo-based 3D-radiobiological dosimetry (3D-RD) in thyroid cancer patients from 124I PET images In: EANM’08: annual congress of the European Association of Nuclear Medicine. Vienna, Austria: EANM; 2008. [Google Scholar]
- Hobbs RF, Wahl RL, Lodge MA, Javadi MS, Cho SY, Chien DT, Ewertz ME, Esaias CE, Ladenson PW, Sgouros G. 124I PET-based 3D-RD dosimetry for a pediatric thyroid cancer patient: real-time treatment planning and methodologic comparison. J Nucl Med 50(11):1844–1847; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs RF, Baechler S, Wahl RL, He B, Song H, Esaias CE, Sgouros G. Arterial wall dosimetry for non-Hodgkin lymphoma patients treated with radioimmunotherapy. J Nucl Med 51(3):368–375; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs RF, McNutt T, Baechler S, He B, Esaias CE, Frey EC, Loeb DM, Wahl RL, Shokek O, Sgouros G. A treatment planning method for sequentially combining radiopharmaceutical therapy and external radiation therapy. Int J Radiat Oncol Biol Phys 80(4):1256–1262; 2011a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs RF, Baechler S, Fu DX, Esaias C, Pomper MG, Ambinder RF, Sgouros G. A model of cellular dosimetry for macroscopic tumors in radiopharmaceutical therapy. Med Phys 38(6):2892–2903; 2011b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs RF, Song H, Huso DL, Sundel MH, Sgouros G. A nephron-based model of the kidneys for macro-to-micro α-particle dosimetry. Phys Med Biol 57(13):4403–4424; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs RF, Wahl RL, Frey EC, Kasamon Y, Song H, Huang P, Jones RJ, Sgouros G. Radiobiologic optimization of combination radiopharmaceutical therapy applied to myeloablative treatment of non-Hodgkin lymphoma. J Nucl Med 54(9):1535–1542; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolbert KS, Pentlow KS, Pearson JR, Sheikh A, Finn RD, Humm JL, Larson SM. Prediction of absorbed dose to normal organs in thyroid cancer patients treated with 131I by use of 124I PET and 3-dimensional internal dosimetry software. J Nucl Med 48(1):143–149; 2007. [PubMed] [Google Scholar]
- Ljungberg M, Celler A, Konijnenberg MW, Eckerman KF, Dewaraja YK, Sjögreen-Gleisner; SNMMI MIRD Committee, Bolch WE, Brill AB, Fahey F, Fisher DR, Hobbs R, Howell RW, Meredith RF, Sgouros G, Zanzonico P; EANM Dosimetry Committee, Bacher K, Chiesa C, Flux G, Lassmann M, Strigari L, Walrand S. MIRD pamphlet no. 26: joint EANM/MIRD guidelines for quantitative 177Lu SPECT applied for dosimetry of radiopharmaceutical therapy. J Nucl Med, 2016. 57(1): p. 151–62. [DOI] [PubMed] [Google Scholar]
- Niemierko A Reporting and analyzing dose distributions: a concept of equivalent uniform dose. Med Phys 24(1):103–110; 1997. [DOI] [PubMed] [Google Scholar]
- Parker C, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, Fosså SD, Chodacki A, Wiechno P, Logue J, Seke M, Widmark A, Johannessen DC, Hoskin P, Bottomley D, James ND, Solberg A, Syndikus I, Kliment J, Wedel S, Boehmer S, Dall’Oglio M, Franzén L, Coleman R, Vogelzang NJ, O’Bryan-Tear CG, Staudacher K, Garcia-Vargas J, Shan M, Bruland ØS, Sartor O. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 369(3):213–223; 2013. [DOI] [PubMed] [Google Scholar]
- Prideaux AR, Song H, Hobbs RF, He B, Frey EC, Ladenson PW, Wahl RL, Sgouros G. Three-dimensional radiobiologic dosimetry: application of radiobiologic modeling to patient-specific 3-dimensional imaging-based internal dosimetry. J Nucl Med 48(6):1008–1016; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senthamizhchelvan S, Hobbs RF, Song H, Frey EC, Zhang Z, Armour E, Wahl RL, Loeb DM, Sgouros G. Tumor dosimetry and response for 153Sm-ethylenediamine tetramethylene phosphonic acid therapy of high-risk osteosarcoma. J Nucl Med 53(2):215–224; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgouros G, Frey E, Wahl R, He B, Prideaux A, Hobbs R. Three-dimensional imaging-based radiobiological dosimetry. iSemin Nucl Med 38(5):321–334; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, Mittra E, Kunz PL, Kulke MH, Jacene H, Bushnell D, O’Dorisio TM, Baum RP, Kulkarni HR, Caplin M, Lebtahi R, Hobday T, Delpassand E, Van Cutsem E, Benson A, Srirajaskanthan R, Pavel M, Mora J, Berlin J, Grande E, Reed N, Seregni E, Öberg K, Lopera Sierra M, Santoro P, Thevenet T, Erion JL, Ruszniewski, Kwekkeboom D, Krenning E Phase 3 trial of 177Lu-dotatate for midgut neuroendocrine tumors. N Engl J Med. 376(2):125–135; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]